Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Soybean Material and Chemicals
2.2. General Procedures for Preparing Soymilk
2.3. Specific Processing (Soaking and Grinding) Methods
2.4. Solvent Extraction of Phenolic Substances
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of Total Flavonoid Content (TFC)
2.7. Determination of Condensed Tannin Content (CTC)
2.8. Quantification of Major Soy Phenolic Acids
2.9. Quantification of Isoflavones by High Performance Liquid Chromatography (HPLC) Analysis
2.10. Chemical Antioxidant Assays
2.11. Anti-Proliferation Assays
2.12. Statistical Analysis
3. Results
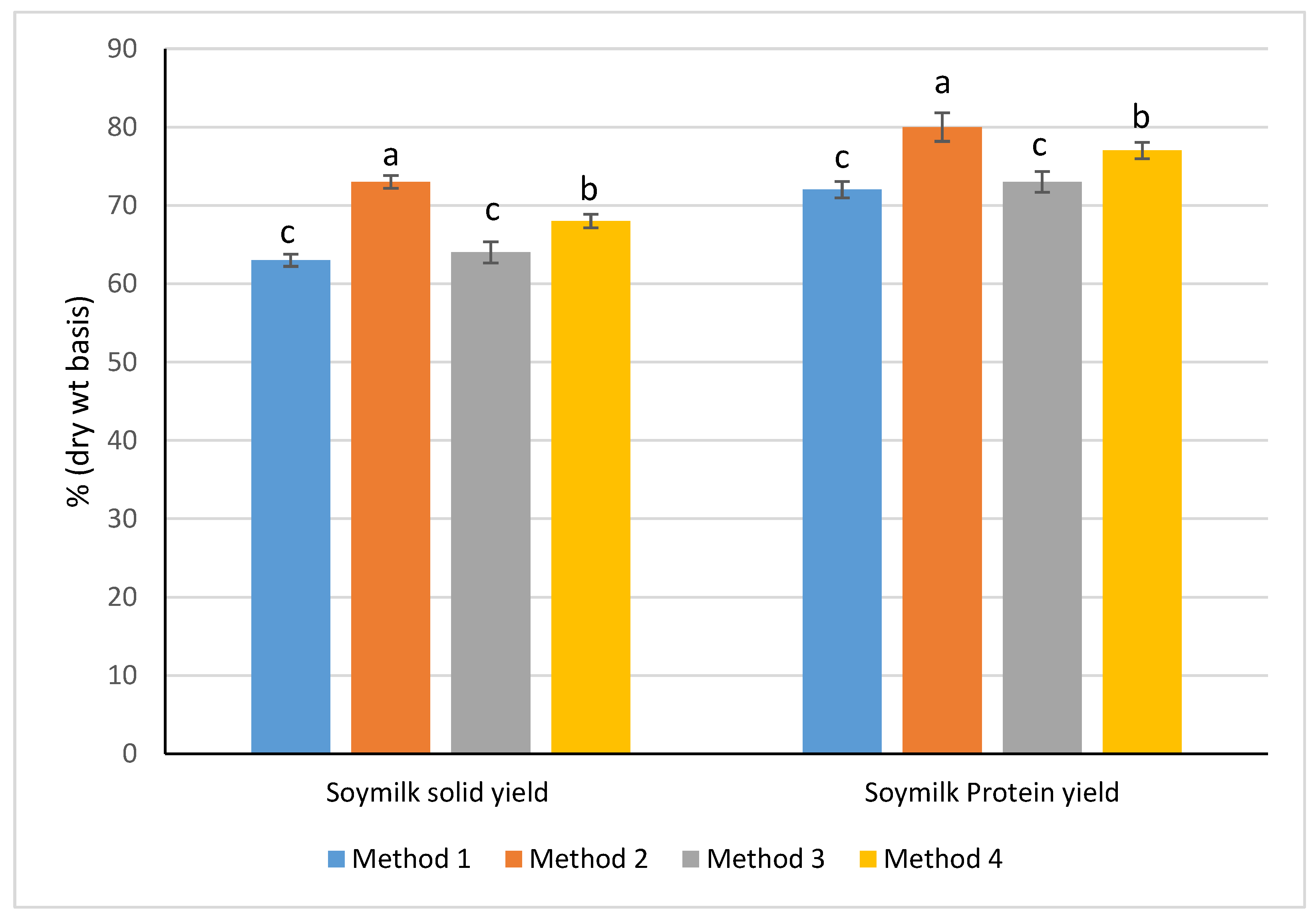
3.1. Yield of Soymilk and Recoveries of Solids and Protein from Soybean
3.2. Phenolic Processing Yield and Composition
3.2.1. Total phenolic content (TPC)
3.2.2. Total Flavonoid Content (TFC)
3.2.3. Condensed Tannin Content (CTC) Determination
3.2.4. Free Phenolic Acid Composition
3.3. Isoflavone Compositions
3.4. Antioxidant Activity Profiles
3.5. Anti-Proliferative Properties of Soymilk against Human Prostate Cancer Cell Line
4. Discussion
4.1. Processing Yields and Solids and Protein Recoveries
4.2. Phenolic Processing Yield and Composition
4.3. Isoflavone Composition
4.4. Antioxidant Activity Profile
4.5. Anti-DU145 Prostate Cancer Cell Proliferation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Chang, S.K. Science and technology of soymilk and tofu manufacturing. In Handbook of vegetable preservation and processing; CRC Press: Florida, United States, 2015; pp. 817–840. [Google Scholar]
- Nakai, S.; Fujita, M.; Kamei, Y. Health promotion effects of soy isoflavones. J. Nutr. Sci. Vitaminol. (Tokyo) 2020, 66, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-de-Almeida, C.A.; Ferraz, I.S.; Ued, F.d.V.; Almeida, A.C.F.; Ciampo, L.A.D. Impact of soy consumption on human health: Integrative review. Braz. J. Food Technol. 2020, 23, e2019129. [Google Scholar] [CrossRef]
- Douglas, C.C.; Johnson, S.A.; Arjmandi, B.H. Soy and its isoflavones: The truth behind the science in breast cancer. Anticancer Agents Med. Chem. 2013, 13, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Woo, J.; Chen, Y.M.; Wong, C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause 2014, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Raynal, N.J.; Momparler, L.; Charbonneau, M.; Momparler, R.L. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2008, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Blanco Mejia, S.; Chiavaroli, L.; Viguiliouk, E.; Li, S.S.; Kendall, C.W.C.; Vuksan, V.; Sievenpiper, J.L. Cumulative meta-analysis of the soy effect over time. J. Am. Heart Assoc. 2019, 8, e012458. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S. The dilemma with the soy protein health claim. J. Am. Heart Assoc. 2019, 8, e013202. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Characterization of phenolic substances and antioxidant properties of food soybeans grown in the North Dakota-Minnesota region. J. Agric. Food Chem. 2008, 56, 9102–9113. [Google Scholar] [CrossRef] [PubMed]
- Poysa, V.; Woodrow, L. Stability of soybean seed composition and its effect on soymilk and tofu yield and quality. Food Res. Int. 2002, 35, 337–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K.; Liu, Z. Isoflavone profile in soymilk as affected by soybean variety, grinding, and heat-processing methods. J. Food Sci. 2015, 80, C983–C988. [Google Scholar] [CrossRef]
- Li, S.Z. (Ming Dynasty). Soybean. In ‘Ben Cao Gang Mu’, 1’st ed.; People's Health Publishing House: Beijing, China, 1999; Volume 2, pp. 1344–1350. [Google Scholar]
- U.S. Cancer Statistics Working Group. US Cancer statistics data visualizations tool, based on 2022 submission data (1999-2020): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Released in November 2023. Accessed on February 14, 2024. Available online: https://www.cdc.gov/cancer/dataviz.
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients. 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Kumi-Diaka, J.; Merchant, K.; Haces, A.; Hormann, V.; Johnson, M. Genistein-selenium combination induces growth arrest in prostate cancer cells. J. Med. Food 2010, 13, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Yun, H.; Lee, E.B.; Min, B.I.; Bae, H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D.J. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo 2010, 24, 393–400. [Google Scholar]
- Allred, C.D.; Twaddle, N.C.; Allred, K.F.; Goeppinger, T.S.; Churchwell, M.I.; Ju, Y.H.; Helferich, W.G.; Doerge, D.R. Soy processing affects metabolism and disposition of dietary isoflavones in ovariectomized BALB/c mice. J. Agric. Food Chem. 2005, 53, 8542–8550. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, B.H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Kim, H.A.; Jeong, K.S.; Kim, Y.K. Soy extract is more potent than genistein on tumor growth inhibition. Anticancer Res. 2008, 28, 2837–2841. [Google Scholar]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp. Biol. Med. (Maywood) 2010, 235, 90–97. [Google Scholar] [CrossRef]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of processing technologies on isoflavones, phenolic acids, and antioxidant capacities of soymilk prepared from 15 soybean varieties. Food Chem. 2021, 345, 128612. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.; Liu, Z.; Xu, B. Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J. Agric. Food Chem. 2008, 56, 7957–7963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, S.K.C. Trypsin inhibitor activity, phenolic content and antioxidant capacity of soymilk as affected by grinding temperatures, heating methods and soybean varieties. LWT 2022, 153, 112424. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.; Zhang, Y. Innovative soaking and grinding methods and cooking affect the retention of isoflavones, antioxidant and antiproliferative properties in soymilk prepared from black soybean. J. Food Sci. 2016, 81, H1016–H1023. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Isoflavones, Flavan-3-ols, phenolic acids, total phenolic profiles, and antioxidant capacities of soy milk as affected by ultrahigh-temperature and traditional processing methods. J. Agric. Food Chem. 2009, 57, 4706–4717. [Google Scholar] [CrossRef] [PubMed]
- Helms, T.C.; Nelson, B.D.; Goos, R.J.; Chang, K.C. Registration of 'ProSoy' soybean. Crop Sci. 2006, 46, 470–471. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Vicentini, F.T.; Yokoyama, C.Y.; Borin, M.F.; Spadaro, A.C.; Fonseca, M.J. Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different beta-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Polar-Cabrera, K.; Watkins, C.B. Fruit quality, antioxidant contents and activity, and antiproliferative activity of strawberry fruit stored in elevated CO2 atmospheres. J. Food Sci. 2008, 73, S339–S344. [Google Scholar] [CrossRef]
- Hou, H.J.; Chang, K.C. Interconversions of isoflavones in soybeans as affected by storage. J. Food Sci. 2002, 67, 2083–2089. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kong, F.; Chang, S.K.; Liu, Z.; Wilson, L.A. Changes of soybean quality during storage as related to soymilk and tofu making. J. Food Sci. 2008, 73, S134–S144. [Google Scholar] [CrossRef]
- Slavin, M.; Lu, Y.; Kaplan, N.; Yu, L.L. Effects of baking on cyanidin-3-glucoside content and antioxidant properties of black and yellow soybean crackers. Food Chem. 2013, 141, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Boye, J. Protein-polyphenol interactions in fruit juices. Recent research developments in agricultural & food chemistry (Vol 3. Part I), 1999; 85–107. [Google Scholar]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar]
- Yu, L.; Blackburn, G.L.; Zhou, J.R. Genistein and daidzein downregulate prostate androgen-regulated transcript-1 (PART-1) gene expression induced by dihydrotestosterone in human prostate LNCaP cancer cells. J. Nutr. 2003, 133, 389–392. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M.T.; Franceschi, S.; Lerici, C.R. Loss and/or formation of antioxidants during food processing and storage. Cancer Lett. 1997, 114, 71–74. [Google Scholar] [CrossRef]
- Manzocco, L.; Anese, M.; Nicoli, M.C. Antioxidant properties of tea extracts as affected by processing. LWT - Food Sci. Technol. 1998, 31, 694–698. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.; Liu, Z.; Yuan, S.; Zou, Y.; Tan, Y. Comparative studies on the chemical and cell-based antioxidant activities and antitumor cell proliferation properties of soy milk manufactured by conventional and commercial UHT methods. J. Agric. Food Chem. 2010, 58, 3558–3566. [Google Scholar] [CrossRef]
| TPC values (mg of GAE/g ) | |||
| Grinding | Raw soymilk | Cooked soymilk | Okara |
| Method 1 | 1.94±0.04cA | 1.68±0.14bB | 1.09±0.17aC |
| Method 2 | 2.09±0.03bcA | 1.74±0.04bB | 0.97±0.03aC |
| Method 3 | 2.11±0.04bA | 1.84±0.09abA | 1.02±0.22aC |
| Method 4 | 2.61±0.11aA | 2.17±0.25aB | 1.30±0.03aC |
| Grinding | Raw | Cooked | Okara | Total | Loss |
| Method 1 | 43.47±1.32cA | 37.14±3.39bB | 12.49±1.67aC | 55.95±2.86b | 44.05±2.86b |
| Method 2 | 54.03±1.20bA | 44.45±1.21abB | 10.90±0.67aC | 64.94±1.62ab | 35.06±1.62ab |
| Method 3 | 48.06±1.92cA | 41.63±1.16bB | 11.45±2.46aC | 59.51±3.15ab | 40.49±3.15ab |
| Method 4 | 62.90±2.81aA | 52.19±6.86aB | 12.47±0.48aC | 75.37±2.56a | 24.63±2.56a |
| TFC values (mg of CAE/g) | |||
| Grinding | Raw | Cooked | Okara |
| Method 1 | 0.25±0.03bC | 0.38±0.03aB | 0.63±0.06bA |
| Method 2 | 0.35±0.03bB | 0.42±0.04aB | 0.65±0.10bA |
| Method 3 Method 4 |
0.26±0.05bB 0.37±0.04aB |
0.38±0.03aB 0.43±0.03aB |
0.76±0.10bA 0.89±0.05aA |
| CTC values (mg of CE/g) | |||
| Grinding | Raw | Cooked | Okara |
| Method 1 | 0.21±0.02dBA | 0.14±0.02bB | 0.46±0.05cA |
| Method 2 | 0.37±0.05bC | 0.27±0.03aB | 0.49±0.01cA |
| Method 3 Method 4 |
0.29±0.05cC 0.48±0.02aC |
0.13±0.01bB 0.32±0.03aB |
0.60±0.03bA 0.70±0.07aA |
| GA | VA | CA | CLA | p-HBA | Total | |
| Raw | ||||||
| M1 | 29.5±1.4b | 17.3±1.6ab | 1.5±0.1a | 35.9±4.0a | nd | 84.2±7.0b |
| M2 | 31.4±1.5b | 15.7±1.1ab | nd | 37.4±3.4a | nd | 84.5±6.0b |
| M3 | 37.1±1.2a | 20.2±1.3a | 1.3±0.1a | 42.9±5.5a | nd | 101.4±8.2a |
| M4 | 41.1±0.9a | 14.0±1.2b | 1.4±0.2a | 48.5±4.3a | 1.5±0.2 | 106.5±6.8a |
| Cooked | ||||||
| M1 | 22.0±2.0b | 12.7±0.6b | 0.9±0.1c | nd | nd | 35.7±2.7b |
| M2 | 23.3±1.1b | 12.3±1.3b | nd | nd | nd | 35.6±2.3b |
| M3 | 29.4±1.5a | 21.8±1.3a | 2.5±0.1a | nd | 2.1±0.1 | 55.8±3.0a |
| M4 | 30.4±1.1a | 24.6±1.3a | 1.4±0.1b | nd | nd | 56.4±2.5a |
| Okara | ||||||
| M1 | 18.0±0.9a | nd | nd | nd | nd | 18.0±0.9a |
| M2 | 17.2±1.3a | nd | nd | nd | nd | 17.2±1.3a |
| M3 | 16.9±1.3a | nd | nd | nd | nd | 16.9±1.3a |
| M4 | 13.5±1.4b | nd | nd | nd | nd | 13.5±1.4b |
| Din | Gly | Gin | MDin | MGly | MGin | MGly | Dein | Gein | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | ||||||||||
| M1 | 274.7± 2.3c | 43.9± 4.1a | 314.7±17.1b | 625.4± 5.1d | 113.5±7.7a | 1743.4± 49.5a | 119.5± 2.2b | 49.7± 4.9a | 62.0± 2.5a | 3346.8±90.3b |
| M2 | 260.5± 1.9d | 42.8± 1.1a | 317.0± 3.6b | 790.3± 52.8c | 110.7±0.2a | 1705.9± 33.0a | 134.9± 6.7b | 53.6± 4.6a | 65.0± 3.3a | 3480.9±34.1b |
| M3 | 289.1± 3.8b | 48.3± 3.9a | 346.9± 6.9ab | 974.8± 3.7ab | 128.3±1.2a | 1818.8± 16.5a | 130.3± 8.8b | 57.3± 0.6a | 63.7± 7.1a | 3857.6±37.4a |
| M4 | 333.1± 1.9a | 48.9± 0.5a | 372.3± 15.5a | 985.9± 22.0a | 134.5±13.0a | 1812.5± 16.5a | 180.8± 5.3a | 62.9± 4.3a | 67.8± 5.9a | 3998.8±84.9a |
| Cooked | ||||||||||
| M1 | 540.5± 5.7b | 69.6± 6.9a | 648.5± 12.1b | 653.3± 22.0b | 111.9±5.0a | 1351.3± 18.1ab | 157.2± 4.8a | 39.9± 2.6b | 52.3± 2.2a | 3624.7±65.4b |
| M2 | 572.6± 24.5ab | 62.7± 6.9a | 636.5± 17.5b | 677.6± 22.4ab | 108.7±2.5a | 1337.9± 19.8b | 162.0± 5.0a | 44.7± 4.3ab | 58.4± 3.3a | 3666.1±42.7b |
| M3 | 610.0± 20.0ab | 79.2± 1.4a | 672.0± 9.0ab | 715.9± 21.2ab | 120.8±5.0a | 1384.2± 19.7ab | 173.3± 7.0a | 50.0± 3.9ab | 60.7± 2.2a | 3866.0±29.5a |
| M4 | 641.9± 12.6a | 78.1± 5.7a | 716.0± 3.1a | 754.5± 4.4a | 123.6±2.5a | 1429.4± 28.0a | 183.9±10.1a | 56.6± 2.9a | 58.5± 7.7a | 4042.5±46.7a |
| Okara | ||||||||||
| M1 | 49.7± 0.6b | 22.1± 1.2a | 103.7± 5.9a | 353.1± 22.0b | 62.5±2.5a | 749.8± 18.5a | 67.2± 6.6a | 124.3± 5.7a | 172.2± 12.0a | 1704.6±71.9a |
| M2 | 73.9± 3.7a | 24.6± 1.2a | 103.6± 3.1a | 352.8± 20.2b | 58.6±3.5a | 661.4± 16.5b | 59.1± 5.3a | 106.9± 5.0ab | 152.3± 4.3ab | 1593.4±12.1a |
| M3 | 71.7± 3.8a | 25.1± 2.5a | 112.7± 5.8a | 338.1± 12.0b | 59.3±1.5a | 714.3± 11.4ab | 62.6± 3.2a | 105.2± 1.4ab | 138.2± 7.6b | 1627.6±14.9a |
| M4 | 76.5±3.8a | 22.0± 2.2a | 78.5± 6.6b | 239.1± 19.8 | 47.8±0.7b | 477.3± 26.4c | 36.9± 5.4b | 101.5± 7.1b | 149.2± 4.3ab | 1229.1±39.5b |
| ORAC values (µmol TE/g) | |||||||||
| Grinding | Raw | Cooked | Okara | ||||||
| Method 1 | 71.49±4.26bB | 89.89±1.68cA | 67.50±6.13aB | ||||||
| Method 2 | 75.36±3.92abC | 101.80±4.67bcB | 59.77±2.14aA | ||||||
| Method 3 | 88.25±7.96aC | 109.65±9.54bB | 56.40±3.31aA | ||||||
| Method 4 | 86.45±6.20abC | 132.56±4.26aB | 59.94±5.00aA | ||||||
| FRAP Values (mmol Fe2+ equivalents/100 g) | |||||||||
| Grinding | Raw | Cooked | Okara | ||||||
| Method 1 | 0.92±0.02dC | 1.08±0.04bB | 0.78±0.03aA | ||||||
| Method 2 | 1.07±0.04cB | 1.10±0.03bB | 0.79±0.02aA | ||||||
| Method 3 Method 4 |
1.01±0.01bC 1.13±0.04aC |
1.25±0.06aB 1.30±0.09aB |
0.77±0.04aA 0.71±0.03aA |
||||||
| DPPH Assay (µmol TE/g) | |||||||||
| Grinding | Raw | Cooked | Okara | ||||||
| Method 1 | 0.58±0.09cB | 1.13±0.04cA | 0.43±0.09bB | ||||||
| Method 2 | 0.83±0.03bC | 1.78±0.07baB | 0.37±0.04bA | ||||||
| Method 3 Method 4 |
0.77±0.08bB 1.45±0.12aC |
1.57±0.05bA 1.96±0.21aB |
0.73±0.09aB 0.47±0.07bA |
||||||
| IC50 values (mg/mL) of MTT Assay | |||
| Grinding | Raw | Cooked | Okara |
| Method 1 | 8.5±0.7cA | 8.1±0.6bA | 9.6±0.7aA |
| Method 2 | 7.7±0.7bcA | 7.7±0.9bA | 8.4±0.3aA |
| Method 3 Method 4 |
7.0±0.6bB 4.9±0.2aB |
10.1±0.6aA 6.8±0.1bA |
9.5±1.2aA 9.4±0.7aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




