Submitted:
08 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cottonseed Sample
2.2. Preparation of Seed Material
2.3. Extraction with Petroleum Ether/Diethyl Ether
2.4. Precipitation of Gossypol as GAA
2.5. Purification and Recrystallization
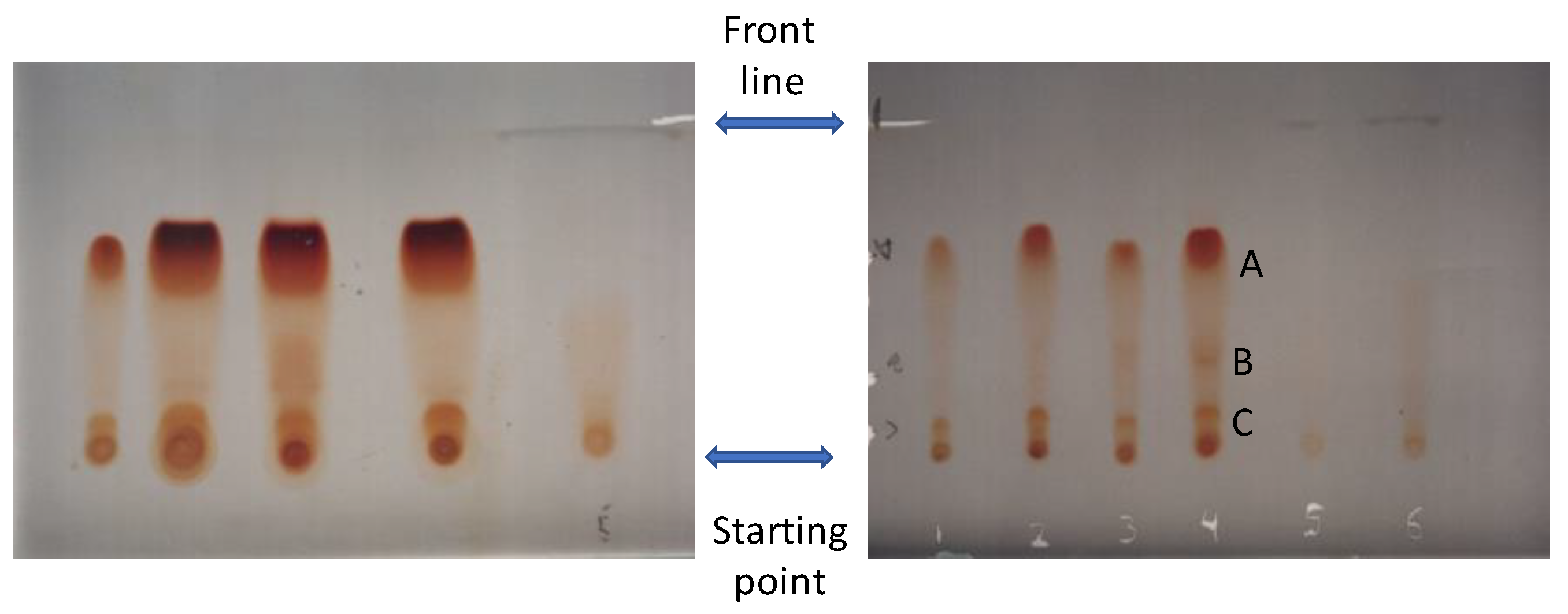
2.7. Preparative TLC for Purification of GAA
2.8. UV Spectrophotometer Analysis for the Spectra of GAA
2.9. GAA Standard Curve
2.10. Determination of the GAA (Separated by TLC) Using HPLC
2.11. Silica Gel Column Isolation and HPLC Quantification of Gossypol from Egyptian and Chinese Cottonseeds
2.12. HPLC Method for Analysis of GAA in Egyptian and Chinese Cottonseeds
2.13. Melting Point of GAA
2.14. Infrared Spectral Measurements for GAA
3. Results and Discussion
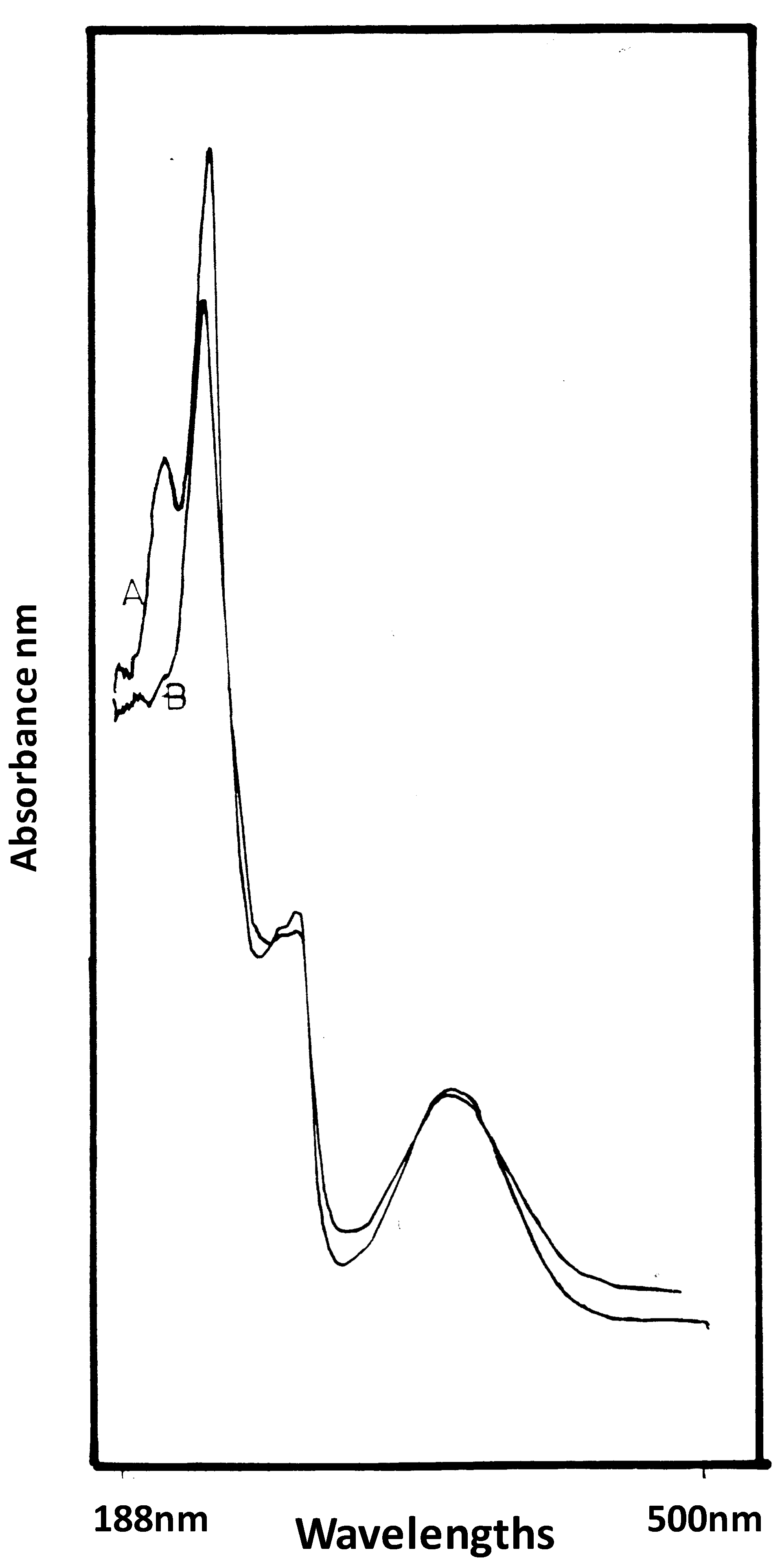
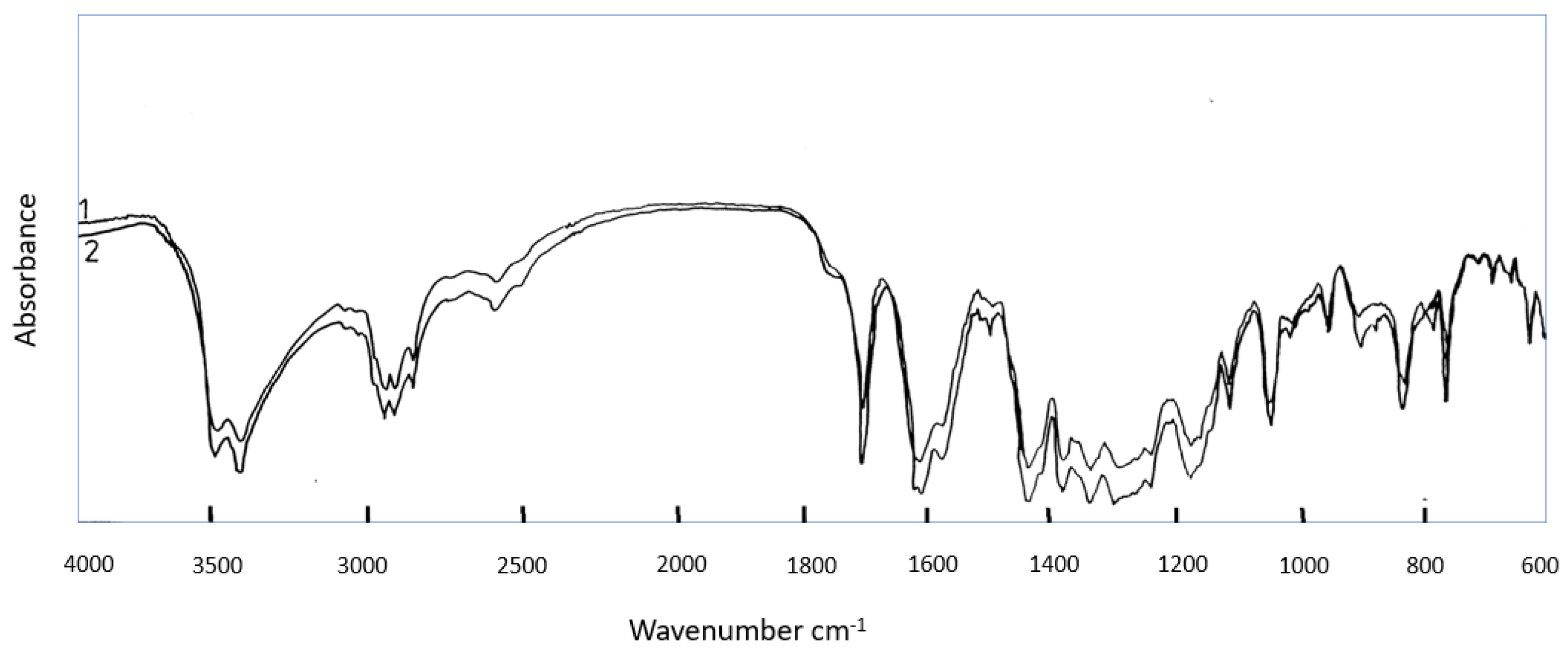
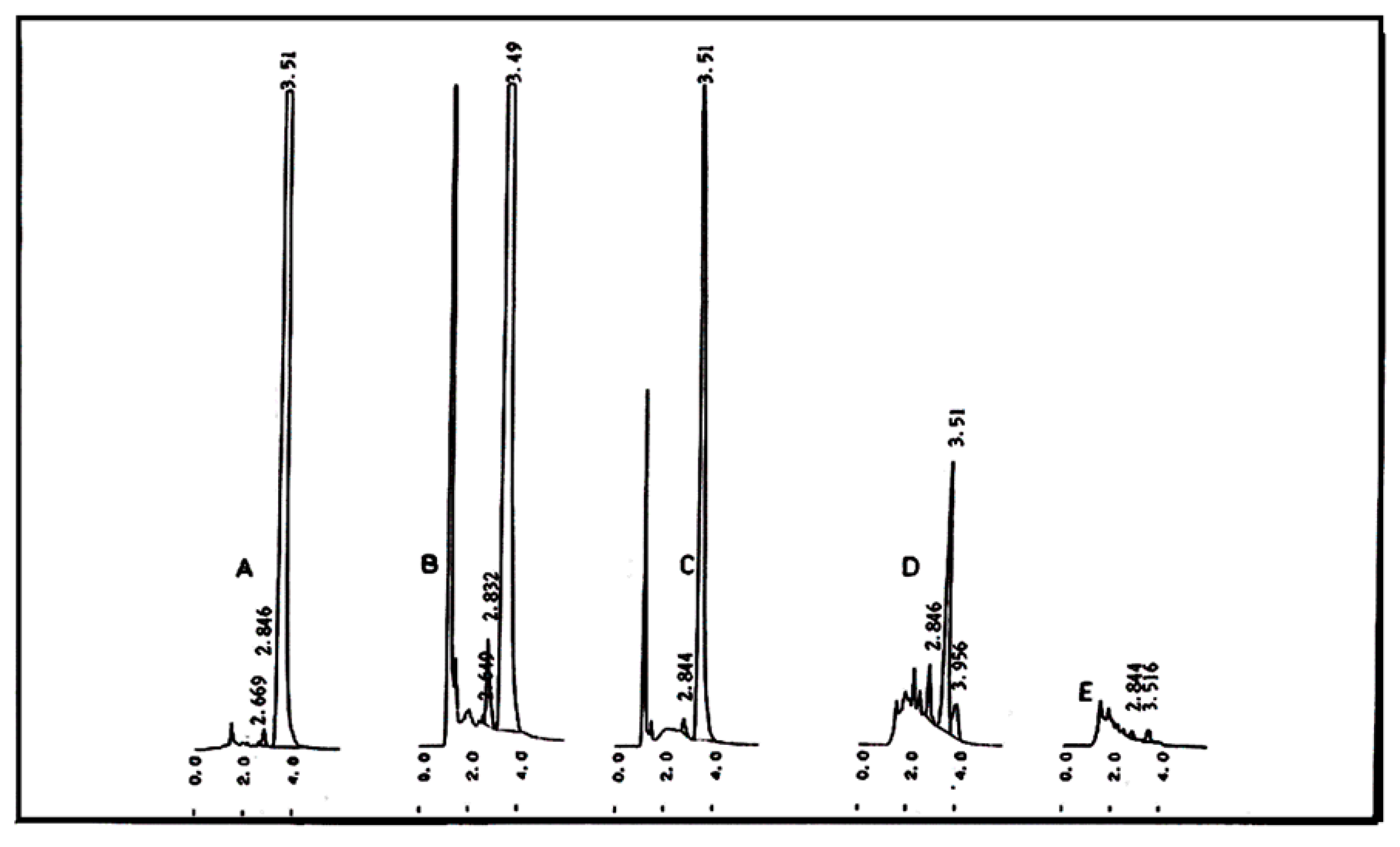
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Jiang, H.; Cao, X.; Zhao, H.; Wang, F.; Cui, Y.; Jiang, B. Chiral gossypol derivatives: Evaluation of their anticancer activity and molecular modeling. European Journal of Medicinal Chemistry 2009, 44, 3961-3972. [CrossRef]
- Borovskaya, T.G. Preclinical and clinical evidence of safety of antiviral drug with immunomodulatory activity. Serbian Journal of Experimental and Clinical Research 2018, 19, 271-276. [CrossRef]
- Borovskaya, T.G. Safety of the russian antiviral drug kagocel. Terapevticheskii arkhiv 2017, 89, 93-99. [CrossRef]
- Chang, Q.; Liu, Z.; Ma, W.Z.; Hei, C.C.; Shen, X.S.; Qian, X.J.; Xu, Z.L. Drug synergistic antifertility effect of combined administration of low-dose gossypol with steroid hormones in rats. Chinese medical journal 2011, 124, 1678-1682.
- Kalla, N.R.; Gadru, N.; Foo, T.W. Studies on the male antifertility agent gossypol acetic acid vii. Effect of motility stimulated factors on the revival of human spermatozoal motility after gossypol treatment in vitro. Andrologia 2009, 18, 393-397. [CrossRef]
- Waites; Wang; Griffin. Gossypol: Reasons for its failure to be accepted as a safe, reversible male antifertility drug. International Journal of Andrology 1998, 21, 8-12.
- Lin, J.; Wu, Y.; Yang, D.; Zhao, Y. Induction of apoptosis and antitumor effects of a small molecule inhibitor of bcl-2 and bcl-xl, gossypol acetate, in multiple myeloma in vitro and in vivo. Oncology reports 2013, 30, 731-738. [CrossRef]
- Amara, A.A.; El-masry, M.H.; Bogdady, H.H. Plant crude extracts could be the solution: Extracts showing in vivo antitumorigenic activity. Pak. J. Pharm. Sci. (2008, 21, 159-171.
- Awny, H.A.; Amara, A.A.; El-Masry, M.H.; Baghdadi, H.H. Effect of 12 plant extract on hepatic microsomal penzo(a) pyrene hydroxylation and hydrogen peroxide production in mice treated with lendane. J. Environmental Nutritional Interaction 1997, 1, 129-142.
- Blasko, G.; Cordell, G.A. Recent developments in the chemistry of plant-derived anticancer agents. Academic Press, U.K.: 1988.
- Budavari, S.; Maryadele, J.; Neil, O. The merek index. Eleventh edition on encyclopedia of chemicals, drugs and biologicals. (ed. Pp. . . Merck & Co. Inc. Rahway, N. J. U.S.A. : 1989.
- Martinez, F.; R., M.; Espinosa, G.T.; Pardo, J.P. The antifirtility agent, gossypol, releases calcium from rat liver mitochondria. Comp. Biochem. Physiol. C. 1993, 104, 165-169.
- Rosenberg, L.J.; Adlakha, R.C.; Desai, D.M.; Rao, P.N. Inhibition of DNA polymerase α by gossypol. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1986, 866, 258-267.
- Zeng, Y.; Ma, J.; Xu, L.; Wu, D. Natural product gossypol and its derivatives in precision cancer medicine. Current Medicinal Chemistry 2019, 26, 1849-1873. [CrossRef]
- Bulut, G.; Atmaca, H.; Karaca, B. Trastuzumab in combination with at-101 induces cytotoxicity and apoptosis in her2 positive breast cancer cells. Future oncology (London, England) 2020, 16, 4485-4495. [CrossRef]
- Degirmenci, M.; Erdogan, A.P.; Bulut, G.; Atmaca, H.; Uzunoglu, S.; Karaca, B.; Karabulut, B.; Uslu, R. Octreotide in combination with at-101 induces cytotoxicity and apoptosis through up-regulation of somatostatin receptors 2 and 5 in du-145 prostate cancer cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2016, 37, 4939-4944. [CrossRef]
- Majumdar, S.K.; Daly, E.P.; Kleemeyer, K.M.; Daehler, C.C.; Baker, M.A. Genotoxic effects of gossypol acetic acid on cultured murine erythroleukemia cells. Environmental and molecular mutagenesis 1991, 18, 212-219. [CrossRef]
- Mayer, M.; Berger, A.; Leischner, C.; Renner, O.; Burkard, M.; Böcker, A.; Noor, S.; Weiland, T.; Weiss, T.S.; Busch, C., et al. Preclinical efficacy and toxicity analysis of the pan-histone deacetylase inhibitor gossypol for the therapy of colorectal cancer or hepatocellular carcinoma. Pharmaceuticals 2022, 15, 438. [CrossRef]
- Benvenuto, M.; Mattera, R.; Masuelli, L.; Taffera, G.; Andracchio, O.; Tresoldi, I.; Lido, P.; Giganti, M.G.; Godos, J.; Modesti, A., et al. (±)-gossypol induces apoptosis and autophagy in head and neck carcinoma cell lines and inhibits the growth of transplanted salivary gland cancer cells in balb/<i>c</i> mice. International Journal of Food Sciences and Nutrition 2016, 68, 298-312.
- Cao, H.; Sethumadhavan, k.; Cao, F.; Wang, T. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. American Chemical Society (ACS): 2021. [CrossRef]
- Chang, C.J.; Ghosh, P.K.; Hu., Y.F.; Brueggemeier, R.W.; Lin., Y.C. Antiproliferative and antimetastatic effects of gossypol on dunning prostata cell-bearing copenhagen rats. Res. Commun .Chem. Pathol. Pharmacol 1993, 79, 293-312.
- Flack, M.R.; Pyle, R.G.; Mullen, N.M.; Lorenzo, B.; Wu, Y.W.; Knazek, R.A. Oral gossypol in the treatment of metastatic adrenal cancer. . J. Clin. Endocarin. Matab. 1993, 74, 1019-1024. [CrossRef]
- Orhan, G.; Ekmekçi, A.; Menev§e, S. The effect of the male contraceptive agent gossypol acetic acid on mouse bone marrow cells in vivo: Micronuclei and mitotic index. Contraception 1993, 47, 377-385. [CrossRef]
- Zaidi, R.; Hadi, S.M. Strand scission in DNA by gossypol and cu(ii): Role of cu(i) and oxygen-free radicals. Journal of Biochemical Toxicology 1992, 7, 213-217. [CrossRef]
- Zaidi, R.; Hadi, S.M. Complexes involving gossypol, DNA and cu (2). Biochemi .Int. 1992, 28, 1135-1143.
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta medica 2010, 76, 1075-1079. [CrossRef]
- Atmaca, H.; Gorumlu, G.; Karaca, B.; Degirmenci, M.; Tunali, D.; Cirak, Y.; Purcu, D.U.; Uzunoglu, S.; Karabulut, B.; Sanli, U.A., et al. Combined gossypol and zoledronic acid treatment results in synergistic induction of cell death and regulates angiogenic molecules in ovarian cancer cells. European Cytokine Network 2009, 20, 121-130. [CrossRef]
- Huang, Y.W.; Wang, L.S.; Dowd, M.K.; Wan, P.J.; Lin, Y.C. (-)-gossypol reduces invasiveness in metastatic prostate cancer cells. Anticancer Res 2009, 29, 2179-2188.
- Tai-Shun, L.; Schinazi, R.F.; Zhu, J.; Birks, E.; Carbone, R.; Yikang, S.; Kemei, W.; Liang, H.; Prusoff, W.H. Anti-hiv-1 activity and cellular pharmacology of various analogs of gossypol. Biochemical Pharmacology 1993, 46, 251-255. [CrossRef]
- Yang, J.; Li, L.-L.; Li, J.-R.; Yang, J.-X.; Zhang, F.; Chen, G.; Yu, R.; Ouyang, W.-J.; Wu, S.-W. Synthesis and biological evaluation of water-soluble derivatives of chiral gossypol as hiv fusion inhibitors targeting gp41. Bioorganic & Medicinal Chemistry Letters 2018, 28, 49-52. [CrossRef]
- González-Garza, M.T.; Matlin, S.A.; Mata-Cárdenas, B.D.; Said-Fernández, S. Differential effects of the (+)- and (–)-gossypol enantiomers upon <i>entamoeba histolytica</i> axenic cultures. Journal of Pharmacy and Pharmacology 1993, 45, 144-145.
- Lin, T.S.; Schinazi, R.; Griffith, B.P.; August, E.M.; Eriksson, B.F.; Zheng, D.K.; Huang, L.A.; Prusoff, W.H. Selective inhibition of human immunodeficiency virus type 1 replication by the (-) but not the (+) enantiomer of gossypol. Antimicrobial Agents and Chemotherapy 1989, 33, 2149-2151. [CrossRef]
- Polsky, B.; Segal, S.J.; Baron, P.A.; Gold, J.W.M.; Ueno, H.; Armstrong, D. Inactivation of human immunodeficiency virus in vitro by gossypol. Contraception 1989, 39, 579-587. [CrossRef]
- Radloff, R.J.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L. Antiviral activities of gossypol and its derivatives against herpes simplex virus type ii. Pharmacological Research Communications 1986, 18, 1063-1073. [CrossRef]
- Royer, R.; Mills, R.; Deck, L.; Mertz, G.; Vanderjagt, D. Inhibition of human immunodeficiency virus type i replication by derivatives of gossypol. Pharmacological Research 1991, 24, 407-412. [CrossRef]
- Vagabov, R.M.; Barinskii, I.F.; Dushanbieva, S. [comparative study of the effectiveness of the interferon inducers, poly(i) : Poly(c) and gss (gossypol-beta-aminoethylsodium sulfate), in combination with a specific vaccine in experimental infection with the human acute encephalomyelitis virus]. Vopr Virusol 1980, 334-337.
- Wichmann, K.; Vaheri, A.; Luukkainen, T. Inhibiting herpes simplex virus type 2 infection in human epithelial cells by gossypol, a potent spermicidal and contraceptive agent. American Journal of Obstetrics and Gynecology 1982, 142, 593-594. [CrossRef]
- Carruth, F.E. Contribution to the chemistry of gossypol, the toxic principle of cottonseed. Journal of the American Chemical Society 1918, 40, 647-663.
- Dehe, J.; Xiuhong, X.; Haibao, S.; Jinfang, Z. Thin-layer chromatoghraphy of gossypol. YaoxueTongbao 1981, 16, 14.
- Frampton, V.L.; Edwards, J.D.; Henze, H.R. The ultraviolet absorption spectrum of gossypol<sup>1</sup>. Journal of the American Chemical Society 1948, 70, 3944-3945.
- Stipanovic, R.D.; Altman, D.W.; Begin, D.L.; Greenblatt, G.A.; Benedict, J.H. Terpenoid aldehydes in upland cottons: Analysis by aniline and hplc methods. Journal of Agricultural and Food Chemistry 1988, 36, 509-515. [CrossRef]
- Jaroszewski, J.; Strøm-Hansen, T.; Hansen, S.; Thastrup, O.; Kofod, H. On the botanical distribution of chiral forms of gossypol. Planta medica 1992, 58, 454-458. [CrossRef]
- Botsoglou, N.A. Liquid chromatographic determination of unbound and acetone-soluble bound gossypol in cottonseed meals and mixed feeds. Journal of AOAC INTERNATIONAL 1992, 75, 815-823. [CrossRef]
- O`Conner, R.T.; Von, D.H.; Dufre, E.F.; Brown, L.E.; Pominski, C.H. The infra-red spectra of gossypol. J. Amer. Chem. Soc. 1954, 76, 2368.
- Nakanishik. Infra-red absorption spectroscopy. Holden Day Inc., San Francisco, USA.: 1962.
- Câmara, A.C.; Gadelha, I.C.; Borges, P.A.; de Paiva, S.A.; Melo, M.M.; Soto-Blanco, B. Toxicity of gossypol from cottonseed cake to sheep ovarian follicles. PloS one 2015, 10, e0143708. [CrossRef]
- Fash, R.H. The effect of crude bollie cottonseed oil upon the color of refined oil from stored crude cottonseed oil. Oil & Soap 1934, 11, 106-109. [CrossRef]
- Kramer, R.Y.; Garner, D.L.; Ericsson, S.A.; Wesen, D.A.; Downing, T.W.; Redelman, D. The effect of cottonseed components on testicular development in pubescent rams. Veterinary and human toxicology 1991, 33, 11-16.
- Tso, W.W.; Lee, C.S. Cottonseed oil as a vaginal contraceptive. Archives of andrology 1982, 8, 11-14. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




