Introduction
Gastric cancer (GC) is a highly aggressive and heterogeneous tumor. In connection, it remains a therapeutic problem despite new treatment options [
1]. GC is the 5th most common cancer worldwide. There were more than 1 million new cases of GC in 2020. Men are almost twice as likely to suffer from GC than women. Moreover, it is the 4th most common cancer in men and the 7th most common cancer in women [
2]. Several factors are known to be associated with an increased risk of GC, there are smoking, Helicobacter pylori infection and exposure to industrial chemical [
3]. Treatment of GC depends on the clinical stage and is based on surgery, chemotherapy, or radiochemotherapy. The preoperative and postoperative examination of lymph node metastases in GC is important as it determines the use of perioperative or postoperative chemotherapy [
4]. Furthermore, the presence of lymph node metastases is associated with a worse prognosis [
5].
Lymphovascular invasion (LVI) plays a key role in metastasis to regional lymph nodes and distant organs [
6,
7]. LVI is defined as invasion of blood or lymphatic vessels by tumor cells and/or tumor embolism in an endothelium-lined space. They constitute an independent risk factor for metastases, recurrences, and mortality [
7]. Pre-operative assessment of lymph node staging is based on diagnostic imaging, CT scans being the most used for this purpose [
8]. Postoperative assessment depends on surgical technique and appropriate pathological examination of the surgical specimen. The surgical treatment of GC remains controversial. Gastrectomy is performed as an open procedure (OG). Recently, minimally invasive methods (MIS) have become more and more common, i.e. laparoscopic gastrectomy (LAG) and robot-assisted gastrectomy (RG). Data show that MIS procedures have lower mortality, faster recovery, and better aesthetic results [
9,
10,
11]. To date, the minimum number of nodes to be harvested during surgery has not been established [
12,
13]. The current classification of the Union for International Cancer Control (UICC) only recommends the presence of at least 16 lymph nodes to accurately assess the pathological grade of GC [
14,
15,
16]. Endoscopic resection may be used for early GC. This method has even more positives compared to minimally invasive methods. Endoscopic resection of early GC does not require long hospital stays, has fewer complications, and is therefore more economical. However, it requires very precise qualification of the patient, as no lymph nodes are collected during the endoscopic procedure, which may lead to incorrect staging and incorrect therapeutic procedures, which will worsen the patient's prognosis [
17,
18].
LVI assessment could be helpful in assessing the likelihood of lymph node metastasis and assessing the collection of the correct number of lymph nodes during gastrectomy. Moreover, in GC in early stage, if will use endoscopic resection without collecting lymph nodes. Therefore, the LVI assessment could indicate whether there is a need to resect lymph nodes during a endoscopic procedure and for example conversion to gastrectomy. Our analysis will allow us to approximate and understand the above-described value of LVI assessment during gastrectomy.
Materials and Methods
We collected 146 histologically confirmed LAGC patients (pathological stage with or without NAC p/ypT1, p/ypT2, p/ypT3, p/ypT4, p/ypN0, p/ypN1, p/ypN2, p/ypN3 and p/ypM0 on admission) after gastrectomy between Jan. 2018 and Dec. 2023. The inclusion criterion was patients with histologically confirmed GC and absence of distant metastases on admission, who underwent 3-4 cycles of NAC followed by gastrectomy with lymph node dissection at our institution or only gastrectomy without NAC. The exclusion criteria were as follows: incomplete pathological data and metastasis to other solid organs. Finally, 31 patients with GC were included in the analysis. The subjects were divided into three groups. The first group included all patients (
Table 1), the second group included 20 patients who received NAC followed by gastrectomy (
Table 1), and the last group was 11 patients without NAC before gastrectomy (
Table 1). Studies were carried out on a group of 16 men and 15 women with GC. Patients' ages ranged from 33 to 80 years, and the mean age of patients was 63,26 years.
Statistical Analysis
The statistical analyses were performed using Statistica v13.3 (Statsoft) and Microsoft Excel 2021. Variables were tested for normality using the Kolmogorov-Smirnov test. The relation intergroup differences were analyzed using the Mann-Witney U Test and the Kruskal-Wallis ANOVA. If applicable, 95% confidence intervals were applied. The correlation was evaluated using Spearman’s rank correlation coefficient. The significance level was set at p<0.05.
Ethical Issues
The study was carried out with the approval of the local ethics committee.
Result
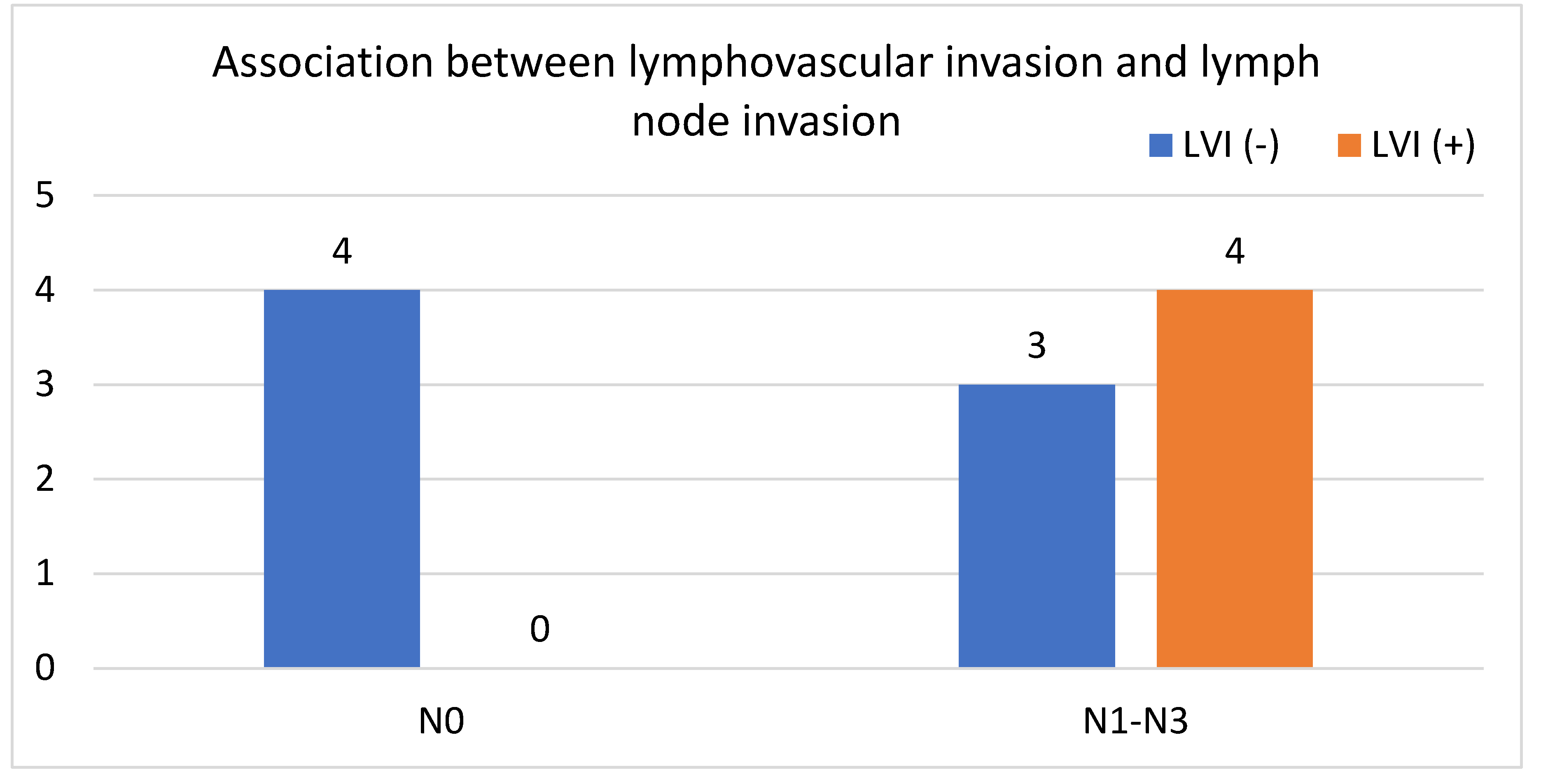
In our study, we analyzed a group of patients with GC after gastrectomy. We analyzed three groups of patients; the first group included all selected patients, the second group included only patients who received NAC and subsequent gastrectomy, and the third group included patients who did not receive NAC. Subsequently, we examined LVI in each group and categorized patients as either LVI (+) or LVI (-) based on histopathology. We then assessed the correlation between the presence of LVI (+) and lymph node metastasis. Furthermore, we assessed the correlation between the presence of LVI (+) and the number of lymph nodes evaluated in patients in each group. In the group of all patients, significantly more LVI (+) than LVI (-) specimens had node metastases (p=0,0003,
Figure 1). There was no statistically significant difference between the number of assessed nodes in both cases (22.4 for LVI (+) and 20.75 for LVI (-), p>0.86). Additionally, there was no statistically significant difference between the ages of patients with and without LVI (65 years vs 62.55 years, p>0.05) (
Table 2). In the second group of GC patients with perioperative NAC before gastrectomy, we showed no significant differences in the number of lymph nodes involved between the LVI (+) and LVI (-) cases (p=0.09). In addition, we showed a strong positive correlation between LVI (+) and the presence of lymph node metastasis (
Figure 2). In the last group of GC patients without NAC before gastrectomy, significantly more LVI (+) specimens had node metastases (p=0,002). In addition, we found a strong positive correlation between LVI (+) and lymph node metastasis (
Figure 3).
Discussion
LVI is an independent predictor of poor prognosis in GC patients, although the pathophysiological behavior of LVI is still unclear. It is still unknown whether LVI affects the long-term survival of GC patients or not. Therefore, this factor is not included as a mandatory standard in the recommendations for diagnostic and therapeutic procedures in GC. Our research should bring us closer to doing the right thing and striving to regulate this issue [
19,
20,
21,
22,
23]. Our results showed that the presence of LVI correlated strongly with lymph node involvement, and the result was statistically significant in the group of all patients and in the group of patients treated with NAC. The difference was not statistically significant in the group not treated by NAC, but this is due to the small number of patients. Furthermore, in our study, we did not find a relationship between the presence of LVI and the number of metastatic lymph nodes.
The current method for assessing LVI is an immunohistochemical examination of postoperative histopathological material with a tumor or after a biopsy with a tumor. This is a very good method to assess the risk of lymph node metastasis in patients with GC, particularly in patients with early GC stage, and an endoscopic method was used [
14,
15,
22]. By assessing the LVI, we can assess the risk of recurrence and possibly extend the surgery to include the removal of the lymph nodes. Interestingly, our study showed no statistical significance in the correlation of LVI and lymph node metastasis in patients who did not receive NAC. However, this study should be extended due to the likely small number of patients. LVI assessment is now standard in the pathology report. However, the prognostic value of LVI in GC is still undefined [
22]. Some data in scientific publications show that LVI is an independent prognostic factor for GC [
23,
24,
25,
26,
27,
28]. Some studies included the assessment of both lymphatic and nerve invasion, which was associated with a significant prognostic impact on disease-free survival and overall survival in patients with GC with stage II or III. The study patients had a worse prognosis compared to patients who did not have lymphatic vessels and nerve invasion [
23,
24]. Additionally, GC patients with coexisting LVI and PNI after preoperative treatment have more advanced disease than patients without these factors or with only one of them [
24]. Lu et al. showed that the invasion of lymphatic vessels is an independent prognostic factor in patients who have no lymph node metastases, i.e., N0 according to the TNM classification. Moreover, in patients with pT3N0M0 stage and LVI (+), adjuvant chemotherapy improves survival [
25]. On the other hand, data indicate that LVI should not be considered a prognostic factor [
28,
29,
30]. Liu et al. showed that lymphatic invasion was not an independent prognostic factor in the case of GC with lymph node involvement, however, in the case of GC with lymph node metastases, LVI had a prognostic significance [
28].
Our research confirms that LVI should be used as a prognostic factor in GC, especially now when we want to use minimally invasive techniques for GC resection. Most data to date regarding LVI have been based on its role as a predictive factor for the eventual selection of effective radical treatment with surgery. Moreover, Fujikawa et al. showed that LVI in GC is an independent prognostic factor, and its impact is particularly strong in advanced cancer with lymph node metastases. These patients may, therefore, require more effective adjuvant therapy [
18]. Our study also confirms this because in patients with LAGC after preoperative NAC administration or without NAC administration, the presence of LVI correlated with the occurrence of lymph node metastases. The group of patients with GC without NAC had no statistical significance, but it could have been related to the small number of patients. This information should lead us to use adjuvant NAC in such patients, even if it turns out that there are no node metastases., because this may be related to too few lymph nodes collected and not to the lack of lymph node metastases.
Interestingly, we did not demonstrate a relationship between the presence of LVI and the number of lymph node metastases. This would mean that the presence of LVI may even suggest the presence of single metastases in lymph nodes or even micrometastases. Additionally, Fujikawa et al. showed that patients with LVI had a higher percentage of people aged 65 years and older. This fact is also confirmed by our research, although no statistical significance was found in the age difference between LVI (+) and LVI (-) patients. The above data clearly confirm the great importance of LVI assessment in GC. However, due to ambiguous data on the use of LVI as a prognostic factor, large-scale international data are needed to clearly resolve this issue and introduce it into diagnostic and therapeutic practice in GC.
Conclusion
The presence of LVI strongly correlated with lymph node involvement, and the result was statistically significant in the group of all patients and in the group of patients treated with NAC. In the group not treated with NAC, the difference was not statistically significant, but this could be due to the small number of patients. Assessment of LVI may help identify potential patients with lymph node metastasis. Due to the ongoing problem of determining the appropriate number of lymph nodes to be harvested during gastrectomy, LVI assessment may be helpful. If LVI is positive and there are no lymph node metastases, it should be considered whether the problem is that too few lymph nodes were collected. Positive LVI may also be a factor suggesting the need for adjuvant NAC. In patients with early GC who underwent endoscopic resection without lymphadenectomy, the LVI assessment may indicate the need to extend the procedure to gastrectomy and/or lymphadenectomy. Our studies clearly indicate the key role of LVI in assessing the presence of lymph node metastases in patients with GC. However, we need large-scale multicentre studies to clearly introduce LVI assessment into the guidelines for the diagnostic and therapeutic management of patients with GC.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest for this article.
References
- Li Liu and Wenhong Deng, Gastric Cancer: Innovations in Screening, Diagnosis and Treatment, J Pers Med. 2023 Jan; 13(1): 127.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249.
- Kwang-Pil Ko, Risk Factors of Gastric Cancer and Lifestyle Modification for Prevention,J Gastric Cancer. 2024 Jan; 24(1): 99–107.
- Wen-Long Guan, Ye He and Rui-Hua Xu, Gastric cancer treatment: recent progress and future perspectives, Journal of Hematology & Oncology volume 16, Article number: 57 (2023).
- Deng JY, Liang H. Clinical Significance of Lymph Node Metastasis in Gastric Cancer. World J Gastroenterol (2014) 20(14):3967–75. [CrossRef]
- Amin MB, Greene FL, Edge SB et al.. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9.
- Fujikawa H, Koumori K, Watanabe H et al., The Clinical Significance of Lymphovascular Invasion in Gastric Cancer. In Vivo. 2020 May-Jun;34(3):1533-1539. [CrossRef] [PubMed]
- Chrysovalantis Vergadis, Dimitrios Schizas, Is Accurate N – Staging for Gastric Cancer Possible?, Front. Surg., 31 May 2018 Sec. Surgical Oncology.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer staging manual, 8. Aufl: Springer International Publishing; 2017.
- Shuo Li, Kecheng Zhang, Lin Chen, Minimally invasive surgery for gastric cancer: Robotic or laparoscopic?, Intelligent Surgery, Volume 6,2023,Pages 31-33, ISSN 2666-6766. [CrossRef]
- Nickel F, Studier-Fischer A, Hausmann D et al., Minimally invasive versus open total Gastrectomy (MEGA): study protocol for a multicentre randomised controlled trial (DRKS00025765). BMJ Open. 2022 Oct 31;12(10):e064286. [CrossRef] [PubMed]
- Dinescu, V.-C., Gheorman, V., Georgescu et al. Uncovering the Impact of Lymphadenectomy in Advanced Gastric Cancer: A Comprehensive Review. Life 2023, 13, 1769. [CrossRef]
- Yong-He Chen, Jun Lu, Run- Cong Nie et al., Retrieval of 30 Lymph Nodes Is Mandatory for Selected Stage II Gastric Cancer Patients, Front Oncol. 2021; 11: 593470.
- Amin MB, Greene FL, Edge SB et al.. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9.
- Stanley, P. Leong, Kamila Naxerova, Laura Keller et al., Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels, Clin Exp Metastasis. 2022; 39(1): 159–179.
- Miftode, S.; Bruns, H. Misclassification of nodal stage in gastric cancer: 16 lymph nodes is not enough. Surg. Exp. Pathol. 2022, 5, 7.
- Park CH, Yang DH, Kim JW et al., Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intest Res. 2021 Apr;19(2):127-157. [CrossRef] [PubMed]
- Yao, K., Uedo, N., Kamada et al., (2020), Guidelines for endoscopic diagnosis of early gastric cancer. Digestive Endoscopy, 32: 663-698. [CrossRef]
- Amin MB, Greene FL, Edge SB et al.. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9.
- Fujikawa H, Koumori K, Watanabe H et al., The Clinical Significance of Lymphovascular Invasion in Gastric Cancer. In Vivo. 2020 May-Jun;34(3):1533-1539. [CrossRef] [PubMed]
- Zhong, YM. , Tong, F. & Shen, J. Lympho-vascular invasion impacts the prognosis in breast-conserving surgery: a systematic review and meta-analysis. BMC Cancer. [CrossRef]
- Lee, J. , Cha, S., Kim, J. et al. Ensemble Deep Learning Model to Predict Lymphovascular Invasion in Gastric Cancer. Cancers 2024, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Liu W, Zhou Z, Dong D, Sun L, Zhang G. Prognostic Value of Lymphovascular Invasion in Node-Negative Upper Urinary Tract Urothelial Carcinoma Patients Undergoing Radical Nephroureterectomy. Yonsei Med J. 2019 Feb;60(2):174-181. [CrossRef]
- Park YS, Kook MC, Kim BH et al., Gastrointestinal Pathology Study Group of the Korean Society of Pathologists. A Standardized Pathology Report for Gastric Cancer: 2nd Edition. J Gastric Cancer. 2023 Jan;23(1):107-145. [CrossRef] [PubMed]
- Hwang JE, Hong JY, Kim JE, et al. Prognostic significance of the concomitant existence of lymphovascular and perineural invasion in locally advanced gastric cancer patients who underwent curative gastrectomy and adjuvant chemotherapy. Jpn J Clin Oncol. 2015; 45(6): 541-546.
- Blumenthaler AN, Newhook TE, Ikoma N, Estrella JS, Blum Murphy M, Das P, Minsky BD, Ajani JA, Mansfield PF, Badgwell BD. Concurrent lymphovascular and perineural invasion after preoperative therapy for gastric adenocarcinoma is associated with decreased survival. J Surg Oncol. 2021 Mar;123(4):911-922. [CrossRef] [PubMed]
- Lu J, Dai Y, Xie JW, et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: results from a high-volume institution. BMC Cancer. 2019; 19(1): 216.
- Zhao B, Huang X, Zhang J, et al. Clinicopathologic factors associated with recurrence and long-term survival in node-negative advanced gastric cancer patients. Rev Esp Enferm Dig. 2019; 111(2): 111-120.
- Zhang F, Chen H, Luo D, Xiong Z, Li X, Yin S, Jin L, Chen S, Peng J, Lian L. Lymphovascular or perineural invasion is associated with lymph node metastasis and survival outcomes in patients with gastric cancer. Cancer Med. 2023 Apr;12(8):9401-9408. [CrossRef] [PubMed]
- Liu E, Zhong M, Xu F, et al. Impact of lymphatic vessel invasion on survival in curative resected gastric cancer. J Gastrointest Surg. 2011; 15(9): 1526-1531.
- Kim JH, Park SS, Park SH, et al. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010; 162(2): 177-183.
- Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014; 17(1): 43-53.
- Fujikawa H, Koumori K, Watanabe H et al., The Clinical Significance of Lymphovascular Invasion in Gastric Cancer. In Vivo. 2020 May-Jun;34(3):1533-1539. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).