Submitted:
09 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling and Dendrochronological Data
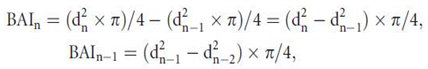
2.3. Sample Preparation
2.4. Statistical Analyses
2.5. Solid Phase Micro Extraction Analyses
3. Results and Discussion
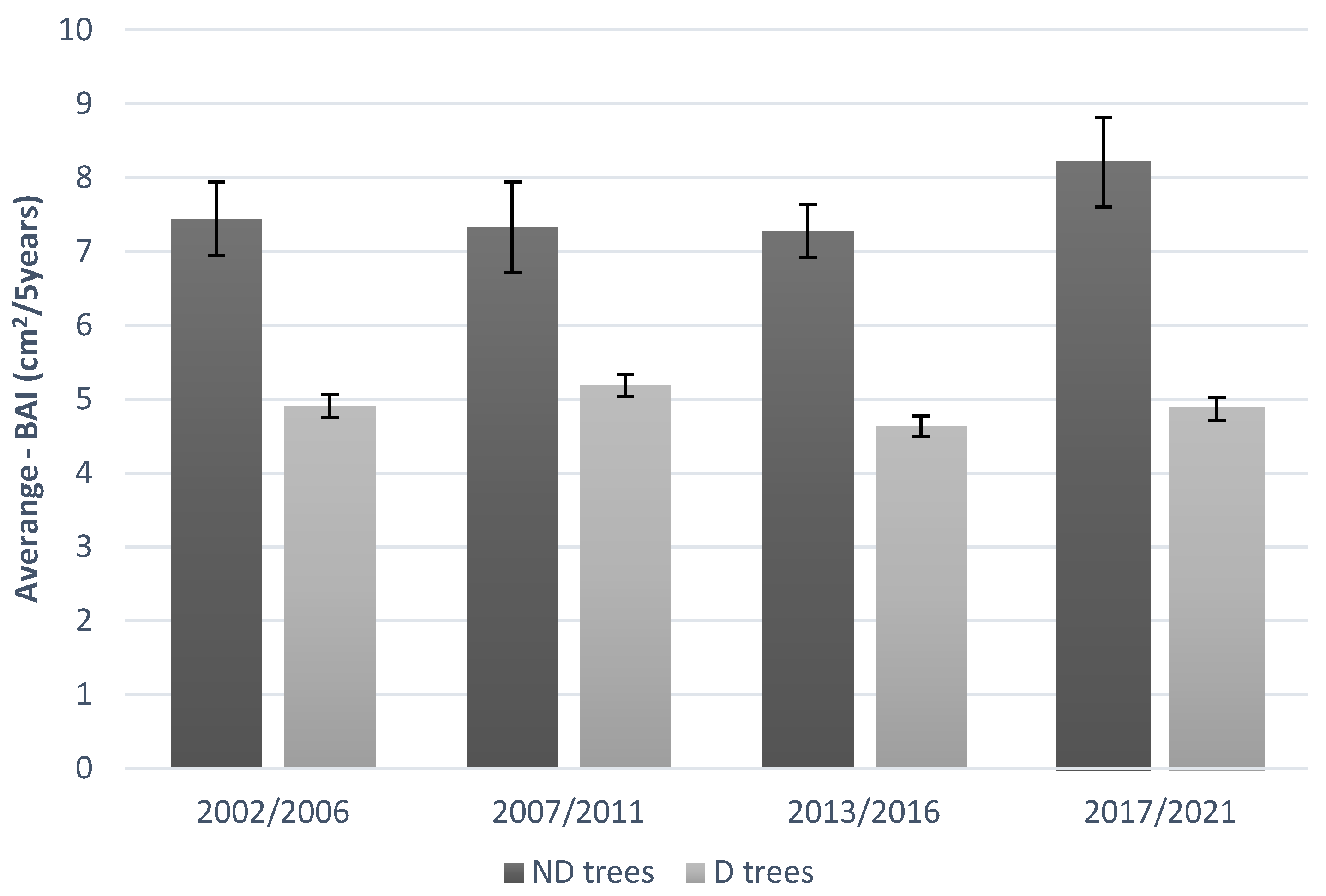
3.1. Dendrochronological Analysis
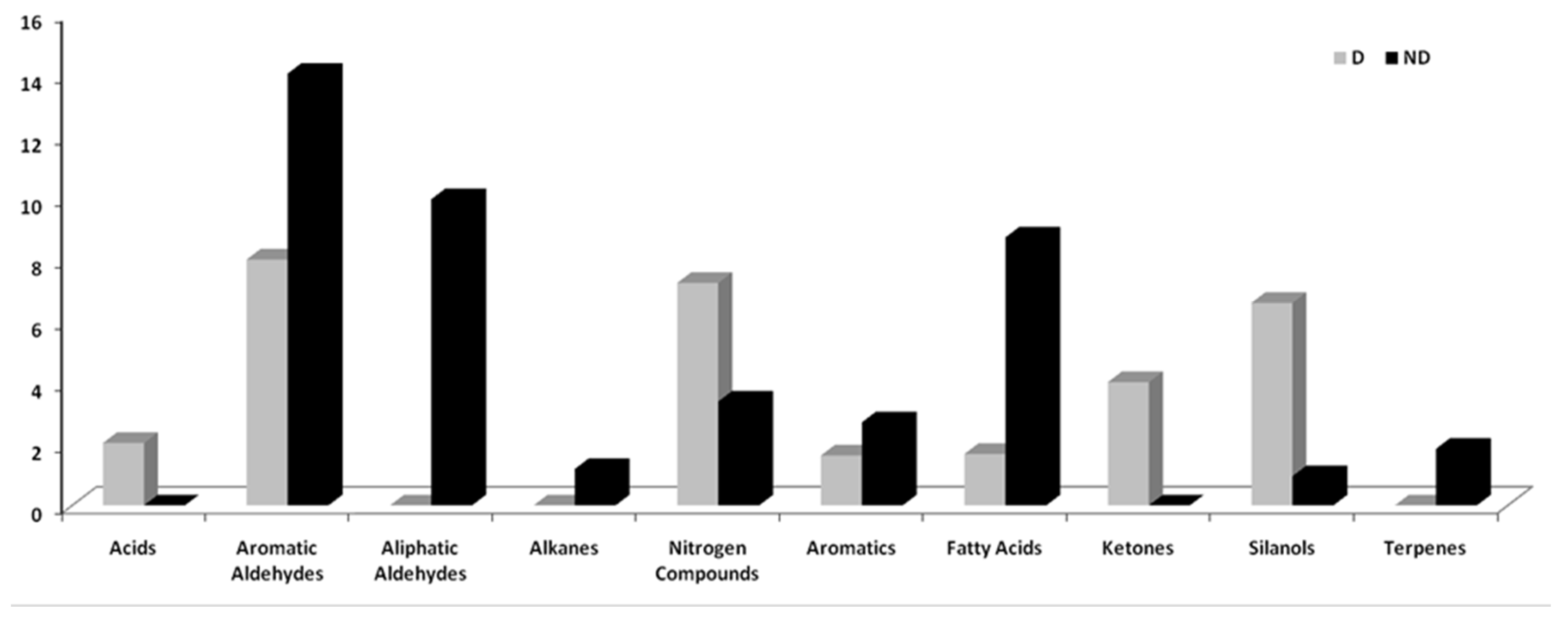
3.2. SPME Analysis
| Class of Compounds | Compound | 1D | 2D | 3D | |||||||||
| Acids | Acetic acid | I | II | III | IV | I | II | III | IV | I | II | III | IV |
| 2.75 | 1.35 | 1.37 | 1.27 | 1.37 | 1.83 | 4.15 | 3.42 | 2.83 | 1.35 | 1.38 | 1.28 | ||
| Aromatic Aldehydes |
Sinapaldehyde | 1.27 | 4.17 | 1.42 | |||||||||
| Coniferaldehyde | |||||||||||||
| Syringaldehyde | 4.15 | 3.15 | 2.05 | 1.90 | 3.95 | 4.00 | 2.60 | 2.43 | 1.90 | 1.82 | 1.72 | 1.63 | |
| Vanillin | 6.88 | 0.92 | 10.95 | 2.95 | 18.90 | 6.53 | 2.68 | 8.08 | |||||
| Aliphatic Aldehydes |
3-Methylbutanal | ||||||||||||
| Decanal | |||||||||||||
| Furfural | |||||||||||||
| Heptanal | |||||||||||||
| Nonanal | |||||||||||||
| Octanal | |||||||||||||
| Alkanes | Eicosane | ||||||||||||
| Nitrogen Compounds |
N-p-Bromophenylselenoacetamide | 1.05 | |||||||||||
| Diethyltoluamide | 6.30 | 4.75 | 4.48 | 20.70 | 20.10 | 6.52 | 11.58 | 11.38 | |||||
| Dimethyl palmitamine | |||||||||||||
| Aromatics | 1,1,3-Trimethyl-3-phenyl-2H-indene | ||||||||||||
| 2,6-Di-tert-butylphenol | |||||||||||||
| Methyl N-hydroxybenzimidate | 1.05 | 0.83 | 2.45 | 1.27 | 1.35 | 2.88 | 4.70 | ||||||
| 2,6-Dimethoxyphenol | 2.05 | 3.25 | |||||||||||
| Xylene | |||||||||||||
| Fatty acids | Pentanoic acid | 0.60 | |||||||||||
| Nonanoic acid | 0.37 | ||||||||||||
| Tetradecanoic acid | |||||||||||||
| Hexadecanoic acid | 2.62 | 2.75 | 2.15 | 2.45 | |||||||||
| Hexanedioic acid | 2.22 | 2.03 | 1.98 | ||||||||||
| Octadecanoic acid | 1.55 | 1.32 | |||||||||||
| Ketones | 4-Oxo-β-dihydroionone | 12.37 | 3.75 | ||||||||||
| 3-Oxo-7,8-dihydro-α-ionone | 1.52 | 1.70 | 5.75 | 6.35 | 12.22 | 2.10 | 2.35 | ||||||
| Silanols | dimethylsilanediol | 8.22 | 9.33 | 7.43 | 7.05 | 3.43 | 3.72 | 7.03 | 5.48 | 5.50 | 7.02 | 7.63 | 7.28 |
| Terpenes | α-Bergamotene | ||||||||||||
| Cyperene | |||||||||||||
| γ-Eudesmol | |||||||||||||
| β-Panasinsene | |||||||||||||
| Pinocarvone | |||||||||||||
| α-Pinene | |||||||||||||
| Class of Compounds | Compound | 1ND | 2ND | 3ND | |||||||||
| I | II | III | IV | I | II | III | IV | I | II | III | IV | ||
| Acids | Acetic acid | ||||||||||||
| Aromatic Aldehydes |
Sinapaldehyde | ||||||||||||
| Coniferaldehyde | 17.13 | 20.38 | 12.40 | 8.35 | |||||||||
| Syringaldehyde | 14.45 | 13.20 | 12.20 | 6.40 | |||||||||
| Vanillin | 0.43 | 11.73 | 18.20 | 16.05 | 13.20 | 2.32 | 2.13 | ||||||
| Aliphatic Aldehydes |
3-Methylbutanal | 0.93 | |||||||||||
| Decanal | 0.58 | 16.45 | 3.27 | 4.77 | 4.27 | 2.13 | 1.77 | 7.92 | 5.57 | ||||
| Furfural | 0.52 | 2.07 | 0.70 | ||||||||||
| Heptanal | 1.02 | 2.05 | 2.65 | 2.45 | 4.40 | 0.97 | 4.25 | 2.43 | 1.88 | ||||
| Nonanal | 1.25 | 7.90 | 9.63 | 4.82 | 7.08 | ||||||||
| Octanal | 0.62 | 8.10 | 3.17 | 3.32 | |||||||||
| Alkanes | Eicosane | 2.67 | 7.20 | 4.28 | |||||||||
| Nitrogen Compounds |
N-p-Bromophenylselenoacetamide | ||||||||||||
| Diethyltoluamide | 5.87 | 8.47 | |||||||||||
| Dimethyl palmitamine | 5.93 | ||||||||||||
| Aromatics | 1,1,3-Trimethyl-3-phenyl-2H-indene | 0.52 | 0.43 | 0.48 | |||||||||
| 2,6-Di-tert-butylphenol | 2.27 | 1.73 | |||||||||||
| Methyl N-hydroxybenzimidate | 1.03 | 1.60 | 0.45 | 2.72 | 1.30 | 0.72 | |||||||
| 2,6-Dimethoxyphenol | 5.93 | 4.70 | 1.35 | ||||||||||
| Xylene | 0.53 | 4.72 | 1.93 | ||||||||||
| Fatty acids | Pentanoic acid | 0.30 | 4.05 | 2.87 | 2.87 | 1.93 | 3.55 | 2.40 | |||||
| Nonanoic acid | 1.88 | 4.90 | |||||||||||
| Tetradecanoic acid | 1.37 | 1.10 | 0.30 | 1.23 | |||||||||
| Hexadecanoic acid | 20.75 | 0.98 | 1.92 | 1.55 | 2.08 | 2.12 | 1.63 | ||||||
| Hexanedioic acid | 1.87 | 2.10 | 1.77 | 5.07 | 3.88 | ||||||||
| Octadecanoic acid | 17.57 | 1.40 | 1.60 | 9.63 | |||||||||
| Ketones | 4-Oxo-β-dihydroionone | ||||||||||||
| 3-Oxo-7,8-dihydro-α-ionone | |||||||||||||
| Silanols | dimethylsilanediol | 0.17 | 2.30 | 0.28 | 2.70 | 3.43 | 2.52 | ||||||
| Terpenes | α-Bergamotene | 0.60 | 1.08 | 0.98 | |||||||||
| Cyperene | 1,18 | 1.37 | 1.13 | ||||||||||
| γ-Eudesmol | 1.27 | 1.70 | |||||||||||
| β-Panasinsene | 1.80 | 0.43 | 1.62 | ||||||||||
| Pinocarvone | 1.02 | 1.40 | |||||||||||
| α-Pinene | 3.62 | 2.87 | |||||||||||
| Wood Type | Acids | Aromatic Aldehydes |
Aliphatic Aldehydes |
Alkanes | Nitrogen Compounds |
Aromatics | Fatty Acids | Ketones | Silanols | Terpenes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| average | D | 2.0 | 4.5 | n.d. | n.d. | 9.7 | 2.2 | 1.8 | 5.3 | 6.6 | n.d. | |
| st. dev | 1.0 | 4.1 | 6.5 | 1.2 | 0.7 | 4.1 | 1.7 | |||||
| average | ND | n.d. | 10.6 | 3.8 | 4.7 | 6.8 | 1.9 | 3.6 | n.d. | 1.9 | 1.5 | |
| st. dev | 5.9 | 3.4 | 1.9 | 1.2 | 1.6 | 4.6 | 1.2 | 0.8 | ||||
| t Test (prob) | 0.008 | n.d. | n.d. | 0.502 | 0.649 | 0.220 | n.d. | 0.000 | n.d. | |||
| significance | ** | n.s. | n.s. | n.s. | *** | |||||||
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M., ... Cobb, N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest ecology and management 2010, 259, 660–684. [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer; A., Barbati, A.; Garcia-Gonzalo, J.; ... Marchetti, M. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest ecology and management 2010, 259, 698–709. [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolã, A.; Ripullone, F.; Nole, A. Drought-induced oak decline in the western Mediterranean region: an overview on current evidences, mechanisms and management options to improve forest resilience. IForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; ... Woods, A.J. Climate change and forest diseases. Plant pathology 2011, 60, 133–149.
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. Forest Pathology 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Rita, A.; Camarero, J. J.; Nolè, A.; Borghetti, M.; Brunetti, M.; Pergola, N.; ... Ripullone, F. Global change biology 2020, 26, 851–863.
- Quentin, A.G.; Beadle, C.L.; O’grady, A.P.; Pinkard, E.A. Effects of partial defoliation on closed canopy Eucalyptus globulus Labilladière: growth, biomass allocation and carbohydrates. Forest Ecology and Management 2011, 261, 695–702. [Google Scholar] [CrossRef]
- Iqbal, N.; Masood, A.; Khan, N.A. Analyzing the significance of defoliation in growth, photosynthetic compensation and source-sink relations. Photosynthetica 2012, 50, 161–170. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; ... Arend, M. Recovery of trees from drought depends on belowground sink control. Nature plants 2016, 2, 1–5.
- Schmid, S.; Palacio, S.; Hoch, G. Growth reduction after defoliation is independent of CO2 supply in deciduous and evergreen young oaks. New Phytologist 2017, 214, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Furze, M.E.; Wainwright, D.K.; Huggett, B.A.; Knipfer, T.; McElrone, A.J.; Brodersen, C.R. Ecologically driven selection of nonstructural carbohydrate storage in oak trees. New Phytologist 2021, 232, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Zeppel, M.J.; Anderegg, W.R.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; ... Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: processes and trade-offs. Ecological research 2018, 33, 839–855. [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: drought-affected Italian oaks are smaller and show lower growth prior to tree death. Frontiers in Plant Science 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Ripullone, F.; Camarero, J.J.; Colangelo, M.; Voltas, J. Variation in the access to deep soil water pools explains tree-to-tree differences in drought-triggered dieback of Mediterranean oaks. Tree Physiology 2020, 40, 591–604. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Battipaglia, G.; Borghetti, M.; De Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree physiology 2017, 37, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cailleret, M.; Dakos, V.; Jansen, S.; Robert, E.M.; Aakala, T.; Amoroso, M.M.; ... Martinez-Vilalta, J. Early-warning signals of individual tree mortality based on annual radial growth. Frontiers in plant science 2019, 9, 1964. [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. European Journal of Forest Research 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant biology 2013, 15, 483–495. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.; ... Martínez-Vilalta, J. Low growth resilience to drought is related to future mortality risk in trees. Nature communications 2020, 11, 545. [CrossRef] [PubMed]
- Mecca, M.; Todaro, L.; Lo Giudice, V.; Lovaglio, T.; D’Auria, M. GC-MS and SPME techniques highlighted contrasting chemical behaviour in the water extractives of modified Castanea sativa mill. and Fagus sylvatica L. wood. Forests 2021, 12, 986. [Google Scholar] [CrossRef]
- Levanič, T.; Čater, M.; McDowell, N.G. Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree physiology 2011, 31, 298–308. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camele, I.N.; Colangelo, M.; Lauteri, M.; Lapolla, A.; Ripullone, F. Oak forest decline in southern Italy: the study case of Gorgoglione forest. In ATTI del Secondo Congresso Internazionale di Selvicoltura 2015 (Vol. 2, pp. 123–129). ACCADEMIA ITALIANA DI SCIENZE FORESTALI.
- Marques, I.G.; Campelo, F.; Rivaes, R.; Albuquerque, A.; Ferreira, M.T.; Rodríguez-González, P.M. Tree rings reveal long-term changes in growth resilience in Southern European riparian forests. Dendrochronologia 2018, 52, 167–176. [Google Scholar] [CrossRef]
- Duval, C.J.; Gourrat, K.; Perre, P.; Prida, A.; Gougeon, R.D. A HS–SPME–GC–MS analysis of IR heated wood: Impact of the water content on the depth profile of oak wood aromas extractability. Food research international 2013, 54, 277–284. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate change effects on secondary compounds of forest trees in the northern hemisphere. Frontiers in plant science 2018, 9, 395200. [Google Scholar] [CrossRef]
- Wink, M. Introduction: biochemistry, physiology and ecological functions of secondary metabolites. Annual plant reviews volume 40: Biochemistry of plant secondary metabolism 2010, 1-19.
- Lindroth, R.L. Atmospheric change, plant secondary metabolites and ecological interactions. The ecology of plant secondary metabolites: from genes to global processes. Cambridge University Press 2012, Cambridge, 120-153.
- Lämke, J.S.; Unsicker, S.B. Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia 2018, 187, 377–388. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. International journal of molecular sciences 2018, 19, 2539. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Current opinion in plant biology 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Role of organic acids in the integration of cellular redox metabolism and mediation of redox signalling in photosynthetic tissues of higher plants. Free Radical Biology and Medicine 2018, 122, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kretovich, V.L. Molecular mechanisms of nitrogen assimilation by plants. Molecular mechanisms of nitrogen assimilation by plants 1980. [Google Scholar]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Frontiers in plant science 2017, 8, 255703. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; ... Yepez, E.A. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New phytologist 2008, 178, 719–739. [CrossRef] [PubMed]
- Bartram, S.; Jux, A.; Gleixner, G.; Boland, W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 2006, 67, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Balakrishnan, R.S.; Shamsudeen, S.; Bahwani, S.A.; Adam, F. A concise review of the natural existance, synthesis, properties, and applications of syringaldehyde. BioResources 2012, 7, 4377–4399. [Google Scholar] [CrossRef]
- Sangeetha, C.; Krishnamoorthy, A.S.; Amirtham, D. Antifungal bioactive compounds from Chinese caterpillar fungus (Ophiocordyceps sinensis (Berk.) GH Sung et al.) against plant pathogens. Madras Agricultural Journal 2015, 102, 1. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest management science 2011, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Janaki, S.; Zandi-Sohani, N.; Ramezani, L.; Szumny, A. Chemical composition and insecticidal efficacy of Cyperus rotundus essential oil against three stored product pests. International Biodeterioration Biodegradation 2018, 133, 93–98. [Google Scholar] [CrossRef]
- Langsi, J.D.; Nukenine, E.N.; Oumarou, K.M.; Moktar, H.; Fokunang, C.N.; Mbata, G.N. Evaluation of the insecticidal activities of α-Pinene and 3-Carene on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Insects 2020, 11, 540. [Google Scholar] [CrossRef]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. Journal of medical entomology 2014, 46, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, R.; Triadiati, T.; Falah, S. Induction of agarwood in Aquilaria malaccensis using nitrogen fertilizer and Fusarium solani. Jurnal Penelitian Kehutanan Wallacea 2018, 7, 165–171. [Google Scholar] [CrossRef]
- Martinez, R.G.; De La Serrana, H.L.G.; Mir, M.V.; Granados, J.Q.; Martinez, M.L. Influence of wood heat treatment, temperature and maceration time on vanillin, syringaldehyde, and gallic acid contents in oak wood and wine spirit mixtures. American Journal of Enology and Viticulture 1996, 47, 441–446. [Google Scholar] [CrossRef]
- Abbas, F.; O'Neill Rothenberg, D.; Zhou, Y.; Ke, Y.; Wang, H.C. Volatile organic compounds as mediators of plant communication and adaptation to climate change. Physiologia Plantarum 2022, 174, e13840. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; D'Alessandro, M.; Jourdie, V.; Jakab, G.; Karlen, D.; Held, M.; ... Turlings, T.C. Priming by airborne signals boosts direct and indirect resistance in maize. The Plant Journal 2007, 49, 16–26. [CrossRef]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior height, growth, and wood anatomy differently predispose to drought-induced dieback in two Mediterranean oak speciesk. Annals of Forest Science 2016, 73, 341–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





