1. Introduction
Lung cancer remains the primary cause of death from cancer [
1]. Non-small cell lung cancer (NSCLC) accounts for most cases of lung cancer. Although lobectomy and lymphadenectomy has been the standard treatment for NSCLC patients with early-stage disease [
2], more than 50% patients relapse after surgery alone [
3]. Previous clinical trials investigating patients with stage I-III NSCLC post-surgery have shown that cisplatin-based adjuvant therapy significantly lowered mortality risk, particularly in stages II and III diseases [
4]. The efficacy of cytotoxic therapy is grounded in an intensive cisplatin-based regimen to eradicate micrometastatic lesions [
5].
In recent years, several clinical trials have confirmed the non-inferior efficacy of sublobar resection compared to lobectomy in small-sized NSCLC. In patients diagnosed with peripheral NSCLC with tumors up to 2 cm in size and verified absence of nodal involvement in the hilar and mediastinal regions, sublobar resection demonstrated comparable outcomes to lobectomy in terms of disease-free survival (DFS) [
6]. In addition, a recent clinical trial in Japan comparing lobectomy and segmentectomy for small-sized (≤ 20 mm) peripheral NSCLC revealed that DFS for segmentectomy was not inferior to lobectomy, and overall survival (OS) was even significantly longer in the segmentectomy group compared to lobectomy [
7]. As the number of patients with early-stage NSCLC suitable for sublobar resection is expected to increase, necessitating increased focus on the clinical role of adjuvant chemotherapy for these patients with pathological lymph node (LN) metastasis. Patients with NSCLC who have undergone lobectomy are indicated for postoperative adjuvant chemotherapy if LN metastases are found postoperatively. Strictly speaking, however, there is no evidence for the efficacy of adjuvant chemotherapy after sublobar resection of small-sized NSCLC. Thus, the aim of the current study was to examine the prognostic significance of adjuvant chemotherapy in patients with small-sized (≤ 20 mm) NSCLC and pathological LN metastasis who underwent sublobar resection.
2. Materials and Methods
2.1. National Cancer Database (NCDB)
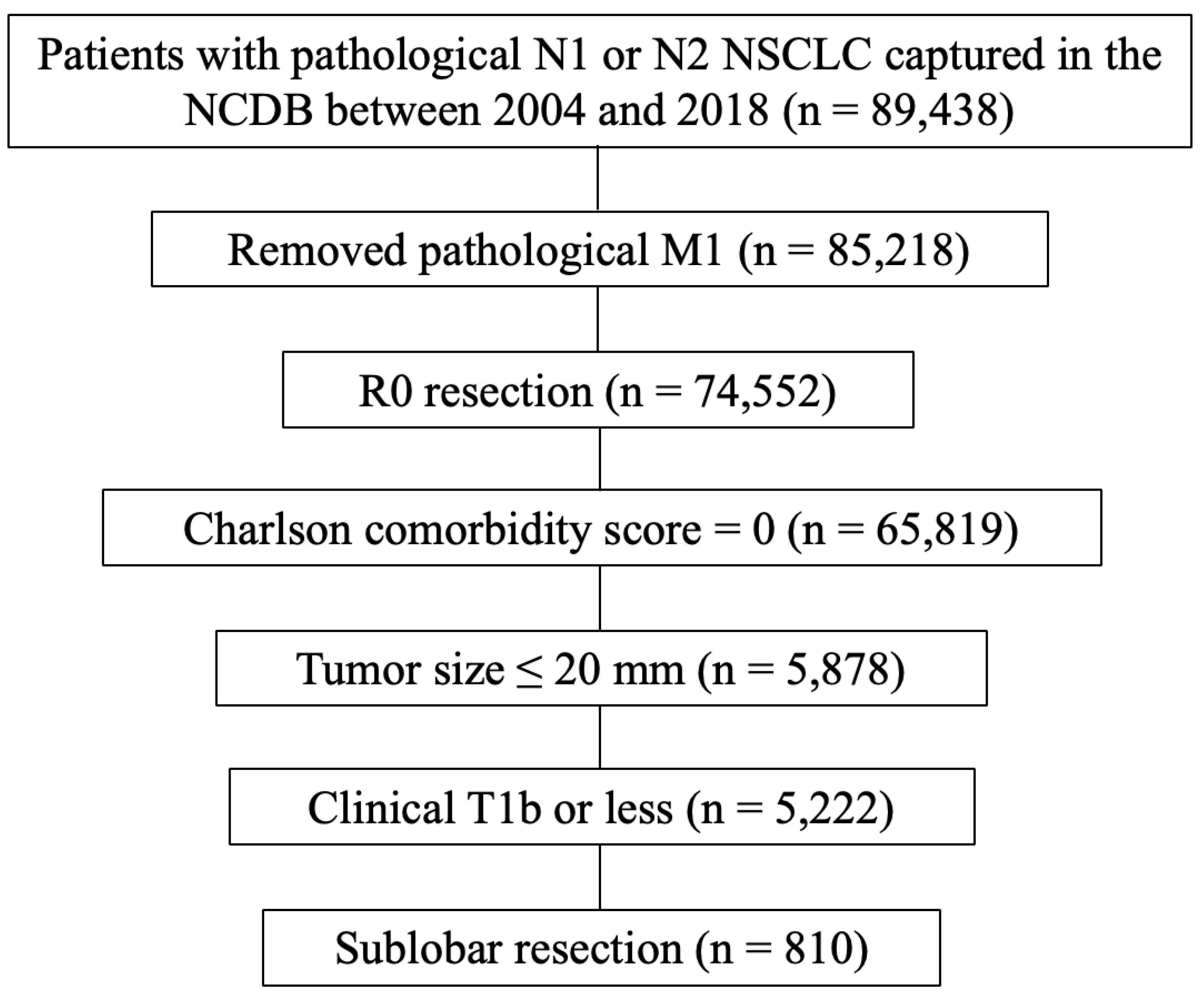
The NCDB represents a collaborative initiative between the Commission on Cancer (CoC) at the American College of Surgeons and the American Cancer Society. The data utilized in this study, provided by hospitals participating in the CoC's NCDB, are de-identified and thus the institutions mentioned do not confirm the accuracy or endorse the conclusions of the data analysis presented by the authors. This dataset is considered hospital-based rather than population-based. The Penn State College of Medicine institutional review board granted an exemption for this study. The process for selecting eligible cases is shown in (
Figure 1). Patients diagnosed with pathological N1 or N2 NSCLC and captured in the NCDB database between 2004 and 2018 were selected (n = 89,438). Of these, patients with pathological M1 disease were excluded (n = 4,220). Patients with R0 resection (n = 74,552), Charlson comorbidity score = 0 (n = 65,819), tumor size ≤ 20 mm (n = 5,878), clinical T1b or less disease (n = 5,222), and sublobar resection (n = 810) were eligible for final analysis. The number of LN dissections were classified into 3 groups (≥ 10, ≤ 9, and unknown) according to the CoC’s recommendation [
8].
2.2. Statistical Analysis
Patients’ demographics and basic clinical characteristics were compared between the adjuvant chemotherapy groups using Chi-square tests. Overall survival (OS) was defined as the time (years) from diagnosis to death from any cause. Kaplan-Meier curves according to the use of adjuvant chemotherapy were compared using the log-rank test. Univariate and multivariable Cox proportional hazards analyses were performed using JMP® 14.0 (SAS Institute Inc., Cary, NC, USA). Analyses using propensity score matching (PSM) were conducted with the aim of reducing the bias of the observational study. The propensity scores included matching of the following variables: age, sex, histology, and pathological N stage. PSM was performed using “MatchIt” package version 4.5.5 running on R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria). 1:1 nearest neighbor matching was done with propensity scores estimated through logistic regression. Finally, 243 matched patients from each group were included in the PSM analyses. All tests were two-sided and p < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
Patient characteristics (n = 810) are summarized (
Table 1). In total, 567 (70%) received adjuvant chemotherapy, 651 (80%) underwent wedge resection, and 526 (65%) had pathological N2 disease. Patients who received adjuvant chemotherapy were significantly associated with younger age (
p < 0.0001), non-academic institution (
p = 0.0060), late years of diagnosis (
p = 0.0026), right laterality (
p = 0.0241), wedge resection (
p = 0.0058), pathologic N2 stage (
p < 0.0001), and adjuvant chest radiation therapy (
p < 0.0001). After PSM adjusting age, sex, histology and pathological N status, these factors were well-balanced between two groups with or without adjuvant chemotherapy.
3.2. Univariate Analyses of OS in NSCLC Patients with Pathological LN Metastasis who Underwent Sublobar Resection According to the Use of Adjuvant Chemotherapy
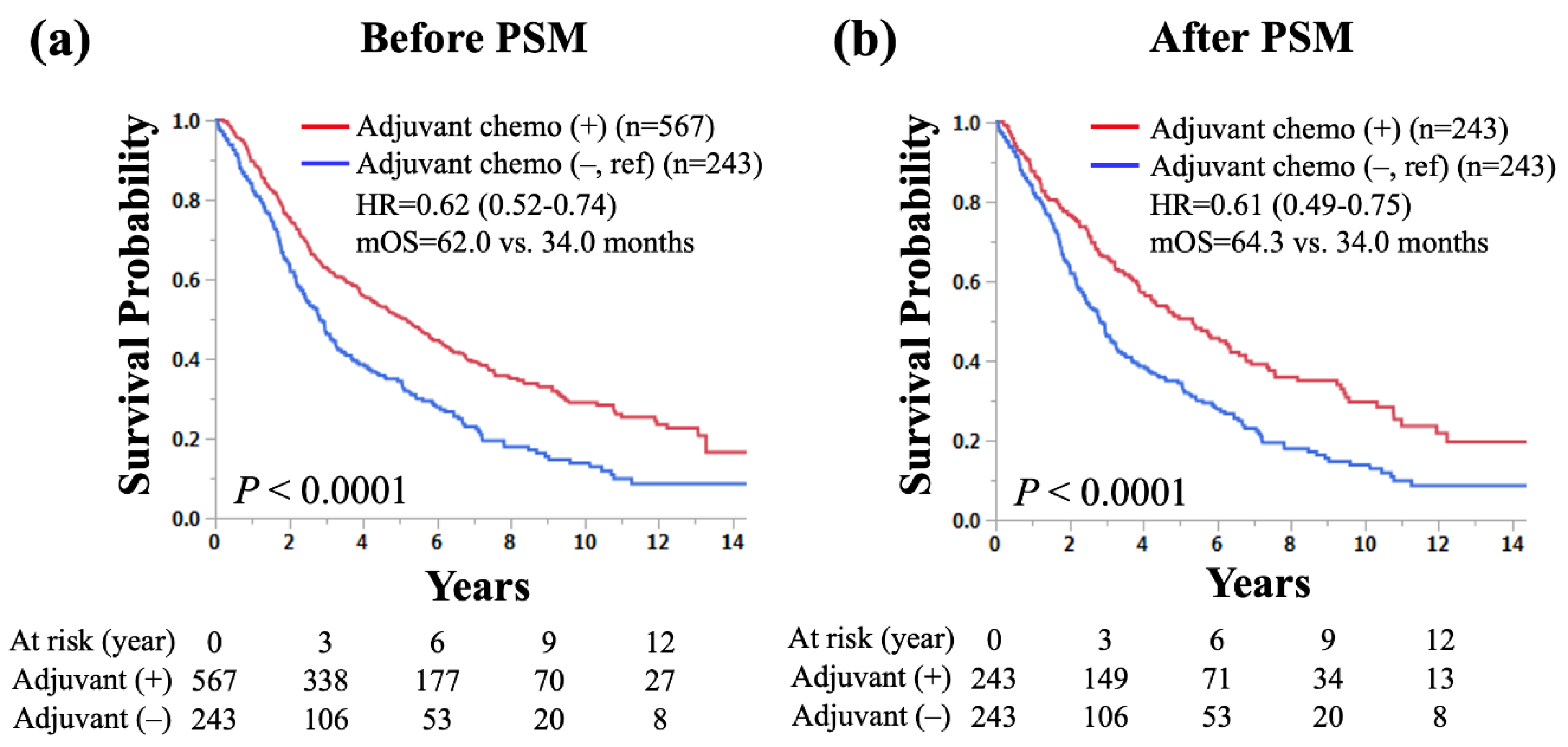
The Kaplan-Meier curves comparing OS according to the use of adjuvant chemotherapy in patients who underwent sublobar resection with pathological LN metastasis are shown (
Figure 2). Patients with adjuvant chemotherapy had a significantly longer OS than those without (5-year survival rate: 50.3% vs. 34.2%, median OS: 62.0 vs. 34.0 months, hazard ratio [HR] for death: 0.62, 95% confidence interval [CI]: 0.52–0.74,
p < 0.0001;
Figure 2a). After PSM for age, sex, histology, and pathological LN metastasis, patients with adjuvant chemotherapy had a significantly longer OS than those without (5-year survival rate: 50.6% vs. 34.2%, median OS: 64.3 vs. 34.0 months, HR for death: 0.61, 95% CI: 0.49–0.75,
p < 0.0001;
Figure 2b).
3.3. Univariate and Multivariable Analyses of OS in NSCLC Patients with Pathological LN Metastasis who Underwent Sublobar Resection
The results of univariate and multivariable analyses for OS in small-sized NSCLC patients who underwent sublobar resection are shown (
Table 2). Univariate analysis showed that younger age (
p = 0.0026), female (
p < 0.0001), number of LN dissected ≥ 10 (
p = 0.0073), pathological N1 disease (
p = 0.0342), and adjuvant chemotherapy (HR for death: 0.62, 95% CI: 0.52–0.74,
p < 0.0001) were significantly associated with longer OS. In multivariable analysis, younger age (
p = 0.0101), female sex (
p < 0.0001), race other than Caucasian (
p = 0.0294), pathological N1 disease (
p = 0.0173), and adjuvant chemotherapy (HR for death: 0.58, 95% CI: 0.48–0.71,
p < 0.0001) were independent factors for predicting longer OS. After PSM for age, sex, histology, and pathological LN metastasis, multivariate analysis of OS showed that younger age (
p = 0.0206), female (
p = 0.0005), and adjuvant chemotherapy (HR for death: 0.58, 95% CI: 0.46–0.72,
p < 0.0001) were independent factors for predicting longer OS (
Table 3).
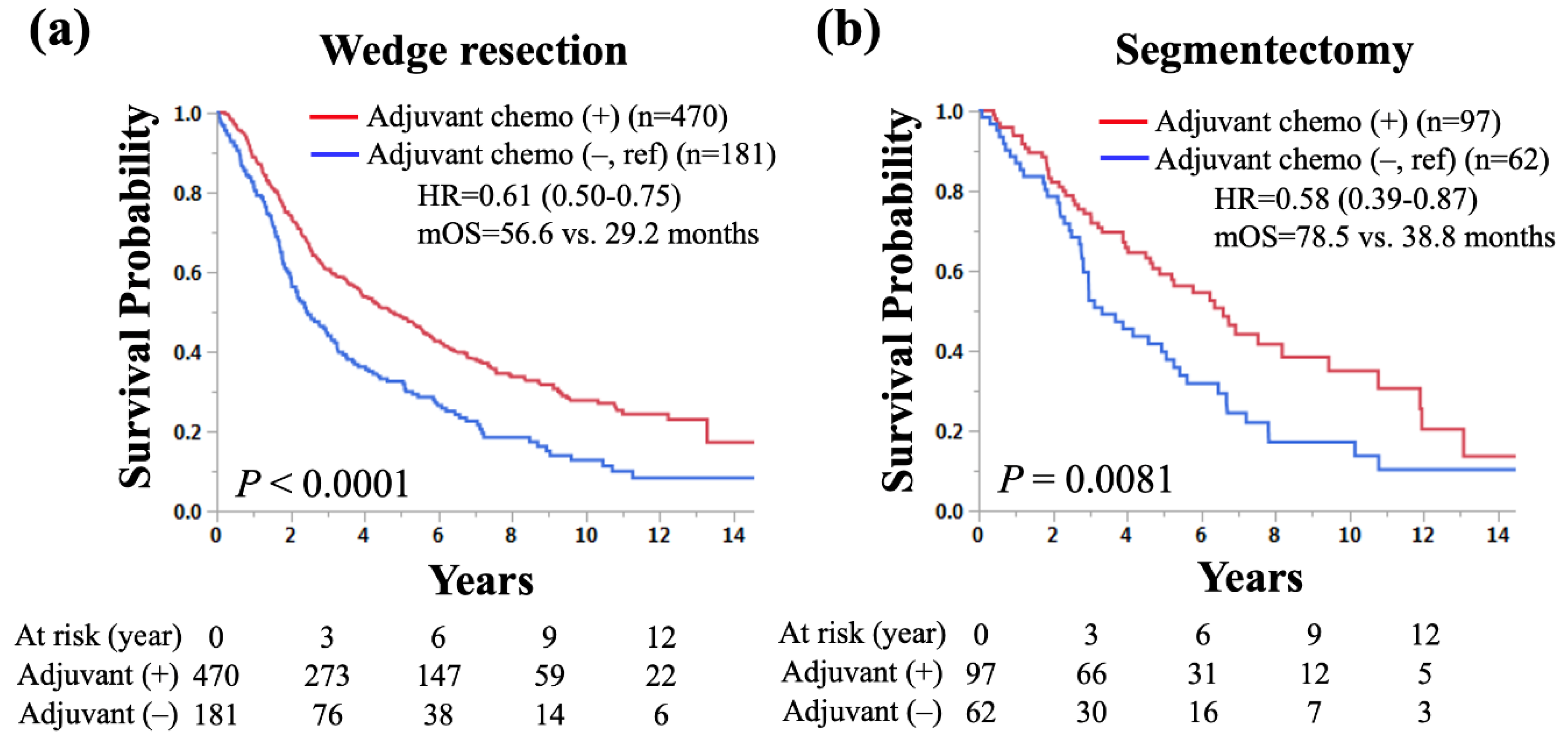
3.4. Subgroup Analyses of OS by Surgical Procedures in NSCLC Patients who Underwent Sublobar Resection with Pathological LN Metastasis According to the Use of Adjuvant Chemotherapy
In total cohort, 651 (80%) and 159 (20%) underwent wedge resection and segmentectomy, respectively. To find out if wedge resection and segmentectomy have the same tedencies with respect to survival benefit of adjuvant chemotherapy, subgroup analyses according to surgical procedure were performed. In NSCLC patients who had wedge resection and pathological LN metastasis, adjuvant chemotherapy was significantly associated with longer OS than those without (median OS: 56.6 vs. 29.2 months, HR for death: 0.61, 95% CI: 0.50–0.75,
p < 0.0001;
Figure 3a). Similarly, in NSCLC patients who had segmentectomy and pathological LN metastasis, adjuvant chemotherapy was significantly associated with longer OS than those without (median OS: 78.5 vs. 38.8 months, HR for death: 0.58, 95% CI: 0.39–0.87,
p = 0.0081;
Figure 3b).
4. Discussion
In patients with NSCLC of 20 mm or smaller, hilar and mediastinal lymph node metastases occur in 10-15% of cases [
9]. Such patients experience stage migration from stage I to stage II or III and an increased frequency of recurrence. In the current study, adjuvant chemotherapy was suggested to have a clinically significant survival benefit in patients with NSCLC ≤ 20 mm with pathologic LN metastases who underwent sublobar resection. Since the application of surgical resection and postoperative adjuvant chemotherapy is influenced by patient age, comorbidities, and pathological N stage, only patients with Charlson comorbidity score = 0 were included in this study to reduce such biases, and PSM was also performed. After PSM adjusting age, sex, histology, and pathological N stage, adjuvant chemotherapy was an independent prognostic factor of longer OS, with a clinically meaningful low HR (0.58), and 5-year survival rate was 16.4% higher with adjuvant chemotherapy than without. Moreover, the subgroup analysis by surgical procedure suggested a similar trend regarding the survival benefit of adjuvant chemotherapy regardless of wedge resection or segmentectomy.
There is no existing evidence on the effectiveness of adjuvant chemotherapy after sublobar resection of small-sized NSCLC with pathological LN metastasis. Recently, two major clinical trials on sublobar resection for small-sized NSCLC provided different protocols on when to use adjuvant chemotherapy. The CALGB 140503 study left the decision to perform adjuvant chemotherapy up to the physician's choice [
6]. In contrast, the JCOG0802/WJOG4607L trial generally recommended administering adjuvant chemotherapy for pathological stage II-III disease [
7]. In the current real-world data, 567 out of 810 (70%) patients received adjuvant chemotherapy. Considering that our results suggested a survival benefit of adjuvant chemotherapy and that 30% of patients did not receive it, it may be important to consider expanding the indication for adjuvant chemotherapy to more patients with small-sized NSCLC and pathological LN metastasis after sublobar resection. The results of the current study also suggested the importance of intraoperative LN dissection to detect potential LN metastasis. Our previous retrospective study regarding the required extent of thoracic lymphadenectomy in patients with small-sized NSCLC who undergo sublobar resection reported that performing ≥ 10 LN dissections had a survival benefit [
10].
Recently, adjuvant chemotherapy has dramatically developed in the area of targeted drugs and immune checkpoint inhibitors (ICI) [
11,
12]. The ADAURA trial on adjuvant osimertinib, a third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors has demonstrated a survival benefit [
13]. Moreover, the ongoing ADAURA2 trial has been assessing the efficacy of adjuvant osimertinib in early-stage (IA2-IA3) NSCLC [
14]. With regard to ICIs, the IMpower010 trial demonstrated significantly improved DFS with adjuvant atezolizumab versus best supportive care in the programmed death-ligand 1-positive populations. Although the use of targeted drugs and ICI has been developed as adjuvant treatment, they were primarily investigated in the setting of post adjuvant chemotherapy except for ALINA trial. Until future studies clearly determine lack of OS benefit, the use of adjuvant chemotherapy prior to targeted drugs/ICI should be strongly considered in routine practice. Therefore, for patients with genetic mutations or programmed death-ligand 1 expression who undergo sublobar resection and have pathological LN metastases, considering platinum-based chemotherapy as an initial adjuvant treatment is advisable.
The current study had several limitations. First, it was a retrospective analysis subject to biases related to the surgeon's decision-making and patient characteristics such as performance status. Future research should aim to verify our results through well-planned randomized trials. However, the rarity of pathological LN metastasis in patients with small-sized NSCLC makes it challenging to conduct such prospective studies. Despite its retrospective nature, the NCDB provided a substantial dataset that facilitated our study. Second, the current study lacked comprehensive data on recurrence or cause of death, which are crucial for assessing the clinical impact of adjuvant chemotherapy on cancerspecific outcomes. Third, the NCDB lacked the detailed data (station and number of metastasis) on pathological LN metastasis. The IASLC Lung Cancer Staging Project proposed the addition of new sub-descriptors to N2 for single-station and multiple-station involvement based on the results that NSCLC patients with multiple-station N2 had a significantly shorter OS compared with those with single-station N2 (5-year survival rate: 38% vs. 49%) [
15]. Survival benefit of adjuvant chemotherapy may differ depending on the station and number of pathological LN metastases, which should be investigated further studies. Fourth, the NCDB lacks in molecular data (i.e.
EGFR and
ALK). Since the prognostic impact of driver genes on survival is significant, they should be added to the survival analysis and require statistical processing.
5. Conclusions
Our retrospective study investigating patients with small-sized (≤ 20 mm) NSCLC with pathological LN metastasis who underwent sublobar resection elucidated that adjuvant chemotherapy was an independent factor of longer OS. Adjuvant chemotherapy may be recommended in patients with small-sized NSCLC with pathological LN metastasis even if the resection is sublobar. Further studies are warranted to validate these findings.
Author Contributions
Conceptualization, S.T. and T.K.; methodology, T.K.; formal analysis, J.Z. and T.K.; investigation, S.T. and A.H.; data curation, T.K.; writing—original draft preparation, S.T. and A.H.; writing—review and editing, J.Z. and T.K.; supervision, J.Z. and T.K.; project administration, S.T. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Penn State College of Medicine institutional review board granted an exemption for this study.
Informed Consent Statement
Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Takefumi Komiya received advisory fees from G1 Therapeutics and Regenerone, and institutional research funding from Gilead. All the other authors declare no conflicts of interest in association with this study.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage i and ii non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013, 143 (5 Suppl), e278S–e313S. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; et al. The iaslc lung cancer staging project: Proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the tnm classification for lung cancer. J Thorac Oncol 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.; Sivajohanathan, D.; Chan, A.; Kulkarni, S.; Ung, Y.; Ellis, P.M. Postoperative adjuvant systemic therapy in completely resected non-small-cell lung cancer: A systematic review. Clinical lung cancer 2017, 18, 259–273.e8. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the lace collaborative group. J Clin Oncol 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; et al. Lobar or sublobar resection for peripheral stage ia non-small-cell lung cancer. N Engl J Med 2023, 388, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (jcog0802/wjog4607l): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (London, England) 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- American college of surgeons coc quality of care measures 2020 surveys. Available at: Https://www.Facs.Org/quality-programs/cancer/ncdb/qualitymeasurescocweb.
- Okada, M.; Koike, T.; Higashiyama, M.; Yamato, Y.; Kodama, K.; Tsubota, N. Radical sublobar resection for small-sized non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg 2006, 132, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Komiya, T.; Shimokawa, M.; Powell, E. Lymph node dissections and survival in sublobar resection of non-small cell lung cancer ≤ 20 mm. Gen Thorac Cardiovasc Surg 2023, 71, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Amini, A.; Govindarajan, A.; et al. Targeted therapies in early-stage resectable non-small-cell lung cancer: New kids on the block. JCO Precis Oncol, 2023; 7, e2200445. [Google Scholar]
- Wu, Y.L.; Dziadziuszko, R.; Ahn, J.S.; et al. Alectinib in resected alk-positive non-small-cell lung cancer. N Engl J Med 2024, 390, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; et al. Overall survival with osimertinib in resected egfr-mutated nsclc. N Engl J Med 2023, 389, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tsutani, Y.; Goldman, J.W.; Dacic, S.; et al. Adjuvant osimertinib vs. Placebo in completely resected stage ia2-ia3 egfr-mutated nsclc: Adaura2. Clin Lung Cancer 2023, 24, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Osarogiagbon, R.U.; Giroux, D.J.; et al. The iaslc lung cancer staging project: Proposals for the revision of the n descriptors in the forthcoming 9th edition of the tnm classification for lung cancer. J Thorac Oncol 2024, 19, 766–785. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).