1. Introduction
Crude Oil is a naturally occurring petroleum product composed of hydrocarbon deposits and other organic materials. It is mainly of hydrogen and carbon composition. Though it may contain some nitrogen, sulphur and oxygen. Crude oils are customarily characterized by the type of hydrocarbon compound that is most prevalent in them; paraffins, naphthenes and aromatics. Paraffins are the most common hydrocarbons in crude oil; certain liquid paraffins are the major constituents of premium motor spirit (gasoline) and are therefore highly valued. Naphtenes are an important part of all liquid refinery products but there is also some of the heavy asphalt like residues of the refinery processes. Aromatics generally constitute only a small percentage of most crude. The most common aromatic in crude oil is benzene, a popular building block in the petrochemical industry (Robert 2005).
Asphaltenes are defined as the nonpolar and nonvolatile components of crude oil. They are insoluble in alkanes (normal pentane and normal heptane) but soluble in aromatics (benzene and toluene). In general, asphaltenes are known as a complex organic matter, containing carbon, oxygen, nitrogen, and sulfur. The black color of some crude oils and residuals is related to the presence of asphaltenes, which have not flocculated or precipitated. Asphaltenes have polar molecules with very high molecular weights.
The asphaltenes are arguably the most complex fractional part of oil. In variation pressure, temperature or composition of oil, asphaltenes tend to related and precipitate raising several costly operational challenges from transport to refining (Trejo, Ancheyta et al. 2007)(Trejo et al., 2007). Many efforts have been done on improving the information about this mixture, deepening their knowledge of the chemical structures involved, characterizing the functions and establishing their behavior against solvents. They also tend to reveal the way their molecules are stabilized and also dispersed in the oil. It’s been concluded that the level and nature of asphaltenes in a sample is as a result of series of parameters which include origin of oil, the flocculating agent, the time used for precipitation, temperature, as well as procedure used and ratio of oil flocculant agent. All these combined parameters not only influenced the amount of asphaltene precipitated, but also account details in its composition, which can be obtained from an asphaltene solid dark brown to a black (Silva, 2003).
2. Materials and Methods
2.1. Materials
The methods that were employ on this research to extracts asphaltene from Nigerian crude oil from Forcados off-shore was soxhlet extractor with the use of condenser follow by separating funnel from which the separation will take place, n-hexane is used in the extraction of asphaltene as solvent. The solubility parameter is the bases that present the interaction in the condensed materials that evaluate and provides a numerical method for describing the degree of interaction. The most common well known method consists of dissolving the sample in various solvents whose solubility parameter is known. In this case, the solubility parameter of the material must be equal to the solubility parameter of the selected solvent which will be subjected to larger interaction.
2.2. Method
The sample was collected from Forcados off-shore in Burutu local government area of Delta state, in the southern part of Nigeria. One liter (1L) of the crude sample was colleceted and put into a clean sample bottle that was kept away from sunlight. The sample of was labeled; Forcados crude sample.
2.3. Sample Preparation
100ml of the crude oil sample was measured into a 500ml round bottom flask with standard tapper joint, a thermometer, distillation receiver with 0.05 graduations, a drying trap and a liebig condenser. The flask is connected to a heating mantle. The heating mantle was used to heat the crude sample to a temperature of 260℃ and the mass of the residue was determined by weighing.
10g of the dried sample was mixed with n-hexane and the mixture heated under reflux and the precipitated asphaltene, waxy substances and inorganic materials collected on a filter paper. The waxy substance was removed by washing with hot n-hexane in an extractor.
2.4. Proximate Analysis of extracted Asphaltene
2.4.1. Moisture Content
The method of the Association of Official Analytical Chemists (AOAC,1990) was employed for the moisture content determination using hot air drying oven at 105℃ for one hour, cooled in a desiccator and weighed. Two grams of the fresh sample was put into the crucible dried in an oven at 105℃ overnight and weighed; heating and cooling was repeated several times at 30 minutes interval until a constant weight is obtained.
The percentage moisture was determined by using the equation;
Weight of Fresh sample
2.4.2. Ash Content of Forcados Asphaltene
The ash content of the sample was determined by heating porcelain crucibles in a muffle furnace at 500℃, cooled in a desiccator and weighed. Two grams of the oven dried sample was transferred into the crucible and weighed. The crucible containing the samples was then placed in the muffle furnace and temperature was allowed to rise to 550℃ for three hours. The crucible containing the ash was allowed to cool and weighed (AOAC, 1990), the percentage was calculated using the equation;
Weight of Sample
2.4.3. Fuel Properties Test
The cloud point; ASTM D 2500 was used using automated Lawler direct refrigeration machine to determine the cloud point. ASTM D4052 was used to determine the API gravity of the asphaltene sample on Kyoto DA645 machine while ASTM D93 was used to determine the flash point on Pensky Martins manual apparatus and ASTM D975 was used to determine the cetane number on Eraspec F191 machine.
2.4.4. Elemental Analysis
The Shimadzu AAF508F machine was set up according to the manufacturer’s instructions which include the selection of fuel gas and oxidant gas, burner type, optimum wavelength and slide-width settings. The sample solution was then aspirated into the instrument and concentration of each metal determined separately with reference to the calibration curve for each metal.
The concentration of each metal was determined by using the equation;
where;
a= concentration of metal in the sample (ppm)
b = concentration of metal in the blank (ppm)
v = total volume of the mixture at the end of the digestion procedure
w = original weight of the sample (g)
2.4.5. Asphaltene Characterization
The asphaltene was characterized using Shimadzu 2010. Gas chromatography-mass spectroscopy (GC-MS) MS- QP 2010 ultra-machine.
3. Result and Discussion
Table 1.
Proximate Analysis of the Extracted Forcados Asphaltene.
Table 1.
Proximate Analysis of the Extracted Forcados Asphaltene.
| Parameters |
Composition |
| Moisture content (%) |
26.0 |
| Ash content (%) |
1.0 |
The carbon content of the asphaltene fraction has shown a great proportion of the total composition of forcados Asphaltenes as a result, the energy content of the generated asphaltene demonstrate some strong heavy aromatic that will lead to energy release during combustion to give high energy efficiency such as marine engine.
The low water content boosts the energy possibilities.
3.1. Fuel Properties Test Result
Table 2.
Fuel Preoperties Result Of Extracted Forcados Asphaltene.
Table 2.
Fuel Preoperties Result Of Extracted Forcados Asphaltene.
| S/N |
Parameters |
Method |
Composition of Extracted Asphaltene |
| 1. |
API GRAVITY |
ASTM D4052 |
26.7 |
| 2. |
FLASH POINT ℃ |
ASTM D93 |
48.9 |
| 3. |
CLOUD POINT ℃ |
ASTM D2500 |
18 |
| 4. |
CETANE NUMBER |
ASTM D975 |
51 |
3.2. Atomic Absorption Spectometry Result For Forcados Asphaltene (AAS)
Table 3.
Present the concentration in ppm of the heavy and trace metals.
Table 3.
Present the concentration in ppm of the heavy and trace metals.
| TSN |
Metal Element |
Concentration in PPM |
| 1 |
Cr |
0125 |
| 2 |
Ni |
0.398 |
| 3 |
Fe |
60.7 |
| 4 |
Pb |
60.425 |
| 5 |
Cd |
26 |
| 6 |
Cu |
165.75 |
| 7 |
Mn |
49.9 |
| 8 |
Zn |
48.825 |
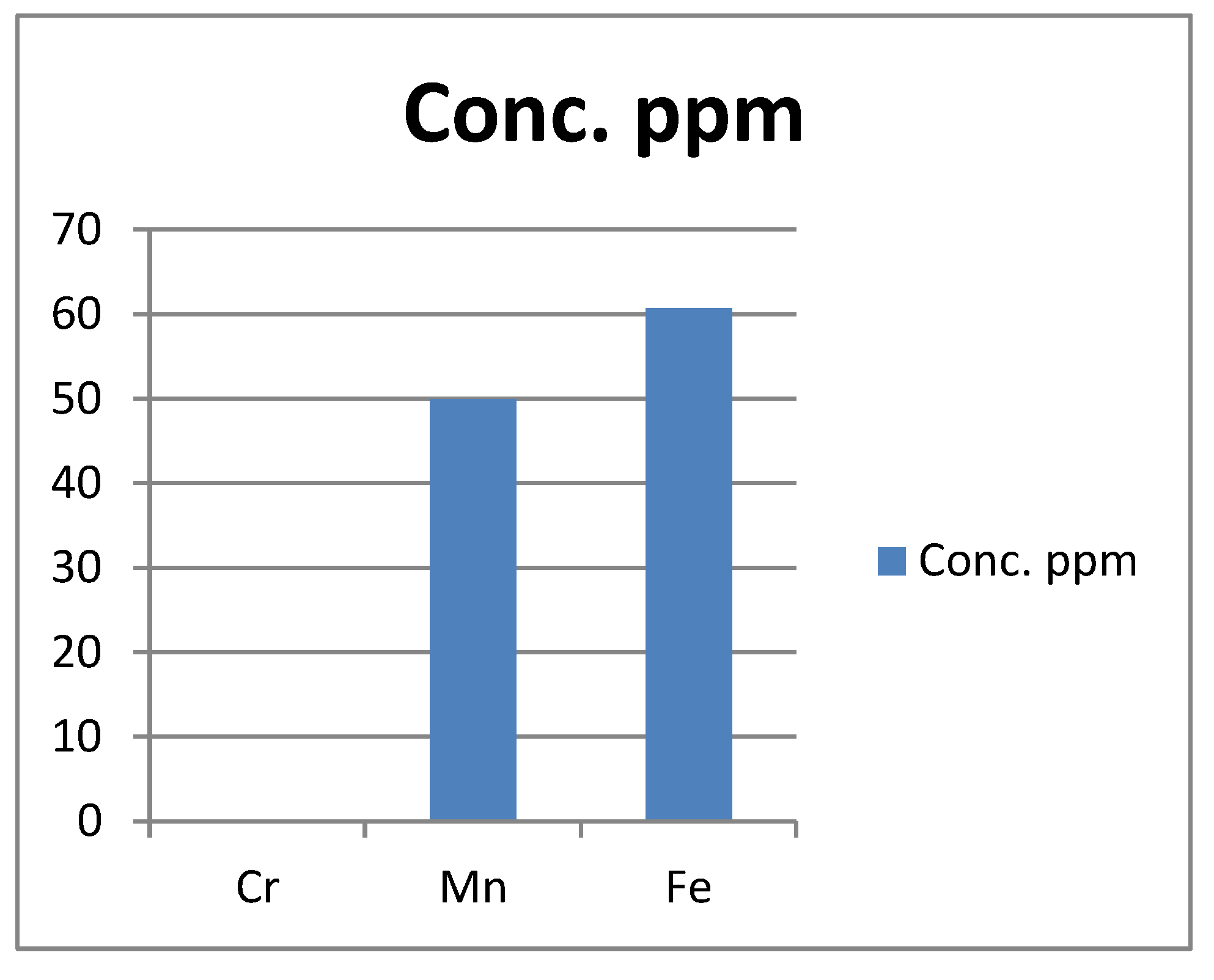
Figure 1.
Present the actual concentration of Cr, Mn, Fe metals in the Forcados asphaltene in ppm.
Figure 1.
Present the actual concentration of Cr, Mn, Fe metals in the Forcados asphaltene in ppm.
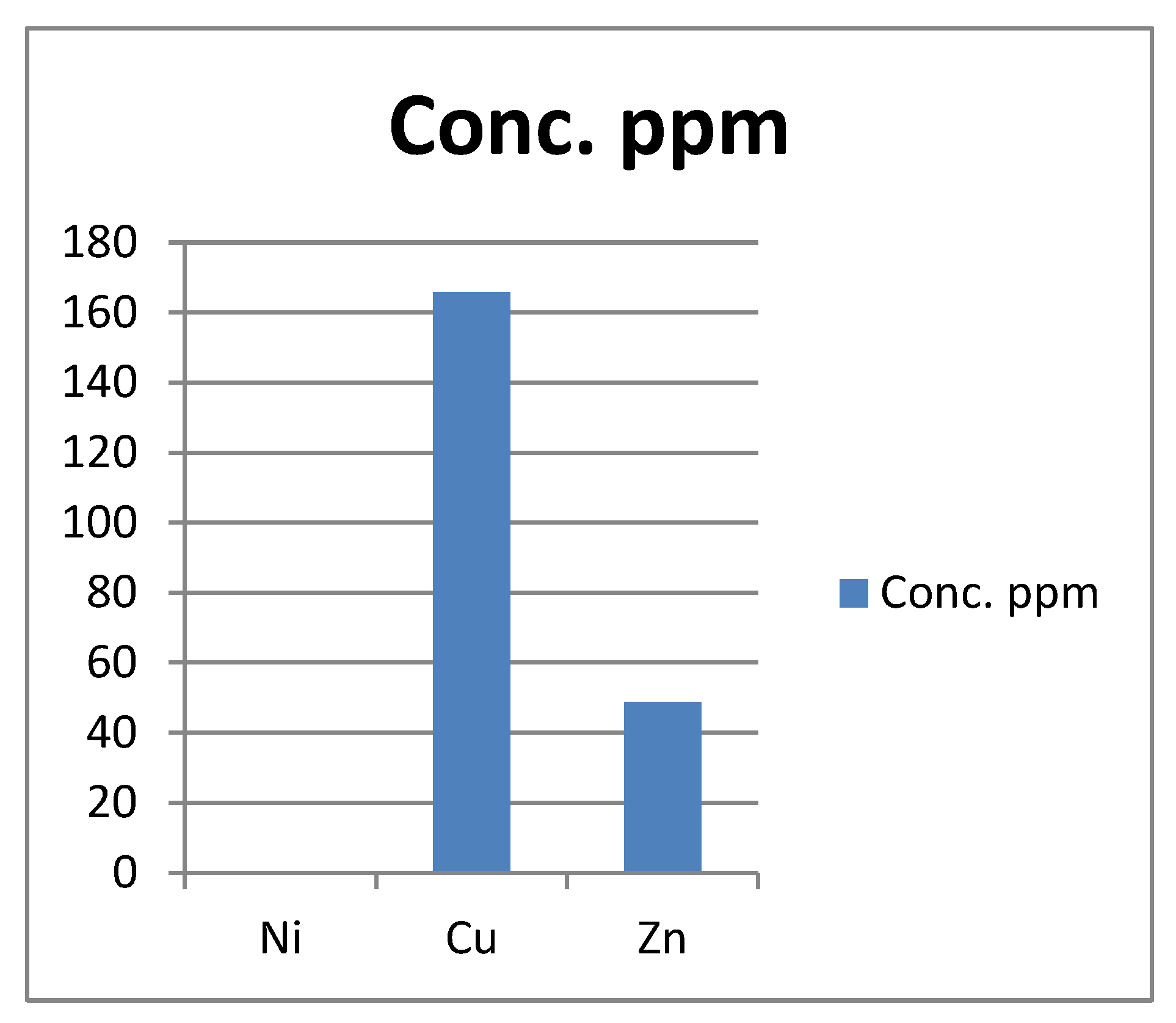
Figure 2.
Present the actual concentration of Ni, Cu, Zn metals in the Forcados asphaltene in ppm.
Figure 2.
Present the actual concentration of Ni, Cu, Zn metals in the Forcados asphaltene in ppm.
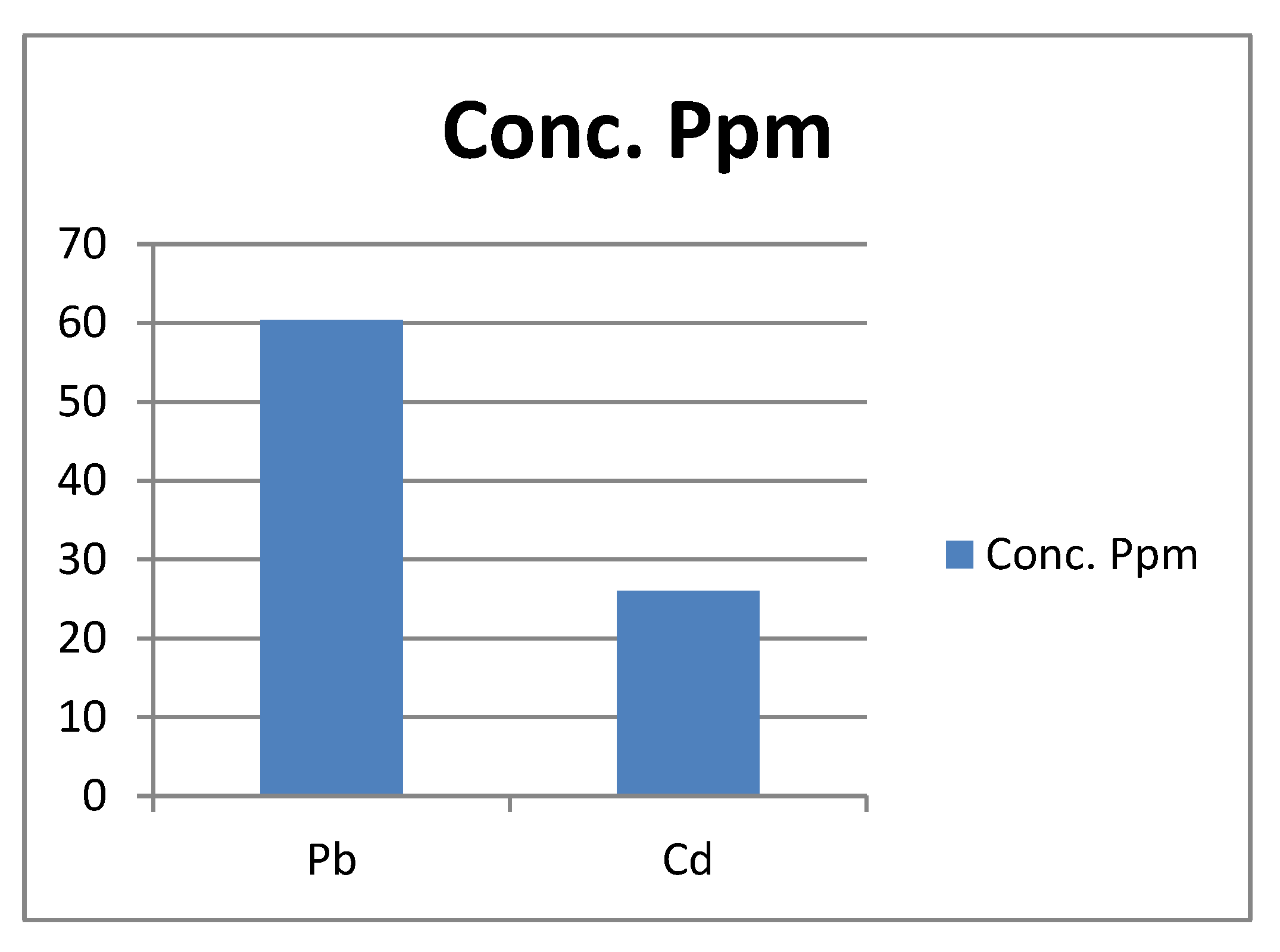
Figure 3.
Present the actual concentration of Pb and Cd metals in the Forcados asphaltene in ppm.
Figure 3.
Present the actual concentration of Pb and Cd metals in the Forcados asphaltene in ppm.
4. GCMS Result
4.1. Fourier Transform Infrared Spectroscopy
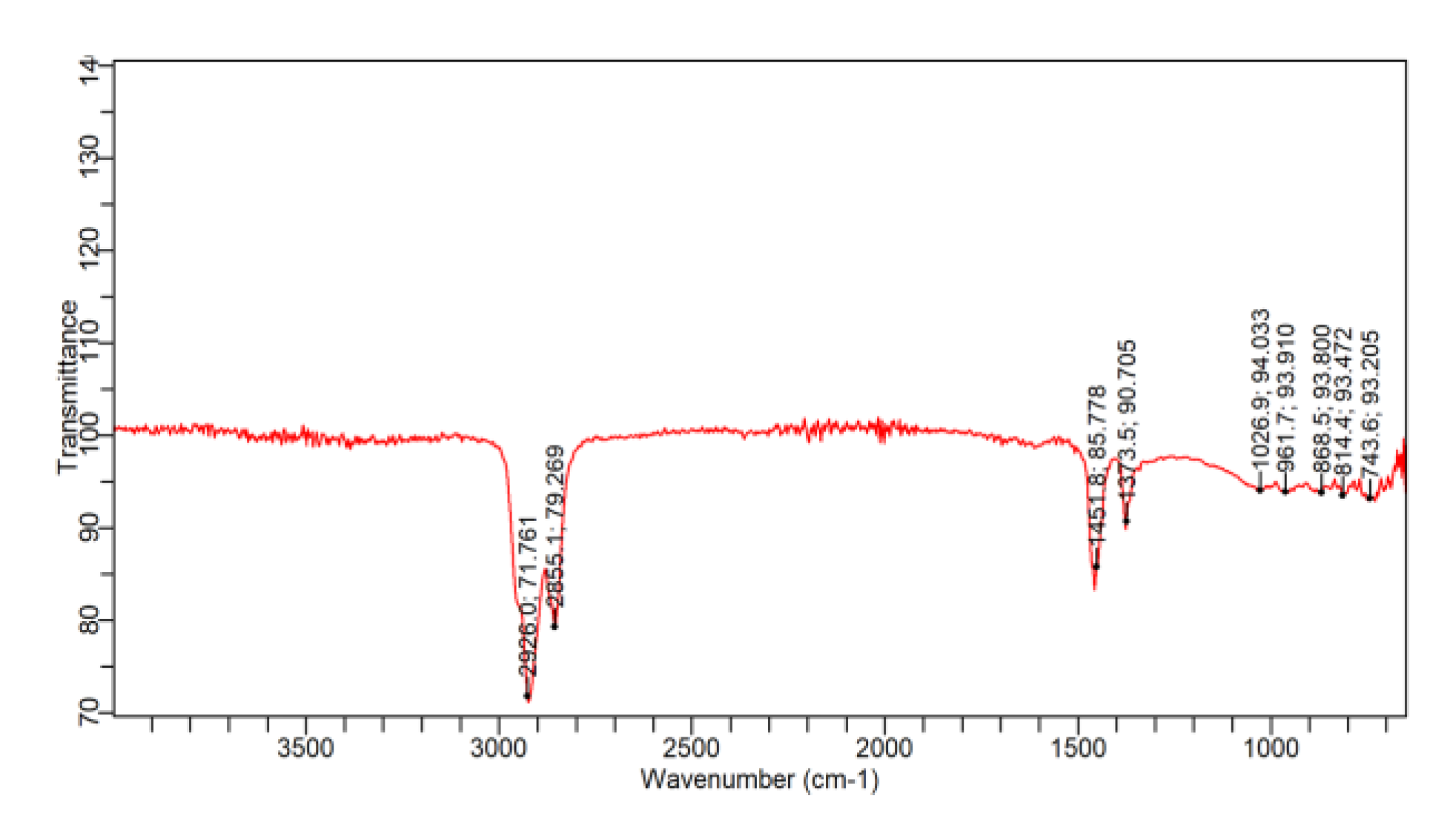
Figure 5.
The FTIRS Result of the Forcados Asphaltene.
Figure 5.
The FTIRS Result of the Forcados Asphaltene.
The FTIR spectra in Figure 8 show that the strongest peaks in the Forcados asphaltene belongs to those which corresponds to the alkane compounds. The peaks 2926, 2855, 1900, 1026, 961, 868, 814 and 743 cm-1 are standing C-H3 asymmetric stretch, symmetrical C-H2, asymmetrical C-H2, C-H bend, C-H scissoring. From 4000cm-1 to 2926cm-1 shows the presence of amine group in the Forcados asphaltene. The region of 3400-3250cm-1 represents the primary amine. Besides, only the pry amine has a peak in the region of 1650 to 1580 cm-1. This confirms the amine group existence in the forcados asphaltene molecule structure.
4.2. Ultraviolet Visible Spectroscopy
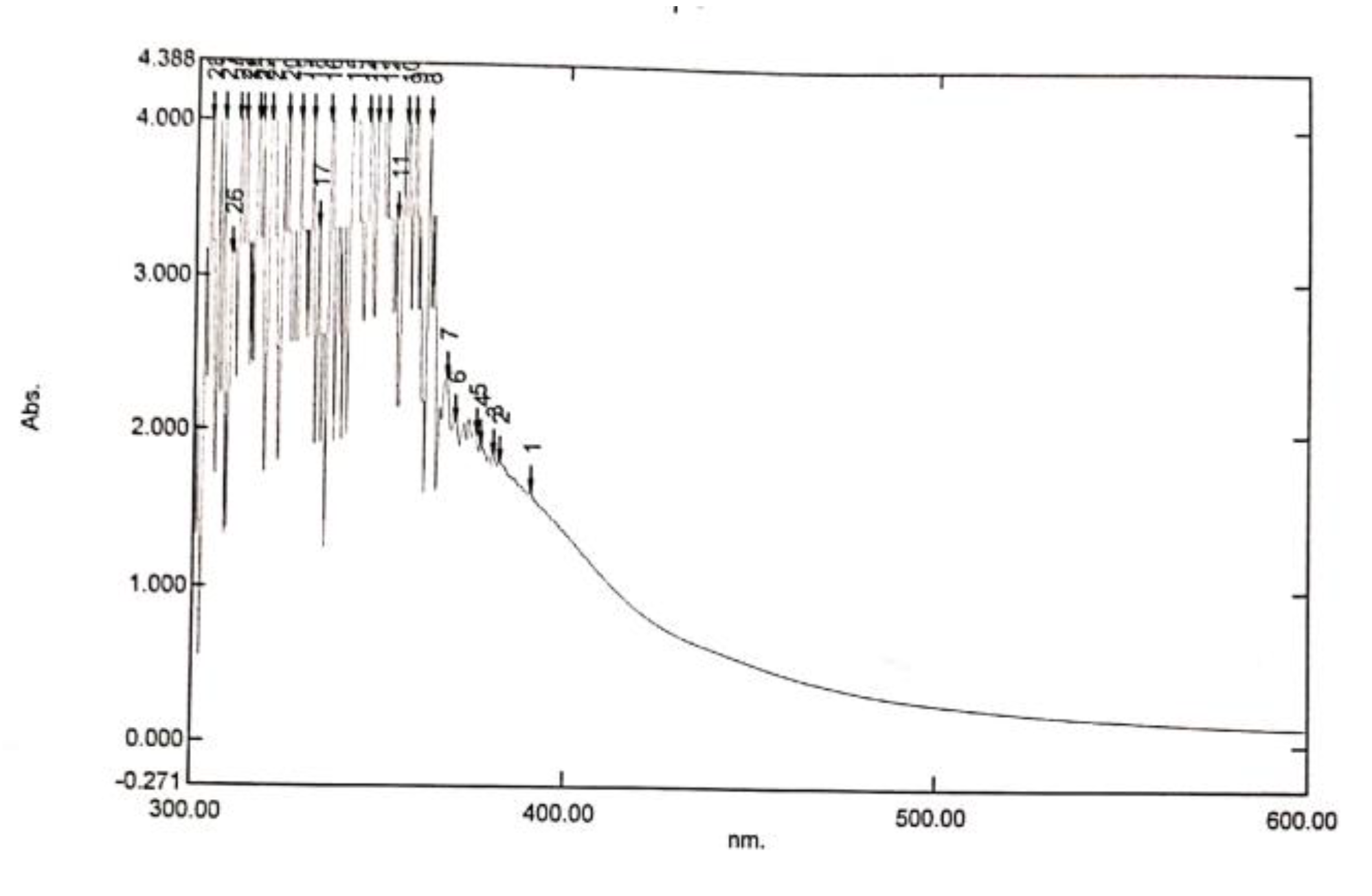
Figure 6.
The UV Result of the Forcados Asphaltene.
Figure 6.
The UV Result of the Forcados Asphaltene.
The UV-VIS spectrum of the Forcados asphaltene in cyclohexane ranging from 100 to 40mg/L are shown in Figure 9. The analyzed wavelength range of 300 to 600 nm. The wavelength of range of 300nm to 380nm contains a greater number of aromatic groups like the benzene, naphthalene and other aromatic compounds as observed by the different spectra of the UV-VIS (Igor N. Evdokimov et al. (2017)).
5. Discussion
From Table 4.1; the moisture content of the extracted oil sample is 26.0 which is quite high relatively comparing it to the accepted moisture content of good heavy fuel like diesel (AGO) which is within the range of 5 to 10. The presence of high water content in the extracted asphaltene sample will result in the corrosion of the engine system, sputtering around the silencer of the engine system, higher cost of the storage of the product as a result of its corrosion potential and other side effects which might not be desirous of a good fuel.
With an ash content of 1, it shows that there is a high amount of inorganic materials in the extracted oil sample. These inorganic materials will largely give an indication of the formation of residues after the combustion process which will not be a desirable feature of a good fuel as this can clog around the engine casing and pose a hazard to the engine system and also high cost of treatment, storage and transportation of the extracted asphaltene sample.
From
Table 2; The API gravity of the extracted asphaltene is 26.7 which is greater 10. This signifies that the oil is lighter than water and hence will float on water which is a good feature of oil as most oil floats on water when they come in contact.
From
Table 2; The flash point of the extracted asphaltene is 48.9°C which is below the accepted limit for heavy fuel oil fractions, 60°C minimum. This means that the asphaltene might not be able to give up enough vapour to form ignition with the air around it to initiate ignition.
The cloud point of the extracted asphaltene; 18°C, which is low for heavy fuel oil as heavy fuel oil cannot be pumped at a temperature of 18°C and therefore must be preheated to a temperature of about 40°C to prevent the formation of wax. This means that there is a tendency of the fuel to form wax crystals when cold at a temperature of 18°C. These formed wax crystals can clog the atomizer that injects fuel into the combustion chamber of the heavy fuel engine.
From the same
Table 2; The cetane number for the extracted asphaltene is 51. Most modern day diesel engines are designed to require fuel with a cetane number in the range of 45 to 55. Since, the higher the cetane number, the better the fuel, the shorter the ignition delay and the earsier the starting of the combustion of the fuel. So, with a cetane number of 51, the extracted asphaltene oil can be said to have a good cetane number for a quality heavy fuel oil.
From
Table 3; the chromium. Cr content of the extracted asphaltene is 0.125 ppm (mg/kg) which exceeded the WHO limit for chromium content in soil (0.01 mg/kg). Cr do not really have a biological function linking it to biological activities in humans and mammals generally. Cr in its compound forms such as chromates of calcium, Zinc, Strontium and lead are very soluble in water, toxic and carcinogens (Omobolaji O. Afolabi et al.).
The Ni content of the extracted asphaltent is 0.398 ppm (mg/kg), which is above the WHO limit of 0.05 (mg/kg) in soil. There are some negative health impact that human exposure to Nickel (Ni) can result to. Some of these health impacts includes allergies, cardiovascular and kidney diseases, lung fibrosis, lung and anal cancer (Omobolaji O. Afolabi et al.)
The Fe content of the extracted oil is 60.7 ppm (mg/kg) which if far higher than the WHO limit of 0,05 mg/kg. According to Adiele et al., the amount of Fe in the soil can be influence by the soil texture. Sequel to this the soil texture of the location where the crude sample was collected in Forcados must have greatly influence the Fe content of crude and hence the extracted asphaltene. When excess oil is stored in the human body organs, especially in the human liver, heart and pancreas, it can lead to life threatning conditions such as liver disease, heart problems and diabetes.
The Lead, Pb content of the asphatene is 60.425 ppm (mg/kg) which is in excess of the WHO limit of 0.1 mg/kg. This is in consistence with the report of Udon and Chukwu and Uduetok et al. where Pb from soil sample exceeded the allowable WHO limit. Lead, Pb can enter the human system via bioaccumulation in plants and animals. Pb poisoning in humans can damage the kidney, liver, heart, brain, skeleton and nervous system, which chronic exposure to low levels of Pb is capable of limiting the intelligence capacity in children. Over exposure to Pb can also cause fatality in human as Nigeria cannot forget the Pb poisoning incident of Zamfara state in 2010 in a hurry, where it was detected that a lot of the dead casualties actually had Pb poisoning from illegal gold mining operations.
The Cadmium, Cd content of the asphaltene is 26 is far above the WHO limit of Cd in soil (0.003). According to Pinto et al. and Lichtfouse, Cd can impede a plant’s growth and development. It’s also also a vital contaminant due to its toxicity level. A long term exposure to cadmium through air, water, soil and food can lead to cancer and organ system toxicity such as skeletal, uninery, reproductive, cardiovascular, central and peripheral nervous and respiratory systems. According to koons AL and Rajasurya V et al. (2022); Inhaled cadmium fumes are readily bioavailable. Once in the bloodstream, Cadmium binds to alpha -2- macroglobulin and albumin and gets distributed to the liver and kidneys. Aside these two main organs, cadmium also concentrates in the pancreas, spleen, heart, lung and testes.
The copper, Cu content of the asphaltene is 165.75 which is above the WHO limit of 2.0. The proper daily intake of Copper, Cu in human is 2.5mg/kg. Howevwer, an intake above this is capable of causing anaemia. Liver and kidney damage, irritation of both stomach and intestine.
With Mn at 49.9, which is a very high concentration in the asphaltene. Different epidemiological studies of workers exposed to manganese of an average levels below 5mg/m3 have shown neurobehavioural, reproductive and respiratory effects both by objective testing methods and by workers self reported symptoms on questionaires. Some neurobehavioural effects have reflected issues with the control of the hand movements e.g tremor, reduced hand steadiness and/or the speed of movement e.g longer reaction time, slower finger-tapping speed. Some sexual dysfunction too have also been observed in some studies of occupational exposure to manganese.
The concentration of zinc; 48.82ppm is below the WHO limit for zinc; 50ppm. So, the zinc content of the ashaltene is still within the accepted limit. Toxicological manifestistation of zinc include stomach pain, loss of appetite and vomiting (Osobamiro .T., Awolesi .O. et al. (2019))
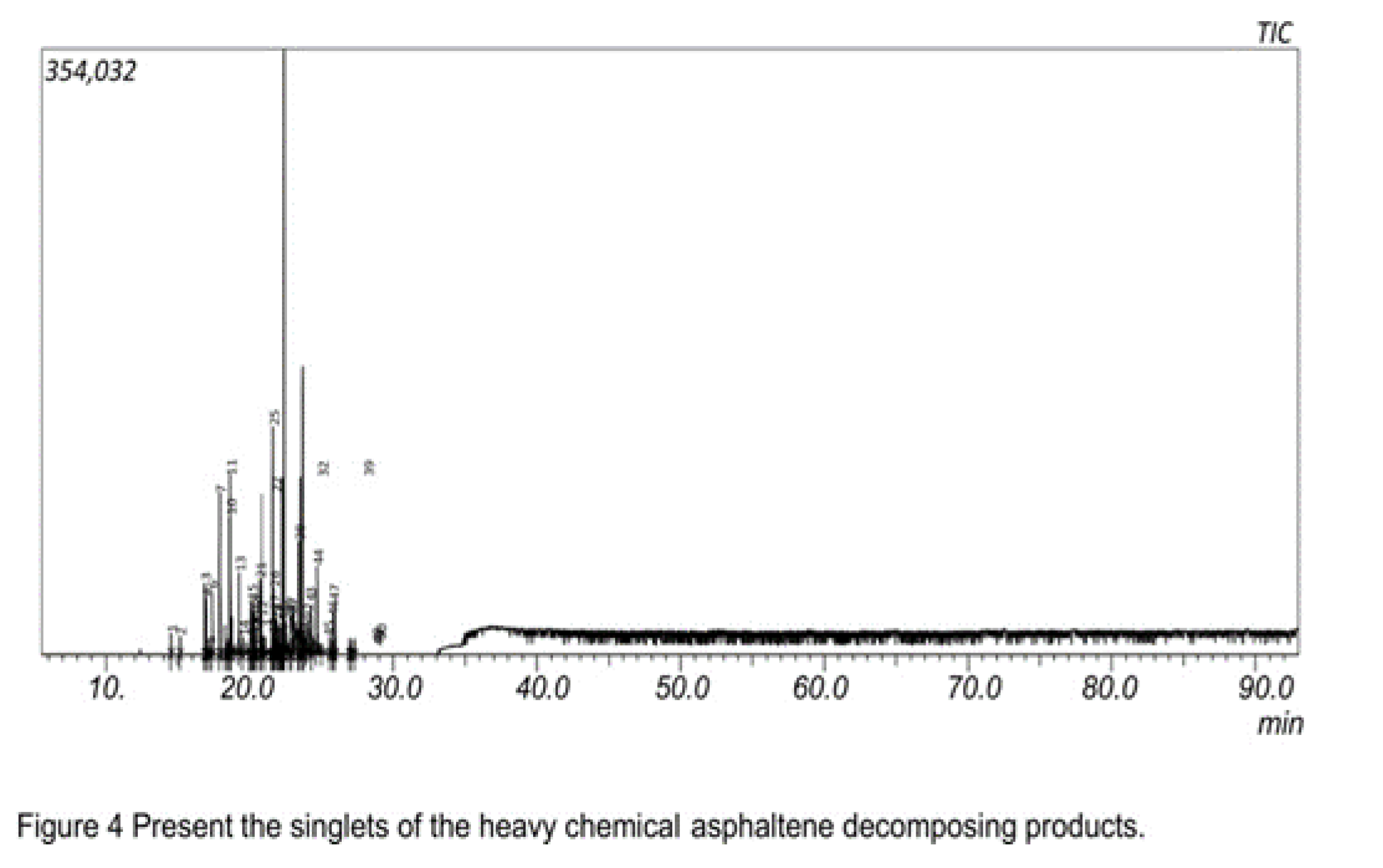
From
Figure 3 above, As the asphaltene passes through the GC column, the volatile compounds in the asphaltene where first eluted as it was observed that they burnt out first within the retention time of 10 mins to 22 mins and displayed long peaks from 1 to 50 on the chromatogram.
The presence of short continuous peaks from retention time 33 minutes indicates the presence of heavy fuel fractions hydrocarbons of the asphaltene as it is continuous all through 90 minutes and even beyond. Xinyan Pei et al. in their work on the Swirling Flame Combustion of Heavy Fuel Oil Blended with Diesel: Effect of Asphaltene Concentration said that heavy fuel fraction asphaltene was difficult to burn due to the inability of the heat to accumulate well. Previous investigation on suspended droplet experiment also showed that the ignation delay time of high asphaltene was higher than that of the low asphaltene fuel under the same droplet size. Therefore the higher hydrocarbon composition of the asphaltene needed more time to combust. Xinyan Pei et al. in the same experiment highlighted above also inferred that higher hydrocarbon asphaltene will leave a high mass carbon after burning due to the effect of asphaltene and the high rate of metal composition in asphaltene. This suggests that the asphaltene was the origin for carbon formation; meaning that the higher the asphaltene the higher the carbon residues formed. That is to say, the formation of cenospheres is caused by the asphaltene during heavy fuel oil combustion.
Acknowledgements
This work was supported by the authors from the department of Pure and Applied Chemistry, Kebbi State University of Science and Technology, Aliero, Kebbi State.
Declaration of Conflict of Interest
The authors declare no conflict of interest.
References
- Afdhol, M. K., et al. (2019). “The Prospect of Electrical Enhanced Oil Recovery for Heavy Oil: A Review.” Journal of Earth Energy Engineering 8(2): 73-94.
- Al-Muhareb, E., et al. (2007). “Characterization of petroleum asphaltenes by size exclusion chromatography, UV-fluorescence and mass spectrometry.” Petroleum science and technology 25(1-2): 81-91.
- Alrashidi, H., et al. (2019). “Application of natural fatty acids as asphaltenes solvents with inhibition and dispersion effects: A mechanistic study.” Journal of Petroleum Science and Engineering 172: 724-730.
- Atiku, F. A., et al. (2016). “A study of the combustion chemistry of petroleum and bio-fuel oil asphaltenes.” Fuel 182: 517-524.
- Bartle, K., et al. (2013). “The combustion of droplets of high-asphaltene heavy oils.” Fuel 103: 835-842.
- Buenrostro-Gonzalez, E., et al. (2001). “Characterization of asphaltenes and resins from problematic Mexican crude oils.” Petroleum science and technology 19(3-4): 299-316.
- Chamkalani, A. (2016). “A novel technique for screening of asphaltene deposition by the pattern recognition method.” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 38(3): 450-457.
- da Silva Ramos, A. C., et al. (2001). “Interfacial and colloidal behavior of asphaltenes obtained from Brazilian crude oils.” Journal of Petroleum Science and Engineering 32(2-4): 201-216.
- Ding, M., et al. (2015). “Mutual interactions of CO2/oil and natural gas/oil systems and their effects on the EOR process.” Petroleum science and technology 33(23-24): 1890-1900.
- Gao, Y., et al. (2018). “Impact of minerals and water on bitumen-mineral adhesion and debonding behaviours using molecular dynamics simulations.” Construction and Building Materials 171: 214-222.
- Hassanv, M., et al. (2013). “Study of temperature effect on asphaltene precipitation by visual and quantitative methods.” Journal of Petroleum Technology and Alternative Fuels 3(2): 1-18.
- Herod, A., et al. (2012). “Analytical methods for characterizing high-mass complex polydisperse hydrocarbon mixtures: an overview.” Chemical reviews 112(7): 3892-3923.
- Huffman, G. P., et al. (2000). “Characterization of fine particulate matter produced by combustion of residual fuel oil.” Journal of the Air & Waste Management Association 50(7): 1106-1114.
- Imanbayev, Y., et al. (2017). “High temperature transformation of tar-asphaltene components of oil sand bitumen.” Journal of the Serbian Chemical Society 82(9): 1063-1073.
- Kalantari-Dahaghi, A., et al. (2008). “Formation damage through asphaltene precipitation resulting from CO2 gas injection in Iranian carbonate reservoirs.” SPE Production & Operations 23(02): 210-214.
- Karimi, A., et al. (2011). “Quantitative evidence for bridged structures in asphaltenes by thin film pyrolysis.” Energy & fuels 25(8): 3581-3589.
- Li, D. D. and M. L. Greenfield (2011). “High internal energies of proposed asphaltene structures.” Energy & fuels 25(8): 3698-3705.
- Petrova, L., et al. (2011). “Structural features of asphaltene and petroleum resin fractions.” Petroleum Chemistry 51(4): 252-256.
- Robert, A. M. (2005). Handbook of Petrochemicals Production Processes. New York, McGraw-Hill Education.
- This unique reference is the only one-stop source for details on licensed petrochemical processes for the major organic chemicals, a $200 billion annual market. With chapters prepared by some of the largest petrochemical and petroleum companies in the world, Handbook of Petrochemicals Production Processes provides in-depth process detail for commercial evaluation and covers plastics and polymers such as ethylene and polyethylene; propylene; ethylbenzene, styrene, and polystyrenes; vinyl chloride and polyvinyl chloride; and many others. This handbook answers questions on yields, unit operations, chemical and physical values, economics, and much more.
- Snape, J., et al. (1984). “Methods for estimating gene numbers for quantitative characters using doubled haploid lines.” Theoretical and applied genetics 67(2): 143-148.
- Speight, J. (2004). “Petroleum Asphaltenes-Part 1: Asphaltenes, resins and the structure of petroleum.” Oil & gas science and technology 59(5): 467-477.
- Speight, J. (2004). “Petroleum asphaltenes-Part 2: The effect of asphaltene and resin constituents on recovery and refining processes.” Oil & gas science and technology 59(5): 479-488.
- Spiecker, P. M., et al. (2003). “Aggregation and solubility behavior of asphaltenes and their subfractions.” Journal of colloid and interface science 267(1): 178-193.
- Trejo, F., et al. (2007). “Characterization of asphaltenes from hydrotreated products by SEC, LDMS, MALDI, NMR, and XRD.” Energy & fuels 21(4): 2121-2128.
- Veisi, S., et al. (2018). “Adsorption behavior of petroleum asphaltenes dissolved in Toluene by low-cost mineral adsorbents.” Journal of Oil, Gas and Petrochemical Technology 5(1): 1-24.
- Osobamiro, T., Awolesi, O. et al. (2019) ‘‘ Heavy Metal level of Soil Samples Collected From a Major Industrial Areab in Abeokuta, Southwestern Nigeria.’’.
- Xinyan Pei, et al. (2022). ‘‘the Swirling Flame Combustion of Heavy Fuel Oil Blended with Diesel: Effect of Asphaltene Concentration’’ Energies 17 (9) 15-6156.
- Bartle Keithet al (2013) ‘‘The combustion of droplets of high-asphaltene heavy oils’’.
- Agbaeze, E. K1*, Udeh, S. N2 and I. O. Onwuka (2015) ‘‘Resolving Nigeria’s dependency on oil – The derivation model’’.
- 31. Kamran Akbarzadeh et al. 2007. “Asphaltenes—Problematic but Rich in Potentia”.
- Afdhol, M. K., et al. (2019). “The Prospect of Electrical Enhanced Oil Recovery for Heavy Oil: A Review.” Journal of Earth Energy Engineering 8(2): 73-94.
- Al-Muhareb, E., et al. (2007). “Characterization of petroleum asphaltenes by size exclusion chromatography, UV-fluorescence and mass spectrometry.” Petroleum science and technology 25(1-2): 81-91.
- Alrashidi, H., et al. (2019). “Application of natural fatty acids as asphaltenes solvents with inhibition and dispersion effects: A mechanistic study.” Journal of Petroleum Science and Engineering 172: 724-730.
- Atiku, F. A., et al. (2016). “A study of the combustion chemistry of petroleum and bio-fuel oil asphaltenes.” Fuel 182: 517-524.
- Bartle, K., et al. (2013). “The combustion of droplets of high-asphaltene heavy oils.” Fuel 103: 835-842.
- Buenrostro-Gonzalez, E., et al. (2001). “Characterization of asphaltenes and resins from problematic Mexican crude oils.” Petroleum science and technology 19(3-4): 299-316.
- Chamkalani, A. (2016). “A novel technique for screening of asphaltene deposition by the pattern recognition method.” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 38(3): 450-457.
- da Silva Ramos, A. C., et al. (2001). “Interfacial and colloidal behavior of asphaltenes obtained from Brazilian crude oils.” Journal of Petroleum Science and Engineering 32(2-4): 201-216.
- Ding, M., et al. (2015). “Mutual interactions of CO2/oil and natural gas/oil systems and their effects on the EOR process.” Petroleum science and technology 33(23-24): 1890-1900.
- Gao, Y., et al. (2018). “Impact of minerals and water on bitumen-mineral adhesion and debonding behaviours using molecular dynamics simulations.” Construction and Building Materials 171: 214-222.
- Hassanv, M., et al. (2013). “Study of temperature effect on asphaltene precipitation by visual and quantitative methods.” Journal of Petroleum Technology and Alternative Fuels 3(2): 1-18.
- Herod, A., et al. (2012). “Analytical methods for characterizing high-mass complex polydisperse hydrocarbon mixtures: an overview.” Chemical reviews 112(7): 3892-3923.
- Huffman, G. P., et al. (2000). “Characterization of fine particulate matter produced by combustion of residual fuel oil.” Journal of the Air & Waste Management Association 50(7): 1106-1114.
- Imanbayev, Y., et al. (2017). “High temperature transformation of tar-asphaltene components of oil sand bitumen.” Journal of the Serbian Chemical Society 82(9): 1063-1073.
- Kalantari-Dahaghi, A., et al. (2008). “Formation damage through asphaltene precipitation resulting from CO2 gas injection in Iranian carbonate reservoirs.” SPE Production & Operations 23(02): 210-214.
- Karimi, A., et al. (2011). “Quantitative evidence for bridged structures in asphaltenes by thin film pyrolysis.” Energy & fuels 25(8): 3581-3589.
- Li, D. D. and M. L. Greenfield (2011). “High internal energies of proposed asphaltene structures.” Energy & fuels 25(8): 3698-3705.
- Petrova, L., et al. (2011). “Structural features of asphaltene and petroleum resin fractions.” Petroleum Chemistry 51(4): 252-256.
- Robert, A. M. (2005). Handbook of Petrochemicals Production Processes. New York, McGraw-Hill Education.
- This unique reference is the only one-stop source for details on licensed petrochemical processes for the major organic chemicals, a $200 billion annual market. With chapters prepared by some of the largest petrochemical and petroleum companies in the world, Handbook of Petrochemicals Production Processes provides in-depth process detail for commercial evaluation and covers plastics and polymers such as ethylene and polyethylene; propylene; ethylbenzene, styrene, and polystyrenes; vinyl chloride and polyvinyl chloride; and many others. This handbook answers questions on yields, unit operations, chemical and physical values, economics, and much more.
- Snape, J., et al. (1984). “Methods for estimating gene numbers for quantitative characters using doubled haploid lines.” Theoretical and applied genetics 67(2): 143-148.
- Speight, J. (2004). “Petroleum Asphaltenes-Part 1: Asphaltenes, resins and the structure of petroleum.” Oil & gas science and technology 59(5): 467-477.
- Speight, J. (2004). “Petroleum asphaltenes-Part 2: The effect of asphaltene and resin constituents on recovery and refining processes.” Oil & gas science and technology 59(5): 479-488.
- Spiecker, P. M., et al. (2003). “Aggregation and solubility behavior of asphaltenes and their subfractions.” Journal of colloid and interface science 267(1): 178-193.
- Trejo, F., et al. (2007). “Characterization of asphaltenes from hydrotreated products by SEC, LDMS, MALDI, NMR, and XRD.” Energy & fuels 21(4): 2121-2128.
- Veisi, S., et al. (2018). “Adsorption behavior of petroleum asphaltenes dissolved in Toluene by low-cost mineral adsorbents.” Journal of Oil, Gas and Petrochemical Technology 5(1): 1-24.
- Osobamiro, T., Awolesi, O. et al. (2019) “ Heavy Metal level of Soil Samples Collected From a Major Industrial Areab in Abeokuta, Southwestern Nigeria”.
- Xinyan Pei, et al. (2022). ‘‘the Swirling Flame Combustion of Heavy Fuel Oil Blended with Diesel: Effect of Asphaltene Concentration’’ Energies 17 (9) 15-6156.
- Bartle Keithet al (2013) ‘‘The combustion of droplets of high-asphaltene heavy oils’’.
- Agbaeze, E. K1*, Udeh, S. N2 and I. O. Onwuka (2015) ‘‘Resolving Nigeria’s dependency on oil – The derivation model’’.
- Kamran Akbarzadeh et al. 2007. ’’Asphaltenes—Problematic but Rich in Potentia’’.
- Morteza Asemani et al. ‘‘Detailed FTIR spectroscopy characterization of crude oil extracted asphaltenes: Curve resolve of overlapping bands’’.
- Zeinab Taherian et al. (2021) ‘‘A new insight to the assessment of asphaltene characterization by using fortier transformed infrared spectroscopy’’.
- Igor N. Evdokimov et L (2017) ‘‘Asphaltenes - Absorbers and Scatterers at NUV-Vis-NIR Wavelengths’’.
- E. E. Banda-Cruz et al. (2020) ‘‘derivative Uv-Vis Spectroscopy Of Crude Oil And Asphaltene Solutions For Composition Determination’’.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).