1. Introduction
Undergraduate chemistry, biochemistry, and other related courses require students to understand absorption spectrophotometry, Beer-Lambert law, and the quantification of analytes in solutions[
1,
2]. However, the high cost of analytical instruments employed in scientific inquiry and instruction constitutes an obstacle for schools and instructors in settings with limited resources. This limitation impairs instructors’ ability to offer practical science training, which limits students’ engagement and comprehension of fundamental scientific principles, with implications for students’ future achievements. [
3].
Several strategies have been proposed to address the financial barriers faced by schools in low-income settings. One of these strategies is the virtual laboratory. However, studies have shown that virtual laboratories have limitations and, therefore, cannot fully replace real laboratories; instead, they can supplement them [
4]. Real laboratory experience contributes significantly to building scientific and research skills in students with real-world applications. Reports have shown that classes that incorporate real-world practical experience. [
5,
6], especially in a collaborative project [
7], help students immensely in their later career path, and eventually contribute to society's general development.
To limit the impact of budgetary constraints in building real laboratories and providing practical science education in low-income schools, the improvisation of scientific instruments and materials for instruction has emerged as a viable option [
8,
9]. Using local resources and creative instructional approaches, educators may close the gap to costly instruments and enable students to receive practical training and technical know-how in conducting scientific experiments. The idea is to improvise scientific instruments utilizing familiar tools. Familiar resources used in learning foster authentic and lasting experiences and consequently improve engagement, comprehension, and interest in education [
10]. Complex ideas can be broken down into sequential steps for easy assimilation by leveraging familiar learning tools.
Most students Because students have smartphones [
11]Therefore, integrating smartphones into student learning is expected to greatly support science education in low-budget schools. According to reports, smartphones are becoming increasingly popular in developing countries, significantly impacting people’s lives [
12] and with the potential for long-term growth [
13]. However, despite the growing use of smartphones among the youth in developing countries, their incorporation into learning in higher education is just emerging [
14]. Unfortunately, students’ misuse of smartphones has become a source of time waste, distraction, emotional alienation, technostress, insomnia [
15], and decreased academic performance [
16]. Therefore, introducing smartphones into practical science training can substantially impact students, allowing them to use them better in their educational endeavors. Teaching Beer's Law and UV-vis spectroscopy using smartphones has been proposed [
17]. Here, we review and discuss the use of smartphones for quantitative colorimetric analysis, commonly known as smartphone digital image colorimetry (SDIC). We aim to show how students in low-budget schools can benefit from SDIC by gaining critical scientific skills and an evidence-based approach to scientific investigation.
2. Smartphone Digital Image Colorimetry
Conventional spectrophotometers, while necessary for quantitative analysis, are usually encased, thus limiting students’ capacity to investigate their interior components and comprehend their functioning principles. Like a “black box,” the traditional enclosed spectrophotometers limit students’ opportunity for a broader comprehension of the workings of the machine, the analytical method, and principles. SDIC has emerged as a viable alternative to conventional spectrophotometers. SDIC is a unique technique that takes advantage of smartphones’ availability and affordability to transform analytical instrumentation, with enormous advantages in science education (
Table 1). Furthermore, the analytical usage of SDIC has also been improved by the availability of free apps and easy-to-use programs for data processing [
18].
SDIC is based on colorimetric principles, where light is absorbed or transmitted by a sample solution at specific wavelengths to estimate the concentration of analyte present. The concentration of analyte in solutions and their absorbance exhibit a linear relationship. Although different based on how measurements are taken and data are processed, nonetheless, the readings of SDIC, like the traditional UV-vis spectrophotometry, are proportional to absorbance, obeying Beer-Lambert’s law [
18].
The fundamental law underlying all spectrophotometric estimations is the Beer-Lambert Law. According to Beer-Lambert law, the absorbance (A) of light by a solution is directly proportional to the concentration (c) of the solution and the path length (l) by which the light passes through the solution (that is, the cuvette width).
That is:
Where:
path length by which the light passes the solution (usually 1 cm)
intensity of incident light
intensity of emergent light
Equation (1) is called the Beer-Lambert Law. The ratio , is the transmittance (T), the ratio of the intensity of light transmitted by a solution to the intensity of light incident on the solution. So, absorbance is the logarithm (to the base 10) of the inverse of the transmittance.
This relationship is pivotal for estimating the analyte concentration in solution. The absorbance of one solution (unknown solution) is compared to a set of solutions with known concentration. The Beer-Lambert Law is only valid under specific conditions, such as the requirement for using monochromatic light and the fact that it is only applicable at low concentrations, meaning in practice, it usually implies up to an absorbance of approximately 0.6 [
19].
In SDIC, colorimetric measurement is taken using smartphones’ built-in cameras. Even though digital images can be captured using a broad range of technologies (such as scanning devices, webcams, and digital cameras), smartphone cameras have emerged as popular devices in colorimetric analyses due to their convenience, availability, as well as cost-effectiveness compared to spectrophotometers [
18]. In recent years, smartphone cameras have seen significant advancements in technology. Two major image sensor technologies, charged coupled devices (CCDs) and complementary metal-oxide semiconductors (CMOS), are foreseen to continue to make an impact and expand the digital imaging industry [
20]. However, while CCDs have long been the dominant image-sensor technology, advances in CMOS technology render them a compelling alternative to CCD sensors [
21]. Currently, CMOS image sensors have become prevalent, especially in high-volume products such as smartphones [
21]. CMOS sensors operate by converting light into an electrical charge and are equipped with appropriate filters for detecting every color separately [
20,
21]. CMOS sensors are more efficient, affordable, and consume less energy than CCDs [
20], which are commonly used in UV-vis spectroscopy [
22].
2.1. Basic Components of Smartphone Digital Image Colorimetry
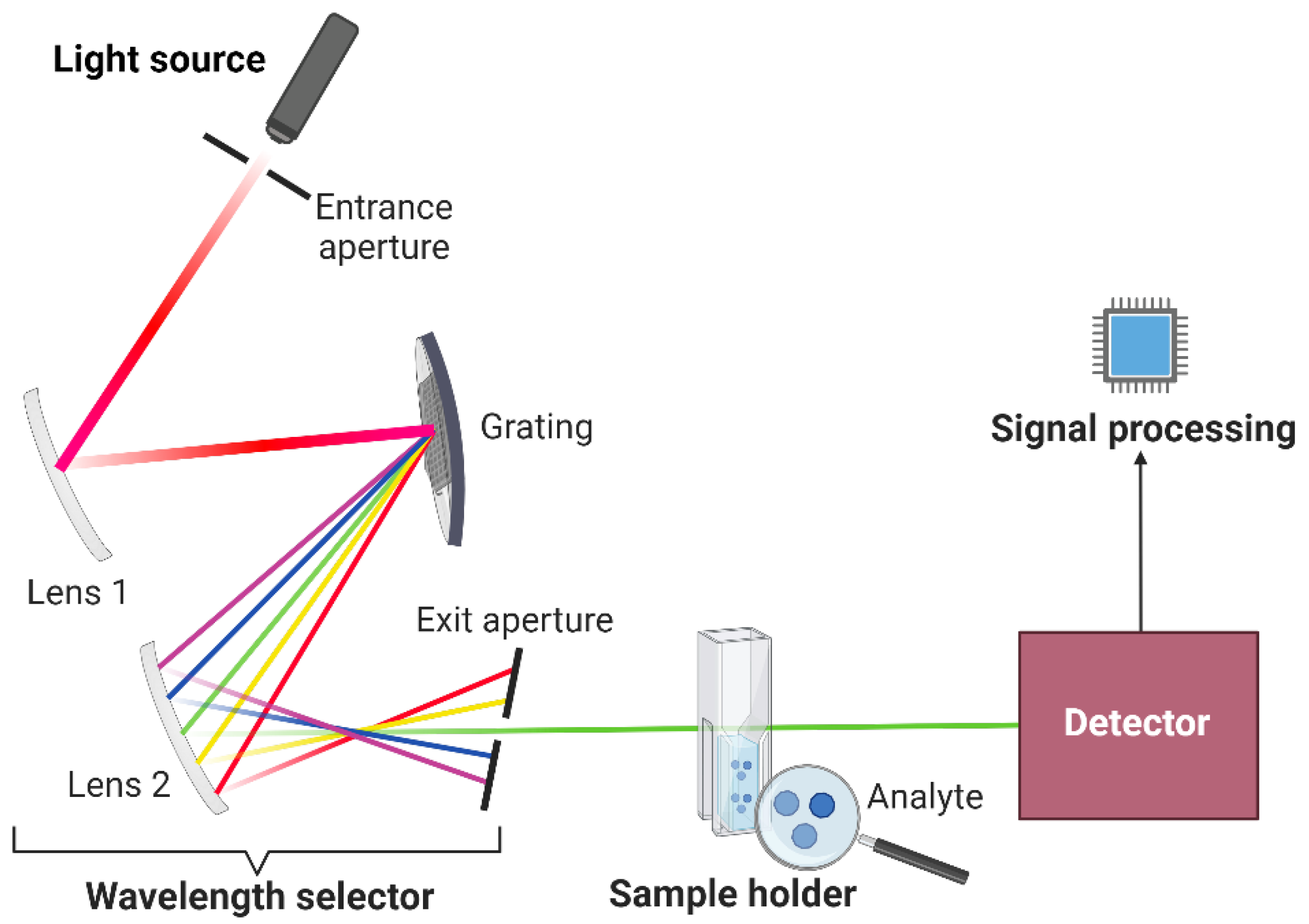
Optical instruments operate within the electromagnetic spectrum's regions, encompassing X-rays, ultraviolet, visible, and infrared. SDIC operates exclusively in the visible spectrum, where the human eye operates. The fundamental optical instrument components will be a reference when constructing the colorimetric box, which will function as the detector in SDIC. While optical instruments generally consist of comparable fundamental components (
Figure 1), their configuration may differ slightly depending on the specific region of the spectrum in which they are utilized. [
23]. The basic components of an optical instrument include:
A stable light source that produces radiant energy.
A transparent sample holder.
A wavelength selector that isolates a specific region of the electromagnetic spectrum for measurement.
A detector that converts radiant energy to usable electric signals.
A signal processor that displays the signal on a scale.
2.2. Constructing a Simple Laboratory-Made Smartphone-Based Colorimetric Instrument
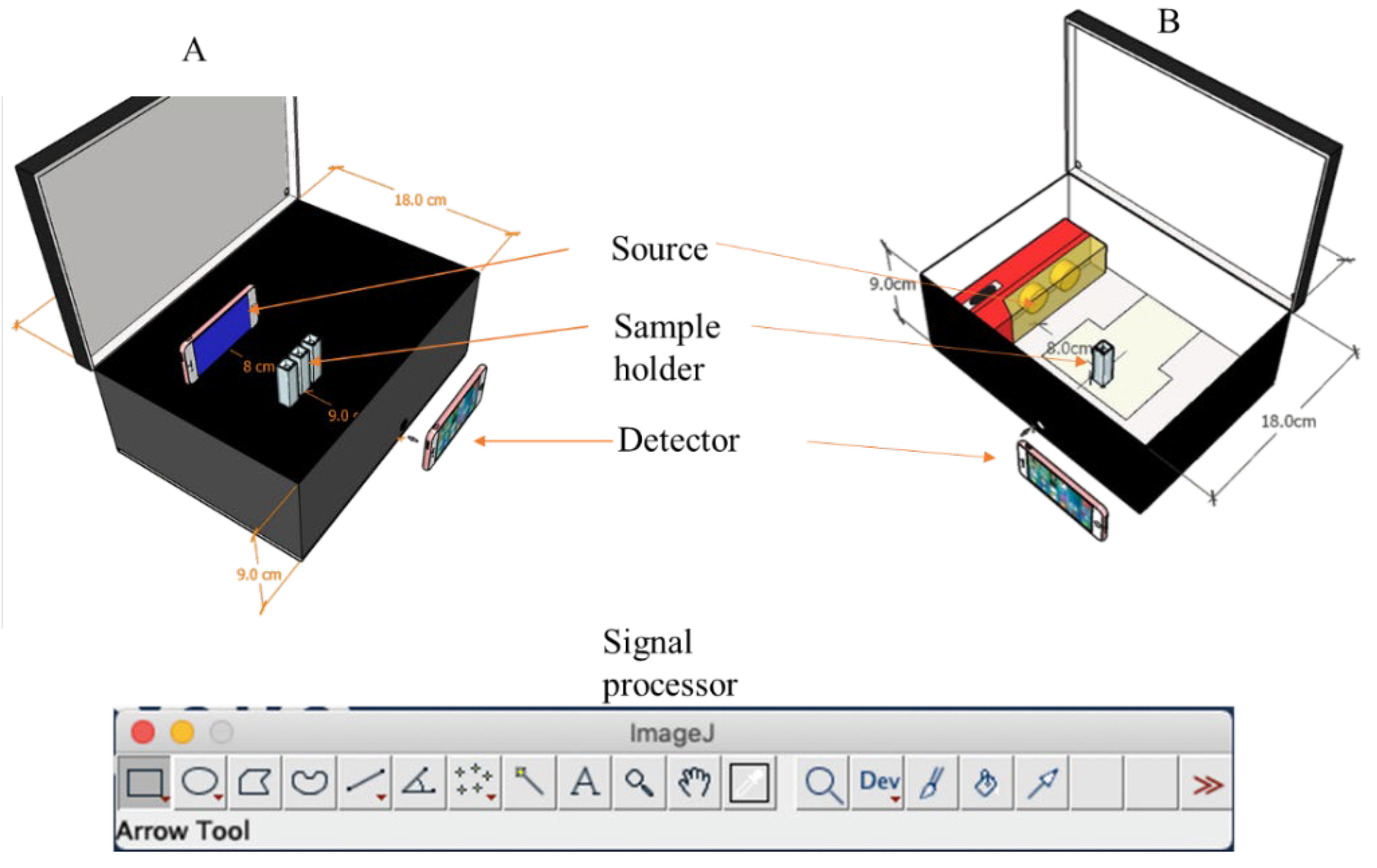
The colorimetric box can be constructed from readily available materials to ensure the reproducible capture of images. For instance, a simple rectangular box with 25 × 18 × 9 cm dimensions could be used. In
Figure 2, two different designs, A and B, can be used for the construction of the colorimetric box.
In
Figure 2A, the interior of the box is painted black to minimize reflection. A monochromatic light source that emits a specific wavelength of radiant energy is used in the form of the backscreen of a smartphone that illuminates a specific wavelength corresponding to the wavelength of maximum absorption of the analyte. The image used for the backscreen can be generated from free online sources such as Wolfram Wavelength to Color Converter (
https://www.wolframalpha.com/widgets/gallery/view.jsp?id=5072e9b72faacd73c9a4e4cb36ad08d). This works by simply inputting the desired wavelength within the visible region of the electromagnetic spectrum. The wavelength's color equivalent will be generated immediately, which can be transferred to the smartphone as an image to be used as a monochromatic light source. In
Figure 2B, instead of using a monochromatic light source, a continuum light source that emits a wide range of wavelengths can be used, such as a light-emitting diode (LED) lamp readily available in local stores. This configuration, however, is prone to inferences and poor selectivity and requires the blank and sample solution to have a wide color contrast, preferably a colorless blank [
24]. A colorful blank will necessitate the use of a monochromatic light source to improve selectivity [
25].
The sample holder can be a regular UV-Vis cuvette [
26]. Depending on the configuration of the box, single or multiple cuvettes can be used at a time. In
Figure 2A, for example, multiple cuvettes can be used to capture the blank and sample solutions simultaneously because the monochromatic light source is placed just behind the sample holder, and all the cuvettes experience the same exposure to the light source. In
Figure 2B, however, the light source is placed beside the sample holder since placing it behind the sample holder can result in the saturation of the detection camera, as the LED lamp brightness cannot be controlled. This setup implies that only a single cuvette can be used at a time.
The detector can be a smartphone's camera that can capture reproducible images. The quality of the photos depends on the specifications of the different components of the phone camera. The better the camera quality, the better the image quality and, hence, the quality of the result.
The signal processor can be an image processing software such as the simple open-source ImageJ software [
27]. Mobile applications can also be used, such as the customized WeChat applet WASDIC [
28], RGB color detector [
29], colorgrab [
30], and Photometrix [
31]. The software generates a histogram by converting the pixel intensity of the sample solution to a number that is correlated with the analyte concentration.
2.3. Optimizing Parameters
The optimization of different parameters is necessary to ensure that the SDIC gives results comparable to those of a typical optical instrument.
2.3.1. Dimensions of the Colorimetric Box
Care must be taken in selecting the size of the colorimetric box to ensure that it is large enough to contain all the components inside but potable enough to be potentially used for onsite analysis. The components should have the right spacing within the colorimetric box to ensure optimal interaction between the components that will produce maximum sensitivity and selectivity.
2.3.2. The Light Source
The effect of the brightness of the light source needs to be optimized if the light source is directly facing the detection camera. If the brightness is high, it can saturate the detection camera. If an LED lamp is used, the brightness cannot be adjusted except if the lamp has the brightness adjustment function. In the case of a smartphone used as a monochromatic light source, the brightness needs to be optimized by changing the setting sequentially and testing the effect of the change. Care should be taken to turn off automatic brightness in the phone settings since when that setting is enabled, the screen's brightness changes according to the brightness of the environment. Fluctuation in brightness between the environments can affect the reproducibility of measurement.
Another critical parameter that needs optimization is the wavelength of the light source. The correct wavelength will improve the selectivity and sensitivity of the measurement.
2.3.3. The Detection Camera
The effect of different detection cameras can be studied to find the one that gives the best sensitivity. Moreover, when capturing the photograph, the sensor needs to be ensured that it is stable. Some studies have optimized the effect of the detection camera. Generally, the latest phone models with the most advanced cameras give the best sensitivity regardless of the brand [
32].
2.4. Image Processing and Data Collection
The RGB color model is the most widely used model for analytical applications [
33]. A previous review gave a detailed discussion of various color models [
34]. In the RGB model, the image is split into R, G, and B channels. Histogram values are assigned from 0 to 255 for each of these three channels, such that [0, 0, 0] corresponds to absolute black and [255, 255, 255] to absolute white [
35]. The implication is that, as the intensity of the color increases, the value of the most intense RBG channel will linearly drop, whilst the other two may slightly increase or even stay constant [
36]. These values can be used directly for quantitation since they are linearly correlated with concentration; however, a negative slope will be obtained in the calibration curve [
37]. To obtain a positive slope, several mathematical operations can be utilized, such as R = 255 – I [
38]. Where R is the response, and 255 represents the blank presumed to be absolute white, which might not be the case for most practical applications. I is the mean histogram value for the analyte from the most intense R, G, or B channel. A more practical approach is to use R = I
0 – I, where I
0 is the intensity of the blank for a specific channel, with other variables being the same as in the previous example [
24]. Alternatively, Beers’ law can be used by inputting the values of the mean histogram values of the blank and analyte in the Beers’ equation [
39].
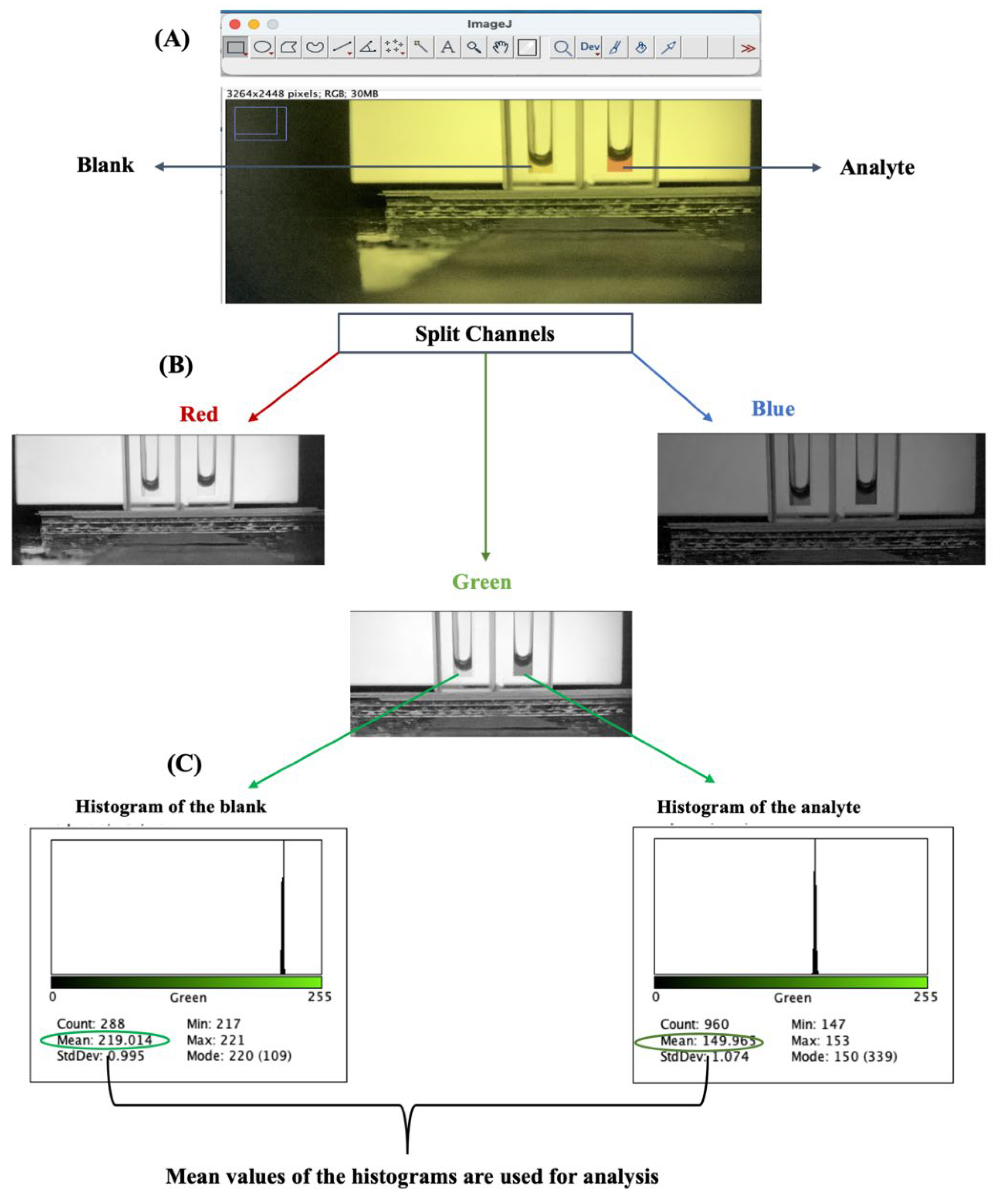
An illustration is presented in
Figure 3 using ImageJ software. In
Figure 3A, the image is opened from ImageJ using the CNTR O (CMD O for Mac) command. The image is opened from the location it is stored in the PC in jpg format. To spit the image into the three channels, in the menu bar, “image” is clicked, then “color”, and split channels. The image is split into R, G, and B channels, as shown in
Figure 3B. The channel that gives the widest contrast between the blank and the analyte is selected – in this example, the green channel. The region of interest is selected by simply highlighting the area within the solution as a square that will be used to generate the histogram.
Figure 3A shows the histogram of the blank and analyte for the green channel used for quantitation by the command CNTR H (CMD H for Mac). The mean histogram values for the blank and analyte can be inputted into a spreadsheet to calculate absorbance using the Beers’ equation.
2.5. Data Analysis and Results Presentation
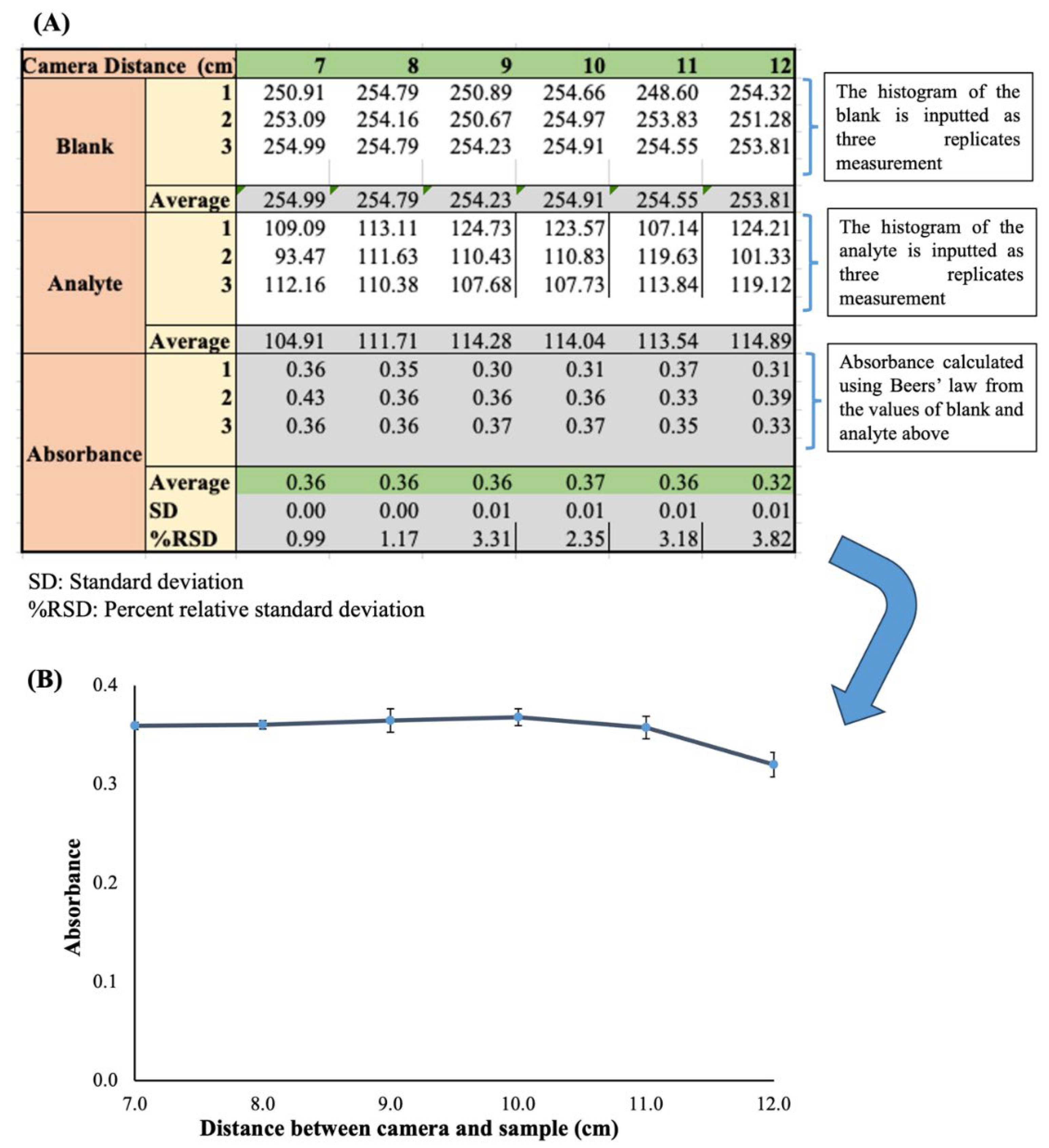
The data collected can be processed with a simple spreadsheet such as Microsoft Office Excel or Apple Numbers. The illustration in
Figure 4 is a simple template designed for optimization of the distance between the sample holder and detection camera for SDIC. The template can be modified slightly to fit any optimization parameter or calibration graph by simply duplicating the sheet and changing the x-axis to accommodate the parameter of interest. In the template in
Figure 4A, the rows show the input box for the mean histogram values for the blank analyte as three replicates each. The absorbance is calculated automatically by typing the Beers’ equation command using the reference cells corresponding to each replicate of the blank and analyte. The average absorbance is calculated by inputting the average command and highlighting the appropriate cells. This average is used as the y-axis. The corresponding standard deviation (SD) is calculated in a like manner and used as the error bar. Typically, a good error bar will be obtained if the percent relative standard deviation (%RSD) is below 5%.
Figure 4B is a line chart plotted from the data obtained for a graphical illustration of the result. Each axis is labeled, and the appropriate significant figures are applied for each axis. The charts can be used in the result and discussion section for clear elaboration. A bar chart can alternatively be used depending on the nature of the parameter that is being optimized.
3. Discussion
The process of constructing a colorimetric box and carrying out SDIC analysis is relatively easy and straightforward. The only condition is for the analyte to be colored [
25] or derivatizing the analyte with a suitable reaction that can change a colorless analyte to a colorful derivative that is distinguishable from a blank solution void of the analyte [
40]. The remaining process is synonymous with carrying out colorimetric measurements using UV/VIS spectrophotometer. Although the sensitivity of SDIC is less than that of a UV/Vis spectrophotometer, a preconcentration step is often applied before detection to improve the sensitivity of SDIC [
41]. The analytical figures of merit can be calculated from calibration graphs as with any typical analytical instrument to validate the result. However, an independent study with another instrument is often required to evaluate the accuracy of a proposed SDIC method by statistical analysis. Typically, SDIC is most comparable to UV/Vis spectrometry, and as such, it is one of the most used instruments for comparison study [
42,
43]. Other instruments that have been used include flame atomic absorption spectrometry [
44], high-performance liquid chromatography [
37], and fluorescence spectroscopy [
45]. Because of the portability of SDIC, it has been applied for on-field analysis [
28].
3.1 Precautions in Smartphone Digital Image Colorimetry
A major precaution that should be considered in a typical SDIC experiment is to ensure that the camera sensor is stable before capturing the image to ensure that the camera's autofocus is constant. This precaution will ensure a reproducible result. It is strongly recommended that the colorimetric box be designed in such a way that the blank and analyte are captured simultaneously. This requirement will serve to correct for any fluctuations in the autofocus efficiency since Beers’ law is a logarithmic ratio of the blank and analyte, and any fluctuations will affect both blank and analyte to the same degree. For a colorful blank, it is strongly recommended to improvise a filter or wavelength selector that is simple and affordable to maintain the main merits of SDIC, which are accessibility, simplicity, and affordability. It is important to ensure that the sample holder is always clean to avoid interference from contamination. Suppose a chemical reaction is required for derivatization purposes to convert colorless analytes to colorful derivatives. In that case, the stability of the reaction and the kinetics need to be evaluated to ensure the right timing is applied.
3.2. Application of Smartphone Digital Image Colorimetry
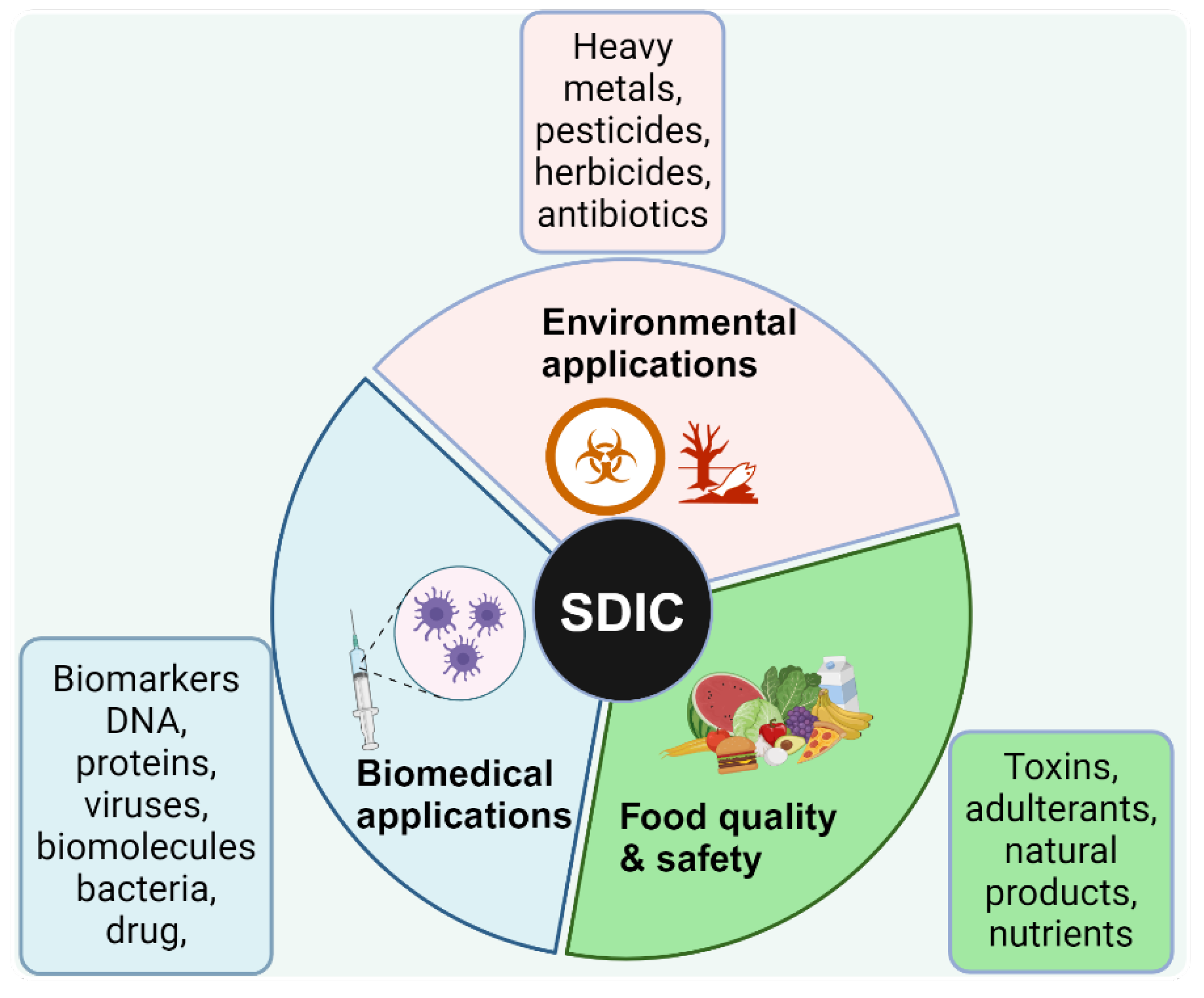
The ubiquitous and exponentially increasing use of smartphones has inspired a wide community of scientists to innovate by finding ways to use them as analytical instruments
. SDIC has become a powerful, low-cost, and rapid analytical tool for the quantitative determination of analytes using visual assessment of color changes of obtained digital images, especially for users who desire to perform colorimetric analyses with reduced effort, low costs, and independent of location. Consequently, SDIC has emerged as an ideal approach for point-of-need analytical methods, especially in resource-limited settings. The application of SDIC cuts across different fields, including environmental monitoring [
46,
47], detection of hazardous substances (such as chemical weapons and explosives) [
48], healthcare and clinical diagnosis [
49,
50,
51], food quality and safety monitoring [
52,
53], (
Figure 5).
In biomedical applications, one of the notable areas SDIC has been applied is the quantification of the drug’s active pharmacologic ingredients [
54] and point-of-care antibiotic residual [
54]. Mermer and colleagues reported standardization experiments on an SDIC method for the determination of vancomycin (antibiotics) content in drugs based on the colorimetric reaction of vancomycin with copper(II) in an acidic pH [
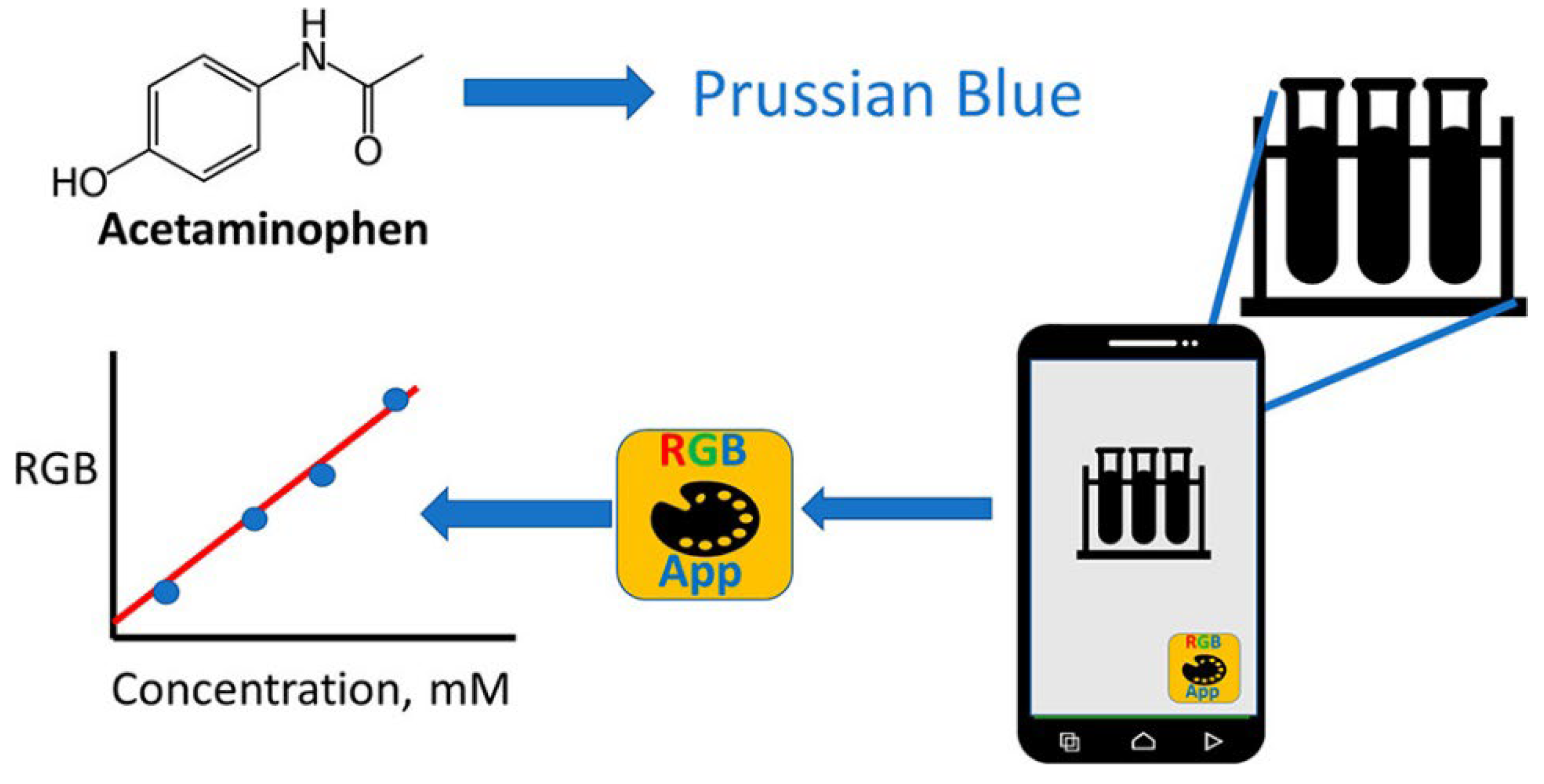
55]. Exploring the simplicity of SDIC, James and Honeychurch [
56] described the colorimetric determination of acetaminophen via its reduction of ferricyanide to give ferrocyanide, which is further converted to Prussian Blue upon the addition of iron(III), as shown in
Figure 6. This approach is seamless and affordable. The diagnostic application of SDIC methods has continued to expand due to its low-cost instruments, portability, simplicity, and practicality. In biomolecular detection, Firdaus and colleagues developed a non-enzymatic nanoparticle-based glucose quantification in urine samples of normal people and diabetic patients [
57]. Other biomolecules analyzed with SDIC include urinary albumin [
58], DNA [
59], tyrosinase [
60], and urea [
61]. Similarly, SDIC for chemical analysis is well documented in recent years, such as in the study of Cu
2+ [
62], Hg
2+ [
63], paraoxon herbicides [
64], pesticides (paraoxon, malathion, and methyl parathion) [
65,
66], tetracycline [
67], methylene blue [
68], Pb
2+ [
63], among others.
In the aspect of food processing and safety, SDIC has been developed for the quantitative detection of nitrite in food samples (cabbage, pickle, and ham) leveraging the colorimetric oxidation chemistry between nitrite and 3,3′,5,5′-tetramethylbenzidine (TMB) [
69]. In their study, the authors [
69] monitored the direct quantitative oxidation of TMB by nitrite to form a yellow TMB diimine (oxTMB); the image of an inherent color variation was captured using a smartphone and analyzed [
69]. This approach was a simple, sensitive, and accessible alternative method of nitrite detection in food samples. Similarly, using a simple, inexpensive lab-made apparatus, SDIC was employed to determine the amount of protein (in milk, lentils, and beans) [
58], sterols in vegetable oils [
59], and alkaline phosphatase in raw milk samples [
70]. Furthermore, the applicability of SDIC to detect contaminants and adulterants in complex food matrices has been reported [
71,
72,
73].With the proliferation of smartphones in low—and middle-income countries and the rapid advances in SDIC's analytical applications, SDIC holds a high prospect of providing an accessible approach for analytical measurements across different disciplines in resource-limited settings for research and pedagogical uses.
4. Future Perspectives
SDIC has the potential to immensely improve scientific training in resource-constrained regions, thus making it possible to build and empower future generations of leaders, researchers, and creative thinkers, irrespective of their background or geographical location. Despite their relevance and use in various fields, factors that affect accurate image acquisition or processing in SDIC are seldom reported and discussed [
74]. These factors are essential in the repeatability and reproducibility of measurements using SDIC. Also, there is a need for standards in analytical parameters such as accuracy, specificity, selectivity, limits of quantification (LOQs), and limits of detection (LODs) [
75]. The Ministry or Department of Education in countries and relevant professional bodies could play essential roles in laying guidelines or providing recommendations and setting standards for incorporating emerging technologies such as SDIC into educational training. The application of SDIC in kinetic studies has a promising future. Using digital videoing to monitor colorimetric reactions in real-time and exploiting SDIC as a kinetic analytical method are attractive prospects. Educational training can extend the use of SDIC for time-dependent kinetic studies, leveraging smartphones’ ability to take high-quality videos. Currently, traditional UV-Vis spectrophotometers can monitor colorimetric reactions and rapid variations in the concentration of analyte over time. However, no known kinetic studies are available using SDIC so far. Thus, SDIC’s versatility and educational applications could be expanded with digital videoing, where recording all phases of a colorimetric reaction will allow for holistic kinetic measurement and estimating reaction rates, constants, and other kinetic parameters. Additionally, SDIC’s future will most likely include the use of machine learning and artificial intelligence for faster and more accurate colorimetric measurement or data analysis [
76], thereby opening up new opportunities for a variety of educational applications and optimizations of the technique or specialized software that may reach even resource-constraint regions that would typically not reap the dividend of technological advancement.
5. Conclusions
Experimental instruments and instructional material are an essential part of science training. Hence, an inadequate supply of these instruments and materials could seriously affect the standard of science education, making it difficult for teachers to give practical science instruction efficiently. Consequently, students’ acquisition of practical skills and hands-on research experiences is hampered. However, SDIC is opening exciting opportunities for students in resource-constraint settings to learn the principles and practices of colorimetric analyses, with the prospect of allowing more and more students and researchers from various fields of study and places in the world, especially in the low-resource regions, to gain quality hands-on research experience and education. Notably, the application of SDIC is increasingly widening. Some of the fields of study SDIC have been applied to include analytical chemistry, chemical sciences, biological sciences, medical sciences, nutritional sciences, agricultural sciences, and environmental sciences. This development is mainly due to the availability, portability, low energy demand, affordability, and user-friendliness of SDIC, enabling colorimetric measurements requiring little reagent utilization and coupled with green microextraction. This review highlights how students can gain practical science knowledge and research skills in absorption spectrophotometry using SDIC. The suitability of SDIC application in education stems from its components, which students can relate to or are already familiar with. We hope this review will contribute to the democratization of science education, ensuring that even in environments with limited resources, students and educators can constructively engage with scientific concepts and techniques. SDIC is a revolutionary innovation that promises to improve science education quality in resource-constraint settings. By adopting SDIC, instructors can break down barriers to hands-on science instruction and empower students to understand and explore the exciting possibilities in quantitative colorimetric analysis and absorption spectroscopy.
Author Contributions
Conceptualization, V.M., and J.C.; writing—original draft preparation, V.M., J.C., AAP.; writing—review and editing, V.M., J.C., AAP., R.S.M.; project administration, V.M., R.S.M., and J.C. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to extend our gratitude to Ms. Kathelina Kristollari, a PhD candidate in the Department of Biotechnology Engineering, for her valuable assistance with creating Biorender illustrations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuntzleman, T.S.; Jacobson, E.C.J.J.o.c.e. Teaching Beer’s law and absorption spectrophotometry with a smart phone: A substantially simplified protocol. 2016, 93, 1249–1252.
- Spitha, N.; Doolittle, P.S.; Buchberger, A.R.; Pazicni, S.J.J.o.C.E. Simulation-Based guided inquiry activity for deriving the Beer–Lambert law. 2021, 98, 1705–1711.
- Owens, A.J.S.o.e. Income segregation between school districts and inequality in students’ achievement. 2018, 91, 1–27.
- Lestari, D.P.; Supahar; Paidi; Suwarjo; Education, H.J.; Technologies, I. Effect of science virtual laboratory combination with demonstration methods on lower-secondary school students’ scientific literacy ability in a science course. 2023, 28, 16153–16175.
- Sella, A.J.N.R.C. Rethinking practical classes. 2017, 1, 0090.
- Marley, S.A.; Siani, A.; Sims, S.J.E.; Evolution. Real-life research projects improve student engagement and provide reliable data for academics. 2022, 12, e9593.
- Mebert, L.; Barnes, R.; Dalley, J.; Gawarecki, L.; Ghazi-Nezami, F.; Shafer, G.; Slater, J.; Yezbick, E.J.H.E.P. Fostering student engagement through a real-world, collaborative project across disciplines and institutions. 2020, 5, 30–51.
- Ndirangu, M.; Kathuri, N.; Mungai, C.J.I.J.o.E.D. Improvisation as a strategy for providing science teaching resources: An experience from Kenya. 2003, 23, 75–84.
- Gabunilas, L.M.; Santos, K.J.M.; Buar, C.L.; Castillo, J.M.L.; Pili, U.B.J.P.E. Improvising an apparatus for teaching sound waves using smartphones. 2022, 58, 015014.
- Teng, Y.; Wang, X.J.I.J.o.E.T.i.H.E. The effect of two educational technology tools on student engagement in Chinese EFL courses. 2021, 18, 27.
- Tai, Z.; Dai, C.J.B.p. College students’ attachment to their smartphones: A subjective operant approach. 2022, 10, 145.
- Duncombe, R.A.J.D.P.R. Understanding the impact of mobile phones on livelihoods in developing countries. 2014, 32, 567–588.
- Rotondi, V.; Kashyap, R.; Pesando, L.M.; Spinelli, S.; Billari, F.C.J.P.o.t.N.A.o.S. Leveraging mobile phones to attain sustainable development. 2020, 117, 13413–13420.
- Iqbal, S.; Bhatti, Z.A.J.I.J.o.E.T.i.H.E. A qualitative exploration of teachers’ perspective on smartphones usage in higher education in developing countries. 2020, 17, 29.
- Sohn, S.Y.; Krasnoff, L.; Rees, P.; Kalk, N.J.; Carter, B.J.F.i.p. The association between smartphone addiction and sleep: A UK cross-sectional study of young adults. 2021, 12, 629407.
- Sapci, O.; Elhai, J.D.; Amialchuk, A.; Montag, C.J.L.; Differences, I. The relationship between smartphone use and studentsacademic performance. 2021, 89, 102035.
- Hosker, B.S.J.J.o.C.E. Demonstrating principles of spectrophotometry by constructing a simple, low-cost, functional spectrophotometer utilizing the light sensor on a smartphone. 2018, 95, 178–181.
- Soares, S.; Fernandes, G.M.; Rocha, F.R.J.T.T.i.A.C. Smartphone-based digital images in analytical chemistry: Why, when, and how to use. 2023, 117284.
- Moorthy, K. Fundamentals of biochemical calculations; CRC press: 2007.
- Gove, R. Complementary metal-oxide-semiconductor (CMOS) image sensors for mobile devices. In High performance silicon imaging; Elsevier: 2014; pp. 191–234.
- Ginhac, D. Smart cameras on a chip: Using complementary metal-oxide-semiconductor (CMOS) image sensors to create smart vision chips. In High Performance Silicon Imaging; Elsevier: 2014; pp. 165–188.
- Galbán, J.; de Marcos, S.; Sanz, I.; Ubide, C.; Zuriarrain, J.J.A. CCD detectors for molecular absorption spectrophotometry. A theoretical and experimental study on characteristics and performance. 2010, 135, 564–569.
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of instrumental analysis; Cengage learning: 2017.
- Caleb, J.; Alshana, U.; Ertas, N. Smartphone digital image colorimetry combined with solidification of floating organic drop-dispersive liquid-liquid microextraction for the determination of iodate in table salt. Food Chemistry 2021, 336. [CrossRef]
- Caleb, J.; Alshana, U. Supramolecular solvent-liquid-liquid microextraction followed by smartphone digital image colorimetry for the determination of curcumin in food samples. Sustainable Chemistry and Pharmacy 2021, 21. [CrossRef]
- Markus, V.; Dalmizrak, O.; Edebal, O.H.; Al-Nidawi, M.; Caleb, J. Smartphone digital image colorimetry for quantification of serum proteins. Analytical Methods 2023, 15, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. Bmc Bioinformatics 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Hu, J.; Ji, F.; Chi, H.; Liu, Y.; Hu, K.; Hao, F.; Wen, X. Portable one-step effervescence tablet-based microextraction combined with smartphone digital image colorimetry: Toward field and rapid detection of trace nickel ion. Talanta 2024, 126036. [Google Scholar] [CrossRef] [PubMed]
- Thanayutsiri, T.; Charoenying, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P.; Ngawhirunpat, T.; Rojanarata, T. Facile, sensitive and reagent-saving smartphone-based digital image colorimetric assay of captopril tablets enabled by long-pathlength RGB acquisition. Pharmacia 2023, 70, 1511–1519. [Google Scholar] [CrossRef]
- Silva, A.F.S.; Rocha, F.R.P. A novel approach to detect milk adulteration based on the determination of protein content by smartphone-based digital image colorimetry. Food Control 2020, 115. [Google Scholar] [CrossRef]
- Soares, S.; Fernandes, G.M.; Moraes, L.M.B.; Batista, A.D.; Rocha, F.R.P. Single-phase determination of calcium and magnesium in biodiesel using smartphone-based digital images. Fuel 2022, 307. [Google Scholar] [CrossRef]
- Abughrin, S.; Alshana, U.; Caleb, J. Smartphone Digital Image Colorimetry for the Determination of Aluminum in Antiperspirant Products. Turkish Journal of Pharmaceutical Sciences 2022, 19, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Fernandes, G.M.; Rocha, F.R.P. Smartphone-based digital images in analytical chemistry: Why, when, and how to use. Trac-Trends in Analytical Chemistry 2023, 168. [Google Scholar] [CrossRef]
- Fan, Y.J.; Li, J.W.; Guo, Y.P.; Xie, L.W.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171. [Google Scholar] [CrossRef]
- Quesada-Gonzalez, D.; Merkoci, A. Mobile phone-based biosensing: An emerging "diagnostic and communication" technology. Biosensors & Bioelectronics 2017, 92, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molinero, A.; Cubero, V.T.; Irigoyen, R.D.; Piazuelo, D.S. Feasibility of digital image colorimetry-Application for water calcium hardness determination. Talanta 2013, 103, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Vakh, C.; Mallabaeva, Z.; Tobiszewski, M. Smartphone-based digital image colorimetry for the determination of total capsaicinoid content in chili pepper extracts. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2024, 124238.
- Porto, I.S.A.; Neto, J.H.S.; dos Santos, L.O.; Gomes, A.A.; Ferreira, S.L.C. Determination of ascorbic acid in natural fruit juices using digital image colorimetry. Microchemical Journal 2019, 149. [Google Scholar] [CrossRef]
- Abdullahi, A.B.; Ismail, S.; Alshana, U.; Ertas, N. Smartphone digital image colorimetry combined with deep eutectic solvent-liquid-liquid microextraction for the determination of cobalt in milk and dairy products. Journal of Food Composition and Analysis 2023, 119. [Google Scholar] [CrossRef]
- Caleb, J.; Alshana, U.; Ertas, N.; Bakirdere, S. Smartphone digital image colorimetry combined with dispersive solid-phase microextraction for the determination of boron in food samples. Food Chemistry 2023, 426. [Google Scholar] [CrossRef]
- Al-Nidawi, M.; Alshana, U. Reversed-phase switchable-hydrophilicity solvent liquid-liquid microextraction of copper prior to its determination by smartphone digital image colorimetry. Journal of Food Composition and Analysis 2021, 104. [Google Scholar] [CrossRef]
- Yue, X.Y.; Fu, L.; Wu, C.Y.; Xu, S.; Bai, Y.H. Rapid Trace Detection of Sulfite Residue in White Wine Using a Multichannel Colorimetric Nanozyme Sensor. Foods 2023, 12. [Google Scholar] [CrossRef]
- Minh-Huy, D.; Anh-Dao, L.; Thanh-Nho, N.; Nhon-Duc, L.; Cong-Hau, N. Smartphone-based digital images as a low-cost and simple colorimetric approach for the assessment of total phenolic contents in several specific Vietnamese dried tea products and their liquors. Food Chemistry 2023, 401. [Google Scholar] [CrossRef]
- Ismail, S.; Abdullahi, A.B.; Alshana, U.; Ertaş, N. Switchable-hydrophilicity solvent liquid–liquid microextraction combined with smartphone digital image colorimetry for the determination of palladium in catalytic converters. Analytical Sciences 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Huang, J.; Zhang, H.; Zhang, N.; Li, F.; Zhou, P.; Zhou, L.; Pu, Q. Natural flavonols as probes for direct determination of borax: From conventional fluorescence analysis to paper-based smartphone sensing. Talanta 2024, 126053. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric Detection of Heavy Metal Ions With Various Chromogenic Materials: Strategies and Applications. Journal of Hazardous Materials 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liang, X.; Guo, X.; Yang, X.; Guo, J.; Zhou, X.N.; Huang, X.; Zhang, W.; Wang, Y.; Liu, Z.; et al. Smartphone-Based Colorimetric Sensor Array Using Gold Nanoparticles for Rapid Distinguishment of Multiple Pesticides in Real Samples. Food Chemistry 2023. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E.J.C.r.i.a.c. Colorimetric sensor arrays for the detection and identification of chemical weapons and explosives. 2017, 47, 138–153.
- Kap, Ö.; Kılıç, V.; Hardy, J.G.; Horzum, N.J.A. Smartphone-based colorimetric detection systems for glucose monitoring in the diagnosis and management of diabetes. 2021, 146, 2784–2806.
- Alawsi, T.; Mattia, G.P.; Al-Bawi, Z.; Beraldi, R. Smartphone-Based Colorimetric Sensor Application for Measuring Biochemical Material Concentration. Sensing and Bio-Sensing Research 2021. [CrossRef]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G.J.M. Digital image colorimetry on smartphone for chemical analysis: A review. 2021, 171, 108829.
- Abdelbasset, W.K.; Savina, S.V.; Mavaluru, D.; Shichiyakh, R.A.; Bokov, D.O.; Mustafa, Y.F. Smartphone Based Aptasensors as Intelligent Biodevice for Food Contamination Detection in Food and Soil Samples: Recent Advances. Talanta 2023. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajšlová, J.; Elliott, C.T. Smartphone-Based Optical Assays in the Food Safety Field. Trac Trends in Analytical Chemistry 2020. [Google Scholar] [CrossRef]
- Lin, B.; Yu, Y.; Cao, Y.; Guo, M.; Zhu, D.; Dai, J.; Zheng, M.J.B.; Bioelectronics. Point-of-care testing for streptomycin based on aptamer recognizing and digital image colorimetry by smartphone. 2018, 100, 482–489.
- Mermer, K.; Paluch, J.; Kozak, J.J.M.f.C.-C.M. Smartphone-based digital image colorimetry for the determination of vancomycin in drugs. 2022, 153, 801–809.
- James, H.; Honeychurch, K.C.J.J.o.C.E. Digital Image Colorimetry Smartphone Determination of Acetaminophen. 2023, 101, 187–196.
- Firdaus, M.L.; Saputra, E.; Ginting, S.M.; Wyantuti, S.; Eddy, D.R.; Rahmidar, L.; Yuliarto, B.J.S.; Research, B.-S. Smartphone-based digital image colorimetry for non-enzymatic detection of glucose using gold nanoparticles. 2022, 35, 100472.
- Mathaweesansurn, A.; Maneerat, N.; Choengchan, N.J.S.; Chemical, A.B. A mobile phone-based analyzer for quantitative determination of urinary albumin using self-calibration approach. 2017, 242, 476–483.
- Wu, T.-H.; Chang, C.-C.; Vaillant, J.; Bruyant, A.; Lin, C.-W.J.L.o.a.C. DNA biosensor combining single-wavelength colorimetry and a digital lock-in amplifier within a smartphone. 2016, 16, 4527–4533.
- Moonrungsee, N.; Peamaroon, N.; Boonmee, A.; Suwancharoen, S.; Jakmunee, J.J.C.P. Evaluation of tyrosinase inhibitory activity in Salak (Salacca zalacca) extracts using the digital image-based colorimetric method. 2018, 72, 2729–2736.
- Choi, C.-K.; Shaban, S.M.; Moon, B.-S.; Pyun, D.-G.; Kim, D.-H.J.A.c.a. Smartphone-assisted point-of-care colorimetric biosensor for the detection of urea via pH-mediated AgNPs growth. 2021, 1170, 338630.
- Kumar, A.; Bera, A.; Kumar, S.J.C. A smartphone-assisted sensitive, selective and reversible recognition of copper ions in an aqueous medium. 2020, 5, 1020–1028.
- Wongthanyakram, J.; Masawat, P.J.A.L. Rapid low-cost determination of lead (II) in cassava by an iPod-based digital imaging colorimeter. 2019, 52, 550–561.
- Pohanka, M.; Zakova, J.; Sedlacek, I.J.S.; Chemical, A.B. Digital camera-based lipase biosensor for the determination of paraoxon. 2018, 273, 610–615.
- Guo, J.; Wong, J.X.; Cui, C.; Li, X.; Yu, H.-Z.J.A. A smartphone-readable barcode assay for the detection and quantitation of pesticide residues. 2015, 140, 5518–5525.
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.J.W.r. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. 2015, 70, 360–369.
- Masawat, P.; Harfield, A.; Namwong, A.J.F.c. An iPhone-based digital image colorimeter for detecting tetracycline in milk. 2015, 184, 23–29.
- Permana, M.D.; Sakti, L.K.; Luthfiah, A.; Firdaus, M.L.; Takei, T.; Eddy, D.R.; Rahayu, I.J.T.i.S. A Simple Methods for Determination of Methylene Blue using Smartphone-Based as Digital Image Colorimetry. 2023, 20, 5149–5149.
- Wang, H.; Jing, X.; Bi, X.; Bai, B.; Wang, X.J.C. Quantitative detection of nitrite in food samples based on digital image colourimetry by smartphone. 2020, 5, 9952–9956.
- Mahato, K.; Chandra, P.J.B.; Bioelectronics. based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. 2019, 128, 9–16.
- Lima, M.J.; Sasaki, M.K.; Marinho, O.R.; Freitas, T.A.; Faria, R.C.; Reis, B.F.; Rocha, F.R.J.M.J. Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry. 2020, 157, 105042.
- Silva, A.F.S.; Rocha, F.R.J.F.C. A novel approach to detect milk adulteration based on the determination of protein content by smartphone-based digital image colorimetry. 2020, 115, 107299.
- Sáez-Hernández, R.; Ruiz, P.; Mauri-Aucejo, A.R.; Yusa, V.; Cervera, M.J.F.C. Determination of acrylamide in toasts using digital image colorimetry by smartphone. 2022, 141, 109163.
- Soares, S.; Fernandes, G.M.; Rocha, F.R.P. Smartphone-based digital images in analytical chemistry: Why, when, and how to use. TrAC Trends in Analytical Chemistry 2023, 168, 117284. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Samanidou, V.F.J.C.A.C. Sample preparation in smartphone-based analysis: Current status and challenges. 2023, 101, 59–72.
- Li, Y.; Zhang, J.; Chen, J.; Zhu, F.; Liu, Z.; Bao, P.; Shen, W.; Tang, S. Detection of SARS-CoV-2 based on artificial intelligence-assisted smartphone: A review. Chinese Chemical Letters 2023, 109220. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).