1. Introduction
Forest pests are causing negative impacts on forest resources worldwide, mainly in coniferous forests. Conifer bark beetles (Coleoptera: Curculionidae: Scolytinae.) are one of the major factors of biological disturbance in temperate forests [
1,
2,
3]. Native forest insect pests are a natural phenomenon that helps control the density and spatial distribution of trees; however, in some instances, they are one of the main causes of disturbance. About 70 species of insects and pathogens affecting forests are recorded in Mexico [
4]. According to a regular monitoring carried out in forest areas in Mexico, during 1990–2014, the average forest area affected each year by pests and diseases was 50 483 ha. Most of this area was affected by bark beetles (39 %) [
4].
Some of the main pests of temperate forests are the bark beetles of the genus
Dendroctonus [
5]. Species of this genus are specific parasites of pine and other conifer species (Coniferales); therefore, their geographic distribution is associated with these plant communities [
6].
Over the past few decades, billions of trees from native coniferous forests have been devastated by bark beetles, spanning a wide geographic scale from Mexico to Alaska; currently, several outbreaks have been considered among the largest and most severe in history [
7]. The dynamics of insect populations are affected by climate variability, as the life cycle of insects accelerates with increasing temperatures [
8,
9].
Several studies have been conducted on the genus
Dendroctonus. Some of them have addressed population fluctuations through traps baited with pheromones [
10], and others have focused on the influence of climatic variables on the increase of populations. It has been documented that the populations of these bark beetles are partially regulated by environmental conditions [
11].
The spatio-temporal dispersal of pest species, such as bark beetles, plays a central role in their ecology and population dynamics. Understanding the underlying patterns is critical to the implementation of appropriate management strategies [
12]. Therefore, monitoring is a primary procedure in the management of bark insects, as it provides information about their presence, population abundance, diversity, development states, and spatial and temporal distribution, among other aspects [
13]. Regular monitoring information and assessment of the damage caused by pests are basic parameters for evaluating forest health [
14].
This study aims to know the community of Dendroctonus species (the ecological guild), the fluctuations of the populations of the main species throughout the year, and the effects of relative humidity, temperature, and precipitation on the fluctuations of these species in three sites of mixed forests in the municipality of Bocoyna, Chihuahua.
2. Materials and Methods
2.1. Study Area
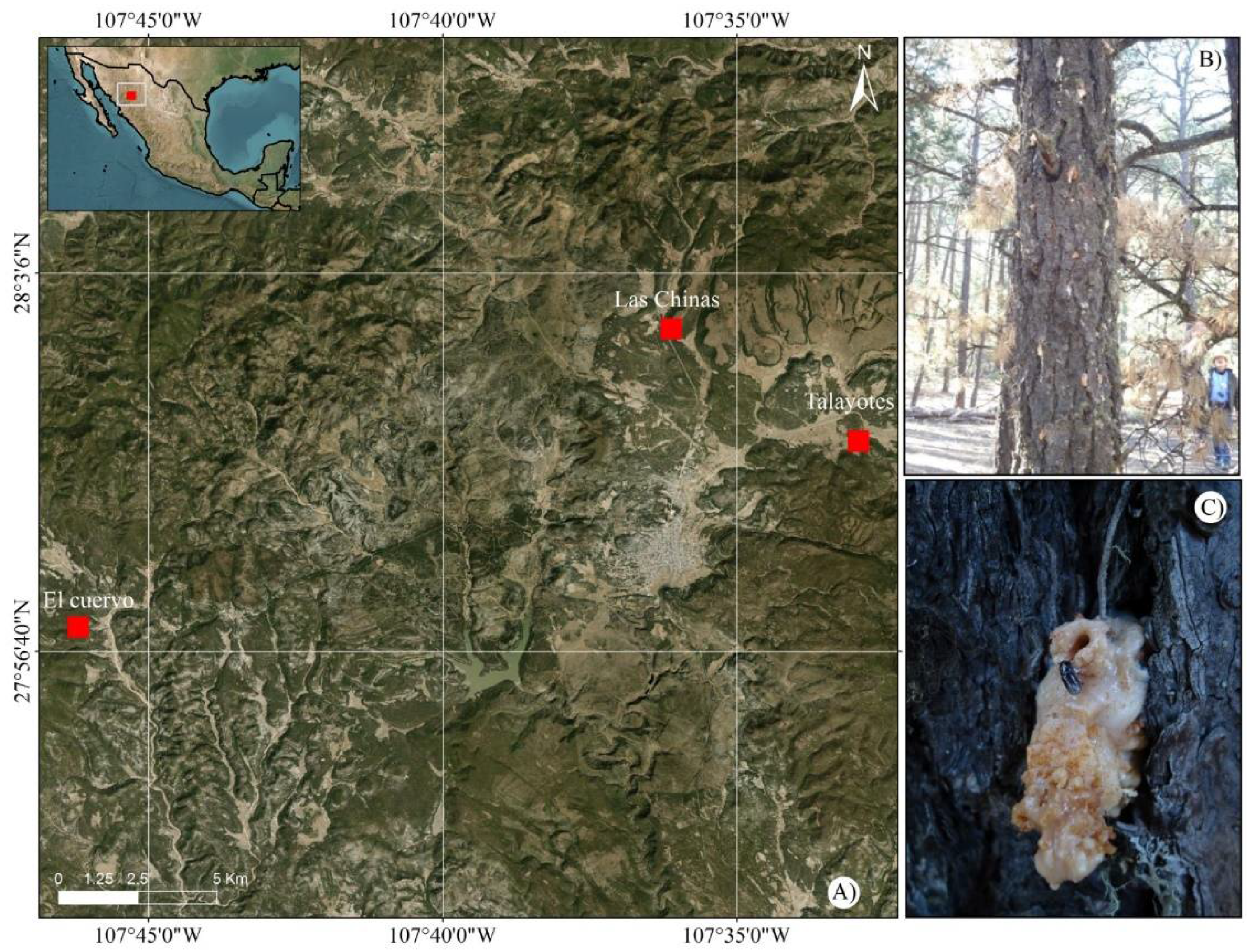
The study was conducted in the municipality of Bocoyna Chihuahua, Mexico, located at 27°51' N and 107°35' W and an altitude of 2348 m asl (
Figure 1). This municipality comprises an area of 2801.80 km², ranking fifth in surface in the state. It is completely surrounded by the Sierra Madre Occidental, reaching altitudes above 2800 m asl. The highest peaks are Ojitos, Nichupiachi, Sojáhuachi, and Rumúrachi. The main land uses are livestock and forestry. Land tenure is ejidal, with 227,744 ha, representing 78.2 % of the municipal area. The private regime comprises 8.6 % of the total area (253 150 ha), and 0.16 % (469 ha) corresponds to urban use.
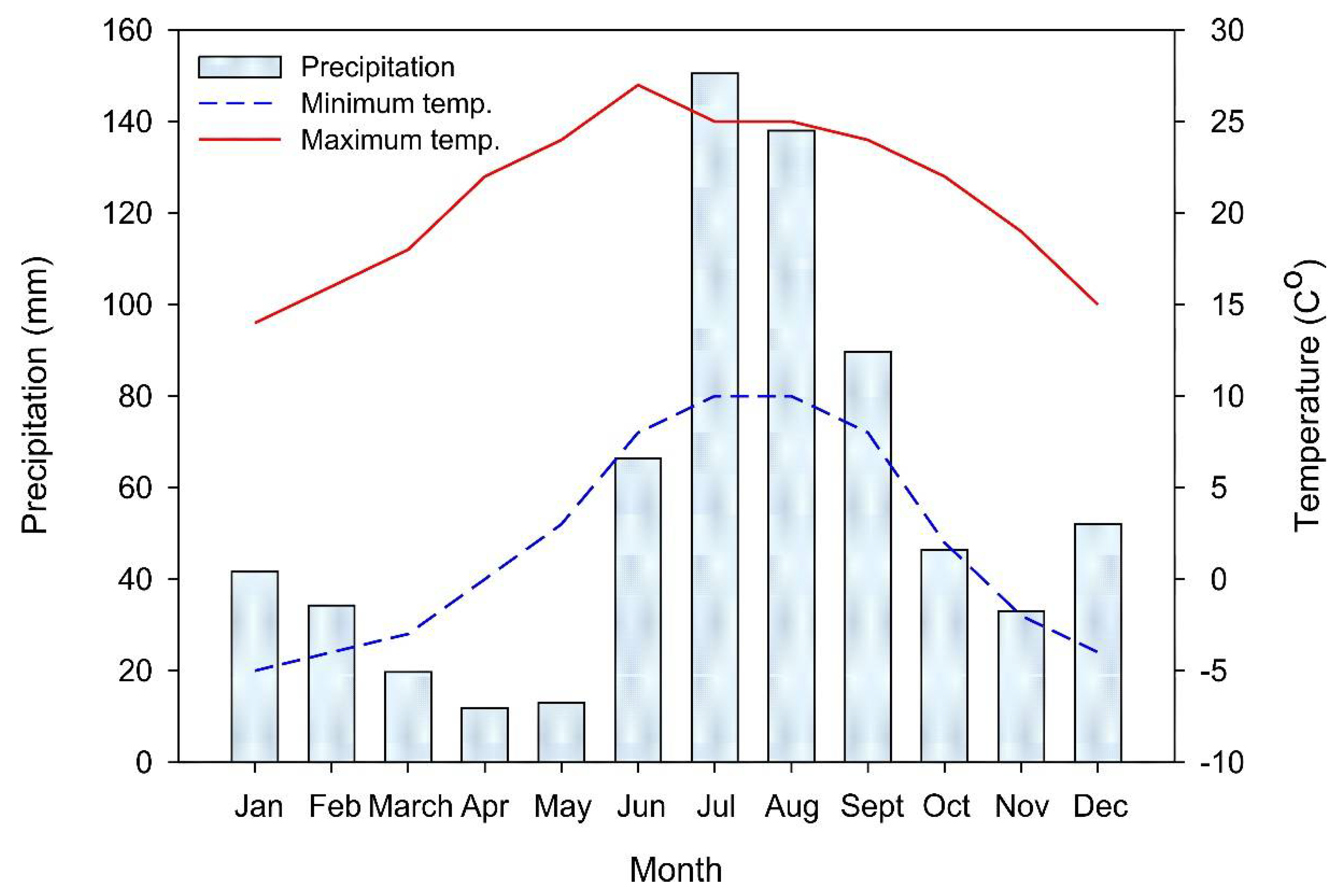
The local climate in the municipality of Bocoyna is cold subhumid. The maximum and minimum annual temperatures are 31.1 ºC and -17.8 ºC, respectively; the mean annual precipitation is 683.3 mm with an annual mean of 85 rainy days and heavy snowfall during winter (
Figure 2). The main soil types are Regosol, Luvisol, Umbrisol, and Leptosol [
15].
The study area is covered by a mixed pine forest with several species, such as Pinus arizonica Engelm, P. engelmannii Carr., P. ayacahuite Ehrenb. Ex Schltdl., P. durangensis Martínez, Picea chihuahuana Martínez, Pseudotsuga menziesii (Mirb.) Franco, Arbutus xalapensis Kunth, Juniperus deppeana Steud., and Quercus spp.
2.2. Monitoring of Bark Beetles
To determine the population fluctuations of bark beetles (Dendroctonus spp.) in the municipality of Bocoyna, we selected the following three sites for monitoring: 1) Las Chinas private land (PPC), (28° 36’ 40.18”N and 107° 36’ 5.70” W; altitude: 2536 m asl); 2) El Cuervo (CUE) (27° 57’ 4.80” N and 107° 46’ 90” W; altitude: 2458 m asl); and 3) Talayotes (TAL) (27° 59’ 24.79” N and 107° 32’ 54.00” W; altitude: 2648 m asl). At each site, we installed Lindgren traps with eight funnels and a collector vessel [
16] baited with semiochemicals (pheromones and allelochemicals). Three different semiochemicals were placed in each baited trap: frontalin and endo-brevicomin (pheromones), and alpha/beta-pinene (kairomone) (Synergy Semiochemicals Corp®), which were replaced every two months. Eight traps (four with attractant and four control traps) were distributed in each site, covering an altitudinal gradient of 100 m and separated by a distance of at least 20 m between traps, a standard method proposed by Macías-Sámano et al. [
17]. The three monitored sites have mixed forests distributed at similar altitudes.
The insects were collected every 15 days for two years. The monitoring started in March 2015 and ended in March 2017. The sampling period used is recommended as a standard method by Macías-Sámano et al. [
17].
The collected insects were separated, identified, and counted monthly; all were kept in 70 % alcohol. These specimens were identified based on their external morphology and using the keys of Wood [
18] and Cibrián et al. [
19]. To confirm the correct identification of
Dendroctonus mexicanus Hopkins
and Dendroctonus frontalis Zimmerman (externally similar species), the seminal rod of a subsample of both species was extracted, mounted, examined, and compared according to the illustrations of the seminal rod of these species in Lanier et al. [
1]. This process was carried out in the Laboratory of Forest and Agricultural Health of the Pabellón Experimental Field of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (National Institute of Forestry, Agricultural, and Livestock Research; INIFAP, in Spanish) at Pabellón de Arteaga, Aguascalientes, Mexico. To accomplish this, the abdomen was separated from the rest of the body of male specimens and placed in a 4.0 mL Eppendorf tube containing 10 % KOH. The tubes were immersed in a water bath at 80 °C for 10 minutes. Subsequently, the abdomens were rinsed with distilled water and dehydrated in alcohol. Each abdomen was dissected with entomological needles under a stereo microscope to extract the seminal rod; this was placed on a glass slide with a drop of Canadian balm and covered with a coverslip. The samples were observed under the microscope and compared with the reference images [
1].
2.3. Climate Data and Analysis
Climate variability data (temperature and relative humidity) were obtained from climate data loggers (Hobbos 16 H01-001-01; Onset Computer Corporation, Pocasset, MA, USA), which recorded data every 30 minutes. Each Hobbo logger was placed between the attractant trap and the control trap. This equipment stores up to 16,382 temperature and relative humidity records within -35 °C to 80 ºC and 0 % to 100 % relative humidity. Data were downloaded to a PC every two months using the Easy Log USB 5.45 program provided by the Lascar manufacturer; the information was monitored for two years. Likewise, climate data were collected and reviewed from databases of the meteorological stations closest to the study areas. This process was carried out to understand the historical climatic conditions in this area.
The monthly number of bark beetles from each site was associated with monthly temperature (minimum and maximum) and relative humidity (minimum and maximum) data recorded by the Hobbos logger and with monthly precipitation data obtained from meteorological stations. The data were analyzed using descriptive statistics. Fluctuation patterns were observed for the populations of Dendroctonus mexicanus, D. frontalis, and D. brevicomis LeConte, the species with the highest number of catches at each site.
The relationship between the abundance of D. frontalis, D. mexicanus, and D.brevicomis and the climatic variables was assessed using Spearman’s correlation tests. The non-parametric Kruskal-Wallis test was used because the data were not normally distributed. Finally, linear regression analyses were performed between the abundances of D. frontalis, D. mexicanus, and D. brevicomis and the climatic variables with an ANOVA test. The best model was determined based on the coefficient of determination (r2).
3. Results
3.1. Monitoring of Bark Beetles
A total of 40,517 bark insects were collected at the three sites during the 24 months, with six species identified: Dendroctonus mexicanus, D. brevicomis, D. frontalis, D. adjunctus Blandford, D. valens LeConte, and D. approximatus Dietz.
From 01 March to 31 December 2015, 25,712 bark insects were collected at the three study sites (Las Chinas, El Cuervo, and Talayotes). The most abundant species was D. mexicanus with 12,136 specimens, followed by D. brevicomis with 8,865; D. frontalis, 4,219; D. adjunctus, 328; D. valens, 164: and D. approximatus, 37. From 01 January to 31 December 2016, 13,773 bark insects were collected. The most abundant species was D. mexicanus with 9419 specimens, followed by D. brevicomis with 2,480 and D. frontalis with 1,258. Finally, from 01 January to 31 March 2017, a total of 1,032 bark insects were collected, with 933 corresponding to D. mexicanus, 57 to D. brevicomis, and 39 to D. frontalis.
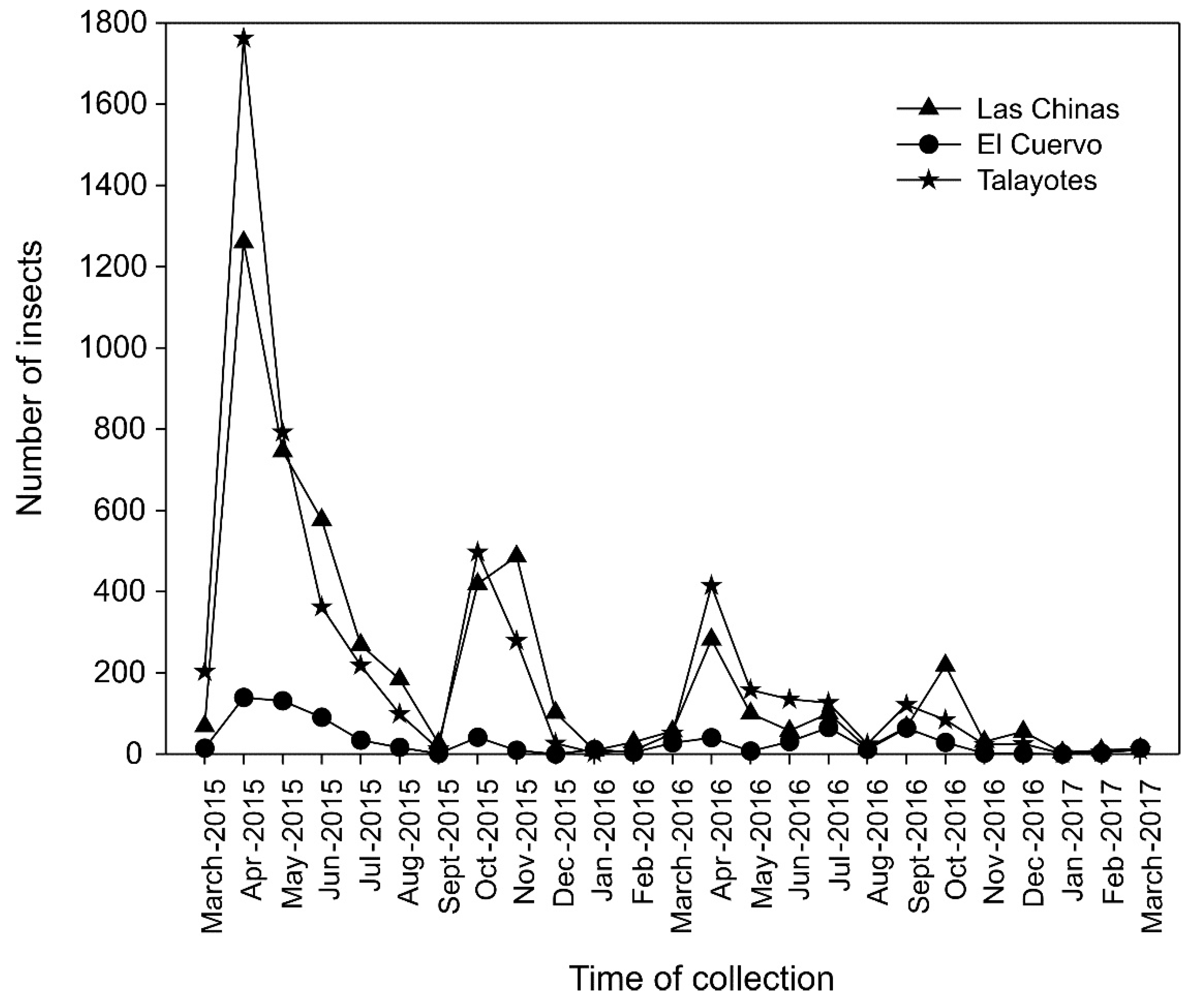
3.2. Fluctuations of Dendroctonus Mexicanus Populations
The populations of
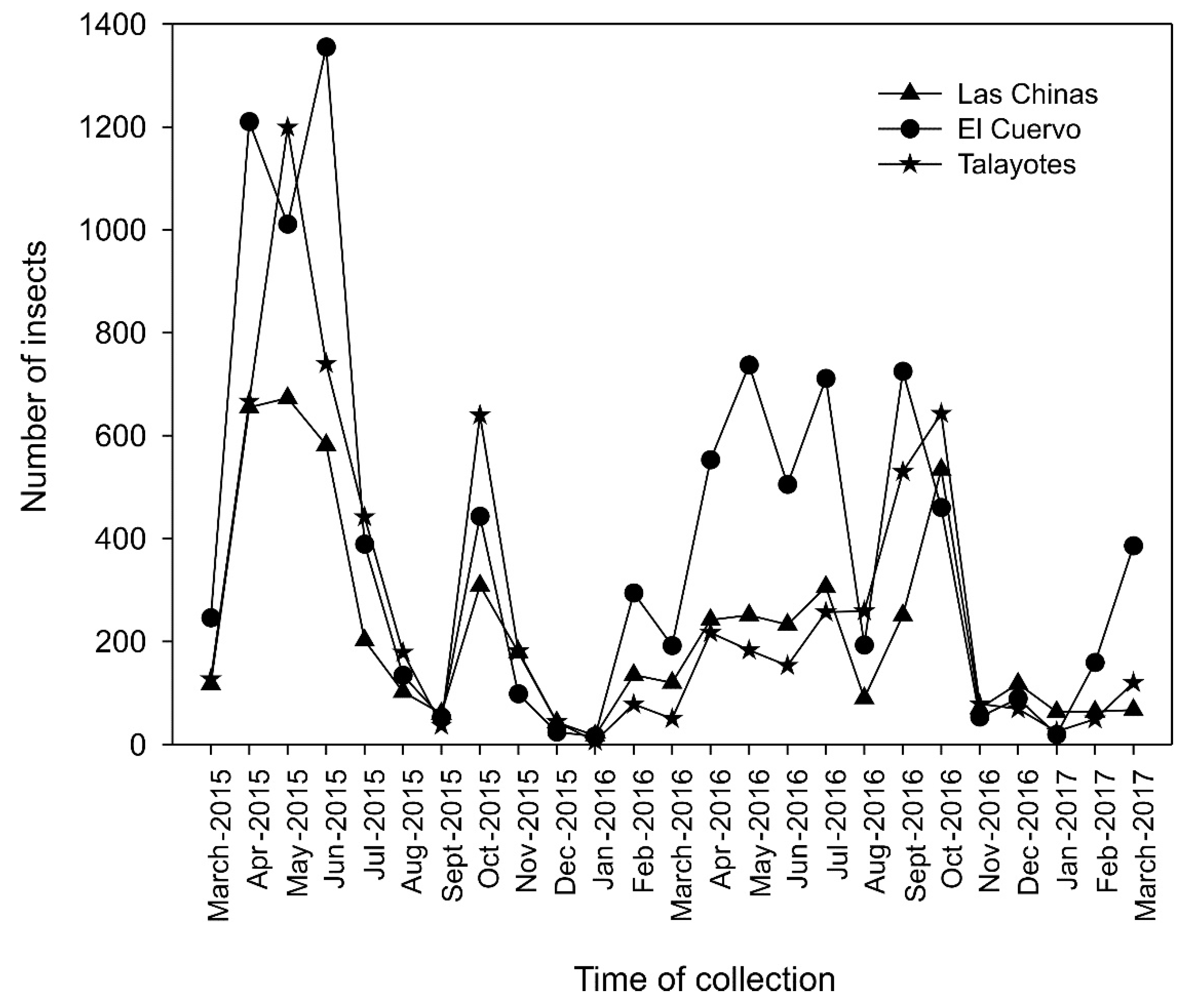
D. mexicanus varied over the monitoring time, showing similar behavior in the three sites. The bark beetle populations showed increased flight activity from April to June that lasted until mid-July in both monitoring years (2015 and 2016) (
Figure 3). Flight activity started decreasing from August onward, and the period with the lowest records for 2015 and 2016 ranged from mid-August to December.
At Las Chinas, we observed three dispersal peaks in D. mexicanus catches each year: April-May, June, and October 2015 and April-May, July, and October 2016. At El Cuervo, three peaks per year were also observed: April, June, and October 2015, and May, July, and September-October 2016. At Talayotes, two peaks were observed each year: April-May and October 2015, and April and October 2016 (
Figure 3). In general, the site with the highest number of catches was El Cuervo, followed by Talayotes, and the site with the lowest number of beetles captured was Las Chinas.
In 2017, catches were scarce at the three sites, as flight activity was minimal in January and February, beginning to rebound in March, except in El Cuervo, where activity started increasing in late January and peaked in March (
Figure 3).
3.3. Fluctuations of Dendroctonus Frontalis Populations
The populations of
D. frontalis showed variations over the monitoring period, with a similar behavior observed at the three sites (
Figure 4). The highest flight activity of these populations was recorded from April to June in both years (2015 and 2016), followed by a rebound in mid-September and a decline in November. The highest peak occurred in April-May and October 2015 and in May, July, and from mid-September to mid-October 2016.
August recorded low activity, and the periods of lowest activity were mid-September and December in both years. The lowest flight activity was observed in January, February, mid-August, and December (
Figure 4).
At Las Chinas, three dispersal peaks were observed in the catches of D. frontalis each year: April-May, June, and October 2015, and April-May, July, and October 2016. At El Cuervo, two peaks were observed in 2015: April-June and October; in 2016, three peaks were recorded: April, July, and September-October. At Talayotes, three peaks of insect populations were observed each year: April-May, July, and October 2015, and April, June-July, and September 2016.
In 2017, catches were minimal at the three sites, as flight activity in January and February was minimal, beginning to rebound in March. The site with the highest number of catches was El Cuervo, followed by Talayotes, and the site with the lowest recorded population was Las Chinas.
3.4. Fluctuations of Dendroctonus Brevicomis Populations
The populations of
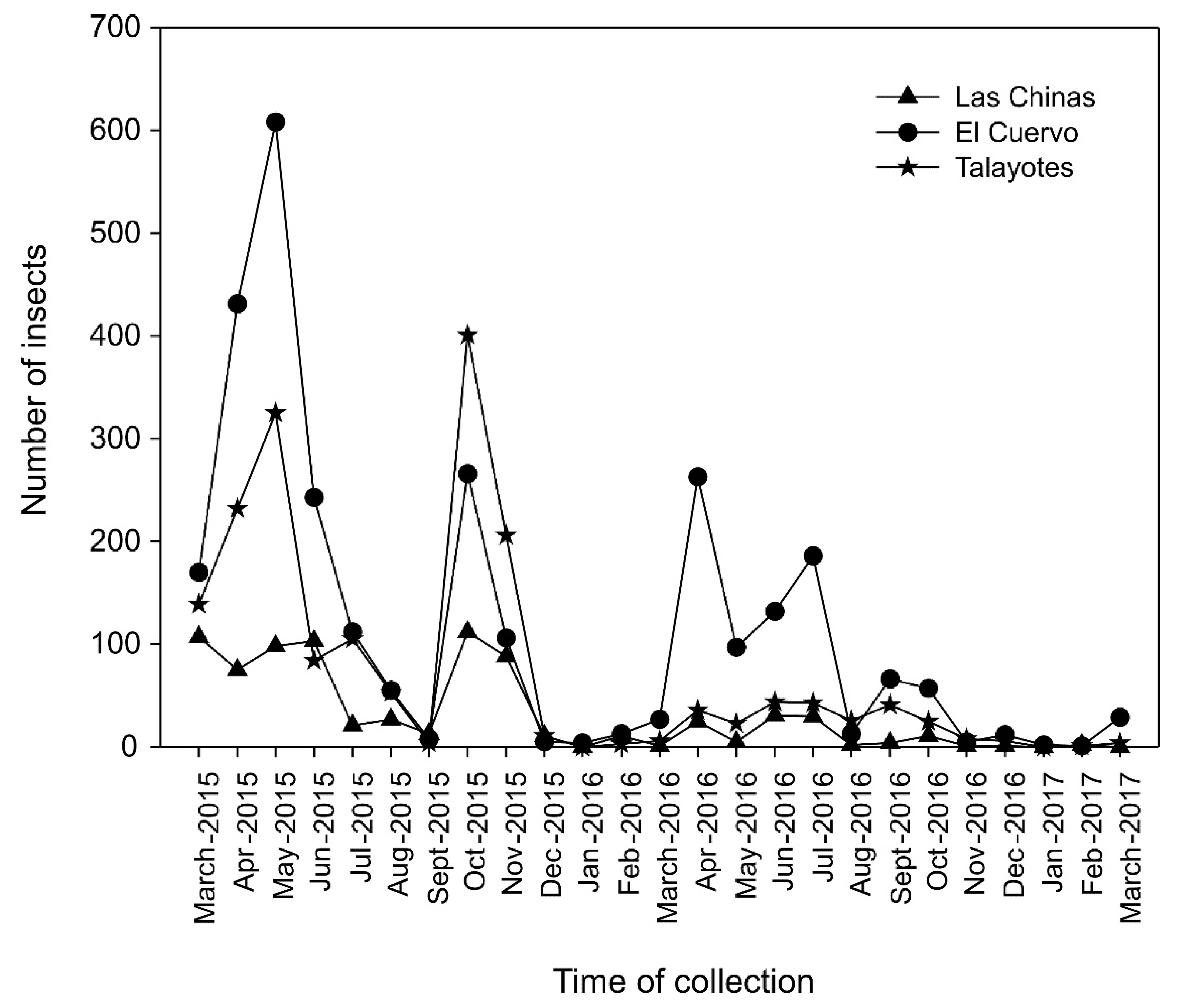
D. brevicomis showed variations over the monitoring time, which were similar at the three sites (
Figure 5). The highest flight activity occurred in March-May and October-November 2015, and in April-May, July, and September-October 2016. The highest peaks were observed in April-May and October 2015 and in April-May, July, and from mid-September to mid-October 2016. The lowest activity was recorded in mid-September and December 2015, and in January, mid-August, and December 2016.
At Las Chinas, two dispersal peaks were observed in D. brevicomis populations in 2015: April-May and October-November; in 2016, three dispersal peaks were observed: April-May, July, and October; the peak of greatest dispersal was observed in April-May. At El Cuervo, two peaks were observed in 2015, April-May and October, and three in 2016, April, July, and October. At Talayotes, two peaks were observed in 2015, April-May and October-November, and three in 2016, April-May, July, and September-October (
Figure 5).
In 2017, catches were minimal at the three sites, as flight activity in January and February was minimal, beginning to rebound in March. Talayotes was the site with the highest number of catches for this species, followed by Las Chinas, and the site with the lowest number of catches was El Cuervo.
3.5. Relationship between Dendroctonus Population Density and Climatic Variables
The Kruskal-Wallis statistical test showed significant differences (p < 0.05) between the total number of insects collected per year (2015 and 2016) (
Table 1).
Considering individual species, significant differences (p < 0.01) were observed between years for D. frontalis and D. brevicomis, and non-significant differences for D. mexicanus. Likewise, non-significant differences were observed in the total number of insects between sites. However, the analysis of differences between sites for individual species reveals significant differences (p < 0.05) for D. mexicanus and highly significant differences (p < 0.01) for D. frontalis and D. brevicomis.
The relationship between the number of
D. mexicanus and temperature (minimum, maximum, and mean) was highly significant (p < 0.01); relative humidity showed a non-significant negative association (p > 0.05), and precipitation, a non-significant positive relationship (p > 0.05), with fluctuations in insect density (
Table 1). The correlation coefficient between the number of
D. frontalis individuals and the minimum temperature showed a positive and highly significant association (p < 0.01) and a non-significant relationship with the rest of the variables (
Table 1). The number of
D. brevicomis showed a highly significant positive relationship (p < 0.01) with the minimum temperature and a highly significant negative relationship (p < 0.01) with the maximum and mean humidity (
Table 1).
The analysis of population dynamics and correlation coefficients showed that other factors affect the behavior of each species, indicating that these are independent of relative humidity and precipitation.
The correlations between the total number of insects and the climatic parameters of temperature (minimum, maximum, and mean), relative humidity (minimum, maximum, and mean), and precipitation determined a highly significant relationship (p < 0.01) with temperature, a significant negative relationship (p < 0.05) with the maximum relative humidity, and a non-significant relationship with the other variables (
Table 1,
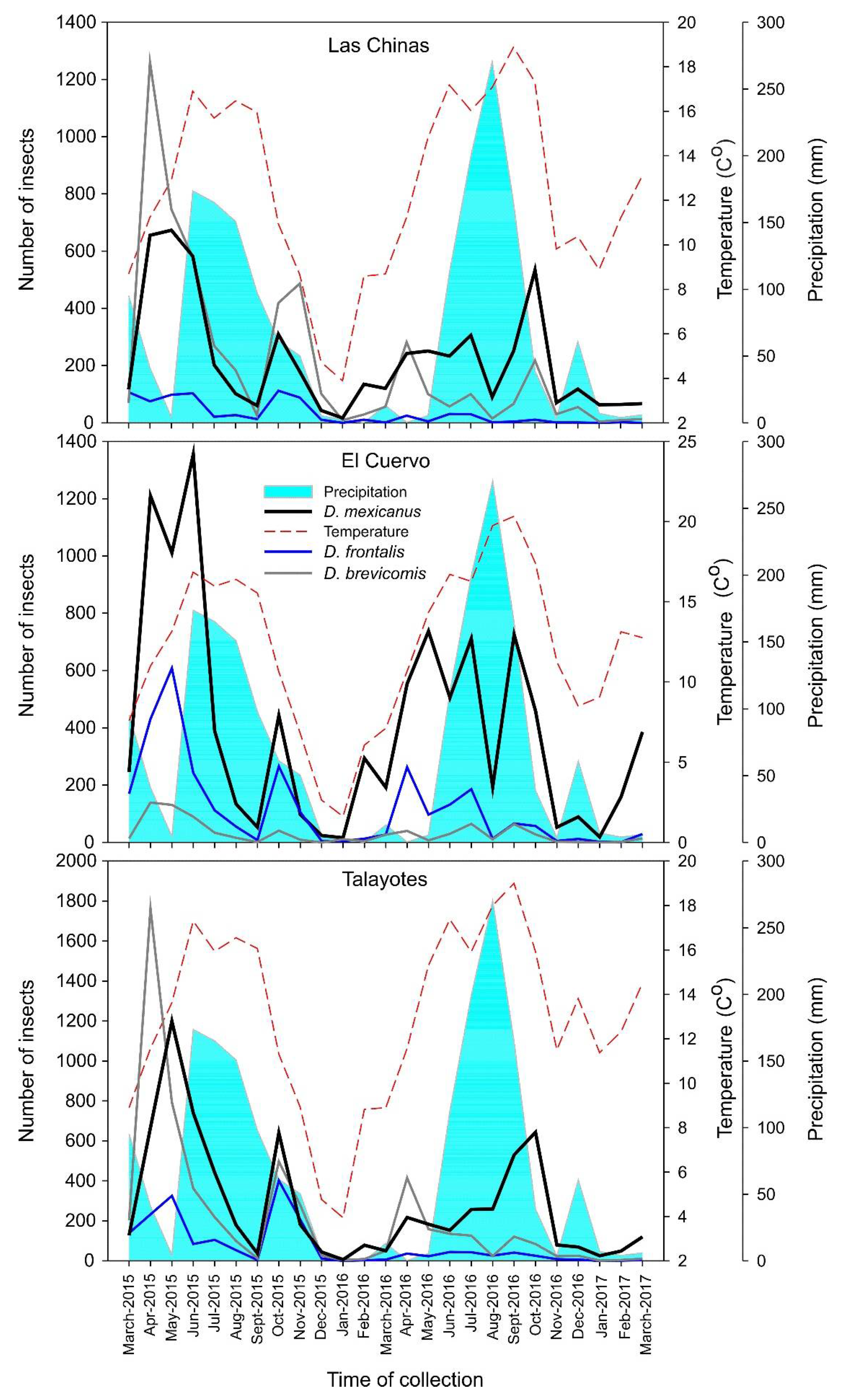
Figure 6).
4. Disssioncu
4.1. Bark Beetles
The number of Dendroctonus species was the same in the three sites studied. The D. mexicanus populations at the three sites of Bocoyna, Chihuahua, showed marked population fluctuations on dates corresponding to the dry season and warmer temperatures. The observed dispersal pattern of
D. mexicanus is similar to that observed in the Sierra de Galena, Nuevo León, which occurs in September-December (autumn) and March-May (spring) [
13]. Similarly, this pattern is consistent with what was reported by Sánchez and Torres [
20], who reported that
D. mexicanus shows one peak of activity from October to December and another between March and June. Rodríguez [
21] also mentioned that May is the month of peak activity for this species, in contrast to Cuéllar et al. [
22], who reported more individuals of
D. mexicanus in the winter months than in any other period, coinciding with reports for the central state of Nuevo León [
13] and the southern United States [
23].
The flight activity of the
D. frontalis populations coincides with the information reported by Avilés et al. [
24] for this species in the state of Hidalgo, showing a seasonal behavior with the highest values in spring. However, the periods with the lowest values were recorded in autumn, which differs from the observations in our study area.
Morales et al. [
25] observed a greater abundance of this species in April, November, and December in the municipality of Landa de Matamoros, Querétaro, associated with the maximum temperature (20 °C). These findings are consistent with the results observed in this study in April. Thompson and Moser [
26] mentioned that the optimal flight temperature for this species is 27 °C. This usually occurs in April and May and continues until the end of September and October. There are periods in which activity increases due to the emergence of adults in summer.
The dispersal pattern of
D. brevicomis in the three study sites is similar to the patterns reported for Sierra de Arteaga in the state of Coahuila [
13]. Ruiz-González et al. [
27] observed that the time of greatest flight activity in
D. brevicomis occurs in spring and summer, which is consistent with the results obtained in this study in spring. However, there is a discrepancy in autumn, as these authors reported that this season shows low flight activity, while this work recorded a second peak of dispersal in autumn. Ortega-Ortíz [
28] reported four dispersal peaks throughout the year (March, July, and October) for this species in El Salto, Durango.
4.1. Population Density of Dendroctonus and Climatic Variables
The variable that influences the fluctuations of bark beetle populations is temperature. This coincides with what has been documented by several authors who mention that temperature directly affects insects through development rates, reproduction, voltinism, flight activity, and associations with other organisms [
29,
30,
31,
32]. As for relative humidity and precipitation, there is no significant positive relationship.
When each species was analyzed separately, we observed a differential response to temperature (minimum, maximum, and mean). In the three sites, the fluctuations in
D. mexicanus catches are related to the monthly minimum temperature, consistent with observations by Alvarado [
33], who reported significant correlations between the minimum temperature and
D. mexicanus catches in the Sierra Gorda de Guanajuato, although
D. mexicanus is also related to the maximum and mean temperatures (
Table 1,
Figure 6).
The highest abundance of bark beetles was determined to occur in April, May, and June (
Figure 6). Pereda-Breceda [
34] reported that the highest abundance of bark insects of the genus
Dendroctonus spp. in the forest region of San Dimas, Durango, occurs in April, May, and June. Additionally, this author indicates a positive correlation between the increase in abundance and the rise in annual temperature, while the frequency of individuals decreases as precipitation increases. These results closely agree with our findings in the present study (
Figure 6).
Logan and Bentz [
35] and Werner et al. [
36] mention that higher temperatures favor the flight activity of bark beetles and that this activity decreases as temperature drops, since this climatic variable plays a central role in the emergence of bark beetles from the host and their flight. The opposite is observed in relation to precipitation because the flight activity of these insects decreases as precipitation increases.
The activity of
D. mexicanus is lower in the winter months [
37], which is consistent with the fluctuations observed during the monitoring period in our study area (
Figure 6). Ortega-Ortíz et al. [
28] observed that the greatest abundance of bark beetles occurred in June, related to the highest temperature. In addition, these authors indicate that the number of insects decreases significantly as precipitation increases. This variability in the populations of bark beetles is similar to the results recorded in the present study for the municipality of Bocoyna, Chihuahua (
Figure 6).
In general, we observed that the species of Dendroctonus show a differential response to temperature.
D. mexicanus shows a significant response to temperature (minimum, maximum, and mean), while
D. frontalis and
D. brevicomis present a significant response only to the minimum temperature (
Table 1). This is attributed to all bark beetles having a specific and evolved response to temperature, which can differ dramatically from one species to another; they respond to different temperature thresholds [
7].
5. Conclusions
Six species of bark beetles were recorded in the monitoring carried out over 24 months in this study: Dendroctonus mexicanus, D. frontalis, D. brevicomis, D. adjunctus, D. valens, and D. approximatus. The most abundant species was D. mexicanus, followed by D. frontalis and D. brevicomis; although the semiochemicals used in the study attract mainly those three species. The highest abundance of these species of bark beetles occurs in spring and summer, and the lowest in winter.
Temperature was the variable that had the greatest influence on the fluctuations in the population of these insects. However, these results may be related to other environmental factors that were not monitored in this study.
Author Contributions
R.C.M. and J.C.P., conceptualization, field collection, data analysis and original draft preparation; G.S.M., draft preparation, review and editing; V.H.C.S., draft review and editing; G.E.A., field collection and draft review. All authors have read and agreed to the published version of the manuscript.
Funding
This study was made possible thanks to CONAFOR-CONACYT funding through the project “Climate variability and interaction with other factors affecting the population dynamics of bark beetles in threatened forests in Mexico” with record CONAFOR-2014, C01- 234547.
Acknowledgments
We thank Saul Silva, Enrique Rascón Camuñez and Abelardo Guevara Rascón for site access and logistical support. We thank numerous students for assistance in field collections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lanier, G.N.; Hendrichs, J.P.; Flores, J.E. Biosystematics of the Dendroctonus frontalis (Coleoptera: Scolytidae) Complex. Ann. Entomol. Soc. Am. 1988, 81(3): 403-418.
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of climate change for forest herbivores and pathogens. The Science of the Total Environment. 2000, 262:263–286.
- Price, T.S.; Dogett, H.C.; Pye, J.M.; Holmes, T.P. 1990. A history of southern pine beetle outbreaks in the southeastern United States. The Georgia Forestry Commission, Macon, GA. 1990; p. 66.
- SEMARNAT. Cambio Climático. En Informe de la Situación del Medio Ambiente En México. Compendio de Estadísticas Ambientales. Indicadores Clave, de Desempeño Ambiental y de Crecimiento Verde. Edición 2015. 2016; pp. 1–63. México. http://apps1.semarnat.gob.mx/dgeia/informe15/tema/cap2.html.
- Billings, R.F.; Clarke, S.R.; Espino-Mendoza, V.; Cordón-Cabrera, P.; Meléndez-Figuera, B.; Ramón-Campos, J.; Baeza, G. Bark beetle outbreaks and fire: adevastating combination for Central America´ s pine forests. Unasylva. 2004, 55, 15–21. [Google Scholar]
- Zúñiga, G.; Mendoza, C.G.; Cisneros, R.; Salinas-Moreno, Y. Zonas de sobreposición en las áreas de Distribución Geográfica de las especies mexicanas de Dendroctonus Erichson (Coleoptera: Scolytidae) y sus implicaciones Ecológico-evolutivas. Acta Zool. Mex. 1999, 77: 1-22.
- Bentz, B.; Logan, J.; MacMahon, J.; Allen, C.D.; Ayres, M.; Berg, E.; Macfarlane, W. Bark beetle outbreaks in western North America: Causes and consequences. In Bark Beetle Symposium; Snowbird, Utah; November 2005. Salt Lake City, UT: University of Utah Press. 2009; p. 42.
- Hódar, J.A.; Castro, J.; Zamora, R. Pine processionary caterpillar Thaumetopoea pityocampa as a new threat for relict Mediterranean Scots pine forests under climatic warming. Biological conservation. 2003, 110(1), 123–129. [Google Scholar]
- Bentz, B.J.; Jönsson, A.M. Modeling Bark Beetle Responses to Climate Change. Pp. 533−553. In: Vega, F. E. and R. W. Hofstetter (Eds.). Bark Beetles: Biology and Ecology of Native and Invasive Species. Elsevier Inc, USA. 2015. [CrossRef]
- Rodríguez-Ortega, A.; Equihua-Martínez, A.; Cibrián-Tovar, J.; Estrada-Venegas, E.G. Fluctuación de Dendroctonus adjunctus Blandford (Curculioniade: Scolytinae) y sus depredadores atraídos por Frontalina+ Alfa-Pineno, la Estación Experimental de Zoquiapan, Edo. De México. Boletín del Museo de Entomología de la Universidad del Valle. 2010, 11(1), 20-27.
- Olivera, L.N. Fluctuación poblacional de Dendroctonus mexicanus Hopkins y variación estacional de la temperatura y humedad relativa, en San Juan del Estado, Etla, Oaxaca. Tesis de Maestría en Ciencias. Instituto de Enseñanza e Investigación en Ciencias Agrícolas. Colegio de Postgraduados. Montecillo, Edo. de Méx., México. 2014; p. 73.
- Kautz, M.; Dworschak, K.; Gruppe, A.; Schopf, R. Quantifying spatio-temporal dispersion of bark beetle infestations in epidemic and non-epidemic conditions. Forest Ecology and Management. 2011, 262(4), 598–608. [Google Scholar]
- Sánchez-Martínez, G.; Torres-Espinosa, L.M.; Vázquez-Collazo, I.; González-Gaona, E.; Narvaéz-Flores, R. Monitoreo y Manejo de insectos descortezadores de coníferas. Aguascalientes, México. INIFAP-CIRNOC. Campo Experimental Pabellón. 2007; p. 107. (Libro Técnico No. 4, campo experimental Pabellón).
- Macías-Sámano, J.E.; Niño-Domínguez, A. Protocolo para monitoreo de descortezadores de coníferas mediante el uso de atrayentes y semioquímicos. ECOSUR. 2016.
- INEGI. Instituto Nacional de Estadística y Geografía. 2015. Disponible en www.inegi.com.mx.
- Lindgren, B.S. A multiple funnel trap for scolytid beetles (Coleoptera). Canadian Entomologist. 1983, 155 (3): 299-302.
- Macías-Sámano, J.E.; Domínguez, A.N.; López, J.C.; Mérida, R.A. Monitoreo de descortezadores y sus depredadores mediante el uso de semioquímicos: Manual operativo. Ecosur-Conafor-Comisión Nacional de Areas Naturales Protegidas-USDA Forest Service. Tapachula, Chiapas, México. 2004.
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs, Number 6. 1982; pp. 1–1359. Idaho: Brigham Young University.
- Cibrián-Tovar, D.; Méndez-Montiel, J.T.; Campos-Bolaños, R.; Yates, H.O.III.; Flores, J. Insectos forestales de México. Universidad Autónoma Chapingo, Secretaría de Agricultura y Recursos Hidráulicos, United States Departament of Agriculture, Natural Resources Canadá y Comisión Forestal de América del Norte. Chapingo, Edo. de Méx., México. 1995; p. 455.
- Sánchez, S.J.A.; Torres, E.L.M. Biología y hábitos del descortezador Dendroctonus mexicanus Hopkins y estrategias de control en Pinus teocote en Nuevo León. CIRNE. Campo Experimental Saltillo. Folleto técnico Núm. 29. Coahuila, México. 2007; p. 35.
- Rodríguez, L.R. Plagas forestales y su control en México. Universidad Autónoma Chapingo. México. 1990; p. 217.
- Cuéllar-Rodríguez, G.; Equihua-Martínez, A.; Estrada-Venegas, E.; Méndez-Montiel, T.; Romero-Nápoles, J. Fluctuación poblacional de Dendroctonus mexicanus Hopkins (Coleoptera: Curculionidae: Scolytinae) atraídos a trampas en el noreste de México y su correlación con variables climáticas. Boletín del Museo de Entomología de la Universidad del Valle. 2012, 13(2): 12-19.
- Moser, J.C.; Firzgibbon, B.A.; Klepzig, K.D. The Mexican pine beetle, Dendroctonus mexicanus: first record in the United States and co-occurrence with the southern pine beetle Dendroctonus frontalis (Coleoptera: Scolytidae or Curculionidae: Scolytinae) Entomological News. 2005, 116(4): 253-243.
- Avilés, C.I.; Vergara, P.S.; Cambrón, S.V.H.; Obregón, Z.A. Fluctuación Poblacional de Dendroctonus frontalis Zimmerman, 1868 y Dendroctonus mexicanus Hopkins, 1909 (Curculionidae: Scolytinae) en Relación a la Variación en la Atitud y Factores Climáticos en un Bosque de Pino en Zimapán, Hidalgo. Revista Entomología Mexicana. 2016, 3, 649–655. http://www.entomologia.socmexent.org/entomologia.php.
- Morales, R.A.; Cambrón, S.V.H.; Vergara, P.S.; Obregón, Z.A. Fluctuación Poblacional de Dendroctonus frontalis Zimmerman, 1868 Y Dendroctonus mexicanus Hopkins, 1909 (Coleoptera: Curculionidae: Scolytinae) y su Asociación con Variables Climáticas en Bosques de Pino en el Municipio de Landa de Matamoros, Querétaro. Entomología mexicana. 2016, 3, 633–638. [Google Scholar]
- Thompson, W.A.; Moser, J.C. Temperature thresholds related to flight of Dendroctonus frontalis Zimm.(Col.: Scolytidae). Agronomie. 1986, 6(10), 905-910.
- Ruiz-González, C.G.; Méndez-González, J.; Cambrón-Sandoval, V.H.; García-Aranda, M.A.; Montoya-Jiménez, J.C.; Sosa-Díaz, L. Distribución altitudinal y estacional de Dendroctonus adjunctus Blandford y Dendroctonus brevicomis Leconte en Coahuila, México. Rev. Fitotec. Mex. 2018, 41 (4-A): 519 - 526.
- Ortega-Ortíz, D.S.; Flores-Ortega, G.; Nájera-Luna, J.A.; Cruz-Cobos, F.; Hernández, F.J. Dinámica poblacional de escarabajos descortezadores en la región forestal de El Salto, Durango, México. Instituto Tecnológico de Cd. Victoria, Tamaulipas. 2018.
- Bentz, B. J.; Logan, J.A.; Amman, G.D. Temperature dependent development of the mountain pine beetle (Coleoptera: Scolytidae) and simulations of its phenology. Canadian Entomologist. 1991, (123): 1083-1094.
- Logan, J.A.; Powell, J.A. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). American Entomologist. 2001, 47(3): 160-172.
- Powell, J.A.; Bentz, B.J. Connecting phenological predictions with population growth rates for mountain pine beetle, an outbreak insect. Landsc. Ecol. 2009, 24, 657–672. [Google Scholar]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecological Monographs. 2013, 83(4), 441–470. [Google Scholar]
- Alvarado, V.O. Evaluación de los factores asociados a las infestaciones de descortezadores (Coleóptera: Scolytinae) en bosques de piñones (Pinus cembroides) en la Reserva de la Biosfera Sierra Gorda de Guanajuato. Tesis de Maestría. Centro Universitario. Querétaro, Qro., México. 2013; p. 58.
- Pereda-Breceda, V. 2016. Fluctuación poblacional de Dendroctonus spp, asociada a variables climáticas y de sitio en bosques de coníferas de la región de San Dimas Durango (tesis de maestría). Instituto Tecnológico de El Salto. El Salto, Pueblo Nuevo, Durango, México. 2016; p. 63.
- Logan, J.A.; Bentz, B.J. Model analysis of mountain pine beetle seasonality. Environmental Entomology. 1999, (28): 924-934.
- Werner, R.A.; Holsten, E.H.; Matsuoka, S.M.; Burnside, R.E. Spruce beetles and forest ecosystems in south-central Alaska: a review of 30 years of research. For. Ecol. Manage. 2006, 227, 195–206. [Google Scholar]
- Negrón, J.; Willhite, E. Observaciones y Recomendaciones para el Manejo y Monitoreo del Descortezador Mexicano del Pino, Dendroctonus mexicanus, en la Reserva de la Biosfera Sierra Gorda de Guanajuato. USDA Forest Service. 2012.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).