1. Introduction
At present, most of the conventional masks on the market use ordinary non-woven fabrics as base fabrics with essence. However, some unstable active ingredients in the liquid components of facial masks are easily oxidized and deteriorated by the external environment, causing skin allergies and other problems [
1]. Electrospinning, an emerging facial mask fabrication technology, can generate nanofiber masks that effectively tackle the previously mentioned issues. This technique allows for the production of continuous nanoscale fibers derived from both synthetic and natural polymers [
2,
3,
4]. The fibres generate by electrostatic spinning has the characteristics of high porosity, large specific surface area, high air permeability and small pore size within the fiber [
5,
6]. Therefore, electrospinning has received widespread attention and has been used in tissue engineering [
7], filtration [
8], energy [
9], and sensors [
10], food engineering [
11] and cosmetics [
12]. As a novel type of facial mask, there are relatively few nanofiber mask products available on the market [
13]. With the increasing market demand for high-end facial mask products, the development of a nanofiber facial mask amenable to large-scale production via electrospinning technology holds great significanc. Nanofiber masks based on electrospinning technology can efficiently capture active ingredients within fibers or meshes. Moreover, the nanofiber mask is stored in a solid-state and will absorb water and become moist when applied to the skin, which greatly enhances product stability. When the facial mask becomes wet, the components within it rapidly dissolve and release active ingredients, ensuring maximum penetration into the skin and restoring its youthful state. In addition, the nanostructure of the nanofiber mask can ensure maximum fit with the skin surface. The nanofiber mask is not only softer than traditional masks, but its nutrients are also more readily released onto the skin's surface, enhancing skin permeability and restoring its youthful state. According to different consumer needs, different raw materials can be used to produce nanofiber masks with different functions through electrospinning technology to achieve personalized care and precise skin care. Currently, many different types of nanofiber membranes have been produced based on electrospinning technology. Activlayr technology has prepared a quick-dissolving nanofiber mask. This mask is made from deep-sea puffer fish in New Zealand. It contains a variety of natural collagen extracts and active ingredients. It will quickly dissolve in just 3 to 5 seconds and is ready for use. It brings an extremely fast skin care experience [
14,
15]. In addition, nanofibers can load a variety of cosmetic ingredients, such as glycerin, vitamin C, okra polysaccharides, active polypeptides, tea polyphenols and small molecule sodium hyaluronate. When the quick-dissolving nanofiber mask comes into contact with water, these functional ingredients will quickly be absorbed by skin. YANG [
16] et al. prepared a multifunctional cellulose mask containing hindered phenol groups through electrospinning technology,which has excellent free radical scavenging properties and can effectively remove excessive free radicals while shielding ultraviolet damage and delaying the aging of skin tissue. TANG [
17] et al. provide a facile approach to develop biocompatible polymer-based electrospinning fiber masks to effectively deliver herbal extracts for topical skin treatment, in It has shown significant clinical efficacy in the treatment of mild to moderate acne, opening up new prospects for a new generation of beauty textiles.
Therefore, in order to solve the problems of traditional facial masks such as poor comfort, poor breathability, insufficient absorption of nutrient solution and solution dripping. This paper develops a high-performance nanofiber facial mask through needleless electrospinning technology, explores the optimal preparation process of nanofiber facial mask, and comprehensively analyzes the performance of nanofiber facial mask to highlight its advantages as a new nanofiber facial mask.
2. Materials and Methods
Collagen peptide powder for the experiment was sourced from Shandong Xinsheng Biotechnology Co., Ltd., while polyethylene oxide (PEO) with a molecular weight of 500,000 was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Nonwoven fabrics were purchased from Shijiazhuang Tianjinsheng Nonwoven Technology Co, Ltd. Deionized water was purchased from Aite (Shandong) New Materials Co, Ltd. At room temperature, combine PEO with deionized water to create a transparent solution with a mass fraction of 9% w/v. Used an electric stirrer to stir for 6 hours until the solution was uniform. Then, mixed the collagen peptide powder and deionized water solvent to form a facial mask essence solution with a mass fraction of 10% w/v, and stirred at room temperature for 4 hours until the solution was uniform. Finally, mixed the prepared PEO solution and collagen peptide essence to make 5 groups of solutions. The proportions of collagen peptide solution in PEO solution were 0%, 10%, 15%, 20%, and 25% respectively. Each set of solutions was thoroughly stirred for 4 hours until uniform and transparent, which was used as the spinning solution.
3. Preparation of Nanofiber Facial Mask
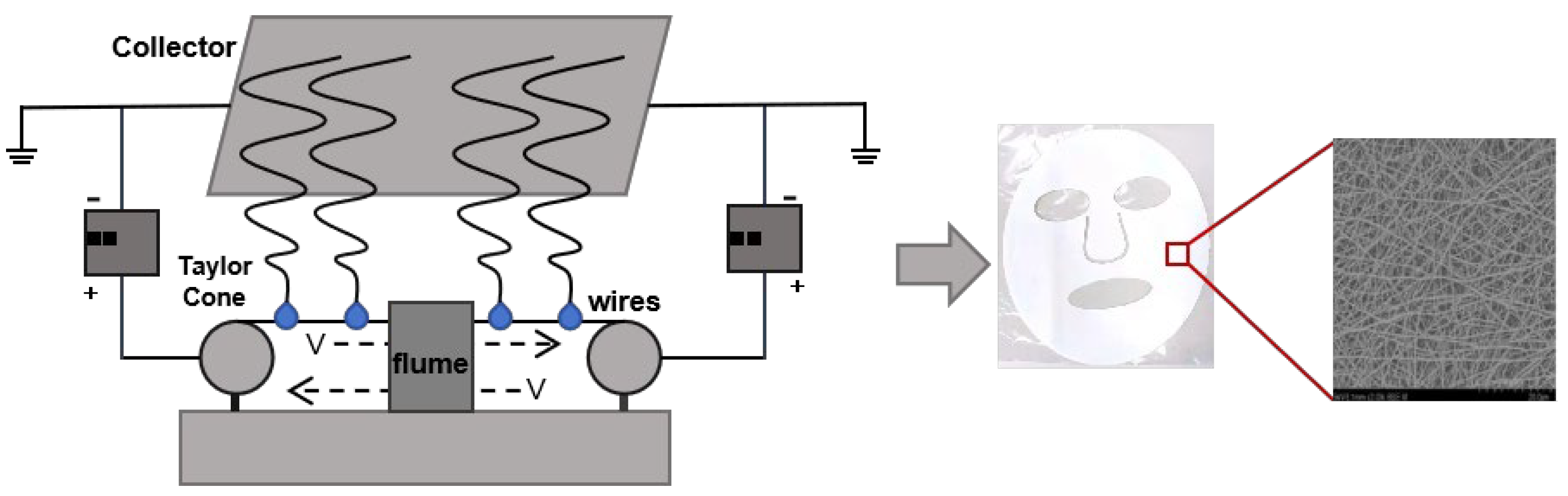
The needleless electrospinning device mainly consists of a liquid supply system, a high-voltage power supply, a metal wire and a collection platform. Its working principle is shown in
Figure 1. Before preparing the nanofiber mask, wind the steel wire tightly onto the spinning electrode. Turn on the environmental control module of the device. Wait for two hours. Adjust the humidity to 40±2%, and the temperature to 26±2℃. When the temperature and humidity inside the spinning equipment are stable, fit the base material to the collection platform and adjust the appropriate liquid supply speed. The receiving speed is 0.5m/min. Pour the spinning liquid of PEO/collagen peptide essence into the liquid tank, and evenly apply the spinning liquid to the steel wire through the reciprocating motion of the liquid tank. After the steel wire is wetted, a uniform solution film is formed on the surface. A high-voltage electrostatic field is applied between the steel wire electrode and the receiving device. When the charge density on the surface of the solution thin layer reaches a critical value, the electrostatic force overcomes the surface tension of the solution and the solution is stretched. A jet is formed and ejected. As the jet flies toward the receiving device, it undergoes solvent evaporation and stretching, and finally deposits on the receiving device to form nanofibers. After spinning for a period of time, the nanofiber masks formed by different process parameters (voltage, liquid supply speed, collection distance) are collected by adjusting the process parameters. The preparation process is shown in
Figure 1. Finally, the prepared nanofiber mask was cut and placed in a drying box for 6 hours, dried at room temperature, and then sealed and stored.
4. Characterization Method
First, the nanofiber facial mask prepared by the needleless electrospinning device was cut and sampled in a targeted manner, and then a plasma thin film sputtering instrument (GSL1100X-SPC-12) was used for gold spraying treatment, and then a Hitachi electron microscope equipment (TM4000) was used for detection. In order to prevent the fiber membrane from falling off during the vacuum extraction process, a mask sample of appropriate size needs to be fixed on a metal specimen stage, and the fiber morphology magnified 1500 times is observed through an accelerating voltage of 5kV. After taking the picture, use ImageJ software to randomly select 50 fibers for measurement, and use Origin software to count the fiber diameter distribution.
5. Results and Discussion
5.1. Effect of Different Mixing Ratios of PEO/Collagen Peptide Solutions on Fiber Morphology
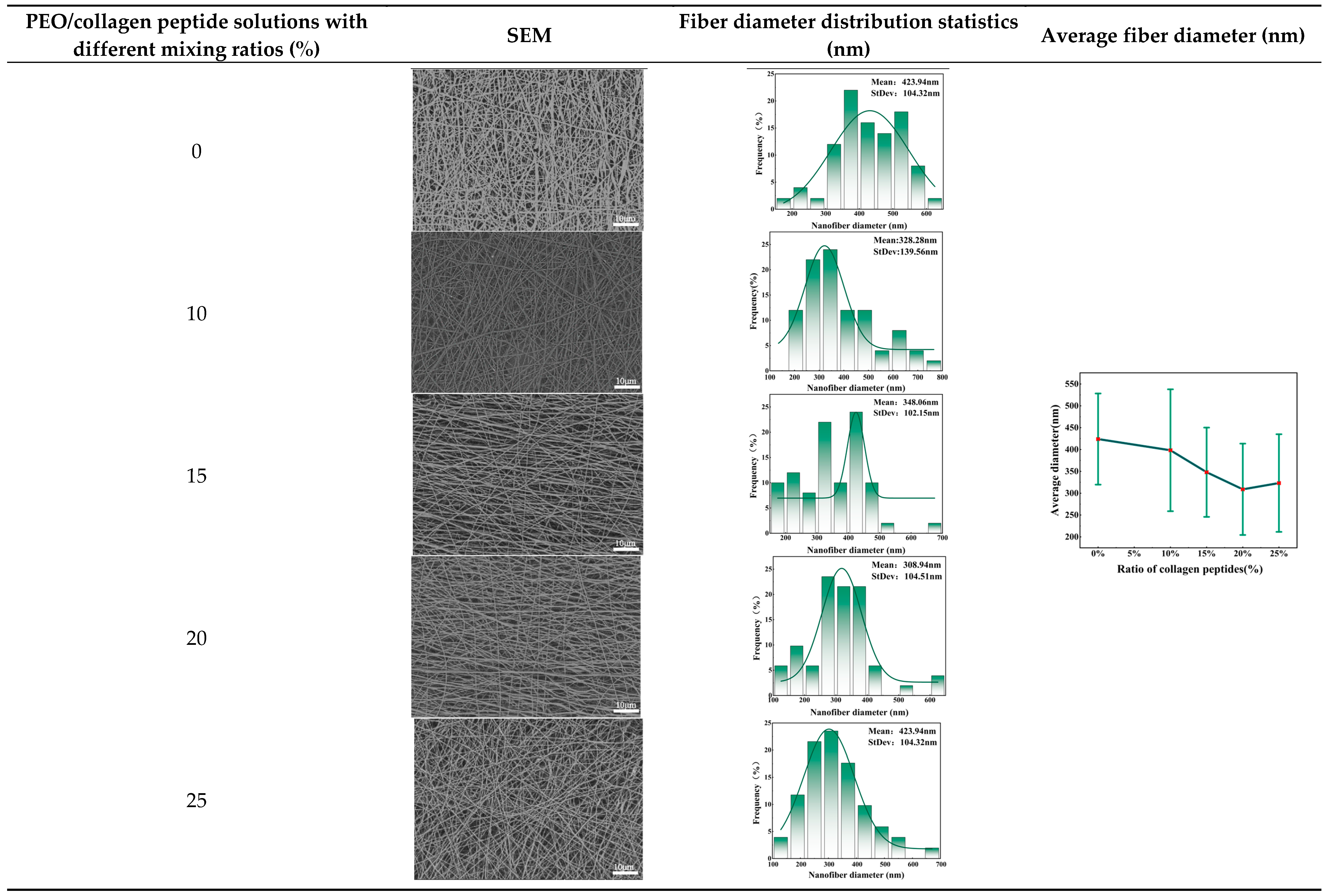
In order to explore the effect of different ratios of PEO/collagen peptide solutions on fiber morphology, in this set of experiments, the liquid supply speed was 130ml/h. The voltage was 30kV. The collection distance was 19cm, and the receiving speed was 0.5m/min. Then prepared a PEO solution with a mass fraction of 9% w/v and mixed it with collagen peptide essence solutions in different proportions. Set the collagen peptide solution proportions to 0%, 10%, 15%, 20%, and 25% respectively. After spinning, 5 kinds of nanofiber facial masks with PEO/collagen peptide combinations were obtained.
Table 1 shows the SEM images, fiber diameter distribution statistics and average fiber diameter of facial masks prepared from solutions with different collagen peptide proportions. Among the five collagen solutions with different ratios, when the proportion of collagen peptide solution was 0%, the average diameter of the fibers prepared was the largest. When the proportion of collagen peptide solution was 20%, the average fiber diameter was smaller. The standard deviation was small and the distribution was relatively uniform. This difference is mainly due to the viscosity characteristics of the spinning solution. As the proportion of collagen peptide solution increases, the viscosity of the spinning solution decreases, and the fibers are more obviously stretched during the forming process, causing the fibers to gradually become thinner. When the proportion of collagen peptide solution was increased to 25%, due to the low viscosity and insufficient intermolecular interaction, continuous fibers could not be formed and a fiber membrane with good morphology could not be obtained. When the proportion of collagen peptide solution was increased to 25%, due to the low viscosity and insufficient intermolecular interaction, continuous fibers could not be formed and a fiber membrane with good morphology could not be obtained.
5.2. Effect of Different Spinning Voltages on Fiber Morphology
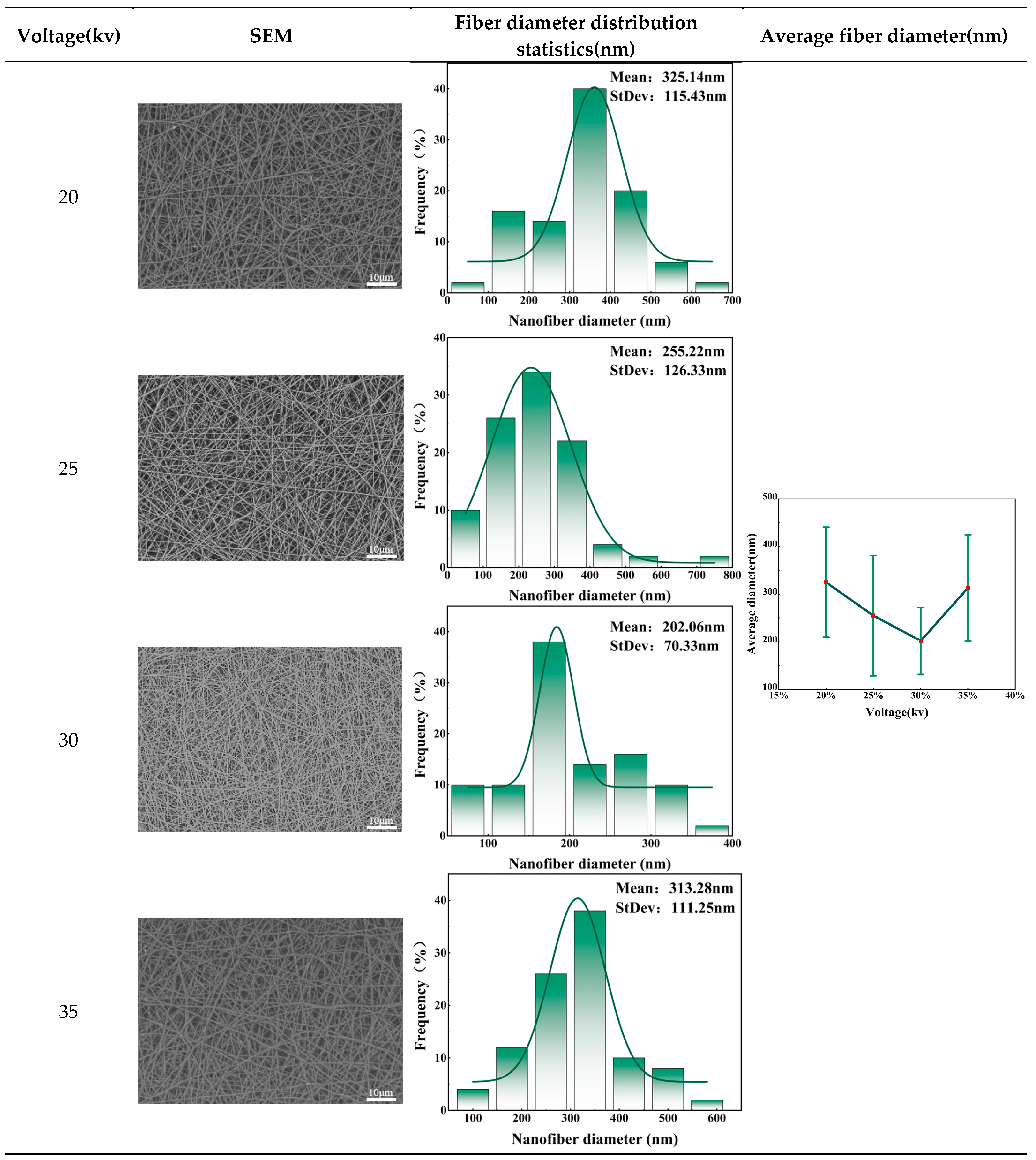
The formation of fibers requires exceeding a specific spinning voltage threshold. Only when the voltage threshold is exceeded can jets be generated and eventually refined into fibers. Therefore, voltage is identified as one of the key factors affecting fiber morphology. In order to explore the effect of voltage on fiber morphology, 20% collagen peptide solution was selected as the spinning solution. The liquid supply speed was set to 130ml/h. The receiving speed was 0.5m/min, and the voltage gradient was set to 20kV, 25kV, 30kV and 35kV to further find the optimal voltage parameters. Presented below are nanofiber masks prepared under different voltages.
Table 2 shows the SEM images, fiber diameter distribution statistics and average fiber diameter of the facial mask prepared under different voltage values measured. From the above figure, it could be seen that the electric field strength of electrospinning increases with the increase of applied voltage, causing the traction force of the multi-jet to increase, which in turn causes the fibers to become smaller and denser. However, when the voltage rose to a certain value, the traction force on the jet will be further enhanced, increasing the instability of the jet, causing the average diameter of the fiber to begin to increase, and the standard deviation to become larger, resulting in uneven fiber distribution. Therefore, within the range of 20kV to 35kV, the voltage selection should avoid being too high or too low, so as not to cause the average diameter of the mask fibers to increase and the distribution to be uneven. The optimal voltage value is 30kV. At this time, the average diameter and standard deviation of the fiber are smallest and the distribution is most uniform.
5.3. Effect of Different Receiving Distances on Fiber Morphology
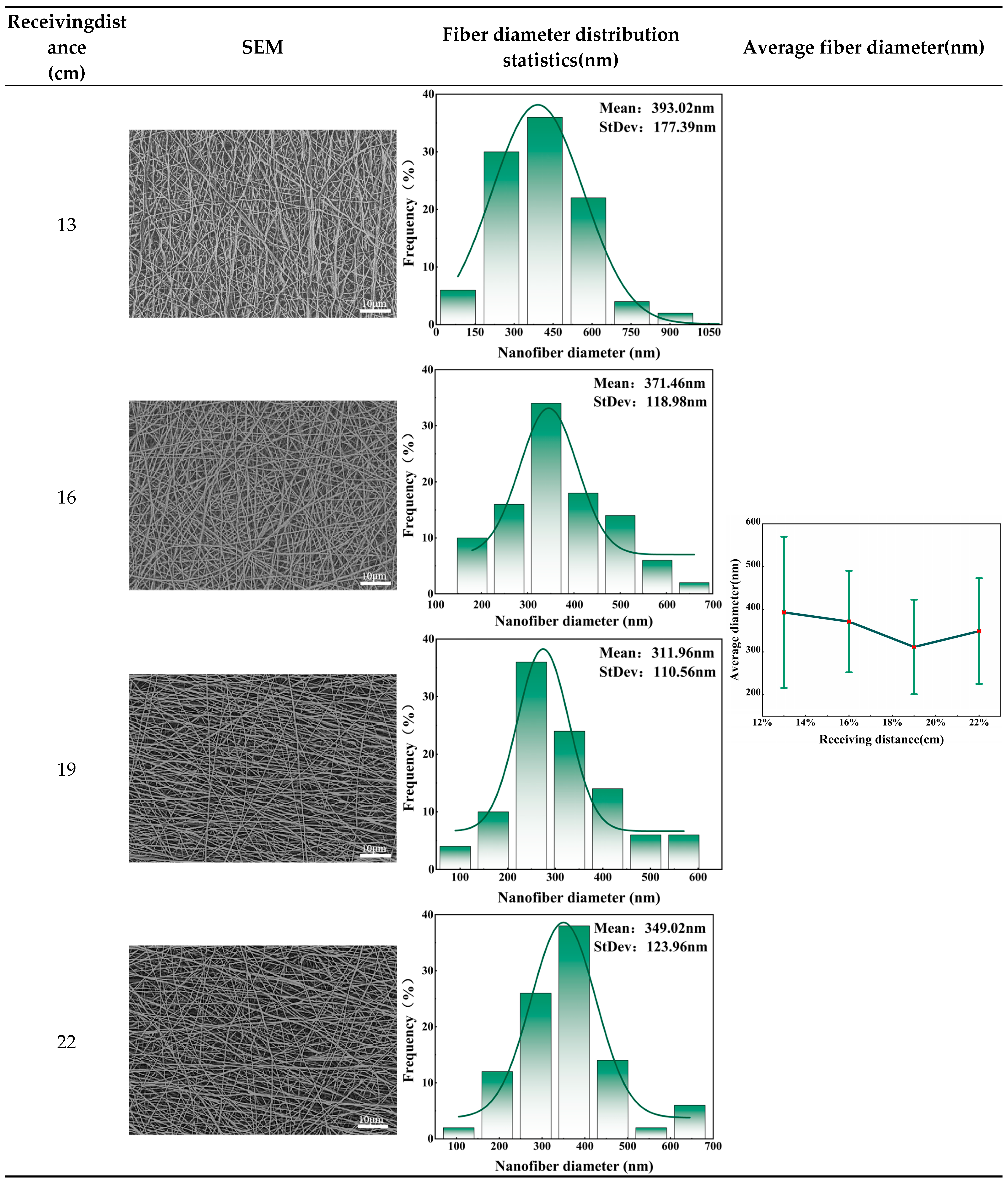
Different receiving distances will affect solvent volatilization and multi-jet electric field intensity, resulting in changes in fiber morphology. In order to study the effect of receiving distance on fiber morphology, 20% collagen solution was used as spinning solution. The liquid supply speed was set to 130ml/h. The voltage was 30kV. The receiving speed was 0.5m/min, and the receiving distance gradient was set to 13cm, 16cm, 19cm and 22cm to further determine the optimal collection distance. Below are the nanofiber masks at different collection distances.
Table 3 shows the SEM images, fiber diameter distribution statistics and average fiber diameter of the facial masks prepared at different receiving distances. Combining the above figure, it can be seen that the morphology of the facial mask fiber is significantly affected by the receiving distance. This is because changes in the receiving distance will cause changes in two key factors in the electrospinning process: the multi-jet electric field intensity and the degree of solvent volatilization. In this experiment, when the receiving distance was short, there are two factors acting simultaneously. When the electric field intensity was high, the traction force on the jet was sufficient, which resulted in a reduction in the flight time of the jet and insufficient solvent volatilization, thus making the diameter of the fiber larger. As the distance increases, the flight time of the jet gradually increases, the degree of solvent volatilization gradually increases, and the diameter of the fiber becomes thinner. However, when the receiving distance was far, the volatilization degree of the solvent did not change much. At this time, the electric field intensity becomes the dominant factor. When the electric field intensity of the multi-jet decreases, the traction force of the jet was insufficient, which in turn makes the diameter of the fiber larger.
Therefore, when conducting nanofiber mask experiments, the receiving distance needs to be adjusted according to the intensity of the jet electric field and the volatility intensity of the solvent to ensure that the best morphological fibers are obtained. The solvent used in this study is deionized water, which has high volatility, so a large receiving distance is required to fully evaporate the solvent. As can be seen from the above figure, when the receiving distance is 19cm, the average diameter and standard deviation of the fibers are small and the distribution is relatively uniform, so 19cm is the best receiving distance.
5.4. Effect of Different Liquid Supply Speeds on Fiber Morphology
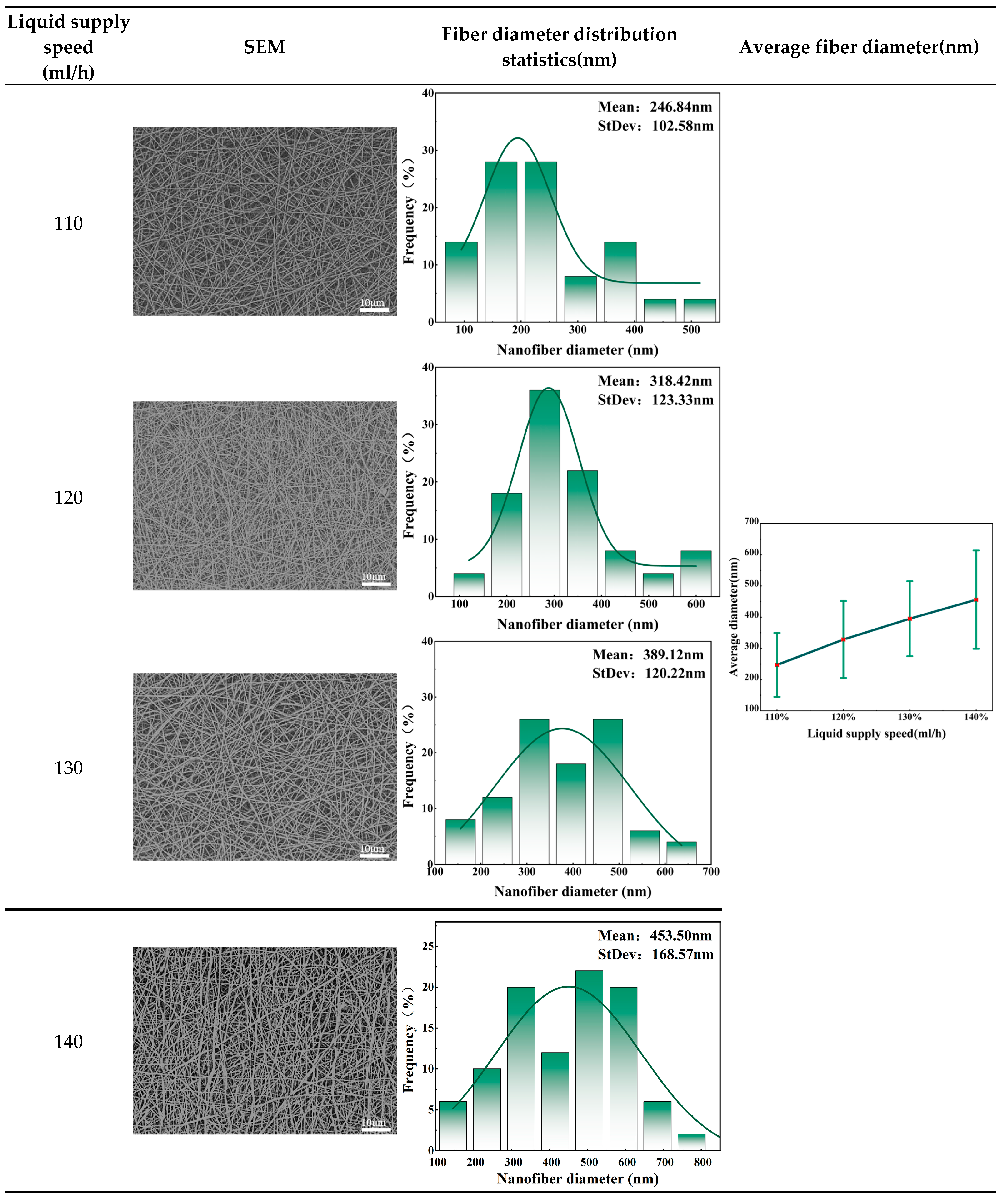
The liquid supply speed will have a significant impact on the degree of solvent volatilization and preparation efficiency. Therefore, the liquid supply speed also plays a key role in needleless electrospinning. In order to explore the effect of liquid supply speed on fiber morphology, 20% collagen solution was selected as the spinning liquid, and the collection distance was set to 19cm. The voltage was 30kV, and the collection speed was 0.5m/min. By setting the liquid supply speed gradient of 110ml/h, 120ml/h, 130ml/h and 140ml/h, the optimal liquid supply speed was further found. In this study, a series of nanofiber facial masks at different liquid supply speeds were prepared to explore the effect of liquid supply speed on their performance.
It can be seen from
Table 4 that as the liquid supply rate increases, the average diameter of the prepared facial mask fibers shows an increasing trend. This trend may be due to the fact that as the liquid supply rate increases, the solution flow rate also increases during the same time. However, the electric field strength is not enough to fully affect the jet flow, resulting in multi-jet instability, thereby causing an increase in fiber diameter and standard deviation. On the other hand, the liquid supply speed also affects the production efficiency of electrospinning. Therefore, taking production efficiency and fiber morphology into consideration, the liquid supply speed is preferably 130ml/h.
6. Performance Analysis of Needleless Electrospinning Nano-Mask
6.1. Fourier Transform Infrared Spectroscopy (FTIR) Measurement
In order to analyze the chemical composition of the PEO/collagen peptide nanofiber facial mask, five groups of PEO/collagen peptide nanofiber facial masks with different ratios were tested using infrared spectroscopy using a NicoletIS50 Fourier transform infrared spectrometer. Set the number of scans to 32 and the resolution to 4. And export the data from OMNIC infrared spectrum software, using Origin software to process and analyze the data.
6.2. Moisturizing Performance Experimental Design
In order to analyze the moisturizing and hydrating performance of the PEO/collagen peptide nanofiber facial mask, the SK-IV digital skin moisture detector was used to test the moisturizing performance of five groups of PEO/collagen peptide nanofiber facial masks with different ratios. The specific testing process is as follows:
Expose the target skin area to the air for 10 minutes at room temperature (25℃), and then use an instrument to measure the moisture content and oil content of the skin at that time.
Apply 5 groups of PEO/collagen peptide nanofiber masks with different ratios evenly to the skin test site, and use a spray bottle containing deionized water to apply the mask to melt and be absorbed by the skin. Then, gently massage the skin area, and after 10 minutes, measure the moisture content and oil content of the mask area. (Before each experiment, a certain period of time should be passed and the skin's moisture content and oil content should be re-measured).
As a control experiment, while performing step 2, spray deionized water on the adjacent parts of the skin where the mask is applied, and massage gently. After 10 minutes, measure the skin moisture and oil content of the area at the same time.
6.3. Quick-Dissolving Performance Experimental Design
In order to study the quick-dissolving effect of the PEO/collagen peptide nanofiber mask, the PEO/collagen peptide nanofber mask was cut into a rectangular shape, and then the mask sample was picked up with tweezers and placed in deionized water. In order to record the dissolution process of the PEO/collagen peptide nanofiber mask, a camera was used to film it. The structure of the quick-dissolving dissolving effect test device is shown in the figure.
7. Experimental Results and Analysis
7.1. Infrared Spectral Analysis
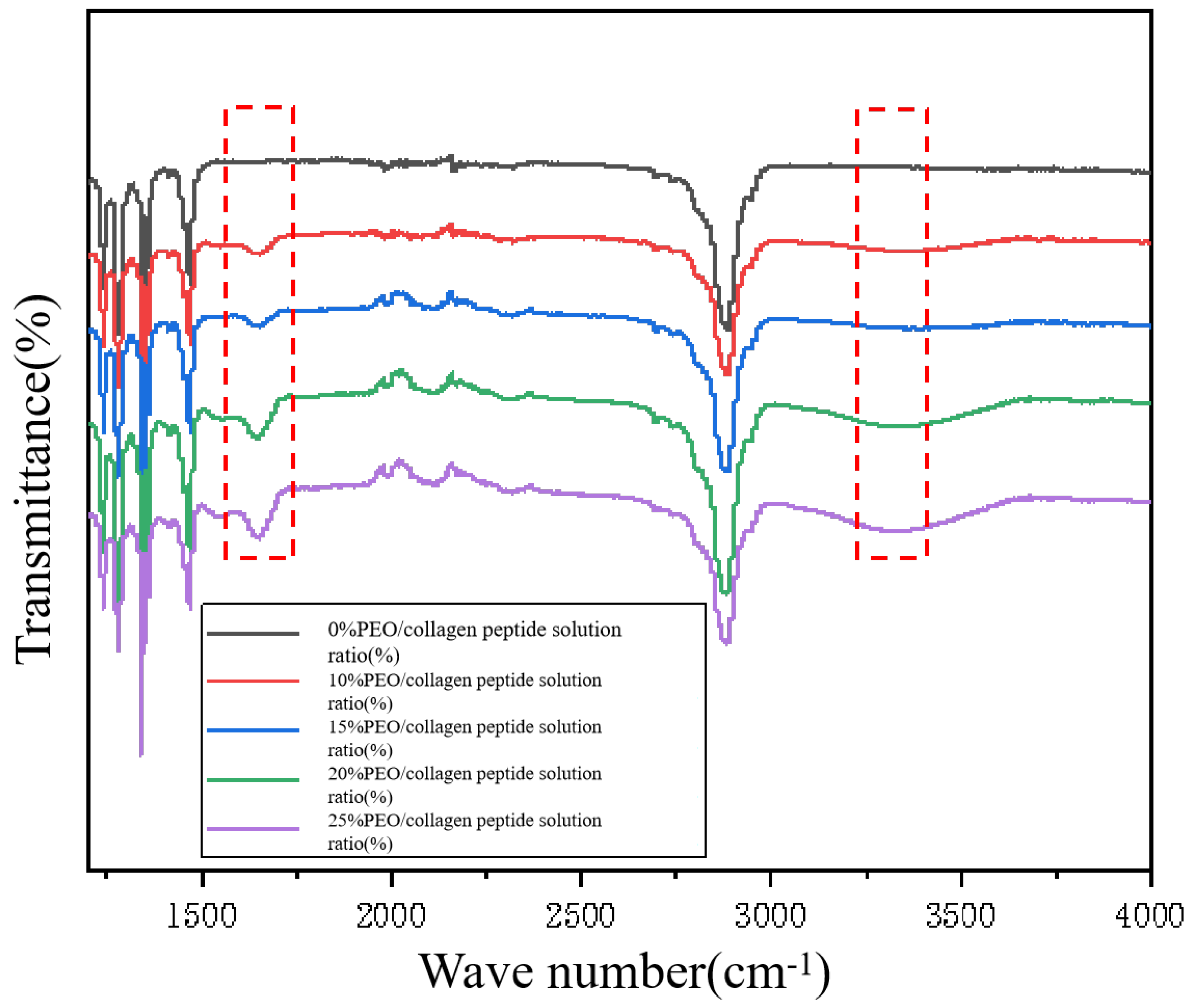
Nicolet IS50 Fourier transform infrared spectrometer is a device that uses substances to analyze the absorption characteristics of infrared radiation of different wavelengths. This equipment can quickly analyze the chemical structure and composition of the PEO/collagen peptide nanofiber mask without damaging it. In this experiment, five groups of PEO/collagen peptide nanofiber facial masks prepared with different ratios were subjected to infrared spectrum detection. The results treated with Origin are shown in
Figure 2.
As can be seen from
Figure 2, after adding collagen peptide, compared with the PEO nanofiber mask, the infrared spectrum has obvious changes at 1650cm1 and 3400cm1, which correspond to the characteristic peaks of collagen peptide. Among them, the characteristic peak of C=O of (-CO-NH-) in the collagen peptide molecule was observed at 1650cm1, which is the amide I region. A characteristic peak appears at 3400cm1, corresponding to the stretching vibration of hydroxyl groups and N-H in collagen peptides. The analysis results in Figure2 show that collagen peptides are successfully loaded into the PEO/collagen peptide nanofiber mask.
7.2. Moisturizing Performance Analysis
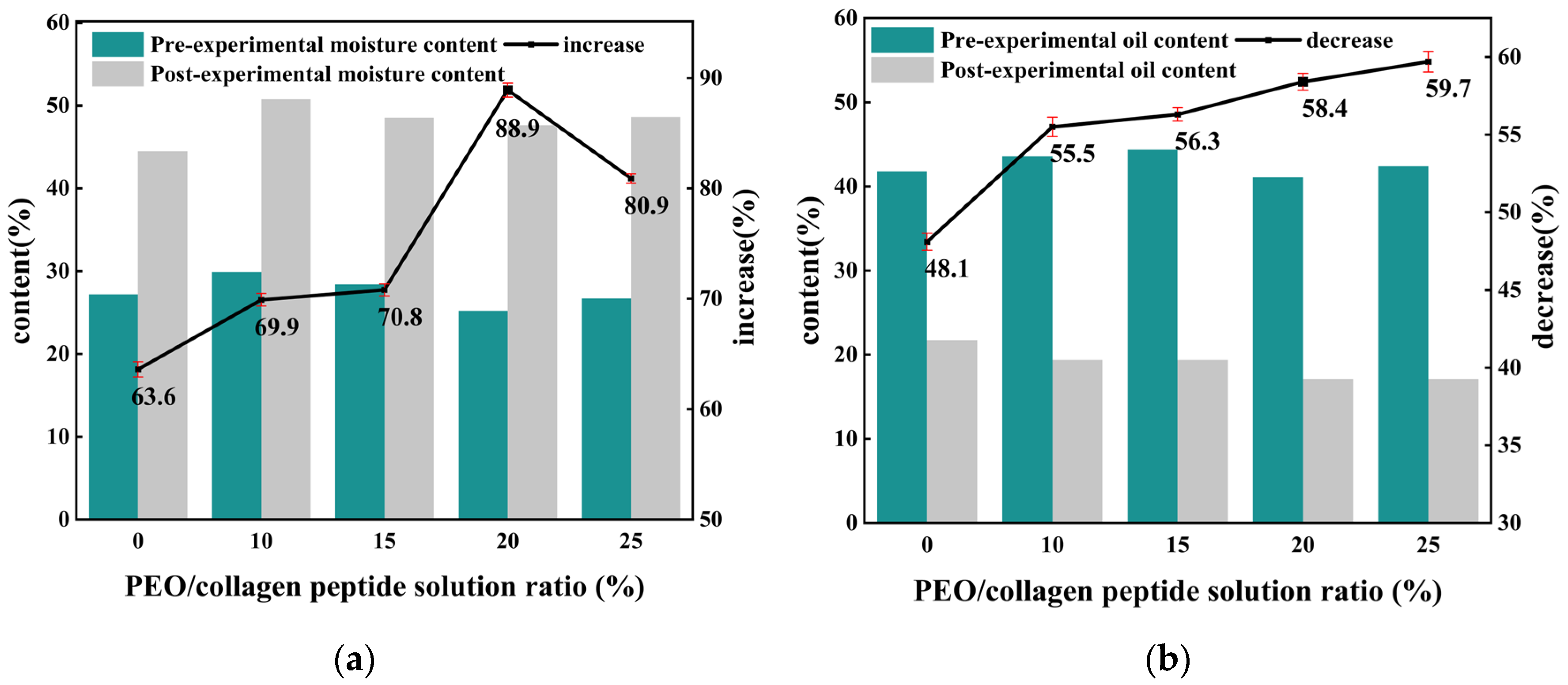
Figure 3 shows the results of testing the moisturizing performance of PEO/collagen peptide masks with different ratios. As can be seen from the table, compared with spraying ionized water directly onto the skin, applying the PEO/collagen peptide mask to the skin can significantly increase the moisture content of the skin and reduce the oil content of the skin, with better results.
As the content of the functional molecule collagen peptide loaded in the nanofiber mask increases, the skin moisture content increases by more than 60%, and the oil content decreases by more than 45%. The increase in skin moisture increased from 63.6% to 88.9%, while the decrease in oil content also increased from 48.1% to 59.7%. This shows that the moisturizing ability of the nanofiber mask is gradually enhanced, and the moisturizing effect on the skin is more obvious. Among them, the skin moisture of the nanofiber mask prepared with 20% collagen peptide solution increased by 88.9% and decreased by 58.4%, which was close to the 59.7% decrease of the 25% collagen peptide solution, and had better moisturizing performance. This may be because the nanofiber mask fibers prepared with 20% collagen peptide solution have the smallest average fiber diameter, are relatively uniform, and have the characteristics of high porosity and large specific surface area. After spraying deionized water, the collagen peptides can be dissolved and absorbed faster. The skin absorbs it, resulting in enhanced moisturizing properties at this time.
7.3. Quick-Dissolving Performance Analysis
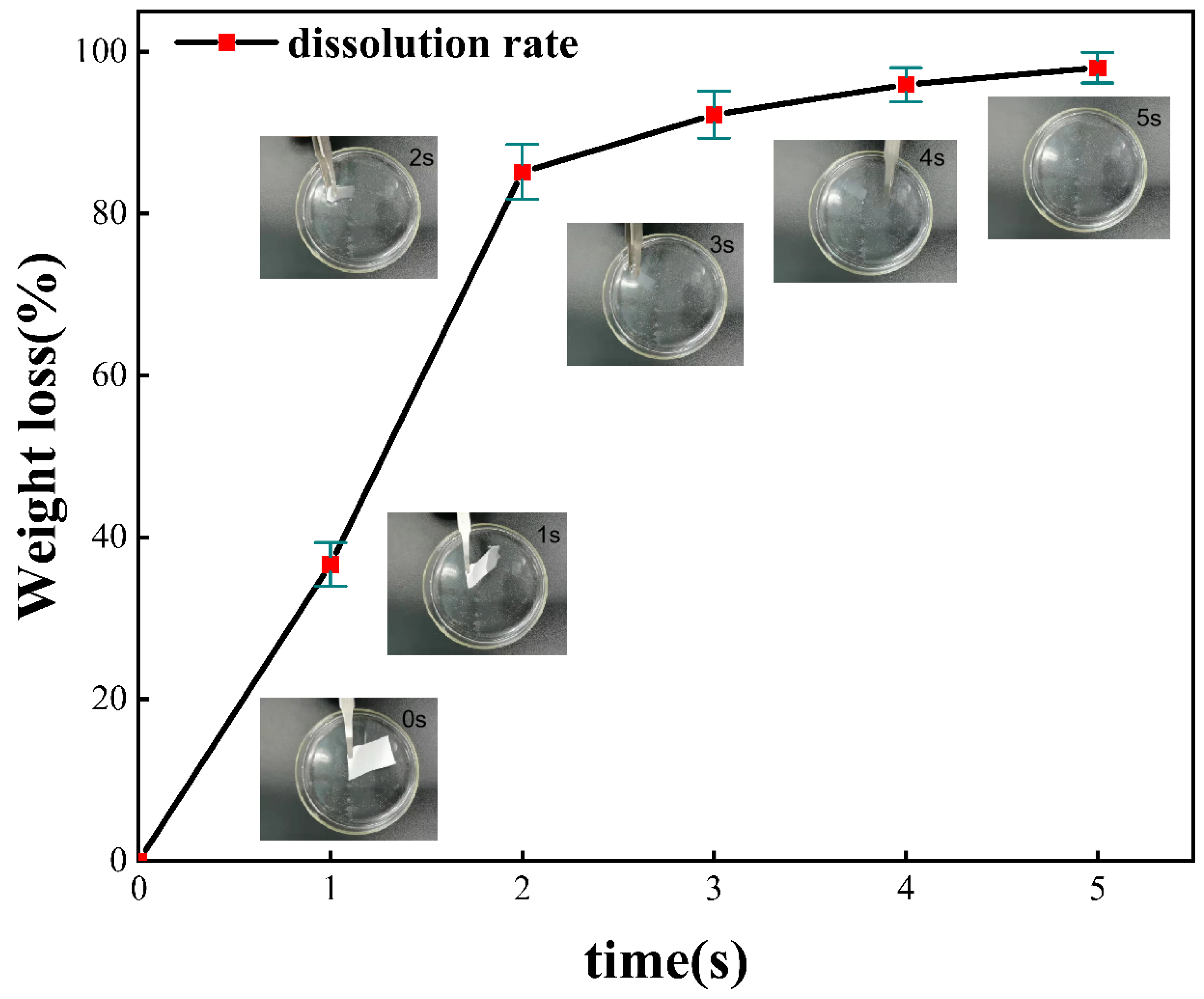
The quick-dissolving dissolution experimental process of PEO/collagen peptide nanofiber mask is shown in
Figure 4. The time in the upper right corner indicates the dissolution of the nanofiber mask at this time. It can be clearly seen that the PEO/collagen peptide nanofiber mask sample begins to dissolve the moment it is put into the ionized water, and quickly spreads around. Within 3 seconds, most of the main body of the mask disappeared visible to the naked eye, leaving some tiny particles floating in the water. At this time, the dissolution rate gradually slowed down. At 5 s, the fiber particles had completely dissolved. During the whole process, it can be clearly seen that the PEO/collagen peptide nanofiber mask dissolves very quickly, and as time goes by, the dissolution rate gradually decreases.
The reason why the PEO/collagen peptide nanofiber mask can dissolve quickly is related to the following factors: First of all, the carrier component PEO of the PEO/collagen peptide nanofiber mask and the functional component collagen peptide are both hydrophilic, making the PEO/collagen peptide nanofiber mask easily soluble in water. Secondly, electrospinning fiber has the advantages of large specific surface area and high porosity, which makes the contact area between the fiber and water larger and makes it easier to dissolve in water.
Therefore, the PEO/collagen peptide nanofiber mask is a quick-dissolving nanofiber mask. A unique advantage of this solid-state nanofiber mask is that after using a spray bottle, spraying water or essence, the water-soluble polymer can be quick-dissolving, allowing the small molecules of efficacy to be quickly absorbed. It is easy to use, can save a lot of time, the quick-dissolving process is visualized, and has super visual effects.
8. Discussion
In this article, a new type of PEO/collagen peptide nanofiber quick-dissolving facial mask was prepared based on needleless electrospinning technology. The influence of different process parameters on the micromorphology of nanofiber facial masks was also explored, including the influence of different mixing ratios of PEO/collagen peptide solutions, spinning voltage, collection distance and liquid supply speed on the mask morphology. Through research, the optimal process parameters for preparing nanofiber masks were obtained, 5 groups of PEO/collagen peptide nanofiber masks with different ratios were prepared, and the composition, moisturizing performance and quick-dissolving effect of the PEO/collagen nanofiber masks were comprehensively analyzed. The result is a high-performance nanofiber mask that is preservative-free, has effective molecules that are quickly absorbed by the skin, and is easy to use.
According to the single factor experiment, it is concluded that: When the spinning liquid is 20% collagen peptide solution, the spinning voltage is 30kV. The collection distance is 19cm, and the liquid supply speed is 130ml/h, the mask morphology is optimal. Five groups of PEO/collagen peptide nanofiber masks with different ratios were tested for infrared spectrum. It was found that the infrared spectrum had characteristic peaks corresponding to collagen peptides, proving that collagen peptides exist in the nanofiber masks. Then, the moisturizing performance of 5 groups of PEO/collagen peptide nanofiber facial masks with different ratios was tested. The results showed that as the amount of collagen peptide added increased, the moisturizing performance became stronger. Finally, the quick-dissolving effect of the solid-state nanofiber mask was explored. The mask was completely dissolved after being soaked for 5 seconds, and the dissolution speed was extremely fast. Compared with traditional facial masks, nanofiber facial masks have more advantages in preservative addition, essence adsorption capacity, compatibility, breathability and production cost, and are more cost-effective.
Author Contributions
Conceptualization, H.W., Q.X. and T.Z; investigation, R.M. , T.Z and X.C.; data curation, X.C., J.H and M.J.; writing—original draft preparation, J.H. and M.J.; writing—review and editing, J.H., X.C., M.J., R.M., Q.W, T.Z and H.W.; visualization, J.H., R.M., Q.X and M.J.; project administration, H.W. Q.X. and T.Z.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y. W.; Chan, S. Y. Novel Vitamin and Gold-Loaded Nanofiber Facial Mask for Topical Delivery. AAPS PharmSciTech 2010, 11 (3), 1164–1170. [CrossRef]
- Subbiah, T.; Bhat, G. S.; Tock, R. W.; Parameswaran, S.; Ramkumar, S. S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96 (2), 557–569. [CrossRef]
- Fang, F.; Chen, X.; Du, Z.; Zhu, Z.; Chen, X.; Wang, H.; Wu, P. Controllable Direct-Writing of Serpentine Micro/Nano Structures via Low Voltage Electrospinning. Polymers 2015, 7 (8), 1577–1586. [CrossRef]
- Liu, C.; Shen, J.; Yeung, K. W. K.; Tjong, S. C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared By Electrospinning. ACS Biomater. Sci. Eng. 2017, 3 (3), 471–486. [CrossRef]
- Su, W.-T.; Wu, P.-S.; Huang, T.-Y. Osteogenic Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth on Poly(ε-Caprolactone) Nanofibers Containing Strontium Phosphate. Mater. Sci. Eng. C 2015, 46, 427–434. [CrossRef]
- Su, W. T.; Liu, Y. J.; Huang, T. Y. Nanofibers Promote HepG2 Aggregate Formation and Cellular Function. Genet. Mol. Res. 2016, 15 (3). [CrossRef]
- Yeo, M.; Kim, G. H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small 2018, 14 (48), 1803491. [CrossRef]
- Park, J. H.; Yoon, K. Y.; Na, H.; Kim, Y. S.; Hwang, J.; Kim, J.; Yoon, Y. H. Fabrication of a Multi-Walled Carbon Nanotube-Deposited Glass Fiber Air Filter for the Enhancement of Nano and Submicron Aerosol Particle Filtration and Additional Antibacterial Efficacy. Sci. Total Environ. 2011, 409 (19), 4132–4138. [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent Advances in Energy Materials by Electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [CrossRef]
- Sinatra, N. R.; Ranzani, T.; Vlassak, J. J.; Parker, K. K.; Wood, R. J. Nanofiber-Reinforced Soft Fluidic Micro-Actuators. J. Micromechanics Microengineering 2018, 28 (8), 084002. [CrossRef]
- Wongsasulak, S.; Patapeejumruswong, M.; Weiss, J.; Supaphol, P.; Yoovidhya, T. Electrospinning of Food-Grade Nanofibers from Cellulose Acetate and Egg Albumen Blends. J. Food Eng. 2010, 98 (3), 370–376. [CrossRef]
- Zhang, Y. Z.; Venugopal, J.; Huang, Z.-M.; Lim, C. T.; Ramakrishna, S. Crosslinking of the Electrospun Gelatin Nanofibers. Polymer 2006, 47 (8), 2911–2917. [CrossRef]
- Su, W.-T.; Hsu, M.-S. Nanofiber Mask Fabrication by Electrospun and Its Application. J. Phys. Conf. Ser. 2020, 1637 (1), 012102. [CrossRef]
- Zheng, H.; Kannan, B.; Chand, N. A.; Blake, A.; Chong, J.; Hosie, I.; Lepe, P. Chapter 9 - ActiVLayr Nanofiber Technology. In Handbook of Nanomaterials for Manufacturing Applications; Hussain, C. M., Ed.; Micro and Nano Technologies; Elsevier, 2020; pp 225–246. [CrossRef]
- Tahir, R.; Albargi, H. B.; Ahmad, A.; Qadir, M. B.; Khaliq, Z.; Nazir, A.; Khalid, T.; Batool, M.; Arshad, S. N.; Jalalah, M.; Alsareii, S. A.; Harraz, F. A. Development of Sustainable Hydrophilic Azadirachta Indica Loaded PVA Nanomembranes for Cosmetic Facemask Applications. Membranes 2023, 13 (2), 156. [CrossRef]
- Yang, T.; Xiao, P.; Zhang, J.; Jia, R.; Nawaz, H.; Chen, Z.; Zhang, J. Multifunctional Cellulose Ester Containing Hindered Phenol Groups with Free-Radical-Scavenging and UV-Resistant Activities. ACS Appl. Mater. Interfaces 2019, 11 (4), 4302–4310. [CrossRef]
- Tang, Y.; Liu, L.; Han, J.; Zhang, Z.; Yang, S.; Li, S.; Fan, Z.; Zhao, H. Fabrication and Characterization of Multiple Herbal Extracts-Loaded Nanofibrous Patches for Topical Treatment of Acne Vulgaris. Fibers Polym. 2021, 22 (2), 323–333. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).