Submitted:
08 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
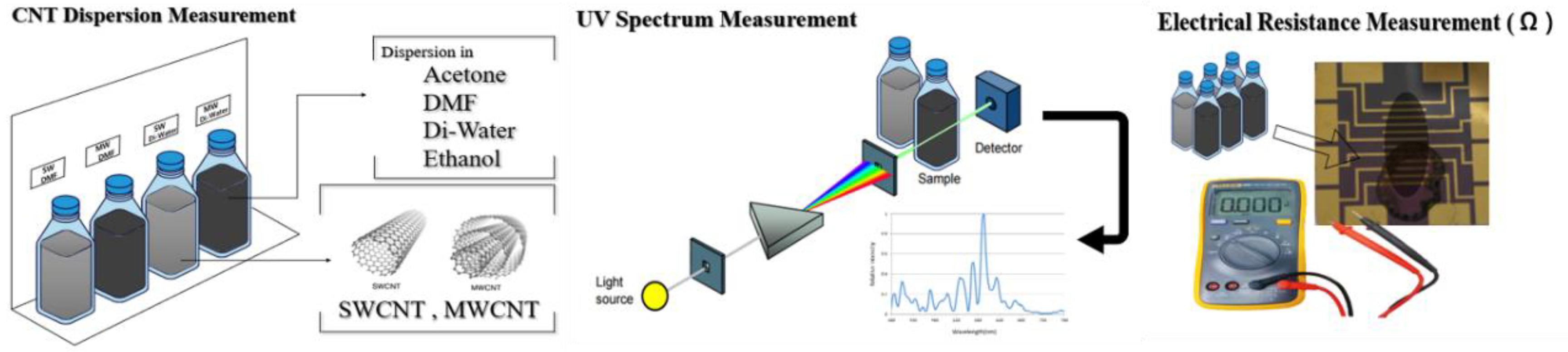
2.2. Solvent suspension and Dispersion of CNTs
2.3. Biosensor Development with SWCNTs
2.4. Detection of Glucose by the Fabricated Biosensor
3. Results and Discussion
3.1. Dispersion Characteristics of CNTs in Various Solvents
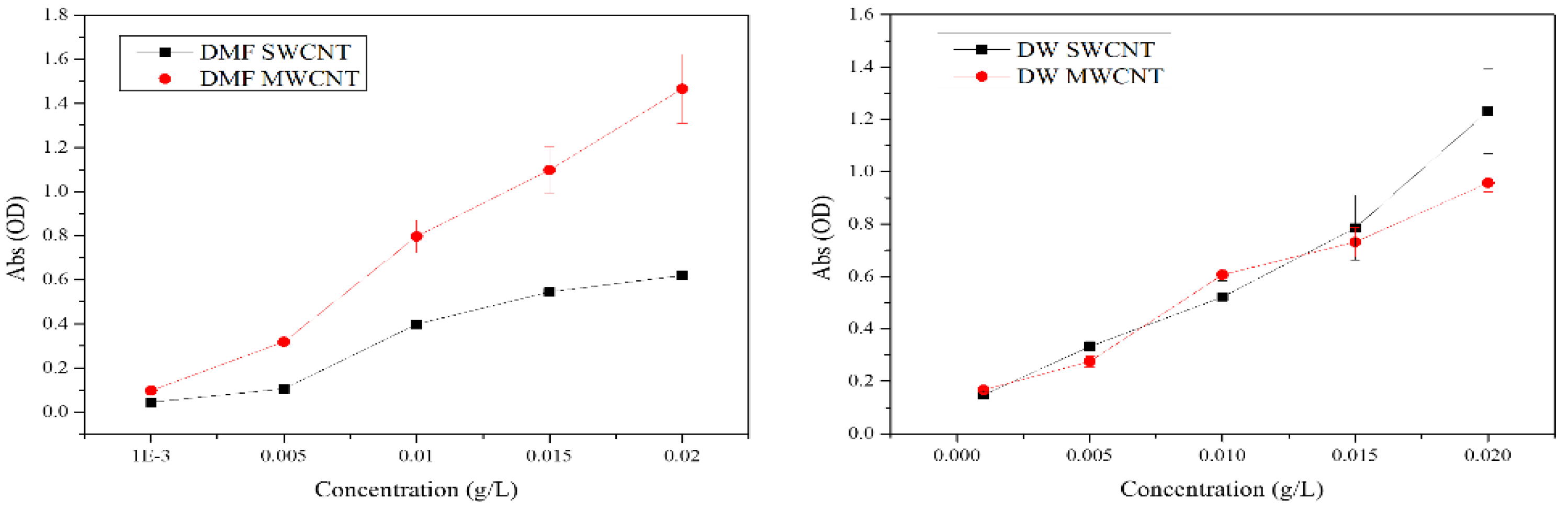
3.2. Dispersion Analysis of CNTs in Various Solvents
3.3. UV-Visible Spectral Analysis of CNTs Dispersions
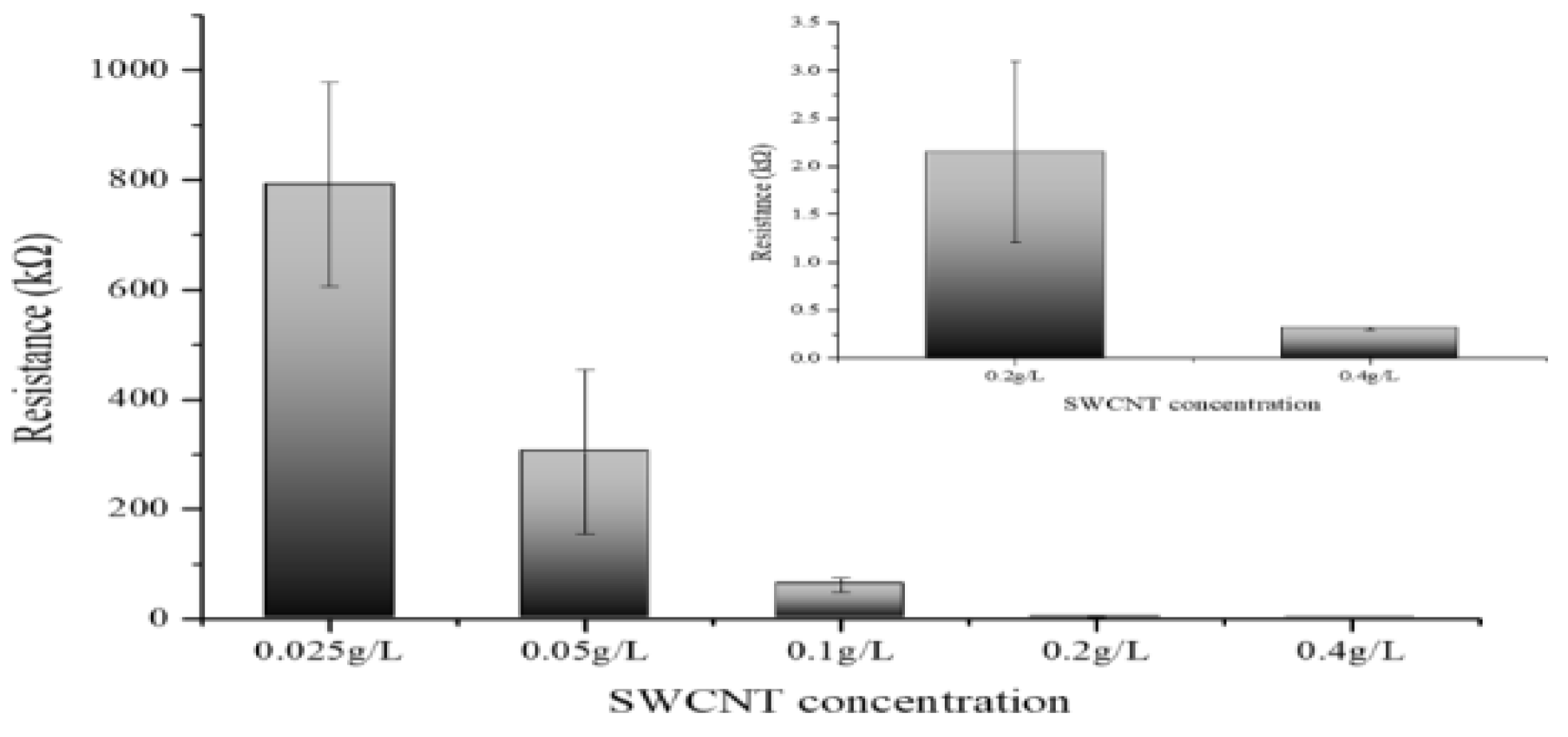
3.4. Quantitative Analysis of Electrical Resistance in CNT Dispersions
3.5. Quantitative Analysis of Electrical Resistance in CNT Dispersions
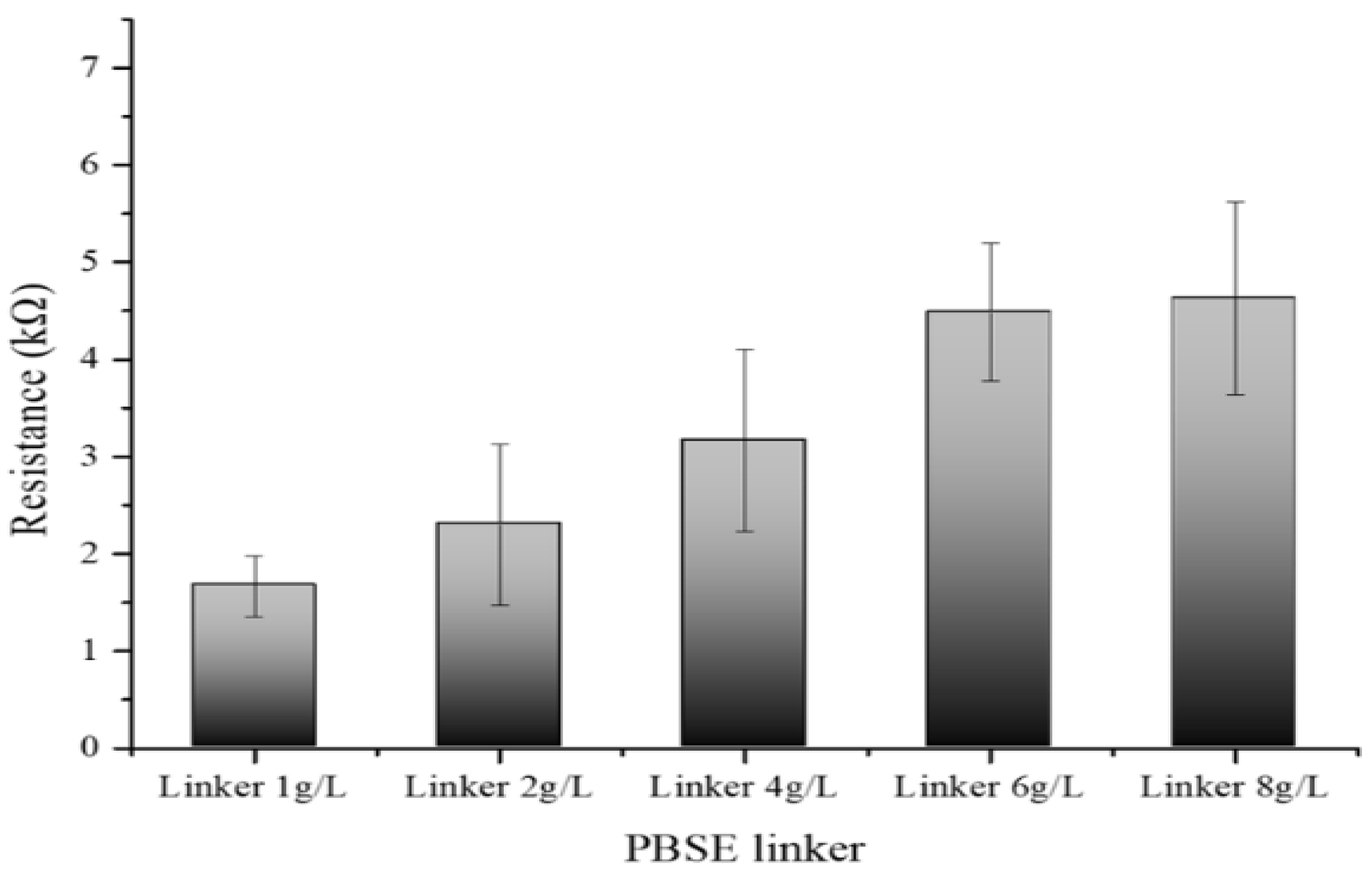
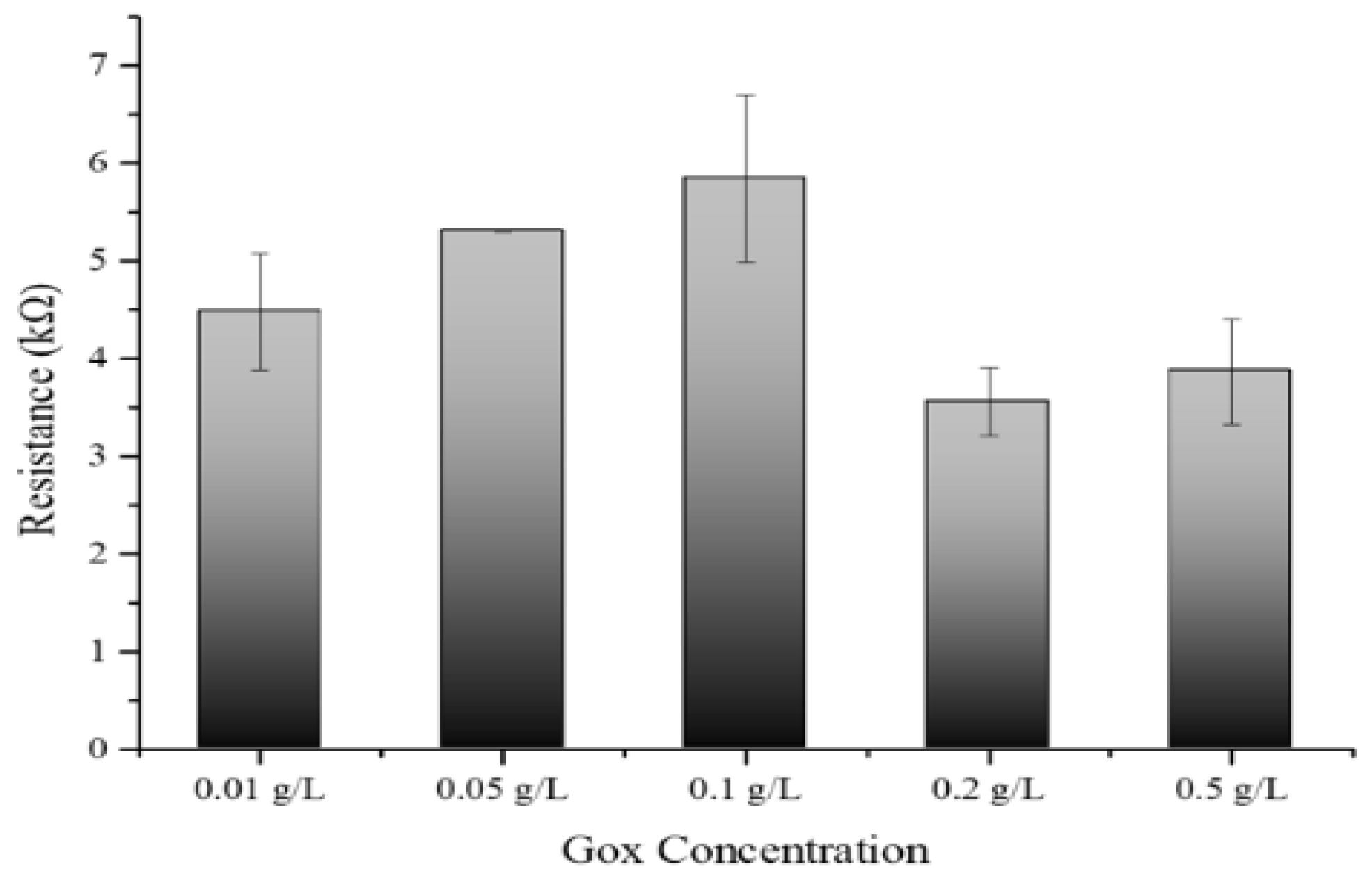
3.6. Optimization of Gox Concentration with Developed Biosensors
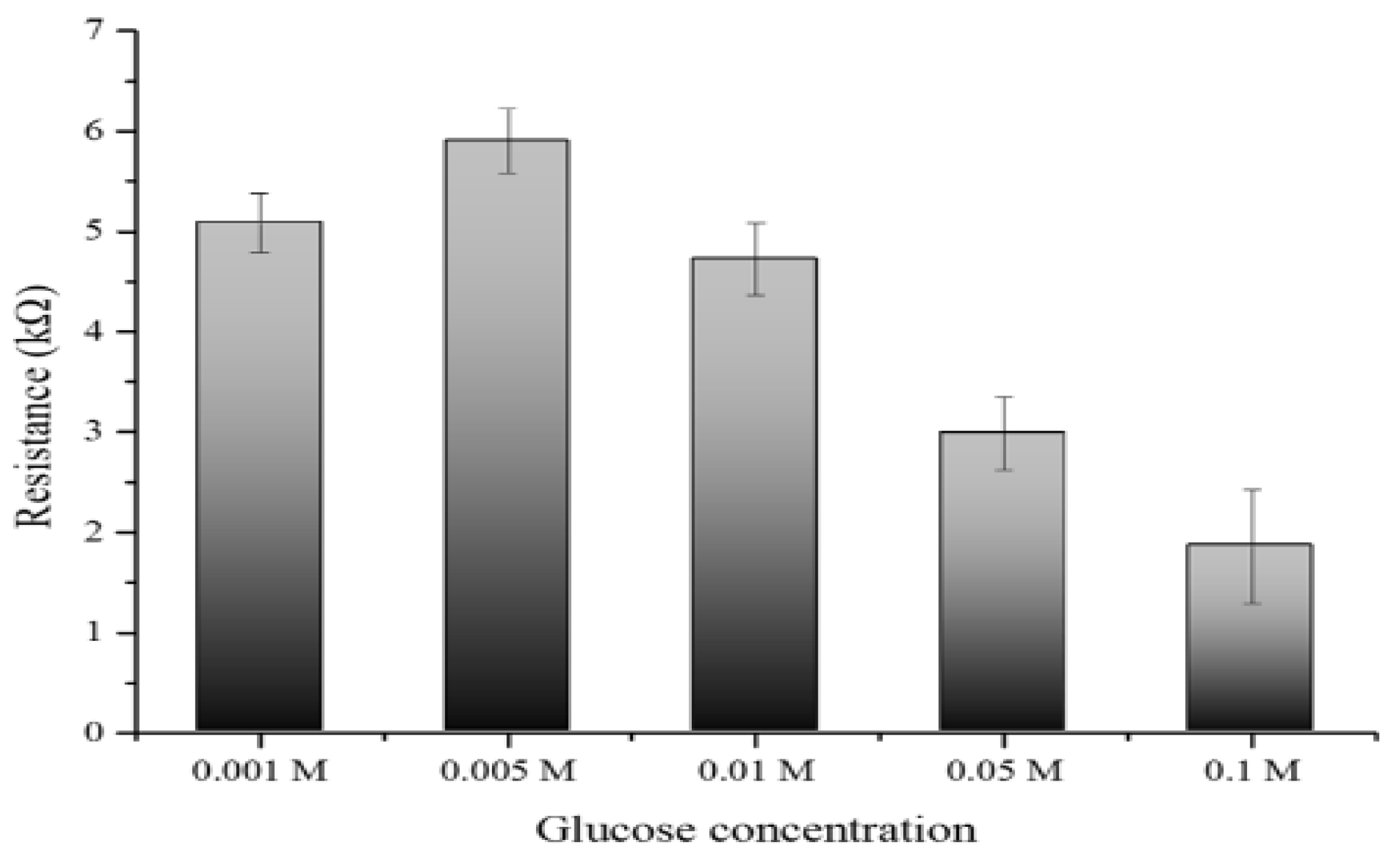
3.7. Glucose concentration Impact on Electrical Resistance in the Developed Biosensors
4. Conclusions
Author Contributions
Declaration of competing interest
Acknowledgements
References
- M. Lotfy, J. Adeghate, H. Kalasz, J. Singh, E. Adeghate, Chronic Complications of Diabetes Mellitus: A Mini Review, Curr Diabetes Rev. 13 (1) (2017) 3-10. [CrossRef]
- Diabetes Care in the Hospital: Standards of Medical Care in Diabetes, Diabetes Care. 44, supplement 1 S211-S220. [CrossRef]
- M. Erbach, G. Freckmann, R. Hinzmann, B. Kulzer, R. Ziegler, L. Heinemann, and O. Schnell, Interferences and Limitations in Blood Glucose Self-Testing, J Diabetes Sci Technol. 10 (2016) 1161-1168.
- F. Gao, C. Liu, L. Zhang, T. Liu, Z. Wang, Z. Song, H. Cai, Z. Fang, J. Chen, J. Wang, M. Han, J. Wang, K. Lin, R. Wang, M. Li, Q. Mei, X. Ma, S. Liang, G. Gou & N. Xue, Wearable and flexible electrochemical sensors for sweat analysis: a review, Microsystems & Nanoengineering. 9 (2023) 1. [CrossRef]
- M. Younus Wani, N.A. Ganie, K.A. Dar, S.Q. Dar, A. Husain Khan, N. A. Khan, S. Zahmatkesh, M. Saood Manzar, R. Banerjee, Nanotechnology future in food using carbohydrate macromolecules: A state-of-the-art review, Int. J. Biol. Macromol. 239 (2023) 124350. [CrossRef]
- Sobhan, J. H. Oh, P. Mi-Kyung, L. Jinyoung, Reusability of a single-walled carbon nanotube-based biosensor for detecting peanut allergens and Y. enterocolitica, Microelectronic Engineering. 225 (2020) 111281. [CrossRef]
- Hina W. Saadeh, Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review, Sensors. 22 (2022) 485. [CrossRef]
- S. D. Psoma, C. Kanthou, Wearable Insulin Biosensors for Diabetes Management: Advances and Challenges, Biosensors, 13 (2023) 719. [CrossRef]
- Sobhan, L. Jinyoung, P. Mi-Kyung, O. Jun-Hyun, Rapid detection of Yersinia enterocolitica using a single–walled carbon nanotube-based biosensor for Kimchi product, Lwt. 108 (2019) 48-54. [CrossRef]
- W. Joseph, Carbon-Nanotube Based Electrochemical Biosensors: A Review, Electroanalysis. 17(1) (2005) 7-14. [CrossRef]
- M. Sireesha,V. Jagadeesh Babu, A. Sandeep Kranthi Kiran & S. Ramakrishna, A review on carbon nanotubes in biosensor devices and their applications in medicine, Nanocomposites. 4 (2018) 2. [CrossRef]
- J. Janssen, M. Lambeta, P. White.A. Byagowi, Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection, Biosensors. 9 (2019) 4 144. [CrossRef]
- P. Liu, Y. Jiao, X. Chai, Y. Ma, S. Liu, X. Fang, F. Fan, L. Xue, J. Han, Q. Liu, High-performance electric and optical biosensors based on single-walled carbon nanotubes, Journal of Luminescence. 250 (2022) 119084. [CrossRef]
- R. Nißler, J. Ackermann, C. Ma, & S. Kruss, Prospects of fluorescent single-chirality carbon nanotube-based biosensors. Analytical Chemistry. 94(28) (2022) 9941-9951. [CrossRef]
- Alatzoglou, E. I. Tzianni, M. Patila, M. G. Trachioti, M. I. Prodromidis, H. Stamatis, Nanomaterials.14 (2024) 85. [CrossRef]
- Y. Hyungsub, T. Russ, H. Byungil, Colloids Interface Sci. 52 (2023) 100686. [CrossRef]
- L. Jinyoung, Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment. Biosensors, 13(2) (2023) 183. [CrossRef]
- Y. Gao, J. Luo, Z. Li, F. Teng, J. Zhang, S. Gao, M. Ma, X. Zhou and X. Tao, Dispersion of carbon nanotubes in aqueous cementitious materials: A review, Nanotechnology Reviews. 12 (2023) 20220560. [CrossRef]
- Q.Pan, Q. Wu, Q. Sun, X. Zhou, L. Cheng, S. Zang,Y. Yuan, Z. Zhang, J. Ma, Y. Zhang, B. Zhu, Biomolecule-friendly conducting PEDOT interface for long-term bioelectronic devices, Sensors and Actuators B: Chemical, 373 (2022). [CrossRef]
- Z. Hameed, S. A. Raj, J. Kandasamy, M. A. Baghdadi and M. A. Shahzad, Chitosan: A Sustainable Material for Multifarious Applications, polymers, 14 (2022) 2335 . [CrossRef]
- S. Kim, X. Yang, J.H. Lee, H. Y. Yoo, C. Park,S. W. Kim, and J. Lee, Development of GO/Co/Chitosan-Based Nano-Biosensor for Real-Time Detection of D-Glucose, biosensors, 12 (2022) 464 . [CrossRef]
- M. Rizvi, H. Gerengi, and P. Gupta, Functionalization of Nanomaterials: Synthesis and Characterization, ACS Publications. 1 (2022) 1-26. [CrossRef]
- N. Kumar & S. Sinha Ray, Synthesis and Functionalization of Nanomaterials, Processing of Polymer-based Nanocomposites. 277 (2018) 15-55. [CrossRef]
- X. Yang, Y. Chen, C. Zhang, G. Duan, S. Jiang, Electrospun carbon nanofibers and their reinforced composites: Preparation, modification, applications, and perspectives, Composites Part B: Engineering. 249 (2022) 110386. [CrossRef]
- Devi Thongam, H. Chaturvedi, Functionalization of Pristine, Metallic, and Semiconducting-SWCNTs by ZnO for Efficient Charge Carrier ransfer: Analysis through Critical Coagulation Concentration, ACS OMEGA. 7 (2022) 14784-14796. [CrossRef]
- Y. Hyungsub, T. Russ, H. Byungil, Dispersibility study of carbon nanotubes using multiple light scattering: A mini-review, Colloid and Interface Science Communications. 52 (2023) 100686. [CrossRef]
- R. Chamorro, L. de Juan-Fernández, B. Nieto-Ortega, Maria J. Mayoral, S. Casado, L. Ruiz-González, Emilio M. Pérez and D. González-Rodríguez, Reversible dispersion and release of carbon nanotubes via cooperative clamping interactions with hydrogen-bonded nanorings, Chem. Sci. 17 (2018) 4176-4184. [CrossRef]
- Daneshvar, H. Chen, K. Noh, H. J. Sue, Critical challenges and advances in the carbon nanotube–metal interface for next-generation electronics. Nanoscale Adv. 3 (2021) 942-962. [CrossRef]
- Stando, S. Han, B. Kumanek, D. Łukowiec, and D. Janas, Tuning wettability and electrical conductivity of single-walled carbon nanotubes by the modified Hummers method. Sci Rep. 12 (2022) 4358. [CrossRef]
- Krasulina, Y. Myasnikova, V. Saik, M. Predtechensky, and S. N. Smirnov, Improved Characterization of Aqueous Single-Walled Carbon Nanotube Dispersions Using Dynamic Light Scattering and Analytical Centrifuge Methods, ACS OMEGA. 8 (2023) 39233-39241. [CrossRef]
- K. Metze, S. Sant, Z. Meng, H. A. Klok, and K. Kaur, Swelling-Activated, Soft Mechanochemistry in Polymer Materials, Langmuir. 39 (2023) 10 3546-3557. [CrossRef]
- N. Hoang Hiep, L. Sun Hyeok, L. Ui Jin, F. D. Cesar, K. Moonil, Immobilized Enzymes in Biosensor Applications, Materials. 12 (2019) 121. [CrossRef]
- D. K. Manish, Z. Andleeb, A. Mohd, M. Mukesh, A. Laxmi, S. Siddhartha, S. Shruti, U. S. Ram, M. B. Ruben, B. K. Vivek, Improvement Strategies, Cost Effective Production, and Potential Applications of Fungal Glucose Oxidase (GOD): Current Updates, Sec. Food Microbiology. 8 (2017) 1032. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




