1. Introduction
Trichoderma-based bio-pesticides and bio-fungal fertilizers demonstrate significant benefits in managing soil-borne diseases. Their systematic induction of disease resistance and stress resilience aids in promoting crop growth and mitigating the challenges related to continuous cropping. Consequently, they have emerged as the world’s leading biocontrol fungi for plant diseases and growth promotion.
Metarhizium, an entomopathogenic fungus, has a widespread presence in both soil and insects [
1]. Currently, the majority of
Metarhizium strains utilized for research have been isolated from the carcasses of insects they had infected, including species such as scarabs, fall armyworms, Blattella germanica, and Dichocrocis punctiferalis [
2,
3,
4]. Characterized by its eco-friendly nature and potent pathogenicity to its target insects, Metarh-izium has rightfully earned its status as one of the key biocontrol microorganisms for insect management [
5].
Maize, one of the three principal food crops in China, is severely compromised by Fusarium graminearum. This pathogen is primarily responsible for maize root rot, stem rot, and ear rot. In addition, it produces toxins DON and ZEN, significantly impacting both the yield and quality of maize crops.
The co-culture of
Trichoderma in conjunction with fungi or biocontrol fungus has been demonstrated to play a commendable role in controlling plant diseases. In one study conducted by Tingting Li and colleagues, Bacillus B22 was co-cultured with
Trichoderma 3403 [
6] below. The findings revealed that, in contrast to a single culture, the fermentation broth resulting from the co-culture showed an upsurge in antifungal and plant growth-promoting metabolites. Similarly, Zhang [
7] and his team discovered that the fermentation broth derived from the co-culture of
Trichoderma and Bacillus Tpb55 remarkably outperformed the single culture, especially in mitigating tobacco black shank. In another study, Cong Yunzhe [
8] determined that the combined fermentation of Rhizopus nigricans and
Trichoderma pseudokoningii exhibited inhibitory effects on an array of plant soil-borne pathogens, as evidenced through bacteriostatic experiments. Ma [
9] revealed that the co-culture of Bacillus amyloliquefaciens and
Trichoderma longibrachiatum substantially heightened the control effect on tomato Fusarium wilt.
Therefore, in this study, we co-cultured a strain of Trichoderma and Metarhizium anisopliae, and analyzed the differential metabolism of co-culture compared to single culture.
1. Materials and Methods
1.1. Material
Metarhizium anisopliae (hereinafter referred to as M3) was purchased from Ningbo Mingzhou Biological Company, and Trichoderma atroviride (hereinafter referred to as D1)and Fusarium graminearum were provided by Shanghai Jiao Tong University Culture Collection Center.
Potato dextrose (PD) medium: potato 200 g, glucose 20 g, distilled water to 1 L, pH natural. Potato dextrose agar (PDA) medium: potato 200 g, glucose 20 g, agar 20 g, distilled water to 1 L, pH natural.
1.2. Methods
The metabolome detection was conducted through four distinct treatments: Trichoderma monoculture fermentation filtrate, Metarhizium monoculture fermentation filtrate, a co-cultured fermentation filtrate of Trichoderma and Metarhizium, and a blank control using 30% PD medium. Fermentation filtrate was produced by washing the spores of Trichoderma and Metarhizium on the plate with sterile water and adjusting the spore concentration to 106 spores/ml. The co-culture medium was formulated by incorporating 1 ml of Trichoderma and Metarhizium into the sterilized PD medium. This medium was then incubated in a constant temperature shaker set at 28 °C and 180 r/min for a duration of 5 days, with the respective monocultures of Trichoderma and Metarhizium serving as controls. The resulting fermentation broth was processed through centrifugation at 8000 rpm for 5 minutes, with the supernatant subsequently isolated. The isolated supernatant was filtered and sterilized via a syringe through a 0.22-micron microporous membrane, yielding the fermentation filtrate. The filtrate was then rapidly cooled using liquid nitrogen and stored in a refrigerator set at -80 ° C. Each treatment was replicated at least four times. The samples collected were forwarded to Shanghai Pasenuo Biotechnology Co., Ltd. for metabolomics sequencing analysis.
2. Results and Analysis
2.1. The Plate Inhibition Rate of Fermentation Broth against Fusarium graminearum
As shown in
Table 1, the inhibition rate of D1-M3 on
Fusarium graminearum was more than 60%, so it could be found that D1-M3 have antibacterial effect on
Fusarium graminearum.
2.2. Metabolite Differential Screening Results
2.2.1. OPLS-DA Analysis
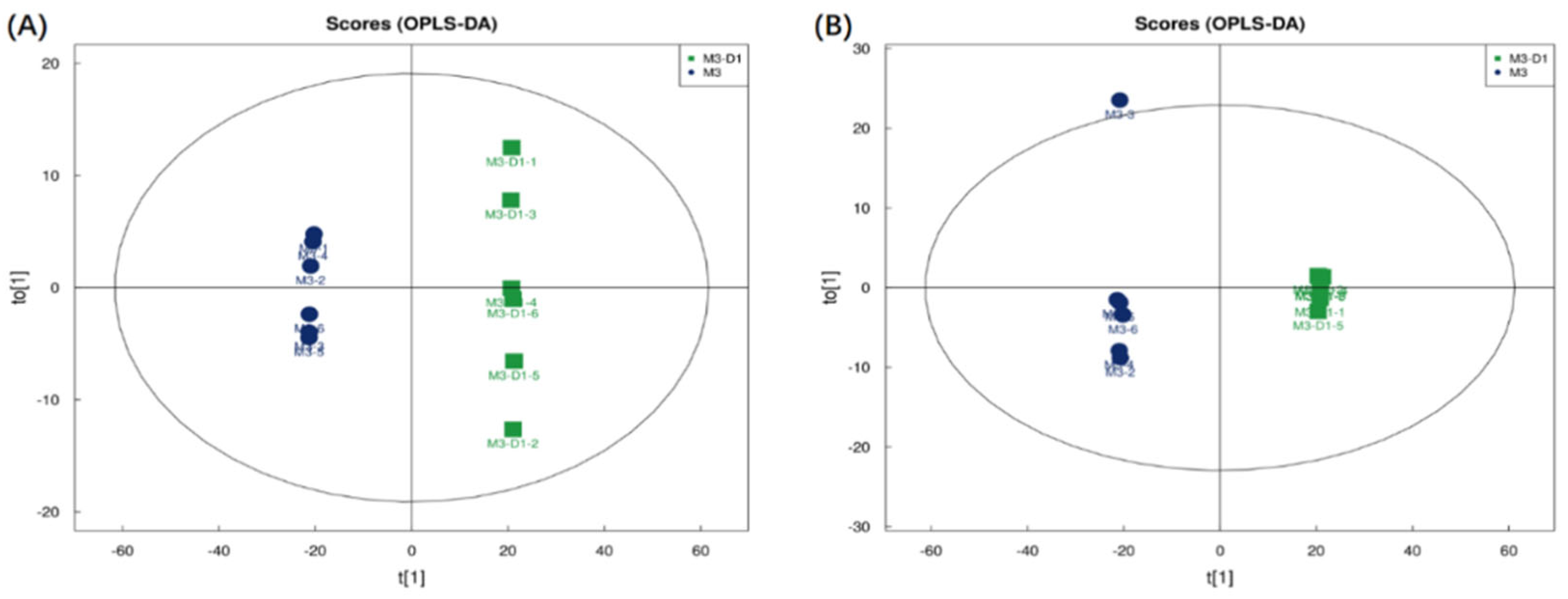
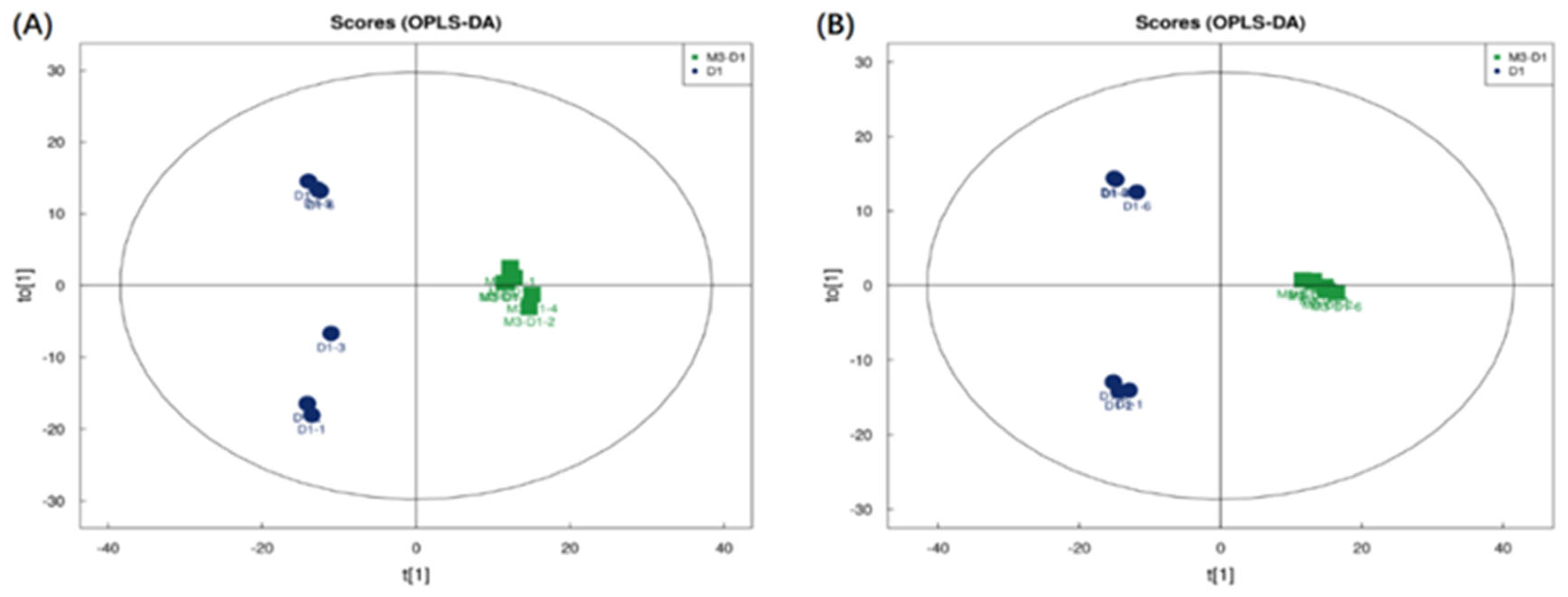
As depicted in
Figure 1 and
Figure 2, the filtrates from co-culture fermentation and single culture fermentation are distributed across different quadrants. The comparisons of M3_D1 vs M3 and M3_D1 vs D1 show a larger intergroup variation and a smaller intragroup variation, which were obtained through the OPLS-DA model. The importance of each variable, denoted as the VIP (Variable Importance in the Projection) value, holds significant influence and explanatory power over the classification of each sample group. Significant difference metabolites were screened based on the criteria of OPLS-DA VIP > 1 and P value < 0.05.
2.2.2. Analysis of Differential Metabolites
As depicted in
Table 2,
Metarhizium anisopliae served as the control group with the co-culture acting as the treatment group. A screening process was undertaken, yielding 241 VIPs. The differentially expressed metabolites (DEMs) that had P values less than 0.05 included 91 metabolites that were up-regulated and 150 that were down-regulated. Subsequently, with
Trichoderma as the control group and the co-culture as the treatment group, a total of 78 differential metabolites were screened, all of which had a VIP greater than 1 and a P value less than 0.05. These DEMs comprised 63 up-regulated metabolites and 15 down-regulated metabolites. In an effort to enhance understanding of the differential metabolites produced by co-culture in comparison to a single culture, the single culture of M3 was utilized. The metabolites of fermentation and
Trichoderma D1 single culture fermentation with significant differences, served as the control group, and the co-culture as the treatment group, underwent normalization processing. Subsequently, four clustering heat maps were created.
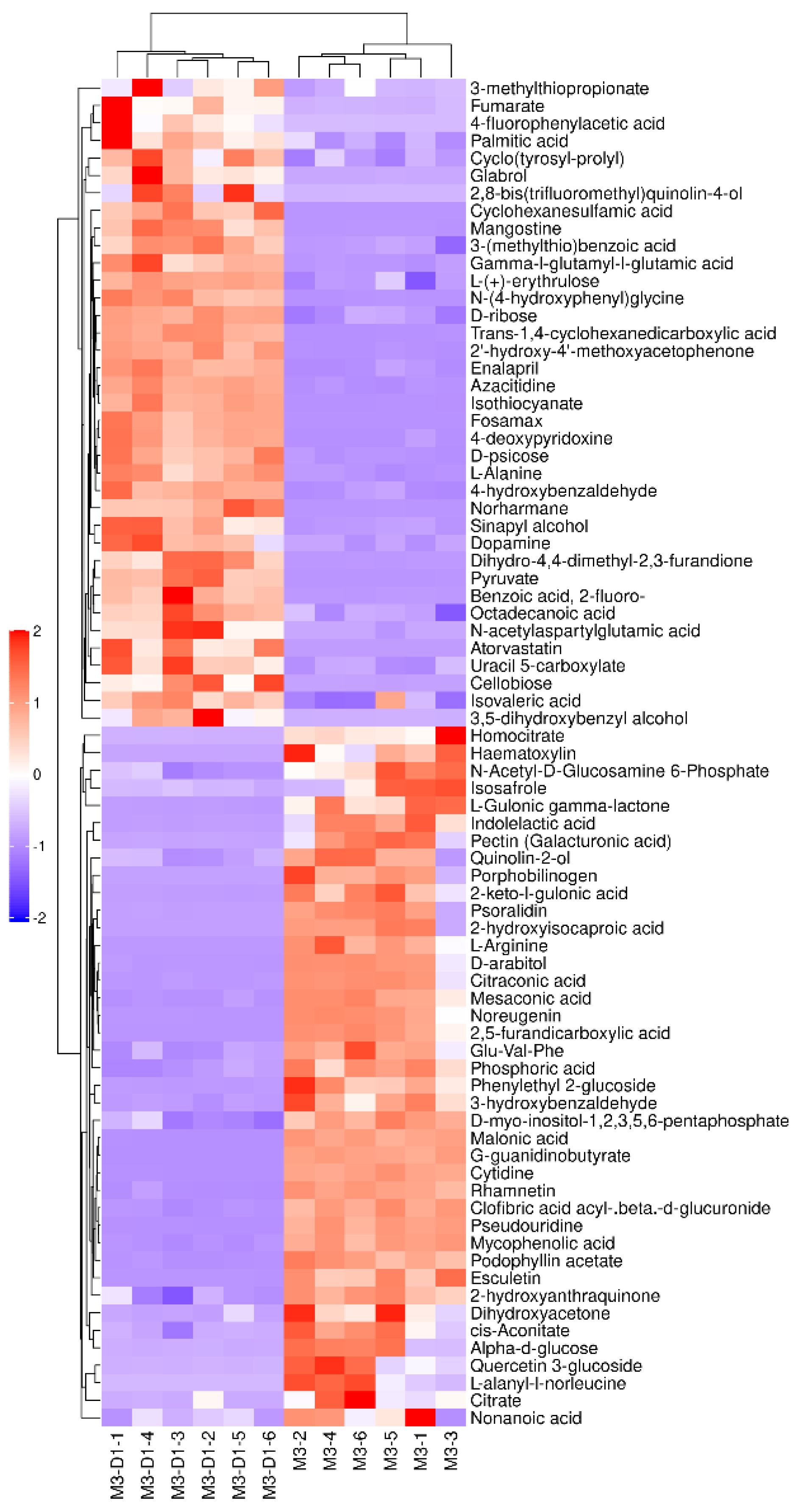
The alterations in M3 _ D1 and M3 metabolites are clearly depicted in
Figure 3 and
Figure 4. In the negative ion mode, metabolites such as Azacitidine, 2’ -hydroxy-4’ -methoxyacetophenone, trans-1, cyclohexanedicarboxylic acid, and isosulfur were identified. The study also identified 37 other metabolites, including Cyanate ester, that were significantly up-regulated and 40 metabolites, such as clofibric acid acyl-β-D-glucuronide, mycophenolic acid, podophyllum acetate, and rhamnetin, that were significantly down-regulated. In the positive ion mode, short bromide amine, O-toluidine, cyclohexylamine, indole, γ-aminobutyric acid, adenosine, N-α-acetyl-L-ornithine, lupine, among others, were detected as significantly up-regulated metabolites. In contrast, 110 metabolites, including trigonelline and pretilachlor, were observed to be significantly down-regulated.
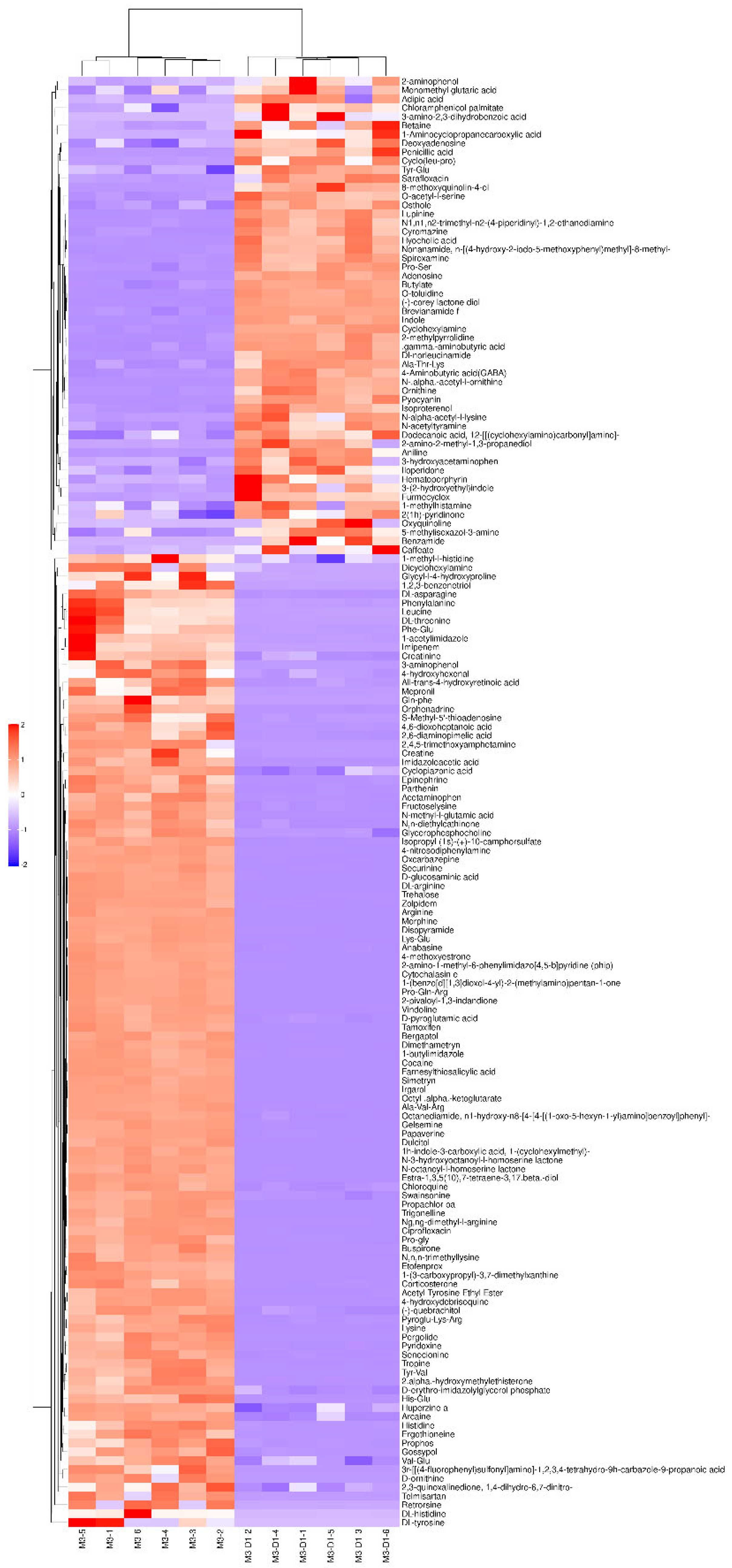
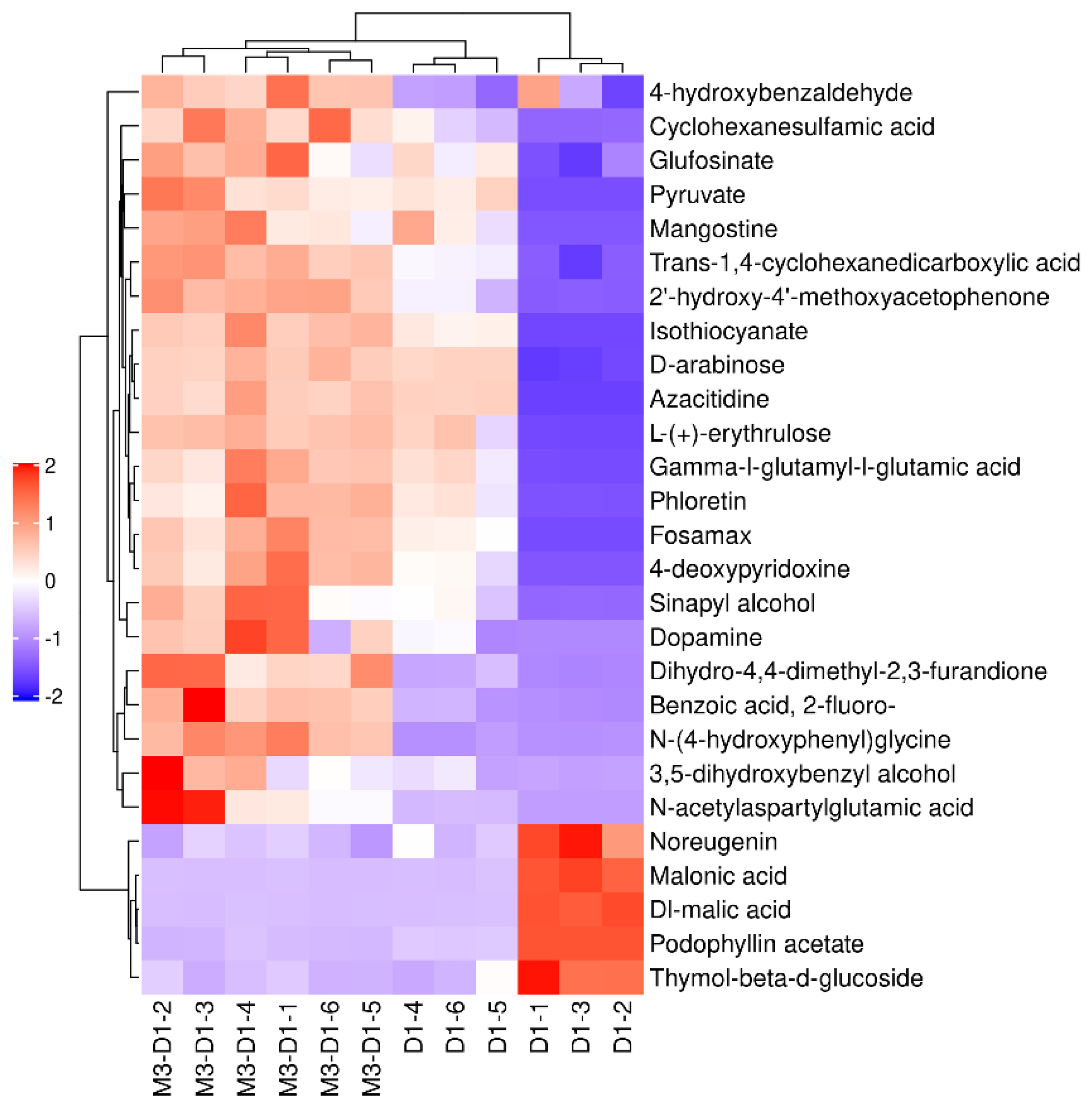
Figure 5 and
Figure 6 illustrate the alterations in metabolites between M3 _ D1 and D1. In the negative ion mode, a significant upregulation was observed in 23 metabolites, including but not limited to benzoic acid, 4-hydroxybenzaldehyde, 2’ -hydroxy-4’ -methoxyacetophenone, and cyclohexane aminosulfonic acid. Conversely, a marked downregulation was noted in 4 metabolites, namely podophylloacetate, thymol-β-d-glucoside, DL-maleic acid, and malonic acid. In the positive ion mode, there was a substantial upregulation in 40 differential metabolites such as butachlor, lupine, spirocyclam, deoxyadenosine, γ-aminobutyric acid, and dodecacyclomorpholine. Meanwhile, 11 metabolites such as tert-butylamine and isovaletine demonstrated a significant downregulation.
2.2.3. Metabolite Pathway and Classification Annotation
Using the KEGG Pathway database as a resource, an analysis was conducted on the metabolites of M3_D1 VS D1 and M3_D1 VS M3, following the P value. Post metabolic classification and annotation, the metabolites of M3_D1 VS M3 were categorized into five distinct groups. The primary group encompasses metabolism, inclusive of a global overview, amino acid metabolism, biosynthesis of alternate secondary metabolites, carbohydrate metabolism, metabolism of diverse amino acids, energy metabolism, and biodegradation and metabolism of varying biomass. Therein, 33 metabolites participate in the global overview, 32 in amino acid metabolism, 19 in biosynthesis of alternate secondary metabolites, 8 in carbohydrate metabolism, and 5 partake in the metabolism of diverse amino acids. Energy metabolism involves four metabolites, while three metabolites are engaged in the biodegradation and metabolism of differing biomass. The second category is centered on the tissue system, comprising the digestive system, endocrine system, circulatory system, and nervous system, each holding a secondary classification. 17 metabolites are involved in the digestive system, 8 in the endocrine system, 2 in the circulatory system, and 4 in the nervous system. The third division relates to environmental information processing, embracing membrane transport, signal molecules and their interactions, as well as signal transduction. Therein, membrane transport involves 20 metabolites, signal transduction 6, and signal molecules and interactions also 6. The fourth grouping pertains to genetic information processing, encompassing translation, folding, sorting and degradation. Nine metabolites partake in the translation pathway, and two are involved in the folding, sorting and degradation pathway. The final, fifth grouping of cellular processes, includes a secondary classification pathway of cellular communities-prokaryotes, with five associated metabolites.
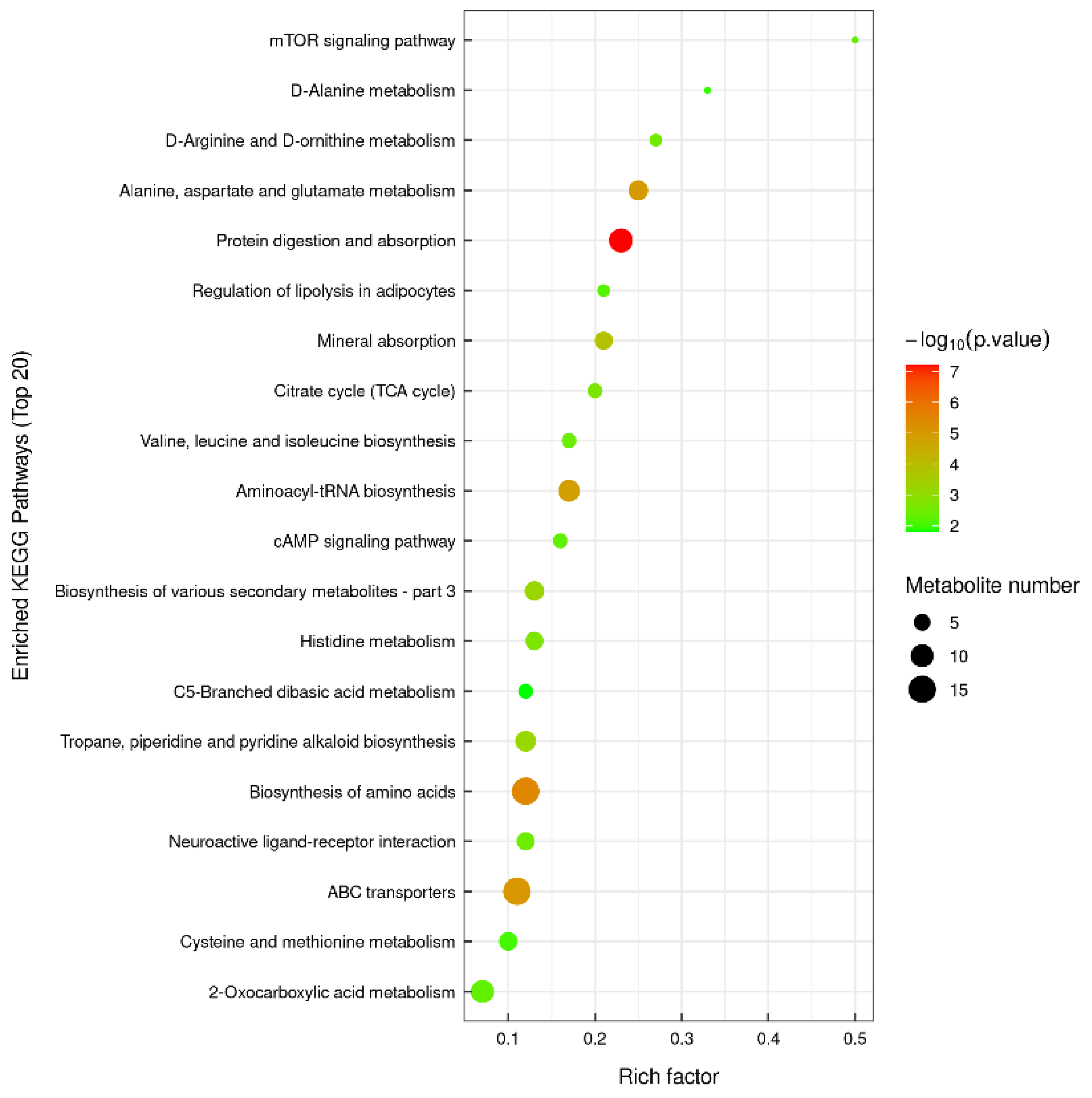
Figure 7 depicts a KEGG enrichment analysis that was conducted following the co-culture of
Metarhizium and
Trichoderma. The analysis highlights several pathways that exhibited substantial alterations when compared to the fermentation of
Metarhizium in isolation. These changes spanned a broad range of categories, including: metabolic pathways, environmental information processing, tissue system, genetic information processing and cellular processes. Within the realm of metabolic pathways, significant changes were observed in the biosynthesis of amino acids, the metabolism of alanine, aspartic acid and glutamic acid, the biosynthesis of secondary metabolites, the biosynthesis of tropane, piperidine and pyridine alkaloids, histidine metabolism, the citric acid (TCA) cycle, and several other pathways. Environmental information processing changes were notable in areas such as ABC transporters, neuroactive ligand-receptor interaction, and the mTOR and cAMP signaling pathways. In the tissue system, changes were detected in the regulation of mineral absorption and adipocyte lipolysis. Genetic information processing changes were most evident in aminoacyl-tRNA biosynthesis. Finally, cellular process changes were seen in the Quorum sensing regulatory system.
The metabolites derived from D1 _ M3 VS D1 can be categorically partitioned into three groups, according to the annotation classifications of metabolites. The initial category pertains to environmental information processing, encompassing two sub-classifications: signal transduction, and interactions of signaling molecules. Within this category, three metabolites partake in signal transduction pathways, while four additional metabolites are implicated in the interaction across signaling molecules. The second category, termed as Organismal Systems, incorporates a dual-tier classification of the Nervous system. Within this context, two metabolites are found to play a role in the nervous system. The third and final category is Metabolism, which encapsulates 6 secondary classifications: amino acid metabolism, biodegradation and metabolism of Xenobiotics, carbohydrate metabolism, metabolism of other amino acids, metabolism of terpenoids and polyketides, as well as global and overview maps. In this category, six metabolites were found to be engaged in the metabolic pathway of amino acids, three were associated with the carbohydrate metabolic pathway, five were linked to the biodegradation and metabolic pathway of heterogeneous biomass, two were correlated with the metabolic pathway of other amino acids, four were implicated in the global and overview map, and two were found to be part of the metabolic pathway of terpenoids and polyketides.
The findings are depicted in
Figure 8. Our KEGG enrichment analysis reveals substantial alterations in certain metabolic pathways of
Trichoderma in isolation, post co-cultivation with
Metarhizium. These include: metabolism of alanine, aspartic acid, and glutamic acid, butyric acid metabolism, metabolism of cysteine and methionine, degradation of caprolactam, biosynthesis of amino acid and β-alanine metabolism, degradation of aminobenzoate, and zeatin biosynthesis. Furthermore, substantial transformations have been observed in environmental information processing, specifically in the interaction between neuroactive ligands and receptors, as well as the cAMP signaling pathway. Notably, the synaptic vesicle cycle within the tissue system has also exhibited significant modifications.
3. Discussion
In the comparison between the co-culture fermentation filtrate as the treatment group and the single culture fermentation filtrate of Metarhizium and Trichoderma as the control group,it can be found that the differential metabolites screened with Metarhizium as the control group are more than those with Trichoderma as the control group, which indicates that the co-culture process may have a greater impact on Metarhizium.
Through the analysis of differential metabolites in the metabolome, the focus was placed on the metabolites with up-regulated relative content after co-culture, and it was found that the content of some substances with antibacterial activity was up-regulated.In the co-culture and
Trichoderma D1 fermentation filtrate metabolome comparison, the metabolite isothiocyanate was up-regulated in the negative ion mode. Some data showed that isothiocyanate in the key decomposition product of glucose in rape had the effect of anti-phytopathogenic fungi. Some key steps in the apoptotic pathway have also been shown to be affected by isothiocyanate: isosulfate may induce apoptosis through the JNK / MAPK signaling pathway [
10]. In this chapter, transcriptome sequencing analysis also found that co-culture fermentation filtrate treatment caused changes in the MAPK signaling pathway of Fusarium graminearum; liu Feng [
11] found that curcumin and phloretin synergistic application of Staphylococcus aureus antibacterial efficacy of scientific research ; in the positive ion mode, the sterol biosynthesis inhibitor spirocyclam with internal absorption activity can be used to control powdery mildew [
12], and 12-cyclomorpholine can also be used as a fungicide [
13]; dodecanoic acid has a significant inhibitory effect on the radial growth of mycelia, the production of zoosporangia and the formation and germination of zoospores [
14].
In the co-culture and
Metarhizium M3 fermentation filtrate metabolome comparison,in the positive ion mode: indole can be used as an antibacterial substance against Ralstonia solanacearum [
15]; 4-Aminobutyric acid (Gaba) can be obtained by plant enrichment, microbial fermentation and other methods. It can be used as a plant growth regulator to help plants resist adversity [
16]; as a mature pesticide currently used in the market, osthole can control peach brown rot [
17], rice sheath blight [
18], rice blast [
19], wheat powdery mildew [
20], cucumber powdery mildew [
21]; penicillic acid has growth inhibitory effect on wheat sharp eyespot and rice sharp eyespot [
22]. Studies have shown that the addition of exogenous betaine can improve the disease resistance of apple to apple leaf blight [
23], indicating that betaine has a certain role in participating in watermelon resistance to Fusarium wilt [
24]. In addition, the contents of metabolites of spirolactam and dodecanoic acid were up-regulated in the two groups, but there was no research on Fusarium graminearum in the current literature. How the co-culture fermentation filtrate affects Fusarium graminearum needs further verification.
Following the KEGG annotation of the metabolites, significant changes were observed in the metabolic pathways of the co-culture fermentation filtrate, compared to the single culture fermentation filtrate of Metarhizium, which served as the control group. The pathways encompassed amino acid biosynthesis, metabolism of alanine, aspartic acid, glutamic acid, and biosynthesis of various secondary metabolites. This suggests that new secondary metabolites might have been introduced or created in the co-culture fermentation filtrate when compared to the single culture fermentation filtrate of Metarhizium. In addition, every metabolite produced by the co-culture and the single culture of the two strains can be separately identified for a more in-depth exploration of the changes in secondary metabolites.
Beyond the presence of antibacterial substances, significant changes were identified in the metabolic pathway - particularly, the synthesis of zeatin - when comparing the monoculture fermentation filtrate of Trichoderma (used as a control group) with the co-culture group (regarded as the treatment group). Numerous studies have elucidated that zeatin serves as an effective plant growth regulator, thus bolstering crop yields and enhancing disease resistance. However, it remains to be validated whether the co-culture fermentation filtrate contributes positively to the growth of corn.
Author Contributions
Conceptualization, X.W., J.C.; Supervision, X.W., J.C., Y.L.; Data curation, Y.W., L.C.; Formal analusis, Y.W., J.L.; Funding acquisition, X.W.; Investigation, Y.W., Y.H., B.L.; Methodology, Y.W.; Project administration, X.W.; Resources, X.W., Y.L.; Software, H.L.; Supervision, X.W., J.C., Y.L.; Validation, J.L., Y.H.; Visualization, Y.W.; Writing - original draft, J.L., Y.W.; Writing - review & editing, J.L., L.C. All authors contributed to the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Research and Demonstration of Key Technologies for Green Control of Corn Diseases and Insect Pests by Trichoderma spp. and Complex Fungi in Hilly Areas of Yan Mountains (2022sj005), China Agriculture Research System of MOF and MARA (CARS-02), Science and Technology Innovation Team of Hetao College, the National Key R&D Program of China (2023YFD1401500).
References
- Travis R Glare, Yvonne Scholte Op Reimer, Nicholas Cummings; et al. Diversity of the insect pathogenic fungi in the genus Metarhizium in New Zealand. New Zealand J. Bot. 2021, 59, 440–456. [CrossRef]
- Senthil Kumar, C.M., Jacob T.K., Devasahayam S.; et al. Characterization and biocontrol potential of a naturally occurring isolate of Metarhizium pingshaense infecting Conogethes punctiferalis. Microbiol. Res. 2021, 243.
- Chen Mingjun, Wang Ting, Bian Linmeng; et al. Identification and biological characteristics of a Metarhizium guizhouense strain isolated from Blattaria germanica. Chin. J. Biol. Control. 2021, 37, 1344–1352.
- Long Xiuzhen, Gao Xuyuan, Zeng Xianru et al. Screening of a Metarhizium rileyi strain and its virulences to spodoptera frugiperda (Lepidoptera: Noctuidae). Chin. J. Biol. Control. 2021, 37, 1111–1119.
- Jin Yurong, Yin Hong, Luo Jianxun. Research progress on the biocontrol fungi Metarhizium. J. Anhui Agri. Sci. 2009, 27, 2060–2062.
- Li T, Tang J, Karuppiah V; et al. Co-culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 improves the production of antifungal secondary metabolites. Biol. Control. 2020, 140, 104122.
- Li Q, Lin W, Zhang X; et al. Transcriptomics integrated with metabolomics reveal the competitive relationship between co-cultured Trichoderma asperellum HG1 and Bacillus subtilis Tpb55. Microbiol. Res. 2024, 280, 127598.
- Cong Y, Fan H, Ma Q; et al. Mixed culture fermentation between Rhizopus nigricans and Trichoderma pseudokoningii to control cucumber Fusarium wilt. Crop Prot. 2019, 124, 104857. [CrossRef]
- Ma Q, Cong Y, Feng L; et al. Effects of mixed culture fermentation of Bacillus amyloliquefaciens and Trichoderma longibrachiatum on its constituent strains and the biocontrol of tomato Fusarium wilt. J. Appl. Microbiol. 2022, 132, 532–546. [CrossRef] [PubMed]
- R. Hu, K. B. Ryang, C. Chen, et al. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [CrossRef] [PubMed]
- Liu Feng, Wu Xian, Xu Yan; et al. Study on the bacteriostatic effect of curcumin combined with phloretin on Staphylococcus aureus. Chin. J. Anim. Sci. [CrossRef]
- Bayer bactericide spiramylamine is to be approved and registered in Canada. Agrochemicals 2015, 54, 402.
- Wang Qunqing, Xu Qian, Liang Changhui; et al. Application of lauric acid in phytophthora control. Chinese invention patent CN112400878B.
- Kazuhiko Matsuda,Hideyoshi Toyoda, Koji Kakutani; et al. Indole as an antibacterial substance against Pseudomonas solanacearum. Agric. Biol. Chem. 2009, 54, 3039–3040.
- Yan Zihong, Zhao Yanliang, Fan Dongsheng; et al. Application of γ-aminobutyric acid(GABA) in agricultural production. Chem. Fertil. Des. 2021, 59, 18.
- Gu Guifei. Bioactivity of osthole on Monilinia fructicola and preliminary study on its antifungal mechanism. Guizhou University, 2022.
- Wang Kaifeng. The application of Bacillus biolcontrol agents and 6% validamycin·osthole to control rice sheath blight disease. Nanjing Agricultural University, 2013.
- Qi Zhongqiang, Xue Yanfeng, Zhang Meng; et al. Effect of osthol on the invasion of Magnaporthe oryzae. Jiangsu J. Agr. Sci. 2015, 31, 1265–1269.
- Sun Guangzhong, Liu Yuanming, Deng Jinsong; et al. Field experiment on the control of wheat powdery mildew with osthol. Hubei Plant Prot. 2016, 6-7, 22.
- Peng Zhiguo, Li Gen, Xu Zhonggui. Experiment on control of cucumber powdery mildew by greenhouse pesticide osthole. Hortic. Seed 2020, 40, 7–8.
- Shen Nanxing, Liang Zhaoyang, Liu Qing; et al. Antifungal secondary metabolites isolated from Mangrove Rhizosphere soil-derived penicillium fungi. J. Ocean. Univ. China 2020, 19, 717–721. [CrossRef]
- Liu Yutong, Xu Ruixuan, Wang Hongtao; et al. Exogenous glycine betaine improved the resistance of apple to glomerella leaf spot. J. Fruit Sci. 2022, 39, 1252–1261.
- Xu Zijian, Sun Mengli, Jiang Xuefei; et al. Preliminary study of betaine mediated resistance against Fusarium wilt in watermelon. Chin. J. Trop. Crops 2018, 39, 355–360.
- Li Ming, Long Houru, Liu Xiangjiang; et al. Experiment and demonstration of new plant growth regulator zeatin. 2004-03–2005-02.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).