Submitted:
15 May 2024
Posted:
15 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
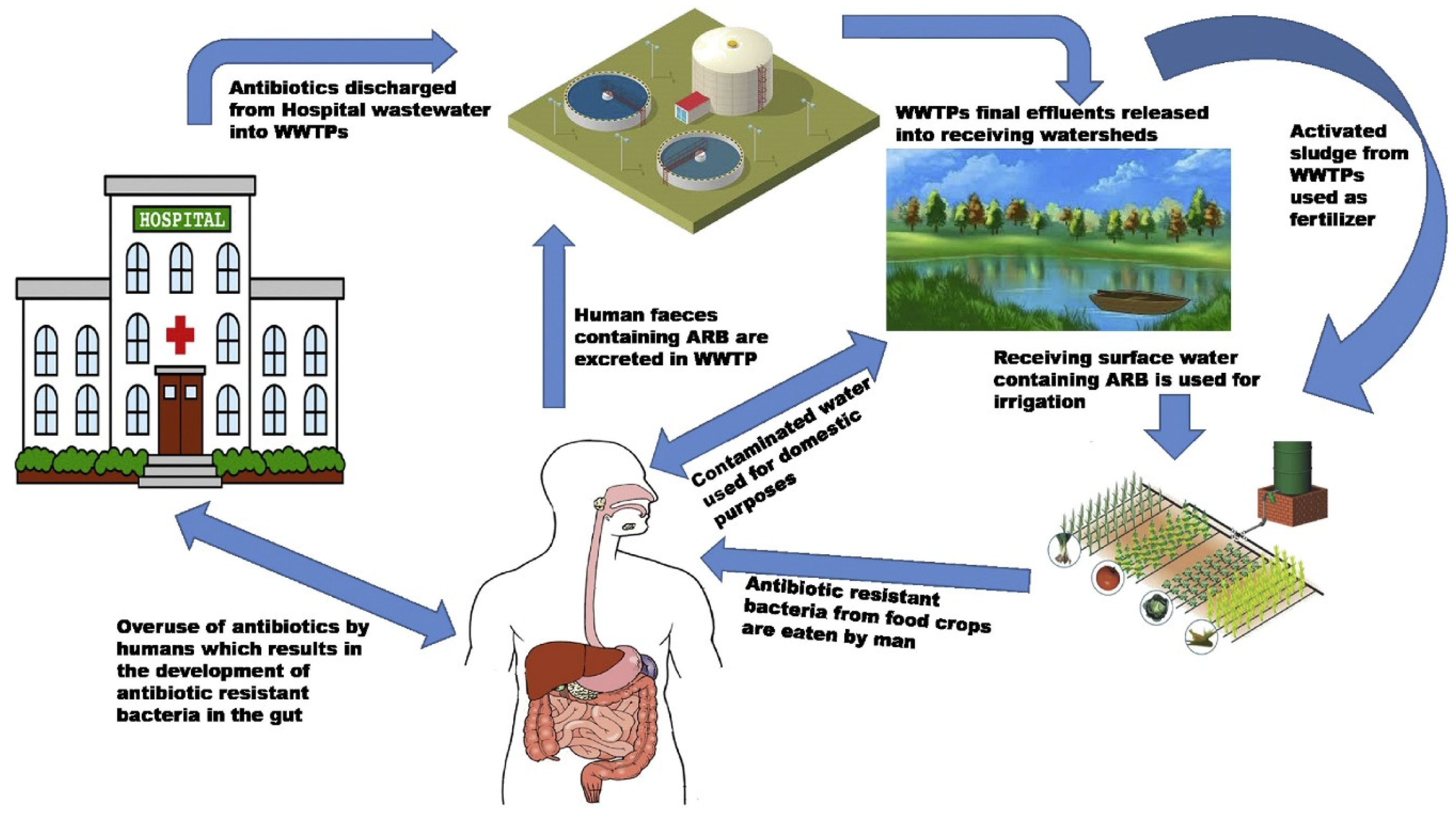
Hospital Waste Disposal
Antibiotics and Antibiotic-Resistant Bacteria in Hospital Wastewater
Origins of Antibiotics Found in Wastewater
Antibiotics in Hospital Wastewater and Their Dissemination in the Environment
| Antibiotics | Concentration in HWw (µg L-1) | Concentration in UWw (µg L-1) |
| Ciprofloxacin | 0,03-125 | 0,01-5,88 |
| Clarithromycin | 0,2-3 | 0,1-4,8 |
| Coprofloxacin | 0,85-2 | |
| Doxycycline | 0,1-6,7 | |
| Erythromycin | 27-83 | 0,04-2,7 |
| Lincomycin | 0,3-2 | |
| Metronidazole | 0,1-90 | |
| Norfloxacin | 0,029-44 | 0,01-0,96 |
| Ofloxacin | 0,353-35,5 | 0,01-31,70 |
| Oxytetracycline | 0,01-3,75 | |
| Penicillin G | 0,85-5,2 | 0,03 |
| Sulfamethoxazole | 0,04-83 | 0,01-6,0 |
| Tetracycline | 0,01-4,2 | 0,01-1,30 |
| Trimethoprim | 0,01-15 | 0,02-7,90 |
Quantification of Antibiotic Residues Encountered in Samples from Certain Rivers Worldwide
| Activity against the Isolates | ||||
|---|---|---|---|---|
| Antibiotics | Resistance Prevalence (%) | MIC (mg L-1) | ||
| Rank | 50% | 90% | ||
| Ampicillin (AMP) | 100 | 64 to ≥ 1024 | 1024 | 1024 |
| Amoxicillin (AMO) | 100 | 1 to ≥ 1024 | 1024 | 1024 |
| Streptomycin Sulfate (STR) | 92.3 | 1 to ≥ 1024 | 128 | 52 |
| Trimethoprim (TRI) | 98 | 8 to ≥ 1024 | 1024 | 1024 |
| Chloramphenicol (CHL) | 100 | 256 to ≥ 1024 | 1024 | 1024 |
| Sulfonamide (SUL) | 100 | 512 ≥ 1024 | 1024 | 1024 |
| Tetracycline (OXY) | 90.6 | 2 to ≥ 512 | 128 | 256 |
| Oxytetracycline (OXY) | 90.6 | 1 to ≥ 1024 | 256 | 512 |
| Nalidixic Acid (NAL) | 73.6 | 1 to ≥ 1024 | 512 | 1024 |
| Erythromycin (ERY) | 92.5 | 4 to ≥ 1024 | 128 | 512 |
| Spiramycin (SPIRA) | 90.6 | 2 to ≥ 1024 | 512 | 1024 |
| Kanamycin (KAN) | 54.7 | 1 to ≥ 1024 | 128 | 1024 |
Bacteria Found in Hospital
| Pathogenic species | Load of bacteria detected in 100 ml water effluent | Estimated minimal infecting Dosis |
|---|---|---|
| Campylobacter spp. | 104-105 | 1.103 |
| Clostridium perfringens | 6.104-8.104 | 103-105 spores |
| Escherichia coli | 104-107 | 106-107 ; 10-102 |
| Entérocoques intestinaux | 4.7.103-108 | 60-102 |
| Salmonella spp. | 0.2-8.103 | 104-1010 |
| Vibrio spp. | ND | 1.106 |
| Shigella spp. | 0.1-103 | 10-104 |
Bacteria Isolated in Community Wastewater and Rivers
| country | Source | Host (s) | Reference |
|---|---|---|---|
| Benin | Urban wastewater | Eschérichia coli, | [66] |
| RC | Household Wastewater |
Escherichia coli, Salmonella spp., Shigella spp., Klebsiella spp., Enterobacter aerogenes, Enterobacter cloacae , Arizona spp., Proteus spp. |
[67] |
| Gabon | River |
Citrobacter freundii, Enterobacter sp, Escherichia coli, Klebsiella pneumoniae, Kluyvera ascorbata, Leclercia adecarboxylata, Pantoea dispersa; Serratia marcescens; and Yokenella regensburgei, Salmonella enterica |
[64] |
| Tunisia | Urban wastewater | Escherichia coli, Salmonella spp. | [68] |
| Ethiopia | Hospital sewage | Klebsiella spp., P. aeruginosa | [69] |
| DRC | River | Escherichia coli | [70] |
The Bacteria Isolated from Both Hospital Effluents and Rivers
| Species | Country | Water source | Reference |
|---|---|---|---|
| Pseudomonas aeruginosa, salmonella spp. | Ivory Coast | Hospital sewage | [38] |
| Escherichia coli | DRC | Hospital sewage, river | [45] |
| Klebsiella spp. | South Africa | Hospital sewage | [46] |
| Salmonella spp., Pseudomonas spp, and Escherichia coli | Burkina Faso | Hospital sewage | [47] |
|
Klebsiella pneumoniae, Aeromonas spp. and Escherichia coli |
Benin | Hospital sewage | [48] |
| Escherichia coli, Enterococcus faecium, Enterobacter cloacae and Pseudomonas aeruginosa, Acinetobacter townrii | Benin & Burkina Faso | Hospital sewage | [49] |
| E. coli, Klebsiella spp., Salmonella spp, Shigella spp., Citrobacter spp.; Bacillus spp., Proteus spp. | Ethiopia | Hospital sewage | [50] |
|
Klebsiella spp., Pseudomonas spp., E. coli, Citrobacter spp., |
Ethiopia | Hospital sewage | [51] |
Antibiotic-Resistant Bacteria Found in Rivers Surrounding Hospitals
Antibiotic-Resistant Bacteria (Gram-Negative Bacilli) in Water
Characterization of ESBLs in Wastewater
| Country | Source | Host | ESBLs | Reference |
| Ghana | River waters | Escherichia coli | P, E. coli | [83] |
| South Africa | Sewage | Escherichia coli | P, E. coli | [84] |
| Burkina Faso | Sewage | Klebsiella oxytoca, Serratia spp, Citrobacter spp, | P, Klebsiella oxytoca | [85] |
| Cameroun | Well water | E. Coli, Salmonella spp, P. aeroginosa, Klebsiella pneumoniae et Bulkholderia cepaceae | P, E. coli | [86] |
| Ivory Coast | Hospital sewage | E. coli, K. pneumoniae, P. aeruginosa et Acinetobacter baumanii | P, Acinetobacter baumanii | [115] |
| Nigeria | Hospital sewage | E. coli, Klebsiella pneumoniae, E. coli, K. pneumoniae | P, E. coli & K. pneumoniae | [87] |
| Ethiopia | Sewage |
E.coli, Salmonella, Klebsiella pneumonia, Enterobacter aerogenes, Citrobacter, Klebsiella oxytoca and Enterobacter cloacae, |
P, K. pneumonia & E. coli | [88] |
Characterization of Antibiotic Resistance Genes in Bacteria Isolated from Wastewater
The Fate of Hospital Antibiotics in Natural Environments
Risk of Contaminated Water in Aquatic Animals
Consequences of Contamination Related to Hospital Wastewater
Consuming Untreated Water Can Lead to Waterborne Illnesses
Conclusions
Funding
References
- Darsy, Coralie, Lescure, Irène, Payot, Véronique, G Rouland. Effluents des établissements hospitaliers: teneur en microorganismes pathogènes, risques sanitaires, procédures particulières d’épuration et de gestion des boues. Mémoire. Office international de l’eau, service national d’information et de documentation sur l’eau, 2002, p 3. Available online: https: // http://greeqs.free.fr/siteeqs/eau/textes/nonregl/aut-02-02a.pdf (accessed on 25 june 2023).
- Julien Passerat, Fatima Tamtam, Barbara Le Bot, Joëlle Eurin, Marc Chevreuil et Pierre Servais. Rejets hospitaliers d’antibiotiques et de bactéries fécales antibiorésistantes dans les rivières du bassin de la Seine. Eur. J. Water Qual. 2010; 26.41, 1–13. [CrossRef]
- Bréchet, C., Hocquet, D., et Bertrand, X. Eaux usées et Escherichia coli producteur de β-lactamases à spectre étendu. J Anti-infect, 2015; 17 (2), 53-59. [CrossRef]
- Kolpin, Dana W., Skopec, Mary, Meyer, Michael T., et al. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ, 2004; 328, 1-3, 119-130. [CrossRef]
- Watkinson, A. J., Murby, E. J., Costanzo, S. D. Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res, 2007; 41, (18), 4164-4176. [CrossRef]
- Mbog, Mbog S., Nguidjoe, Evrard Marcel, Djocgoue, Pierre F., et al. Évaluation de la gestion des déchets liquides hospitaliers du Centre Hospitalier Universitaire de Yaoundé I (CHU): cas des eaux usées. Sci. Nat. Appl, 2019; 38, 2.
- Luhua Zhang, Xinyue Ma, Li Luo, Nan Hu, Jiayao Duan, Zhongjian Tang, Rujie Zhong and Ying LI. The Prevalence and Characterization of Extended-Spectrum -Lactamase- and Carbapenemase-Producing Bacteria from Hospital Sewage, Treated Effluents and Receiving Rivers. Int. J. Environ. Res. Public Health 2020; 17, 1183. [CrossRef]
- Parveau P, Bactéries multi résistantes dans l’environnement : recherche dans les effluents de la ville de TOULOUSE, Université de Limoges, France. 2011; p 55. Available online: https:// https://scholar.google.fr/scholar?hl=fr&as_sdt=0%2C5&q=Parveau+P%2C+Bact%C3%A9ries (accessed on 25 April 2023).
- Julia Baudart and Nathalie Paniel. Sources et devenir des micro-organismes pathogènes dans les environnements aquatiques Sources and fate of pathogenic microorganisms in aquatic environments. Rev Fran des Lab, 2014; 459, 29-39. [CrossRef]
- Peralta, Denisse Archundia. Etude du devenir et de l’impact des antibiotiques à l’échelle d’un bassin versant: application au bassin versant du Katari (Bolivie). Diss. Université Grenoble Alpes, 2016.
- Orias Frédéric et Perrodin, Yves. Characterisation of the ecotoxicity of hospital effluents: a review. Sci. Total Environ, 2013; 454, 250-276. [CrossRef]
- Silva B, Costa F, Neves IC, Tavares T. Psychiatric Pharmaceuticals as Emerging Contaminants in Wastewater. Springer International Publishing; 2015. Available online: https:// link.springer.com/book/10.1007/978-3-319-20493-2.
- Chamkal N., Lhlou I., Bandadi L., Ounine K. Hospital Antibiotics Usage: Environmental Hazard and Promotion of Antibiotic Resistant Bacteria. Ann Ig, 2022; 34 (3): 266-278. [CrossRef]
- Haguenoer Jean-Marie. Does Pharmaceutical Waste and Drug Residue Pose a Risk to Public Health? Sante Publique, 2010; 22 (3), 325-342.
- Akoua-Koffi, C., Guessennd, N., Gbonon, V., et al. La méticillino-résistance de Staphylococcus aureus isolés à Abidjan (1998–2001): un nouveau problème en milieu hospitalier. Méd Mal Infect, 2004, 34 (3), 132-136. [CrossRef]
- Guessennd, N., Bremont, S., Gbonon, V., et al. Résistance aux quinolones de type qnr chez les entérobactéries productrices de bêta-lactamases à spectre élargi à Abidjan en Côte d’Ivoire. Pathol. Biol. 2008; 56, (7-8), 439-446. [CrossRef]
- Lamprecht C, Romanis M, Huisamen N, Carinus A, Schoeman N, Sigge Go, et al. Escherichia coli with virulence factors and multidrug resistance in the Plankenburg River. S Afr J Sci, 2014; 110: 1–6. [CrossRef]
- Fadare Ft, Okoh Anthony Ifeanyi, Distribution and molecular characterization of ESBL, pAmpC β-lactamases, and non-β-lactam encoding genes in Enterobacteriaceae isolated from hospital wastewater in Eastern Cape Province, South Africa, Plos One, 2021; 16 (7), e0254753. [CrossRef]
- Corvaisier, N. Les substances médicamenteuses rejetées dans les eaux urbaines. Ecole Nationale du Génie Rural des Eaux et des Forets. Centre de Montpellier-Février, France, 2000.
- Davies, Julian et Davies, Dorothy. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev., 2010; 74 (3), 417-433. [CrossRef]
- Garric, Jeanne et Ferrari, Benoît. Les substances pharmaceutiques dans les milieux aquatiques. Niveaux d’exposition et effet biologique: que savons-nous?. Rev. Sci. Eau, 2005; 18 (3), 307-330. [CrossRef]
- Heberer Thomas. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. lett, 2002; 131, (1-2), 5-17. [CrossRef]
- Mater, Nicolas, Geret, Florence, Castillo, Luis, et al. In vitro tests aiding ecological risk assessment of ciprofloxacin, tamoxifen and cyclophosphamide in range of concentrations released in hospital wastewater and surface water. Environ. Int., 2014; 63, 191-200. [CrossRef]
- Oberlé K, Capdeville Mj, Berthe T, Budzinski H, Petit F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli: from medical center patients to a receiving environment. Environ Sci Technol, 2012; 46: 1859-68.ORIAS. [CrossRef]
- Berthe T, Ratajczak M, Clermont O, Denamur E, Petit F. Evidence for coexistence of distinct Escherichia coli populations in various aquatic environments and their survival in estuary eater. Appl Environ Microbiol, 2013; 79: 4684-93. [CrossRef]
- Fabienne Petit. L’antibiorésistance dans les environnements aquatiques: une problématique d’écologie microbienne et de santé publique, Environ Risque Sante, 2018; 17 (1), 40-46.
- Kingsley Ehi Ebomah and Anthony Ifeanyi Okoh. An African perspective on the prevalence, fate and effects of carbapenem resistance genes in hospital effluents and wastewater treatment plant (WWTP) final effluents: A critical review. Heliyon 6, 2020; e03899. [CrossRef]
- Emmanuel, Evens, Perrodin, Yves, Keck, Gérard, Blanchard, J. M., & Vermande, P. Effects of hospital wastewater on aquatic ecosystem. In: Proceedings of the XXVIII Congreso Interamericano de Ingenieria Sanitaria y Ambiental. Cancun, México. 2002. 27-31.
- Ngankem Ngankem Ii, Aurelien Flavien. Evaluation de la gestion des déchets biomédicaux liquides dans les centres hospitaliers universitaires du Point G et Gabriel Touré. Thèse de doctorat. Université des Sciences des Techniques et des Technologies de BAMAKO, Mali. 2014. Available online: https://bibliosante.ml/handle/123456789/736 (accessed on 25 April 2023).
- Akin, Beril Salman, et al. Contaminant properties of hospital clinical laboratory wastewater: a physiochemical and microbiological assessment. J. Environ, 2016, 7 (5), 635. [CrossRef]
- Al-Ajlouni, Kholoud, Shakhatreh, Saleh, Al-Ibraheem, N., Jawarneh, M. Evaluation of wastewater discharge from hospitals in Amman–Jordan. J Basic Appl Sci, 2013, 13 (4), 44-50.
- Mahvi, A. H., Ghanbarian, M., Nasseri, S., et al. Mineralization and discoloration of textile wastewater by TiO2 nanoparticles. Desalination, 2009; 239 (1-3), 309-316. [CrossRef]
- Ibeh, I. N. and Omoruyi, M. I. Seasonal Dynamics in the physiochemical parameters of hospital effluent from a university teaching hospital based in Southern Nigeria. Asian J. Sci. Res, 2011; 1 (1), 7.
- Qadouri Asmaa, Mouhir Latifa, Belkadi MS. Application d’une méthode d’étude quantitative et qualitative des rejets liquides hospitaliers au niveau de la Région de Marrakech Tensift El Haouz, Maroc. Eur Sci. J, 2016; 12 (32), 110-130. [CrossRef]
- Koutchika, Olga, SALOU, Souwébatou, et DEGBEY, Comlan Cyriaque. Gestion des déchets biomédicaux liquides dans les maternités des formations sanitaires publiques de la commune de Sème-Podji. EPAC/CAP/UAC, 2019.
- Benneni Halima, Bouarissa Besma. Epuration des eaux usées, analyse et synthèse des données scientifiques. Cas de la station d’épuration des eaux de la wilaya de Bordj Bou Arreridj: Prospection, évaluation du rendement épuratif. Diss. Mémoire de Master. 2020. m596. pdf (univ-bba.dz) Available online: (accessed on 15 March 2023).
- Hocquet Didier, Muller Allison, et Bertrand, Xavier. What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect, 2016; 93 (4), 395-402.
- Amarachukwu Obayiuwana, Adeniyi Ogunjobi, Min Yang and Mark Ibekwe. Characterization of bacterial communities and their antibiotic resistance profiles in wastewaters obtained from pharmaceutical facilities in Lagos and Ogun States, Nigeria. Int. J. Environ. Res. Public Health, 2018, 15 (7), 1365. [CrossRef]
- Placide, Sadia Sahi, Ollo, Kambiré, Quand-Même, Gnamba Corneil, et al. Mineralization of Wastewater from the Teaching Hospital of Treichville by a Combination of Biological Treatment and Advanced Oxidation Processes. Asian J Chem Sci, 2021, 10 (2): 1-10. [CrossRef]
- Derolez Valérie. Méthode de caractérisation de la fragilité microbiologique des zones conchylicoles. Rapport ENSP, 2003. Available online: https: // https://documentation.ehesp.fr/memoires/2003/igs/derolez.pdf (accessed on 25 April 2023).
- World Health Organization. Guidelines for safe recreational water environments: Coastal and fresh waters. Vol. 1. World Health Organization, 2003.
- Gotkowska-Płachta, A. The prevalence of virulent and multidrug-resistant enterococci in river water and in treated and untreated municipal and hospital wastewater. Int. J. Environ. Res. Public Health, 2021; 18 (2), 563. [CrossRef]
- Rouquet, R. M. La légionellose: la bactérie et les données épidémiologiques. Lett. pneumol, 2005; 8 (1), 17-20.
- Walia, S., Murleedharn, C., Band, J., Kanwar, M., & Kumar, A. Quantitation of antibiotic resistance genes pollution in hospital waste water effluent and Urban Clinton River Water, Michigan, USA. Curr. Med. Res. Pract, 2016; 6 (4), 149-151. [CrossRef]
- Lévi Yves. Inquiétudes sur la présence d’antibiotiques et de bactéries antibiorésistantes dans les eaux. Environ. Risques Santé, 2006; 5 (4), 261-265.
- Laffite, Amandine, Kilunga, Pitchouna I., Kayembe, John M., et al. Hospital effluents are one of several sources of metal, antibiotic resistance genes, and bacterial markers disseminated in Sub-Saharan urban rivers. Front. Microbiol, 2016; 7, 1128. [CrossRef]
- King Tlb, Schmidt S, Essack Sy. Antibiotic resistant Klebsiella spp. from a hospital, hospital effluents and wastewater treatment plants in the uMgungundlovu District, KwaZulu-Natal, South Africa. Sci Total Environ, 2020; 712: 135550. [CrossRef]
- Ouedraogo, Ganamé Abasse, Kone, Souleymane, Ouedraogo, Arouna, et al. Ecotoxicity of hospital wastewaters and their impact on bacterial multi-drug resistance: a review. Jlife Sci. Biomed, 2021; 11 (4), 58- 71. [CrossRef]
- Koudokpon, H., Dougnon, V., Lougbegnon, C., Agbankpe, A. J., Avodagbe, G., Saidou, S., ... & Baba- Moussa, L. Emergence of Multidrug-resistance Bacteria Isolated of Drinking Water, Groundwater and Hospital Wastewater in Southern Benin: Antibiotic Resistance Profile and Resistomes Determination. Res Sq. 2021. [CrossRef]
- Markkanen, Melina A., Haukka, Kaisa, Pärnänen, Katariina MM, et al. Metagenomic analysis of the abundance and composition of antibiotic resistance genes in hospital wastewater in Benin, Burkina Faso, and Finland. Msphere, 2023; 8, 1, e00538-22. [CrossRef]
- Fekadu, Sintayehu, Merid, Yared, Beyene, Hunachew, et al. Assessment of antibiotic-and disinfectantresistant bacteria in hospital wastewater, south Ethiopia: a cross-sectional study. J. Infect. Dev. Ctries, 2015; 9, (2), 149-156. [CrossRef]
- Moges, Feleke, Endris, Mengistu, Belyhun, Yeshambel, et al. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res. Notes, 2014; 7, 1-6. [CrossRef]
- Radhakrishna L, Nagarajan P. Isolation and Preliminary Characterization of Bacterial from Liquid Hospital Wastes. Int J Pharmtech Res, 2015; 8 (2), 308–314.
- Dhafer Mmas, Georgette Nn, Amandine L, Jean-Paul O, Crispin M, John P. Ecotoxicology and Environmental Safety Hospital wastewaters: A reservoir and source of clinically relevant bacteria and antibiotic resistant genes dissemination in urban river under tropical conditions. Ecotoxicol. Environ. Saf. 2020; 200, 9.
- Jyothirmai Vk, Sharmila Smr, Arun S. Degradation of ciprofloxacin using fenton process and its effect on biodegradability. Rasayan J. Chem, 2020; 13 (4), 2274–2280.
- Thomas, Kevin V., Dye, Christian, Schlabach, Martin, Langford, K. H. Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J Environ Monitor, 2007; 9 (12), 1410-1418. [CrossRef]
- Dong Li, Tao Yu, Yu Zhang, Min Yang, Zhen Li, Miaomiao Liu, and Rong Qi. Antibiotic Resistance Characteristics of Environmental Bacteria from an Oxytetracycline Production Wastewater Treatment Plant and the Receiving River. Appl. Environ. Microbiol. 2010; 76 (11), 3444–3451. [CrossRef]
- Petit F, Denamur E, Clermont O, et al. Fate of antibiotic and antibiotic-resistant fecal bacteria in water and sediments from the contamination source to the estuary: impact and/or resilience? In: Marine productivity: perturbation and resilience of socio-ecosystem. Springer Verlag, 2014. Available online: hps://link.springer.com/chapter/10.1007/978-3-319-13878-7_9 (accessed on 25 May 2023).
- Kapepula L, Ndikumanat, Dieu-Donné M, Alconero Pl, Tamungang Neb, Tarimo I, Bruggen Bvd. Qualitative and quantitative analysis of the pollutant load of effluents discharged Northwestern of Lake Tanganyika, in the Democratic Republic of Congo. Afr. J. Environ. Sci. Technol. 2020; 14, 11, 361–373. [CrossRef]
- Dubois-Brissonnet F, Guillier L. Les maladies microbiennes d’origine alimentaire. Cah. de Nutr. Diet. 2020; 55 (1), 30–38. [CrossRef]
- Siourimè, Somda Namwin, Isidore, Bonkoungou Ouindgueta Juste, Oumar, Traoré, et al. Serotyping and antimicrobial drug resistance of Salmonella isolated from lettuce and human diarrhea samples in Burkina Faso. Afr J Infect Dis, 2017; 11 (2), 24-30. [CrossRef]
- K’oreje Kold, Vergeynst D, Ombaka P, De Wispelaere M, Okoth Ch, Langenhove Kv. Chemosphere Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Elsevier (Chemosphere). 2016; 149 (238–244), 7. [CrossRef]
- Asmaa Qlm, Said B. M. Application d’une méthode d’étude quantitative et qualitative des rejets liquides hospitaliers au niveau de la Région de Marrakech Tensift El Haouz, Maroc. Eur. Sci. J. 2016 ; 12 (32), 110–130. [CrossRef]
- Elias A, Kanhounnon Sa, Gbèdodé W, Boni Cc, Dogbè Ca, Koumolou L, Bonou B, Fiogbe Ed, Aléodjrodo Pe. Physicochemical and microbiological characterization of effluents from the “Centre Hospitalier Universitaire de la Mère et d e l’Enfant Lagune (CHU-MEL)” discharged in the Cotonou lagoon in Benin. Int. J. Biol. Chem. Sci. 2018; 12 (1991–8631), 1955–1964. [CrossRef]
- Ehrhardt, Jonas, Alabi, Abraham S., Kremsner, Peter G., Rabsch, W., Becker, K., Foguim, F. T.. Bacterial contamination of water samples in Gabon, 2013. J Microbiol, Immunol Infect, 2017; 50 (5), 718-722. [CrossRef]
- Yala, Jean-Fabrice, Souza, Alain, Lebamba, JudicaëL, et al. Etude préliminaire de l’évaluation des paramètres physico-chimiques, détection et dénombrement des coliformes totaux et fécaux dans quelques lacs de la ville de Franceville [Preliminary study of the evaluation of physicochemical parameters, detection and enumeration of total and faecal coliforms in some lakes of the town of Franceville (Gabon). Int. J. Innov Appl. Stud, 2017; 20 (3), 963-974. [CrossRef]
- Mathieu Hounsou, Euloge K. Agbossou, Bernard Ahamide et Irenikatche Akponikpe, Qaulité bactériologique de l’eau du bassin de l’Ouémé: cas de coliformes totaux et fécaux dans les retenues d’eau de l’Okpara, de Djougou et de Savalou au Bénin. Int. J. Chem. Sci, 2010, 4 (2), 377-390.
- Moyen, Rachel, Ngoulou, Tarcisse Baloki, Nguimbi, Etienne, et al. Antibiotic Resistance Phenotypes of Enterobacteriaceae Isolated from Household Wastewater in Brazzaville, Republic of Congo. Adv. Microbiol, 2021; 11 (1), 27-36. [CrossRef]
- Salem, Imen Ben, Ouardani, Imen, Hassine, Mouna, et al. Bacteriological and physico-chemical assessment of wastewater in different region of Tunisia: impact on human health. BMC Res Notes, 2011; 4 (1), 1-11. [CrossRef]
- Asfaw, Tsegahun, Negash, Letemichael, Kahsay, Amlsha, et al. Antibiotic resistant bacteria from treated and untreated hospital wastewater at Ayder Referral Hospital, Mekelle, North Ethiopia. Adv. Microbiol, 2017, 7 (12), 871-886. [CrossRef]
- Tshibanda, Joseph B., Devarajan, Naresh, Birane, Niane, et al. Microbiological and physicochemical characterization of water and sediment of an urban river: N’Djili River, Kinshasa, Democratic Republic of the Congo. Sustain. Water Qual. Ecol, 2014; 3, 47-54. [CrossRef]
- Touati A, Benallaoua S, Kecha M Et Idres N. Etude des phénotypes de résistance aux β-lactamines des souches d’entérobactéries isolées, en milieu hospitalier : cas de l’hôpital d’Amizouk (W. Bejaia). Sci. Technol, 2003; 19: 92-7.
- Alexy Radka, Kümpel Tina, Kümmerer Klaus. Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere. 2004; 57, (6), 505-51. [CrossRef]
- Aminata Maiga, Nathalie Aya Nguessend, Oumar Agaly Dicko, Modibo Fofana, Ibrahim Izetiégouma Maiga, Koumba Soumahoro Man Agbo, Mireille Dosso. Les résidus d’antibiotiques dans les effluents hospitaliers de BAMAKO (MALI), Mali medical, 2018; 33 (3).
- Boillot, Clotilde. Évaluation des risques écotoxicologiques liés aux rejets d’effluents hospitaliers dans les milieux aquatiques. Contribution à l’amélioration de la phase «caractérisation des effets». Diss. INSA de Lyon. 2008. Thèse de doctorat. 2008. Available online:https://www.bing.com/search?q=Billau+Pascal%2C+2008.+Estimation+des+dangers+de+d%C3%A9 chets+ (accessed on 2 May 2023).
- Somda, Namwin Siourime, Isidore Juste O. Bonkoungou, Bissoume Sambe-Ba, Moustapha Soungalo Drabo, Abdoul Aziz Wane, Hagr’etou Sawadogo-Lingani, Aly Savadogo. Diversity and antimicrobial drug resistance of non-typhoid Salmonella serotypes isolated in lettuce, irrigation water and clinical samples in Burkina Faso. J. Agric. Food Res; 2021; 5; , 100-167. [CrossRef]
- Frédéric, Orias and Yves, Perrodin. Pharmaceuticals in hospital wastewater: their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere, 2014, 115, 31-39. [CrossRef]
- Lévi Yves. Contamination des eaux par les résidus de médicaments et stratégies de prévention Contamination of waters by drug residues and prevention strategies. Elsevier, 2020; 59; 18-23.
- Kolpin, Dana W., Furlong, Edward T., Meyer, Michael T., et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999− 2000: A national reconnaissance. Environ Sci. Technol., 2002; 36 (6), 1202-1211. [CrossRef]
- Besse, Jean-Philippe et Garric, Jeanne. Médicaments à usage humain : risque d’exposition et effets sur les milieux récepteurs. Proposition d’une liste de médicaments à usage humain à surveiller dans les eaux de surface continentales. irstea. 2007, pp.260. ⟨hal-02590157.
- Abdoul-Salam Ouedraogo. Prévalence, circulation et caractérisation des bactéries multirésistantes au Burkina Faso. Université Montpellier, France, 2016Ladjal, Salima, and Amira Baghdadi. Mécanismes de résistance aux β-lactamines chez Escherichia coli au niveau des hôpitaux algériens. Thèse de doctorat. Universite Mohamed Boudiaf-M’sila, Algérie, 2021.
- Ruppé, E., Lixandru, B., Cojocaru, R., Büke, Ç., Paramythiotou, E., Angebault, C., ... & Andremont, A. Relative fecal abundance of extended-spectrum-β-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrobial agents and chemotherapy. 2013; 57 (9), 4512-4517. [CrossRef]
- Banu, Regina Ama, Alvarez, Jorge Matheu, REID, Anthony J., et al. Extended Spectrum Beta-Lactamase Escherichia coli in river waters collected from two cities in Ghana, 2018–2020. Trop. Med. Infect, 2021, 6 (2), 105. [CrossRef]
- Gumede, S. N., Abia, A. L., Amoako, D. G., & Essack, S. Y. Analysis of wastewater reveals the spread of diverse extended-spectrum β-lactamase-producing E. coli strains in uMgungundlovu District, South Africa. Antibiotics. 2021; 10 (7), 860. [CrossRef]
- Soré, Souleymane, Sawadogo, Yacouba, Bonkoungou, Juste Isidore, et al. Detection, identification and characterization of extended-spectrum beta-lactamases producing Enterobacteriaceae in wastewater and salads marketed in Ouagadougou, Burkina Faso. Int J. Biol. Chem. Sci. 2020; 14 (8), 2746-2757. [CrossRef]
- Viban, Tangwa Bernard, Herman, Okah-Nnane Ndode, Layu, Tangwa Clotilda, et al. Risk factors contributing to microbiological contamination of boreholes and hand dug wells water in the Vina Division, Adamawa, Cameroon. Adv. Microbiol, 2021; 11 (2), 90-108. [CrossRef]
- Atta, H. I., Idris, S. M., Gulumbe, B. H., et al. Detection of extended spectrum beta-lactamase genes in strains of Escherichia coli and Klebsiella pneumoniae isolated from recreational water and tertiary hospital waste water in Zaria, Nigeria. Int. J. Environ Health Res, 2022, 32 (9), 2074-2082. [CrossRef]
- Tesfaye, Hemen, Alemayehu, Haile, Desta, Adey F., et al. Antimicrobial susceptibility profile of selected Enterobacteriaceae in wastewater samples from health facilities, abattoir, downstream rivers and a WWTP in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control, 2019; 8 (1), 1-11. [CrossRef]
- Khaled Hassine and Ridha Hamza. Les Risques Lies Aux Eléments Biologiques Et Minéraux Des Eaux Usées Urbaines. Institut d’Aménagement et d’Urbanisme de la Région Ile de France. 2004. Available online: https:// http://193.95.84.5/revue_sante_pub/3-4.pdf (accessed on 25 April 2022).
- Puckowski, Alan, Mioduszewska, Katarzyna, Łukaszewicz, Paulina, et al. Bioaccumulation and analytics of pharmaceutical residues in the environment: A review. J. Pharm. Biomed. Anal, 2016; 127, 232-255. [CrossRef]
- Bilal, M., Ashraf, S. S., Barceló, D., & Iqbal, H. M. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ, 2019, 691, 1190-1211. [CrossRef]
- Tamtam F, Le Bot B, Dinh T, et al. A 50-year record of quinolone and sulphonamide antimicrobial agents in Seine River sediments. J Soils Sed, 2011; 11: 852-9. [CrossRef]
- Coutu S, Rossi L, Barry DA, Rudaz S, Vernaz N. Temporal variability of antibiotics fluxes in wastewater and contribution from hospitals. PLoS One, 2013;8:e53592. [CrossRef]
- Montezzi LF, Campana EH, Correa LL, et al. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int J Antimicrob Agents 2015;45: 174–7. [CrossRef]
- Paschoal RP , Campana EH , Correa LL , et al. Concentration and variety of carbapenemase producers in recreational coastal waters showing distinct levels of pollution. Antimicrob Agents Chemother 2017;61:e01963 -17. [CrossRef]
- Gomi R, Matsuda T, Yamamoto M, et al. Characteristics of carbapenemase-producing Enterobacteriaceae in wastewater revealed by genomic analysis. Antimicrob Agents Chemother 2018;62:e02501 -17. [CrossRef]
- Mathys DA, Mollenkopf DF, Feicht SM, et al. Carbapenemase-producing Enterobacteriaceae and Aeromonas spp. present in wastewater treatment plant effluent and nearby surface waters in the US. PLoS One, 2019;14:e0218650. [CrossRef]
- Teixeira P, Tacao M, Pureza L, et al. Occurrence of carbapenemase-producing Enterobacteriaceae in a Portuguese river: bla NDM , bla KPC and bla GES among the detected genes. Environ Pollut 2020;260: 113913.
- Manageiro V, Ferreira E, Canica M, et al. GES-5 among the β-lactamases detected in ubiquitous bacteria isolated from aquatic environment samples. FEMS Microbiol Lett 2014;351: 64–9. [CrossRef]
- Sivalingam, Periyasamy, Poté, John, et Prabakar, Kandasamy. Environmental prevalence of carbapenem resistance Enterobacteriaceae (CRE) in a tropical ecosystem in India: human health perspectives and future directives. Pathogens, 2019; 8 (4), 174. [CrossRef]
- Jean Lesne, Sandrine Baron. La résistance bactérienne aux antibiotiques : stratégies de lutte One Health ou Global Health et normes sociales de comportement individuel. Environ. Risques Santé. 2022; 4 ; 4 (21), 303-309. [CrossRef]
- Briet, Arnaud. “Étude de la flore bactérienne et de sa résistance aux antibiotiques des produits de la pêche et de l’aquaculture.” Université du Littoral Côte d’Opale, 2018. Thèse de doctorat. 2018. Available online: https: //scholar. google.com /scholar?hl =fr&as_sdt =0%2C5&q= Briet+Arnaud.+%22%C3%89tude+de+la+flore+bact%C3%A9rienne+et+de+ (accessed on 2 May 2023).
- Valdés, F., Camiloti, P. R., Rodriguez, R. P., Delforno, T. P., Carrillo-Reyes, J., Zaiat, M., & Jeison, D. Sulfideoxidizing bacteria establishment in an innovative microaerobic reactor with an internal silicone membrane for sulfur recovery from wastewater. Biodegradation. 2016; 27, 119-130. [CrossRef]
- Peralta, Denisse Archundia. Etude du devenir et de l’impact des antibiotiques à l’échelle d’un bassin versant: application au bassin versant du Katari (Bolivie). Université Grenoble Alpes, France. 2016. Available online: https://theses.hal.science/tel-01530466/ (accessed on 20 May 2023).
- Hébert, Serge et Légaré, Stéphane. Suivi de la qualité de l’eau des rivières et des petits cours d’eau. 2000. Available online: https:// belsp.uqtr.ca/id/eprint/1288/ (accessed on 2 April 2023).
- Ivanowsky A. Ouvrages d’assainissement des eaux et qualité du milieu récepteur en zone urbaine. Cas de rejets dans la Marque à Villeneuve d’Ascq. Thèse de Doctorat. Université de Lille 1, France, 2016; p 229. Available online: https:// hal.science/tel-01581755/ (accessed on 25 April 2021).
- Ndahama, Henri, Cishibanji, Pierre Batumike, Mashimago, Jean Jacques Bagalwa, et al. Inventaire de la Biodiversité Aquatique du Bassin du Lac Kivu: Cas Spécifique des Poissons de la Rivière Nyabarongo RD Congo [Inventory of the Biodiversity of Kivu Lake Bassin: Specific Case of Nyabarongo River Fishes]. Int J Innovat Appl Stud, 2014; 7 (4), 1298.
- World Health Organization, et al. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. WHO, 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255874/97892415?sequence=1 (accessed on 25 February2023).
- Some Ysc, Soro Td, Ouedraogo S. Étude de la prévalence des maladies liées à l’eau et influences des facteurs environnementaux dans l’arrondissement de Nomgr-Masson: cas du quartier Tanghin (Ouagadougou-Burkina Faso). Int J. Biol. Chem. Sci., 2014; 8: 289–303. [CrossRef]
- Rouamba J, Nikiema E, Rouamba S, et al. Accès à l’eau potable et risques sanitaires en zone périphérique d’Ouagadougou, Burkina Faso. Rev Épidémiol Santé Publique, 2016; 64: S211. [CrossRef]
- Benkaddour Batoul. Contribution à l’étude de la contamination des eaux et des sédiments de l’Oued Chéliff (Algérie). Thèse de doctorat. Université de Perpignan; Université Abdelhamid Ibn Badis Mostaganem (Mostaganem, Algérie), 2018. Available online: Benkaddour Batoul. Contribution à l’étude Abdelhamid -Google Scholar (accessed on 25 April 2020).
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. In Review on Antimicrobial Resistance; Chaired by Jim O’Neill, December 2014; Wellcome Trust: London, UK, 2016.
- Singer, R. S., Finch, R., Wegener, H. C., Bywater, R., Walters, J., & Lipsitch, M. Antibiotic resistance—the interplay between antibiotic use in animals and human beings. The Lancet infectious diseases, 2003; 3 (1), 47-51. [CrossRef]
- Ouattara, N. K., Passerat, J., & Servais, P. Faecal contamination of water and sediment in the rivers of the Scheldt drainage network. Environ. Monit. Assess, 2011; 183, 243-257. [CrossRef]
- Guessennd, N. K., Ouattara, M. B., Ouattara, N. D., Nevry, R. K., Gbanon, V., Tiekoura, K. B., ... & Ger, B. M. R. Étude des bactéries multirésistantes des effluents hospitaliers d’un centre hospitalier et universitaire (CHU) de la ville d’Abidjan (Côte d’Ivoire). J. Appl. Biosci, 2013, 69, 5456-5464. [CrossRef]
- Hernando, M. D., Mezcua, M., Fernández-Alba, A. R., & Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta, 2006, 69 (2), 334-342. [CrossRef]
- Zineb Cherak, Lotfi Loucif, Abdelhamid Moussi, Jean-Marc Rolain, Carbapenemase producing Gramnegative bacteria in aquatic environments: a review. J. Glob. Antimicrob. Resist, 2021, 25, 287–309. Montezzi LF, Campana EH, Correa LL, et al. Occurrence of carbapenemase- producing bacteria in coastal recreational waters. Int J Antimicrob Agents, 2015; 45:174–7.
- Weingarten RA, Johnson RC, Conlan S, et al. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio, 2018;9:e02011 -17. [CrossRef]
- Picao RC, Cardoso JP, Campana EH, et al. The route of antimicrobial resistance from the hospital effluent to the environment: focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn Microbiol Infect Dis 2013;76:80–5. [CrossRef]
- de Araujo CF, Silva DM, Carneiro MT, et al. Detection of carbapenemase genes in aquatic environments in Rio de Janeiro, Brazil. Antimicrob Agents Chemother 2016; 60:4380–3. [CrossRef]
- Yang F, Mao D, Zhou H, et al. Prevalence and fate of carbapenemase genes in a wastewater treatment plant in Northern China. PLoS One 2016; 11:e0156383. [CrossRef]
- Paschoal RP, Campana EH, Correa LL, et al. Concentration and variety of carbapenemase producers in recreational coastal waters showing distinct levels of pollution. Antimicrob Agents Chemother 2017; 61:e01963 -17. [CrossRef]
- Mathys DA, Mollenkopf DF, Feicht SM, et al. Carbapenemase-producing Enterobacteriaceae and Aeromonas spp. present in wastewater treatment plant effluent and nearby surface waters in the US. PLoS One 2019;14:e0218650. [CrossRef]
- Mathers AJ, Vegesana K, German Mesner I, et al. Intensive care unit wastewater interventions to prevent transmission of multispecies Klebsiella pneumonia carbapenemase-producing organisms. Clin Infect Dis 2018;67:171–8. [CrossRef]
- Sekizuka T, Inamine Y, Segawa T, et al. Potential KPC-2 carbapenemase reservoir of environmental Aeromonas hydrophila and Aeromonas caviae isolates from the effluent of an urban wastewater treatment plant in Japan. Environ Microbiol Rep 2019;11:589–97. [CrossRef]
- Xu H, Wang X, Yu X, et al. First detection and genomics analysis of KPC-2-producing Citrobacter isolates from river sediments. Environ Pollut 2018;235:931–7. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





