1. Introduction
Microglia, the immune cells of the central nervous system (CNS), are macrophages originating from yolk-sac progenitors during embryogenesis. These microglial cells exhibit distinct signature genes from other CNS macrophages [
1]. Their development follows a stepwise process, indicating differences between prenatal, postnatal, and adult microglia [
2]. Initially, thought to have hematopoietic origins due to similarities with dendritic cells and peripheral monocytes, recent studies, including fate mapping, confirmed their embryonic yolk sac derivation. While irradiation-induced myeloablation can lead to monocyte infiltration into the CNS parenchyma, it remains essential to distinguish them from true resident microglia [
3]. Overall, microglia precursors migrate from the yolk sac during embryonic development, proliferate, and acquire their signature genes, contributing to CNS homeostasis through dynamic surveillance and interaction with various components [
4]. They colonize the CNS, self-renewing throughout life [
5], with minimal contribution from bone marrow-derived monocytes [
6]. These cells play crucial roles, including supporting neurogenesis, pruning synapses, phagocytosing apoptotic neurons, defending against infections, producing and remodeling the extracellular matrix, maintaining myelin health, and removing protein aggregates linked to neurodegenerative diseases [
7,
8,
9,
10]. While homeostatic microglia exhibit a highly ramified morphology, responding to stimuli, aging, or CNS pathology triggers morphological changes, as noted by Pío del Río-Hortega over a century ago [
11].
The intricate role of factors like colony-stimulating factor 1 (CSF1) and its receptor CSF1R in microglia development and maintenance highlights the complexity of their regulation [
12]. The absence of yolk-sac macrophages and the failure of microglia colonization in CSF1R-mutant mice emphasize the importance of this signaling pathway [
13]. Additionally, the significant reduction of microglia in response to CSF1R inhibition further underscores the critical role of these factors in microglial population dynamics. The findings from microglia-specific Csf1r knockout mice and interleukin-34 ablation in neuronal progenitors emphasize the specific and dose-dependent nature of factors influencing microglia [
14]. The involvement of transcription factors, interferon regulatory factor 8 (IRF8) and PU.1, along with the anti-inflammatory cytokine transforming growth factor-β (TGF-β), further underscores the complexity of regulatory mechanisms governing microglial development and homeostatic functions [
3]. Homeostasis is defined as a relative constancy of set point formed in certain conditions, and maintaining homeostatic microglial function demonstrate an effort to restore the deviating set point due to aging in the CNS environment [
3].
In the early nineties, there were evidences that indicated that aged microglia had greater phagocytosis activity [
15]. Different studies have corroborated that aged microglia have a primed phenotype [
16] and a tendency to react more intensely to stimulation arises, attributed to their downregulation of homeostasis and inhibitory genes and increased expression of activating ones [
5,
17]. These modifications have been documented in various areas of the aged brain, particularly notable in white matter-resident microglia, indicating their responsiveness to age-related changes in myelin [
5].
Aging microglia respond to an inflammatory stimulus can induce heightened levels of pro-inflammatory cytokines like interleukins (IL-1β and IL-6) or tumor necrosis factor-α (TNF-α) as reported [
18]. Nevertheless, research indicates that while aged microglia still express IL-10 and IL4R-α at levels akin to young microglia, these interleukins fail to mitigate inflammation due to diminished cellular responsiveness [
19]. Consequently, aging microglia fail to restore homeostasis, exacerbating cognitive impairment, sickness, and depressive-like behaviors to levels typically observed in neuroinflammation scenarios [
5,
19].
An important microglia function is to phagocytose synapses, essential process during development in order to ensure correct neuronal connectivity [
20]. However, this occurs as a mechanism, mediated by microglia, to ensure neuronal plasticity and depends on neuronal activity [
5,
21]. It is still unknown whether microglia-mediated synaptic phagocytosis is responsible for the decline in synaptic loss that correlates with cognitive decline, as observed in aging human brains [
22].
The present review article summarizes our current knowledge on functional and phenotypic properties of aging microglia, with a special focus on the contribution of aged microglia to the development and progression of neurodegenerative diseases such as Alzheimer’s Disease (AD) and Parkinson’s disease (PD).
3. Characteristics and Phenotypes of Aged Microglia
It has been shown in studies carried out in healthy rodents that microglia constitutes between 5% and 12% of all specific cells in the CNS. However, the distribution is diverse and some brain areas display higher densities of microglia [
28]. Interestingly, higher microglia densities are exhibit compared to adjacent brain regions by the nigrostriatal system, comprising the substantia nigra (SN) and the caudate putamen (CPu) [
29]. In humans, microglia represent 0.5%–16.6% of all brain parenchymal cells, with higher numbers observed in white matter than in gray matter [
30].
Different works have tried to address age-related changes in microglial cells numbers in diverse species with different results. While there have been no obvious changes in the number of Iba1+ microglia in the hippocampus of aged rats [
31], reductions in the number of microglia were detected in the aged nigrostriatal system and cerebral cortex [
30]. Conversely, in aged rhesus monkeys (25 to 35 years old), there is an increase in the number of microglia, accompanied by heterogeneous intracellular inclusions suggesting heightened phagocytic activity but reduced particle digestion ability [
32]. Aging human microglia display dystrophic morphologies, characterized by residual process fragmentation, reduced branching, deramified dendritic arbors, and cytoplasmic beading, varying across brain regions [
33]. Dystrophic microglia are differentiated at a morphological and functional level from dark microglia, with highly branched morphology, which shows condensation of their cytoplasm and nucleoplasm, accompanied by contraction of the cytoplasm, Golgi apparatus and dilation of the endoplasmic reticulum [
34].
Homeostatic microglial functions decrease with aging, along with these morphological changes. Maintaining homeostatic function involves timely and appropriate responses at each life stage. Excessive or insufficient microglial responses can hinder tissue repair after CNS damage, underscoring the importance of tightly regulating the transition from steady-state homeostasis to an immunomodulatory mode during pathological conditions. Microglial immune checkpoints, mechanisms that prevent uncontrolled responses, have been proposed [
35]. The fractalkine receptor, CX3CR1, expressed on dendritic cells, monocytes, and microglial cells, plays a crucial role. due to its nature as transmembrane protein and chemokine for leukocyte migration [
36]. Its ligand, CX3CL1, expressed on neurons, regulates microglial functional phenotype and prevents hyperactivation under inflammatory conditions. For instance, mice lacking CX3CR1 showed exaggerated neurotoxic microglial responses and increased neuronal death when exposed to lipopolysaccharide (LPS) stimuli in the CNS. Additionally, CD200 receptor (CD200R) interaction with its ligand on neighboring cells, including neurons, astrocytes, oligodendrocytes, and endothelial cells, attenuates microglial activation, particularly in inflammatory contexts [
3].
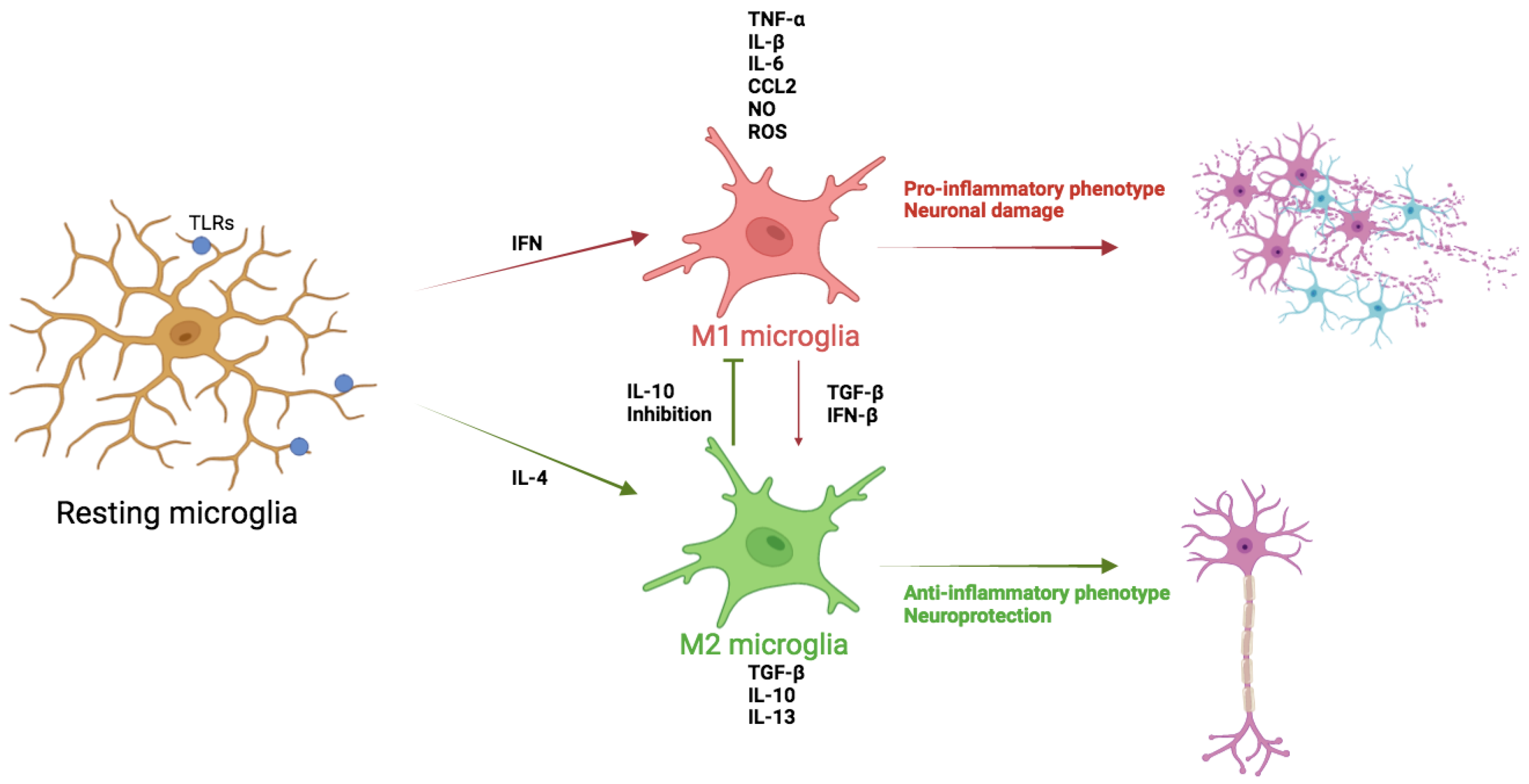
There are some works that have analyzed the changes of microglial polarization responses associated with age. In 2019, Wang et al. compared microglial markers in 2, 6, 18 and 28-month-old rat brains. Markers associated with different microglial phenotypes were examined, including M1 markers such as IL-1β and TNF-α, M2 markers such as IL-10 and arginase 1 (Arg1), A1 markers such as lipocalin-2 (Lcn2) and complement C3 (C3), and A2 markers such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF). Besides, M1 markers increased and M2 markers decreased in aged rats.
In aged rats, A2 markers (BDNF and GDNF) were decreased while A1 markers (Lcn2 and C3) were increased, suggesting an alteration in midbrain glial cell phenotypic polarization, potentially contributing to neurodegenerative diseases like PD due to age-induced DA neuron loss.(
Figure 1).
In aged mice, a loss of the M2-like phenotype has been demonstrated, evidenced by suppressed anti-inflammatory IL-4/IL-13 signaling [
37]. There is also an observed increase in the upregulation of TLRs, activation markers (MHCII, CD68, and CD86), and microglia/macrophage-specific inflammatory receptors CD11b in aging brains across various species [
38], indicating a predominant M1-like phenotype associated with neurotoxic responses (
Figure 1).
One mechanism to demonstrate the presence of reactive microglia in the aging brain is the age-associated loss of endogenous microglia regulatory pathways. For example, the signaling pathway that promotes the inactivity of microglial cells in which TGFβ is involved, is related to a reduction in the protective function of microglia [
39].
Furthermore, notable downregulation of microglial receptors involved in microglia-neuron interactions, such as the purinergic P2Y receptor 12 (P2Y12R), which is crucial for regulating microglial activation and phenotypic transformation, is evident in the aging brain [
40]. Additionally, age-related neurodegeneration leads to the depletion of neuron-derived CX3CL1 ligand, which normally maintains microglial cells in a quiescent state [
23]. These alterations in endogenous regulatory factors contribute to microglial dystrophy, neurodegeneration and chronic activation [
23].
Transcriptomic studies have helped advance the study of global changes in gene expression associated with aging. Different works such as a study carried out by Soreq et al. (2017) in which the transcriptomic profiles of different types of microglia and neuronal cells in different regions of the human brain, were analyzed in 480 subjects of widely varied ages (between 16 and 106 years) and an increase in cell-specific genes was observed. microglial cells in all brain regions that strongly predict biological aging [
41]. In other studies, signature genes associated with normal aging were identified, including high expression of genes encoding TNF family ligands, vesicle release proteins, and proinflammatory cytokine high mobility group box 1 (HMGB1) [
42].
HMGB1 mediates microglia priming in aging brain, and desensitize aged microglia to an inflammatory insult through its inhibition [
43]. Holtman et al. (2015) conducted a co-expression analysis, revealing changes in primed microglia from healthy aged animals and models of accelerated aging and neurodegeneration. They identified a common expression profile characterized by the upregulation of immune receptors, phagosome- and lysosome-related genes, and lipoprotein Apoe [
44]. This signature differed from the acute inflammatory gene set induced in animals treated with lipopolysaccharide (LPS), where genes related to the NF-κb pathway, Toll-like and NOD-like receptor (TLRs and NLRs) signaling were highly enriched. Microglia cells characterized by increased mammalian target of rapamycin (mTOR) signaling had higher transcript levels of ribosomal genes and interferon (IFN) α/β signaling [
44].
In Zhang and colleagues' 2021 research, they compared typical elderly mice, Ercc1ko-knockout mice lacking DNA repair mechanisms, and transgenic models of Alzheimer’s disease and Amyotrophic Lateral Sclerosis. Their study revealed shared microglial inflammatory gene networks between normal aging and pathological conditions, which contribute to age-related microglial priming. Unlike the acute inflammatory gene networks induced by LPS, such as NFκB signaling activation, primed microglia in these aging models exhibited increased expression of MHCII and other genes encoding CD11c integrins and CXC-chemokine receptor 4 on cell surfaces. Gene expression analysis demonstrated that Ercc1 deletion in microglia led to a temporary aging signature, distinct from a priming or disease-related microglia gene expression profile.
One of the main functions of microglial cells is synapse pruning through a phagocytic elimination process in a healthy developing brain. This is mediated through a mechanism that involves the recognition of complement proteins at synapses via microglial receptors [
23]. Complement-mediated clearance mechanisms are known to be involved in the aging process although not observed in healthy adult microglia, leading to an altered microglial phenotype that may contribute to neurodegeneration [
23].
In the elderly brain and accumulate on synapses are present CIq and C3 proteins, activating phagocytic complement receptors C1qR and C3R expressed in microglia, initiating the removal of healthy synapses [
45]. These findings suggest a harmful role for complement-mediated phagocytosis in aging, contributing to neurodegeneration.
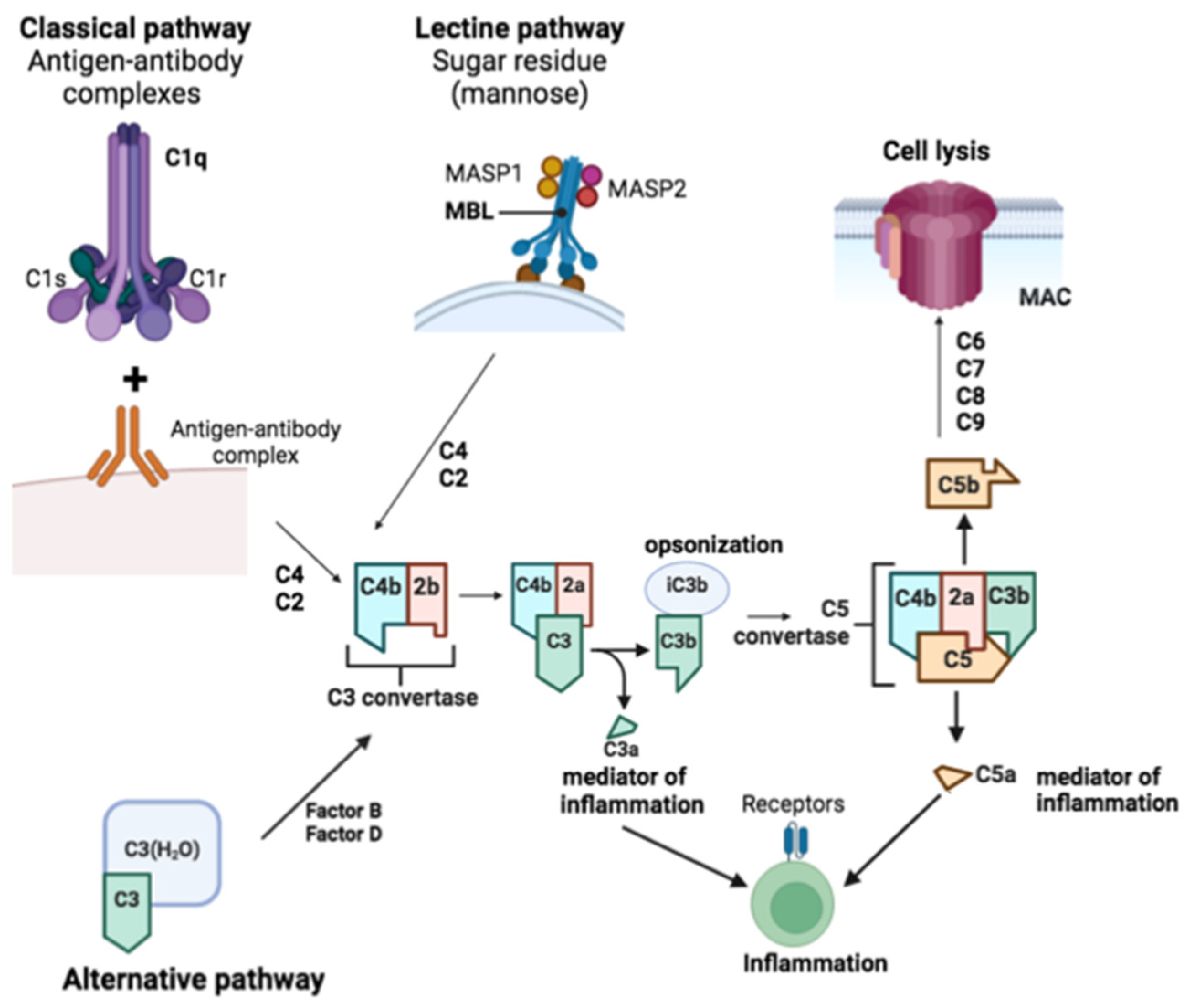
Through the classical or alternative pathways, the complement system can be activated. The classical pathway is initiated when C1q, C1r, and C1s of the C1 complex bind to apoptotic cells, hyperphosphorylated tau, or antigen-antibody complexes via C1q. The lectin pathway is triggered when mannan-binding lectin (MBL) forms a complex with MASP1 and MASP2, binding to microbial carbohydrates. C3 convertase is formed by cleaving C4 and C2 to generate C4b2b in both pathways [
46]. C3 is cleaved to produce C3a, promoting chemotaxis and microglial activation via C3aR, while C3b can be cleaved to iC3b to facilitate opsonization. C5 is cleaved into C5a, inducing a potent inflammatory response and acting through C5aR1 to enhance chemotaxis and glial activation, and C5b, which binds to C6, C7, C8, and C9 to form the membrane attack complex, leading to lysis. The alternative pathway can be activated by spontaneous hydrolysis of C3 to C3-H2O, enabling factors B and D to generate C3 convertase (C3(H2O)Bb), which then cleaves other C3 molecules into C3b. This pathway forms an amplification loop, where factor B binds to C3b and is cleaved by factor D to produce C3bBb, continuing the cleavage of C3 to enhance activation [
46] (
Figure 2).
Age-related functional change in the brain is also related to dysregulation of signaling of Ca
2+ [
47], which can produce a variety of neuropathological conditions. Dysregulation of Ca
2+ channels and dysfunction in mitochondria and the endoplasmic reticulum impede Ca
2+ homeostasis in the aging brain [
47]. Changes in intracellular Ca2+ levels are produced and microglia express various plasma membrane ionotropic and metabotropic receptors and P2X receptors and P2Y receptors (nucleotide receptors) [
47].
Additional signaling pathways that play a crucial role in regulating various key functions of microglia, such as phagocytosis and inflammatory responses, active also these receptors [
23]. Live visualization of microglial Ca2+ dynamics is being utilized to investigate functional and structural changes in the intact brain [
47]. In a recent and interesant study by Olmedillas del Moral and colleagues,
in vivo two-photon imaging, along with intracellular Ca2+ signaling and process extension analysis of cortical microglia, was conducted in young adult (2-4-month-old), middle-aged (9-11-month-old), and old (18-21-month-old) mice. They characterized a complex and nonlinear relationship between the properties of intracellular Ca2+ signals and the age of the animals. Furthermore, in old mice, microglial processes extending toward an ATP source exhibited faster but more disorganized movement compared to young adult mice. These findings highlight two distinct phenotypes of aging microglia: a reactive phenotype that increases with age, and a bell-shaped relationship between the frequencies and durations of spontaneous Ca2+ transients in middle-aged mice.
Other phenotype showed an ATP source moved faster in a more disorganized manner in old mice. Ca2+ dysregulation is also prominent in response to injury, inflammation, and neurodegenerative diseases [
47]. In another study by Brawek and colleagues, a microglia-specific microRNA-9-regulated viral vector was employed to express a genetically-encoded ratiometric Ca2+ sensor, Twitch-2B, in microglial cells. In intact
in vivo microglia, steady-state intracellular Ca2+ levels were found to be very homogeneous and low. However, these levels increased significantly after acute slice preparation and cell culturing, accompanied by an upregulation in the expression of activation markers CD68 and IL-1β. These findings highlight the steady-state intracellular Ca2+ level as a versatile marker for microglial activation, which is highly responsive to the cellular environment [
48].
In general, these data reviewed have evidenced that age-associated damage of microglial responses throughout life contributes to the development of neurodegenerative diseases.
4. Aged-Microglia and Neurodegeneration
Aged microglia exhibit both damage neuroprotective capacity such as low and sustained secretion of molecules that drive inflammation observed in neurodegenerative diseases [
49]. Microglial cells with dystrophia have been observed in both aging brains and the brains of patients with neurodegenerative diseases. They are commonly found near areas of tau pathology and amyloid plaques in AD brains, as well as near Lewy bodies in brains affected by dementia with Lewy bodies [
50].
Aging microglia in models with a β-amyloid burden, exhibiting reduced phagocytic capability, were also noted to express the cytokines TNF-α and IL-1β, unlike microglial cells that successfully phagocytosed β-amyloid [
51]. The inflammatory cytokines expression is believed to exacerbate microglial dysfunction and perpetuate AD pathology. Additionally, microglial cells have demonstrated the ability to internalize and degrade extracellular α-synuclein aggregates in cell culture studies, a process apparently regulated by the activation state of the cells, as activation with LPS reduced this activity [
51].
The role of iron-rich microglia in neurodegenerative diseases is significant. Recent studies have identified ferritin-positive dystrophic microglial cells associated with amyloid plaques and neurofibrillary tangles [
51]. Iron accumulation has also been observed in the hippocampus and, notably, in the amyloid plaques of AD patients. The interaction between iron and other metal ions contributes to the toxicity of β-amyloid oligomers [
51]. Excessive iron accumulation occurs in regions affected by Parkinson's disease (PD), such as the substantia nigra, and is associated with Lewy bodies, where dystrophic ferritin-positive microglia have been analyzed [
52].
A higher secretion of pro-inflammatory cytokines in microglial cells
in vitro has been related to disruption in iron homeostasis [
51]. Iron exposure has been related to a higher risk of developing PD [
53], and short-term exposure to iron has been shown to enhance neurotoxicity in rat primary microglia cells [
51]. Neuromelanin, a protein that stores iron in neuron cells, can be engulfed by microglia cells attracted to degenerating neurons, leading to an increase in the iron content of these cells. Rathnasamy et al. in 2013 has studied that neuromelanin phagocytosis can induce increased release of pro-inflammatory cytokines and reactive oxygen species (ROS), thus driving inflammatory processes that can contribute to neuronal degeneration further [
54].
It's conceivable that the accumulation of iron in microglial cells could initially serve as a protective mechanism against iron toxicity in the brain. However, this accumulation may eventually impair the microglia themselves, contributing to the accelerated aging signature observed in neuronal degeneration [
51].
5. Metabolism and Oxidative Stress in Aged Microglia
A finely energy metabolism is crucial for immune cells to maintain balance and fulfill the requirements of bio precursors essential for mounting an effective immune response [
55]. However, aging produces the decay of nutrient-sensing networks and a decline in mitochondrial efficiency and integrity [
55]. Notably, aged microglia also exhibit altered metabolism, with a downregulation of genes involved in oxidative phosphorylation [
55]. This regulation could be a response to changes in energy metabolism or due to an imbalance of growth factors in the brain environment, leading to heightened levels of mTOR signaling in aging microglia [
18]. mTOR plays a pivotal role in regulating cell growth and metabolism across all cell types, with its inhibition associated with increased longevity [
55]. In aged microglia, augmented mTOR signaling adds another layer of regulation to the primed phenotype by boosting mRNA translation [
18].
Activation of the PI3K-AKT-mTOR signaling pathway in microglia is triggered by growth factors and cytokines [
18]. AKT activation drives the sequestration of glucose into glycogen, resulting in energy depletion and succinate accumulation [
56]. Succinate, stabilized by mTOR signaling, promotes the transcription of pro-inflammatory genes via hypoxia-inducible factor 1α (HIF1α) [
56]. Concurrently, mTOR complex 1 (mTORC1) stimulates ribosomal gene transcription through various mechanisms [
5], culminating in heightened translation of pro-inflammatory factors like TNF, IL-1β, and IL-6 in aged microglia [
18]. Transcription factors like PPARγ facilitate the formation of lipid droplets in myeloid cells, suggesting a similar mechanism in microglia, downstream of mTOR [
5]. COX2, located on the droplet membrane, enhances PGE2 production, creating a positive feedback loop [
5]. The PI3K-AKT-mTOR pathway, crucial for microglial response to amyloid-β (Aβ) deposition, peptide is considered a critical neurotoxic agent in AD pathology, is activated by TREM2 [
56]. Reduced expression of TREM2 variants is associated with neurodegeneration [
56].
Antignano and co-researchers recently unveiled that aged microglia heighten mTORC1 signaling and downstream mRNA translation via the 4EPB1-EIF4E axis [
18]. The heightened mTOR-dependent phosphorylation of 4EBP1 leads to increased translation and subsequently elevated protein levels of inflammatory receptors and cytokines in aged microglial cells compared to their younger counterparts, an effect that could be mitigated by the loss of Rheb1, the upstream positive regulator of mTORC1. In a interesting study, Holtman et al. reported that microglial cells with heightened mTOR signaling exhibit higher transcript levels of ribosomal genes [
18,
44].
Metabolic shifts can directly produce mild inflammation in aged microglial cells, and upregulation of pro-inflammatory prostaglandin E2 (PGE2) signaling [
56]. EP2 receptor permit PGE2 acts through this receptor, promoting the sequestration of glucose into glycogen via the AKT–GSK3β–GYS1 pathway, resulting in decreased mitochondrial respiration, production of ATP and reduced levels of glucose. The decreased
de novo NAD + synthesis is produced by this energy-depleted state, damaging the NAD-dependent Sirt3-mediated deacetylation of mitochondrial complex II subunits, thereby reducing succinate dehydrogenase activity [
57]. Consequently, the accumulation of a TCA cycle metabolite, succinate, stabilizes the activity of hypoxia-inducible factor 1α, which activates pro-inflammatory cytokines [
56]. Inhibition PGE2, either pharmacologically or genetically, decreases the expression of pro-inflammatory cytokines in the blood and hippocampus of aging animals, enhances hippocampal plasticity and memory function and reverses this phenotype towards a more anti-inflammatory profile in peripheral macrophages and microglial cells [
56]. Importantly, peripheral block of the EP2 receptor with a brain-impermeant EP2 antagonist in aging animals yields similar outcomes, suggesting that age-associated brain inflammation and cognitive decline are not irreversible processes and can be altered by intervening in peripheral cells [
18].
Microglial lipid metabolism undergoes alterations with aging, evident through the accumulation of cytoplasmic inclusions such as lipid droplets [
18]. Marschallingeret al. have elucidated the functional implications of this aged phenotype. Analysis of the transcriptome in Lipid Droplets-Associated Microglia (LDAM) shown a significant deficiency in phagocytosis and an elevation in ROS and inflammatory cytokine production. Analysys of RNA-sequence of LDAM uncovered a transcriptional profile predominantly driven by innate inflammation, differing from previously documented states of microglial cells. An impartial CRISPR-Cas9 screen pinpointed genetic modifiers of lipid droplet formation; surprisingly, variants of several of these genes are implicated in autosomal-dominant forms of human neurocognitive disorders. Notably, LDAM exhibited a gene signature partially overlapping with that of microglia from LPS-treated mice [
58]. In this study it have been proposed that LDAM contribute to age-related and genetic forms of neurodegeneration [
58].
6. Studies of Aged Microglia in Alzheimer’s and Parkinson’s Disease
In mouse models of AD, microglia seem to construct a barrier that diminishes the neurotoxic effects of protofibrillar Aβ in amyloid plaques [
59]. Employing high-resolution confocal and
in vivo two-photon imaging in AD mouse models, Condello et al. demonstrated that this microglial barrier inhibits outward plaque expansion, leading to compact plaque microregions with low Aβ42 affinity. Regions devoid of microglial cells display less compactness but possess high Aβ42 affinity, fostering the development of protofibrillar Aβ42 hotspots linked to severe axonal dystrophy. With aging, microglia coverage diminishes, resulting in enlarged protofibrillar Aβ42 hotspots and more severe neuritic dystrophy [
59]. Anti-Aβ immunotherapy or deletion of the CX3CR1 gene promotes expansion of microglial cells coverage and reduces dystrophy of the neurites [
59]. The breakdown of the microglial barrier and the accumulation neurotoxic protofibrillar Aβ hotspots accumulation represent potential targets for therapy and clinical imaging in AD.
Recent research highlights the potential of young microglia in restoring amyloid clearance in aged microglia, as demonstrated in ex vivo brain slice co-cultures by Daria et al. [
60]. To explore the role of microglia in phagocytosis of amyloid plaque, a novel
ex vivo model was devised, co-culturing organotypic brain slices from amyloid-bearing AD mouse models with young, neonatal wild-type mice. Curiously, co-culturing induced the recruitment, proliferation, and clustering of old microglia around amyloid plaques, resulting in the clearance of the plaque halo. There is a synergistic effect of old or young microglia due to depletion of either old or young microglial cells that impeded amyloid plaque clearance. Old microglial cells have been exposed to conditioned media from young microglial cells or the addition of granulocyte-macrophage colony-stimulating factor that induced reduced amyloid plaque size and microglial cells proliferation [
60]. There is reversal of mitochondrial dysfunction in AD that is suggested by these data and their ability to phagocytize can be modulated to restrict accumulation of amyloid. This innovative
ex vivo model provides a important platform for identifying, screening, and testing compounds aimed at enhancing microglial phagocytosis therapeutically.
In transgenic AD mice, exacerbated age-dependent microglial activation and disturbances in cytoskeletal regulations contribute to further neurodegeneration [
61]. This research provides a comprehensive analysis of the gene expression patterns in the APP23 model for AD and control mice, exploring the impact of aging on these patterns. These results are linked to different symptomatic and pathological features of the model such as changes in soluble Aβ levels [
61]. A distinct two-phase expression profile was observed, with the first phase resembling features found in young carriers of familial AD mutations, while the second phase mirrors the progression of human AD pathology. There is a noticeable increase in microglial activation and lysosomal pathways during this later phase, alongside a decrease in neuron differentiation and axon guidance pathways. Curiously, these alterations are associated with aging but are more pronounced in APP23 mice [
61].
Moreover, complement factor C3 secreted by reactive astrocytes interacts with microglial C3a receptor (C3aR), mediating Aβ pathology and neuroinflammation in AD mouse models [
62].
In this study, it has been reported that astrocytic complement activation also regulates Aβ dynamics
in vitro and amyloid pathology in AD mouse models through microglial C3aR [
62]. In primary microglial cultures, it has been demonstrated that acute activation of C3 or C3a promotes microglial phagocytosis, while chronic treatment with C3/C3a diminishes it. The impact of chronic exposure to C3 can be mitigated by co-treatment with a C3aR antagonist and by genetically deleting C3aR. Furthermore, it is demonstrated that, neuroinflammation and Aβ pathology worsen in transgenic mice due to astroglial NF-κB hyperactivation and resulting C3 elevation [
62]. Besides therapy with a C3aR antagonist reduces microgliosis and plaque load, indicating a complement-dependent intercellular communication between Aβ and astroglial NF-κB activated, triggering the extracellular release of C3 that interacts with neuronal and microglial C3aR to impede Aβ phagocytosis and modify cognitive function [
62]. This feedback loop can be disrupted by C3aR inhibition, suggesting therapeutic potential in chronic neuroinflammation conditions.
However, lack of C3aR in APP transgenic mice results in decreased Aβ deposition, suggesting a complex role of microglia in AD pathogenesis [
63]. Additionally, the regulation of the clearance of soluble Aβ independently of phagocytosis is a new duty for the microglial complement receptor 3 (CR3). Remarkably, leads to, with cultured microglia lacking CR3 exhibiting greater efficiency in degrading extracellular Aβ. Moreover, a small molecule modulator of CR3 reduces extracellular soluble Aβ levels and Aβ half-life in brain interstitial fluid, indicating a potential new therapeutic target in AD [
63].
Interestingly, studies utilizing microglia ablation in APP transgenic mouse models revealed that neither Aβ plaque formation nor amyloid-associated neuron dystrophy depended on the presence of microglia [
64]. Further investigations are crucial to comprehensively understand how microglia contribute to AD onset and progression, with particular emphasis on aged microglia to elucidate the impact of aging on microglial functions in AD.
In PD, pronounced microglial reactions have been observed in the human substantia nigra (SN) associated with extraneuronal neuromelanin deposits [
29]. Human neuromelanin can induce microglia-mediated neuroinflammation and neurodegeneration in rodents [
29], suggesting its role as a potent trigger for microglial activation. Rodent toxin-based PD models, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), have elucidated the contribution of microglia to mDA neuron degeneration [
65]. Machado and col. have conducted analyses of transgenic mice deficient for chemokines, cytokines, as well as neurotrophic factors and their respective receptors in the MPTP model of PD, expanding our understanding of neurotrophic support and neuroinflammation. Their work explains the role of microglia-mediated neuroinflammation in MPTP-induced neurodegeneration and highlights the contribution of neurotrophic factors in slowing the progression of midbrain dopaminergic neurons [
65].
Microglia priming increases susceptibility to toxins, exacerbating mDA neurodegeneration [
66], with aged monkeys displaying heightened and persistent microglial reactivity after MPTP application [
67]. Age-dependent microglia priming involves epigenetic modifications, as evidenced by the role of histone H3K27me3 demethylase Jumonji domain containing 3 (Jmjd3) in M2-like microglia activation [
68]. Tang et al. studied that the suppression of Jmjd3 inhibited M2 polarization and concurrently amplified M1 microglial inflammatory responses, resulting in widespread neuron death
in vitro. Additionally, in vivo suppression of Jmjd3 in the SN markedly induced microglial overactivation and worsened dopamine neuron death in the MPTP-intoxicated mouse model of PD [
68]. Furthermore, a lower level of Jmjd3 in the midbrain of aged mice, accompanied by an increased level of H3K27me3 and a higher ratio of M1 to M2 markers, suggested that aging plays a crucial role in altering microglia phenotypes [
68]. Overall, these findings suggest that Jmjd3 can enhance M2 microglia polarization by modifying histone H3K27me3, thereby playing a crucial role in the switch of microglia phenotypes that may contribute to the immune pathogenesis of PD.
Aging has influence on the severity of MPTP-induced neurodegeneration and gains additional backing from a study utilizing Senescence-accelerated mouse prone 8, a mouse strain with premature senility onset, demonstrating heightened microglial cells activation and increased neurodegeneration post-MPTP intoxication [
69]. Apart from toxin-based PD models, αSyn transgenic mice are frequently employed to elucidate how αSyn aggregates contribute to neuroinflammation and neurodegeneration in PD. Non-aggregated αSyn can trigger TLR-mediated immune responses of microglia, potentially contributing to sporadic and/or familial forms of αSyn-related PD [
70].
Scheffold et al. have studied telomere shortening, a consequence of incomplete replication of chromosome ends, that is a recognized hallmark of aging [
71]. However, the precise role of telomere dysfunction in neurological diseases and the aging brain remains unclear, with ongoing debate regarding its association with PD. In this study, it has been investigated a mouse model of PD (Thy-1 [A30P] α-synuclein transgenic mouse model) in the context of telomere shortening using the Terc knockout mouse model. It has been found that α-synuclein transgenic mice with shortened telomeres (αSYN(tg/tg) G3Terc(-/-)) exhibited accelerated disease progression, resulting in significantly reduced survival.
This expedited phenotype in mice with truncated telomeres was marked by deteriorated motor performance and enhanced formation of α-synuclein aggregate formation [
71]. Quantification studies of mRNA expression and analysis of immunohistochemica revealed that in the late stages of the disease, brain stem microglia exhibited damaged responses in αSYN(tg/tg) G3Terc(-/-) microglial cell animals. These data offer initial experimental proof that telomere shortening accelerates the pathology of α-synuclein related to compromised microglial cell function in the brainstem [
71]. Extracellular alpha-synuclein (αsyn) oligomers have a important duty play in PD pathogenesis. Growing evidence demonstrates that these extracellular entities activate microglia, leading to heightened neuronal impairment [
72]. Despite the studies that are being carried out, little is known about the age impact on phagocytosis and microglial cell activation, particularly of extracellular αsyn oligomers. This study demonstrates that microglia isolated from adult mice, unlike those from young mice, exhibit phagocytosis deficiencies of free and exosome-associated αsyn oligomers along with increased TNFα secretion [
72]. Additionally, in this work it is described, a dysregulation of monocyte subpopulations in aging mice and humans. Human monocytes from elderly donors also displayed reduced phagocytic activity of extracellular αsyn. These results explain that these age-related alterations may contribute to enhances susceptibility to pathogens or abnormally aged folded proteins in neurodegenerative diseases [
72].
In summary, the involvement of aged microglia in the progressive nature of PD appears likely, especially considering the high density of microglia in the nigrostriatal system [
29], further bolstering the notion of microglial involvement in PD pathogenesis. However, the molecular and functional alterations of aged microglia are only partially understood, and their role in neurodegeneration and neuroinflammation in aged individuals requires further investigation in future studies.
7. Conclusions
Microglial senescence appears to underlie the transition of microglia from being neuroprotective in the young brain to neurotoxic in the aged brain. Microglial functions have been clearly affected by aging and activation states both in vitro and in vivo. Aging-related changes in microglial activation and neuroinflammation enhance their neurotoxicity and additionally, it seems that the distinctive nature of microglia contributes to their age-dependent functional impairment. However, it remains unclear whether aged microglia are responsible for exacerbating neurodegeneration in aged individuals or if aged neurons themselves are more susceptible to degenerative cues. The development of models for incorporating aged microglia into both in vitro and in vivo studies of neurodegenerative diseases and aging itself, has a significant potential impact in pioneering the reversal of aging effects in microglia. For example, successful reversal of the iron-overloaded phenotype could not only deepen the understanding of the aging process but also facilitate the development of potent brain anti-aging therapies, offering protection against debilitating neurodegenerative diseases in older individuals.
Overall, further analysis of the effect of aging on microglia is necessary to better understand the molecular mechanisms underlying age-related changes in microglial phenotypes and functions.
Furthermore, the onset, severity, and progression of neurodegenerative diseases such as AD and PD are influenced by aging and the aging-associated changes in microglial functions. The accumulation of senescent cells in the aging brain might create favorable proinflammatory conditions that promote the onset of AD alongside other risk factors. Subsequently, feedback loops between senescent cells and AD-related neuropathological features, such as Aβ plaques and Neurofibrillary tangles-accumulating neurons, could hasten neurodegeneration, worsening cognitive decline.
Aging is associated with shifts in the number of non-classical monocytes and diminished phagocytosis of both free and exosome-associated α-synuclein in PD. These changes in monocytes among older adults could potentially increase susceptibility to pathogens or misfolded proteins in aging humans. Further investigation is needed to fully understand the mechanisms underlying dysregulated microglial and myeloid cells during aging and their influence on the progression of neurocognitive disorders.
Notably, research into age-related diseases beyond the central nervous system increasingly indicates causal connections between cellular senescence and disease progression. These areas of study could provide a valuable framework for delving deeper into the roles of senescence in both healthy and pathological brain aging.
Targeting the regulation of microglial activation emerges as a promising therapeutic strategy, as inhibiting them can ameliorate some neurodegenerative changes. However, further research is necessary to comprehend the regulation of beneficial or deleterious microglial activation in disease progression. Rather than a broad inhibition of microglia, the therapeutic aim should focus on promoting their protective functions while mitigating their harmful effects.