Submitted:
10 May 2024
Posted:
10 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
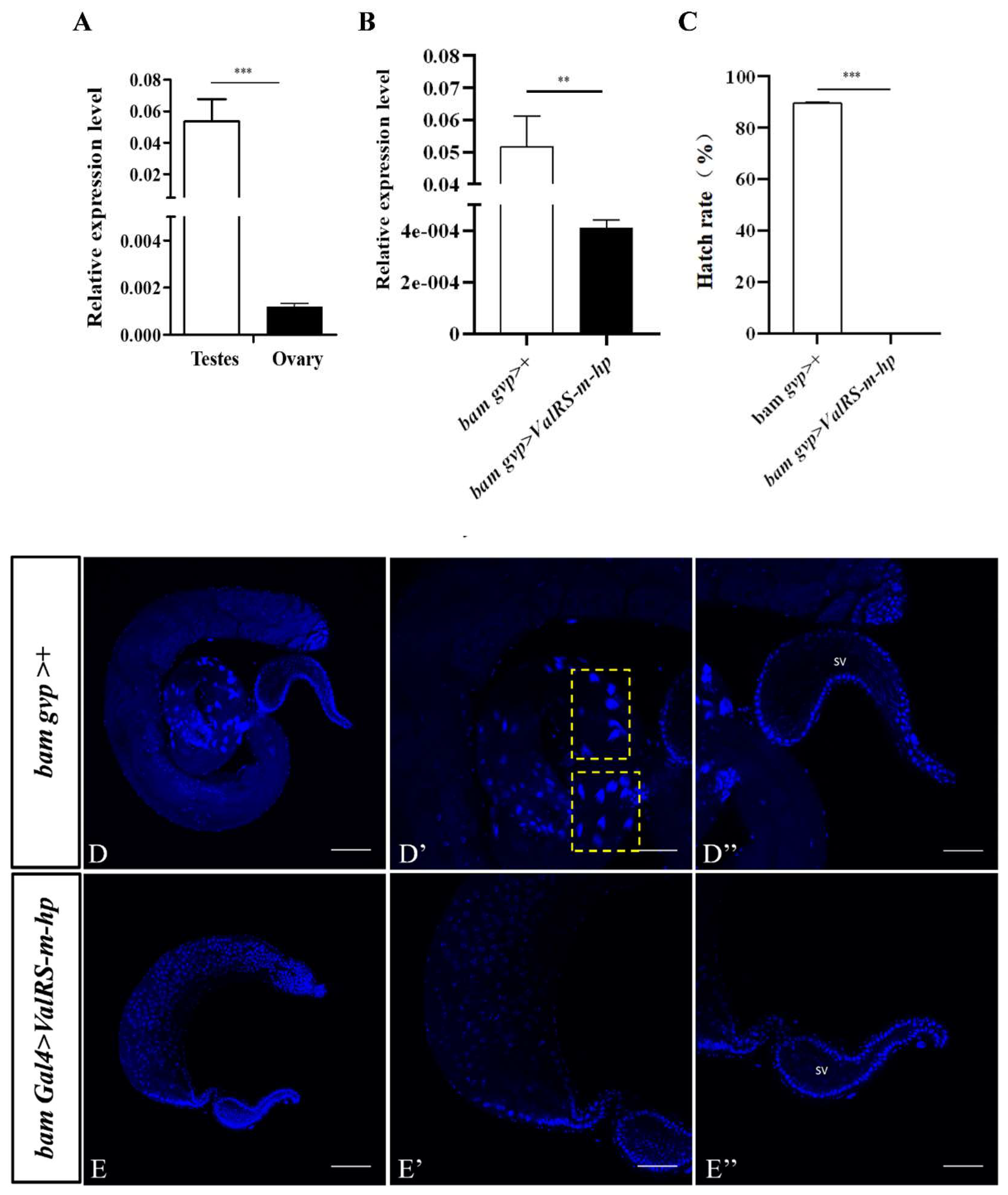
2.1. Knockdown of ValRS-m in Testes Caused Male Infertility
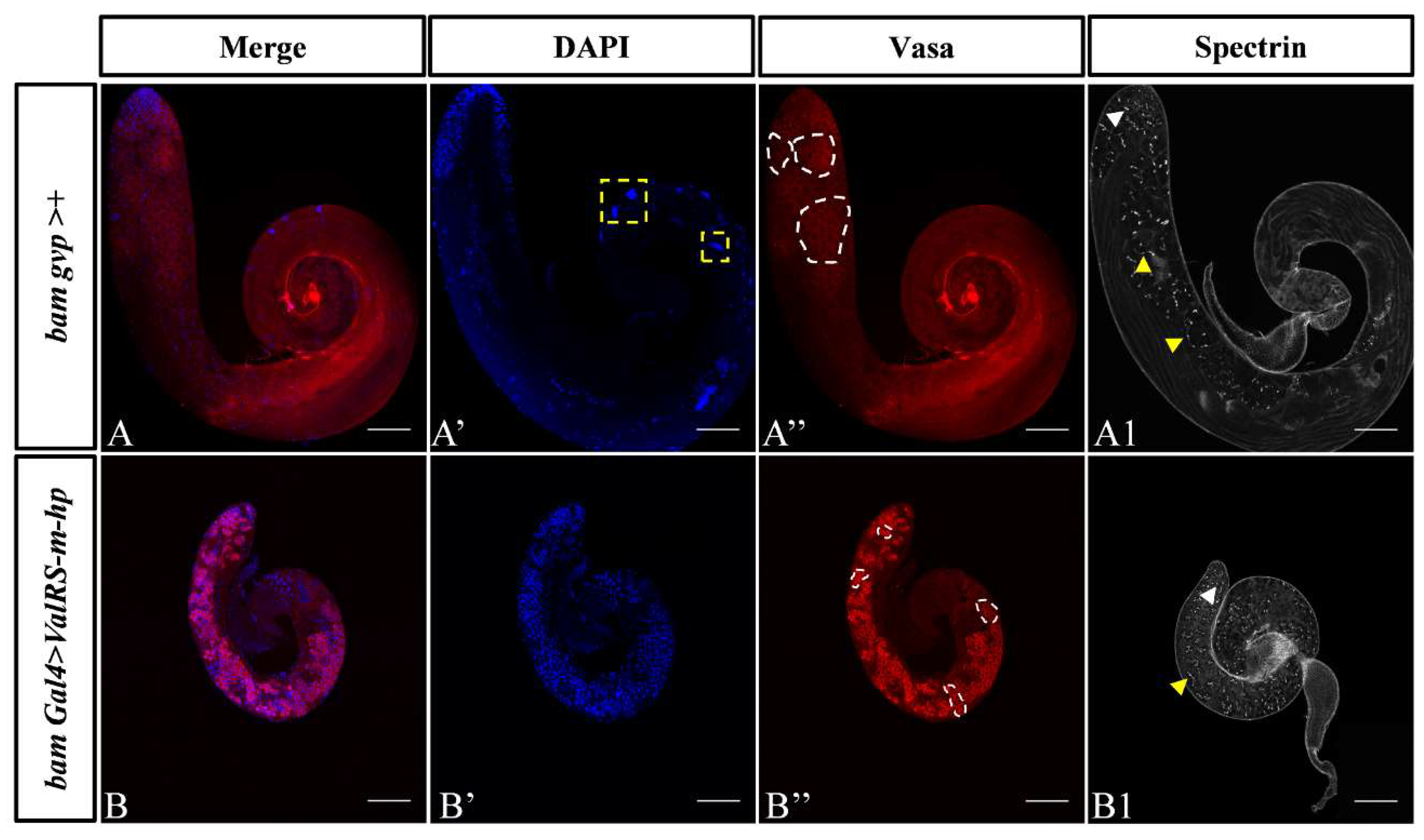
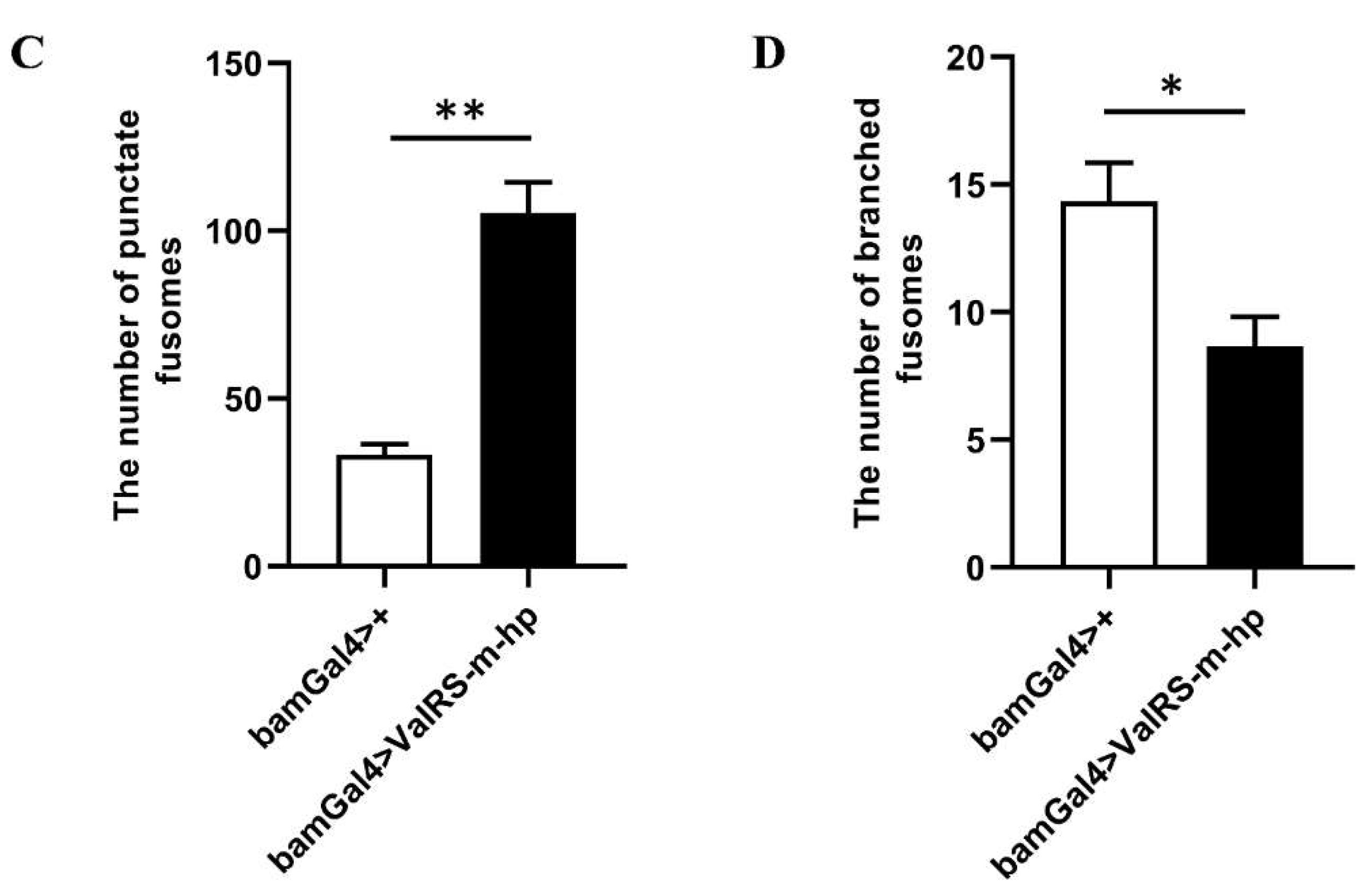
2.2. ValRS-m RNAi Inhibits Spermatogonia Differentiation and Caused Abnormal Proliferation
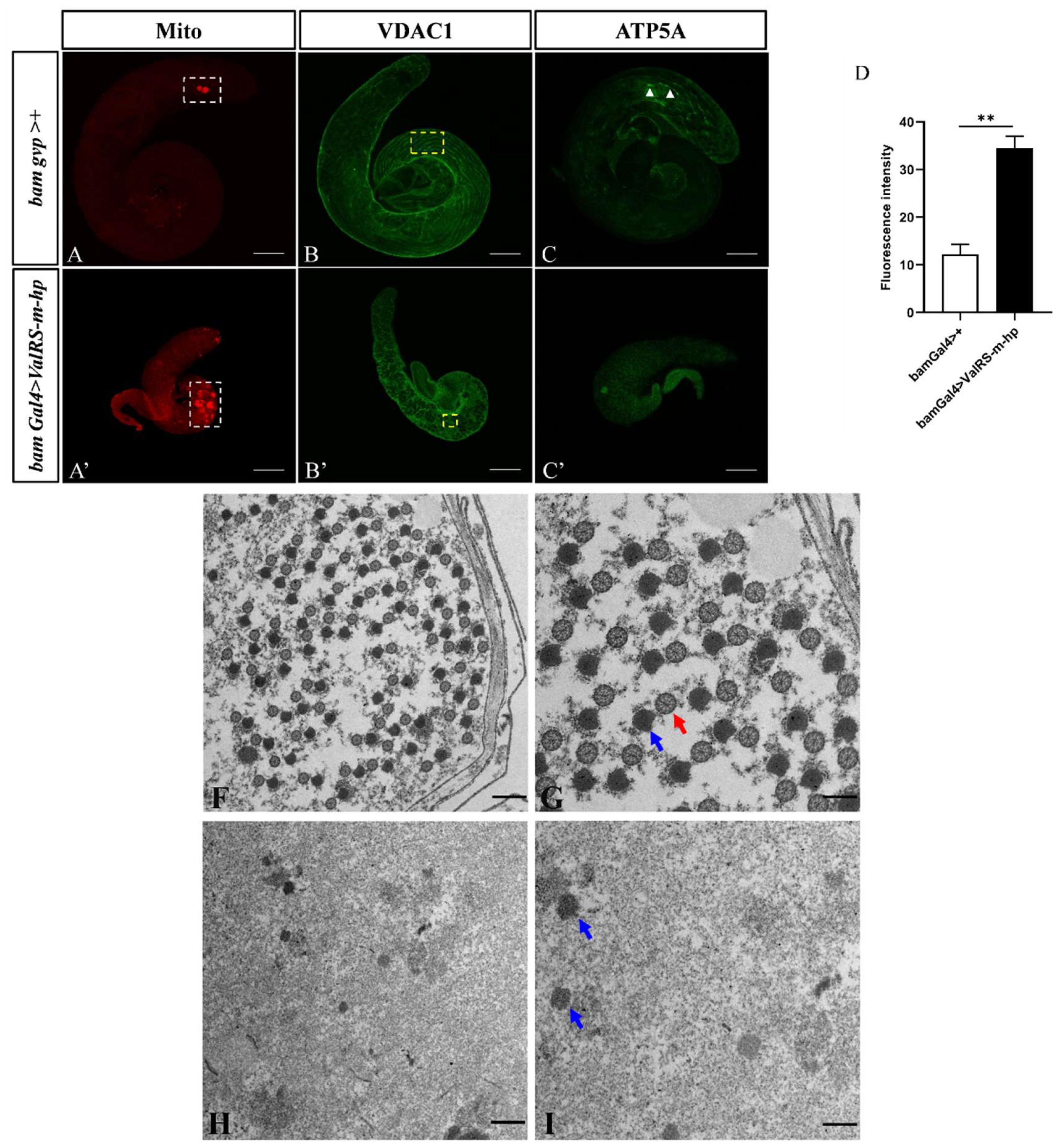
2.3. Knockdown of ValRS-m Disrupts the Mitochondrial Fusion and ATP Synthesis
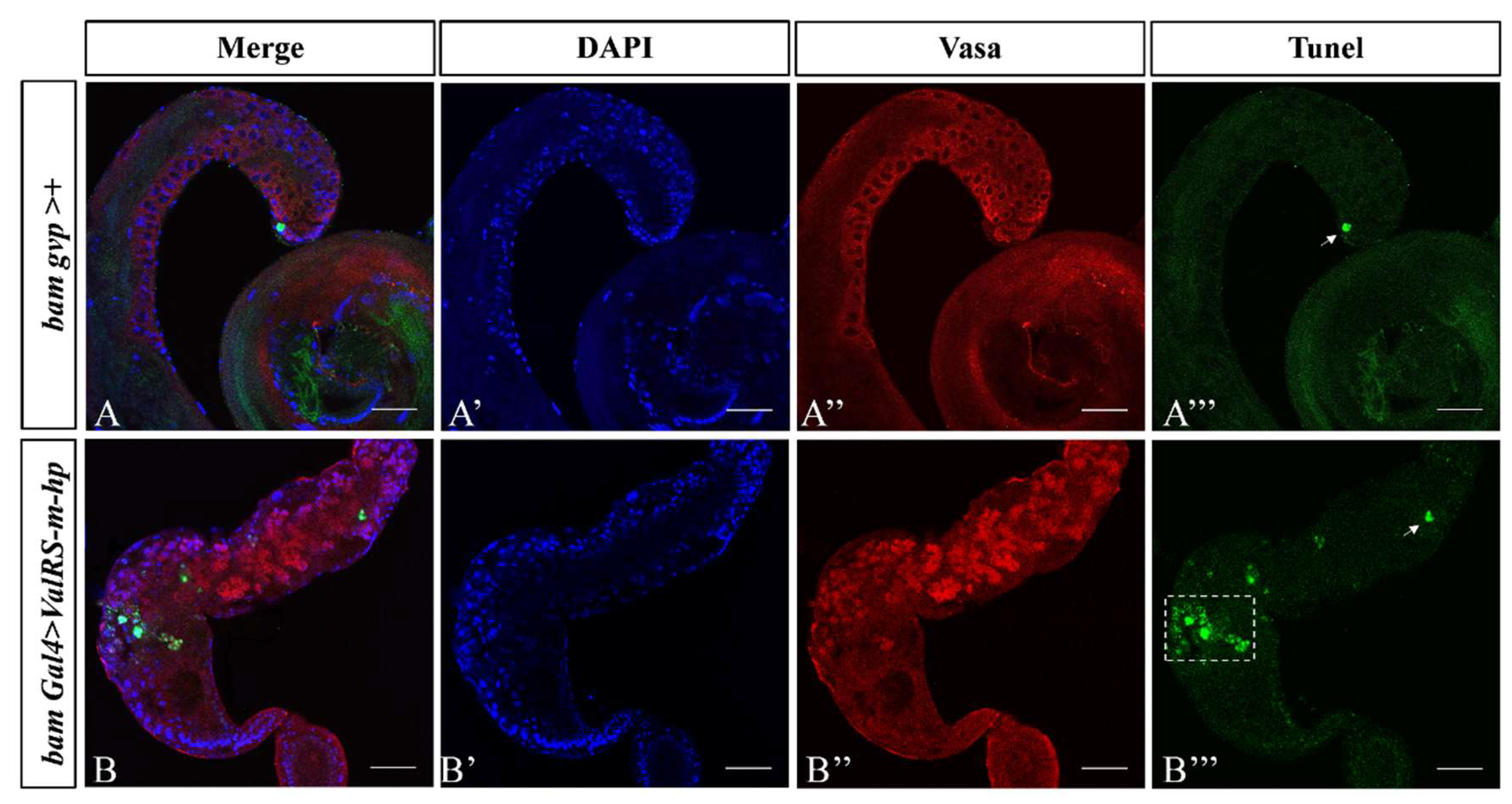
2.4. Knockdown of ValRS-m Leads to Excessive Apoptosis in Testes
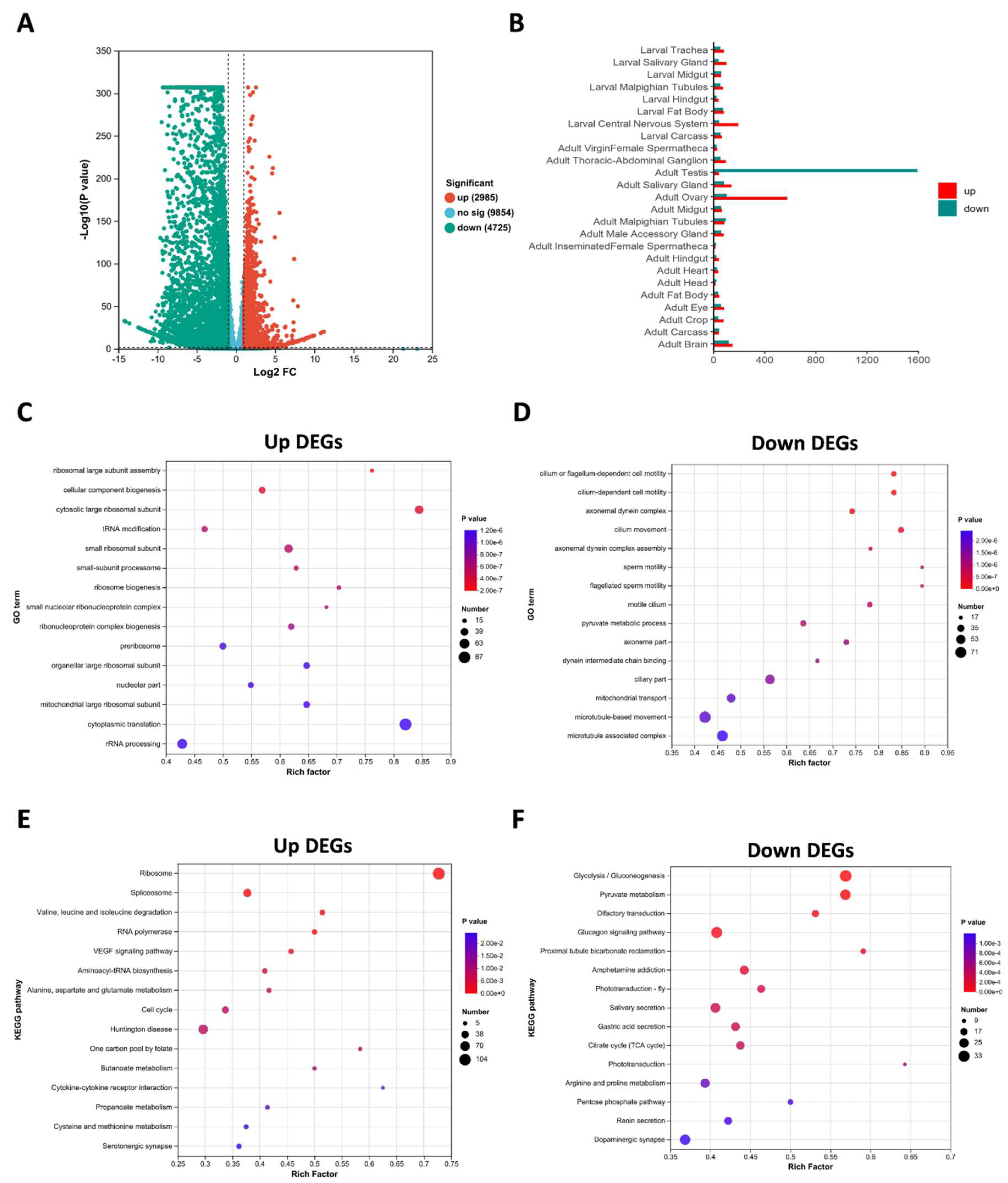
2.5. Absence of ValRS-m Alters the Expression Profiles of Genes in Testes
3. Discussion
4. Materials and Methods
4.1. Fly Stocks
4.2. Fertility Test
4.3. Immunofluorescence Staining
4.4. Transmission Electron Microscopy
4.5. TUNEL Staining
4.6. RNA Extraction, Library Preparation and Sequencing
4.7. Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sucato, A.; Buttà, M.; Bosco, L.; Di, Gregorio. L.; Perino, A.; Capra, G. Human Papillomavirus and Male Infertility: What Do We Know? Int J Mol Sci 2023, 24,17562.
- Fabian, L.; Brill J.A. Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis 2012, 2, 197-212.
- Chen, H.; Ren, S.; Clish, C.; Jain, M.; Mootha, V.; McCaffery, J.M.; Chan, D.C. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J Cell Biol 2015, 211, 795-805. [CrossRef]
- Demarco, R.S.; Eikenes, Å.H.; Haglund, K.; Jones, D.L. Investigating spermatogenesis in Drosophila melanogaster. Methods 2014, 68, 218-227. [CrossRef]
- Yu, J.; Zheng, Q.; Li, Z.; Wu, Y.; Fu, Y.; Wu, X.; Lin, D.; Shen, C.; Zheng, B.; Sun, F. CG6015 controls spermatogonia transit-amplifying divisions by epidermal growth factor receptor signaling in Drosophila testes. Cell Death Dis 2021, 12, 491. [CrossRef]
- Lim, C.; Tarayrah, L.; Chen, X. Transcriptional regulation during Drosophila spermatogenesis. Spermatogenesis 2012, 2, 158-166. [CrossRef]
- Shi, Z.; Lim, C.; Tran, V.; Cui, K.; Zhao, K.; Chen, X. Single-cyst transcriptome analysis of Drosophila male germline stem cell lineage. Development 2020, 147, dev184259.
- Trost, M.; Blattner, A.C.; Leo, S.; Lehner, C.F. Drosophila dany is essential for transcriptional control and nuclear architecture in spermatocytes. Development 2016,143, 2664-2676.
- Laktionov, P. P., Maksimov, D. A., Romanov, S. E., Antoshina, P. A., Posukh, O. V., White-Cooper, H., Koryakov, D. E., & Belyakin, S. N. Genome-wide analysis of gene regulation mechanisms during Drosophila spermatogenesis. Epigenet & chromatin 2018,11, 14. [CrossRef]
- White-Cooper, H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010, 139, 11–21. [CrossRef]
- Li, A. Y. Z.; Di, Y.; Rathore, S.; Chiang, A. C.; Jezek, J.; Ma, H. Milton assembles large mitochondrial clusters, mitoballs, to sustain spermatogenesis. Proc Natl Acad Sci U S A 2023, 120, e2306073120. [CrossRef]
- St John, J. C.; Facucho-Oliveira, J.; Jiang, Y.; Kelly, R.; Salah, R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update 2010, 16, 488-509. [CrossRef]
- Hales, K. G.; Fuller, M. T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 1997, 90, 121-129. [CrossRef]
- Pereira, S.; Adrião, M.; Sampaio, M.; Basto, MA.; Rodrigues, E.; Vilarinho, L.; Teles, EL.; Alonso I.; Leão M. Mitochondrial Encephalopathy: First Portuguese Report of a VARS2 Causative Variant. JIMD reports 2018, 42, 113-119.
- Alsemari, A.; Al-Younes, B.; Goljan, E.; Jaroudi, D.; BinHumaid, F.; Meyer, B.F.; Arold, S.T.; Monies, D. Correction to: Recessive VARS2 mutation underlies a novel syndrome with epilepsy, mental retardation, short stature, growth hormone deficiency, and hypogonadism. Hum Genomics 2017, 11, 33. [CrossRef]
- Ruzman, L.; Kolic, I.; Radic Nisevic, J.; Ruzic Barsic, A.; Skarpa Prpic, I.; Prpic, I. A novel VARS2 gene variant in a patient with epileptic encephalopathy. Upsala J Med Sci 2019, 124, 273–277. [CrossRef]
- Begliuomini, C.; Magli, G.; Di Rocco, M.; Santorelli, F. M.; Cassandrini, D.; Nesti, C.; Deodato, F.; Diodato, D.; Casellato, S.; Simula, D. M.; Dessì, V.; Eusebi, A.; Carta, A.; Sotgiu, S. VARS2-linked mitochondrial encephalopathy: two case reports enlarging the clinical phenotype. BMC Medical Genetics 2019, 20,77-93. [CrossRef]
- Zheng, Y.; Bi, J.; Hou, M.Y.; Shen, W.; Zhang. W.; Ai, H.; Yu, X.Q.; Wang, Y.F. Ocnus is essential for male germ cell development in Drosophila melanogaster. Insect Mol Biol 2018, 27, 545-555. [CrossRef]
- Carbonell, A.; Pérez-Montero, S.; Climent-Cantó, P.; Reina, O.; Azorín, F. The Germline Linker Histone dBigH1 and the Translational Regulator Bam Form a Repressor Loop Essential for Male Germ Stem Cell Differentiation. Cell reports 2017, 21, 3178–3189. [CrossRef]
- Yu, J.; Yan, Y.; Luan, X.; Qiao, C.; Liu, Y.; Zhao, D.; Xie, B.; Zheng, Q.; Wang, M.; Chen, W.; et al. Srlp is crucial for the self-renewal and differentiation of germline stem cells via RpL6 signals in Drosophila testes. Cell Death Dis 2019, 10, 294. [CrossRef]
- Chen, W. Y.; Yan, Y. D.; Luan, X. J.; Wang, M.; Fang, J. Functional analysis of CG8005 gene in Drosophila testis. Hereditas 2020, 42, 1122–1132.
- Wang, M.; Chen, X.; Wu, Y.; Zheng, Q.; Chen, W.; Yan, Y.; Luan, X.; Shen, C.; Fang, J.; Zheng, B.; Yu, J. RpS13 controls the homeostasis of germline stem cell niche through Rho1-mediated signals in the Drosophila testis. Cell proliferat 2020, 53, e12899. [CrossRef]
- Eikenes, A. H.; Brech, A.; Stenmark, H.; Haglund, K. Spatiotemporal control of Cindr at ring canals during incomplete cytokinesis in the Drosophila male germline. Dev. Biol 2013, 377, 9–20. [CrossRef]
- Jiang, L.; Li, T.; Zhang, X.; Zhang, B.; Yu, C.; Li, Y.; Fan, S.; Jiang, X.; Khan, T.; Hao, Q.; et al. RPL10L Is required for male meiotic division by compensating for RPL10 during meiotic sex chromosome inactivation in mice. Curr. Biol 2017, 27, 1498–1505. [CrossRef]
- Demarco, R.S.; Jones, D. L. Mitochondrial fission regulates germ cell differentiation by suppressing ROS-mediated activation of Epidermal Growth Factor Signaling in the Drosophila larval testis. Scientific reports 2019, 9, 19695. [CrossRef]
- Joubert, F.; Puff, N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes 2021, 11, 465. [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M. E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; Perales-Clemente, E.; Salviati, L.; Fernandez-Silva, P.; Enriquez, J. A.; Scorrano, L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160-171. [CrossRef]
- Maaroufi, H. O.; Pauchova, L.; Lin, Y. H.; Wu, B. C.; Rouhova, L.; Kucerova, L.; Vieira, L. C.; Renner, M.; Sehadova, H.; Hradilova, M.; Zurovec, M. Mutation in Drosophila concentrative nucleoside transporter 1 alters spermatid maturation and mating behavior. Front. Cell Dev. Biol. 2022, 10, 945572. [CrossRef]
- Chen, M. Y.; Duan, X.; Wang, Q.; Ran, M. J.; Ai, H.; Zheng, Y.; Wang, Y. F. Cytochrome c1-like is required for mitochondrial morphogenesis and individualization during spermatogenesis in Drosophila melanogaster. J Exp Biol 2023, 226, jeb245277.
- Wu, J.; Li, X.; Gao, Z.; Pang, L.; Liu, X.; Huang, X.; Wang, Y.; Wang, Z. RNA kinase CLP1/Cbc regulates meiosis initiation in spermatogenesis. Hum mol genet. 2021, 30, 1569-1578.
- Parisi, M.; Nuttall, R.; Edwards, P.; Minor, J.; Naiman, D.; Lü, J.; Doctolero, M.; Vainer, M.; Chan, C.; Malley, J.; Eastman, S.; Oliver, B. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome biol 2004, 5, R40.
- Chen, X.; Lu, C.; Morillo Prado, J. R.; Eun, S. H.; Fuller, M. T. Sequential changes at differentiation gene promoters as they become active in a stem cell lineage. Development 2011, 138, 2441-2450. [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol 2013, 6, 19. [CrossRef]
- Costa, J.; Braga, P. C.; Rebelo, I.; Oliveira, P. F.; Alves, M. G. Mitochondria Quality Control and Male Fertility. Biology 2023,12, 827. [CrossRef]
- Horbay, R., Bilyy, R. Mitochondrial dynamics during cell cycling. Apoptosis 2016, 21, 1327–1335. [CrossRef]
- Madan, S.; Uttekar, B.; Chowdhary, S.; Rikhy, R. Mitochondria lead the way: mitochondrial dynamics and function in cellular movements in development and disease. Front Cell Dev Biol 2021, 9, 781933. [CrossRef]
- Al-Zubaidi, U.; Liu, J.; Cinar, O.; Robker, R. L.; Adhikari, D.; Carroll, J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod 2019, 25, 695–705. [CrossRef]
- Fleck, D.; Kenzler, L.; Mundt, N.; Strauch, M.; Uesaka, N.; Moosmann, R.; Bruentgens, F.; Missel, A.; Mayerhofer, A.; Merhof, D.; Spehr, J.; Spehr, M. ATP activation of peritubular cells drives testicular sperm transport. Elife 2021,10, e62885.
- Detmer, S. A.; Chan, D. C. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Bio 2007, 8, 870-879. [CrossRef]
- Dorogova, N. V.; Bolobolova, E. U.; Akhmetova, K. A.; Fedorova, S. A. Drosophila male-sterile mutation emmenthal specifically affects the mitochondrial morphogenesis. Protoplasma 2013, 250, 515-520. [CrossRef]
- Beall, E. L.; Lewis, P. W.; Bell, M.; Rocha, M.; Jones, D. L.; Botchan, M. R. Discovery of tMAC: a Drosophila testis-specific meiotic arrest complex paralogous to Myb-Muv B. Genes Dev 2007, 21, 904-919. [CrossRef]
- Chishiki, M.; Takagi, K.; Sato, A.; Miki, Y.; Yamamoto, Y.; Ebata, A.; Shibahara, Y.; Watanabe, M.; Ishida, T.; Sasano, H.; Suzuki, T. Cytochrome c1 in ductal carcinoma in situ of breast associated with proliferation and comedo necrosis. Cancer Sci 2017, 108, 1510-1519. [CrossRef]
- Crofts, A.R. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol 2004, 66, 689-733.
- Chen, J.V.; Megraw, T. L. Spermitin: a novel mitochondrial protein in Drosophila spermatids. PLoS One 2014, 9, e108802. [CrossRef]
- Meng, H., Yamashita, C., Shiba-Fukushima, K., Inoshita, T., Funayama, M., Sato, S., Hatta, T., Natsume, T., Umitsu, M., Takagi, J., Imai, Y., & Hattori, N. Loss of Parkinson's disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c. Nat. Commun 2017, 8, 15500-15518. [CrossRef]
- Sawyer, E. M.; Brunner, E. C.; Hwang, Y.; Ivey, L. E.; Brown, O.; Bannon, M.; Akrobetu, D.; Sheaffer, K. E.; Morgan, O.; Field, C. O.; Suresh, N.; Gordon, M. G.; Gunnell, E. T.; Regruto, L. A.; Wood, C. G.; Fuller, M. T.; Hales, K. G. Testis-specific ATP synthase peripheral stalk subunits required for tissue-specific mitochondrial morphogenesis in Drosophila. BMC Cell Bio 2017, 18, 16-31. [CrossRef]
- Bhavsar, R.B.; Makley, L.N.; Tsonis, P. A. The other lives of ribosomal proteins. Hum Genomics 2010, 4, 327-344. [CrossRef]
- Yadavilli, S.; Hegde, V.; Deutsch, W. A. Translocation of human ribosomal protein S3 to sites of DNA damage is dependant on ERK-mediated phosphorylation following genotoxic stress. DNA Repair 2007, 6, 1453-1462. [CrossRef]
- Fang, Y.; Zhang, F.; Zhan, Y.; Lu, M.; Xu, D.; Wang, J.; Li, Q.; Zhao, L.; Su, Y. RpS3 Is Required for Spermatogenesis of Drosophila melanogaster. Cells 2023, 12, 573. [CrossRef]
- Ajore, R.; Raiser, D.; McConkey, M.; Jöud, M.; Boidol, B.; Mar, B.; Saksena, G.; Weinstock, D. M.; Armstrong, S.; Ellis, S. R.; Ebert, B. L.; Nilsson, B. Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations. EMBO Mol. 2017, 9, 498-507. [CrossRef]
- Fang, Y.; Zong, Q.; He, Z.; Liu, C.; Wang, Y. F. Knockdown of RpL36 in testes impairs spermatogenesis in Drosophila melanogaster. J Exp Zool 2021, 336, 417-430. [CrossRef]
- Storey, B.T. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol 2008, 52, 427-437. [CrossRef]
- Hudry, B.; de Goeij, E.; Mineo, A.; Gaspar, P.; Hadjieconomou, D.; Studd, C.; Mokochinski, J. B.; Kramer, H. B.; Plaçais, P. Y.; Preat, T.; Miguel-Aliaga, I. Sex Differences in Intestinal Carbohydrate Metabolism Promote Food Intake and Sperm Maturation. Cell 2019, 178, 901-918. [CrossRef]
- Di Giorgio, M. L.; Morciano, P.; Bucciarelli, E.; Porrazzo, A.; Cipressa, F.; Saraniero, S.; Manzi, D.; Rong, Y. S.; Cenci, G. The Drosophila Citrate Lyase Is Required for Cell Division during Spermatogenesis. Cells 2020, 9, 206-218. [CrossRef]
- Greenspan, L. J.; de Cuevas, M.; Matunis, E. Genetics of gonadal stem cell renewal. Annu. Rev. Cell Dev. Biol 2015, 31, 291-315. [CrossRef]
- Yang, X.; Liu, X.; Li, Y.; Huang, Q.; He,W.; Zhang, R.; Feng, Q.; Benayahu, D. The negative effect of silica nanoparticles on adipogenic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci 2017, 81, 341. [CrossRef]
- Li, B.; Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011, 12, 323. [CrossRef]
- Xu, J.; Du, R.; Wang, Y.; Chen, J. RNA-sequencing reveals the involvement of sesquiterpene biosynthesis genes and transcription factors during an early response to mechanical wounding of Aquilaria sinensis. Genes (Basel). 2023, 14, 464. [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C. Y.; Wei, L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 2011, 39, W316-322. [CrossRef]
- Livak, K. J.; Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 2001, 25, 402–408.
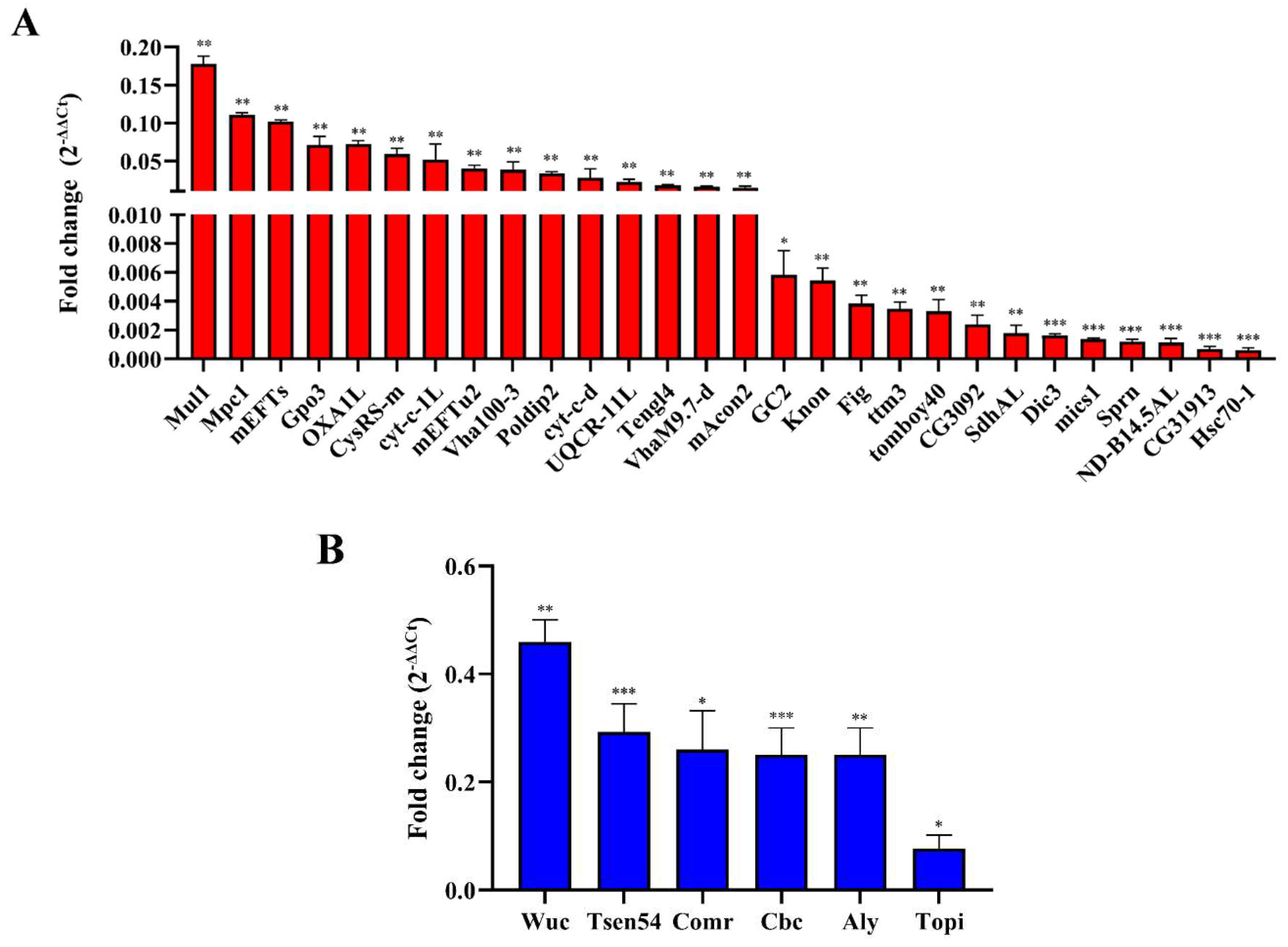
| Annotation ID | Gene Name | Description |
|---|---|---|
| Male meiosis-related | ||
| CG2075 | Aly (always early) | the onset of spermatid differentiation |
| CG13493 | Comr (cookie monster) | involved in spermatogenesis and transcription regulation |
| CG8484 | Topi (matotopetli) | male meiotic division and spermatid differentiation |
| CG12442 | Wuc (Wake-up-call) | male meiotic nuclear division |
| CG5970 | Cbc (crowded by cid) | required at the transition to meiosis in spermatogenesis |
| CG5626 | Tsen54 (tRNA splicing endonuclease subunit 54) | tRNA processing |
|
Mitochondria-related | ||
| CG14128 | Sprn (Spermitin) | testis-specific mitochondrial lumen protein |
| CG1287 | mics1 (Mitochondrial morphology and cristae structure 1) | maintenance of mitochondrial morphology |
| CG11196 | Dic3 (Dicarboxylate carrier 3) | mitochondrial dicarboxylate carrier |
| CG8330 | tomboy40 | import of protein precursors into mitochondria |
| CG6691 | ttm3 (tiny tim 3) | mitochondrial protein translocation |
| CG12201 | GC2 (Glutamate Carrier 2) | catalyzes the transport of L-glutamate across the inner mitochondrial membrane |
| CG4706 | mAcon2 (Mitochondrial aconitase 2) | enable 4 irons, 4 sulfur cluster binding activity |
| CG30354 | UQCR-11L (Ubiquinol-cytochrome c reductase 11 kDa subunit-like) | mitochondrial electron transport |
| CG13263 | cyt-c-d (Cytochrome c distal) | Electron carrier protein, sperm individualization |
| CG12736 | mEFTu2 (mitochondrial translation elongation factor Tu 2) | bring aminoacyl-tRNA to the ribosome during the elongation phase of mRNA translation |
| CG14508 | cyt-c1L (Cytochrome c1-like) | enable ubiquinol-cytochrome-c reductase activity |
| CG8257 | CysRS-m (Cysteinyl-tRNA synthetase, mitochondrial) | enable ATP binding activity and cysteine-tRNA ligase activity |
| CG6404 | OXA1L (OXA1L mitochondrial inner membrane protein) | mitochondrial proton-transporting ATP synthase complex assembly |
| CG7311 | Gpo3 (Glycerophosphate oxidase 3) | enable glycerol-3-phosphate dehydrogenase (quinone) activity |
| CG6412 | mEFTs (mitochondrial translation elongation factor Ts) | recharge the products of mEFTu1 and mEFTu2 with GTP |
| CG14290 | Mpc1 (Mitochondrial pyruvate carrier) | transports pyruvate across the mitochondrial inner membrane |
| CG1134 | Mul1 (Mitochondrial E3 ubiquitin protein ligase 1) | in the control of mitochondrial morphology by promoting mitochondrial fission |
| CG6914 | ND-B14.5AL (NADH dehydrogenase (ubiquinone) B14.5 A subunit-like) | mitochondrial electron transport, NADH to ubiquinone |
| CG5718 | SdhAL (Succinate dehydrogenase, subunit A (flavoprotein)-like) | mitochondrial respiratory chain complex II |
| CG7813 | Knon (knotted onions) | Nebenkern assembly |
| CG14909 | VhaM9.7-d (Vacuolar H+ ATPase M9.7 subunit d) | proton-transporting ATPase activity |
| CG4683 | Tengl4 (Testis EndoG-Like 4) | active in mitochondrial inner membrane and nucleus |
| CG30329 | Vha100-3 (Vacuolar H+ ATPase 100kD subunit 3) | ATPase binding activity |
| CG12162 | Poldip2 (Polymerase (DNA-directed), delta interacting protein 2) | active in mitochondrial nucleoid and nucleus |
| CG7615 | Fig (fos intronic gene) | active in mitochondrion |
| CG8937 | Hsc70-1 (Heat shock protein 70 cognate 1) | ATP hydrolysis activity |
| CG3092 | CG3092 | protein insertion into mitochondrial inner membrane from matrix |
| CG31913 | CG31913 | mitochondrial cytochrome c oxidase assembly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




