1. Introduction
Erythrocytes, as the most numerous formed elements of human blood, are a standard model object in the study of the biological effects of laser irradiation on living organisms [
1,
2,
3,
4,
5,
6,
7].
According to the findings of the international scientific community, the exposure to laser irradiation has been shown to stimulate the proliferation and differentiation processes of erythrocyte cells [
8,
9] and regulate blood microcirculation [
8,
10,
11]. To a greater extent, these physiological phenomena are observed upon exposure to low-level laser irradiation at the wavelength of the infrared spectrum of light [
12].
The exposure of donor blood to infrared laser irradiation significantly slows down the aging process of erythrocytes [
12]. Scanning electron microscopy, the express thick blood smear method and morphometry have been utilized to examine the modifications in the shape of red blood cells from donor blood during storage and subsequent to irradiation with the He-Ne and infrared lasers. It has been established that laser irradiation can delay the appearance of pathological erythrocytes in the blood. A more significant effect was observed when erythrocytes were subjected to He–Ne laser irradiation [
13].
According to I.M. Baibekov et al., the peculiarities of the biological response of tissues to laser irradiation depend not only on the wavelength, but also on the time of exposure to photon energy [
4,
8,
12].
Peter Kassák et al., report that the impact of a Nd:YAG laser with a wavelength of 532 nm and a power of 30 mW on erythrocytes results in an enhancement of the activity of Na+/K+–adenosine triphosphatase, an enzyme belonging to the transport adenosine triphosphatases present in the plasma membrane, thereby exerting a biostimulating effect on cells [
14].
The administration of laser irradiation intravenously enhances the activity of tissue respiration mediators in erythrocytes, specifically the enzymes glucose-6-phosphate dehydrogenase, pyruvate kinase and lactate dehydrogenase [
8]. The exposure to laser irradiation with a wavelength of 635 nm has been shown to catalase parameters in erythrocytes [
8]. It has also been demonstrated that exposure to laser irradiation in the near-infrared range significantly decreases catalase activity and lipid peroxidation in erythrocytes [
7].
Scientists Dan G. Siposan and Adalbert Lukacs examined the effect of helium-neon laser irradiation with a wavelength of 632.8 nm on blood cells. The exposure of whole blood to laser irradiation was carried out in continuous mode at a laser irradiation power of 6 mW and a flux density of 180 mW/cm2. The researchers discovered that laser irradiation induces biostimulating effects in the cell membrane of erythrocytes. These phenomena are based on a non-resonance mechanism [
15].
As per the findings of Poludnikova et al., the exposure of erythrocytes to high-level infrared laser irradiation results in an elevation in the processes of lipid peroxidation of red blood cell membranes and the activity of cellular enzymes of antioxidant protection. During the course of the experiment, the researchers exposed a suspension of rat red blood cells with a wavelength of 1265 nm in continuous mode and a power of 5.5 W at doses of 7.8 J/cm2, 10.8 J/cm2, 39 J/cm2, 54 J/cm2, 78 J/cm2, 108 J/cm2, 156 J/cm2 and 216 J/cm2. In all instances of observation, an increase in the level of activity of the enzyme superoxide dismutase was observed (p<0.05), which indicated the activation of the antioxidant system. There was also a dose-dependent increase in the activity of other enzymes, specifically lipid peroxidation products such as malondialdehyde, catalase and glutathione-S-transferase. At doses of 156 J/cm2 and 216 J/cm2, a decrease in the hemoglobin level in the suspension was noted. The authors attribute this phenomenon to the thermal denaturation of hemoglobin in response to high doses of laser irradiation [
2].
Erythrocytes in a physiological state can be represented in various forms. Normally, erythrocytes in the blood are dominated by discocytes, which make up 98% of all types of red blood cells [
12]. The most prevalent red blood cells in pathological forms are erythrocytes with a crest, stomatocytes, erythrocytes with a significant depression on one side of the disc, and echinocytes (erythrocytes with processes) [
16].
In hypoxia, there is a change in the ratio of normal erythrocytes to their pathological forms in the blood [
16]. Changes in the ratio between normal erythrocytes and their pathological counterparts are also observed in stress, all types of acute and chronic diseases, bleeding and poisoning of the body with exo- and endotoxins [
6,
12].
Changes in the shape of erythrocytes are caused not only by the effects of various endogenous and exogenous factors, but also by shifts in constants such as pH and osmoticity. These changes can manifest themselves in the form of outgrowths on the surface of the cell membrane, which is called echinocytic transformation, or in the form of intussusceptions on the cell surface, which are defined as stomatocytic transformation [
12].
During the initial phases of all types of transformation, following the cessation of the aggressive factors, it is feasible to completely restore erythrocytes from their pathological forms back to discocytes [
6].
The exposure to low-level laser irradiation (LLLI) increases the fluidity of erythrocytes by 18%, reduces their aggregation capacity, reduces the number of pathological forms of erythrocytes [
12], and slows down their sedimentation rate [
3].
I.M. Baibekov and M.M. Irkhanov found that even in such a superficial pathological condition as prosthetic stomatitis, the ratio of discocytes and pathological forms of erythrocytes (PFE) changes towards the latter [
16]. However, when the oral mucosa is exposed to low-level laser irradiation in the area of inflammation, the percentage of red blood cells normalizes: the number of discocytes increases from 59% to 88%, and the proportion of pathological forms of erythrocytes decreases from 41% to 12% (p<0.05) [
16].
Pathological forms of erythrocytes lose their ability to deform and traverse through narrow capillaries, which causes pronounced microcirculation disorders [
12].
Due to the anatomical features of red blood cells, the ability to deform is one of the unique properties of erythrocytes [
17].
Erythrocytes (or red blood cells) are blood cells without a nucleus or mitochondria [
8,
12]. The cytoplasm of erythrocytes has an average volume of 94 μm3 and is rich in the protein hemoglobin, each tetramer of which is capable of binding four oxygen molecules (O2). The formation of red blood cells is derived from hematopoietic stem cells located within the bone marrow, and their volume is estimated to be approximately 2.4 million cells per second [
12]. This process is regulated by the hormone erythropoietin. Erythrocytes consist of 60% water, 30–35% hemoglobin, and 5–7% other substances, including non-hemoglobin proteins, fats, carbohydrates, minerals. The mass of hemoglobin does not exceed a third of the weight of an erythrocyte. The erythrocyte membrane is composed of proteins (49%), fats (43%) and carbohydrates (8%) [
12].
Erythrocytes have a disc-shaped biconcave shape with a diameter of 7–10 μm and a thickness of 1.7–2.4 μm [
12,
16]. The thickness of the central part of an erythrocyte is 1 μm [
12]. The unique shape contributes to an increase in the total surface area of erythrocytes by 20% compared to a sphere of the same volume [
8]. The absence of a nucleus and the biconcave shape determine their good deformability and passage through narrow capillaries with subsequent complete restoration of the original shape. These properties and integrity are essential for the full performance of their specific functions: the transport of O2 into tissues, the transport of carbon dioxide (CO2) into the lungs, and the buffering of hydrogen ions (H+).
The erythrocyte membrane consists of a double layer of phospholipids and an underlying two-dimensional network of spectrin molecules [
19]. The spectrin protein, which possesses several functions, assembles a three-dimensional structure and forms the submembrane cytoskeleton of the erythrocyte. The combined properties of the double phospholipid layer and the protein network of spectrin contribute to the discocytic morphology of healthy erythrocytes and give the membrane its elasticity and biorheological properties [
19,
20]. The surface of erythrocytes has a negative charge that prevents them from aggregating.
The lumen of the exchange capillaries in the papillary gingiva may be less than 7 mm in size. In small arteries, arterioles, and venules, the movement of blood cells can be significantly altered by regulatory constriction and vascular dilatation. In exchange capillaries, these phenomena are impracticable due to the absence of muscular components in the wall of vessels of this type. Therefore, in exchange capillaries, the flow deformation of erythrocytes is crucial for tissue perfusion [
20].
The duration of erythrocyte functioning is 100–120 days. During this period, erythrocytes circulate 1.7x105 times and travel a continuous path of 500,000 km [
21]. With age, the deformability of erythrocytes undergoes significant changes [
21]. Mature erythrocytes have reduced deformability compared to the young ones, which is due to the deterioration of membrane elasticity and an increase in the internal viscosity of the cytoplasm as a result of an increase in the intracellular content of hemoglobin and a decrease in the concentration of ATP [
22]. For young erythrocytes, the average intracellular hemoglobin concentration is 317 g/l, and for mature erythrocytes, it is 375 g/l, which is equivalent to internal viscosity values of 9 centipoises (cP) and 54 cP, respectively. According to Arman Namvar et al., the diminished deformability of mature erythrocytes is also associated with a decrease in the ratio of erythrocyte surface area to its volume [
23].
It is widely acknowledged that the inflammatory process in the oral mucosa and periodontal tissues is accompanied by impaired blood microcirculation and tissue hypoxia [
10]. Erythrocytes are the most important component of microcirculation [
6]. The efficiency of microcirculation is determined by the shape of red blood cells [
24].
The findings of the study conducted by Gang-Yue Luo et al. indicate that the exposure to low-level helium-neon laser irradiation with a wavelength of 632.8 nm at a power of 4.4 mW/cm2 for a duration of 5 minutes has a significant impact on the process of glycolysis of erythrocytes. This is reflected in a decrease in the activity of the energy-consuming enzyme phosphofructokinase (PFK) and an increase in the activity of the energy-generating enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which in turn enhances the deformability of red blood cells [
11].
It has been previously established that the exposure of blood to low-level laser irradiation in the red and infrared ranges of the light spectrum increases the ability of red blood cells to be deformed [
8,
14,
15].
At present, there is an active introduction of modern, innovative blue laser technology into worldwide dental practice. The active medium of a blue laser is represented by a semiconductor material consisting of a mixture of gallium nitride (GaN) and indium nitride (InN), designated as InGaN [
25]. Hemoglobin, water and melanin are absorbed by blue light laser irradiation with a wavelength of 445±40 nm. It is imperative to investigate the peculiarities of the biological response of erythrocytes to 445 nm laser irradiation during the procedure of low-level laser therapy.
The aim of the study was to determine the capacity of erythrocytes to undergo deformation upon exposure to a blue laser.
2. Materials and Methods
We investigated the effect of 445 nm laser irradiation on the formed elements of blood, specifically red blood cells. The study examined the impact of laser irradiation at 445 nm on erythrocytes during a medical procedure involving exposure to low-level laser irradiation on the gum area, designated as state code A22.07.008 (as per Order 804n of the Ministry of Health of the Russian Federation, dated October 13, 2017).
In this experimental study, the object of study was 24 sexually mature male laboratory rats of the WISTAR breed, weighing between 170 and 200 grams. All the animals were issued with sanitary passports. The laboratory rats were maintained in the Central Vivarium of Sechenov University in accordance with the laboratory protocols of practice during preclinical studies adopted in the Russian Federation (All-Union State Standards R50258-92, 351000.3-96 and 51000.4-96). Laboratory animals were handled in accordance with the standards of Good Clinical Practice and the principles of the Declaration of Helsinki of the World Medical Association (1964). Medical manipulations were granted approval by the Local Ethics Committee of the I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia on October 07, 2021 (LEC Protocol No. 17–21).
Prior to being exposed to low-level laser irradiation (LLLI) in the vicinity of the attached keratinized gingiva, for the purpose of general anesthesia, the rats were administered intramuscularly with Zoletil (a combination of tiletamine hydrochloride and zolazepam hydrochloride; manufactured by Virbac, France) at the rate of 5 mg/kg of animal weight and Xyla (xylazine hydrochloride; manufactured by Interchemie, the Netherlands) at the rate of 0.2 ml/kg of animal weight.
Low–Level Laser Therapy
A prototype of the ALTA BLUE diode semiconductor laser manufactured by the IRE-Polus Scientific and Technical Association (Russia) was used as a source of laser irradiation with a wavelength of 445 nm. The procedure of exposing the gingival region of the lower jaw of the rats was performed with contactless laser irradiation at a power of 0.5 W using the dynamic technique in continuous mode (CW) and an uninitiated fiber with a diameter of 400 μm. The distance between the tip of the light guide and the gingiva was 4.5–5 mm.
To detect the phenomenon of erythrocyte deformability upon exposure to a laser treatment with a wavelength of 445 nm, the first venous blood sampling was carried out prior to the LLLT procedure. This procedure was performed using the ALTA BLUE laser device with a wavelength of 445 nm and a duration of 5 minutes. The eyes of laboratory animals were protected from exposure to blue light with a sterile gauze pad. 25 minutes after the end of LLLT, a drop of blood was taken from the lateral vein of the tail of the laboratory animal using a needle and a syringe. A drop of blood was transferred to a sterile laboratory glass and stretched across its entire smooth surface.
The LLLT procedure was repeated within 24 and 48 hours. Venous blood sampling was performed on the 1st, 2nd, 3rd, and 5th days.
Cytological Examination
In the clinical laboratory, blood smears were stained according to the Romanovsky-Giemse method, and blood cytological examination was carried out by bright-field light microscopy using a Leica DM 4000 B LED microscope (Germany). The photometry of the objects was conducted by utilizing a Leica DM 4000 B LED light microscope equipped with a Leica DFC 7000 T camera (Germany). The number of altered shapes of red blood cells was measured in the ImageJ program on microscopy images at 400x magnification. Each specimen contained a total of 10 visual fields.
Statistical Analysis
The statistical processing was carried out using the Jamovi project R programming environment [
26]. The study involved the evaluation of the results of counting deformable forms of red blood cells in laboratory rats of the WISTAR breed before and during exposure to laser irradiation at a wavelength of 445 nm. Normal-shaped erythrocytes, such as discocytes, reversible red blood cells, and dome-shaped erythrocytes, were detected. For an objective assessment, a comparative analysis of the percentage of dome-shaped erythrocytes was carried out before exposure to laser irradiation with a wavelength of 445 nm, during the LLLT procedure, and on the 5th day from the beginning of the experiment. For each stage, the distribution of indicators (minimum, 1-, 2.5-, 10-, 25-, 50-; (median), 75-, 90-, 97.5-, 99-percentile, maximum), mean and standard deviation, as well as the 95% confidence interval (CI) of the mean and median were assessed. The level of significance for comparative and regression analyses corresponded to 0.05. For quantitative measures, the Shapiro–Wilk test was used to determine the nature of the distribution, as well as the mean, standard deviation, median, interquartile interval, minimum and maximum values. For both categorical and qualitative traits, the proportion and absolute number of values were determined. A comparative analysis of normally distributed quantitative traits was conducted utilizing the Student's t-test, followed by a pairwise comparison between the two groups.
3. Results
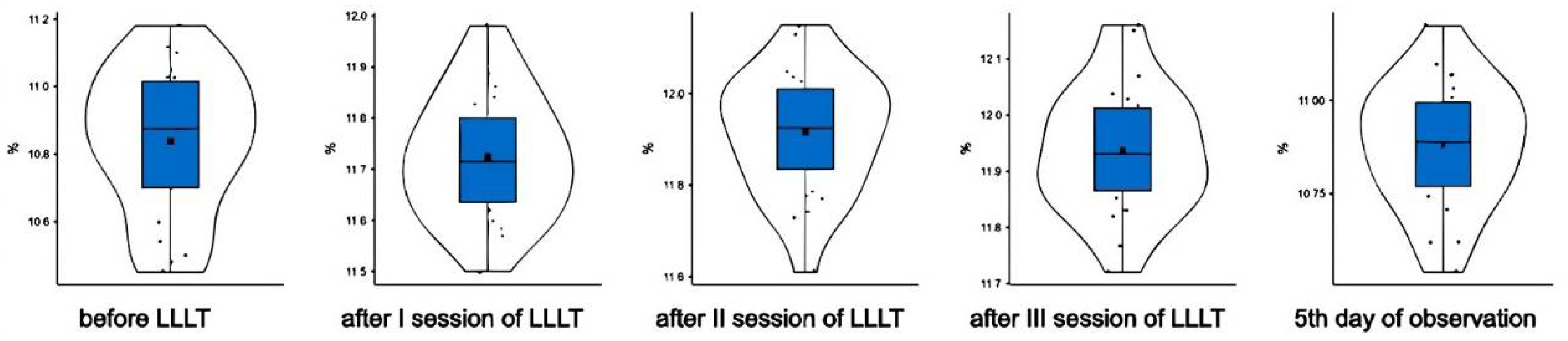
Prior to the LLLT procedure, deformable erythrocytes accounted for 10.8+0.213% (M+sd) of the total number of red blood cells in the WISTAR rats (
Figure 1).
Discocytes were represented by 89.1%. Pathological forms of erythrocytes (irreversible conditions) accounted for a volume of 0.1%. We have revealed the appearance of many deformed red blood cells in stomatocyte form.
After the first LLLT session, the proportion of deformable erythrocytes was 11.7+0.122% (M+sd); after the second session – 11.9+0.133% (M+sd), after the third session – 11.9+0.111% (M+sd) (
Figure 1).
On the fifth day of observation, the population of deformed erythrocytes was 10.9+0.166% (M+sd) (
Figure 1).
Erythrocytes have a high capacity for deformability [
27,
28,
29]. According to Badr Kaoui et al., depending on the diameter of the capillaries and the direction of the pressure gradient in the bloodstream, erythrocytes can deform into axisymmetric parachute or asymmetrical shoe shapes during circulation [
30]. These forms enable erythrocytes to move more actively in narrow capillaries and carry out oxygen transfer in the peripheral circulatory system. Such anatomical conditions are observed in the papillary and marginal gingiva of the upper and lower jaw.
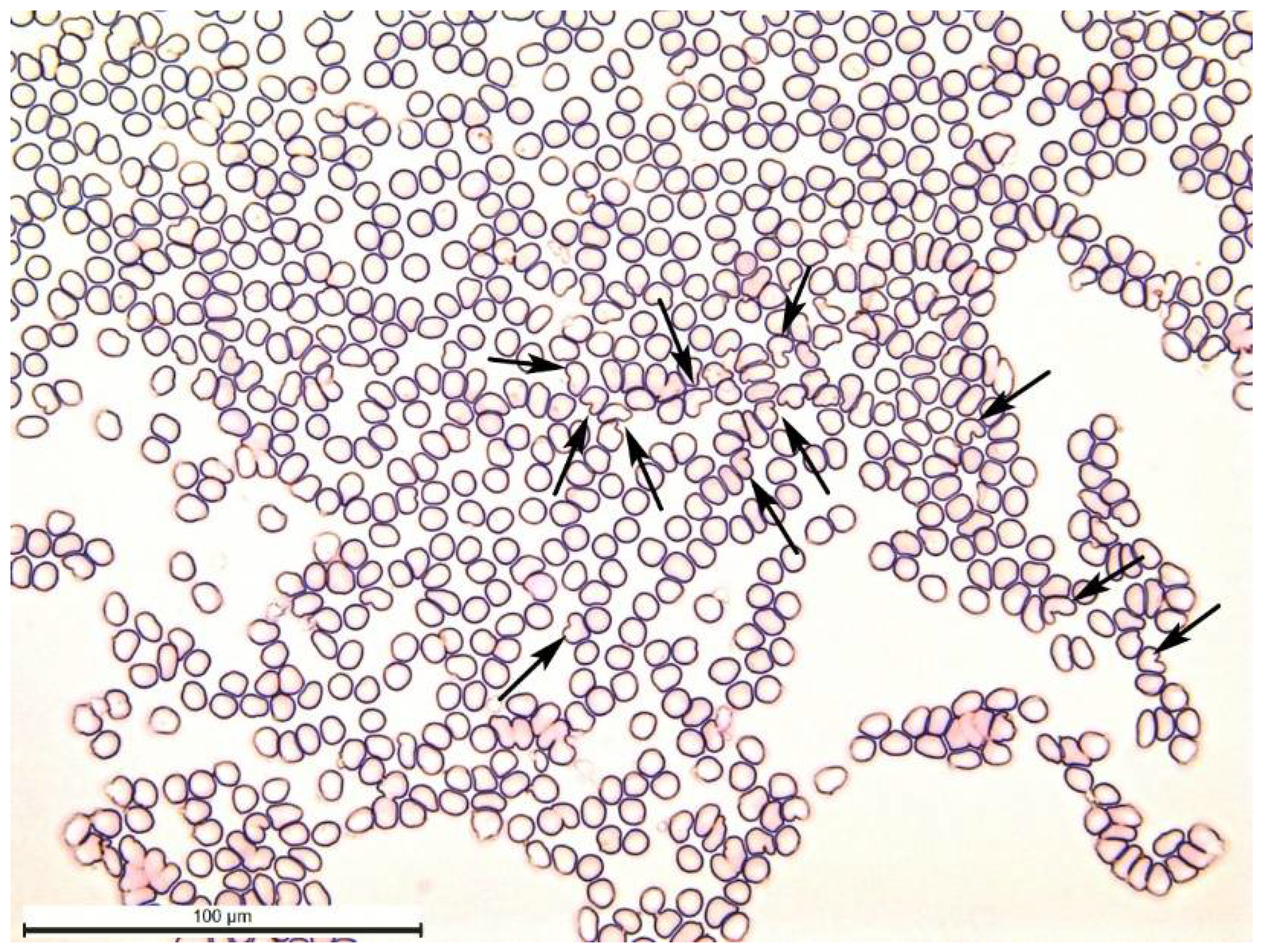
Furthermore, we counted the slipper-shaped red blood cells (
Table 1).
The exposure to laser irradiation at a wavelength of 445 nm resulted in a significant increase in the number of slipper-shaped deformable red blood cells in laboratory rats of the WISTAR breed (
Table 2). Each LLLT maintained a high number of slipper-shaped red blood cells. The slipper-shaped red blood cells are depicted in
Figure 2.
The comparative evaluation of the quantitative parameters of slipper-shaped erythrocytes in the peripheral blood of the laboratory rats prior to exposure to 445 nm laser irradiation, 25 minutes after the initial LLLT, and 2 days after the conclusion of the LLLT course is presented in
Table 2 and
Table 3.
Two days subsequent to the conclusion of the LLLT course, we observed a significant decrease in the quantity of slipper-shaped erythrocytes present in the peripheral blood of laboratory rats (
Table 5). However, the value of this parameter remained significantly higher than the control value (
Table 3).
We did not observe any significant variance in the quantitative parameters of dome-shaped forms of erythrocytes in the peripheral blood of laboratory rats following exposure to 445 nm laser irradiation after the three LLLT sessions (
Table 4).
Table 4.
Paired Samples T–Test. Comparative evaluation of the quantitative parameters of slipper-shaped erythrocytes in the peripheral blood of the laboratory rats during the exposure to laser irradiation at a wavelength of 445 nm in the course of three LLLT sessions.
Table 4.
Paired Samples T–Test. Comparative evaluation of the quantitative parameters of slipper-shaped erythrocytes in the peripheral blood of the laboratory rats during the exposure to laser irradiation at a wavelength of 445 nm in the course of three LLLT sessions.
| |
|
|
statistic |
df |
p |
| Session II |
Session I |
Student’s t |
0.0398 |
23.0 |
0.969 |
| Session III |
Session II |
Student’s t |
0.0436 |
23.0 |
0.966 |
| Session III |
Session I |
Student’s t |
0.1279 |
23.0 |
0.899 |
Table 5.
Paired Samples T–Test.
Table 5.
Paired Samples T–Test.
| |
|
|
statistic |
df |
p |
| Session III |
5th day |
Student’s t |
5.99 |
23.0 |
<.001 |
4. Discussion
We have selected a technique for exposing the gingival region to low-level laser irradiation, comprising a course of three days of 5 minutes each, in accordance with both clinical recommendations and practical experience [
31]. During this procedure, there is an elevation in the temperature of gingival tissue by 8.37+0.296°C, and the average temperature value is 37.48+0.043°C [
32], which does not exceed the LLLI safety threshold of 42°C [
33]. Therefore, we have determined this method to be safe.
As per the findings of the study conducted by Amal Yousif Al-Yasiri, it has been demonstrated that the direct exposure of a red blood cell suspension to irradiation from a diode semiconductor laser for a duration of up to 20 minutes is safe and does not result in the denaturation of cell membrane proteins. The scientist from Iraq used laser irradiation with a wavelength of 650 nm and a power of 50 mW [
34].
In our research, we utilized laser irradiation to treat healthy periodontal tissues. We have noted a slight but significant increase in the number of deformable forms of erythrocytes. The non-contact exposure of tissues to laser irradiation at low power levels results in a variety of physiological processes at the cellular and tissue levels [
35]. Since 2015, the global dental community has adopted the term photobiomodulation (PBM) for this procedure [
36]. PBM is a potentially effective and non-invasive method for improving blood viability and microcirculation processes.
The blood test conducted on the fifth day of the experiment was motivated by our curiosity regarding the duration of the effect of laser irradiation. Upon the fifth day of follow-up, the slipper-shaped erythrocytes accounted for 2.06+0.335% of the total volume of red blood cells, which was significantly higher than the value we had established before the LLLT (p=0.023). This fact permits us to assert that, at least until the fifth day following the procedure, the capacity of erythrocytes to exhibit active deformability is maintained after three exposures to laser irradiation at a wavelength of 445 nm.
Erythrocytes have a unique ability to undergo repetitively large deformations [
37], which allows them to move through blood vessels with diameters of up to 2–3 μm. Erythrocytes possess the capability to traverse microvessels with a diameter of 2–2.7 μm without causing any harm or rupture to the cell membrane. In capillaries with a diameter of less than 5 μm, erythrocytes sequentially move in a synchronized manner, encompassing the entire lumen of the vessel.
According to Timothy J. McMahon, in order to exert an effect on endothelial and vascular smooth muscle cells during the formation of mechanical stress, erythrocytes export vasodilator mediators, specifically the S-nitrosothiol (SNO) group, which is synthesized on hemoglobin from nitric oxide (NO) [
22].
Under normal circumstances, the deformability of erythrocytes is attributed to the elastic properties of the cell membrane. The viscosity of the internal contents of the erythrocyte also plays a significant role in its deformability when the hemoglobin concentration exceeds 50 g/dL [
20]. As the deformability of erythrocytes decreases, local tissue perfusion is impaired.
A.V. Muravyov et al. discovered that the ability of erythrocytes to undergo deformation is contingent upon the age of an individual and the presence or absence of concomitant pathology. Hence, the degree of deformability of red blood cells in athletes is higher than in individuals with metabolic disorders and vascular disorders [
20].
Low deformability of erythrocytes is observed when the functioning of intracellular signaling pathways is impaired, which accompanies a decrease in membrane elasticity. This process is regulated by the enzyme adenylate cyclase (AC). Stimulation of AC by incubation of erythrocytes with labdan diterpenoid – forskolin reduces the viscosity of erythrocyte suspensions by 12% and increases the degree of their deformability by 33% (p<0.05) [
20].
The biconcave disc-shaped form of the erythrocyte is responsible for its good deformability and passage through narrow capillaries, with subsequent complete restoration of its original configuration [
37]. Erythrocytes that circulate within capillaries undergo significant deformations, resulting in a diverse range of shapes. According to the principles of hydrodynamics, erythrocytes in the capillary are situated along its axis. However, while their rotation ceases, their stretch-type deformation increases. Improving the deformability properties of erythrocytes increases the transport of oxygen to tissues.
The results of our study confirm the discovery of an international team of scientists from Europe and Asia. According to Ruixue Zhu et al., exposure to laser irradiation with a wavelength of 450 nm at an energy density below 9.5 J/cm2 causes an increase in the deformability of human erythrocytes and a weakening of their intercellular interaction, which leads to a decrease in the aggregation capacity of cells [
38]. The researchers observed a linear stretching of the red blood cells, their narrowing and an increase in their length of 20%.
The ability of erythrocytes to deform improves with an increase in the temperature of the surrounding environment. However, with prolonged exposure and an increase in tissue temperature above 40°C, the ability of red blood cells to deform significantly decreases [
39]. The LLLT technique proposed by us is characterized by a comfortable warmth resulting from a distance of 4.5–5 mm between the tip of the fiberglass and the gingival surface [
40].
In order to maintain the normal physicochemical characteristics of the erythrocyte membrane, the presence of ATP is imperative [
4]. A decrease in the concentration of ATP causes a change in the shape of red blood cells and the formation of echinocytes [
41]. With an increase in the concentration of ATP, there is a stomatocytic transformation of discocytes and the formation of slipper-shaped forms of erythrocytes [
12]. This phenomenon has been observed during both the LLLT and PBM procedures [
31]. As is widely acknowledged, the fundamental mechanism behind LLLT and PBM is the activation of thermodynamic Ca2+-dependent processes, as a result of which the synthesis of DNA and RNA is enhanced, the synthesis and accumulation of ATP in the mitochondria increases, the release of nitric monoxide increases, while the level of Ca2+ in the Golgi apparatus is maintained due to the work of the Ca2+–ATP enzyme [
8,
35]. The phenomenon of increasing the energy capacity of cells and the entire body in the process of laser therapy is of key importance, since there is an elimination of chronic energy deficiency inherent in the sick body and the appearance of free energy necessary to start processes aimed at healing [
42].
Milene Castilhos de Oliveira et al. discovered that LLLT induces a rise in the quantity of red blood cells in laboratory rats, even after a course of irradiation therapy and the appearance of signs of anemia [
9].
Author Contributions
Conceptualization, N.V.R. and N.B.S.; methodology, N.V.R., N.B.S., A.A.D., and S.V.T.; software, N.V.R., N.B.S., E.V.S., V.E.A., and A.S.S.; validation, N.V.R., N.B.S., and S.V.D.; formal analysis, A.A.D., and S.V.T.; investigation, N.V.R., N.B.S., S.V.D., A.A.Der., E.V.S., V.E.A., A.S.S., and T.L.P.; resources, N.V.R., N.B.S., S.V.D., A.A.Der., E.V.S., V.E.A., A.S.S., and T.L.P.; data curation, N.V.R., E.V.S., V.E.A., and A.S.S.; writing—original draft preparation, N.V.R., S.V.T, A.A.D., and T.L.P.; writing—review and editing, N.V.R., N.B.S., A.A.D., and S.V.T.; visualization, N.V.R., N.B.S., A.A.D., and S.V.T.; supervision, S.V.T.; project administration, N.V.R. All authors have read and agreed to the published version of the manuscript.