1. Introduction
Graphene, a two-dimensional (2D) allotrope of carbon, has captured the imagination of scientists and engineers since its discovery in 2004 by Andre Geim and Konstantin Novoselov [
1]. This remarkable material, consisting of a single layer of carbon atoms arranged in a hexagonal lattice, exhibits extraordinary properties that have led to groundbreaking advancements in various fields, including electronics, energy, materials science, and biomedical engineering [
2].
The unique properties of graphene stem from its atomic structure, where each carbon atom is bonded to three neighboring atoms, forming strong covalent bonds and a highly stable structure [
3]. This arrangement results in exceptional mechanical strength, high electrical and thermal conductivity, optical transparency, and flexibility. Moreover, graphene possesses intriguing quantum mechanical properties, such as massless Dirac fermions, which enable novel electronic and optoelectronic phenomena [
3,
4].
Given its remarkable properties, graphene has emerged as a promising candidate for a wide range of applications [
5]. In electronics, graphene-based transistors, sensors, and interconnects offer the potential for faster, more efficient devices with reduced energy consumption [
6,
7]. In energy storage and conversion, graphene-based materials show promise for high-capacity batteries, supercapacitors, and fuel cells due to their large surface area and fast charge transport properties [
7,
8]. In energy storage and conversion, graphene-based materials show promise for high-capacity batteries, supercapacitors, and fuel cells due to their large surface area and fast charge transport properties [
7,
9].
However, the realization of graphene’s full potential hinges on the development of scalable and cost-effective synthesis methods that can produce high-quality graphene with tailored properties [
10]. Various synthesis techniques have been developed, each offering distinct advantages and challenges. Chemical vapor deposition (CVD) allows for the scalable production of graphene films on metal substrates, while mechanical exfoliation produces pristine graphene flakes but lacks scalability. Epitaxial growth enables precise control over graphene’s crystal structure, while chemical reduction methods offer a route to producing reduced graphene oxide (rGO) with tunable properties [
11].
In this review, we aim to provide a comprehensive overview of recent advances in graphene synthesis techniques, including principles, advantages, challenges, and applications associated with each method. Furthermore, we will explore emerging trends and future prospects in graphene synthesis, with a focus on scalability, cost-effectiveness, and environmental sustainability. By synthesizing the latest research findings and technological developments, this review aims to contribute to the understanding and advancement of graphene synthesis for diverse applications in the 21st century and beyond.
2. Synthesis Methods
Graphene synthesis methods can be broadly categorized into several techniques, each offering unique advantages and challenges [
12]. The choice of synthesis method depends on factors such as desired graphene properties, scalability, cost-effectiveness, and application requirements. Here, we discuss four primary synthesis methods: Chemical Vapor Deposition (CVD), Mechanical Exfoliation, Epitaxial Growth, and Chemical Reduction.
A). Chemical Vapor Deposition (CVD)
B). Mechanical Exfoliation
C). Epitaxial Growth
D). Chemical Reduction
3. Chemical Vapor Deposition (CVD)
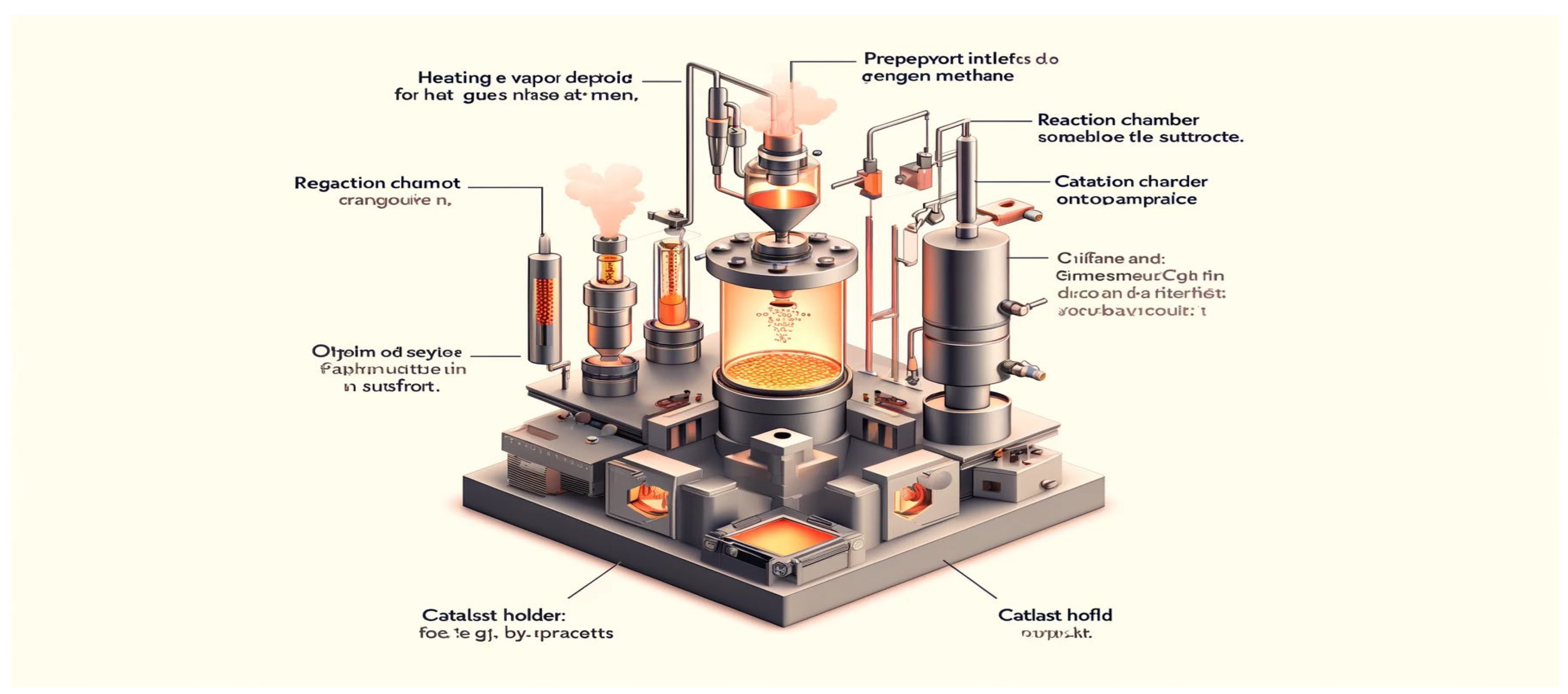
Chemical Vapor Deposition (CVD) is a versatile and widely used technique for synthesizing graphene films with precise control over thickness, morphology, and quality [
13]. In CVD, graphene growth occurs through the catalytic decomposition of hydrocarbon precursors in the presence of a metal catalyst substrate at elevated temperatures [
14]. The process involves several key steps, including precursor decomposition, carbon atom nucleation, and graphene layer growth, all of which are influenced by factors such as temperature, pressure, precursor gas composition, and catalyst material [
14].
3.1. Principle of Operation
The principle of CVD involves the thermal decomposition of carbon-containing precursor gases, such as methane (CH
4) or ethylene (C
2H
4), in a controlled environment [
15]. The precursor gas is introduced into a reaction chamber containing a metal catalyst substrate, typically copper (Cu), nickel (Ni), or cobalt (Co). The catalyst substrate acts as a template for carbon atom nucleation and graphene growth, facilitating the formation of graphene layers on its surface [
16].
At elevated temperatures (typically 800°C to 1100°C), the precursor gas molecules decompose into carbon atoms, which diffuse and adsorb onto the catalyst surface [
17]. The carbon atoms then undergo surface-mediated processes, such as diffusion and aggregation, leading to the formation of graphene nuclei. These nuclei continue to grow laterally, merging with neighboring nuclei to form continuous graphene layers [
18].
The growth kinetics of graphene in CVD are influenced by various factors, including temperature, pressure, gas flow rate, substrate morphology, and catalyst properties [
19]. By controlling these parameters, researchers can tailor the properties of the synthesized graphene, such as layer thickness, crystallinity, grain size, and doping level, for specific applications.
Figure 1.
Schematic diagram of Chemical Vapor Deposition (CVD).
Figure 1.
Schematic diagram of Chemical Vapor Deposition (CVD).
3.2. Advantages of CVD
CVD offers several advantages for synthesizing graphene films, making it a preferred technique for both research and industrial applications:
a. Scalability: CVD is inherently scalable and capable of producing large-area graphene films on various substrates, including metals, insulators, and flexible substrates. This scalability makes CVD suitable for industrial-scale production of graphene for commercial applications [
20].
b. Control over Thickness and Morphology: CVD allows precise control over the thickness, morphology, and orientation of graphene films by adjusting growth parameters such as temperature, pressure, and precursor gas composition. This control enables the synthesis of graphene with tailored properties for specific applications [
13].
c. Compatibility with Substrates: CVD can be performed on a wide range of substrate materials, including silicon, silicon dioxide, glass, and polymers. This compatibility with diverse substrates enables the integration of graphene into various device architectures and applications [
21].
d. High Quality and Uniformity: CVD-grown graphene exhibits high crystallinity, uniform thickness, and low defect density, resulting in superior electronic and mechanical properties compared to other synthesis methods [
13]. This high quality and uniformity make CVD-grown graphene suitable for advanced electronic, optoelectronic, and sensor applications.
e. Tunable Doping and Functionalization: CVD allows for the incorporation of dopant atoms (e.g., nitrogen, boron) and functional groups (e.g., hydrogen, oxygen) into the graphene lattice during growth, enabling the tuning of electronic, chemical, and mechanical properties for specific applications [
22]
3.3. Challenges and Future Directions
Despite its advantages, CVD-based graphene synthesis still faces several challenges and opportunities for improvement:
a. Catalyst Contamination and Substrate Effects: The choice of catalyst material and substrate properties can affect graphene nucleation, growth kinetics, and quality. Contaminants and impurities from the catalyst or substrate may introduce defects and impede graphene growth. Developing strategies to minimize catalyst contamination and substrate effects is crucial for improving the quality and reproducibility of CVD-grown graphene.
b. Uniformity and Defect Engineering: Achieving uniform growth and controlling defect density in CVD-grown graphene remain challenges for large-area synthesis. Strategies such as substrate engineering, gas phase doping, and in-situ monitoring techniques are being explored to enhance the uniformity and quality of CVD-grown graphene films.
c. Scalability and Cost-Effectiveness: While CVD is scalable, optimizing production processes to reduce costs and increase efficiency is essential for widespread commercialization of graphene-based technologies. Research efforts focus on developing cost-effective catalysts, precursor gases, and growth techniques to enhance the economic viability of CVD for industrial applications.
d. Heterostructure Integration: Integrating graphene with other 2D materials and functional layers to form heterostructures offers opportunities for developing novel devices with enhanced functionalities. CVD provides a versatile platform for synthesizing graphene heterostructures with tailored properties for applications in electronics, photonics, and energy conversion.
e. Environmental and Safety Considerations: Addressing environmental and safety concerns associated with precursor gases, catalyst materials, and by-products generated during CVD processes is essential for sustainable graphene production. Green synthesis approaches, waste recycling, and environmentally friendly growth techniques are being explored to minimize the environmental impact of CVD-based graphene synthesis.
4. Mechanical Exfoliation
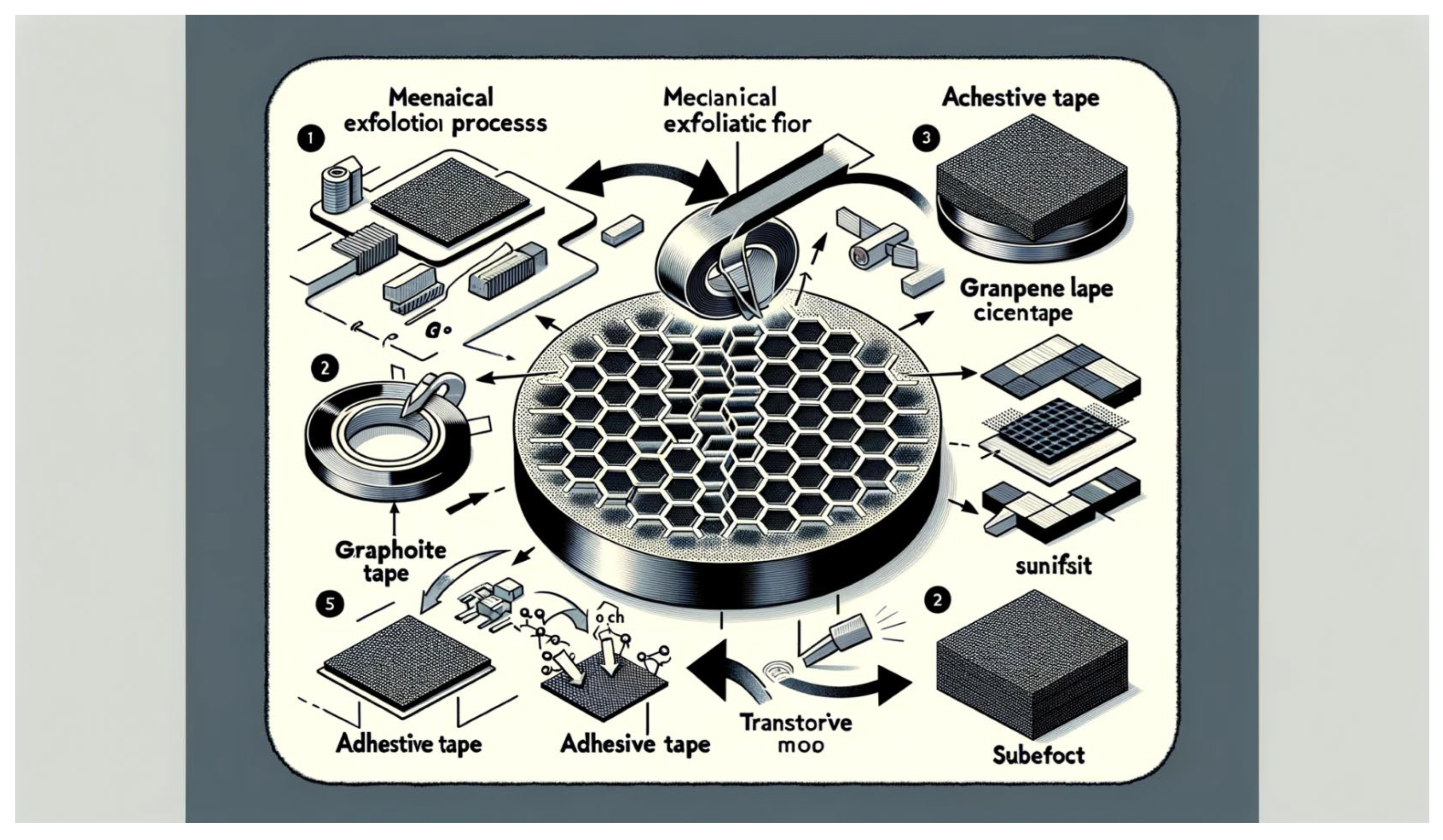
Mechanical exfoliation, often referred to as the “Scotch tape” method, is a simple yet powerful technique for producing graphene flakes with atomic-scale thicknesses [
23]. This method, which was instrumental in the initial discovery of graphene, involves the mechanical cleavage of graphite crystals to isolate individual graphene layers [
24]. Despite its simplicity, mechanical exfoliation remains a fundamental tool for producing high-quality graphene samples for research and device prototyping.
4.1. Principle of Operation
The principle of mechanical exfoliation is based on the mechanical cleavage of layered materials, such as graphite, to obtain thin flakes with atomic-scale thicknesses [
25]. Graphite, a naturally occurring form of carbon, consists of stacked layers of graphene held together by weak van der Waals forces. By applying adhesive tape to a freshly cleaved surface of graphite and peeling it off, individual layers of graphene can be separated and transferred onto a target substrate.
The mechanical exfoliation process begins by depositing a bulk graphite crystal onto a flat surface, such as a silicon wafer or glass slide [
26]. A piece of adhesive tape, typically Scotch tape or polydimethylsiloxane (PDMS), is then pressed onto the surface of the graphite crystal and firmly attached. Upon peeling off the tape, some graphene layers adhere to the tape due to its strong adhesive properties, while others remain on the substrate.
By repeatedly applying and peeling off the tape, thinner and thinner graphene flakes can be obtained, eventually leading to the isolation of single-layer graphene. The resulting graphene flakes can be transferred onto various substrates for further characterization and device fabrication.
Figure 2.
Schematic diagram of Mechanical Exfoliation.
Figure 2.
Schematic diagram of Mechanical Exfoliation.
4.2. Advantages of Mechanical Exfoliation
Mechanical exfoliation offers several advantages for producing graphene flakes with atomic-scale thicknesses:
a. Pristine Quality: Mechanical exfoliation produces graphene flakes with minimal defects and high crystallinity, as they are obtained directly from the bulk graphite crystal [
26]. This pristine quality makes mechanical exfoliation ideal for fundamental studies and exploring the intrinsic properties of graphene.
b. Thickness Control: By controlling the number of tape peeling steps, researchers can precisely control the thickness of the exfoliated graphene flakes, ranging from monolayer to few-layer thicknesses [
27]. This thickness control is essential for investigating thickness-dependent properties and fabricating devices with specific functionalities.
c. Low Cost and Accessibility: Mechanical exfoliation requires minimal equipment and materials, making it a low-cost and accessible technique for producing graphene samples in research laboratories [
28]. The simplicity of the method allows researchers to quickly obtain graphene flakes without the need for specialized equipment or expertise.
d. Rapid Prototyping: Mechanical exfoliation enables rapid prototyping of graphene-based devices by providing immediate access to high-quality graphene flakes [
29]. Researchers can use exfoliated graphene samples to fabricate devices, such as field-effect transistors, sensors, and photodetectors, for proof-of-concept experiments and device optimization.
e. Compatibility with Substrates: Exfoliated graphene flakes can be transferred onto various substrates, including silicon, silicon dioxide, polymers, and even flexible substrates [
30]. This compatibility with diverse substrates allows researchers to integrate graphene into different device architectures and applications.
4.3. Limitations and Challenges
Despite its advantages, mechanical exfoliation has limitations and challenges that restrict its scalability and applicability for industrial-scale production:
a. Low Yield and Efficiency: Mechanical exfoliation has a low yield and efficiency, as only a small fraction of the exfoliated graphene flakes are usable for further experiments or device fabrication. The manual nature of the process limits its throughput and scalability, making it unsuitable for large-scale production.
b. Lack of Uniformity: The size, shape, and quality of exfoliated graphene flakes can vary significantly from sample to sample and even within the same sample. This lack of uniformity poses challenges for reproducibility and consistency in research studies and device fabrication processes.
c. Sample Contamination: Mechanical exfoliation can introduce contaminants and impurities from the adhesive tape or substrate onto the surface of exfoliated graphene flakes. These contaminants may affect the electronic, optical, and chemical properties of graphene, necessitating thorough cleaning and characterization procedures.
d. Limited Scalability: The manual and labor-intensive nature of mechanical exfoliation limits its scalability for large-area graphene production. Alternative synthesis methods, such as Chemical Vapor Deposition (CVD) and Liquid Phase Exfoliation (LPE), offer higher throughput and scalability for industrial-scale production of graphene-based materials.
e. Lack of Control over Lateral Dimensions: Mechanical exfoliation provides limited control over the lateral dimensions (e.g., size and shape) of exfoliated graphene flakes, which can vary widely depending on the specific cleavage process. Achieving uniformity and reproducibility in the lateral dimensions of graphene flakes remains a challenge for mechanical exfoliation.
4.4. Future Directions and Research Opportunities
Despite its limitations, mechanical exfoliation continues to be a valuable technique for producing high-quality graphene flakes for research and development purposes. Ongoing research efforts aim to address the limitations of mechanical exfoliation and explore new approaches for enhancing its scalability, throughput, and controllability:
a. Automation and Robotics: Developing automated systems and robotic platforms for mechanical exfoliation can improve the throughput, reproducibility, and efficiency of the process. Robotic systems can perform repetitive tape peeling steps with higher precision and consistency than manual methods, enabling higher throughput and scalability.
b. Advanced Characterization Techniques: Advancements in microscopy and spectroscopy techniques, such as atomic force microscopy (AFM), scanning electron microscopy (SEM), and Raman spectroscopy, allow researchers to characterize exfoliated graphene flakes with higher resolution and sensitivity. These advanced characterization techniques provide insights into the structural, electronic, and optical properties of graphene at the nanoscale.
c. Hybrid Approaches: Combining mechanical exfoliation with other synthesis methods, such as CVD and LPE, offers opportunities for enhancing the scalability and controllability of graphene production. Hybrid approaches allow researchers to leverage the advantages of each technique while mitigating their respective limitations, leading to improved quality, yield, and uniformity of graphene materials.
d. Tailored Substrate Engineering: Engineering substrate surfaces with specific properties, such as surface roughness, chemistry, and adhesion strength, can enhance the efficiency and reproducibility of mechanical exfoliation. Tailored substrate designs and surface treatments can promote more efficient graphene transfer and reduce contamination during the exfoliation process.
e. Multiscale Modeling and Simulation: Computational modeling and simulation techniques provide valuable insights into the underlying mechanisms of mechanical exfoliation and help optimize process parameters for improved performance. Multiscale modeling approaches, combining atomistic simulations with continuum mechanics, enable researchers to predict and control the mechanical properties of exfoliated graphene flakes at various length scales.
5. Epitaxial Growth
Epitaxial growth is a versatile technique for synthesizing high-quality graphene films with precise control over crystal structure, orientation, and electronic properties [
31]. In epitaxial growth, graphene layers are grown on single-crystal metal substrates through processes such as thermal decomposition or chemical vapor deposition (CVD) [
32]. This method offers several advantages for producing large-area graphene films with tailored properties for various applications.
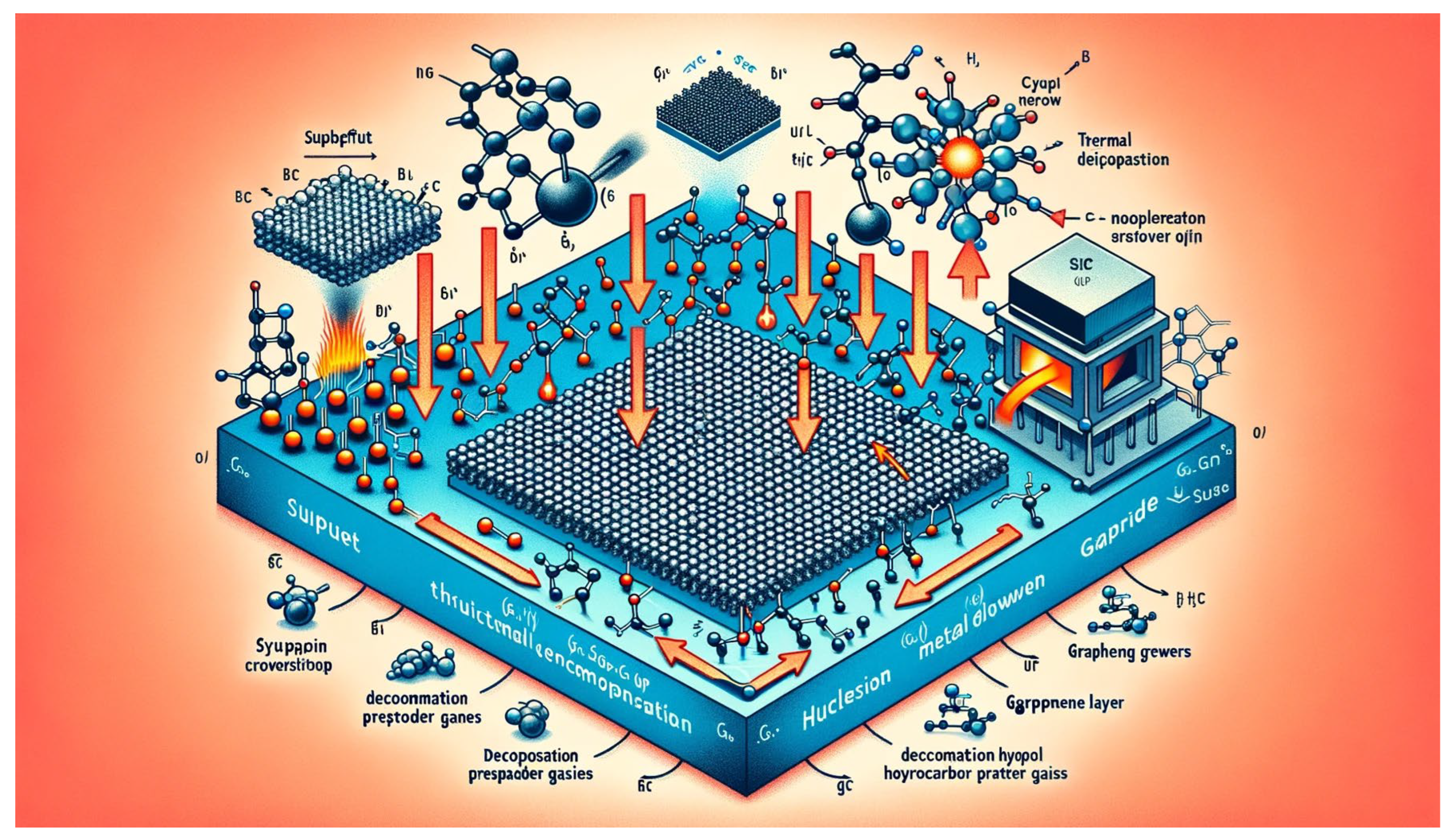
5.1. Principle of Operation
The principle of epitaxial growth involves the growth of crystalline layers (epitaxial layers) on a substrate surface with a well-defined crystal lattice orientation [
33]. In the context of graphene, epitaxial growth typically occurs on single-crystal metal substrates, such as silicon carbide (SiC) or transition metal surfaces (e.g., copper, nickel).
Epitaxial growth of graphene on SiC substrates involves the thermal decomposition of silicon atoms from the SiC surface, leaving behind a carbon-rich surface layer that undergoes graphene nucleation and growth [
34]. The choice of SiC polytype (e.g., 6H-SiC, 4H-SiC) and growth conditions (temperature, pressure) influences the thickness, quality, and domain size of the epitaxially grown graphene.
Alternatively, epitaxial growth of graphene on transition metal surfaces, such as copper or nickel, can be achieved through processes similar to CVD. In this approach, hydrocarbon precursor gases are decomposed on the metal surface, leading to the formation of graphene layers via carbon atom adsorption, diffusion, and aggregation. Epitaxial graphene growth on metal substrates offers precise control over the crystal structure, domain orientation, and doping level of the synthesized graphene.
Figure 3.
Schematic diagram of Epitaxial Growth.
Figure 3.
Schematic diagram of Epitaxial Growth.
5.2. Advantages of Epitaxial Growth
Epitaxial growth offers several advantages for synthesizing graphene films with tailored properties for specific applications:
a. Precise Control over Crystal Structure: Epitaxial growth enables precise control over the crystal structure and orientation of graphene layers by selecting appropriate substrate materials and growth conditions. This control allows researchers to tailor the electronic properties and functionalities of graphene for desired applications [
31].
b. Large-Area Growth: Epitaxial growth techniques, such as thermal decomposition on SiC substrates or CVD on metal surfaces, facilitate the large-area synthesis of graphene films with uniform thickness and high crystallinity [
34]. This scalability makes epitaxial growth suitable for industrial-scale production of graphene-based materials.
c. High Quality and Uniformity: Epitaxially grown graphene films exhibit high crystallinity, uniform thickness, and low defect density, resulting in superior electronic and mechanical properties compared to other synthesis methods [
35]. This high quality and uniformity make epitaxial graphene well-suited for advanced electronic, optoelectronic, and sensor applications.
d. Precise Control over Doping: Epitaxial growth techniques allow for the precise control of graphene doping levels by incorporating dopant atoms (e.g., nitrogen, boron) during growth. Doping graphene can modulate its electrical, optical, and chemical properties, enabling the design of graphene-based devices with enhanced functionalities [
36].
e. Compatibility with Heterostructures: Epitaxial growth enables the integration of graphene with other 2D materials and functional layers to form heterostructures with tailored properties. Graphene heterostructures offer opportunities for developing novel devices with enhanced functionalities, such as field-effect transistors, photodetectors, and sensors [
37].
5.3. Challenges and Future Directions
Despite its advantages, epitaxial growth of graphene faces several challenges and opportunities for improvement:
a. Substrate Compatibility and Surface Quality: The choice of substrate material and surface quality can significantly impact the nucleation, growth kinetics, and quality of epitaxially grown graphene. Developing compatible substrate materials and surface treatments is essential for optimizing epitaxial growth processes and enhancing graphene quality and uniformity [
31].
b. Interfacial Interactions and Strain Engineering: Interactions between graphene and the substrate surface, as well as lattice mismatches and strain effects, can influence the electronic and mechanical properties of epitaxially grown graphene [
38]. Understanding and controlling interfacial interactions and strain engineering are key research areas for optimizing epitaxial growth techniques and tailoring graphene properties.
c. Scalability and Cost-Effectiveness: While epitaxial growth techniques offer scalability and control over graphene properties, optimizing production processes to reduce costs and increase efficiency is essential for widespread commercialization [
39]. Research efforts focus on developing cost-effective growth methods, precursor gases, and substrate materials for industrial-scale production of epitaxially grown graphene.
d. Defect Engineering and Surface Functionalization: Despite its high quality, epitaxially grown graphene may still contain defects and impurities that affect its performance in certain applications. Strategies for defect engineering and surface functionalization, such as post-growth treatments and chemical modification techniques, are being explored to enhance the properties and functionalities of epitaxially grown graphene [
40].
e. Integration with Device Fabrication: Integrating epitaxially grown graphene with device fabrication processes poses challenges related to material transfer, lithography, and patterning [
41]. Developing compatible processing techniques and fabrication methodologies for epitaxial graphene-based devices is crucial for transitioning from synthesis to practical applications.
5.4. Emerging Trends and Applications
Epitaxial growth of graphene continues to drive advancements in various fields and applications, including:
a. Electronics and Photonics: Epitaxial graphene films serve as building blocks for high-performance electronic and photonic devices, such as field-effect transistors, photodetectors, and integrated circuits [
42]. The precise control over graphene properties and interfaces enables the development of next-generation electronic devices with improved performance and functionality.
b. Quantum Devices and Spintronics: Graphene heterostructures, formed by combining epitaxial graphene with other 2D materials (e.g., transition metal dichalcogenides, boron nitride), offer opportunities for exploring quantum phenomena and spintronics applications [
43]. Epitaxial growth techniques provide a platform for engineering novel device architectures with tailored electronic and spin properties for quantum computing and spin-based information processing [
44].
c. Energy Conversion and Storage: Epitaxial graphene-based materials are being investigated for applications in energy conversion and storage, such as photovoltaics, catalysis, and batteries [
45]. The high conductivity, chemical stability, and large surface area of epitaxial graphene make it attractive for enhancing the efficiency and performance of energy conversion and storage devices.
d. Sensors and Flexible Electronics: Epitaxial graphene films are promising candidates for sensor applications, including gas sensors, biosensors, and strain sensors, due to their high sensitivity, low noise, and compatibility with flexible substrates [
46]. The scalability and uniformity of epitaxial graphene enable the development of large-area sensor arrays and flexible electronics for healthcare, environmental monitoring, and wearable technology.
6. Chemical Reduction
Chemical reduction is a versatile approach for synthesizing reduced graphene oxide (rGO) from graphene oxide (GO) or graphene oxide derivatives [
47]. Graphene oxide, derived from the oxidation of graphite, contains oxygen-containing functional groups (such as hydroxyl, epoxy, and carboxyl groups) that disrupt the π-conjugated structure of graphene and render it insulating [
48]. Chemical reduction methods aim to remove these oxygen functional groups and restore the sp² carbon network of graphene, resulting in rGO with enhanced electrical conductivity, mechanical strength, and chemical stability [
49].
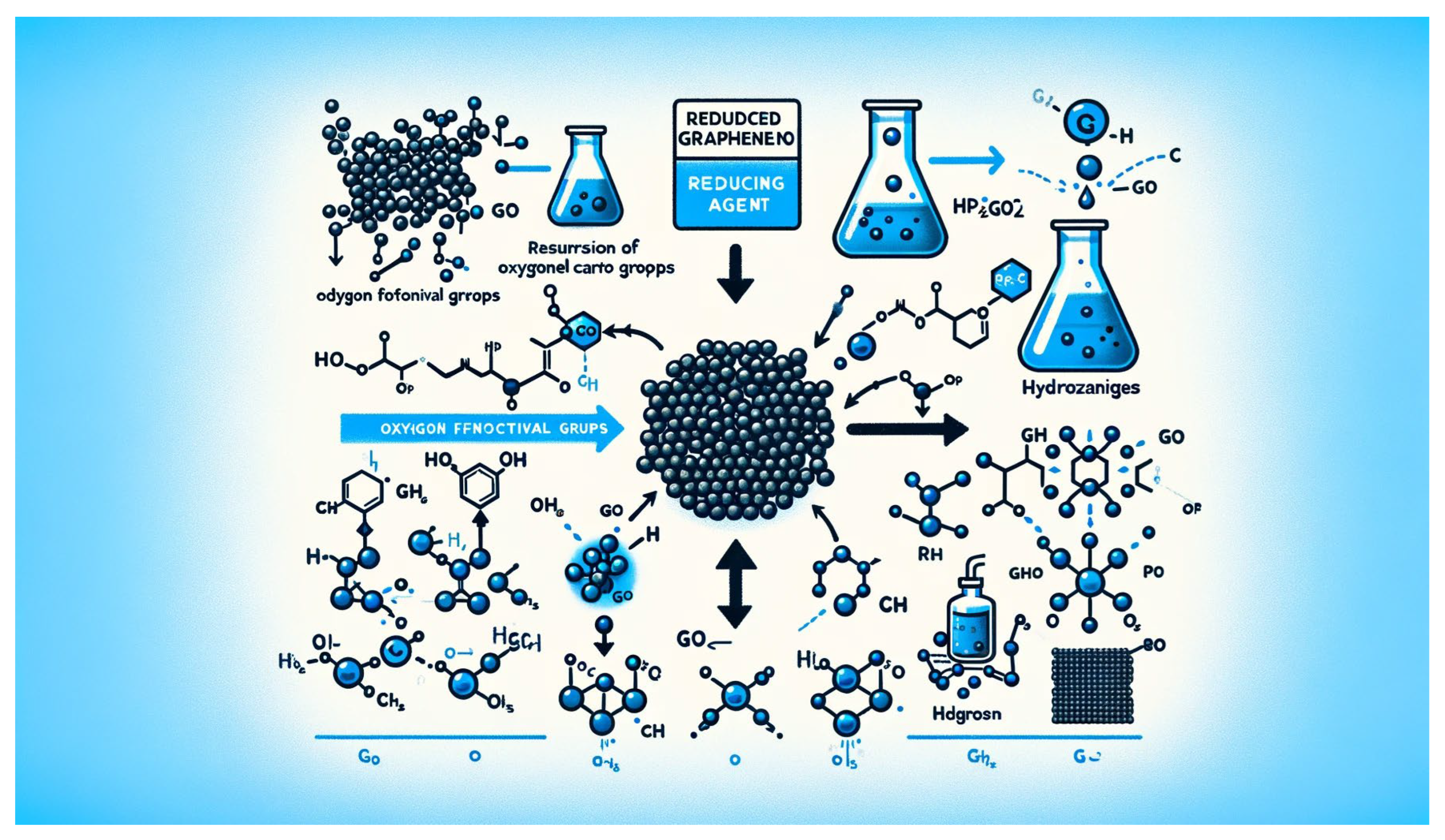
6.1. Principle of Operation
Chemical reduction involves the use of reducing agents to remove oxygen functional groups from graphene oxide and restore the sp² carbon-carbon bonds characteristic of graphene [
50]. Common reducing agents used in chemical reduction methods include hydrazine, hydrazine hydrate, sodium borohydride, and hydrogen gas. These reducing agents react with oxygen functional groups on the graphene oxide surface, leading to the reduction of graphene oxide to rGO [
51].
The chemical reduction process typically occurs in solution, where graphene oxide is dispersed in a solvent such as water or alcohol, and the reducing agent is added under controlled conditions [
51]. The reduction reaction proceeds at elevated temperatures and may require additional catalysts or additives to enhance reaction kinetics and efficiency. Following reduction, the resulting rGO can be collected by filtration, centrifugation, or other separation techniques and further processed for various applications [
52].
Figure 4.
Schematic diagram of Chemical Reduction.
Figure 4.
Schematic diagram of Chemical Reduction.
6.2. Advantages of Chemical Reduction
Chemical reduction methods offer several advantages for synthesizing rGO with tailored properties for specific applications:
a. Enhanced Electrical Conductivity: Chemical reduction removes oxygen functional groups from graphene oxide and restores the sp² carbon network, resulting in rGO with significantly improved electrical conductivity compared to pristine graphene oxide [
52]. This enhanced conductivity makes rGO suitable for applications in electronics, energy storage, and conductive coatings.
b. Mechanical Strength and Flexibility: The reduction of graphene oxide leads to the restoration of π-conjugated carbon-carbon bonds, enhancing the mechanical strength and flexibility of rGO compared to graphene oxide. rGO exhibits improved tensile strength, elasticity, and toughness, making it suitable for applications in composites, membranes, and flexible electronics [
53].
c. Chemical Stability and Compatibility: rGO exhibits improved chemical stability and compatibility with a wide range of solvents, substrates, and functional materials compared to graphene oxide [
54]. This stability allows for the facile integration of rGO into various device architectures and composite materials for diverse applications.
d. Tunable Properties: Chemical reduction methods offer flexibility in tailoring the properties of rGO by adjusting reduction conditions, such as reaction temperature, duration, and choice of reducing agent. This tunability enables the optimization of rGO properties, such as electrical conductivity, mechanical strength, and surface chemistry, for specific applications.
e. Scalability and Cost-Effectiveness: Chemical reduction methods are relatively simple, scalable, and cost-effective compared to other graphene synthesis techniques, such as chemical vapor deposition (CVD) or mechanical exfoliation [
39]. The use of inexpensive starting materials and readily available reducing agents makes chemical reduction attractive for large-scale production of rGO for commercial applications.
6.3. Challenges and Considerations
Despite its advantages, chemical reduction of graphene oxide presents several challenges and considerations:
a. Residual Functional Groups and Defects: Chemical reduction may not completely remove all oxygen functional groups from graphene oxide, resulting in residual defects and impurities in the synthesized rGO. Residual functional groups and defects can affect the properties and performance of rGO in certain applications, necessitating post-reduction treatments and purification steps to improve material quality [
55].
b. Environmental and Safety Concerns: Some chemical reduction methods involve the use of hazardous or toxic reducing agents, such as hydrazine, which pose environmental and safety risks. Developing environmentally friendly and sustainable reduction methods, using safer reducing agents and green solvents, is essential for mitigating these concerns and promoting the widespread adoption of rGO in various industries [
56].
c. Control over Structural and Morphological Properties: Chemical reduction methods may result in variations in the structural and morphological properties of rGO, depending on reduction conditions and synthesis parameters. Achieving precise control over the size, shape, and distribution of rGO flakes is important for ensuring uniformity and reproducibility in material properties and device performance [
57].
d. Stability and Long-Term Performance: rGO synthesized via chemical reduction methods may exhibit reduced stability and long-term performance compared to pristine graphene due to residual defects and functional groups [
58]. Understanding the degradation mechanisms and developing strategies to enhance the stability and durability of rGO-based materials is critical for extending their operational lifespan and reliability in real-world applications [
59].
e. Integration with Other Materials and Processes: Incorporating rGO into composite materials, coatings, and device architectures requires compatibility with other materials and processing techniques [
60]. Optimizing material compatibility, interfacial interactions, and processing conditions is essential for achieving synergistic effects and enhancing the performance of rGO-based materials in practical applications [
61].
6.4. Applications and Future Directions
rGO synthesized via chemical reduction methods finds applications in various fields, including:
a. Energy Storage and Conversion: rGO-based materials are used in energy storage and conversion devices, such as supercapacitors, batteries, and fuel cells, due to their high electrical conductivity, large surface area, and chemical stability [
61]. rGO electrodes exhibit enhanced charge storage capacity, cycling stability, and rate capability compared to conventional carbon materials, making them promising candidates for next-generation energy storage technologies [
62].
b. Sensors and Biosensors: rGO-based sensors and biosensors are employed for detecting gases, biomolecules, and environmental pollutants with high sensitivity, selectivity, and response speed [
63]. The large surface area and biocompatibility of rGO facilitate the immobilization of sensing elements and enable rapid and selective detection of analytes in real-time applications.
c. Composite Materials: rGO serves as a reinforcing filler in composite materials for enhancing mechanical strength, thermal conductivity, and electrical properties [
64]. rGO-based composites find applications in automotive components, aerospace structures, and sporting goods, where lightweight, high-performance materials are required.
d. Membranes and Separation Technologies: rGO-based membranes are used in water purification, gas separation, and desalination processes due to their high permeability, selectivity, and chemical stability [
65]. rGO membranes exhibit superior performance compared to conventional polymer membranes, offering opportunities for improving the efficiency and sustainability of membrane-based separation technologies.
e. Flexible Electronics and Wearable Devices: rGO-based materials are integrated into flexible electronics and wearable devices for applications such as flexible displays, electronic textiles, and biomedical sensors [
66]. The mechanical flexibility and electrical conductivity of rGO enable the development of lightweight, conformable, and stretchable electronics for wearable and portable applications [
67].
7. Applications and Future Outlook
The synthesis of graphene has spurred a wide range of applications across various fields, ranging from electronics and energy storage to biomedical devices and environmental remediation. As research progresses and technology advances, the potential applications of graphene continue to expand, driven by its unique properties, versatility, and potential for innovation. Here, we discuss some key applications of graphene and provide insights into future directions and emerging trends in graphene research and technology.
7.1. Electronics and Photonics
Graphene’s exceptional electrical conductivity, high carrier mobility, and optical transparency make it an attractive material for next-generation electronic and photonic devices [
68]. Graphene-based transistors, sensors, and interconnects offer superior performance, energy efficiency, and miniaturization compared to conventional silicon-based devices [
21]. Future research aims to explore novel device architectures, such as flexible and stretchable electronics, quantum devices, and optoelectronic devices, to unlock the full potential of graphene in advanced electronics and photonics applications.
Figure 5.
Schematic diagram of Electronics and Photonics.
Figure 5.
Schematic diagram of Electronics and Photonics.
7.2. Energy Storage and Conversion
Graphene’s large surface area, high electrical conductivity, and chemical stability enable its use in energy storage and conversion devices, including batteries, supercapacitors, and fuel cells [
7]. Graphene-based electrodes and catalysts offer improved charge storage capacity, cycling stability, and catalytic activity, leading to enhanced energy efficiency and performance [
69]. Future research focuses on developing graphene-based materials for high-capacity batteries, fast-charging supercapacitors, and efficient hydrogen production technologies to address global energy challenges and accelerate the transition to renewable energy sources.
Figure 6.
Schematic diagram of Energy Storage and Conversion.
Figure 6.
Schematic diagram of Energy Storage and Conversion.
7.3. Composite Materials
Graphene’s mechanical strength, lightweight, and barrier properties make it an ideal reinforcement filler in composite materials for enhancing mechanical, thermal, and electrical properties [
70]. Graphene-based composites find applications in aerospace, automotive, construction, and sporting goods industries, where lightweight and high-performance materials are essential [
71]. Future research aims to optimize composite processing techniques, tailor material properties, and explore new applications, such as 3D printing, nanocomposite coatings, and structural materials, to expand the use of graphene composites in various industrial sectors.
Figure 7.
Schematic diagram of Composite Materials.
Figure 7.
Schematic diagram of Composite Materials.
7.4. Biomedical Devices and Sensors
Graphene’s biocompatibility, large surface area, and electrical sensitivity make it promising for biomedical applications, including biosensors, drug delivery systems, and tissue engineering scaffolds [
72]. Graphene-based biosensors offer rapid, label-free detection of biomolecules with high sensitivity and selectivity, enabling early diagnosis and monitoring of diseases [
73]. Future research focuses on integrating graphene with biological systems, developing wearable and implantable devices, and exploring therapeutic applications, such as drug delivery and tissue regeneration, to revolutionize healthcare and improve patient outcomes.
Figure 8.
Schematic diagram of Biomedical Devices and Sensors.
Figure 8.
Schematic diagram of Biomedical Devices and Sensors.
7.5. Environmental and Sustainable Technologies
Graphene’s adsorption capacity, photocatalytic activity, and barrier properties make it useful for environmental remediation, water purification, and pollution control applications [
74]. Graphene-based membranes, adsorbents, and photocatalysts offer efficient removal of pollutants, heavy metals, and pathogens from air and water sources, contributing to environmental sustainability and public healthGraphene-based membranes, adsorbents, and photocatalysts offer efficient removal of pollutants, heavy metals, and pathogens from air and water sources, contributing to environmental sustainability and public health [
75]. Future research aims to develop scalable and cost-effective graphene-based technologies, such as water filtration systems, air purifiers, and solar-powered devices, to address environmental challenges and promote sustainable development.
Figure 9.
Schematic diagram of Environmental and Sustainable Technologies.
Figure 9.
Schematic diagram of Environmental and Sustainable Technologies.
8. Conclusion
In conclusion, the synthesis of graphene represents a remarkable advancement in materials science and nanotechnology, offering a wealth of opportunities for innovation and discovery across diverse fields and industries. From its humble beginnings as a theoretical concept to its realization in the laboratory, graphene has captured the imagination of scientists, engineers, and entrepreneurs worldwide, driven by its exceptional properties, versatility, and potential for transformative impact.
Throughout this review, we have explored various methods for synthesizing graphene, including mechanical exfoliation, chemical vapor deposition (CVD), epitaxial growth, and chemical reduction, each with its unique advantages, challenges, and applications. These synthesis techniques have enabled the production of graphene materials with tailored properties, ranging from high-quality monolayers to functionalized derivatives, to meet the demands of specific applications in electronics, energy, biomedical devices, and environmental technologies.
Looking ahead, the future of graphene research and technology holds great promise, with ongoing efforts focused on addressing key challenges, expanding the scope of applications, and realizing the full potential of graphene-based materials and devices. Collaborative interdisciplinary research, technological innovation, and sustainable development are essential for unlocking new opportunities and overcoming barriers to progress in graphene science and technology.
As we continue to explore the frontiers of graphene research, we envision a future where graphene-based materials and devices play a central role in advancing science, improving quality of life, and addressing pressing global challenges. By harnessing the remarkable properties of graphene and leveraging its unique advantages, we can pave the way for a more sustainable, interconnected, and innovative world, where graphene fuels the next wave of technological revolution and drives meaningful change for generations to come.
References
- Warner, J.H.; Schaffel, F.; Rummeli, M.; Bachmatiuk, A. Graphene: Fundamentals and emergent applications; Newnes: 2012.
- Huang, H.; Feng, W.; Chen, Y. Two-dimensional biomaterials: Material science, biological effect and biomedical engineering applications. Chemical Society Reviews 2021, 50, 11381–11485. [Google Scholar] [PubMed]
- Zhen, Z.; Zhu, H. Structure and properties of graphene. In Graphene, Elsevier: 2018; pp. 1–12.
- Gupta, G.K.; Sagar, P.; Srivastava, M.; Singh, A.K.; Singh, J.; Srivastava, S.; Srivastava, A. Hydrothermally synthesized nickel ferrite nanoparticles integrated reduced graphene oxide nanosheets as an electrode material for supercapacitors. Journal of Materials Science: Materials in Electronics 2024, 35, 255. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P. The chemistry and promising applications of graphene and porous graphene materials. Advanced Functional Materials 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Kang, J.; Cao, W.; Xie, X.; Sarkar, D.; Liu, W.; Banerjee, K. Graphene and beyond-graphene 2D crystals for next-generation green electronics. In Proceedings of Micro-and Nanotechnology Sensors, Systems, and Applications VI; pp. 20–26.
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Physical Chemistry Chemical Physics 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Gupta, G.K.; Shandilya, K.K. Hierarchical Ni-Mn Double Layered/Graphene Oxide with Excellent Energy Density for Highly Capacitive Supercapacitors. Mater Sci 2023, 11, 004. [Google Scholar]
- Sagar, P.; Gupta, G.K.; Srivastava, M.; Srivastava, A.; Srivastava, S. Tagetes erecta as an organic precursor: Synthesis of highly fluorescent CQDs for the micromolar tracing of ferric ions in human blood serum. RSC advances 2021, 11, 19924–19934. [Google Scholar] [CrossRef] [PubMed]
- Albalushi, R.A.R.; Yussuf, M.A.M. A short review on graphene derivatives towards photoelectrochemical water splitting. in E3S Web of Conferences. 2024. EDP Sciences.
- Gupta, G.K.; Sagar, P.; Srivastava, M.; Singh, A.K.; Singh, J.; Srivastava, S.; Srivastava, A. Excellent supercapacitive performance of graphene quantum dots derived from a bio-waste marigold flower (Tagetes erecta). International Journal of Hydrogen Energy 2021, 46, 38416–38424. [Google Scholar] [CrossRef]
- Gupta, G.K.; Sagar, P.; Pandey, S.K.; Srivastava, M.; Singh, A.; Singh, J.; Srivastava, A.; Srivastava, S.; Srivastava, A. In situ fabrication of activated carbon from a bio-waste desmostachya bipinnata for the improved supercapacitor performance. Nanoscale research letters 2021, 16, 85. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Seah, C.-M.; Chai, S.-P.; Mohamed, A.R. Mechanisms of graphene growth by chemical vapour deposition on transition metals. Carbon 2014, 70, 1–21. [Google Scholar] [CrossRef]
- Spear, K.E. Chemical and energetic aspects of CVD diamond growth. Preprints of Papers, American Chemical Society, Division of Fuel Chemistry;(USA) 1989. 34CONF-8904167-).
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Materials Science in Semiconductor Processing 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Ahmed, S.; Aitani, A.; Rahman, F.; Al-Dawood, A.; Al-Muhaish, F. Decomposition of hydrocarbons to hydrogen and carbon. Applied Catalysis A: General 2009, 359, 1–24. [Google Scholar] [CrossRef]
- Li, J.; Deepak, F.L. In situ kinetic observations on crystal nucleation and growth. Chemical reviews 2022, 122, 16911–16982. [Google Scholar] [CrossRef]
- Fauzi, F.B.; Ismail, E.; Ani, M.H.; Bakar, S.N.S.A.; Mohamed, M.A.; Majlis, B.Y.; Din, M.F.M.; Abid, M.A.A.M. A critical review of the effects of fluid dynamics on graphene growth in atmospheric pressure chemical vapor deposition. Journal of Materials Research 2018, 33, 1088–1108. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nature Reviews Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Akinwande, D.; Huyghebaert, C.; Wang, C.-H.; Serna, M.I.; Goossens, S.; Li, L.-J.; Wong, H.-S.P.; Koppens, F.H. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Ullah, S.; Shi, Q.; Zhou, J.; Yang, X.; Ta, H.Q.; Hasan, M.; Ahmad, N.M.; Fu, L.; Bachmatiuk, A.; Rümmeli, M.H. Advances and trends in chemically doped graphene. Advanced Materials Interfaces 2020, 7, 2000999. [Google Scholar] [CrossRef]
- Russo, P. Graphene and related materials: From" scotch tape" to advanced production methods. 2014.
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R. Graphene synthesis, characterization and its applications: A review. Results in Chemistry 2021, 3, 100163. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Z.B.; Tian, J.G. Stacking of exfoliated two-dimensional materials: A review. Chinese Journal of Chemistry 2020, 38, 981–995. [Google Scholar] [CrossRef]
- Sumdani, M.; Islam, M.; Yahaya, A.; Safie, S. Recent advances of the graphite exfoliation processes and structural modification of graphene: A review. Journal of Nanoparticle Research 2021, 23, 1–35. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J. Mechanisms of liquid-phase exfoliation for the production of graphene. ACS nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. Journal of Materials Chemistry A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Islam, A.; Mukherjee, B.; Pandey, K.K.; Keshri, A.K. Ultra-fast, chemical-free, mass production of high quality exfoliated graphene. ACS nano 2021, 15, 1775–1784. [Google Scholar] [CrossRef]
- Kang, J.; Shin, D.; Bae, S.; Hong, B.H. Graphene transfer: Key for applications. Nanoscale 2012, 4, 5527–5537. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the gap between reality and ideal in chemical vapor deposition growth of graphene. Chemical reviews 2018, 118, 9281–9343. [Google Scholar] [CrossRef]
- Ago, H.; Ito, Y.; Mizuta, N.; Yoshida, K.; Hu, B.; Orofeo, C.M.; Tsuji, M.; Ikeda, K.-i.; Mizuno, S. Epitaxial chemical vapor deposition growth of single-layer graphene over cobalt film crystallized on sapphire. ACS nano 2010, 4, 7407–7414. [Google Scholar] [CrossRef]
- Capper, P.; Irvine, S.; Joyce, T. Epitaxial crystal growth: Methods and materials. Springer Handbook of Electronic and Photonic Materials 2017, 1–1. [Google Scholar]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial graphene on SiC: A review of growth and characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Jia, K.; Sun, L.; Peng, H.; Liu, Z. Controlled growth of single-crystal graphene films. Advanced Materials 2020, 32, 1903266. [Google Scholar] [CrossRef]
- Lee, H.; Paeng, K.; Kim, I.S. A review of doping modulation in graphene. Synthetic Metals 2018, 244, 36–47. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Kirchner, M.M.; Wang, H.; Ren, Y.; Lei, W. Two-dimensional heterostructures and their device applications: Progress, challenges and opportunities. Journal of Physics D: Applied Physics 2021, 54, 433001. [Google Scholar] [CrossRef]
- Deng, B.; Hou, Y.; Liu, Y.; Khodkov, T.; Goossens, S.; Tang, J.; Wang, Y.; Yan, R.; Du, Y.; Koppens, F.H. Growth of ultraflat graphene with greatly enhanced mechanical properties. Nano Letters 2020, 20, 6798–6806. [Google Scholar] [CrossRef] [PubMed]
- Safian, M.T.-u.; Umar, K.; Ibrahim, M.N.M. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. Journal of Cleaner Production 2021, 318, 128603. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Aljarb, A.; Shi, Y.; Li, L.-J. Epitaxial growth of two-dimensional layered transition-metal dichalcogenides: Growth mechanism, controllability, and scalability. Chemical reviews 2017, 118, 6134–6150. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, J.; Zhang, Y.; Qu, Y.; Jia, B.; Chen, Z.; Moss, D.J. Fabrication technologies for the on-chip integration of 2D materials. Small Methods 2022, 6, 2101435. [Google Scholar] [CrossRef]
- Chen, X.; Shehzad, K.; Gao, L.; Long, M.; Guo, H.; Qin, S.; Wang, X.; Wang, F.; Shi, Y.; Hu, W. Graphene hybrid structures for integrated and flexible optoelectronics. Advanced Materials 2020, 32, 1902039. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R. Recent advances in two-dimensional materials beyond graphene. ACS nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef]
- Laucht, A.; Hohls, F.; Ubbelohde, N.; Gonzalez-Zalba, M.F.; Reilly, D.J.; Stobbe, S.; Schröder, T.; Scarlino, P.; Koski, J.V.; Dzurak, A. Roadmap on quantum nanotechnologies. Nanotechnology 2021, 32, 162003. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xin, L.; Hu, J.; Li, C.; Qi, J.; Hou, Y.; Wei, X. Advances in the applications of graphene-based nanocomposites in clean energy materials. Crystals 2021, 11, 47. [Google Scholar] [CrossRef]
- Mehmood, A.; Mubarak, N.; Khalid, M.; Walvekar, R.; Abdullah, E.; Siddiqui, M.T.H.; Baloch, H.A.; Nizamuddin, S.; Mazari, S. Graphene based nanomaterials for strain sensor application—A review. Journal of Environmental Chemical Engineering 2020, 8, 103743. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. Rsc Advances 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Ray, S. Applications of graphene and graphene-oxide based nanomaterials; William Andrew: 2015.
- Eigler, S.; Dimiev, A.M. Functionalization and reduction of graphene oxide. Graphene oxide: Fundamentals and applications.
- Wei, G. Graphite Oxide: Structure, Reduction and Applications. RICE UNIVERSITY, 2012.
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chemical Society Reviews 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Trimm, D. Minimisation of carbon monoxide in a hydrogen stream for fuel cell application. Applied Catalysis A: General 2005, 296, 1–11. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the oxygen content of reduced graphene oxide and effects on its properties. ACS omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Composites Part B: Engineering 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically conductive “alkylated” graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Advanced materials 2010, 22, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chemical Society Reviews 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Cui, T.; Tong, G.; Wu, W. Enhanced photocatalytic properties of ZnO/reduced graphene oxide sheets (rGO) composites with controllable morphology and composition. Applied Surface Science 2017, 412, 58–68. [Google Scholar] [CrossRef]
- Cai, L.; Yu, G. Recent advances in growth and modification of graphene-based energy materials: From chemical vapor deposition to reduction of graphene oxide. Small Methods 2019, 3, 1900071. [Google Scholar] [CrossRef]
- Shalini, S.; Naveen, T.; Durgalakshmi, D.; Balakumar, S.; Rakkesh, R.A. Progress in flexible supercapacitors for wearable electronics using graphene-based organic frameworks. Journal of Energy Storage 2024, 86, 111260. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Composites Part B: Engineering 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Malik, M.T.U.; Sarker, A.; Rahat, S.S.M.; Shuchi, S.B. Performance enhancement of graphene/GO/rGO based supercapacitors: A comparative review. Materials Today Communications 2021, 28, 102685. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/metal oxide nanocomposites as promising gas-sensing materials—A review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Zhang, Z.; Xian, Y.; Lin, Y.; Ji, X.; Lu, Y.; Zhang, L. Construction of interconnected Al2O3 doped rGO network in natural rubber nanocomposites to achieve significant thermal conductivity and mechanical strength enhancement. Composites Science and Technology 2020, 186, 107930. [Google Scholar] [CrossRef]
- Han, Z.-y.; Huang, L.-j.; Qu, H.-j.; Wang, Y.-x.; Zhang, Z.-j.; Rong, Q.-l.; Sang, Z.-q.; Wang, Y.; Kipper, M.J.; Tang, J.-g. A review of performance improvement strategies for graphene oxide-based and graphene-based membranes in water treatment. Journal of Materials Science 2021, 56, 9545–9574. [Google Scholar] [CrossRef]
- Diniz, F.L.; Lima, T.; Araujo, E.S.; Araujo, P.L. Graphene-Based Flexible and Eco-Friendly Wearable Electronics and Humidity Sensors. Materials Research 2024, 27, e20230480. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Advanced materials 2019, 31, 1801072. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, H. Graphene-based nanomaterials: Synthesis, properties, and optical and optoelectronic applications. Advanced Functional Materials 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Advanced Energy Materials 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Verma, D.; Gope, P.; Shandilya, A.; Gupta, A. Mechanical-thermal-electrical and morphological properties of graphene reinforced polymer composites: A review. Transactions of the Indian Institute of Metals 2014, 67, 803–816. [Google Scholar] [CrossRef]
- Valorosi, F.; De Meo, E.; Blanco-Varela, T.; Martorana, B.; Veca, A.; Pugno, N.; Kinloch, I.A.; Anagnostopoulos, G.; Galiotis, C.; Bertocchi, F. Graphene and related materials in hierarchical fiber composites: Production techniques and key industrial benefits. Composites Science and Technology 2020, 185, 107848. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Lin, Z. Graphene and graphene-based nanocomposites: Biomedical applications and biosafety. Journal of Materials Chemistry B 2016, 4, 7813–7831. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Abu Serea, E.S.; Salah-Eldin, R.E.; Al-Hafiry, S.A.; Ali, M.K.; Shalan, A.E.; Lanceros-Méndez, S. Recent progress in graphene-and related carbon-nanomaterial-based electrochemical biosensors for early disease detection. ACS Biomaterials Science & Engineering 2022, 8, 964–1000. [Google Scholar]
- Perreault, F.; De Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chemical Society Reviews 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Tamil Elakkiya, V.; Rajeswari, R. Graphene-based materials for environmental applications: A review. Environmental Chemistry Letters 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).