4. Discussion
While BMI is a widely used to assess and classify obesity globally, its efficacy in accurately predicting body fat percentage is limited by its curvilinear association with body fat in both men and women. Recognizing this limitation, VAI emerges as a gender-specific equation designed to overcome these challenges by providing a more nuanced assessment of fat content. Importantly, VAI evaluates the functionality of AT to offer a more comprehensive understanding of its impact on health. The development of the VAI equation took into consideration populations of both normal and overweight individuals. This inclusive approach enhances the generalizability of the index, making it applicable to a broader spectrum of individuals. This emphasis on diverse populations contributes to the robustness of VAI as a tool for assessing adiposity and underscores its relevance in capturing variations in body composition beyond what BMI can offer [
18,
19].
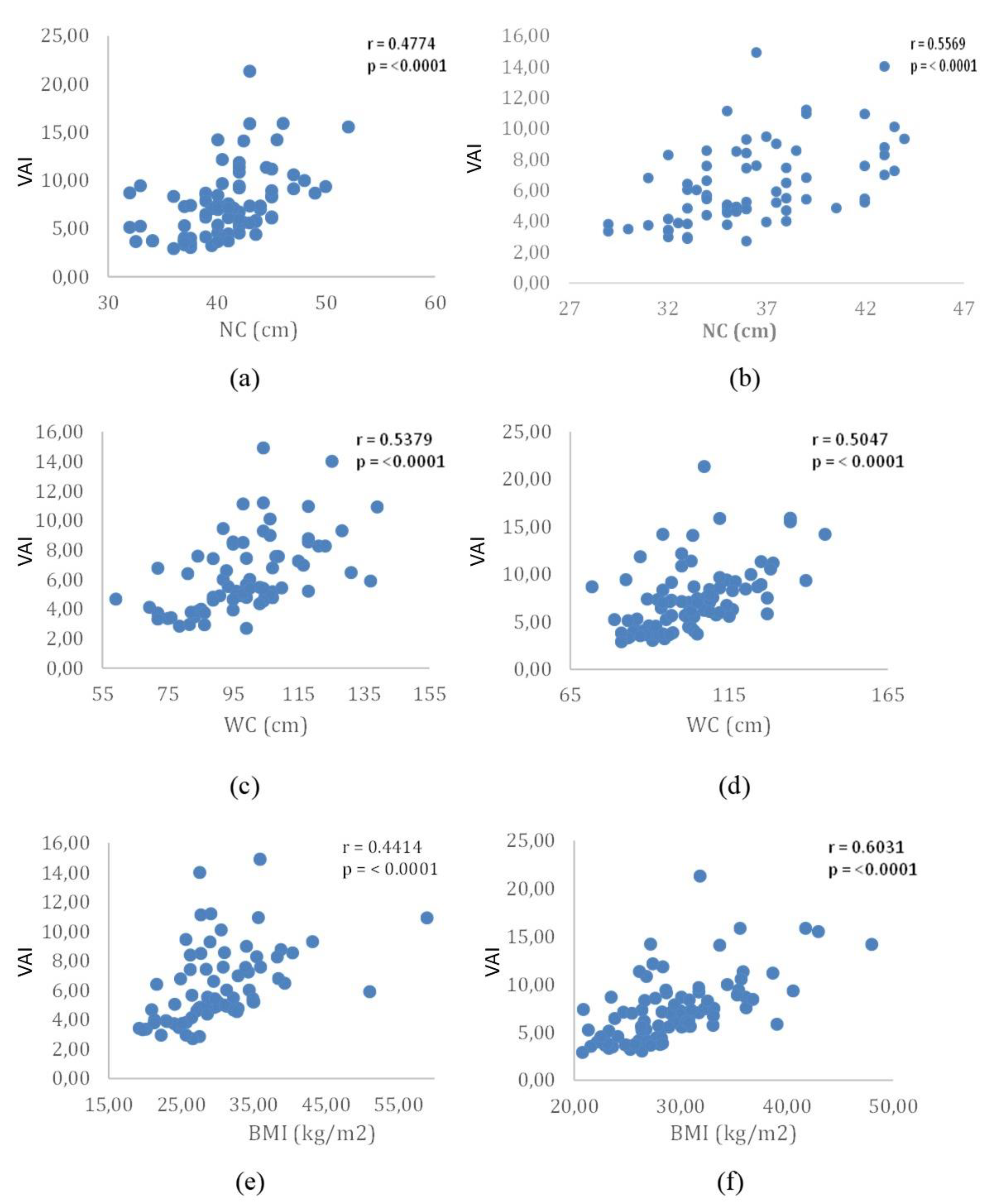
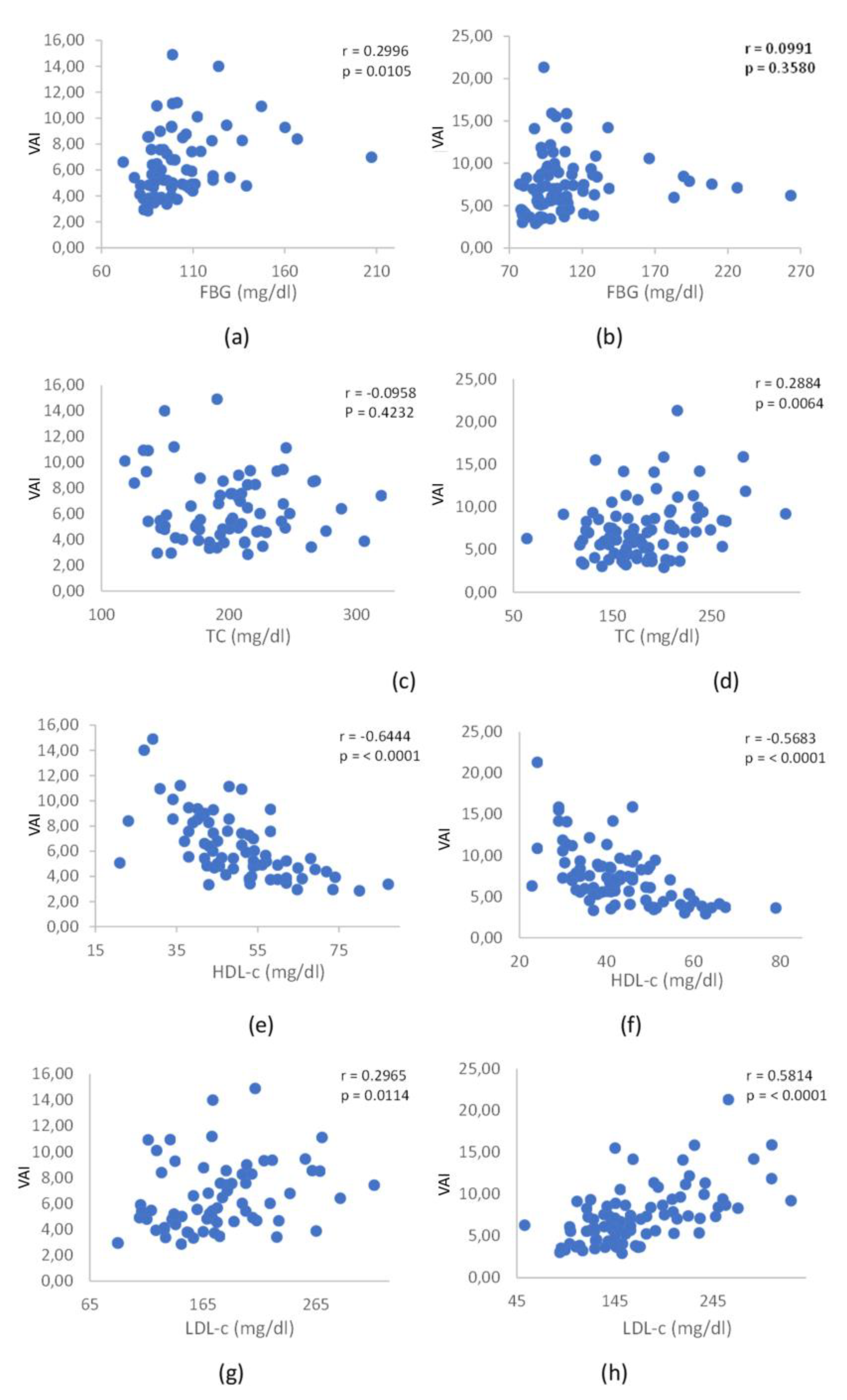
Our study reveals that VAI exhibits positive and significant correlations with both biochemical and anthropometric parameters, pointing towards the identification of individuals at increased risk of CVD. These correlations became evident when examining the concurrent influence of VAI with TC in men, FBG in women, LDL-c in both sexes, and TG in both sexes. Significantly, these outcomes are in harmony with observations from other studies, underscoring the strength and reliability of these correlations across varied populations, with a specific focus on the Brazilian population in this instance. The observed correlations underscore the utility of VAI as a comprehensive metric that captures various facets of cardiovascular functionality. This study contributes to the growing body of evidence supporting the relevance of VAI in identifying individuals at risk for cardiovascular complications, emphasizing its potential as a valuable tool to be used in clinical assessments and risk stratification.
Gu et al. [
20] conducted an extensive study to explore the relationship between the VAI and prediabetes. The study employed a cross-sectional epidemiological survey, categorizing all participants into four groups based on VAI and WC levels: VAI and WC both within the normal range, increased VAI (postcritical VAI) with normal WC, VAI with increased WC (normal VAI with postcritical WC), and increased VAI with increased WC (both postcritical VAI and WC), respectively. The researchers employed multivariate logistic analysis to analyze the associations between these groups and prediabetes, as well as diabetes. Results indicated that both VAI and WC emerged as independent risk factors for prediabetes. In males, the Odds Ratios (ORs) for prediabetes were 1.641 (95% CI 1.146–2.349), P=0.007, in the increased VAI with normal WC group and 1.454 (95% CI 1.055–2.005), P=0.022, in the normal VAI and increased WC group, both in Model 2. In females, the ORs for prediabetes were 2.305 (95% CI 1.623–3.273), P=0.000, in the increased VAI and normal WC group and 1.997 (95% CI 1.529–2.608), P=0.000, in the normal VAI and increased WC group. Furthermore, the study assessed the accuracy of predictions for prediabetes using Receiver Operating Characteristic (ROC) curve analysis. For men, the area under the curve (AUC) value of VAI was 0.601 (95% CI 0.568–0.634), P=0.000, and for women, it was 0.645 (95% CI 0.618–0.672), P=0.000. Notably, WC exhibited the highest AUC value of 0.605 (95% CI 0.571–0.638), P=0.000, in prediabetes for men and the highest AUC value of 0.673 (95% CI 0.648–0.697), P=0.000, in prediabetes for women. These findings underscore the significance of VAI and WC in predicting prediabetes, with WC demonstrating notable efficacy in both genders.
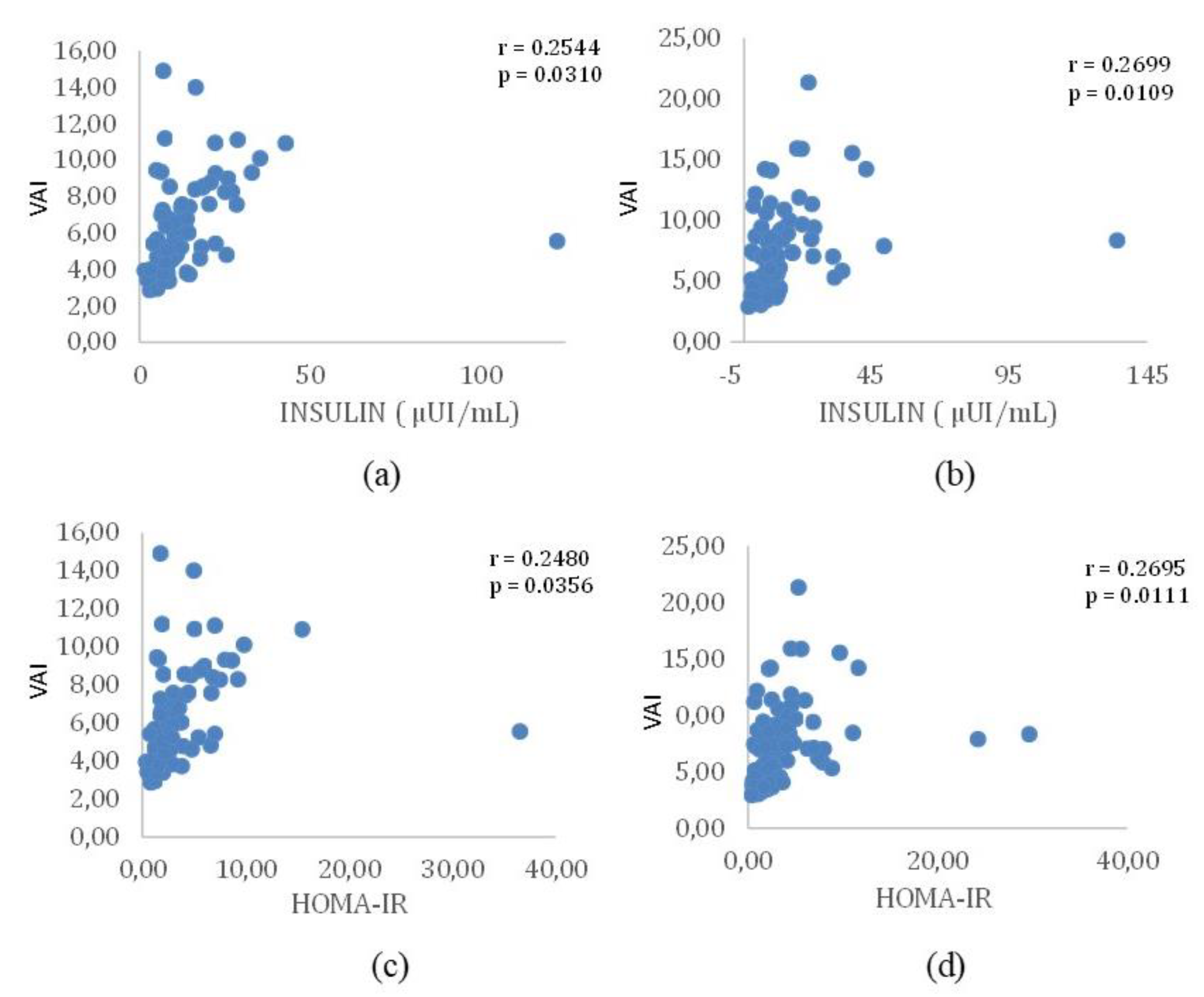
Pekgor et al. [
21] conducted a comprehensive investigation into the positive and significant relationship between HOMA-IR and VAI levels. Their study discussed the exploration of VAI levels in obese patients, both with and without MetS, shedding light on its association with IR. Additionally, the researchers aimed to establish a cutoff value for VAI in identifying patients with MetS. In this study, 92 obese patients, ranging in age from 18 to 65, were included. The levels of HOMA-IR and VAI were meticulously calculated for each participant. Notably, among the 92 patients, 41 (44.6%) with MetS exhibited significantly higher levels of both HOMA-IR and VAI (P < 0.001 for both). The determined cutoff value for VAI in predicting MetS was identified as 2.205. Intriguingly, the prevalence of MetS was 22.2% when VAI was below this threshold, but it surged to 66% when VAI exceeded 2.205. Furthermore, the study unveiled a positive correlation between VAI and HOMA-IR levels. In cases where HOMA-IR was equal to or greater than 2.5, VAI was observed to be higher in 39.1% of individuals, and concurrently, levels of high-density lipoprotein-cholesterol were lower. The established cutoff value for VAI in predicting IR was determined as 2.31. Within this context, the prevalence of IR was 23.4% in patients with VAI below 2.31, contrasting with a notably higher frequency of 55% in individuals with VAI equal to or greater than 2.31. These findings underscore the significance of VAI as a valuable indicator for assessing both MetS and IR in obese patients, providing clinicians with a practical tool for risk stratification and management.
Jafari and colleagues [
22] investigated the correlation between IR indices and the 5- and 10-year incidence of CVD. The study included 1888 and 1450 healthy adults aged 15 to 75 years, selected from the 5895 participants of the KERCADR study in 2012. Participants were monitored for five and ten years, and baseline Lipid Accumulation Product (LAP), triglyceride-glucose index (TyG), and VAI were computed. Logistic regression models were employed to evaluate their association with CVD incidence during the specified follow-up periods. Additionally, the predictive accuracy of these indices in predicting CVD development was assessed using the area under the ROC AUC. Over the 5- and 10-year follow-ups, 399 and 476 CVD cases (21.1% and 32.8%) were recorded, respectively. The adjusted odds ratio (AOR, 95% CI) for 5-year CVD risk showed LAP (2.24 [1.44, 3.50]), VAI (1.58 [1.08, 2.33]), and TyG (1.57 [1.02, 2.42]). For 10-year CVD risk, AOR was LAP (1.61 [1.04, 2.49]), TyG (1.57 [1.02, 2.41]), and VAI (1.41 [0.96, 2.09]). In summary, when considering CVD risk prediction, LAP, TyG, and VAI emerged as more effective predictors compared to conventional single risk factors, which demonstrates insulin resistance indexes’ superiority compared to conventional clinical techniques to assess CVD risk.
The presence of the MetS is linked to a twofold rise in cardiovascular outcomes and a 1.5-fold increase in overall mortality. There is a crucial need for research to determine whether the prognostic significance of the MetS surpasses the risk associated with the cumulative impact of its individual components. Additionally, investigations are imperative to unravel the mechanisms through which the metabolic syndrome amplifies the risk of cardiovascular events [
23].
In 2021, a cross-sectional observational cohort study in Brazil aimed to evaluate the diagnostic accuracy of eight IR indicators in identifying MetS within the Brazilian population. This study specifically aimed to evaluate the diagnostic accuracy of eight IR indicators in identifying MetS within the Brazilian population. The studied IR indicators included the metabolic score for IR (METS-IR), triglycerides to high-density lipoprotein cholesterol index (TG-HDL-c), TyG, triglyceride glucose index body mass-index (TyG-BMI), triglyceride glucose index-neck circumference (TyG-NC), triglyceride glucose index-neck circumference to height ratio (TyG-NHtR), triglyceride glucose index-waist circumference (TyG-WC), and triglyceride glucose index-waist to height ratio (TyG-WHtR). The research cohort comprised 268 participants, consisting of 152 men and 116 women aged between 53 and 59 years. Among them, 111 individuals were diagnosed with MetS according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria. The study findings demonstrated significant diagnostic accuracy across all eight IR indicators. TyG-WC emerged as the leader with the highest AUC value at 0.849 (95% confidence interval [CI]: 0.800–0.889), followed closely by TyG (AUC: 0.837, 95% CI: 0.787–0.879) and TG-HDL-c (AUC: 0.817, 95% CI: 0.765–0.861). Notably, these values underscore the robust discriminatory ability of these indicators in identifying MetS within the Brazilian population. Further analysis revealed that TG-HDL-c exhibited the most elevated diagnostic sensitivity at 90.99%, closely followed by TyG-WC (89.19%) and TyG-NC (84.68%). Conversely, TyG demonstrated the highest diagnostic specificity at 73.89%, followed by TyG-WHtR (72.61%) and TyG-WC (66.88%). These sensitivity and specificity metrics highlight the capacity of these IR indicators to accurately identify individuals with MetS [
24]. It is noteworthy that VAI was not included in the scope of this study.
In our study, we observed an insignificance association between VAI values and CRP levels. This contrasts with findings by Ferreira et al. [
25], who conducted a cross-sectional population-based study involving 854 adults in Viçosa, Minas Gerais, Brazil. Their investigation incorporated a questionnaire, anthropometric measurements, body composition analysis, and biochemical data collection. The analysis, employing ordinal logistic regression, aimed to identify factors associated with VAI. Interestingly, in the study by Ferreira et al., a positive association between VAI and the increase in the percentage of body fat, uric acid concentration, and ultra-sensitive C-reactive protein in the blood was noted among males. Among females, the factors associated with VAI included an increase in age, NC, elevated serum uric acid concentration, and higher levels of CRP. The divergence in findings between our study and that of Ferreira et al. underscores the complexity of factors influencing VAI associations. Variability in study populations, methodologies, and regional characteristics may contribute to these differences. It is important to highlight that the participants in our study were drawn from the São Paulo state of Brazil, while Ferreira et al.'s study sample originated from Minas Gerais, another Brazilian state. This geographical distinction between the two studies emphasizes the potential existence of regional variability. The diverse demographic, lifestyle, and environmental factors across different Brazilian states could contribute to variations in health-related parameters and, consequently, impact the associations between VAI and biomarkers like CRP. Further research and exploration are essential to gain a comprehensive understanding of the multifaceted relationships between VAI and various regional factors affecting adiposity and inflammation.
With regards lipid assessments, Jabłonowska-Lietz et al [
26] conducted an investigation into the potential of VAI as a reliable predictor for lipid abnormalities among 106 obese adults. This diverse study group comprised 72 women and 34 men, with an average age of 39.0 ± 5.9 years, and a mean BMI of 32.6 ± 2.4 kg/m2. These participants were admitted for body weight reduction. The comprehensive evaluation encompassed various anthropometric measures, including body weight (BW), height, WC, hip circumference (HC), BMI, waist-to-hip ratio (WHR), VAI, body adiposity index (BAI), and waist-to-height ratio. Additionally, bioelectrical impedance was used to determine visceral adipose tissue (VAT) levels and body fat percentage (FM%). Serum concentrations of key lipid parameters, including TC, HDL, LDL, TG, glucose, insulin, and HOMA-IR, were also meticulously assessed. The results revealed significant associations between several anthropometric indicators and VAT estimated by bioimpedance. Notably, VAI, WC, and WHR emerged as robustly correlated with glucose and lipid abnormalities in the obese population. Conversely, BAI and BMI exhibited correlations with total FM%. Furthermore, WC, WHR, and VAI demonstrated correlations with total body weight. These findings underscore the potential of VAI, along with specific anthropometric measures, as valuable indicators for predicting lipid disturbances in obese individuals.
Goldani et al. [
27] explored the utility of VAI in predicting components of MetS among elderly individuals in Brazil. This cross-sectional study involved 221 elderly participants with a mean age of 70.65 ± 7.34 years, comprising 53.4% females and 46.4% males. The research gathered comprehensive data, including weight, height, WC, FBG, TG, TC, HDL-c, LDL-c, and blood pressure (BP), along with lifestyle information. Anthropometric indicators such as BMI and WHR were calculated. Adiposity measures were then compared with MetS components, and the predictive capability of VAI for MetS component occurrence was assessed. The analysis of associations among biochemical and MetS components revealed direct and significant correlations of BMI, weight, and VAI with blood glucose, HDL-c, and TG (p<0.01). Remarkably, VAI exhibited the strongest correlation with all parameters. In terms of VAI's applicability in determining the relative risk of MetS component occurrence, it emerged as a robust predictor. Specifically, VAI demonstrated good predictive ability for abdominal obesity (OR = 1.27, p<0.001), hyperglycaemia (OR = 1.10, p=0.043), hypertriglyceridemia (OR = 3.64, p<0.001), and low HDL-c (OR = 2.26, p<0.001).
In another Brazilian investigation, researchers explored the use of VAI for predicting components of MetS, focusing on a younger population. Schuster et al. [
28] conducted a cross-sectional survey involving 444 individuals, with a mean age of 25.6 ± 6.5, and a predominant representation of 77.7% females. This study delved into the applicability of VAI in forecasting MetS components in young adults. Comprehensive data on weight, height, WC, BF%, FBG, TG, TC, HDL-c, LDL-c, and BP were collected, alongside information on participants' lifestyles. BMI and WHR were also computed. The results revealed correlations between VAI and glucose (r=0.136), HDL-c (r=-0.436), and TG (r=0.825) in females. Among males, VAI correlated with glucose (r=0.258), HDL-c (r=-0.550), TG (r=0.897), and diastolic BP (r=0.290). Elevated VAI was associated with an increased risk of abdominal obesity (OR=1.86), hypertriglyceridemia (OR=30.74), and low HDL-c (OR=3.95). Notably, among obesity indicators, VAI exhibited a larger AUC for increased TG and low HDL-c. The study contributes valuable information for clinical assessments and risk stratification in the context of metabolic health among the younger demographic.
By delving into these various aspects, we can unravel the intricate relationship between VAI and the risk of CVD. Considering its practicality, simplicity, and non-invasive nature, integrating VAI into daily clinical practice holds considerable promise. The incorporation of VAI into routine assessments could offer clinicians a reliable tool to gauge the potential cardiovascular risks associated with adiposity. This approach not only enhances our ability to identify individuals at risk but also lays the foundation for the development of targeted interventions and preventive strategies to mitigate the impact of CVD. Thus, a thorough evaluation of VAI across multiple parameters is essential for maximizing its clinical utility and advancing its integration into everyday healthcare practices.
5. Conclusions and Future Research Directions
In the course of our investigation, the VAI revealed a notable and positive correlation with various biochemical and anthropometric parameters, along with IR, which transform into CVD risk factors when dysregulated. The findings from our sample indicate that VAI serves as a straightforward and non-invasive tool, proving to be exceptionally valuable in detecting metabolic alterations stemming primarily from AT dysfunction, notably dyslipidemia. Given its simplicity and efficacy, VAI should be routinely incorporated into clinical practice, becoming an integral part of cardiovascular risk assessment. Moreover, its continued application in diverse research studies is warranted, contributing to the establishment of a comprehensive cardiovascular routine for patients identified to be at risk for cardiovascular events. This proactive approach holds significant promise in advancing our understanding of the intricate interplay between VAI and various metabolic parameters, ultimately enhancing cardiovascular risk management strategies.
Embark on longitudinal investigations to meticulously track the trajectory of the VAI over an extended timeframe is of extreme importance. This in-depth examination aims to unravel the dynamic evolution of VAI and its profound implications for long-term cardiovascular health. By systematically monitoring VAI trends over an extended period, researchers can glean valuable insights into the progressive changes associated with this adiposity index and discern its potential impact on cardiovascular outcomes. This comprehensive analysis will contribute to a more nuanced understanding of the temporal patterns of VAI, offering critical information for refining risk assessment strategies and guiding personalized interventions in the realm of cardiovascular health.
Researchers must also examine the suitability and dependability of VAI across a broad spectrum of populations, considering variables such as age, ethnicity, geographical location, and regional susceptibilities. Delving into the potential variances in these factors will contribute to a more comprehensive understanding of VAI's generalizability, ensuring its effectiveness across diverse demographic groups.
Scientists must also embark on a thorough exploration of the intricate mechanisms that establish the connection between VAI and inflammation, delving into the molecular pathways and cellular interactions that underlie this association. By unraveling the intricacies of these biological processes, researchers can gain profound insights into the molecular underpinnings of VAI-related inflammation, opening avenues for innovative therapeutic interventions. Within this research framework, it is essential to scrutinize the molecular pathways involved in the inflammatory response triggered by elevated VAI. Investigate the signaling cascades, cytokine profiles, and immune cell activations that contribute to the inflammatory milieu associated with visceral adiposity. This in-depth exploration may lead to the identification of novel therapeutic targets for mitigating cardiovascular risk associated with elevated VAI. By pinpointing specific molecules or cellular processes that play pivotal roles in VAI-induced inflammation, researchers can envision the development of precision-targeted therapies. These interventions may range from pharmacological agents that modulate key inflammatory mediators to lifestyle interventions that address the cellular and molecular drivers of VAI-related inflammation.
Researchers must delve into a comprehensive exploration of the genetic factors that exert influence on the VAI, thereby unraveling the heritability patterns inherent in this index. By scrutinizing the genetic determinants associated with VAI, researchers can uncover valuable insights into the extent to which individual genetic makeup contributes to the susceptibility to cardiovascular risks linked with visceral adiposity. This line of inquiry involves conducting detailed investigations into the hereditary aspects of VAI, aiming to discern the specific genetic variations or polymorphisms that may predispose individuals to alterations in their visceral adiposity levels. The research should encompass large-scale genomic studies, employing advanced techniques such as genome-wide association studies (GWAS). Moreover, this investigation may lead to the identification of gene-environment interactions, shedding light on how lifestyle factors interact with genetic predispositions to influence VAI. Pinpointing these gene-environment interactions is instrumental in developing personalized approaches to cardiovascular risk assessment and management, as it allows for a more nuanced and precocious understanding of individual susceptibility.
Author Contributions
Conceptualization, L.F.L., S.M.B., M.D.B., A.C.A.d.C, M.O., P.C.d.S.B., T.L.M.Z., and K.Q.; methodology, L.F.L., S.M.B., L.A.S., K.P.S., M.D.B., and K.Q.; software, F.C.C., A.P., B.B.C., C.T.C., G.A.A., I.d.N.V., B.L.B., and J.C.A.; validation, L.F.L., S.M.B., R.J.T., C.R.P.D., J.F.d.S.H., M.D.B., and K.Q.; formal analysis, L.F.L., S.M.B., M.D.B., and K.Q.; investigation, L.F.L., F.C.C., A.P., B.B.C., C.T.C., G.A.A., I.d.N.V., J.C.A., S.M.B., L.A.S., K.P.S., M.D.B., and K.Q.; resources, L.F.L. and S.M.B.; data curation, L.F.L., S.M.B., M.D.B., and K.Q.; writing—original draft preparation, L.F.L. and S.M.B.; writing—review and editing, L.F.L., S.M.B., M.D.B., and K.Q.; visualization, L.F.L., S.M.B., M.D.B., and K.Q.; supervision, L.F.L., S.M.B., M.D.B., and K.Q.; project administration, L.F.L., S.M.B., and K.Q.; funding acquisition, L.F.L. and S.M.B. All authors have read and agreed to the published version of the manuscript.