Submitted:
12 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
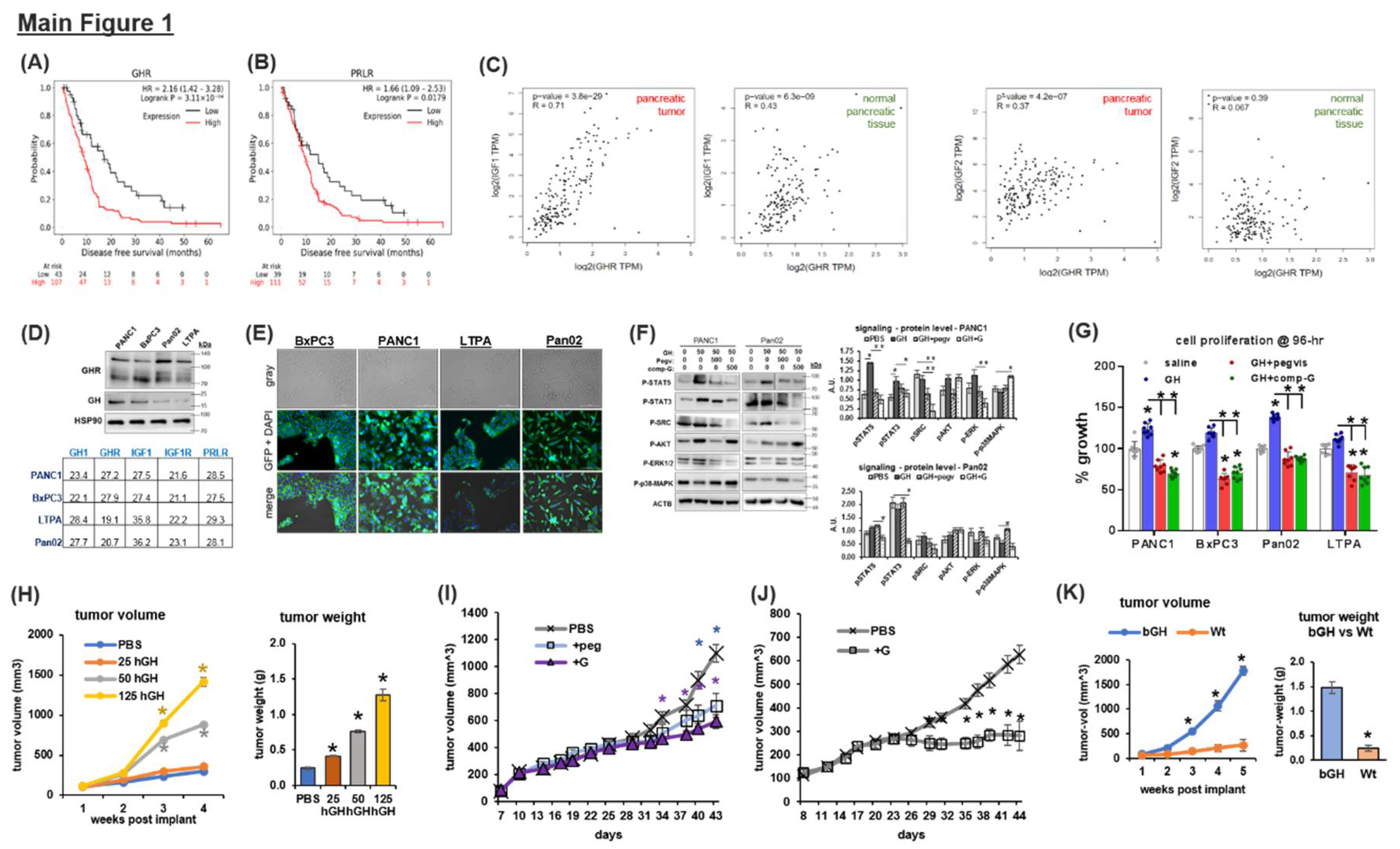
GHR Expression in PDAC and Correlation with Patient Survival:
GH Drives Pancreatic Cancer Cell Growth:
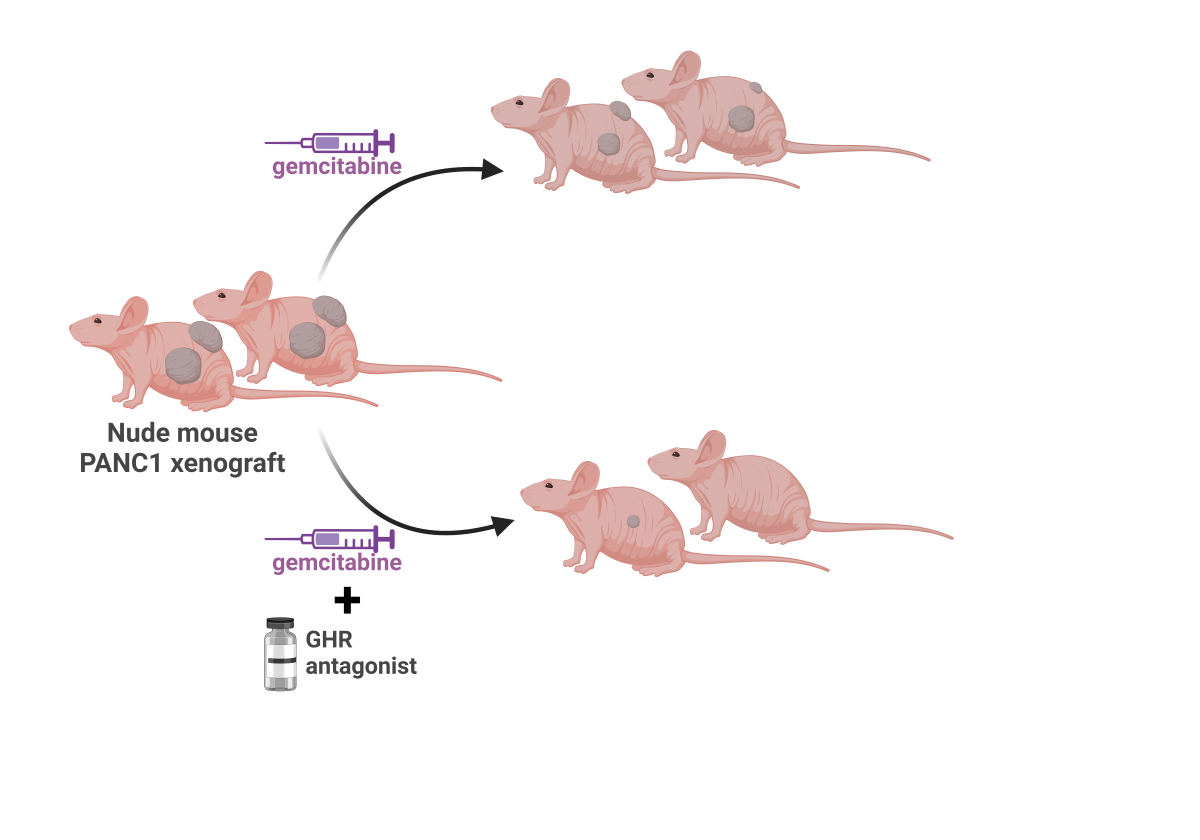
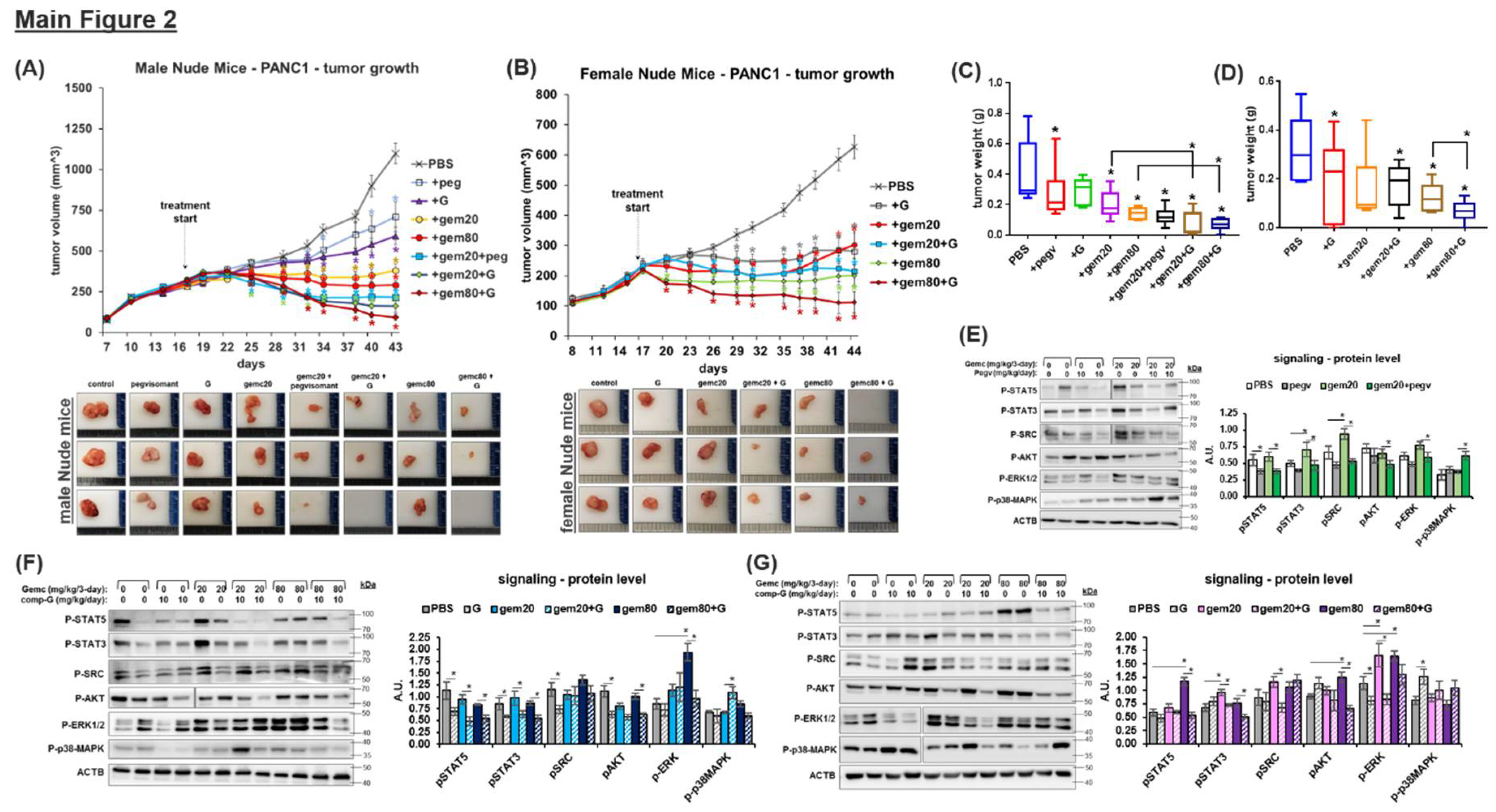
GHR Antagonism Markedly Improves Chemotherapeutic Efficacy against PDAC:
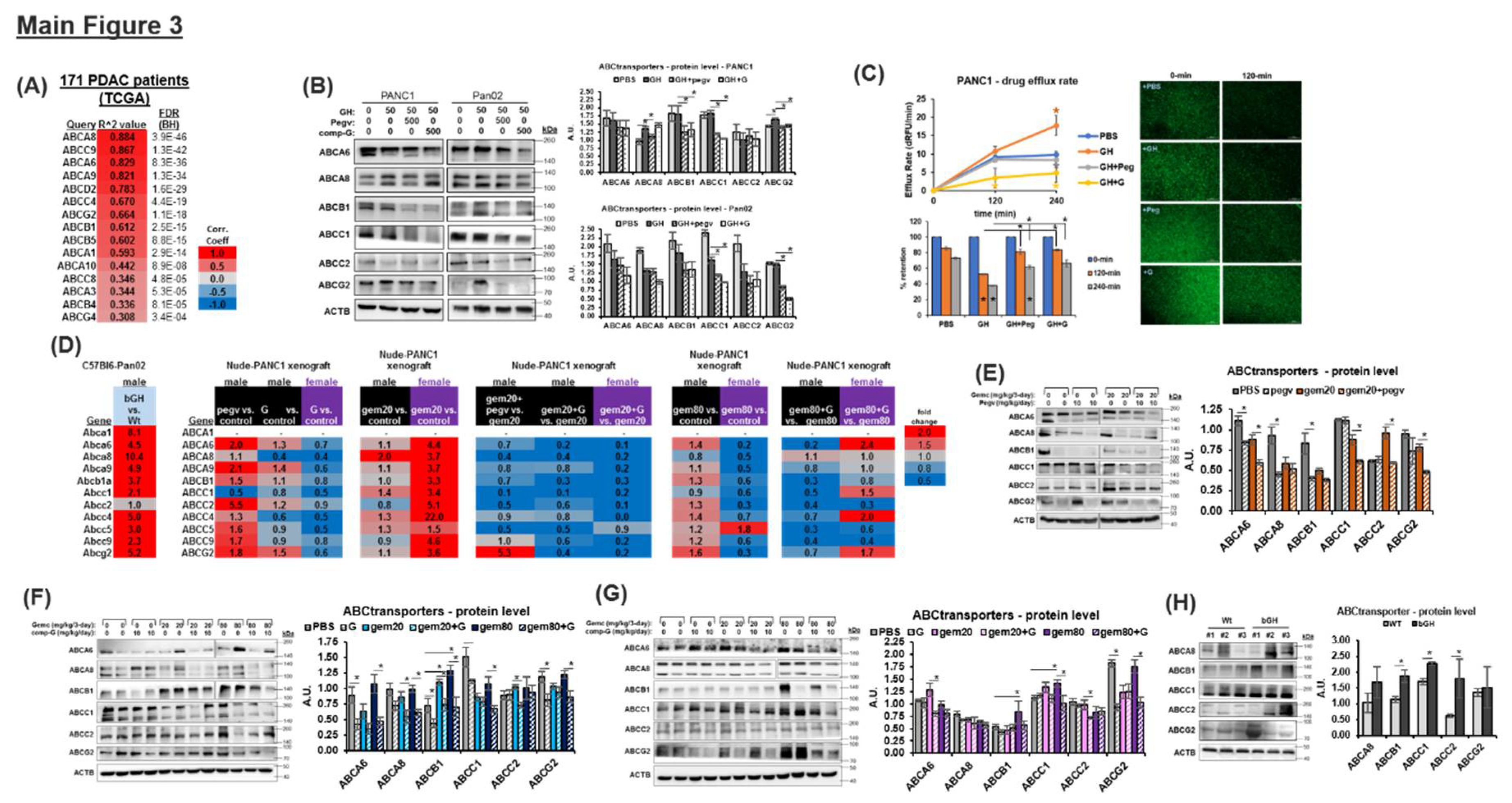
Combination of Chemotherapy and GHR Antagonism Suppresses ABC Multidrug Transporter Expression and Drug Efflux Activity in PDAC Cells and Xenografts
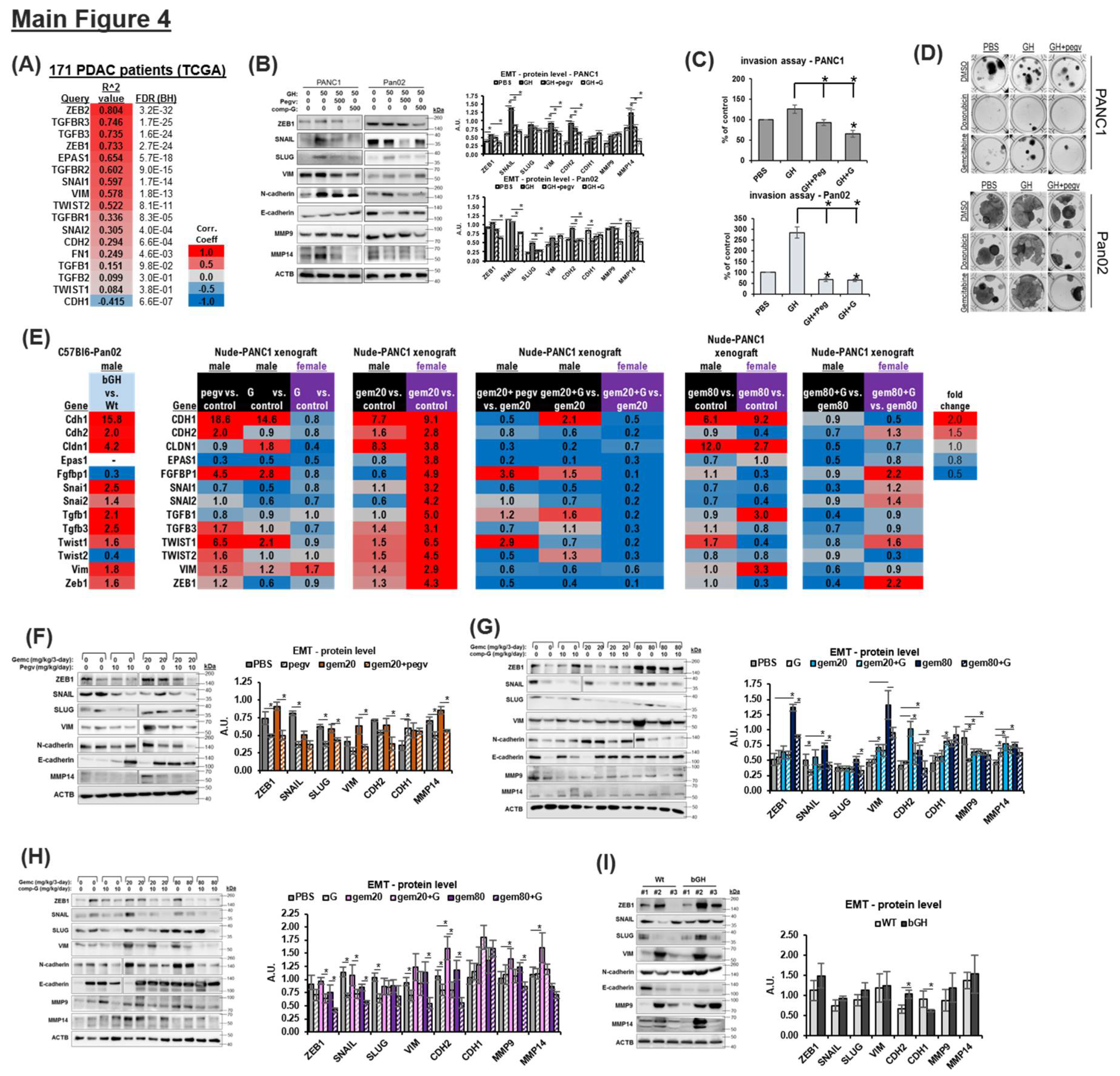
Combination of Chemotherapy and GHR Antagonism Suppresses EMT Induction in PDAC Cells and Xenografts
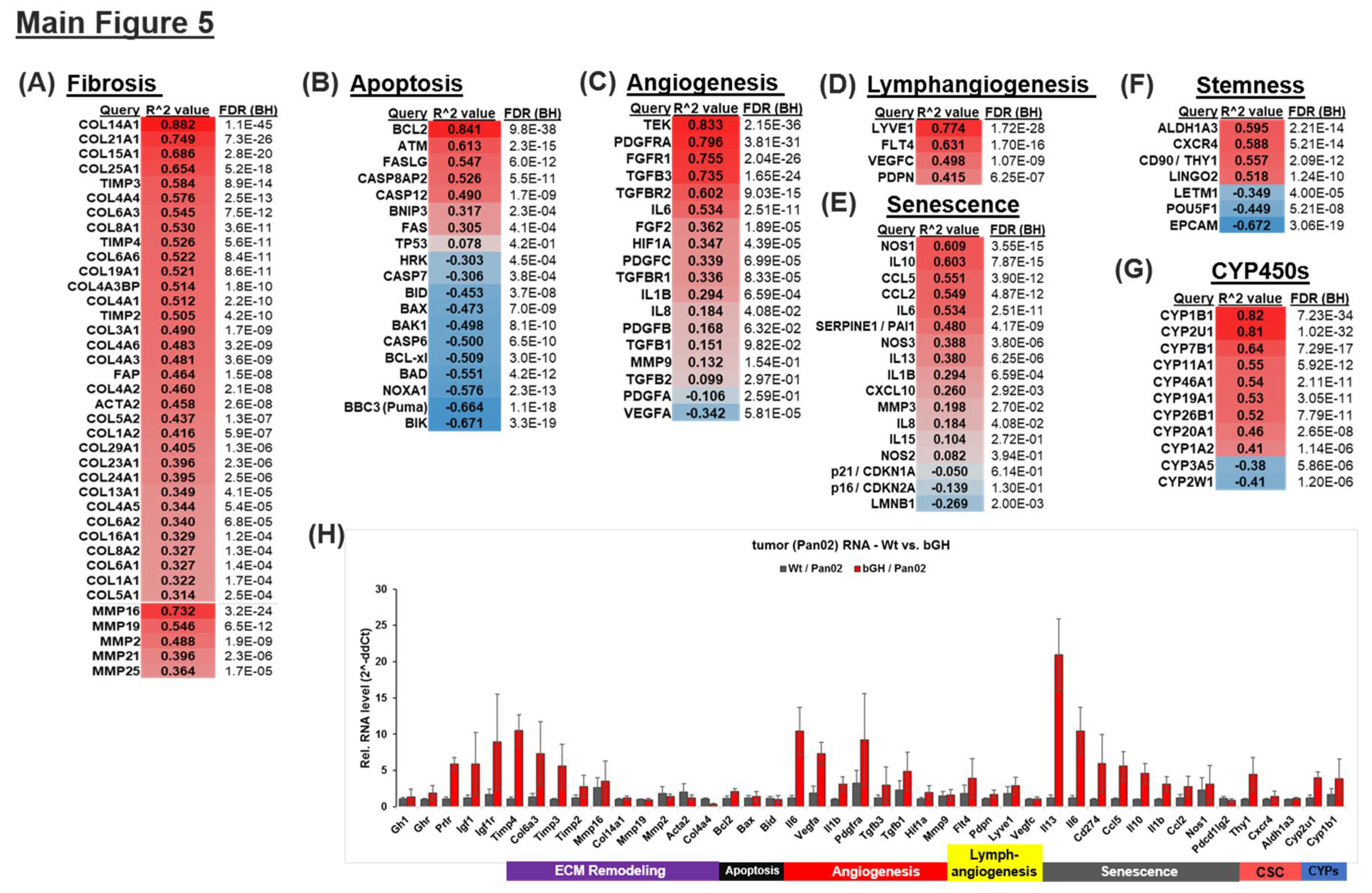
The Combination of Chemotherapy and GHR Antagonism Suppresses Markers of Apoptosis, Fibrosis, Lymphangiogenesis, Angiogenesis, Senescence and Stemness in PDAC Cells and Xenografts
3. Discussion
4. Materials and Methods
Figure Legends
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Müller, P.C.; Frey, M.C.; Ruzza, C.M.; Nickel, F.; Jost, C.; Gwerder, C.; Hackert, T.; Z’graggen, K.; Kessler, U. Neoadjuvant Chemotherapy in Pancreatic Cancer: An Appraisal of the Current High-Level Evidence. Pharmacology 2021, 106, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Schnittert, J.; Bansal, R.; Prakash, J. Targeting Pancreatic Stellate Cells in Cancer. Trends in cancer 2019, 5, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Grasso, C.; Jansen, G.; Giovannetti, E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit. Rev. Oncol. Hematol. 2017, 114, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Gu, J.; Li, J. Mechanisms of drug resistance of pancreatic ductal adenocarcinoma at different levels. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Basu, R.; Berryman, D.E.; Jorgensen, J.O.L.; Johannsson, G.; Puri, V. Covert actions of growth hormone: fibrosis, cardiovascular diseases and cancer. Nat. Rev. Endocrinol. 2022, 18, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Qian, Y.; Kopchick, J.J. MECHANISMS IN ENDOCRINOLOGY: Lessons from growth hormone receptor gene disrupted mice: Are there benefits of endocrine defects? Eur. J. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Basu, R.; Kopchick, J. GH and IGF1 in cancer therapy resistance. Endocr. Relat. Cancer 2023. [Google Scholar] [CrossRef]
- Basu, R.; Kopchick, J.J. The effects of growth hormone on therapy resistance in cancer. Cancer Drug Resist. (Alhambra, Calif.) 2019, 2, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Melmed, S. Non-pituitary GH regulation of the tissue microenvironment. Endocr. Relat. Cancer 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Zonis, S.; Apostolou, A.; Estrada, H.Q.; Knott, S.; Wawrowsky, K.; Michelsen, K.; Ben-Shlomo, A.; Barrett, R.; Gorbunova, V.; et al. Local non-pituitary growth hormone is induced with aging and facilitates epithelial damage. Cell Rep. 2021, 37, 110068. [Google Scholar] [CrossRef]
- Chesnokova, V.; Melmed, S. Growth hormone in the tumor microenvironment. Arch. Endocrinol. Metab. 2020, 63, 568–575. [Google Scholar] [CrossRef]
- Chesnokova, V.; Zonis, S.; Zhou, C.; Recouvreux, M.V.; Ben-Shlomo, A.; Araki, T.; Barrett, R.; Workman, M.; Wawrowsky, K.; Ljubimov, V.A.; et al. Growth hormone is permissive for neoplastic colon growth. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E3250–E3259. [Google Scholar] [CrossRef]
- Lombardi, S.; Honeth, G.; Ginestier, C.; Shinomiya, I.; Marlow, R.; Buchupalli, B.; Gazinska, P.; Brown, J.; Catchpole, S.; Liu, S.; et al. Growth Hormone Is Secreted by Normal Breast Epithelium upon Progesterone Stimulation and Increases Proliferation of Stem/Progenitor Cells. Stem Cell Reports 2014, 2, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.E.; Banerjee, I.; Murray, P.G.; Renehan, A.G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 2011, 7, 11–24. [Google Scholar] [CrossRef] [PubMed]
- The Tabula Muris Consortium Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 2018, 562, 367–372. [CrossRef]
- Nie, R.-C.; Zou, X.-B.; Yuan, S.-Q.; Chen, Y.-B.; Chen, S.; Chen, Y.-M.; Chen, G.-M.; Chen, X.-J.; Luo, T.-Q.; Li, S.-M.; et al. Disease-free survival as a surrogate endpoint for overall survival in adjuvant trials of pancreatic cancer: a meta-analysis of 20 randomized controlled trials. BMC Cancer 2020, 20, 421. [Google Scholar] [CrossRef]
- Posta, M.; Győrffy, B. Analysis of a large cohort of pancreatic cancer transcriptomic profiles to reveal the strongest prognostic factors. Clin. Transl. Sci. 2023, 16, 1479–1491. [Google Scholar] [CrossRef]
- Xu, J.; Sun, D.; Jiang, J.; Deng, L.; Zhang, Y.; Yu, H.; Bahl, D.; Langenheim, J.F.; Chen, W.Y.; Fuchs, S.Y.; et al. The role of prolactin receptor in GH signaling in breast cancer cells. Mol. Endocrinol. 2013, 27, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Tandon, M.; Coudriet, G.M.; Criscimanna, A.; Socorro, M.; Eliliwi, M.; Singhi, A.D.; Cruz-Monserrate, Z.; Bailey, P.; Lotze, M.T.; Zeh, H.; et al. Prolactin Promotes Fibrosis and Pancreatic Cancer Progression. Cancer Res. 2019, 79, 5316–5327. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A.; Biller, B.M.K. Pegvisomant: a growth hormone receptor antagonist used in the treatment of acromegaly. Pituitary 2017, 20, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Brody, R.; Sandbhor, U.; Kulkarni, P.; Davis, E.; Swegan, D.; Caggiano, L.J.; Brenya, E.; Neggers, S.; Kopchick, J.J. Structure and function of a dual antagonist of the human growth hormone and prolactin receptors with site-specific PEG conjugates. J. Biol. Chem. 2023, 105030. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Tanno, S.; Nakano, Y.; Habiro, A.; Izawa, T.; Mizukami, Y.; Okumura, T.; Kohgo, Y. Activation of p38 mitogen-activated protein kinase is necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cells. Anticancer Res. 2005, 25, 3347–3353. [Google Scholar] [PubMed]
- SUMIYOSHI, H.; MATSUSHITA, A.; NAKAMURA, Y.; MATSUDA, Y.; ISHIWATA, T.; NAITO, Z.; UCHIDA, E. Suppression of STAT5b in pancreatic cancer cells leads to attenuated gemcitabine chemoresistance, adhesion and invasion. Oncol. Rep. 2016, 35, 3216–3226. [Google Scholar] [CrossRef]
- Poh, A.R.; Ernst, M. Functional roles of SRC signaling in pancreatic cancer: Recent insights provide novel therapeutic opportunities. Oncogene 2023, 42, 1786–1801. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Su, G.H.; Lin, J. Dasatinib can enhance paclitaxel and gemcitabine inhibitory activity in human pancreatic cancer cells. Cancer Biol. Ther. 2019, 20, 855–865. [Google Scholar] [CrossRef]
- Peisl, S.; Mellenthin, C.; Vignot, L.; Gonelle-Gispert, C.; Bühler, L.; Egger, B. Therapeutic targeting of STAT3 pathways in pancreatic adenocarcinoma: A systematic review of clinical and preclinical literature. PLoS One 2021, 16, e0252397. [Google Scholar] [CrossRef]
- Sasaki, N.; Ishiwata, T.; Hasegawa, F.; Michishita, M.; Kawai, H.; Matsuda, Y.; Arai, T.; Ishikawa, N.; Aida, J.; Takubo, K.; et al. Stemness and anti-cancer drug resistance in ATP-binding cassette subfamily G member 2 highly expressed pancreatic cancer is induced in 3D culture conditions. Cancer Sci. 2018, 109, 1135–1146. [Google Scholar] [CrossRef]
- Hagmann, W.; Jesnowski, R.; Löhr, J.M. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia 2010, 12, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yuan, H.; Gu, J.; Xu, D.; Wang, M.; Qiao, J.; Yang, X.; Zhang, J.; Yao, M.; Gu, J.; et al. ABCA8-mediated efflux of taurocholic acid contributes to gemcitabine insensitivity in human pancreatic cancer via the S1PR2-ERK pathway. Cell death Discov. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.G.; Monte, M.J.; Macias, R.I.R.; Romero, M.R.; Herraez, E.; Asensio, M.; Ortiz-Rivero, S.; Cives-Losada, C.; Di Giacomo, S.; Gonzalez-Gallego, J.; et al. Expression of Chemoresistance-Associated ABC Proteins in Hepatobiliary, Pancreatic and Gastrointestinal Cancers. Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- Weadick, B.; Nayak, D.; Persaud, A.K.; Hung, S.W.; Raj, R.; Campbell, M.J.; Chen, W.; Li, J.; Williams, T.M.; Govindarajan, R. EMT-Induced Gemcitabine Resistance in Pancreatic Cancer Involves the Functional Loss of Equilibrative Nucleoside Transporter 1. Mol. Cancer Ther. 2021, 20, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ashrafizadeh, M.; Zhang, W.; Zou, R.; Sethi, G.; Zhang, X. Molecular profile of metastasis, cell plasticity and EMT in pancreatic cancer: a pre-clinical connection to aggressiveness and drug resistance. Cancer Metastasis Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Brittain, A.L.; Basu, R.; Qian, Y.; Kopchick, J.J. Growth Hormone and the Epithelial-to-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2017, 102, 3662–3673. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Lopez-Valdez, R.; Salcido, A.; Boopalan, T.; Arumugam, A.; Nandy, S.; Lakshmanaswamy, R. Growth hormone receptor inhibition decreases the growth and metastasis of pancreatic ductal adenocarcinoma. Exp. Mol. Med. 2014, 46, e117. [Google Scholar] [CrossRef]
- DeClerck, Y.A. Desmoplasia: A Response or a Niche? Cancer Discov. 2012, 2, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting Fibrosis: The Bridge That Connects Pancreatitis and Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Myo Min, K.K.; Ffrench, C.B.; Jessup, C.F.; Shepherdson, M.; Barreto, S.G.; Bonder, C.S. Overcoming the Fibrotic Fortress in Pancreatic Ductal Adenocarcinoma: Challenges and Opportunities. Cancers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Modi, S.; Kir, D.; Banerjee, S.; Saluja, A. Control of Apoptosis in Treatment and Biology of Pancreatic Cancer. J. Cell. Biochem. 2016, 117, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers (Basel). 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-N.; Goh, K.-S.; Huang, C.-R.; Chiang, T.-C.; Lee, C.-Y.; Jeng, Y.-M.; Peng, S.-J.; Chien, H.-J.; Chung, M.-H.; Chou, Y.-H.; et al. Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EBioMedicine 2019, 47, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.; Zanoni, M.; Pirini, F.; Tumedei, M.M.; Ravaioli, S.; Rapposelli, I.G.; Frassineti, G.L.; Bravaccini, S. Pancreatic Cancer and Cellular Senescence: Tumor Microenvironment under the Spotlight. Int. J. Mol. Sci. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Bubin, R.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Yada, E.; Kasajima, R.; Niida, A.; Imoto, S.; Miyano, S.; Miyagi, Y.; Sasada, T.; Wada, S. Possible Role of Cytochrome P450 1B1 in the Mechanism of Gemcitabine Resistance in Pancreatic Cancer. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Pandol, S.; Edderkaoui, M.; Gukovsky, I.; Lugea, A.; Gukovskaya, A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2009, 7, S44–S47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Wang, X.; Li, C.; Ma, Z.; Wan, Q.; Peng, F. Pancreatic cancer and fibrosis: Targeting metabolic reprogramming and crosstalk of cancer-associated fibroblasts in the tumor microenvironment. Front. Immunol. 2023, 14, 1152312. [Google Scholar] [CrossRef] [PubMed]
- Banziger-Tobler, N.E.; Halin, C.; Kajiya, K.; Detmar, M. Growth hormone promotes lymphangiogenesis. Am. J. Pathol. 2008, 173, 586–597. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef]
- Chesnokova, V.; Melmed, S. GH and Senescence: A New Understanding of Adult GH Action. J. Endocr. Soc. 2022, 6, bvab177. [Google Scholar] [CrossRef]
- Ronchi, C.L.; Sbiera, S.; Volante, M.; Steinhauer, S.; Scott-Wild, V.; Altieri, B.; Kroiss, M.; Bala, M.; Papotti, M.; Deutschbein, T.; et al. CYP2W1 is highly expressed in adrenal glands and is positively associated with the response to mitotane in adrenocortical carcinoma. PLoS One 2014, 9, e105855. [Google Scholar] [CrossRef]
- Greenhill, C. Gemcitabine confirmed as the first-line therapy for pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 3–3. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Büchler, P.; Henne-Bruns, D.; Manzini, G. Adjuvant gemcitabine after resection of pancreatic cancer without significant difference in overall survival: a retrospective cohort study. Ann. Med. Surg. 2023, 85, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Mutgan, A.C.; Besikcioglu, H.E.; Wang, S.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol. Cancer 2018, 17, 66. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Zhao, X.; Cao, L.; Karnad, A.; Kumar, A.P.; Freeman, J.W. Gemcitabine resistance of pancreatic cancer cells is mediated by IGF1R dependent upregulation of CD44 expression and isoform switching. Cell Death Dis. 2022, 13, 682. [Google Scholar] [CrossRef]
- Yin, D.; Vreeland, F.; Schaaf, L.J.; Millham, R.; Duncan, B.A.; Sharma, A. Clinical Pharmacodynamic Effects of the Growth Hormone Receptor Antagonist Pegvisomant: Implications for Cancer Therapy. Clin. Cancer Res. 2007, 13, 1000–1009. [Google Scholar] [CrossRef]
- Werner, H.; LeRoith, D. Hallmarks of cancer: The insulin-like growth factors perspective. Front. Oncol. 2022, 12, 1055589. [Google Scholar] [CrossRef]
- Beckwith, H.; Yee, D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol. Endocrinol. 2015, 29, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, V.; Osipenko, L.; Scannell, J.W.; Hickman, J.A. Costs and Causes of Oncology Drug Attrition With the Example of Insulin-Like Growth Factor-1 Receptor Inhibitors. JAMA Netw. open 2023, 6, e2324977. [Google Scholar] [CrossRef]
- Flyvbjerg, A.; Bennett, W.F.; Rasch, R.; Kopchick, J.J.; Scarlett, J.A. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes 1999, 48, 377–382. [Google Scholar] [CrossRef]
- Stalnecker, C.A.; Grover, K.R.; Edwards, A.C.; Coleman, M.F.; Yang, R.; DeLiberty, J.M.; Papke, B.; Goodwin, C.M.; Pierobon, M.; Petricoin, E.F.; et al. Concurrent Inhibition of IGF1R and ERK Increases Pancreatic Cancer Sensitivity to Autophagy Inhibitors. Cancer Res. 2022, 82, 586–598. [Google Scholar] [CrossRef]
- Basu, R.; Nahar, K.; Kulkarni, P.; Kerekes, O.; Sattler, M.; Hall, Z.; Neggers, S.; Holub, J.M.; Kopchick, J.J. A novel peptide antagonist of the human growth hormone receptor. J. Biol. Chem. 2021, 100588. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Qian, Y.; Mathes, S.; Terry, J.; Arnett, N.; Riddell, T.; Stevens, A.; Funk, K.; Bell, S.; Bokal, Z.; et al. Growth hormone receptor antagonism downregulates ATP-binding cassette transporters contributing to improved drug efficacy against melanoma and hepatocarcinoma in vivo. Front. Oncol. 2022, 12, 936145. [Google Scholar] [CrossRef]
- Qian, Y.; Basu, R.; Arnett, N.A.; Mathes, S.C.; Duran-Ortiz, S.; Funk, K.R.; Brittain, A.L.; Singerman, J.T.; Terry, J.; Henry, B.E.; et al. Elevated growth hormone action preferentially upregulates mediators of drug efflux and epithelial-to-mesenchymal transition in a syngeneic melanoma mouse model. Mol. Cancer Res. 2019. [Google Scholar]
- Basu, R.; Baumgaertel, N.; Wu, S.; Kopchick, J.J. Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps. Horm. Cancer 2017, 8, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.I.; Liang, D.; Yuan, Y.; Zeng, D.; Chen, J.; Yang, J. Effect of combination therapy of siRNA targeting growth hormone receptor and 5-fluorouracil in hepatic metastasis of colon cancer. Oncol. Lett. 2015, 10, 3505–3509. [Google Scholar] [CrossRef]
- Shiau, C.; Su, J.; Guo, J.A.; Hong, T.S.; Wo, J.Y.; Jagadeesh, K.A.; Hwang, W.L. Treatment-associated remodeling of the pancreatic cancer endothelium at single-cell resolution. Front. Oncol. 2022, 12, 929950. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.N.; Paragomi, P.; Wang, R.; Huang, J.Y.; Adams-Haduch, J.; Midttun, Ø.; Ulvik, A.; Nguyen, T.C.; Brand, R.E.; Gao, Y.; et al. The Association between Serum Serine and Glycine and Related-Metabolites with Pancreatic Cancer in a Prospective Cohort Study. Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- Qian, Y.; Berryman, D.E.; Basu, R.; List, E.O.; Okada, S.; Young, J.A.; Jensen, E.A.; Bell, S.R.C.; Kulkarni, P.; Duran-Ortiz, S.; et al. Mice with gene alterations in the GH and IGF family. Pituitary 2021. [Google Scholar] [CrossRef]
- Young, J.A.; Duran-Ortiz, S.; Bell, S.; Funk, K.; Tian, Y.; Liu, Q.; Patterson, A.D.; List, E.O.; Berryman, D.E.; Kopchick, J.J. Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice. Metabolites 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Vreeland, F.; Schaaf, L.J.; Millham, R.; Duncan, B.A.; Sharma, A. Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: implications for cancer therapy. Clin. Cancer Res. 2007, 13, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Pivonello, R.; Auriemma, R.S.; De Martino, M.C.; Bidlingmaier, M.; Briganti, F.; Tortora, F.; Burman, P.; Kourides, I.A.; Strasburger, C.J.; et al. Efficacy of 12-month treatment with the GH receptor antagonist pegvisomant in patients with acromegaly resistant to long-term, high-dose somatostatin analog treatment: effect on IGF-I levels, tumor mass, hypertension and glucose tolerance. Eur. J. Endocrinol. 2006, 154, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Urbani, C.; Sardella, C.; Calevro, A.; Rossi, G.; Scattina, I.; Lombardi, M.; Lupi, I.; Manetti, L.; Martino, E.; Bogazzi, F. Effects of medical therapies for acromegaly on glucose metabolism. Eur. J. Endocrinol. 2013, 169, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Giampietro, A.; Visconti, F.; Rossi, L.; Donfrancesco, F.; Fleseriu, C.M.; Mirra, F.; Pontecorvi, A.; Giustina, A.; Fleseriu, M.; et al. Glucose metabolism outcomes in acromegaly patients on treatment with pasireotide-LAR or pasireotide-LAR plus Pegvisomant. Endocrine 2021, 73, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Pirchio, R.; Auriemma, R.S.; Montini, M.E.; Vergura, A.; Pivonello, R.; Colao, A. Control of acromegaly in more than 90% of patients after 10 years of pegvisomant therapy: an European referral centre real-life experience. J. Endocrinol. Invest. 2023, 46, 1027–1038. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, M.; Buckley, C.; Maynard, H.D.; Langley, R.J.; Perry, J.K. Growth hormone receptor agonists and antagonists: From protein expression and purification to long-acting formulations. Protein Sci. 2023, 32, e4727. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kulkarni, P.; Qian, Y.; Walsh, C.; Arora, P.; Davis, E.; Duran-Ortiz, S.; Funk, K.; Ibarra, D.; Kruse, C.; et al. Growth Hormone Upregulates Melanocyte-Inducing Transcription Factor Expression and Activity via JAK2-STAT5 and SRC Signaling in GH Receptor-Positive Human Melanoma. Cancers (Basel). 2019, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Wu, S.; Kopchick, J. Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways. Oncotarget 2017, 5. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: an integrated repository portal for tumor–immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




