1. Introduction
The pathogenesis of myocardial fibrosis is complicated and metabolic changes are important process of myocardial fibrosis [
1,
2]. Metabolic targeting therapy has become an important strategy for fibrosis treatment. Metabolic enzymes are the leading role in the regulation of metabolic remodeling, which not only meet the needs of cell energy metabolism, but also have non-classical metabolic enzyme functions. Among them, the metabolic enzymes in glycolysis process affect cell proliferation and differentiation through non-enzymatic functions, which is particularly interesting[
3,
4]. Previous studies have found that various metabolic enzymes such as hexokinase (HK), pyruvate kinase M2 (PKM2) and pyruvate dehydrogenase kinase 1 (PDK1) can regulate the proliferation, survival and apoptosis of tumor cells through non-enzymatic functions. Our previous study has demonstrated that the post-translation modification and nuclear translocation of PKM2 plays a key role myocardial infarction[
5,
6]. However, Vander Heiden et al. proposed that the glycolysis process of rapidly proliferating cells depends on phosphoglycerate mutase 1(PGAM1) pathway rather than PKM2 activity[
7]. Over activated fibroblasts may bypass PKM2 and continue to proliferate through bypass metabolism by targeting PKM2 alone. In addition, PGAM1 could directly interacts with α-smooth muscle actin (ACTA2) and participate in cell migration regulation [
8,
9].Thus, we speculate that targeting PGAM1 maybe a new direction for fibrosis therapy.
PGAM1 catalyzes the conversion of 3-phosphoglyceric acid (3-PG) to 2-phosphoglyceric acid (2-PG) in glycolysis pathway. It promotes glucose metabolism and energy generation, participates in the synthesis of biomacromolecules in cells, maintains redox stability and promotes cell proliferation[
8,
10]. Traditional view holds that PGAM1 is not involved in the rate-limiting step of glycolysis. However, recent studies have found that PGAM1 is a conditional rate-limiting enzyme in glycolysis of tumor cells, heart tissues and white blood cells, and participates in NAD+-dependent deacetylation regulation[
7]. The human
Pgam gene contains two subtypes,
Pgam1 (also known as
Pgam-B) and
Pgam2 (also known as
Pgam-M).
Pgam1 is expressed in brain, heart and other tissues, while
Pgam2 is mainly expressed in muscle tissues[
11]. Muscle tissue is an important part of the heart, and
Pgam1 and
Pgam2 are all expressed in the heart. Previous study reported that heart specific knockout of
Pgam1 can alleviate myocardial fibrosis [
12]. Therefore, screening new ligands based on PGAM1 target may provide a new way for the treatment of myocardial fibrosis.
Natural products have been a rich source of compounds for drug discovery[
13,
14]. Recently, natural products have continued to enter clinical trials or to provide leads for compounds that have entered clinical trials, particularly as anticancer and antimicrobial agents[
15]. However, the process of developing a new drug can be long, complex, and uncertain[
16]. Virtual screening is a rapid, cost-effective method, plays an important role in drug discovery processes. However, databases comprising different chemical structures are crucial for virtual screening [
17].
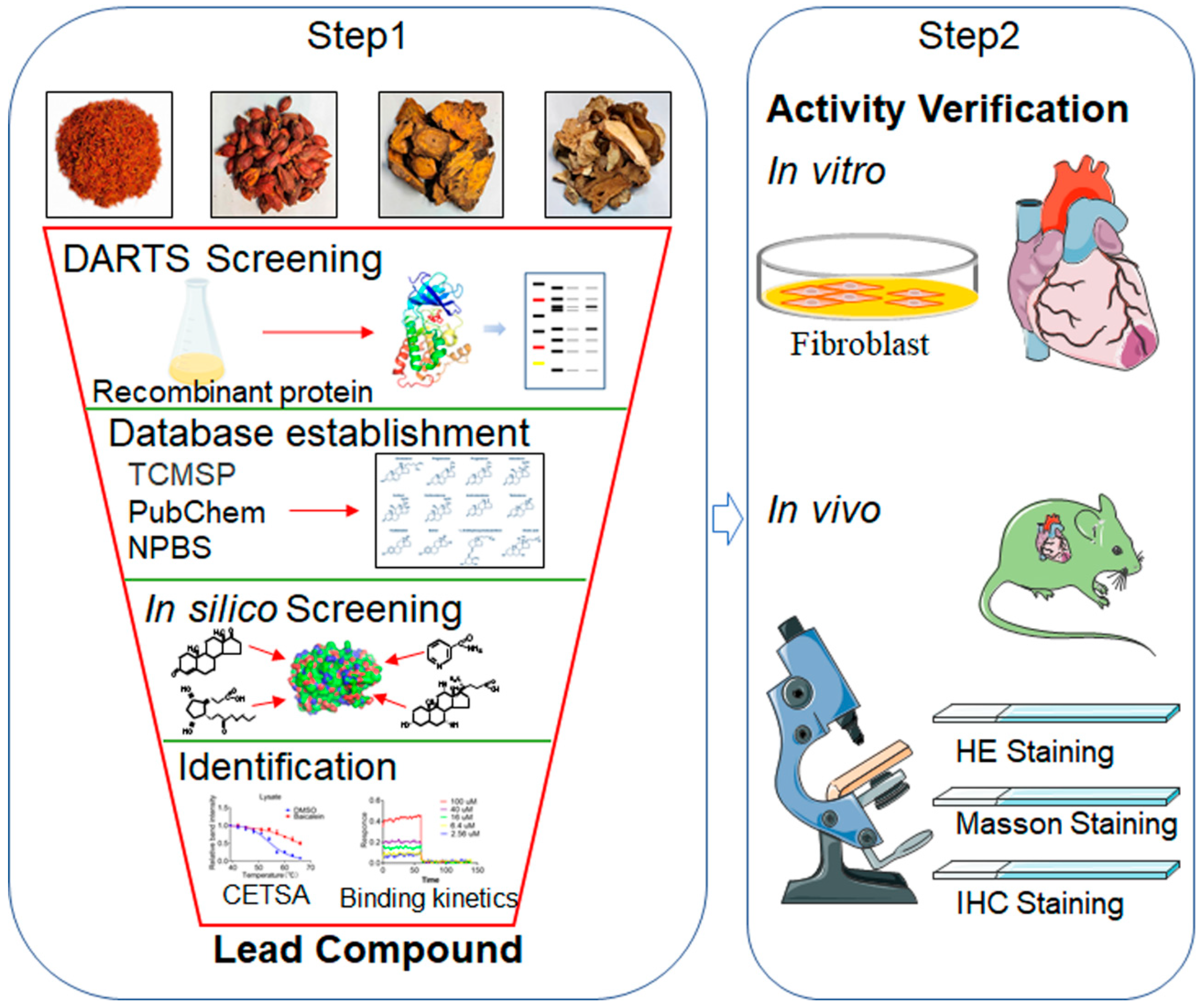
In this study, we propose a novel strategy that combines the DARTS strategy and in silico screening methods to identify novel PGAM1 ligand. Firstly, DARTS screeing, in house database establishment, in silico screening assay were performed and baicalein was identified as lead candidate. Then, the interaction of PGAM1 and baicalein was identified. Furthermore, the cardioprotective effects against myocardial fibrosis were analyzed in vivo (
Figure 1). This novel methodology and strategy may help uncover novel templates for PGAM1, even uncover new therapeutic strategy for myocardial fibrosis.
2. Results
2.1. The Expression of PGAM1 Increased in Myocardial Fibrotic Tissue
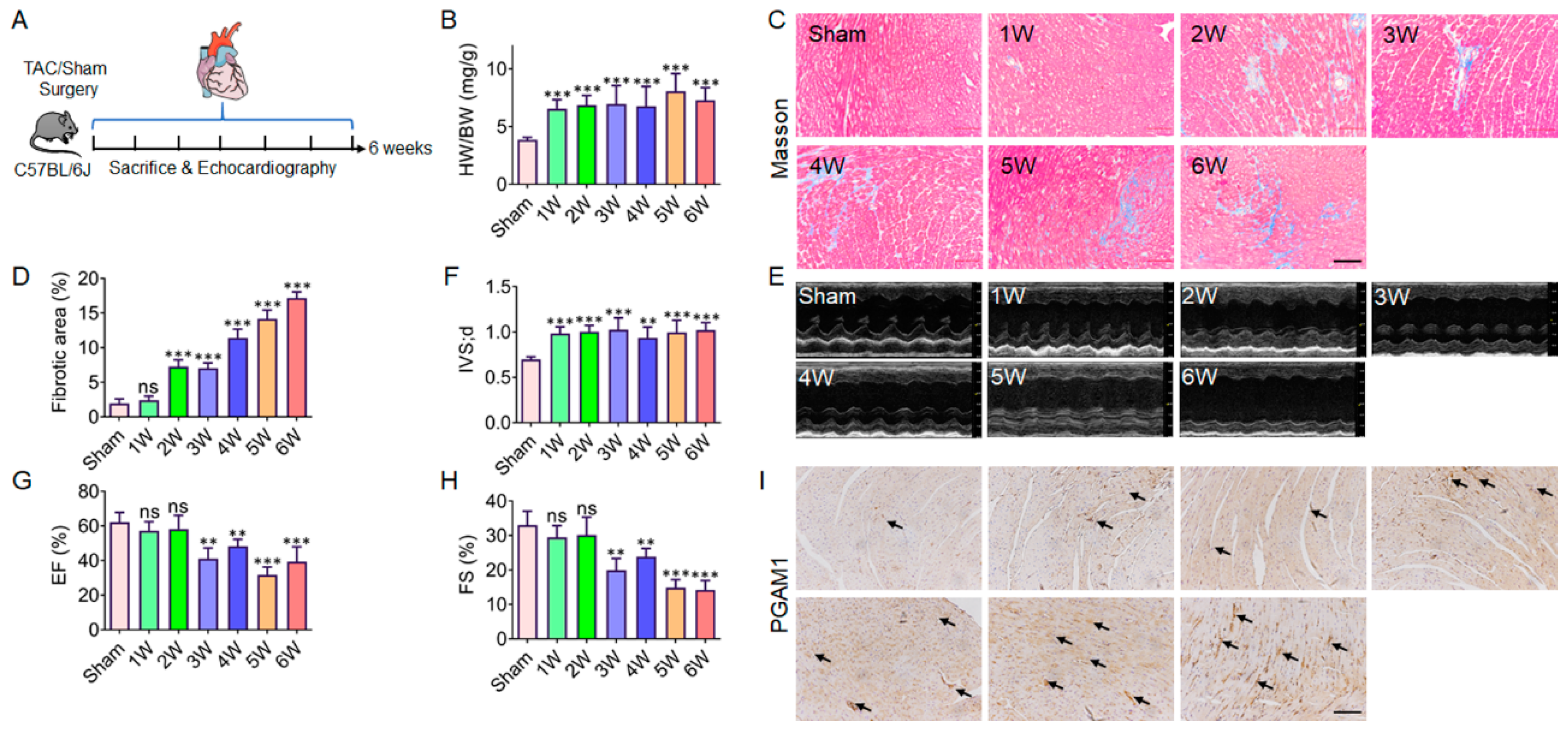
Previous study reported that PGAM1 expression is increased after myocardial infarction. However, the relationship between the expression of PGAM1 and myocardial fibrosis is unknown. In order to further determine the key role of PGAM1 in myocardial fibrosis, TAC models at different times (1-6 weeks) was established to explore the dynamic changes of myocardial fibrosis and PGAM1 (
Figure 2A). From the first week, the ratio of heart weight to body weight (HW/BW) was increased significantly (
Figure 2B). In addition, obvious fibrotic lesions appeared in myocardial tissue from the second week (
Figure 2C, 2D). The thickness of ventricular septum (IVS; D) was increased significantly from the first month (
Figure 2E, 2F). From the third week, the ejection fraction (EF) and shortening fraction (FS) of the left ventricle of mice decreased significantly, indicating that the cardiac function decreased significantly (
Figure 2G, 2H). In addition, the expression changes of PGAM1 in different stages of fibrosis was assayed by immunohistochemical method. The results showed that the expression level of PGAM1 increased from the first week (
Figure 2I). The above results further confirm that PGAM1 may be an important driving factor of myocardial fibrosis, and targeting PGAM1 may develop a new way for myocardial fibrosis treatment.
2.2. Screening of Active Ingredient Targets on PGAM1 through DARTS and In Silico Assay
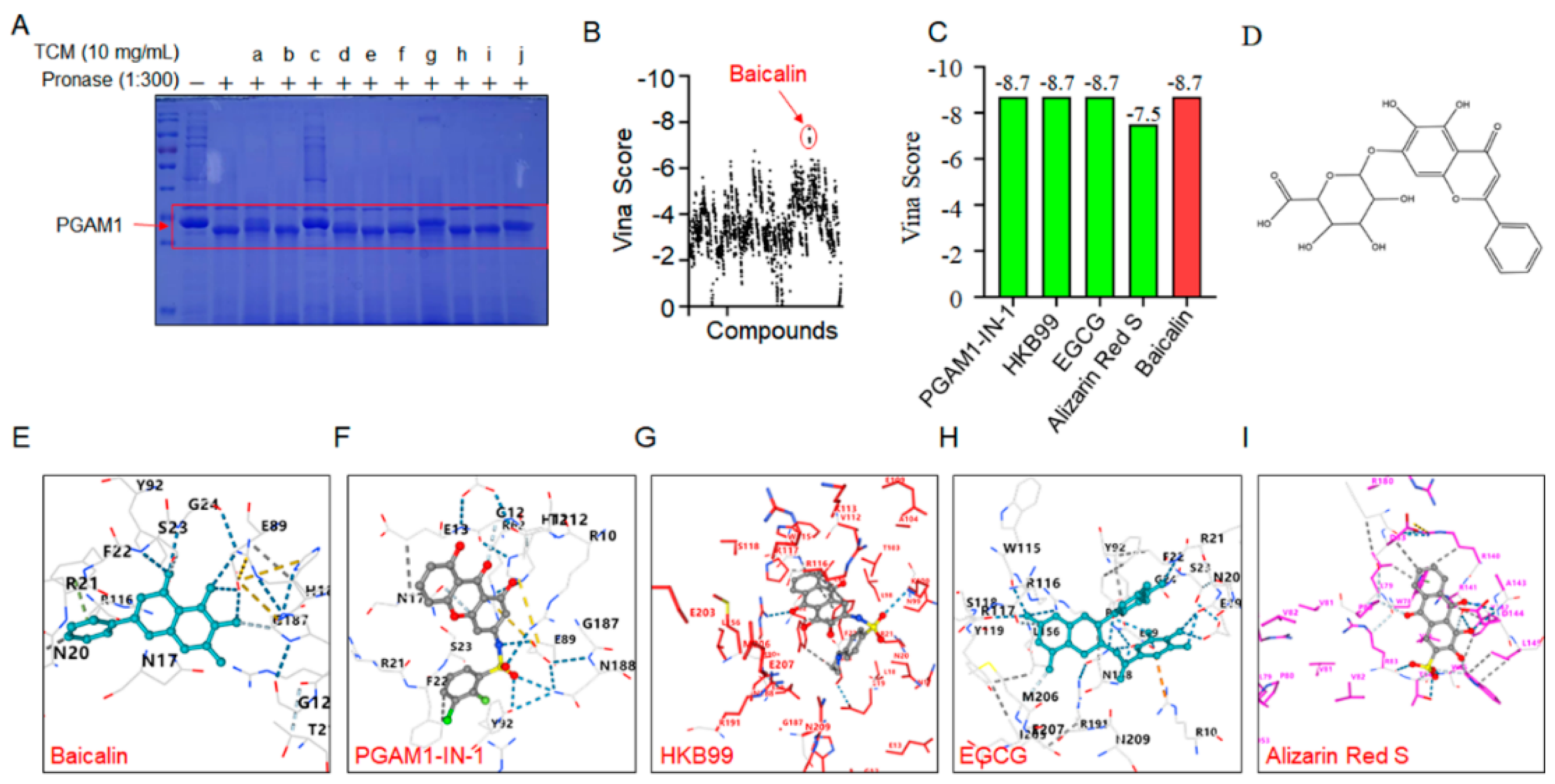
Many natural products used in traditional medicine have been reported to have anti-myocardial fibrosis properties. Considering the availability and sustainability of natural products, many TCM extracts, such as Taraxacum, Radix Puerariae, Fructus aurantii, Rhizoma gastrodiae, Polygonum multiflorum, Liquorice, Fructus Gardeniae, Rhizoma Atractylodis Macrocephalae, Radix sophorae flavescentis and other natural products were evaluated. DARTS screening assay with recombinant PGAM1 protein was performed. Results showed that the extracts of Carthamus tinctorius, Liquorice and Taraxacum could increase the resistance to proteolysis, in which the extract of Carthamus tinctorius present the best effect (
Figure 3A). Thus, Carthamus tinctorius was selected for the further screening. However, a library containing Carthamus tinctorius substances was established for docking studies. As a basis for this, the TCMSP, PubChem, NPBS database were used to obtain the stucture. Herein, the biological active conformations of 191 curated molecules were obtained through ligand preparation process and the molecule structures were sketched with ChemDraw. Then, Docking analyses were performed on Ledock system and throughout the cavity that is built by all pockets. The 1959 docking results obtained in the docking comprise different structures from different groups of insects. Taken together, flavonoids compounds baicalin was top ranked from the docking analysis (
Figure 3B, 3D, Supplementary Table-S1). In addition, four verified PGAM1 inhibitor (PGAM1-IN-1, HKB99, EGCG, Alizarin Red S) were selected as positive control. Docking analysis was performed on baicalin and the verified PGAM1 inhibitor. Results showed that baicalin has the same score with PGAM1-IN-1, HKB99 and EGCG, indicate that baicalin might be an ideal ligand for PGAM1(
Figure 3C, 3E-I).
2.3. Interaction Mode of Baicalin and PGAM1
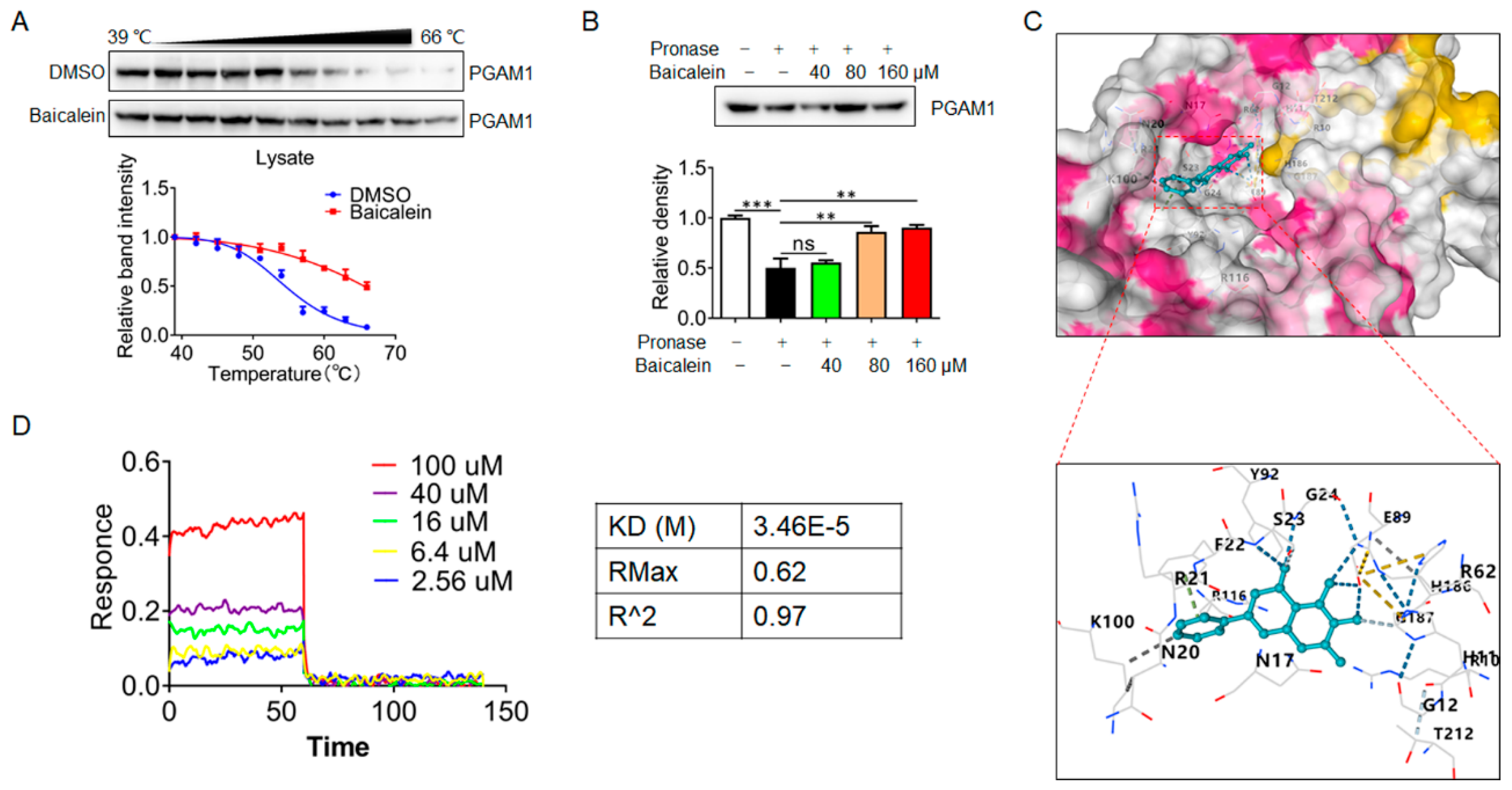
Next, we sought to reveal the interaction mode of baicalin with PGAM1. Firstly, the baicalin–PGAM1 interaction was demonstrated by CETSA assay. CETSA recognizes baicalin–PGAM1 engagement with increased stability of the PGAM1 proteins towards heat-induced precipitation. As expected, baicalin incubation stabilized PGAM1 in heat-denatured NIH-3T3 cell lysates (
Figure 4A). Similarly, a DARTS approach was employed to identify the PGAM1 that interact with baicalin. DARTS detects ligand-bound PGAM1 in lysates basing on their increased resistance to proteolysis. After incubated baicalin with cells lysates at concentrations of 80 and 160 μmol/L, increased stability of the PGAM1 proteins was showed (
Figure 4B). Collectively, these data suggest that baicalin interacted with PGAM1 in NIH-3T3 cells. In addition, molecular docking analysis was conducted, and results showed that baicalin is likely bind to a highly conserved residue N20, R21, S23, G24, and E89 of PGAM1 (
Figure 4C). Previous structural comparison supports that the conformational changes of residues 13-21 determine phosphoglycerate mutase activity differences [
18]. Based on the structural information, we speculate that phosphoglycerate mutase activity regulation might be the molecular mechanism of baicalin. To investigate the affinity kinetic of baicalin and PGAM1 protein interactions, bio-layer interferometry analyses were performed. Our results demonstrated that baicalin could directly bind to the PGAM1 protein with a KD value of 3.46E-05M (
Figure 4D). These findings indicate that PGAM1 is a direct pharmacological target of baicalin.
2.4. Molecular Dynamics Simulation
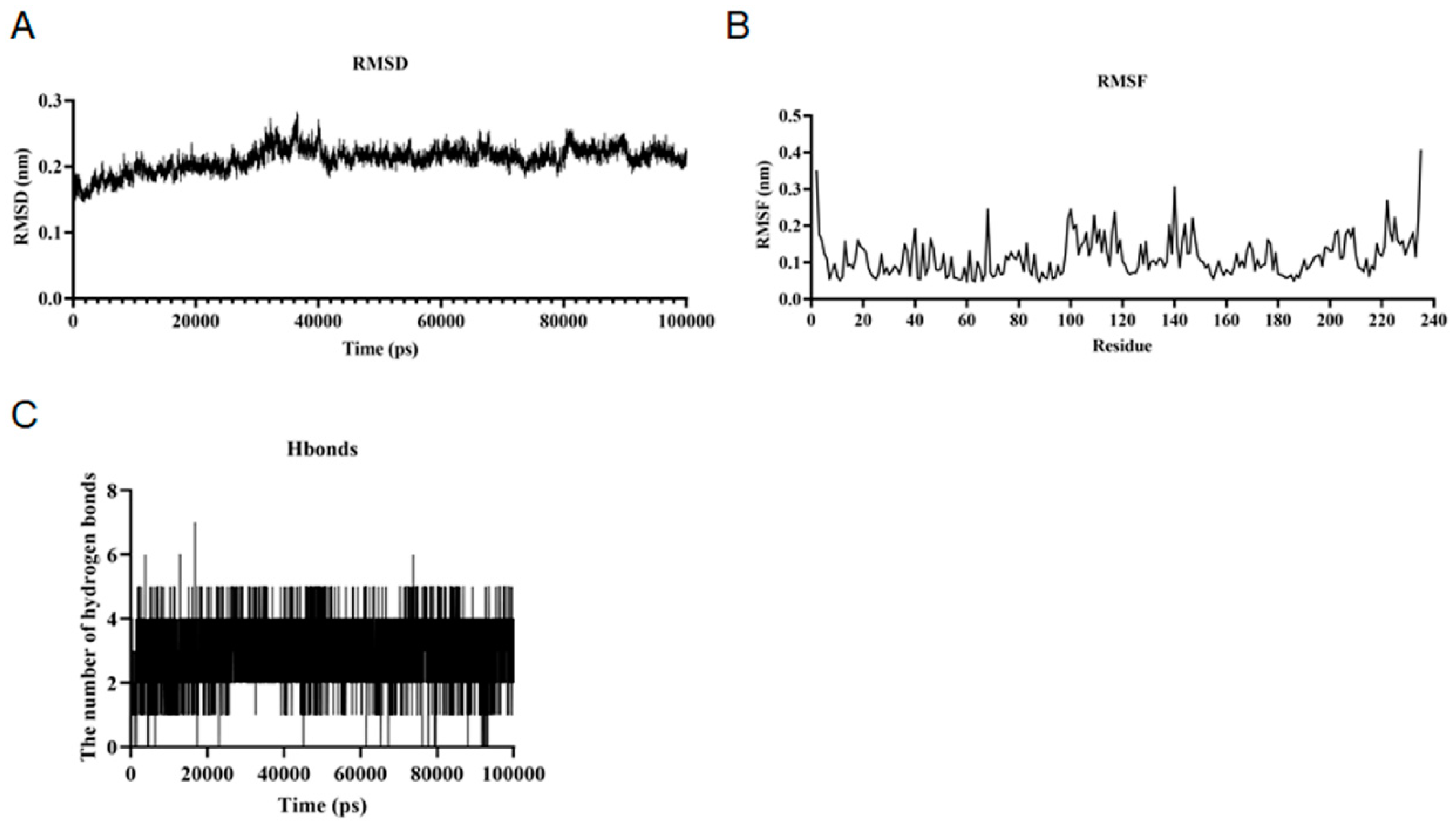
Since the structures obtained by conventional experimental techniques are fixed in the lattice, the experimentally observed conformations of macromolecules show a single static structure. The conventional macromolecule is regarded as a rigid entity. Molecular dynamics simulations differ from conventional experimental methods in that they can capture the position and motion of each atom at each time point[
19,
20].The RMSD values of baicalin and PGAM1 remained stable during the simulation, and the overall trend showed a gentle decrease. The RMSD value of complex the overall trend remained the same during the simulation, indicating that the baicalin and PGAM1 interactions have little effect on the stability of the overall structure of the protein molecules (
Figure 5A). The RMSF values of baicalin and PGAM1 were stable during the simulation and the overall trend remained the same. The RMSF value of PGAM1 decreased from the initial 0.35 nm to 0.25 nm during the simulation, which indicates that the baicalin and PGAM1 interactions have less influence on the stability of the internal structure of protein molecules (
Figure 5B). The number of hydrogen bonds between baicalin and PGAM1 remained stable during the simulation and the overall trend remained unchanged. This indicates that the interaction characteristics and stability of baicalin and PGAM1 interactions are good (
Figure 5C). The MMGBSA values for baicalin and PGAM1 interactions remained stable during the simulation and the overall trend remained constant. The MMGBSA value of baicalin and PGAM1 interactions in the MMGBSA value of baicalin and PGAM1 interactions remained around -23.08 kJ/mol during the simulation. This indicates that the strength and stability of baicalin and PGAM1 interactions are good. Herein, baicalin and PGAM1 interactions were simulated by Gromacs software and the simulation results were analyzed in several ways (
Table 1). The results showed that during the simulation of 100ns, the baicalin and PGAM1 interactions on the stability of the overall structure of the protein molecule, the stability of the internal structure, the compactness, the surface characteristics and the strength of the interactions are stable.
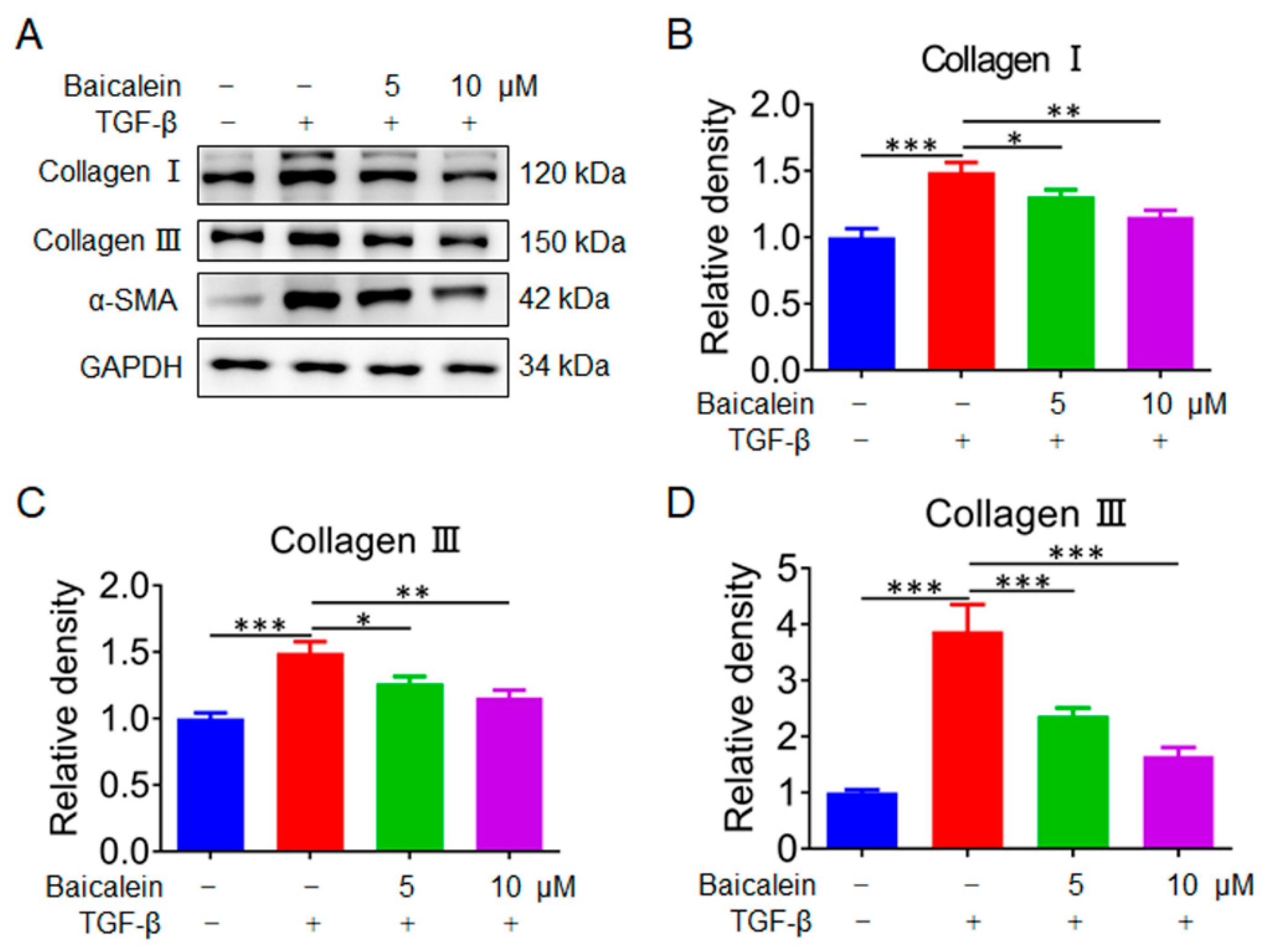
2.5. Baicalin Inhibits the Activation of Fibroblasts In Vitro
To further investigate the anti-myocardial fibrosis mechanism of baicalin, Collagen Ⅰ, Collagen Ⅲ and α-SMA were further examined by immunoblotting after treatment with TGF-β. As shown in
Figure 6, the protein level of Collagen Ⅰ, Collagen Ⅲ and α-SMA were significantly increased in the model group. After treatment with baicalin in different dosage, the protein level of Collagen Ⅰ, Collagen Ⅲ and α-SMA were significantly decreased in dose-dependent trend. Thus, these results suggested that baicalin was capable of blocking the cardiac fibrosis response
in vitro.
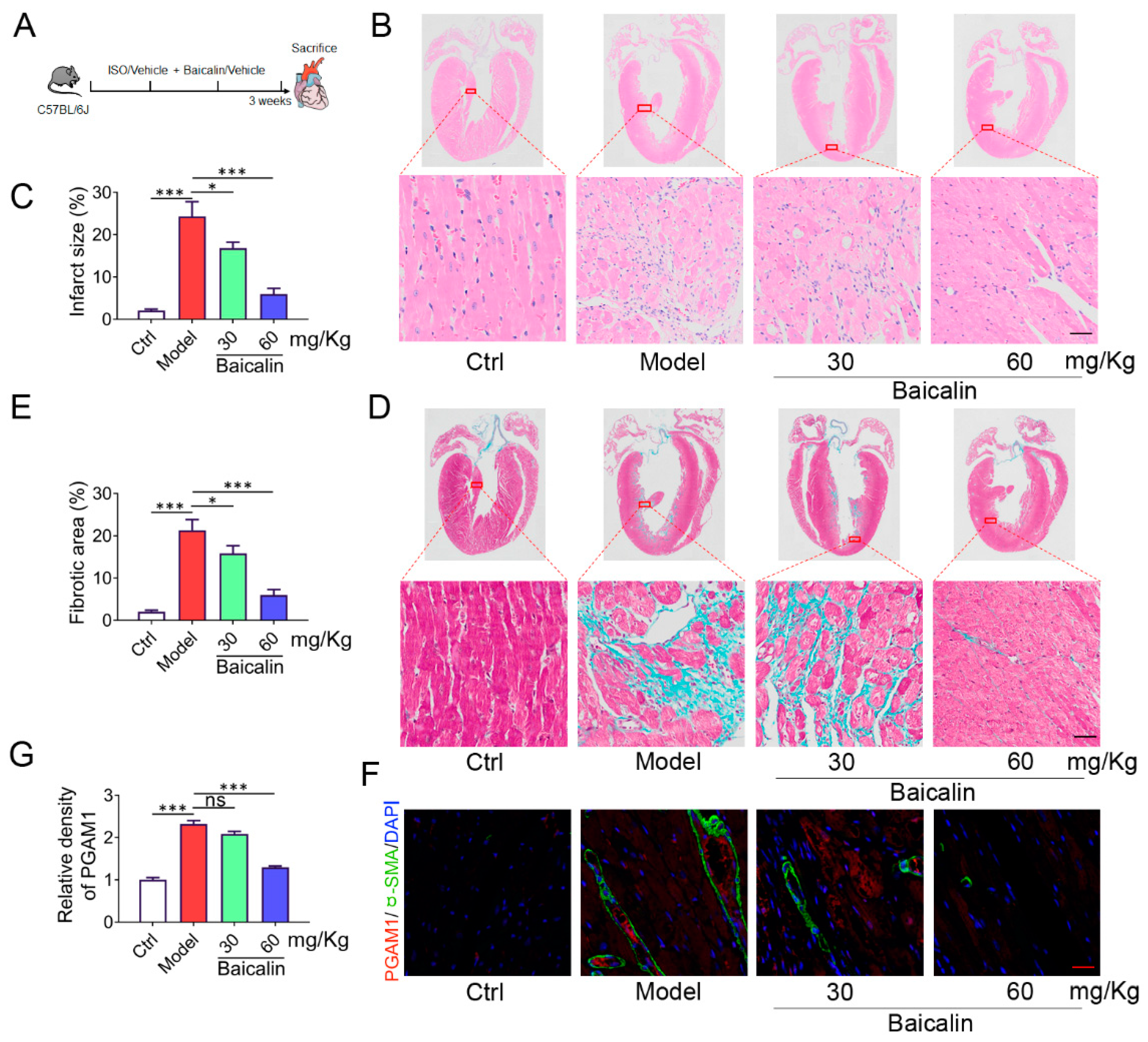
2.6. Baicalin Inhibits ISO-Induced Cardiac Fibrosis in Mice
Isoprenaline (ISO) is a persistent β-adrenergic receptor stimulation induces heart injury owing to the convergence of more contributing factors, including oxidative stress, inflammation and thrombosis[
21]. Finally, the cardioprotective effect of baicalin in mice infused with the hypertrophic agonist ISO was assessed. Mice were challenged with ISO and baicalin (30 mg/kg and 60 mg/kg) for 21 days (
Figure 7A). HE staining shows that ISO induced cardiac injury with fiber broken, but baicalin administration reduced the myocardial infarct surface with structure normalization, indicating the potential to attenuate myocardial injury (
Figure 7B, C). In addition, masson trichrome stain results demonstrated that administration with baicalin effectively improved the degree of myocardial fibrosis in ISO mice (
Figure 7D, E). It is unknown whether baicalin could directly modulate the level of PGAM1. To address this question, we examined the level of PGAM1 with immunofluorescence histochemistry assay on an ISO induced mouse fibrosis model. ISO induction resulted in significant PGAM1 compared to mice in the control group. Treatment with baicalin significantly reversed the change (
Figure 7F, G). Collectively, these results suggested that baicalin was capable of blocking the cardiac fibrosis response induced by ISO in mice.
3. Discussion
Phosphoglycerate mutase 1 (PGAM1) is a crucial glycolytic enzyme, and its expression status has been confirmed to be associated with tumor progression and metastasis[
22]. Previous study has demonstrated target PGAM1 could inhibit the cell migration [
8]. Therefore, many drug researchers are trying to develop novel PGAM1 inhibitors. However, there is still a lack of diverse PGAM1 inhibitors. Herein, baicalein was screened and identified as a new PGAM1 ligand. In addition, the anti-myocardial fibrosis effect and the expression of PGAM1 were verified
in vivo. Our study provides new insight into the regulatory effect of PGAM1 in myocardial fibrosis and might shed new light on strategies for myocardial fibrosis treatment.
The process of developing a new drug can be long, complex, and uncertain, but it can also be highly rewarding from a societal, scientific, and economic perspective[
23,
24]. High-throughput screening (HTS) is one of the strategies used to discover novel template for small-molecule drug design[
25,
26]. Despite the reinforced interest in natural products for anti-myocardial fibrosis lead discovery, natural products and in particular insect products have been basically neglected in such screenings[
27]. However, the natural products contain huge compound database, which present us new options for lead screening process. Motivated by our pilot studies that revealed DARTS sceening assay could remedy this gap and performed a virtual screening in a small library. Herein, to explore the ligand of PGAM1 in natural product, seveal natural product were screened and baicalein was identified as a PGAM1 ligand. The effective components of Carthamus tinctorius L. include many compounds such as quinone chalcone glycosides, flavonoids, spermidine, alkaloids and organic acids, in which quinone chalcone glycosides and flavonoids are the main components of Carthamus tinctorius L. in water extract. Modern pharmacological research shows that
Carthamus tinctorius L. has a wide range of biological activities such as anticoagulation, anti-fibrosis and anti-inflammation[
28]. The extract of
Carthamus tinctorius L can inhibit myocardial fibrosis by regulating ERK1/2 and other pathways[
29,
30]. The in water extract of
Carthamus tinctorius L contains many effective components, include quinoxalone glycosides, flavonoids, such as hydroxysafflor yellow A, safflor yellow A, carthamin and baicalein [
31]. Among them, flavonoids can improve cardiovascular and cerebrovascular diseases through anti-inflammatory, anti-apoptosis and other ways. In this study, we found that PGAM1 is a new target for baicalein. This study not only clarified the anti-myocardial fibrosis mechanism of safflower, it also provided new lead compounds for PGAM1. However, the types of herbs selected in this study were limited, and future studies may be able to screen for better active ingredients if the types of herbs can be increased.
4. Material and Methods
4.1. Reagents
Baicalin (purity ≥ 98%) was obtained from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). Isoprenaline hydrochloride (#I5627), Pronase was purchased from Sigma-aldrich (St. Louis, MO, USA). Antibody against PGAM1 Rabbit mAb (#16126-1-AP), Smooth muscle actin specific monoclonal antibody (67735-1-Ig) were purchased from Proteintech (Beverly, MA, USA). RIPA lysis buffer (#P0013D) and BCA assay (#P0010) were obtained from Beyotime (Suzhou, China).
4.2. Extraction of Plant Molecules
The active ingredients of Taraxacum, Radix Puerariae, Carthamus tinctorius, Fructus aurantii, Rhizoma gastrodiae, Polygonum multiflorum, Liquorice, Fructus Gardeniae, Rhizoma Atractylodis Macrocephalae, Radix sophorae flavescentis were extracted (solid-liquid ratio at 1:10) in water bath at 100 ℃ for 2 h. Then, the supernatant was concentrated and stored in 4 ℃ before use.
4.3. Identification of Candidate Ligands of PGAM1
The database of active components in Carthamus tinctorius was established according to TCMSP[
32], NPBS[
33] and other databases.The structure of active components was drew by ChemBio3D Ultra 14.0 software, and the sdf format structure of active components was uploaded, Then, results were presented after completing screening and scoring protocol for each ligands set by Ledock[
34].
4.4. Bio-Layer Interferometry
Bio-layer interferometry analyses were performed using a GatorPrime biosensor system (GatorBio) with streptavidin probes. In brief, PGAM1 protein was mixed with biotin for 30 min, and then a desalting column was adopted to remove the excess biotin. Biotinylated PGAM1 (10 μg/ml) was immobilized onto the streptavidin biosensor (GatorBio). The tips were washed with PBS buffer for 60s, then different concentrations of baicalin were assayed by the biosensors for 120s. Data analysis was performed with GraphPad PRISM 9 using a standard 1:1 binding model.
4.5. Molecular Dynamics Simulation
Gromacs 2022.3 software was used for molecular dynamics simulation and the experimental methodology and steps referenced to references[
35].
4.6. Cell Culture
NIH-3T3 cell line was obtained from Procell Life Science&Technology Co.,Ltd.. NIH-3T3 cells were cultured in the medium of DMEM supplemented with 10% (v/v) FBS with a condition of 37 °C with 5% CO2 in air atmosphere.
4.7. Drug Affinity–Responsive Target Stabilization Assay (DARTS)
In brief, NIH-3T3 cells were lysed with NP-40 buffer on ice for 30 min. Indicated concentrations of baicalin was added into the aliquoted cell lysate. Then, samples were gently mixed and incubated for 2 h at room temperature. Then, pronase was added and digested for precisely 30 min. Then, loading buffer was added and boiled for 10 min. Samples were separated and analysised with Coomassie blue or western-blotting assay.
4.8. Cellular Thermal Shift Assay (CETSA)
For CETSA experiments, NIH-3T3 cells were lysed with a freeze–thawed method. The resultant cell lysates were then divided into two fractions, one incubated with the solvent as the control group and the other incubated with baicalin for 30 min at room temperature. Then, lysates were aliquoted and heating at sequentially increased temperature (39–66 °C with a 3 °C interval). Then, loading buffer was added and boiled for 10 min. Samples were separated and analysised with western-blotting assay.
4.9. Animals and Treatments
C57BL-6J mice (Male, 8-9 week) were purchased from Wushi Animal Center (Fuzhou, China). All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals, following protocols approved by Pharmaceutical Animal Experimental of Huaqiao University (No. A2021039).
The myocardial fibrosis model on mice were established by ISO (10 mg/kg,
s.c., once daily), and control animals were administered with an equal volume of normal saline as described previously[
36]. Baicalin (30, 60 mg/kg,
p.o.) was administrated once daily for 14 consecutive days. Then, mice were euthanized and the heart tissues were collected for further analysis.
4.10. Immunoblotting Experiments
Cell lysates were harvested with lysis buffer and assayed the protein quantitation using BCA assay. Then, samples were separated by SDS-PAGE and transferred onto nitrocellulose filter (NC) membrane. NC membranes were incubated with indicated primary antibody for overnight at 4 °C followed by blocking with 5% skim milk for 1 h, The next day, membranes were washed and incubation with HRP-conjugated secondary antibodies for 1.5 h at room temperature. The protein bands were imaging using Tanon 500 system (Tanon, Shanghai, China).
4.11. Preparation of Recombinant PGAM1 Protein
Pgam1 cDNA was subcloned to pET-28a(+) vector and transformed into E. coli BL-21 competent cell. Then, plate screening method was performed and monoclonal cell was selected. Then, after cultured in LB medium for 12 h, 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added, then induced for 24 h at 16 °C. Purification of the resultant PGAM1 was achieved by Ni-NTA column.
4.12. Molecular Docking
The 3D structure of the PGAM1 was downloaded from the Protein Data Bank (PDB entry 5Y2I). Upon removing water molecules, hydrogen atoms were added to the protein according to the protonation states of chemical groups at the physiological pH. The initial 3D conformer of molecules was generated and docked into the PGAM1 by the program CB-Dock[
37].
4.13. Histological Analysis
Slices of hearts were fixed, dehydrated and embedded. Then, heart sections (5 μm-thick) were stained with hematoxylin and eosin (HE), masson for histopathological evaluation. The protein expression of PGAM1 and α-SMA were measured by immunohistochemistry (IHC) staining. Myocardial injury area and fibrosis area were determined by Image J software.
4.14. Statistical Analysis
All the data used were normally distributed. For GEO data analysis, the differences between groups were calculated using Mann-Whitney Wilcoxon test. For other experiments, the difference between groups (>2 two groups) were performed using one-way ANOVA (Tukey’s multiple comparisons test) with IBM SPSS Statistics 26 (IBM Corp., Armonk, NY). All data are represented as mean±SD, unless otherwise specified.
5. Conclusion
In this study, a novel strategy that combines the DARTS strategy and in silico screening methods was establised. In addition, a novel anti-fibrosis templates were successfully identified. This study will provide theoretical and experimental basis for clarifying the molecular mechanism of safflower in treating myocardial fibrosis. Besides, the baicalein-PGAM1 interaction mode may provide a new strategy of metabolic intervention against myocardial fibrosis.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
XW: RZ, CZ, ML, SW, NY and QSW contributed to experiments, data analysis, and the manuscript writing. XW and YD designed and guided the study. XW and RZ contributed to data analysis guidance. All authors contributed to the revision of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 82304762; Natural Science Foundation of Fujian Province, grant number 2022J05062; Natural Science Foundation of Xiamen, grant number 3502Z20227041; The Scientific Research Funds of Huaqiao University, grant number 21BS126.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.; Zhang, L.; Ye, T.; Ding, B.; Zhang, B.; Yang, J.; Yao, Y., Targeting fibrosis, mechanisms and cilinical trials. Signal transduction targeted therapy 2022, 7, (1), 206.
- López, B.; Ravassa, S.; Moreno, M.U.; José, G.S.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498, . [CrossRef]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2019, 19, 57–75, . [CrossRef]
- Bertero, E.; Maack, C., Metabolic remodelling in heart failure. Nature Reviews Cardiology 2018, 15, (8), 457-470.
- Wu, X.; Liu, L.; Zheng, Q.; Ye, H.; Yang, H.; Hao, H.; Li, P. Dihydrotanshinone I preconditions myocardium against ischemic injury via PKM2 glutathionylation sensitive to ROS. Acta Pharm. Sin. B 2023, 13, 113–127, . [CrossRef]
- Wu, X.; Liu, L.; Zheng, Q.; Hao, H.; Ye, H.; Li, P.; Yang, H. Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharm. Sin. B 2021, 11, 3553–3566, . [CrossRef]
- Hallows, W.C.; Yu, W.; Denu, J.M. Regulation of Glycolytic Enzyme Phosphoglycerate Mutase-1 by Sirt1 Protein-mediated Deacetylation. J. Biol. Chem. 2012, 287, 3850–3858, . [CrossRef]
- Huang, K.; Liang, Q.; Zhou, Y.; Jiang, L.; Gu, W.; Luo, M.; Tang, Y.; Wang, Y.; Lu, W.; Huang, M.; Zhang, S.; Zhuang, G.; Dai, Q.; Shen, Q.; Zhang, J.; Lei, H.; Zhu, L.; Ye, D.; Chen, H.; Zhou, L.; Shen, Y., A Novel Allosteric Inhibitor of Phosphoglycerate Mutase 1 Suppresses Growth and Metastasis of Non-Small-Cell Lung Cancer. Cell metabolism 2019, 30, (6), 1107-1119.e8.
- Al Ashmar, S.; Anlar, G.G.; Krzyslak, H.; Djouhri, L.; Kamareddine, L.; Pedersen, S.; Zeidan, A. Proteomic Analysis of Prehypertensive and Hypertensive Patients: Exploring the Role of the Actin Cytoskeleton. Int. J. Mol. Sci. 2024, 25, 4896, . [CrossRef]
- Hitosugi, T.; Zhou, L.; Elf, S.; Fan, J.; Kang, H.-B.; Seo, J.H.; Shan, C.; Dai, Q.; Zhang, L.; Xie, J.; et al. Phosphoglycerate Mutase 1 Coordinates Glycolysis and Biosynthesis to Promote Tumor Growth. Cancer Cell 2012, 22, 585–600, . [CrossRef]
- Sun, Q.; Li, S.; Wang, Y.; Peng, H.; Zhang, X.; Zheng, Y.; Li, C.; Li, L.; Chen, R.; Chen, X.; Bai, W.; Jiang, X.; Liu, L.; Wei, F.; Wang, B.; Zhang, Y.; Li, H.; Ren, X.; Zhang, H., Phosphoglyceric acid mutase-1 contributes to oncogenic mTOR-mediated tumor growth and confers non-small cell lung cancer patients with poor prognosis. Cell death differentiation 2018, 25, (6), 1160-1173.
- Wu, Y.; Chen, S.; Wen, P.; Wu, M.; Wu, Y.; Mai, M.; Huang, J., PGAM1 deficiency ameliorates myocardial infarction remodeling by targeting TGF-β via the suppression of inflammation, apoptosis and fibrosis. Biochemical biophysical research communications 2021, 534, 933-940.
- Hao, H.; Zheng, X.; Wang, G. Insights into drug discovery from natural medicines using reverse pharmacokinetics. Trends Pharmacol. Sci. 2014, 35, 168–177, . [CrossRef]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16, 577, . [CrossRef]
- Wu, X.; Li, X.; Yang, C.; Diao, Y. Target Characterization of Kaempferol against Myocardial Infarction Using Novel In Silico Docking and DARTS Prediction Strategy. Int. J. Mol. Sci. 2021, 22, 12908, . [CrossRef]
- Gallinger, T.L.; Aboagye, S.Y.; Obermann, W.; Weiss, M.; Grünweller, A.; Unverzagt, C.; Williams, D.L.; Schlitzer, M.; Haeberlein, S. First In Silico Screening of Insect Molecules for Identification of Novel Anti-Parasitic Compounds. Pharmaceuticals 2022, 15, 119, . [CrossRef]
- Braga, R.C.; Alves, V.M.; Silva, A.C.; Nascimento, M.N.; Silva, F.C.; Liao, L.M.; Andrade, C.H. Virtual Screening Strategies in Medicinal Chemistry: The State of the Art and Current Challenges. Curr. Top. Med. Chem. 2014, 14, 1899–1912, . [CrossRef]
- Wang, Y.; Wei, Z.; Liu, L.; Cheng, Z.; Lin, Y.; Ji, F.; Gong, W. Crystal structure of human B-type phosphoglycerate mutase bound with citrate. Biochem. Biophys. Res. Commun. 2005, 331, 1207–1215, . [CrossRef]
- Abraham M J , Murtola T , Schulz R ,et al.GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Softwarex, 2015, 1-2(C):19-25.
- Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005 Dec;26(16):1701-18.
- Garg, M.; Khanna, D. Exploration of pharmacological interventions to prevent isoproterenol-induced myocardial infarction in experimental models. Ther. Adv. Cardiovasc. Dis. 2014, 8, 155–169, . [CrossRef]
- Liu, M.; Li, R.; Wang, M.; Liu, T.; Zhou, Q.; Zhang, D.; Wang, J.; Shen, M.; Ren, X.; Sun, Q. PGAM1 regulation of ASS1 contributes to the progression of breast cancer through the cAMP/AMPK/CEBPB pathway. Mol. Oncol. 2022, 16, 2843–2860, . [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216, doi:10.1038/s41573-020-00114-z.
- Zheng, L.; Luo, M.; Zhou, H.; Chen, J. Natural products from plants and microorganisms: Novel therapeutics for chronic kidney disease via gut microbiota regulation. Front. Pharmacol. 2023, 13, 1068613, . [CrossRef]
- Ajay, A.K.; Chu, P.; Patel, P.; Deban, C.; Roychowdhury, C.; Heda, R.; Halawi, A.; Saad, A.; Younis, N.; Zhang, H.; et al. High-Throughput/High Content Imaging Screen Identifies Novel Small Molecule Inhibitors and Immunoproteasomes as Therapeutic Targets for Chordoma. Pharmaceutics 2023, 15, 1274, . [CrossRef]
- Dong, H.; You, J.; Zhao, Y.; Zheng, D.; Zhong, Y.; Li, G.; Weng, Z.; Luo, H.; Jiang, S. Study on the Characteristics of Small-Molecule Kinase Inhibitors-Related Drug-Induced Liver Injury. Front. Pharmacol. 2022, 13, 838397, . [CrossRef]
- Wang, J.; Wong, Y.; Liao, F., What has traditional Chinese medicine delivered for modern medicine? Expert reviews in molecular medicine 2018, 20, e4.
- Ruyvaran, M.; Zamani, A.; Mohamadian, A.; Zarshenas, M.M.; Eftekhari, M.H.; Pourahmad, S.; Abarghooei, E.F.; Akbari, A.; Nimrouzi, M. Safflower (Carthamus tinctorius L.) oil could improve abdominal obesity, blood pressure, and insulin resistance in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2022, 282, 114590, . [CrossRef]
- Chen, J.; Cao, W.; Asare, P.F.; Lv, M.; Zhu, Y.; Li, L.; Wei, J.; Gao, H.; Zhang, H.; Mao, H.; et al. Amelioration of cardiac dysfunction and ventricular remodeling after myocardial infarction by danhong injection are critically contributed by anti-TGF-β-mediated fibrosis and angiogenesis mechanisms. J. Ethnopharmacol. 2016, 194, 559–570, . [CrossRef]
- Xue, X.; Deng, Y.; Wang, J.; Zhou, M.; Liao, L.; Wang, C.; Peng, C.; Li, Y. Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine 2021, 91, 153694, . [CrossRef]
- Xian, B.; Wang, R.; Jiang, H.; Zhou, Y.; Yan, J.; Huang, X.; Chen, J.; Wu, Q.; Chen, C.; Xi, Z.; Ren, C.; Pei, J., Comprehensive review of two groups of flavonoids in Carthamus tinctorius L. Biomedecine pharmacotherapie 2022, 153, 113462.
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13, doi:10.1186/1758-2946-6-13.
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. NPBS database: a chemical data resource with relational data between natural products and biological sources. Database 2020, 2020, . [CrossRef]
- Zhao, H.; Caflisch, A., Discovery of ZAP70 inhibitors by high-throughput docking into a conformation of its kinase domain generated by molecular dynamics. Bioorganic medicinal chemistry letters 2013, 23, (20), 5721-6.
- Binmujlli, M.A. Radiological and Molecular Analysis of Radioiodinated Anastrozole and Epirubicin as Innovative Radiopharmaceuticals Targeting Methylenetetrahydrofolate Dehydrogenase 2 in Solid Tumors. Pharmaceutics 2024, 16, 616, . [CrossRef]
- Wang, S.-X.; Feng, Y.-N.; Feng, S.; Wu, J.-M.; Zhang, M.; Xu, W.-L.; Zhang, Y.-Y.; Zhu, H.-B.; Xiao, H.; Dong, E.-D. IMM-H007 attenuates isoprenaline-induced cardiac fibrosis through targeting TGFβ1 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2542–2549, . [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.; Cao, Y., CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic acids research 2022, 50, W159-W164.
Figure 1.
The flow diagram of active components screening and characterization target PGAM1 with anti-myocardial fibrosis effects. Step1: Screening and determination of TCM extracts targeting disease PGAM1 targets by DARTS strategy; Target TCM extracts database establishment and in silico docking; affinity kinetic determination of baicalein/PGAM1. Step2: Activity verification using in vivo and in vivo models.
Figure 1.
The flow diagram of active components screening and characterization target PGAM1 with anti-myocardial fibrosis effects. Step1: Screening and determination of TCM extracts targeting disease PGAM1 targets by DARTS strategy; Target TCM extracts database establishment and in silico docking; affinity kinetic determination of baicalein/PGAM1. Step2: Activity verification using in vivo and in vivo models.
Figure 2.
Effects of different induction time (1-6 weeks) of TAC model on myocardial fibrosis and PGAM1 expression. (A) Schedule of animal treatments. Mice were subjected to TAC for 1-6 weeks (n = 6). (B) The ratio of heart weight to body weight in mice (n = 6); (C) Masson staining was used to detect the level of fibrosis, scale bar: 50 μm; (D) Quantification of myocardial fibrosis area (n = 6); (E) Representative echocardiogram of mice; (F) Interventricular septal thickness at diastole in mouse heart (IVS;d) (n = 6); (G) Cardiac ejection fraction (EF) in mice (n=6); (H) Left ventricular shortening fraction (FS) in mice (n=6); (I) Immunohistochemistry was used to detect the changes of PGAM1 protein level in the heart slices of mice, scale bar: 50 μm (n=6). The results were all expressed as mean±SD, *p <0.05, **p <0.01, ***p < 0.001, ns indicated that there was no statistical difference.
Figure 2.
Effects of different induction time (1-6 weeks) of TAC model on myocardial fibrosis and PGAM1 expression. (A) Schedule of animal treatments. Mice were subjected to TAC for 1-6 weeks (n = 6). (B) The ratio of heart weight to body weight in mice (n = 6); (C) Masson staining was used to detect the level of fibrosis, scale bar: 50 μm; (D) Quantification of myocardial fibrosis area (n = 6); (E) Representative echocardiogram of mice; (F) Interventricular septal thickness at diastole in mouse heart (IVS;d) (n = 6); (G) Cardiac ejection fraction (EF) in mice (n=6); (H) Left ventricular shortening fraction (FS) in mice (n=6); (I) Immunohistochemistry was used to detect the changes of PGAM1 protein level in the heart slices of mice, scale bar: 50 μm (n=6). The results were all expressed as mean±SD, *p <0.05, **p <0.01, ***p < 0.001, ns indicated that there was no statistical difference.
Figure 3.
Screening and target identification of active components in Carthamus tinctorius L. (A) DARTS based screening and Coomassie brilliant blue analysis, (a) Taraxacum, (b) Radix Puerariae, (c) Carthamus tinctorius, (d) Fructus aurantii, (e) Rhizoma gastrodiae, (f) Polygonum multiflorum, (g) Liquorice, (h) Fructus Gardeniae, (i) Rhizoma Atractylodis Macrocephalae, (j) Radix sophorae flavescentis; (B) Screening potential ligands of PGAM1 in safflower based on molecular docking; (C) Comparison of binding energy values of known inhibitors of B)PGAM1 and potential ligands. (D) Structure of Baicalin. (E) The interaction mode between Baicalin and PGAM1. (F) The interaction mode between PGAM1-IN-1 and PGAM1. (G) The interaction mode between HKB99 and PGAM1. (H) The interaction mode between EGCG and PGAM1. (I) The interaction mode between Alizarin Red S and PGAM1.
Figure 3.
Screening and target identification of active components in Carthamus tinctorius L. (A) DARTS based screening and Coomassie brilliant blue analysis, (a) Taraxacum, (b) Radix Puerariae, (c) Carthamus tinctorius, (d) Fructus aurantii, (e) Rhizoma gastrodiae, (f) Polygonum multiflorum, (g) Liquorice, (h) Fructus Gardeniae, (i) Rhizoma Atractylodis Macrocephalae, (j) Radix sophorae flavescentis; (B) Screening potential ligands of PGAM1 in safflower based on molecular docking; (C) Comparison of binding energy values of known inhibitors of B)PGAM1 and potential ligands. (D) Structure of Baicalin. (E) The interaction mode between Baicalin and PGAM1. (F) The interaction mode between PGAM1-IN-1 and PGAM1. (G) The interaction mode between HKB99 and PGAM1. (H) The interaction mode between EGCG and PGAM1. (I) The interaction mode between Alizarin Red S and PGAM1.
Figure 4.
Identification of Baicalin as a ligand for PGAM1 (A) PGAM1 degradation in NIH-3T3 cell lysates (n=3). (B) Immunoblot analysis of PGAM1 in pronase-digested cell lysate (n=3). (C) Predicted binding mode of Baicalin to PGAM1. Protein is shown in surface and key amino acids interacting with Baicalin are highlighted in stick representation. Baicalin is colored in cyan. Hydrogen bonds are depicted by blue dashed lines. (D) The binding kinetics between baicalin and PGAM1 were evaluated using the Gator system based on BLI technology. Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4.
Identification of Baicalin as a ligand for PGAM1 (A) PGAM1 degradation in NIH-3T3 cell lysates (n=3). (B) Immunoblot analysis of PGAM1 in pronase-digested cell lysate (n=3). (C) Predicted binding mode of Baicalin to PGAM1. Protein is shown in surface and key amino acids interacting with Baicalin are highlighted in stick representation. Baicalin is colored in cyan. Hydrogen bonds are depicted by blue dashed lines. (D) The binding kinetics between baicalin and PGAM1 were evaluated using the Gator system based on BLI technology. Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 5.
Baicalin-PGAM1 complexes structural stability analysis. (A) During 100 ns simulations, RMSD values of Baicalin-PGAM1 complex; (B) RMSF values; (C) Hbonds values.
Figure 5.
Baicalin-PGAM1 complexes structural stability analysis. (A) During 100 ns simulations, RMSD values of Baicalin-PGAM1 complex; (B) RMSF values; (C) Hbonds values.
Figure 6.
Baicalin Inhibits the activation of fibroblasts in vitro (A) Immunoblot analysis of Collagen Ⅰ, Collagen Ⅲ and α-SMA in NIH-3T3 cell extracts after treatment with baicalin (n=3). (B) Quantitative analysis of Collagen Ⅰ (n=3). (C) Quantitative analysis of Collagen Ⅲ (n=3). (D) Quantitative analysis of α-SMA (n=3). Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.
Baicalin Inhibits the activation of fibroblasts in vitro (A) Immunoblot analysis of Collagen Ⅰ, Collagen Ⅲ and α-SMA in NIH-3T3 cell extracts after treatment with baicalin (n=3). (B) Quantitative analysis of Collagen Ⅰ (n=3). (C) Quantitative analysis of Collagen Ⅲ (n=3). (D) Quantitative analysis of α-SMA (n=3). Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 7.
Baicalein protects mice from an ISO-induced myocardial fibrosis response. (A) Schedule of animal treatments. 50 mg/kg (once daily) ISO was given for 3 weeks by subcutaneous injection (n = 6). (B) HE staining of cardiac sections, scale bar, lower panel: 50 μm (n = 6). (C) Quantification of myocardial infarct size in different groups of mice (n = 6). (D) Sections of hearts stained with Masson’s trichrome to detect fibrosis, scale bar, lower panel: 50 μm (n = 6). (E) Quantification of myocardial fibrosis in different groups of mice (n = 6). (F) Immunofluorescent double-staining of PGAM1 (Red) and α-SMA (Green) of myocardial sections, scale bar, 50 μm, (n = 6). (G) Quantification of PGAM1 density in different groups of mice (n = 6). Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 7.
Baicalein protects mice from an ISO-induced myocardial fibrosis response. (A) Schedule of animal treatments. 50 mg/kg (once daily) ISO was given for 3 weeks by subcutaneous injection (n = 6). (B) HE staining of cardiac sections, scale bar, lower panel: 50 μm (n = 6). (C) Quantification of myocardial infarct size in different groups of mice (n = 6). (D) Sections of hearts stained with Masson’s trichrome to detect fibrosis, scale bar, lower panel: 50 μm (n = 6). (E) Quantification of myocardial fibrosis in different groups of mice (n = 6). (F) Immunofluorescent double-staining of PGAM1 (Red) and α-SMA (Green) of myocardial sections, scale bar, 50 μm, (n = 6). (G) Quantification of PGAM1 density in different groups of mice (n = 6). Results are shown as mean±SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 1.
Calculation of MMGBSA Free Energy.
Table 1.
Calculation of MMGBSA Free Energy.
| Delta (Complex - Receptor - Ligand): |
| Energy Component |
Average |
SD (Prop.) |
SD |
SEM(Prop.) |
SEM |
| ΔBOND |
0.00 |
0.95 |
0.00 |
0.30 |
0.00 |
| ΔANGLE |
0.00 |
1.18 |
0.00 |
0.37 |
0.00 |
| ΔDIHED |
-0.00 |
1.34 |
0.00 |
0.42 |
0.00 |
| ΔVDWAALS |
-28.78 |
0.06 |
1.35 |
0.02 |
0.43 |
| ΔEEL |
-15.00 |
1.64 |
3.25 |
0.52 |
1.03 |
| Δ1-4 VDW |
-0.00 |
0.47 |
0.00 |
0.15 |
0.00 |
| Δ1-4 EEL |
0.00 |
0.81 |
0.00 |
0.26 |
0.00 |
| ΔEGB |
24.74 |
0.09 |
2.89 |
0.03 |
0.91 |
| ΔESURF |
-4.04 |
0.10 |
0.27 |
0.03 |
0.09 |
| ΔGGAS |
-43.78 |
1.64 |
3.75 |
0.52 |
1.18 |
| ΔGSOLV |
20.70 |
0.13 |
2.98 |
0.04 |
0.94 |
| ΔTOTAL |
-23.08 |
1.65 |
2.39 |
0.52 |
0.75 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).