Submitted:
12 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
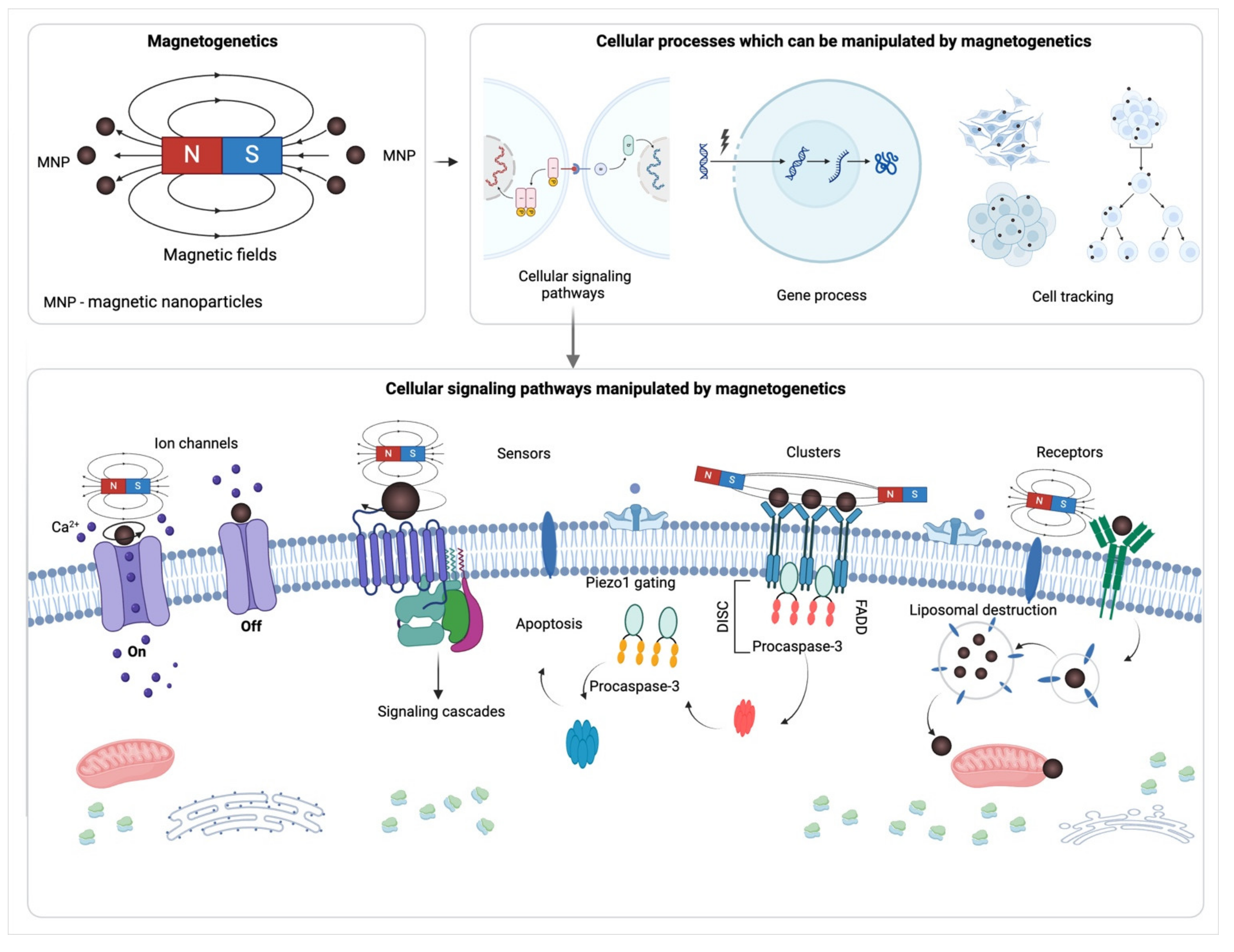
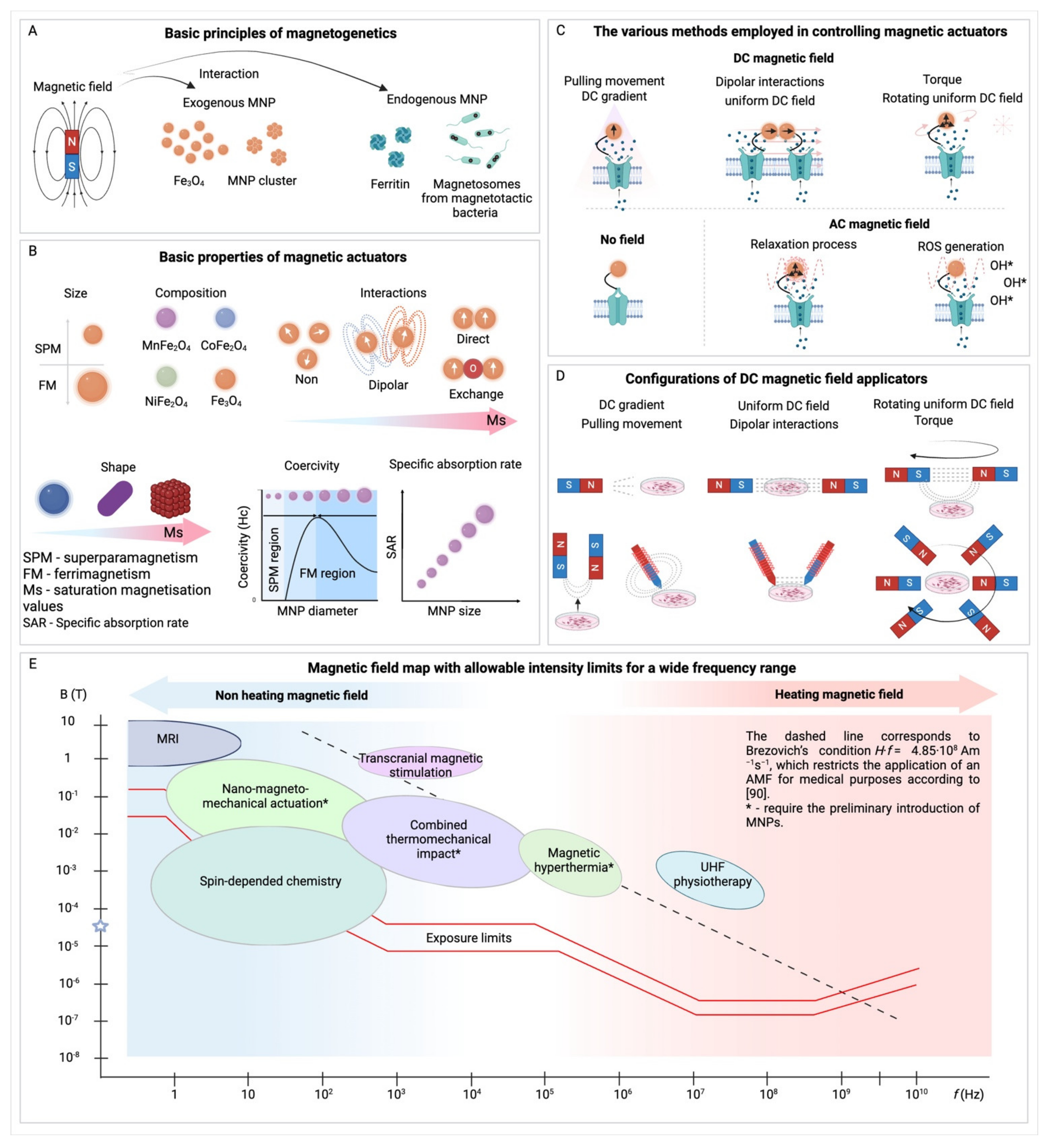
2. Basic Principles of Magnetogenetics
2.1. Exogenous Magnetic Nanoparticles in Magnetogenetics
2.2. Endogenous Magnetic Nanoparticles in Magnetogenetics
2.3. Magnetic Field and Action on a Target in Magnetogenetics
3. Application of Magnetogenetics for Mechano- and Thermosensitivity Associated Pathways Activation
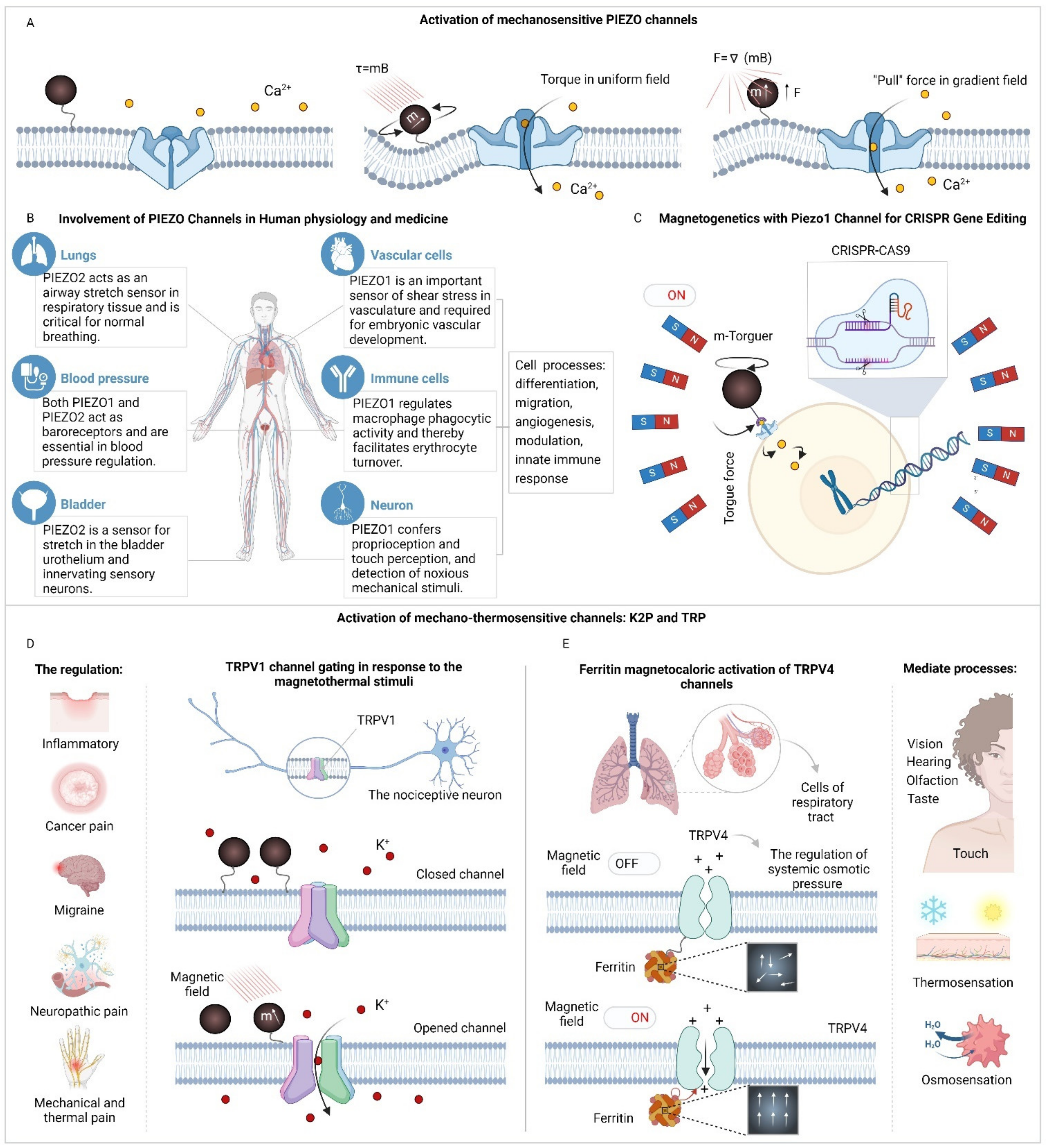
3.1. Activation of Mechano- and Thermosensitive Ion Channels via Magnetogenetics
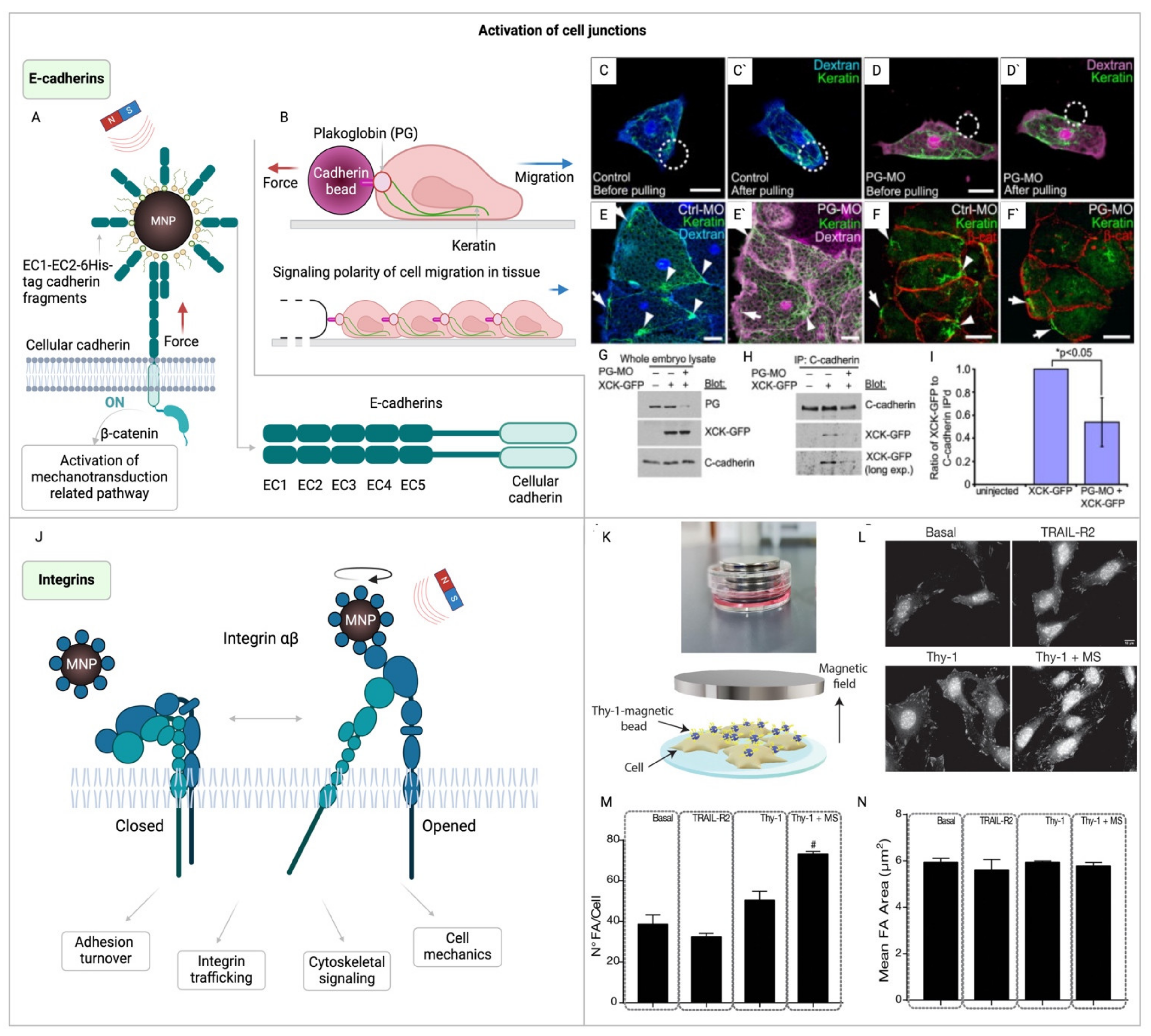
3.2. Magnetogenetic Manipulation of Cell Junctions: Bridging Cellular Mechanics and Signaling
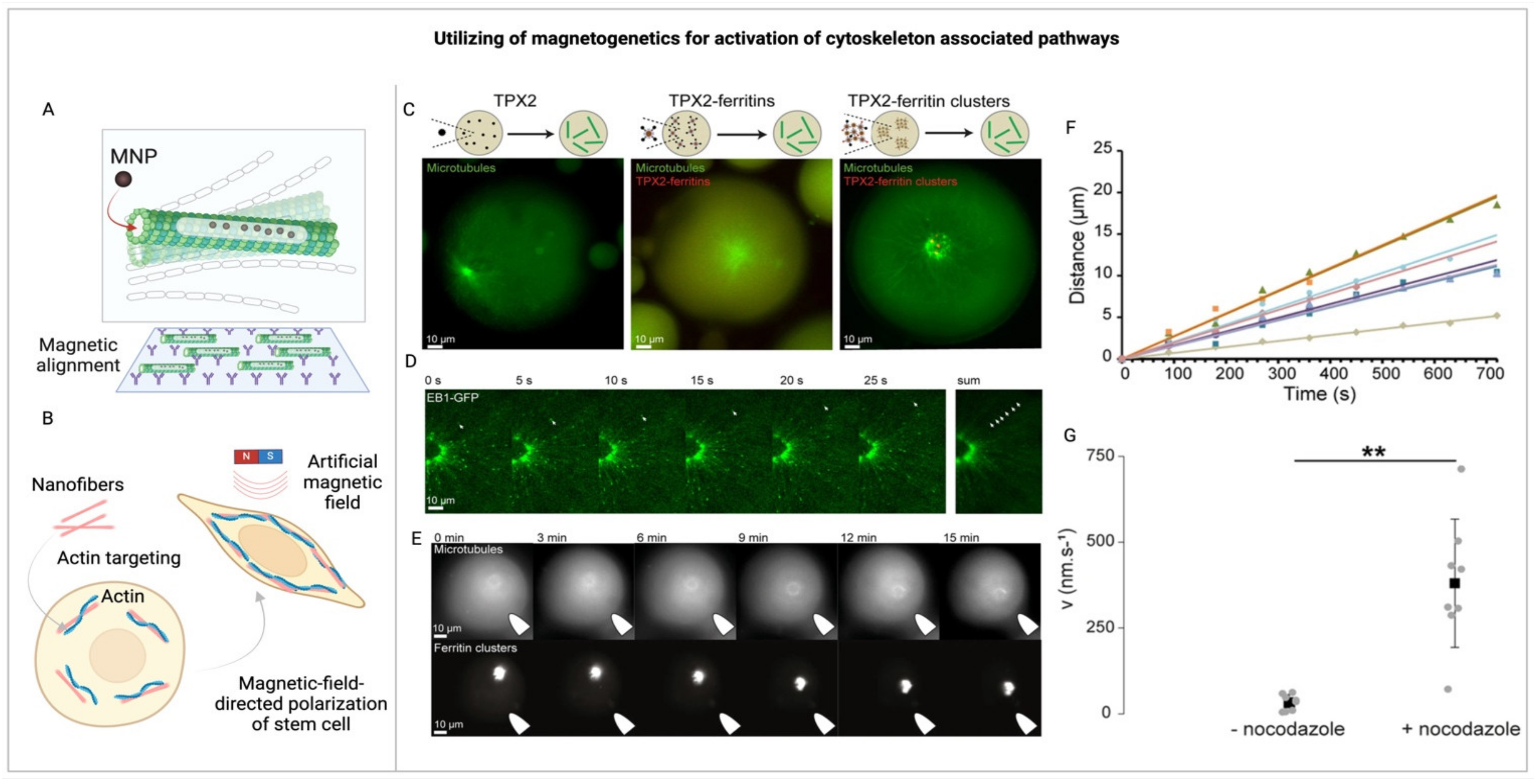
3.3. Utilizing Magnetogenetics to Explore Cytoskeletal Dynamics and Mechanotransduction
4. Ligand-Free Induction of Ligand-Mediated Signaling through Magnetogenetics
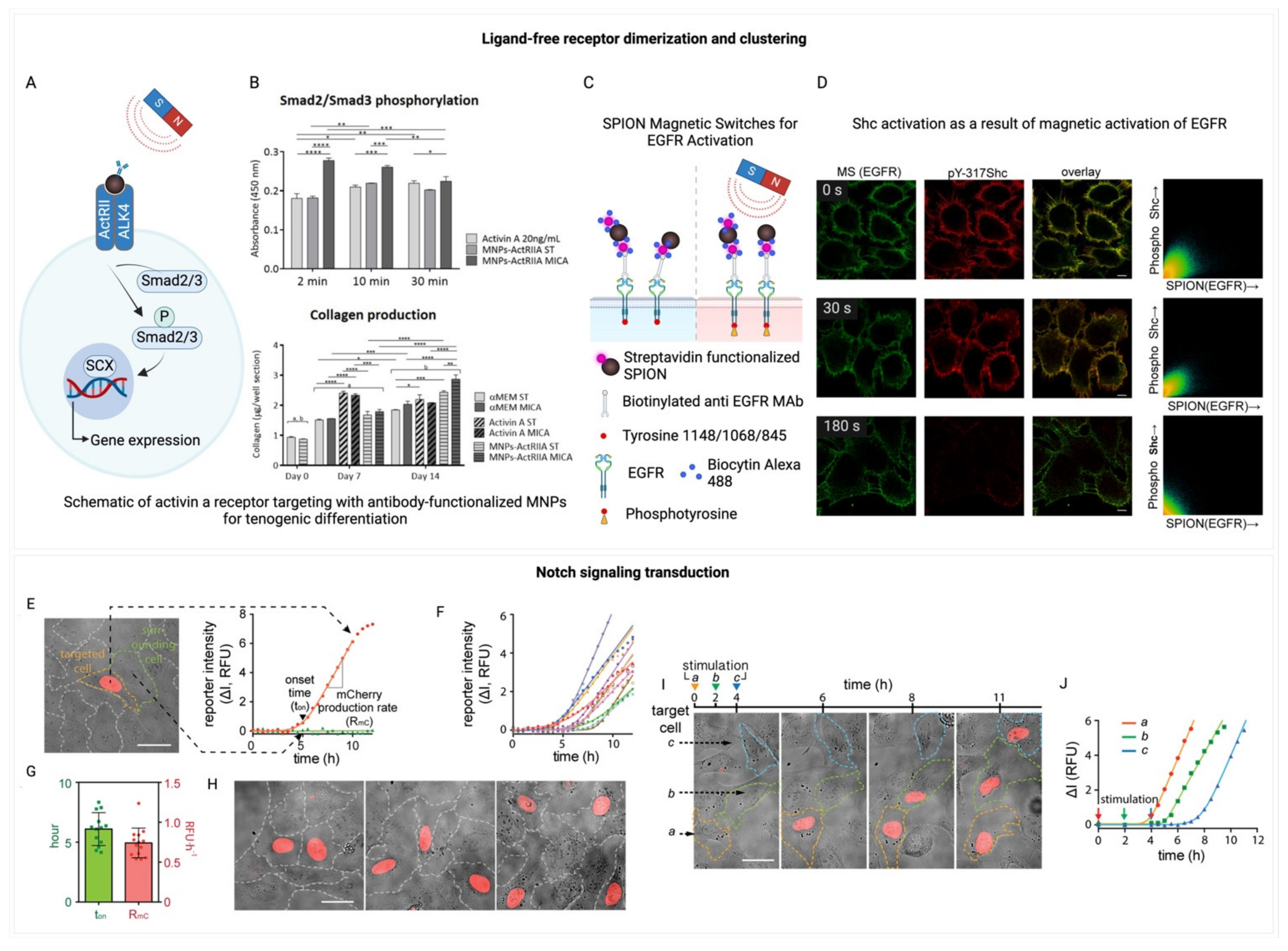
4.1. Exploring Ligand-Free Receptor Dimerization and Clustering via Magnetogenetics
4.2. Magnetogenetic Activation of Notch Signaling Pathways
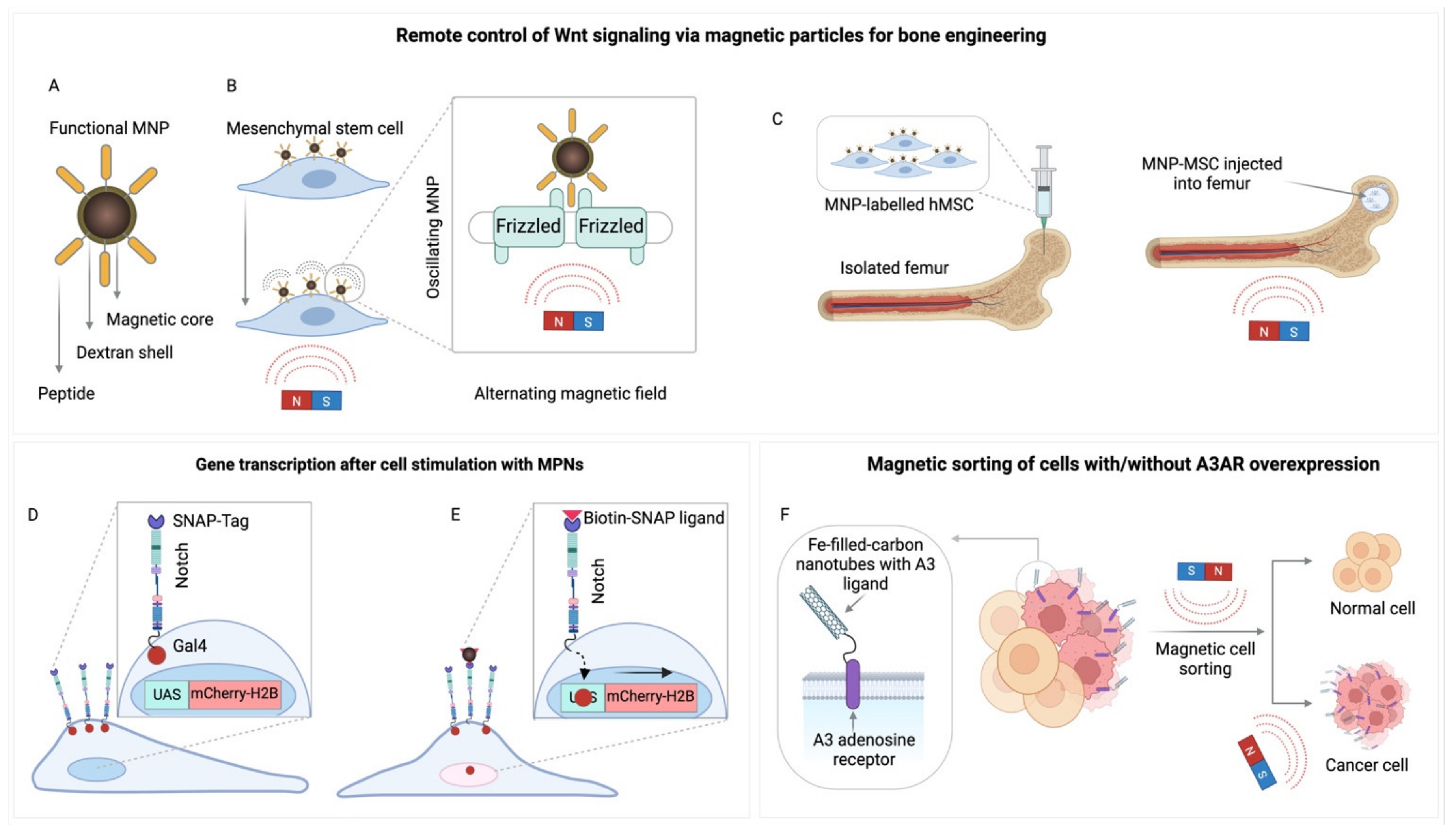
4.3. Magnetogenetic Manipulation of G protein-Coupled Receptors (GPCRs)
5. Conclusion and Future Perspectives
Author Contributions
Acknowledgments
Conflicts Of Interest
References
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal Transduction and Signal Modulation by Cell Adhesion Receptors: The Role of Integrins, Cadherins, Immunoglobulin-Cell Adhesion Molecules, and Selectins. Pharmacol Rev. 1998;50:197–264.
- Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020;587:567–76.
- Xiao R, Xu XZS. Temperature Sensation: From Molecular Thermosensors to Neural Circuits and Coding Principles. Annual Review of Physiology. 2021;83:205–30.
- Lingueglia, E. Acid-sensing Ion Channels in Sensory Perception *. Journal of Biological Chemistry. 2007;282:17325–9.
- Sun Y, Liu W-Z, Liu T, Feng X, Yang N, Zhou H-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of Receptors and Signal Transduction. 2015;35:600–4.
- Yim EK, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem Cell Research & Therapy. 2012;3:41.
- Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2011;10:939–53.
- Schlie-Wolter S, Ngezahayo A, Chichkov BN. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Experimental Cell Research. 2013;319:1553–61.
- Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. Journal of Cellular Physiology. 2011;226:299–308.
- Del Sol A, Balling R, Hood L, Galas D. Diseases as network perturbations. Current Opinion in Biotechnology. 2010;21:566–71.
- Lu, K.P. Pinning down cell signaling, cancer and Alzheimer’s disease. Trends in Biochemical Sciences. 2004;29:200–9.
- Ho G, Drego R, Hakimian E, Masliah E. Mechanisms of Cell Signaling and Inflammation in Alzheimers Disease. CDTIA. 2005;4:247–56.
- Calì T, Ottolini D, Brini M. Calcium signaling in Parkinson’s disease. Cell Tissue Res. 2014;357:439–54.
- Yang X-Z, Li X-X, Zhang Y-J, Rodriguez-Rodriguez L, Xiang M-Q, Wang H-Y, et al. Rab1 in cell signaling, cancer and other diseases. Oncogene. 2016;35:5699–704.
- Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch - Eur J Physiol. 2010;459:807–16.
- Adeshara K, G. Diwan A, S. Tupe R. Diabetes and Complications: Cellular Signaling Pathways, Current Understanding and Targeted Therapies. CDT. 2016;17:1309–28.
- Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integrative Biology. 2011;3:279–96.
- Ardito F, Giuliani M, Perrone D, Troiano G, Muzio LL. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). International Journal of Molecular Medicine. 2017;40:271–80.
- Giancotti, F.G. Deregulation of cell signaling in cancer. FEBS Letters. 2014;588:2558–70.
- Souza JACD, Junior CR, Garlet GP, Nogueira AVB, Cirelli JA. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J Appl Oral Sci. 2012;20:128–38.
- Katoh, M. WNT Signaling in Stem Cell Biology and Regenerative Medicine. CDT. 2008;9:565–70.
- Rotherham M, Nahar T, Broomhall TJ, Telling ND, El Haj AJ. Remote magnetic actuation of cell signalling for tissue engineering. Current Opinion in Biomedical Engineering. 2022;24:100410.
- Hayrapetyan A, Jansen JA, Van Den Beucken JJJP. Signaling Pathways Involved in Osteogenesis and Their Application for Bone Regenerative Medicine. Tissue Engineering Part B: Reviews. 2015;21:75–87.
- Wu R-C, Qin J, Yi P, Wong J, Tsai SY, Tsai M-J, et al. Selective Phosphorylations of the SRC-3/AIB1 Coactivator Integrate Genomic Reponses to Multiple Cellular Signaling Pathways. Molecular Cell. 2004;15:937–49.
- Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. Journal of Biology. 2006;5:14.
- Yao Z, Petschnigg J, Ketteler R, Stagljar I. Application guide for omics approaches to cell signaling. Nat Chem Biol. 2015;11:387–97.
- Roelfsema F, Veldhuis JD. Growth Hormone Dynamics in Healthy Adults Are Related to Age and Sex and Strongly Dependent on Body Mass Index. Neuroendocrinology. 2016;103:335–44.
- Egli M, Leeners B, Kruger THC. Prolactin secretion patterns: Basic mechanisms and clinical implications for reproduction. REPRODUCTION. 2010;140:643–54.
- Satou R, Sato M, Kimura M, Ishizuka Y, Tazaki M, Sugihara N, et al. Temporal Expression Patterns of Clock Genes and Aquaporin 5/Anoctamin 1 in Rat Submandibular Gland Cells. Front Physiol. 2017;8:320.
- Koseska A, Bastiaens PIH. Processing Temporal Growth Factor Patterns by an Epidermal Growth Factor Receptor Network Dynamically Established in Space. Annu Rev Cell Dev Biol. 2020;36:359–83.
- Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA, Emiliani V. Holographic photolysis of caged neurotransmitters. Nat Methods. 2008;5:821–7.
- Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods. 2015;12:969–74.
- Yoon Y, Shin H, Byun D, Woo J, Cho Y, Choi N, et al. Neural probe system for behavioral neuropharmacology by bi-directional wireless drug delivery and electrophysiology in socially interacting mice. Nat Commun. 2022;13:5521.
- Li L, Lu L, Ren Y, Tang G, Zhao Y, Cai X, et al. Colocalized, bidirectional optogenetic modulations in freely behaving mice with a wireless dual-color optoelectronic probe. Nat Commun. 2022;13:839.
- Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Le Cong, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–6.
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001.
- Arias-Gil G, Ohl FW, Takagaki K, Lippert MT. Measurement, modeling, and prediction of temperature rise due to optogenetic brain stimulation. Neurophoton. 2016;3:045007.
- Huang K, Dou Q, Loh XJ. Nanomaterial mediated optogenetics: Opportunities and challenges. RSC Adv. 2016;6:60896–906.
- Gorostiza P, Isacoff EY. Optical Switches for Remote and Noninvasive Control of Cell Signaling. Science. 2008;322:395–9.
- Knöpfel T, Akemann W. Remote control of cells. Nature Nanotech. 2010;5:560–1.
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotech. 2010;5:602–6.
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73.
- Wozniak MA, Chen CS. Mechanotransduction in development: A growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43.
- Cho MH, Kim S, Lee J-H, Shin T-H, Yoo D, Cheon J. Magnetic Tandem Apoptosis for Overcoming Multidrug-Resistant Cancer. Nano Lett. 2016;16:7455–60.
- Cho MH, Lee EJ, Son M, Lee J-H, Yoo D, Kim J, et al. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nature Mater. 2012;11:1038–43.
- Lopez S, Hallali N, Lalatonne Y, Hillion A, Antunes JC, Serhan N, et al. Magneto-mechanical destruction of cancer-associated fibroblasts using ultra-small iron oxide nanoparticles and low frequency rotating magnetic fields. Nanoscale Adv. 2022;4:421–36.
- Chen M, Wu J, Ning P, Wang J, Ma Z, Huang L, et al. Remote Control of Mechanical Forces via Mitochondrial-Targeted Magnetic Nanospinners for Efficient Cancer Treatment. Small. 2020;16:1905424.
- Kim D-H, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nature Mater. 2010;9:165–71.
- Jiang H, Fu H, Min T, Hu P, Shi J. Magnetic-Manipulated NK Cell Proliferation and Activation Enhance Immunotherapy of Orthotopic Liver Cancer. J Am Chem Soc. 2023;145:13147–60.
- Sim T, Choi B, Kwon SW, Kim K-S, Choi H, Ross A, et al. Magneto-Activation and Magnetic Resonance Imaging of Natural Killer Cells Labeled with Magnetic Nanocomplexes for the Treatment of Solid Tumors. ACS Nano. 2021;15:12780–93.
- Gonçalves AI, Rotherham M, Markides H, Rodrigues MT, Reis RL, Gomes ME, et al. Triggering the activation of Activin A type II receptor in human adipose stem cells towards tenogenic commitment using mechanomagnetic stimulation. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14:1149–59.
- Kanczler JM, Sura HS, Magnay J, Green D, Oreffo ROC, Dobson JP, et al. Controlled Differentiation of Human Bone Marrow Stromal Cells Using Magnetic Nanoparticle Technology. Tissue Engineering Part A. 2010;16:3241–50.
- Markides H, McLaren JS, Telling ND, Alom N, Al-Mutheffer EA, Oreffo ROC, et al. Translation of remote control regenerative technologies for bone repair. npj Regen Med. 2018;3:9.
- Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–80.
- Wang L, Hu P, Jiang H, Zhao J, Tang J, Jiang D, et al. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43:101401.
- Bharde AA, Palankar R, Fritsch C, Klaver A, Kanger JS, Jovin TM, et al. Magnetic Nanoparticles as Mediators of Ligand-Free Activation of EGFR Signaling. Xu B, editor. PLoS ONE. 2013;8:e68879.
- Seo D, Southard KM, Kim J, Lee HJ, Farlow J, Lee J, et al. A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time. Cell. 2016;165:1507–18.
- Barnsley LC, Carugo D, Stride E. Optimized shapes of magnetic arrays for drug targeting applications. J Phys D: Appl Phys. 2016;49:225501.
- Häfeli UO, Gilmour K, Zhou A, Lee S, Hayden ME. Modeling of magnetic bandages for drug targeting: Button vs. Halbach arrays. Journal of Magnetism and Magnetic Materials. 2007;311:323–9.
- O’Connell JLG, Robertson WSP, Cazzolato BS. Optimization of the Magnetic Field Produced by Frustum Permanent Magnets for Single Magnet and Planar Halbach Array Configurations. IEEE Trans Magn. 2021;57:1–9.
- Dobson J, Cartmell SH, Keramane A, El Haj AJ. Principles and Design of a Novel Magnetic Force Mechanical Conditioning Bioreactor for Tissue Engineering, Stem Cell Conditioning, and Dynamic In Vitro Screening. IEEE Trans.on Nanobioscience. 2006;5:173–7.
- Lee J, Kim ES, Cho MH, Son M, Yeon S, Shin J, et al. Artificial Control of Cell Signaling and Growth by Magnetic Nanoparticles. Angewandte Chemie. 2010;122:5834–8.
- Lee J, Shin W, Lim Y, Kim J, Kim WR, Kim H, et al. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat Mater. 2021;20:1029–36.
- Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nature Nanotech. 2008;3:36–40.
- Del Sol-Fernández S, Martínez-Vicente P, Gomollón-Zueco P, Castro-Hinojosa C, Gutiérrez L, Fratila RM, et al. Magnetogenetics: Remote activation of cellular functions triggered by magnetic switches. Nanoscale. 2022;14:2091–118.
- Wu C, Shen Y, Chen M, Wang K, Li Y, Cheng Y. Recent Advances in Magnetic-Nanomaterial-Based Mechanotransduction for Cell Fate Regulation. Advanced Materials. 2018;30:1705673.
- Ko MJ, Hong H, Choi H, Kang H, Kim D-H. Multifunctional Magnetic Nanoparticles for Dynamic Imaging and Therapy. Advanced NanoBiomed Research. 2022;2:2200053.
- Chen Y, Guzik S, Sumner JP, Moreland J, Koretsky AP. Magnetic manipulation of actin orientation, polymerization, and gliding on myosin using superparamagnetic iron oxide particles. Nanotechnology. 2011;22:065101.
- Liße D, Monzel C, Vicario C, Manzi J, Maurin I, Coppey M, et al. Engineered Ferritin for Magnetogenetic Manipulation of Proteins and Organelles Inside Living Cells. Advanced Materials. 2017;29:1700189.
- Tay A, Di Carlo D. Magnetic Nanoparticle-Based Mechanical Stimulation for Restoration of Mechano-Sensitive Ion Channel Equilibrium in Neural Networks. Nano Lett. 2017;17:886–92.
- Wu J, Goyal R, Grandl J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat Commun. 2016;7:12939.
- Zhang B, Yu Q, Liu Y. Polarization of Stem Cells Directed by Magnetic Field-Manipulated Supramolecular Polymeric Nanofibers. ACS Appl Mater Interfaces. 2021;13:9580–8.
- Henstock JR, Rotherham M, El Haj AJ. Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells. J Tissue Eng. 2018;9:204173141880869.
- Hu B, Haj A, Dobson J. Receptor-Targeted, Magneto-Mechanical Stimulation of Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. IJMS. 2013;14:19276–93.
- Hu B, Dobson J, El Haj AJ. Control of smooth muscle α-actin (SMA) up-regulation in HBMSCs using remote magnetic particle mechano-activation. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:45–55.
- Su C-L, Cheng C-C, Yen P-H, Huang J-X, Ting Y-J, Chiang P-H. Wireless neuromodulation in vitro and in vivo by intrinsic TRPC-mediated magnetomechanical stimulation. Commun Biol. 2022;5:1166.
- Efremova MV, Veselov MM, Barulin AV, Gribanovsky SL, Le-Deygen IM, Uporov IV, et al. In Situ Observation of Chymotrypsin Catalytic Activity Change Actuated by Nonheating Low-Frequency Magnetic Field. ACS Nano. 2018;12:3190–9.
- Munshi R, Qadri SM, Zhang Q, Castellanos Rubio I, Del Pino P, Pralle A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. eLife. 2017;6:e27069.
- Sebesta C, Torres Hinojosa D, Wang B, Asfouri J, Li Z, Duret G, et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. Nat Mater. 2022;21:951–8.
- Sanchez C, El Hajj Diab D, Connord V, Clerc P, Meunier E, Pipy B, et al. Targeting a G-Protein-Coupled Receptor Overexpressed in Endocrine Tumors by Magnetic Nanoparticles To Induce Cell Death. ACS Nano. 2014;8:1350–63.
- Sung Lee J, Myung Cha J, Young Yoon H, Lee J-K, Keun Kim Y, Myung Cha J, et al. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci Rep. 2015;5:12135.
- Golovin YI, Golovin DY, Vlasova KY, Veselov MM, Usvaliev AD, Kabanov AV, et al. Non-Heating Alternating Magnetic Field Nanomechanical Stimulation of Biomolecule Structures via Magnetic Nanoparticles as the Basis for Future Low-Toxic Biomedical Applications. Nanomaterials. 2021;11:2255.
- Gorobets SV, Gorobets OYu, Chyzh YuM, Sivenok DV. Magnetic dipole interaction of endogenous magnetic nanoparticles with magnetoliposomes for targeted drug delivery. BIOPHYSICS. 2013;58:379–84.
- Suwa M, Uotani A, Tsukahara S. Magnetic and viscous modes for physical rotation of magnetic nanoparticles in liquid under oscillating magnetic field. Applied Physics Letters. 2020;116:262403.
- Shah RR, Davis TP, Glover AL, Nikles DE, Brazel CS. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. Journal of Magnetism and Magnetic Materials. 2015;387:96–106.
- Garanina AS, Nikitin AA, Abakumova TO, Semkina AS, Prelovskaya AO, Naumenko VA, et al. Cobalt Ferrite Nanoparticles for Tumor Therapy: Effective Heating versus Possible Toxicity. Nanomaterials. 2021;12:38.
- Naumenko V, Garanina A, Nikitin A, Vodopyanov S, Vorobyeva N, Tsareva Y, et al. Biodistribution and Tumors MRI Contrast Enhancement of Magnetic Nanocubes, Nanoclusters, and Nanorods in Multiple Mice Models. Contrast Media & Molecular Imaging. 2018;2018:1–12.
- Jarockyte G, Daugelaite E, Stasys M, Statkute U, Poderys V, Tseng T-C, et al. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. IJMS. 2016;17:1193.
- Yaremenko AV, Zelepukin IV, Ivanov IN, Melikov RO, Pechnikova NA, Dzhalilova DSh, et al. Influence of magnetic nanoparticle biotransformation on contrasting efficiency and iron metabolism. J Nanobiotechnol. 2022;20:535.
- Mirkasymov AB, Zelepukin IV, Ivanov IN, Belyaev IB, Sh. Dzhalilova D, Trushina DB, et al. Macrophage blockade using nature-inspired ferrihydrite for enhanced nanoparticle delivery to tumor. International Journal of Pharmaceutics. 2022;621:121795.
- Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem Rev. 2012;112:5818–78.
- Etoc F, Lisse D, Bellaiche Y, Piehler J, Coppey M, Dahan M. Subcellular control of Rac-GTPase signalling by magnetogenetic manipulation inside living cells. Nature Nanotech. 2013;8:193–8.
- Shin W, Jeong S, Lee J, Jeong SY, Shin J, Kim HH, et al. Magnetogenetics with Piezo1 Mechanosensitive Ion Channel for CRISPR Gene Editing. Nano Lett. 2022;22:7415–22.
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of Mechanotransduction. Developmental Cell. 2006;10:11–20.
- Smolková B, Uzhytchak M, Lynnyk A, Kubinová Š, Dejneka A, Lunov O. A Critical Review on Selected External Physical Cues and Modulation of Cell Behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers. JFB. 2018;10:2.
- Etoc F, Vicario C, Lisse D, Siaugue J-M, Piehler J, Coppey M, et al. Magnetogenetic Control of Protein Gradients Inside Living Cells with High Spatial and Temporal Resolution. Nano Lett. 2015;15:3487–94.
- Iacovita C, Hurst J, Manfredi G, Hervieux PA, Donnio B, Gallani JL, et al. Magnetic force fields of isolated small nanoparticle clusters. Nanoscale. 2020;12:1842–51.
- Jin B, Odongo S, Radwanska M, Magez S. Nanobodies: A Review of Generation, Diagnostics and Therapeutics. IJMS. 2023;24:5994.
- Chandler PG, Buckle AM. Development and Differentiation in Monobodies Based on the Fibronectin Type 3 Domain. Cells. 2020;9:610.
- Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med. 2015;21:92–8.
- Liu L, Alizadeh K, Donnelly SC, Dassanayake P, Hou TT, McGirr R, et al. MagA expression attenuates iron export activity in undifferentiated multipotent P19 cells. Rao J, editor. PLoS ONE. 2019;14:e0217842.
- Pereira SM, Williams SR, Murray P, Taylor A. MS-1 magA: Revisiting Its Efficacy as a Reporter Gene for MRI. Mol Imaging. 2016;15:153601211664153.
- Kerans F, Lungaro L, Azfer A, Salter D. The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering. IJMS. 2018;19:3159.
- Elfick A, Rischitor G, Mouras R, Azfer A, Lungaro L, Uhlarz M, et al. Biosynthesis of magnetic nanoparticles by human mesenchymal stem cells following transfection with the magnetotactic bacterial gene mms6. Sci Rep. 2017;7:39755.
- Naumova AV, Vande Velde G. Genetically encoded iron-associated proteins as MRI reporters for molecular and cellular imaging. WIREs Nanomedicine and Nanobiotechnology. 2018;10:e1482.
- Jutz G, Van Rijn P, Santos Miranda B, Böker A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem Rev. 2015;115:1653–701.
- Kilcoyne SH, Cywinski R. Ferritin: A model superparamagnet. Journal of Magnetism and Magnetic Materials. 1995;140–144:1466–7.
- Makhlouf SA, Parker FT, Berkowitz AE. Magnetic hysteresis anomalies in ferritin. Phys Rev B. 1997;55:R14717–20.
- Gilles C, Bonville P, Rakoto H, Broto JM, Wong KKW, Mann S. Magnetic hysteresis and superantiferromagnetism in ferritin nanoparticles. Journal of Magnetism and Magnetic Materials. 2002;241:430–40.
- Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531:647–50.
- Hutson MR, Keyte AL, Hernández-Morales M, Gibbs E, Kupchinsky ZA, Argyridis I, et al. Temperature-activated ion channels in neural crest cells confer maternal fever–associated birth defects. Sci Signal. 2017;10:eaal4055.
- Hutson MR, Keyte AL, Hernández-Morales M, Gibbs E, Kupchinsky ZA, Argyridis I, et al. Temperature-activated ion channels in neural crest cells confer maternal fever–associated birth defects. Sci Signal. 2017;10:eaal4055.
- Hernández-Morales M, Shang T, Chen J, Han V, Liu C. Lipid Oxidation Induced by RF Waves and Mediated by Ferritin Iron Causes Activation of Ferritin-Tagged Ion Channels. Cell Reports. 2020;30:3250-3260.e7.
- Brier MI, Mundell JW, Yu X, Su L, Holmann A, Squeri J, et al. Uncovering a possible role of reactive oxygen species in magnetogenetics. Sci Rep. 2020;10:13096.
- Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, et al. Genetically targeted magnetic control of the nervous system. Nat Neurosci. 2016;19:756–61.
- Hernández-Morales M, Han V, Kramer RH, Liu C. Evaluating methods and protocols of ferritin-based magnetogenetics. iScience. 2021;24:103094.
- Xu F-X, Zhou L, Wang X-T, Jia F, Ma K-Y, Wang N, et al. Magneto is ineffective in controlling electrical properties of cerebellar Purkinje cells. Nat Neurosci. 2020;23:1041–3.
- Wang G, Zhang P, Mendu SK, Wang Y, Zhang Y, Kang X, et al. Revaluation of magnetic properties of Magneto. Nat Neurosci. 2020;23:1047–50.
- Kole K, Zhang Y, Jansen EJR, Brouns T, Bijlsma A, Calcini N, et al. Assessing the utility of Magneto to control neuronal excitability in the somatosensory cortex. Nat Neurosci. 2020;23:1044–6.
- Dziuba MV, Zwiener T, Uebe R, Schüler D. Single-step transfer of biosynthetic operons endows a non-magnetotactic Magnetospirillum strain from wetland with magnetosome biosynthesis. Environmental Microbiology. 2020;22:1603–18.
- Blakemore, R. Magnetotactic Bacteria. Science. 1975;190:377–9.
- Zahn C, Keller S, Toro-Nahuelpan M, Dorscht P, Gross W, Laumann M, et al. Measurement of the magnetic moment of single Magnetospirillum gryphiswaldense cells by magnetic tweezers. Sci Rep. 2017;7:3558.
- Bazylinski DA, Frankel RB. Magnetosome formation in prokaryotes. Nat Rev Microbiol. 2004;2:217–30.
- Radoul M, Lewin L, Cohen B, Oren R, Popov S, Davidov G, et al. Genetic manipulation of iron biomineralization enhances MR relaxivity in a ferritin-M6A chimeric complex. Sci Rep. 2016;6:26550.
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a Quantitative Magnetic Resonance Method for Mouse Whole Body Composition Analysis. Obesity Research. 2004;12:150–60.
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Analytical and Bioanalytical Chemistry. 2003;377:990–1002.
- Bakhteeva IuA, Medvedeva IV, Zhakov SV, Byzov IV, Filinkova MS, Uimin MA, et al. Magnetic separation of water suspensions containing TiO2 photocatalytic nanoparticles. Separation and Purification Technology. 2021;269:118716.
- Manz B, Benecke M, Volke F. A simple, small and low cost permanent magnet design to produce homogeneous magnetic fields. Journal of Magnetic Resonance. 2008;192:131–8.
- Sanz B, Calatayud MP, Cassinelli N, Ibarra MR, Goya GF. Long-Term Stability and Reproducibility of Magnetic Colloids Are Key Issues for Steady Values of Specific Power Absorption over Time. Eur J Inorg Chem. 2015;2015:4524–31.
- Bauer LM, Situ SF, Griswold MA, Samia ACS. High-performance iron oxide nanoparticles for magnetic particle imaging – guided hyperthermia (hMPI). Nanoscale. 2016;8:12162–9.
- Nimpf S, Keays DA. Is magnetogenetics the new optogenetics? The EMBO Journal. 2017;36:1643–6.
- Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Progress in Biophysics and Molecular Biology. 2003;82:11–24.
- Kung C, Martinac B, Sukharev S. Mechanosensitive Channels in Microbes. Annu Rev Microbiol. 2010;64:313–29.
- Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. TRPs et al.: A molecular toolkit for thermosensory adaptations. Pflugers Arch - Eur J Physiol. 2018;470:745–59.
- Digel I, Kayser P, Artmann GM. Molecular Processes in Biological Thermosensation. Journal of Biophysics. 2008;2008:1–9.
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202.
- Walter BA, Purmessur D, Moon A, Occhiogrosso J, Laudier DM, Hecht AC, et al. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123–36.
- Matsumoto K, Ohishi A, Iwatsuki K, Yamazaki K, Takayanagi S, Tsuji M, et al. Transient receptor potential vanilloid 4 mediates sour taste sensing via type III taste cell differentiation. Sci Rep. 2019;9:6686.
- Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–89.
- Zhang M, Ma Y, Ye X, Zhang N, Pan L, Wang B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Sig Transduct Target Ther. 2023;8:261.
- Hof T, Chaigne S, Récalde A, Sallé L, Brette F, Guinamard R. Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol. 2019;16:344–60.
- Rosenbaum T, Morales-Lázaro SL, Islas LD. TRP channels: A journey towards a molecular understanding of pain. Nat Rev Neurosci. 2022;23:596–610.
- Signorelli L, Hescham S-A, Pralle A, Gregurec D. Magnetic nanomaterials for wireless thermal and mechanical neuromodulation. iScience. 2022;25:105401.
- Della Pietra A, Mikhailov N, Giniatullin R. The Emerging Role of Mechanosensitive Piezo Channels in Migraine Pain. International Journal of Molecular Sciences. 2020;21:696.
- Fang X-Z, Zhou T, Xu J-Q, Wang Y-X, Sun M-M, He Y-J, et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell & Bioscience. 2021;11:13.
- Duret G, Polali S, Anderson ED, Bell AM, Tzouanas CN, Avants BW, et al. Magnetic Entropy as a Proposed Gating Mechanism for Magnetogenetic Ion Channels. Biophysical Journal. 2019;116:454–68.
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science. 2010;330:55–60.
- Jin P, Jan LY, Jan Y-N. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci. 2020;43:207–29.
- Delmas P, Parpaite T, Coste B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron. 2022;110:2713–27.
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111:16148–53.
- Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, et al. Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett. 2013;13:2399–406.
- Unda SR, Marongiu R, Pomeranz LE, Dyke JP, Fung EK, Grosenick L, et al. Bidirectional Regulation of Motor Circuits Using Magnetogenetic Gene Therapy [Internet]. Neuroscience; 2023 Jul. Available from: http://biorxiv.org/lookup/doi/10.1101/2023.07.13.548699.
- Rosenfeld D, Senko AW, Moon J, Yick I, Varnavides G, Gregureć D, et al. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci Adv. 2020;6:eaaz3734.
- Moon J, Christiansen MG, Rao S, Marcus C, Bono DC, Rosenfeld D, et al. Magnetothermal Multiplexing for Selective Remote Control of Cell Signaling. Adv Funct Materials. 2020;30:2000577.
- Brier MI, Mundell JW, Yu X, Su L, Holmann A, Squeri J, et al. Uncovering a possible role of reactive oxygen species in magnetogenetics. Sci Rep. 2020;10:13096.
- Meister, M. Physical limits to magnetogenetics. eLife. 2016;5:e17210.
- Gregurec D, Senko AW, Chuvilin A, Reddy PD, Sankararaman A, Rosenfeld D, et al. Magnetic Vortex Nanodiscs Enable Remote Magnetomechanical Neural Stimulation. ACS Nano. 2020;14:8036–45.
- Hughes S, McBain S, Dobson J, El Haj AJ. Selective activation of mechanosensitive ion channels using magnetic particles. J R Soc Interface. 2008;5:855–63.
- Unnithan AR, Rotherham M, Markides H, El Haj AJ. Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels. IJMS. 2023;24:3364.
- Unnithan AR, Sasikala ARK, Shrestha BK, Lincoln A, Thomson T, El Haj AJ. Remotely Actuated Magnetic Nanocarpets for Bone Tissue Engineering: Non-Invasive Modulation of Mechanosensitive Ion Channels for Enhanced Osteogenesis. Adv Funct Materials. 2022;32:2201311.
- Rotherham M, Moradi Y, Nahar T, Mosses D, Telling N, El Haj AJ. Magnetic activation of TREK1 triggers stress signalling and regulates neuronal branching in SH-SY5Y cells. Front Med Technol. 2022;4:981421.
- Liu J, Munshi R, He M, Parker SD, Pralle A. Deep Brain Magnetothermal Silencing of Dopaminergic Neurons via Endogenous TREK1 Channels Abolishes Place Preference in Mice [Internet]. Neuroscience; 2022 Apr. Available from: http://biorxiv.org/lookup/doi/10.1101/2022.04.12.487994.
- Alattia J-R, Tong KI, Takeichi M, Ikura M. Cadherins. Calcium-Binding Protein Protocols [Internet]. New Jersey: Humana Press; 2002 [cited 2024 Jan 20]. p. 199––210. Available from: http://link.springer.com/10.1385/1-59259-183-3:199.
- Mehta S, Nijhuis A, Kumagai T, Lindsay J, Silver A. Defects in the adherens junction complex (E-cadherin/ β-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015;360:749–60.
- Kingsley C, Kourtidis A. Critical roles of adherens junctions in diseases of the oral mucosa. Tissue Barriers. 2023;11:2084320.
- Castro-Hinojosa C, Ovejero JG, Sol-Fernández SD, Fuente JM de la, Grazú V, Fratila RM, et al. Synthesis of a Cadherin-Magnetic Nanoparticle Bioconjugate as a Novel Magneto-Mechanical Cell Actuator. [cited 2024 Mar 24]. Available from: https://www.nanoge.org/proceedings/AMA4MED/6268301ccf01ad021336f2b2.
- Weber GF, Bjerke MA, DeSimone DW. A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Developmental Cell. 2012;22:104–15.
- Morse EM, Brahme NN, Calderwood DA. Integrin Cytoplasmic Tail Interactions. Biochemistry. 2014;53:810–20.
- Marycz K, Sobierajska P, Roecken M, Kornicka-Garbowska K, Kępska M, Idczak R, et al. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J Nanobiotechnol. 2020;18:33.
- Fass JN, Odde DJ. Tensile Force-Dependent Neurite Elicitation via Anti-β1 Integrin Antibody-Coated Magnetic Beads. Biophysical Journal. 2003;85:623–36.
- Pérez LA, Rashid A, Combs JD, Schneider P, Rodríguez A, Salaita K, et al. An Outside-In Switch in Integrin Signaling Caused by Chemical and Mechanical Signals in Reactive Astrocytes. Front Cell Dev Biol [Internet]. 2021 [cited 2024 Mar 24];9. Available from: https://www.frontiersin.org/articles/10.3389/fcell.2021.712627.
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–9.
- Labernadie A, Kato T, Brugués A, Serra-Picamal X, Derzsi S, Arwert E, et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19:224–37.
- McEvoy E, Sneh T, Moeendarbary E, Javanmardi Y, Efimova N, Yang C, et al. Feedback between mechanosensitive signaling and active forces governs endothelial junction integrity. Nat Commun. 2022;13:7089.
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906.
- Cai X, Wang K-C, Meng Z. Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression. Front Cell Dev Biol. 2021;9:673599.
- Cooper J, Giancotti FG. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell. 2019;35:347–67.
- Keely, P.J. Mechanisms by Which the Extracellular Matrix and Integrin Signaling Act to Regulate the Switch Between Tumor Suppression and Tumor Promotion. J Mammary Gland Biol Neoplasia. 2011;16:205–19.
- Dupont, S. Regulation of YAP/TAZ Activity by Mechanical Cues: An Experimental Overview. In: Hergovich A, editor. The Hippo Pathway [Internet]. New York, NY: Springer New York; 2019 [cited 2024 Jan 20]. p. 183–202. Available from: http://link.springer.com/10.1007/978-1-4939-8910-2_15.
- Du H, Bartleson JM, Butenko S, Alonso V, Liu WF, Winer DA, et al. Tuning immunity through tissue mechanotransduction. Nat Rev Immunol. 2023;23:174–88.
- Yang S, Plotnikov SV. Mechanosensitive Regulation of Fibrosis. Cells. 2021;10:994.
- Di X, Gao X, Peng L, Ai J, Jin X, Qi S, et al. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Sig Transduct Target Ther. 2023;8:282.
- Meyer CJ, Alenghat FJ, Rim P, Fong JH-J, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2:666–8.
- Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. Journal of Cell Science. 2006;119:508–18.
- Mousavizadeh R, Hojabrpour P, Eltit F, McDonald PC, Dedhar S, McCormack RG, et al. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci Rep. 2020;10:12644.
- Chen Y, Li Z, Ju LA. Tensile and compressive force regulation on cell mechanosensing. Biophys Rev. 2019;11:311–8.
- Moujaber O, Stochaj U. The Cytoskeleton as Regulator of Cell Signaling Pathways. Trends in Biochemical Sciences. 2020;45:96–107.
- Laisne M-C, Michallet S, Lafanechère L. Characterization of Microtubule Destabilizing Drugs: A Quantitative Cell-Based Assay That Bridges the Gap between Tubulin Based- and Cytotoxicity Assays. Cancers. 2021;13:5226.
- Fujiwara I, Zweifel ME, Courtemanche N, Pollard TD. Latrunculin A Accelerates Actin Filament Depolymerization in Addition to Sequestering Actin Monomers. Current Biology. 2018;28:3183-3192.e2.
- Inaba H, Yamada M, Rashid MstR, Kabir AMdR, Kakugo A, Sada K, et al. Magnetic Force-Induced Alignment of Microtubules by Encapsulation of CoPt Nanoparticles Using a Tau-Derived Peptide. Nano Lett. 2020;20:5251–8.
- Ducasse R, Wang W-A, Navarro MG-J, Debons N, Colin A, Gautier J, et al. Programmed Self-Assembly of a Biochemical and Magnetic Scaffold to Trigger and Manipulate Microtubule Structures. Sci Rep. 2017;7:11344.
- Hillion A, Hallali N, Clerc P, Lopez S, Lalatonne Y, Noûs C, et al. Real-Time Observation and Analysis of Magnetomechanical Actuation of Magnetic Nanoparticles in Cells. Nano Lett. 2022;22:1986–91.
- Gupta R, Sharma D. Manganese-Doped Magnetic Nanoclusters for Hyperthermia and Photothermal Glioblastoma Therapy. ACS Appl Nano Mater. 2020;3:2026–37.
- Kaur H, Kumar S, Kaur I, Singh K, Bharadwaj LM. Low-intensity magnetic fields assisted alignment of actin filaments. International Journal of Biological Macromolecules. 2010;47:371–4.
- Hutchins BM, Platt M, Hancock WO, Williams ME. Directing Transport of CoFe 2 O 4 -Functionalized Microtubules with Magnetic Fields. Small. 2007;3:126–31.
- Karpov OA, Fearnley GW, Smith GA, Kankanala J, McPherson MJ, Tomlinson DC, et al. Receptor tyrosine kinase structure and function in health and disease. AIMSBPOA. 2015;2:476–502.
- Niu G, Chen X. Why Integrin as a Primary Target for Imaging and Therapy. Theranostics. 2011;1:30–47.
- Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR Therapy for Cancer: Reassessing the Target. Cancer Research. 2012;72:1909–14.
- Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–51.
- Finger C, Escher C, Schneider D. The Single Transmembrane Domains of Human Receptor Tyrosine Kinases Encode Self-Interactions. Sci Signal [Internet]. 2009 [cited 2024 Jan 20];2. Available from: https://www.science.org/doi/10.1126/scisignal.2000547.
- Godfroy JI, Roostan M, Moroz YS, Korendovych IV, Yin H. Isolated Toll-like Receptor Transmembrane Domains Are Capable of Oligomerization. Gay N, editor. PLoS ONE. 2012;7:e48875.
- Remy I, Wilson IA, Michnick SW. Erythropoietin Receptor Activation by a Ligand-Induced Conformation Change. Science. 1999;283:990–3.
- Lemmon, M.A. Ligand-induced ErbB receptor dimerization. Experimental Cell Research. 2009;315:638–48.
- Burgess AW, Cho H-S, Eigenbrot C, Ferguson KM, Garrett TPJ, Leahy DJ, et al. An Open-and-Shut Case? Recent Insights into the Activation of EGF/ErbB Receptors. Molecular Cell. 2003;12:541–52.
- Endres NF, Barros T, Cantor AJ, Kuriyan J. Emerging concepts in the regulation of the EGF receptor and other receptor tyrosine kinases. Trends in Biochemical Sciences. 2014;39:437–46.
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic Evidence for Preformed Dimers of Erythropoietin Receptor Before Ligand Activation. Science. 1999;283:987–90.
- Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat Struct Mol Biol. 2010;17:398–402.
- Massague J, Pilch PF, Czech MP. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci USA. 1980;77:7137–41.
- Caré BR, Soula HA. Impact of receptor clustering on ligand binding. BMC Syst Biol. 2011;5:48.
- Matthews EE, Zoonens M, Engelman DM. Dynamic Helix Interactions in Transmembrane Signaling. Cell. 2006;127:447–50.
- Matos AM, Gonçalves AI, Rodrigues MT, Miranda MS, Haj AJE, Reis RL, et al. Remote triggering of TGF-β/Smad2/3 signaling in human adipose stem cells laden on magnetic scaffolds synergistically promotes tenogenic commitment. Acta Biomaterialia. 2020;113:488–500.
- Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic Field-Induced T Cell Receptor Clustering by Nanoparticles Enhances T Cell Activation and Stimulates Antitumor Activity. ACS Nano. 2014;8:2252–60.
- Kopan R, Ilagan MaXG. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell. 2009;137:216–33.
- Kopan, R. Notch Signaling. Cold Spring Harbor Perspectives in Biology. 2012;4:a011213–a011213.
- Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300.
- Lovendahl KN, Blacklow SC, Gordon WR. The Molecular Mechanism of Notch Activation. In: Borggrefe T, Giaimo BD, editors. Molecular Mechanisms of Notch Signaling [Internet]. Cham: Springer International Publishing; 2018 [cited 2024 Jan 20]. p. 47–58. Available from: http://link.springer.com/10.1007/978-3-319-89512-3_3.
- Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy Proteins on Stretchy Substrates: The Important Elements of Integrin-Mediated Rigidity Sensing. Developmental Cell. 2010;19:194–206.
- Sniadecki, N.J. Minireview: A Tiny Touch: Activation of Cell Signaling Pathways with Magnetic Nanoparticles. Endocrinology. 2010;151:451–7.
- Lin H-H, Ng K-F, Chen T-C, Tseng W-Y. Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals. 2022;15:219.
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506.
- Hide M, Fukui H, Watanabe T, Wada H, Yamamoto S. Histamine H1-receptor in endothelial and smooth muscle cells of guinea-pig aorta. European Journal of Pharmacology. 1988;148:161–9.
- Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell. 2018;173:762-775.e16.
- Abdul-Majeed S, Nauli SM. Dopamine Receptor Type 5 in the Primary Cilia Has Dual Chemo- and Mechano-Sensory Roles. Hypertension. 2011;58:325–31.
- Storch U, Blodow S, Gudermann T, Mederos Y Schnitzler M. Cysteinyl Leukotriene 1 Receptors as Novel Mechanosensors Mediating Myogenic Tone Together With Angiotensin II Type 1 Receptors—Brief Report. ATVB. 2015;35:121–6.
- Dos Santos Á, Fili N, Pearson DS, Hari-Gupta Y, Toseland CP. High-throughput mechanobiology: Force modulation of ensemble biochemical and cell-based assays. Biophys J. 2021;120:631–41.
- Pineux F, Federico S, Klotz K-N, Kachler S, Michiels C, Sturlese M, et al. Targeting G Protein-Coupled Receptors with Magnetic Carbon Nanotubes: The Case of the A3 Adenosine Receptor. ChemMedChem. 2020;15:1909–20.
- Araç D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Südhof TC, et al. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis: Cell-adhesion GPCRs mediates autoproteolysis. The EMBO Journal. 2012;31:1364–78.
- Hamann J, Aust G, Araç D, Engel FB, Formstone C, Fredriksson R, et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G Protein–Coupled Receptors. Ohlstein EH, editor. Pharmacol Rev. 2015;67:338–67.
- Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, et al. Common activation mechanism of class A GPCRs. eLife. 2019;8:e50279.
- Latek D, Modzelewska A, Trzaskowski B, Palczewski K, Filipek S. G protein-coupled receptors--recent advances. Acta Biochim Pol. 2012;59:515–29.
- Wingler LM, Lefkowitz RJ. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends in Cell Biology. 2020;30:736–47.
- Rotherham M, Henstock JR, Qutachi O, El Haj AJ. Remote regulation of magnetic particle targeted Wnt signaling for bone tissue engineering. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14:173–84.
- Fu C, Huang W, Tang Q, Niu M, Guo S, Langenhan T, et al. Unveiling Mechanical Activation: GAIN Domain Unfolding and Dissociation in Adhesion GPCRs. Nano Lett. 2023;23:9179–86.
- Zhong BL, Lee CE, Vachharajani VT, Bauer MS, Südhof TC, Dunn AR. Piconewton Forces Mediate GAIN Domain Dissociation of the Latrophilin-3 Adhesion GPCR. Nano Lett. 2023;23:9187–94.
- Erdogmus S, Storch U, Danner L, Becker J, Winter M, Ziegler N, et al. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat Commun. 2019;10:5784.
- Marullo S, Doly S, Saha K, Enslen H, Scott MGH, Coureuil M. Mechanical GPCR Activation by Traction Forces Exerted on Receptor N -Glycans. ACS Pharmacol Transl Sci. 2020;3:171–8.
- Zhang Y-L, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. American Journal of Physiology-Cell Physiology. 2009;296:C1391–9.
- Rotherham M, Nahar T, Goodman T, Telling N, Gates M, El Haj A. Magnetic Mechanoactivation of Wnt Signaling Augments Dopaminergic Differentiation of Neuronal Cells. Adv Biosys. 2019;3:1900091.
- Hu B, Rotherham M, Farrow N, Roach P, Dobson J, El Haj AJ. Immobilization of Wnt Fragment Peptides on Magnetic Nanoparticles or Synthetic Surfaces Regulate Wnt Signaling Kinetics. IJMS. 2022;23:10164.
- Rotherham M, El Haj AJ. Remote Activation of the Wnt/β-Catenin Signalling Pathway Using Functionalised Magnetic Particles. Tang S-J, editor. PLoS ONE. 2015;10:e0121761.
- Marullo S, Doly S, Saha K, Enslen H, Scott MGH, Coureuil M. Mechanical GPCR Activation by Traction Forces Exerted on Receptor N -Glycans. ACS Pharmacol Transl Sci. 2020;3:171–8.
- Dela Paz NG, Melchior B, Frangos JA. Shear stress induces Gα q/11 activation independently of G protein-coupled receptor activation in endothelial cells. American Journal of Physiology-Cell Physiology. 2017;312:C428–37.
- Gao Y, Qian H, Tang X, Du X, Wang G, Zhang H, et al. Superparamagnetic iron oxide nanoparticle-mediated expression of miR-326 inhibits human endometrial carcinoma stem cell growth. IJN. 2019;Volume 14:2719–31.
- Metto AC, Telgkamp P, McLane-Svoboda AK, Gilad AA, Pelled G. Closed-loop neurostimulation via expression of magnetogenetics-sensitive protein in inhibitory neurons leads to reduction of seizure activity in a rat model of epilepsy. Brain Research. 2023;1820:148591.
- Krishnan V, Park SA, Shin SS, Alon L, Tressler CM, Stokes W, et al. Wireless control of cellular function by activation of a novel protein responsive to electromagnetic fields. Sci Rep. 2018;8:8764.
- Faivre D, Schüler D. Magnetotactic Bacteria and Magnetosomes. Chem Rev. 2008;108:4875–98.
- Lefèvre CT, Bazylinski DA. Ecology, Diversity, and Evolution of Magnetotactic Bacteria. Microbiology and Molecular Biology Reviews. 2013;77:497–526.
- Barber-Zucker S, Zarivach R. A Look into the Biochemistry of Magnetosome Biosynthesis in Magnetotactic Bacteria. ACS Chem Biol. 2017;12:13–22.
- Arakaki A, Yamagishi A, Fukuyo A, Tanaka M, Matsunaga T. Co-ordinated functions of Mms proteins define the surface structure of cubo-octahedral magnetite crystals in magnetotactic bacteria. Molecular Microbiology. 2014;93:554–67.
- Uebe R, Schüler D. Magnetosome biogenesis in magnetotactic bacteria. Nat Rev Microbiol. 2016;14:621–37.
- Fromain A, Van De Walle A, Curé G, Péchoux C, Serrano A, Lalatonne Y, et al. Biomineralization of magnetic nanoparticles in stem cells. Nanoscale. 2023;15:10097–109.
| Magnetogenetic task | MNP composition | MNP size | MNP Ms | MNP surface modification | Applied magnetic field (H and f) | Ref. | |
|---|---|---|---|---|---|---|---|
| Permanent magnetic field | Actin filament manipulation in vitro | Fe3O4 | 10 nm | N/A | Streptavidin | N/A | [68] |
| Rapid spatial reorganization of proteins captured to the nanoparticle surface | Engineered ferritin | 20 nm | 87 emu/g | αGFP; TNFα; SBP; TIAM | N/A | [69] | |
| Acute neural stimulation in constant gradient | Fe3O4 | 100 nm | 40 emu/g | Starch | N/A | [70] | |
| Magnetically controlled DR4 apoptosis induction | Zn0.4Fe2.6O | 15 nm | 161 emu/g | DR4 Abs; doxorubicin | N/A | [44] | |
| Piezo1 receptor stimulation in cell culture | Fe3O4 | 75 nm | N/A | A-bungarotoxin | 40 mT – |
[71] | |
| Polarization of stem cells | Fe3O4 | N/A | 70 emu/g | SiO2 – actin binding peptide | 0 – 3.81 mT – |
[72] | |
| Low-frequency magnetic field | External manipulation of activin receptor type IIA in hASC | Fe3O4 | 250 nm | N/A | Dextran@Abs | 25 mT 1Hz |
[51] |
| Activation of TREK-1 in hMSC | Fe3O4 | 300 nm | N/A | Dextran@Abs | 25 mT 1Hz |
[73] | |
| Osteogenic differentiation of bone marrow-derived hMSC | Fe3O4 | 250 nm | N/A | Dextran@Abs | 60 –120 mT 1Hz |
[74] | |
| hBMSC differentiation towards a smooth muscle cell lineage | Fe3O4 | 250 nm | N/A | Dextran@Abs | 60 –120 mT 1Hz |
[75] | |
| Magnetomechanical neuronal stimulation with nanodiscs | Fe3O4 | 280 nm | 110 emu/g | PMAO | 50 mT 10 Hz |
[76] | |
| Chymotrypsin catalytic activity change | Fe3O4 | 25 nm | N/A | Au | 5 – 250 mT 16 – 500 Hz |
[77] | |
| High-frequency magnetic field | Thermal TRPV1 activation in neurons | MnFe2O4 | 6 nm | ~70 emu/g | Streptavidin | 0.84 mT 40 MHz |
[41] |
| Magnetic activation of neurons, heat-sensitized by expressing TRPV1 | CoFe2O4@MnFe2O4 | 10 nm | ~70 emu/g | Neutravidin | 46 mT 412.5 kHz |
[78] | |
| Stimulation of heat sensitive TRPA1-A in fly neurons | Fe3O4@CoFe2O4; Fe3O4 |
15 nm; 40 nm | ~70 emu/g | DSPE-PEG | 10 – 80 mT 0.05 – 5 MHz |
[79] | |
| Cell death activation through internalization of CCK2R | Fe3O4 | 10 nm | 80 emu/g | Gastrine | 24 – 40 mT 275 kHz |
[80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





