Submitted:
07 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
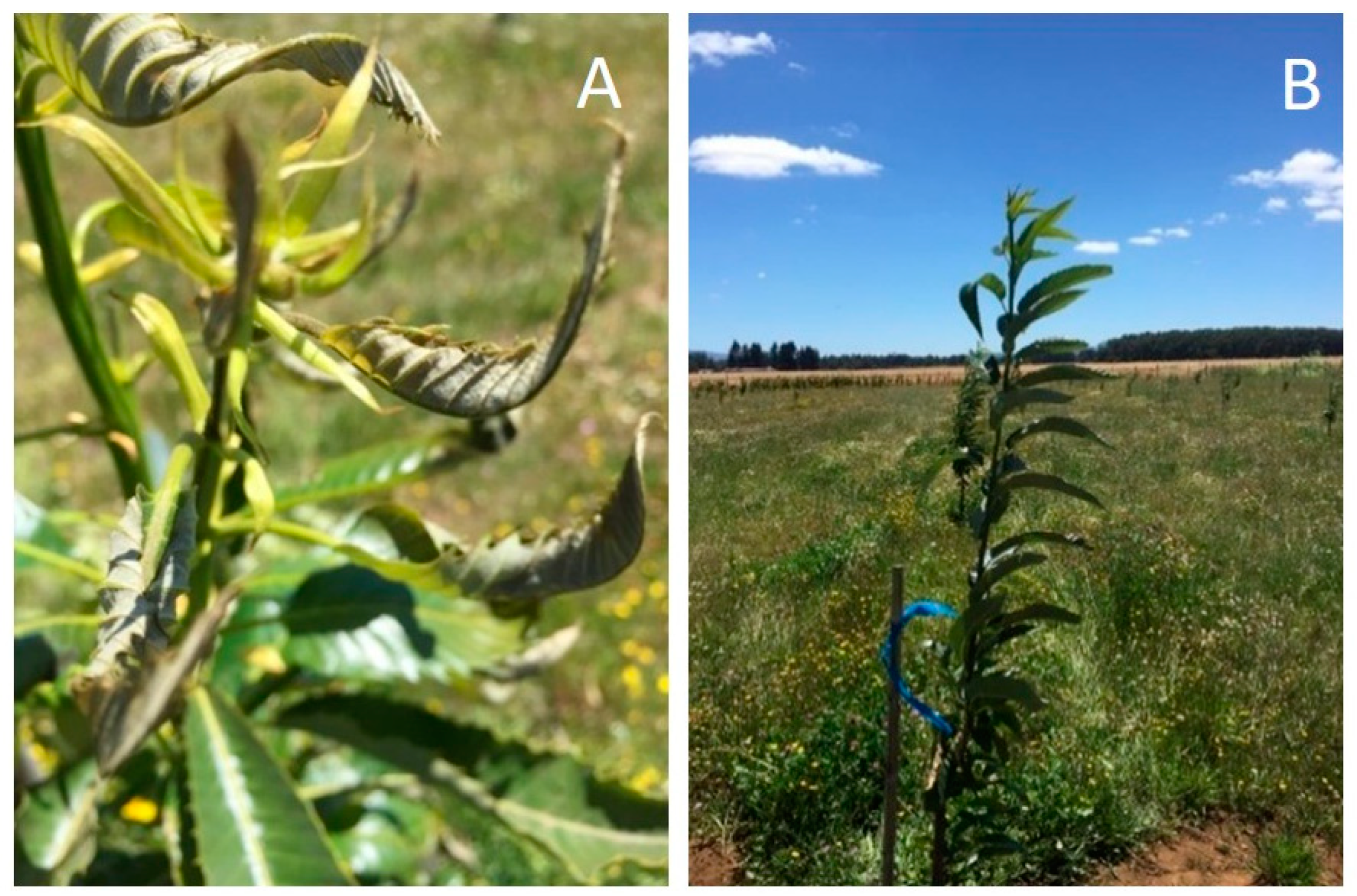
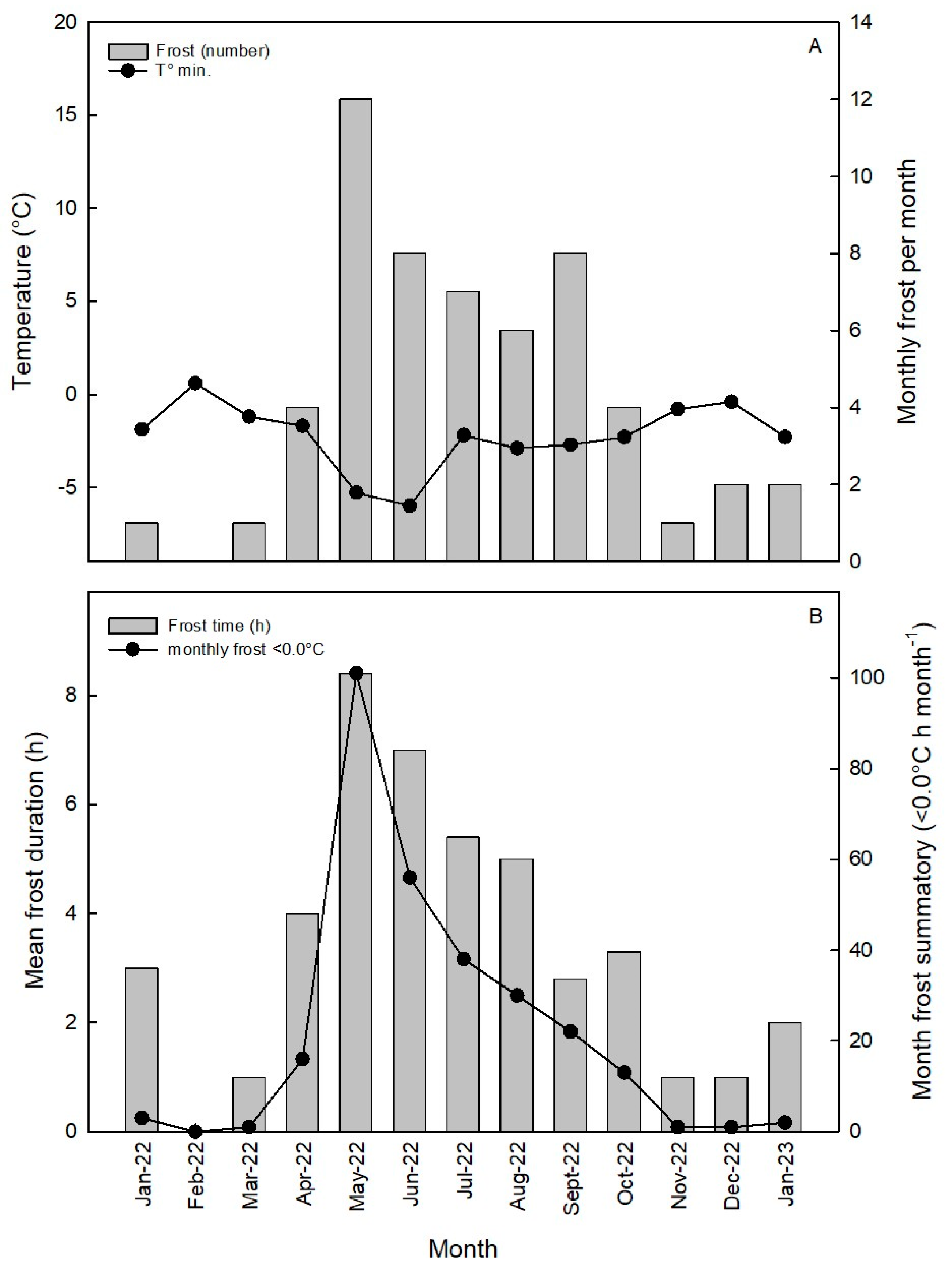
2.1. Experimental Site and Treatments
2.2. Vegetative Growth
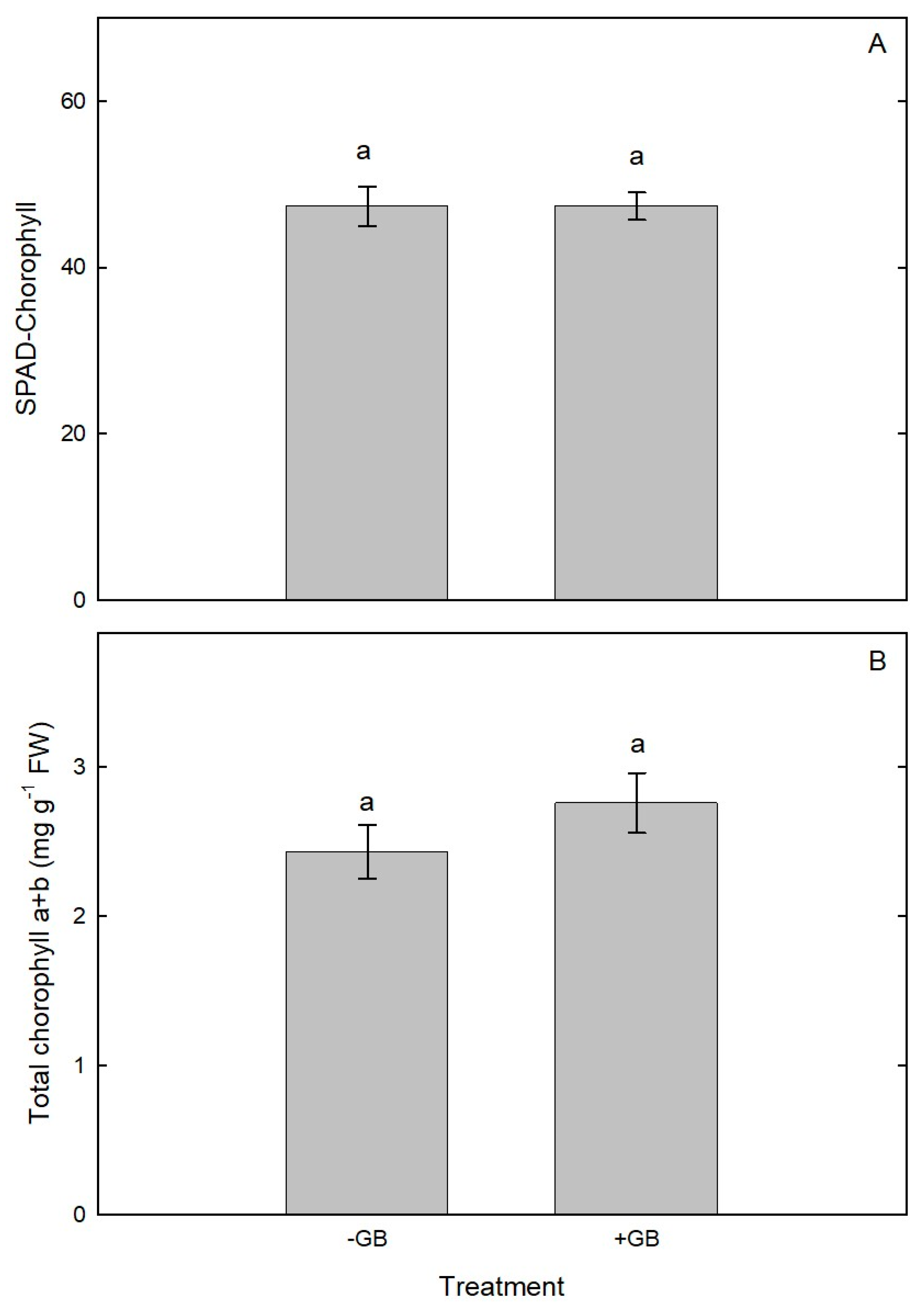
2.3. SPAD-Chlorophyll and Total Chlorophyll Content
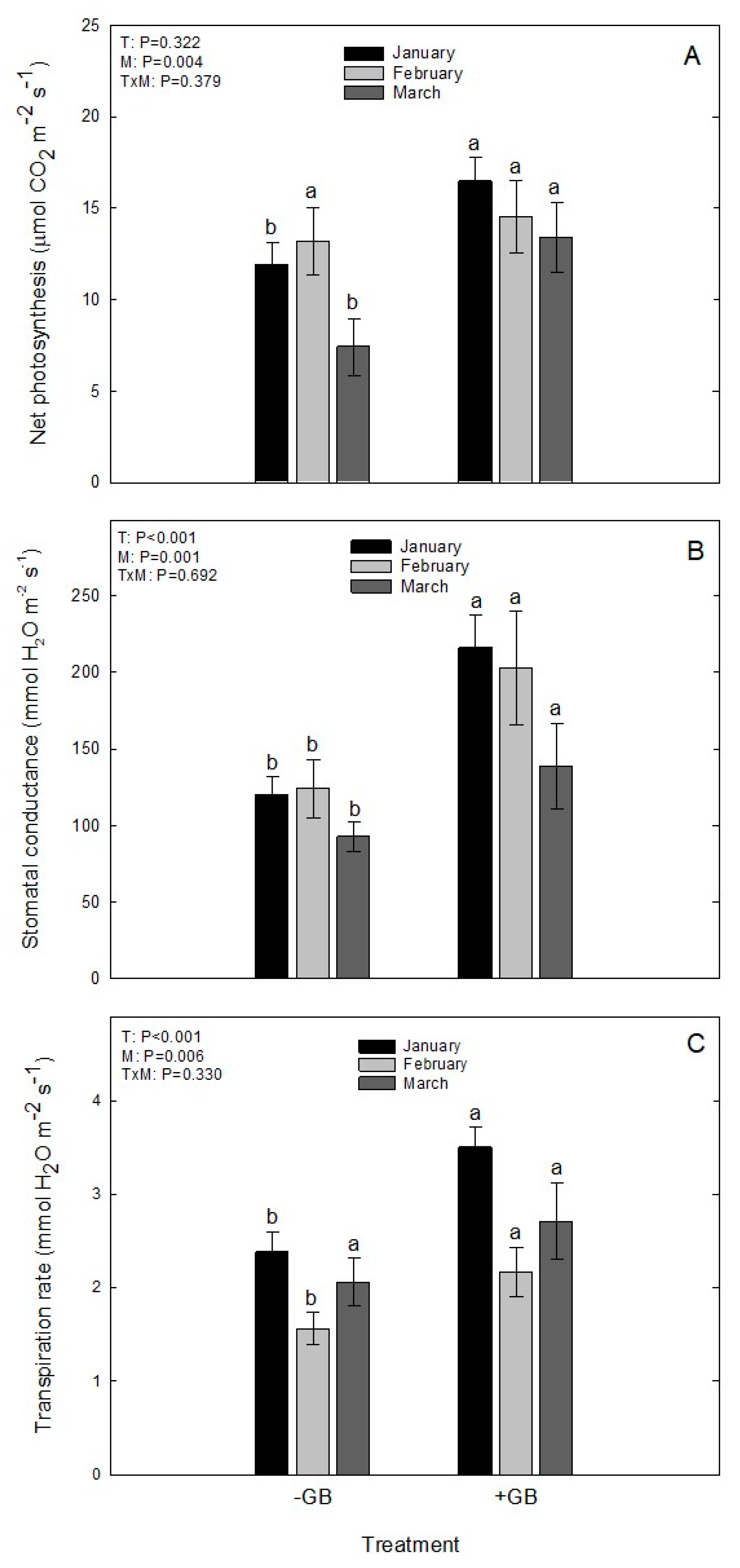
2.4. Net Photosynthesis, Stomatal Conductance and Transpiration
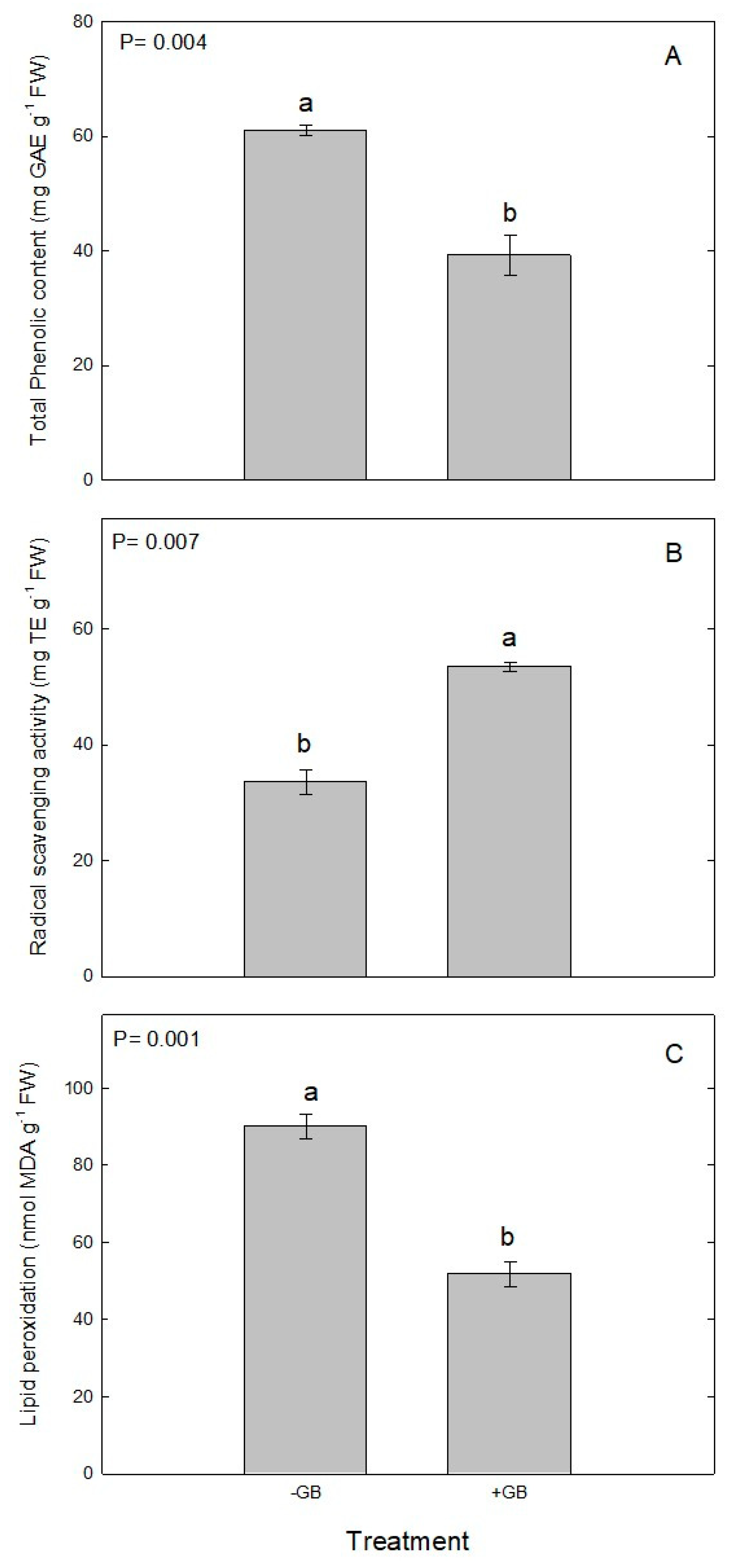
2.5. Total Phenols, Radical Scavenging Activity and Lipid Peroxidation
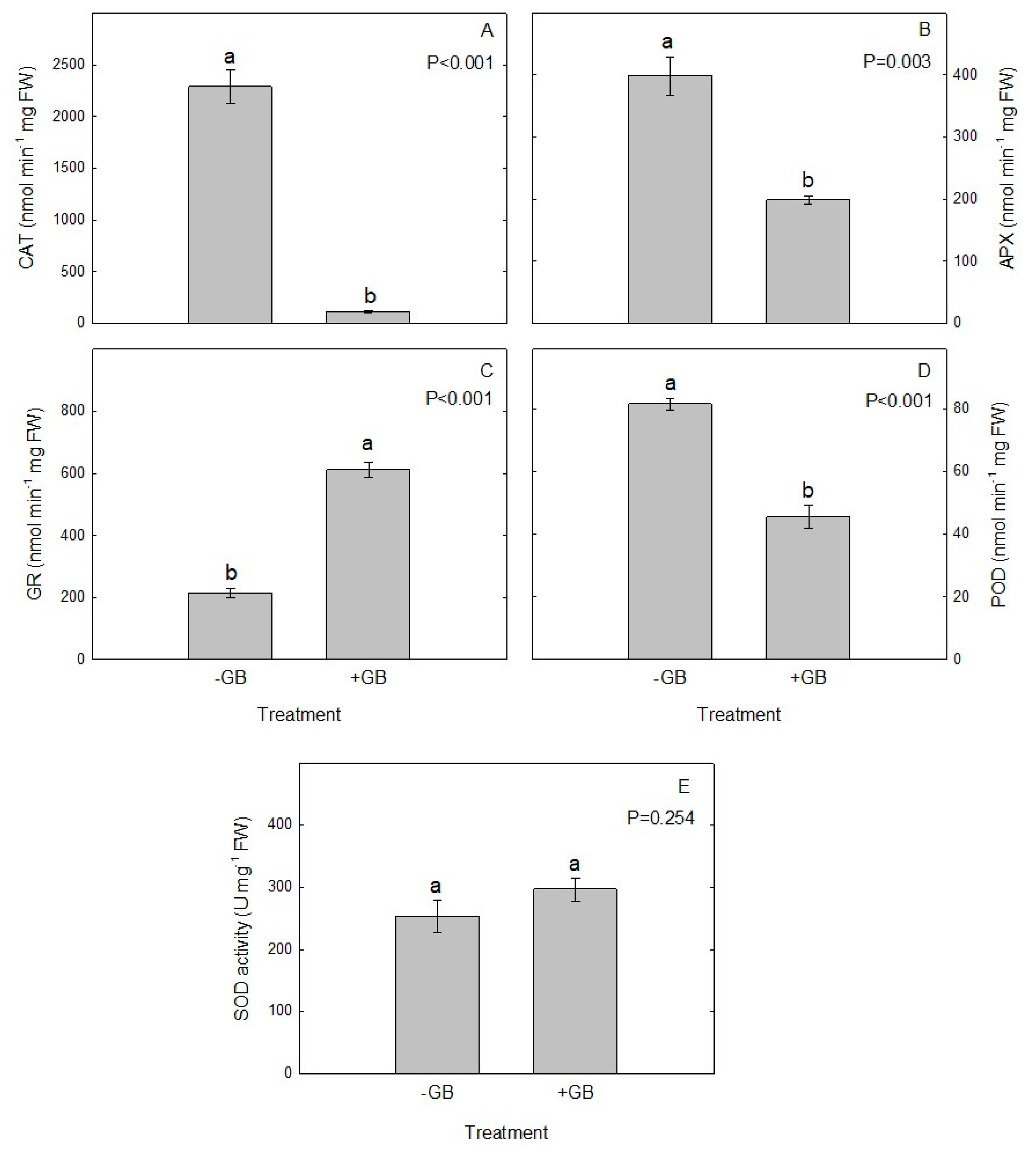
2.6. Antioxidant Enzymes
2.7. Experimental Design and Statistical Anayles
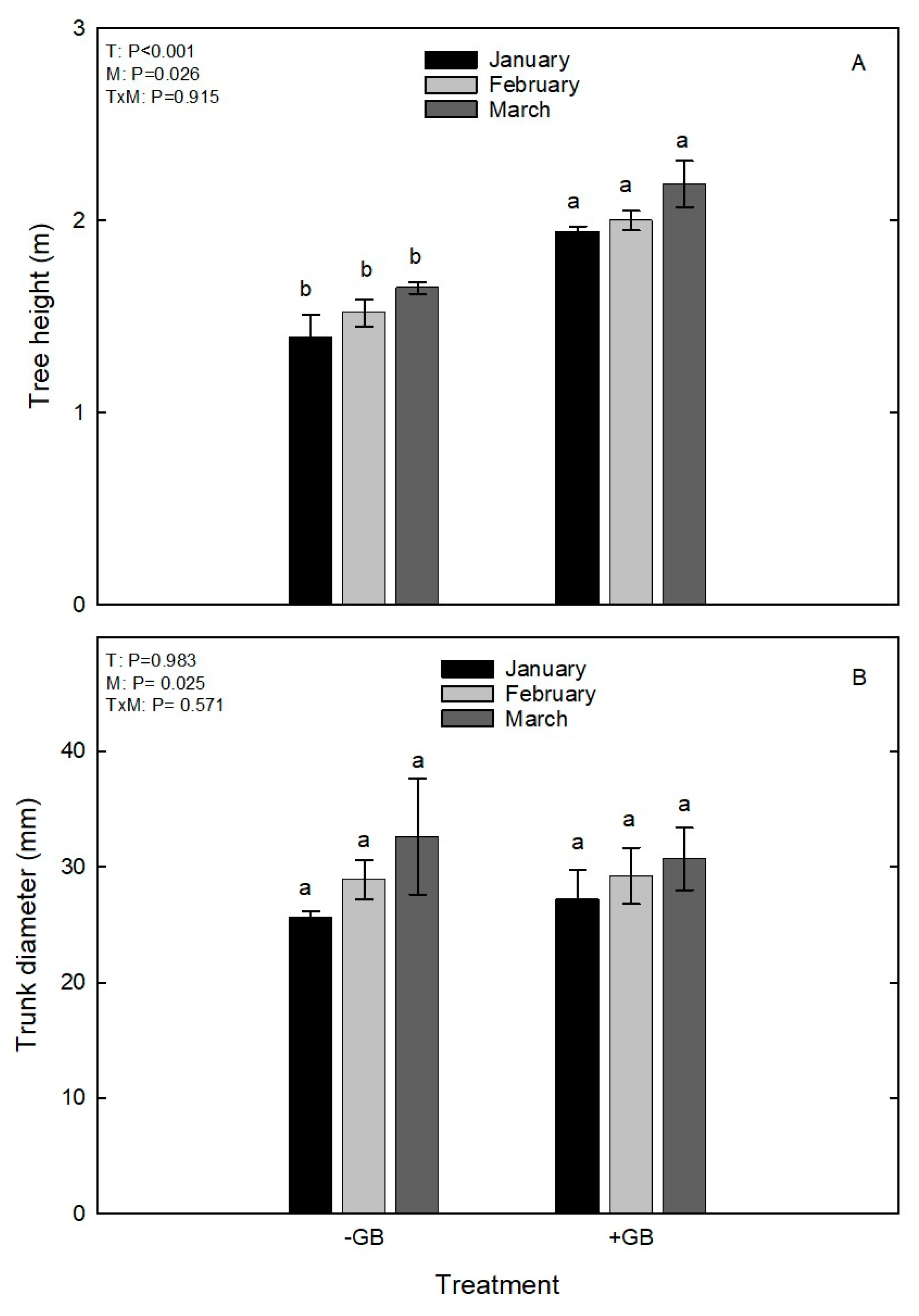
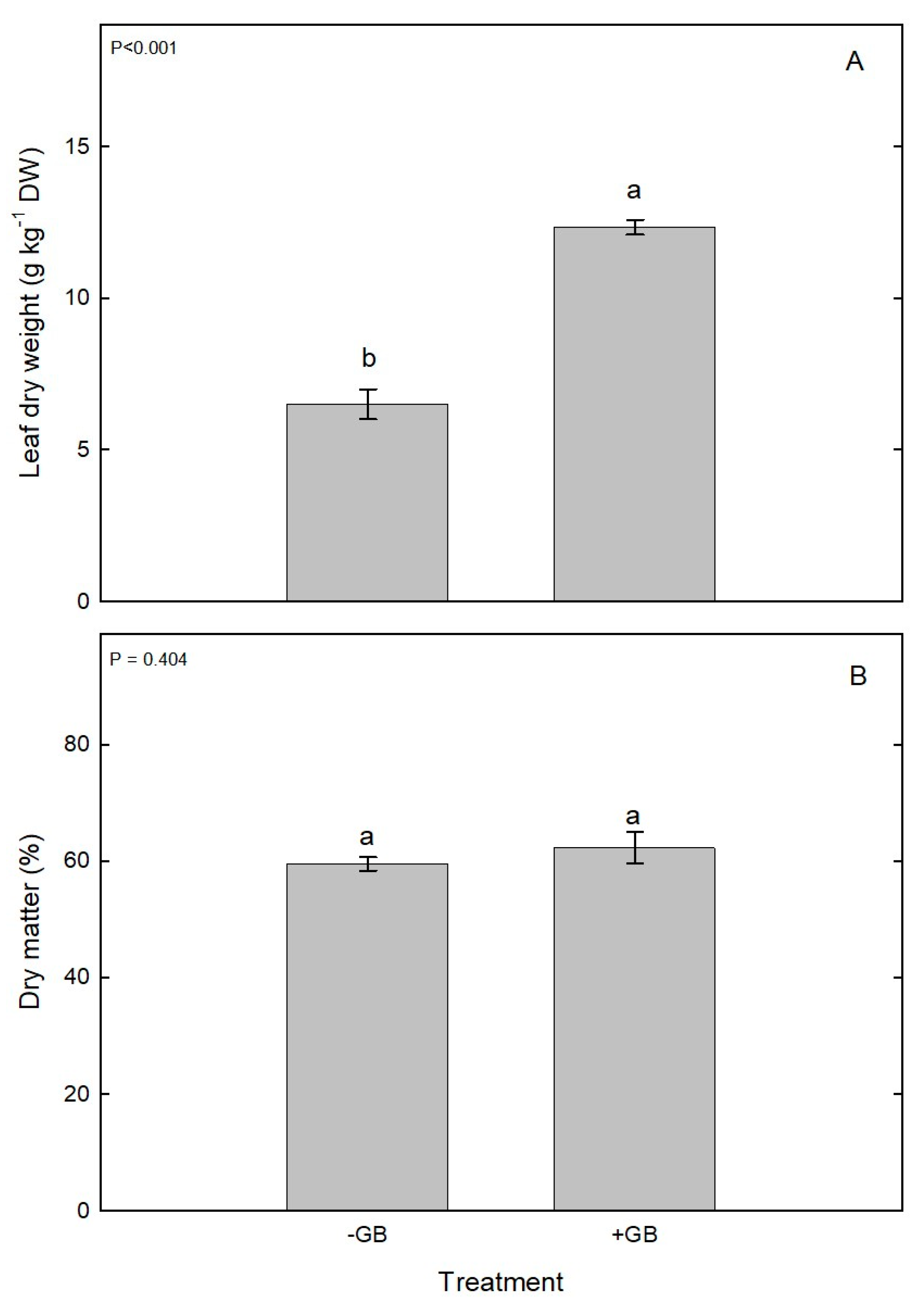
3. Results and discussion
4. Conclusions
Conflicts of Interest Statement
Author Contributions statement
Acknowledgments
References
- Jalili, I.; Ebadi, A.; Ashari, M.; Kalatehjari, S.; Ali-Aazami, M. Foliar application of putrescine, salicylic acid and ascorbic acid mitigates frost stress damage in Vitis vinifera c.v ´Giziluzum. BMC Plant Biol. 2023, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Mechanism of freeze‒thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant. Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarnillo, F.; Shigematsu, S.; Minami, A.; Kawamura, Y. Spatial and temporal profile of glycine‒betaine accumulations in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Li, P.H.; Chen, T.H. Glycine betaine increases chilling tolerance and reduces chilling‒induced lipid peroxidation in Zea mays L. Plant Cell Environ. 2000, 23, 609–618. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Kröl, M. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Koster, K.L.; Lynch, D.V. Solute accumulation and compartmentation during the cold acclimation of Puma rye. Plant Physiol. 1992, 88, 108–113. [Google Scholar] [CrossRef]
- Zulfiqr, F.; Ashraf, M.; Siddique, K.H. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Aras, S.; Eşitken, A. Effects of antifreeze proteins and glycine betaine on strawberry plants for resistance to cold temperature. IPCBEE 2013, 60, 21. [Google Scholar]
- Meriño-Gergichevich, C.; Luengo-Escobar, A.; Alarcón, D.; Reyes-Díaz, M.; Ondrasek, G.; Morina, F.; Ogass, K. Combined spraying of boron and zinc during fruit set and premature stage improves yield and fruit quality of European hazelnut cv. Tonda di Giffoni. Frontiers in Plant Science 2021, 12, 984. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorohyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Slimkard, K.; Singleton, V.L. Total, phenol analysis: Automation and comparison with manual methods. Am. J Enol. Vitic. 1997, 28, 29–55. [Google Scholar]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavening propierties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1556–1570. [Google Scholar] [CrossRef]
- Aguilera, A.; Tereucán, G.; Ercoli, S.; Cornejo, P.; Gomez, M.R.; Uhlmann, L.; Ruiz, A. Influence of organic and chemical fertilisation on antioxidant compounds profiles and activities in fruits of Fragaria ananassa var. Camarosa. J. Soil Sci. Plant Nutr. 2020, 20, 715–724. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Aires, A.; Anjos, R.; Martins, L.; Pinto, T.; Peixoto, F.; Gomes-Laranjo, J. The role of silicon fertilization in the synthesis of phenolic compounds on chestnut plants infected with P. cinnamomi and C. parasitica. J. Plant Dis. Protect. 2020, 127, 211–227. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against stress: Mechanism and biotechnological application. Plant. Cell. Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhang, J.; Jing, L.; Xiaping, G.; Ma, H.; Yanqiang, G.; Chang, Y.; Xie, J. Effects of exogenous glycine betaine and cycloleucine on photosynthetic capacity, amino acid composition, and hormone metabolism in Solanum melongena L. Sci. Rep. 2023, 13, 7626. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Parrey, Z.A.; Shah, S.H.; Mohammed, F. Glycine betaine mediated changes in growth, photosynthetic efficiency, antioxidant system, yield and quality of mustard. Scientia Hortic. 2021, 285, 110170. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Z.; Lu, C. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 2005, 138, 2299–2309. [Google Scholar] [CrossRef]
- He, C.; Zhang, W.; Gao, Q.; Fang, A. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytia 2011, 177, 151–167. [Google Scholar] [CrossRef]
- Che-um, S.; Samphumphiang, T.; Kirddmanee, C. Glycinebetaine alleviates water defficit stress in indica rice using proline accumulation, photosynthetic efficiencies, growth performances and yield attributes. Aust. J. Crop Sci. 2013, 7, 213–218. [Google Scholar]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C. Transgenic poplar expressing codA exhibits enhanced growth and abiotic stress tolerance. Plant Physiol Biochem. 2016, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Manaf, H.H. Benefical effects of exogenous selenium, glycine betaine and seawed extract on salt stressed cowpea plant. Ann Agric Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Ami, A.S.; Wang, S.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.). seedlings. BMC Plant Biol. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, W.; Wang, C. Genetic engineering of the biosyntesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. 2017, 257, 74–83. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, J.; Wang, M. Genetic engineering of the biosynthesis of glycinebetaine enhances the fruit development and size of tomato. Plant Sci. 2019, 280, 355–366. [Google Scholar] [CrossRef]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of elevated ion accumulation, oxidative stress, and lipid peroxidation with salycilic acid-induced accumulation of glycine betaine in salinity-exposed Vigna radiata L. Apply Biochem Biotechnol. 2021, 193, 3301–3320. [Google Scholar] [CrossRef]
- Castro-Duque, N.E.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar glycine betaine or hydrogen peroxide sprays ameliorate waterlogging stress in cape gooseberry. Plants 2020, 9, 644. [Google Scholar] [CrossRef]
- Shams, M.; Yildirim, E.; Ekini, M.; Turan, M.; Dursun, A.; Parlakova, F.; Kul, R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hort. Environ. Biotech. 2016, 57, 225–231. [Google Scholar] [CrossRef]
- Molei, S.; Rabiei, V.; Soleinami, A.; Razave, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv. Malese Saveh during cold storage. J. Food Process Preserv. 2021, 45, e15315. [Google Scholar]
- Khedr, R.A.; Sorour, S.G.R.; Aboukhadrah, S.H.; El Shafey, N.M.; Abd Elsalam, H.E.; El-Sharnouby, M.E.; El-Tahan, M. Alleviation of salinity stress effects on agro-physiological traits of wheat by auxin, glycine betaine, and soil additives. Saudi J. Biol Sci. 2022, 29, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, Y.; Li, K.; Zhou, Y.; Zhang, M.; Li, Z. Exogenous application of glycine betaine improved water use efficiency in winter wheat (Triticum aestivum L.) via modulating photosynthetic efficiency and antioxidative capacity under conventional and limited irrigation conditions. Crop J. 2019, 7, 635–650. [Google Scholar] [CrossRef]
- Min, K.; Cho, Y.; Kim, E.; Lee, M.; Lee, S.R. Exogenous glycine betaine application improves freezing tolerance of cabbage (Brassica oleracea L.) leaves. Plants 2021, 10, 2821. [Google Scholar] [CrossRef] [PubMed]
- Shemi, R.; Wang, R.; Geith, E.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Scientific Reports 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.A.; de Souza, K.R.D.; de Olivera Santos, M.; da Silva, D.M.; Alves, J.D. Hydrogen peroxide promotes the tolerance of soybean to waterlogging. Sci. Hortic. 2018, 232, 40–45. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Shomo, M.; Tobita, S. Effects of proline and betaine in heat inactivation of ribulose-1, 5-bisphosphate carboxylase/oxigenase in crude extract of rice seedlings. Photosynthetica 2000, 36, 557–563. [Google Scholar] [CrossRef]
- Palonem, P.; Buszard, D.; Donnelly, D. Changes in carbohydrates and freezing tolerance during cold acclimation of red raspsberry cultivars grown in vitro and in vivo. Physiol. Plant 2002, 110, 393–401. [Google Scholar] [CrossRef]
- Giri, J. Glycine betaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1745–1751. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the bioma scale. Front. Ecol. Evol 2018, 6, 64. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




