You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Review
Live and Pasteurized Akkermansia muciniphila Mechanisms of Action and Its Potential Use as an Aid in the Treatment of Non-communicable Diseases
Altmetrics
Downloads
130
Views
84
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
This comprehensive review delineates the extensive roles of Akkermansia muciniphila in various health domains, spanning from metabolic and inflammatory diseases to neurodegenerative disorders. A. muciniphila, known for its ability to reside in the mucous layer of the intestine, plays a pivotal role in maintaining gut integrity and interacting with host metabolic processes. Its influence extends to modulating immune responses and potentially easing symptoms across several non-communicable diseases, including obesity, diabetes, and inflammatory bowel disease. Recent studies highlight its capacity to interact with the gut-brain axis, suggesting a possible impact on neuropsychiatric conditions. Despite the promising therapeutic potential of A. muciniphila highlighted in animal and preliminary human studies, challenges remain in its practical application due to stability and cultivation issues. However, the development of pasteurized forms and synthetic mediums offers new avenues for its use in clinical settings, as recognized by regulatory bodies like the European Food Safety Authority. This narrative review serves as a crucial resource for understanding the broad implications of A. muciniphila across different health conditions and its potential integration into therapeutic strategies
Keywords:
Subject: Biology and Life Sciences - Immunology and Microbiology
Introduction
Akkermansia muciniphila (A. muciniphila) constitutes about 1% to 3% of the entire gut microbiota, with a concentration of 109 colony-forming units per gram. This bacterium is abundantly present within the mucus layer of the intestine, enabling it to more readily interact with epithelial cells located at the tips of intestinal villi compared to bacteria that do not degrade mucin [1,2]. The bacterium is gram negative obligatory anaerobe. Not only improves intestinal barrier integrity, but it also causes mucus degradation using mucin as the main energy source [3,4]. However, by doing so, it can exert an inhibitory effect on the growth of pathogenic bacteria using the same energy source [5]. It was first isolated and described in 2004 by the team of scientists from Wageningen University in the Netherlands leading by Prof. Willem de Vos [6]. It was recognised as the first representative of Verrucomicrobiota (former name Verrucomicrobia) in the human intestine [4,7,8]. Moreover, in the last few years also other species belonging to the genus Akkermansia were described, based on human fecal metagenomes datasets and isolated strains. As it was noticed, in samples derived from one host in 99% only one particular Akkermansia sp. was identified, showing mutual exclusion pattern. However, only species Akkermansia municiphila was significantly negatively correlated with BMI [4].
A. muciniphila highest concentration is observed in the cecum and abundance vary with a number of factors including age, with a general decrease with age [5,9], although overrepresentation of this bacterium has been reported in centenarians [10]. A. muciniphila is implicated in various health conditions including metabolic health, obesity, diabetes, and inflammatory bowel disease (IBD). Its role is multifaceted, showing both potential benefits and complex interactions with the host's health. The bacterium's abundance in the gut has been linked to the state of glucose tolerance and type 2 diabetes (T2D), albeit with inconsistent findings across different studies, which may be attributed to the diversity in study populations and methodologies [11,12]. The potential health benefits of A. muciniphila stem from its capacity to degrade mucin, producing short-chain fatty acids (SCFAs) like acetate and propionate. These metabolites are known for their anti-inflammatory and metabolic effects, suggesting A. muciniphila's role in weight regulation and immune modulation [13].
Interventions that alter the gut microbiota, such as calorie restriction or bariatric surgery, have been shown to impact A. muciniphila levels, correlating with improved metabolic health and reduced obesity. This underscores the importance of understanding the bacterium's specific role in metabolic regulation and its potential as a therapeutic target [11].
Moreover, A. muciniphila's influence extends to the intestinal barrier's integrity, crucial in preventing conditions like IBD. Research suggests that A. muciniphila or its components, like the outer membrane protein Amuc_1100, can reduce inflammation and enhance the integrity of the intestinal barrier, highlighting its potential therapeutic role in chronic inflammatory diseases [14].
Despite such interesting potential, A. muciniphila could not find therapeutic application due to the instability of this bacterium resulting from its anaerobic nature, the necessity of using animal-derived components in its cultivation, and the low efficiency of cultivation, it was not possible to develop a suitable preparation that would allow its use in humans. The first step was the application of a synthetic medium for cultivation, followed by pasteurization at 70°C. It turned out that pasteurization of A. muciniphila MucT cultivated on a synthetic medium increased its capabilities to limit the development of fat mass, insulin resistance, and dyslipidemia in experimental models [15].
On July 7, 2021, the European Food Safety Authority (EFSA) adopted a scientific opinion on the safety of pasteurized A. muciniphila as a novel food in accordance with Regulation (EU) 2015/2283 [16]. This opinion opens up numerous possibilities for the use of this form of bacteria as a "novel food," which refers to food that was not consumed to a significant degree in the EU before May 15, 1997. Novel food is recently discovered food sources or food that has been newly developed, innovative, produced using new technologies and manufacturing processes, and traditionally consumed outside the EU but not within it [17]. For this reason, we decided to present current information on the potential application of pasteurized A. muciniphila in various diseases, focusing on the mechanism of action and the results of experimental and clinical studies, using the narrative review format.
Mechanisms of action
Several mechanisms of action of A. muciniphila are highlighted in the literature that contribute to host health. In the case of pasteurized A.muciniphila, the primary mechanism involves improving the intestinal barrier function. A key role of the intestinal barrier is to stabilise the space between intestinal epithelial cells and fenestrae of gut endothelium in order to prevent the transmission of, among other things, bacterial fragments and toxins into the circulatory system [18]. The intestinal barrier also ensures the maintenance of normal mucus levels which translates into both structural and functional stabilisation of the intestinal microbiota [19]. A. muciniphila not only has the ability to degrade mucins, but also to stimulate their synthesis. Importantly, this process depends on the initial mucin thickness and intestinal eubiose [19]. Genome analyses allow to identify gene cluster encoding secretion proteins that very likely constitute the pili-like structures present on the A. muciniphila outer membrane and might be involved in crosstalk with the host [20].. One of the proteins named Amuc_1100, has been shown to modulate the intestinal barrier and its permeability by increasing the expression of genes encoding proteins such as occludin (Ocln) and claudin-3 (Cldn3) and cannabinoid receptor 1 (Cnr1). In addition, the bacterium has been shown to reduce lipopolysaccharide (LPS) synthesis [21,22]. What is also interesting, data obtained in study, where influence of Amuc_1100 was examine in mice treated with recombinant protein produced in E. coli cells has shown greater influence than mice treated with A. muciniphila pasterized cells. This mechanisms appear to be caused through activated Toll-like receptor 2 (TLR-2) [22]. Additionally, extracellular vesicles derived from A. muciniphila (AmEV) have been shown to reduce intestinal permeability by inducing AMPK activation [23].
The evidence is mounting, that A. muciniphila content is correlated with metabolic disease, but the molecular mechanisms of its exact effects on the host have not been well defined. In one study, A. muciniphila was shown to increase thermogenesis and glucagon-like peptide-1 (GLP-1) secretion in C57BL/6J mice fed a high-fat diet (HFD) by inducing uncoupling protein in brown adipose tissue. Based on liquid chromatography and spectrophotometry, the researchers identified the protein that is secreted by A. muciniphila and causes the changes described above - the P9 protein. It interacts with the intercellular adhesion molecule 2 [5]. In addition, IL-6 deficiency abrogates the effect of P9 on glucose haemostasis and reduces the expression of ICAM-2 [24]. Another study found that one of the mechanisms of action of A. muciniphila in obesity is the downregulation of pro-inflammatory cytokines (IL-2, INF-Y, IL12p40 AND MCP-1). In addition, concomitant administration of live A. muciniphila inhibited the overall infiltration of mononuclear leukocytes in the liver with immune-mediated damage and reduced TLR2 and TLR4 levels. [23] By affecting the low density lipoproteins (LDL) receptor pathway, reducing apoB48 and apoB100 on LDL lipoprotein, prebiotic supplementation in HFD-fed mice has a beneficial effect on lipid metabolism. By affecting the production of short-chain fatty acids (SCFAs), A. muciniphila, by stimulating their synthesis, is involved in signal transduction through inhibition of histone deacetylase (HDAC) and activation of G protein-coupled receptors, which translates into stimulation of the immune system [21]. A. muciniphila, through the production of acetate and propionate, as well as the Amuc_1100 protein, interacts with FFAR2, 3 and also the TLR2 receptor of goblet cells, thereby increasing their number and differentiation, which also translates into the amount of mucus produced. These phenomena improve the integrity of the intestinal barrier and also the renewal of the intestinal epithelium [25]. By acting on the ICAM2 receptor in the intestinal epithelial L cell, there is an increase in the synthesis of bioactive lipids such as 2-OG, which translates into an increase in oxidation of TCs, a decrease in energy expenditure and a decrease in epithelial inflammation [25]. It has been shown that, pasteurised Akkermansia acts via the AMUC_1100 protein and the TLR2 receptor, making this action more controllable than that of a probiotic, which requires colonisation and appropriate environmental conditions for its action. The AMUC_1000 protein improves intestinal barrier integrity and crosses the intestinal barrier in most likely microvesilces (MVs) and enters adipose tissue, where it induces lipolysis, inhibits lipogenesis and promotes thermogenesis [26]. In addition, it increases mucus production, which is also an energy expenditure for the body. So we have a triple mechanism of action that reduces inflammation, alters fat metabolism and increases energy expenditure. Very importantly, pasteurisation can increase the bioavailability of AMUC_1100, by affecting the bacterial surface charge.
In addition, A. muciniphila has been shown to reverse the enzymatic expression of moonooxygenase 3 (FMO3) affecting the conversion of TMA to TMAO in the liver [15]. A. muciniphila administration was also associated with increased expression of the antimicrobial peptide Reg3y associated with antimicrobial and anti-inflammatory activity. [23]. Applied A. muciniphila supplementation in diabetic patients increased anti-inflammatory macrophage levels and restored the number of Foxp3+ Treg regulatory cells. [23]. It has also been shown that the Amuc_1100 protein can affect the expression of the enzyme Tph1, which regulates the rate of serotonin (5-HT) synthesis in RIN-14B cells, and reduces the expression of the serotonin reuptake transporter (SERT) through interactions with TLR-2, resulting in improved 5-HT biosynthesis and its extracellular availability [14]. Figure 1 summarises the mechanisms of action described so far for Akkermansia muciniphila
Gut diseases
The impact of intestinal barrier permeability in both inflammatory bowel disease is highlighted in many scientific studies [27]. An association between increased intestinal barrier permeability resulting from tight junctions between the development and progression of many gastrointestinal diseases has been shown. In the case of IBD, there is, among other things, an increase in the internalisation of occludin and claudin-2. A similar phenomenon is observed in colitis (reduction of zonulin-1 and zonulin-2 ). It is also worth noting that an association has been shown between increased intestinal barrier permeability and the risk of developing IBD in healthy relatives suffering from Crohn's disease. Increased intestinal barrier permeability is also observed in IBS patients with predominant diarrhoea, whereas in the constipation type of IBS the intestinal barrier is unaltered. Animal studies have shown that these changes may translate into visceral sensitivity [28]
A. muciniphila shows great potential in the treatment of IBD. Research highlights that transplantation of intestinal microbiota used as therapy for IBD leads to an increase in A.muciniphila in the gut of patients. [25] In human studies, A.muciniphila abundance was shown to be significantly reduced in both ulcerative colitis (UC) and Crohn's disease patients. [25]. A study by Lo Sasso et al. compared changes in the gut microbiota in patients with IBD using 16S rRNA sequencing. A reduction in the abundance of A. muciniphila was observed in these patients. [29] Another study by Earley et al. compared changes in the composition of the gut microbiota in patients with active UC compared to healthy patients or patients in remission of the disease. Patients with the active form of UC showed a significantly reduced abundance of A.muciniphila compared to other groups. The study confirmed an inverse relationship between A. muciniphila abundance and inflammation. [30] Lo Presti et al. compared the abundance of A. muciniphila in healthy individuals, patients with irritable bowel syndrome (IBS) and those with IBD. A significantly reduced abundance of A.muciniphila was observed in patients with IBD compared to the other groups. [31] Lopez-Siles et al. compared the content of A.muciniphila in healthy subjects compared to patients with UC, Crohn's disease, IBS and colorectal cancer. Although no difference was observed in the study with regard to A. muciniphila abundance, its levels were reduced in subjects under 16 years of age with IBD compared to the control group. [32] The relationship between A.muciniphila abundance in pediatric patients struggling with Crohn's disease was also investigated. In children and adolescents in remission, it was observed that the gut microbiota was abundant in A.muciniphila. [33] Therefore, the scientific data points towards a protective role for A. muciniphila in the course of IBD. However, the situation is awkward when the intestinal mucosal barrier is damaged by the influence of pathogenic bacteria. The number of A.muciniphila was significantly reduced in patients with IBD and also in mice that had colitis or colon cancer. Studies using oral supplementation of A. muciniphila or Amuc_1100 significantly reduced the infiltration of macrophages and CD8+ cytotoxic T lymphocytes in the colon of mice with colitis, resulting in a reduced inflammatory reaction. [34] In addition, administration of A.muciniphila attenuated inflammation in UC mice and resulted in protective effects on the function and structure of the intestinal microbiota and reduced levels of pro-inflammatory cytokines, as well as neuroinflammation. [34,35,36]
In addition to the use of A.muciniphila in the treatment of IBD, the use of prostbiotic supplementation in irritable bowel syndrome (IBS) has recently received attention. The abnormalities of the gut-brain axis are major pathophysiological factor [37]. In one study in mouse models (C57BL6/J mice subjected to neonatal maternal separation and Citrobacter rodentinum infection), supplemented with pasteurised A. muciniphila was shown to reduce visceral hypersensitivity by affecting intestinal barrier function. In addition, a reduction in anxiety-like behaviour, as well as memory impairment and an effect on pain sensations, has been shown [38].
Metabolic diseases
Recent studies have emphasized the link between significant damage to intestinal barriers and various health issues, including diabetes, obesity, fatty liver, and cardiovascular diseases. Research suggests that a weakened intestinal barrier may result in endotoxemia, which is connected to systemic inflammation, insulin resistance, diabetes, and lipid buildup, thereby hastening the progression of obesity and fatty liver diseases [39]. The pathologically increased permeability of the intestinal barrier is suspected to allow a significant influx of bacterial metabolites through the portal vein, causing the release of pro-inflammatory cytokines, chemokines and eicosanoids. Both these metabolites and the substances released as a result of their presence induce a chronic inflammatory process, fibrosis of liver tissue and have carcinogenic effects [40]. However, the exact processes by which intestinal barrier damage occurs and the methods to effectively enhance the intestinal barrier are still subjects for further investigation. [39]
Numerous studies related to the risk of type I diabetes due to abnormalities in microbiota composition have shown that patients have a higher abundance of Bacteroides ovatus and Bacteroides uniformis, while Bacteroides fragilis appears to play a protective role [41]. In healthy controls, butyrate-producing Bifidobacteria and mucin-degrading bacteria such as A. muciniphila make up a larger part of the microbiota composition, compared to patients with type I diabetes mellitus [42]. The protective potential of Bifidobacteria is not fully described, but de Goffrau's study suggests that they limit the growth of Bacteriodes or restrict movement across the epithelium, thereby reducing inflammation [41]. However, Brown suggests that a microbiota rich in butyrate producers leads to increased amounts of mucin, tighter junctions and increased intergranularity of the intestinal barrier, thereby creating an attractive environment for the proliferation of A. muciniphila, whose abundance is significantly higher in healthy individuals [42,43].
In type II diabetes, elevated levels of LPS are seen in patients, causing weight gain, insulin resistance, induction of inflammation in the gut and ultimately the development of type II diabetes [44]. Studies have shown that patients have bacterial overgrowth and delayed gastrointestinal transit time, while hyperglycaemia and hyperinsulinaemia affect the motility of the jejunum. A reduced abundance of Faecalibacterium prausnitzii, which are found in abundance in the microflora of healthy individuals, was also noted in patients [45]. The intestines of patients with type II diabetes showed reduced numbers of Firmicutes and Clostridia. It has also been noted that the ratio of Bacteroidetes to Firmicutes is correlated with plasma glucose concentration [46]
It has been shown that disturbance of the intestinal flora can significantly affect liver health. Although the mechanism by which this would occur has not been sufficiently explored, a specific composition of the gut microflora has been found in NAFLD patients. [47]
Studies in mice have linked low intestinal abundance of A. muciniphila to pre-diabetes and diabetes [48]. Mice fed a high-fat diet (HFD) for 16 weeks showed increased expression of inflammatory markers, higher serum leptin levels and developing hyperinsulinaemia and hypoglycaemia. After 3 weeks on the diet, the appearance of peripheral insulin resistance and a decrease in A. muciniphila counts were noted [49]. However, the reduced absolute bacterial count does not appear to affect only the development of pre-diabetic states in mice. In patients with developed type II refractory diabetes, manifested by a serum glycated haemoglobin level less than or equal to 8%, examination of faecal samples showed reduced numbers of A. muciniphila compared to patients who achieved optimal glycaemic control with metformin or other hypoglycaemic drugs. [50]
In an experimental model of streptozotocin-induced type 2 diabetes in rats, live and pasteurised A. muciniphila significantly improved well-being, which was primarily associated with improved liver function, reduced plasma levels of pro-inflammatory factors, prevention of gluco/lipotoxicity and reduction of oxidative stress [51].
An imbalance of the gut microbiota accompanies the development of both type II and type I diabetes, despite differences in the aetiology of the two diseases [52]. In type I diabetes, it is IFN-γ that plays a key role in its pathogenesis. IFN-γ (-) mice show better glucose tolerance and increased numbers of A. muciniphila in the gut compared to wild-type mice. However, IFN-γ (-) mice without A. muciniphila did not show improved glucose tolerance This may indicate that the diabetogenic role of IFN-γ may be related to its ability to induce changes in the composition of the microbiota and, in particular, to reduce the abundance of A. muciniphila [53,54].
In addition, NOD mice (non-odese diabetic, mice with elevated fasting glucose levels) treated with oral vancomycin from 28 days of age showed a reduced incidence of type one diabetes, with A. muciniphila predominating in their microbiota. Furthermore, by observing 2 colonies of NOD mice, it was noted that a lower incidence of type one diabetes was always associated with an increased abundance of A. muciniphila. The above studies suggest a significant effect of A. muciniphila on the development of diabetes of both types, which may put forward the inclusion of a new therapy to combat the disease [55].
In the first of the human proof of concept, clinical trials evaluating the validity of A. muciniphila supplementation, 40 overweight or obese subjects with insulin resistance were included. Patients received live or pasteurised A. muciniphila for 3 months. Primary endpoints included safety of the supplement and its tolerability (i.e. liver function, renal function, inflammation) and metabolic parameters (i.e. insulin resistance, circulating lipid concentrations, visceral fat, body mass index). Secondary endpoints included parameters of intestinal barrier function (i.e. plasma lipopolysaccharide (LPS)/metabolic endotoxaemia), gut microbiota composition and metabolites. After 2 weeks, the safety of the supplement was confirmed, as interpreted by the absence of changes in the measured biochemical parameters. Only a significant (still normal) reduction in GGT and CK activity in the group with pasteurised A. muciniphila was noted. There was also no significant change in the incidence of gastrointestinal disorders. After 3 months, a significant increase in fasting plasma insulin levels was observed in the placebo group (p<0.05, T3 versus T0), in contrast to participants receiving both forms of A. muciniphila, who had reduced plasma insulin levels (by approximately 30%) compared with placebo Insulin sensitivity was significantly reduced at T3 in the placebo group (fig. d). Conversely, the supply of both forms of A. muciniphila improved this parameter. pasteurised A. muciniphila clearly and significantly improved the insulin sensitivity index by approximately 30% compared to the Placebo group, and live A. muciniphila significantly improved the insulin resistance index . Pasteurised A. muciniphila significantly reduced DPP- IV activity after 3 months of the study compared to baseline. WBC counts remained significantly increased compared to baseline and week 2 in the placebo group (fig. f), whereas supplementation with pasteurised A. muciniphila completely abolished this effect, resulting in a significant reduction in WBC counts compared to the placebo group . The magnitude of this difference between T0 and T3 or the placebo group (i.e. 866 cells/µl) is highly significant, as a difference of 300 to 1000 cells/µl is considered clinically significant. Supplementation with the pasteurised form of A. muciniphila significantly reduced both γGT and AST levels after 3 months compared to baseline. Specifically, γGT levels were markedly reduced by approximately 24% in the group supplemented with pasteurised A. muciniphila compared to T3 levels observed in placebo. Neither intervention induced a significant change in microbial community composition, although supplementation with live bacteria had a slightly greater effect (partial dbRDA, adjusted R2=0.03, p =0.095) than pasteurised(partial dbRDA, adjusted R2=0.02, p=0.14), while placebo had the least effect (partial dbRDA, adjusted R2=0.01, p =0.66). Administration of pasteurised A. muciniphila significantly reduced total cholesterol by 8.68% compared with placebo, while LDL cholesterol was 7.53% lower and triglycerides 15.71% lower, although these differences did not reach statistical significance. The administration of pasteurised A. muciniphila slightly reduced body weight by about 2.27 kg, fat mass by about 1.37 kg and hip circumference by 2.63 cm compared with the placebo group. Waist circumference decreased by approximately 1.56 cm. The study thus reports a broad-spectrum effect of A. muciniphila on changes associated with increased body weight [30]. Supplementation with A. muciniphila reverses metabolic disorders induced by a fatty diet, among others, metabolic endotoxaemia, increased body fat and insulin resistance Administration of A. muciniphila decreased postprandial triglycerides and chylomicrons and increased expression of receptors for low-density lipoprotein (LDL), thereby regulating intermediate-density lipoprotein (IDL) through induction of apolipoprotein B 100 and apolipoprotein E [28,29,30].
A. muciniphila has the ability to activate immune signalling through receptors that recognise molecular patterns. There is increased secretion of the chemokine CCL20 binding to the CCR6 receptor on the surface of T and B lymphocytes. A. muciniphila has been shown to have a strong negative correlation with inflammatory markers, adipose tissue homeostasis, insulin levels and glucose levels. [5] Supplementation-induced changes included stimulation of Treg cell proliferation and suppression of the hepatic stress marker ER-glucose-regulated protein [59]. In addition, A. muciniphila restored intestinal barrier function by reducing the high concentration of LPS induced by the Western diet. Consequently, this led to the alleviation of LPS-induced systemic inflammation and arthritis by reducing macrophage transmission to the inner membrane through reduced expression of TNF-α and IL-1β [60].
According to available data, Akkermansia appears to have a positive effect on many metabolic disorders, but the exact mechanism of its action is not fully known. One of the causes of MAFLD (Metabolic Dysfunction-associated fatty liver disease) is damage to the intestinal barrier with endotoxaemia, which translates into the functioning of the microbiota-gut-hepatic axis. In the study by Wenrui Wu et al. the study mice were divided into three groups. Mice were fed a low-fat diet, a high-fat diet or a high-fat diet with additional supplementation of approximately 1.5 x 109 live A. muciniphila per day. It was shown that mice given A. muciniphila had less weight gain compared to the other groups. In addition, serum AST and ALT concentrations and NAS score (the NAS scale assesses the degree of liver fibrosis) were lower than in the group with the same diet without supplementation. Supplementation with A .muciniphila affects the gut microbiome in a way that alters the bile acid profile, improves glucose tolerance, reduces the development of white adipose tissue (WAT) and lowers insulin, resistin and leptin levels [61]. In contrast, Sejeong et al. conducted a study in mice that were fed a diet consisting of 45% or 10% fat. Some of the individuals were also given A. muciniphila. The researchers noted no differences in body weight gain between mice supplemented with A. muciniphila compared to mice without active supplementation. However, serum triglyceride and ALT levels were lower when A. muciniphila was introduced into the diet of intervention group, indicatingit may reduce the liver damage caused by the disease. When the expression levels of chREBP and SREBP were determined in liver tissue, supplementation with A. muciniphilia decreased the expression levels of the genes tested, resulting in a reduction of triglyceride synthesis in the liver compared to mice that were not given the probiotic. The same relationship was found for IL-6. As mentioned earlier, the amount of A .muciniphilia decreases in individuals on a high-fat diet. The study showed that supplementation allows this effect to be reversed and the amount of bacteria to increase. The researchers suspect that supplementation could improve the tightness of the intestinal barrier by maintaining homeostasis of the intestinal microbiome, thereby preventing the development of MAFLD [66]. Selected metabolic studies in which A. muciniphila supplementation was used are included in Table 1.
Cancer
The state of the intestinal barrier is also important for the development of cancer in the body. It has been shown that excessive permeability within the intestinal barrier can lead to increased penetration of toxic and pro-inflammatory substances, which can then cause inflammation in the body and interfere with cell proliferation towards carcinogenesis. An important component of this system are the tight junctions, whose malfunction has been linked to the development of bowel cancer. Since some of the most important factors influencing the proper functioning of the intestinal barrier are diet and the absence of intestinal dysbiosis, correctly selected supplementation supported by appropriate dietary habits may favourably influence the development and course of cancer [28].
The data are mounting that the presence of the bacterium A. muciniphila in the human intestinal microbiome may significantly influence the effectiveness of cancer treatment, particularly for non-small cell lung and colon cancer [69,70,71]. Although not all of its mechanisms of action in the context of cancer have been understood, evidence suggests that this microorganism can exert a beneficial effect on immunity, with potentially important implications for the success of ongoing therapy [72]. Immunotherapy with anti-PD-1 and anti-PD-L1 antibodies is used in the treatment of some cancer types, such as non-small cell lung cancer, renal cell carcinoma and melanoma. The efficacy of cancer immunotherapy depends on the ability of the host to produce tumour antigen-specific Th1 cells that secrete IFNγ and cytotoxic effector T cells (Tc1) [73]. This raises the possibility for commensal gut bacteria having the ability to correct Th1/Tc1 immune responses, which can be defective. This offers considerable hope for the use of microbiota-focused interventions against both primary and secondary resistance to cancer immunotherapy [69,74].
After analysing studies on the correlation of A. muciniphila and the body's response to immunotherapy with an anti-PD-1 antibody, it was shown that the high presence of this bacterial species in the gut microbiota of lung or kidney cancer patients was associated with a positive body response to the administered therapy through increased mobilisation of CCR9+, CXCR3+ and CD4+ T cells in tumour foci. It has been shown that commensal gut bacteria may have an effect on the regulation of endogenous immune stimulation and infiltration of T cells within the tumour environment. Interestingly, no such immune response was observed in patients whose gut microflora was deficient in A. muciniphila. In both cases, faecal microflora was collected from patients and transplanted into sterile mice. Here, too, the results clearly indicated increased T-lymphocyte activity within the tumour focus of mice with transplanted microbiota containing A. muciniphila. In contrast, mice that received transplants from patients who did not respond to treatment also showed no response to the administered therapy. An important fact is that after both transplantation of faeces rich in A. muciniphila and after oral administration of the presented strain, a positive response of mouse organisms to anti-PD-1 antibodies was observed [72,75,76].
Another correlation of A. muciniphila with cancer treatment was noted for immune checkpoint targeting (ICI) - a therapy used in advanced stages of non-small cell lung cancer, melanoma or renal cell carcinoma. The first key point was to prove that the anti-tumour effect of ICI immunotherapy depends on the presence of microorganisms in the gut. The researchers noted that patients who proceeded with ICI treatment responded significantly less well to the immunotherapy they received after the antibiotic treatment. Their life expectancy was shown to be reduced by 6.7 months (95% CI: 5.1-8.4). These observations were confirmed in prospective studies and large meta-analyses suggesting that the gut microbiota may play a key role in the immunostimulatory mechanism of action of ICI [69,77].
Prostate cancer (PCa) is one of the most common malignancies affecting men. Despite the successes achieved by ongoing immunotherapy in other types of cancer, there is still a need to develop a more effective therapeutic strategy for the treatment of PCa. To this end, a study was conducted to test the effect of the extracellular vesicles secreted by A. muciniphila (Akk-EVs) on developing PCa. During the study, isolated Akk-EVs were injected into immunocompetent mice with implanted PCa. Mice were then monitored for immunophenotypic changes in cells, including CD8+ T-cell activation. Wound healing rates were also assessed to see how macrophages affect Akk-Ev-induced PCa cell proliferation and invasion. The body weight of the mice, their food and water intake were recorded throughout the study. After 13 treatments, it was shown that tumour proliferation was significantly slowed in the Akk-EV-treated mice compared to the control group. In addition, major organs such as the liver and kidneys were harvested for histopathology to assess potential systemic toxicity. The researchers' conclusions were very promising; immunocompetent mice after Akk-EV administration showed a reduced prostate tumour burden with no observed toxicity to healthy tissues. The treatment resulted in increased granzyme B-positive (GZMB) and interferon γ-positive (IFN-γ) activity in CD8 + T-cells. An increased proportion of CD8+ T lymphocytes and the accumulation of more macrophages, with an increase in the number of M1 macrophages, which have tumour-fighting capacity, and a decrease in the number of M2 macrophages, which exhibit immunosuppressive effects. The macrophage growth environment conditioned by Akk-EV inhibited the proliferation and invasion of prostate cancer cells. Importantly, healthy cells tolerated the Akk-EV administered to the mice well. This study indicates a promising effect of A. muciniphila in immune therapy of prostate cancer [78].
At the turn of the year, it was noted that the gut microbiota may play a role in both the initiation and inhibition of colorectal cancer. A group of bacteria has been identified whose biofilm and potential to disrupt gut vascular barrier increases the risk of colon cancer [79] and augment liver metastases [80]. Such a relationship is shown, for example, by Bacteroides fragilis or Fusobacterium nucleatum. F. nucleatum also mediates resistance to chemotherapy and increases the bluntness of metastasis through activation of the autophagy pathway increasing chemoresistance of the tumour [81]. With this knowledge, the researchers decided to see if we could simultaneously isolate bacteria carrying a beneficial effect in the prevention and treatment of colorectal cancer. A study focusing on A. muciniphila was promising, as this bacterium showed a mitigating effect in mild and acute dextran sulphate sodium (DSS)-induced colitis by reducing levels of pro-inflammatory cytokines and improving host intestinal barrier function [82]. The first results on the efficacy and type of mechanisms of action of A. muciniphila in colorectal cancer showed that patients with colonic adenocarcinoma or colorectal cancer were characterised by a reduced abundance of this bacterium compared to healthy subjects. Interestingly, it was also observed that a significantly lower amount of A. muciniphila was found within tumour cells relative to adjacent normal cells [83].
Mice with induced spontaneous intestinal adenoma were divided into three different groups and given A. muciniphila, E. coli or phosphate-buffered saline (PBS). They had previously received one week of antibiotic therapy. At week 12 after implementation of the intervention, the group treated with A. muciniphila significantly inhibited tumour growth compared to the PBS control group as assessed by tumour count (5,333 ± 0.7638 vs. 9,714 ± 1.04, P <0.01), tumour volume (10% vs. 20% for tumours larger than 3 mm) and tumour burden (9,056 ± 1,621 vs.15,11 ± 1,654, P <0.05). In addition, tumours in the A. muciniphila had lower levels of the PNCA marker responsible for cell proliferation and an increased number of macrophages present in the tumour growth medium (TAM) type M1 compared to the PBS group (28.1% vs. 11.9%, P <0.05). This suggests that A. muciniphila can stimulate M1-type TAMs to mount an immune response, which have beneficial therapeutic effects within colorectal cancer [83].
One study on the effect of A. muciniphila in the treatment of colorectal cancer indicates that its presence may be beneficial in first-line therapy with FOLFOX (oxaliplatin, fluorouracil and calcium folinate), which in an experiment showed higher efficacy than the administration of oxaliplatin alone. It demonstrated that the abundance of A. muciniphila in patients receiving FOLFOX increased significantly. Most significantly, however, the increase in bacterial abundance in the intestine of patients was associated with increased antitumour efficacy of the administered preparation. A study was conducted in mice that had a significantly weakened and homogeneous intestinal microflora through broad-spectrum antibiotic therapy. They were then administered every two days via gastric tube A. muciniphila until the end of the study. The mice were divided into the following groups: control group, oxaliplatin model group, oxaliplatin treated group, FOLFOX model group and FOLFOX treated group. Before administering the drug to the mice, it was verified, by collecting and testing faecal samples, that the graft of A. muciniphila passed successfully. Oxaliplatin and FOLFOX showed different effects on the composition and metabolism of the intestinal microflora. Oxaliplatin increased carbohydrate and nucleotide metabolism within the intestinal microflora, whereas FOLFOX increased carbohydrate and lipid metabolism. After analysing the differences between the model groups and the groups receiving oxaliplatin and FOLFOX, it was shown that the most increased abundance of A. muciniphila between the model group for FOLFOX and the FOLFOX-treated group. It also increased the anti-cancer effect of this therapy from 36% to 48% and the degree of tumour inhibition from 48% to 76% (p < 0.05). The above results indicate the efficacy of A. muciniphila in this type of colorectal cancer therapy. The group taking oxaliplatin alone had significantly worse results. Its anti-tumour effect was weaker than FOLFOX, so further research was devoted to the leading therapy. Although the study sounds promising, it is worth bearing in mind that it was conducted on mice that had previously received intensive antibiotic therapy to reduce the diversity and abundance of bacteria residing in the gut of the rodents [70].
In 2020, Wang et al. analysed the effect of A. muciniphila injected to mice with sodium dextrasulphate followed by azoxymethane (DAO/AOM) administration. The latter ones induce colitis consequently leading to colorectal cancer. Mice were orally administered with either pasteurised A. muciniphila or Amuc_1100 protein as early as two weeks before the induction of inflammation and continued for a further 23 weeks during which the mice and their faeces were systematically examined. At the same time, faecal samples were collected from patients with colitis and colorectal cancer. After completion of the study and analysis of the faecal samples, it was shown that in both DSS/AOM-infected mice and diagnosed patients, the concentration of Akermanisa in the intestine was reduced. It was noted that mice that received supplementation with pasteurised A. muciniphila or Amuc_1100 protein showed significantly later tumour development (p <0.005) and also reduced tumour abundance (p < 0.05 for pasteurised A. muciniphila and p<0.001 for Amuc_1100 protein) and tumour size (p < 0.01) at week 12 of the study. A reduced expression of tumour-associated markers γH2AX and Ki67 in colonic epithelial cells was also observed in mice treated with pasteurised Akk. or Amuc_1100 protein. The above observations may suggest that supplementation of pasteurised A. muciniphila may inhibit excessive cell proliferation. The results are promising, but should also be carried out fully on the human population [34].
In conclusion, A. muciniphila has a positive effect and contributes to the body's response to certain agents administered in both immunotherapy and chemotherapy. Although the full mechanism of action has not yet been fully studied, we know that it plays a significant role in stimulating M1-type macrophages and increasing the number of CD8+ T lymphocytes. However, most of the studies have been carried out on animals, so further exploration and testing is needed.
Neurodegenerative diseases
Since the discovery of A. muciniphila in 2004 by Derrien et al. [6] evidence has emerged for the great potential of A. muciniphila and its outer membrane proteins in supporting the treatment of neuropsychological diseases. This evidence is supported by studies in animals and clinical trials [84]. The potential of A. muciniphila as a therapeutic support for neurological disorders such as Alzheimer's disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD) or stroke has already been recognised. This suggests a viable role for A. muciniphila in normal brain function [23]. The interest of researchers from various backgrounds in conducting functional studies of the intestinal barrier under various conditions has led to the linking of these diseases to abnormal intestinal permeability, which is a sign of impaired intestinal barrier function. In PD and AD, a biomarker for assessing intestinal permeability is elevated levels of zonulin in the blood. It is therefore necessary to initiate measures leading to the restoration of a normal intestinal barrier, and thus proper absorption of nutrients and fluids, preventing toxins and harmful bacteria from entering the body through the intestinal epithelium [85].
In mouse models of AD, a large decrease in A. muciniphila is observed [86], and this is associated with an impaired intestinal barrier [87]. These findings indicate the potential of A. muciniphila treatment in improving mucosal barrier dysfunction, metabolic dysfunction and memory [88,89]. Mice on a high-fat diet showed a decline in cognitive and mental function, and this was associated with A. muciniphila depletion [90]. It is possible to make up the deficit of this bacterium by, for example, a 16-week ketogenic diet, thus reducing the risk of neurodegeneration by improving the metabolic profile in young, healthy mice [91]. Beneficial effects of A. muciniphila supplementation whether by oral administration or dietary interference are observed in AD mouse models, compared to AD mouse models that do not receive this bacterium and its faecal abundance decreases with age [84]. A mouse model of AD APP/PS1 fed a normal diet or a high-fat diet and treated with A. muciniphila (5 x 109 cfu A. muciniphila in 200µl sterile PBS) by gavage daily for 6 months showed improvement in cognitive deficits and a reduction in amyloid-β (Aβ) protein levels [92]. Human studies have shown that patients with very mild to moderate AD have more A. muciniphila than the control group [93], while its levels are similar in people with mild cognitive impairment (MCI) and control subjects [94]. Studies on the beneficial effects of A. muciniphila on AD have been conducted relatively recently. However, direct evidence is still needed for validation, especially from human studies [95].
Postmortem findings in PD patients suggest that α-synuclein inclusions (a pathological feature of PD) may be transported from the gut to the brain via the vagus nerve [96], which raises the hypothesis that PD may begin in the gut. A growing number of recent studies have demonstrated alterations in the diversity of the gut microbiome in PD patients, which may cause inappropriate α-synuclein folding or disrupt enteric nervous system function [84]. Increased abundance of A. muciniphila was only found in mice with tetrahydropyridine (MPTP)-induced PD [97] and after treatment with rotenone [98]. However, Akkermansia abundance did not increase in most mouse models of PD in other studies [62,63,64]. In contrast, studies of PD patients in clinical settings in different countries have shown increased abundance of A. muciniphila [65,66,67]. In a study that used three machine learning algorithms to analyse metagenomic results from 472 PD patients and 374 healthy controls, 22 bacterial families were identified that helped distinguish between controls and potential PD patients. Among the bacteria in these 22 families, Akkermansia showed high efficiency in distinguishing PD patients from controls [105]. These studies and findings present an important role for Akkermansia in the pathogenesis of PD. The increased abundance of A. muciniphila makes it a potential early biomarker of PD [106].
A study was conducted on the difference in microbial abundance between MS patients and controls, and investigated how specific bacteria associated with MS modulate T-lymphocyte responses using in vitro and in vivo model systems. The results indicate that significantly increased abundance of A. muciniphila in MS patients is associated with a shift towards a pro-inflammatory T-cell profile that exacerbates or perpetuates the immune response. In an in vitro model, A. muciniphila extracts significantly increased the differentiation of peripheral blood mononuclear cells (PBMCs) into Th1 lymphocytes [107]. There are many studies confirming an increase in A. muciniphila abundance with the activation of the pro-inflammatory response in MS patients compared to controls [70,71,72]. In contrast, a reduction in A. muciniphila abundance in MS patients induced an anti-inflammatory immune response in the peripheral immune system [110]. Increased levels of A. muciniphila were also reported in a study in a mouse model of experimental autoimmune encephalomyelitis (EAE) compared with control mice [109]. Additionally, transplantation of the faecal microbiome from MS patients into mice induced a pro-inflammatory environment, which worsened the severity of EAE [107,111]. On the other hand, Cox et al. investigated a negative association between A. muciniphila abundance and disability and a positive correlation between A. muciniphila and brain volume, showing that the bacterium also plays a beneficial role. They sequenced the microflora in healthy controls, patients with relapsing-remitting multiple sclerosis (RRMS) and patients with progressive multiple sclerosis and correlated bacterial levels with clinical features of the disease, quality of life and MRI brain atrophy. They colonised mice with Akkermansia from multiple sclerosis and induced experimental autoimmune encephalomyelitis. By testing RRMS patients with high levels of Akkermansia, they distinguished between three subtypes based on the 16S rRNA V4 sequence. They then isolated the bacteria on culture medium and sequencing identified the strains most similar to A. muciniphila. To illustrate the role of MS-derived Akkermansia, they colonised C57/BL6 mice with three strains and found that they all alleviated autoimmune encephalomyelitis (EAE). In addition, they measured immune responses and found that the Akkermansia sp. BWH-H3 strain reduced the number of RORγT-positive γδ T cells and IL-17-producing γδ T cells [112]. These findings support that increased levels of this battery in MS may be a beneficial compensatory response in the MS microbiome [112,113].
The studies in the case of MS and PD seem at first glance to be worrying and at odds with the previously described positive association between A. muciniphila abundance and health. Nevertheless, the following facts should be taken into account when analysing the work described in this chapter:
- The studies described in MS and PD prove correlation, not causation
- Bacterial contents in tests are given in relative abundances, which in practice means that their number (absolute value) is not necessarily higher [118]
- Pasteurised Akkermansia muciniphil does not colonise the gut so does not induce changes in the composition of the microbiota [68]
- No authority, including EFSA, has denied the product's placing on the market on the basis of these correlative reports alone. [16]
A. muciniphila is negatively associated with amyotrophic lateral sclerosis (ALS), as evidenced by the progressively decreasing abundance of A. muciniphila in ALS mice [119]. Supplementation of pasteurized A. muciniphila in Sod1 transgenic mice (Sod1 -Tg) with ALS improved motor function and brain atrophy through accumulation of nicotinamide in the central nervous system. Nicotinamide is associated with A. muciniphila and its functions are to support dopamine production in neurons, neurotransmission and normal neuronal cellular metabolism [89].
Studies show that bacterial pneumonia is the leading cause of death after stroke. The selective movement of bacteria from the host's gut microbiome to the lungs is an essential factor in causing this pneumonia. This leads to the conclusion that the intestinal mucosa and gut microbiota plays an important role in post-stroke mortality [120]. Studies have shown that stroke can be triggered by disturbances in the gut microbiota, which in turn can affect neuroinflammatory and functional indicators after brain injury. The efficacy of post-stroke treatment may be improved by faecal microbiota transplantation (FMT) [121]. An increase in faecal A. muciniphila levels has been noted in patients following ischaemic stroke in clinical trials [122], leading to proposals to treat it as a microbiological marker [123]. There are other studies confirming that after stroke there is an increase in abundance in mouse models [120,124]. One of these studies analysed the communities of different microorganisms in five gastrointestinal tract docs in mice after stroke: duodenum, jejunum, ileum, cecum and colon. The finding of this study was that A. muciniphila changes its interaction profile after impaction towards activating beneficial bacteria, e.g. Ruminococcus spp., and inhibiting pathogenic bacteria, such as Streptococcus spp. and Staphylococcus spp. [120]. Thus, A. muciniphila contributes to reducing the migration of pathogens into epithelial cells and, consequently, into the lungs of stroke patients [120,125]. Different results were obtained in a faecal study of eight post-stroke humans and a 10-person control group, where the abundance of bacterial groups was compared. The abundance of A. muciniphila was reduced compared with the control group [126,127]. Still, the role of A. muciniphila in post-stroke patients remains unclear, so further studies especially on larger study groups are needed to determine this significance.
Mental illnesses
Research has shown that Akk may also play a role in neuropsychiatric disorders. Its direct influence is seen in the bacterium's effect on the host microbiota-gut-brain axis. The bacterium itself and its metabolites play a role in alleviating neuropsychiatric symptoms by reducing intestinal imbalances, thereby contributing to improving the integrity and structure of the intestinal barrier which translates into reduced inflammation. [88] Additionally, there is evidence in research that links stress response and psychiatric phenotypes to TLR signalling [128,129]. Research is scarce, mainly in animal models. One of the first studies was conducted in male C57BL/6N mice subjected to chronic stress (CRS), in which colitis was induced by administration of dextran sodium sulphate (DSS). The mice were then transplanted with A.muciniphila (1x109 IU/ml) after reducing the diversity of the gut microbiota following the intervention. The study showed that CRS caused behavioural deficits, but did not reduce the diversity of the gut microbiota. In addition, in CRS-treated mice, supplementation with A.muciniphila reduced the intensity of depressive disorders. Supplementation also improved histopathological indices - it improved mucosal barrier structure and function by increasing, among other things, the number of cup cells and also MUC-2 cells [130]. Another study also conducted in an animal model in male C57BL/6 mice aged 6-8 weeks sought to demonstrate the effect of CRS and A.muciniphila supplementation on depressive disorders. The control group received no supplementation, while two of the other groups were subjected to CRS, one of them with A. muciniphila supplementation for 3 weeks (5x108 CFU/ml by oral gavage). The study showed that supplementation had a significant effect on the behaviour of the mice, additionally reducing corticosterone levels and increasing dopamine and BDNF levels. However, no effect was observed on serotonin levels [131]. Another study sought to demonstrate how A.muciniphila supplementation would affect depressive and anxiety behaviours induced in animals by antibiotic use. Mice were supplemented with A.muciniphila for 3 weeks at 1.5 x 109 CFU in one group, while the other group was given Amuc_1100 protein (100ug/200ul) daily. The study showed that supplementation reduced behavioural deficits induced by antibiotic therapy [132].
In a study by Mcgaughey et al. also conducted in mice subjected to social stress, there was a decrease in A.muciniphila, Ruminococcus spp., Mollicutes spp., Paraprevotella spp., Doreta spp.. However, an increase in the amount of Oscillospira spp., Bacteroides spp, Lachnospiraceae spp was observed. In addition, the amount of A. muciniphila was shown to correlate with behavioural behaviour in mice. [133] Attempts were also made to demonstrate the effect of melatonin on modulating the composition of the gut microbiota in response to water stress and sleep deprivation. It was observed that in rodents, exposure to stress leads to a decrease in A.muciniphila and Lactobacillus murinus, but also to an increase in Bacteroides masiliensis. In addition, a decrease in melatonin concentration was observed, presumably as a mechanism of reduced bacterial abundance. [134] A study by Ding et al. sought to demonstrate a link between major depressive disorder (MDD) and the composition and function of the gut microbiota. The main aim of the study was to evaluate the antidepressant effect of A.muciniphila in a mouse model of chronic restraint stress (CRS)-induced depression. Mice were divided into three groups. One group was subjected to CRS, a second group without exposure to CRS and mice given A.muciniphila supplementation for 3 weeks. Subsequently, behavioural tests were conducted and levels of hormones, neurotransmitters including brain-derived neutrophic factor (BDNF) were determined. The composition of the gut microbiota was analysed by 16S rRNA sequencing. Metabolites were also determined. Supplementation with A. muciniphila has been shown to alleviate depressive behaviour and restore normal levels of depression-related hormones ( including dopamine and BDNF). [131] Sun et. al. report using broad-spectrum antibiotics in mice with induced depression. Pharmacotherapy resulted in increased anxiety and depressive behaviour. In addition, reduced levels of 5-hydroxytryptamine (5-HT) were observed in both mouse serum and hippocampus. Mice were supplemented with A. muciniphila and Amuc_1100 for 3 weeks. An alleviation of anxiety and depressive symptoms was observed. In addition, a normalisation of BDNF expression in different brain areas and an increase in serum and hippocampal 5-HT was observed. [132] A study by Guo et al. sought to demonstrate a link between the occurrence of depression associated with alcohol abuse. After alcohol supply in rodents, there was damage to the intestinal barrier, as well as lipopolysaccharide (LPS) translocation, increased inflammatory response (increase in TNF-a and IL-1B) and decreased 5-HT levels. A 2-week supplementation with A. muciniphila resulted in an alleviation of depression-like behaviour. Occludin, BDNF and 5-HT increased and LPS, TNF-a, IL-1b AND IL-6 decreased. [131]
In autism spectrum disorders (ASD), the composition and function of the intestinal microbiota is disrupted. In a study by Zou et al. an increase in Bacteroidetes spp. and Firmicutes spp. was observed in patients with ASD. These patients also showed a reduction in A.muciniphila, E.coli, B.fragilis, H.parainflienzae, among others. [135] In autism spectrum disorders, abnormalities in the composition and function of the gut microbiota are repeatedly highlighted, which translates into gastrointestinal dysfunction. In a mouse model of ASD, BTBRT +tf./j wished to test whether the use of a ketogenic diet would contribute to altering the composition and function of the gut microbiota. C57BL/6 and BTBR mice were fed standard food or a ketogenic diet for 10-14 days. After diet therapy, faecal samples and cecal sections were collected. The composition of the intestinal microbiota was altered in BTBR mice compared to control mice; in addition, the ketogenic diet reduced total faecal and colonic bacterial counts and increased A.muciniophila content. [136] A subsequent study conducted in a mouse model by Liu X. et al. sought to demonstrate the association of A.muciniphila supplementation and melatonin using a mouse model of valproic acid (VPA)-induced autism. It was shown that probiotic therapy increased the activation of dopaminergic neurons during social interactions in the mouse model and also translated into metabolic changes. [137]
Modulating the quantity of A. muciniphila
A number of studies have noted that dietary interventions significantly affect both the health of the subjects and the levels of Akkermansia spp. in the gut. Studies indicate that compounds such as polyphenols, resistant starch, FODMAP products (fermentable oligosaccharides, disaccharides and monosaccharides, and polyols) and individual products e.g. red pitaya, whole grain barley, oat bran can increase A. muciniphila abundance. [60] In a study by Walker et al. 500 mg resveratol was used for 5 weeks in patients with metabolic syndrome. The study did not show significant differences. However, considering the administration of resveratrol to Caucasians resulted in an increase in the number of A. muciniphila [138]. A study in the United States sought to demonstrate the effect of grape juice supplementation on metabolic parameters and the gut microbiota. In the study, C57BL/6J mice were fed a high-fat diet (HFD) supplemented with 1% Concord grape polyphenols. Compared to the control group, it was observed that the addition of polyphenols contributes to a reduction in the effects induced by the HFD diet (e.g. weight gain, inflammatory markers, glucose tolerance) and also improves intestinal barrier function by increasing the expression of genes including occludin, In addition, microbiome analyses showed a significant increase in A. muciniphila and also a reduction in the ratio of Firmicutes spp. To Bacteroidetes spp. [60] In a study conducted on 20 healthy volunteers, pomegranate extract 1000 mg was used for 4 weeks. Data from the study were not presented for the whole population. Interestingly, the level of A. muciniphila depended on whether the subjects produced urolithin A. In this group, A. muciniphila levels were 47-fold higher after 44 weeks [139]. In a study conducted in Iran by Roshanravan, the subjects were divided into 3 groups. However, one of them received 600 mg/d of sodium butyrate, the second group received 10 g of inulin while the third group received a combination of the two substances in the same amounts. Considering the percentage increase in A. muciniphila abundance showed a significant increase in the group receiving sodium butyrate and inulin. A non-significant increase was obtained when both substances were co-administered [140].
Limitations
This narrative review has some limitations. Firstly, it is possible that our search strategy of nor systemic type may have missed some publications. Secondly, the included studies did not always contain explicit and detailed information on the form of A. muciniphila used. In addition, practically all studies are experimental in nature and therefore it is difficult to draw conclusions about the practical use of A. muciniphila in medicine at this stage.
Author Contributions
Conceptualization, K.S.-Ż.; methodology, K.S.-Ż., I.Ł.; software, W. C.; validation, K.S.-Ż., I.Ł., W. M., E.S.; formal analysis, K.S.-Ż., I.Ł.; investigation, all; resources, all; data curation, K.S.-Ż., I.Ł., W. M., E.S.; writing—original draft preparation, K.S.-Ż.; writing—review and editing, all.; visualization, W.C.; supervision, K.S.-Ż., I.Ł., W. M., E.S..; project administration, K.S.-Ż.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
not applicable.
Acknowledgments
none.
Conflicts of Interest
Igor Łoniewski is probiotic company shareholders, Anna Wierzbicka-Woś is employed by probiotic company, Karolina Skonieczna-Żydecka receives remuneration from probiotic company. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia Muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl Environ Microbiol 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghen, F.; De Paepe, K.; Marzorati, M.; Van de Wiele, T. Mucin as a Functional Niche Is a More Important Driver of in Vitro Gut Microbiota Composition and Functionality than Supplementation of Akkermansia m Uciniphila. Appl Environ Microbiol 2021, 87, e02647-20. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schüller, S.; Juge, N.; et al. Experimental Models to Study Intestinal Microbes-Mucus Interactions in Health and Disease. FEMS Microbiol Rev 2019, 43, 457–489. [Google Scholar] [CrossRef]
- Karcher, N.; Nigro, E.; Punčochář, M.; Blanco-Míguez, A.; Ciciani, M.; Manghi, P.; Zolfo, M.; Cumbo, F.; Manara, S.; Golzato, D.; et al. Genomic Diversity and Ecology of Human-Associated Akkermansia Species in the Gut Microbiome Revealed by Extensive Metagenomic Assembly. Genome Biol 2021, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Microbe Profile: Akkermansia Muciniphila: A Conserved Intestinal Symbiont That Acts as the Gatekeeper of Our Mucosa. Microbiology (Reading) 2017, 163, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int J Syst Evol Microbiol 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Hoskins, L.C. Mucin Degradation in Human Colon Ecosystems. Fecal Population Densities of Mucin-Degrading Bacteria Estimated by a “Most Probable Number” Method. Gastroenterology 1981, 81, 759–765. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid Publication of the Names of Forty-Two Phyla of Prokaryotes. International Journal of Systematic and Evolutionary Microbiology 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia Muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl Environ Microbiol 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Loviselli, A.; Velluzzi, F.; Manzin, A. Gut Microbiota Markers and Dietary Habits Associated with Extreme Longevity in Healthy Sardinian Centenarians. Nutrients 2022, 14, 2436. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Frontiers in Immunology 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia Muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The Outer Membrane Protein Amuc_1100 of Akkermansia Muciniphila Promotes Intestinal 5-HT Biosynthesis and Extracellular Availability through TLR2 Signalling. Food Funct 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat Med 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Safety of Pasteurised Akkermansia Muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283 | EFSA Available online:. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6780 (accessed on 9 April 2023).
- Novel Food-European Commission. Available online: https://food.ec.europa.eu/safety/novel-food_en (accessed on 25 April 2024).
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb Pathog 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like Proteins of Akkermansia Muciniphila Modulate Host Immune Responses and Gut Barrier Function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front Microbiol 2020, 11, 219. [Google Scholar] [CrossRef]
- Segers, A.; de Vos, W.M. Mode of Action of Akkermansia Muciniphila in the Intestinal Dialogue: Role of Extracellular Proteins, Metabolites and Cell Envelope Components. Microbiome Res Rep 2023, 2, 6. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Critical Reviews in Microbiology 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia Muciniphila Secretes a Glucagon-like Peptide-1-Inducing Protein That Improves Glucose Homeostasis and Ameliorates Metabolic Disease in Mice. Nat Microbiol 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The Potential of Akkermansia Muciniphila in Inflammatory Bowel Disease. Appl Microbiol Biotechnol 2021, 105, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, W.; Li, Q.; Chen, Y.; Wu, L.; Dong, Y.; Huang, X.; He, X.; Ou, Z.; Peng, Y. Membrane Protein Amuc_1100 Derived from Akkermansia Muciniphila Facilitates Lipolysis and Browning via Activating the AC3/PKA/HSL Pathway. Microbiol Spectr 2023, 11, e0432322. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.A.; Raffals, L.E.; Camilleri, M. Intestinal Barrier Dysfunction in Inflammatory Bowel Disease: Underpinning Pathogenesis and Therapeutics. Dig Dis Sci 2023, 68, 4306–4320. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat Rev Gastroenterol Hepatol 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; Danilova, N.A.; Grigoryeva, T.V.; Markelova, M.I.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease–Associated Changes in the Gut: Focus on Kazan Patients. Inflammatory Bowel Diseases 2021, 27, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The Abundance of Akkermansia Muciniphila and Its Relationship with Sulphated Colonic Mucins in Health and Ulcerative Colitis. Sci Rep 2019, 9, 15683. [Google Scholar] [CrossRef]
- Presti, A.L.; Chierico, F.D.; Altomare, A.; Zorzi, F.; Cella, E.; Putignani, L.; Luca Guarino, M.P.; Monteleone, G.; Cicala, M.; Angeletti, S.; et al. Exploring the Genetic Diversity of the 16S rRNA Gene of Akkermansia Muciniphila in IBD and IBS. Future Microbiology 2019, 14, 1497–1509. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-Occurrence of Akkermansia Muciniphila and Faecalibacterium Prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Frontiers in Cellular and Infection Microbiology 2018, 8, 281. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflammatory Bowel Diseases 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Gewirtz, A.T.; Chassaing, B. Akkermansia Muciniphila Counteracts the Deleterious Effects of Dietary Emulsifiers on Microbiota and Host Metabolism. Gut 2023, 72, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Okullu, S.O.; Catakci, M.; Elmas, M.A.; Pinheiro, Y.; Arbak, S.; Demir, E.; Schaefer, K.H.; Kolgazi, M. Akkermansia Muciniphila Improves Chronic Colitis-Induced Enteric Neuroinflammation in Mice. Neurogastroenterology & Motility 2024, 36, e14745. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized Akkermansia Muciniphila Improves Irritable Bowel Syndrome-like Symptoms and Related Behavioral Disorders in Mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front Nutr 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2022, 10, 83. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal Microbiota Composition Differs between Children with β-Cell Autoimmunity and Those Without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Derrien, M.; Van Baarlen, P.; Hooiveld, G.; Norin, E.; Müller, M.; de Vos, W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia Muciniphila. Front Microbiol 2011, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Zeng, Q. Gut Microbiota and Immunopathogenesis of Diabetes Mellitus Type 1 and 2. Front Biosci (Landmark Ed) 2016, 21, 900–906. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc Natl Acad Sci U S A 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, F.; Grander, C.; Effenberger, M.; Adolph, T.E.; Tilg, H. Gut Dysfunction and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 611. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia Muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci Rep 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-T.; Yeh, Y.-T.; Lin, C.-C.; Yang, L.-Y.; Chiang, C.-P. Akkermansia Muciniphila Is Negatively Correlated with Hemoglobin A1c in Refractory Diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia Muciniphila Can Reduce the Damage of Gluco/Lipotoxicity, Oxidative Stress and Inflammation, and Normalize Intestine Microbiota in Streptozotocin-Induced Diabetic Rats. Pathog Dis 2018, 76, fty028. [Google Scholar] [CrossRef]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is There Any Association between Gut Microbiota and Type 1 Diabetes? A Systematic Review. Gut Pathog 2019, 11, 49. [Google Scholar] [CrossRef]
- Debray-Sachs, M.; Carnaud, C.; Boitard, C.; Cohen, H.; Gresser, I.; Bedossa, P.; Bach, J.F. Prevention of Diabetes in NOD Mice Treated with Antibody to Murine IFN Gamma. J Autoimmun 1991, 4, 237–248. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc Natl Acad Sci U S A 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tong, X.; Sud, N.; Khound, R.; Song, Y.; Maldonado-Gomez, M.X.; Walter, J.; Su, Q. Low-Density Lipoprotein Receptor Signaling Mediates the Triglyceride-Lowering Action of Akkermansia Muciniphila in Genetic-Induced Hyperlipidemia. Arterioscler Thromb Vasc Biol 2016, 36, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia Muciniphila Alleviates High-Fat-Diet-Related Metabolic-Associated Fatty Liver Disease by Modulating Gut Microbiota and Bile Acids. Microb Biotechnol 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia Muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J Mol Endocrinol 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp Mol Med 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front Microbiol 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Katiraei, S.; de Vries, M.R.; Costain, A.H.; Thiem, K.; Hoving, L.R.; van Diepen, J.A.; Smits, H.H.; Bouter, K.E.; Rensen, P.C.N.; Quax, P.H.A.; et al. Akkermansia Muciniphila Exerts Lipid-Lowering and Immunomodulatory Effects without Affecting Neointima Formation in Hyperlipidemic APOE*3-Leiden.CETP Mice. Mol Nutr Food Res 2020, 64, e1900732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia Muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl Environ Microbiol 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Lawenius, L.; Scheffler, J.M.; Gustafsson, K.L.; Henning, P.; Nilsson, K.H.; Colldén, H.; Islander, U.; Plovier, H.; Cani, P.D.; de Vos, W.M.; et al. Pasteurized Akkermansia Muciniphila Protects from Fat Mass Gain but Not from Bone Loss. Am J Physiol Endocrinol Metab 2020, 318, E480–E491. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat Med 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia Muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat Med 2022, 28, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, P.; Du, H.; Chu, W.; Sun, R.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Akkermansia Muciniphila Potentiates the Antitumor Efficacy of FOLFOX in Colon Cancer. Frontiers in Pharmacology 2021, 12, 725583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, Q.; Chen, Q.; Wei, Z.; Liu, S.; Zhang, L.; Zhang, Y.; Li, Z.; Liu, H.; Sui, H. Akkermansia Muciniphila Inhibits Tryptophan Metabolism via the AhR/β-Catenin Signaling Pathway to Counter the Progression of Colorectal Cancer. Int J Biol Sci 2023, 19, 4393–4410. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti–PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I Interferons in Anticancer Immunity. Nat Rev Immunol 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. Journal of thoracic oncology : Official publication of the International Association for the Study of Lung Cancer 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Xia, K.; Liu, Y.-W.; Liu, J.-H.; Rao, S.-S.; Hu, X.-K.; Chen, C.-Y.; Xu, R.; Wang, Z.-X.; Xie, H. Extracellular Vesicles from Akkermansia Muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. IJN 2021, 16, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; Shields, C.E.D.; Hechenbleikner, E.M.; Sears, C.L.; et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science (New York, N.Y.) 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut Vascular Barrier Impairment Leads to Intestinal Bacteria Dissemination and Colorectal Cancer Metastasis to Liver. Cancer Cell 2021, 39, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia Muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Frontiers in Microbiology 2019, 10, 2259. [Google Scholar] [CrossRef]
- Fan, L.; Xu, C.; Ge, Q.; Lin, Y.; Wong, C.C.; Qi, Y.; Ye, B.; Lian, Q.; Zhuo, W.; Si, J.; et al. Muciniphila Suppresses Colorectal Tumorigenesis by Inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunology Research 2021, 9, 1111–1124. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D. Potential of Akkermansia Muciniphila and Its Outer Membrane Proteins as Therapeutic Targets for Neuropsychological Diseases. Front Microbiol 2023, 14, 1191445. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci Rep 2017, 7, 41802. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia Muciniphila in Neuropsychiatric Disorders: Friend or Foe? Front Cell Infect Microbiol 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Si, J.; Kang, H.; You, H.J.; Ko, G. Revisiting the Role of Akkermansia Muciniphila as a Therapeutic Bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-Life High-Fat Diet-Induced Obesity Programs Hippocampal Development and Cognitive Functions via Regulation of Gut Commensal Akkermansia Muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci Rep 2018, 8, 6670. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective Effects of Akkermansia Muciniphila on Cognitive Deficits and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Nutr Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci Rep 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Critical Reviews in Microbiology 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct Evidence of Parkinson Pathology Spread from the Gastrointestinal Tract to the Brain in Rats. Acta Neuropathol 2014, 128, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Bae, C.-H.; Lee, Y.; Kim, H.-Y.; Kim, S. Korean Red Ginseng Suppresses 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Inflammation in the Substantia Nigra and Colon. Brain Behav Immun 2021, 94, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic Stress-Induced Gut Dysfunction Exacerbates Parkinson’s Disease Phenotype and Pathology in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Neurobiol Dis 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martinez, G.; Chin, B.; Camarillo, C.; Herrera, G.V.; Yang, B.; Sarosiek, I.; Perez, R.G. A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J Parkinsons Dis 2020, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-F.; Shan, C.; Zhuang, S.-Y.; Zhuang, Q.-Q.; Ghosh, A.; Zhu, K.-C.; Kong, X.-K.; Wang, S.-M.; Gong, Y.-L.; Yang, Y.-Y.; et al. Gut Microbiota-Derived Propionate Mediates the Neuroprotective Effect of Osteocalcin in a Mouse Model of Parkinson’s Disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Gorecki, A.M.; Preskey, L.; Bakeberg, M.C.; Kenna, J.E.; Gildenhuys, C.; MacDougall, G.; Dunlop, S.A.; Mastaglia, F.L.; Akkari, P.A.; Koengten, F.; et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model. Front Neurosci 2019, 13, 839. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov Disord 2020, 35, 1626–1635. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Parkinsons Dis 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front Aging Neurosci 2021, 13, 636545. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional Implications of Microbial and Viral Gut Metagenome Changes in Early Stage L-DOPA-Naïve Parkinson’s Disease Patients. Genome Med 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc Natl Acad Sci U S A 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.-K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut Microbiota-Specific IgA+ B Cells Traffic to the CNS in Active Multiple Sclerosis. Sci Immunol 2020, 5, eabc7191. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of Cannabinoids, Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD), Mitigates Experimental Autoimmune Encephalomyelitis (EAE) by Altering the Gut Microbiome. Brain Behav Immun 2019, 82, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A Probiotic Modulates the Microbiome and Immunity in Multiple Sclerosis. Ann Neurol 2018, 83, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut Microbiota from Multiple Sclerosis Patients Enables Spontaneous Autoimmune Encephalomyelitis in Mice. Proc Natl Acad Sci U S A 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann Neurol 2021, 89, 1195–1211. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Molanouri Shamsi, M.; Abolhasani, M.; Bakhshi, B.; Sahraian, M.A.; Quinn, L.S.; Negaresh, R. Home-Based Exercise Training Influences Gut Bacterial Levels in Multiple Sclerosis. Complement Ther Clin Pract 2021, 45, 101463. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Gastrointestinal Motility Disorders in Neurologic Disease. J Clin Invest 2021, 131, e143771. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal Colonic Transit Is Linked to Gut Microbiota Diversity and Microbial Fermentation in Humans with Slow Colonic Transit. American Journal of Physiology-Gastrointestinal and Liver Physiology 2020, 318, G361–G369. [Google Scholar] [CrossRef]
- Mruk-Mazurkiewicz, H.; Kulaszyńska, M.; Jakubczyk, K.; Janda-Milczarek, K.; Czarnecka, W.; Rębacz-Maron, E.; Zacha, S.; Sieńko, J.; Zeair, S.; Dalewski, B.; et al. Clinical Relevance of Gut Microbiota Alterations under the Influence of Selected Drugs—Updated Review. Biomedicines 2023, 11, 5022. [Google Scholar] [CrossRef] [PubMed]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J.; Skonieczna-Żydecka, K. Clinical Significance of Microbiota Changes under the Influence of Psychotropic Drugs. An Updated Narrative Review. Frontiers in Microbiology 2023, 14, 1125022. [Google Scholar] [CrossRef] [PubMed]
- Bruijning, M.; Ayroles, J.F.; Henry, L.P.; Koskella, B.; Meyer, K.M.; Metcalf, C.J.E. Relative Abundance Data Can Misrepresent Heritability of the Microbiome. Microbiome 2023, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An Insight into Intestinal Mucosal Microbiota Disruption after Stroke. Sci Rep 2018, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Yao, S.; Xie, H.; Wang, Y.; Shen, N.; Chen, Q.; Zhao, Y.; Gu, Q.; Zhang, J.; Liu, J.; Sun, J.; et al. Predictive Microbial Feature Analysis in Patients with Depression after Acute Ischemic Stroke. Front Aging Neurosci 2023, 15, 1116065. [Google Scholar] [CrossRef]
- Xiang, L.; Lou, Y.; Liu, L.; Liu, Y.; Zhang, W.; Deng, J.; Guan, Y.; She, M.; You, X.; Liu, M.; et al. Gut Microbiotic Features Aiding the Diagnosis of Acute Ischemic Stroke. Front Cell Infect Microbiol 2020, 10, 587284. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants (Basel) 2022, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and Dissemination of Commensal Bacteria in Post-Stroke Infection. Nat Med 2016, 22, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhu, Y.; Kan, P.; Cai, Y.; Wang, Z.; Wu, Z.; Yang, P. Analysis of Intestinal Microbial Communities of Cerebral Infarction and Ischemia Patients Based on High Throughput Sequencing Technology and Glucose and Lipid Metabolism. Mol Med Rep 2017, 16, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Woo, H.G.; Jeong, J.H.; Kim, G.H.; Park, K.D.; Song, T.-J. Microbiota Dysbiosis and Functional Outcome in Acute Ischemic Stroke Patients. Sci Rep 2021, 11, 10977. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.B.; Nieto, S.J.; Haile, C.N.; Kosten, T.A. Immune Receptor Toll-like Receptor 4 Contributes to Stress-Induced Affective Responses in a Sex-Specific Manner. Brain Behav Immun Health 2021, 14, 100248. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Hall, L.K.; Paulus, M.P.; Savitz, J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology 2020, 121, 104843. [Google Scholar] [CrossRef]
- Chen, T.; Wang, R.; Duan, Z.; Yuan, X.; Ding, Y.; Feng, Z.; Bu, F.; Liu, L.; Wang, Q.; Zhou, J.; et al. Akkermansia Muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front Cell Infect Microbiol 2021, 11, 723856. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A Next-Generation Probiotic: Akkermansia Muciniphila Ameliorates Chronic Stress–Induced Depressive-like Behavior in Mice by Regulating Gut Microbiota and Metabolites. Appl Microbiol Biotechnol 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H.; Cheng, R.; Tang, Z.; Zhang, M. Outer Membrane Protein Amuc_1100 of Akkermansia Muciniphila Alleviates Antibiotic-Induced Anxiety and Depression-like Behavior in Mice. Physiology & Behavior 2023, 258, 114023. [Google Scholar] [CrossRef]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia Spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci Rep 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.-H.; Choi, Y.J.; Jun, J.-H.; Jung, H.C.; Kim, E.K. Melatonin in the Colon Modulates Intestinal Microbiota in Response to Stress and Sleep Deprivation. Intest Res 2020, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Research 2020, 13, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Molecular Autism 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, Y.; Zhang, Y.; Xiang, G.; Yu, M.; Wang, X.; Qiu, B.; Li, X.; Liu, W.; Zhang, D. Rescue of Social Deficits by Early-Life Melatonin Supplementation through Modulation of Gut Microbiota in a Murine Model of Autism. Biomedicine & Pharmacotherapy 2022, 156, 113949. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic Effect of Fruit and Vegetable Shots Containing Jerusalem Artichoke Inulin: A Human Intervention Study. Br J Nutr 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs1234. J Nutr 2016, 146, 673–680. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin Transl Gastroenterol 2016, 7, e164. [Google Scholar] [CrossRef]
Figure 1.
Modes of action of A. muciniphila. Tph1 - Tryptophan hydroxylase 1; Ocln – occludin, Cldn 3 – claudin 3; Cnr1 - cannabinoid receptor 1; LPS- Lipopolysaccharide; TLR- Toll-like receptor; FFR- Free fatty acid receptor; GLP- glucagon-like peptide; IL- Interleukin; MCP-MonocyteChemoattractant Protein; Apo- Apolipoprotein; Treg- Regulatory T limfocytes. Created with BioRender.
Figure 1.
Modes of action of A. muciniphila. Tph1 - Tryptophan hydroxylase 1; Ocln – occludin, Cldn 3 – claudin 3; Cnr1 - cannabinoid receptor 1; LPS- Lipopolysaccharide; TLR- Toll-like receptor; FFR- Free fatty acid receptor; GLP- glucagon-like peptide; IL- Interleukin; MCP-MonocyteChemoattractant Protein; Apo- Apolipoprotein; Treg- Regulatory T limfocytes. Created with BioRender.
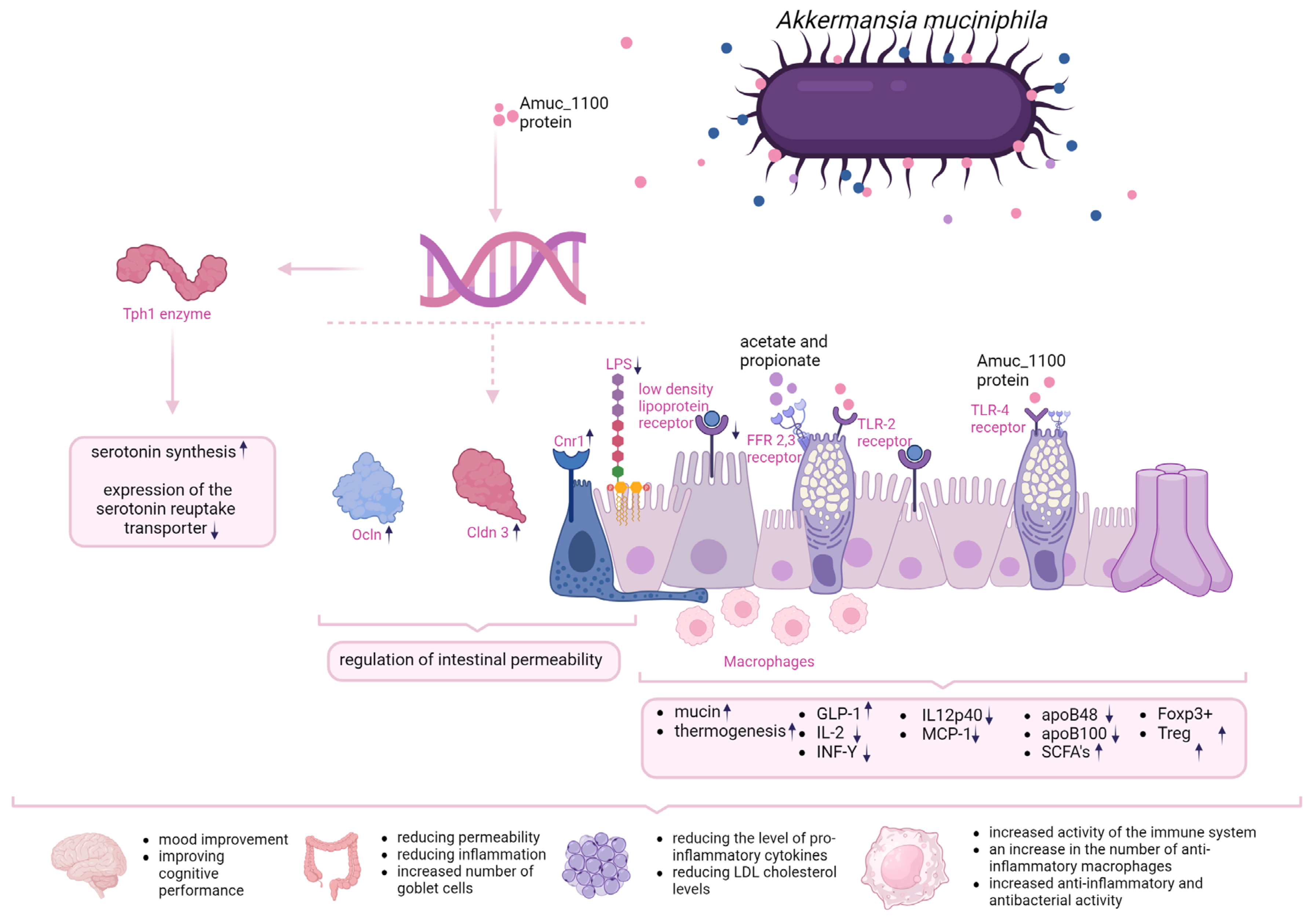
Table 1.
The impact of A. muciniphila intake on metabolic indices in animal models. EV- extracellular vesciles, LPS – lipopolysaccharide, GGT -gamma-glutamyl transpeptidase, AST - aspartate aminotransferase, LDH - lactate dehydrogenase, TG – triglycerides.
Table 1.
The impact of A. muciniphila intake on metabolic indices in animal models. EV- extracellular vesciles, LPS – lipopolysaccharide, GGT -gamma-glutamyl transpeptidase, AST - aspartate aminotransferase, LDH - lactate dehydrogenase, TG – triglycerides.
| References: | Model: | Study groups: | Intervention | Survey results: |
|---|---|---|---|---|
| Wu et al (2020) [61] | Animal | 10-week-old male C57BL/6J mice orally fed | 2x108 per day; live or pasteurised A. muciniphila; 4 weeks | Induction of metabolism, reduction of body weight and improvement of body composition, reduction of insulin resistance, reduction of adipose tissue mass, induction of adipocytes, reduction of serum glucose after its oral administration, restoration of fat layer thickness after a high-fat diet. |
| Li et al. (2016) [57] | Animal | Apoe-/- mice fed orally | 5x109 per day; live or pasteurised A. muciniphila; 9 weeks | Pasteurised A. mucniphila Reduced fat gain, insulin resistance and dyslipidaemia compared to mice supplemented with live A. muciniphila. Glucose tolerance comparable in both groups. |
| Zhao et al. (2017) [62] | Animal | Six-week-old pathogen-free mice on a low-carbohydrate diet | 1x109 for day, live A. muciniphila; 14 weeks; | Reduced weight gain and body fat, improved glucose tolerance and insulin sensitivity, reduced fatty acid-related gene expression, reduced chronic low-grade inflammation, increased anti-inflammatory factors |
| Chelakkot et al. (2018) [63] | Animal | 6-8-week-old male mice C57BL/ 6 | 10 μg EV per day, 2 weeks | Reduced intestinal permeability, weight reduction, improved glucose tolerance |
| Ashrafian et al. (2019) [64] | Animal | 8-week-old male C57BL mice on a low-carbohydrate or high-fat diet | 1x109 live A. muciniphila per day; 10 mg protein/200 ul EV, 5 weeks | Significant weight loss in mice on a fat-rich diet, improved intestinal barrier integrity, improved glucose tolerance and lipid profile |
| Everard et al. (2019) [56] | Animal | 10-week-old male mice on a low-carbohydrate or high-fat diet | 2x108 per day; live A. muciniphila | Reduction in diet-induced obesity, reduced appetite, body weight and fat mass, reduced hyperglycaemia and hyperinsulinaemia |
| Katiraei et al. (2019) [65] | Animal | 9-13-week-old male E3L.CETP mice with an increased lipid profile | 2x108 per day; live A. muciniphila, 4 weeks | Reduction in body weight, reduction in plasma triglyceride and cholesterol levels, |
| Shin et al. (2019) [59] | Animal | 8-week-old female C57BL/6 mice on a high-fat or low-carbohydrate diet treated with Akk growing on medium with (+) or without (-) mucilage addition | 1x108 live A. muciniphila per day, 4 weeks | A. muciniphila (-) attenuated the changes induced by the high-fat diet, reduced adipocyte hypertrophy and increased the proportion of small adipocytes, improved glucose levels and increased insulin tolerance, reduced LPS levels and inhibited diet-induced progressive intestinal inflammation. |
| Wu et al. (2020) [61] | Animal | 8-week-old female C57BL/6 mice on a low-carbohydrate, high-fat diet | 1x109 live A. muciniphila per day, 10 months | Decreased weight gain, decreased appetite, increased expression of the anti-inflammatory factor IL-10 |
| Kim et al. (2020) [66] | Animal | 5-week-old C57BL/6N mice on a high-fat, low-carbohydrate diet | 1x108 -1x109 live A. muciniphila per day, 10 weeks | No difference in weight gain, reduced TG and ALT levels in obese mice, reduced hepatic IL-6 expression in obese mice |
| Lawenius et al. (2020) [67] | Animal | 12-week-old female mice C57BL/6 | Pasteurised A. muciniphila 2x108 per day, 4 weeks | Reduced weight and fat gain, reduced incidence of T lymphocytes in the bone marrow |
| Depommier et al. (2019) [68] | Human | 32 people with overweight, isulin resistance, metabolic diseases | Pasteurised or live A. muciniphila 1x1010 per day, 3 months | Reduced plasma insulin levels and increased insulin sensitivity, reduced cholesterol, GGT, AST, LPS, LDH and serum creatine kinase,slight weight loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
10 May 2024
Posted:
10 May 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
Submitted:
10 May 2024
Posted:
10 May 2024
You are already at the latest version
Alerts
Abstract
This comprehensive review delineates the extensive roles of Akkermansia muciniphila in various health domains, spanning from metabolic and inflammatory diseases to neurodegenerative disorders. A. muciniphila, known for its ability to reside in the mucous layer of the intestine, plays a pivotal role in maintaining gut integrity and interacting with host metabolic processes. Its influence extends to modulating immune responses and potentially easing symptoms across several non-communicable diseases, including obesity, diabetes, and inflammatory bowel disease. Recent studies highlight its capacity to interact with the gut-brain axis, suggesting a possible impact on neuropsychiatric conditions. Despite the promising therapeutic potential of A. muciniphila highlighted in animal and preliminary human studies, challenges remain in its practical application due to stability and cultivation issues. However, the development of pasteurized forms and synthetic mediums offers new avenues for its use in clinical settings, as recognized by regulatory bodies like the European Food Safety Authority. This narrative review serves as a crucial resource for understanding the broad implications of A. muciniphila across different health conditions and its potential integration into therapeutic strategies
Keywords:
Subject: Biology and Life Sciences - Immunology and Microbiology
Introduction
Akkermansia muciniphila (A. muciniphila) constitutes about 1% to 3% of the entire gut microbiota, with a concentration of 109 colony-forming units per gram. This bacterium is abundantly present within the mucus layer of the intestine, enabling it to more readily interact with epithelial cells located at the tips of intestinal villi compared to bacteria that do not degrade mucin [1,2]. The bacterium is gram negative obligatory anaerobe. Not only improves intestinal barrier integrity, but it also causes mucus degradation using mucin as the main energy source [3,4]. However, by doing so, it can exert an inhibitory effect on the growth of pathogenic bacteria using the same energy source [5]. It was first isolated and described in 2004 by the team of scientists from Wageningen University in the Netherlands leading by Prof. Willem de Vos [6]. It was recognised as the first representative of Verrucomicrobiota (former name Verrucomicrobia) in the human intestine [4,7,8]. Moreover, in the last few years also other species belonging to the genus Akkermansia were described, based on human fecal metagenomes datasets and isolated strains. As it was noticed, in samples derived from one host in 99% only one particular Akkermansia sp. was identified, showing mutual exclusion pattern. However, only species Akkermansia municiphila was significantly negatively correlated with BMI [4].
A. muciniphila highest concentration is observed in the cecum and abundance vary with a number of factors including age, with a general decrease with age [5,9], although overrepresentation of this bacterium has been reported in centenarians [10]. A. muciniphila is implicated in various health conditions including metabolic health, obesity, diabetes, and inflammatory bowel disease (IBD). Its role is multifaceted, showing both potential benefits and complex interactions with the host's health. The bacterium's abundance in the gut has been linked to the state of glucose tolerance and type 2 diabetes (T2D), albeit with inconsistent findings across different studies, which may be attributed to the diversity in study populations and methodologies [11,12]. The potential health benefits of A. muciniphila stem from its capacity to degrade mucin, producing short-chain fatty acids (SCFAs) like acetate and propionate. These metabolites are known for their anti-inflammatory and metabolic effects, suggesting A. muciniphila's role in weight regulation and immune modulation [13].
Interventions that alter the gut microbiota, such as calorie restriction or bariatric surgery, have been shown to impact A. muciniphila levels, correlating with improved metabolic health and reduced obesity. This underscores the importance of understanding the bacterium's specific role in metabolic regulation and its potential as a therapeutic target [11].
Moreover, A. muciniphila's influence extends to the intestinal barrier's integrity, crucial in preventing conditions like IBD. Research suggests that A. muciniphila or its components, like the outer membrane protein Amuc_1100, can reduce inflammation and enhance the integrity of the intestinal barrier, highlighting its potential therapeutic role in chronic inflammatory diseases [14].
Despite such interesting potential, A. muciniphila could not find therapeutic application due to the instability of this bacterium resulting from its anaerobic nature, the necessity of using animal-derived components in its cultivation, and the low efficiency of cultivation, it was not possible to develop a suitable preparation that would allow its use in humans. The first step was the application of a synthetic medium for cultivation, followed by pasteurization at 70°C. It turned out that pasteurization of A. muciniphila MucT cultivated on a synthetic medium increased its capabilities to limit the development of fat mass, insulin resistance, and dyslipidemia in experimental models [15].
On July 7, 2021, the European Food Safety Authority (EFSA) adopted a scientific opinion on the safety of pasteurized A. muciniphila as a novel food in accordance with Regulation (EU) 2015/2283 [16]. This opinion opens up numerous possibilities for the use of this form of bacteria as a "novel food," which refers to food that was not consumed to a significant degree in the EU before May 15, 1997. Novel food is recently discovered food sources or food that has been newly developed, innovative, produced using new technologies and manufacturing processes, and traditionally consumed outside the EU but not within it [17]. For this reason, we decided to present current information on the potential application of pasteurized A. muciniphila in various diseases, focusing on the mechanism of action and the results of experimental and clinical studies, using the narrative review format.
Mechanisms of action
Several mechanisms of action of A. muciniphila are highlighted in the literature that contribute to host health. In the case of pasteurized A.muciniphila, the primary mechanism involves improving the intestinal barrier function. A key role of the intestinal barrier is to stabilise the space between intestinal epithelial cells and fenestrae of gut endothelium in order to prevent the transmission of, among other things, bacterial fragments and toxins into the circulatory system [18]. The intestinal barrier also ensures the maintenance of normal mucus levels which translates into both structural and functional stabilisation of the intestinal microbiota [19]. A. muciniphila not only has the ability to degrade mucins, but also to stimulate their synthesis. Importantly, this process depends on the initial mucin thickness and intestinal eubiose [19]. Genome analyses allow to identify gene cluster encoding secretion proteins that very likely constitute the pili-like structures present on the A. muciniphila outer membrane and might be involved in crosstalk with the host [20].. One of the proteins named Amuc_1100, has been shown to modulate the intestinal barrier and its permeability by increasing the expression of genes encoding proteins such as occludin (Ocln) and claudin-3 (Cldn3) and cannabinoid receptor 1 (Cnr1). In addition, the bacterium has been shown to reduce lipopolysaccharide (LPS) synthesis [21,22]. What is also interesting, data obtained in study, where influence of Amuc_1100 was examine in mice treated with recombinant protein produced in E. coli cells has shown greater influence than mice treated with A. muciniphila pasterized cells. This mechanisms appear to be caused through activated Toll-like receptor 2 (TLR-2) [22]. Additionally, extracellular vesicles derived from A. muciniphila (AmEV) have been shown to reduce intestinal permeability by inducing AMPK activation [23].
The evidence is mounting, that A. muciniphila content is correlated with metabolic disease, but the molecular mechanisms of its exact effects on the host have not been well defined. In one study, A. muciniphila was shown to increase thermogenesis and glucagon-like peptide-1 (GLP-1) secretion in C57BL/6J mice fed a high-fat diet (HFD) by inducing uncoupling protein in brown adipose tissue. Based on liquid chromatography and spectrophotometry, the researchers identified the protein that is secreted by A. muciniphila and causes the changes described above - the P9 protein. It interacts with the intercellular adhesion molecule 2 [5]. In addition, IL-6 deficiency abrogates the effect of P9 on glucose haemostasis and reduces the expression of ICAM-2 [24]. Another study found that one of the mechanisms of action of A. muciniphila in obesity is the downregulation of pro-inflammatory cytokines (IL-2, INF-Y, IL12p40 AND MCP-1). In addition, concomitant administration of live A. muciniphila inhibited the overall infiltration of mononuclear leukocytes in the liver with immune-mediated damage and reduced TLR2 and TLR4 levels. [23] By affecting the low density lipoproteins (LDL) receptor pathway, reducing apoB48 and apoB100 on LDL lipoprotein, prebiotic supplementation in HFD-fed mice has a beneficial effect on lipid metabolism. By affecting the production of short-chain fatty acids (SCFAs), A. muciniphila, by stimulating their synthesis, is involved in signal transduction through inhibition of histone deacetylase (HDAC) and activation of G protein-coupled receptors, which translates into stimulation of the immune system [21]. A. muciniphila, through the production of acetate and propionate, as well as the Amuc_1100 protein, interacts with FFAR2, 3 and also the TLR2 receptor of goblet cells, thereby increasing their number and differentiation, which also translates into the amount of mucus produced. These phenomena improve the integrity of the intestinal barrier and also the renewal of the intestinal epithelium [25]. By acting on the ICAM2 receptor in the intestinal epithelial L cell, there is an increase in the synthesis of bioactive lipids such as 2-OG, which translates into an increase in oxidation of TCs, a decrease in energy expenditure and a decrease in epithelial inflammation [25]. It has been shown that, pasteurised Akkermansia acts via the AMUC_1100 protein and the TLR2 receptor, making this action more controllable than that of a probiotic, which requires colonisation and appropriate environmental conditions for its action. The AMUC_1000 protein improves intestinal barrier integrity and crosses the intestinal barrier in most likely microvesilces (MVs) and enters adipose tissue, where it induces lipolysis, inhibits lipogenesis and promotes thermogenesis [26]. In addition, it increases mucus production, which is also an energy expenditure for the body. So we have a triple mechanism of action that reduces inflammation, alters fat metabolism and increases energy expenditure. Very importantly, pasteurisation can increase the bioavailability of AMUC_1100, by affecting the bacterial surface charge.
In addition, A. muciniphila has been shown to reverse the enzymatic expression of moonooxygenase 3 (FMO3) affecting the conversion of TMA to TMAO in the liver [15]. A. muciniphila administration was also associated with increased expression of the antimicrobial peptide Reg3y associated with antimicrobial and anti-inflammatory activity. [23]. Applied A. muciniphila supplementation in diabetic patients increased anti-inflammatory macrophage levels and restored the number of Foxp3+ Treg regulatory cells. [23]. It has also been shown that the Amuc_1100 protein can affect the expression of the enzyme Tph1, which regulates the rate of serotonin (5-HT) synthesis in RIN-14B cells, and reduces the expression of the serotonin reuptake transporter (SERT) through interactions with TLR-2, resulting in improved 5-HT biosynthesis and its extracellular availability [14]. Figure 1 summarises the mechanisms of action described so far for Akkermansia muciniphila
Gut diseases
The impact of intestinal barrier permeability in both inflammatory bowel disease is highlighted in many scientific studies [27]. An association between increased intestinal barrier permeability resulting from tight junctions between the development and progression of many gastrointestinal diseases has been shown. In the case of IBD, there is, among other things, an increase in the internalisation of occludin and claudin-2. A similar phenomenon is observed in colitis (reduction of zonulin-1 and zonulin-2 ). It is also worth noting that an association has been shown between increased intestinal barrier permeability and the risk of developing IBD in healthy relatives suffering from Crohn's disease. Increased intestinal barrier permeability is also observed in IBS patients with predominant diarrhoea, whereas in the constipation type of IBS the intestinal barrier is unaltered. Animal studies have shown that these changes may translate into visceral sensitivity [28]
A. muciniphila shows great potential in the treatment of IBD. Research highlights that transplantation of intestinal microbiota used as therapy for IBD leads to an increase in A.muciniphila in the gut of patients. [25] In human studies, A.muciniphila abundance was shown to be significantly reduced in both ulcerative colitis (UC) and Crohn's disease patients. [25]. A study by Lo Sasso et al. compared changes in the gut microbiota in patients with IBD using 16S rRNA sequencing. A reduction in the abundance of A. muciniphila was observed in these patients. [29] Another study by Earley et al. compared changes in the composition of the gut microbiota in patients with active UC compared to healthy patients or patients in remission of the disease. Patients with the active form of UC showed a significantly reduced abundance of A.muciniphila compared to other groups. The study confirmed an inverse relationship between A. muciniphila abundance and inflammation. [30] Lo Presti et al. compared the abundance of A. muciniphila in healthy individuals, patients with irritable bowel syndrome (IBS) and those with IBD. A significantly reduced abundance of A.muciniphila was observed in patients with IBD compared to the other groups. [31] Lopez-Siles et al. compared the content of A.muciniphila in healthy subjects compared to patients with UC, Crohn's disease, IBS and colorectal cancer. Although no difference was observed in the study with regard to A. muciniphila abundance, its levels were reduced in subjects under 16 years of age with IBD compared to the control group. [32] The relationship between A.muciniphila abundance in pediatric patients struggling with Crohn's disease was also investigated. In children and adolescents in remission, it was observed that the gut microbiota was abundant in A.muciniphila. [33] Therefore, the scientific data points towards a protective role for A. muciniphila in the course of IBD. However, the situation is awkward when the intestinal mucosal barrier is damaged by the influence of pathogenic bacteria. The number of A.muciniphila was significantly reduced in patients with IBD and also in mice that had colitis or colon cancer. Studies using oral supplementation of A. muciniphila or Amuc_1100 significantly reduced the infiltration of macrophages and CD8+ cytotoxic T lymphocytes in the colon of mice with colitis, resulting in a reduced inflammatory reaction. [34] In addition, administration of A.muciniphila attenuated inflammation in UC mice and resulted in protective effects on the function and structure of the intestinal microbiota and reduced levels of pro-inflammatory cytokines, as well as neuroinflammation. [34,35,36]
In addition to the use of A.muciniphila in the treatment of IBD, the use of prostbiotic supplementation in irritable bowel syndrome (IBS) has recently received attention. The abnormalities of the gut-brain axis are major pathophysiological factor [37]. In one study in mouse models (C57BL6/J mice subjected to neonatal maternal separation and Citrobacter rodentinum infection), supplemented with pasteurised A. muciniphila was shown to reduce visceral hypersensitivity by affecting intestinal barrier function. In addition, a reduction in anxiety-like behaviour, as well as memory impairment and an effect on pain sensations, has been shown [38].
Metabolic diseases
Recent studies have emphasized the link between significant damage to intestinal barriers and various health issues, including diabetes, obesity, fatty liver, and cardiovascular diseases. Research suggests that a weakened intestinal barrier may result in endotoxemia, which is connected to systemic inflammation, insulin resistance, diabetes, and lipid buildup, thereby hastening the progression of obesity and fatty liver diseases [39]. The pathologically increased permeability of the intestinal barrier is suspected to allow a significant influx of bacterial metabolites through the portal vein, causing the release of pro-inflammatory cytokines, chemokines and eicosanoids. Both these metabolites and the substances released as a result of their presence induce a chronic inflammatory process, fibrosis of liver tissue and have carcinogenic effects [40]. However, the exact processes by which intestinal barrier damage occurs and the methods to effectively enhance the intestinal barrier are still subjects for further investigation. [39]
Numerous studies related to the risk of type I diabetes due to abnormalities in microbiota composition have shown that patients have a higher abundance of Bacteroides ovatus and Bacteroides uniformis, while Bacteroides fragilis appears to play a protective role [41]. In healthy controls, butyrate-producing Bifidobacteria and mucin-degrading bacteria such as A. muciniphila make up a larger part of the microbiota composition, compared to patients with type I diabetes mellitus [42]. The protective potential of Bifidobacteria is not fully described, but de Goffrau's study suggests that they limit the growth of Bacteriodes or restrict movement across the epithelium, thereby reducing inflammation [41]. However, Brown suggests that a microbiota rich in butyrate producers leads to increased amounts of mucin, tighter junctions and increased intergranularity of the intestinal barrier, thereby creating an attractive environment for the proliferation of A. muciniphila, whose abundance is significantly higher in healthy individuals [42,43].
In type II diabetes, elevated levels of LPS are seen in patients, causing weight gain, insulin resistance, induction of inflammation in the gut and ultimately the development of type II diabetes [44]. Studies have shown that patients have bacterial overgrowth and delayed gastrointestinal transit time, while hyperglycaemia and hyperinsulinaemia affect the motility of the jejunum. A reduced abundance of Faecalibacterium prausnitzii, which are found in abundance in the microflora of healthy individuals, was also noted in patients [45]. The intestines of patients with type II diabetes showed reduced numbers of Firmicutes and Clostridia. It has also been noted that the ratio of Bacteroidetes to Firmicutes is correlated with plasma glucose concentration [46]
It has been shown that disturbance of the intestinal flora can significantly affect liver health. Although the mechanism by which this would occur has not been sufficiently explored, a specific composition of the gut microflora has been found in NAFLD patients. [47]
Studies in mice have linked low intestinal abundance of A. muciniphila to pre-diabetes and diabetes [48]. Mice fed a high-fat diet (HFD) for 16 weeks showed increased expression of inflammatory markers, higher serum leptin levels and developing hyperinsulinaemia and hypoglycaemia. After 3 weeks on the diet, the appearance of peripheral insulin resistance and a decrease in A. muciniphila counts were noted [49]. However, the reduced absolute bacterial count does not appear to affect only the development of pre-diabetic states in mice. In patients with developed type II refractory diabetes, manifested by a serum glycated haemoglobin level less than or equal to 8%, examination of faecal samples showed reduced numbers of A. muciniphila compared to patients who achieved optimal glycaemic control with metformin or other hypoglycaemic drugs. [50]
In an experimental model of streptozotocin-induced type 2 diabetes in rats, live and pasteurised A. muciniphila significantly improved well-being, which was primarily associated with improved liver function, reduced plasma levels of pro-inflammatory factors, prevention of gluco/lipotoxicity and reduction of oxidative stress [51].
An imbalance of the gut microbiota accompanies the development of both type II and type I diabetes, despite differences in the aetiology of the two diseases [52]. In type I diabetes, it is IFN-γ that plays a key role in its pathogenesis. IFN-γ (-) mice show better glucose tolerance and increased numbers of A. muciniphila in the gut compared to wild-type mice. However, IFN-γ (-) mice without A. muciniphila did not show improved glucose tolerance This may indicate that the diabetogenic role of IFN-γ may be related to its ability to induce changes in the composition of the microbiota and, in particular, to reduce the abundance of A. muciniphila [53,54].
In addition, NOD mice (non-odese diabetic, mice with elevated fasting glucose levels) treated with oral vancomycin from 28 days of age showed a reduced incidence of type one diabetes, with A. muciniphila predominating in their microbiota. Furthermore, by observing 2 colonies of NOD mice, it was noted that a lower incidence of type one diabetes was always associated with an increased abundance of A. muciniphila. The above studies suggest a significant effect of A. muciniphila on the development of diabetes of both types, which may put forward the inclusion of a new therapy to combat the disease [55].
In the first of the human proof of concept, clinical trials evaluating the validity of A. muciniphila supplementation, 40 overweight or obese subjects with insulin resistance were included. Patients received live or pasteurised A. muciniphila for 3 months. Primary endpoints included safety of the supplement and its tolerability (i.e. liver function, renal function, inflammation) and metabolic parameters (i.e. insulin resistance, circulating lipid concentrations, visceral fat, body mass index). Secondary endpoints included parameters of intestinal barrier function (i.e. plasma lipopolysaccharide (LPS)/metabolic endotoxaemia), gut microbiota composition and metabolites. After 2 weeks, the safety of the supplement was confirmed, as interpreted by the absence of changes in the measured biochemical parameters. Only a significant (still normal) reduction in GGT and CK activity in the group with pasteurised A. muciniphila was noted. There was also no significant change in the incidence of gastrointestinal disorders. After 3 months, a significant increase in fasting plasma insulin levels was observed in the placebo group (p<0.05, T3 versus T0), in contrast to participants receiving both forms of A. muciniphila, who had reduced plasma insulin levels (by approximately 30%) compared with placebo Insulin sensitivity was significantly reduced at T3 in the placebo group (fig. d). Conversely, the supply of both forms of A. muciniphila improved this parameter. pasteurised A. muciniphila clearly and significantly improved the insulin sensitivity index by approximately 30% compared to the Placebo group, and live A. muciniphila significantly improved the insulin resistance index . Pasteurised A. muciniphila significantly reduced DPP- IV activity after 3 months of the study compared to baseline. WBC counts remained significantly increased compared to baseline and week 2 in the placebo group (fig. f), whereas supplementation with pasteurised A. muciniphila completely abolished this effect, resulting in a significant reduction in WBC counts compared to the placebo group . The magnitude of this difference between T0 and T3 or the placebo group (i.e. 866 cells/µl) is highly significant, as a difference of 300 to 1000 cells/µl is considered clinically significant. Supplementation with the pasteurised form of A. muciniphila significantly reduced both γGT and AST levels after 3 months compared to baseline. Specifically, γGT levels were markedly reduced by approximately 24% in the group supplemented with pasteurised A. muciniphila compared to T3 levels observed in placebo. Neither intervention induced a significant change in microbial community composition, although supplementation with live bacteria had a slightly greater effect (partial dbRDA, adjusted R2=0.03, p =0.095) than pasteurised(partial dbRDA, adjusted R2=0.02, p=0.14), while placebo had the least effect (partial dbRDA, adjusted R2=0.01, p =0.66). Administration of pasteurised A. muciniphila significantly reduced total cholesterol by 8.68% compared with placebo, while LDL cholesterol was 7.53% lower and triglycerides 15.71% lower, although these differences did not reach statistical significance. The administration of pasteurised A. muciniphila slightly reduced body weight by about 2.27 kg, fat mass by about 1.37 kg and hip circumference by 2.63 cm compared with the placebo group. Waist circumference decreased by approximately 1.56 cm. The study thus reports a broad-spectrum effect of A. muciniphila on changes associated with increased body weight [30]. Supplementation with A. muciniphila reverses metabolic disorders induced by a fatty diet, among others, metabolic endotoxaemia, increased body fat and insulin resistance Administration of A. muciniphila decreased postprandial triglycerides and chylomicrons and increased expression of receptors for low-density lipoprotein (LDL), thereby regulating intermediate-density lipoprotein (IDL) through induction of apolipoprotein B 100 and apolipoprotein E [28,29,30].
A. muciniphila has the ability to activate immune signalling through receptors that recognise molecular patterns. There is increased secretion of the chemokine CCL20 binding to the CCR6 receptor on the surface of T and B lymphocytes. A. muciniphila has been shown to have a strong negative correlation with inflammatory markers, adipose tissue homeostasis, insulin levels and glucose levels. [5] Supplementation-induced changes included stimulation of Treg cell proliferation and suppression of the hepatic stress marker ER-glucose-regulated protein [59]. In addition, A. muciniphila restored intestinal barrier function by reducing the high concentration of LPS induced by the Western diet. Consequently, this led to the alleviation of LPS-induced systemic inflammation and arthritis by reducing macrophage transmission to the inner membrane through reduced expression of TNF-α and IL-1β [60].
According to available data, Akkermansia appears to have a positive effect on many metabolic disorders, but the exact mechanism of its action is not fully known. One of the causes of MAFLD (Metabolic Dysfunction-associated fatty liver disease) is damage to the intestinal barrier with endotoxaemia, which translates into the functioning of the microbiota-gut-hepatic axis. In the study by Wenrui Wu et al. the study mice were divided into three groups. Mice were fed a low-fat diet, a high-fat diet or a high-fat diet with additional supplementation of approximately 1.5 x 109 live A. muciniphila per day. It was shown that mice given A. muciniphila had less weight gain compared to the other groups. In addition, serum AST and ALT concentrations and NAS score (the NAS scale assesses the degree of liver fibrosis) were lower than in the group with the same diet without supplementation. Supplementation with A .muciniphila affects the gut microbiome in a way that alters the bile acid profile, improves glucose tolerance, reduces the development of white adipose tissue (WAT) and lowers insulin, resistin and leptin levels [61]. In contrast, Sejeong et al. conducted a study in mice that were fed a diet consisting of 45% or 10% fat. Some of the individuals were also given A. muciniphila. The researchers noted no differences in body weight gain between mice supplemented with A. muciniphila compared to mice without active supplementation. However, serum triglyceride and ALT levels were lower when A. muciniphila was introduced into the diet of intervention group, indicatingit may reduce the liver damage caused by the disease. When the expression levels of chREBP and SREBP were determined in liver tissue, supplementation with A. muciniphilia decreased the expression levels of the genes tested, resulting in a reduction of triglyceride synthesis in the liver compared to mice that were not given the probiotic. The same relationship was found for IL-6. As mentioned earlier, the amount of A .muciniphilia decreases in individuals on a high-fat diet. The study showed that supplementation allows this effect to be reversed and the amount of bacteria to increase. The researchers suspect that supplementation could improve the tightness of the intestinal barrier by maintaining homeostasis of the intestinal microbiome, thereby preventing the development of MAFLD [66]. Selected metabolic studies in which A. muciniphila supplementation was used are included in Table 1.
Cancer
The state of the intestinal barrier is also important for the development of cancer in the body. It has been shown that excessive permeability within the intestinal barrier can lead to increased penetration of toxic and pro-inflammatory substances, which can then cause inflammation in the body and interfere with cell proliferation towards carcinogenesis. An important component of this system are the tight junctions, whose malfunction has been linked to the development of bowel cancer. Since some of the most important factors influencing the proper functioning of the intestinal barrier are diet and the absence of intestinal dysbiosis, correctly selected supplementation supported by appropriate dietary habits may favourably influence the development and course of cancer [28].
The data are mounting that the presence of the bacterium A. muciniphila in the human intestinal microbiome may significantly influence the effectiveness of cancer treatment, particularly for non-small cell lung and colon cancer [69,70,71]. Although not all of its mechanisms of action in the context of cancer have been understood, evidence suggests that this microorganism can exert a beneficial effect on immunity, with potentially important implications for the success of ongoing therapy [72]. Immunotherapy with anti-PD-1 and anti-PD-L1 antibodies is used in the treatment of some cancer types, such as non-small cell lung cancer, renal cell carcinoma and melanoma. The efficacy of cancer immunotherapy depends on the ability of the host to produce tumour antigen-specific Th1 cells that secrete IFNγ and cytotoxic effector T cells (Tc1) [73]. This raises the possibility for commensal gut bacteria having the ability to correct Th1/Tc1 immune responses, which can be defective. This offers considerable hope for the use of microbiota-focused interventions against both primary and secondary resistance to cancer immunotherapy [69,74].
After analysing studies on the correlation of A. muciniphila and the body's response to immunotherapy with an anti-PD-1 antibody, it was shown that the high presence of this bacterial species in the gut microbiota of lung or kidney cancer patients was associated with a positive body response to the administered therapy through increased mobilisation of CCR9+, CXCR3+ and CD4+ T cells in tumour foci. It has been shown that commensal gut bacteria may have an effect on the regulation of endogenous immune stimulation and infiltration of T cells within the tumour environment. Interestingly, no such immune response was observed in patients whose gut microflora was deficient in A. muciniphila. In both cases, faecal microflora was collected from patients and transplanted into sterile mice. Here, too, the results clearly indicated increased T-lymphocyte activity within the tumour focus of mice with transplanted microbiota containing A. muciniphila. In contrast, mice that received transplants from patients who did not respond to treatment also showed no response to the administered therapy. An important fact is that after both transplantation of faeces rich in A. muciniphila and after oral administration of the presented strain, a positive response of mouse organisms to anti-PD-1 antibodies was observed [72,75,76].
Another correlation of A. muciniphila with cancer treatment was noted for immune checkpoint targeting (ICI) - a therapy used in advanced stages of non-small cell lung cancer, melanoma or renal cell carcinoma. The first key point was to prove that the anti-tumour effect of ICI immunotherapy depends on the presence of microorganisms in the gut. The researchers noted that patients who proceeded with ICI treatment responded significantly less well to the immunotherapy they received after the antibiotic treatment. Their life expectancy was shown to be reduced by 6.7 months (95% CI: 5.1-8.4). These observations were confirmed in prospective studies and large meta-analyses suggesting that the gut microbiota may play a key role in the immunostimulatory mechanism of action of ICI [69,77].
Prostate cancer (PCa) is one of the most common malignancies affecting men. Despite the successes achieved by ongoing immunotherapy in other types of cancer, there is still a need to develop a more effective therapeutic strategy for the treatment of PCa. To this end, a study was conducted to test the effect of the extracellular vesicles secreted by A. muciniphila (Akk-EVs) on developing PCa. During the study, isolated Akk-EVs were injected into immunocompetent mice with implanted PCa. Mice were then monitored for immunophenotypic changes in cells, including CD8+ T-cell activation. Wound healing rates were also assessed to see how macrophages affect Akk-Ev-induced PCa cell proliferation and invasion. The body weight of the mice, their food and water intake were recorded throughout the study. After 13 treatments, it was shown that tumour proliferation was significantly slowed in the Akk-EV-treated mice compared to the control group. In addition, major organs such as the liver and kidneys were harvested for histopathology to assess potential systemic toxicity. The researchers' conclusions were very promising; immunocompetent mice after Akk-EV administration showed a reduced prostate tumour burden with no observed toxicity to healthy tissues. The treatment resulted in increased granzyme B-positive (GZMB) and interferon γ-positive (IFN-γ) activity in CD8 + T-cells. An increased proportion of CD8+ T lymphocytes and the accumulation of more macrophages, with an increase in the number of M1 macrophages, which have tumour-fighting capacity, and a decrease in the number of M2 macrophages, which exhibit immunosuppressive effects. The macrophage growth environment conditioned by Akk-EV inhibited the proliferation and invasion of prostate cancer cells. Importantly, healthy cells tolerated the Akk-EV administered to the mice well. This study indicates a promising effect of A. muciniphila in immune therapy of prostate cancer [78].
At the turn of the year, it was noted that the gut microbiota may play a role in both the initiation and inhibition of colorectal cancer. A group of bacteria has been identified whose biofilm and potential to disrupt gut vascular barrier increases the risk of colon cancer [79] and augment liver metastases [80]. Such a relationship is shown, for example, by Bacteroides fragilis or Fusobacterium nucleatum. F. nucleatum also mediates resistance to chemotherapy and increases the bluntness of metastasis through activation of the autophagy pathway increasing chemoresistance of the tumour [81]. With this knowledge, the researchers decided to see if we could simultaneously isolate bacteria carrying a beneficial effect in the prevention and treatment of colorectal cancer. A study focusing on A. muciniphila was promising, as this bacterium showed a mitigating effect in mild and acute dextran sulphate sodium (DSS)-induced colitis by reducing levels of pro-inflammatory cytokines and improving host intestinal barrier function [82]. The first results on the efficacy and type of mechanisms of action of A. muciniphila in colorectal cancer showed that patients with colonic adenocarcinoma or colorectal cancer were characterised by a reduced abundance of this bacterium compared to healthy subjects. Interestingly, it was also observed that a significantly lower amount of A. muciniphila was found within tumour cells relative to adjacent normal cells [83].
Mice with induced spontaneous intestinal adenoma were divided into three different groups and given A. muciniphila, E. coli or phosphate-buffered saline (PBS). They had previously received one week of antibiotic therapy. At week 12 after implementation of the intervention, the group treated with A. muciniphila significantly inhibited tumour growth compared to the PBS control group as assessed by tumour count (5,333 ± 0.7638 vs. 9,714 ± 1.04, P <0.01), tumour volume (10% vs. 20% for tumours larger than 3 mm) and tumour burden (9,056 ± 1,621 vs.15,11 ± 1,654, P <0.05). In addition, tumours in the A. muciniphila had lower levels of the PNCA marker responsible for cell proliferation and an increased number of macrophages present in the tumour growth medium (TAM) type M1 compared to the PBS group (28.1% vs. 11.9%, P <0.05). This suggests that A. muciniphila can stimulate M1-type TAMs to mount an immune response, which have beneficial therapeutic effects within colorectal cancer [83].
One study on the effect of A. muciniphila in the treatment of colorectal cancer indicates that its presence may be beneficial in first-line therapy with FOLFOX (oxaliplatin, fluorouracil and calcium folinate), which in an experiment showed higher efficacy than the administration of oxaliplatin alone. It demonstrated that the abundance of A. muciniphila in patients receiving FOLFOX increased significantly. Most significantly, however, the increase in bacterial abundance in the intestine of patients was associated with increased antitumour efficacy of the administered preparation. A study was conducted in mice that had a significantly weakened and homogeneous intestinal microflora through broad-spectrum antibiotic therapy. They were then administered every two days via gastric tube A. muciniphila until the end of the study. The mice were divided into the following groups: control group, oxaliplatin model group, oxaliplatin treated group, FOLFOX model group and FOLFOX treated group. Before administering the drug to the mice, it was verified, by collecting and testing faecal samples, that the graft of A. muciniphila passed successfully. Oxaliplatin and FOLFOX showed different effects on the composition and metabolism of the intestinal microflora. Oxaliplatin increased carbohydrate and nucleotide metabolism within the intestinal microflora, whereas FOLFOX increased carbohydrate and lipid metabolism. After analysing the differences between the model groups and the groups receiving oxaliplatin and FOLFOX, it was shown that the most increased abundance of A. muciniphila between the model group for FOLFOX and the FOLFOX-treated group. It also increased the anti-cancer effect of this therapy from 36% to 48% and the degree of tumour inhibition from 48% to 76% (p < 0.05). The above results indicate the efficacy of A. muciniphila in this type of colorectal cancer therapy. The group taking oxaliplatin alone had significantly worse results. Its anti-tumour effect was weaker than FOLFOX, so further research was devoted to the leading therapy. Although the study sounds promising, it is worth bearing in mind that it was conducted on mice that had previously received intensive antibiotic therapy to reduce the diversity and abundance of bacteria residing in the gut of the rodents [70].
In 2020, Wang et al. analysed the effect of A. muciniphila injected to mice with sodium dextrasulphate followed by azoxymethane (DAO/AOM) administration. The latter ones induce colitis consequently leading to colorectal cancer. Mice were orally administered with either pasteurised A. muciniphila or Amuc_1100 protein as early as two weeks before the induction of inflammation and continued for a further 23 weeks during which the mice and their faeces were systematically examined. At the same time, faecal samples were collected from patients with colitis and colorectal cancer. After completion of the study and analysis of the faecal samples, it was shown that in both DSS/AOM-infected mice and diagnosed patients, the concentration of Akermanisa in the intestine was reduced. It was noted that mice that received supplementation with pasteurised A. muciniphila or Amuc_1100 protein showed significantly later tumour development (p <0.005) and also reduced tumour abundance (p < 0.05 for pasteurised A. muciniphila and p<0.001 for Amuc_1100 protein) and tumour size (p < 0.01) at week 12 of the study. A reduced expression of tumour-associated markers γH2AX and Ki67 in colonic epithelial cells was also observed in mice treated with pasteurised Akk. or Amuc_1100 protein. The above observations may suggest that supplementation of pasteurised A. muciniphila may inhibit excessive cell proliferation. The results are promising, but should also be carried out fully on the human population [34].
In conclusion, A. muciniphila has a positive effect and contributes to the body's response to certain agents administered in both immunotherapy and chemotherapy. Although the full mechanism of action has not yet been fully studied, we know that it plays a significant role in stimulating M1-type macrophages and increasing the number of CD8+ T lymphocytes. However, most of the studies have been carried out on animals, so further exploration and testing is needed.
Neurodegenerative diseases
Since the discovery of A. muciniphila in 2004 by Derrien et al. [6] evidence has emerged for the great potential of A. muciniphila and its outer membrane proteins in supporting the treatment of neuropsychological diseases. This evidence is supported by studies in animals and clinical trials [84]. The potential of A. muciniphila as a therapeutic support for neurological disorders such as Alzheimer's disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD) or stroke has already been recognised. This suggests a viable role for A. muciniphila in normal brain function [23]. The interest of researchers from various backgrounds in conducting functional studies of the intestinal barrier under various conditions has led to the linking of these diseases to abnormal intestinal permeability, which is a sign of impaired intestinal barrier function. In PD and AD, a biomarker for assessing intestinal permeability is elevated levels of zonulin in the blood. It is therefore necessary to initiate measures leading to the restoration of a normal intestinal barrier, and thus proper absorption of nutrients and fluids, preventing toxins and harmful bacteria from entering the body through the intestinal epithelium [85].
In mouse models of AD, a large decrease in A. muciniphila is observed [86], and this is associated with an impaired intestinal barrier [87]. These findings indicate the potential of A. muciniphila treatment in improving mucosal barrier dysfunction, metabolic dysfunction and memory [88,89]. Mice on a high-fat diet showed a decline in cognitive and mental function, and this was associated with A. muciniphila depletion [90]. It is possible to make up the deficit of this bacterium by, for example, a 16-week ketogenic diet, thus reducing the risk of neurodegeneration by improving the metabolic profile in young, healthy mice [91]. Beneficial effects of A. muciniphila supplementation whether by oral administration or dietary interference are observed in AD mouse models, compared to AD mouse models that do not receive this bacterium and its faecal abundance decreases with age [84]. A mouse model of AD APP/PS1 fed a normal diet or a high-fat diet and treated with A. muciniphila (5 x 109 cfu A. muciniphila in 200µl sterile PBS) by gavage daily for 6 months showed improvement in cognitive deficits and a reduction in amyloid-β (Aβ) protein levels [92]. Human studies have shown that patients with very mild to moderate AD have more A. muciniphila than the control group [93], while its levels are similar in people with mild cognitive impairment (MCI) and control subjects [94]. Studies on the beneficial effects of A. muciniphila on AD have been conducted relatively recently. However, direct evidence is still needed for validation, especially from human studies [95].
Postmortem findings in PD patients suggest that α-synuclein inclusions (a pathological feature of PD) may be transported from the gut to the brain via the vagus nerve [96], which raises the hypothesis that PD may begin in the gut. A growing number of recent studies have demonstrated alterations in the diversity of the gut microbiome in PD patients, which may cause inappropriate α-synuclein folding or disrupt enteric nervous system function [84]. Increased abundance of A. muciniphila was only found in mice with tetrahydropyridine (MPTP)-induced PD [97] and after treatment with rotenone [98]. However, Akkermansia abundance did not increase in most mouse models of PD in other studies [62,63,64]. In contrast, studies of PD patients in clinical settings in different countries have shown increased abundance of A. muciniphila [65,66,67]. In a study that used three machine learning algorithms to analyse metagenomic results from 472 PD patients and 374 healthy controls, 22 bacterial families were identified that helped distinguish between controls and potential PD patients. Among the bacteria in these 22 families, Akkermansia showed high efficiency in distinguishing PD patients from controls [105]. These studies and findings present an important role for Akkermansia in the pathogenesis of PD. The increased abundance of A. muciniphila makes it a potential early biomarker of PD [106].
A study was conducted on the difference in microbial abundance between MS patients and controls, and investigated how specific bacteria associated with MS modulate T-lymphocyte responses using in vitro and in vivo model systems. The results indicate that significantly increased abundance of A. muciniphila in MS patients is associated with a shift towards a pro-inflammatory T-cell profile that exacerbates or perpetuates the immune response. In an in vitro model, A. muciniphila extracts significantly increased the differentiation of peripheral blood mononuclear cells (PBMCs) into Th1 lymphocytes [107]. There are many studies confirming an increase in A. muciniphila abundance with the activation of the pro-inflammatory response in MS patients compared to controls [70,71,72]. In contrast, a reduction in A. muciniphila abundance in MS patients induced an anti-inflammatory immune response in the peripheral immune system [110]. Increased levels of A. muciniphila were also reported in a study in a mouse model of experimental autoimmune encephalomyelitis (EAE) compared with control mice [109]. Additionally, transplantation of the faecal microbiome from MS patients into mice induced a pro-inflammatory environment, which worsened the severity of EAE [107,111]. On the other hand, Cox et al. investigated a negative association between A. muciniphila abundance and disability and a positive correlation between A. muciniphila and brain volume, showing that the bacterium also plays a beneficial role. They sequenced the microflora in healthy controls, patients with relapsing-remitting multiple sclerosis (RRMS) and patients with progressive multiple sclerosis and correlated bacterial levels with clinical features of the disease, quality of life and MRI brain atrophy. They colonised mice with Akkermansia from multiple sclerosis and induced experimental autoimmune encephalomyelitis. By testing RRMS patients with high levels of Akkermansia, they distinguished between three subtypes based on the 16S rRNA V4 sequence. They then isolated the bacteria on culture medium and sequencing identified the strains most similar to A. muciniphila. To illustrate the role of MS-derived Akkermansia, they colonised C57/BL6 mice with three strains and found that they all alleviated autoimmune encephalomyelitis (EAE). In addition, they measured immune responses and found that the Akkermansia sp. BWH-H3 strain reduced the number of RORγT-positive γδ T cells and IL-17-producing γδ T cells [112]. These findings support that increased levels of this battery in MS may be a beneficial compensatory response in the MS microbiome [112,113].
The studies in the case of MS and PD seem at first glance to be worrying and at odds with the previously described positive association between A. muciniphila abundance and health. Nevertheless, the following facts should be taken into account when analysing the work described in this chapter:
- The studies described in MS and PD prove correlation, not causation
- Bacterial contents in tests are given in relative abundances, which in practice means that their number (absolute value) is not necessarily higher [118]
- Pasteurised Akkermansia muciniphil does not colonise the gut so does not induce changes in the composition of the microbiota [68]
- No authority, including EFSA, has denied the product's placing on the market on the basis of these correlative reports alone. [16]
A. muciniphila is negatively associated with amyotrophic lateral sclerosis (ALS), as evidenced by the progressively decreasing abundance of A. muciniphila in ALS mice [119]. Supplementation of pasteurized A. muciniphila in Sod1 transgenic mice (Sod1 -Tg) with ALS improved motor function and brain atrophy through accumulation of nicotinamide in the central nervous system. Nicotinamide is associated with A. muciniphila and its functions are to support dopamine production in neurons, neurotransmission and normal neuronal cellular metabolism [89].
Studies show that bacterial pneumonia is the leading cause of death after stroke. The selective movement of bacteria from the host's gut microbiome to the lungs is an essential factor in causing this pneumonia. This leads to the conclusion that the intestinal mucosa and gut microbiota plays an important role in post-stroke mortality [120]. Studies have shown that stroke can be triggered by disturbances in the gut microbiota, which in turn can affect neuroinflammatory and functional indicators after brain injury. The efficacy of post-stroke treatment may be improved by faecal microbiota transplantation (FMT) [121]. An increase in faecal A. muciniphila levels has been noted in patients following ischaemic stroke in clinical trials [122], leading to proposals to treat it as a microbiological marker [123]. There are other studies confirming that after stroke there is an increase in abundance in mouse models [120,124]. One of these studies analysed the communities of different microorganisms in five gastrointestinal tract docs in mice after stroke: duodenum, jejunum, ileum, cecum and colon. The finding of this study was that A. muciniphila changes its interaction profile after impaction towards activating beneficial bacteria, e.g. Ruminococcus spp., and inhibiting pathogenic bacteria, such as Streptococcus spp. and Staphylococcus spp. [120]. Thus, A. muciniphila contributes to reducing the migration of pathogens into epithelial cells and, consequently, into the lungs of stroke patients [120,125]. Different results were obtained in a faecal study of eight post-stroke humans and a 10-person control group, where the abundance of bacterial groups was compared. The abundance of A. muciniphila was reduced compared with the control group [126,127]. Still, the role of A. muciniphila in post-stroke patients remains unclear, so further studies especially on larger study groups are needed to determine this significance.
Mental illnesses
Research has shown that Akk may also play a role in neuropsychiatric disorders. Its direct influence is seen in the bacterium's effect on the host microbiota-gut-brain axis. The bacterium itself and its metabolites play a role in alleviating neuropsychiatric symptoms by reducing intestinal imbalances, thereby contributing to improving the integrity and structure of the intestinal barrier which translates into reduced inflammation. [88] Additionally, there is evidence in research that links stress response and psychiatric phenotypes to TLR signalling [128,129]. Research is scarce, mainly in animal models. One of the first studies was conducted in male C57BL/6N mice subjected to chronic stress (CRS), in which colitis was induced by administration of dextran sodium sulphate (DSS). The mice were then transplanted with A.muciniphila (1x109 IU/ml) after reducing the diversity of the gut microbiota following the intervention. The study showed that CRS caused behavioural deficits, but did not reduce the diversity of the gut microbiota. In addition, in CRS-treated mice, supplementation with A.muciniphila reduced the intensity of depressive disorders. Supplementation also improved histopathological indices - it improved mucosal barrier structure and function by increasing, among other things, the number of cup cells and also MUC-2 cells [130]. Another study also conducted in an animal model in male C57BL/6 mice aged 6-8 weeks sought to demonstrate the effect of CRS and A.muciniphila supplementation on depressive disorders. The control group received no supplementation, while two of the other groups were subjected to CRS, one of them with A. muciniphila supplementation for 3 weeks (5x108 CFU/ml by oral gavage). The study showed that supplementation had a significant effect on the behaviour of the mice, additionally reducing corticosterone levels and increasing dopamine and BDNF levels. However, no effect was observed on serotonin levels [131]. Another study sought to demonstrate how A.muciniphila supplementation would affect depressive and anxiety behaviours induced in animals by antibiotic use. Mice were supplemented with A.muciniphila for 3 weeks at 1.5 x 109 CFU in one group, while the other group was given Amuc_1100 protein (100ug/200ul) daily. The study showed that supplementation reduced behavioural deficits induced by antibiotic therapy [132].
In a study by Mcgaughey et al. also conducted in mice subjected to social stress, there was a decrease in A.muciniphila, Ruminococcus spp., Mollicutes spp., Paraprevotella spp., Doreta spp.. However, an increase in the amount of Oscillospira spp., Bacteroides spp, Lachnospiraceae spp was observed. In addition, the amount of A. muciniphila was shown to correlate with behavioural behaviour in mice. [133] Attempts were also made to demonstrate the effect of melatonin on modulating the composition of the gut microbiota in response to water stress and sleep deprivation. It was observed that in rodents, exposure to stress leads to a decrease in A.muciniphila and Lactobacillus murinus, but also to an increase in Bacteroides masiliensis. In addition, a decrease in melatonin concentration was observed, presumably as a mechanism of reduced bacterial abundance. [134] A study by Ding et al. sought to demonstrate a link between major depressive disorder (MDD) and the composition and function of the gut microbiota. The main aim of the study was to evaluate the antidepressant effect of A.muciniphila in a mouse model of chronic restraint stress (CRS)-induced depression. Mice were divided into three groups. One group was subjected to CRS, a second group without exposure to CRS and mice given A.muciniphila supplementation for 3 weeks. Subsequently, behavioural tests were conducted and levels of hormones, neurotransmitters including brain-derived neutrophic factor (BDNF) were determined. The composition of the gut microbiota was analysed by 16S rRNA sequencing. Metabolites were also determined. Supplementation with A. muciniphila has been shown to alleviate depressive behaviour and restore normal levels of depression-related hormones ( including dopamine and BDNF). [131] Sun et. al. report using broad-spectrum antibiotics in mice with induced depression. Pharmacotherapy resulted in increased anxiety and depressive behaviour. In addition, reduced levels of 5-hydroxytryptamine (5-HT) were observed in both mouse serum and hippocampus. Mice were supplemented with A. muciniphila and Amuc_1100 for 3 weeks. An alleviation of anxiety and depressive symptoms was observed. In addition, a normalisation of BDNF expression in different brain areas and an increase in serum and hippocampal 5-HT was observed. [132] A study by Guo et al. sought to demonstrate a link between the occurrence of depression associated with alcohol abuse. After alcohol supply in rodents, there was damage to the intestinal barrier, as well as lipopolysaccharide (LPS) translocation, increased inflammatory response (increase in TNF-a and IL-1B) and decreased 5-HT levels. A 2-week supplementation with A. muciniphila resulted in an alleviation of depression-like behaviour. Occludin, BDNF and 5-HT increased and LPS, TNF-a, IL-1b AND IL-6 decreased. [131]
In autism spectrum disorders (ASD), the composition and function of the intestinal microbiota is disrupted. In a study by Zou et al. an increase in Bacteroidetes spp. and Firmicutes spp. was observed in patients with ASD. These patients also showed a reduction in A.muciniphila, E.coli, B.fragilis, H.parainflienzae, among others. [135] In autism spectrum disorders, abnormalities in the composition and function of the gut microbiota are repeatedly highlighted, which translates into gastrointestinal dysfunction. In a mouse model of ASD, BTBRT +tf./j wished to test whether the use of a ketogenic diet would contribute to altering the composition and function of the gut microbiota. C57BL/6 and BTBR mice were fed standard food or a ketogenic diet for 10-14 days. After diet therapy, faecal samples and cecal sections were collected. The composition of the intestinal microbiota was altered in BTBR mice compared to control mice; in addition, the ketogenic diet reduced total faecal and colonic bacterial counts and increased A.muciniophila content. [136] A subsequent study conducted in a mouse model by Liu X. et al. sought to demonstrate the association of A.muciniphila supplementation and melatonin using a mouse model of valproic acid (VPA)-induced autism. It was shown that probiotic therapy increased the activation of dopaminergic neurons during social interactions in the mouse model and also translated into metabolic changes. [137]
Modulating the quantity of A. muciniphila
A number of studies have noted that dietary interventions significantly affect both the health of the subjects and the levels of Akkermansia spp. in the gut. Studies indicate that compounds such as polyphenols, resistant starch, FODMAP products (fermentable oligosaccharides, disaccharides and monosaccharides, and polyols) and individual products e.g. red pitaya, whole grain barley, oat bran can increase A. muciniphila abundance. [60] In a study by Walker et al. 500 mg resveratol was used for 5 weeks in patients with metabolic syndrome. The study did not show significant differences. However, considering the administration of resveratrol to Caucasians resulted in an increase in the number of A. muciniphila [138]. A study in the United States sought to demonstrate the effect of grape juice supplementation on metabolic parameters and the gut microbiota. In the study, C57BL/6J mice were fed a high-fat diet (HFD) supplemented with 1% Concord grape polyphenols. Compared to the control group, it was observed that the addition of polyphenols contributes to a reduction in the effects induced by the HFD diet (e.g. weight gain, inflammatory markers, glucose tolerance) and also improves intestinal barrier function by increasing the expression of genes including occludin, In addition, microbiome analyses showed a significant increase in A. muciniphila and also a reduction in the ratio of Firmicutes spp. To Bacteroidetes spp. [60] In a study conducted on 20 healthy volunteers, pomegranate extract 1000 mg was used for 4 weeks. Data from the study were not presented for the whole population. Interestingly, the level of A. muciniphila depended on whether the subjects produced urolithin A. In this group, A. muciniphila levels were 47-fold higher after 44 weeks [139]. In a study conducted in Iran by Roshanravan, the subjects were divided into 3 groups. However, one of them received 600 mg/d of sodium butyrate, the second group received 10 g of inulin while the third group received a combination of the two substances in the same amounts. Considering the percentage increase in A. muciniphila abundance showed a significant increase in the group receiving sodium butyrate and inulin. A non-significant increase was obtained when both substances were co-administered [140].
Limitations
This narrative review has some limitations. Firstly, it is possible that our search strategy of nor systemic type may have missed some publications. Secondly, the included studies did not always contain explicit and detailed information on the form of A. muciniphila used. In addition, practically all studies are experimental in nature and therefore it is difficult to draw conclusions about the practical use of A. muciniphila in medicine at this stage.
Author Contributions
Conceptualization, K.S.-Ż.; methodology, K.S.-Ż., I.Ł.; software, W. C.; validation, K.S.-Ż., I.Ł., W. M., E.S.; formal analysis, K.S.-Ż., I.Ł.; investigation, all; resources, all; data curation, K.S.-Ż., I.Ł., W. M., E.S.; writing—original draft preparation, K.S.-Ż.; writing—review and editing, all.; visualization, W.C.; supervision, K.S.-Ż., I.Ł., W. M., E.S..; project administration, K.S.-Ż.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
not applicable.
Acknowledgments
none.
Conflicts of Interest
Igor Łoniewski is probiotic company shareholders, Anna Wierzbicka-Woś is employed by probiotic company, Karolina Skonieczna-Żydecka receives remuneration from probiotic company. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia Muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl Environ Microbiol 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghen, F.; De Paepe, K.; Marzorati, M.; Van de Wiele, T. Mucin as a Functional Niche Is a More Important Driver of in Vitro Gut Microbiota Composition and Functionality than Supplementation of Akkermansia m Uciniphila. Appl Environ Microbiol 2021, 87, e02647-20. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schüller, S.; Juge, N.; et al. Experimental Models to Study Intestinal Microbes-Mucus Interactions in Health and Disease. FEMS Microbiol Rev 2019, 43, 457–489. [Google Scholar] [CrossRef]
- Karcher, N.; Nigro, E.; Punčochář, M.; Blanco-Míguez, A.; Ciciani, M.; Manghi, P.; Zolfo, M.; Cumbo, F.; Manara, S.; Golzato, D.; et al. Genomic Diversity and Ecology of Human-Associated Akkermansia Species in the Gut Microbiome Revealed by Extensive Metagenomic Assembly. Genome Biol 2021, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Microbe Profile: Akkermansia Muciniphila: A Conserved Intestinal Symbiont That Acts as the Gatekeeper of Our Mucosa. Microbiology (Reading) 2017, 163, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int J Syst Evol Microbiol 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Hoskins, L.C. Mucin Degradation in Human Colon Ecosystems. Fecal Population Densities of Mucin-Degrading Bacteria Estimated by a “Most Probable Number” Method. Gastroenterology 1981, 81, 759–765. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid Publication of the Names of Forty-Two Phyla of Prokaryotes. International Journal of Systematic and Evolutionary Microbiology 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia Muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl Environ Microbiol 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Loviselli, A.; Velluzzi, F.; Manzin, A. Gut Microbiota Markers and Dietary Habits Associated with Extreme Longevity in Healthy Sardinian Centenarians. Nutrients 2022, 14, 2436. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Frontiers in Immunology 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia Muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The Outer Membrane Protein Amuc_1100 of Akkermansia Muciniphila Promotes Intestinal 5-HT Biosynthesis and Extracellular Availability through TLR2 Signalling. Food Funct 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat Med 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Safety of Pasteurised Akkermansia Muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283 | EFSA Available online:. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6780 (accessed on 9 April 2023).
- Novel Food-European Commission. Available online: https://food.ec.europa.eu/safety/novel-food_en (accessed on 25 April 2024).
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb Pathog 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like Proteins of Akkermansia Muciniphila Modulate Host Immune Responses and Gut Barrier Function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front Microbiol 2020, 11, 219. [Google Scholar] [CrossRef]
- Segers, A.; de Vos, W.M. Mode of Action of Akkermansia Muciniphila in the Intestinal Dialogue: Role of Extracellular Proteins, Metabolites and Cell Envelope Components. Microbiome Res Rep 2023, 2, 6. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Critical Reviews in Microbiology 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia Muciniphila Secretes a Glucagon-like Peptide-1-Inducing Protein That Improves Glucose Homeostasis and Ameliorates Metabolic Disease in Mice. Nat Microbiol 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The Potential of Akkermansia Muciniphila in Inflammatory Bowel Disease. Appl Microbiol Biotechnol 2021, 105, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, W.; Li, Q.; Chen, Y.; Wu, L.; Dong, Y.; Huang, X.; He, X.; Ou, Z.; Peng, Y. Membrane Protein Amuc_1100 Derived from Akkermansia Muciniphila Facilitates Lipolysis and Browning via Activating the AC3/PKA/HSL Pathway. Microbiol Spectr 2023, 11, e0432322. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.A.; Raffals, L.E.; Camilleri, M. Intestinal Barrier Dysfunction in Inflammatory Bowel Disease: Underpinning Pathogenesis and Therapeutics. Dig Dis Sci 2023, 68, 4306–4320. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat Rev Gastroenterol Hepatol 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; Danilova, N.A.; Grigoryeva, T.V.; Markelova, M.I.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease–Associated Changes in the Gut: Focus on Kazan Patients. Inflammatory Bowel Diseases 2021, 27, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The Abundance of Akkermansia Muciniphila and Its Relationship with Sulphated Colonic Mucins in Health and Ulcerative Colitis. Sci Rep 2019, 9, 15683. [Google Scholar] [CrossRef]
- Presti, A.L.; Chierico, F.D.; Altomare, A.; Zorzi, F.; Cella, E.; Putignani, L.; Luca Guarino, M.P.; Monteleone, G.; Cicala, M.; Angeletti, S.; et al. Exploring the Genetic Diversity of the 16S rRNA Gene of Akkermansia Muciniphila in IBD and IBS. Future Microbiology 2019, 14, 1497–1509. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-Occurrence of Akkermansia Muciniphila and Faecalibacterium Prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Frontiers in Cellular and Infection Microbiology 2018, 8, 281. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflammatory Bowel Diseases 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Gewirtz, A.T.; Chassaing, B. Akkermansia Muciniphila Counteracts the Deleterious Effects of Dietary Emulsifiers on Microbiota and Host Metabolism. Gut 2023, 72, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Okullu, S.O.; Catakci, M.; Elmas, M.A.; Pinheiro, Y.; Arbak, S.; Demir, E.; Schaefer, K.H.; Kolgazi, M. Akkermansia Muciniphila Improves Chronic Colitis-Induced Enteric Neuroinflammation in Mice. Neurogastroenterology & Motility 2024, 36, e14745. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized Akkermansia Muciniphila Improves Irritable Bowel Syndrome-like Symptoms and Related Behavioral Disorders in Mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front Nutr 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2022, 10, 83. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal Microbiota Composition Differs between Children with β-Cell Autoimmunity and Those Without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Derrien, M.; Van Baarlen, P.; Hooiveld, G.; Norin, E.; Müller, M.; de Vos, W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia Muciniphila. Front Microbiol 2011, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Zeng, Q. Gut Microbiota and Immunopathogenesis of Diabetes Mellitus Type 1 and 2. Front Biosci (Landmark Ed) 2016, 21, 900–906. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc Natl Acad Sci U S A 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, F.; Grander, C.; Effenberger, M.; Adolph, T.E.; Tilg, H. Gut Dysfunction and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 611. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia Muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci Rep 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-T.; Yeh, Y.-T.; Lin, C.-C.; Yang, L.-Y.; Chiang, C.-P. Akkermansia Muciniphila Is Negatively Correlated with Hemoglobin A1c in Refractory Diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia Muciniphila Can Reduce the Damage of Gluco/Lipotoxicity, Oxidative Stress and Inflammation, and Normalize Intestine Microbiota in Streptozotocin-Induced Diabetic Rats. Pathog Dis 2018, 76, fty028. [Google Scholar] [CrossRef]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is There Any Association between Gut Microbiota and Type 1 Diabetes? A Systematic Review. Gut Pathog 2019, 11, 49. [Google Scholar] [CrossRef]
- Debray-Sachs, M.; Carnaud, C.; Boitard, C.; Cohen, H.; Gresser, I.; Bedossa, P.; Bach, J.F. Prevention of Diabetes in NOD Mice Treated with Antibody to Murine IFN Gamma. J Autoimmun 1991, 4, 237–248. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc Natl Acad Sci U S A 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tong, X.; Sud, N.; Khound, R.; Song, Y.; Maldonado-Gomez, M.X.; Walter, J.; Su, Q. Low-Density Lipoprotein Receptor Signaling Mediates the Triglyceride-Lowering Action of Akkermansia Muciniphila in Genetic-Induced Hyperlipidemia. Arterioscler Thromb Vasc Biol 2016, 36, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia Muciniphila Alleviates High-Fat-Diet-Related Metabolic-Associated Fatty Liver Disease by Modulating Gut Microbiota and Bile Acids. Microb Biotechnol 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia Muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J Mol Endocrinol 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp Mol Med 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front Microbiol 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Katiraei, S.; de Vries, M.R.; Costain, A.H.; Thiem, K.; Hoving, L.R.; van Diepen, J.A.; Smits, H.H.; Bouter, K.E.; Rensen, P.C.N.; Quax, P.H.A.; et al. Akkermansia Muciniphila Exerts Lipid-Lowering and Immunomodulatory Effects without Affecting Neointima Formation in Hyperlipidemic APOE*3-Leiden.CETP Mice. Mol Nutr Food Res 2020, 64, e1900732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia Muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl Environ Microbiol 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Lawenius, L.; Scheffler, J.M.; Gustafsson, K.L.; Henning, P.; Nilsson, K.H.; Colldén, H.; Islander, U.; Plovier, H.; Cani, P.D.; de Vos, W.M.; et al. Pasteurized Akkermansia Muciniphila Protects from Fat Mass Gain but Not from Bone Loss. Am J Physiol Endocrinol Metab 2020, 318, E480–E491. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat Med 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia Muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat Med 2022, 28, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, P.; Du, H.; Chu, W.; Sun, R.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Akkermansia Muciniphila Potentiates the Antitumor Efficacy of FOLFOX in Colon Cancer. Frontiers in Pharmacology 2021, 12, 725583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, Q.; Chen, Q.; Wei, Z.; Liu, S.; Zhang, L.; Zhang, Y.; Li, Z.; Liu, H.; Sui, H. Akkermansia Muciniphila Inhibits Tryptophan Metabolism via the AhR/β-Catenin Signaling Pathway to Counter the Progression of Colorectal Cancer. Int J Biol Sci 2023, 19, 4393–4410. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti–PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I Interferons in Anticancer Immunity. Nat Rev Immunol 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. Journal of thoracic oncology : Official publication of the International Association for the Study of Lung Cancer 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Xia, K.; Liu, Y.-W.; Liu, J.-H.; Rao, S.-S.; Hu, X.-K.; Chen, C.-Y.; Xu, R.; Wang, Z.-X.; Xie, H. Extracellular Vesicles from Akkermansia Muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. IJN 2021, 16, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; Shields, C.E.D.; Hechenbleikner, E.M.; Sears, C.L.; et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science (New York, N.Y.) 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut Vascular Barrier Impairment Leads to Intestinal Bacteria Dissemination and Colorectal Cancer Metastasis to Liver. Cancer Cell 2021, 39, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia Muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Frontiers in Microbiology 2019, 10, 2259. [Google Scholar] [CrossRef]
- Fan, L.; Xu, C.; Ge, Q.; Lin, Y.; Wong, C.C.; Qi, Y.; Ye, B.; Lian, Q.; Zhuo, W.; Si, J.; et al. Muciniphila Suppresses Colorectal Tumorigenesis by Inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunology Research 2021, 9, 1111–1124. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D. Potential of Akkermansia Muciniphila and Its Outer Membrane Proteins as Therapeutic Targets for Neuropsychological Diseases. Front Microbiol 2023, 14, 1191445. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci Rep 2017, 7, 41802. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia Muciniphila in Neuropsychiatric Disorders: Friend or Foe? Front Cell Infect Microbiol 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Si, J.; Kang, H.; You, H.J.; Ko, G. Revisiting the Role of Akkermansia Muciniphila as a Therapeutic Bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-Life High-Fat Diet-Induced Obesity Programs Hippocampal Development and Cognitive Functions via Regulation of Gut Commensal Akkermansia Muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci Rep 2018, 8, 6670. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective Effects of Akkermansia Muciniphila on Cognitive Deficits and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Nutr Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci Rep 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Critical Reviews in Microbiology 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct Evidence of Parkinson Pathology Spread from the Gastrointestinal Tract to the Brain in Rats. Acta Neuropathol 2014, 128, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Bae, C.-H.; Lee, Y.; Kim, H.-Y.; Kim, S. Korean Red Ginseng Suppresses 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Inflammation in the Substantia Nigra and Colon. Brain Behav Immun 2021, 94, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic Stress-Induced Gut Dysfunction Exacerbates Parkinson’s Disease Phenotype and Pathology in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Neurobiol Dis 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martinez, G.; Chin, B.; Camarillo, C.; Herrera, G.V.; Yang, B.; Sarosiek, I.; Perez, R.G. A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J Parkinsons Dis 2020, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-F.; Shan, C.; Zhuang, S.-Y.; Zhuang, Q.-Q.; Ghosh, A.; Zhu, K.-C.; Kong, X.-K.; Wang, S.-M.; Gong, Y.-L.; Yang, Y.-Y.; et al. Gut Microbiota-Derived Propionate Mediates the Neuroprotective Effect of Osteocalcin in a Mouse Model of Parkinson’s Disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Gorecki, A.M.; Preskey, L.; Bakeberg, M.C.; Kenna, J.E.; Gildenhuys, C.; MacDougall, G.; Dunlop, S.A.; Mastaglia, F.L.; Akkari, P.A.; Koengten, F.; et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model. Front Neurosci 2019, 13, 839. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov Disord 2020, 35, 1626–1635. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Parkinsons Dis 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front Aging Neurosci 2021, 13, 636545. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional Implications of Microbial and Viral Gut Metagenome Changes in Early Stage L-DOPA-Naïve Parkinson’s Disease Patients. Genome Med 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc Natl Acad Sci U S A 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.-K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut Microbiota-Specific IgA+ B Cells Traffic to the CNS in Active Multiple Sclerosis. Sci Immunol 2020, 5, eabc7191. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of Cannabinoids, Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD), Mitigates Experimental Autoimmune Encephalomyelitis (EAE) by Altering the Gut Microbiome. Brain Behav Immun 2019, 82, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A Probiotic Modulates the Microbiome and Immunity in Multiple Sclerosis. Ann Neurol 2018, 83, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut Microbiota from Multiple Sclerosis Patients Enables Spontaneous Autoimmune Encephalomyelitis in Mice. Proc Natl Acad Sci U S A 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann Neurol 2021, 89, 1195–1211. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Molanouri Shamsi, M.; Abolhasani, M.; Bakhshi, B.; Sahraian, M.A.; Quinn, L.S.; Negaresh, R. Home-Based Exercise Training Influences Gut Bacterial Levels in Multiple Sclerosis. Complement Ther Clin Pract 2021, 45, 101463. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Gastrointestinal Motility Disorders in Neurologic Disease. J Clin Invest 2021, 131, e143771. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal Colonic Transit Is Linked to Gut Microbiota Diversity and Microbial Fermentation in Humans with Slow Colonic Transit. American Journal of Physiology-Gastrointestinal and Liver Physiology 2020, 318, G361–G369. [Google Scholar] [CrossRef]
- Mruk-Mazurkiewicz, H.; Kulaszyńska, M.; Jakubczyk, K.; Janda-Milczarek, K.; Czarnecka, W.; Rębacz-Maron, E.; Zacha, S.; Sieńko, J.; Zeair, S.; Dalewski, B.; et al. Clinical Relevance of Gut Microbiota Alterations under the Influence of Selected Drugs—Updated Review. Biomedicines 2023, 11, 5022. [Google Scholar] [CrossRef] [PubMed]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J.; Skonieczna-Żydecka, K. Clinical Significance of Microbiota Changes under the Influence of Psychotropic Drugs. An Updated Narrative Review. Frontiers in Microbiology 2023, 14, 1125022. [Google Scholar] [CrossRef] [PubMed]
- Bruijning, M.; Ayroles, J.F.; Henry, L.P.; Koskella, B.; Meyer, K.M.; Metcalf, C.J.E. Relative Abundance Data Can Misrepresent Heritability of the Microbiome. Microbiome 2023, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An Insight into Intestinal Mucosal Microbiota Disruption after Stroke. Sci Rep 2018, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Yao, S.; Xie, H.; Wang, Y.; Shen, N.; Chen, Q.; Zhao, Y.; Gu, Q.; Zhang, J.; Liu, J.; Sun, J.; et al. Predictive Microbial Feature Analysis in Patients with Depression after Acute Ischemic Stroke. Front Aging Neurosci 2023, 15, 1116065. [Google Scholar] [CrossRef]
- Xiang, L.; Lou, Y.; Liu, L.; Liu, Y.; Zhang, W.; Deng, J.; Guan, Y.; She, M.; You, X.; Liu, M.; et al. Gut Microbiotic Features Aiding the Diagnosis of Acute Ischemic Stroke. Front Cell Infect Microbiol 2020, 10, 587284. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants (Basel) 2022, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and Dissemination of Commensal Bacteria in Post-Stroke Infection. Nat Med 2016, 22, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhu, Y.; Kan, P.; Cai, Y.; Wang, Z.; Wu, Z.; Yang, P. Analysis of Intestinal Microbial Communities of Cerebral Infarction and Ischemia Patients Based on High Throughput Sequencing Technology and Glucose and Lipid Metabolism. Mol Med Rep 2017, 16, 5413–5417. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Woo, H.G.; Jeong, J.H.; Kim, G.H.; Park, K.D.; Song, T.-J. Microbiota Dysbiosis and Functional Outcome in Acute Ischemic Stroke Patients. Sci Rep 2021, 11, 10977. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.B.; Nieto, S.J.; Haile, C.N.; Kosten, T.A. Immune Receptor Toll-like Receptor 4 Contributes to Stress-Induced Affective Responses in a Sex-Specific Manner. Brain Behav Immun Health 2021, 14, 100248. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Hall, L.K.; Paulus, M.P.; Savitz, J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology 2020, 121, 104843. [Google Scholar] [CrossRef]
- Chen, T.; Wang, R.; Duan, Z.; Yuan, X.; Ding, Y.; Feng, Z.; Bu, F.; Liu, L.; Wang, Q.; Zhou, J.; et al. Akkermansia Muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front Cell Infect Microbiol 2021, 11, 723856. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A Next-Generation Probiotic: Akkermansia Muciniphila Ameliorates Chronic Stress–Induced Depressive-like Behavior in Mice by Regulating Gut Microbiota and Metabolites. Appl Microbiol Biotechnol 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H.; Cheng, R.; Tang, Z.; Zhang, M. Outer Membrane Protein Amuc_1100 of Akkermansia Muciniphila Alleviates Antibiotic-Induced Anxiety and Depression-like Behavior in Mice. Physiology & Behavior 2023, 258, 114023. [Google Scholar] [CrossRef]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia Spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci Rep 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.-H.; Choi, Y.J.; Jun, J.-H.; Jung, H.C.; Kim, E.K. Melatonin in the Colon Modulates Intestinal Microbiota in Response to Stress and Sleep Deprivation. Intest Res 2020, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Research 2020, 13, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Molecular Autism 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, Y.; Zhang, Y.; Xiang, G.; Yu, M.; Wang, X.; Qiu, B.; Li, X.; Liu, W.; Zhang, D. Rescue of Social Deficits by Early-Life Melatonin Supplementation through Modulation of Gut Microbiota in a Murine Model of Autism. Biomedicine & Pharmacotherapy 2022, 156, 113949. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic Effect of Fruit and Vegetable Shots Containing Jerusalem Artichoke Inulin: A Human Intervention Study. Br J Nutr 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs1234. J Nutr 2016, 146, 673–680. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin Transl Gastroenterol 2016, 7, e164. [Google Scholar] [CrossRef]
Figure 1.
Modes of action of A. muciniphila. Tph1 - Tryptophan hydroxylase 1; Ocln – occludin, Cldn 3 – claudin 3; Cnr1 - cannabinoid receptor 1; LPS- Lipopolysaccharide; TLR- Toll-like receptor; FFR- Free fatty acid receptor; GLP- glucagon-like peptide; IL- Interleukin; MCP-MonocyteChemoattractant Protein; Apo- Apolipoprotein; Treg- Regulatory T limfocytes. Created with BioRender.
Figure 1.
Modes of action of A. muciniphila. Tph1 - Tryptophan hydroxylase 1; Ocln – occludin, Cldn 3 – claudin 3; Cnr1 - cannabinoid receptor 1; LPS- Lipopolysaccharide; TLR- Toll-like receptor; FFR- Free fatty acid receptor; GLP- glucagon-like peptide; IL- Interleukin; MCP-MonocyteChemoattractant Protein; Apo- Apolipoprotein; Treg- Regulatory T limfocytes. Created with BioRender.

Table 1.
The impact of A. muciniphila intake on metabolic indices in animal models. EV- extracellular vesciles, LPS – lipopolysaccharide, GGT -gamma-glutamyl transpeptidase, AST - aspartate aminotransferase, LDH - lactate dehydrogenase, TG – triglycerides.
Table 1.
The impact of A. muciniphila intake on metabolic indices in animal models. EV- extracellular vesciles, LPS – lipopolysaccharide, GGT -gamma-glutamyl transpeptidase, AST - aspartate aminotransferase, LDH - lactate dehydrogenase, TG – triglycerides.
| References: | Model: | Study groups: | Intervention | Survey results: |
|---|---|---|---|---|
| Wu et al (2020) [61] | Animal | 10-week-old male C57BL/6J mice orally fed | 2x108 per day; live or pasteurised A. muciniphila; 4 weeks | Induction of metabolism, reduction of body weight and improvement of body composition, reduction of insulin resistance, reduction of adipose tissue mass, induction of adipocytes, reduction of serum glucose after its oral administration, restoration of fat layer thickness after a high-fat diet. |
| Li et al. (2016) [57] | Animal | Apoe-/- mice fed orally | 5x109 per day; live or pasteurised A. muciniphila; 9 weeks | Pasteurised A. mucniphila Reduced fat gain, insulin resistance and dyslipidaemia compared to mice supplemented with live A. muciniphila. Glucose tolerance comparable in both groups. |
| Zhao et al. (2017) [62] | Animal | Six-week-old pathogen-free mice on a low-carbohydrate diet | 1x109 for day, live A. muciniphila; 14 weeks; | Reduced weight gain and body fat, improved glucose tolerance and insulin sensitivity, reduced fatty acid-related gene expression, reduced chronic low-grade inflammation, increased anti-inflammatory factors |
| Chelakkot et al. (2018) [63] | Animal | 6-8-week-old male mice C57BL/ 6 | 10 μg EV per day, 2 weeks | Reduced intestinal permeability, weight reduction, improved glucose tolerance |
| Ashrafian et al. (2019) [64] | Animal | 8-week-old male C57BL mice on a low-carbohydrate or high-fat diet | 1x109 live A. muciniphila per day; 10 mg protein/200 ul EV, 5 weeks | Significant weight loss in mice on a fat-rich diet, improved intestinal barrier integrity, improved glucose tolerance and lipid profile |
| Everard et al. (2019) [56] | Animal | 10-week-old male mice on a low-carbohydrate or high-fat diet | 2x108 per day; live A. muciniphila | Reduction in diet-induced obesity, reduced appetite, body weight and fat mass, reduced hyperglycaemia and hyperinsulinaemia |
| Katiraei et al. (2019) [65] | Animal | 9-13-week-old male E3L.CETP mice with an increased lipid profile | 2x108 per day; live A. muciniphila, 4 weeks | Reduction in body weight, reduction in plasma triglyceride and cholesterol levels, |
| Shin et al. (2019) [59] | Animal | 8-week-old female C57BL/6 mice on a high-fat or low-carbohydrate diet treated with Akk growing on medium with (+) or without (-) mucilage addition | 1x108 live A. muciniphila per day, 4 weeks | A. muciniphila (-) attenuated the changes induced by the high-fat diet, reduced adipocyte hypertrophy and increased the proportion of small adipocytes, improved glucose levels and increased insulin tolerance, reduced LPS levels and inhibited diet-induced progressive intestinal inflammation. |
| Wu et al. (2020) [61] | Animal | 8-week-old female C57BL/6 mice on a low-carbohydrate, high-fat diet | 1x109 live A. muciniphila per day, 10 months | Decreased weight gain, decreased appetite, increased expression of the anti-inflammatory factor IL-10 |
| Kim et al. (2020) [66] | Animal | 5-week-old C57BL/6N mice on a high-fat, low-carbohydrate diet | 1x108 -1x109 live A. muciniphila per day, 10 weeks | No difference in weight gain, reduced TG and ALT levels in obese mice, reduced hepatic IL-6 expression in obese mice |
| Lawenius et al. (2020) [67] | Animal | 12-week-old female mice C57BL/6 | Pasteurised A. muciniphila 2x108 per day, 4 weeks | Reduced weight and fat gain, reduced incidence of T lymphocytes in the bone marrow |
| Depommier et al. (2019) [68] | Human | 32 people with overweight, isulin resistance, metabolic diseases | Pasteurised or live A. muciniphila 1x1010 per day, 3 months | Reduced plasma insulin levels and increased insulin sensitivity, reduced cholesterol, GGT, AST, LPS, LDH and serum creatine kinase,slight weight loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Novel Dietary Proteins Selectively Affect Intestinal Health In Vitro after Clostridium difficile-Secreted Toxin A Exposure
Paulus Jochems
et al.
Nutrients,
2020
Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study
Jia Yin
et al.
Microorganisms,
2021
Akkermansia muciniphila Suppresses High-Fat Diet-Induced Obesity and Related Metabolic Disorders in Beagles
Xiao-Qi Lin
et al.
Molecules,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








