Introduction
Since early 2020, coronavirus disease (COVID-19) has become a global crisis of the 21
st century, causing a major pandemic that affects human health and activities worldwide and leading to a major international emergency [
1,
2]. In December 2022, the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected people worldwide exceeded 630 million, with the death toll exceeding approximately 6.5 million. In Japan, the cumulative number of infected people exceeded 30 million, and the death toll exceeded 70,000 as of May 2023 [
3]. Notably, the COVID-19 pandemic has severely affected skilled nursing facility residents and healthcare workers [
4,
5]. Healthcare workers are at a high risk of SARS-CoV-2 exposure during patient care [
6,
7] and are among the earliest groups prioritized for COVID-19 vaccination [
8].
The emergence of the Omicron strain, which has stronger infectivity, resulted in an outbreak in the sixth pandemic wave; the seventh wave became the largest outbreak to date, causing the highest number of deaths in Japan [
3]. Several clusters also occurred in medical institutions. The impact on general medical care was serious, with medical workers being infected or coming into close contact with the virus. Considering the impact of COVID-19 outbreak within medical institutions, stricter infection control measures than those for the general public are mandatory.
On the other hand, owing to widespread vaccine use and weakening of the toxicity of the virus in healthy people, behavioral restrictions and infection control measures in the general public are being relaxed, and the society as a whole is returning to the pre-COVID-19 pandemic situation. Simultaneously, the system for reporting all cases of infection in Japan changed, making it difficult to understand the actual COVID-19 status in the community.
Under these circumstances, our hospital introduced a regular rapid antigen test (R-RAT) for healthcare workers during the eighth and ninth pandemic waves.
Methods
Notification System
To understand the health status of the employees, prevent nosocomial infections, and conduct epidemiological investigations, when an employee is absent from work because of an infectious disease, a report must be submitted to the Infection Control Office by the supervisor as an infection control measure in our hospital. Additionally, since the beginning of the COVID-19 pandemic, we decided to notify anyone who has come into close contact with COVID-19 patients and required them to undergo follow-up observation for a designated period.
We referred to the Japanese Ministry of Health, Labour and Welfare and defined ”close contact" as "an infected person within the same household" or "contact for more than 15 min, such as having a meal with an infected person or conversation without wearing a mask [
9].” In such cases, a close contact report must be submitted. Until the seventh wave, the department managers used paper-based notification methods. The Infection Control Office confirmed the report details and made a final decision, provided instructions, and notified when the employee could return to resume work. Since the sixth wave, the number of infected employees increased rapidly; therefore, we started the digitization of notifications using the hospital intra-net system to quickly respond and give instructions to close contacts and infected employees. This study primarily analyzed the eighth and ninth waves after switching to an electronic reporting system.
The reported contents were as follows: department, age, gender, date of onset, symptoms, presence or absence of meals outside with others, presence of family members with symptoms/infection, behavioral history up to 2 days before the onset of symptoms, and free description.
Regular Antigen Qualitative Test
From August 21, 2022, with the end of the seventh wave, we started the R-RAT (once to twice/week), using a rapid COVID-19 antigen test kit provided by the Tokyo Metropolitan Government. The RAT kits were distributed to the employees who tested themselves once or twice a week and reported the results to their supervisor. Subsequently, the supervisor reported the weekly results to the office. The testing dates were not specified and were left to each employee. We used the following three RAT kits approved by the Japanese Ministry of Health, Labour and Welfare: 1) Panbio COVID-19 Antigen Rapid Test (Abott Japan LLC, Tokyo, Japan); 2) Rapid COVID-19 Antigen Self-Test (Siemens Healthinners Diagnostics, Tokyo, Japan); and 3) SARS-CoV-2 Rapid Antigen Test (Roche Diagnostics, Tokyo, Japan). Based on the data provided by the distributors, the sensitivities and specificities of these RAT kits were almost identical.
Criteria for Release from Quarantine
During the eighth and ninth waves, "close contact" employees could work while confirming a negative RAT every day until the third day. "Infected employees" could return to work if a negative RAT result was confirmed after the fifth day with no fever for more than 24 h. Otherwise, infected employees could return to work after the 10th day, even if they did not confirm negative RAT results. For asymptomatic infected employees, the date of symptom onset was defined as the date of a positive test result.
We used the average number of employees during the analysis period, including 263 doctors, 806 nurses, 194 administrative employees, and 218 clinical laboratory technicians.
Statistical Analysis
Chi square and Mann–Whitney analyses were used for statistical analysis, and a p-value<0.05 was considered significant. Statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA).
Results
Analysis from the Infectious Disease Notification Reporting System
Table 1 shows trends in the number of new SARS-CoV-2 infections among employees since the third wave and that in the southern North-Tama Medical Area, where our hospital operates as a core and designated type-2 infectious disease hospital. After the Omicron strain emergence during the sixth wave, the number of infected employees rapidly increased. As the R-RAT for employees was initiated on August 21, 2022, we compared data obtained during the eighth (2022/9/20–2023/5/2; 225 days) and the ninth waves (2023/5/7–2023/10/21; 168 days). The number of reported infections in the eighth and ninth waves was comparable, at 320 and 299, respectively (
Table 1), with no significant difference in the number of reported cases per day (1.42 vs 1.76, odds ratio [OR] 1.25, 95% confidence interval [CI]: 0.97– 1.61, p=0.08). During the COVID-19 pandemic, the total population of the Southern North-Tama Medical Area was approximately 1.06 million people, and the average number of employees at our hospital was 1481 individuals including 263 doctors, 806 nurses, 194 administrative employees, and 218 clinical laboratory technicians; if the infection rate was the same in both population, the ratio was approximately 1.39×10
-3. Therefore, the infection rate of our employees was higher than that in the general population during the third wave; lower during the fourth and fifth waves; comparable during the sixth and seventh waves, after the appearance of the Omicron strain. In the eighth wave, the proportion of infected employees increased significantly compared with that of the general population. However, in the eighth wave, the previous all-case registration reporting system was changed to a voluntary online registration system in which individuals could register online using commercially available RAT without visiting a medical institution. Since the new system allowed voluntary registration, a significant number of asymptomatic and mildly infected people were assumed to not have registered. In the ninth wave, the reporting system was changed to fixed-point reporting, making conducting comparative studies, such as the one above, impossible.
Table 2 presents the initial symptoms of the infected employees during the eighth and ninth waves. During both waves, fever and sore throat were the most common symptoms (60–70%), followed by cough and headache. Several symptoms were milder than those observed during previous epidemics. Asymptomatic cases accounted for 7.8% and 5.7% in the eighth and ninth waves, respectively. In cases of infection through close contact, the proportion of asymptomatic cases was high, at 33.9% and 15.4%, respectively; the rate was significantly higher than that of infected employees without close contact; in the eighth wave (33.9% [19/56] vs. 2.3% [6/264]) and in the ninth wave (15.4% [4/26] vs. 4.8% [13/273]). Our regulation may have effectively detected the number of asymptomatic positive cases.
Among the infected employees, nurses accounted for the majority (
Table 3). The trend was comparable between the eighth and ninth waves. Regarding the infection rate by occupation (infected employees vs. total employees), the infection rate was significantly lower in doctors than in other occupations.
During the eighth wave, 195 people had close contact with SARS-CoV-2; among them, 56 were infected. Durin the ninth wave, 36 of 62 close contacts developed infections (
Table 2). The transmission rate from close contacts was lower in the eighth wave than in the ninth wave, although no significant difference was observed (0.29 vs. 0.42, p=0.052). Regarding the ratio of close contacts to the total infected employees, the number of close contacts was significantly higher in the eighth wave (odds ratio: 2.94, 95%CI: 2.12–4.07, p<0.0001) than in the ninth wave. Upon comparing the number of vaccinations received by infected and non-infected close contacts, no significant difference was observed during each wave (eighth wave: 3.41 vs. 3.56, ninth wave: 3.88 vs. 4.42).
Nevertheless, of the 299 employees infected during the ninth wave, 47 had a history of infection. Subsequently, we compared the number of vaccinations administered to employees with and without a history of infection. We found that 40 of 283 employees vaccinated with ≥3 doses and 7 of 15 employees vaccinated with <3 doses had a history of infection; thus, those vaccinated with ≥3 doses were less frequently infected (OR: 0.30, 95% CI: 0.12–0.79, p<0.0001). In the eighth wave, only 15/320 infected employees had a history of COVID-19.
Regarding the infection route, family infection was the most common (78 employees [24.4%] in the eighth wave and 55 employees [18.4%] in the ninth wave), followed by outside meals (32 employees [10.0%] and 54 employees [18.1%] in the eighth and ninth waves, respectively).
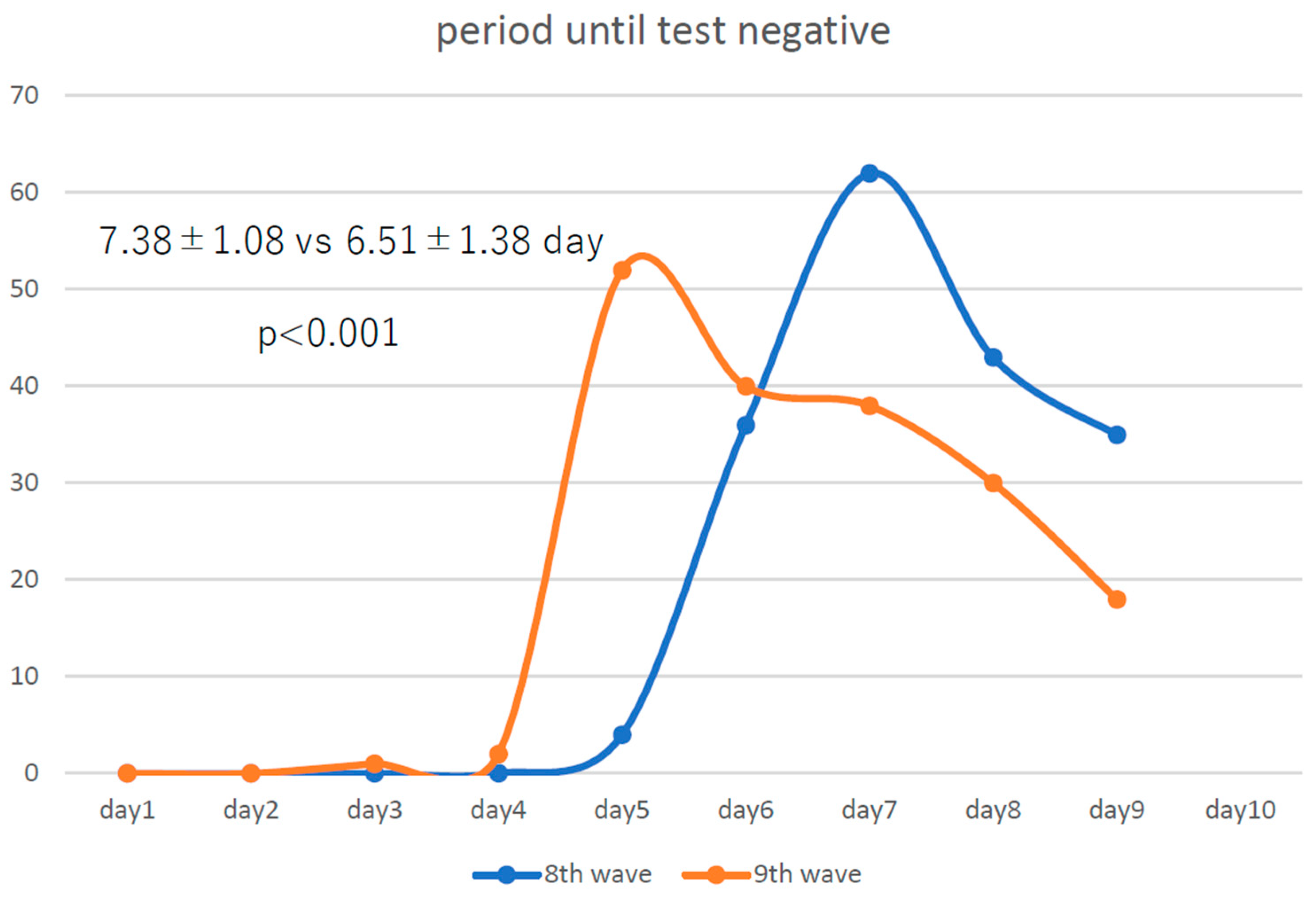
The number of infected employees for whom we were able to examine the trends in the number of days until antigen negativity was 180 and 181 in the eighth and ninth waves, respectively. Compared with those in the eighth wave (
Figure 1), infected medical employees became negative for RAT a little less than a day earlier in the ninth wave, showing that COVID-19 became even more attenuated from the eighth to the ninth wave.
Analysis of Regular Rapid Antigen Testing
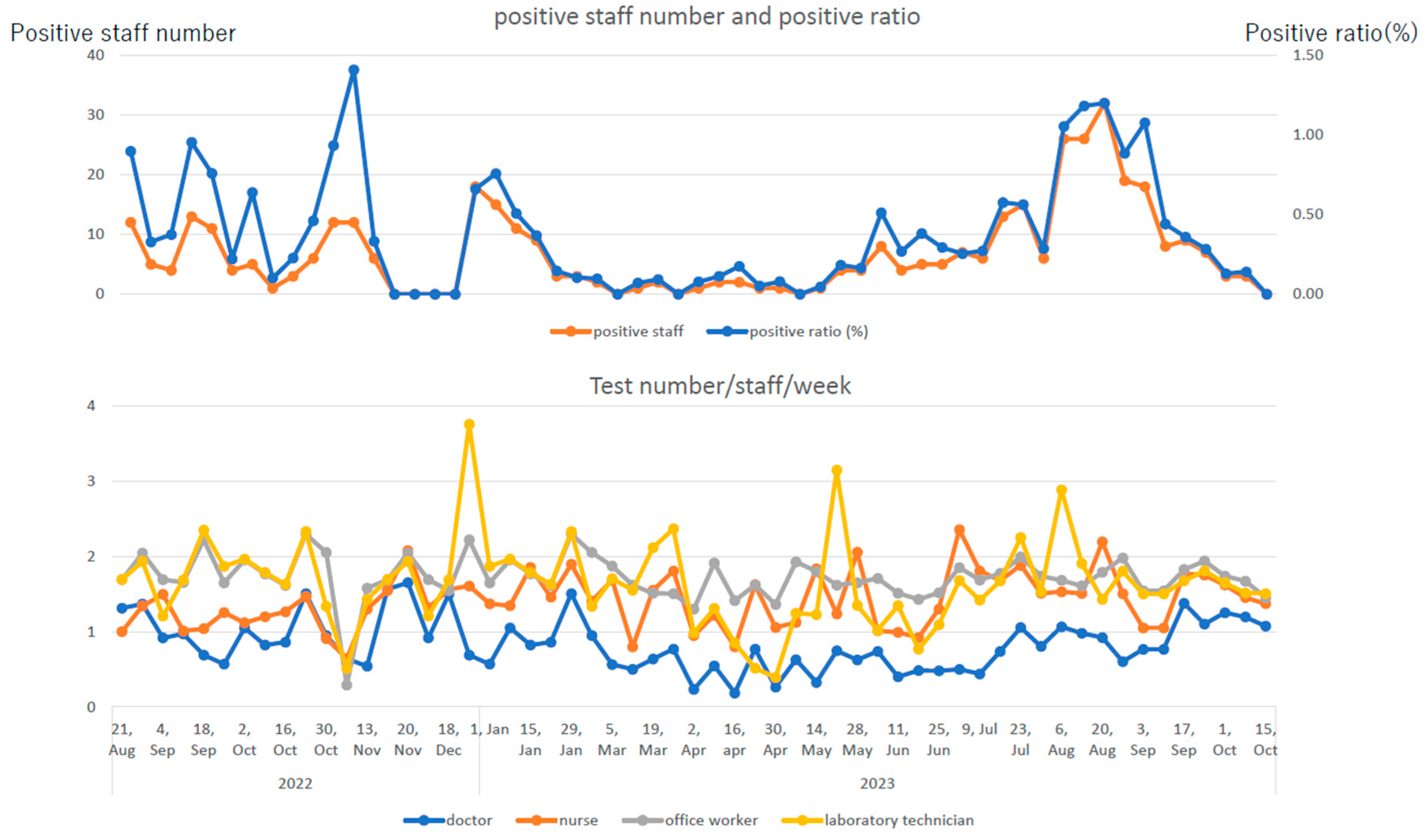
Figure 2 shows trends in the number of positive cases and positivity rates of R-RAT for employees and those in the number of test reports by occupation. At the peak of the pandemic, the positivity rate exceeded 1.00%, and the number of positive cases exceeded 30 per week. Doctors were tested less than once/week (0.84±0.35/week), whereas individuals with other occupations were tested 1.5–2 times/week (nurses: 1.41±0.37/week, administrative employees: 1.72±0.30/week, and clinical laboratory technicians and others: 1.64±0.59/week). Notably, the ratio of the number of positive cases on R-RAT to the number of reported employees with infection was lower in doctors, who underwent less frequent R-RAT than other occupational groups.
Discussion
Digitization of employee infection reports in our hospital resulted in a uniform, qualified, simplified, and faster reporting of the contents, making the reports more detailed and easier to analyze. Therefore, we examined and analyzed the actual COVID-19 status among hospital employees during the COVID-19 pandemic, focusing on the eighth and ninth waves, during which the number of infected employees rapidly increased.
Until the third wave, all employees diagnosed with COVID-19 at our hospital were nurses and doctors working in the COVID-19 wards; thus, these cases were a nosocomial infection occurring because of medical activities. Community transmission of COVID-19 was detected since the fifth wave and expanded widely with the emergence of the Omicron strain during the sixth wave. Consequently, the number of infections among employees, including administrative workers, increased. Immediately before the fifth wave, mRNA vaccines for COVID-19 were first launched for individuals aged ≥65 years and healthcare workers. The vaccine effectiveness may have been one reason why the number of infected people aged ≥65 years during the fifth wave remained low in Japan [
10], and the number of infected employees at our hospital remained low. Nevertheless, nationwide statistics reported that the number of severe cases and death rates among relatively healthy infected people aged 40–65 years, who were not eligible for vaccination, were the highest during the fifth wave [
10].
Since the sixth wave, when community infections increased rapidly owing to the Omicron strain [
11], which have increased infectivity due to viral mutations and stronger ability to evade immunity from vaccines [
12,
13,
14], the chances of community and home infections was speculated to be increased among medical workers and hospital employees, and the probability of infection has become almost the same as that observed in the community (
Table 1). The lower infection rate among doctors than that for other occupations may be due to the thorough infection prevention measures taken when examining patients and in daily life. On the other hand, the high infection rate among nurses may be because they were required to have closer and longer contact with infected patients than doctors, making their infection risk higher. The suppression of infection rate in nurses to the same level as that in the general community or lower than that of administrative workers and clinical laboratory technicians in the same hospital could be attributed to the effectiveness of infection prevention measures among nurses.
After the sixth wave, the pneumonia symptoms of COVID-19 tended to be milder than those before [
15,
16,
17,
18,
19]. Since the eighth wave, the number of beds for critically ill patients has decreased, indicating that fewer COVID-19 patients require hospitalization among healthy individuals. Nevertheless, the number of patients requiring long-term hospitalization owing to worsening of pre-existing complications increased during this period. For the general public, the vaccine may be able to reduce the severity of COVID-19 symptoms; however, it is not sufficient to prevent the infection. Upon comparison of close contacts (
Table 2), we found no significant difference in the number of vaccinations between infected and non-infected individuals, indicating that vaccination was ineffective in preventing infections due to intense exposure. Similar results were reported in in a previous report [
20]. However, during the ninth wave, employees who had been fully vaccinated (≥3 times) had significantly fewer number of previous infections, indicating that vaccination had a certain preventive effect.
Infections caused by the Omicron strain and its derivatives, which have acquired mutations allowing them to evade vaccines [
12,
13,
14], do not cause serious illness in the general public but may cause severe infection in older people with comorbidities and those with severe immunodeficiency [
21]. After the sixth wave, infection control measures at medical institutions and nursing homes treating this specific population have become increasingly difficult, and mass infections in elderly welfare facilities and welfare facilities for people with disabilities have increased rapidly [
10]. However, when the reported mortality rate due to COVID-19 among older people during the seventh wave was equal to or lower than that of seasonal influenza, the relaxation of behavior and infection control measures in general social life was recommended. Until then, the national registration system was changed to allow voluntary personal online registration for people who were diagnosed at home using a commercially available RAT kit, although patients diagnosed at medical institutions remained registered by doctors. Owing to the lowered sense of crisis in the society and relaxation of social behavioral norms, there would have been an increase in cases where asymptomatic or mild symptoms were not tested or diagnosed, causing concerns that the actual situation could not be ascertained. This may be related to our finding that the ratio of infection rate among hospital employees to the infection rate in the community increased by approximately three times in the eighth wave (
Table 1). With the relaxation of infection control measures, R-RAT was started with cooperation from the Tokyo Metropolitan Government. We found that some employees were completely asymptomatic (
Table 2). When employees come into close contact, the detection rate of asymptomatic infected people increased if the RAT was performed daily during the incubation period. Our results suggested that there must have been a considerable number of asymptomatic infected individuals in the community. Additionally, the infection rate among close contacts increased from 28.7% (56/195) in the eighth wave to 41.9% (26/62) in the ninth wave, indicating that the virus became even more infectious during the ninth wave. The ratio of close contacts to infected people in the eighth wave (195 vs. 320) was significantly higher than in that the ninth wave (62 vs. 299) (odds ratio: 2.94, 95%CI: 2.12–4.07, p<0.0001). This may be because in the ninth wave, there were many asymptomatic infected people, and close contacts in several cases were not recognized. As shown in
Table 3 and
Figure 2, R-RAT twice/week had a better infection-detection rate than R-RAT once/week. Asymptomatic infected individuals without close contact accounted for a small percentage of all infected employees (
Table 2). However, several initial symptoms were relatively mild and temporary, and many infected cases might have been overlooked without the R-RAT. During the eighth and ninth waves, when infectivity increased and clinical symptoms became milder, the R-RAT for employees was considered to have had a certain effect from the perspective of preventing nosocomial infections. Notably, during the ninth wave, the proportion of infected employees identified through the R-RAT increased compared with that during the eighth wave in all occupations.
Although the risk to healthy people has decreased to the same level as that of seasonal influenza, COVID-19 remains an infectious disease that requires caution in medical institutions where older people with complications are hospitalized. Therefore, understanding the actual state of infection among medical workers involved in patient treatment and care is important. This observational study showed that regular testing using the RAT kit was effective in detecting infections among healthcare workers.
This study had some limitations. First, this was a retrospective observational study. Second, the implementation of the R-RAT is left to each medical employee, making it impossible to confirm whether each person has been tested evenly and equally. The notification system was based on interviews with the head of each department, and there may have been variations in the content and accuracy. Finally, the timing of confirmation of a negative RAT is not mandatory and is based on each employee’s discretion.
Conclusion
The R-RAT effectively detects mild or asymptomatic COVID-19 at an early stage and at a high rate in healthcare employees during the eighth and ninth pandemic waves in Japan.
Author Contributions
Masayuki Nagasawa: Conceptualization, study design, writing-original draft, review and editing, data analysis., Tomoyuki Kato: Data curation, writing-review., Hayato Sakaguchi: Data curation, Ippei Tanaka: Data curation, Asami Watanabe: Data curation and analysis, Yoko Hiroshima: Data curation, Mie Sakurai:. Data curation and analysis.
Funding
We do not receive any fundings for this project.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
We would like to thank all employees who cooperated with the regular rapid antigen testing. We would like to thank Honyaku Center Inc. for English language editing.
Conflicts of Interest
We have nothing to declare.
Authorship Statement
All the authors met the International Committee of Medical Journal Editors authorship criteria, and approved the contents of this paper.
Ethical Approval
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and the study protocol was approved by the Musashino Red Cross Hospital Clinical Research Ethics Committee (registration number: 5107).
Patient Consent Statement
Informed consent was secured by the opt-out method.
Abbreviations
COVID-19; coronavirus disease; SARS-CoV-2; severe acute respiratory syndrome coronavirus 2; R-RAT; regular rapid antigen test; OR; odds ratio; CI; confidence interval
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020, 382, 727–733. [CrossRef] [PubMed]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–1062. [CrossRef] [PubMed]
- Ministry of Health LaW, Japan.https://covid19.mhlw.go.jp/. 2023[accessed 2024/3/14].
- Institute. APP. Long-term services and supports and family caregiving. p. https://www.aarp.org/ppi/issues/caregiving/info-2020/nursing-home-coviddashboard.html. 2020 [accessed 2024/3/14].
- Foundation KF. Coronavirus (COVID-19). Nursing homes experienced steeper increase in COVID-19 cases and deaths in August 2021 than the rest of the country https://www.kff.org/coronaviruscovid-19/issue-brief/nursing-homes-experienced-steeperincrease-in-covid--cases-and-deaths-in-august-2021-thanthe-rest-of-the-country. 2023[accessed 2024/3/14].
- Roth A, Feller S, Ruhnau A, Plamp L, Viereck U, Weber K, et al. Characterization of COVID-19 outbreaks in three nursing homes during the first wave in Berlin, Germany. Sci Rep 2021, 11, 24441. [CrossRef] [PubMed]
- Taylor J, Carter RJ, Lehnertz N, Kazazian L, Sullivan M, Wang X, et al. Serial Testing for SARS-CoV-2 and Virus Whole Genome Sequencing Inform Infection Risk at Two Skilled Nursing Facilities with COVID-19 Outbreaks - Minnesota, April-June 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 1288–1295. [CrossRef] [PubMed]
- Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The Advisory Committee on Immunization Practices' Updated Interim Recommendation for Allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep 2021, 69, 1657–1660. [CrossRef] [PubMed]
- WHO. NCP: Close Contact Management Protocol.https://www.who.int/docs/default-source/wpro---documents/countries/china/covid-19-briefing-nhc/6-annex-3-protocol-for-management-of-close-contacts-v5.pdf?sfvrsn=eb6bca3a_2. 2020[accessed 2024/3/14].
- Ministry of Health LaW, Japan. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00395.html#h2_free16. 2022 [accessed 2024/3/14].
- Hayakawa K, Asai Y, Matsunaga N, Tsuzuki S, Terada M, Suzuki S, et al. Evaluation of the representativeness of data in the COVID-19 Registry Japan during the first six waves of the epidemic. Glob Health Med 2022, 4, 204–209. [CrossRef] [PubMed]
- Cui Z, Liu P, Wang N, Wang L, Fan K, Zhu Q, et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell 2022, 185, 860–71 e13. [CrossRef] [PubMed]
- Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [CrossRef] [PubMed]
- Feikin DR, Higdon MM, Andrews N, Collie S, Deloria Knoll M, Kwong JC, et al. Assessing COVID-19 vaccine effectiveness against Omicron subvariants: Report from a meeting of the World Health Organization. Vaccine 2023, 41, 2329–2338. [CrossRef] [PubMed]
- Abdullah F, Myers J, Basu D, Tintinger G, Ueckermann V, Mathebula M, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis 2022, 116, 38–42. [CrossRef] [PubMed]
- Nagasawa M, Kato T, Yamaguchi Y, Sugita Y, Kajiwara H. Rapid decrease of nasopharyngeal SARS-CoV-2 antigen in an outbreak of the Omicron strain. J Med Virol 2023, 95, e28179. [CrossRef] [PubMed]
- Ullrich F, Hanoun C, Turki AT, Liebregts T, Breuckmann K, Alashkar F, et al. Early report on the severity of COVID-19 in hematologic patients infected with the SARS-CoV2 omicron variant. Eur J Haematol 2022, 109, 364–372. [CrossRef] [PubMed]
- Schuller M, Ginthör NE, Paller A, Waller M, Köstenbauer M, Schreiber NGO, et al. Reduced COVID-19 morbidity and mortality in hemodialysis patients across the various Omicron sublineages-A retrospective analysis. Front Public Health 2023, 11, 1218188. [CrossRef] [PubMed]
- Di Chiara C, Boracchini R, Sturniolo G, Barbieri A, Costenaro P, Cozzani S, et al. Clinical features of COVID-19 in Italian outpatient children and adolescents during Parental, Delta, and Omicron waves: a prospective, observational, cohort study. Front Pediatr 2023, 11, 1193857. [CrossRef] [PubMed]
- Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022, 376, e069761. [CrossRef] [PubMed]
- Hu C, Liu YK, Sun QD, Du Z, Fang YQ, Guo F, et al. Clinical characteristics and risk factors for a prolonged length of stay of patients with asymptomatic and mild COVID-19 during the wave of Omicron from Shanghai, China. BMC Infect Dis 2022, 22, 947. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 Infected
Infected Infected
Infected




