1. Introduction
The general decline of pollinating insects over the last past three decades as the results of human activities [
1] is of particular concern to naturalists, conservation biologists, and agroeconomists [
2,
3,
4,
5]. Indeed, the decrease in the abundance and diversity of pollinating insects has direct and negative effects agricultural production [
6,
7] as about 90% of flowering plant species depend upon animal pollination [
8,
9]. Pollinating insects also contribute to maintain high levels of floral and animal diversity within both natural and anthropized ecosystems [
10,
11].
Various factors can contribute to the decline of pollinating insects such as agricultural practices [
12], invasive species [
13], pollution [
14] and climate change [
15,
16]. However, pollinating insects are particularly vulnerable to change in land use [
17], driven by intensive agriculture and urbanization. Ample evidence shows that agricultural intensification negatively affects both pollinator diversity and pollination services [
18,
19]. Similarly, urbanization generally reduces pollinator diversity [
20,
21,
22], although to a lesser extent than intensive agriculture [
23]. Given the rapid increase in global food demand [
24], the expected development of urbanization in the future [
25], and their projected impacts on land use and natural environments [
26], pollinating insects may decline even more strongly in the years to come.
In this context, an important question is to what extent pollinating insects can benefit from protected areas (PAs). There is compelling evidence that PAs directly contribute to the conservation of various species [
27,
28,
29,
30,
31]. However, most protected areas have been designed to protect charismatic species or landscapes, and might be less efficient for the conservation of little conspicuous or poorly known species such as insects [
32,
33]. Assessing to what extent insects benefit from PAs remains difficult to assess as there has been so far limited research and monitoring programs comparing insect assemblages within and outside of protected areas, and the few existing studies have focused on specific orders or families. For instance, several beetle species of conservation concern are not represented in protected areas in Italy [
34]. Similarly, about one quarter of orthopteran species are not present in protected sites in Greece [
35]. On the other hand, PAs appear to contribute efficiently to the conservation of dragonfly species of conservation interest [
36,
37]. Similar, albeit limited, evidence exists for pollinating insects. Butterfly species richness was found to be highest in PAs in Germany, but was declining at the same rate than in non-PAs over an 11-year period [
38]. In Mexico, 66% of endemic Erebidae moth species are not present in PAs [
39]. In Serbia, one of the main hotspots of hoverfly (Syrphidae) diversity in Europe, PAs are only partly efficient in the conservation of this family of pollinating insects [
40]. A recent study [
41] assessing insect pollinator diversity in protected vs. unprotected areas in a hotspot of biodiversity in Nigeria found that pollinator diversity varied more in relation to land-use type rather than area protection per se.
Assessing to what extent PAs effectively protect insect pollinators is further complicated by lack of knowledge about the existing diversity and geographical distribution of pollinator insect species, particularly in tropical regions [
42,
43]. Whereas the large majority of insect species is located in the tropics [
44], biodiversity research is mainly conducted on vertebrates in temperate regions [
45]. Several factors may contribute to this situation. First, scientists from developing countries tend to have reduced access to funding, organizational support and logistics for field work activities, training and expertise in entomology, and biological collections [
46,
47,
48,
49]. Second, invertebrate taxonomy appears to remain poorly appealing to young scientists and scientific institutions [
50], particularly in in the Southern Hemisphere [
51,
52].
The situation is particularly exacerbated in Haiti, which is centrally located in the Caribbean hotspot of biodiversity. This is mainly due to extreme poverty [
53], ongoing environmental degradation and growing urbanization [
54,
55,
56], weak institutions and public governance [
57], lack of public funding for research and higher education [
58], and very limited expertise in conservation biology [
48]. Several protected areas have however been created in Haiti, accounting for about 6.75% of land territory and et 4.72% of maritime territory (
https://www.biodiversite.ht/2020/08/03/aires-protegees/). Their location and boundaries have been essentially established from the known or supposed distribution of vertebrate endemics of conservation concern [
59], and little if any information is available on their relevance to the conservation of insects, including pollinating species. Still, the Haitian government and foreign institutions plan on agricultural development to meet increasing basic food needs associated with human population growth [
60]. Given the high dependence of major crops on pollination by insects [
61], improving knowledge on the conservation status of major groups of insect pollinators in Haiti thus appears to be primordial. More specifically, given the overall level of environmental degradation [
62], the effectiveness of Haitian PAs for the conservation of insect pollinators deserves particular attention.
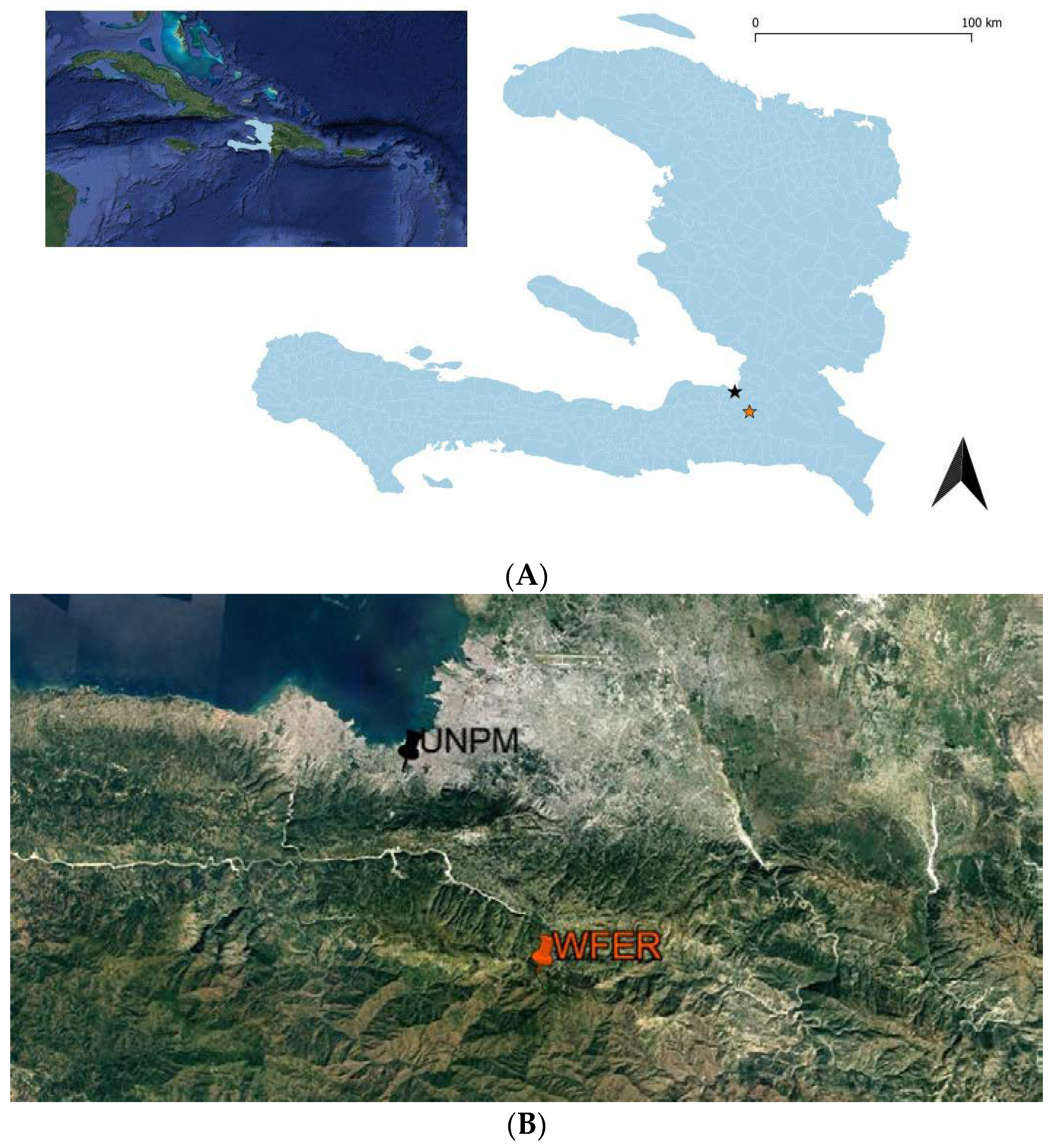
Here we report results from a study comparing assemblage composition and relative abundance of pollinating insects between two different protected areas in Haiti, the Wynne Farm Ecological Reserve (hereafter WFER) and the Urban National Park of Martissant (hereafter UNPM). WFER is situated on the heights of the capital city of Port-au-Prince, at a distance of about 20km. By contrast, UNPM is located in the middle of Port-au-Prince, with surrounding densely populated and built-up neighborhood, one of the poorest in the city. We relied on the use of colored pan traps to collect pollinating insects [
63,
64], and subsequently assessed the influence of site, habitat type, and their interaction on the relative abundance and assemblage composition of insect pollinators. Based on observed differences in both the number and diversity of pollinating insects between rural and urban areas e.g. [
65,
66,
67], we expected higher diversity and abundance of pollinating insects at WFER compared to UNPM, as well as differences in order and family composition between the two sites.
2. Materials and Methods
The study was carried out between January 2018 and April 2018 at two different and contrasted protected areas in the metropolitan area of Port-au-Prince, Western Haiti (
Figure 1).
Created by Victor A. Wynne in 1956, the WFER is located in the suburban area of Kenscoff (18° 26'35.70''N, 72° 17'39.45''W). It covers an area of 15.4 hectares, at an elevation of 1800 meters, in the heart of a 400-hectare area, which was declared as a protected area in 2020 based on its importance for the protection and conservation of the biodiversity of the Kenscoff region. WFER is a private reserve, open to the general public since 1992. Its management is entrusted to the Wynne family together with a team on site, including agronomists and farmers. Its main mission is to raise awareness about environmental issues and sustainable agriculture.
The UNPM is located in the densely populated capital of Port-au-Prince (18° 31'32.66''N, 72° 21'45.66''W), and covers an area of 17 hectares at an elevation of 58 meters above sea level. Also known as "Habitation Leclerc", it was declared of public utility in 2007. Ten years later, it was declared as a protected area, in recognition of its role in environmental awareness in the urban environment of Port-au-Prince and its large wooded area which serves as a refuge for many animal species. It is still today the first and only urban national park in Haiti. Its management is entrusted to the “Fondasyon Konesans ak Libète” (FOKAL). UNPM, includes, among other things, medicinal herb gardens, a library, a cultural center and play areas for children.
According to the IUCN classification [
68], WFER falls into two categories, taking into account the strict use of its space: Category I-a as an ecological reserve and Category II as a naturel park, where human visitation, use and impacts are strictly controlled and limited to ensure protection of conservation values. UNPM strictly belongs to category II as a national park, as it protects the ecological processes and the characteristics of the ecosystems of its region. Both sites are characterized by a mosaic of green spaces, including forest patches, agroforestry and agricultural crops. At WFER, the forest habitat is dominated by
Pinus occidentalis L.,
Eucalyptus spp.,
Ligustrum lucidum L.,
Eryobotrya japonica L.,
Acacia mearnsii L.,
Acacia melanoxylon L.,
Persea americana L., while the agricultural habitat is dominated by crops such as
Beta vulgaris L.,
Passiflora ligularis,
Ipomoea batatas L.,
Pepino dulce,
Rubus spp.. The agroforestry habitat essentially consists of
Pinus occidentalis and
Acacia melanoxylon L. associated with the same agricultural crops. At UNPM, the forest habitat is dominated by
Hura crepitans L.,
Swietenia mahogony L.,
Delonix regia Raf, and
Azadirachta indica, while the agricultural habitat corresponds to various crops such as
Zea mays L.,
Cajanus cajan,
Ipomoea batatas L.,
Dioscorea spp.,
Musa spp.,
Phaseolus spp.,
araceae spp. The agroforestry habitat is largely domination by
Swietenia mahogony L. in association with the crops.
Pollinating insects were sampled with a passive collection method using colored “pan traps”. We used three sets of colored pan traps at each site. Each set consisted of three plastic, non-fluorescent pan traps (22 cm diameter) of three different colors: blue, white, yellow. The combination of three colors is particularly efficient for sampling a large diversity of pollinating insects [
63,
64,
69,
70]. We placed one set of pan traps in each habitat type (agricultural, agroforestry and forest) at each site (WFER and UNPM) on six consecutive sessions (14 and 11 days of average time space, respectively, WFER and UNPM). Trap arrangement and sampling effort were therefore the same for each habitat type in each site. The traps were set 1 meter above the ground using a support, and filled with 300 ml of soapy water. They remained active for 24 hours. Then after, we collect all trapped insects using a sieve (size: 0.1 mm) and stored them in 70% ethanol. We then identified specimens in the laboratory using a stereo binocular microscope (Bresser Advance ICD 10-160x). In the absence of reference collections and detailed identification keys for the Haitian entomofauna, the evaluation of the diversity of insect assemblages at the family or species level was relatively difficult [
71]. We therefore identified collected insects morphologically to the order or lowest taxonomic categories, from available references [
72,
73,
74,
75], photographs, and identification guides [
76]. Because of damaged insects and limited taxonomic expertise, we were unable to identify all specimens at the family level with enough confidence. However, we managed to identified some specimens of conservation interest at the species level from their conspicuous and distinctive characteristics. After examination, all specimens collected from both sites were maintained in 70% ethanol and stored at WFER to allow more detailed investigations in the future.
The frequency distribution of the number of collected insects per replicate did not follow a normal distribution (Shapiro-Wilk test,
W = 0.80,
P < 0.0001). However, log-transformation of data resulted in a normal distribution (
W = 0.95,
P = 0.1019; UNPM only:
W = 0.96,
P = 0.0963; WFER only:
W = 0.98,
P = 0.1117). We therefore relied on a one-way ANOVA to first assess the overall influence of pan trap colour on the number of insects collected per replicate. We then relied on model selection to assess the influence of site, habitat type, pan trap colour and their interactions on the number of collected insects per replicate using GLMs. We used Akaike's information criterion to rank models, and models with AIC
c < 2 were considered to be equally informative [
77]. To further check complementarity in the use of different pan trap colors, we examined the overall proportions of each insect order between the three pan trap colors using a Chi-square test. We then assessed pan-trap color dominance,
D (as a measure of how much one or few orders dominate the composition) as the proportional abundance of the three more abundant orders [
78], with
D = (
p1 +
p2 +
p3) x 100, where
pj is the proportion of j
th most abundant order.
After pooling data from the six replicates, we first relied on nominal logistic regression to assess the influence of site, habitat type and their interaction on order composition. As the site*habitat interaction was significant (
see results), we compared the frequency distribution of the four more abundant orders among habitats between sites using chi-square tests. Then, following recommendations from [
79], we measured relative abundance-based similarities in order composition between sites, between habitats within sites and between sites for each habitat, using the Renkonen index [
80], with
Sp = Σ min (
p1i,
p2i), where
pji the proportion of species
i in sample
j =
nji/
Nj. Following [
81], we applied a logarithm transformation to order frequencies to reduce the dominance of the most abundant orders. The index thus measures the extent to which two samples are alike in composition, and ranges from zero for samples with no orders in common to 1 for identical samples.
As only a fraction of specimens could be identified at the family level (see results), we restricted additional analyses to the comparison of the total number of families observed at each site and in each site/habitat combination. We first drew a Venn diagram to visualize the overall overlap in family distribution between pan-trap colours. Because both the total number of identified families and the total number of insects collected in pan traps differed between the two sites and between habitats within sites (see results), we relied on a simple index of proportional dominance to establish comparisons between sites and among habitats. The index was equal to the ratio between the minimum number of families accounting for 50% of the total number of collected individuals and the total number of observed families. We then compared the obtained proportions using Chi-square tests.
We performed all statistical tests using JMP 10 (SAS Institute Inc., Cary, NC, USA) and considered results to be significant at the 0.05 level.
3. Results
Overall, we collected 3722 insects belonging to six different Orders (Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera and Thysanoptera;
Table 1) during the course of the study, out of which 1212 were sampled at UNPM and 2510 at WFER.
Table 2 shows results from the six best models testing for the influence of site, habitat, pan-trap colour, and their triple and double interactions on the number of insect collected per replicate.
The best model retained only the effects of site, pan-trap colour, and the interaction between site and habitat. The mean number of insects collected per replicate at WFER was more than twice that at UNPM (back-transformed mean value and 95% confidence interval, WFER = 109.4, [75.8-157.9] and UNPM = 55,1 [40.3-74.9]). It also differed between the three pan trap colors (back-transformed mean and 95% confidence interval, Blue: 13.0 [9.0-18.8], White: 23.5 [17.6-31.5], Yellow: 32.4 [23.5-44.8]; One way ANOVA, F2,105 = 8.14, P = 0.0005). Finally, the number of collected insect par replicate differed significantly between habitats at UNPM (F2,51 = 16.97, P < 0.0001), whereas the difference was not quite significant at WFER (F2,51 = 3.00, P = 0.0588). Post-hoc Tukey HSD tests showed that the difference at UNPM was essentially due to a higher number of insects collected per replicate in the forest habitat compared to the two other habitats (P < 0.0001 for both comparisons), whereas there was no significant difference between the agroforest and agricultural habitats (P = 0.9383).
Individuals from all six orders were collected in pan traps of each color, although their overall relative proportions differed, as expected, between colors (Chi-square test, X² = 170.22, d.f. = 10, P < 0.0001). Three orders (Diptera, Hemiptera and Hymenoptera) accounted for 77.7% of the total number of collected insects. Order dominance, D, based on the three most abundant orders was 85.4%, 75.3% and 71.6% for the white, yellow and blue colors, respectively.
3.1. Analysis of Assemblage Composition at the Order Level
Insects from the six orders were collected at each site, but not systematically in each habitat (
Table 1). The effects of site (nominal logistic regression,
X² = 325.59, d.f. = 5,
P < 0.0001), habitat (
X² = 369.81, d.f. = 10,
P < 0.0001) and their interaction (
X² = 39.10, d.f. = 10,
P < 0.0001) on order composition were all significant. In addition, the relative abundance of insects among habitats varied between the two sites for the four most abundant and ubiquitous orders (Chi-square test, Diptera:
X² = 261,16, d.f. = 2,
P < 0.0001; Hemiptera:
X² = 261,16, d.f. = 2,
P < 0.0001; Hymenoptera:
X² = 32.71, d.f. = 2,
P < 0.0001; Lepidoptera:
X² = 63.02, d.f. = 2,
P < 0.0001). Differences in order composition were to some extent reflected in the obtained values for the Renkonen index of similarity (
Table 3).
Overall similarity in order composition between the two sites (
Sp = 0.856) was relatively high. However, similarity in order composition between the two sites was higher for both the forest (
Sp = 0.851) and agroforest habitats (
Sp = 0.828) than for the agricultural one (
Sp = 0.753). In addition, similarity in order composition between pairs of habitats differed between the two sites (
Table 3).
3.2. Analysis of Assemblage Composition at the Family Level
Overall, we identified 2538 specimens at the family level (852 from UNPM and 1686 from WFER), accounting to 68.2% of the total number of collected insects. The percentage of identified specimens did not differ between the two sites (UNPM: 70.3%, WFER: 67.2%,
X² = 0.69, d.f. = 1,
P = 0.4055). However, the proportion of specimens identified at the family level differed significantly between orders (
Table 4; logistic nominal regression,
X² = 403,17, d.f. = 5,
P < 0.0001).
Overall, we identified 118 different families (
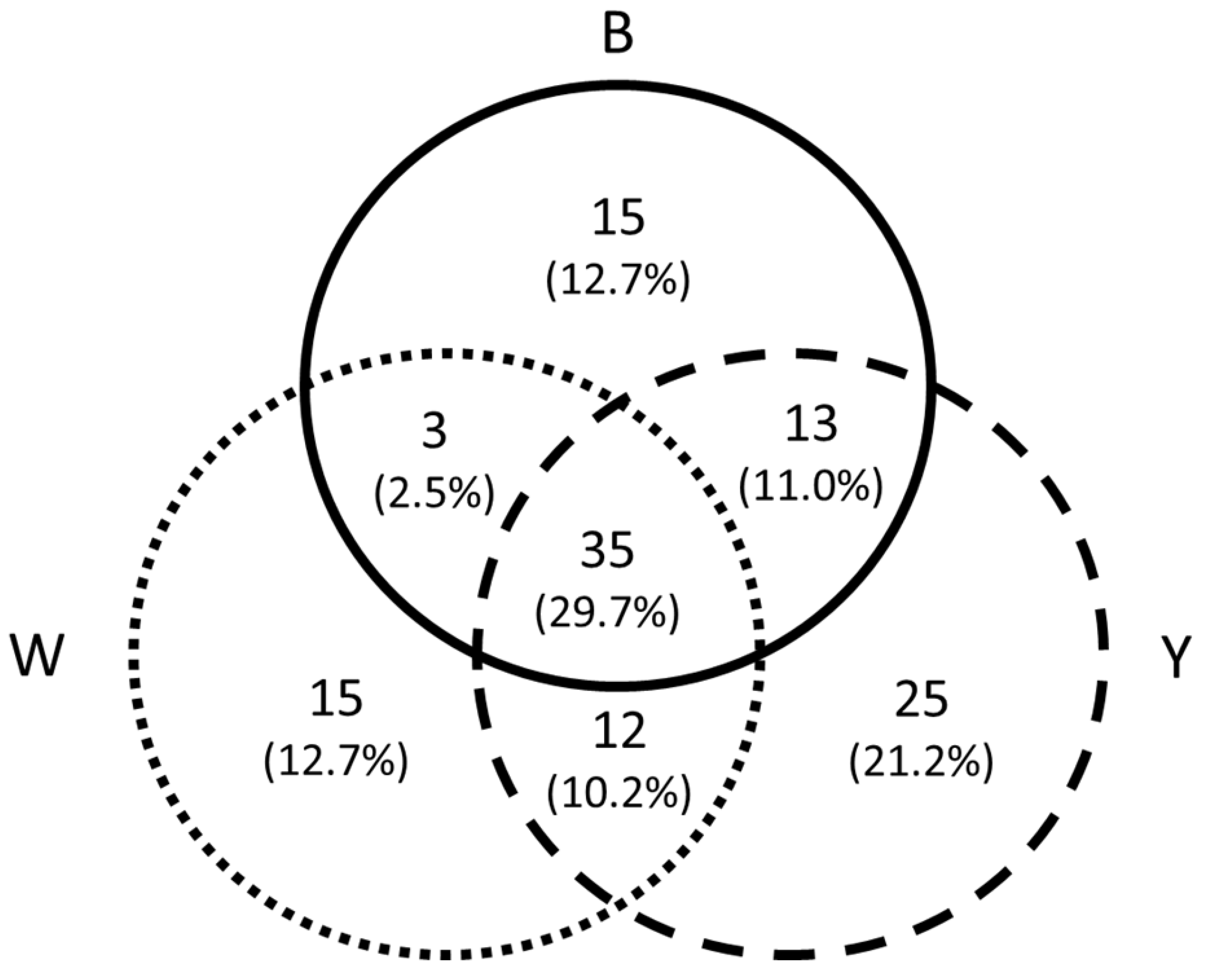
Table 4), out of which 36 (30.51%) were observed at both sites, 20 (16.95%) were observed only at UNPM, and 62 (52.54%) were observed only at WFER. Overall, 46.6% of all identified families were captured in only a single colour trap, whereas about 30% of them were captured in the three colored pan-traps (
Figure 2).
Families observed at both sites were significantly more abundant at both UNPM (one-way analysis of variance on log-transformed data, F1,54 = 11.63, P = 0.0012) and WFER (F1,96 = 13.26, P = 0.0004). However, their abundance was not significantly correlated between the two sites (Pearson correlation coefficient, r² = 0.03, n = 36, P = 0.3063). The ratio of the number of observed families to total sample size differed significantly between the three habitat types at UNPM (Chi-square test, X² = 6.58, d.f. = 2, P = 0.0372), whereas no such difference was found for WFER (X² = 3.98, d.f. = 2, P = 0.1368). The minimum number of families accounting for 50% of all captured insect was 7 (12.5% of all identified families) and 13 (12.3%) at UNPM and WFER, respectively, with no significant difference in proportional dominance between the two sites (X² = 0.02, P = 0.8919). Similarly, proportional dominance did not differ between habitats within each site (UNPM: X² = 0.76, d.f. = 2, P = 0.6827; WFER: X² = 0.28, d.f. = 2, P = 0.8679), nor between sites for each habitat (Agroforestry: X² = 0.06, d.f. = 1, P = 0.8020; Agriculture: X² = 0.24, d.f. = 1, P = 0.6246; Forest: X² = 0.65, d.f. = 1, P = 0.4194).
3.3. Identified Species of Patrimonial Interest
Although we could not identify all collected specimens to genus or species level, we captured in pan traps several species of patrimonial interest during the course of our study, particularly Lepidopterans and Hymenopterans endemic to Hispaniola.
The endemic lepidopteran
Calisto thomasi [
82] was present at WFER in both the agroforest habitat (four and two specimens collected in the yellow and white pan traps, respectively) and the agriculture habitat (three and one specimens collected in the yellow and white pan traps, respectively). A second endemic lepidopteran,
Pyrisitia pyro (formerly called
Eurema pyro) [
83], was only found in the agroforest habitat at WFER (four and one specimens collected in the yellow and white pan traps, respectively). Another Nymphalidae, the near-threatened
Anetia jaegeri [
84], endemic from Hispaniola and Jamaica [
85], was collected and observed at WFER in the agroforest habitat (three and two individuals in the yellow and white traps, respectively). The least concern Prickly ash Swallowtail,
Papilio pelaus, endemic from the Greater Antilles, was observed in the forest habitat at both WFER (two and one individuals in the blue and white traps, respectively) and UNPM (one individual in the blue trap). Finally, the recently identified Arctiinae species,
Cosmosoma odilae [
86] was observed at WFER in the agriculture habitat (one individual in the yellow trap).
Two endemic hymenopterans, the mason wasps
Odynerus haitiensis [
87] and
Omicron lacerum [
88], were collected during the course of the study. The first species was observed in the agroforest habitat at both WFER (five and two individuals in the yellow and white traps, respectively) and UNPM (one and two individuals in the yellow and white traps, respectively). The second species was only found in the agroforest habitat at WFER (four individuals in the yellow pan trap).
4. Discussion
Although we were not able to provide detailed taxonomic identification for all collected specimens, the present study adds to our knowledge of pollinating insects in Haiti and provides a firm basis for easy replicate monitoring. Combining pan traps of three different colors allowed us to better assess the presence and relative abundance of insect orders and families as previously reported [
63,
69,
70,
89,
90]. Overall, attractiveness differed between pan-trap colours, with blue ones attracting lower numbers of insects and yellow ones attracting higher numbers compared to white ones.
As expected, the abundance of pollinating insects at the suburban WFER was significantly higher, more than twice that observed at the urban UNPM. However, differences in abundance among habitats were not consistent between the two sites. This was mainly due to insects being more abundant in the forest site at UNPM whereas there was a tendency for the reverse at WFER. This contrast is most probably due to differences in tree and vegetation composition between the two forest habitats, although additional data should be collected over a longer time-period to better understand variation in abundance between sites and among habitats.
The overall difference in order composition between the two sites was mainly due to the large predominance of Diptera and very low abundance of both Coleoptera and Thysanoptera at UNPM compared to WFER (
Table 1). In particular, about two thirds of dipterans, hemipterans and lepidopterans were captured in the forest habitat at UNPM, whereas the distributions of the same orders were more even among the three habitats at WFER. Although we could not fully examine diversity at the family level, only about 30% of identified families were observed at both sites. Overall, then, our results indicate that the combination of the two protected areas conserves a wider and more even diversity of pollinating insects than either area on its own.
Importantly, we observed several species of patrimonial interest at both sites. In particular, two endemic Lepidopterans,
C. thomasi from the Nymphalidae family and
P. pyro from the Pieridae family, were collected at WFER. Interestingly, the presence of both species had only been documented so far from south-western Haiti [
82,
91]. In addition, the distribution of
P. pyro has been reported to be extremely restricted in the Dominican Republic Hispaniola, where the species has been observed in mesic habitat at elevation of about 450 m [
92]. Their presence in agricultural crops and agroforestry habitat might be the consequence of the intensive deforestation of pine forests in many parts of Haiti. Several characteristics of WFER, such as relatively high elevation, adjacent could forest and, possibly, the non-use of chemicals in agricultural crops, may also contribute to explain their presence. Although the danaid butterfly
Anetia jaegeri is considered as rare [
85] and was listed as Near-Threatened in the IUCN red list about 25 years ago [
93], we collected five individuals at WFER in the agroforestry habitat, suggesting that the species might be relatively abundant there. We also provide first evidence for the presence of
Cosmosoma odilae in Haiti. The species was only known from the Dominican Republic, where it appears to widely distributed at medium and high altitudes [
86].
The observation of two collected endemic hymenopterans of patrimonial interest belonging to the Vespidae family
, O. haitiensis (observed at both sites) and
O. lacerum (observed only at WFER), confirms the importance of the two protected sites for the conservation of pollinating insects in Haiti. Although very little is known about their biology and ecology, most species the genus
Odynerus are known for hunting on caterpillars (Lepidoptera: Gelechiidae) or weevil larvae (Coleoptera: Curculionidae) to provision their nests, while adults feed on nectar [
94,
95]. Omicron larvae are known to provision their nests with microlepidopteran larvae, whereas adults feed on nectar [
96]. The relative abundance and distribution of both species in Haiti deserves further consideration.
Our results then confirm the relevance of protected areas for the conservation of pollinating insect taxa, such as butterflies, ground beetles, hoverflies and wild bees [
97,
98,
99]. Given the high importance of pollinating insects for ecosystem services, we recommend to extend investigations to other protected and non-protected green areas in urban and suburban areas of Haiti. In particular, a more inclusive assessment of the diversity of pollinating insects could be achieved through setting pan traps at various heights [
100], increase spatial replicates within habitat type, and relying on bar-coding for family and species identification [
101]. Improving on techniques and methods would in particular allow for a better assessment of species' richness and diversity using appropriate indices [
78]. This information is of particular importance to increase basic knowledge on insect biodiversity in the country, and to develop management plans for green areas, in the light of habitat requirements of the various taxa of pollinating insects.
Unfortunately, taxonomic expertise on insects remains restricted to a very few people in Haiti, and mainly in the field of medical [
102] or agricultural entomology [
103]. Consequently, taxonomic specialists are lacking and several insect species, including pollinators, may remain undescribed, thus limiting the assessment and monitoring of biodiversity in Haiti, with direct negative consequences in terms of conservation. In addition, local researchers are particularly isolated and have very limited access to molecular tools and modern equipment for the preservation of biological samples [
48]. This situation is unlikely to improve in the coming years, unless non-governmental organizations invest massively in the training of Haitian entomologists with a strong academic background in biological conservation. In the meantime, preserving the few existing protected urban and suburban areas from degradation should be a national priority.
Author Contributions
Conceptualization, PMB and FC; methodology, PMB and FC; software, FC; validation, PMB and FC; formal analysis, PMB and FC; investigation, PMB; resources, PMB and FC; data curation, PMB and FC; writing—original draft preparation, PMB and FC; writing—review and editing, PMB and FC; visualization, PMB and FC; supervision, PMB and FC; project administration, FC; funding acquisition, FC.
Funding
This research was funded by Caribaea Initiative (Grant no.: 201901A_PMB).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank the Fokal Foundation and Caribaea Initiative for funding and moral support. We are particularly grateful to Jean-Marry Exantus for help in the field, and to Michèle Pierre-Louis and Jane Wynne for grating access to UNPM and WFER, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; Settele, J.; Kunin, W.E. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Slles, J.M.; Settele, J.; Viassière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecolo. Econo. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010a, 25, 345–353. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; Hill, M.J.; Hochkirch, A.; Mackenzie, L.; Kwak, M.L.; Mammola, S.; Noriega, J.A.; Orfinger, A.B.; Pedraza, F.; James, S.; Pryke, J.S.; Roque, F.O.; Settele, J.; Michael, J.; Samways, M.J. Scientists warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tanveer, M.; Ahmad, S.; Mars, M.; Naeem, M.; Naveed, Z.; Schuett, W.; Drees, C.; Goulson, D. Declining abundance of pollinating insects drives falls in loquat (Eriobotrya japonica) fruit yields in the Potwar region of Pakistan. Agr. Ecosyst. Environ. 2022, 339, 108138. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010b, 49, 15–22. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Albrecht, M.; Duelli, P.; Schmid, B.; Mueller, C.B. Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. J. Anim. Ecol. 2007, 76, 1015–1025. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator diversity: distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Nuttman, C.V.; Otieno, M.; Kwapong, P.K.; Combey, R.; Willmer, P.; Potts, S.G. The utility of aerial pan-trapping for assessing insect pollinators across vertical strata. J. Kans. Entomol. Soc. 2011, 84, 260–270. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Espíndola, A.; Aizen, M.A. Risks to pollinators and pollination from invasive alien species. Nat. Ecol. Evol. 2017, 2, 16–25. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Lanford, B.; Mullinger, N.J.; Bromfield, L.M.; Nemitz, E.; Pfrang, C.; Girling, R.D. Anthropogenic air pollutants reduce insect-mediated pollination services. Environ. Pollut. 2022, 297, 118847. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Giannini, T.C.; Costa, W.F.; Cordeiro, G.D.; Imperatriz-Fonseca, V.L.; Saraiva, A.M.; Biesmeijer, J.; Garibaldi, L.A. Projected climate change threatens pollinators and crop production in Brazil. PLoS One 2017, 12, e0182274. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; Ren, Z.-X.; Newbold, T. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Deguines, N.; Jono, C.; Baude, M.; Henry, M.; Julliard, R.; Fontaine, C. Large-scale trade-off between agricultural intensification and crop pollination services. Front. Ecol. Environ. 2014, 12, 212–217. [Google Scholar] [CrossRef]
- Paiva, I.G.; Auad, A.M.; Veríssimo, B.A.; Silveira, L.C.P. Differences in the insect fauna associated to a monocultural pasture and a silvopasture in Southeastern Brazil. Sci. Rep. 2020, 10, 12112. [Google Scholar] [CrossRef] [PubMed]
- Geslin, B.; Gauzens, B.; Thébault, E.; Dajoz, I. Plant pollinator networks along a gradient of urbanisation. PLoS One 2013, 8, e63421. [Google Scholar] [CrossRef]
- Iserhard, C.A.; Duarte, L.; Seraphim, N.; Freitas, A.V.L. How urbanization affects multiple dimensions of biodiversity in tropical butterfly assemblages. Biodivers. and Conserv. 2019, 28, 621–638. [Google Scholar] [CrossRef]
- González-Césped, C.; Alaniz, A.J.; Vergara, P.M.; Chiappa, E.; Zamorano, J.; Mandujano, V. Effects of urban environmental conditions and landscape structure on taxonomic and functional groups of insects. Urban For. Urban Green. 2021, 58, 126902. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination - A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. PNAS, 2011; 108, 20260–20264. [Google Scholar]
- Seto, K.C.; Guneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; He, C.; Huang, Q.; Shi, P.; Zhang, D.; Güneralp, B. Impacts of urban expansion on natural habitats in global drylands. Nat. Sustain. 2022, 5, 869–878. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Dinerstein, E.; Boucher, T. Pinpointing and preventing imminent extinctions. PNAS 2005, 51, 18497–18501. [Google Scholar] [CrossRef]
- Abellán, M.D.; Martínez, J.E.; Palazón, M.A.; Esteve, J.A.; Calvo, J.F. Efficiency of a protected-area network in a Mediterranean region: a multispecies assessment with raptors. Environ. Manag. 2011, 47, 983–991. [Google Scholar] [CrossRef]
- Watson, J.E.; Dudley, N.; Segan, D.B.; Hockings, M. The performance and potential of protected areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; DiMarco, M.; Watson, J.E.M. Protected areas are now the last strongholds for many imperiled mammal species. Conserv. Lett. 2020, 13, e12748. [Google Scholar] [CrossRef]
- Floigl, K.; Benedetti, Y.; Reif, J.; Vŏríšek, P.; Morelli, F. Assessing protected area network effectiveness through the temporal change in avian communities’ compositionJ. Nat. Conserv. 2022, 68, 126222. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Cazalis, V.; Dudley, N.; Hoffmann, M.; Rodrigues, A.S.; Stolton, S.; Watson, J.E. Area-based conservation in the twenty-first century. Nature 2020, 586, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Delso, Á.; Fajardo, J.; Muñoz, J. Protected area networks do not represent unseen biodiversity. Sci. Rep. 2021, 11, 12275. [Google Scholar] [CrossRef] [PubMed]
- D'Amen, M.; Bombi, P.; Campanaro, A.; Zapponi, L.; Bologna, M.A.; Mason, F. Protected areas and insect conservation: questioning the effectiveness of Natura 2000 network for saproxylic beetles in Italy. Anim. Conserv. 2013, 16, 370–378. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.; Fuller, R.A. Protected areas and the future of insect conservation. Trends Ecol. Evol. 2022, 38, 85–95. [Google Scholar]
- Monteiro-Júnior, C.S.; Esposito, M.C.; Juen, L. Are the adult odonate species found in a protected area different from those present in the surrounding zone? A case study from eastern Amazonia. J. Insect Conserv. 2016, 20, 643–652. [Google Scholar] [CrossRef]
- Ribeiro, C.; Elio-Rodrigues, M.; Sahlén, G.; de Oliveira Roque, F. Dragonflies within and outside a protected area: a comparison revealing the role of well-preserved atlantic forests in the preservation of critically endangered, phytotelmatous species. J. Insect Conserv. 2022, 26, 271–282. [Google Scholar] [CrossRef]
- Rada, S.; Schweiger, O.; Harpke, A.; Kühn, E.; Kuras, T.; Settele, J.; Musche, M. (2019) Protected areas do not mitigate biodiversity declines: A case study on butterflies. Divers. Distrib. 2019, 25, 217–224. [Google Scholar] [CrossRef]
- Falćon-Brindis, A.; León-Cortés, J.L.; Montañez-Reyna, M. How effective are conservation areas to preserve biodiversity in Mexico? Perspect. in Ecol. Conser. 2021, 19, 399–410. [Google Scholar] [CrossRef]
- Jankovic, M.; Miličić, M.; Ačanski, J.; Vujic, A. Protected areas and prime hoverfly areas: Safe haven for hoverflies or not? Entomol. Sci. 2020, 23, 173–182. [Google Scholar] [CrossRef]
- Balogun, I.; Eluyeba, O.; Adedoja, O.; Samways, M.J.; Polašek, O.; Kehinde, T. Open habitats in a tropical biodiversity hotspot support pollinator diversity in both protected and unprotected areas. Biotropica 2022, 54, 947–957. [Google Scholar] [CrossRef]
- Rodger, J.G.; Balkwill, K.; Gemmill, B. African pollination studies: where are the gaps? Int. J. Trop. 2004, 24, 5–28. [Google Scholar] [CrossRef]
- Ramírez, F.; Kallarackal, J. Climate change, tree pollination and conservation in the tropics: a research agenda beyond IPBES. Wiley Interdiscip. Rev. Clim. 2018, 9, e502. [Google Scholar] [CrossRef]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Titley, M.A.; Snaddon, J.L.; Turner, E.C. Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS One 2017, 12, e0189577. [Google Scholar] [CrossRef] [PubMed]
- Wemmer, C.; Rudran, R.; Dallmeier, F.; Wilson, D.E. (1993) Training developing-country nationals is the critical ingredient to conserving global biodiversity. Bioscience 1993, 43, 762–767. [Google Scholar] [CrossRef]
- Paknia, O.; Rajaei Sh, H.; Koch, A. Lack of well-maintained natural history collections and taxonomists in megadiverse developing countries hampers global biodiversity exploration. Org. Divers. Evol. 2015, 15, 619–629. [Google Scholar] [CrossRef]
- Vallès, H.; Labaude, S.; Bezault, E.; Browne, D.; Deacon, A.; Guppy, R.; Aimara Pujadas, C.; Cézilly, F. Low contribution of Caribbean-based researchers to academic publications on biodiversity conservation in the insular. Caribb. Perspect. Ecol. Conserv. 2021, 19, 443–453. [Google Scholar] [CrossRef]
- Asase, A.; Mzumara-Gawa, T.I.; Owino, J.O.; Peterson, A.T.; Saupe, E. Replacing "parachute science" with "global science" in ecology and conservation biology. Conserv. sci. pract. 2022, 4, e517. [Google Scholar] [CrossRef]
- Giangrande, A. Biodiversity, conservation, and the ‘Taxonomic impediment’. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2003; 13, 451–459. [Google Scholar]
- Kehinde, T.O. Conserving insect diversity in the tropics: Challenges and prospects. Niger. J. Entomo. 2017, 33, 7–15. [Google Scholar] [CrossRef]
- Dourojeanni, M.J. Conservación de insectos en la Amazona. Ecol. Appl. 2019, 18, 189–202. [Google Scholar] [CrossRef]
- Sutton, P. Poverty in Haiti: Essays on underdevelopment and post disaster prospects. J. Lat. Am. Stud. 2013, 45, 597–599. [Google Scholar] [CrossRef]
- Woods, C.A.; Sergile, F.E. (Eds) Biogeography of the West Indies: patterns and perspectives. CRC Press: Boca Raton, Florida, United States, 2011; pp. 547.
- Klose, C.D. Evidence for higher tropical storm risks in Haiti due to increasing population density in hazard prone urban areas. Environ. Res. Lett. 2011, 6, 044020. [Google Scholar] [CrossRef]
- Hedges, S.B.; Cohen, W.B.; Timyan, J.; Yang, Z. Haiti’s biodiversity threatened by nearly complete loss of primary forest. Proc. Am. Acad. Arts Sci. 2018, 115, 11850–11855. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B. Domestic institutions and foreign assistance in Haiti: Requisites for economic development. Dev. Policy Rev. 2018, 36, O514–O530. [Google Scholar] [CrossRef]
- Jacob, S. Massification and the public financing of higher education in Haiti: issues and challenges. Int. Rev. Adm. Sci. 2018, 86, 002085231878145. [Google Scholar] [CrossRef]
- Latta, S.C. Complementary areas for conserving avian diversity on Hispaniola. In Anim. Conserv. Forum 2005, 8, 69–81. [Google Scholar] [CrossRef]
- Moore, K.; Swisher, M.; Koenig, R.; Monval, N.; Tarter, A.; Milord, E.; Delva, L. Capitalizing on the strengths of farmer organizations as potential change agents in Haiti. J. Rural Stud. 2021, 85, 68–78. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Magrach, A.; Dainese, M.; Garibaldi, L.A.; Kleijn, D.; Rader, R.; Reilly, J.R. CropPol: A dynamic, open and global database on crop pollination. Ecology 2022, 103, e3614. [Google Scholar] [CrossRef] [PubMed]
- Exantus, J.M.; Beaune, D.; Cézilly, F. The relevance of urban agroforestry and urban remnant forest for avian diversity in a densely-populated developing country: the case of Port-au-Prince, Haiti. Urban For. Urban Green. 2021, 63, 127217. [Google Scholar] [CrossRef]
- Saunders, M.E.; Luck, G.W. Pan trap catches of pollinator insects vary with habitat. Aust. J. Entomol. 2013, 52, 106–113. [Google Scholar] [CrossRef]
- Moreira, E.F.; Santos, R.L.D.S.; Penna, U.L.; Angel-Coca, C.; de Oliveira, F.F.; Viana, B.F. Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- Persson, A.S.; Ekroosa, J.; Olsson, P.; Smith, H.G. Wild bees and hoverflies respond differently to urbanisation, human population density and urban form. Landsc. Urban Plan. 2020, 204, 103901. [Google Scholar] [CrossRef]
- Theodorou, P.; Radzevičiūté, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; Murray, T.E.; Paxton, R.J. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Zaninotto, V.; Perrard, A.; Babiar, O.; Hansart, A.; Hignard, C.; Dajoz, I. Seasonal variations of pollinator assemblages among urban and rural habitats: A comparative approach using a standardized plant community. Insects 2021, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.; Parrish, J.D.; Redford, K.H.; Stolton, S. The revised IUCN protected area management categories: the debate and ways forward. Oryx 2010, 44, 485–490. [Google Scholar] [CrossRef]
- Vrdoljak, S.M.; Samways, M.J. Optimising coloured pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Bashir, M.A.; Saeed, S.; Sajjad, A. Monitoring Hymenoptera and Diptera pollinators in a subtropical forest of southern Punjab, Pakistan. Pak. J. Agric. Sci. 2013, 50, 359–366. [Google Scholar]
- Beaujour, P.M.; Cézilly, F. The importance of urban green spaces for pollinating insects: The case of the metropolitan area of Port-au-Prince, Haiti. Caribb. J. Sci. 2022, 52, 238–249. [Google Scholar] [CrossRef]
- Woodruff, R.E.; Sanderson, M.W. Revision of the Phyllophaga of Hispaniola (Coleoptera: Scarabaeidae: Melolonthinae) - PART 2. Insecta Mundi 2004, 18. [Google Scholar]
- Perez-Gelabert, D.E. Arthropods of Hispaniola (Dominican Republic and Haiti): A checklist and bibliography. Zootaxa 2008, 1831, 1–530. [Google Scholar] [CrossRef]
- Perez-Gelabert, D.E. Checklist, bibliography and quantitative data of the arthropods of Hispaniola. Zootaxa 2020, 4749, 1–668. [Google Scholar] [CrossRef]
- Racheli, T. An updated list to the Butterflies of Hispaniola, with notes on the classification of Calisto Hübner, 1823 (Lepidoptera, Hesperioidea, Papilionoidea). Neue Ent. Nachr. 2019, 76, 1–135. [Google Scholar]
- Delvare, G.; Aberlenc, H.P. Les insectes d'Afrique et d'Amérique tropicale : clés pour la reconnaissance des familles. Editions Quae: Montpellier, France, 1989.
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Maurer, B.A.; McGill, B.J. Measurement of species diversity. In: Magurran, A.E. and McGill, B.J. (eds) Biological Diversity: Frontiers in Measurement and Assessment. Oxford University Press: Oxford, England, 2011; 55-65 pp.
- Jost, L.; Chao, A.; Chazdon, R.L. Compositional similarity and β (beta) diversity. In: Magurran, A.E. and McGill, B.J. (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press: New York, USA, 2011; 66–84 pp.
- Renkonen, O. Statistisch-ökologische Untersuchungen über die terrestrische Käferwelt der finnischen Bruchmoore. Doctoral dissertation, Societas zoologica-botanica Fennica Vanamo, Germany, 1938.
- Wolda, H. Similarity indices, sample size and diversity. Oecologia 1981, 50, 296–302. [Google Scholar] [CrossRef]
- Johnson, K.; Hedges, S.B. Three new species of Calisto from southwestern Haiti (Lepidoptera: Nymphalidae: Satyrinae). Trop. Lepid. Res. 1998, 45–53. [Google Scholar]
- Bland, K.P. Name-bearing types of butterflies (Lepidoptera, Papilionoidea). in the National Museums of Scotland, Edinburgh. Zootaxa, 2019; 4559, 57–89. [Google Scholar]
- Lepidoptera Specialist Group. Available online: https://www.iucnredlist.org/species/1288/3393996 (accessed on 1 May 2023).
- Vane-Wright, R.I.; Ackery, P.R.; Turner, T. Anetia Jaegeri, Danaus cleophile and Lycorea Cleobaea from Jamaica (Nymphalidae: Danainae). J. Lepid. Soc. 1992, 46, 273–279. [Google Scholar]
- Laguerre, M. Description of a new species of Euchromiina from Dominican Republic (Lepidoptera Erebidae Arctiinae Ctenuchini). Antenor 2014, 1, 3–10. [Google Scholar]
- Bequaert, J.; Salt, G. New West Indian Diploptera. Ann. Entomol. Soc.Am. 1931, 24, 765–797. [Google Scholar] [CrossRef]
- Giordani Soika, A. Revisione degli Eumenidi neotropicali appartenenti ai generi Eumenes Latr., Omicron (Sauss.), Pararhaphidoglossa Schulth. ed affini. Bolletino della Museo Civico di Storia Naturale di Venezia, 1978; 29, 1–420. [Google Scholar]
- Campbell, J.W.; Hanula, J.L. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Freitas-Moreira, E.; da Silva Santos, R.L.; Lopes Penna, U.; Angel-Coca, C.; Freitas de Oliveira, F.; Viana, B.F. (2016) Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- Gali, F.; Schwartz, A. The butterflies (Lepidoptera: Rhopalocera) of Morne La Visite and Pic Macaya, Haiti. US Agency for International Development, Gainesville, Florida 1986.
- Racheli, T.; Stefanelli, E.; Racheli, L. Parsimony analysis of butterflies communities in the Dominican Republic: assessing relationships among butterflies assemblages (Lepidoptera: Papilionoidea). SHIL. Rev. lepidopterol. 2017, 45, 533–549. [Google Scholar]
- Baillie, J.; Groombridge, B. (eds). IUCN Red List of Threatened Animals. International Union for Conservation
of Nature, Gland, Switzerland and Cambridge, UK, 1996; pp. 378.
- Bohart, G.E.; Parker, F.D.; Tepedino, V.J. Notes on the biology of Odynerus dilectus [Hym. : Eumenidae], a predator of the falafa weevil, Hypera postica [Col. : Curculionidae]. Entomophaga, 1982; 27, 23–31. [Google Scholar]
- Fateryga, A.V. Nesting biology of Odynerus albopictus calcaratus (Morawitz, 1885) and Odynerus femoratus de Saussure, 1856 (Hymenoptera: Vespidae: Eumeninae). J. Insects 2013, 597583. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Genaro, J.A. Vespidae (Insecta: Hymenoptera) of Puerto Rico, West Indies. Insecta Mundi 2011, 0202, 1–35. [Google Scholar]
- Connor, E.F.; Hafernik, J.; Levy, J.; Lee Moore, V.; Rickman, J.K. Insect conservation in an urban biodiversity hotspot: the San Francisco Bay Area. J. Insect Conserv. 2002, 6, 247–259. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alam, S.; Chowdhury, S.U.; Rokonuzzaman, M.; Shahriar, S.A.; Shome, A.R.; Fuller, R.A. Butterflies are weakly protected in a mega-populated country. Bengaldesh Global. Ecol. Conserv. 2021, 26, e01484. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Park; K. E.; Çakmak, I.; Hranitz, J.M.; Barthell, J.F. Pan traps and bee body size in unmanaged urban habitats. J. Hymenopt. Res. 2016, 51, 241–247. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Gong, Y.-B.; Adamowicz, A.J.; Packer, L. Forecasting pollination declines through DNA barcoding: the potential contributions of macroecological and macroevolutionary scales of inquiry. New Phytol. 2017, 214, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.; Saint Jean, Y.; Lemoine, J.F.; Dotson, E.M.; Mace, K.E.; Chang, M.; Slutsker, L.; Le Menach, A.; Beier, J.C.; Eisele, T.P.; Okech, B.A.; Beau de Rochars, V.M.; Carter, K.H.; Keating, J.; Impoinvil, D.E. Malaria vector research and control in Haiti: a systematic review. Malar. J. 2016, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Exilien, R.; Brodeur, J.; Fournier, V.; Martini, X. Host range and phenology of sugarcane aphid (Hemiptera: Aphididae) and natural enemy community in sorghum in Haiti. J. Econ. Entomol. 2022, 115, 1956–1963. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).