1. Introduction
Extravasation in the context of cancer treatment refers to the unintended leakage of chemotherapy drugs, embolic agents, or other intravenously administered substances from the blood vessel into surrounding tissues. This phenomenon can occur during the infusion process or as a result of damage to the blood vessel or surrounding tissue. Extravasation events can lead to tissue damage, inflammation, pain, and in severe cases, necrosis and permanent scarring [
1].
As an example,
Figure 1 shows extravasation, or bleeding, from a polycystic kidney. Identifying the precise bleeding site on the computed tomography (CT) image presented a challenge. However, upon reviewing the angiography findings, it became evident that the bleeding originated from the area depicted in the CT image. In
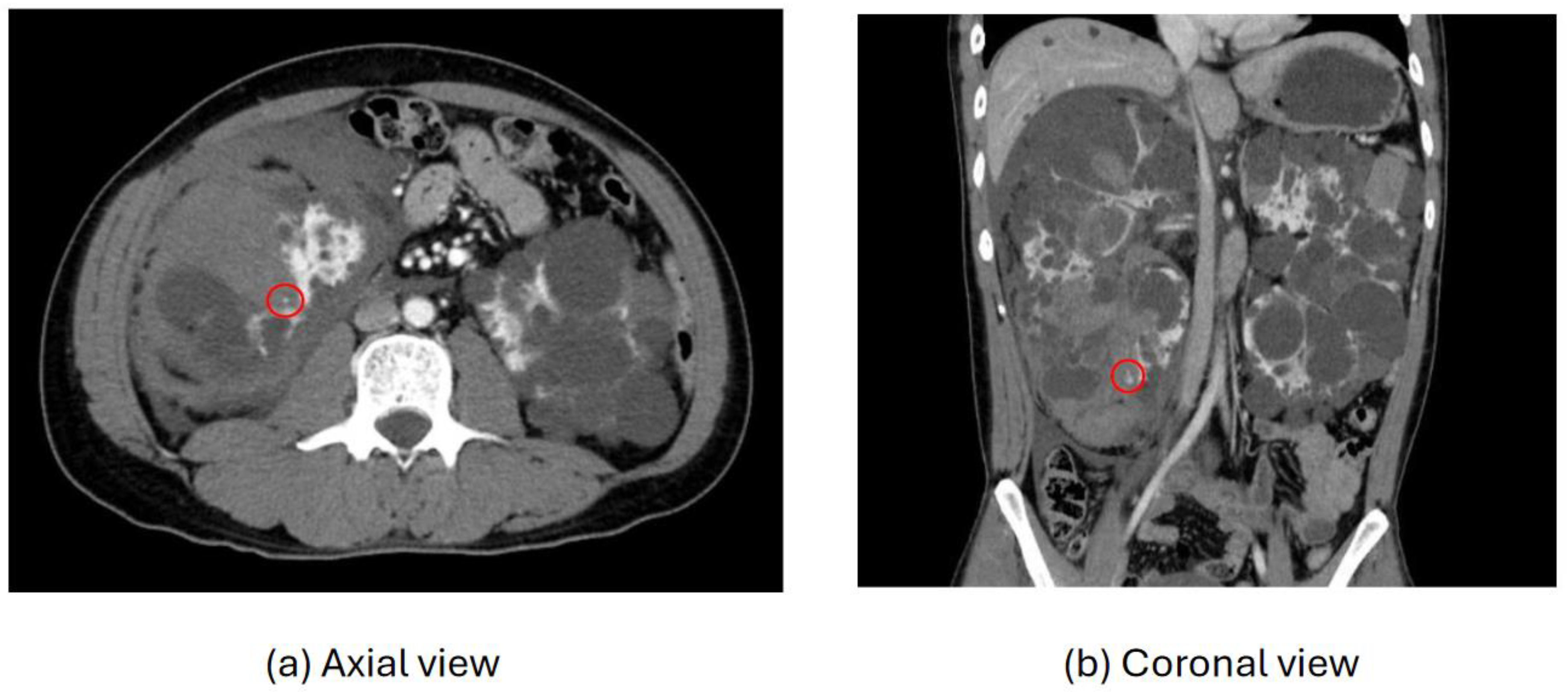
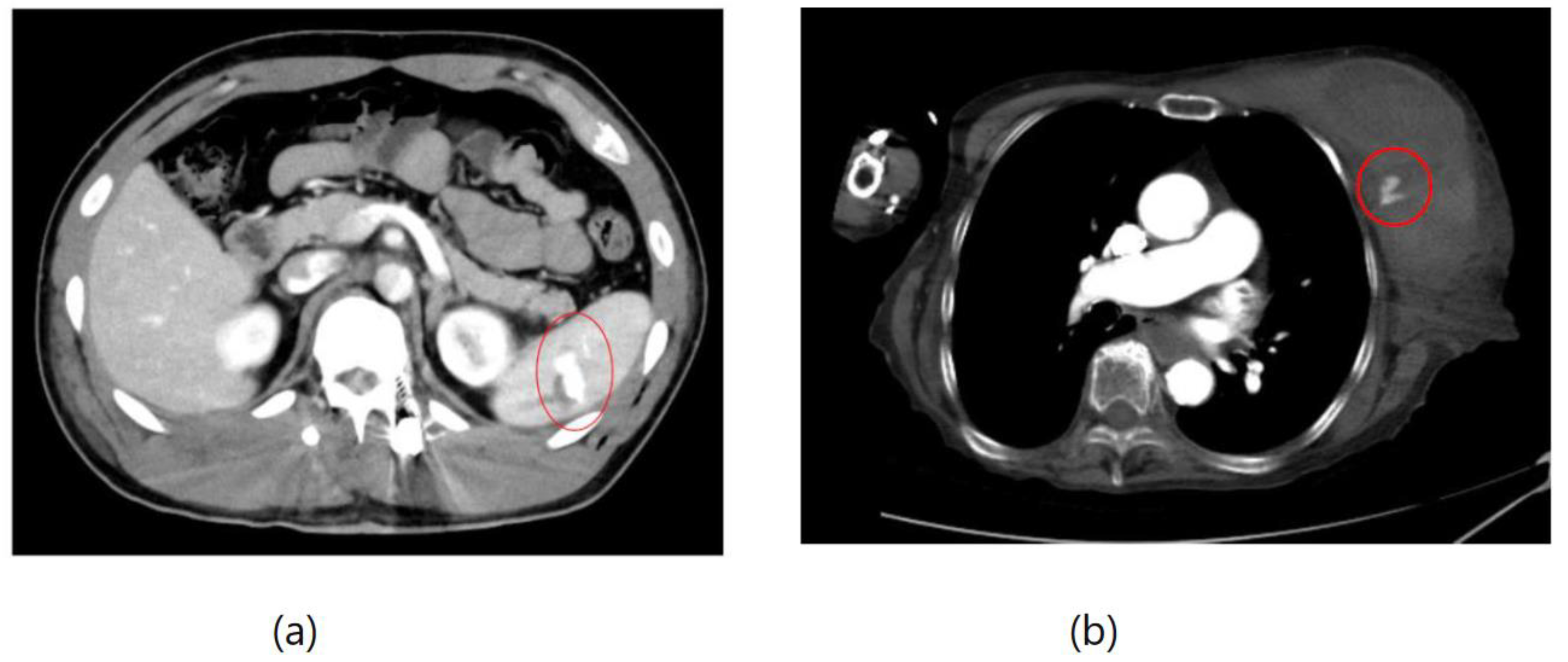
Figure 2, two instances of extravasation are depicted marked with the circles. Firstly, (a) displays a pseudoaneurysm visible post-spleen injury. Secondly, (b) illustrates a muscle hematoma in the chest region. In
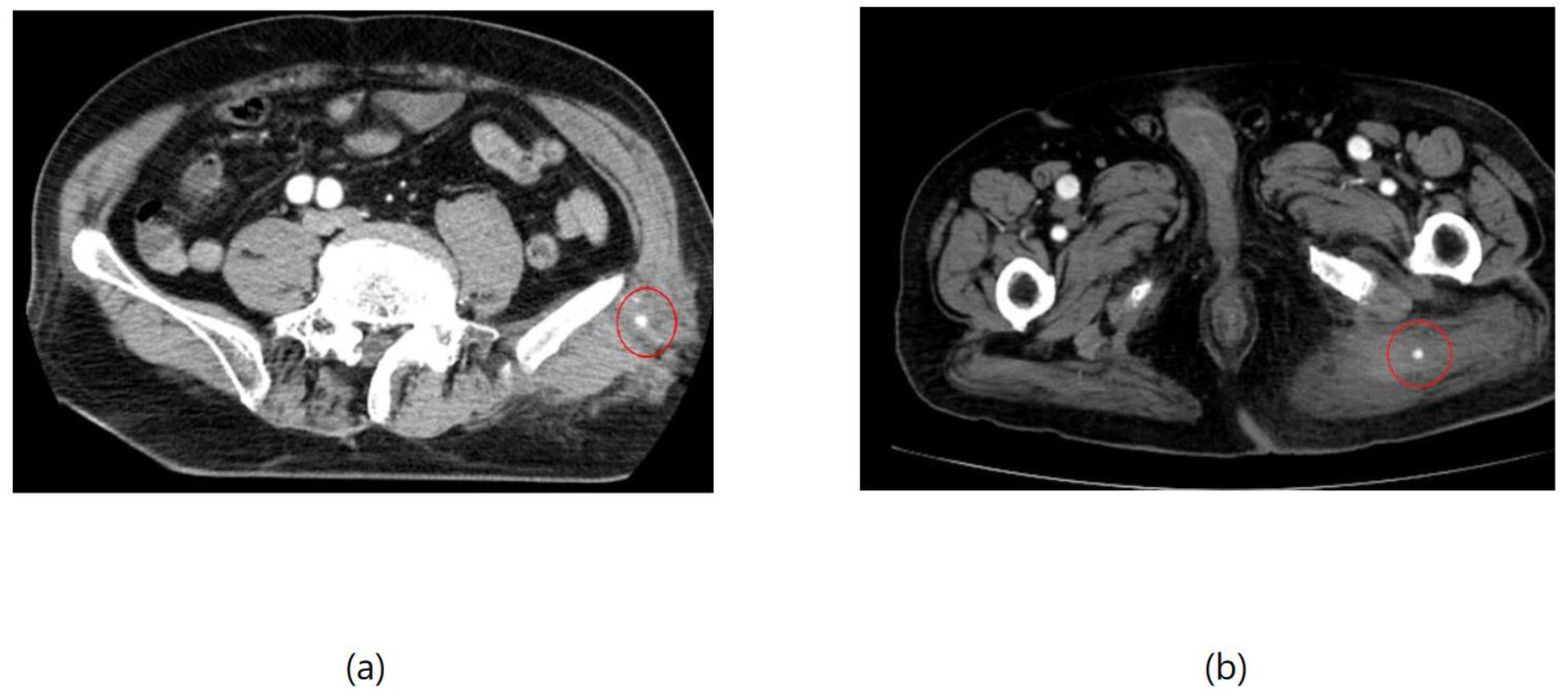
Figure 3, two cases of extravasation are evident. In (a), circles mark extravasation on a fractured pelvic region, indicating a significant injury. Meanwhile, (b) showcases an intragluteal hematoma, highlighting the varied presentations of extravasation across different anatomical contexts. These CT images provide valuable insights into the varied presentations of extravasation across different anatomical contexts.
Chemotherapy agents are powerful medications used to treat cancer by targeting rapidly dividing cells. However, many of these drugs are vesicants, meaning they can cause severe tissue damage if they leak outside the blood vessel [
2,
3]. Extravasation can occur due to various factors [
4], including poorly functioning intravenous catheters, improper drug administration techniques, or fragile blood vessels in cancer patients [
5,
6].
In addition to chemotherapy, extravasation can also occur in the context of radiotherapy treatment [
7,
8,
9,
10]. Radiotherapy involves the targeted delivery of high-energy radiation to cancerous tissues to destroy cancer cells or inhibit their growth. While extravasation events during radiotherapy are less common than with chemotherapy, they can still occur, particularly when using brachytherapy or external beam radiation techniques. Radiotherapy-induced extravasation can lead to similar local tissue damage and complications, including inflammation, pain, and tissue necrosis [
7].
The consequences of extravasation, both in chemotherapy and radiotherapy, can vary depending on factors such as the type and amount of drug involved, the location of the extravasation, and the promptness of intervention [
2,
8]. Mild cases may cause local discomfort, erythema, and swelling, while severe cases can lead to tissue necrosis, ulceration, and long-term functional impairment. In radiotherapy, extravasation events can result in similar local tissue damage and complications, depending on the type and dose of radiation delivered and the duration of exposure.
Extravasation not only poses immediate risks to patients’ health and well-being but can also impact the effectiveness of cancer treatment and compromise future treatment options [
11]. Therefore, prevention, early detection, and prompt management of extravasation events are crucial components of comprehensive cancer care.
Healthcare providers involved in cancer treatment must be vigilant in assessing patients for signs of extravasation during and after chemotherapy infusion [
4,
12]. Timely intervention, which may include stopping the infusion, applying appropriate antidotes or treatments, and providing supportive care, can help minimize tissue damage and mitigate the impact of extravasation on patients' outcomes. Understanding extravasation in the context of cancer treatment is essential for healthcare professionals to optimize patient safety, ensure the effectiveness of cancer therapy, and provide comprehensive supportive care to individuals undergoing treatment for cancer.
As an illustration,
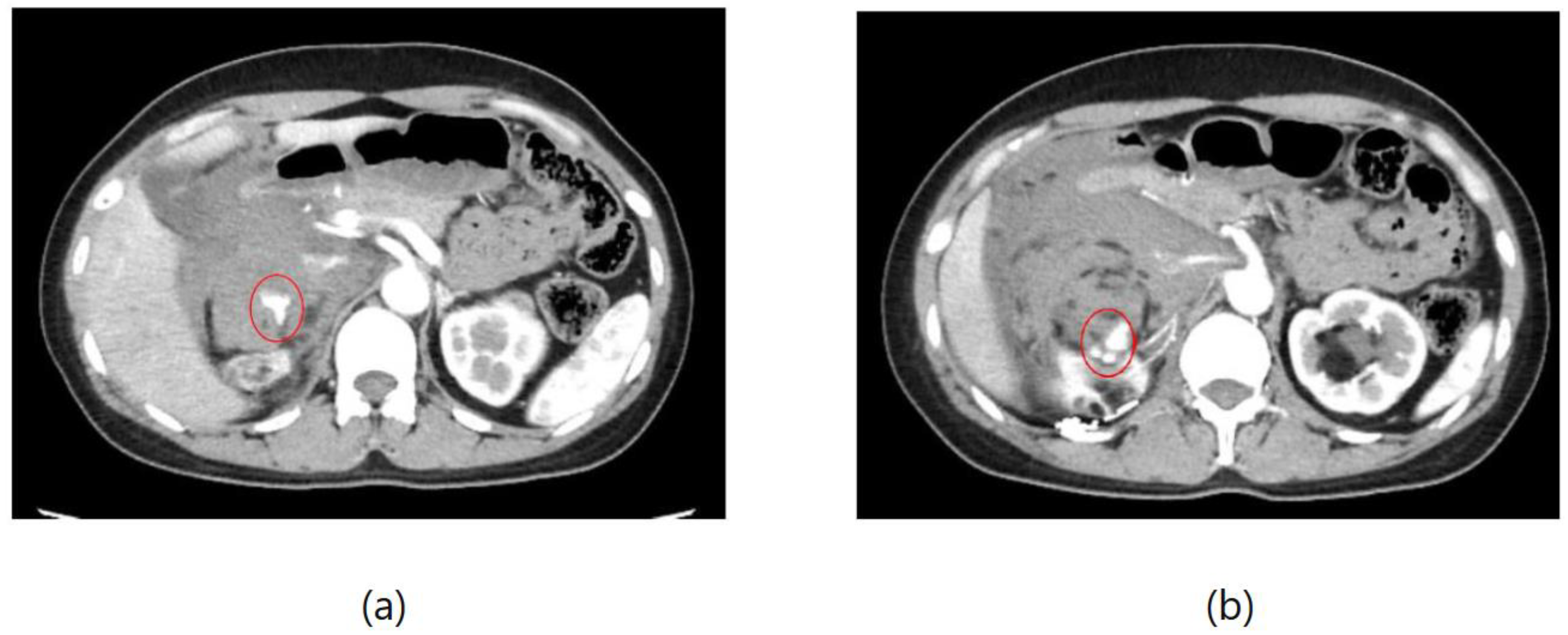
Figure 4 depicts extravasation from kidney angiomyolipoma, a condition characterized by the formation of benign tumors in the kidney. Patients with this condition may experience a range of symptoms, including anemia, fever, pain, or high blood pressure. In cases where tumors grow, treatment such as embolization or surgery may be necessary to mitigate the risk of bleeding.
Figure 5 illustrates extravasation resulting from the rupture of hepatocellular carcinoma in a 68-year-old male patient. This individual had previously undergone transcatheter arterial chemoembolization, a minimally invasive and targeted treatment utilized for managing certain advanced liver tumors that are not amenable to surgical removal. Following the rupture, the patient was admitted to the hospital in a state of hypotension, characterized by low blood pressure. This case underscores the importance of closely monitoring patients with hepatocellular carcinoma, particularly those who have undergone chemoembolization, for potential complications such as extravasation.
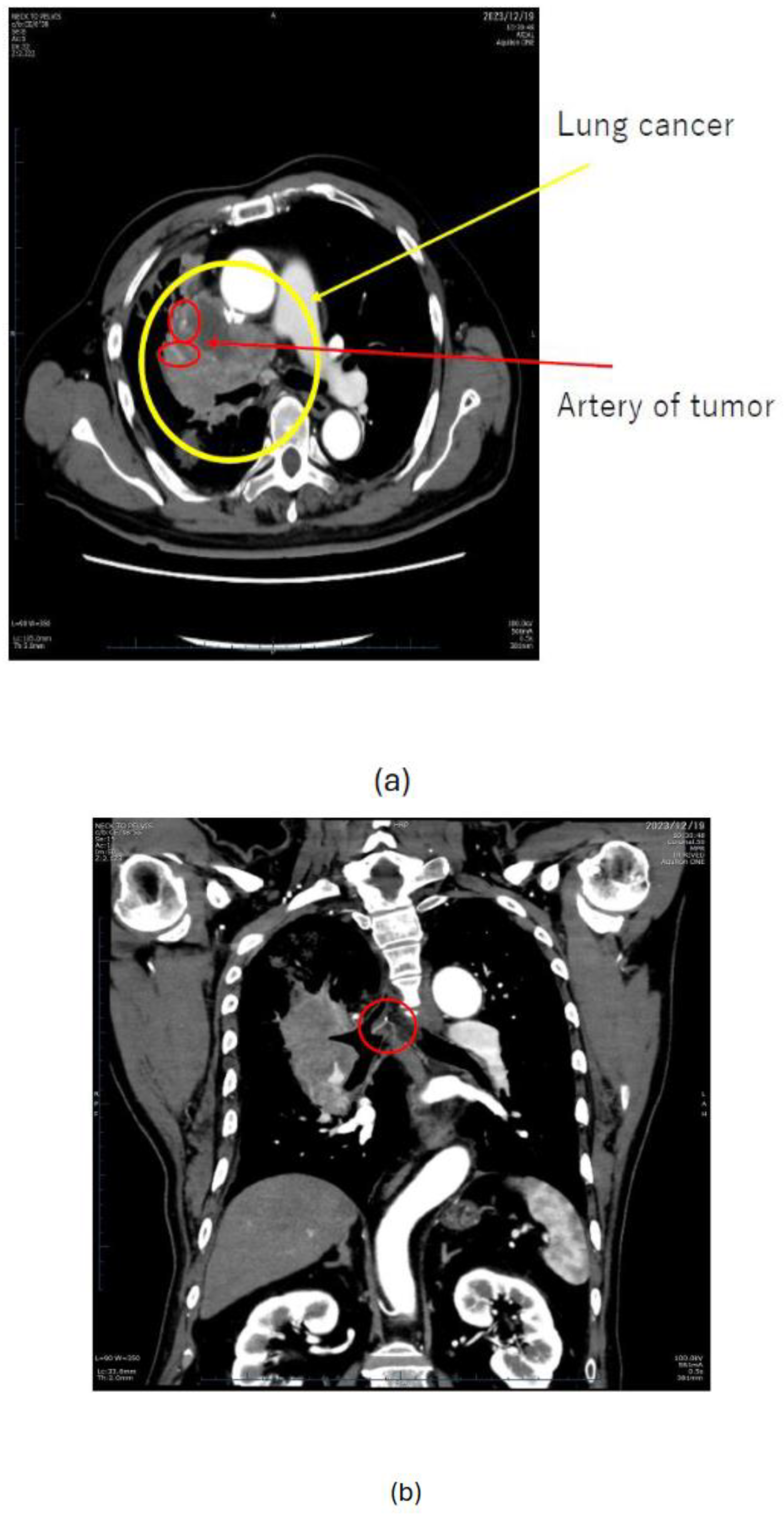
Additionally,
Figure 6 delineates the locations of extravasation detected in a patient’s lung cancer as visualized on CT images. Specifically, in panel (b), one of the feeding arteries of the tumor (the right bronchial arterial) is depicted. Identifying the responsible vessel on CT proved challenging, leading to the decision to embolize this artery. The patient had previously experienced hemoptysis prior to initiating chemotherapy.
2. Extravasation and Its Significance in Cancer Care
The implications of extravasation extend beyond immediate physical discomfort, encompassing both chemotherapy and radiotherapy. Extravasation events can impact the effectiveness of cancer treatment by reducing the delivery of chemotherapy or radiation to the intended target site. This can compromise the therapeutic efficacy of the treatment regimen and potentially affect treatment outcomes [
13]. Furthermore, severe extravasation injuries may necessitate treatment interruptions or modifications, leading to delays in cancer therapy and potentially limiting future treatment options [
14].
In addition to the direct physical effects, extravasation can also have psychological implications for patients undergoing cancer treatment [
15]. The experience of pain, disfigurement, or long-term complications resulting from extravasation can contribute to increased anxiety, distress, and decreased quality of life for affected individuals.
Given its potential to cause significant harm and disrupt cancer treatment, extravasation prevention, early detection, and prompt management are critical components of comprehensive cancer care [
2,
16]. Healthcare providers must be vigilant in assessing patients for signs of extravasation during chemotherapy or radiotherapy infusion, and prompt intervention is essential to minimize tissue damage and mitigate the impact on patients' outcomes [
2,
4,
12]. Strategies for preventing extravasation include proper vascular access device selection and placement, computerized detection methods, meticulous administration techniques, and patient education on recognizing and reporting signs of extravasation.
3. Review Scope and Objectives
The scope of this review covers an in-depth exploration of extravasation in patients undergoing cancer treatment, encompassing both chemotherapy-induced and radiotherapy-associated extravasation events. The review aims to provide a comprehensive understanding of extravasation, including its pathophysiology, incidence, risk factors, clinical presentation, diagnosis, prevention strategies, management approaches, complications, and long-term effects.
The objectives of this review include the following aspects.
Elucidating the pathophysiology of extravasation: This includes examining the mechanisms by which chemotherapy agents and radiotherapy cause tissue damage upon extravasation and identifying factors that influence the severity of extravasation reactions.
Evaluating the incidence and risk factors associated with extravasation: This involves analyzing available data on the prevalence of extravasation in cancer patients and identifying patient-related and treatment-related factors that predispose individuals to extravasation events.
Clinical presentation and diagnosis of extravasation: This includes describing the typical signs and symptoms of extravasation, as well as reviewing diagnostic tools and techniques used in confirming extravasation.
Reviewing preventive strategies for extravasation: This part outlines measures aimed at reducing the risk of extravasation during chemotherapy and radiotherapy administration, such as proper vascular access device selection, administration techniques, and patient education.
Exploring management approaches for extravasation events: This involves presenting current guidelines and protocols for managing extravasation injuries, including pharmacological and non-pharmacological interventions.
Complications and long-term effects of extravasation: This includes exploring potential complications arising from extravasation, as well as the impact of extravasation on patients’ quality of life and treatment outcomes.
Patient education and support: This involves highlighting the importance of patient education regarding extravasation risks and early symptom recognition, as well as strategies for providing psychological support to affected individuals.
Identifying future research directions and opportunities: This includes identifying gaps in current knowledge, areas for further investigation, and emerging technologies or therapies that may improve extravasation prevention and management.
By addressing these objectives, this review aims to provide healthcare professionals and researchers with a comprehensive resource for understanding, preventing, and managing extravasation in patients undergoing cancer treatment, including both chemotherapy and radiotherapy, ultimately contributing to enhanced patient safety and improved clinical outcomes.
4. Pathophysiology of Extravasation
Both chemotherapy and radiotherapy can induce extravasation through complex mechanisms involving direct tissue toxicity, vascular damage, altered tissue perfusion, and secondary effects from tumor response. Understanding these mechanisms is crucial for effective management of extravasation events in cancer treatment, thereby minimizing patient morbidity and optimizing treatment outcomes.
4.1. Extravasation in Chemotherapy
Many chemotherapy drugs are highly potent cytotoxic agents designed to target rapidly dividing cancer cells. However, these drugs can also be highly toxic to healthy tissues if they inadvertently leak into the surrounding extracellular space. Chemotherapy agents can induce chemical irritation and tissue toxicity upon extravasation, leading to local inflammation, pain, and tissue necrosis. The severity of tissue damage depends on factors such as the concentration and potency of the drug, the volume extravasated, and the duration of exposure [
2,
17].
Chemotherapy agents can directly damage endothelial cells lining the blood vessels, leading to disruption of vascular integrity [
18,
19]. This damage can manifest as endothelial cell death, increased vascular permeability, and impaired endothelial function. As a result, blood vessel walls become more fragile and prone to leakage, facilitating extravasation of chemotherapy drugs into surrounding tissues.
Some chemotherapy drugs, particularly those administered in high concentrations or volumes, can exert mechanical pressure on blood vessels, leading to vascular compression and compromised blood flow [
20]. This vascular compression can induce localized ischemia, further exacerbating tissue damage and increasing the risk of extravasation [
21]. Additionally, ischemic tissues may be more susceptible to the toxic effects of chemotherapy agents, amplifying the severity of extravasation injuries, and increasing cardiovascular risk in cancer patients [
22].
Extravasation events can occur due to various vascular access-related issues, including catheter displacement, infiltration, or leakage around the insertion site [
23]. Improper placement, maintenance, or malfunction of vascular access devices such as central venous catheters or peripherally inserted central catheters can increase the risk of chemotherapy leakage into surrounding tissues. Additionally, patient factors such as poor venous access, vascular fragility, or obesity may further predispose individuals to extravasation events [
24,
25].
4.2. Extravasation in Radiotherapy
Radiotherapy delivers ionizing radiation precisely to tumor tissues while minimizing exposure to surrounding healthy tissues. However, unintended radiation exposure to adjacent blood vessels can cause direct damage to the endothelial cells lining the vessel walls [
26,
27]. Radiation-induced endothelial cell death, inflammation, and fibrosis can weaken blood vessel integrity and increase permeability, facilitating the extravasation of blood or fluid into surrounding tissues [
28].
Radiation therapy can induce secondary effects on vascular integrity through inflammatory responses and fibrotic changes in the irradiated tissues [
29]. Chronic inflammation and fibrosis can lead to progressive vascular remodeling and sclerosis [
30], compromising blood vessel function and increasing the risk of extravasation [
31]. Additionally, radiation-induced endothelial dysfunction and thrombosis can further exacerbate vascular damage, predisposing vessels to leakage and extravasation events [
28,
32].
Radiation therapy can induce complex alterations in tissue perfusion and microvascular architecture within the irradiated field. Radiation-induced microvascular damage, capillary rarefaction, and thrombotic occlusion can disrupt tissue perfusion dynamics, leading to localized ischemia and hypoxia [
33]. These microvascular changes can compromise vascular integrity and increase the susceptibility of blood vessels to extravasation, particularly in tissues with pre-existing vascular compromise or impaired healing capacity [
34].
The tumor’s response to radiation therapy, such as tumor shrinkage, necrosis, or vascular regression [
35,
36], can indirectly contribute to extravasation events. Radiation-induced tumor cell death and vascular changes can alter the structural integrity and perfusion dynamics of the tumor microenvironment, increasing the risk of vascular leakage and extravasation. Additionally, radiation-induced tumor regression may lead to changes in tissue architecture and vascular distribution [
37], further predisposing vessels to extravasation injuries.
4.3. Mechanisms of Tissue Damage Induced by Chemotherapy and Radiation Extravasation
Extravasation of chemotherapy drugs and radiation therapy can cause tissue damage through multiple mechanisms, including chemical toxicity, vascular injury, ischemic injury, direct cellular damage, inflammatory response, and vascular fibrosis. Understanding these mechanisms is crucial for implementing preventive measures, early detection, and prompt management of extravasation injuries to minimize patient morbidity and optimize treatment outcomes in cancer care.
Chemotherapy-induced tissue damage encompasses a spectrum of factors that contribute to adverse effects upon extravasation. Chemotherapy agents, known for their high cytotoxicity, exert direct harm on cells and tissues upon inadvertent leakage [
38]. These agents induce chemical irritation and toxic effects on the surrounding tissues, eliciting inflammatory responses characterized by pain and tissue necrosis. Furthermore, chemotherapy drugs disrupt the integrity of blood vessel walls, resulting in endothelial cell damage and heightened vascular permeability [
39]. This vascular injury compromises normal vascular function, facilitating the extravasation of chemotherapy agents into the surrounding tissues [
40]. Additionally, certain chemotherapy drugs can induce local vasoconstriction or impair tissue perfusion, leading to tissue ischemia and hypoxia. The resultant ischemic injury exacerbates tissue damage and impedes wound healing processes, amplifying the severity of extravasation injuries [
21].
On the other hand, radiation-induced tissue damage arises from various factors intrinsic to radiotherapy. Ionizing radiation, utilized in radiotherapy, directly inflicts damage to cells and tissues by inducing DNA damage and oxidative stress [
41]. When blood vessels are exposed to radiation, endothelial cells undergo apoptosis, culminating in vascular damage and increased permeability. Concurrently, radiation therapy prompts an inflammatory response within irradiated tissues, prompting the release of pro-inflammatory cytokines and recruitment of immune cells. Prolonged inflammation exacerbates tissue damage and hampers wound healing, thereby prolonging the effects of extravasation [
42]. Moreover, radiation-induced fibrosis of blood vessels and surrounding tissues can occur due to the activation of fibroblasts and deposition of extracellular matrix proteins. This vascular fibrosis compromises vascular function, exacerbating tissue ischemia and elevating the risk of necrosis and impaired tissue repair [
43].
In cases where patients undergo both chemotherapy and radiation therapy concurrently or sequentially, the combined effects of chemotherapy and radiation extravasation can synergistically exacerbate tissue injury. Extravasation of chemotherapy drugs and radiation-induced tissue damage mutually potentiate each other, resulting in more severe and prolonged effects. Furthermore, compromised healing processes, stemming from chemotherapy-induced immunosuppression and radiation-induced tissue fibrosis, impede the normal wound healing cascade following extravasation injuries. Delayed or impaired wound healing heightens the risk of infection, scarring, and long-term functional impairment, underscoring the importance of timely intervention and comprehensive management strategies in mitigating the adverse consequences of extravasation in cancer care [
44].
4.4. Factors Influencing the Severity of Extravasation Reactions in Both Treatment Modalities
The severity of extravasation reactions in chemotherapy and radiotherapy is influenced not only by patient characteristics and treatment variables but also by a range of environmental and systemic factors that interact dynamically throughout the treatment process [
45].
Patient-related factors encompass a spectrum of considerations that impact extravasation reactions. Patients with pre-existing conditions affecting vascular integrity, such as diabetes or peripheral vascular disease, may be at heightened risk of severe reactions due to compromised tissue perfusion and healing capacity [
45,
46]. Additionally, factors such as smoking history, nutritional status, and immune function can influence tissue resilience and susceptibility to injury, further complicating the assessment and management of extravasation events [
47].
Treatment-related factors play an important role in shaping the severity and trajectory of extravasation reactions. The choice of chemotherapy regimen, including the specific agents used and their concentration, formulation, and infusion rate, can significantly impact tissue tolerance and susceptibility to injury. Certain chemotherapy drugs, such as anthracyclines or vinca alkaloids, are notorious for their vesicant properties and high potential for tissue damage if extravasated [
2]. Similarly, in radiotherapy, factors such as the total radiation dose, fractionation schedule, and beam energy distribution can influence the depth of tissue penetration and the extent of radiation-induced damage, with higher doses and prolonged treatment courses increasing the risk of severe reactions [
49].
Environmental and procedural factors also contribute to the complexity of extravasation management [
11,
12,
44]. The type and placement of vascular access devices, such as central venous catheters or peripherally inserted central catheters (PICCs), can influence the ease of drug administration and the likelihood of extravasation. Moreover, the skill and experience of healthcare providers in catheter insertion, maintenance, and monitoring play a critical role in preventing and mitigating extravasation events. Adequate patient education and counseling regarding the signs and symptoms of extravasation, as well as the importance of early reporting and intervention, are essential for empowering patients to actively participate in their care and minimize the risk of complications.
The severity of extravasation reactions in chemotherapy and radiotherapy is influenced by interconnected factors spanning patient characteristics, treatment variables, and environmental considerations. A comprehensive understanding of these factors is essential for healthcare professionals to anticipate, recognize, and effectively manage extravasation events, thereby minimizing patient morbidity and optimizing treatment outcomes in cancer care. By adopting a multidisciplinary approach that addresses the complex interplay of patient, treatment, and environmental factors, healthcare teams can enhance patient safety and quality of care throughout the cancer treatment journey.
5. Incidence and Risk Factors
In oncology practice, the occurrence of extravasation, which is the unintended leakage of chemotherapy drugs or radiation into surrounding tissues, is a concern for healthcare providers and patients alike. While precise incidence rates vary, existing data offer valuable insights into the frequency of extravasation events in cancer patients undergoing chemotherapy and radiotherapy.
For chemotherapy, past studies indicated that the overall incidence of extravasation ranges from 0.1% to 6% [
49]. Another study found that the occurrence rates of chemotherapy extravasation varied significantly, with estimates ranging from as low as 0.01% to as high as 7% [
4]. This variability can be attributed to factors such as the specific chemotherapy regimen used, the route of administration (intravenous, intramuscular, or subcutaneous), and the type of vascular access device employed. Certain chemotherapy agents, notably anthracyclines like doxorubicin and vinca alkaloids such as vincristine, are more commonly associated with extravasation due to their vesicant or irritant properties.
In contrast, the incidence of extravasation during radiotherapy is relatively rare, typically falling below 1% [
11]. The occurrence rate of extravasation among cancer patients undergoing CT is relatively high, with reported incidences ranging from 0.25% to 1.2% [
51], influenced by factors such as the type and location of radiation treatment, as well as patient-specific characteristics. External beam radiation therapy is generally associated with a lower risk of extravasation compared to internal radiation techniques like brachytherapy. However, extravasation incidents can still occur, particularly in areas where radiation fields intersect with major blood vessels or superficial tissues.
The administration of intravenous contrast plays a significant role in diagnostic radiology, yet it is not devoid of risks. These risks include local and systemic allergic reactions, as well as the potential for subcutaneous extravasation of contrast media. While subcutaneous extravasation of contrast medium is a relatively uncommon complication, it is widely acknowledged. Fortunately, the majority of incidents are minor and can be addressed through conservative management. However, there are rare cases that necessitate immediate surgical intervention [
52].
While extravasation is infrequent, its occurrence can have significant implications for patient safety and treatment outcomes. Prompt recognition and management are crucial in minimizing tissue damage and optimizing patient care. Healthcare providers must remain vigilant in monitoring patients for signs and symptoms of extravasation, implementing preventive measures, and promptly intervening when an extravasation event is suspected.
6. Clinical Presentation and Diagnosis
6.1. Typical Signs and Symptoms of Extravasation in Patients Receiving Chemotherapy and Radiotherapy
In chemotherapy, patients undergoing infusion may report sensations of pain or a burning feeling at the site of administration. This discomfort can range from mild irritation to severe, debilitating pain, often accompanied by swelling and edema around the infusion site. Visible changes in the skin, such as erythema or redness, may also occur, indicating inflammation and tissue damage. In more severe cases, blistering or the formation of vesicles may be observed, signifying significant injury to the surrounding tissues. Patients may experience necrosis, where skin cells die, leading to the development of ulcers or necrotic lesions [
4,
5]. Importantly, decreased mobility or impaired function in the affected limb may occur, particularly in instances of substantial swelling or tissue damage [
5].
In radiotherapy, patients may experience a range of symptoms indicative of extravasation. Skin changes, such as redness, warmth, or localized inflammation, may develop in the treatment area, reflecting radiation-induced tissue damage [
53,
54]. Patients may report pain, tenderness, or discomfort at the site of irradiation, varying in intensity based on the radiation dose and duration of exposure [
54]. Severe cases of extravasation can lead to the formation of skin ulcers or open wounds, indicating significant tissue necrosis and damage. Delayed healing of wounds in the radiation treatment area may prolong the recovery process, with changes in skin texture, such as thickening or scarring, observed over time [
55].
Prompt recognition and response to signs and symptoms of extravasation can minimize patient morbidity and optimize treatment outcomes. Early intervention, including discontinuation of infusion, application of cold compresses, and administration of appropriate antidotes or supportive care measures, can help mitigate the effects of extravasation and prevent long-term complications. Regular assessment and communication with patients regarding any discomfort or changes at the treatment site are essential to ensure timely intervention and optimal management of extravasation events.
6.2. Challenges in Diagnosing Extravasation and Distinguishing It from Other Complications
Diagnosing extravasation, particularly in the context of cancer treatment with chemotherapy and radiotherapy, presents a multifaceted challenge for healthcare providers. The overlapping symptoms and potential complications associated with extravasation make it crucial to differentiate it from other treatment-related reactions or conditions accurately.
One of the primary challenges in diagnosing extravasation is the variability in presenting symptoms [
44,
56]. While some patients may experience immediate pain, swelling, or erythema at the infusion site, others may exhibit more subtle or delayed signs, complicating the diagnostic process. Moreover, extravasation reactions can manifest differently depending on the type of chemotherapy drugs or radiation techniques used, further complicating diagnosis.
Distinguishing extravasation from other treatment-related reactions, such as infusion reactions or dermatitis, requires a comprehensive assessment of clinical signs and symptoms [
57]. For example, infusion reactions may present with symptoms like fever, chills, or allergic manifestations, which may not be typical of extravasation. Similarly, dermatitis or radiation dermatitis may cause skin changes such as erythema, itching, or peeling, resembling extravasation in some cases.
Furthermore, extravasation must be differentiated from other complications, such as cellulitis, thrombophlebitis, or compartment syndrome, which may present with similar clinical features [
58,
59]. Cellulitis, characterized by localized inflammation and infection of the skin and subcutaneous tissues, can mimic the erythema and swelling seen in extravasation. Thrombophlebitis, inflammation of the vein with associated pain and tenderness, may occur concurrently with extravasation or independently, complicating diagnosis. Compartment syndrome, characterized by increased pressure within a muscle compartment leading to tissue ischemia, can result from severe extravasation injuries, necessitating prompt recognition and intervention.
Additionally, the timing of symptom onset and progression may provide valuable clues in differentiating extravasation from other complications [
60]. Extravasation reactions typically occur during or shortly after infusion, with symptoms worsening over time if left untreated [
61]. In contrast, other conditions may present with delayed or persistent symptoms, necessitating careful monitoring and follow-up assessments.
Given the complexity of diagnosing extravasation and distinguishing it from other complications, healthcare providers must rely on a combination of clinical judgment, patient history, and diagnostic tests, such as ultrasound or tissue biopsy, when necessary. Timely recognition and intervention are essential to minimize tissue damage and optimize patient outcomes, highlighting the importance of vigilant monitoring and communication among multidisciplinary healthcare teams involved in cancer care.
6.3. Diagnostic Tools and Techniques Used in Confirming Extravasation for Both Treatment Modalities
Confirming extravasation in cancer treatment, whether during chemotherapy or radiotherapy, requires a versatile approach involving clinical assessment, diagnostic imaging, and sometimes tissue sampling. Healthcare providers utilize a variety of diagnostic tools and techniques to accurately identify extravasation events and assess the extent of tissue damage.
In the context of chemotherapy, clinical assessment plays an important role in the initial identification of extravasation [
57,
62]. Healthcare providers carefully inspect the infusion site for signs of extravasation, including pain, swelling, erythema, and blistering. Patient-reported symptoms, such as discomfort or changes in sensation at the infusion site, also inform the diagnostic process. While clinical evaluation provides valuable insights, additional diagnostic tools may be necessary to confirm extravasation and assess tissue damage.
Ultrasound imaging is commonly employed to visualize the extent of extravasation and assess tissue involvement [
63,
64,
65]. Ultrasonography enables healthcare providers to identify fluid accumulation, tissue edema, and structural changes in the affected area, aiding in the characterization of extravasation injuries. Doppler ultrasound may also be utilized to assess vascular integrity and blood flow, particularly in cases where vascular compromise is suspected.
In some instances, more invasive diagnostic techniques, such as tissue biopsy, may be warranted to confirm extravasation and evaluate tissue necrosis [
66]. Tissue biopsy allows for histological examination of the affected tissues, providing definitive evidence of extravasation and guiding treatment decisions. While less commonly utilized due to its invasive nature, tissue biopsy may be indicated in cases of diagnostic uncertainty or suspicion of severe tissue damage.
In radiotherapy, diagnostic tools and techniques for confirming extravasation differ slightly from those used in chemotherapy. Clinical assessment remains paramount, with healthcare providers closely monitoring patients for signs and symptoms of tissue damage, such as skin changes, pain, or ulceration, in the radiation treatment area. Imaging modalities, including CT and magnetic resonance imaging (MRI), may be utilized to visualize tissue changes and assess the extent of radiation-induced injury [
67].
In cases where extravasation is suspected but not definitively confirmed through clinical assessment and imaging, consultation with specialists, such as dermatologists or plastic surgeons, may be beneficial [
57]. These specialists can provide expertise in evaluating tissue damage and guiding management strategies, particularly in cases of severe extravasation requiring surgical intervention or advanced wound care.
Overall, confirming extravasation in cancer treatment requires a comprehensive approach that integrates clinical assessment, diagnostic imaging, and, when necessary, tissue sampling. By utilizing a combination of diagnostic tools and techniques, healthcare professionals can accurately identify extravasation events, assess tissue damage, and tailor treatment interventions to optimize patient outcomes. Vigilant monitoring and prompt intervention are essential to minimize the risk of complications and ensure optimal care for patients undergoing chemotherapy and radiotherapy.
7. Prevention Strategies
7.1. Preventive Measures Aimed at Reducing the Risk of Extravasation During Chemotherapy and Radiotherapy
Preventing extravasation during chemotherapy and radiotherapy is a critical aspect of patient care, aimed at minimizing tissue damage and potential complications. Healthcare professionals employ a variety of preventive measures to reduce the risk of extravasation outlined as follows.
Education and Training: Healthcare providers undergo comprehensive training on recognizing, managing, and preventing extravasation incidents. Patients should also be educated about the signs and symptoms of extravasation, emphasizing the importance of immediate reporting [
12,
68,
69,
70].
Vein Assessment: Prior to treatment initiation, healthcare professionals conduct a thorough assessment of the patient's veins to identify suitable access points [
57]. For patients with fragile or difficult-to-access veins, alternative access sites such as central venous catheters may be considered [
71].
Proper Catheter Placement: Skilled healthcare professionals ensure the proper placement of intravenous catheters, minimizing the risk of extravasation [
72]. Techniques such as ultrasound guidance may be used to verify catheter placement, especially in patients with challenging vascular access.
Use of Vein Visualization Technology: Vein visualization devices are employed to enhance the visualization of peripheral veins [
73,
74], aiding in accurate catheter insertion and reducing the risk of extravasation.
Regular Monitoring and Assessment: Infusion sites are frequently monitored during chemotherapy or radiotherapy sessions to detect early signs of extravasation [
75]. Symptoms such as pain, swelling, redness, or blanching around the infusion site are assessed promptly.
Chemotherapy Agents and Diluents: Healthcare providers select chemotherapy agents and diluents with lower vesicant properties whenever possible to minimize tissue damage in case of extravasation [
12,
57].
Temperature Regulation: Proper temperature control of chemotherapy solutions is ensured to minimize the risk of tissue injury if extravasation occurs [
76].
Prompt Recognition and Intervention: Healthcare providers are trained to promptly recognize signs of extravasation and differentiate between irritants and vesicants. Clear protocols are established for the immediate cessation of infusion upon suspicion of extravasation and initiation of appropriate interventions [
12,
57].
Extravasation Kits: Healthcare facilities maintain availability of extravasation kits containing specific antidotes, such as hyaluronidase for certain vesicant agents, to facilitate prompt treatment if extravasation occurs [
12,
57].
Documentation and Reporting: All incidents of extravasation are documented meticulously, including details such as the type and volume of infusate, site of extravasation, and actions taken. This information is essential for quality improvement and risk management purposes [
57,
77].
7.2. Importance of Proper Vascular Access Device Selection, Administration Techniques, and Patient Education for Both Modalities
Proper vascular access device selection, administration techniques, and patient education are crucial aspects of ensuring safe and effective delivery of chemotherapy and radiotherapy. These aspects are delineated as follows.
Vascular Access Device Selection: Choosing the appropriate vascular access device is essential to minimize the risk of extravasation and ensure optimal treatment outcomes. For patients undergoing chemotherapy or radiotherapy, the selection of the vascular access device depends on various factors such as treatment duration, frequency, and the patient's vascular status. Peripheral venous catheters are commonly used for short-term treatments, while central venous catheters, including peripherally inserted central catheters and implanted ports, are preferred for long-term therapy or when peripheral access is challenging [
71,
72,
78,
79]. Proper selection reduces the risk of complications such as thrombosis, infection, and extravasation.
Administration Techniques: Skilled administration techniques are essential to prevent extravasation and ensure accurate delivery of chemotherapy and radiotherapy. Healthcare professionals must receive adequate training in catheter insertion and maintenance to minimize the risk of complications. Techniques such as ultrasound-guided catheter insertion can improve success rates and reduce the risk of vessel injury [
80]. Additionally, proper flushing and locking protocols help maintain catheter patency and reduce the risk of occlusion and infection [
81]. Regular assessment of the catheter site and monitoring for signs of complications during treatment sessions are essential components of safe administration techniques.
Patient Education: Educating patients about vascular access devices, administration procedures, and potential complications is vital for their active involvement in their care and treatment adherence [
82]. Patients should be informed about the purpose of the vascular access device, its maintenance requirements, and signs of complications such as infection or thrombosis. Patient education also includes instructions on recognizing early signs of extravasation and the importance of timely reporting to healthcare professionals. Moreover, patients with central venous catheters need to understand the proper care and maintenance of their device to prevent complications and ensure its longevity [
83].
8. Complications and Long-Term Effects
Extravasation during chemotherapy or radiotherapy administration can lead to a spectrum of complications [
45,
58,
84], varying from mild discomfort to severe tissue damage and long-term sequelae. Immediate tissue damage is often evident at the infiltration site, manifesting as pain, swelling, redness, and, in severe cases, tissue necrosis. This damage can extend to nerves, resulting in neurotoxicity symptoms like numbness, tingling, weakness, or neuropathic pain, which may become permanent in severe cases. Moreover, muscles and joints may be affected, leading to stiffness, limited range of motion, and impaired mobility, potentially resulting in chronic pain and functional impairment if not promptly addressed.
Severe extravasation injuries can also cause scarring and fibrosis of affected tissues [
58,
85,
86], leading to long-term cosmetic deformities and functional limitations. Breakdown of tissue integrity following extravasation increases the risk of secondary infection, leading to cellulitis, abscess formation, or systemic infection if untreated. Additionally, irritation of blood vessels by extravasated agents can trigger thrombus formation [
57,
87], resulting in local thrombophlebitis or thromboembolic events like deep vein thrombosis or pulmonary embolism.
Moreover, suboptimal delivery of chemotherapy or radiotherapy agents to the intended target site due to extravasation can compromise treatment efficacy and impact patient outcomes [
88]. Extravasation events can also cause distress and anxiety for patients, affecting their emotional well-being and quality of life [
89]. Fear of recurrence or future complications may further impact treatment adherence.
In severe cases, extravasation injuries may lead to long-term disability, requiring extensive medical intervention, rehabilitation, and supportive care. Functional impairment and chronic pain may persist, significantly affecting the patient's quality of life. Additionally, extravasation events may result in legal and ethical dilemmas, particularly if complications arise due to negligence or inadequate management [
90]. Healthcare providers must adhere to professional standards of care, ensuring comprehensive documentation and reporting of extravasation incidents.
9. Future Directions and Research Opportunities
9.1. Gaps in Current Knowledge and Areas for Future Research in Extravasation Prevention and Management for Both Chemotherapy and Radiotherapy
While significant progress has been made in extravasation prevention and management for both chemotherapy and radiotherapy, there are still several gaps in current knowledge and areas for future research, which are outlined as follows.
Risk Factors and Predictive Models: Further research is needed to identify additional risk factors for extravasation, including patient-specific factors such as age, comorbidities, and vascular status. Developing predictive models that incorporate these factors could help stratify patients based on their risk of extravasation and guide personalized prevention strategies.
Novel Prevention Strategies: Current prevention strategies primarily focus on proper vascular access device selection and administration techniques. Future research could explore the efficacy of novel preventive interventions, such as vein mapping technologies, protective dressings, or pharmacological agents, in reducing the incidence of extravasation.
Early Detection Methods: Research into non-invasive or minimally invasive methods for early detection of extravasation is warranted. Developing innovative imaging techniques or biomarkers that can accurately detect extravasation at its earliest stages could facilitate timely intervention and prevent tissue damage.
Optimal Management Protocols: There is a need for standardized management protocols for extravasation events, including clear guidelines on the selection and administration of antidotes, wound care techniques, and follow-up strategies. Comparative studies evaluating the effectiveness of different management approaches and interventions are essential for establishing evidence-based protocols.
Patient Education and Support: Research focusing on the effectiveness of patient education programs and supportive interventions in enhancing patient awareness, self-management, and coping strategies following extravasation events is needed. Understanding patients' perspectives, experiences, and information needs can inform the development of tailored educational resources and support services.
Long-Term Outcomes and Quality of Life: Limited research has examined the long-term consequences of extravasation on patients' quality of life, functional outcomes, and psychological well-being. Longitudinal studies investigating the impact of extravasation-related complications, such as scarring, neuropathy, or chronic pain, on patients' long-term health outcomes are necessary.
Healthcare Provider Training and Competency: Research into effective strategies for training healthcare providers in extravasation prevention, recognition, and management is essential. Assessing the impact of educational interventions, simulation training, and competency assessments on healthcare providers' knowledge, skills, and confidence in managing extravasation events can help improve patient safety and outcomes.
Health Economics and Resource Utilization: Evaluating the economic burden of extravasation-related complications, including healthcare resource utilization, hospitalization costs, and productivity losses, is crucial for healthcare decision-making and resource allocation. Cost-effectiveness analyses of different prevention and management strategies can inform policy and practice guidelines.
9.2. Emerging Technologies or Therapies that May Improve Extravasation Prevention and Management in Both Treatment Modalities
Emerging technologies and therapies hold great promise for improving extravasation prevention and management in both chemotherapy and radiotherapy. These advancements encompass a wide range of approaches, including innovative medical devices, targeted therapies, and artificial intelligence (AI) applications for image analysis. These promising technologies are outlined as follows.
Vein Visualization Technologies: Advanced vein visualization devices, such as near-infrared imaging and augmented reality systems, offer real-time visualization of peripheral veins, enhancing the accuracy of vascular access device placement and reducing the risk of extravasation. These technologies provide healthcare providers with improved guidance for catheter insertion, especially in patients with difficult-to-access veins.
Smart Catheters and Infusion Systems: The development of smart catheters and infusion systems equipped with sensors and feedback mechanisms enables real-time monitoring of infusion parameters, including flow rates, pressure, and drug compatibility. These systems can alert healthcare providers to potential extravasation events and automatically adjust infusion parameters to minimize the risk of tissue damage.
Targeted Drug Delivery Systems: Targeted drug delivery systems, such as liposomes, nanoparticles, or drug-eluting implants, offer localized and controlled release of chemotherapy or radiotherapy agents, reducing systemic toxicity and the risk of extravasation-related complications. These technologies allow for precise delivery of therapeutic agents to tumor tissues while minimizing exposure to healthy tissues.
Topical Treatments and Wound Care Products: Novel topical treatments and wound care products specifically designed for extravasation injuries offer promising avenues for improving tissue healing and minimizing scarring. Advanced wound dressings incorporating growth factors, antimicrobial agents, or tissue-engineered scaffolds to promote tissue regeneration and accelerate wound closure.
AI for Image Analysis: AI-based image analysis algorithms can enhance the detection and diagnosis of extravasation events on imaging studies, such as ultrasound, MRI, and CT. These algorithms can automatically identify subtle signs of extravasation, assist healthcare providers in interpreting imaging findings, and facilitate timely intervention. This suggestion is subsequently extended as a separate discussion.
10. AI for Image Analysis in Extravasation Detection
Detecting extravasation on medical images poses several challenges, particularly when dealing with small abnormal objects or subtle changes in tissue appearance. Conventional image analysis techniques may struggle to accurately identify these abnormalities among complex anatomical structures and imaging artifacts. Moreover, the subjective interpretation of imaging findings by healthcare professionals can introduce variability and may lead to delays in diagnosis and intervention.
In oncology, there is a growing emphasis on precision medicine, tailoring treatments to each patient's and tumor’s specific characteristics [
91]. This shift enhances the personalized chemotherapy and radiotherapy, while advancements in imaging technologies offer improved tissue characterization and response evaluation [
88]. However, oncology treatments can lead to both local and systemic changes, underscoring the need for accurate post-treatment imaging interpretation [
92,
93]. AI shows great promise in rapidly analyzing vast amounts of imaging data, identifying subtle patterns, aiding in early detection, precise tumor characterization, and predicting treatment response [
94,
95,
96]. Moreover, AI can integrate imaging data with clinical information, enabling personalized treatment strategies and ultimately enhancing patient outcomes [
97,
98]. Thus, AI holds significant potential in advancing oncology imaging, potentially enabling personalized chemotherapy and radiotherapy.
AI algorithms, especially those based on deep learning architectures like convolutional neural networks (CNNs) [
99], hold promise in identifying subtle deviations in tissue appearance, such as faint discolorations or irregular contrast patterns, indicative of extravasation. By training CNNs on annotated datasets of images depicting extravasation events of varying sizes and characteristics, researchers can develop models capable of accurately detecting small abnormal objects with high precision. By automating this detection process, AI can significantly enhance the efficiency and precision of extravasation diagnosis.
The utilization of AI in extravasation detection represents a new frontier in this domain. Advancements in AI algorithms, particularly in the domain of small object detection and segmentation, hold promise for heightened accuracy and sensitivity in identifying extravasation events. Moreover, integrating AI with three-dimensional reconstruction could unlock new dimensions for comprehensive extravasation assessment and characterization.
AI-driven image analysis not only can flag potential extravasation events but also offers invaluable assistance to healthcare providers in interpreting imaging findings. By highlighting regions of interest and providing quantitative assessments of extravasation severity and extent, these algorithms can empower clinicians to make informed decisions regarding patient care. Leveraging this information, healthcare providers can formulate tailored treatment strategies and initiate timely interventions to mitigate potential complications.
For small and transient object detection, particularly in medical imaging applications like detecting extravasation, pretrained CNN models that have demonstrated effectiveness in possessing high sensitivity to complex patterns and subtle features are preferable. Below are some pretrained CNN models suitable for this task.
RetinaNet [
100]: This CNN is a single-stage object detection model known for its effectiveness in detecting small and transient objects. It addresses the challenge of class imbalance in dense detection tasks by employing a focal loss function. This enables RetinaNet to assign higher weights to hard-to-detect objects, making it particularly suitable for detecting subtle abnormalities like extravasation.
Mask R-CNN [
101]: This model extends Faster R-CNN by adding a mask prediction branch, enabling pixel-level segmentation in addition to object detection. This makes it well-suited for tasks requiring precise localization of small and transient objects. Mask R-CNN has been successfully applied to various medical imaging tasks and can be adapted for detecting extravasation with high accuracy.
YOLOv4 [
102]: It is an advanced version of the YOLO (You Only Look Once) object detection algorithm known for its speed and accuracy. It utilizes a single neural network to predict bounding boxes and class probabilities for small and transient objects in real-time. YOLOv4's efficiency and effectiveness make it suitable for detecting extravasation events in medical images.
EfficientDet [
103]: This pretrained CNN is a family of efficient and accurate object detection models based on the EfficientNet backbone architecture. These models achieve state-of-the-art performance with significantly fewer parameters compared to traditional models, making them well-suited for resource-constrained environments like medical imaging applications. EfficientDet's lightweight design and high accuracy make it suitable for detecting small and transient objects like extravasation.
CenterNet [
104]: This net is a simple and efficient object detection model that directly predicts object centers and regresses bounding boxes from them. This approach makes CenterNet particularly effective for detecting small and transient objects with high accuracy. CenterNet's simplicity and effectiveness make it a viable option for detecting extravasation events in medical images.
These pretrained CNN models provide a strong foundation for detecting small and transient objects like extravasation in medical imaging. Fine-tuning these models on annotated datasets of medical images containing extravasation cases can further enhance their performance and adaptability to specific clinical applications.
11. Conclusions
This review emphasizes the critical importance of proactive management and prevention strategies in minimizing the impact of extravasation on cancer patients undergoing both chemotherapy and radiotherapy. Extravasation events can lead to a spectrum of complications, ranging from local tissue damage to long-term disability, significantly affecting patients' quality of life and treatment outcomes. Thus, prompt recognition, appropriate intervention, and comprehensive follow-up are essential to mitigate the potential adverse effects of extravasation.
To achieve optimal extravasation care, healthcare professionals must prioritize prevention and management efforts as part of comprehensive oncology care. This includes implementing standardized protocols and guidelines, providing comprehensive training and education, fostering multidisciplinary collaboration, investing in research and development of innovative technologies and therapies, and enhancing patient education and support services.
Recommendations for healthcare professionals and policymakers also include advocating for policy initiatives aimed at improving extravasation care standards and promoting patient safety. By integrating AI technologies and proactive management strategies into clinical practice, healthcare providers can effectively mitigate the impact of extravasation on cancer patients, ultimately improving treatment outcomes and enhancing patient quality of life.
Author Contributions
TDP led the conceptualization of the review, conducted extensive literature review, analyzed findings from selected studies, drafted the manuscript, and coordinated revisions based on feedback. TT contributed to the literature review, provision of images, critical insights and feedback during manuscript drafting. Both TDP and TT approved the submission of the manuscript,.
Funding
This research was funded by The Great Britain Sasakawa Foundation (Grant Application No. B152).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nguyen M, Borders L, Wesolow JT, Greene J. Chemotherapy extravasation causing soft-tissue necrosis mimicking infection: A longitudinal case study. Cureus 2024, 16, e55333. [CrossRef] [PubMed]
- Kreidieh FY, Moukadem HA, El Saghir NS. Overview, prevention and management of chemotherapy extravasation. World J Clin Oncol. 2016, 7, 87–97. [CrossRef] [PubMed]
- Pluschnig U, Haslik W, Bartsch R, Mader RM. Extravasation emergencies: state-of-the-art management and progress in clinical research. Memo. 2016, 9, 226–230. [CrossRef] [PubMed]
- Fidalgo JAP, Fabregat LG, Cervantes A, Margulies A, Vidall C, Roila F. ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012, 23, vii167–vii173. [CrossRef] [PubMed]
- Al-Benna S, O'Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013, 2013, 856541. [CrossRef] [PubMed]
- Apisarnthanarax N, Duvic MM. Extravasation Reactions. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.
- Schaverien MV, Evison D, McCulley SJ. Management of large volume CT contrast medium extravasation injury: technical refinement and literature review. J Plast Reconstr Aesthet Surg. 2008, 61, 562–565. [CrossRef]
- van der Pol J, Voo S, Bucerius J, Mottaghy FM. Consequences of radiopharmaceutical extravasation and therapeutic interventions: a systematic review. Eur J Nucl Med Mol Imaging 2017, 44, 1234–1243. [CrossRef] [PubMed]
- Belzunegui T, Louis CJ, Torrededia L, Oteiza J. Extravasation of radiographic contrast material and compartment syndrome in the hand: a case report. Scand J Trauma Resusc Emerg Med. 2011, 19, 9. [CrossRef] [PubMed]
- Tonolini M, Campari A, Bianco R. Extravasation of radiographic contrast media: prevention, diagnosis, and treatment. Curr Probl Diagn Radiol. 2012, 41, 52–55. [CrossRef]
- Alexander, L. Extravasation injuries: A trivial injury often overlooked with disastrous consequences. World J Plast Surg. 2020, 9, 326–330. [Google Scholar] [CrossRef]
- Kim JT, Park JY, Lee HJ, Cheon YJ. Guidelines for the management of extravasation. J Educ Eval Health Prof. 2020, 17, 21. [CrossRef] [PubMed]
- Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol. 2004, 15, 858–862. [CrossRef]
- Keritam O, Juhasz V, Schofer C, Thallinger C, Aretin MB, Schabbauer G, Breuss J, Unseld M, Uhrin P. Determination of extravasation effects of nal-iri and trabectedin and evaluation of treatment options for trabectedin extravasation in a preclinical animal model. Front Pharmacol. 2022, 13, 875695. [CrossRef] [PubMed]
- Amjad MT, Chidharla A, Kasi A. Cancer Chemotherapy. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564367/.
- O’Malley DM, Alfano CM, Doose M, Kinney AY, Lee SJC, Nekhlyudov L, Duberstein P, Hudson SV. Cancer prevention, risk reduction, and control: opportunities for the next decade of health care delivery research. Transl Behav Med. 2021, 11, 1989–1997. [CrossRef] [PubMed]
- Rudolph R, Larson DL. Etiology and treatment of chemotherapeutic agent extravasation injuries: A review. JCO 1987, 5, 1116–1126. [CrossRef] [PubMed]
- Hsu PY, Mammadova A, Benkirane-Jessel N, Desaubry L, Nebigil CG. Updates on anticancer therapy-mediated vascular toxicity and new horizons in therapeutic strategies. Front Cardiovasc Med. 2021, 8, 694711. [CrossRef] [PubMed]
- Terwoord JD, Beyer AM, Gutterman DD. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol Ther. 2022, 237, 108116. [CrossRef] [PubMed]
- Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014, 16, 321–346. [CrossRef] [PubMed]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012, 298, 229–317. [CrossRef]
- Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol. 2016, 32, 852–862. [CrossRef]
- Barton, A. Extravasation and infiltration: Under-recognised complications of intravenous therapy. Br J Nurs. 2024, 33, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Armenteros-Yeguas V, Garate-Echenique L, Tomas-Lopez MA, Cristobal-Domínguez E, Moreno-de Gusmao B, Miranda-Serrano E, Moraza-Dulanto MI. Prevalence of difficult venous access and associated risk factors in highly complex hospitalised patients. J Clin Nurs. 2017, 26, 4267–4275. [CrossRef] [PubMed]
- Billingham MJ, Mittal R. Peripheral venous extravasation injury. BJA Educ. 2023, 23, 42–45. [CrossRef] [PubMed]
- Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J. 2014, 32, 103–115. [CrossRef] [PubMed]
- Nepon H, Safran T, Reece EM, Murphy AM, Vorstenbosch J, Davison PG. Radiation-Induced Tissue Damage: Clinical Consequences and Current Treatment Options. Semin Plast Surg. 2021, 35, 181–188. [CrossRef] [PubMed]
- Wijerathne H, Langston JC, Yang Q, Sun S, Miyamoto C, Kilpatrick LE, Kiani MF. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother Oncol. 2021, 158, 21–32. [CrossRef] [PubMed]
- Purkayastha A, Sharma N, Sarin A, Bhatnagar S, Chakravarty N, Mukundan H, Suhag V, Singh S. Radiation fibrosis syndrome: The evergreen menace of radiation therapy. Asia Pac J Oncol Nurs. 2019, 6, 238–245. [CrossRef]
- Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [CrossRef] [PubMed]
- Wu B, Sodji QH, Oyelere AK. Inflammation, fibrosis and cancer: Mechanisms, therapeutic options and challenges. Cancers (Basel) 2022, 14, 552. [CrossRef]
- Venkatesulu BP, Mahadevan LS, Aliru ML, Yang X, Bodd MH, Singh PK, Yusuf SW, Abe JI, Krishnan S. Radiation-induced endothelial vascular injury: A review of possible mechanisms. JACC Basic Transl Sci. 2018, 3, 563–572. [CrossRef]
- Yang EH, Marmagkiolis K, Balanescu DV, Hakeem A, Donisan T, Finch W, Virmani R, Herrman J, Cilingiroglu M, Grines CL, Toutouzas K, Iliescu C. Radiation-induced vascular disease-A state-of-the-art review. Front Cardiovasc Med. 2021, 8, 652761. [CrossRef]
- LeBlanc AJ, Krishnan L, Sullivan CJ, Williams SK, Hoying JB. Microvascular repair: post-angiogenesis vascular dynamics. Microcirculation 2012, 19, 676–695. [CrossRef] [PubMed]
- Fernandes GNC. Immunotherapy as a turning point in the treatment of melanoma brain metastases. Discoveries (Craiova) 2023, 11, e169. [CrossRef] [PubMed]
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020, 77, 1745–1770. [CrossRef] [PubMed]
- Guipaud O, Jaillet C, Clement-Colmou K, François A, Supiot S, Milliat F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br J Radiol. 2018, 91, 20170762. [CrossRef] [PubMed]
- van den Boogaard WMC, Komninos DSJ, Vermeij WP. Chemotherapy side-effects: Not all DNA damage is equal. Cancers (Basel) 2022, 14, 627. [CrossRef] [PubMed]
- Hsu PY, Mammadova A, Benkirane-Jessel N, Désaubry L, Nebigil CG. Updates on anticancer therapy-mediated vascular toxicity and new horizons in therapeutic strategies. Front Cardiovasc Med. 2021, 8, 694711. [CrossRef] [PubMed]
- Terwoord JD, Beyer AM, Gutterman DD. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol Ther. 2022, 237, 108116. [CrossRef] [PubMed]
- Borrego-Soto G, Ortiz-Lopez R, Rojas-Martínez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015, 38, 420–432. [CrossRef]
- Zhang C, Liang Z, Ma S, Liu X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front Oncol. 2021, 11, 670464. [CrossRef]
- Yu Z, Xu C, Song B, Zhang S, Chen C, Li C, Zhang S. Tissue fibrosis induced by radiotherapy: Current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J Transl Med. 2023, 21, 708. [CrossRef] [PubMed]
- Corbett M, Marshall D, Harden M, Oddie S, Phillips R, McGuire W. Treatment of extravasation injuries in infants and young children: a scoping review and survey. Health Technol Assess. 2018, 22, 1–112. [CrossRef]
- Al-Benna S, O’Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013, 2013, 856541. [CrossRef] [PubMed]
- Liu W, Wang P, Zhu H, Tang H, Wang X, Guan H, Wang C, Qiu Y, Peng A, He L. Risk factors for contrast media extravasation in intravenous contrast-enhanced computed tomography: An observational cohort study. Academic Radiology 2024, S1076-633200507-X. [CrossRef]
- Chiu K, Tindholdt TT, Tonseth KA. Extravasation injuries. Tidsskr Nor Laegeforen. 2016, 136, 233–235. [CrossRef]
- Russ E, Davis CM, Slaven JE, Bradfield DT, Selwyn RG, Day RM. Comparison of the medical uses and cellular effects of high and low linear energy transfer radiation. Toxics 2022, 10, 628. [CrossRef]
- Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol. 2004, 15, 858–862. [CrossRef] [PubMed]
- Jordan K, Behlendorf T, Mueller F, Schmoll HJ. Anthracycline extravasation injuries: management with dexrazoxane. Ther Clin Risk Manag. 2009, 5, 361–366. [CrossRef]
- Silva HCS, Bitencourt AGV, Chojniak R. Extravasation of iodinated contrast medium in cancer patients undergoing computed tomography. Radiol Bras. 2018, 51, 236–241. [CrossRef]
- Nicola R, Shaqdan KW, Aran S, Prabhakar AM, Singh AK, Abujudeh HH. Contrast media extravasation of computed tomography and magnetic resonance imaging: management guidelines for the radiologist. Curr Probl Diagn Radiol. 2016, 45, 161–164. [CrossRef]
- Wei J, Meng L, Hou X, Qu C, Wang B, Xin Y, Jiang X. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2018, 11, 167–177. [CrossRef]
- van der Pol J, Voo S, Bucerius J, Mottaghy FM. Consequences of radiopharmaceutical extravasation and therapeutic interventions: A systematic review. Eur J Nucl Med Mol Imaging 2017, 44, 1234–1243. [CrossRef] [PubMed]
- Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005, 2, 112–127. [CrossRef] [PubMed]
- Roditi G, Khan N, van der Molen AJ, Bellin MF, Bertolotto M, Brismar T, Correas JM, Dekkers IA, Geenen RWF, Heinz-Peer G, Mahnken AH, Quattrocchi CC, Radbruch A, Reimer P, Romanini L, Stacul F, Thomsen HS, Clement O. Intravenous contrast medium extravasation: Systematic review and updated ESUR Contrast Media Safety Committee Guidelines. Eur Radiol. 2022, 32, 3056–3066. [CrossRef] [PubMed]
- West Midlands Expert Advisory Group for Chemotherapy. Guideline for the management of extravasation of a systemic anti-cancer therapy including cytotoxic agents. West Midlands Expert Advisory Group for Systemic Anti-Cancer Therapy (SACT) 2017, available at: https://www.england.nhs.uk/midlands/wp-content/uploads/sites/46/2019/05/management-extravasation-of-a-systemic-anti-cancer-therapy-including-cytotoxic-agents.pdf.
- Alexander, L. Extravasation Injuries: A Trivial Injury Often Overlooked with Disastrous Consequences. World J Plast Surg. 2020, 9, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Belzunegui T, Louis CJ, Torrededia L, Oteiza J. Extravasation of radiographic contrast material and compartment syndrome in the hand: a case report. Scand J Trauma Resusc Emerg Med. 2011, 19, 9. [CrossRef] [PubMed]
- Barton, A. Extravasation and infiltration: Under-recognised complications of intravenous therapy. Br J Nurs. 2024, 33, S18–S26. [Google Scholar] [CrossRef]
- IV Infiltrations and Extravasations: Causes, Signs, Side Effects, and Treatment. ivWatch. Date of access: 06 May 2024. Available at: https://www.ivwatch.com/2020/05/27/iv-infiltrations-and-extravasations-causes-signs-side-effects-and-treatment/.
- Bahrami M, Karimi T, Yadegarfar G, Norouzi A. Assessing the quality of existing clinical practice guidelines for chemotherapy drug extravasation by appraisal of guidelines for research and evaluation II. Iran J Nurs Midwifery Res. 2019, 24, 410–416. [CrossRef]
- Spinnato P, Patel DB, Di Carlo M, Bartoloni A, Cevolani L, Matcuk GR, Crombé A. Imaging of musculoskeletal soft-tissue infections in clinical practice: A comprehensive updated review. Microorganisms 2022, 10, 2329. [CrossRef]
- Yemane PT, Aslund AKO, Snipstad S, Bjorkoy A, Grendstad K, Berg S, Morch Y, Torp SH, Hansen R, Davies CL. Effect of ultrasound on the vasculature and extravasation of nanoscale particles imaged in real time. Ultrasound Med Biol. 2019, 45, 3028–3041. [CrossRef]
- Klibanov AL, Hossack JA. Ultrasound in radiology: From anatomic, functional, molecular imaging to drug delivery and image-guided therapy. Invest Radiol. 2015, 50, 657–670. [CrossRef]
- Okuda H, Masatsugu A, Sijimaya T, Arai R. Skin necrosis due to the extravasation of irritant anticancer agents. Intern Med. 2018, 57, 757–760. [CrossRef] [PubMed]
- Jeraj R, Cao Y, Ten Haken RK, Hahn C, Marks L. Imaging for assessment of radiation-induced normal tissue effects. Int J Radiat Oncol Biol Phys. 2010, 76, S140–S144. [CrossRef] [PubMed]
- Coyle CE, Griffie J, Czaplewski LM. Eliminating extravasation events: A multidisciplinary approach. J Infus Nurs. 2015, 38, Suppl 6:S43–S50. [CrossRef]
- Mohammed HS, Mohammad ZA, Azer SZ, Khallaf SM. Impact of in- service training program on nurses' performance for minimizing chemotherapy extravasation. Asian Pac J Cancer Prev. 2023, 24, 3537–3542. [CrossRef] [PubMed]
- Bellin MF, Jakobsen JA, Tomassin I, Thomsen HS, Morcos SK, Thomsen HS, Morcos SK, Almen T, Aspelin P, Bellin MF, Clauss W, Flaten H, Grenier N, Idee JM, Jakobsen JA, Krestin GP, Stacul F, Webb JA; Contrast Media Safety Committee Of The European Society Of Urogenital Radiology. Contrast medium extravasation injury: guidelines for prevention and management. Eur Radiol. 2002, 12, 2807–2812. [CrossRef] [PubMed]
- Sugawara S, Sone M, Sakamoto N, Sofue K, Hashimoto K, Arai Y, Tokue H, Takigawa M, Mimura H, Yamanishi T, Yamagami T. Guidelines for central venous port placement and management (abridged translation of the Japanese version). Interv Radiol (Higashimatsuyama) 2023, 8, 105–117. [CrossRef] [PubMed]
- Chan KM, Chau JPC, Choi KC, Fung GPG, Lui WW, Chan MSY, Lo SHS. Clinical practice guideline on the prevention and management of neonatal extravasation injury: A before-and-after study design. BMC Pediatr. 2020, 20, 445. [CrossRef] [PubMed]
- Vyas V, Sharma A, Goyal S, Kothari N. Infrared vein visualization devices for ease of intravenous access in children: Hope versus hype. Anaesthesiol Intensive Ther. 2021, 53, 69–78. [CrossRef] [PubMed]
- Chiao FB, Resta-Flarer F, Lesser J, Ng J, Ganz A, Pino-Luey D, Bennett H, Perkins C Jr, Witek B. Vein visualization: Patient characteristic factors and efficacy of a new infrared vein finder technology. Br J Anaesth. 2013, 110, 966–971. [CrossRef]
- Hirata I, Mazzotta A, Makvandi P, Cesini I, Brioschi C, Ferraris A, Mattoli V. Sensing technologies for extravasation detection: A review. ACS Sens. 2023, 8, 1017–1032. [CrossRef]
- Matsui Y, Murayama R, Tanabe H, Oe M, Motoo Y, Wagatsuma T, Michibuchi M, Kinoshita S, Sakai K, Konya C, Sugama J, Sanada H. Evaluation of the predictive validity of thermography in identifying extravasation with intravenous chemotherapy infusions. J Infus Nurs. 2017, 40, 367–374. [CrossRef] [PubMed]
- Hackenberg RK, Kabir K, Müller A, Heydweiller A, Burger C, Welle K. extravasation injuries of the limbs in neonates and children—Development of a treatment algorithm. Dtsch Arztebl Int. 2021, 118, 547–554. [CrossRef] [PubMed]
- Bahoush G, Salajegheh P, Anari AM, Eshghi A, Aski BH. A review of peripherally inserted central catheters and various types of vascular access in very small children and pediatric patients and their potential complications. J Med Life. 2021, 14, 298–309. [CrossRef] [PubMed]
- Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Central line catheters and associated complications: a review. Cureus 2019, 11, e4717. [CrossRef] [PubMed]
- Villa A, Hermand V, Bonny V, Preda G, Urbina T, Gasperment M, Gabarre P, Missri L, Baudel JL, Zafimahazo D, Joffre J, Ait-Oufella H, Maury E. Improvement of central vein ultrasound-guided puncture success using a homemade needle guide-a simulation study. Crit Care 2023, 27, 379. [CrossRef] [PubMed]
- Goossens, GA. Flushing and locking of venous catheters: Available evidence and evidence deficit. Nurs Res Pract. 2015, 2015, 985686. [Google Scholar] [CrossRef] [PubMed]
- Vahdat S, Hamzehgardeshi L, Hessam S, Hamzehgardeshi Z. Patient involvement in health care decision making: a review. Iran Red Crescent Med J. 2014, 16, e12454. [CrossRef]
- Central venous line - care of. NHS Cambridge University Hospitals. Date of access: 17 May 2024. Available at: https://www.cuh.nhs.uk/patient-information/central-venous-line-care-of/#:~:text=Caring%20for%20your%20central%20venous%20line,looks%20mucky%20or%20is%20bleeding.
- Ding S, Meystre NR, Campeanu C, Gullo G. Contrast media extravasations in patients undergoing computerized tomography scanning: A systematic review and meta-analysis of risk factors and interventions. JBI Database System Rev Implement Rep. 2018, 16, 87–116. [CrossRef] [PubMed]
- Gundeşlioglu AO, Ozen EC. Necrotizing fasciitis of the cervical region following extravasation injury. Case Rep Med. 2012, 2012, 941578. [CrossRef]
- Ghanem AM, Mansour A, Exton R, Powell J, Mashhadi S, Bulstrode N, Smith G. Childhood extravasation injuries: improved outcome following the introduction of hospital-wide guidelines. J Plast Reconstr Aesthet Surg. 2015, 68, 505–518. [CrossRef]
- Extravasation injuries: Prevention and management. MCN for Neonatology West of Scotland Neonatal Guideline 2019, 11 pages. Date of access: 07 May 2024. Available at: https://perinatalnetwork.scot/wp-content/uploads/2022/06/Extravasation-Neonates_WoS.pdf.
- Albano D, Benenati M, Bruno A, Bruno F, Calandri M, Caruso D, Cozzi D, De Robertis R, Gentili F, Grazzini I, Micci G, Palmisano A, Pessina C, Scalise P, Vernuccio F, Barile A, Miele V, Grassi R, Messina C; Young SIRM Working Group. Imaging side effects and complications of chemotherapy and radiation therapy: a pictorial review from head to toe. Insights Imaging 2021, 12, 76. [CrossRef] [PubMed]
- Harding-Lister S. Common injuries in iron extravasation. Apex Health Associate. Date of access: 08 May 2024. Available at: https://www.apexhealth.net/post/common-injuries-in-iron-extravasation#:~:text=Anxiety%20and%20Fear%20of%20Medical,a%20shadow%20over%20future%20treatments.
- Duarte ACDS, Chicharo SCR, Silva TASMD, Oliveira AB. Ethical dilemmas and illicit acts in nursing: Reflections on the legal (dis)order. Rev Bras Enferm. 2023, 76, e20220558. [CrossRef] [PubMed]
- Le Tourneau C, Borcoman E, Kamal M. Molecular profiling in precision medicine oncology. Nat Med. 2019, 25, 711–712. [CrossRef]
- Cuccia F, Mortellaro G, Trapani G, Valenti V, Ognibene L, De Gregorio G, Quartuccio E, Luca N, Tripoli A, Serretta V, Lo Casto A, Ferrera G. Acute and late toxicity and preliminary outcomes report of moderately hypofractionated helical tomotherapy for localized prostate cancer: a mono-institutional analysis. Radiol Med. 2020, 125, 220–227. [CrossRef] [PubMed]
- Ngo D, Jia JB, Green CS, Gulati AT, Lall C. Cancer therapy related complications in the liver, pancreas, and biliary system: an imaging perspective. Insights Imaging 2015, 6, 665–677. [CrossRef]
- Hunter B, Hindocha S, Lee RW. The role of artificial intelligence in early cancer diagnosis. Cancers (Basel) 2022, 14, 1524. [CrossRef]
- Zhang B, Shi H, Wang H. Machine learning and AI in cancer prognosis, prediction, and treatment selection: a critical approach. J Multidiscip Healthc. 2023, 16, 1779–1791. [CrossRef]
- Koh DM, Papanikolaou N, Bick U, Illing R, Kahn CE Jr, Kalpathi-Cramer J, Matos C, Martí-Bonmatí L, Miles A, Mun SK, Napel S, Rockall A, Sala E, Strickland N, Prior F. Artificial intelligence and machine learning in cancer imaging. Commun Med (Lond). 2022, 2, 133. [CrossRef]
- Gala D, Behl H, Shah M, Makaryus AN. The role of artificial intelligence in improving patient outcomes and future of healthcare delivery in cardiology: A narrative review of the literature. Healthcare (Basel). 2024, 12, 481. [CrossRef]
- Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA, Al Yami MS, Al Harbi S, Albekairy AM. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med Educ. 2023 Sep 22;23, 689. [CrossRef]
- Yamashita R, Nishio M, Do RKG, Togashi K. Convolutional neural networks: an overview and application in radiology. Insights Imaging 2018, 9, 611–629. [CrossRef]
- Nakada A, Niikura R, Otani K, Kurose Y, Hayashi Y, Kitamura K, Nakanishi H, Kawano S, Honda T, Hasatani K, Sumiyoshi T, Nishida T, Yamada A, Aoki T, Harada T, Kawai T, Fujishiro M. Improved object detection artificial intelligence using the revised RetinaNet model for the automatic detection of ulcerations, vascular lesions, and tumors in wireless capsule endoscopy. Biomedicines 2023, 11, 942. [CrossRef] [PubMed]
- Zhang Y, Chu J, Leng L, Miao J. Mask-refined R-CNN: A network for refining object details in instance segmentation. Sensors (Basel) 2020, 20, 1010. [CrossRef] [PubMed]
- Guo Z, Wang J, Wang J, Yuan J. Lightweight YOLOv4 with multiple receptive fields for detection of pulmonary tuberculosis. Comput Intell Neurosci. 2022, 2022, 9465646. [CrossRef] [PubMed]
- Tan M, Pang R, Le QV. EfficientDet: Scalable and efficient object detection. 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 2020, pp. 10778–10787. [CrossRef]
- K. Duan, S. K. Duan, S. Bai, L. Xie, H. Qi, Q. Huang and Q. Tian, CenterNet: Keypoint triplets for object detection. 2019 IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Korea (South), 2019, pp. 6568–6577. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).