Submitted:
08 May 2024
Posted:
09 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing Processes Used
2.2.1. Manufacturing via Sintering of the β-TCP Ceramics Used
2.2.2. Additive manufacturing of the β-TCP Ceramics Used
2.3. Characterization of the Resulting β-TCP Scaffolds
2.3.1. Characterization of Porosity
2.3.2. Mechanical Testing
2.3.3. Degradation tests
2.3.4. Biocompatibility
2.4. Statistics
3. Results
3.1. Porosity
3.2. Mechanical Properties
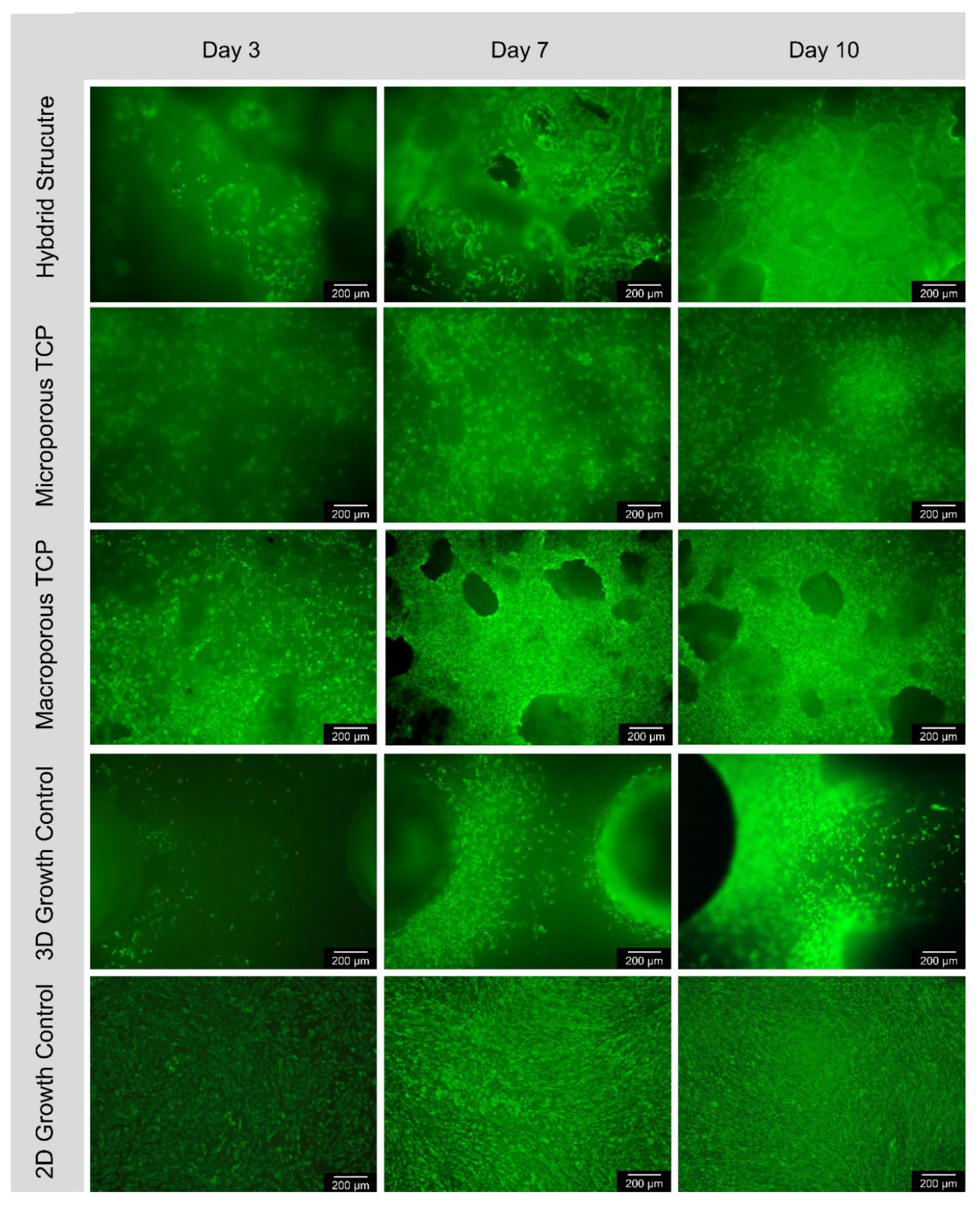
3.3. Bicompatibility
3.3.1. Live/Dead-Assay
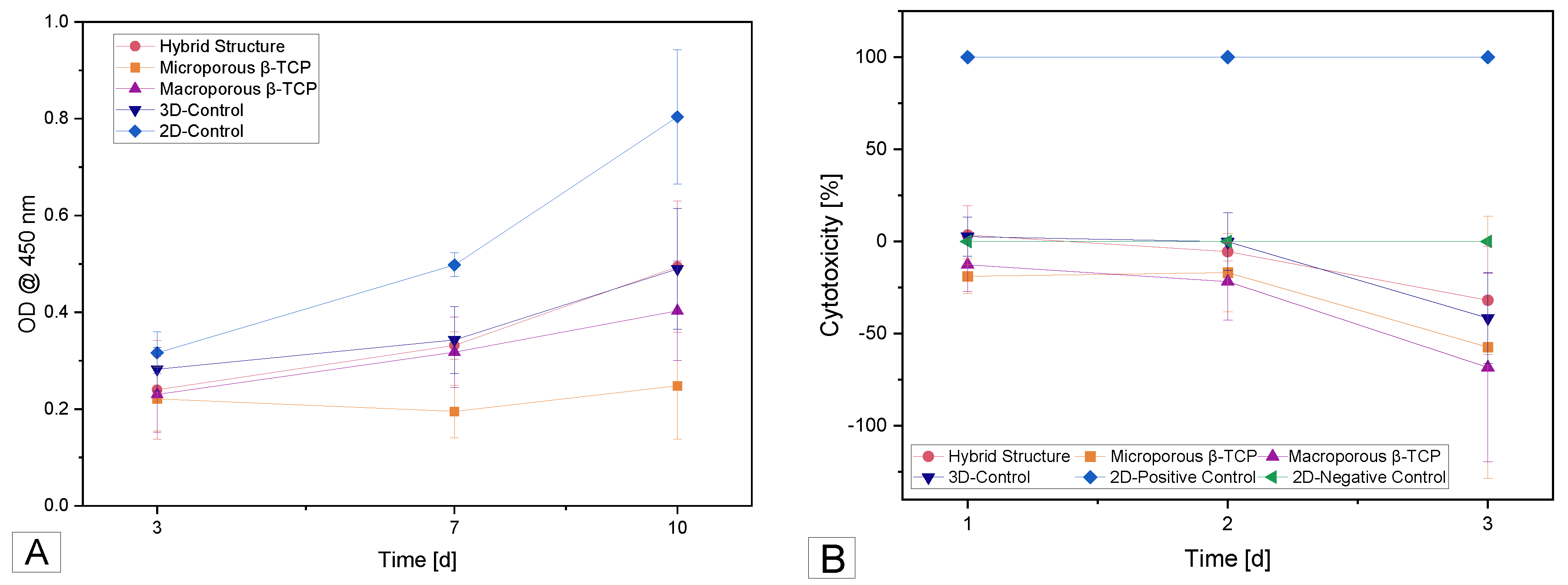
3.3.2. Cell Proliferation (WST-I)
3.3.3. Cytotoxicity (LDH)
4. Discussion
4.1. Porosity
4.2. Mechanical Properties
4.3. Biocompatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-size bone defects: Is there a consensus for diagnosis and treatment? J Orthop Trauma 2018, 32 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J Bone Joint Surg Br 2005, 87, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat Rev 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the bone in cancer metastasis. J. Bone Miner. Res. 2018, 33, 2099–2113. [Google Scholar] [CrossRef]
- Omar, M.; Graulich, T.; von Falck, C.; Bruns, N.; Krettek, C.; Ettinger, M. Versorgungsstrategien bei tumorbedingten pathologischen frakturen der extremitäten. Der Unfallchirurg 2021, 124, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Azi, M.L.; Aprato, A.; Santi, I.; Kfuri, M., Jr.; Masse, A.; Joeris, A. Autologous bone graft in the treatment of post-traumatic bone defects: A systematic review and meta-analysis. BMC Musculoskelet Disord 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- destatis. Fallpauschalenbezogene krankenhausstatistik (drg-statistik) operationen und prozeduren der vollstationären patientinnen und patienten in krankenhäusern (4-steller) für 2021. Office, G.F.S., Ed. Wiesbaden, 2022.
- Kim, D.H.; Rhim, R.; Li, L.; Martha, J.; Swaim, B.H.; Banco, R.J.; Jenis, L.G.; Tromanhauser, S.G. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J 2009, 9, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the ria: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Chung, F. Complications of general anesthesia. Clin Plast Surg 2013, 40, 503–513. [Google Scholar] [CrossRef]
- Lohmann, H.; Grass, G.; Rangger, C.; Mathiak, G. Economic impact of cancellous bone grafting in trauma surgery. Arch Orthop Trauma Surg 2007, 127, 345–348. [Google Scholar] [CrossRef]
- Rupp, M.; Klute, L.; Baertl, S.; Walter, N.; Mannala, G.K.; Frank, L.; Pfeifer, C.; Alt, V.; Kerschbaum, M. The clinical use of bone graft substitutes in orthopedic surgery in germany-a 10-years survey from 2008 to 2018 of 1,090,167 surgical interventions. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef] [PubMed]
- Khijmatgar, S.; Panda, S.; Das, M.; Arbildo-Vega, H.; Del Fabbro, M. Recombinant factors for periodontal intrabony defects: A systematic review and network meta-analysis of preclinical studies. J Tissue Eng Regen Med 2021, 15, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Tampieri, A.; Cabezas-Rodríguez, R.; Di Martino, A.; Fini, M.; Giavaresi, G.; Lelli, M.; Martínez-Fernández, J.; Martini, L. , et al. New bio-ceramization processes applied to vegetable hierarchical structures for bone regeneration: An experimental model in sheep. Tissue Eng Part A 2014, 20, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Manfrini, M.; Di Bona, C.; Canella, A.; Lucarelli, E.; Pellati, A.; D’Agostino, A.; Barbanti-Bròdano, G.; Tognon, M. Mesenchymal stem cells from patients to assay bone graft substitutes. J Cell Physiol 2013, 228, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, X. Bioceramics in endodontics: Updates and future perspectives. Bioengineering (Basel, Switzerland) 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. Β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Galois, L.; Mainard, D.; Delagoutte, J.P. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop 2002, 26, 109–115. [Google Scholar] [CrossRef]
- Köster, K.; Karbe, E.; Kramer, H.; Heide, H.; König, R. [experimental bone replacement with resorbable calcium phosphate ceramic (author’s transl)]. Langenbecks Arch Chir 1976, 341, 77–86. [Google Scholar] [CrossRef]
- Hirata, M.; Murata, H.; Takeshita, H.; Sakabe, T.; Tsuji, Y.; Kubo, T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop 2006, 30, 510–513. [Google Scholar] [CrossRef]
- Seto, S.; Muramatsu, K.; Hashimoto, T.; Tominaga, Y.; Taguchi, T. A new β-tricalcium phosphate with uniform triple superporous structure as a filling material after curettage of bone tumor. Anticancer Res. 2013, 33, 5075–5081. [Google Scholar] [PubMed]
- Bernstein, A.; Nobel, D.; Mayr, H.O.; Berger, G.; Gildenhaar, R.; Brandt, J. Histological and histomorphometric investigations on bone integration of rapidly resorbable calcium phosphate ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.O.; Suedkamp, N.P.; Hammer, T.; Hein, W.; Hube, R.; Roth, P.V.; Bernstein, A. Β-tricalcium phosphate for bone replacement: Stability and integration in sheep. J. Biomech. 2015, 48, 1023–1031. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Mrestani, Y.; Neubert, R.H.H.; Bernstein, A.; Mayr, H.O. Release kinetics and antibacterial efficacy of microporous β-tcp coatings. Journal of Nanomaterials 2013, 2013, 8. [Google Scholar] [CrossRef]
- Ahlhelm, M.; Latorre, S.H.; Mayr, H.O.; Storch, C.; Freytag, C.; Werner, D.; Schwarzer-Fischer, E.; Seidenstücker, M. Mechanically stable β-tcp structural hybrid scaffolds for potential bone replacement. Journal of Composites Science 2021, 5, 281. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Schmeichel, T.; Ritschl, L.; Vinke, J.; Schilling, P.; Schmal, H.; Bernstein, A. Mechanical properties of the composite material consisting of β-tcp and alginate-di-aldehyde-gelatin hydrogel and its degradation behavior. Materials 2021, 14, 1303. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakai, H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials 1992, 13, 308–312. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- Schmidleithner, C.; Malferrari, S.; Palgrave, R.; Bomze, D.; Schwentenwein, M.; Kalaskar, D.M. Application of high resolution dlp stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed Mater 2019, 14, 045018. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, T.; Zeng, Y. Fabrication of trabecular-like beta-tricalcium phosphate biomimetic scaffolds for bone tissue engineering. Ceramics International 2021, 47, 13187–13198. [Google Scholar] [CrossRef]
- Peralta, L.; Maeztu Redin, J.D.; Fan, F.; Cai, X.; Laugier, P.; Schneider, J.; Raum, K.; Grimal, Q. Bulk wave velocities in cortical bone reflect porosity and compression strength. Ultrasound Med Biol 2021, 47, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Perilli, E.; Baleani, M.; Öhman, C.; Fognani, R.; Baruffaldi, F.; Viceconti, M. Dependence of mechanical compressive strength on local variations in microarchitecture in cancellous bone of proximal human femur. J Biomech 2008, 41, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.O.; Suedkamp, N.P.; Hammer, T.; Hein, W.; Hube, R.; Roth, P.V.; Bernstein, A. Beta-tricalcium phosphate for bone replacement: Stability and integration in sheep. J. Biomech. 2015, 48, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qiang, Z.; Zhao, H.; Piao, H.; Ren, L. Mechanical properties of cortical bones related to temperature and orientation of haversian canals. Materials Research Express 2020, 7, 015408. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of cap and cap/polymer composites for applications in bone replacement and repair. Acta Biomater 2011, 7, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Südkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Görz, L. , et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater 2013, 9, 7490–7505. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F.; Silva, A.P.; Lopes, L.; Pires, I.; Correia, I.J. Design and production of sintered β-tricalcium phosphate 3d scaffolds for bone tissue regeneration. Mater Sci Eng C 2012, 32, 1293–1298. [Google Scholar] [CrossRef]
- Tarafder, S.; Balla, V.K.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Microwave-sintered 3d printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med 2013, 7, 631–641. [Google Scholar] [CrossRef]
- Miranda, P.; Pajares, A.; Saiz, E.; Tomsia, A.P.; Guiberteau, F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J Biomed Mater Res A 2008, 85A, 218–227. [Google Scholar] [CrossRef]
- Bertrand, E.; Zankovic, S.; Vinke, J.; Schmal, H.; Seidenstuecker, M. About the mechanical strength of calcium phosphate cement scaffolds. Designs 2023, 7, 87. [Google Scholar] [CrossRef]
- Zerbo, I.R.; Zijderveld, S.A.; de Boer, A.; Bronckers, A.L.; de Lange, G.; ten Bruggenkate, C.M.; Burger, E.H. Histomorphometry of human sinus floor augmentation using a porous beta-tricalcium phosphate: A prospective study. Clin Oral Implants Res 2004, 15, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F. , et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater 2010, 6, 4476–4487. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M.; Lange, S.; Esslinger, S.; Latorre, S.H.; Krastev, R.; Gadow, R.; Mayr, H.O.; Bernstein, A. Inversely 3d-printed β-tcp scaffolds for bone replacement. Materials 2019, 12, 3417. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M. Herstellung eines kompositmaterials bestehend aus einer porösen β -tricalciumphosphatkeramik und einem drug-release-system mit kontinuierlicher wirkstofffreisetzung. Dissertation, Universität Freiburg, 2015. [CrossRef]
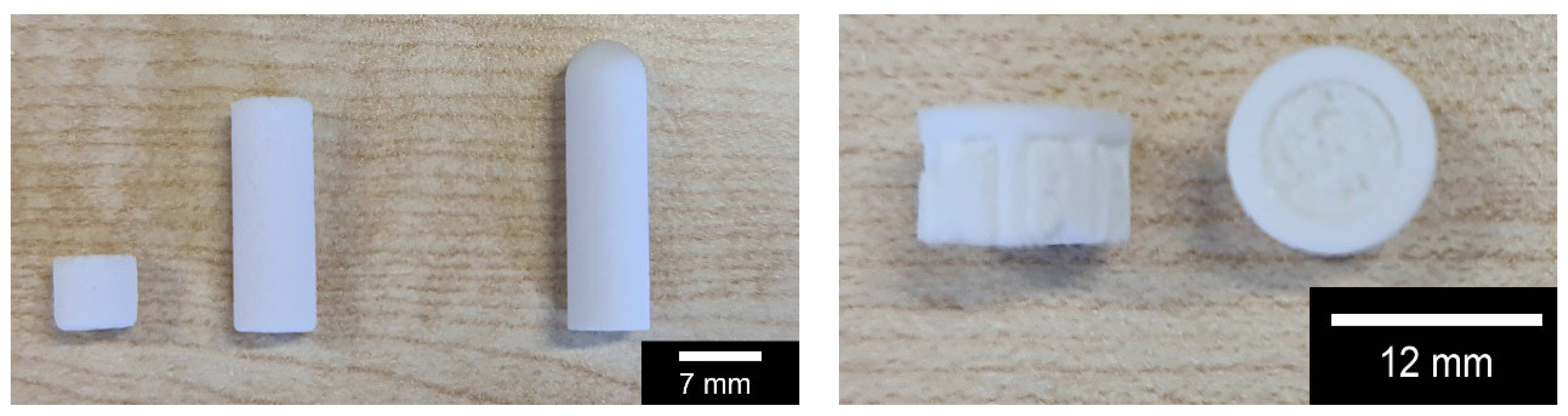
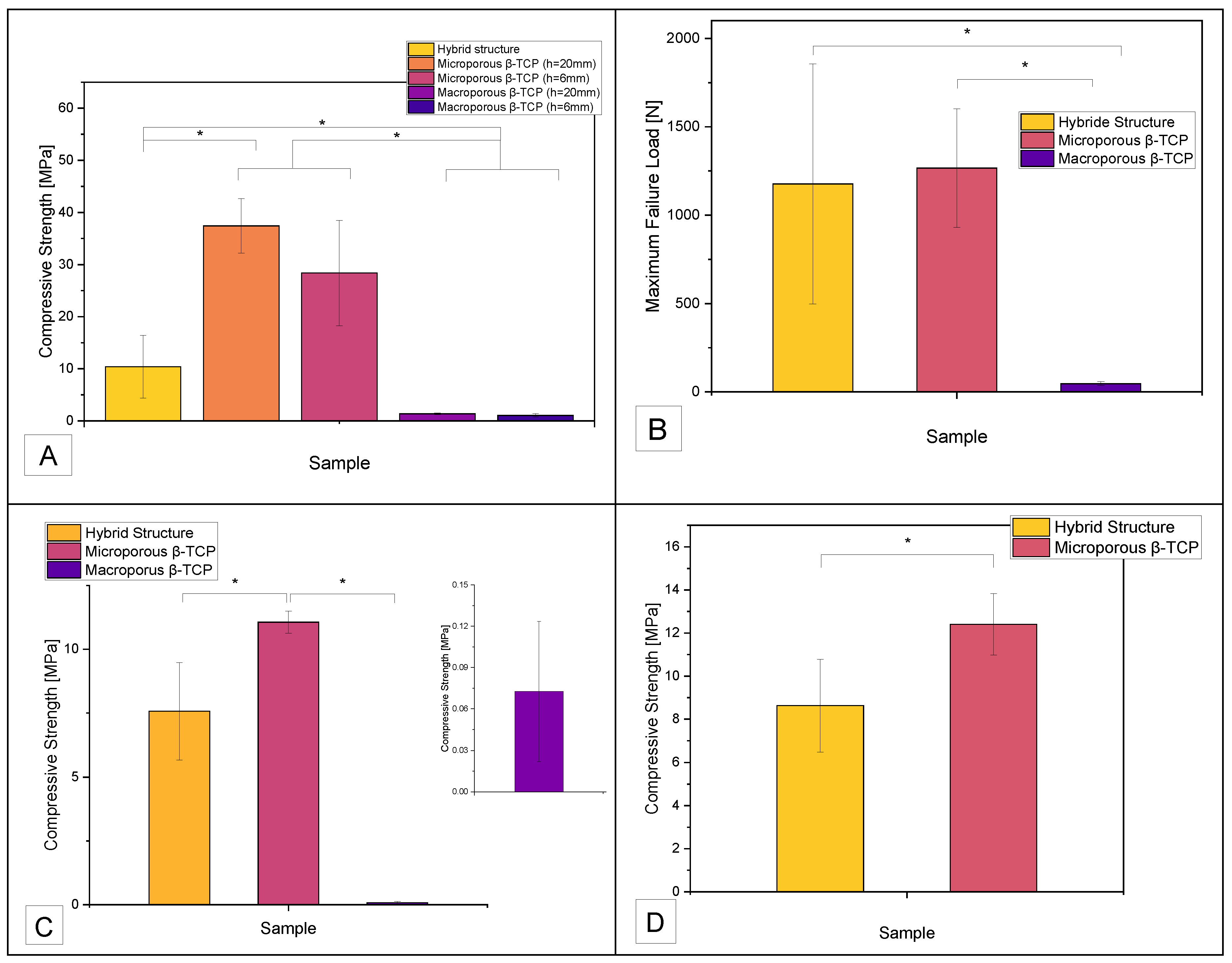
| Compressive Strength [MPa] | |||
| Sample | Hybrid Structure | Microporous β-TCP | Macroporous β-TCP |
| No Tris buffer | 10.4 ± 6 | 32.9 ± 8.7 | 1.2 ± 0.3 |
| Tris buffer pH 7.4 | 7.6 ± 1.9 | 11.1 ± 0.4 | 0.07 ± 0.05 |
| Tris buffer pH 5 | 8.6 ± 2.1 | 12.4 ± 1.4 | n.a. |
| Maximum Failure Load [N] | |||
| Sample | Hybrid Structure | Microporous β-TCP | Macroporous β-TCP |
| No Tris buffer | 1176.6 ± 678.7 | 1266.4 ± 336.1 | 46.6 ± 11.1 |
| Tris buffer pH 7.4 | 856.9 ± 215.4 | 425.7 ± 16.7 | 2.8 ± 2 |
| Tris buffer pH 5 | 975.7 ± 243.1 | 477.5 ± 55 | n.a. |
| Surface | day 3 | day 7 | day 10 | |||
|---|---|---|---|---|---|---|
| Cells/mm² | living | dead | living | dead | living | dead |
| Hybrid structure | 66 ± 22 | 2 ± 4 | 131 ± 66 | 3 ± 5 | 240 ± 84 | 1 ± 1 |
| Microporous β-TCP | 128 ± 136 | 1 ± 1 | 266 ± 270 | 1 ± 1 | 624 ± 462 | 1 ± 3 |
| Macropoous β-TCP | 256 ± 299 | 1 ± 1 | 520 ± 520 | 0 | 993 ± 748 | 3 ± 9 |
| 3D-control curasan | 64 ± 70 | 9 ± 11 | 166 ± 101 | 12 ± 22 | 380 ± 216 | 6 ± 4 |
| 2D-control Thermanox | 862 ± 548 | 3 ± 4 | 1697 ± 403 | 12 ± 15 | 2468 ± 420 | 50 ± 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





