1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is an endemic disease in all countries with large pig holdings [
1]. This disease affects pigs of all ages and has a strong economic impact on the pig industry, with losses reaching up to
$2.5 billion a year in the US and Europe alone [
2]. The disease is caused by the similarly named Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), which is a small, enveloped virus measuring 45-70 nm in diameter. Its genome consists of a polyadenylated, single-stranded, non-segmented, positive-sense RNA polycystronic molecule ranging from 15.1-15.5 kb. PRRSV belongs to the family
arteriviridae, in the genus Arterivirus, order Nidovirales [
3]. Two genotypes of PRRSV have been reported: type 1 or European and type 2 or American [
4]. PRRSV has shown a high capacity for infection and transmission through the oronasal and reproductive routes [
5]. It causes high rates of abortions, mummified fetuses, and post-weaning mortality due to the birth of weak piglets. Perinatal mortality reaches 70% [
6].
Several vaccine formulations have been developed against PRRS; some of these have shown low efficacy, while others are still in the experimental phase [
7]. Current commercial vaccines against PRRSV include modified live viruses (MLV) or inactivated viruses [
8,
9]. These vaccines provide limited protection against homologous strains and little or no protection against heterologous strains, which are circulating and mutating in the field [
10]. For instance, the antibody protection following vaccination is low and may result in antibody-dependent enhancement, facilitating virus entry [
11]. The high genetic and antigenic diversity presents a significant impediment to developing an effective vaccine to control PRRS. In addition, it is known that in some cases, attenuated vaccine viruses revert virulence under field conditions [
9], and are also associated with the appearance of severe outbreaks due to the vaccine virus reverting to a pathogen status [
12].
Therefore, research into safe and effective antigens is necessary to induce an effective immune response.
Structural glycoprotein GP5 has been reported as a promising candidate for vaccine development, as it is one of the immunodominant epitopes inducing a high concentration of neutralizing antibodies, likely containing B cell epitopes [
13,
14,
15,
16,
17]. To induce effective anti-PRRSV immunity, exposing immunogenic epitopes is crucial for the induction of efficient innate and adaptive immune response mediated by specific-antibodies, cytokines, and T cell responses. Compared to commercial vaccines, peptide vaccines do not contain nucleic acid substances; therefore, they are considered safer. Peptides contain only B or T epitopes that induce a specific response. Besides, adjuvants can enhance T or B cells responses by enhancing phagocyting activity and secreting a variety of cytokines that boost the specific immune response to the vaccine. The goal of this work was to determine the immunogenic response in swine elicited by two synthetic peptides derived from the GP5 of PRRSV.
2. Materials and Methods
2.1. Peptides
The GP5-B and GP5-B3 peptides contain epitopes that match the peptide sequence within the ectodomain of the GP5 protein (residues 30 to 62) from PRRSV type 2 (Sequence ID: UTS56108.1). Our research group has previously studied the GP5-B peptide, while the GP5-B3 peptide encompasses a pair of epitopes previously described [
17]. Both peptides demonstrated high antigenicity in silico, according to the Immunoepitope database IEDB and CCL Main Workbench 20.0 (unpublished data).
The synthesis method to obtain the peptides was solid-phase standard synthesis (GenScript, Piscataway, NJ, USA). The GP5-B peptide sequence, ASNDSSSHLQLIYNLTLCELNGTDWLANKF (30 aa), and the GP5-B3 peptide sequence, SSSNLQLIYNLTTPVTRVSAEQWGRPC (27 aa), exhibited 95% and 98% purity, respectively. We modified the GP5-B3 peptide by adding a cysteine at the terminal carboxyl end to facilitate binding with a carrier protein. The stock solution (1 mg/mL), for each peptide, was prepared in dimethyl sulfoxide (DMSO).
Using the Protein BLAST database, we conducted a comparison of the GP5-B and GP5-B3 peptide sequences against types -1, -2, and high pathogenic PRRSV. This analysis showed that GP5-B peptide sequences matched PRRSV type 1 between 93-100%, PRRSV type 2 between 96-100%, and high pathogenic PRRSV (HP-PRRSV) between 94-100%. In contrast, GP5-B3 peptide sequences exhibited query coverage ranges of 77-96% for PRRSV type 1, 92-96% for PRRSV type 2, and 90-96% for HP-PRRSV.
2.2. Experimental Design, Animals, and Housing
The research was conducted on piglets procured from a certified farm (El DAPO farm, which conducts Good Livestock Practices), where periodic testing ensured a negative status for PCV2 and PRRSV. Piglets, 21 days old, weaned, of indistinct sex, and interbreed large white x pietrain, selected randomly from six different litters. All piglets received iron supplementation and vaccines as described previously [
18].
Twenty-eight piglets were moved to alternative experimental stockyards larger than 33.6 m2. Each pig was ear-tagged and distributed randomly across two stockyards. Using the GraphPad-Random number tool, each animal was randomly allocated to one of four treatment groups, with each group comprising seven individuals.
The immunization scheme was conducted as previously outlined by Calderon-Rico et al., 2023 [
18] with seven pigs per group and 800 µl of inoculum. Briefly, the control group (PBS), the vehicle group (1: 1 PBS and aluminum hydroxide). The experimental groups, GP-5 and GP5-B3, received 200 µg of peptide (1 mg/mL), 200 µg of Maleimide Activated BSA (1 mg/mL) (Thermo Fisher), and 400 µL alhydrogel (InvivoGen, San Diego, CA, USA).
Pigs were visually monitored daily and weighed every 21 days and maintained as previously described, following the applicable guidelines under the approved study protocol CICUMSNH-A101-FMVZ [
18].
2.3. Collection and Analysis of Blood and Serum Samples
At 2- and 42-days post-treatment, 15 ml blood samples were drawn via the jugular vein, with and without EDTA (Vacutainer, BD, NJ, USA) from the control, vehicle, GP5-B, and GP5-B3 peptide groups at “El DAPO farm”. The samples were used no later than 3 hours after collection. The protocol to isolate Peripheral Blood Mononuclear Cells (PBMC) was carried out as described previously [
18].
2.4. Serum Sampling from Immunized Pigs with a Commercial Vaccine
Serum samples from vaccinated piglets were donated from “El DAPO farm”. Animals were maintained on the same farm throughout the experiment. The Ingelvac-PRRSV, MLV® (Modified-Live Virus) group was vaccinated with a single dose. According to the manufacturer’s manual, the active substance contained the strain virus ATCC VR 2332, and the amount of the virus in the vaccine was 104.9DICC50, 2ml/piglet. Serum samples were collected at 42 days post-immunization (dpi).
2.5. Serum Samples from PRRSV Infected Pigs
Serum samples from naturally infected and non-vaccinated pigs were donated by “El Limon Farm”, located in Tarimbaro, Michoacan, Mexico, during July 2023. A total of seven 16-week-old pigs were sampled.
2.6. Quantification of Cytokine Concentrations in Serum
The cytokine concentration in the serum was determined as described before [
18].
2.7. Antibody Detection
Peptide-specific antibody analysis was performed using an indirect Enzyme-Linked Immunosorbent Assay (ELISA) to quantify immunoglobulins G (IgGs) against each of the GP5-B and GP5-B3 peptides. The commercial Peptide Coating Kit (Takara, Shiga, Japan) was used for the ELISA test. Following the manufacturer’s protocols, the 96-well plate was coated with each peptide at a concentration of 4 µg/ml for 2 h at room temperature (RT) and then the plate was blocked with a block solution for 2 h (solution provided in the kit) at RT. The plates were washed three times with distilled water. Then, serum samples from piglets were diluted 1:100 in phosphate-buffered saline-T (PBS containing Tween-20 at 0.05%) containing 0.3% gelatin and added to the plate. After incubating overnight at 4 ℃, three washes were performed and the plates were incubated with the goat anti-porcine-IgG (H+L)-HRP antibody (SouthernBiotech, Birmingham, AL, USA) in dilution 1:1000 for 2 h at RT. The plate was washed three times with PBS-T, after which the substrate was added, and the plate was read at 15 min. The reading was done at OD450 in a plate microreader (Bio-Rad iMARK microplate reader). The results are shown in [ng/ml] according to the IgG´s calibration curve.
2.8. Immunoreactivity of Peptides against Serum Antibodies
The immunoreactivity assay was performed using an indirect ELISA with the Peptide Coating Kit, as described above. Immunoreactivity was assessed in serum samples from both the vaccinated group and the non-vaccinated, naturally infected group. Results are presented in [ng/ml], based on the IgG´s calibration curve.
2.9. Flow Cytometry
Cytometry was performed with approximately 1×106 cells/ml of PBS per sample. The instrument used was the Attune NxT acoustic focusing cytometer (Thermo Fisher Scientific, Carlsbad, CA, USA). The cytometer contains a violet (405 nm) and a blue laser (488 nm). Cells were stained with antibodies coupled to fluorochromes to detect specific surface markers of antibody-secreting B cells. The analysis targeted three subpopulations of B cells: IgM+/CD2+/CD21+ (Naïve); CD2+/CD21- (Plasmocytes) and CD2-/CD21+ (Primed). Surface markers were used for triple staining enabling primary antibodies to form combinations of goat-anti-pig IgM-FitC (MBS224956, MyBioSource, USA) with anti-pig-CD2-PECy7 (RPA-2.10, eBioscience, San Diego, CA, USA) and anti-pig-CD21-PE (BB6-11C9.6, Invitrogen, Carlsbad, CA, USA).
For the analysis, 10,000 individual event packages were captured within the singlets gate, and a dot plot contrasting forward scatter-A with forward scatter-H was generated to exclude doubles. Additionally, a linear dot plot comparing side scatter to linear forward scatter was utilized to delineate the lymphocytes among the singlets. Another panel from the lymphocytes compared BL1-IgM+ cells against the linear scatter side. Finally, a quadrant panel analysis of IgM+ identified four subpopulations of IgM+ B cells containing CD2-/CD21-, CD2+/CD21+CD2+/CD21- and CD2-/CD21+ (
Figure 4). The results from the subpopulations are presented as the percentage of cells that tested positive.
2.10. Statistical Analysis
The data represent three different measurements for each animal and are presented as means ± standard error of the mean (SEM). Differences between groups were estimated using a one-way ANOVA test with Tukey´s pos hoc analysis. A P-value < 0.05 was considered statistically significant. The GraphPad Prism software (version 9.1.1, GraphPad Software, San Diego, USA) was used. Software (BD®)FlowJo V10 was used to analyze cytometry data.
3. Results
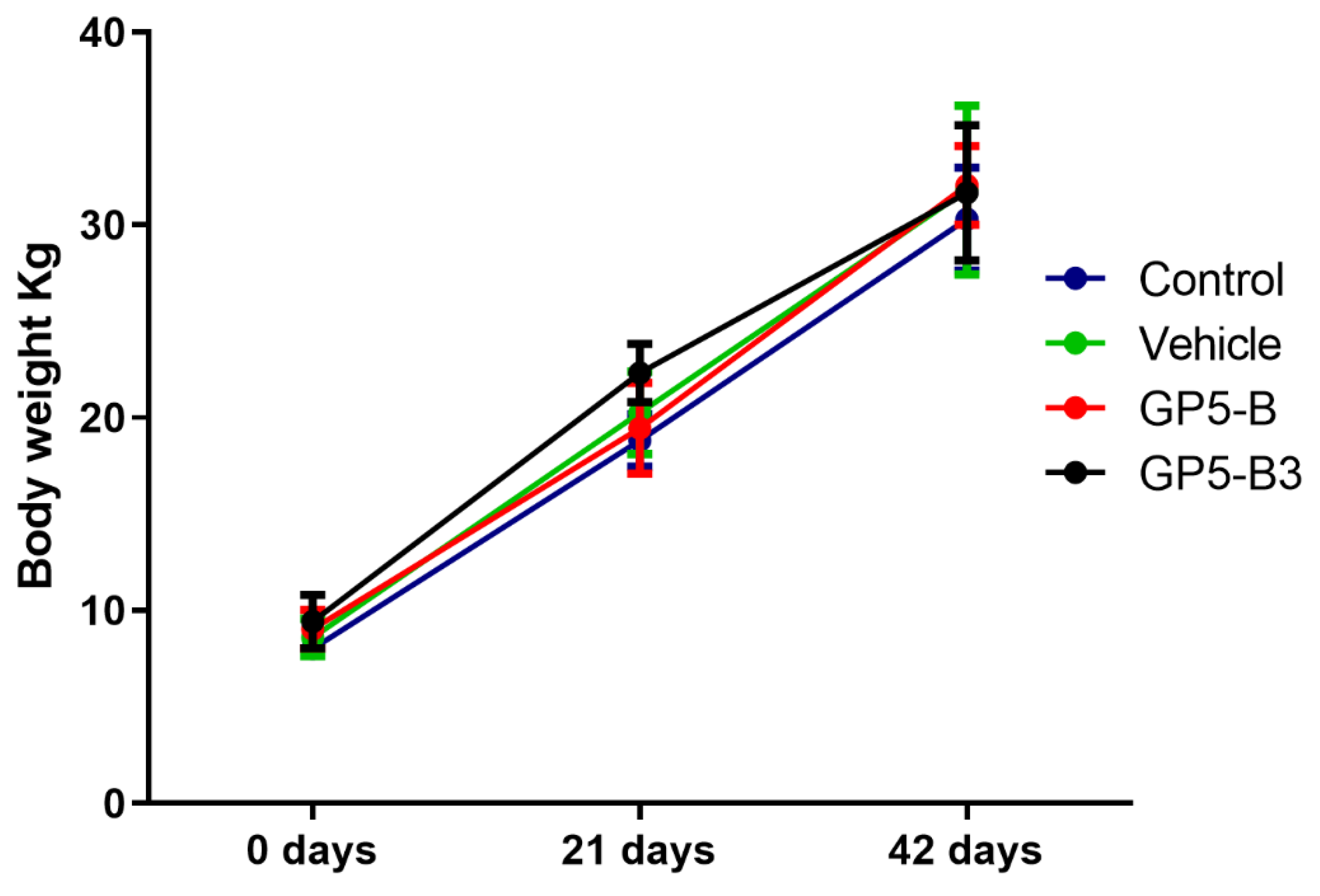
3.1. Body Weight Monitoring after Immunization
Body weight was monitored from Day 0 to Day 42. All animals maintained a constant body condition and gained weight throughout the study (
Figure 1). There are non-significant differences between the control and peptide-immunized groups. Furthermore, no adverse reactions to immunization were observed in any of the four groups.
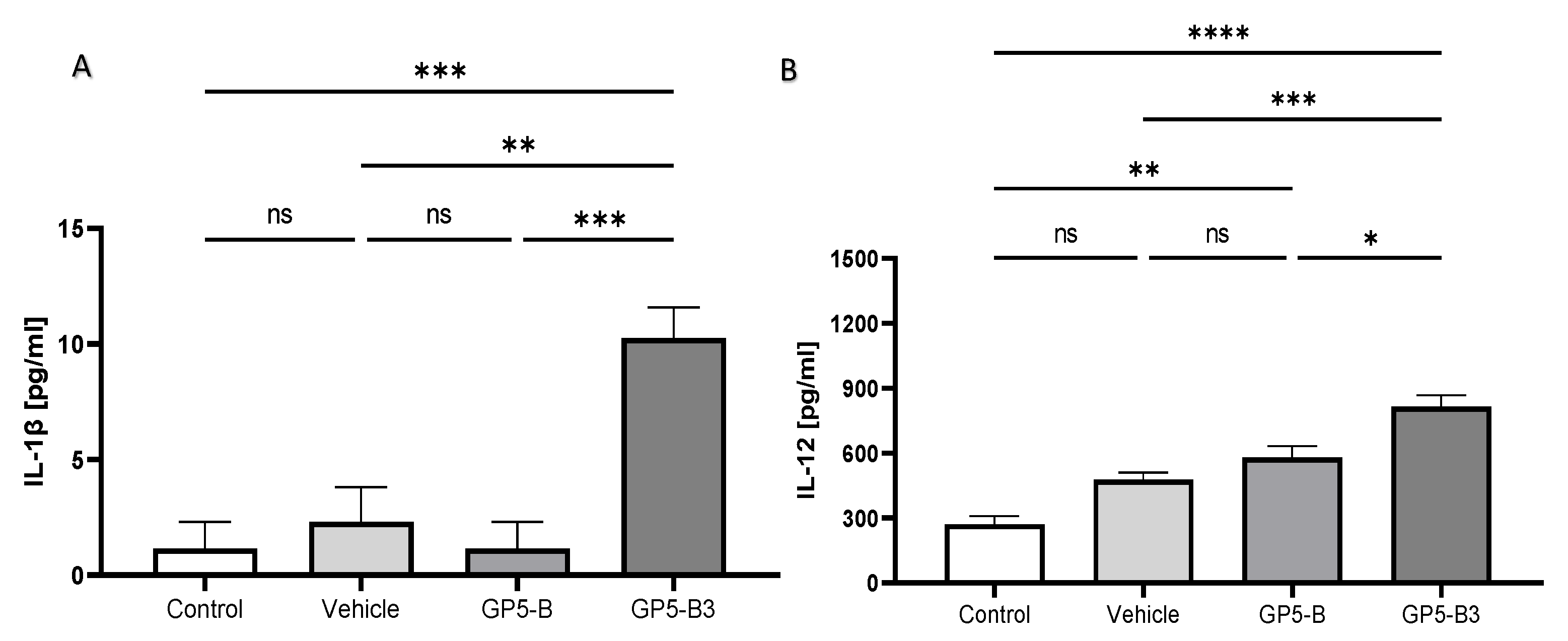
3.2. GP5-B3 Peptide Enhanced the Level of Proinflammatory Cytokines in Sera
Cytokines concentrations were measured in serum samples collected at 2 dpi to determine the proinflammatory state. Pigs immunized with GP5-B3 exhibited significantly higher serum concentrations of IL-1β and IL-12 than the control and vehicle groups (
Figure 2A,B). There were no significant differences in cytokine serum concentrations between the GP5-B group and the control group. This observation implies that the GP5-B3 peptide might trigger a proinflammatory response following immunization. To assess the immunomodulatory effects at the onset of antigen processing and presentation, cytokine levels were measured 48 hours post-immunization.
3.3. GP5-B and GP5-B3 Induce Specific IgG-Mediated Response upon Immunization
The group immunized with the GP5-B peptide showed a significant increase in anti-GP5-B IgGs at 21 and 42 dpi compared to Day 0 (pre-immune). Additionally, a significant increase was observed at 42 dpi compared to 21 dpi. In the case of the GP5-B group, a sustained increase in IgGs concentration was observed (
Figure 3A). Conversely, in the group immunized with the GP5-B3 peptide, a significant induction of anti-GP5-B3 IgGs was observed only at 21 dpi relative to Day 0. No induction of IgGs was observed at 42 dpi; thus, the IgGs increase observed at 21 dpi remained at the same concentration until 42 dpi (
Figure 3B). The data demonstrate a clear IgG-mediated response following immunization with the peptides GP5-B or GP5-B3.
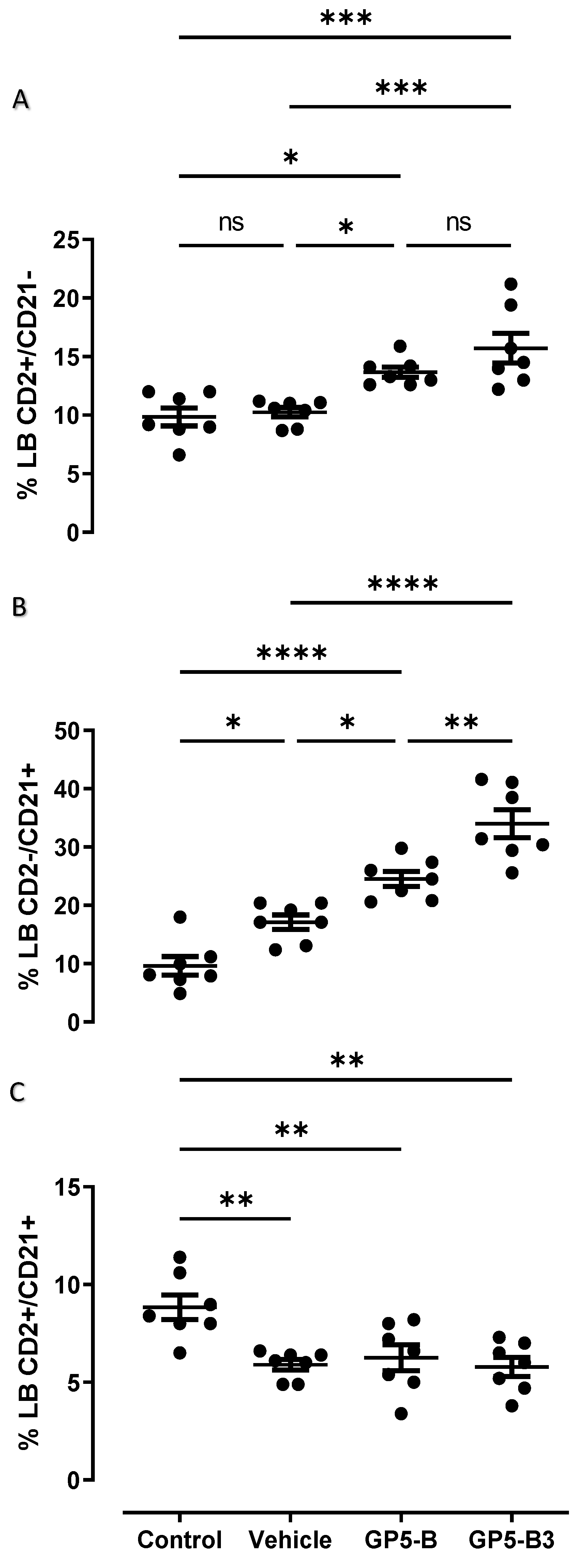
3.4. Peptides Prime B Cells and Induce the Generation of Antibody Secreting Cells
Immunotyping of B cells was performed at 42 dpi, and percentages of IgM cells and subpopulations (CD2+/CD21-; CD2-/CD21+; CD2+/CD21+) were evaluated for four experimental groups: control, vehicle, GP5-B and GP5-B3 groups. The results showed that, in the case of B cells, the groups immunized with GP5-B or GP5-B3 peptides increased the percentage of antibody-forming/plasma B cells (CD2+/CD21-) compared to the control group and the vehicle group; however, no differences were observed between the two peptide-immunized groups (
Figure 5A). This suggests that the peptides GP5-B and GP5-B3 induce the generation of antibody-secreting cells.
On the other hand, the primed B cells subpopulation CD2-/CD21+ showed a statistically significant increase in the groups immunized with the peptides GP5-B and GP5-B3 compared to the control and vehicle groups. Additionally, in the case of the GP5-B3 group, there was a statistically significant increase compared to the group immunized with the GP5-B peptide (
Figure 5B), indicating that the GP5-B3 peptide was the most effective inducer of primed B cells.
Finally, the evaluation of naïve B cells (CD2+/CD21+) showed a statistically significant reduction across all experimental groups compared to the control group. However, no differences were observed in the percentage of these cells among the three groups (
Figure 5C). This suggests that, in response to peptide immunization, the naïve cell subpopulation was reduced, but this reduction was also seen in response to the BSA carrier in equal proportions.
Figure 4.
Panel for analysis of B cell subpopulations. Humoral immune response was induced in B cell subpopulations of piglets immunized with synthetic peptides. The cells were recovered at day 42 dpi. The marking was made with anti-CD2 and anti-CD21 markers. The graphs show the selection strategy for analyzing CD2+/CD21+; CD2-/CD21+ and CD2+/CD21- B cells.
Figure 4.
Panel for analysis of B cell subpopulations. Humoral immune response was induced in B cell subpopulations of piglets immunized with synthetic peptides. The cells were recovered at day 42 dpi. The marking was made with anti-CD2 and anti-CD21 markers. The graphs show the selection strategy for analyzing CD2+/CD21+; CD2-/CD21+ and CD2+/CD21- B cells.
3.5. GP5 Epitopes Elicit In Vitro Immunoreactivity in Sera from Naturally Infected and Vaccinated Pigs
Our next question was whether the B epitopes contained in GP5-B or GP5-B3 peptides could be conserved epitopes capable of inducing antibodies during natural infection with PRRSV or after MLV vaccination.
Figure 6 illustrates the immunoreactivity of both GP-B and GP5-B3 peptides against serum antibodies from naturally infected and MLV vaccinated animals. The response against the two peptides was significantly higher in the group of vaccinated animals than in those naturally infected. However, the naturally infected group also exhibited significant immunoreactivity against the two peptides compared to the immunized control group. Interestingly, this indicates that the B epitopes in GP5-B or GP5-B3 are conserved in circulating wild viruses.
4. Discussion
PRRSV-specific IgG neutralizing antibodies are produced 3–4 weeks after infection [
19], which is too late to stop the acute phase of viremia [
19]. The use of epitopes that are potential inducers of neutralizing antibodies is a topic of great importance in the research of safe and effective vaccines [
19].
The humoral immune response to PRRSV infection has been frequently studied, especially the one directed to different envelope proteins (GP3, GP4, GP5 or M) or linear epitopes from these proteins [
10,
13,
20,
21]. In the present study, we evaluated the humoral response induced by two synthetic peptides derived from the GP5 protein of PRRSV-2 (GP5-B and GP5-B3) in a relevant model. These peptides contain linear epitopes recognized by B cells. In particular, the response mediated by peptide-specific antibodies and B-cell activation was analyzed. This allowed us to evaluate immunogenic epitopes within the GP5 protein.
Immunization of piglets with the GP5-B and GP5-B3 peptides showed a significant increase in the concentration of specific IgGs after 21 days after primary immunization in both cases. However, only the GP5-B group showed a statistically significant increase after secondary immunization, suggesting a strong and sustained response, indicating the presence of active antibody producing cells. Conversely, GP5-B3 group did not show a specific increase in IgGs after secondary immunization; nevertheless, its IgG levels remained elevated until 42 dpi, indicating the presence of antibody-forming/Plasma B cells [
22].
The presence of memory B cells allows antigen-specific antibodies to be rapidly generated when the antigen that induced them comes into contact with these cells a second time, resulting in a faster and stronger humoral response [
23].
Figure 3A,B show that at Day 0, the animals, in a pre-immune state, exhibited a basal concentration of specific antibodies against GP5-B (
Figure 3A) and GP5-B3 (
Figure 3B). This could be explained by nonspecific binding of antibodies to the ELISA plate or a cross-reaction with the assay´s microarrangement [
24,
25]. Another potential explanation for these readings at Day 0 is the presence of maternal antibodies, which were likely transferred from the mother to the piglets via colostrum. According to Guzman-Bautista et al
., 2013 [
26], maternal antibodies in pigs can provide protection against respiratory infections through IgG, the predominant isotype in pig breast milk. These maternal antibodies also provide protection through the neutralization of pathogens and the recruitment of innate immunity effector molecules through the Fc domain of antibodies [
27]. Thus, it is plausible that maternal antibodies could recognize the epitopes of both peptides, given that the mothers of the piglets had been vaccinated.
To corroborate the antibodies-mediated humoral response, we evaluate active B cells subpopulations in pigs. The humoral response mediated by B cells was also evaluated through three subpopulations: i) antibody-forming/Plasma B cells (CD2+/CD21-), which are B cells corresponds to B cells circulating within the peripheral blood with the function of secreting antibodies that can potentially recognize the specific antigen (anti-peptide antibody) that stimulated the cell [
28,
29]. ii) primed B cells (CD2-/CD21+), which present their B receptors (BCR) charged with the antigen and remain in circulation waiting to contact with T cells that provide the activation signal [
30]. iii) naïve B cells (CD2+/CD21+), which circulate through the peripheral blood and have not been exposed to an antigen [
29,
31]. Thus, this work evaluated the induction of subpopulations of B cells as follows: plasmatic cells (CD2+/CD21-), trained cells (CD2-/CD21+), and naïve cells (CD2+/CD21+). All these subpopulations were analyzed based on the selection of B cells with reduced IgM expression (IgM low).
The data obtained showed that both peptides can induce antibody-secreting B cells, which directly correlate with the increase in anti-peptide specific antibodies observed in the IgG evaluation. Surprisingly, the results of trained B cells showed that the group immunized with the peptide GP5-B3 induced a significant increase in trained B cells, greater than the group immunized with GP5-B peptide. This could explain why the same level of anti-GP5-B3 IgGs did not change from 21 to 42 dpi. Although these B cells contain the charged antigen in their BCR, they are not activated and therefore have not differentiated into secreting or memory B cells. On the other hand, the group immunized with GP5-B peptide also revealed a significant increase in comparison to the vehicle and control groups, indicating that there are also antigen-charged cells waiting to be activated to differentiate into plasmatic cells or memory cells.
Finally, the evaluation of the subpopulation of naïve B cells showed a decrease in these cells in the groups immunized with peptides, which correlates with the literature. It has been reported that naïve cells are cells that have not been stimulated or come into contact with an antigen; thus, these cells circulate in the bloodstream and lymph nodes waiting to come into contact with an antigen. When Naïve B cells encounter an antigen, this subpopulation of B cells diminishes as they differentiate into trained B cells, antibody secretory, or memory cells [
32]. Together, these data indicate that the peptides GP5-B and GP5-B3 can stimulate naïve B cells to differentiate into trained cells that will later become antibody-specific secretory B cells or CD2+/CD21- memory B cells.
The PRRSV exhibits significant genetic variability, which influences its interaction with the host’s immune response and the antigenic characteristics of viral proteins [
33]. This virus has a significant capacity to evade the immune response through various mechanisms, including internalization into cells [
34]. In contrast, the host response includes neutralizing antibodies against B epitopes in the protein complex involved in the virus´s interaction with the CD163 receptor, which the virus uses to infect [
7,
34,
35].
PRRSV glycoprotein GP5 has been widely studied as a highly antigenic protein and is also the principal target for broadly neutralizing antibodies [
36]. In fact, the main neutralization epitope is located in a hypervariable region of the ectodomain from the GP5 protein [
37]. Thus, vaccines probably have low efficiency in providing protection against homologous wild strains. Other researchers have studied B epitopes from GP5 protein and found that they induce a humoral immune response [
13,
14,
15,
16,
17,
38].
As shown in
Figure 6, both peptides GP5-B and GP5-B3 were also immunoreactive against the sera of vaccinated animals with a commercial vaccine based on the model strain VR-2332 of the American genotype and against sera of animals naturally infected by wild strains, which are largely dominated by genotype 2 strains. However, as the Ministry of Agriculture and Rural Development reported in 2017, circulating strains of PRRSV-1 were also present, so cross-immunoreactivity against heterologous strains is not ruled out. This can be attributed to the fact that the epitopes contained in the peptides are short sequences that were selected because they were conserved across different in silico reference strains, which was experimentally tested in this immunoreactivity test. In this test, the presence of anti-peptide antibodies was again observed (
Figure 4) in the control group of this experiment (pre-immune animal sera), which can also be attributed to the same conditions as explained above in
Figure 3.
5. Conclusions
In conclusion, the data obtained in the present study show that immunization with GP5-B or GP5-B3 peptides induces a sustained humoral response of peptide-specific IgG antibodies. In addition, both peptides induced B-cell activation and their differentiation into peptide-specific IgG antibody-forming/Plasma B cells. The two peptides, both GP5-B and GP5-B3, were immunoreactive with the sera of animals vaccinated with the commercial vaccine and with the sera from animals naturally infected by circulating strains of PRRSV. These data suggest that B epitopes contained in peptides GP5-B and GP5-B3 are conserved across various strains of PRRSV. This work provides evidence of the capacity of B epitopes in the synthetic peptides GP5-B and GP5-B3 to induce a humoral response. Additionally, it was experimentally demonstrated that these are epitopes preserved in circulating wild strains, which could be included in the future development of a safe and effective vaccine model to prevent PRRSV infection.
Author Contributions
Conceptualization, F.P.-D., R.E.N.-A., F.C.-R., I.H.-M., R.C.-V. and A.B.-P.; methodology, F.P.-D., R.E.N.-A., F.C.-R., I.H.-M., R.O.-F., D.D.-H. and R.C.-V.; formal analysis, F.P.-D., R.E.N.-A. and F.C.-R.; investigation, F.P.-D., R.E.N.-A., F.C.-R., I.H.-M., R.C.-V., R.O.-F.,D.D.-H., L.E.F.-C. and A.G.Z.-A.; resources, R.E.N.-A., A.B.-P. and I.H.-M.; writing—original draft preparation, F.P.-D., I.H.-M., R.C.-V. and R.E.N.-A.; writing—review and editing, F.P.-D., I.H.-M., R.C.-V., A.B.-P. and R.E.N.-A.; visualization, F.P.-D.; supervision, R.E.N.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded and supported by grant CONAHCYT; grant number A1-S-43236. We thank CIC-UMSNH funding (2021–2023), which made part of this research possible. We thank PAPPIT-UNAM IA207519 for partial funding. We thank the Proteomic and Cellular Bioengineering Unit, INFR-2015-01 255010 (CONAHCYT). We thank ICTI Michoacan project FCCHTI23_ME-4.1.-0014 for partial funding. We also express thanks for the grant from CONAHCYT, “FOMENTO A LA INFRAESTRUCTURA CIENTÍFICA” 2021, project No. 317189. F.P.-D. received a fellowship from the 804387 CONAHCYT.
Institutional Review Board Statement
The animal study protocol was approved by the Institu-tional Animal Research and Ethics Committee of Facultad de Medicina Veterinaria y Zootecnia from the Universidad Michoacana de San Nicolas de Hidalgo (protocol code: CI-CUMSNH-A101-FMVZ and approval of 1 September 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors give thanks to “El DAPO farm” and “El LIMON farm” for donation of serum samples, vaccinated (Ingelvac-PRRSV, MLV®), non-vaccinated and naturally infected groups respectively. We thank technical support to Fredy Garcia Luna D.V.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nieuwenhuis, N.; Duinhof, T.F.; van Nes, A. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet Rec 2012, 170, 225. [Google Scholar] [CrossRef]
- Chase-Topping, M.; Xie, J.; Pooley, C.; Trus, I.; Bonckaert, C.; Rediger, K.; Bailey, R.I.; Brown, H.; Bitsouni, V.; Barrio, M.B.; et al. New insights about vaccine effectiveness: Impact of attenuated PRRS-strain vaccination on heterologous strain transmission. Vaccine 2020, 38, 3050–3061. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu Rev Anim Biosci 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Mardassi, H.; Mounir, S.; Dea, S. Identification of major differences in the nucleocapsid protein genes of a Québec strain and European strains of porcine reproductive and respiratory syndrome virus. J Gen Virol 1994, 75 ( Pt 3), 681–685. [Google Scholar] [CrossRef]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet Res 2016, 47, 108. [Google Scholar] [CrossRef]
- López-Heydeck, S.M.; Alonso-Morales, R.A.; Mendieta-Zerón, H.; Vázquez-Chagoyán, J.C. Síndrome reproductivo y respiratorio del cerdo (PRRS). Revisión. Revista Mexicana de Ciencias Pecuarias 2015, 6, 69–89. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Rowland, R.R.R. Challenges for Porcine Reproductive and Respiratory Syndrome (PRRS) Vaccine Design: Reviewing Virus Glycoprotein Interactions with CD163 and Targets of Virus Neutralization. Vet Sci 2019, 6. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front Microbiol 2017, 8, 1635. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; O’Connell, C.M.; Costa, A.; Pan, Y.; Smyth, J.A.; Verardi, P.H.; Burgess, D.J.; Van Kruiningen, H.J.; Garmendia, A.E. A PRRSV GP5-Mosaic vaccine: Protection of pigs from challenge and ex vivo detection of IFNγ responses against several genotype 2 strains. PLoS One 2019, 14, e0208801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, Q.; He, Y.; Zheng, Y.; Sha, H.; Li, G.; Kong, W.; Liao, J.; Zhao, M. Research Progress on the Development of Porcine Reproductive and Respiratory Syndrome Vaccines. Vet Sci 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral Strategies against PRRSV Infection. Trends Microbiol 2017, 25, 968–979. [Google Scholar] [CrossRef]
- Montaner-Tarbes, S.; Del Portillo, H.A.; Montoya, M.; Fraile, L. Key Gaps in the Knowledge of the Porcine Respiratory Reproductive Syndrome Virus (PRRSV). Front Vet Sci 2019, 6, 38. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Bi, Y.; Yang, L.; Meng, S.; Zhou, Y.; Jia, X.; Meng, S.; Sun, L.; Liu, W. Synthetic B- and T-cell epitope peptides of porcine reproductive and respiratory syndrome virus with Gp96 as adjuvant induced humoral and cell-mediated immunity. Vaccine 2013, 31, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.N.; Trible, B.R.; Chen, N.; Rowland, R.R.R. GP5 of porcine reproductive and respiratory syndrome virus (PRRSV) as a target for homologous and broadly neutralizing antibodies. Vet Microbiol 2017, 209, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.M.V.; Ni, Y.; Meng, X.; Zhang, C. Production and Evaluation of Virus-Like Particles Displaying Immunogenic Epitopes of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). International Journal of Molecular Sciences 2015, 16, 8382–8396. [Google Scholar] [CrossRef]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol 2002, 76, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Rico, F.; Bravo-Patiño, A.; Mendieta, I.; Perez-Duran, F.; Zamora-Aviles, A.G.; Franco-Correa, L.E.; Ortega-Flores, R.; Hernandez-Morales, I.; Nuñez-Anita, R.E. Glycoprotein 5-Derived Peptides Induce a Protective T-Cell Response in Swine against the Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2023, 16. [Google Scholar] [CrossRef]
- Lopez, O.J.; Osorio, F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol 2004, 102, 155–163. [Google Scholar] [CrossRef]
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: where are we headed? Porcine Health Manag 2021, 7, 1. [Google Scholar] [CrossRef]
- Charerntantanakul, W. Adjuvants for swine vaccines: Mechanisms of actions and adjuvant effects. Vaccine 2020, 38, 6659–6681. [Google Scholar] [CrossRef] [PubMed]
- Rahe, M.C.; Dvorak, C.M.T.; Patterson, A.; Roof, M.; Murtaugh, M.P. The PRRSV-Specific Memory B Cell Response Is Long-Lived in Blood and Is Boosted During Live Virus Re-exposure. Front Immunol 2020, 11, 247. [Google Scholar] [CrossRef]
- Braun, R.O.; Python, S.; Summerfield, A. Porcine B Cell Subset Responses to Toll-like Receptor Ligands. Front Immunol 2017, 8, 1044. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, M.; Albo, J.; Rao, Q. Non-Specific Binding and Cross-Reaction of ELISA: A Case Study of Porcine Hemoglobin Detection. Foods 2021, 10. [Google Scholar] [CrossRef]
- Engvall, E. The ELISA, enzyme-linked immunosorbent assay. Clin Chem 2010, 56, 319–320. [Google Scholar] [CrossRef]
- Guzman-Bautista, E.R.; Garcia-Ruiz, C.E.; Gama-Espinosa, A.; Ramirez-Estudillo, C.; Rojas-Gomez, O.I.; Vega-Lopez, M.A. Effect of age and maternal antibodies on the systemic and mucosal immune response after neonatal immunization in a porcine model. Immunology 2014, 141, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, B.; Mwangi, W.; Rijal, P.; Schwartz, J.C.; Noble, A.; Shaw, A.; Sealy, J.E.; Bonnet-Di Placido, M.; Graham, S.P.; Townsend, A.; et al. Fc-Mediated Functions of Porcine IgG Subclasses. Front Immunol 2022, 13, 903755. [Google Scholar] [CrossRef] [PubMed]
- Sinkora, M.; Sinkorova, J. B cell lymphogenesis in swine is located in the bone marrow. J Immunol 2014, 193, 5023–5032. [Google Scholar] [CrossRef] [PubMed]
- Sinkora, M.; Butler, J.E.; Lager, K.M.; Potockova, H.; Sinkorova, J. The comparative profile of lymphoid cells and the T and B cell spectratype of germ-free piglets infected with viruses SIV, PRRSV or PCV2. Vet Res 2014, 45, 91. [Google Scholar] [CrossRef]
- Turner, J.S.; Marthi, M.; Benet, Z.L.; Grigorova, I. Transiently antigen-primed B cells return to naive-like state in absence of T-cell help. Nat Commun 2017, 8, 15072. [Google Scholar] [CrossRef]
- Kienzler, A.-K.; Eibel, H. Human B Cell Development and Tolerance. In Encyclopedia of Immunobiology, Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, 2016; pp. 105–121. [Google Scholar]
- Thorarinsdottir, K.; Camponeschi, A.; Cavallini, N.; Grimsholm, O.; Jacobsson, L.; Gjertsson, I.; Mårtensson, I.L. CD21(-/low) B cells in human blood are memory cells. Clin Exp Immunol 2016, 185, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, M.; Van Breedam, W.; Costers, S.; Geldhof, M.; Noppe, Y.; Nauwynck, H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine 2011, 29, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Rowland, R.R.R.; Yoo, D. Recent Advances in PRRS Virus Receptors and the Targeting of Receptor-Ligand for Control. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Li, J.N.; Su, C.M.; Yoo, D. Molecular and Cellular Mechanisms for PRRSV Pathogenesis and Host Response to Infection. Virus Res 2020, 286, 197980. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E.; Dvorak, C.M.T.; Graham, S.P.; Murtaugh, M.P. Isolation of Porcine Reproductive and Respiratory Syndrome Virus GP5-Specific, Neutralizing Monoclonal Antibodies From Hyperimmune Sows. Front Immunol 2021, 12, 638493. [Google Scholar] [CrossRef]
- Thaa, B.; Sinhadri, B.C.; Tielesch, C.; Krause, E.; Veit, M. Signal peptide cleavage from GP5 of PRRSV: a minor fraction of molecules retains the decoy epitope, a presumed molecular cause for viral persistence. PLoS One 2013, 8, e65548. [Google Scholar] [CrossRef]
- Hou, Y.H.; Chen, J.; Tong, G.Z.; Tian, Z.J.; Zhou, Y.J.; Li, G.X.; Li, X.; Peng, J.M.; An, T.Q.; Yang, H.C. A recombinant plasmid co-expressing swine ubiquitin and the GP5 encoding-gene of porcine reproductive and respiratory syndrome virus induces protective immunity in piglets. Vaccine 2008, 26, 1438–1449. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).