Submitted:
09 May 2024
Posted:
09 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples
- Cohort 1
- Cohort 2
- Infant formula
2.2. Fatty Acid Determination
2.3. Statistics
3. Results and Discussion
3.1. Samples Data
3.1.1. TFA, Diet and Lactation Characteristics
- Cohort 1
- Cohort 2
3.1.2. TFA across Samples
- Cohort 1
- Cohort 1 versus cohort 2
- Breast milk versus infant formula
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hennet, T.; Borsig, L. Breastfed at Tiffany’s. Trends Biochem Sci 2016, 41, 508–518. [Google Scholar] [CrossRef]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast Milk Composition and Infant Nutrient Intakes during the First 12 Months of Life. Eur J Clin Nutr 2016, 70. [Google Scholar] [CrossRef]
- Barreiro, R.; Díaz-Bao, M.; Cepeda, A.; Regal, P.; Fente, C.A. Fatty Acid Composition of Breast Milk in Galicia (NW Spain): A Cross-Country Comparison. Prostaglandins Leukot Essent Fatty Acids 2018, 135, 102–114. [Google Scholar] [CrossRef]
- Craig-Schmidt, M.C. World-Wide Consumption of Trans Fatty Acids. Atheroscler Suppl 2006, 7. [Google Scholar] [CrossRef]
- Ganguly, R.; Pierce, G.N. The Toxicity of Dietary Trans Fats. Food and Chemical Toxicology 2015, 78, 170–176. [Google Scholar] [CrossRef]
- Mennitti, L. V; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; do Nascimento, C.M.O.; Pisani, L.P. Type of Fatty Acids in Maternal Diets during Pregnancy and/or Lactation and Metabolic Consequences of the Offspring. J Nutr Biochem 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Baker, E.J.; De Souza, C.O.; Miles, E.A.; Calder, P.C. Differential Effects of Ruminant and Industrial 18-Carbon Trans-Monounsaturated Fatty Acids (Trans Vaccenic and Elaidic) on the Inflammatory Responses of an Endothelial Cell Line. Molecules 2021, 26, 5834. [Google Scholar] [CrossRef]
- Fan, H.; Xia, S.; Xiang, J.; Li, Y.; Ross, M.O.; Lim, S.A.; Yang, F.; Tu, J.; Xie, L.; Dougherty, U. Trans-Vaccenic Acid Reprograms CD8+ T Cells and Anti-Tumour Immunity. Nature 2023, 1–10. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.S. Trans-Palmitoleic Acid, Metabolic Risk Factors, and New-Onset Diabetes in US Adults: A Cohort Study. Ann Intern Med 2010, 153, 790–799. [Google Scholar] [CrossRef]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A. V; Yang, W.-S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.-A.; Wong, K. Fatty Acid Biomarkers of Dairy Fat Consumption and Incidence of Type 2 Diabetes: A Pooled Analysis of Prospective Cohort Studies. PLoS Med 2018, 15, e1002670. [Google Scholar] [CrossRef]
- Guillocheau, E.; Penhoat, C.; Drouin, G.; Godet, A.; Catheline, D.; Legrand, P.; Rioux, V. Current Intakes of Trans-Palmitoleic (Trans-C16: 1 n-7) and Trans-Vaccenic (Trans-C18: 1 n-7) Acids in France Are Exclusively Ensured by Ruminant Milk and Ruminant Meat: A Market Basket Investigation. Food Chem X 2020, 5, 100081. [Google Scholar] [CrossRef]
- Wolff, R.L. Content and Distribution of Trans-18: 1 Acids in Ruminant Milk and Meat Fats. Their Importance in European Diets and Their Effect on Human Milk. J Am Oil Chem Soc 1995, 72, 259–272. [Google Scholar] [CrossRef]
- Varela, G.; Moreiras, O.; Ansón, R. Consumo de Alimentos En Galicia-La Dieta Atlántica. Madrid, Spain: Fundación Española de Nutrición 2004. [Google Scholar]
- Oliveira, A.; Lopes, C.; Rodríguez-Artalejo, F. Adherence to the Southern European Atlantic Diet and Occurrence of Nonfatal Acute Myocardial Infarction. Am J Clin Nutr 2010, 92, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillón, P.; Oliveira, A.; Lopes, C.; López-García, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet Is Associated with Lower Concentrations of Markers of Coronary Risk. Atherosclerosis 2013, 226, 502–509. [Google Scholar] [CrossRef]
- Nasser, R.; Stephen, A.M.; Goh, Y.K.; Clandinin, M.T. The Effect of a Controlled Manipulation of Maternal Dietary Fat Intake on Medium and Long Chain Fatty Acids in Human Breast Milk in Saskatoon, Canada. Int Breastfeed J 2010, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mosley, E.E.; McGuire, M.K.; Williams, J.E.; McGuire, M.A. Cis-9, Trans-11 Conjugated Linoleic Acid Is Synthesized from Vaccenic Acid in Lactating Women. J Nutr 2006, 136, 2297–2301. [Google Scholar] [CrossRef]
- Aumeistere, L.; Beluško, A.; Ciproviča, I.; Zavadska, D. Trans Fatty Acids in Human Milk in Latvia: Association with Dietary Habits during the Lactation Period. Nutrients 2021, 13, 2967. [Google Scholar] [CrossRef]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.E.P.; Huber, M.; Van Ree, R.; Simões-Wüst, A.P.; Dagnelie, P.C.; Van Den Brandt, P.A. Fatty Acids in Breast Milk and Development of Atopic Eczema and Allergic Sensitisation in Infancy. Allergy 2011, 66, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chisaguano, A.M.; Montes, R.; Castellote, A.I.; Morales, E.; Júlvez, J.; Vioque, J.; Sunyer, J.; López-Sabater, M.C. Elaidic, Vaccenic, and Rumenic Acid Status during Pregnancy: Association with Maternal Plasmatic LC-PUFAs and Atopic Manifestations in Infants. Pediatr Res 2014, 76, 470–476. [Google Scholar] [CrossRef]
- Sánchez, C.; Fente, C.; Barreiro, R.; López-Racamonde, O.; Cepeda, A.; Regal, P. Association between Breast Milk Mineral Content and Maternal Adherence to Healthy Dietary Patterns in Spain: A Transversal Study. Foods 2020, 9, 659. [Google Scholar] [CrossRef]
- Barreiro, R.; Regal, P.; López-Racamonde, O.; Cepeda, A.; Fente, C. Evolution of Breast Milk Fatty Acids in Spanish Mothers after One Year of Uninterrupted Lactation. Prostaglandins Leukot Essent Fatty Acids 2020, 159, 102141. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, C.; Garcia-Ortiz, L.; Rodriguez-Sanchez, E.; Martin-Cantera, C.; Soriano-Cano, A.; Arietaleanizbeaskoa, M.S.; Magdalena-Belio, J.F.; Menendez-Suarez, M.; Maderuelo-Fernandez, J.A.; Lugones-Sanchez, C. The Relationship of the Atlantic Diet with Cardiovascular Risk Factors and Markers of Arterial Stiffness in Adults without Cardiovascular Disease. Nutrients 2019, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Roibás, L.; Martínez, I.; Goris, A.; Barreiro, R.; Hospido, A. An Analysis on How Switching to a More Balanced and Naturally Improved Milk Would Affect Consumer Health and the Environment. Science of the Total Environment 2016, 566, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Flores, B.; Matencio, A.; Navarro-Orcajada, S.; Martínez-Lede, I.; Conesa, I.; Vidal-Sánchez, F.J.; García-Carmona, F.; López-Nicolás, J.M. Development If Healthy Milk and Yogurt Products for Reducing Metabolic Diseases Using Cyclodextrin and Omega-3 Fatty Acids from Fish Oil. Food Funct 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Sanjulián, L.; Lamas, A.; Barreiro, R.; Cepeda, A.; Fente, C.A.; Regal, P. Bacterial Diversity of Breast Milk in Healthy Spanish Women: Evolution from Birth to Five Years Postpartum. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Jahreis, G.; Pritsche, J.; Steinhart, H. Conjugated Linoleic Acid in Milk Fat: High Variation Depending on Production System. Nutrition Research 1997, 17. [Google Scholar] [CrossRef]
- Bergamo, P.; Fedele, E.; Iannibelli, L.; Marzillo, G. Fat-Soluble Vitamin Contents and Fatty Acid Composition in Organic and Conventional Italian Dairy Products. Food Chem 2003, 82. [Google Scholar] [CrossRef]
- van Wijlen, R.P.J.; Colombani, P.C. Grass-Based Ruminant Production Methods and Human Bioconversion of Vaccenic Acid with Estimations of Maximal Dietary Intake of Conjugated Linoleic Acids. Int Dairy J 2010, 20, 433–448. [Google Scholar] [CrossRef]
- Rist, L.; Mueller, A.; Barthel, C.; Snijders, B.; Jansen, M.; Simões-Wüst, A.P.; Huber, M.; Kummeling, I.; von Mandach, U.; Steinhart, H.; et al. Influence of Organic Diet on the Amount of Conjugated Linoleic Acids in Breast Milk of Lactating Women in the Netherlands. British Journal of Nutrition 2007, 97. [Google Scholar] [CrossRef]
- Simões-Wüst, A.P.; Rist, L.; Mueller, A.; Huber, M.; Steinhart, H.; Thijs, C. Consumption of Dairy Products of Biodynamic Origin Is Correlated with Increased Contents of Rumenic and Trans-Vaccenic Acid in the Breast Milk of Lactating Women. Organic Agriculture 2011, 1. [Google Scholar] [CrossRef]
- Turpeinen, A.M.; Mutanen, M.; Aro, A.; Salminen, I.; Basu, S.; Palmquist, D.L.; Griinari, J.M. Bioconversion of Vaccenic Acid to Conjugated Linoleic Acid in Humans. American Journal of Clinical Nutrition 2002, 76. [Google Scholar] [CrossRef] [PubMed]
- Mosley, E.E.; McGuire, M.K.; Williams, J.E.; McGuire, M.A. Cis-9, Trans-11 Conjugated Linoleic Acid Is Synthesized from Vaccenic Acid in Lactating Women. J Nutr 2006, 136, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.E.P.; Huber, M.; Van Ree, R.; Simões-Wüst, A.P.; Dagnelie, P.C.; Van Den Brandt, P.A. Fatty Acids in Breast Milk and Development of Atopic Eczema and Allergic Sensitisation in Infancy. Allergy 2011, 66, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, R.; Regal, P.; López-Racamonde, O.; Cepeda, A.; Fente, C.A. Comparison of the Fatty Acid Profile of Spanish Infant Formulas and Galician Women Breast Milk. J Physiol Biochem 2018, 74. [Google Scholar] [CrossRef]
- Crawford, M.A.; Wang, Y.; Forsyth, S.; Brenna, J.T. The European Food Safety Authority Recommendation for Polyunsaturated Fatty Acid Composition of Infant Formula Overrules Breast Milk, Puts Infants at Risk, and Should Be Revised. Prostaglandins Leukot Essent Fatty Acids 2015, 102, 1–3. [Google Scholar] [CrossRef]

| Maternal Data | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|
| Pregnancy Time (weeks) | 39.77 | 40.00 | 1.34 | 36.00 | 42.29 |
| Maternal Age (years) | 35.43 | 35.00 | 4.06 | 26.00 | 46.00 |
| Maternal BMI (kg/m2) | 24.47 | 24.36 | 3.83 | 17.85 | 35.03 |
| Lactating time (months) | 7.77 | 3.00 | 11.12 | 0.5 | 58.97 |
| AD adherence (score) | 3.80 | 4.00 | 1.48 | 0.00 | 7.00 |
| Time lactation | 7.77 | 3.00 | 11.12 | 0.5 | 58.97 |
| Infant gender: n♂(%)/n♀(%) | 43 (46.73)/49 (53.26) | C-section delivery (%) | 11.96 | Parity number 1st child (%) | 60.87 |
| Cohort 1 | Cohort 2 | Infant formula | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid (%wt/wt of total fatty acids) | LT < 6 | LT≥ 6 | BMI< 25 | BMI≥ 25 | Infant 0 | Infant 1 | SEAD <5 | SEAD ≥5 | Milk <3 | Milk ≥3 | Meat <3 | Meat ≥3 | LT<6 | FSF | FOF | GUF | |
| n= 67 | n= 26 | n= 47 | n= 46 | n= 41 | n= 52 | n= 64 | n= 29 | n= 39 | n= 41 | n= 63 | n= 18 | n= 16 | n= 5 | n= 13 | n= 5 | ||
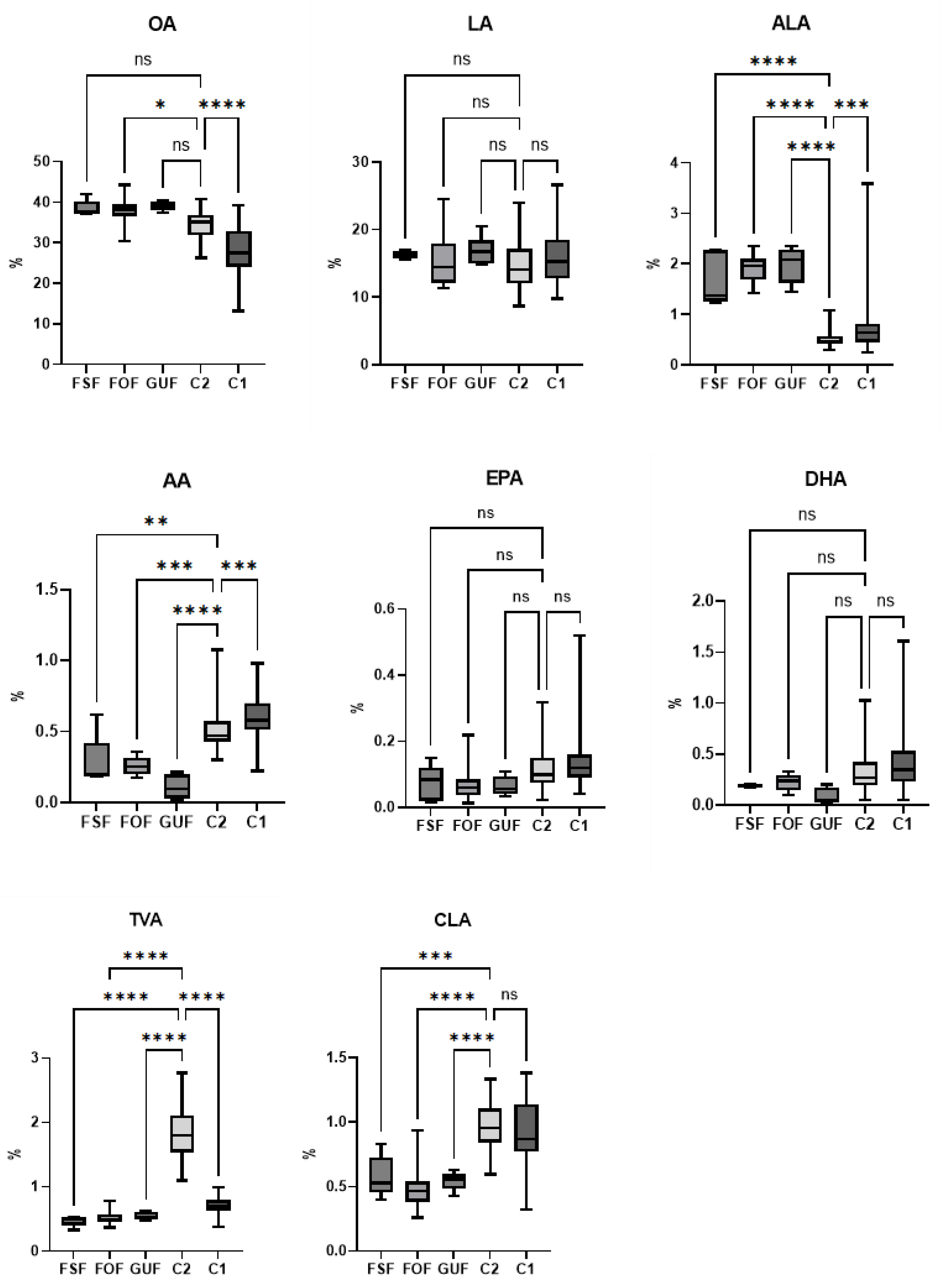
| 18:1(n-9) OA | Mean | 28.35* | 29.38 | 30.02 | 27.34 | 30.12 | 27.47 | 28.17 | 29.67 | 28.41 | 29.49 | 27.74 | 31.88 | 34.49* | 38.49 | 38.06* | 39.07 |
| SD | 5.87 | 8.37 | 6.85 | 6.20 | 6.81 | 6.30 | 6.86 | 6.05 | 6.96 | 6.00 | 6.20 | 6.58 | 3.22 | 2.06 | 3.44 | 1.18 | |
| Min | 13.11 | 14.52 | 14.52 | 13.11 | 19.76 | 13.11 | 13.11 | 20.03 | 13.11 | 20.15 | 13.11 | 22.50 | 26.21 | 37.10 | 36.45 | 37.32 | |
| Max | 39.28 | 43.19 | 42.57 | 43.19 | 43.19 | 41.58 | 43.19 | 42.11 | 42.11 | 43.19 | 43.19 | 42.11 | 40.67 | 42.04 | 44.20 | 40.43 | |
| 18:2(n-6) LA | Mean | 15.96 | 15.78 | 15.63 | 16.18 | 15.68 | 16.10 | 15.84 | 16.08 | 16.64 | 15.23 | 16.50 | 14.53 | 14.60 | 16.26 | 15.64 | 16.96 |
| SD | 3.84 | 4.17 | 4.13 | 3.73 | 4.03 | 3.85 | 3.77 | 4.27 | 4.10 | 3.85 | 3.99 | 2.44 | 3.41 | 0.58 | 3.91 | 2.32 | |
| Min | 9.75 | 8.73 | 8.73 | 9.42 | 8.73 | 9.75 | 8.73 | 9.42 | 9.42 | 8.73 | 8.73 | 9.42 | 8.66 | 15.50 | 11.54 | 14.90 | |
| Max | 26.64 | 23.86 | 26.64 | 24.80 | 24.80 | 26.64 | 24.80 | 26.64 | 26.64 | 22.68 | 26.64 | 19.62 | 23.97 | 16.94 | 24.53 | 20.51 | |
| 18:3(n-3) ALA | Mean | 0.75* | 0.91 | 0.91 | 0.68 | 0.66 | 0.89 | 0.83 | 0.70 | 0.94 | 0.71 | 0.79 | 0.70 | 0.50* | 1.69* | 1.88* | 1.98* |
| SD | 0.49 | 0.75 | 0.74 | 0.33 | 0.29 | 0.71 | 0.66 | 0.31 | 0.69 | 0.37 | 0.52 | 0.29 | 0.13 | 0.54 | 0.28 | 0.36 | |
| Min | 0.25 | 0.31 | 0.31 | 0.25 | 0.25 | 0.31 | 0.31 | 0.25 | 0.31 | 0.25 | 0.25 | 0.40 | 0.30 | 1.23 | 1.69 | 1.45 | |
| Max | 3.59 | 4.12 | 4.12 | 1.69 | 1.55 | 4.12 | 4.12 | 1.54 | 4.12 | 1.67 | 3.59 | 1.54 | 1.08 | 2.27 | 2.35 | 2.35 | |
| 20:4(n-6) AA | Mean | 0.60* | 0.60 | 0.58 | 0.61 | 0.59 | 0.60 | 0.59 | 0.61 | 0.58 | 0.60 | 0.59 | 0.56 | 0.45* | 0.28* | 0.26* | 0.11* |
| SD | 0.14 | 0.19 | 0.16 | 0.16 | 0.16 | 0.15 | 0.16 | 0.15 | 0.15 | 0.18 | 0.15 | 0.15 | 0.11 | 0.19 | 0.07 | 0.09 | |
| Min | 0.22 | 0.30 | 0.30 | 0.22 | 0.22 | 0.31 | 0.30 | 0.22 | 0.30 | 0.22 | 0.22 | 0.35 | 0.28 | 0.18 | 0.28 | 0.02 | |
| Max | 0.98 | 1.00 | 0.98 | 1.00 | 1.00 | 0.98 | 1.00 | 0.98 | 1.00 | 0.98 | 1.00 | 0.88 | 0.72 | 0.62 | 0.36 | 0.21 | |
| 20:5(n-3) EPA | Mean | 0.14 | 0.12 | 0.15 | 0.13 | 0.12 | 0.15 | 0.14 | 0.13 | 0.14 | 0.15 | 0.14 | 0.10 | 0.11 | 0.07 | 0.07 | 0.06 |
| SD | 0.09 | 0.08 | 0.09 | 0.09 | 0.08 | 0.09 | 0.09 | 0.09 | 0.07 | 0.12 | 0.08 | 0.05 | 0.06 | 0.05 | 0.05 | 0.03 | |
| Min | 0.04 | 0.05 | 0.06 | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 0.04 | 0.05 | 0.02 | 0.02 | 0.01 | 0.03 | |
| Max | 0.52 | 0.35 | 0.51 | 0.52 | 0.52 | 0.51 | 0.52 | 0.51 | 0.35 | 0.52 | 0.52 | 0.29 | 0.32 | 0.15 | 0.22 | 0.11 | |
| 22:6(n-3) DHA | Mean | 0.42 | 0.50 | 0.48 | 0.40 | 0.39 | 0.48 | 0.44 | 0.43 | 0.44 | 0.47 | 0.41 | 0.37 | 0.32 | 0.21 | 0.22 | 0.09 |
| SD | 0.32 | 0.30 | 0.33 | 0.29 | 0.27 | 0.34 | 0.30 | 0.34 | 0.28 | 0.37 | 0.29 | 0.24 | 0.18 | 0.05 | 0.08 | 0.08 | |
| Min | 0.05 | 0.17 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.18 | 0.28 | 0.01 | |
| Max | 1.61 | 1.23 | 1.61 | 1.41 | 1.41 | 1.61 | 1.41 | 1.61 | 1.23 | 1.61 | 1.41 | 0.96 | 1.03 | 0.30 | 0.33 | 0.20 | |
| 18:1(n-7) TVA | Mean | 0.71* | 0.69 | 0.70 | 0.71 | 0.70 | 0.71 | 0.71 | 0.70 | 0.69 | 0.75 | 0.70 | 0.72 | 1.84* | 0.47* | 0.53* | 0.54* |
| SD | 0.13 | 0.13 | 0.12 | 0.13 | 0.14 | 0.12 | 0.12 | 0.14 | 0.12 | 0.15 | 0.12 | 0.14 | 0.41 | 0.08 | 0.11 | 0.06 | |
| Min | 0.38 | 0.41 | 0.43 | 0.38 | 0.38 | 0.42 | 0.42 | 0.38 | 0.41 | 0.38 | 0.38 | 0.41 | 1.10 | 0.33 | 0.45 | 0.48 | |
| Max | 0.99 | 0.87 | 0.99 | 0.94 | 0.95 | 0.99 | 0.95 | 0.99 | 0.94 | 0.99 | 0.95 | 0.94 | 2.77 | 0.53 | 0.79 | 0.63 | |
| CLAs | Mean | 0.93 | 0.86 | 0.89 | 0.93 | 0.88 | 0.93 | 0.93 | 0.85 | 0.96 | 0.85 | 0.94 | 0.85 | 0.97* | 0.58* | 0.50* | 0.54* |
| SD | 0.23 | 0.30 | 0.26 | 0.24 | 0.26 | 0.24 | 0.25 | 0.24 | 0.26 | 0.25 | 0.26 | 0.23 | 0.19 | 0.16 | 0.19 | 0.07 | |
| Min | 0.32 | 0.50 | 0.41 | 0.32 | 0.32 | 0.59 | 0.41 | 0.32 | 0.41 | 0.32 | 0.32 | 0.57 | 0.60 | 0.40 | 0.42 | 0.43 | |
| Max | 1.38 | 1.42 | 1.42 | 1.38 | 1.42 | 1.38 | 1.42 | 1.31 | 1.42 | 1.29 | 1.42 | 1.33 | 1.33 | 0.83 | 0.94 | 0.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





