Submitted:
10 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
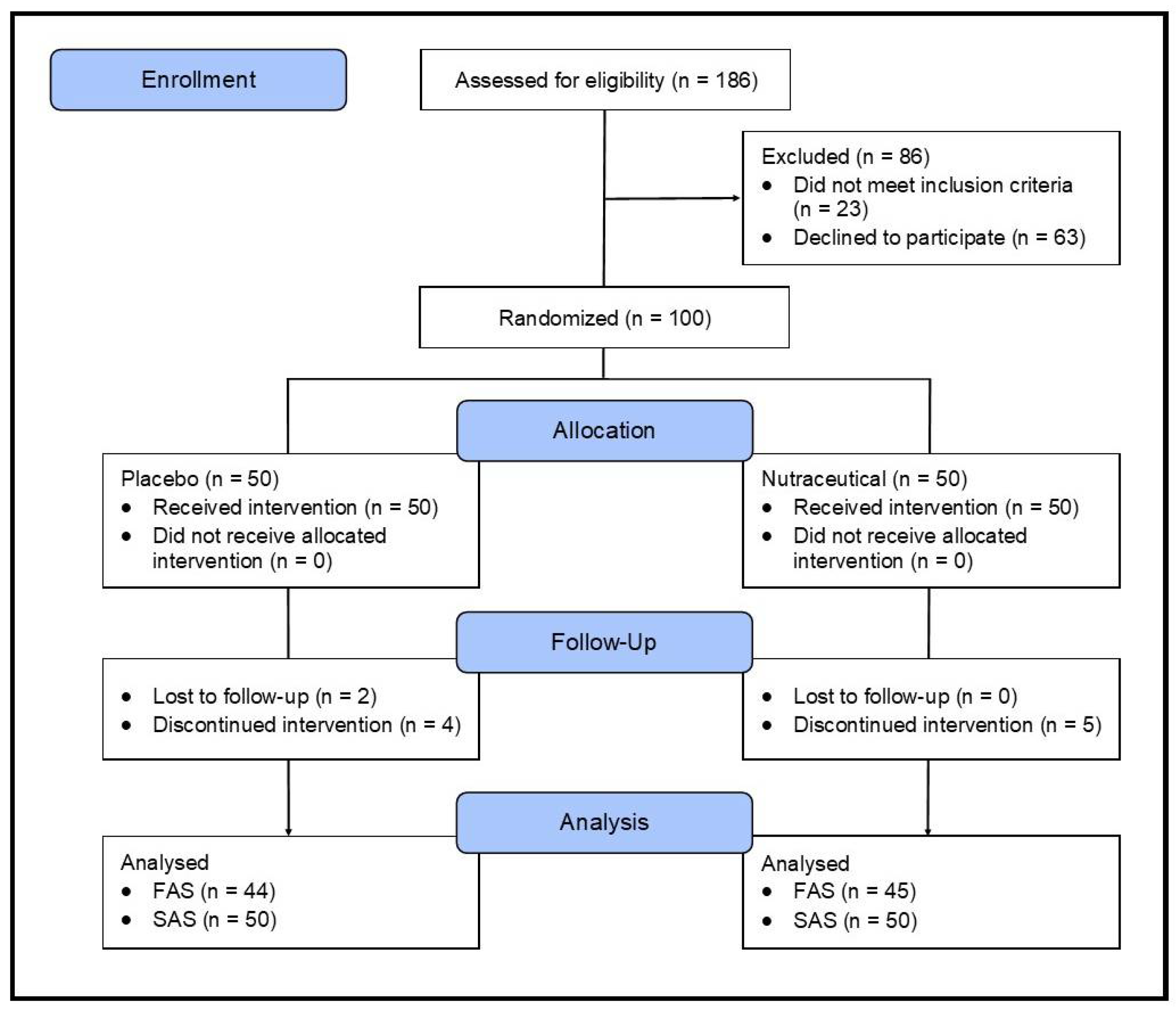
2.1. Study Design
2.2. Sample Size Calculation
2.3. Recruitment and Randomization
2.4. Participants
2.4.1. Inclusion Criteria.
2.4.2. Exclusion Criteria.
2.5. Intervention
2.6. Outcome Measures
2.6.1. Primary Outcome Measures
- Change in Episodic Memory from baseline to week 12 as measured by the numeric working memory, location learning (delayed recall), and RAVLT delayed recall task (calculations are detailed in Supplementary Table S2).
- Change in Working Memory from baseline to week 12 as measured by the corsi blocks and numeric working memory tasks (calculations are detailed in Supplementary Table S2).
- Change in Verbal Learning and Memory from baseline to week 12 as measured by the Rey Auditory Verbal Learning Test (RAVLT). The RAVLT is a neuropsychological assessment designed to evaluate a wide diversity of cognitive functions, including short-term auditory-verbal memory, rate of learning, learning strategies, retroactive and proactive interference, presence of confabulation of confusion in memory processes, retention of information, and differences between learning and retrieval [16]. In the RAVLT, the examiner reads aloud a list of 15 words at the rate of one word per second. The participant is then asked to repeat all words from the list that he/she can remember. This procedure is repeated a total of five times. The examiner then presents a second list of 15 words (interference list), allowing the participant only one attempt to recall this new list. Immediately following this, the participant is asked to remember as many words as possible from the first list. After a 20-minute delay, the participant is again asked to recall as many words as possible from the first list. Changes in the total number of words recalled on trials 1 to 5 scores were used to measure verbal learning and memory.
2.6.2. Secondary Outcome Measures
- Change in the Everyday Memory Questionnaire – revised (EMQ) total score. The EMQ is a 13-item, reliable and valid self-report measure of memory failure associated with everyday life [17]. Respondents indicate how often they have experienced specific memory problems over the last month.
- Change in the Perceived Stress Questionnaire (PSQ) total score. The PSQ is a 30-item self-report questionnaire that assesses a person’s subjective experiences of perceived stressful situations and their stress reactions [18].
- Change in the total score on the World Health Organization - 5 Wellbeing Index (WHO-5). The WHO-5 is among the most widely used self-report questionnaires assessing subjective psychological wellbeing [19]. It consists of 5 items that provide a generic global rating of subjective wellbeing.
- Change in the Speed of Information Processing from baseline to week 12 as measured by the simple reaction time, choice reaction time, numeric working memory, and digit vigilance task (calculations are detailed in Supplementary Table S2).
- Change in the Accuracy of Attention from baseline to week 12 as measured by the choice reaction time task and digit vigilance task (calculations are detailed in Supplementary Table S2).
- Change in Visuospatial learning from baseline to week 12 as measured by displacement scores during the 5 learning trials of the computerized location learning task.
- A fasting venous blood sample was collected between 8 and 11 am to measure changes in plasma brain-derived neurotrophic factor (BDNF), malondialdehyde (MDA), Tumor Necrosis Factor-alpha (TNF-α), and Interleukin-6 (IL-6). BDNF plays an important role in learning, memory, and neuronal survival and growth. Disturbances in BDNF have been linked with Alzheimer’s disease and cognitive impairment [20]. MDA is a secondary by-product of cellular lipid peroxidation of polyunsaturated fatty acids and is often used as a biomarker of oxidative stress [21]. TNF-α is an inflammatory cytokine produced by macrophages/monocytes during acute inflammation. It is responsible for various cell signaling events, leading to necrosis or apoptosis [22]. IL-6 is rapidly and transiently produced in response to infections and tissue injuries and contributes to host defense by stimulating acute phase responses, hematopoiesis, and immune reaction [23].
2.6.3. Exploratory Outcome Measures:
- Change in theOcular Surface Disease Index (OSDI)total score. The OSDI assesses ocular irritation symptoms in dry eye disease and how they affect vision-related functions [24]. This 12-item questionnaire assesses dry eye symptoms and their effects on vision-related function in the past week of the patient’s life. A total score is calculated, which ranges from 0 to 100, with scores 0 to 12 representing normal, 13 to 22 representing mild dry eye disease, 23 to 32 representing moderate dry eye disease, and greater than 33 representing severe dry eye disease.
- Change inSkin Health Satisfactionratings. On a scale from 0 (none) to 4 (severe), participants rated their skin appearance based on the following criteria: (1) lines, (2) firmness, (3) radiance, (4) texture, (5) hydration, and (6) overall skin appearance.
- Change in Skin Carotenoidconcentrations measured using resonance Raman spectroscopy (BioPhotonic Scanner; NSE Products, Provo, UT). In this measurement, blue LED light from the BioPhotonic Scanner was directed on the palm of the hand to measure concentrations of skin carotenoids, measured in Raman Intensity Units (RIUs). Resonance Raman spectroscopy has been shown to accurately measure total carotenoids in human skin with less intra-individual variability than measurements of carotenoids in serum [25]. Skin carotenoids were measured at baseline and week 12.
2.6.4. Safety Measures
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Outcome Measures
3.2.1. Primary Outcome Measures
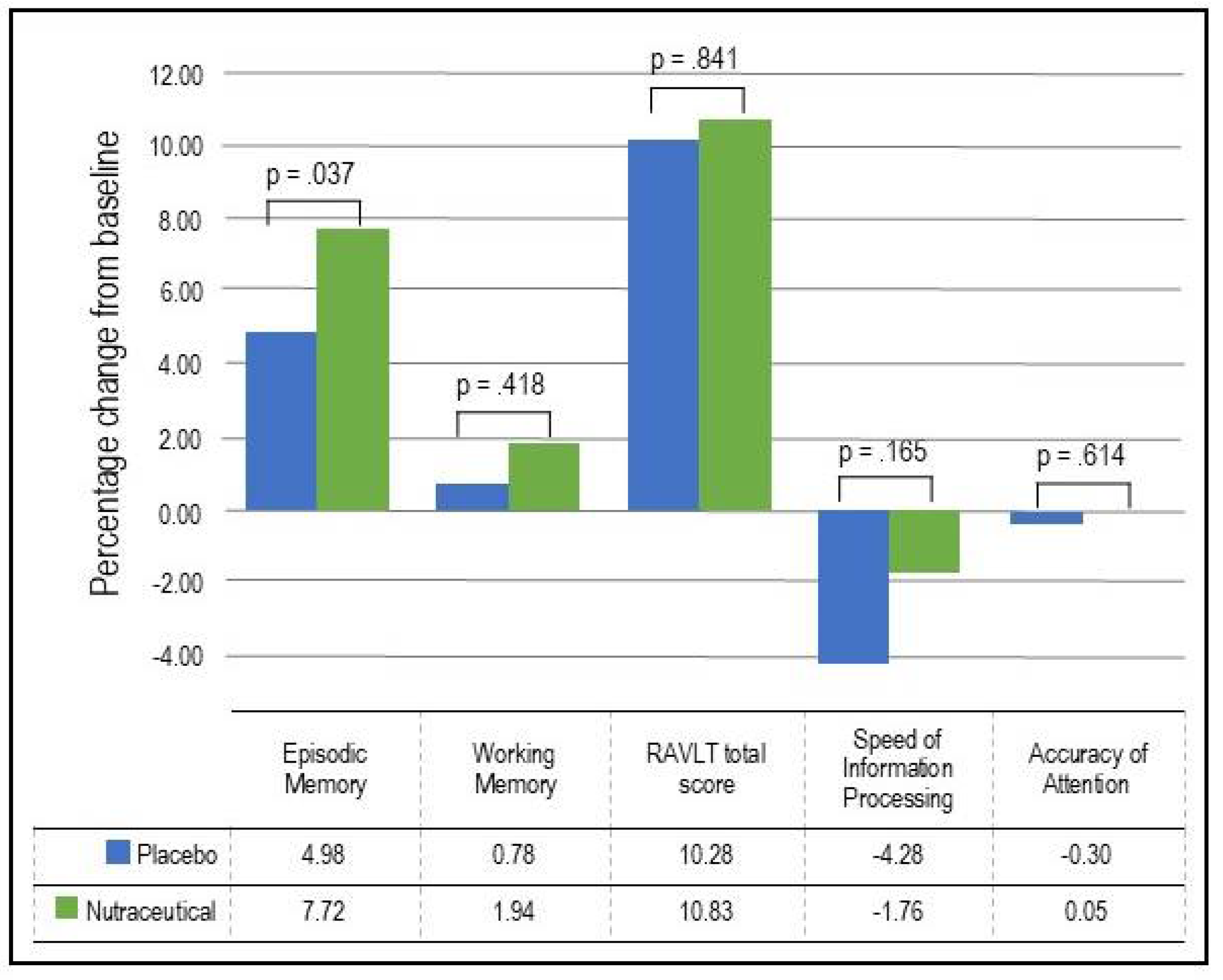
- Episodic Memory: As demonstrated in Table 3 and Figure 2, based on the GLMM, there was a statistically significant time x group interaction in episodic memory (p = .037). In the nutraceutical group, there was a statistically significant 7.72% increase in episodic memory from baseline to week 12 (p < .001) compared to a statistically significant but smaller 4.98% improvement in the placebo group (p < .001).
- Working Memory: As demonstrated in Table 3 and Figure 2, based on the GLMM, there was no statistically significant time x group interaction in working memory (p = .418). In the nutraceutical group, there was a near statistically significant 1.94% increase in working memory from baseline to week 12 (p = .055) compared to a non-significant 0.78% improvement in the placebo group (p = .443).
- Verbal Learning and Memory: As demonstrated in Table 3 and Figure 2, based on the GLMM, there was no statistically significant time x group interaction in the RAVLT total score (p = .841). In the nutraceutical group, there was a statistically significant 10.83% increase in the RAVLT total score from baseline to week 12 (p < .001) compared to a 10.28% improvement in the placebo group (p < .001).
3.2.2. Secondary Outcome Measures
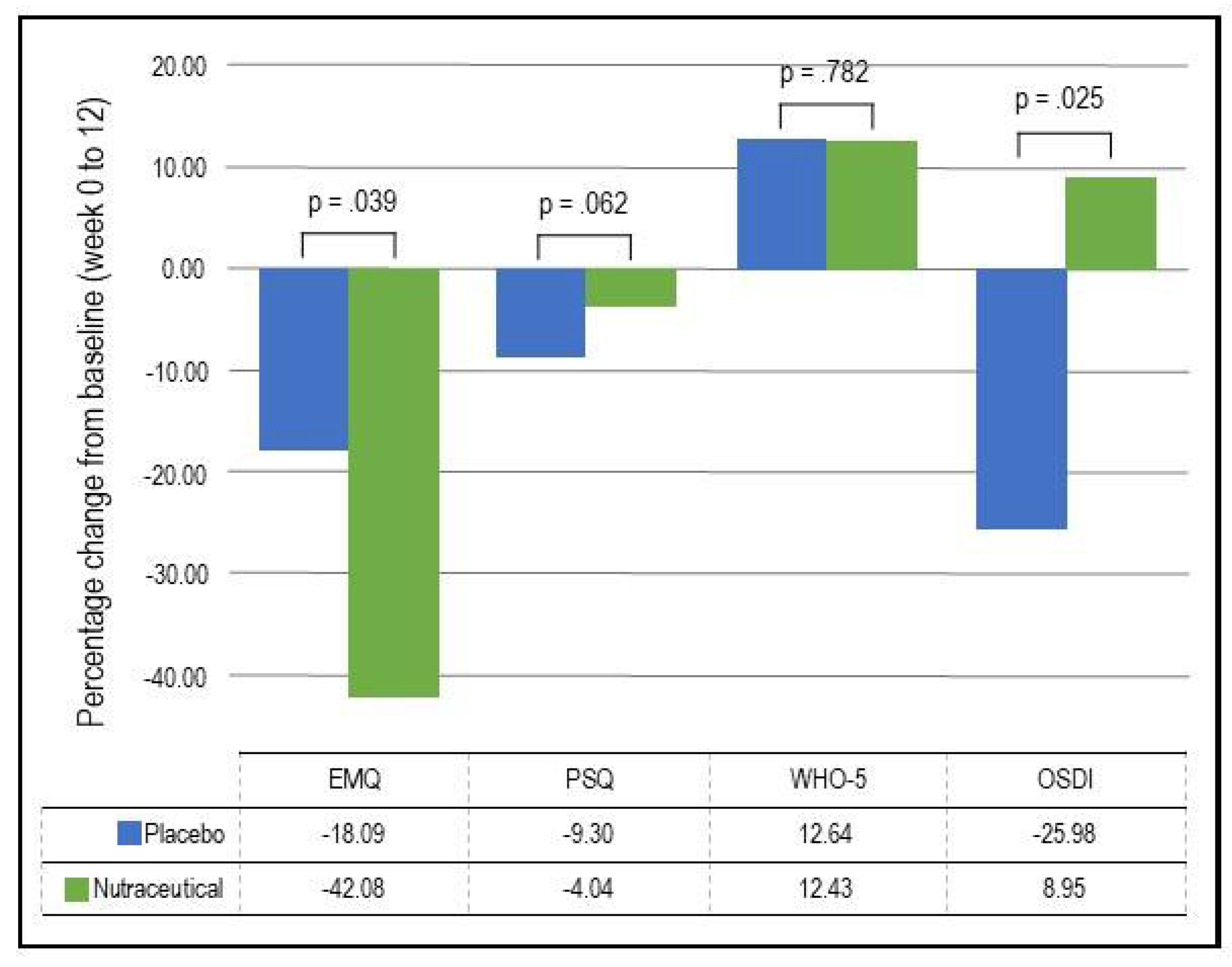
- EMQ: As demonstrated in Table 4 and Figure 3, based on the GLMM, there was a statistically significant time x group interaction in EMQ scores (p = .039). In the nutraceutical group, there was a statistically significant 42.08% decrease in EMQ scores from baseline to week 12 (p < .001) compared to a statistically significant but smaller 18.09% decrease in the placebo group (p = .022).
- Visuospatial learning (Computerized Location Learning Task): Based on the GLMM, there was no statistically significant time x group interaction in the Location Learning total displacement score from trials 1 to 5 (p = .715).
- Blood markers: As demonstrated in Table 5, based on the GLMM, there were statistically significant time x group interactions for BDNF (p = .030) and MDA concentrations (p = .040). However, there were no statistically significant time x group interactions for TNF-α (p = .445) and IL-6 (p = .691). In the nutraceutical group, there was a statistically significant 46.10% increase in BDNF concentrations from baseline to week 12 (p < .001) compared to a 24.41% increase in the placebo group (p < .001). Regarding changes in MDA concentrations over time, there was a non-significant 12.49% decrease in the nutraceutical group (p = .294) and a near-significant 26.52% increase in the placebo group (p = .072).
3.2.3. Exploratory Outcome Measures
- OSDI: As demonstrated in Table 4 and Figure 3, based on the GLMM, there was a statistically significant time x group interaction in OSDI scores (p = .025). In the placebo group, there was a statistically significant 25.98% decrease (improvement) in OSDI scores from baseline to week 12 (p = .002) compared to a non-significant 8.95% increase in the nutraceutical group (p = .297).
- Skin Carotenoid concentrations: As demonstrated in Table 5, based on the GLMM, there was a statistically significant time x group interaction in skin carotenoid concentrations (p = .006). In the nutraceutical group, there was a statistically significant 9.16% increase in carotenoid concentrations from baseline to week 12 (p = .014) compared to a non-significant 5.13% decrease in the placebo group (p = .143).
- Skin Health Satisfaction: Based on the GLMM, there was a statistically significant time x group interaction in ratings of facial radiance (p = .014). In the placebo group, there was a statistically significant 6.48% decrease in self-ratings (improvement) from baseline to week 12 (p < .001) compared to a non-significant 3.09% increase in the nutraceutical group (p = .487). There were no statistically significant time x group interactions on other skin satisfaction ratings.
- Individual Cognitive Tasks: As demonstrated in Supplementary Table S3, based on the GLMM, there was a statistically significant time x group interaction in the Numeric Working Memory (NWM) task for percentage correct responses (p =.014) and Computerized Location Learning (CLL) recall displacement score (p < .001). In the placebo group, there was a non-significant 0.30% increase in correct responses on the NWM task (p = .755) and a non-significant 23.03% decrease in the displacement score (indicating an improvement) on the CLL recall task (p = .148). However, in the nutraceutical group, there was a statistically significant 3.65% increase in correct responses on the NWM task (p < .001) and a statistically significant 75.04% decrease in the displacement score on the CLL recall task (p < .001). There were no between-group differences in the performance of other cognitive tasks.
3.2.4. Safety and Tolerability
3.2.5. Treatment Discontinuation
3.2.6. Efficacy of Participant Blinding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montejo, P.; Montenegro, M.; Fernández, M.A.; Maestú, F. Memory complaints in the elderly: Quality of life and daily living activities. A population based study. Arch. Gerontol. Geriatr. 2011, 54, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Shpigelman, S.R.; Sternberg, S.; Maeir, A. Beyond memory problems: Multiple obstacles to health and quality of life in older people seeking help for subjective memory complaints. Disabil. Rehabilitation 2017, 41, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Dhana, K.; Leurgans, S.E.; Shea, K.; Booth, S.L.; Rajan, K.B.; Schneider, J.A.; Barnes, L.L. Association of Dietary Intake of Flavonols With Changes in Global Cognition and Several Cognitive Abilities. Neurology 2023, 100, e694–e702. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M. Dietary Intake of Antioxidants and Risk of Alzheimer Disease. JAMA J. Am. Med Assoc. 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, R.; Sharma, M.; Batra, K.; Beatty, F.B. The Role of Vitamin E in Slowing Down Mild Cognitive Impairment: A Narrative Review. Healthcare 2021, 9, 1573. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.J.; Hoggard, N.; Aceves-Martins, M. The effect of grape interventions on cognitive and mental performance in healthy participants and those with mild cognitive impairment: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 367–380. [Google Scholar] [CrossRef]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Nguyen, G.; Torres, A. Systemic antioxidants and skin health. J Drugs Dermatol. 2012, 11, e1–e4. [Google Scholar]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. de Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Majeed, M.; Drummond, P.D. Effects of an Oroxylum indicum Extract (Sabroxy((R))) on Cognitive Function in Adults With Self-reported Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Study. Front Aging Neurosci. 2021, 13, 728360. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The Effects of Lutein and Zeaxanthin Supplementation on Cognitive Function in Adults With Self-Reported Mild Cognitive Complaints: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef] [PubMed]
- Bentvelzen, A.C.; Crawford, J.D.; Theobald, A.; Maston, K.; Slavin, M.J.; Reppermund, S.; Kang, K.; Numbers, K.; Brodaty, H.; Sachdev, P.; et al. Validation and Normative Data for the Modified Telephone Interview for Cognitive Status: The Sydney Memory and Ageing Study. J. Am. Geriatr. Soc. 2019, 67, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.E.; Marsiske, M.; McCoy, K.J.M. The Use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the Detection of Amnestic Mild Cognitive Impairment. J. Geriatr. Psychiatry Neurol. 2009, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.R.; Dawson, K.A.; Duff, K.; Patton, D.; Scott, J.G.; Adams, R.L. Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Arch. Clin. Neuropsychol. 2006, 21, 693–703. [Google Scholar] [CrossRef]
- Royle, J.; Lincoln, N.B. The Everyday Memory Questionnaire – revised: Development of a 13-item scale. Disabil. Rehabilitation 2008, 30, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.; Berto, E.; Luzi, C.; Andreoli, A. Development of the perceived stress questionnaire: A new tool for psychosomatic research. J. Psychosom. Res. 1993, 37, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer's disease and its pharmaceutical potential. Transl Neurodegener 2022, 11, 4. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990, 186, 421–431. [Google Scholar] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- A Zidichouski, J.; Mastaloudis, A.; Poole, S.J.; Reading, J.C.; Smidt, C.R. Clinical Validation of a Noninvasive, Raman Spectroscopic Method to Assess Carotenoid Nutritional Status in Humans. J. Am. Coll. Nutr. 2009, 28, 687–693. [Google Scholar] [CrossRef]
- Nyberg, L.; Mclntosh, A.R.; Houle, S.; Nilsson, L.-G.; Tulving, E. Activation of medial temporal structures during episodic memory retrieval. Nature 1996, 380, 715–717. [Google Scholar] [CrossRef]
- Chatzikostopoulos, A.; Moraitou, D.; Tsolaki, M.; Masoura, E.; Papantoniou, G.; Sofologi, M.; Papaliagkas, V.; Kougioumtzis, G.; Papatzikis, E. Episodic Memory in Amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s Disease Dementia (ADD): Using the “Doors and People” Tool to Differentiate between Early aMCI—Late aMCI—Mild ADD Diagnostic Groups. Diagnostics 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yuan, Q.; Xue, C.; Qi, W.; Ge, H.; Yan, Z.; Chen, S.; Song, Y.; Wu, H.; Xiao, C.; et al. Convergent functional changes of the episodic memory impairment in mild cognitive impairment: An ALE meta-analysis. Front. Aging Neurosci. 2022, 14, 919859. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Eichenbaum, H. The Episodic Memory System: Neurocircuitry and Disorders. Neuropsychopharmacology 2009, 35, 86–104. [Google Scholar] [CrossRef]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Singh, P.; Barman, B.; Thakur, M.K. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front. Aging Neurosci. 2022, 14, 944697. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M.; Gałecki, P.; Maes, M.; Gardner, A.; Chamielec, M.; Orzechowska, A.; Bobińska, K.; Kowalczyk, E. Malondialdehyde plasma concentration correlates with declarative and working memory in patients with recurrent depressive disorder. Mol. Biol. Rep. 2011, 39, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
| Placebo (n=50) | Nutraceutical (n=50) | p-value | ||
| Age | Mean | 58.93 | 59.82 | 0.564^ |
| SE | 1.18 | 0.99 | ||
| Sex | Female (n) | 40 | 39 | 0.806# |
| Male (n) | 10 | 11 | ||
| Height (m) | Mean | 1.69 | 1.69 | 0.690^ |
| SE | 0.01 | 0.01 | ||
| Weight (kg) | Mean | 77.26 | 75.92 | 0.826^ |
| SE | 2.54 | 2.20 | ||
| BMI | Mean | 26.76 | 26.48 | 0.765^ |
| SE | 0.69 | 0.64 | ||
| Systolic blood pressure (mmHg) | Mean | 130.82 | 131.00 | 0.956^ |
| SE | 1.93 | 2.63 | ||
| Diastolic blood pressure (mmHg) | Mean | 81.26 | 81.54 | 0.895^ |
| SE | 1.30 | 1.68 | ||
| Marital status | Single | 28 | 17 | 0.027^ |
| Married/ defacto | 22 | 33 | ||
| Educational level | Secondary | 25 | 28 | 0.626# |
| Tertiary | 12 | 13 | ||
| Post-graduate | 13 | 9 | ||
| IPAQ category | Low | 29 | 20 | 0.005# |
| Moderate | 21 | 21 | ||
| High | 0 | 9 | ||
| Occupation | Retired | 15 | 11 | 0.697# |
| Professional | 12 | 10 | ||
| Services and sales worker | 6 | 9 | ||
| Unemployed | 5 | 4 | ||
| Technicians and associate professionals | 2 | 2 | ||
| Elementary occupation | 1 | 3 | ||
| Plant and machine operators and assemblers | 0 | 2 | ||
| Clerical support worker | 2 | 5 | ||
| Craft and related trades worker | 1 | 1 | ||
| Student | 1 | 0 | ||
| Manager | 5 | 3 |
| Placebo (n=50) | Nutraceutical (n=50) | p-value | ||
| EMQ | Mean | 14.28 | 16.50 | 0.376^ |
| SE | 1.20 | 1.40 | ||
| PSQ | Mean | 64.62 | 67.90 | 0.310^ |
| SE | 1.96 | 2.54 | ||
| WHO-5 | Mean | 13.62 | 12.54 | 0.311^ |
| SE | 0.63 | 0.85 | ||
| OSDI | Mean | 18.78 | 18.42 | 0.906^ |
| SE | 2.29 | 2.01 | ||
| BioPhotonic Scanner score (Raman Intensity Units (RIUs)) | Mean | 31040 | 32600 | 0.464^ |
| SE | 1451.80 | 1550.77 | ||
| RAVLT – total recalled (trials 1 to 5) | Mean | 54.10 | 51.00 | 0.085^ |
| SE | 1.28 | 1.24 |
| Placebo (n=44) | Nutraceutical (n=45) | p-valueb | ||||||||
| Week 0 | Week 12 | % change | p-valuea | Week 0 | Week 12 | % change | p-valuea | |||
| Episodic Memory | Mean | 85.87 | 90.15 | 4.98 | < .001 | 85.22 | 91.80 | 7.72 | < .001 | 0.037 |
| SE | 0.48 | 0.54 | 0.48 | 0.54 | ||||||
| Working Memory | Mean | 65.10 | 65.61 | 0.78 | 0.443 | 65.21 | 66.47 | 1.94 | 0.055 | 0.418 |
| SE | 0.84 | 0.87 | 0.83 | 0.86 | ||||||
| RAVLT - Total score (Trials 1 to 5) |
Mean | 53.54 | 59.05 | 10.28 | < .001 | 50.85 | 56.36 | 10.83 | < .001 | 0.841 |
| SE | 1.36 | 1.54 | 1.26 | 1.43 | ||||||
| Speed of Information Processing | Mean | 575.66 | 551.05 | -4.28 | 0.001 | 574.50 | 564.40 | -1.76 | < .001 | 0.165 |
| SE | 12.25 | 11.93 | 11.92 | 11.90 | ||||||
| Accuracy of Attention | Mean | 95.39 | 95.10 | -0.30 | 0.545 | 95.89 | 95.94 | 0.05 | 0.917 | 0.614 |
| SE | 0.90 | 0.91 | 0.88 | 0.89 | ||||||
| Placebo (n=44) | Nutraceutical (n=45) | p-valueb | ||||||||||||
| Week 0 | Week 4 | Week 8 | Week 12 | % change | p-valuea | Week 0 | Week 4 | Week 8 | Week 12 | % change | p-valuea | |||
| EMQ | Mean | 12.22 | 10.22 | 9.70 | 10.01 | -18.09 | 0.022 | 14.59 | 10.53 | 10.06 | 8.45 | -42.08 | < .001 | 0.039 |
| SE | 1.33 | 1.13 | 1.08 | 1.11 | 1.54 | 1.13 | 1.08 | 0.92 | ||||||
| PSQ | Mean | 62.67 | 61.63 | 59.28 | 56.84 | -9.30 | < .001 | 66.66 | 66.90 | 67.56 | 63.97 | -4.04 | 0.044 | 0.062 |
| SE | 2.33 | 2.32 | 2.24 | 2.15 | 2.42 | 2.44 | 2.48 | 2.35 | ||||||
| WHO-5 | Mean | 14.24 | 14.94 | 14.75 | 16.04 | 12.64 | 0.01 | 12.87 | 12.92 | 12.68 | 14.47 | 12.43 | 0.011 | 0.782 |
| SE | 0.87 | 0.93 | 0.92 | 1.01 | 0.77 | 0.78 | 0.77 | 0.88 | ||||||
| OSDI | Mean | 17.01 | 14.94 | 13.95 | 12.59 | -25.98 | 0.002 | 16.75 | 15.76 | 15.91 | 18.25 | 8.95 | .297 | 0.025 |
| SE | 2.37 | 2.39 | 2.40 | 2.41 | 2.32 | 2.32 | 2.34 | 2.35 | ||||||
| Placebo | Nutraceutical | p-valueb | ||||||||
| Week 0 | Week 12 | % change | p-valuea | Week 0 | Week 12 | % change | p-valuea | |||
| N | 40 | 43 | ||||||||
| BDNF pg/mL | Mean | 666.37 | 829.06 | 24.41 | < .001 | 575.45 | 840.74 | 46.1 | < .001 | 0.030 |
| SE | 37.92 | 46.93 | 31.38 | 46.51 | ||||||
| MDA ng/mL | Mean | 27.02 | 34.18 | 26.52 | 0.072 | 28.56 | 25 | -12.49 | 0.294 | 0.040 |
| SE | 3.94 | 4.96 | 3.99 | 3.53 | ||||||
| TNF-α pg/mL | Mean | 25.99 | 19.14 | -26.37 | 0.049 | 20.21 | 17.09 | -15.43 | 0.217 | 0.445 |
| SE | 7.4 | 5.44 | 5.49 | 4.66 | ||||||
| IL-6 pg/mL | Mean | 32.87 | 33.59 | 2.21 | 0.962 | 38.33 | 50.63 | 32.09 | 0.55 | 0.691 |
| SE | 11.05 | 11.21 | 12.48 | 16.82 | ||||||
| Skin carotenoid concentrations (RIU) | N | 44 | 45 | |||||||
| Mean | 29874 | 28341 | -5.13 | 0.143 | 31498 | 34383 | 9.16 | 0.014 | 0.006 | |
| SE | 1644 | 1590 | 1689 | 1872 | ||||||
| AE Class | Diagnosis or symptom | Placebo (n=50) | Nutraceutical (n=50) |
| Gastrointestinal | n | 0 (0%) | 4 (10%) |
| Stomach Pains | 0 (0%) | 1 (2%) | |
| Increased bowel movements | 0 (0%) | 1 (2%) | |
| Nausea | 0 (0%) | 2 (4%) | |
| Neurological | n | 1 (2%) | 2 (4%) |
| Low mood | 0 (0%) | 1 (2%) | |
| Tiredness | 1 (2%) | 0 (0%) | |
| Worsened sleep | 0 (0%) | 1 (2%) | |
| Dermatological | n | 0 (0%) | 1 (2%) |
| Minor skin discoloring (orange) | 0 (0%) | 1 (2%) | |
| All Adverse events | n | 1 (2%) | 4 (10.0%) |
| Number of participants experiencing treatment-related AE* | 1 (2%) | 5 (10%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





