Submitted:
09 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
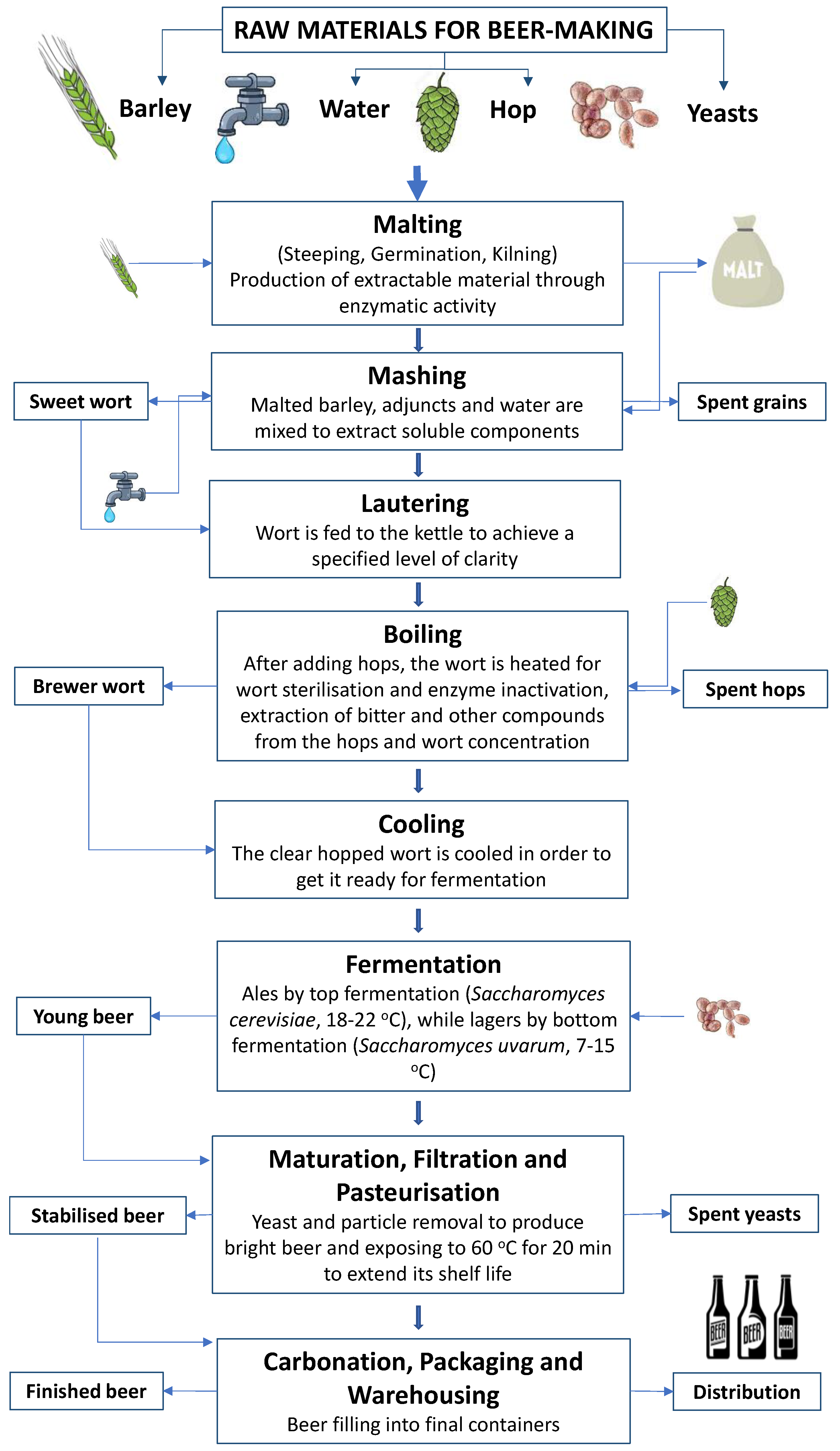
2. Methodology
3. Behaviour and Fate of the Most Important Chemical Pollutants in the Course of the Beer Production Process
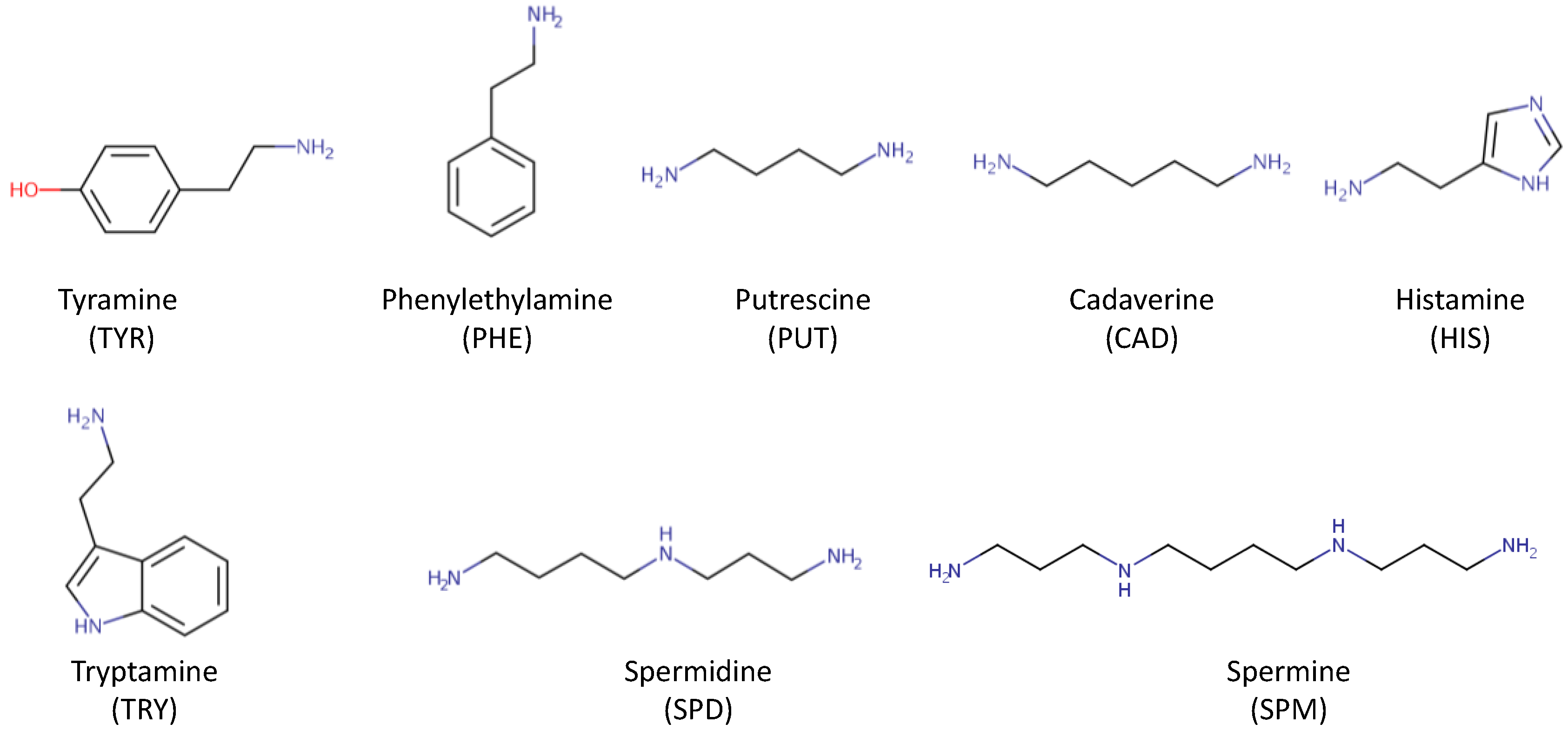
3.1. Biogenic Amines
3.2. Heavy Metals
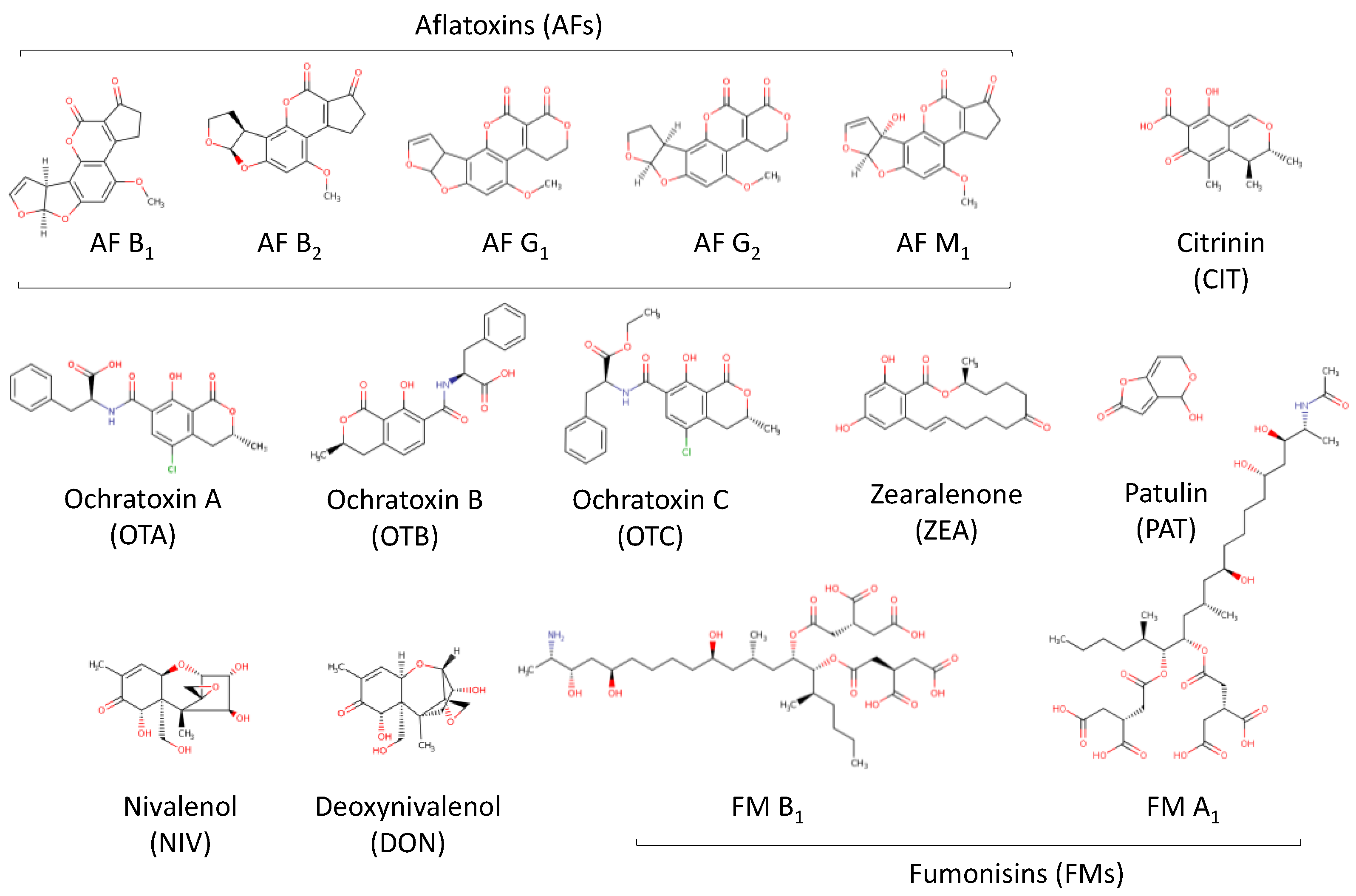
3.3. Mycotoxins
3.4. Nitrosamines
3.5. Pesticides
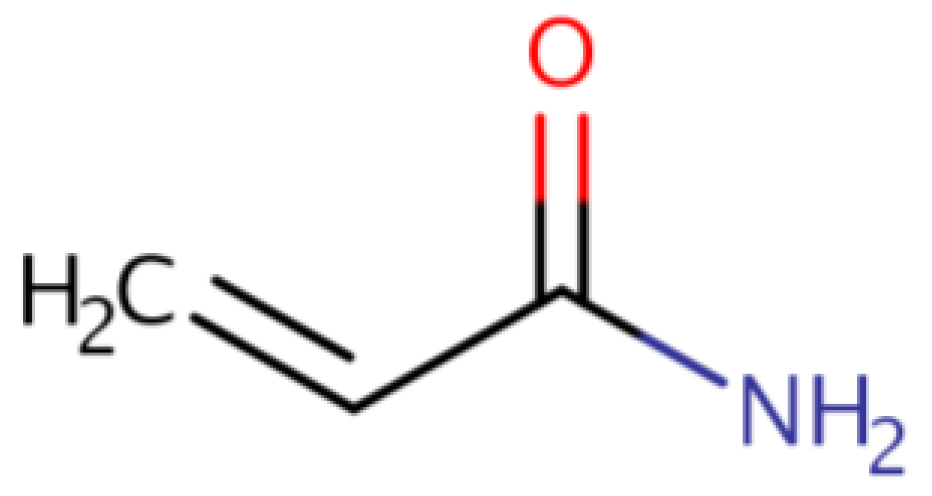
3.6. Acrylamide
3.7. Micro- and Nanoplastics
3.7.1. Chemical Pollutants Related to Plastics (Phthalates and Bisphenols)
3.8. Other Minority Pollutants
3.8.1. Polychlorinated Biphenyls
3.8.2. Aliphatic and Aromatic Hydrocarbons
3.8.3. Carbonyl and Furan Compounds
3.8.4. Trihalomethanes
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zion Market Research. 2023. Beer market-size, share, trends by product type (Lager, Ale, Stout, Porter, Malt, and Others), by packaging (Metal Can and Glass Bottles), by category (Premium Beer And Regular Beer), by distribution channel (Off-Trade Channels And On-Trade Channels) and by region–global and regional industry overview, market intelligence, comprehensive analysis, historical data, and forecasts 2022-2028. Salt Lake City, UT. https://www.zionmarketresearch.com/sample/beer-market-size.
- Goldammer, T. 2022. The Brewer’s Handbook. A Complete Book to Brewing Beer. (3rd ed). Apex Publishers. Centreville, VI.
- Mosher, M., Trantham, K. 2017. Brewing science. A multidisciplinary approach. Springer Cham. Switzerland. [CrossRef]
- Poli, A., Marangoni, F., Avogaro, A., Barba, G., Bellentani, S., Bucci, M., Cambieri, R., Catapano, A. L., Costanzo, S., Cricelli, C., de Gaetano, G., Di Castelnuovo, A., Faggiano, P., Fattirolli, F., Fontana, L., Forlani, G., Frattini, S., Giacco, R., et al. 2013. Moderate alcohol use and health: a consensus document. Nutr. Metab. Cardiovasc. Dis. 23, 487-504. [CrossRef]
- Wagner, E. M., Thalguter, S., Wagner, M., Rychli, K. 2021. Presence of microbial contamination and biofilms at a beer can filling production line. J. Food Prot. 84, 896-902. [CrossRef]
- Matoulková, D., Vontrobová, E., Brožová, M., Kubizniaková, P. 2018. Microbiology of brewery production–bacteria of the order Enterobacterales. Kvasny Prum. 64, 161-166. [CrossRef]
- Navarro, S., Vela, N. 2009. Fate of pesticide residues during brewing. In Beer in health and disease prevention (pp. 415-428). Academic Press. [CrossRef]
- Grumezescu, A. M., Holba, A. M. 2019. Quality control in the Beverage industry. Academic Press. NY. [CrossRef]
- Birkle, C., Pendlebury, D. A., Schnell, J., Adams, J. 2020. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 1, 363-376. [CrossRef]
- Williams, A. J., Grulke, C. M., Edwards, J., McEachran, A. D., Mansouri, K., Baker, N. C., Richard, A. M. 2017. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Chem. Inf. 9, e61. [CrossRef]
- Wójcik, W., Łukasiewicz, M., Puppel, K. 2021. Biogenic amines: formation, action and toxicity–a review. J. Sci. Food Agric. 101, 2634-2640. [CrossRef]
- Santos, M. S. 1996. Biogenic amines: their importance in foods. Int. J. Food Microbiol. 29, 213-231. [CrossRef]
- Spano, G., Russo, P., Lonvaud-Funel, A., Lucas, P., Alexandre, H., Grandvalet, C., Coton, E., Coton, M., Barnavon, L., Bach, B., Rattray, F., Bunte, A., Magni, C., Ladero, V., Alvarez, M., Fernández, M., Lopez, P., de Palencia, P.F., Corbi, A., Lolkema, J. S. 2010) Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 64, S95-S100. [CrossRef]
- Kalac, P., Krízek, M. 2003. A review of biogenic amines and polyamines in beer. J. Inst. Brew, 109, 123-128. [CrossRef]
- Romero, R., Bagur, M. G., Sánchez-Viñas, M., Gázquez, D. 2003. The influence of the brewing process on the formation of biogenic amines in beers. Anal. Bioanal. Chem. 376, 162-167. [CrossRef]
- Kiss, J., Korbász, M., Sass-Kiss, A. 2006. Study of amine composition of botrytized grape berries. J. Agric. Food Chem. 54, 8909-8918. [CrossRef]
- Tiris, G., Yanikoğlu, R. S., Ceylan, B., Egeli, D., Tekkeli, E. K., Önal, A. 2023. A review of the currently developed analytical methods for the determination of biogenic amines in food products. Food Chem. 398, e133919. [CrossRef]
- Koller, H., Perkins, L. B. 2022. Brewing and the chemical composition of amine-containing compounds in beer: A review. Foods. 11, 257. [CrossRef]
- Buňka, F., Budinský, P., Čechová, M., Drienovský, V., Pachlová, V., Matoulková, D., Buňková, L. 2012. Content of biogenic amines and polyamines in beers from the Czech Republic. J. Inst. Brew. 118, 213-216. [CrossRef]
- Nalazek-Rudnicka, K., Wojnowski, W., Wasik, A. 2021. Occurrence and levels of biogenic amines in beers produced by different methods. Foods, 10, e2902. [CrossRef]
- Tang, T., Shi, T., Qian, K., Li, P., Li, J., Cao, Y. 2009. Determination of biogenic amines in beer with pre-column derivatization by high performance liquid chromatography. J. Chromatogr. B, 877, 507-512. [CrossRef]
- Pereira, C. I., Matos, D., San Romão, M. V., Barreto Crespo, M. T. 2009. Dual role for the tyrosine decarboxylation pathway in Enterococcus faecium E17: response to an acid challenge and generation of a proton motive force. Appl. Environ. Microbiol. 75, 345-352. [CrossRef]
- Pérez, M., Calles-Enríquez, M., Nes, I., Martin, M. C., Fernandez, M., Ladero, V., Alvarez, M. A. 2015. Tyramine biosynthesis is transcriptionally induced at low pH and improves the fitness of Enterococcus faecalis in acidic environments. Appl. Microbiol. Biotechnol. 99, 3547-3558. [CrossRef]
- Steinkraus, K. H. 1992. Lactic acid fermentations. In: F.R. Ruskin (Ed.), Applications of biotechnology to traditional fermented foods, (pp. 43-51). The National Academies Press. [CrossRef]
- Duruibe, J.O., Ogwuegbu, M.O.C., Egwurugwu, J.N. 2007. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2, 112-118.
- Deka, A. K., Kumar, K. J., Basumatary, S. 2023. Monitoring Strategies for Heavy Metals in Foods and Beverages: Limitations for Human Health Risks. In B. A. Almayyahi, (Ed.). Heavy metals. IntechOpen, London. [CrossRef]
- Čejka, P., Horák, T., Dvořák, J., Čulík, J., Jurkova, M., Kellner, V., Hašková, D. 2011. Monitoring of the distribution of some heavy metals during brewing process. Ecol. Chem. Eng. S. 18, 67-74.
- Pohl, P. 2008. Determination and fractionation of metals in beer: A review. Food Addit. Contam. Part A. 25, 693-703. [CrossRef]
- Iwegbue, C. M., Overah, L. C., Bassey, F. I., Martincigh, B. S. 2014. Trace metal concentrations in distilled alcoholic beverages and liquors in Nigeria. J. Inst. Brew. 120, 521-528. [CrossRef]
- Passaghe, P., Bertoli, S., Tubaro, F., Buiatti, S. 2015. Monitoring of some selected heavy metals throughout the brewing process of craft beers by inductively coupled plasma mass spectrometry. Eur. Food Res. Technol. 241, 199-215. [CrossRef]
- EC. 2006. Commission Regulation (EC) No 1881/2006. OJEU. L364, 5. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881.
- Eticha, T., Hymete, A. 2014. Health risk assessment of heavy metals in locally produced beer to the population in Ethiopia. J. Bioanal. Biomed. 6, 65-68. [CrossRef]
- Filippini, T., Tancredi, S., Malagoli, C., Cilloni, S., Malavolti, M., Violi, F., Vinceti, M. 2019. Aluminum and tin: Food contamination and dietary intake in an Italian population. J. Trace Elem. Med. Biol. 52, 293-301. [CrossRef]
- Han, R., Li, H., Li, Y., Zhang, J., Xiao, H., Shi, J. 2006. Biosorption of copper and lead ions by waste beer yeast. J. Hazard. Mat. 137, 1569-1576. [CrossRef]
- Zufall, C., Tyrell, T. 2008. The influence of heavy metal ions on beer flavour stability. J. Inst. Brew. 114, 134-142. [CrossRef]
- WHO. 2023. Mycotoxins. World Health Organisation. Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/mycotoxins.
- Asao, T., Buchi, G., Abdel-Kader, M. M., Chang, S. B., Wick, E. L., Wogan, G. N. 1963. Aflatoxins B and G. J. Am. Chem. Soc. 85, 1706-1707. [CrossRef]
- Rushing, B. R., Selim, M. I. 2019. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 124, 81-100. [CrossRef]
- Creppy, E. E. 2002. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett., 127(1-3), 19-28. [CrossRef]
- Benešová, K., Čumová, M., Běláková, S., Mikulíková, R., Svoboda, Z. 2015. The occurrence of “Emerging“ mycotoxins in brewing raw materials. Kvasny Prum. 61, 114-119. [CrossRef]
- IARC. 1993. IARC Monograph on the evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer. Lyon.
- Gupta, R. 2017. Reproductive and developmental toxicology. (2nd ed). Academic Press, NY.
- Eskola, M., Kos, G., Elliott, C. T., Hajšlová, J., Mayar, S., Krska, R. 2020. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nut. 60, 2773-2789. [CrossRef]
- Şenyuva, H. Z., Gilbert, J. 2010. Immunoaffinity column clean-up techniques in food analysis: A review. J. Chromatogr. B, 878, 115-132. [CrossRef]
- Běláková, S., Benešová, K., Mikulíková, R., Svoboda, Z. 2011. Determination of ochratoxin A in brewing materials and beer by ultra performance liquid chromatography with fluorescence detection. Food Chem. 126, 321-325. [CrossRef]
- Krstanovic, V., Šarkanj, B., Velic, N., Mastanjevic, K., Šantek, B., Mastanjevic, K. 2017. Mycotoxins in malting and brewing by-products used for animal feed. J. Biotechnol, 256, S68-S69. https://doi.org/10.1016/j.jbiotec.2017.06.1033.(https://www.sciencedirect.com/science/article/pii/S0168165617313299). [CrossRef]
- EC. 2023. Commission Regulation (EC) No 915/2023. OJEU. L119, 103-157. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915.
- EC. 2005. Commission Regulation (EC) No 123/2005. OJEU. L25, 3-5. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R0123.
- Thellmann, A., & Weber, W. (1997). Determination of ochratoxin A in cereals, malt and beer after accumulation and separation on immunoaffinity columns and following high-pressure liquid chromatography with fluorescence detection. DEUTSCHE LEBENSMITTEL-RUNDSCHAU. 93, 1-3.
- Wolff, J. 2000. Ochratoxin A in cereals and cereal products. Archiv für Lebensmittelhygiene, 51, 85-88.
- Gumus, T., Arici, M., Demirci, M. 2004. A survey of barley, malt and beer contamination with ochratoxin A in Turkey. J. Inst. Brew. 110, 146-149. [CrossRef]
- Anli, E., Alkis, İ. M. 2010. Ochratoxin A and brewing technology: a review. J. Inst. Brew. 116, 23-32. [CrossRef]
- Lund, F., Frisvad, J. C. 2003. Penicillium verrucosum in wheat and barley indicates presence of ochratoxin A. J. Appl. Microbiol. 95, 1117-1123. [CrossRef]
- Magan, N., Hope, R., Cairns, V., Aldred, D. 2003. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. In: X. Xu, J.A. Bailey, B.M. Cooke (eds) Epidemiology of Mycotoxin Producing Fungi (pp. 723-730) Springer, Dordrecht. [CrossRef]
- Malachová, A., Cerkal, R., Ehrenbergerova, J., Hajslova, J. 2010. The fate of mycotoxins during malting and brewing. In Proceedings of the International Ph. D. Students Conference on MendelNet, pp. 708-715.
- Simpson, D. R., Weston, G. E., Turner, J. A., Jennings, P., Nicholson, P. 2001. Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin contamination of grain. Eur. J. Plant Pathol. 107, 421-431. [CrossRef]
- Heier, T., Jain, S. K., Kogel, K. H., Pons-Kühnemann, J. 2005. Influence of N-fertilization and fungicide strategies on Fusarium head blight severity and mycotoxin content in winter wheat. J. Phytopathol. 153, 551-557. [CrossRef]
- Šafránková, I., Marková, M., Kmoch, M. 2010. Seed mycoflora of malting varieties and lines of spring barley in localities Kroměříž and Žabčice. Kvasny Prum. 56, 138-144. [CrossRef]
- Rodhouse, L., Carbonero, F. 2019. Overview of craft brewing specificities and potentially associated microbiota. Crit. Rev. Food Sci. Nutr. 59, 462-473. [CrossRef]
- Hoff, S., Lund, M. N., Petersen, M. A., Jespersen, B. M., Andersen, M. L. 2014. Quality of pilsner malt and roasted malt during storage. J. Inst.Brew. 120, 331-340. [CrossRef]
- Kensler, T. W., Roebuck, B. D., Wogan, G. N., Groopman, J. D. 2011. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 120, S28-S48. [CrossRef]
- Wolff-Hall, C.E., Schwarz, P.B. 2002. Mycotoxins and Fermentation - Beer Production. In: J.W. DeVries, M.W. Trucksess, L.S. Jackson (eds). Mycotoxins and Food Safety. Adv. Exp. Med. Biol, vol 504 (pp 217–226). Springer. [CrossRef]
- Gumus, T., ARICI, M., Demirci, M. 2003. Okratoksin A’n in Bira Fermentasyonundaki Durumu ve Fermentasyona Etkisi. Trakya Üniversitesi Fen Bilimleri Dergisi, 4, 181-186.
- Scott, P. M. 1996. Mycotoxins transmitted into beer from contaminated grains during brewing. J. AOAC Int. 79, 875-882. [CrossRef]
- Bullerman, L. B., Bianchini, A. 2007. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 119, 140-146. [CrossRef]
- Krogh, P., Hald, B., Gjertsen, P., Myken, F. 1974. Fate of ochratoxin A and citrinin during malting and brewing experiments. Appl. Microbiol 28, 31-34. [CrossRef]
- Scott, P. M., Kanhere, S. R. 1995. Determination of ochratoxin A in beer. Food Addit. Contam. 12, 591-598. [CrossRef]
- Payen, J., Girard, T., Gaillardin, M., Lafont, P. 1983. Sur la presence de mycotoxines dans des bieres. Microbiol. Alim. Nutr. 1, 143-146.
- Mateo, R., Medina, Á., Mateo, E. M., Mateo, F., Jiménez, M. 2007. An overview of ochratoxin A in beer and wine. Int. J. Food Microbiol. 119, 79-83. [CrossRef]
- Odhav, B., Naicker, V. 2002. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 19, 55-61. [CrossRef]
- Chu, F. S., Chang, C. C., Ashoor, S. H., Prentice, N. 1975. Stability of aflatoxin B1 and ochratoxin A in brewing. Appl. Microbiol. 29, 313-316. [CrossRef]
- Scott, P. M., Kanhere, S. R., Lawrence, G. A., Daley, E. F., Farber, J. M. 1995. Fermentation of wort containing added ochratoxin A and fumonisins B1 and B2. Food Addit. Contam. 12, 31-40. [CrossRef]
- Legarda, T., Burdaspal, P. A. 1998. Ocratoxina A en cervezas elaboradas en España y en otros países europeos. Alimentaria. 291, 115-122.
- Deetae, P., Perello, M. C., De Revel, G. 2013. Occurrence of ochratoxin A and biogenic amines in Asian beers sold in French markets. J. Inst. Brew. 119, 57-63. [CrossRef]
- Medina, Á., Valle-Algarra, F. M., Gimeno-Adelantado, J. V., Mateo, R., Mateo, F., Jiménez, M. 2006. New method for determination of ochratoxin A in beer using zinc acetate and solid-phase extraction silica cartridges. J. Chromatogr. A. 1121, 178-183. [CrossRef]
- Nip, W. K., Chang, F. C., Chu, F. S., Prentice, N. 1975. Fate of ochratoxin A in brewing. Appl. Microbiol. 30, 1048-1049. [CrossRef]
- Guerra, M. C., Galvano, F., Bonsi, L., Speroni, E., Costa, S., Renzulli, C., Cervellati, R. 2005. Cyanidin-3-O-β-glucopyranoside, a natural free-radical scavenger against aflatoxin B1-and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br. J. Nutr. 94, 211-220. [CrossRef]
- Gjertsen, P., Myken, F., Krogh, P Hald, B. 1973. Malting and brewing experiments with ochratoxin and citrinin. Proceedings of the European Brewery Convention Congress, Salzburg, Elsevier Scientific, Amsterdam, pp. 373-380.
- Schothorst, R. C., Jekel, A. A. 2003. Determination of trichothecenes in beer by capillary gas chromatography with flame ionisation detection. Food Chem. 82, 475-479. [CrossRef]
- Piacentini, K. C., Savi, G. D., Pereira, M. E., Scussel, V. M. 2015. Fungi and the natural occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum vulgare L.). Food Chem. 187, 204-209. [CrossRef]
- Piacentini, K. C., Rocha, L. O., Fontes, L. C., Carnielli, L., Reis, T. A., Corrêa, B. 2017. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 218, 64-69. [CrossRef]
- Wall-Martínez, H. A., Pascari, X., Ramos, A. J., Marin, S., Sanchis, V. 2019. Frequency and levels of mycotoxins in beer from the Mexican market and exposure estimate for deoxynivalenol mycotoxins. Mycotoxin Res. 35, 207-216. [CrossRef]
- Peters, J., van Dam, R., van Doorn, R., Katerere, D., Berthiller, F., Haasnoot, W., Nielen, M. W. 2017. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS One 12, e0185887.
- Djoulde, D.R. 2011. Deoxynivanol (DON) and fumonisins B1 (FB1) in artisanal sorghum opaque beer brewed in north Cameroon. Afr. J. Microbiol. Res. 5, 1565-1567. [CrossRef]
- Lulamba, T. E., Stafford, R. A., Njobeh, P. B. 2019. A sub-Saharan African perspective on mycotoxins in beer–a review. J. Inst. Brew. 125, 184-199. [CrossRef]
- Bertuzzi, T., Rastelli, S., Mulazzi, A., Donadini, G., Pietri, A. 2018. Known and emerging mycotoxins in small-and large-scale brewed beer. Beverages. 4, e46. [CrossRef]
- Bogdanova, E., Rozentale, I., Pugajeva, I., Emecheta, E. E., Bartkevics, V. 2018. Occurrence and risk assessment of mycotoxins, acrylamide, and furan in Latvian beer. Food Addit. Contam: Part B, 11, 126-137. [CrossRef]
- Olšovská, J., Jandovská, V., Běláková, S., Kubizniaková, P., Vrzal, T., Štěrba, K. 2019. Monitoring of potential contaminants in beer from the Czech Republic. Kvasny Prum. 65, 84-96. [CrossRef]
- Inoue, T., Nagatomi, Y., Uyama, A., Mochizuki, N. 2013. Fate of mycotoxins during beer brewing and fermentation. Biosci. Bbiotechnol. Biochem. 77, 1410-1415. [CrossRef]
- Pietri, A., Bertuzzi, T., Agosti, B., Donadini, G. 2010. Transfer of aflatoxin B1 and fumonisin B1 from naturally contaminated raw materials to beer during an industrial brewing process. Food Addit. Contam, Part A. 27, 1431-1439. [CrossRef]
- Gushgari, A. J., Halden, R. U. 2018. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere, 210, 1124-1136. [CrossRef]
- Havery, D. C., Hotchkiss, J. H., Fazio, T. 1981. Nitrosamines in malt and malt beverages. J. Food Sci. 46, 501-505. https://doi.org/10.1111/j.1365-2621.1981.tb04896.x. [CrossRef]
- Wainwright, T. 1986. Nitrosamines in malt and beer. J. Inst. Brew. 92, 73-80. [CrossRef]
- Vrzal, T., Olšovská, J. 2016. N-nitrosamines in 21th Century. Kvasny Prum. 62, 2-8. [CrossRef]
- Khorolskiy, M., Ramenskaya, G., Vlasov, A., Perederyaev, O., Maslennikova, N. 2021. Development and validation of four nitrosamine impurities determination method in medicines of valsartan, losartan, and irbesartan with HPLC-MS/MS (APCI). Iran. J. Pharm. Res. 20, 541-552. [CrossRef]
- Fan, C. C., Lin, T. F. 2018. N-nitrosamines in drinking water and beer: Detection and risk assessment. Chemosphere, 200, 48-56. [CrossRef]
- Baxter, E. D., Slaiding, I. R., Travers, V. 2007. Current incidence of N-nitrosodimethylamine in beers worldwide. Food Addit. Contam. 24, 807-811. [CrossRef]
- FAO-WHO. 2014. The International code of conduct on pesticide management. Food and Agriculture Organization (FAO) of the United Nations and the World Health Organization (WHO). Rome.
- Hengel, M. J., Shibamoto, T. 2002. Method development and fate determination of pesticide-treated hops and their subsequent usage in the production of beer. J. Agric. Food Chem, 50, 3412-3418. [CrossRef]
- Wong, J. W., Webster, M. G., Bezabeh, D. Z., Hengel, M. J., Ngim, K. K., Krynitsky, A. J., Ebeler, S. E. 2004. Multiresidue determination of pesticides in malt beverages by capillary gas chromatography with mass spectrometry and selected ion monitoring. J. Agric. Food Chem. 52, 6361-6372. [CrossRef]
- Omote, M., Harayama, K., Sasaki, T., Mochizuki, N., Yamashita, H. 2006. Analysis of simultaneous screening for 277 pesticides in malt and beer by liquid chromatography with tandem mass spectrometry. J. Am. Soc. Brew. Chem. 64, 139-150. [CrossRef]
- Vela, N., Pérez, G., Navarro, G., Navarro, S. 2007. Gas chromatographic determination of pesticide residues in malt, spent grains, wort, and beer with electron capture detection and mass spectrometry. J. AOAC Int. 90, 544-549.
- Inoue, T., Nagatomi, Y., Suga, K., Uyama, A., Mochizuki, N. 2011. Fate of pesticides during beer brewing. J. Agric. Food Chem. 59, 3857-3868. [CrossRef]
- Hengel, M. J., Miller, D., Jordan, R. 2016. Development and validation of a method for the determination of pesticide residues in beer by liquid chromatography-mass spectrometry. J. Am. Soc. Brew. Chem. 74, 49-52. [CrossRef]
- Bedassa, T., Megersa, N., Gure, A. 2017. Salting-out assisted liquid-liquid extraction for the determination of multiresidue pesticides in alcoholic beverages by high performance liquid chromatography. Sci. J. Anal. Chem. 5, 38-45. [CrossRef]
- Dušek, M., Jandovská, V., Olšovská, J. 2018. Tracking, behavior and fate of 58 pesticides originated from hops during beer brewing. J. Agric. Food Chem. 66, 10113-10121. [CrossRef]
- He, N. X., Bayen, S. (2020). An overview of chemical contaminants and other undesirable chemicals in alcoholic beverages and strategies for analysis. Compr. Rev. Food Sci. Food Saf., 19, 3916-3950. [CrossRef]
- Pires, N. A., Goncalves De Oliveira, M. L., Goncalves, J. A., Faria, A. F. 2021. Multiclass analytical method for pesticide and mycotoxin analysis in malt, brewers’ spent grain, and beer: development, validation, and application. J. Agric. Food Chem. 69, 4533-4541. [CrossRef]
- Biendl, M. (2017). Systematic monitoring of residues. Brauwelt Int. 4, 257-260.
- Pérez-Lucas, G., Navarro, G., Navarro, S. 2023. Comprehensive review on monitoring, behavior, and impact of pesticide residues during beer-making. J. Agric. Food Chem. 71, 1820-1836. [CrossRef]
- Kunze, W. 2019. Technology of Brewing & Malting. (6th ed). VLB, Berlin.
- Miyake, Y., Hashimoto, K., Matsuki, H., Ono, M., Tajima, R. 2002. Fate of insecticide and fungicide residues on barley during storage and malting. J. Am. Soc. Brew. Chem. 60, 110-115. [CrossRef]
- Navarro, S., Perez, G., Navarro, G., Vela, N. 2007. Decline of pesticide residues from barley to malt. Food Addit. Contam. 24, 851-859. [CrossRef]
- Navarro, S., Vela, N., Navarro, G. 2011. Fate of triazole fungicide residues during malting, mashing and boiling stages of beermaking. Food Chem. 124, 278-284. [CrossRef]
- Hack, M., Nitz, S., Parlar, H. 1997. Behavior of [14C] atrazine, [14C] terbutylazine, and their major metabolites in the brewing process. J. Agric. Food Chem. 45, 1375-1380. [CrossRef]
- Miyake, Y., Koji, K., Matsuki, H., Tajima, R., Ono, M., Mine, T. 1999. Fate of agrochemical residues, associated with malt and hops, during brewing. J. Am. Soc. Brew. Chem. 57, 46-54. [CrossRef]
- Navarro, S., Pérez, G., Vela, N., Mena, L., Navarro, G. 2005. Behavior of myclobutanil, propiconazole, and nuarimol residues during lager beer brewing. J. Agric. Food Chem. 53, 8572-8579. [CrossRef]
- Navarro, S., Pérez, G., Navarro, G., Mena, L., Vela, N. 2006. Decay of dinitroaniline herbicides and organophosphorus insecticides during brewing of lager beer. J. Food Prot. 69, 1699-1706. [CrossRef]
- Kong, Z., Li, M., Chen, J., Gui, Y., Bao, Y., Fan, B., Dai, X. 2016. Behavior of field-applied triadimefon, malathion, dichlorvos, and their main metabolites during barley storage and beer processing. Food Chem. 211, 679-686. [CrossRef]
- Hakme, E., Nielsen, I. K., Madsen, J. F., Storkehave, L. M., Pedersen, M. S. E., Schulz, B. L., Poulsen, M. E., Hobley, T. J., Duedahl-Olesen, L. 2023. Fate of pesticide residues in beer and its by-products. Food Addit. Contam: Part A. 41, 45-59. [CrossRef]
- Jones, R. D., Kavanagh, T. E., Clarke, B. J. 1988. Determination of carbaryl residues in malt and beer and their impact on beer quality. J. Am. Soc. Brew. Chem. 46, 43-50. [CrossRef]
- Navarro, S., Pérez, G., Navarro, G., Mena, L., Vela, N. 2007. Influence of fungicide residues on the primary fermentation of young lager beer. J. Agric. Food Chem. 55, 1295-1300. [CrossRef]
- Navarro, S., Pérez, G., Navarro, G., Mena, L., Vela, N. 2007. Variability in the fermentation rate and colour of young lager beer as influenced by insecticide and herbicide residues. Food Chem. 105, 1495-1503. [CrossRef]
- Regueiro, J., López-Fernández, O., Rial-Otero, R., Cancho-Grande, B., Simal-Gandara, J. 2015. A review on the fermentation of foods and the residues of pesticides-biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. Nutr. 55, 839-863. [CrossRef]
- Bartkiene, E., Juodeikiene, G., Zadeike, D., Baliukoniene, V., Bakutis, B., Cizeikiene, D. 2019. Influence of microbial and chemical contaminants on the yield and quality of ethanol from wheat grains. J. Sci. Food Agric. 99, 2348-2355. [CrossRef]
- Wei, Q., Zhong, B., Zhu, J., Hu, S., He, J., Hong, Q., He, Q. 2020. Effect of pesticide residues on simulated beer brewing and its inhibition elimination by pesticide-degrading enzyme. J. Biosci. Bioeng. 130, 496-502. [CrossRef]
- Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., Törnqvist, M. 2000. Acrylamide: a cooking carcinogen?. Chem. Res. Toxicol. 13, 517-522. [CrossRef]
- Törnqvist, M. 2005. Acrylamide in Food: The Discovery and Its Implications. In: M. Friedman, D. Mottram (eds) Chemistry and Safety of Acrylamide in Food. Advances in Experimental Medicine and Biology, vol 561. Springer, Boston, MA. [CrossRef]
- US FDA. 2022. Acrylamide: Questions and Answers. United States Food and Drug Administration. https://www.fda.gov/food/process-contaminants-food/acrylamide-questions-and-answers.
- Rice, J. M. 2005. The carcinogenicity of acrylamide. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 5802), 3-20. [CrossRef]
- Miyake, Y., Tajima, R., Ono, M. 2003. Fate of pesticide metabolites on malt during brewing. J. Am. Soc. Brew. Chem. 61, 33-36. [CrossRef]
- EFSA. 2015. Acrylamide in food. European Food Safety. https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/acrylamide150604.pdf.
- EC. 2017. Commission Regulation (EU) 2017/2158. OJEU. L304, 24-44. https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32017R2158.
- Elbashir, A. A., Omar, M. M. A., Ibrahim, W. A. W., Schmitz, O. J., Aboul-Enein, H. Y. 2014. Acrylamide analysis in food by liquid chromatographic and gas chromatographic methods. Crit. Rev. Anal. Chem. 44, 107-141. [CrossRef]
- Mikuliková, R., Svoboda, Z., Belakova, S., Macuchova, S. 2008. Monitoring of acrylamide in the course of malting and in beer. Kvasny Prum. 54, 181-185. [CrossRef]
- Mottram, D. S., Wedzicha, B. L., Dodson, A. T. 2002. Acrylamide is formed in the Maillard reaction. Nature, 419, 448-449. [CrossRef]
- Arisseto, A. P., Toledo, M. C., Govaert, Y., Loco, J. V., Fraselle, S., Weverbergh, E., Degroodt, J. M. 2007. Determination of acrylamide levels in selected foods in Brazil. Food Addit. Contam. 24, 236-241. [CrossRef]
- Van Boekel, M., Fogliano, V., Pellegrini, N., Stanton, C., Scholz, G., Lalljie, S., Eisenbrand, G. 2010. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 54, 1215-1247. [CrossRef]
- Pflaum, T., Hausler, T., Baumung, C., Ackermann, S., Kuballa, T., Rehm, J., Lachenmeier, D. W. 2016. Carcinogenic compounds in alcoholic beverages: an update. Arch. Toxicol, 90, 2349-2367. [CrossRef]
- Dupire, S. 2003. Highlights symposium “mycotoxins and other contaminants in the malting and brewing industries. In Proceedings of the Congress-European Brewery Convention 29th (Vol. 29, pp. 129-131). Fachverlag Hans Carl.
- EC. 2013. Commission Recommendation (EU) No 2013/647. OJEU. L301, 15-17. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0647.
- Arthur, C., Baker, J. E., Bamford, H. A. 2009. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, (pp. 7-179). NOAA Marine Debris Program. https://repository.library.noaa.gov/view/noaa/2509.
- ECHA. 2020. ECHA/RAC/RES-O−0000006790−71−01/F. European Chemical Agency. https://echa.europa.eu/documents/10162/2842450/rest_microplastics_opinion_rac_16339_en.pdf/b4d383cd-24fc-82e9-cccf-6d9f66ee9089s_opinion_rac_16339_en.
- Gigault, J., Ter Halle, A., Baudrimont, M., Pascal, P. Y., Gauffre, F., Phi, T. L., & Reynaud, S. (2018). Current opinion: what is a nanoplastic?. Environ. Pollut. 235, 1030-1034. [CrossRef]
- Barceló, D. 2020. Microplastics analysis. MethodsX, 7, e100884. [CrossRef]
- Rainieri, S., Barranco, A. 2019. Microplastics, a food safety issue?. Trends Food Sci. Technol. 84, 55-57. [CrossRef]
- Bond, T., Ferrandiz-Mas, V., Felipe-Sotelo, M., Van Sebille, E. 2018. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: a review. Crit. Rev. Environ. Sci. Technol. 48, 685-722. [CrossRef]
- Ivleva, N. P. 2021. Chemical analysis of microplastics and nanoplastics: challenges, advanced methods, and perspectives. Chem. Rev. 121, 11886-11936. [CrossRef]
- Picó, Y., Barceló, D. (2019). Analysis and prevention of microplastics pollution in water: current perspectives and future directions. ACS Omega, 4, 6709-6719. [CrossRef]
- Prata, J. C., da Costa, J. P., Duarte, A. C., Rocha-Santos, T. 2019. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal. Chem. 110, 150-159. [CrossRef]
- Rocha-Santos, T., Duarte, A. C. 2015. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 65, 47-53. [CrossRef]
- Blair, R. M., Waldron, S., Phoenix, V. R., Gauchotte-Lindsay, C. 2019. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 26, 12491-12504. [CrossRef]
- Peñalver, R., Arroyo-Manzanares, N., López-García, I., Hernández-Córdoba, M. 2020. An overview of microplastics characterization by thermal analysis. Chemosphere, 242, 125170. [CrossRef]
- Liebezeit, G., Liebezeit, E. 2014. Synthetic particles as contaminants in German beers. Food Addit. Contam.: Part A, 31, 1574-1578. [CrossRef]
- Li, Y., Peng, L., Fu, J., Dai, X., Wang, G. 2022. A microscopic survey on microplastics in beverages: the case of beer, mineral water and tea. Analyst, 147, 1099-1105. [CrossRef]
- Kortenkamp, A., Martin, O., Faust, M., Evans, R., McKinlay, R., Orton, F., Rosivatz, E. 2011. State of the art assessment of endocrine disrupters. Final Rep. (pp 1-135). European Commission. https://op.europa.eu/en/publication-detail/-/publication/cf507642-3862-11ec-8daf-01aa75ed71a1/language-en.
- Wams, T. J. 1987. Diethylhexylphthalate as an environmental contaminant-a review. Sci. Total Environ. 66, 1-16. [CrossRef]
- Huang, J., Nkrumah, P.N., Li, Y., Appiah-Sefah, G. 2013. Chemical Behavior of Phthalates Under Abiotic Conditions in Landfills. In: D. Whitacre (eds). Reviews of Environmental Contamination and Toxicology Volume 224. Springer, New York, NY. [CrossRef]
- Talsness, C. E., Andrade, A. J., Kuriyama, S. N., Taylor, J. A., Vom Saal, F. S. 2009. Components of plastic: experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. B: Biol. Sci. 364, 2079-2096. [CrossRef]
- Mariana, M., Feiteiro, J., Verde, I., Cairrao, E. 2016. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 94, 758-776. [CrossRef]
- Meeker, J. D., Calafat, A. M., Hauser, R. 2012. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J. Expo. Sci. Environ. Epidemiol. 22, 376-385. [CrossRef]
- Frederiksen, H., Skakkebaek, N. E., Andersson, A. M. 2007. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 51, 899-911. [CrossRef]
- Zoeller, R. T., Brown, T. R., Doan, L. L., Gore, A. C., Skakkebaek, N. E., Soto, A. M., Vom Saal, F. S. 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology, 153, 4097-4110. [CrossRef]
- Muscogiuri, G., Colao, A. 2017. Phtalates: new cardiovascular health disruptors?. Arch. Toxicol. 91, 1513-1517. [CrossRef]
- EC. 2006. Regulation (EC) No. 1907/2006. OJEU. L 396, 1-520. https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32006R1907.
- Leebowitz, J. N., Sarmiento, R., Dugar, S. M., Ethridge, M. W. (1995). Determination of six common phthalate plasticizers in grain neutral spirits and vodka. J. AOAC Int., 78(3), 730-735.
- Del Carlo, M., Pepe, A., Sacchetti, G., Compagnone, D., Mastrocola, D., Cichelli, A. 2008. Determination of phthalate esters in wine using solid-phase extraction and gas chromatography-mass spectrometry. Food Chem. 111, 771-777. [CrossRef]
- Gao, J., Yang, C., Ye, C., Li, X. 2009. Determination of trace phthalates in beer by gas chromatography coupled with solid-phase microextraction using a calix [6] arene fiber. Chin. J. Chrom. 27, 356-358.
- Russo, M. V., Notardonato, I., Avino, P., Cinelli, G. 2014. Determination of phthalate esters at trace levels in light alcoholic drinks and soft drinks by XAD-2 adsorbent and gas chromatography coupled with ion trap-mass spectrometry detection. Anal. Met. 6, 7030-7037. [CrossRef]
- Gemenetzis, E. G., Alygizakis, N. A. 2023. Development and Validation of an HPLC-UV Method for the Determination Bis (2-ethylhexyl) Phthalate Ester in Alcoholic Beverages. Appl. Sci. 13, 3194. [CrossRef]
- Horák, T., Olšovská, J. 2020. Phthalates in beverages-A review. Kvasny Prum. 66, 264-269. [CrossRef]
- Sendón, R., Sanches-Silva, A., Bustos, J., Martín, P., Martínez, N., Cirugeda, M. E. 2012. Detection of migration of phthalates from agglomerated cork stoppers using HPLC-MS/MS. J. Sep. Sci. 35, 1319-1326. [CrossRef]
- Fasano, E., Bono-Blay, F., Cirillo, T., Montuori, P., Lacorte, S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Cont. 27, 132-138. [CrossRef]
- Carnol, L., Schummer, C., Moris, G. 2017. Quantification of six phthalates and one adipate in Luxembourgish beer using HS-SPME-GC/MS. Food Anal. Met. 10, 298-309. [CrossRef]
- Ye, C. W., Gao, J., Yang, C., Liu, X. J., Li, X. J., Pan, S. Y. 2009. Development and application of an SPME/GC method for the determination of trace phthalates in beer using a calix [6] arene fiber. Anal. Chim. Acta, 641, 64-74. [CrossRef]
- Habschied, K., Kartalović, B., Lazić, D., Krstanović, V., Mastanjević, K. 2023. Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles. Fermentation, 9, 125. [CrossRef]
- Pereira, C., Cunha, S. C., Fernandes, J. O. 2023. Commercial beers: a source of phthalates and di-ethylhexyl adipate. Food Chem. X. 19, e100768. [CrossRef]
- Priovolos, I., Samanidou, V. 2023. Βisphenol a and its analogues migrated from contact materials into food and beverages: An updated review in sample preparation approaches. J. Sep. Sci. e2300081. [CrossRef]
- EC. 2010. Joint Research Centre. Institute for Health Consumer Protection (2010). Updated European Union risk assessment report: 4,4’-isopropylidenediphenol (bisphenol-A) environment addendum of February 2008. Publications Office. (p. 6). [CrossRef]
- Cadogan, D. F., Howick, C. J. 2000. Plasticizers. Ullmann’s Encyclopedia of Industrial Chemistry. [CrossRef]
- Geens, T., Aerts, D., Berthot, C., Bourguignon, J. P., Goeyens, L., Lecomte, P., Covaci, A. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 50, 3725-3740. [CrossRef]
- Chen, D., Kannan, K., Tan, H., Zheng, Z., Feng, Y. L., Wu, Y., Widelka, M. 2016. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity- a review. Environ. Sci. Technol. 50, 5438-5453. [CrossRef]
- Taskeen, A., Naeem, I. 2010. Analysis of bisphenol A in canned food: A mini review. As. J. Chem. 22, 4133-4135.
- HCD. 2010. Survey of Bisphenol A in Soft Drink and Beer Products from Canadian Markets. Bureau of Chemical Safety Food Directorate Health Products and Food Branch. Health Canada Department. http://www.hc-sc.gc.ca/fn-an/securit/packag-emball/bpa/bpa_survey-summ-enquete-soft-drink-boisson-gazeuse-eng.php.
- Erickson, M. D., Kaley, R. G. 2011. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 18, 135-151. [CrossRef]
- UN. 2023. The 12 initial POPs under the Stockholm Convention. United Nations Environment Program. https://chm.pops.int/TheConvention/ThePOPs/%20The12InitialPOPs/tabid/296/%20Default.aspx.
- Djordjevic, A. B., Antonijevic, E., Curcic, M., Milovanovic, V., Antonijevic, B. 2020. Endocrine-disrupting mechanisms of polychlorinated biphenyls. Curr. Opin. Toxicol. 19, 42-49. [CrossRef]
- Zabelina, O. N., Saloutin, V. I., Chupakhin, O. N. 2010. Analysis of polychlorinated biphenyl mixtures by gas chromatography. J. Anal. Chem. 65, 1098-1108. [CrossRef]
- Reddy, A. V. B., Moniruzzaman, M., Madhavi, G., Aminabhavi, T. M. 2020. Modern approaches in separation, identification and quantification of polychlorinated biphenyls. Curr. Opin. Environ. Sci. Health. 18, 26-39. [CrossRef]
- Thabit, T. M., El-Hefny, D. E., Elgeddawy, D. I., El-Naggar, M. A., Serageldin, F. M. 2022. Monitoring of Some Chemical Contaminants Residue in Imported Wheat and Barley Grains Using QuEChERS Method and GC-MS/MS. J. AOAC Int. 105, 115-128. [CrossRef]
- Witczak, A., Abdel-Gawad, H. 2012. Comparison of organochlorine pesticides and polychlorinated biphenyls residues in vegetables, grain and soil from organic and conventional farming in Poland. J. Environ.l Sci. Health, Part B, 47, 343-354. [CrossRef]
- Liu, S., Zhao, T., Xing, Z., Yang, X., Wang, E. 2018. Advances in biotic and abiotic mutual promoting mechanism for chlorinated aliphatic hydrocarbons degradation. Chin. J. Biotechnol. 34, 510-524. [CrossRef]
- Sahoo, B. M., Ravi Kumar, B. V., Banik, B. K., Borah, P. 2020. Polyaromatic hydrocarbons (PAHs): structures, synthesis and their biological profile. Curr. Org. Synth. 17, 625-640. [CrossRef]
- Patel, A. B., Shaikh, S., Jain, K. R., Desai, C., Madamwar, D. 2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front. Microbiol. 11, e562813. [CrossRef]
- Mojiri, A., Zhou, J. L., Ohashi, A., Ozaki, N., Kindaichi, T. 2019. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 696, e133971. [CrossRef]
- Bolden, A. L., Rochester, J. R., Schultz, K., Kwiatkowski, C. F. 2017. Polycyclic aromatic hydrocarbons and female reproductive health: a scoping review. Reprod Toxicol. 73, 61-74. [CrossRef]
- Horák, T., Čulík, J., Jurková, M., Kellner, V. 1999. The determination of aliphatic chlorocarbons in beer. Kvasný Průmysl 45(12): 317–320. [CrossRef]
- Hernandes, K. C., Souza-Silva, É. A., Assumpção, C. F., Zini, C. A., Welke, J. E. 2019. Validation of an analytical method using HS-SPME-GC/MS-SIM to assess the exposure risk to carbonyl compounds and furan derivatives through beer consumption. Food Addit. Contam: Part A. 36, 1808-1821. [CrossRef]
- Hernandes, K. C., Souza-Silva, É. A., Assumpção, C. F., Zini, C. A., Welke, J. E. 2020).Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam: Part A. 37, 61-68. [CrossRef]
- Sun, J., He, Y., Ning, Y., Xue, Z., Wang, H., Zhang, Y., Ma, J., Chen, X., Chai, F. 2023. Pollution characteristics and sources of carbonyl compounds in a typical city of Fenwei Plain, Linfen, in summer. Environ. Pollut. 320, e120913.
- Knutsen, H. K., Alexander, J., Barregård, L., Bignami, M., Brüschweiler, B. et al. 2017. Risks for public health related to the presence of furan and methylfurans in food. EFSA J. 15, e05005. [CrossRef]
- WHO. 2019. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. World Health Organisation. Geneva, Switzerland. https://monographs.iarc.fr/monographs-and-supplements-available-online.
- Vanderhaegen B, Neven H, Verstrepen KJ, Delvaux FR, Verachtert H, Derdelinckx G. 2004. Influence of the brewing process on furfuryl ethyl ether formation during beer aging. J. Agric. Food Chem. 52, 6755-6764. [CrossRef]
- Webersinke, F., Klein, H., Flieher M., Urban, A., Jäger, H., Forster, C. H. 2018. Control of fermentation by-products and aroma features of beer produced with scottish ale yeast by variation of fermentation temperature and wort aeration rate. J. Am. Soc. Brew. Chem. 76, 147-155. [CrossRef]
- Burcham, P.C. 2017. Acrolein and human disease: untangling the knotty exposure scenarios accompanying several diverse disorders. Chem. Res. Toxicol. 30, 145-161. [CrossRef]
- Sheridan, M.K., Elias RJ. 2016. Reaction of acetaldehyde with wine flavonoids in the presence of sulfur dioxide. J. Agric. Food Chem. 64, 8615-8624. [CrossRef]
- EFSA. 2011. Update on furan levels in food from monitoring years 2004-2010 and exposure assessment. EFSA J., 9, 2347-2380. [CrossRef]
- Nie, S., Huang, J., Hu, J., Zhang, Y., Wang, S., Li, C., Xie, M. 2013. Effect of pH, temperature and heating time on the formation of furan in sugar–glycine model systems. Food Sci. Hum. Wellness, 2, 87-92. [CrossRef]
- Eumann, M., Schildbach, S. 2012. 125th Anniversary Review: Water sources and treatment in brewing. J. Inst. Brew., 118, 12-21S. 2012. 125th Anniversary Review: Water sources and treatment in brewing. [CrossRef]
- Montesinos, I., Gallego, M. 2014. How the inclusion of treated water in beverages influences the appearance of halogenated volatile organic compounds. J. Agric. Food Chem. 62, 10240-10247. [CrossRef]
- Caon, A., Conte, G., Skoronski, E. 2022. Use of tannin-based coagulant and chlorine dioxide in treating brewing water: reduction of trihalomethanes and impact on physicochemical and sensory quality. J. Environ. Sci. Health, Part A, 57, 858-868. [CrossRef]
- Sfynia, C., Bond, T., Kanda, R., Templeton, M. R. (2022). Simultaneous prediction of trihalomethanes, haloacetic acids, haloacetonitriles and haloacetamides using simulated distribution system tests. Environ. Sci. Water Res. Technol. 8, 742-756. [CrossRef]
- Sinha, R., Gupta, A. K., Ghosal, P. S. 2021. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies. J. Environ. Chem. Eng. 9, 106511. [CrossRef]
- Valdivia-García, M., Weir, P., Graham, D. W., Werner, D. 2019. Predicted impact of climate change on trihalomethanes formation in drinking water treatment. Sci. Rep. 9, 9967. [CrossRef]
- adiq, R., Rodriguez, M. J. 2004. Disinfection by-products (DBPs) in drinking water and predictive models for their occurrence: a review. Sci. Total Environ. 321, 21-46. [CrossRef]
- Padhi, R. K., Subramanian, S., Mohanty, A. K., Satpathy, K. K. 2019. Comparative assessment of chlorine reactivity and trihalomethanes formation potential of three different water sources. J. Water Proc. Eng. 29, e100769. [CrossRef]
- Richardson, S. D., Plewa, M. J., Wagner, E. D., Schoeny, R., DeMarini, D. M. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. - Rev. Mutat. Res., 636, 178-242. [CrossRef]
- US EPA. 1998. National primary drinking water regulations: Disinfectants and disinfection byproducts; final rule. Federal Register, 63, 69390-69476. https://www.govinfo.gov/content/pkg/FR-1998-12-16/pdf/98-32887.pdf.
- de Castro Medeiros, L., de Alencar, F. L. S., Navoni, J. A., de Araujo, A. L. C., do Amaral, V. S. 2019. Toxicological aspects of trihalomethanes: a systematic review. Environ. Sci. Pollut. Res. 26, 5316-5332. [CrossRef]
- EC. 2020. The European parliament and the council of the European union. Directive (EU) 2020/2184 of the European parliament and of the council of 16 December 2020 on the quality of water intended for human consumption. OJEU. L 435, 1–62. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184.
- Pérez-Pavón, J.L., Herrero-Marín, S., García-Pinto, C., Moreno-Cordero, B. 2008. Determination of trihalomethanes in water samples: A review. Anal. Chim. Acta, 629, 6-23. [CrossRef]
- Thurnau, R. C., Clark, R. M. 2017. Determination of volatilisation rate constants of trihalomethanes from heated distilled and finished tap water. Water Environ. J. 31, 252-261. [CrossRef]
- Pérez-Lucas, G., Martínez-Menchón, M., Vela, N.,Navarro, S. 2022. Removal assessment of disinfection by-products (DBPs) from drinking water supplies by solar heterogeneous photocatalysis: A case study of trihalomethanes (THMs). J. Environ. Manag, 321, 115936. [CrossRef]
- Gati, L., Gordon, A., Asiedu, G. 2017. Investigation into trihalomethanes in lager beers brewed in Ghana. Chem. Mat. Res. 9, 54-59.
- Wu, Q.J.;,Lin, H., Fan, W., Dong, J.J. Chen, H.L. 2006. Investigation into benzene, trihalomethanes and formaldehyde in Chinese lager beers. J. Inst. Brew. 112, 291-294. [CrossRef]

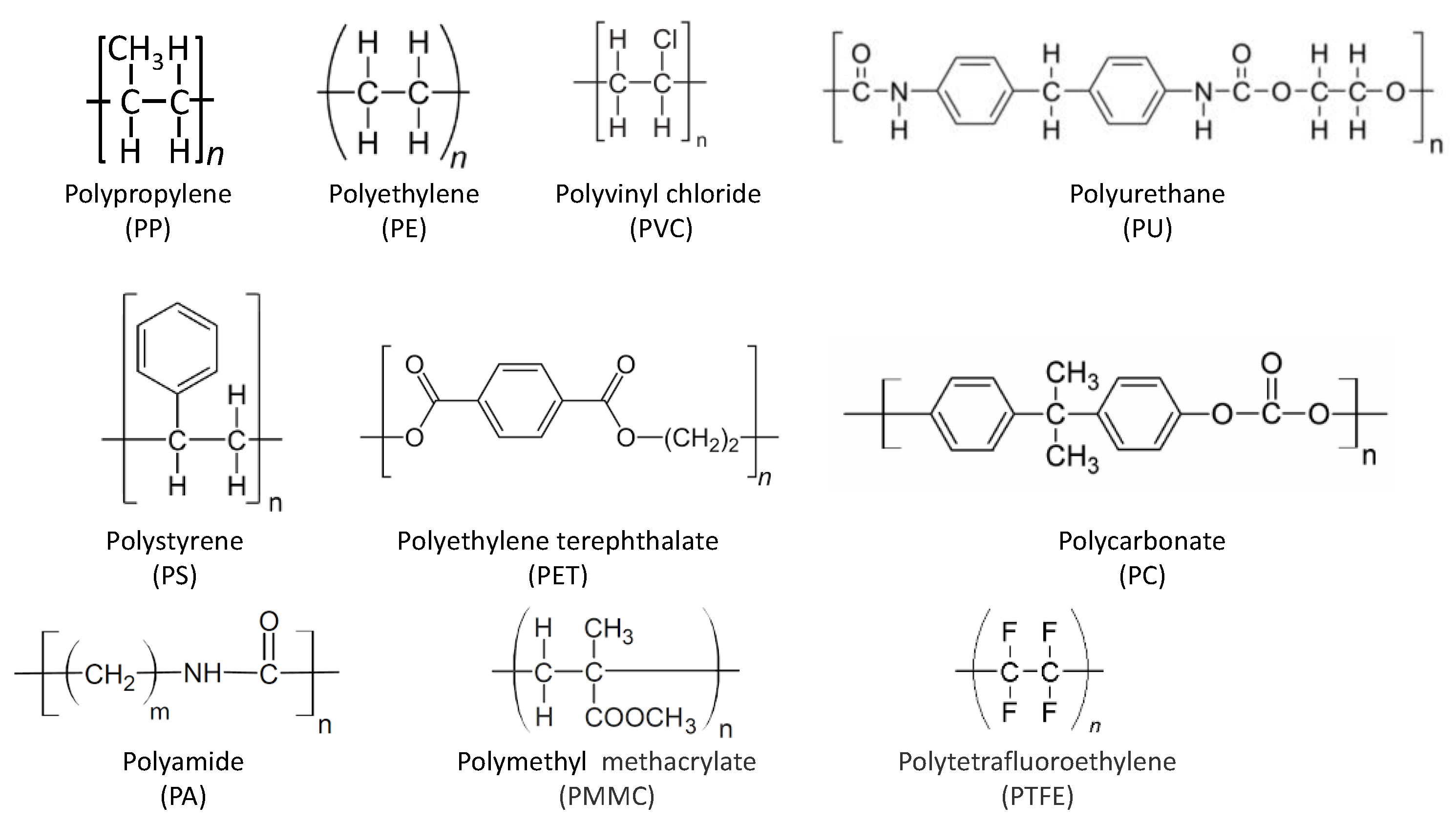
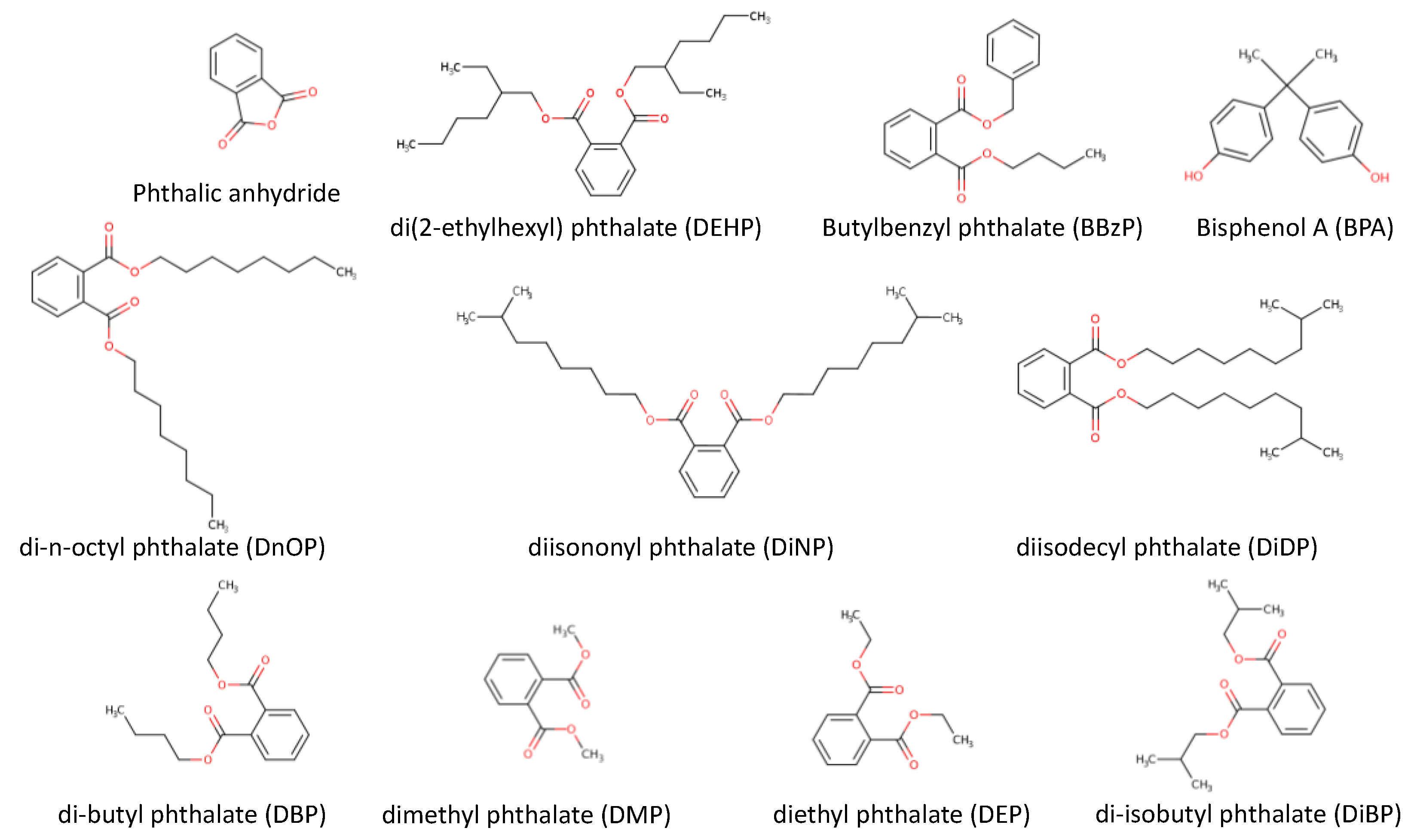
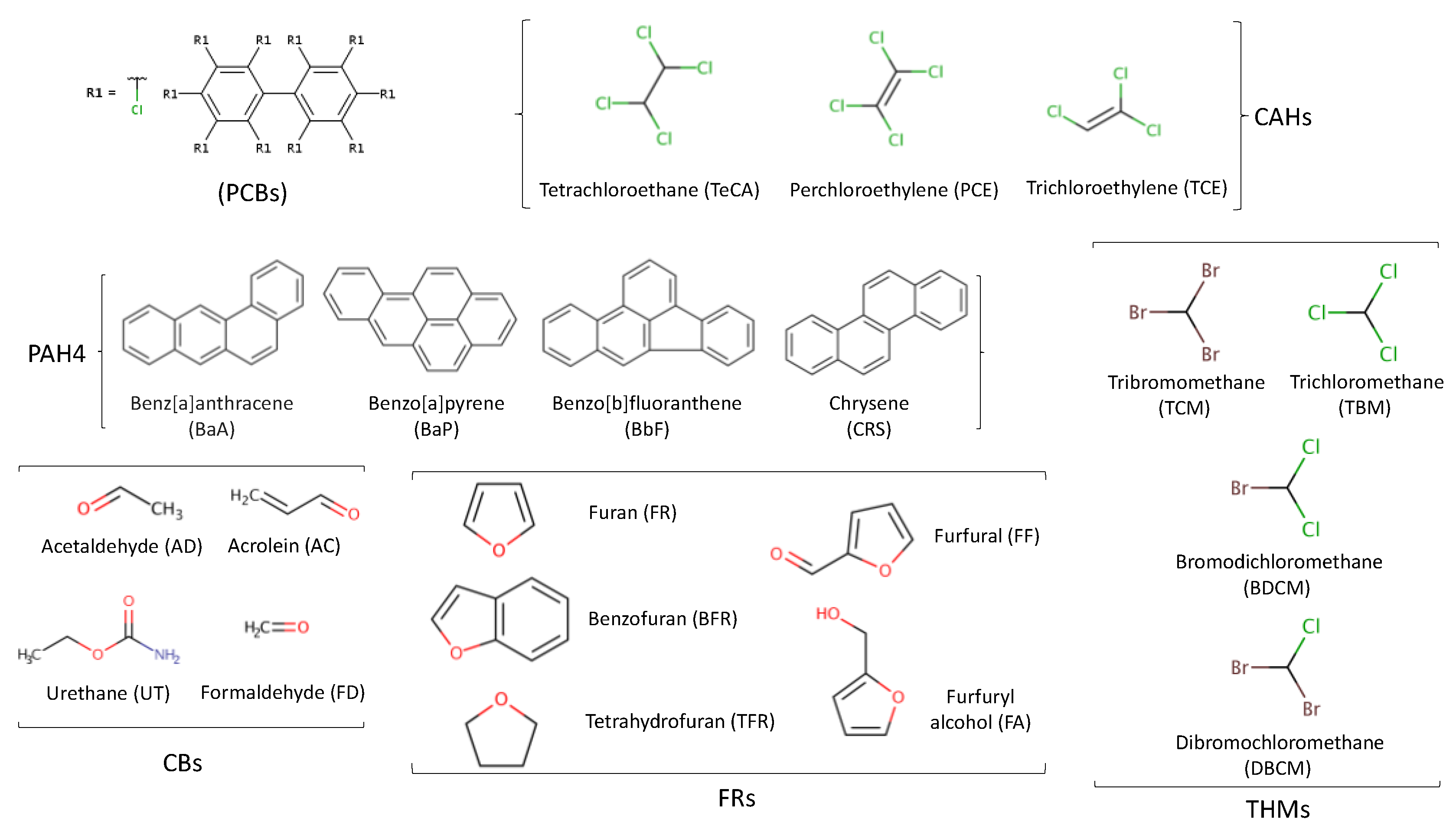
| Pollutant | Pollution source | Toxicological remarks* |
|---|---|---|
| Acrylamide (α, β-unsaturated (conjugated) reactive molecule (C3H5NO)) |
Thermal processing | A range of adverse health effects, including mutagenicity, genotoxicity, carcinogenicity, neurotoxicity and reproductive toxicity. |
| Aliphatic chlorinated hydrocarbons (Chlorinated derivatives of non-cyclic hydrocarbons) |
Ground- or surface water. Technological treatment of drinking water in breweries | They are dangerous because of their persistence, toxicity and ability to accumulate in biological systems. They are stored in fat tissue in the human body and cause carcinogenic diseases with prolonged exposure. |
| Biogenic amines (Organic nitrogen compounds formed by decarboxylation of free amino acids) |
Microbial contamination in the brewery. Decarboxylation of free amino acids | They have a toxic effect on the human body above-limit concentrations. |
| Bisphenols (Group of chemical compounds related to diphenylmethane based on two hydroxyphenyl functional groups linked by a methylene bridge, with the exception of bisphenol S, P and M). |
Migration from plastic contact materials to raw material and beer | Bisphenol A is a xenoestrogen, which has hormone-like properties that mimic the effects of oestrogen in the body. |
| Carbonyls (Carbonyl compounds (carbonyls), mainly including aldehydes and ketones, are a crucial class of oxygen-containing volatile organic compounds (VOCs) in the troposphere) |
Atmospheric carbonyls come from both primary emissions (vehicle exhaust, solvent volatilization, industry and plants) and secondary production (carbonyls generated by the photooxidation of VOCs) from anthropogenic and natural sources | Some carbonyls harm human health due to their potential mutagenic and carcinogenic properties. |
| Furans and derivatives (Furan is a 5-membered heterocyclic, oxygen-containing, unsaturated ring compound. Compounds containing the furan ring (as well as the tetrahydrofuran ring) are usually referred to as furans). |
They are low molecular weight compounds with high volatility found in heat-treated commercial foods and produced through thermal degradation of natural food precursors such as ascorbic acid, amino acids, carbohydrates, unsaturated fatty acids and carotenoids. | Several of these compounds cause necrosis of target cells within certain organs, including the liver, the kidneys, and the lungs. |
| Heavy metals (Metal of relatively high density, or of high relative atomic weight) |
Barley, hop and water. Brewing equipment (pipes, tanks, containers, filtration equipment) or containers for transporting or storing the final product | As, Pb, Cd, Cr, Hg and others. Possibly carcinogenic and accumulates in the human body. Cancer of the skin, lungs, liver, prostate, bones and bladder. Kidney and liver dysfunction, high blood pressure, liver damage and bone fragility. Neurotoxicity, respiratory and digestive effects, neurodegenerative diseases such as Alzheimer’s disease. |
| Microplastics (Extremely small pieces (< 5 mm) of plastic debris) |
Disposal and breakdown of consumer products and industrial waste | The nature of the human health effects and the ultimate damage cannot be predicted at this time. |
| Mycotoxins (Toxins of natural origin produced as secondary metabolites by microscopic filamentous fungi) |
Cultivation of cereals in the field, as well as during storage | Chemically and thermally very stable compounds. The adverse health effects of mycotoxins range from acute poisoning to long term effects such as weakening of the immune system and cancer. |
| Nitrosamines and ATNC (sum of all N-nitroso compounds) (Substances belonging to a group of N-nitroso compounds, i.e., substances which have a covalently bonded nitroso group (NO) to a nitrogen atom in their molecule) |
Bacterial contamination. Product of reaction of amines naturally found in barley with nitrogen oxides from drying air, or they can also be transformed from pesticides | N-nitrosamines are highly toxic, including carcinogenic, mutagenic, embryopathic and teratogenic effects. |
| Pesticides (Crop protection products for a wide range of diseases, pests and weeds, as well as plant growth regulators, including insecticides, fungicides and others.) |
Barley, hops, water and soil | They can cause many acute and chronic diseases, such as endocrine disruption, infertility and abnormal foetal development. Pesticides can also affect the development of the nervous system, leading to problems with coordination, behavioural problems or delayed physical development. Some pesticides have a negative effect on the immune system and cause allergies. Others are proven carcinogens and teratogens. |
| Phthalates (Esters of phthalic acids, plasticizers) |
Raw materials, but they can also be released from plastic materials, that are in a direct contact with beer or intermediates | They have been shown to be carcinogenic, affect the endocrine system and can cause premature birth or asthma. |
| Polychlorinated biphenyls (PCBs) (A mixture of biphenyl molecules substituted with chlorine atoms) |
Soil, air and water. Used widely in electrical equipment like capacitors and transformers | High chemical, thermal and biological stability PCBs are very harmful substances that cause liver damage, affect endocrine function and cognitive function, are carcinogenic and immunotoxic, and cause reproductive and developmental problems. |
| Polycyclic aromatic hydrocarbons PAHs) (Compounds composed of two or more condensed benzene rings in different configurations with different substituents) |
Soil, air and water. Volcanic eruptions and forest fires | Smoking, grilling and roasting increase the levels of PAHs in drinks. PAHs have been shown to have mutagenic and carcinogenic effects, but these effects and their severity depend on the chemical structure. |
| Trihalomethanes (Compounds with single-carbon substituted halogens (CHX3), where X can be fluorine, chlorine, bromine or iodine or a group of these) |
Source of water (municipal-, surface- or groundwater used in breweries) and system used for water sterilisation | Exposure to higher amounts of trihalomethanes may cause reproductive problems and birth defects with DNA damage. |
| Year | MTs | Samples (No) | + Samples (No) | + Samples (%) | Levels* | Below limit** |
|---|---|---|---|---|---|---|
| 2014 | AFs | 35 | 0 | 0 | - | |
| DON | 43 | 28 | 65 | 2.33-22.5 | √ | |
| ZEA | 27 | 0 | 0 | - | ||
| T-2 and HT-2 | 24 | 8 | 33 | 0.17-0.71 | √ | |
| OTA | 47 | 33 | 70 | 1.4-141 | √ | |
| 2015 | AFs | 37 | 0 | 0 | - | |
| DON | 47 | 14 | 30 | 2.01-29.3 | √ | |
| ZEA | 35 | 0 | 0 | - | ||
| T-2 and HT-2 | 35 | 18 | 51 | 0.04-0.9 | √ | |
| OTA | 50 | 35 | 70 | 1.8-28.8 | √ | |
| 2016 | AFs | 38 | 0 | 0 | - | |
| DON | 73 | 24 | 33 | 2.12-10.7 | √ | |
| ZEA | 25 | 1 | 4 | 0.41 | ||
| T-2 and HT-2 | 25 | 4 | 16 | 0.04-0.82 | √ | |
| OTA | 78 | 62 | 80 | 1.0-134 | √ | |
| 2017 | AFs | 35 | 0 | 0 | - | |
| DON | 50 | 29 | 58 | 2.09-13.9 | √ | |
| ZEA | 3 | 0 | 0 | - | ||
| T-2 and HT-2 | 29 | 7 | 24 | 0.3-0.85- | √ | |
| OTA | 4955 | 45 | 92 | 1.3-77.3 | √ | |
| 2018 | AFs | 57 | 0 | 0 | - | |
| DON | 40 | 44 | 77 | 2.03-17.0 | √ | |
| ZEA | 43 | 0 | 0 | - | ||
| T-2 and HT-2 | 67 | 38 | 88 | 0.05-1.8 | √ | |
| OTA | 51 | 76 | 1.4-56.1 |
| Pesticides | Log KOW | Stage | References | ||
| Steeping | Germination | Kilning | |||
| Cyproconazole | 3.1 | 47 | 38 | 31 | [114] |
| Diniconazole | 4.3 | 70 | 61 | 39 | [114] |
| Epoxiconazole | 3.4 | 62 | 53 | 38 | [114] |
| Ethiofencarb | 2.0 | 3 | 1 | 5 | [112] |
| Fenitrothion | 3.4 | 52 | 31 | 13 | [113] |
| Flutriafol | 2.3 | 43 | 35 | 30 | [113] |
| Malathion | 2.8 | 45 | 20 | 14 | [113] |
| Mepronil | 3.8 | 24 | 6 | 30 | [112] |
| Myclobutanil | 2.9 | 59 | 42 | 36 | [113] |
| Nuarimol | 3.2 | 64 | 57 | 51 | [113] |
| Pendimethalin | 5.2 | 85 | 67 | 49 | [113] |
| Phentoate | 3.7 | 27 | 4 | 18 | [112] |
| Propiconazole | 3.6 | 50 55 |
10 43 |
55 30 |
[112,113] |
| Tebuconazole | 3.7 | 56 | 45 | 37 | [114] |
| Triadimefon | 3.1 | 24 | 5 | 30 | [112] |
| Triadimenol | 3.1 | 36 | 13 | 47 | [112] |
| Triflumizole | 4.4 | 38 | 11 | 9 | [112] |
| Trifluralin | 5.3 | 80 | 65 | 50 | [113] |
| Pesticide | Log KOW | Sweet wort | Spent grains | Brewer wort | Spent hops | References |
| Atrazine | 2.5 | 45 | 55 | 42 | 20 | [115] |
| α-BHC | 4.0 | 8 | 54 | 30 | 15 | [116] |
| Captafol | 3.8 | BDLa | 3 | BDL | BDL | [116] |
| Chlorpyrifos | 4.7 | 17 | 3 | 4 | 32 | [116] |
| Cyproconazole | 3.1 | 10 | 40 | 9 | NDb | [117] |
| Deltamethrin | 4.6 | BDL | 45 | 3 | 37 | [116] |
| Dichlorvos | 1.9 | 8 | BDL | BDL | BDL | [116] |
| Diclofuanid | 3.7 | 10 | 10 | BDL | BDL | [116] |
| Dicofol | 4.3 | BDL | 70 | 18 | 60 | [116] |
| Diniconazole | 4.3 | 4 | 49 | 3 | ND | [117] |
| Epoxiconazole | 3.4 | 8 | 44 | 7 | ND | [117] |
| Fenitrothion | 3.4 | 4 | 30 | 3 | ND | [118] |
| Fenobucarb | 2.8 | 35 | 30 | 64 | 1 | [116] |
| Fenvalerate | 5.0 | BDL | 50 | 3 | 7 | [116] |
| Flucythrinate | 6.2 | BDL | 60 | BDL | 10 | [116] |
| Flutriafol | 2.3 | 13 | 36 | 10 | ND | [114] |
| Glyphosate | -3.2 | 97 | 3 | 95 | 2 | [116] |
| Malathion | 2.7 | 20 7 |
35 40 |
15 4 |
5 ND |
[116,118] |
| Myclobutanil | 2.9 | 9 | 38 | 8 | ND | [117] |
| Nuarimol | 3.2 | 6 | 26 | 6 | ND | [117] |
| Oxamyl | 0.4 | 1 | BDL | 20 | BDL | [116] |
| Parathion-methyl | 3.0 | 1 | BDL | 10 | 3 | [116] |
| Pemdimethalin | 5.2 | 1 | 21 | 1 | ND | [118] |
| Permethrin | 6.1 | BDL | 70 | BDL | 50 | [116] |
| Pirimicarb | 1.7 | 84 | 14 | 50 | 3 | [116] |
| Pirimiphos-methyl | 4.2 | 2 | 68 | 6 | 12 | [116] |
| Propiconazole | 3.6 | 4 | 42 | 4 | ND | [117] |
| Tebuconazole | 3.7 | 8 | 44 | 7 | ND | [114] |
| Terbutylazine | 3.2 | 12 | 80 | 7 | 40 | [115] |
| Triadimenol | 3.1 | 36 | ND | ND | ND | [131] |
| Trifluralin | 5.3 | 1 | 17 | 1 | ND | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





