2. Case Description
A 3-month-old female Ragdoll cat weighing 1.98 kg (body condition score [BCS]: 4/9) was presented for consultation. No apparent cardiogenic clinical signs were observed; however, a heart murmur was observed before acquisition. During vaccination, cardiac enlargement was noted at a referral hospital, leading to further examination at the Azabu University Veterinary Teaching Hospital. The heart murmur was most pronounced at the right cardiac apex, with an audible grade 5/6 systolic murmur. The body temperature was 38.2 °C, heart rate was 138 bpm, and respiratory rate was 48 breaths/min. Chest radiography indicated a vertebral heart size of 9.0 vertebrae (v), which is within the normal range for a kitten (median 9.5 v; range: 7.7–10.8 v) [
8]; however, an enlarged pulmonary artery shadow was noted. Electrocardiography revealed increased R waves, but the electrical axis was +80 °with a normal sinus rhythm (180 bpm), and no arrhythmias were observed. Transthoracic echocardiography (TTE) was performed using an ultrasound unit (Vivid E9; GE Healthcare Co., Ltd., Tokyo, Japan) equipped with 6–12 MHz phased-array transducers. TTE identified a defect just below the aortic valve in the right parasternal left ventricular outflow tract view. Additionally, at the level of the aortic valve in the right parasternal short-axis view, the defect was located between the pulmonary artery outflow tract and tricuspid valve, leading to a diagnosis of a combination of Type 2 and Type 3 VSD according to the Kirklin classification. The VSD flow was 4.97 m/s. The left atrial diameter was 1.45 cm, enlarged compared with the normal value for a 2 kg kitten (0.96 cm; range: 0.82–1.13 cm) [
8]. The left ventricular end-diastolic diameter was 1.94 cm, which was larger than the normal value of 1.25 cm (range: 1.04–1.49 cm) [
8]. The pulmonary artery was significantly dilated, and the pulmonary-to-systemic blood flow ratio (Qp:Qs) [
9] calculated using Doppler imaging was 2.45. The owner expressed interest in surgical treatment for the patient and visited our hospital, but decided to wait for the kitten to grow up because of its small size.
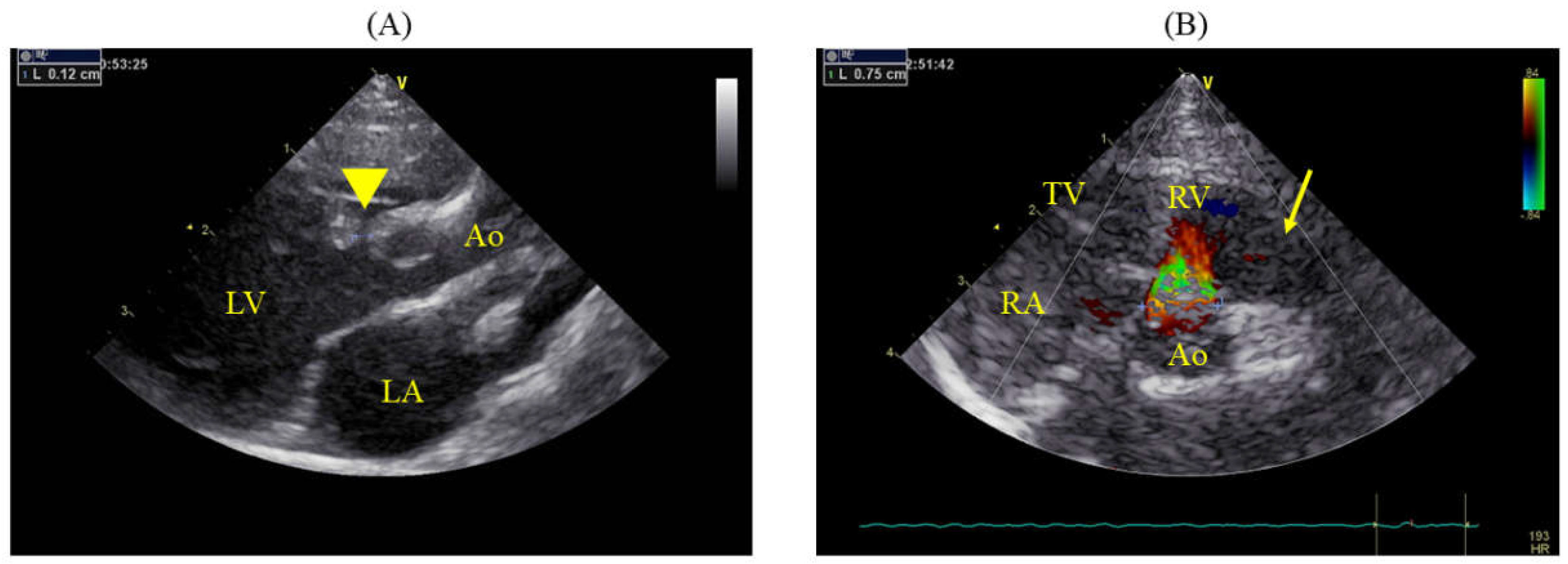
On day 383, the cat weighed 4.44 kg (BCS: 4/9). Echocardiography revealed that the VSD had a minor axis of 1.2 mm and major axis of 8.1 mm (
Figure 1) with a VSD/Ao ratio of 95.3%. Color Doppler imaging in the right parasternal short-axis view at the level of the aortic valve revealed a shunt flow at the 12 o’clock position (
Figure 1B). The LA/Ao ratio was 1.69 (1.43/0.85 cm; normal range < 1.5 [
10]), which was considerably enlarged, and the left ventricular internal diameter in diastole (LVIDd) was 1.8 cm, at the upper limit of the normal range for an adult cat weighing 4.5 kg (1.8 cm, range: 1.27–1.98 cm [
11]).
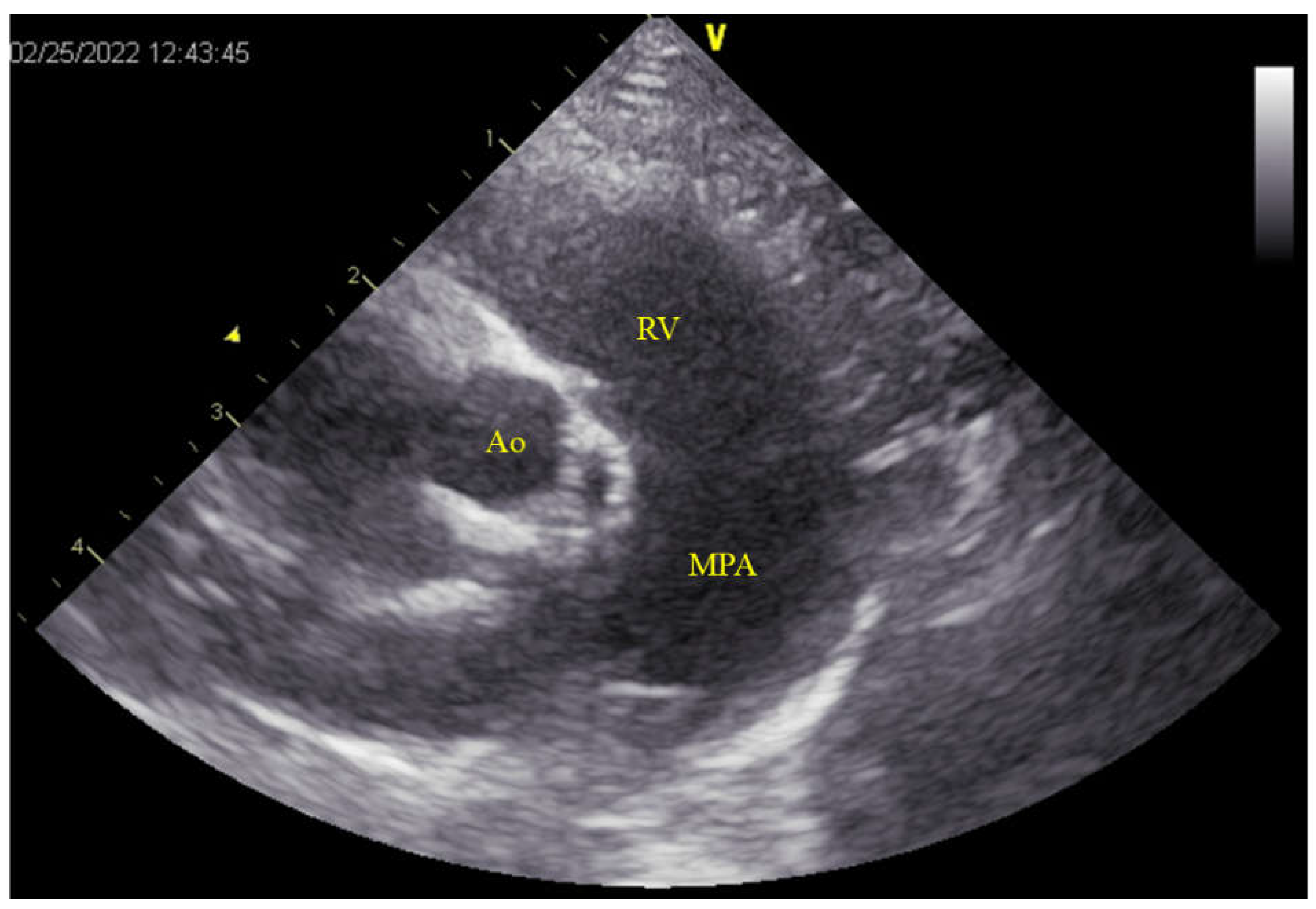
Significant enlargement of the pulmonary artery was evident (main pulmonary artery-to-aorta diameter ratio 1.29 (11.0/8.5 mm); normal value in dogs < 1.0 [
12] (
Figure 2). Although no tricuspid or pulmonary valve regurgitation and no evident signs of pulmonary hypertension (PH) were noted [
12,
13], the pulmonary to systemic blood flow ratio calculated by using Doppler was 2.96. Given the enlargement of the left side of the heart, surgical intervention was deemed necessary, and the cat underwent surgical correction under cardiopulmonary bypass. Pre-anesthesia included subcutaneous administration of atropine (0.025 mg/kg; Atropine Sulfate; NIPRO ES PHARMA Co., Ltd., Osaka, Japan), followed by intravenous administration of cefazolin (20 mg/kg, with additional doses every 2 h during surgery; Cefazolin Sodium for Injection, Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan ), and slow intravenous injection of dexamethasone (0.2 mg/kg; dexamethasone injection A; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan). The cat was oxygenated with 100% oxygen for 5 min and received an intravenous injection of fentanyl (2 μg/kg; Fentanyl injection 0.5 mg; Janssen Pharmaceutical K.K., Tokyo, Japan). Subsequently, alfaxalone (5 mg/kg; Alfaxan multidose; Meiji Animal Health Co., Ltd., Kumamoto, Japan) was administered intravenously, and anesthesia was maintained with isoflurane (1–2%; Isoflurane; Mylan Pharma Co., Ltd., Osaka, Japan). During surgery, a continuous rate infusion (CRI) of fentanyl (5 μg/kg/h) was administered for pain management. Rocuronium bromide (0.3 mg/kg; Eslax intravenous; MSD K.K., Tokyo, Japan) was administered intravenously to halt respiration, with additional doses of 0.1 mg/kg administered every 40 min as needed. If the blood pressure dropped during surgery, isoflurane was discontinued, and anesthesia was maintained with a CRI of alfaxalone (5–8 mg/kg/h).
After anesthesia, the cat was positioned in dorsal recumbency. Arterial pressure was measured invasively after exposing the femoral artery and vein via cutdown. A median sternotomy was performed, followed by a median pericardiotomy, and the pericardium was retracted to either side to create a pericardial tent. At this stage, palpation of the thrill caused by the VSD revealed a defect in the right ventricular outflow tract, and the VSD was approached by incising the pulmonary artery.
The periaortic adipose tissue was removed to expose the aorta for the placement of a perfusion cannula distally and a myocardial protection solution root cannula proximally. Before inserting the perfusion cannula, double purse-string sutures were placed using non-absorbable monofilament 6-0 polyvinylidene fluoride sutures (Asflex; Konoseisakusyo Co., Ltd., Tokyo, Japan). Similarly, a single purse-string suture was used to secure the root cannula. For venous drainage, the cranial vena cava, azygos vein, and caudal vena cava were dissected, and taping was performed on the cranial and caudal vena cava using an expanded polytetrafluoroethylene suture (CV-0; ethylene oxide gas before surgery). A purse-string suture with 6-0 polyvinylidene fluoride was placed before the venous drainage cannula was inserted. A purse-string suture of the same material was used to vent the left atrial appendage. During cardiac incision, carbon dioxide was infused into the thoracic cavity to prevent air embolism.
Subsequently, heparin (heparin sodium injection; NIPRO ES PHARMA Co., Ltd., Osaka, Japan) was administered intravenously at a dose of 200 IU/kg, and an activated coagulation time (ACT) exceeding 300 s was confirmed. An 8 Fr arterial perfusion cannula (DLP™ One-Piece Pediatric Arterial Cannula, MEDTRONIC JAPAN CO., LTD., Tokyo, Japan) and a 16 G root cannula (DLP™ Pediatric Aortic Root Cannulae, MEDTRONIC JAPAN CO., LTD., Tokyo, Japan) were then inserted and secured with tourniquets. An 8 Fr curved venous return cannula was inserted into the cranial vena cava and a 10 Fr straight venous return cannula (Flexmate, Senko Medical Instrument MFG. Co. Ltd., Tokyo, Japan) was placed in the caudal vena cava, inserted distal to the taped ePTFE sutures, and secured with a tourniquet. A vent cannula was placed in the left atrium and secured using a tourniquet.
Partial bypass perfusion was initiated, followed by cross-clamping of the aorta. Myocardial protection solution (Miotector coronary vascular injection, Kyowa Criticare Co., Ltd., Kanagawa, Japan) was slowly administered via a root cannula at 34 ml/kg (150 ml) to achieve cardiac arrest and transition to total bypass perfusion. After 30 min, an additional dose of 15 ml/kg (65 ml) was administered, totaling 215 ml. Initially, the plan was to tape the azygos vein together with the cranial vena cava. However, owing to the distance, a suction cannula was inserted through the right atrial incision to perform suction venous drainage from the blood perfused from the azygos vein.
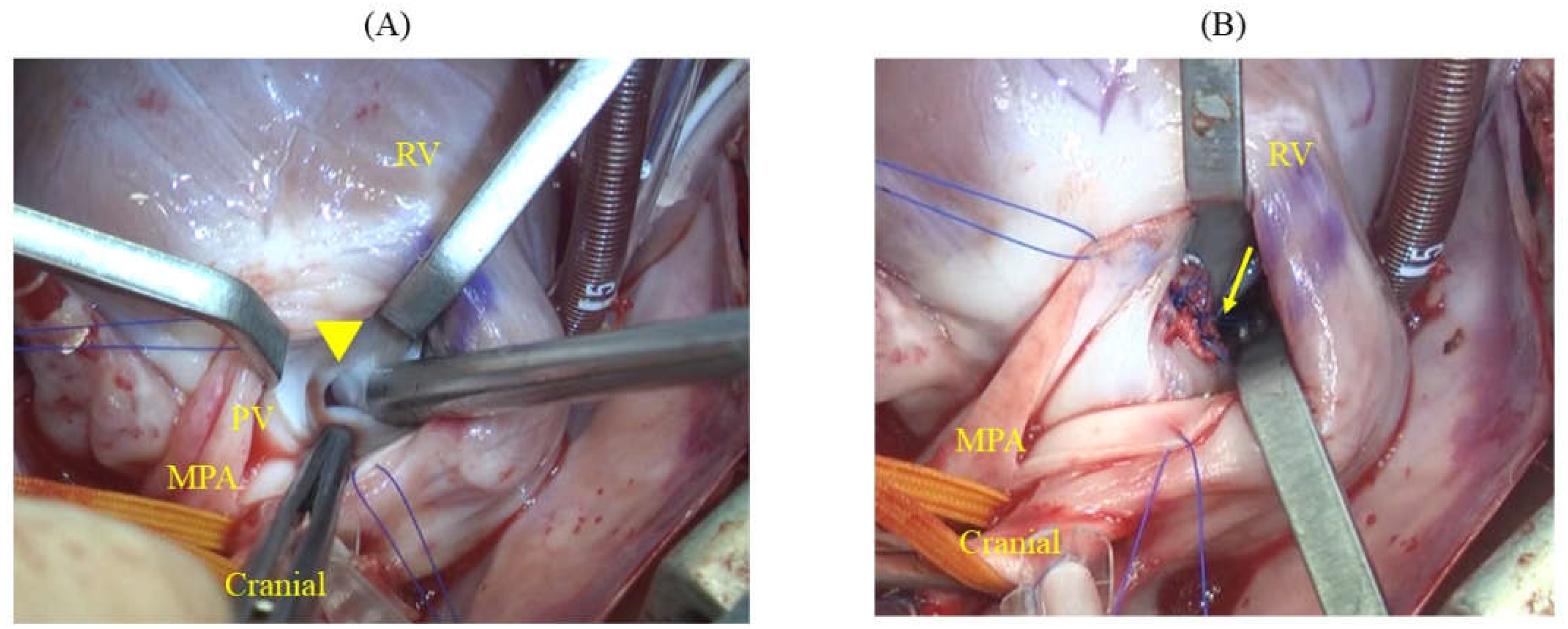
After cardiac arrest was induced, the main pulmonary artery was incised, and the pulmonary valve was incised to the right ventricle at the commissure to expose the ventricular septum. This revealed an elliptical defect surrounded by muscular tissue, measuring 9 mm in length and approximately 2–3 mm in width (
Figure 3A). The full extent of the defect was visualized by inserting a right-angle clamp into the defect and pulling it towards the operator. The rim of the defect was sutured using six double-armed sutures with polypropylene pledgets (Oval-M; Matsudaika Inc., Tokyo, Japan). A patch (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) was used to close the defect, which was tailored to fit the size of the hole. Double-armed needles previously sewn into the rim were then threaded through the circular patch in sequence, and closure was completed once it was confirmed that no blood had leaked from the left ventricle (
Figure 3). Subsequently, blood was evacuated from the left atrial vent cannula, and the pulmonary artery and right ventricle were continuously sutured using 6-0 polyvinylidene fluoride sutures. The right atrium was similarly sutured continuously, and upon release of the aortic cross-clamp, spontaneous beating of heart resumed. Partial cardiopulmonary bypass continued until the patient was rewarmed to 36.5 °C. The total aortic cross-clamp time was 56 min and cardiopulmonary bypass duration was 105 min. Subsequently, a chest drain was placed and the chest was closed in the standard manner. Postoperative pain was managed with fentanyl (2 microg/kg/h) administration continuously for 24 h.
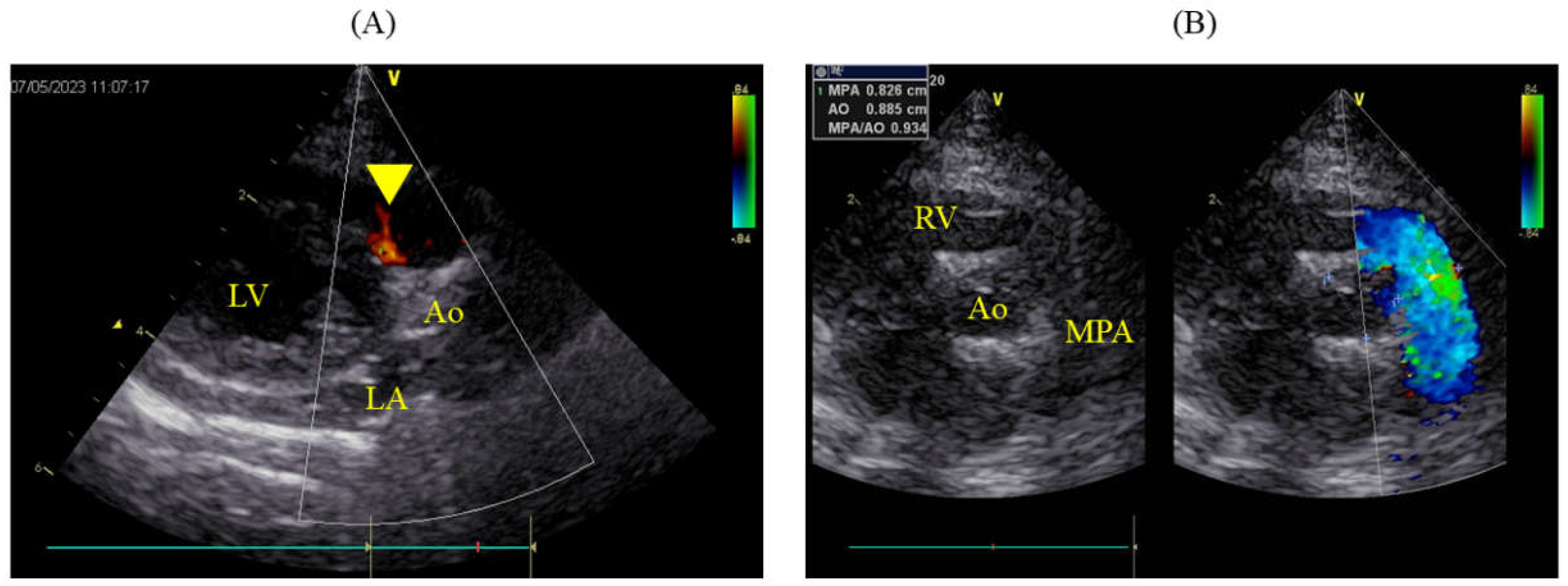
The packed cell volume (PCV) decreased to 15.7% postoperatively, necessitating a transfusion of 49 ml of blood, after which the PCV on the following day was 24.3%. Urine output immediately post-surgery was maintained at 2 ml/kg/hr but subsequently decreased, and by the second postoperative day, azotemia was observed (blood urea nitrogen, BUN: 78.5 mg/dL; creatinine, Cre: 3.6 mg/dL). Blood potassium level remained normal at 4.03 mmol/L. Continuous fluid therapy resulted in normalization by postoperative day 12 (BUN: 26.0 mg/dL; Cre: 1.2 mg/dL). However, by postoperative day 7, the pleural effusion had turned milky, requiring approximately 100 ml/day to be drained. The triglyceride (TG) and total cholesterol (T-Chol) levels in the pleural fluid were 60.0 mg/dL, with serum levels at TG 59.0 mg/dL and T-Chol 183.0 mg/dL. From postoperative day 8, oral administration of rutin 500 mg three times daily was initiated. A contrast-enhanced CT scan under anesthesia was performed on the postoperative day 12. The thoracic duct was highlighted using an iodinated contrast (iohexol 300 mg/ml; Fuji Pharma Co., Ltd., Tokyo, Japan) injected subcutaneously at 1.8 ml/kg around the anus, followed by 5 min of massage. This revealed significant collateral development in the anterior chest and leakage of the contrast agent into the pleural cavity. No thrombi or embolic material was found in the cranial vena cava. The milky effusion gradually decreased and resolved by postoperative day 15, allowing removal of the chest drain on the following day and discharge on postoperative day 17. Antithrombotic therapy was not implemented in this case. On the postoperative day 490, the patient remained asymptomatic and lively, with no recurrence of the chylothorax. Echocardiography showed slight residual shunting from the VSD (
Figure 4A), but the pulmonary artery had returned to normal (MPA: Ao 0.93; 8.3/8.9 mm), and both LA/Ao and LVIDd were within normal ranges at 1.41 (1.25/0.89 cm) and 1.18 cm, respectively (
Figure 4B).
4. Discussion
We encountered a cat with an infundibular muscular VSD classified according to the Soto classification, and surgically closed the defect using total bypass perfusion via a median sternotomy approach with a heart-lung machine. To the best of our knowledge, there are no prior reports of the surgical closure of VSD in cats, nor are there any reports specifically detailing infundibular muscular VSD. Additionally, while cardiac surgeries in cats using cardiopulmonary bypass, including left atrial-to-atrial septal defects, have been reported, these were conducted via an intercostal approach and involved partial bypass perfusion without cardiac arrest induced by cardioplegia [
14,
15].
Initially, the cat was diagnosed with the most common type of VSD in felines, perimembranous VSD, via echocardiographic examination. However, upon opening the chest and palpating the heart, the thrill observed in the pulmonary outflow tract led to a diagnosis of infundibular muscular VSD. In felines, VSDs are generally classified according to the Kirklin classification, where Type 1 VSDs are supracristal, Type 2 are either membranous or perimembranous, and Types 3 and 4 are atrioventricular canal and muscular VSDs, respectively, with 79% diagnosed as membranous or perimembranous [
3].
However, in this case, the VSD was located in the outflow septum and surrounded entirely by muscular tissue, making it difficult to classify using the Kirklin classification. The Soto classification, commonly used in humans, categorizes VSDs into four types: (1) infundibular defects situated in the infundibular septum, often part of which abuts the semilunar valves; (2) membranous defects centered around the membranous septum and extending to areas near the atrioventricular or aortic valves; (3) defects between the membranous part and the inflow tract, similar to those seen in atrioventricular septal defects; and (4) muscular defects, where the surrounding tissue of the defect is entirely muscular [
7]. Furthermore, muscular defects are sub-classified based on their location: infundibular, inflow tract, and trabecular septums.
According to the Kirklin classification, a Type 1 defect located above the crista supraventricularis corresponds to an infundibular defect according to the Soto classification, where the aortic valve may deviate. In the Kirklin classification, Type 1 defects are located above the crista supraventricularis and correspond to infundibular defects in the Soto classification. These defects are large and occur directly below the pulmonary valve, potentially causing deviations in the aortic valve. However, in the present case, although the defect was near the crista supraventricularis, it was not an infundibular defect because of its distance from the pulmonary artery. Consequently, it was ultimately classified as a muscular defect according to the Soto classification and diagnosed as an infundibular muscular VSD. This type of defect is advantageous for suturing because unlike perimembranous VSDs, it does not have a nearby conduction system.
In the case of membranous defects, color Doppler imaging using the clock-face method typically reveals a defect between the 10 and 11 o’clock positions. Conversely, a defect under the aortic valve (subaortic VSD) appears between the 11 and 1 o’clock positions, and in the Kirklin classification, a Type 1 defect is seen between the 1 and 2 o’clock positions [
16]. In the present case, the absence of a defect directly beneath the pulmonary artery and the shunt flow originating from the 12 o’clock direction suggested that it was unlikely to be an infundibular or membranous defect. Thus, a preoperative diagnosis of infundibular muscular VSD may have been possible.
Additionally, there was a slight discrepancy between the measurements of VSD size by using color Doppler imaging and those obtained visually during surgery. This discrepancy could be attributed not only to the difference between the beating heart and the heart relaxed by cardioplegia but also to the elliptical shape of the defect. Studies on humans have reported that three-dimensional (3D) echocardiography can measure the size and location of defects more accurately than that measured using two-dimensional echocardiography [
17].
Henceforth, particularly in cats undergoing closure procedures, it would be beneficial to use preoperative 3D echocardiography to better understand the size and location of defects. Additionally, the Soto classification appears to be more appropriate for categorizing VSDs, especially when traditional methods such as the Kirklin classification encounter limitations owing to anatomical variations specific to felines.
Shunt volume in VSD is determined by the size of the defect, pulmonary vascular resistance, and systemic vascular resistance, with severity typically assessed by the Qp:Qs. When Qp:Qs is less than 1.5, between 1.5 and 2.5, and greater than 2.5, the VSD is defined as small (restrictive), moderate (moderately restrictive), or large (nonrestrictive), respectively, correlating with the VSD: Ao ratio [
3]. A higher Qp:Qs ratio is associated with pulmonary hypertension (PH) and heart failure; in this case, both high Qp:Qs and high VSD: Ao ratio were noted, along with significant dilation of the main pulmonary artery. When Qp:Qs is < 1.5, it is advisable to monitor the patient’s condition without any intervention. However, if Qp:Qs exceeds 2.5 and there are clinical signs such as cardiac enlargement or indicators suggestive of PH, aggressive therapeutic intervention is warranted [
3].
Surgical correction using a cardiopulmonary bypass is less common in cats than in dogs. In dogs, it is common to establish a cardiopulmonary bypass by inserting a perfusion cannula into the common carotid artery; however, in cats, this artery is too narrow to accommodate the necessary equipment. Additionally, cats have a smaller blood volume, which complicates securing sufficient blood for transfusion, and leads to anemia due to blood dilution during cardiopulmonary bypass.
In cats, surgical corrections for conditions such as Cor Triatriatum Sinister (CTS) and atrial septal defect (ASD) have been reported, utilizing intercostal approaches without the use of cardioplegia, thereby avoiding blood dilution [
14,
15]. In ASD repair, a 5 Fr feeding tube is used as a substitute for a perfusion cannula and inserted into the common carotid artery [
15]. For the CTS, an 8 Fr perfusion cannula was inserted into the descending aorta [
14]. In our case, because a median sternotomy approach was used, we were able to insert an appropriately sized perfusion cannula into the ascending aorta without difficulty, maintaining cerebral circulation due to the direction of blood flow. For venous drainage, the ASD cases involved the insertion of 8 Fr straight-type cannulas into both the cranial and caudal vena cava [
15], whereas in CTS, a 16 Fr cannula was inserted into the right atrium [
14]. In this case, we chose a curved venous return cannula for the cranial vena cava to facilitate insertion at the tip of the cannula.
Unlike the previous two cases, the use of cardioplegia in this case provided a stable field for surgery, making the procedure easier. However, 215 ml of cardioplegia was administered, which resulted in postoperative anemia. Transient renal impairment observed in this case may have been associated with anemia [
18]. Given that hypothermia during cardiopulmonary bypass can reduce heart rate, surgery under pulsatile conditions might have been possible [
15,
19]. Furthermore, although not explored in cats, techniques such as electrically induced ventricular fibrillation have been reported in dogs [
20]. In cats, where the donor blood supply is often insufficient, future considerations may need to include using less or avoiding cardioplegia altogether.
In veterinary medicine, open-heart surgery typically involves intercostal thoracotomy approach. The right intercostal approach is appropriate for treating common membranous VSDs in cats. This allows the insertion of venous drainage cannulae into both the cranial and caudal vena cava, enabling VSD closure through an incision in the right atrium or ventricle. However, in this case, an infundibular muscular VSD was diagnosed after thoracotomy at the left ventricular outflow tract. Such a diagnosis would likely have resulted in an unsuccessful outcome with the right intercostal approach, justifying the use of a median sternotomy approach to prepare for unforeseen circumstances.
Additionally, the patient exhibited transient chylothorax postoperatively, which resolved with conservative treatment. Chylothorax is a rare complication in humans following thoracotomy, primarily caused by thoracic duct damage, with no significant difference in incidence between the intercostal and median sternotomy approaches [
21]. In cats, the thoracic duct runs along the left side of the aorta and drains into the venous system [
22]. The possibility of thoracic duct damage cannot be excluded in this case because the ascending aorta was dissected to insert perfusion and root cannulas using the median sternotomy approach. Thus, careful handling of major vessels is necessary when performing a median sternotomy.
Postoperative chylothorax can also be associated with elevated central venous pressure during surgery [
23]. In this case, the central venous pressure might have increased due to taping of the cranial vena cava, potentially contributing to thoracic duct damage. However, similar to other cases in cats in which chylothorax does not necessitate surgery unless the thoracic duct is severed, this condition improved with the administration of rutin and regular drainage from a chest drain [
24]. Therefore, conservative treatment and observation over 1–2 weeks are suitable for managing postoperative chylothorax following open-heart surgery.