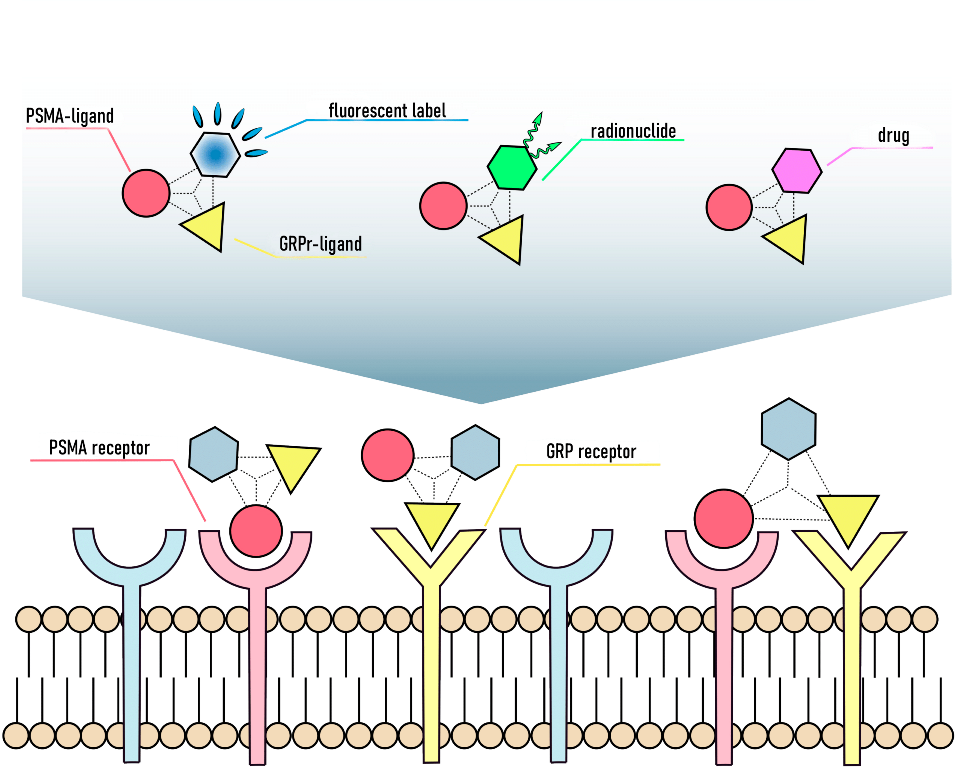
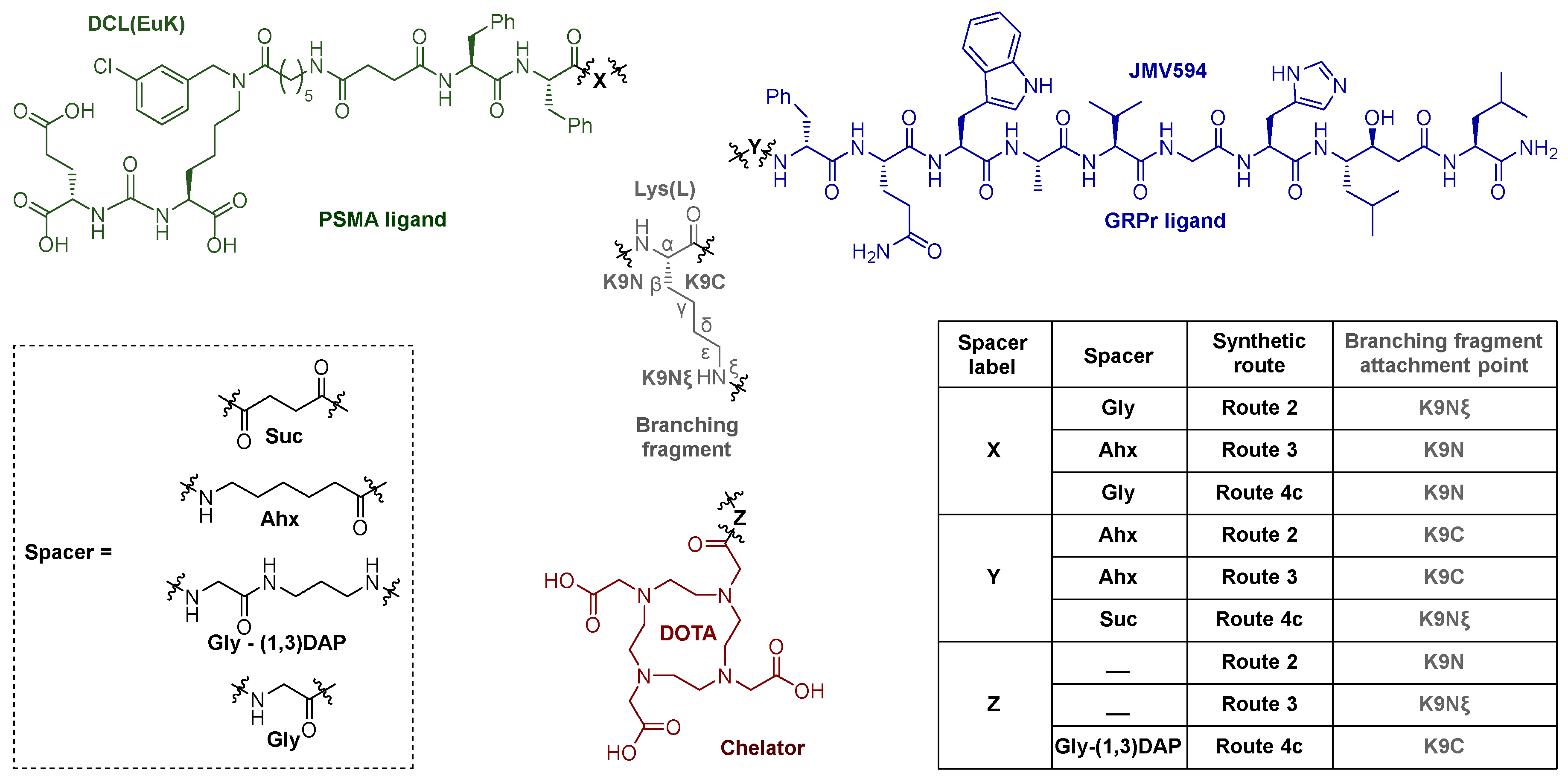
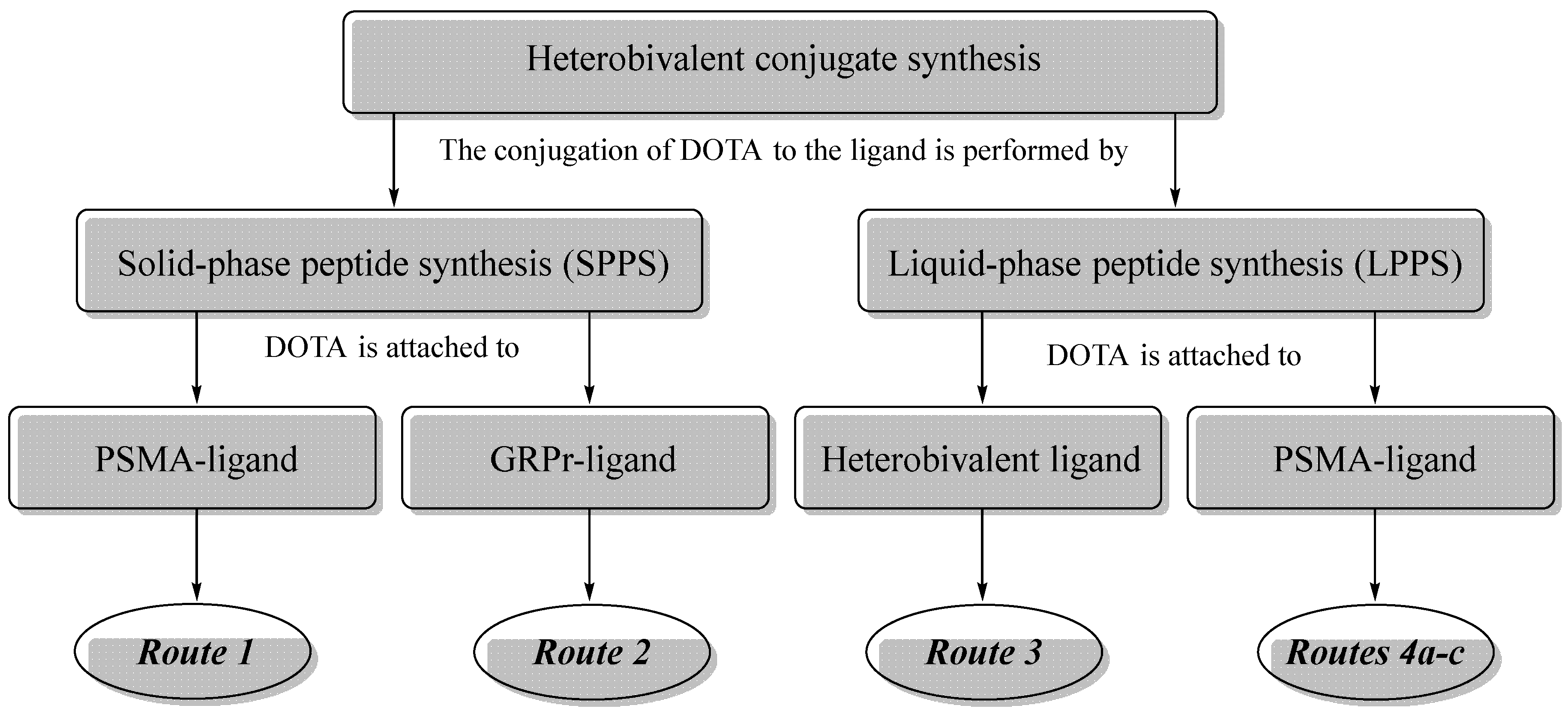
4. Material and methods
All solvents used were purified according to the described procedures [
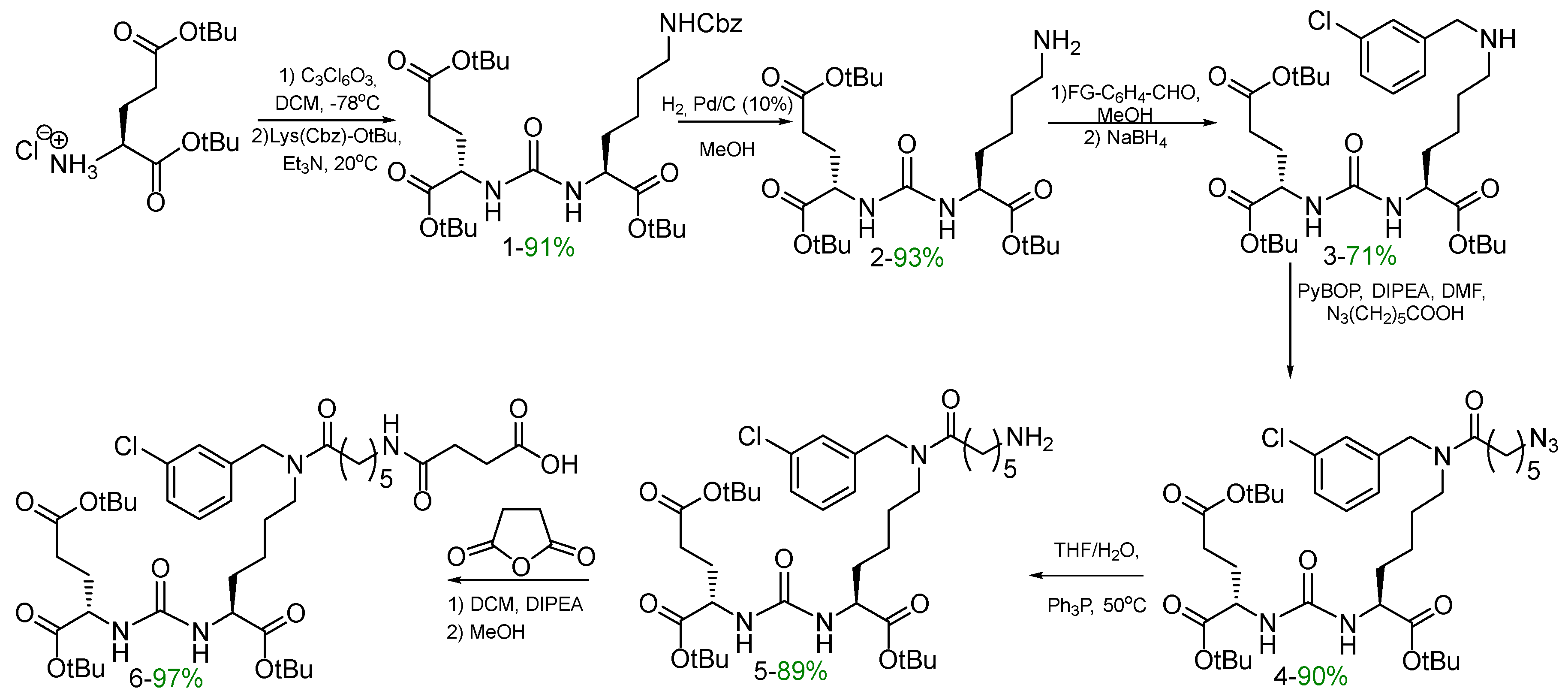
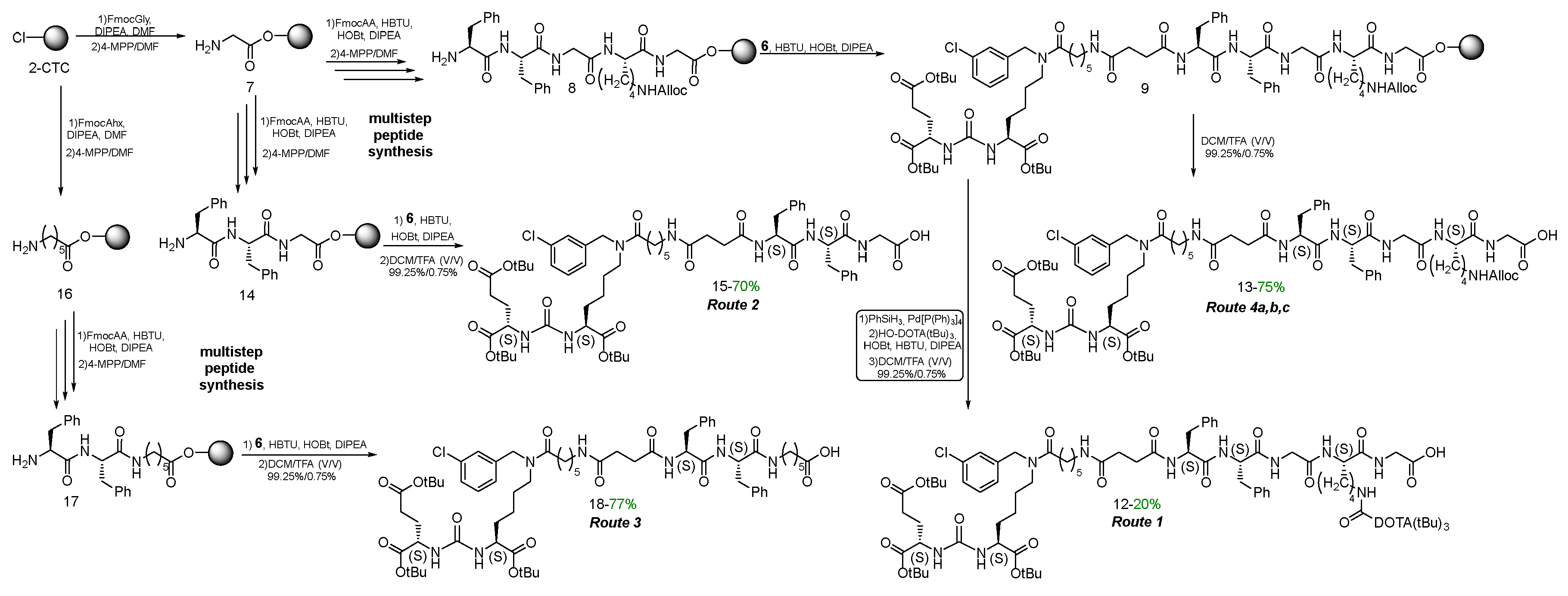
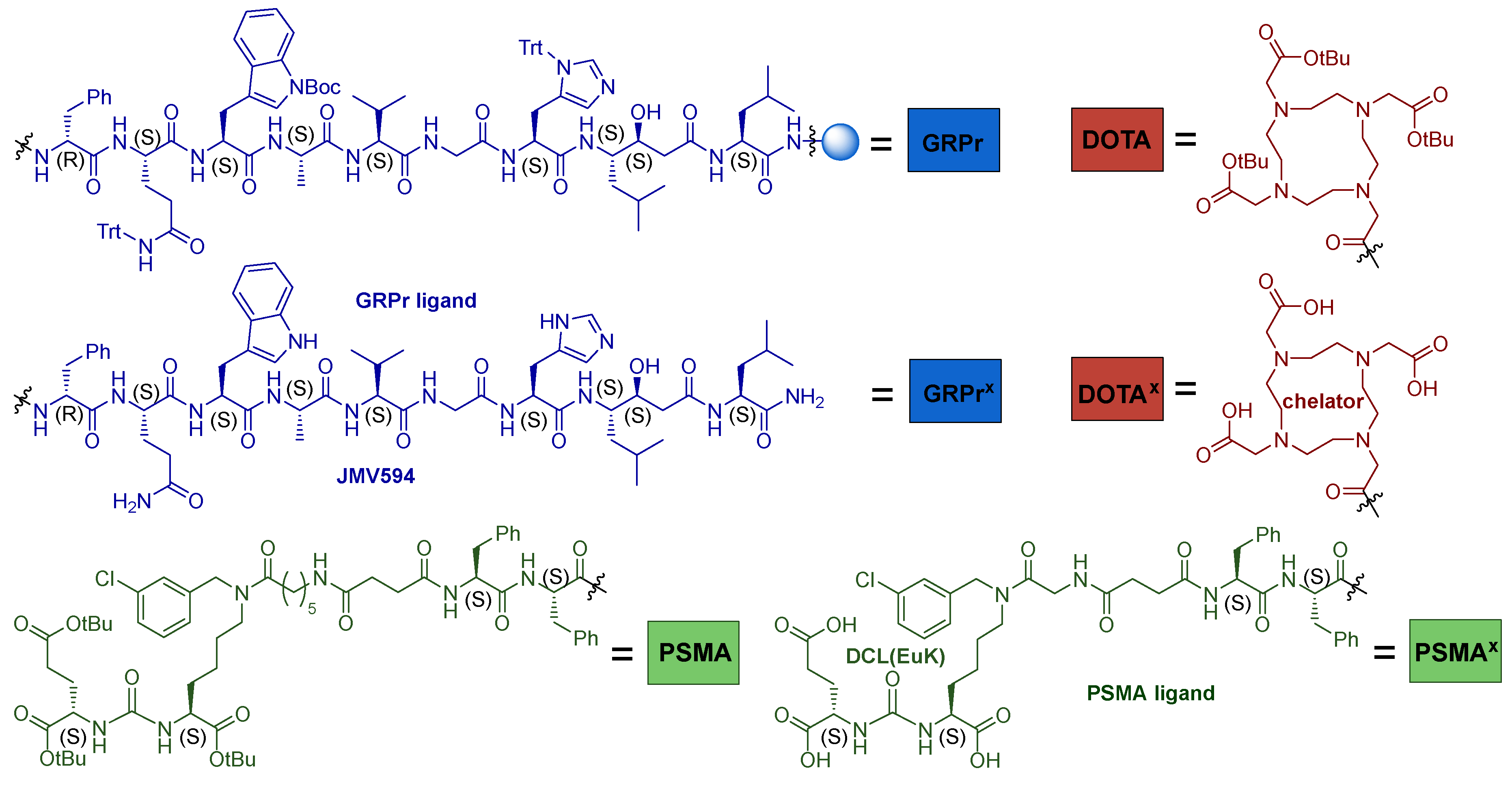
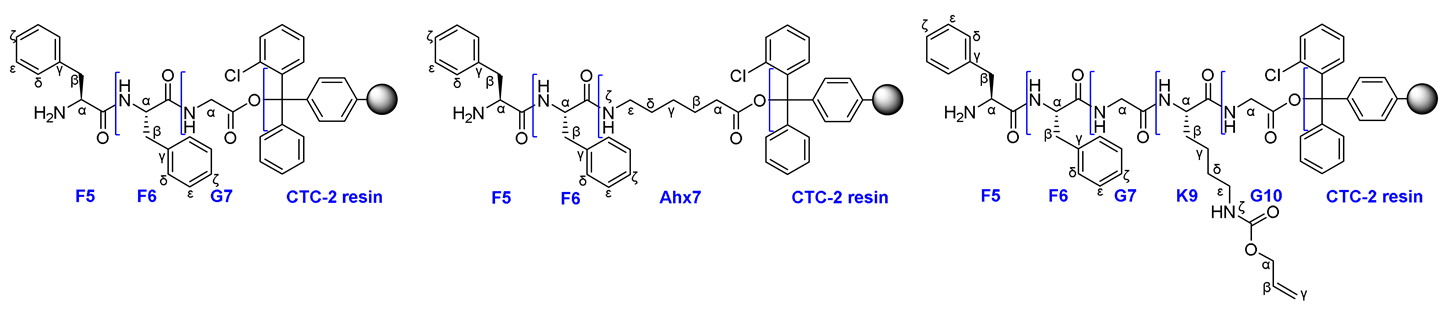
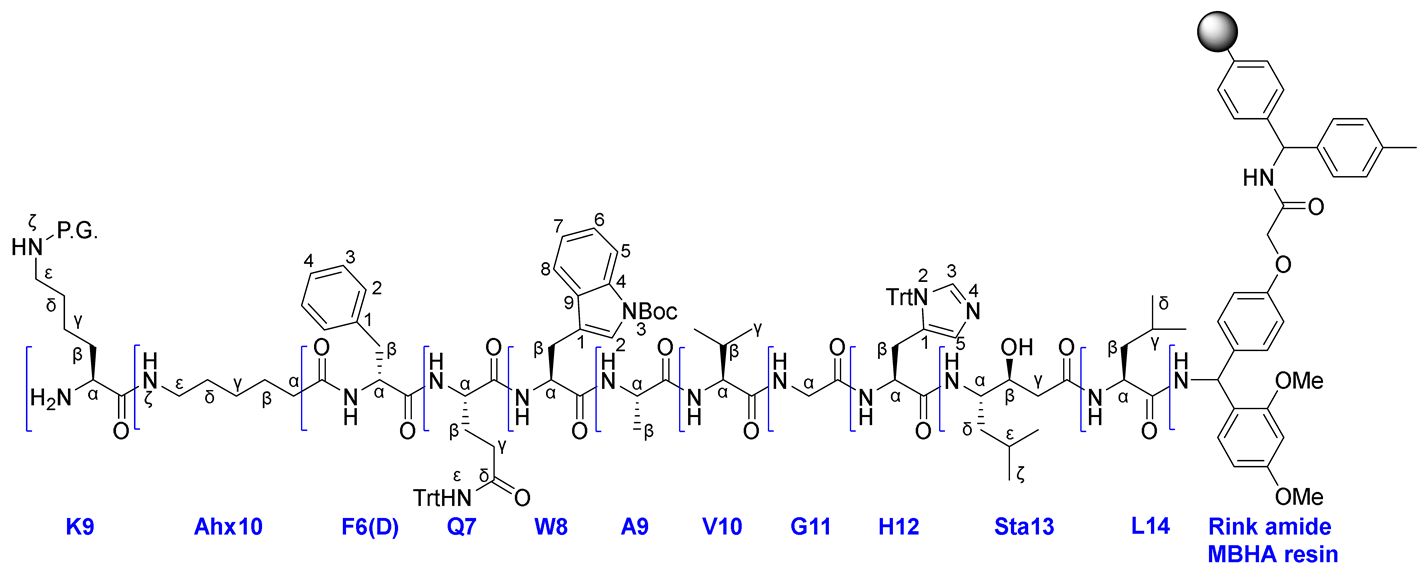
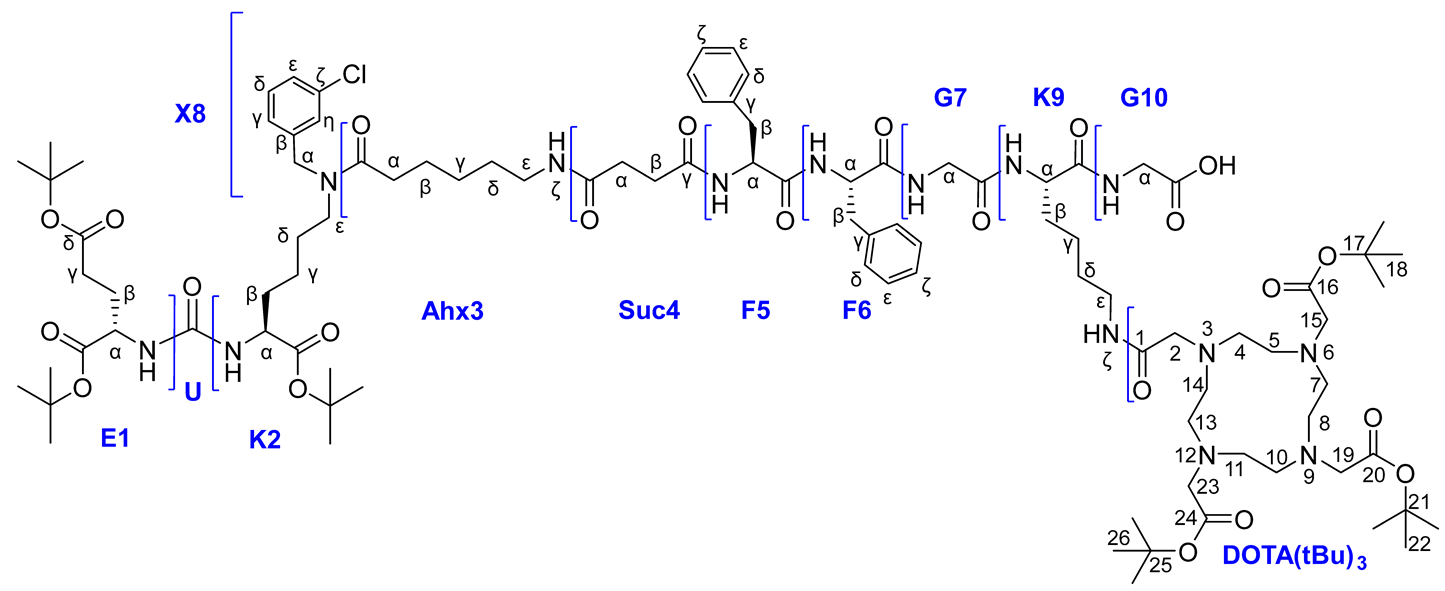
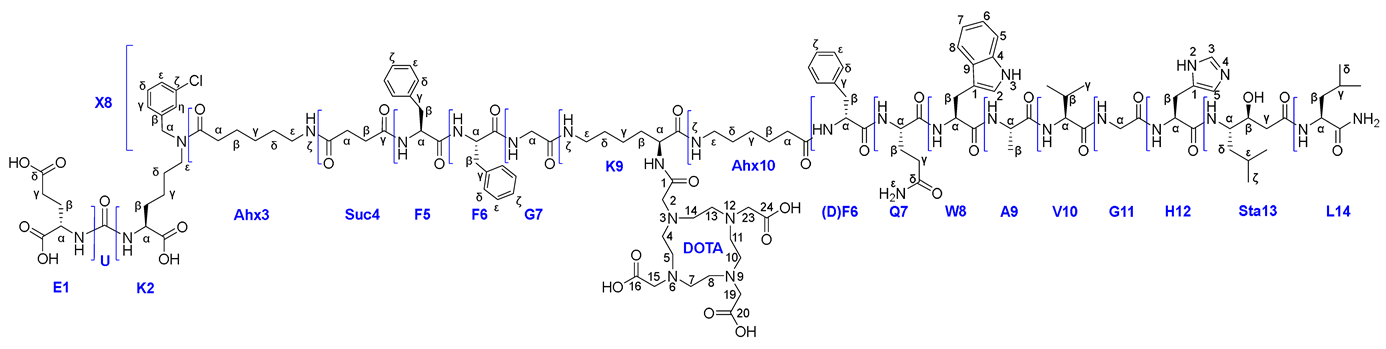
35]. All reagents were obtained from commercial suppliers (Sigma-Aldrich, Fluka®Analytical, abcr, Carbosynth, Lumiprobe) and used as received. The initial stages of the synthesis of vector fragments 1–6 (
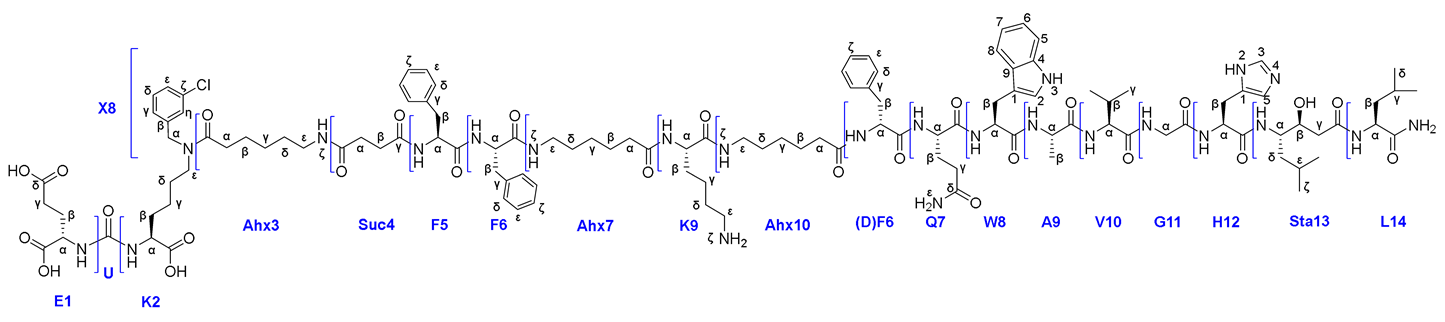
Scheme 1) were carried out using methods previously developed by our scientific group [
23].
1H NMR spectra were measured at Bruker Avance spectrometer operating at 400 MHz using DMSO-d
6 as a solvent. Chemical shifts are reported in δ units to 0.01 ppm precision with coupling constants reported to 0.1 Hz precision using residual solvent as an internal reference.
13C NMR spectra were measured at Bruker Avance spectrometer operating at 100.6 MHz using DMSO-d
6 as solvents. Chemical shifts are reported in δ units to 0.1 ppm precision using residual solvent as an internal reference. 2D NMR was measured using an Agilent 400 spectrometer operating at 400 MHz for
1H and 100.6 MHz for
13C using DMSO-d
6 as the solvent. As 2D NMR methods were used: ROESY,
13C-
1H HSQC,
13C-
1H HMBC and
15N-
1H HSQC. NMR spectra were processed and analyzed using Mnova software (Mestrelab Research, Spain).
For HPLC analysis system with Shimadzu Prominence LC-20 column and a convection fraction collector connected with a single quadrupole mass spectrometer Shimadzu LCMS-2020 with dual ionization source DUIS-ESI-APCI were used. The analytical and preparative column was Phenomenex Luna 3 µm C18 90A (150 x 4.6 mm) with column thermostat at 40 °C and fraction collector. High-resolution mass spectra (HRMS) were recorded on a TripleTOF 5600+ quadrupole time-of-flight mass spectrometer (AB Sciex, Canada) equipped with a TurboIon Spray electrospray ionization source and an LC-30 “Nexera” liquid chromatograph (Shimadzu, Japan). Solutions of samples in acetonitrile with 1% formic acid were introduced into the ionization source by electrospray.
Preparative chromatographic separation of the reaction mixtures was carried out using the INTERCHIM puriFlash 4250 chromatograph. Evaporation of the solvent was carried out using a rotary evaporator, under reduced pressure at a bath temperature of 20-50°C; Flash column chromatography was performed using Merck silica gel 60 (230-400 ASTM mesh).
Synthesis of PSMA ligands
synthesis of peptide sequences on CTC-2 resin
General procedure for obtaining peptide sequences by SPPS on CTC-2 resin.
Activation of 2-CTC. The mixture of 2-CTC (1 eq.; 1 g; 1.0–1.6 mmol/g; 100–200 mesh) in DCM (10 ml/1 g) was stirred for 10 min, purged with Ar and SOCl2 (3 eq.) was added dropwise. The resulting mixture was charged with DMF (5%V/V to SOCl2) and then stirred at 40 °C for 4 h. After that, the resin was filtered and transferred to a polypropylene reactor, washed with DMF (3 × 10 ml, 1 min) and DCM (3 × 10 ml, 1 min). The amount of solvent for the reaction and washing is 10 ml/1 g of resin.
Addition of the first amino acid residue. To the CTC-2 resin (1 eq.; 1 g; 1.2–1.6 mmol/g; 100–200 mesh) in DMF (10 ml/1 g), Fmoc-protected amino acid (2 eq. relative to the upper capacity of CTC-2 resin) and DIPEA (10 eq.) were added, and the mixture was stirred for 4 h. Then, the resin was filtered and washed with MeOH*3 (10 ml/1 g, 5 min), DCM*3 (10 ml/1 g, 1 min), DMF*3 (10 ml/1 g, 1 min), and DCM*3 (10 ml/1 g, 1 min).
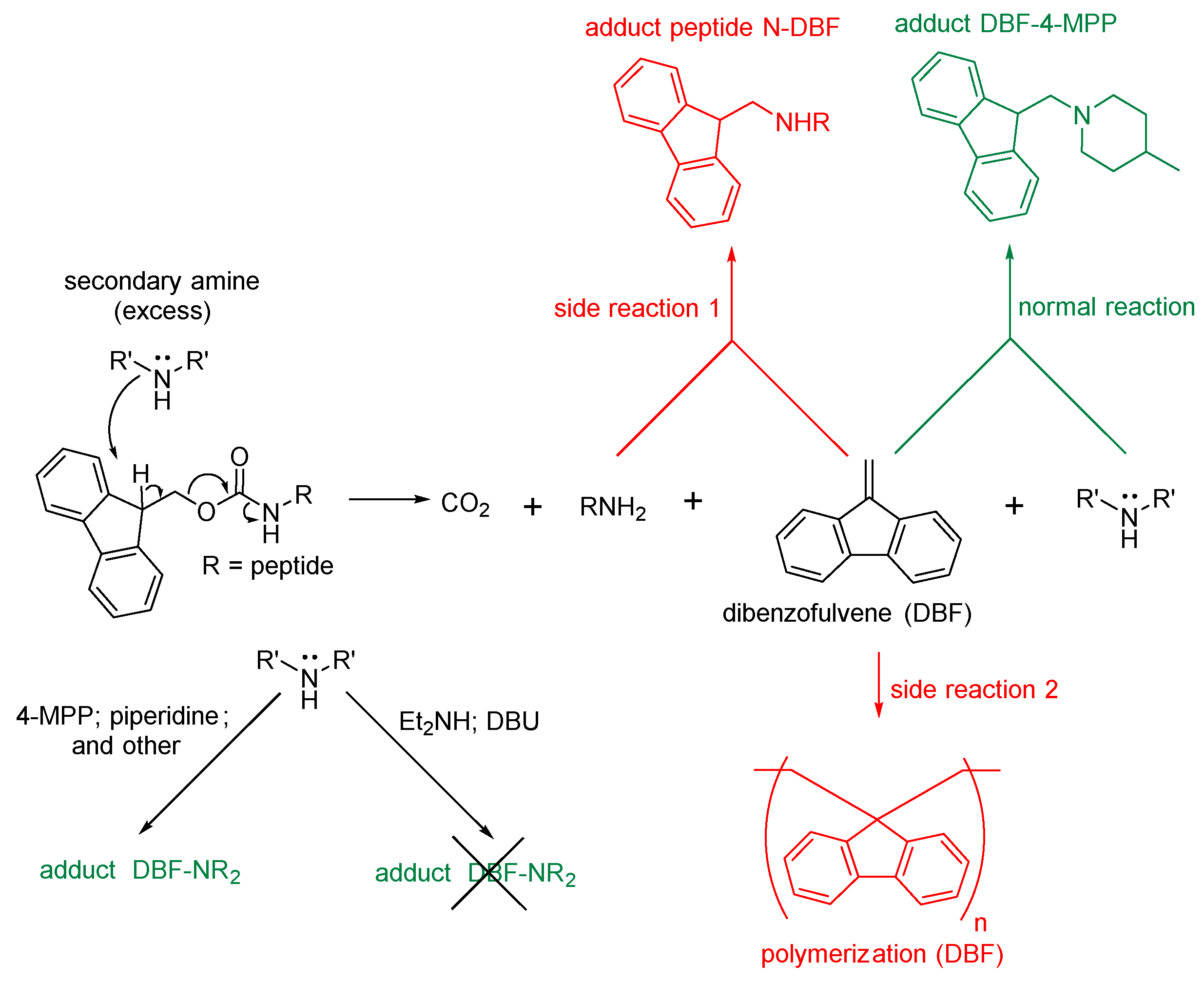
Deprotection of Fmoc. Peptide sequence on a 2-CTC resin (1 eq.) was washed with DMF*2 (10 ml/1 g, 1 min), then 4-methylpiperidine in DMF (20%/80% V/V, 15 ml) was added and stirred for 15 min, then the resin was filtered and washed with DMF*3 (10 ml/1 g, 1 min), then 4-methylpiperidine in DMF (20%/80% V/V, 10 ml/1 g) was added and stirred for 15 min. After the resin was filtered and washed with DMF*3 (10 ml/1 g, 1 min) and DCM*3 (10 ml/1 g, 1 min).
Deprotection of Alloc. Peptide sequence on a Rink Amide MBHA resin (1eq.) was washed with DCM*1 (10 ml/1 g, 1 min) for resin swelling, then DCM (10 ml/1 g) and PhSiH3 (8 eq.) were added and the mixture was stirred under inert atmosphere of Ar for few min; then Pd[P(Ph)3]4 (0.1 eq.) was added and the mixture was stirred under argon atmosphere for 60 min. Then the resin was filtered and washed DCM*3 (10 ml/1 g, 2 min) DMF*3 (10 ml/1 g, 2 min) and DCM*3 (10 ml/1 g, 2 min).
Addition of the second and subsequent amino acid residues. To the mixture of CTC-2 (1 eq.) in DMF (10 ml/1 g) Fmoc-protected amino acid (2 eq), HOBt (0.5 eq.), HBTU (2 eq.) and DIPEA (3 eq.) were added and the mixture was stirred for 4 h. Then the resin was filtered and washed DMF*3 (10 ml/1 g, 1 min) and DCM*3 (10 ml/1 g, 1 min).
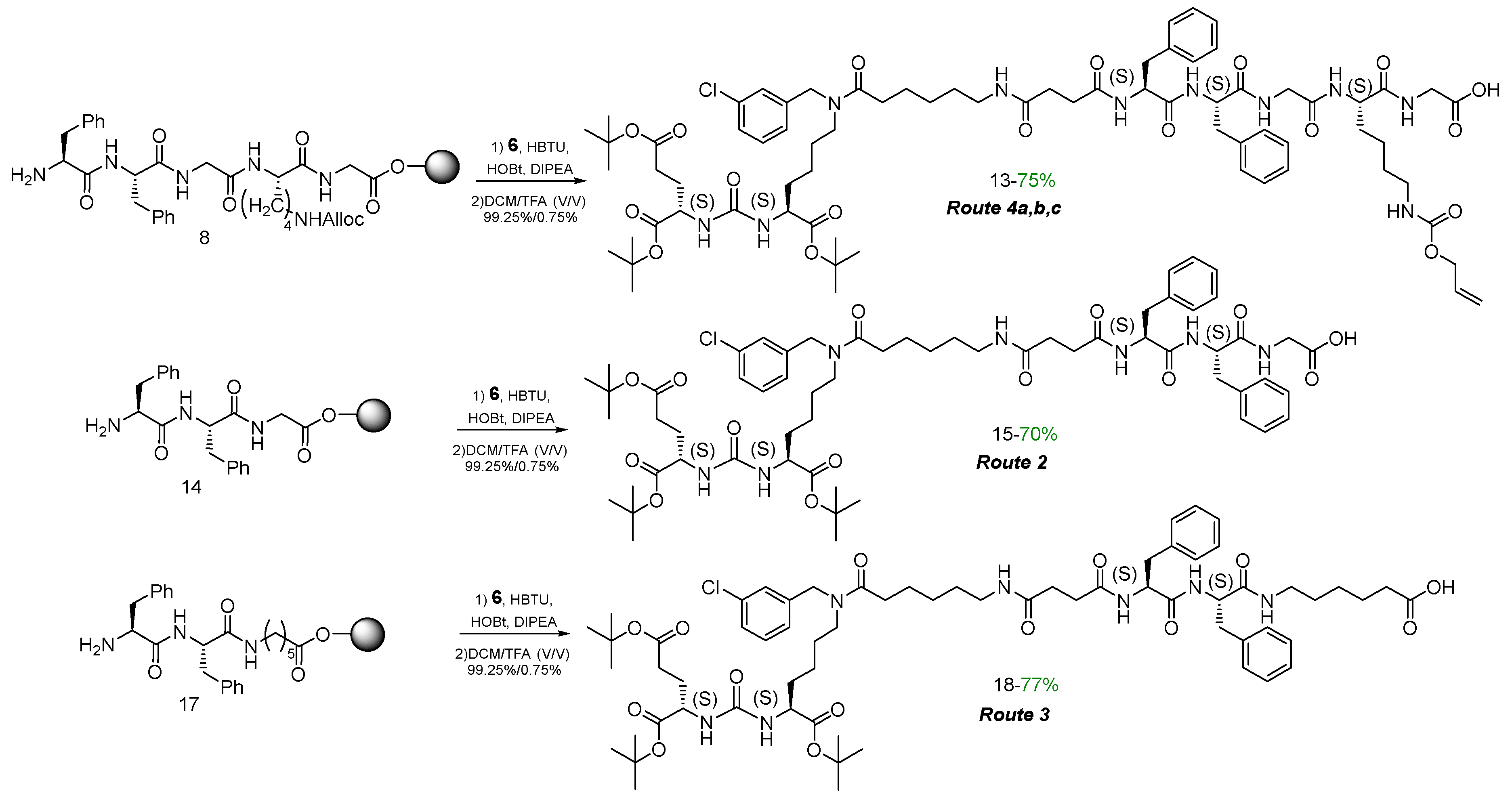
Synthesis of pentapeptide NH2-FFGK(Alloc)G on 2-CTC resin - 8.
Using 2-CTC resin (1000 mg, 1.0-1.6 mmol), FmocGly-OH (951 mg; 3.2 mmol), DIPEA (2.78 ml; 16 mmol) to add the first amino acid, FmocLys(L)(Alloc)-OH (1448 mg; 3.2 mmol), HBTU (1214 mg; 3.2 mmol), HOBt (108 mg; 0.8 mmol) and DIPEA (836 μl; 4.8 mmol) to add the second amino acid, FmocGly-OH (951 mg; 3.2 mmol), HBTU (1214 mg; 3.2 mmol), HOBt (108 mg; 0.8 mmol) and DIPEA (836 μl; 4.8 mmol) to add the third amino acid, FmocPhe(L)-OH (1240 mg; 3.2 mmol), HBTU (1214 mg; 3.2 mmol), HOBt (108 mg; 0.8 mmol) and DIPEA (836 μl; 4.8 mmol) to add the fourth amino acid, FmocPhe(L)-OH (1240 mg; 3.2 mmol), HBTU (1214 mg; 3.2 mmol), HOBt (108 mg; 0.8 mmol) and DIPEA (836 μl; 4.8 mmol) to add the fifth amino acid, the pentapeptide NH2-FFGK(Alloc)G on 2-CTC resin was obtained.
Synthesis of tripeptide NH2-FFG on 2-CTC resin - 14.
Using 2-CTC resin (500, 0.5-0.8 mmol), FmocGly-OH (476 mg; 1.6 mmol), DIPEA (1.394 ml; 8 mmol) to add the first amino acid, FmocPhe(L)-OH (620 mg; 1.6 mmol), HBTU (607 mg; 1.6 mmol), HOBt (54 mg; 0.4 mmol) and DIPEA (418 μl; 2.4 mmol) to add the second amino acid, FmocPhe(L)-OH (620 mg; 1.6 mmol), HBTU (607 mg; 1.6 mmol), HOBt (54 mg; 0.4 mmol) and DIPEA (418 μl; 2.4 mmol) to add the third amino acid, the tripeptide NH2-FFG on 2-CTC resin was obtained.
Synthesis of tripeptide NH2-FFAhx on 2-CTC resin - 17.
Using 2-CTC resin (500 mg, 0.5-0.8 mmol), FmocAhx-OH (565 mg; 1.6 mmol), DIPEA (1.394 ml; 8 mmol) to add the first amino acid, FmocPhe(L)-OH (620 mg; 1.6 mmol), HBTU (607 mg; 1.6 mmol), HOBt (54 mg; 0.4 mmol) and DIPEA (418 μl; 2.4 mmol) to add the second amino acid, FmocPhe(L)-OH (620 mg; 1.6 mmol), HBTU (607 mg; 1.6 mmol), HOBt (54 mg; 0.4 mmol) and DIPEA (418 μl; 2.4 mmol) to add the third amino acid, the tripeptide NH2-FFKAhx on 2-CTC resin was obtained.
Compound 13
To a mixture of pentapeptide NH2-Phe(L)Phe(L)GlyLys(Alloc)(L)Gly on 2-CTC resin 8 (1 eq.; 0.47 mmol) in DMF (10 ml) in a reactor compound 6 (1.1 eq.; 426 mg; 0.517 mmol), HOBt (0.5 eq.; 32 mg; 0.235 mmol), HBTU (2 eq.; 357 mg; 0.94 mmol), DIPEA (3 eq.; 246 μl; 1.41 mmol) were added. The mixture was stirred for 12 h. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (10 ml), three times with DCM (10 ml), then dried from traces of solvents. After that, DCM/TFA system (99.25%-0.75%, 12 ml) was added to the resin and left under stirring for 15 min, then the resin was filtered off and washed with DCM. The solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM. Product was purified by column chromatography (Puriflash PF-15C18HP-F0020 + PF-15C18HP-F0040 (15 μ 32 g + 15 μ 60 g), eluent: H2O(80%)/MeCN(20%) => H2O(0%)/MeCN(100%) for 40 min, after MeCN(100%) for 5 min). Compound 13 was obtained as a white amorphous solid (513 mg, 75% yield).
1H NMR (400 MHz, DMSO-d6, δ): 12.53 (br.s, 1H, G10COOH), 8.25 (t, J = 6 Hz, 1H, G10NH), 8.20-8.14 (m, 1Н, F5NHmn), 8.12 (d, J = 8 Hz, 1Н, F6NH), 8.06 (t, J = 5.9 Hz, 1H, G7NH), 7.91 (d, J = 8.0 Hz, 1H, K9NH), 7.82 (t, J = 5.4 Hz, m) & 7.79 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.42-7.09 (m, 15H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.19 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.82 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.42 (m, 5H, X8αnm + F6α + Allocα), 4.42-4.34 (m, 1H, F5Hα), 4.32-4.20 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.65 (m, 4Н, G7α + G9α), 3.21 (t, J = 7.3 Hz, n) & 3.17 (t, J = 7.3 Hz, m) (2H, K2ε, m/n = 3/2), 3.12-3.04 (m, 1Н, F6β(a)), 3.03-2.90 (m, 5Н, F6β(b) + Ahx3ε + K9ε), 2.90-2.82 (m, 1Н, F5β(a)), 2.71-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.40-1.35 (m, 27H, tBu).
LCMS 100% in positive ion mode, 100% in negative ion mode
ESI-MS C73H10537ClN10O18: m/z calc. for [M+2H+]2+: 724.37, found: 724.05
HRMS (m/z, ESI): calc. for C73H10535ClN10O18 - [M+2Na]2+ 745.3541, found: 745.3531.
Compound 15
To a mixture of tripeptide NH2-Phe(L)Phe(L)Gly on 2-CTC resin 14 (1 eq.; 0.21 mmol) in DMF (5 ml) in a reactor compound 6 (1.1 eq.; 190 mg; 0.231 mmol), HOBt (0.5 eq.; 28 mg; 0.1 mmol), HBTU (2 eq.; 159 mg; 0.42 mmol), DIPEA (3 eq.; 110 μl; 0.63 mmol) were added. The mixture was stirred for 12 h. The solvent was then removed by filtration on a porous reactor filter and the resin was washed three times with DMF (5 ml), three times with DCM (5 ml), then dried from traces of solvents. A DCM/TFA system (99.25%-0.75%, 10 ml) was added to the resin and left under stirring for 15 min, after the solution was filtered from the resin. The solvent was removed under reduced pressure and the residue was re-evaporated with DCM. The crude product was purified by column chromatography (Puriflash PF-15C18HP-F0040 (15 μ 60 g), eluent: H2O(80%)/MeCN(20%) => H2O(0%)/MeCN(100%) for 30 min, after MeCN(100%) for 5 min). Compound 15 was obtained as a white amorphous solid (173 mg, 70% yield).
1H NMR (400 MHz, DMSO-d6, δ): 12.54 (s, 1H, COOH), 8.20-8.07 (m, 3Н, F5NHmn + F6NHmn + G7NH), 7.84 (t, J = 5.4 Hz, m) & 7.81 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.42-7.08 (m, 14H, X8Hδn + X8Hεn + X8Hδm + X8Hεm + F6Hε + F6Hδ + X8Hηmn + F5Hε + F6Hζ + F5Hζ + F5Hδ + X8Hγmn), 6.34-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.59-4.45 (3H, X8Hαn + F6Hα + X8Hαm), 4.41-4.32 (m, 1H, F5Hα), 4.07-3.90 (m, 2H, E1Hα + K2Hαm + K2Hαn), 3.75 (d, J = 5.7 Hz, 2H, G7α), 3.26-3.13 (m, 2H, K2Hεmn), 3.12-3.05 (m, 1H, F6Hβ(a)), 3.04-2.90 (m, 3H, Ahx3Hε(mn) + F6Hβ(b)), 2.90-2.81 (m, 1Н, F5Hβ(a)), 2.69-2.59 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 8H, Ahx3Hαm + Suc4Hβmn + E1Hγ + Suc4Hαmn + Ahx3Hαn), 1.92-1.80 (m, 1Н, E1Hβ(a)), 1.72-1.62 (m, 1Н, E1Hβ(b)), 1.62-1.10 (m, 12Н, K2Hβ(a) + Ahx3Hβ + K2Hβ(b) + Ahx3Hδ + K2Hδ + K2Hγ + Ahx3Hγ, m + n), 1.40-1.35 (m, 27H, tBu).
HRMS (m/z, ESI): calc. for C61H86ClN7O14 - [M+2Na+]2+ 610.7853, found: 610.7863.
Compound 18
To a mixture of tripeptide NH2-Phe(L)Phe(L)Ahx on 2-CTC resin 17 (1 eq.; 0.339 mmol) in DMF (5 ml) in a reactor compound 6 (1.1 eq.; 308 mg; 0.373 mmol), HOBt (0.5 eq.; 23 mg; 0.17 mmol), HBTU (2.5 eq.; 322 mg; 0.85 mmol), DIPEA (3.75 eq.; 220 μl; 1.27 mmol) were added. The mixture was stirred for 12 h. The solvent was then removed by filtration on a porous reactor filter and the resin was washed three times with DMF (7 ml), three times with DCM (7 ml), and then dried from traces of solvents. A DCM/TFA system (99.25%-0.75%, 10 ml) was added to the resin and left under stirring for 15 min, after the solution was filtered from the resin. The solvent was removed under reduced pressure and the residue was re-evaporated with DCM. The crude product was purified by column chromatography (Puriflash PF-15C18HP-F0040 (15 μ 60 g), eluent: H2O(80%)/MeCN(20%) => H2O(0%)/MeCN(100%) for 30 min, after MeCN(100%) for 5 min). Compound 18 was obtained as a white amorphous solid (322 mg, 77% yield).
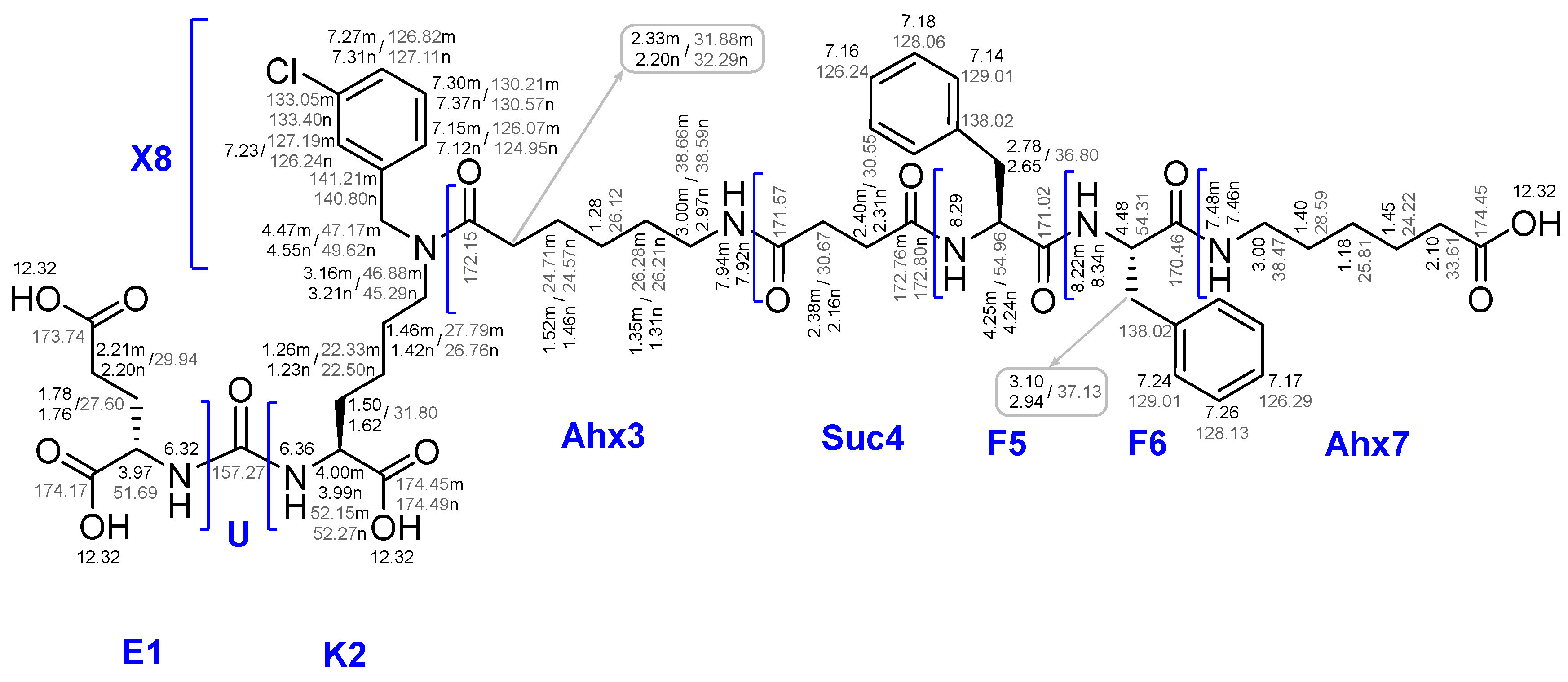
1H NMR (400 MHz, DMSO-d6, δ): 11.99 (s, 1H, COOH), 8.31 (d, J = 7.3 Hz, 1Н, F5NHmn), 8.17 (d, J = 8.4 Hz 1Н, F6NHmn), 7.96 (t, J = 5.4 Hz, m) & 7.93 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.55-7.44 (m, 1H, Ahx7NHζ), 7.42-7.08 (m, 14H, X8Hδn + X8Hεn + X8Hδm + X8Hεm + F6Hε + F6Hδ + X8Hηmn + F5Hε + F6Hζ + F5Hζ + F5Hδ + X8Hγmn), 6.35-6.19 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.55 (s, n) & 4.47 (s, m) (2H, X8Hα, m + n), 4.44-4.34 (m, 1H, F6Hα), 4.34-4.24 (m, 1H, F5Hα), 4.08-3.90 (m, 2H, E1Hα + K2Hαm + K2Hαn), 3.26-3.12 (m, 2H, K2Hεmn), 3.11-2.84 (m, 7H, F6Hβ(a) + Ahx7Hε + Ahx3Hε(mn) + F6Hβ(b) + F5Hβ(a)), 2.69-2.59 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 10H, Ahx3Hαm + Suc4Hβmn + E1Hγ + Suc4Hαmn + Ahx7Hα + Ahx3Hαn), 1.91-1.80 (m, 1Н, E1Hβ(a)), 1.72-1.62 (m, 1Н, E1Hβ(b)), 1.62-1.10 (m, 18Н, K2Hβ(a) + Ahx3Hβ + Ahx7Hβ + K2Hβ(b) + Ahx3Hδ + Ahx7Hδ + K2Hδ + K2Hγ + Ahx3Hγ + Ahx7Hγ, m + n), 1.40-1.35 (m, 27H, tBu).
13C NMR (101 MHz, DMSO-d6, δ): 174.48 (Ahx7C), 172.89 (Suc4Cγ(n)), 172.83 (Suc4Cγ(m)), 172.24 (K2C(n)), 172.20 (K2C(m)), 172.11 (Ahx3C(nm)), 171.92 (E1C), 171.59 (Suc4C(mn)), 171.44 (E1Cδ), 171.05 (F5C), 170.46 (F6C), 157.13 (U), 141.17 (X8Cβ(m)), 140.76 (X8Cβ(n)), 138.18 (F6Cγ), 138.05 (F5Cγ), 133.42 (X8Cζ(n)), 133.07 (X8Cζ(m)), 130.59 (X8Cδ(n)), 130.24 (X8Cδ(m)), 129.02 (F6Cδ + F5Cδ), 128.14 (F6Cε), 128.07 (F5Cε), 127.20 (X8Cη(m)), 127.15 (X8Cε(n)), 126.86 (X8Cε(m)), 126.30 (F6Cζ), 126.24 (X8Cη(n) + F5Cζ), 126.05 (X8Cγ(m)), 124.95 (X8Cγ(n)), 80.58 (E1tBu), 80.40 (K2tBu(m)), 80.32 (K2tBu(n)), 79.75 (E1δtBu), 55.05 (F5Cα), 54.33 (F6Cα), 52.99 (K2Cα(n)), 52.86 (K2Cα(m)), 52.17 (E1Cα), 49.60 (X8Cα(n)), 47.08 (X8Cα(m)), 46.79 (K2Cε(m)), 45.19 (K2Cε(n)), 38.65 (Ahx3Cε(m)), 38.59 (Ahx3Cε(n)), 38.47 (Ahx7Cε), 37.11 (F6Cβ), 36.78 (F5Cβ), 33.62 (Ahx7Cα), 32.31 (Ahx3Cα(n)), 31.94 (Ahx3Cα(m)), 31.81 (K2Cβ), 30.91 (E1Cγ), 30.64 (Suc4Cα), 30.52 (Suc4Cβ), 29.06 (Ahx3Cδ(m)), 28.96 (Ahx3Cδ(n)), 28.63 (Ahx7Cδ), 27.74 (tBuE1), 27.65 (tBuK2 + K2Cδ(m)), 27.62 (tBuE1δ), 27.53 (E1Cβ), 26.70 (K2Cδ(n)), 26.33 (Ahx3Cγ(m)), 26.24 (Ahx3Cγ(n)), 25.83 (Ahx7Cγ), 24.73 (Ahx3Cβ(m)), 24.59 (Ahx3Cβ(n)), 24.24 (Ahx7Cβ), 22.43 (K2Cγ(n)), 22.25 (K2Cγ(m)).
HRMS (m/z, ESI): calc. for C65H94ClN7O14 - [M+Na+]+ 1254.6439, found: 1254.6429.
Compound 18x
Compound 18 (1 eq.; 45 mg; 36.52 μmol) was dissolved in system of TFA/DCM/TIPS (47.5%/47.5%/5%, V = 3 ml). The mixture was stirred for 3 h. The solvent was removed under reduced pressure. Crude product was precipitated by Et2O, washed twice with Et2O (2 ml) and purified by column chromatography (Puriflash PF-15C18HP-F0012 (15 μ 20 g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 30 min, after MeCN(100%) for 5 min). Compound 18x was obtained as a white amorphous solid (31.6 mg, 81% yield).
1H NMR (400 MHz, DMSO-d6, δ): 12.32 (br.s., 4H, COOH), 8.29 (d, J = 7.3 Hz, 1Н, F5NHmn), 8.21-8.11 (m, 1Н, F6NHmn), 7.94 (t, J = 5.4 Hz, m) & 7.92 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.55-7.44 (m, 1H, Ahx7NHζ), 7.42-7.08 (m, 14H, X8Hδn + X8Hεn + X8Hδm + X8Hεm + F6Hε + F6Hδ + X8Hηmn + F5Hε + F6Hζ + F5Hζ + F5Hδ + X8Hγmn), 6.40-6.25 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.55 (s, n) & 4.47 (s, m) (2H, X8Hα, m + n), 4.45-4.35 (m, 1H, F6Hα), 4.34-4.24 (m, 1H, F5Hα), 4.15-3.99 (m, 2H, E1Hα + K2Hαm + K2Hαn), 3.26-3.12 (m, 2H, K2Hεmn), 3.11-2.84 (m, 7H, F6Hβ(a) + Ahx7Hε + Ahx3Hε(mn) + F6Hβ(b) + F5Hβ(a)), 2.69-2.59 (m, 1Н, F5Hβ(b)), 2.40-2.10 (m, 10H, Ahx3Hαm + Suc4Hβmn + E1Hγ + Suc4Hαmn + Ahx7Hα + Ahx3Hαn), 1.99-1.85 (m, 1Н, E1Hβ(a)), 1.78-1.67 (m, 1Н, E1Hβ(b)), 1.67-1.10 (m, 18Н, K2Hβ(a) + Ahx3Hβ + Ahx7Hβ + K2Hβ(b) + Ahx3Hδ + Ahx7Hδ + K2Hδ + K2Hγ + Ahx3Hγ + Ahx7Hγ, m + n).
13C NMR (101 MHz, DMSO-d6, δ): 174.49 (K2C(n)), 174.45 (K2C(m) + Ahx7C), 174.17 (E1C), 173.74 (E1Cδ), 172.80 (Suc4Cγ(n)), 172.76 (Suc4Cγ(m)), 172.15 (Ahx3C(nm)), 171.57 (Suc4C(mn)), 171.02 (F5C), 170.46 (F6C), 157.27 (U), 141.21 (X8Cβ(m)), 140.80 (X8Cβ(n)), 138.12 (F6Cγ), 138.02 (F5Cγ), 133.40 (X8Cζ(n)), 133.05 (X8Cζ(m)), 130.57 (X8Cδ(n)), 130.21 (X8Cδ(m)), 129.01 (F6Cδ + F5Cδ), 128.13 (F6Cε), 128.06 (F5Cε), 127.19 (X8Cη(m)), 127.11 (X8Cε(n)), 126.82 (X8Cε(m)), 126.29 (F6Cζ), 126.24 (X8Cη(n) + F5Cζ), 126.07 (X8Cγ(m)), 124.95 (X8Cγ(n)), 54.96 (F5Cα), 54.31 (F6Cα), 52.27 (K2Cα(n)), 52.15 (K2Cα(m)), 51.69 (E1Cα), 49.62 (X8Cα(n)), 47.17 (X8Cα(m)), 46.88 (K2Cε(m)), 45.29 (K2Cε(n)), 38.66 (Ahx3Cε(m)), 38.59 (Ahx3Cε(n)), 38.47 (Ahx7Cε), 37.13 (F6Cβ), 36.80 (F5Cβ), 33.61 (Ahx7Cα), 32.29 (Ahx3Cα(n)), 31.88 (Ahx3Cα(m)), 31.80 (K2Cβ), 30.67 (Suc4Cα), 30.55 (Suc4Cβ), 29.94 (E1Cγ), 29.04 (Ahx3Cδ(m)), 28.93 (Ahx3Cδ(n)), 28.59 (Ahx7Cδ), 27.79 (K2Cδ(m)), 27.60 (E1Cβ), 26.76 (K2Cδ(n)), 26.28 (Ahx3Cγ(m)), 26.21 (Ahx3Cγ(n)), 25.81 (Ahx7Cγ), 24.71 (Ahx3Cβ(m)), 24.57 (Ahx3Cβ(n)), 24.22 (Ahx7Cβ), 22.50 (K2Cγ(n)), 22.33 (K2Cγ(m)).
Synthesis of GRPr ligands
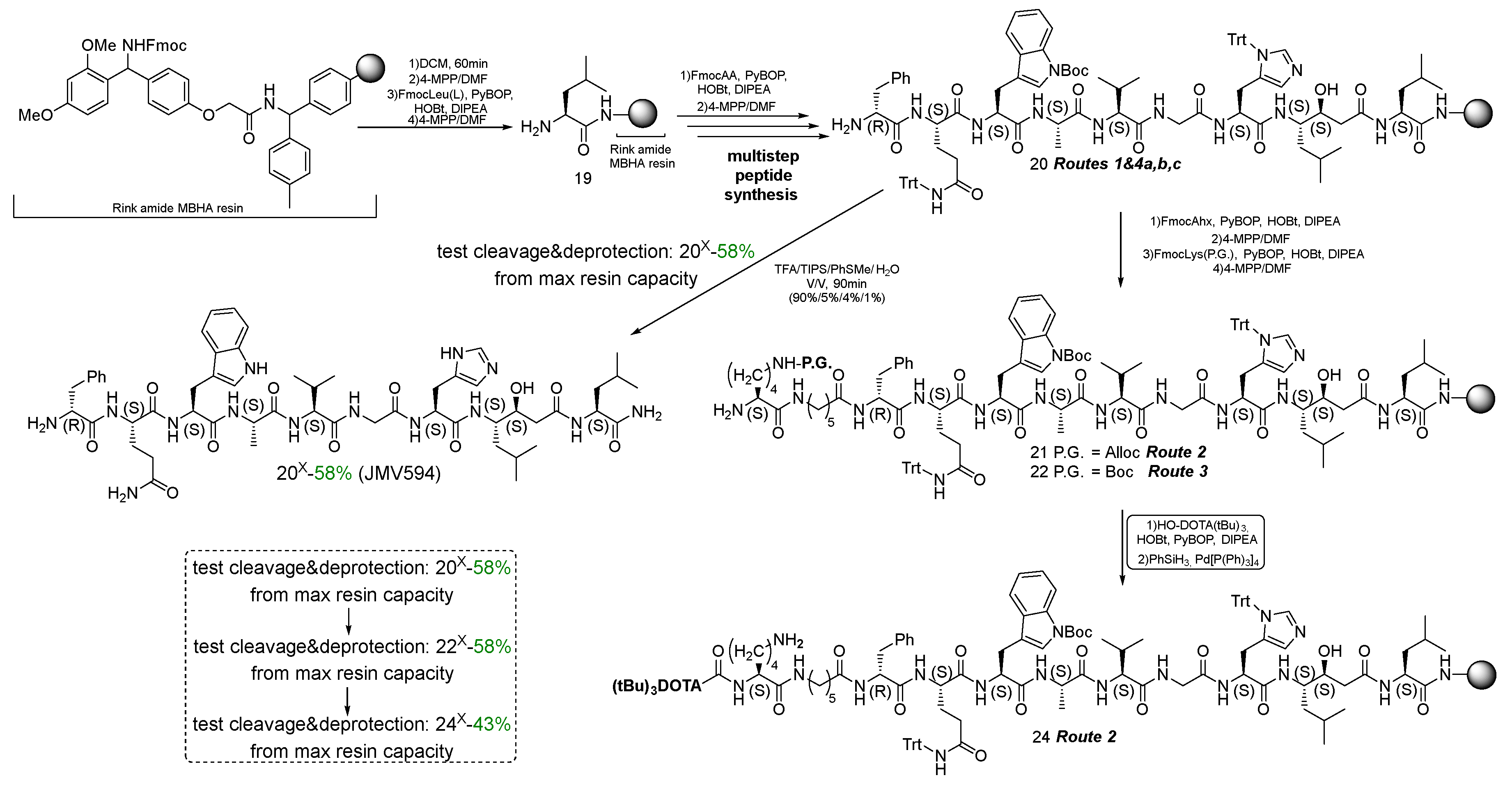
Synthesis of peptide sequences on Rink Amide MBHA resin.
General procedure for obtaining peptide sequences by SPPS on Rink Amide MBHA resin.
Activation of Rink Amide MBHA resin. To a Rink Amide MBHA resin (1 eq., 0.3-0.8 mmol/g, 100-200 mesh) in polypropylene reactor DCM (10 ml/1 g) was added and the mixture was left under stirring for 60 min. Then the Fmoc protection was removed by the standard protocol.
Addition of the first amino acid residue. To a Rink Amide MBHA resin (1 eq., 0.3-0.8 mmol/g, 100-200 mesh) in DMF (10 ml/1 g) Fmoc-protected amino acid (4 eq. relative to the upper capacity of Rink Amide MBHA resin), PyBOP (4 eq.), HOBt (4 eq.), DIPEA (8 eq.) were added in atmosphere of Ar and the mixture was stirred overnight. Then, the resin was filtered and washed with DMF*3 (10 ml/1 g, 2 min), and DCM*3 (10 ml/1 g, 2 min).
Deprotection of Fmoc. Peptide sequence on a Rink Amide MBHA resin (1 eq.) was washed with DCM (10 ml/1 g, 1 min) for resin swelling. The mixture was charged with 4-methylpiperidine in DMF (20%/80% V/V, 15 ml) and stirred for 15 min, then the resin was filtered and washed with DMF (10 ml/1 g, 1 min) - this series of operations was performed 3 times. After that, the resin was filtered and washed with DMF*3 (10 ml/1 g, 2 min) and DCM*3 (10 ml/1 g, 2 min).
Deprotection of Alloc. Peptide sequence on a Rink Amide MBHA resin (1 eq.) was washed with DCM (10 ml/1 g, 1 min) for resin swelling, then DCM (10 ml/1 g), PhSiH3 (8 eq.) were and the mixture was stirred under inert atmosphere of Ar for few min; then added Pd[P(Ph)3]4 (0.1 eq.) and the mixture was stirred under inert atmosphere of Ar for 60 min, then the resin was filtered and washed DCM*3 (10 ml/1 g, 2 min) DMF*3 (10 ml/1 g, 2 min), and DCM*3 (10 ml/1 g, 2 min).
Addition of the second and subsequent amino acid residues. To a peptide sequence on Rink Amide MBHA resin (1 eq.) in DMF (10 ml/1 g) Fmoc-protected amino acid (2 eq. relative to the upper capacity of Rink Amide MBHA resin), PyBOP (2 eq.), HOBt (0.5 eq.), DIPEA (4 eq.) were added under argon atmosphere and the mixture was stirred overnight. Then the resin was filtered and washed with DMF*3 (10 ml/1 g, 1 min) and DCM*3 (10 ml/1 g, 1 min).
Using Rink Amide MBHA resin (500 mg, 0.15-0.4 mmol), FmocLeu-OH (565 mg, 1.6 mmol), PyBOP (832 mg, 1.6 mmol), HOBt (216 mg, 1.6 mmol), DIPEA (557 μl, 3.2 mmol) to add the first amino acid, Fmoc(S,S)Sta-OH (198 mg, 0.5 mmol), PyBOP (260.2 mg, 0.5 mmol), HOBt (16.9 mg, 0.125 mmol), DIPEA (174 μl, 1.0 mmol) to add the second amino acid, FmocHis(Trt)-OH (372 mg, 0.6 mmol), PyBOP (312 mg, 0.6 mmol), HOBt (20.3 mg, 0.15 mmol), DIPEA (210 μl, 1.2 mmol) to add the third amino acid, FmocGlu-OH (238 mg, 0.8 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the fourth amino acid, FmocVal-OH (272 mg, 0.8 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the fifth amino acid, FmocAla-OH (249 mg, 0.8 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the sixth amino acid, FmocTrp(Boc)-OH (316 mg, 0.8 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the seventh amino acid, FmocGln(Trt)-OH (305 mg, 0.5 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the eighth amino acid, FmocPhe(D)-OH (233 mg, 0.6 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the ninth amino acid, the amino acid sequence NH2-F(D)QWAVGHStaL on Rink Amide MBHA resin 20 was obtained.
Using FmocAhx-OH (212 mg, 0.6 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the tenth amino acid, FmocLys(Alloc)-OH (226 mg, 0.5 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the eleventh amino acid, the amino acid sequence NH2-K(Alloc)AhxF(D)QWAVGHStaL on Rink Amide MBHA resin 21 was obtained.
Using FmocLys(Boc)-OH (234 mg, 0.5 mmol), PyBOP (416 mg, 0.8 mmol), HOBt (27 mg, 0.2 mmol), DIPEA (279 μl, 1.6 mmol) to add the eleventh amino acid, the amino acid sequence NH2-K(Boc)AhxF(D)QWAVGHStaL on Rink Amide MBHA resin 22 was obtained.
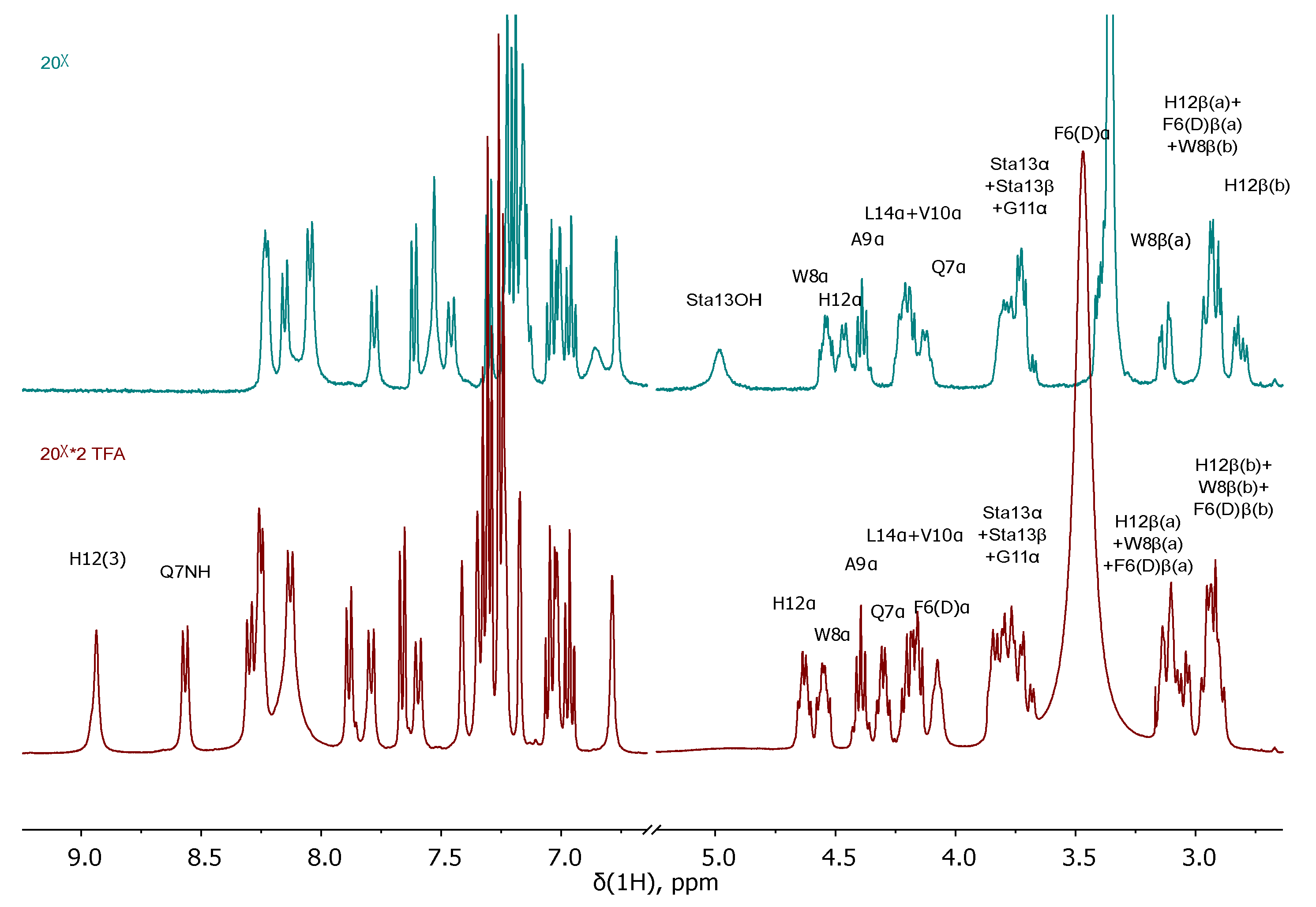
Compound 20x
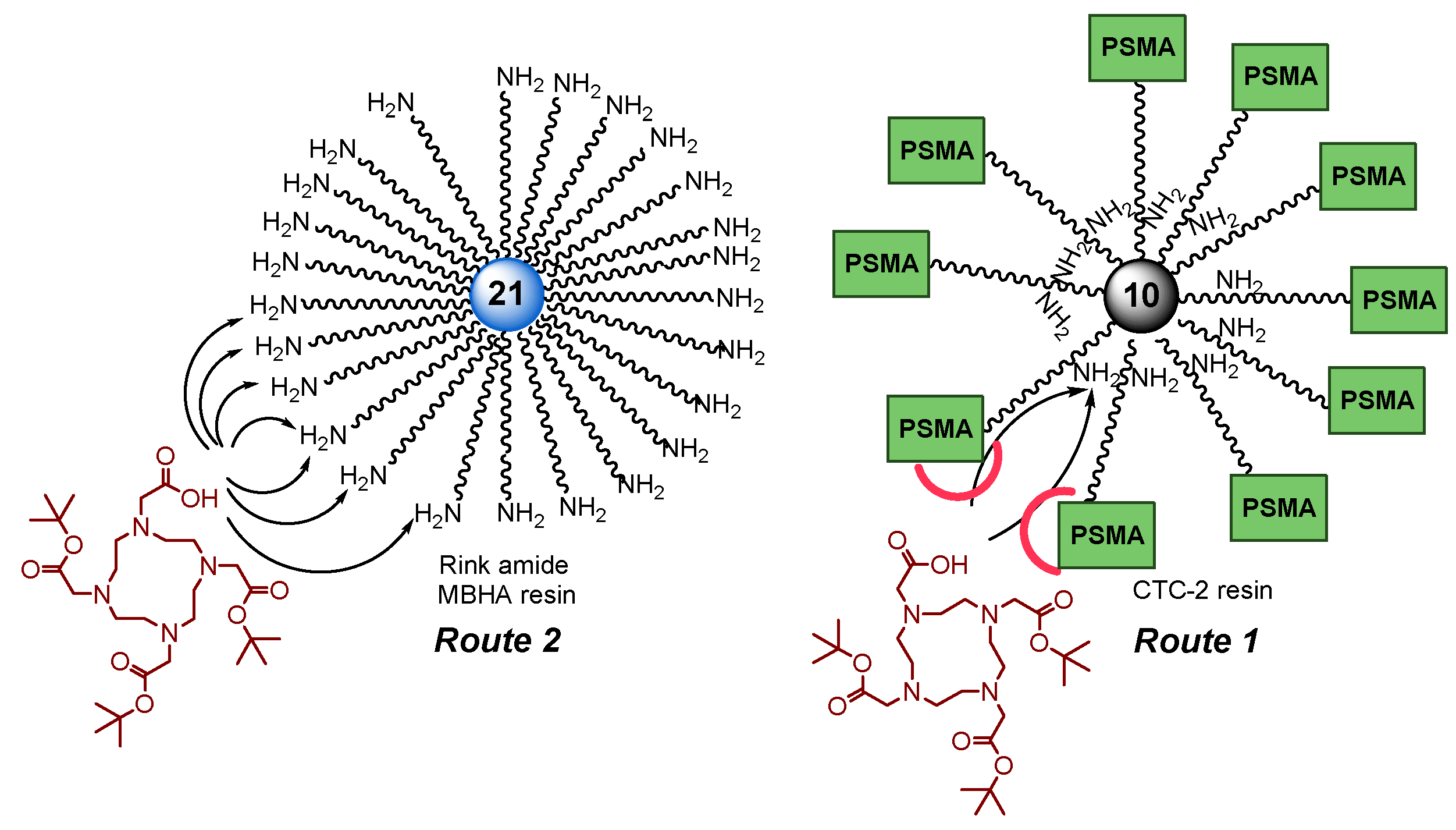
To establish the chemical purity and evaluate the actual capacity of Rink Amide MBHA resin coated with the peptide sequence NH2-F(D)QWAVGHStaL 20, removal of part of the peptide sequence from the polymer substrate was carried out.
Part of the resin was transferred to a 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 2 ml) and stirred for 1.5 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, crude product was precipitated by Et2O and purified by column chromatography (Puriflash PF-15C18HP-F0012 (15 μ 20 g), eluent: H2O(90%)/MeCN(10%) => H2O(40%)/MeCN(60%) for 40 min, after MeCN(100%) for 10 min). Compound 20x was obtained in several forms: *2TFA; free NH2 (23 mg, 58% yield from max resin capacity).
1H NMR (400 MHz, DMSO-d6, δ) for *2TFA form: 14.34 (br.s., 2H, H12(2)<=>(4) + H+), 10.85 (s, 1H, W8(3)), 8.95 (s, 1H, H12(3)), 8.57 (d, J = 8.0 Hz, 1H, Q7NH), 8.30 (d, J = 7.7 Hz, 1H, W8NH), 8.28-8.21 (m, 2H, G11NH + A9NH), 8.20-8.00 (m, 4H, F6(D)NH3+ + H12NH), 7.88 (d, J = 8.0 Hz, 1H, L14NH), 7.79 (d, J = 8.4 Hz, 1H, V10NH), 7.66 (d, J = 7.8 Hz, 1H, W8(8)), 7.60 (d, J = 8.9 Hz, 1H, Sta13NH), 7.41 (s, 1H, L14NH2(a)), 7.35 (s, 1H, H12(5)), 7.34-7.28 (m, 3H, W8(5) + F6(D)ε), 7.28-7.20 (m, 4H, F6(D)δ + F6(D)ζ + Q7NH2(a)), 7.17 (d, J = 2.1 Hz, 1H, W8(2)), 7.08-7.00 (m, 2H, W8(6) + L14NH2(b)), 7.00-6.93 (m, 1H, W8(7)), 6.79 (s, 1H, Q7NH2(b)), 4.94 (br.s., 1H, Sta13OH), 4.67-4.59 (m, 1H, H12α), 4.59-4.50 (m, 1H, W8α), 4.45-4.35 (m, 1H, A9α), 4.35-4.25 (m, 1H, Q7α), 4.25-4.12 (m, 2H, L14α + V10α), 4.12-4.02 (m, 1H, F6(D)α), 3.89-3.65 (m, 4H, Sta13α + Sta13β + G11α), 3.16-3.00 (m, 3H, H12β(a) + W8β(a) + F6(D)β(a)), 3.00-2.85 (m, 3H, H12β(b) + W8β(b) + F6(D)β(b)), 2.24-2.04 (m, 2H, Sta13γ), 2.03-1.87 (m, 3H, V10β + Q7γ), 1.84-1.69 (m, 1H, Q7β(a)), 1.68-1.55 (m, 2H, Q7β(b) + L14γ), 1.54-1.40 (m, 3H, Sta13ε + L14β), 1.40-1.22 (m, 2H, Sta13δ(a) + Sta13δ(b)), 1.20 (d, J = 7.0 Hz, 3H, A9β), 0.91-0.76 (m, 18H, L14δ(a) + Sta13ζ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(b)).
1H NMR (400 MHz, DMSO-d6, δ) for free NH2 form: 11.86 (br.s., 1H, H12(2)<=>(4)), 10.83 (s, 1H, W8(3)), 8.31-7.90 (m, 6H, G11NH + A9NH + W8NH + H12(3) + Q7NH + H12NH), 7.78 (d, J = 8.5 Hz, 1H, V10NH), 7.61 (d, J = 7.8 Hz, 1H, W8(8)), 7.59-7.36 (m, 3H, L14NH2(a) + L14NH + Sta13NH), 7.30 (d, J = 7.8 Hz, 1H, W8(5)), 7.26-7.10 (m, 7H, F6(D)δ + Q7NH2(a) + F6(D)ε + W8(2) + F6(D)ζ), 7.08-6.93 (m, 3H, W8(6) + L14NH2(b) + W8(7)), 6.86 (br.s., 1H, H12(5)), 6.77 (s, 1H, Q7NH2(b)), 4.98 (br.s., 1H, Sta13OH), 4.58-4.50 (m, 1H, W8α), 4.50-4.43 (m, 1H, H12α), 4.43-4.34 (m, 1H, A9α), 4.26-4.16 (m, 2H, L14α + V10α), 4.16-4.06 (m, 1H, Q7α), 3.86-3.64 (m, 4H, Sta13α + Sta13β + G11α), 3.43-3.36 (m, 1H, F6(D)α), 3.18-3.07 (m, 1H, W8β(a)), 3.00-2.86 (m, 3H, H12β(a) + F6(D)β(a) + W8β(b)), 2.86-2.76 (m, 1H, H12β(b)), 2.54-2.45 (m, 1H, F6(D)β(b)), 2.25-1.92 (m, 5H, Sta13γ + V10β + Q7γ), 1.87-1.76 (m, 1H, Q7β(a)), 1.72-1.55 (m, 2H, Q7β(b) + L14γ), 1.51-1.27 (m, 4H, Sta13ε + L14β + Sta13δ(a)), 1.26-1.12 (m, 4H, A9β + Sta13δ(b)), 0.90-0.73 (m, 18H, L14δ(a) + Sta13ζ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(b)).
13C NMR (101 MHz, DMSO-d6, δ) for *2TFA form: 174.70 (L14C), 173.74 (Q7δ) 172.29 (A9C), 171.42 (W8C + V10C), 170.91 (Sta13C), 170.77 (Q7C), 169.66 (H12C), 168.82 (G11C), 167.82 (F6(D)C), 158.95 (TFA), 158.64 (TFA), 158.32 (TFA), 158.01 (TFA), 136.13 (W8(4)), 134.96 (F6(D)γ), 133.79 (H12(3)), 129.56 (F6(D)δ), 129.37 (H12(1)), 128.56 (F6(D)ε), 127.24 (F6(D)ζ), 127.22 (W8(9)), 124.04 (W8(2)), 121.67 (TFA), 120.94 (W8(6)), 118.70 (TFA), 118.65 (W8(8)), 118.27 (W8(7)), 116.95 (H12(5)), 115.72 (TFA), 112.75 (TFA), 111.37 (W8(5)), 109.89 (W8(1)), 69.04 (Sta13β), 57.78 (V10α), 53.36 (F6(D)α + W8α), 52.00 (Q7α), 51.75 (H12α), 51.05 (L14α), 50.87 (Sta13α), 48.28 (A9α), 41.99 (G11α), 40.86 (L14β), 39.60 (Sta13γ), 39.16 (Sta13δ), 37.31 (F6(D)β), 31.18 (Q7γ), 30.64 (V10β), 28.72 (Q7β), 27.72 (W8β), 27.45 (H12β), 24.27 (L14γ + Sta13ε), 23.32 (Sta13ζ(a)), 23.14 (L14δ(a)), 21.95 (Sta13ζ(b)), 21.56 (L14δ(b)), 19.23 (V10γ(a)), 18.03 (V10γ(b)), 17.76 (A9β).
15N NMR (41 MHz, DMSO-d6, δ) for *2TFA form: 180.27 (H12N4), 177.08 (H12N2), 131.30 (W8N3), 124.72 (L14N), 121.69 (A9N), 121.57 (Q7N), 120.25 (W8N), 120.06 (Sta13N), 115.48 (H12N), 113.99 (V10N), 108.74 (Q7Nε), 108.32 (G11N), 104.81 (L14NH2).
LCMS 100% in positive ion mode
ESI-MS (m/z) C55H80N14O11: calc. for [M+2H+]2+: 557.31, found: 557.75
HRMS (m/z, ESI): calc. for C55H80N14O11 - [M+2H] 2+ 557.3138, found: 557.315
Compound 22x
To establish the chemical purity and evaluate the actual capacity of Rink Amide MBHA resin coated with the peptide sequence NH2-KAhxF(D)QWAVGHStaL 22, removal of part of the peptide sequence from the polymer substrate was carried out.
Part of the resin was transferred to a 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 5 ml) and stirred for 1.5 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, crude product was precipitated by Et2O and purified by column chromatography (Puriflash PF-15C18HP-F0012 (15 μ 20 g), eluent: H2O(90%)/MeCN(10%) => H2O(40%)/MeCN(60%) for 40 min, after MeCN(100%) for 10 min). Compound 22x was obtained as *3TFA form (32.3 mg, 58% yield from max resin capacity).
1H NMR (400 MHz, DMSO-d6, δ) for *3TFA form: 14.16 (br.s., 2H, H12(2) + (4) + H+), 10.80 (s, 1H, W8(3)), 8.91 (s, 1H, H12(3)), 8.40 (t, J = 5.6 Hz, 1H, Ahx10NHζ), 8.36 (d, J = 7.2 Hz, 1H, F6(D)NH), 8.25 (t, J = 5.7 Hz, 1H, G11NH), 8.22-8.00 (m, 6H, W8NH + Q7NH + H12NH + K9NH3+), 7.98 (d, J = 7.3 Hz, 1H, A9NH), 7.88 (d, J = 8.0 Hz, 1H, L14NH), 7.79 (br.s., 3H, K9NH3+), 7.64 (d, J = 8.4 Hz, 1H, V10NH), 7.61-7.54 (m, 2H, W8(8) + Sta13NH), 7.41 (s, 1H, L14NH2(a)), 7.34 (s, 1H, H12(5)), 7.31 (d, J = 7.8 Hz, 1H, W8(5)), 7.28-7.20 (m, 4H, F6(D)ε + F6(D)ζ + Q7NH2(a)), 7.20-7.12 (m, 2H, F6(D)δ + W8(2)), 7.08-7.00 (m, 2H, W8(6) + L14NH2(b)), 7.00-6.92 (m, 1H, W8(7)), 6.78 (s, 1H, Q7NH2(b)), 4.96 (br.s., 1H, Sta13OH), 4.67-4.59 (m, 1H, H12α), 4.52-4.41 (m, 2H, F6(D)α + W8α), 4.40-4.30 (m, 1H, A9α), 4.23-4.05 (m, 3H, Q7α + L14α + V10α), 3.89-3.62 (m, 5H, K9α + Sta13α + Sta13β + G11α), 3.20-2.87 (m, 7H, H12β(a) + K9ε + W8β(a) + F6(D)β(a) + H12β(b) + W8β(b)), 2.81-2.68 (m, 3H, Ahx10ε + F6(D)β(b)), 2.20-2.03 (m, 4H, Sta13γ + Ahx10α), 2.03-1.87 (m, 3H, V10β + Q7γ), 1.85-1.72 (m, 1H, Q7β(a)), 1.71-1.63 (m, 2H, Q7β(b) + L14γ), 1.63-1.23 (m, 15H, K9β(а) + K9δ + Sta13ε + K9β(b) + Ahx10β + L14β + Ahx10δ + Sta13δ(a) + K9γ + Sta13δ(b)), 1.20 (d, J = 7.0 Hz, 3H, A9β), 1.16-1.05 (m, 2H, Ahx10γ), 0.91-0.76 (m, 18H, L14δ(a) + Sta13ζ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(b)).
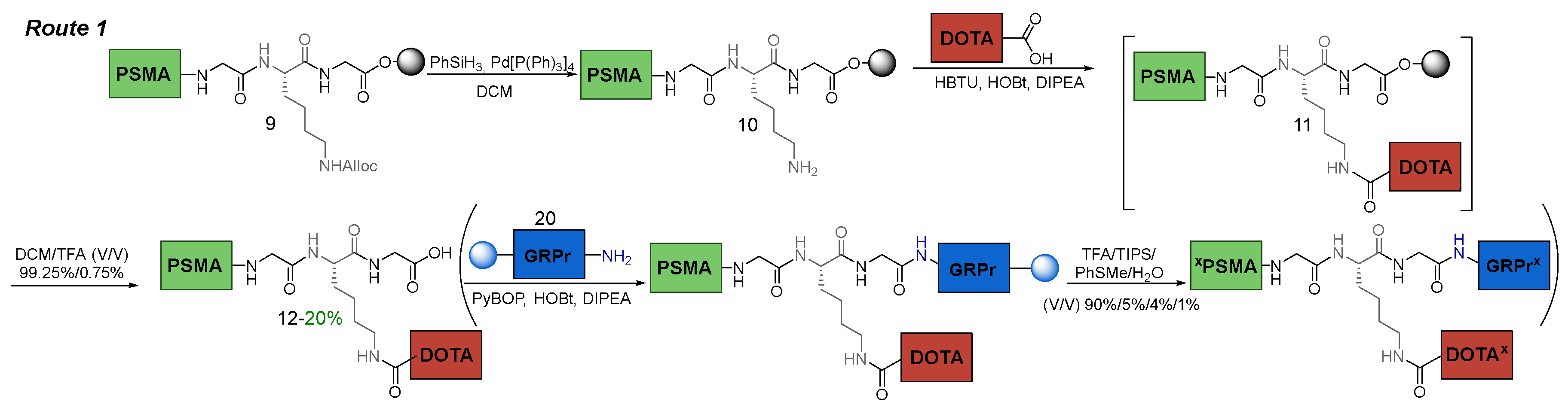
Route 1
Compound 12
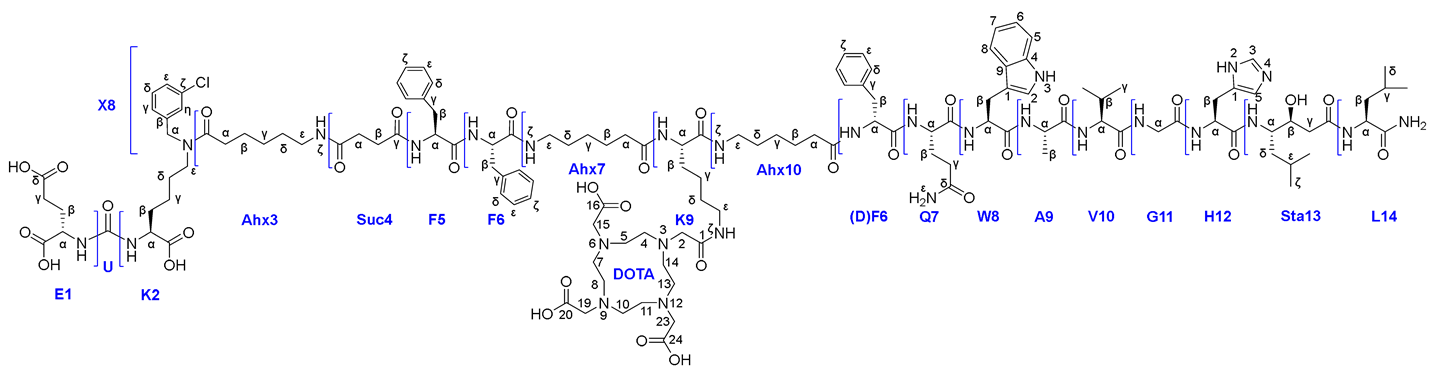
To a mixture of Alloc-deprotected compound 9 on 2-CTC resin (1 eq.; 0.15 mmol) in DMF (10 ml) in reactor HO-DOTA(tBu)3 (1 eq.; 86 mg; 0.15 mmol), HOBt (0.5 eq.; 10 mg; 0.075 mmol), HBTU (2 eq.; 114 mg; 0.3 mmol), DIPEA (3 eq.; 78 μl; 0.45 mmol) were added. The mixture was stirred 24 h under inert atmosphere of Ar. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (10 ml), three times with DCM (10 ml), then dried from traces of solvents.
A DCM/TFA system (99%-1%, 10 ml) was added to the resin and left under stirring for 15 min, after that, the solution was filtered from the resin. The solvent was removed under reduced pressure and the residue was re-evaporated with DCM. Next, the residue was dissolved in DCM (20 ml) and washed with saturated NaHCO3 solution (2*20 ml). The organic fraction was dried over Na2SO4. Afterwards, the solvent was removed under reduced pressure. The crude residue was purified by column chromatography (Puriflash PF-15C18HP-F0012 + PF-15C18HP-F0012 (15 μ 20 g + 15 μ 20 g), eluent: H2O(90%)/MeCN(10%) => H2O(0%)/MeCN(100%) for 30 min, after MeCN(100%) for 5 min). Compound 12 was obtained as a white amorphous solid (58 mg, 20% yield).
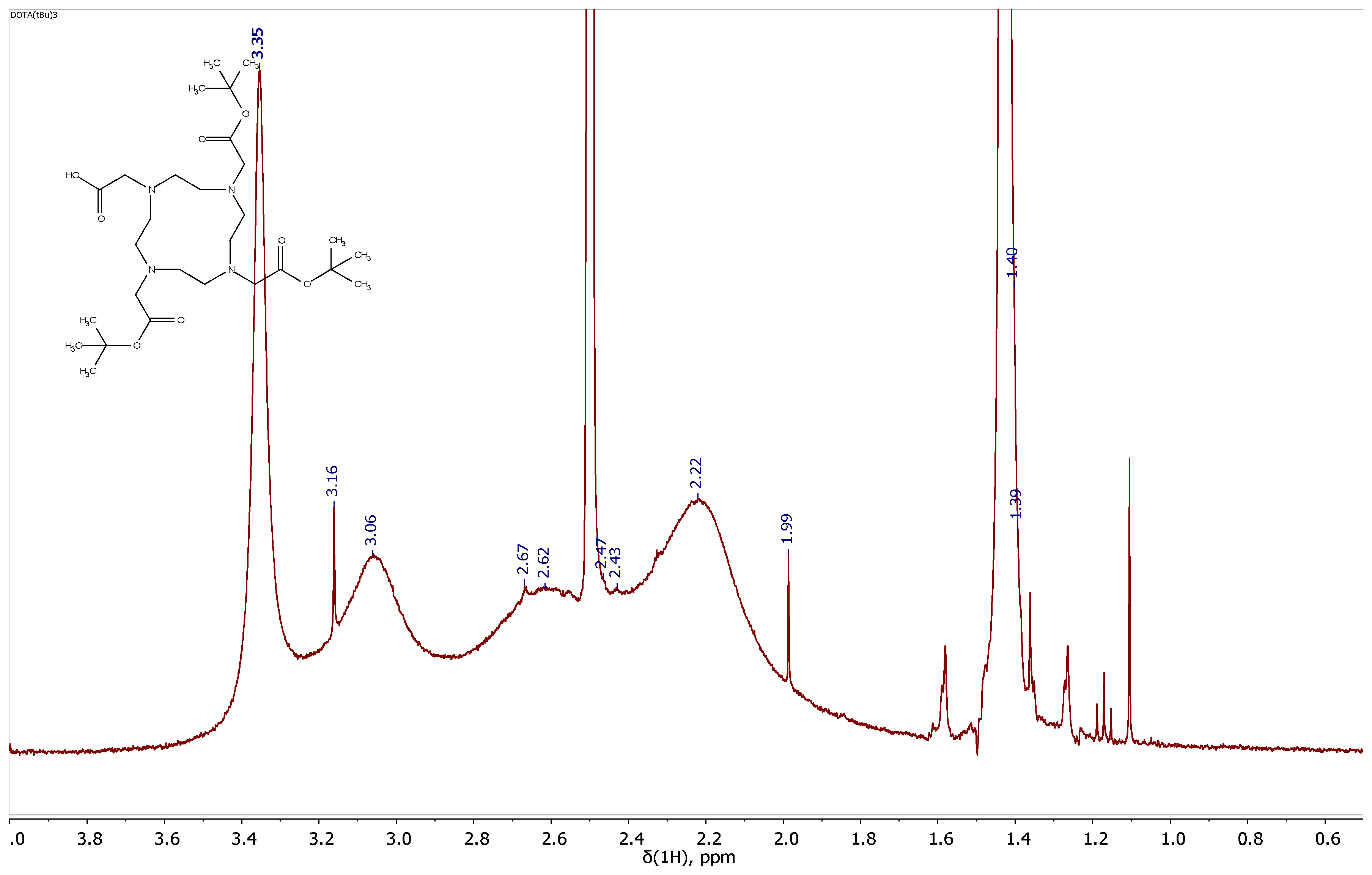
1H NMR (400 MHz, DMSO-d6, δ): 8.32-8.22 (m, 1H, G10NH), 8.21-8.14 (m, 2Н, F5NHmn + F6NH), 8.12-8.02 (m, 1H, G7NH), 7.92 (d, J = 8.0 Hz, 1H, K9NH), 7.89-7.79 (m, 1H, Ahx3NHζ, mn), 7.42-7.09 (m, 15H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.19 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.60-4.42 (m, 3H, X8αnm + F6α), 4.42-4.34 (m, 1H, F5Hα), 4.33-4.20 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.58 (m, 4Н, G7α + G9α), 3.80-1.60 (br.m., 24H, DOTA), 3.25-2.81 (m, 9Н, K2εnm + F6β(a) + K9ε + F6β(b) + Ahx3ε + F5β(a)), 2.70-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 19Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.47 (s, 9H, 22), 1.40-1.35 (m, 45H, 18 + 26 + tBu).
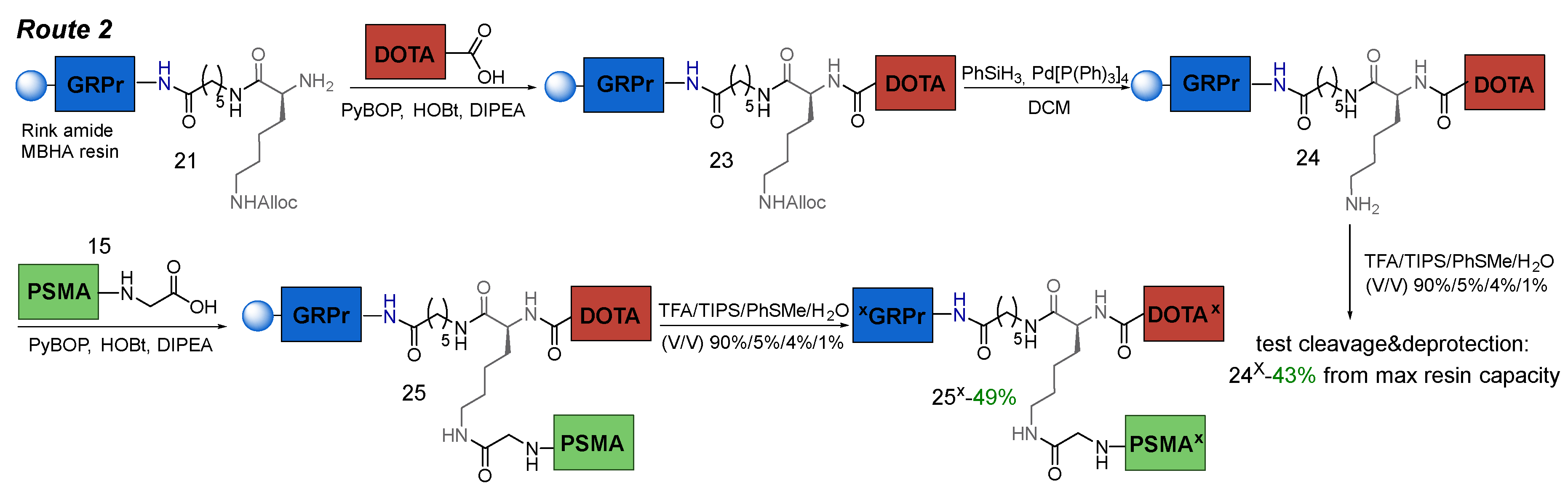
Route 2
Compound 24x
To a mixture of compound 21 on Rink Amide MBHA resin (1 eq.; 0.153 mmol = 58% from max capacity) in DMF (10 ml) in reactor HO-DOTA(tBu)3 (1.5 eq.; 131 mg; 0.228 mmol), HOBt (0.5 eq.; 10 mg; 0.076 mmol), PyBOP (2.45 eq.; 194 mg; 0.3735 mmol), DIPEA (5 eq.; 133 μl; 0.765 mmol) were added. The mixture was stirred for 24 h under inert atmosphere of Ar. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (10 ml), three times with DCM (10 ml), then dried from traces of solvents. Afterwards, Alloc was removed according to the standard protocol.
To establish the chemical purity and evaluate the actual capacity of Rink Amide MBHA resin coated with a peptide sequence DOTA-K(NH2)AhxF(D)QWAVGHStaL 24, removal of part of the peptide sequence from the polymer substrate was carried out.
Part of the resin was transferred to a 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 5 ml) and stirred for 3 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, crude product was precipitated by Et2O and purified by column chromatography (Puriflash PF-15C18HP-F0012 (15 μ 20 g), eluent: H2O(95%)/MeCN(5%) => H2O(50%)/MeCN(50%) for 40 min, after MeCN(100%) for 10 min). Compound 22x was obtained as *3TFA form (18.2 mg, 22% of total capacity) and target compound 24x (70 mg, 78% of total capacity), where total capacity = 55% of max resin capacity.
1H NMR (400 MHz, DMSO-d6, δ): 10.86 (s, 1H, W8(3)), 8.63-8.43 (m, 3H, Q7NH + G11NH + F6(D)NH), 8.42-8.30 (m, 2H, W8NH + H12NH), 8.20-8.10 (m, 2H, L14NH + A9NH), 8.10-7.97 (m, 2H, Ahx10NHζ + K9NH), 7.90-7.76 (m, 1H, V10NH), 7.61-7.47 (m, 4H, W8(8) + H12(3) + L14NH2(a) + Sta13NH), 7.30 (d, J = 7.8 Hz, 1H, W8(5)), 7.27-7.12 (m, 7H, F6(D)ε + F6(D)ζ + Q7NH2(a) + F6(D)δ + W8(2)), 7.08-6.92 (m, 3H, W8(6) + L14NH2(b) + W8(7)), 6.83 (br.s., 1H, H12(5)), 6.76 (s, 1H, Q7NH2(b)), 5.10 (br.s., 1H, Sta13OH), 4.55-4.30 (m, 4H, W8α + H12α + F6(D)α + A9α), 4.22-4.04 (m, 4H, K9α + V10α + L14α + Q7α), 3.85-3.62 (m, 4H, Sta13α + Sta13β + G11α), 3.80-1.60 (br.m., 24H, DOTA), 3.20-2.87 (m, 10H, H12β(a) + K9ε + W8β(a) + F6(D)β(a) + H12β(b) + W8β(b) + Ahx10ε + F6(D)β(b)), 2.20-2.03 (m, 4H, Sta13γ + Ahx10α), 2.03-1.86 (m, 3H, V10β + Q7γ), 1.85-1.71 (m, 2H, Q7β(a) + K9β(a)), 1.70-1.52 (m, 2H, Q7β(b) + L14γ + K9δ), 1.52-1.13 (m, 11H, K9β(b) + L14β + Sta13ε + Ahx10β + Sta13δ(a) + Ahx10δ + K9γ), 1.26-1.15 (m, 4H, A9β + Sta13δ(b)), 1.14-1.02 (m, 2H, Ahx10γ), 0.91-0.73 (m, 18H, L14δ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(a) + Sta13ζ(b)).
LCMS 92% in negative ion mode
ESI-MS (m/z) C83H129N21O20: calc. for [M-2H+]2-: 868.97, found: 868.96
Compound 25X
To a mixture of compound 24 on Rink Amide MBHA resin (1 eq.; 97 μmol) in DMF (7 ml) in reactor under Ar atmosphere compound 15 (1 eq.; 114 mg; 97 μmol), HOBt (0.5 eq.; 6.5 mg; 48.5 μmol), PyBOP (3 eq.; 152 mg; 291 μmol), DIPEA (4.5 eq.; 76 μl; 436 μmol) were added. The mixture was stirred for 24 h under inert atmosphere of Ar. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (5 ml), three times with DCM (5 ml), then dried from traces of solvents.
Part of the resin was transferred to a 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 5 ml) and stirred for 3 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, crude product was precipitated by Et2O. Compound 25X (49% according to LCMS) was obtained in mixture with compound 24x (37% according to LCMS) and other impurities.
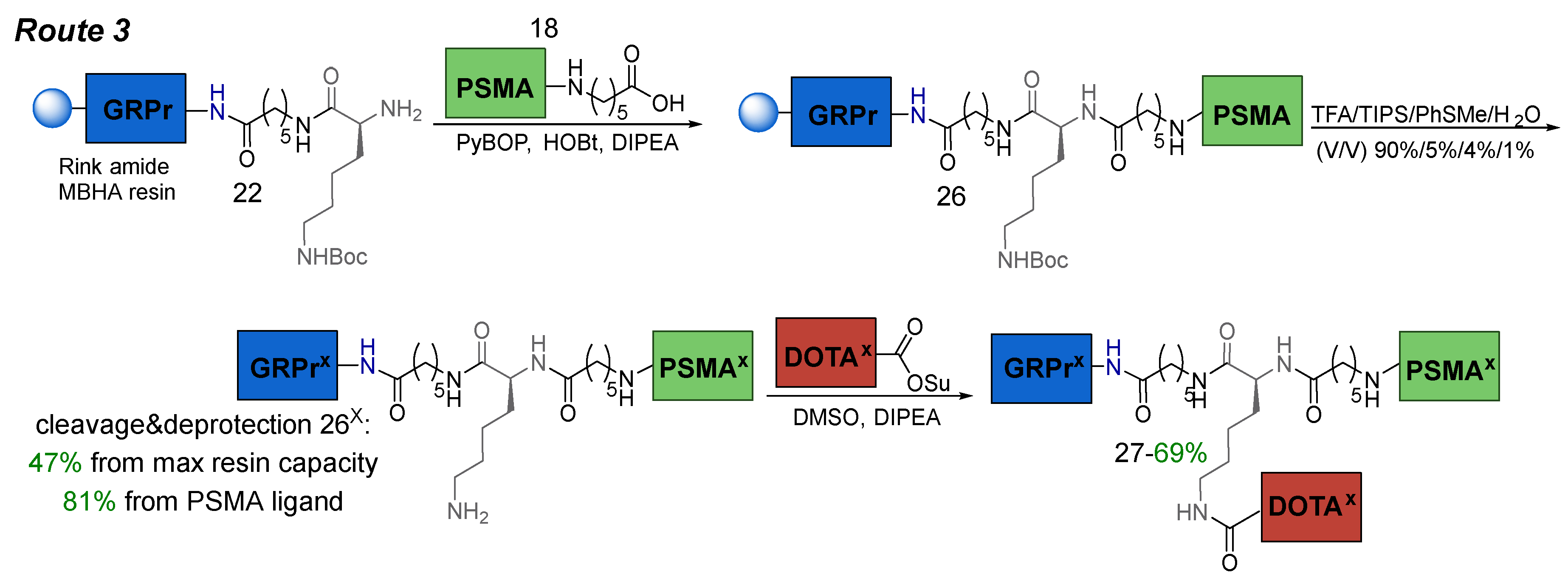
Route 3
Compound 26x
To compound 22 on Rink Amide MBHA resin (1 eq.; 57 μmol) in DMF (5 ml) in reactor under Ar atmosphere compound 18 (1 eq.; 70 mg; 57 μmol), HOBt (1 eq.; 7.7 mg; 57 μmol), PyBOP (3 eq.; 65 mg; 171 μmol), DIPEA (6 eq.; 59 μl; 342 μmol) were added. The mixture was stirred for 24 h under inert atmosphere of Ar. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (5 ml), three times with DCM (5 ml), then dried from traces of solvents.
Part of the resin was transferred to 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 5 ml) and stirred for 3 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, crude product was precipitated by Et2O and purified by column chromatography (Puriflash PF-15C18HP-F0040 (15 μ 60 g), eluent: H2O(90%)/MeCN(10%) => H2O(50%)/MeCN(50%) for 40 min, after MeCN(100%) for 10 min) Compound 26x was obtained as free NH2 form (110 mg, 47% of max resin capacity, 81% of PSMA fragment)
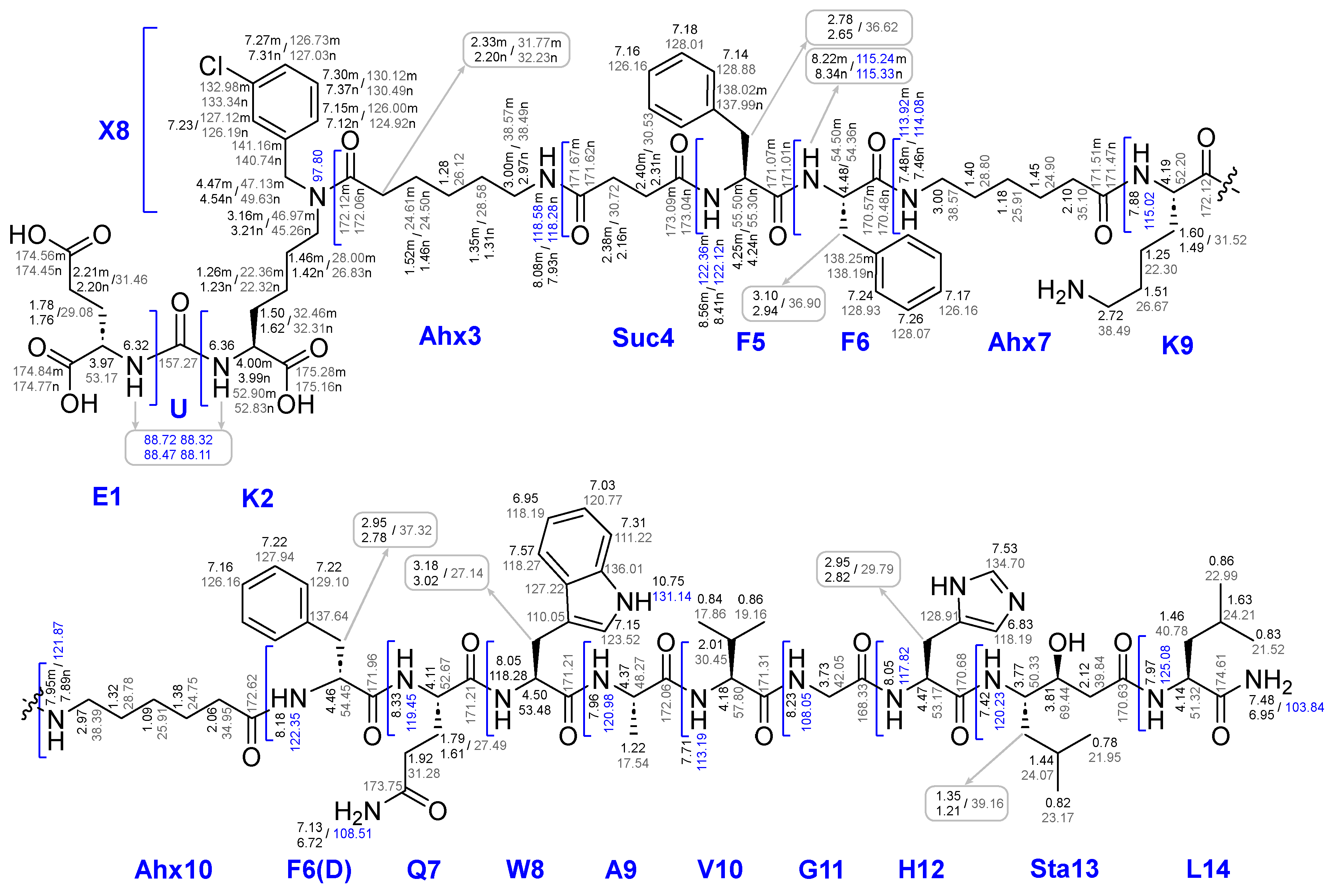
1H NMR (400 MHz, DMSO-d6, δ) for free NH2 form: 10.75 (s, 1H, W8(3)), 8.56 (d, J = 7.2 Hz, m) & 8.41 (d, J = 7.2 Hz, n) (1H, F5NH, m + n), 8.38-8.13 (m, 4H, F6NHm + Q7NH + G11NH + F6NHn + F6(D)NH), 8.13-7.80 (m, 7H, Ahx3NHζm + H12NH + W8NH + Ahx3NHζn + L14NH + A9NH + Ahx10NHζm + Ahx10NHζn + K9NH), 7.71 (d, J = 8.5 Hz, 1H, V10NH), 7.57 (d, J = 7.8 Hz, 1H, W8(8)), 7.55-7.40 (m, 4H, H12(3) + L14NH2(a) + Ahx7NHζm + Ahx7NHζn + Sta13NH), 7.40-7.07 (m, 22H, X8Hδn + X8Hεn + W8(5) + X8Hδm + X8Hεm + F6Hε + F6Hδ + X8Hηmn + F6(D)δ + F6(D)ε + F5Hε + F6Hζ + F6(D)ζ + F5Hζ + W8(2) + X8Hγm + F5Hδ + Q7NH2(a) + X8Hγn), 7.03 (t, J = 7.5 Hz, 1H, W8(6)), 7.00-6.89 (m, 2H, L14NH2(b) + W8(7)), 6.83 (br.s., 1H, H12(5)), 6.72 (s, 1H, Q7NH2(b)), 6.48-6.17 (m, 2Н, K2NHmn + E1NHmn), 4.60-4.30 (m, 7H, X8αn + W8α + H12α + X8αm + F6(D)α + F6α + A9α), 4.29-4.06 (m, 5H, F5αmn + K9α + V10α + L14α + Q7α), 4.05-3.90 (m, 2H, E1Hα + K2Hαm + K2Hαn), 3.86-3.64 (m, 4H, Sta13β + Sta13α + G11α), 3.26-2.59 (m, 20H, K2εn + W8β(a) + K2εm + F6β(a) + Ahx7ε + W8β(b) + Ahx3εm + Ahx10ε + Ahx3εn + F6(D)β(a) + F6β(b) + H12β(a) + F5β(a) + H12β(b) + F6(D)β(b) + K9ε + F5β(b)), 2.46-1.95 (m, 15H, Suc4βm + Suc4αm + Ahx3αm + Suc4βn + E1γ + Ahx3αn + Suc4αn + Sta13γ + Ahx7α + Ahx10α + V10β), 1.95-1.86 (m, 2H, Q7γ), 1.86-1.70 (m, 3H, E1β(a) + Q7β(a) + E1β(b)), 1.70-1.56 (m, 4H, L14γ + K2β(a) + Q7β(b) + K9β(a)), 1.56-1.13 (m, 34H, K9δ + Ahx3βm + K2β(b) + K9β(b) + Ahx3βn + K2δm + Ahx7β + L14β + Sta13ε + K2δn + Ahx7δ + Ahx10β + Ahx3δm + Sta13δ(a) + Ahx10δ + Ahx3δn + Ahx3γmn + K9γ + K2γmn + A9β + Sta13δ(b) + Ahx7γ), 1.13-1.04 (m, 2H, Ahx10γ), 0.90-0.75 (m, 18H, L14δ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(a) + Sta13ζ(b)).
13C NMR (101 MHz, DMSO-d6, δ) for free NH2 form: 175.28 (K2Cm), 175.16 (K2Cn), 174.84 (E1Cm), 174.77 (E1Cn), 174.61 (L14C), 174.56 (E1Cδm), 174.45 (E1Cδn), 173.75 (Q7Cδ), 173.09 (Suc4Cγm), 173.04 (Suc4Cγn), 172.62 (Ahx10C), 172.12 (K9Cmn + Ahx3Cm), 172.06 (A9C + Ahx3Cn), 171.96 (F6(D)C), 171.67 (Suc4Cm), 171.62 (Suc4Cn), 171.51 (Ahx7Cm), 171.47 (Ahx7Cn), 171.31 (V10C), 171.21 (W8C + Q7C), 171.07 (F5Cm), 171.01 (F5Cn), 170.68 (H12C), 170.63 (Sta13C), 170.57 (F6Cm), 170.48 (F6Cn), 168.33 (G11C), 157.27 (U), 141.16 (X8Cβm), 140.74 (X8Cβn), 138.25 (F6Cγm), 138.19 (F6Cγn), 138.02 (F5Cγm), 137.99 (F5Cγn), 137.64 (F6(D)Cγ), 136.01 (W8C4), 134.70 (H12C3), 133.34 (X8Cζn), 132.98 (X8Cζm), 130.49 (X8Cδn), 130.12 (X8Cδm), 129.10 (F6(D)Cδ), 128.93 (F6Cδ), 128.91 (H12C1), 128.88 (F5Cδ), 128.07 (F6Cε), 128.01 (F5Cε), 127.94 (F6(D)Cε), 127.22 (W8C9), 127.12 (X8Cηm), 127.03 (X8Cεn), 126.73 (X8Cεm), 126.19 (X8Cηn + F5Cζ), 126.16 (F6Cζ + F6(D)Cζ), 126.00 (X8Cγm), 124.92 (X8Cγn), 123.52 (W8C2), 120.77 (W8C6), 118.27 (W8C8), 118.19 (W8C7 + H12C5), 111.22 (W8C5), 110.05 (W8C1), 69.44 (Sta13Cβ), 57.80 (V10Cα), 55.50 (F5Cαm), 55.30 (F5Cαn), 54.50 (F6Cαm), 54.45 (F6(D)Cα), 54.36 (F6Cαn), 53.48 (W8Cα), 53.17 (E1Cαm + H12Cα), 53.07 (E1Cαn), 52.90 K2Cαm), 52.83 (K2Cαn), 52.67 (Q7Cα), 52.20 (K9Cα), 51.32 (L14Cα), 50.33 (Sta13Cα), 49.63 (X8Cαn), 48.27 (A9Cα), 47.13 (X8Cαm), 46.97 (K2Cεm), 45.26 (K2Cεn), 42.05 (G11Cα), 40.78 (L14Cβ), 38.57 (Ahx3Cεm + Ahx7Cε), 38.49 (Ahx3Cεn + K9Hε), 38.39 (Ahx10Cε), 37.32 (F6(D)Cβ), 36.90 (F6Cβ), 36.62 (F5Cβ), 35.09 (Ahx7Cα), 34.95 (Ahx10Cα), 32.46 (K2Cβm), 32.31 (K2Cβn), 32.23 (Ahx3Cαn), 31.77 (Ahx3Cαm), 31.52 (K9Cβ), 31.46 (E1Cγ), 31.28 (Q7Cγ), 30.72 (Suc4Cα), 30.53 (Suc4Cβ), 30.45 (V10Cβ), 29.79 (H12Cβ), 29.08 (E1Cβ), 28.80 (Ahx7Cδ), 28.78 (Ahx10Cδ), 28.58 (Ahx3Cδ), 28.00 (K2Cδm), 27.49 (Q7Cβ), 27.14 (W8Cβ), 26.83 (K2Cδn), 26.67 (K9Cδ), 26.12 (Ahx3Cγ), 25.91 (Ahx7Cγ + Ahx10Cγ), 24.90 (Ahx7Cβ), 24.75 (Ahx10Cβ), 24.61 (Ahx3Cβm), 24.50 (AhxCβn), 24.21 (L14Cγ), 24.07 (Sta13Cε), 23.17 (Sta13Cζ(a)), 22.99 (L14Cγ(a)), 22.36 (K2Cγm), 22.32 (K2Cγn), 22.30 (K9Cγ), 21.95 (Sta13Cζ(b)), 21.52 (L14Cγ(b)), 19.16 (V10Cγ(a)), 17.86 (V10Cγ(b)), 17.54 (A9Cβ).
15N NMR (41 MHz, DMSO-d6, δ) for free NH2 form: 131.14 (W8N3), 125.08 (L14N), 122.36 (F5Nm + F6(D)N), 122.12 (F5Nn), 121.87 (Ahx10Nζmn), 120.98 (A9N), 120.23 (Sta13N), 119.45 (Q7N), 118.58 (Ahx3Nζm), 118.28 (Ahx3Nζn + W8N), 117.82 (H12N), 115.24 (F6Nnm), 115.02 (K9N), 114.08 (Ahx7Nζn), 113.92 (Ahx7Nζm), 113.19 (V10N), 108.51 (Q7Nε), 108.05 (G11N), 103.84 (L14NH2), 97.80 (K2Nε), 88.72 (K2Nm), 88.47 (K2Nn), 88.32 (E1Nm), 88.11 (E1Nn).
LCMS 97% in negative ion mode
ESI-MS (m/z) C120H17137ClN24O26: calc. for [M-2H+]2-: 1199.62, found: 1199.12.
HRMS (m/z, ESI): calc. for C120H17137ClN24O26 - [M-2H]2- 1199.6227, found: 1199.4351
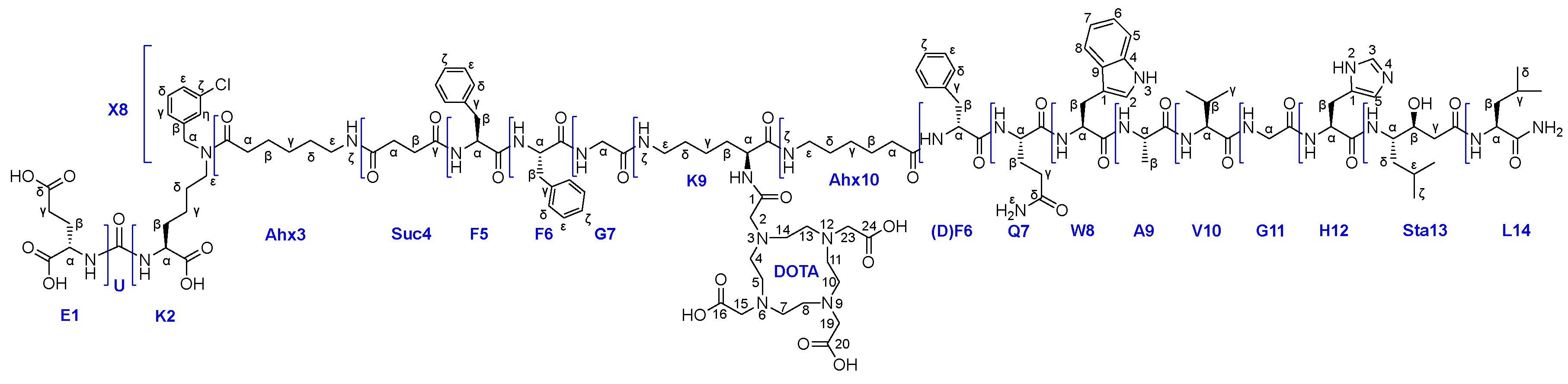
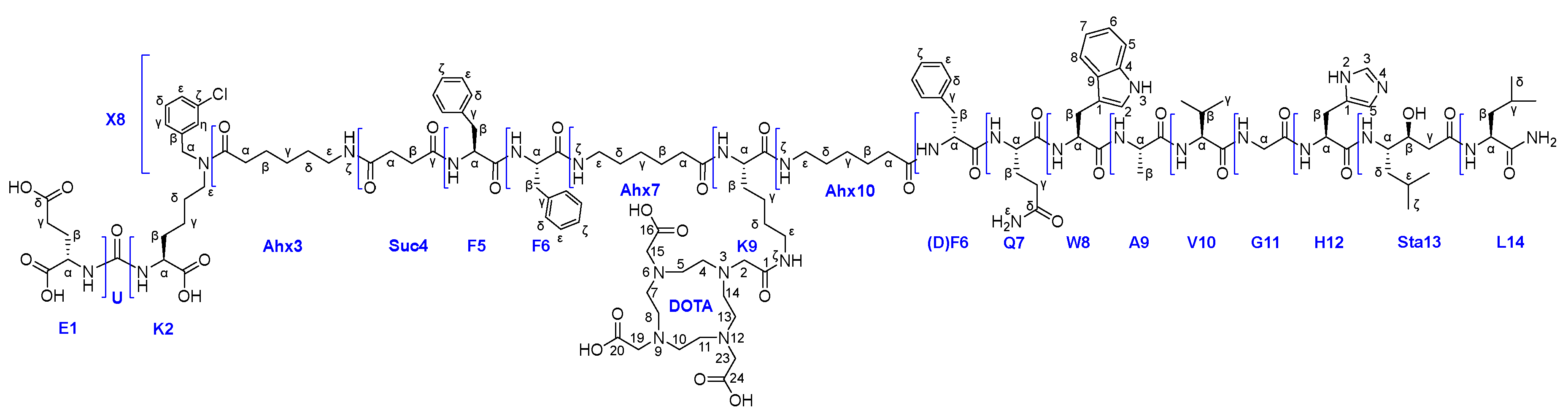
Compound 27
Compound 26x (1 eq.; 39 mg; 15.5 μmol) was dissolved in DMSO (4 ml) (CGC 20-25 mg/ml, complete dissolution was performed under ultrasound bath action for 10 min at 40°C) and purged with Ar. DIPEA (5 eq.; 10 mg; 77.5 μmol), DOTA-NHS ester (1 eq.; 12 mg; 15.5 μmol) were sequentially added. The mixture was stirred for 12 h, afterwards the solvent was removed under reduced pressure. Crude product was purified by column chromatography (PF-15C18HP-F0012 (15 μ 20 g), eluent: H2O(90%)/MeCN(10%) => H2O(50%)/MeCN(50%) for 30 min, after MeCN(100%) for 10 min). Compound 27 was obtained as a white powder (30 mg, 69% yield, 88% purity according to LCMS) in a mixture (m = 34.2 mg) with the initial HBV ligand 26x (10% according to LCMS).
1H NMR (400 MHz, DMSO-d6, δ): 10.80 (s, 1H, W8(3)), 8.58-7.78 (m, 13H, F5NHmn + F6NHm + Q7NH + G11NH + F6NHn + F6(D)NH + Ahx3NHζm + H12NH + K9NHε + W8NH + Ahx3NHζn + L14NH + A9NH + Ahx10NHζm + Ahx10NHζn + K9NH), 7.71 (d, J = 8.5 Hz, 1H, V10NH), 7.65-7.41 (m, 5H, W8(8) + H12(3) + L14NH2(a) + Ahx7NHζm + Ahx7NHζn + Sta13NH), 7.40-7.07 (m, 22H, X8δn + X8εn + W8(5) + X8δm + X8εm + F6ε + F6δ + X8ηmn + F6(D)δ + F6(D)ε + F5ε + F6ζ + F6(D)ζ + F5ζ + W8(2) + X8γm + F5δ + Q7NH2(a) + X8γn), 7.07-6.90 (m, 3H, W8(6) + L14NH2(b) + W8(7)), 6.86 (br.s., 1H, H12(5)), 6.77 (s, 1H, Q7NH2(b)), 6.48-6.17 (m, 2Н, K2NHmn + E1NHmn), 4.60-4.30 (m, 7H, X8αn + W8α + H12α + X8αm + F6(D)α + F6α + A9α), 4.29-3.96 (m, 7H, F5αmn + K9α + V10α + L14α + Q7α + E1α + K2αm + K2αn), 3.86-3.64 (m, 4H, Sta13β + Sta13α + G11α), 3.60-2.40 (br.m, 44H, 2 + 19 + 15 + 23 + Hcyclic + K2εn + W8β(a) + K2εm + F6β(a) + Hcyclic + Ahx7ε + W8β(b) + Ahx3εm + Ahx10ε + Ahx3εn + F6(D)β(a) + K9ε + F6β(b) + H12β(a) + F5β(a) + H12β(b) + F6(D)β(b) + F5β(b)), 2.40-1.95 (m, 15H, Suc4βm + Suc4αm + Ahx3αm + Suc4βn + E1γ + Ahx3αn + Suc4αn + Sta13γ + Ahx7α + Ahx10α + V10β), 1.95-1.83 (m, 3H, Q7γ + E1β(a)), 1.86-1.69 (m, 2H, Q7β(a) + E1β(b)), 1.69-1.55 (m, 4H, L14γ + K2β(a) + Q7β(b) + K9β(a)), 1.55-1.13 (m, 34H, K9δ + Ahx3βm + K2β(b) + K9β(b) + Ahx3βn + K2δm + Ahx7β + L14β + Sta13ε + K2δn + Ahx7δ + Ahx10β + Ahx3δm + Sta13δ(a) + Ahx10δ + Ahx3δn + Ahx3γmn + K9γ + K2γmn + A9β + Sta13δ(b) + Ahx7γ), 1.11-1.01 (m, 2H, Ahx10γ), 0.90-0.75 (m, 18H, L14δ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(a) + Sta13ζ(b)).
13C NMR (101 MHz, DMSO-d6, δ): 174.70 (L14C), 174.54 (K2Cn), 174.52 (K2Cm), 174.22 (E1C), 173.81 (E1Cδ + Q7Cδ), 172.72 (Suc4Cγnm + Ahx10C), 172.15 (Ahx3Cnm + A9C), 172.06 (F6(D)C), 171.79 (K9C), 171.56 (Suc4Cnm + Ahx7C), 171.36 (V10C), 171.30 (W8C), 171.28 (Q7C), 171.03 (F5C), 170.70 (Sta13C), 170.64 (H12C), 170.48 (F6C), 170.13 (DOTA), 169.72 (DOTA), 168.39 (G11C), 157.28 (U), 141.21 (X8Cβm), 140.80 (X8Cβn), 138.12 (F6Cγ), 138.01 (F5Cγ), 137.67 (F6(D)Cγ), 136.05 (W8C4), 134.68 (H12C3), 133.39 (X8Cζn), 133.04 (X8Cζm), 130.59 (X8Cδn), 130.22 (X8Cδm), 129.17 (F6(D)Cδ), 129.02 (F6Cδ + H12C1 + F5Cδ), 128.13 (F6Cε), 128.05 (F5Cε), 128.02 (F6(D)Cε), 127.25 (W8C9), 127.18 (X8Cηm), 127.12 (X8Cεn), 126.82 (X8Cεm), 126.28 (X8Cηn + F5Cζ), 126.25 (F6Cζ + F6(D)Cζ), 126.06 (X8Cγm), 124.95 (X8Cγn), 123.58 (W8C2), 120.85 (W8C6), 118.33 (W8C8), 118.26 (W8C7 + H12C5), 111.30 (W8C5), 110.11 (W8C1), 69.42 (Sta13Cβ), 58.25 (DOTA2), 57.79 (V10Cα), 55.28 (DOTA19), 54.95 (F5Cα), 54.90 (DOTA15+23), 54.54 (F6(D)Cα), 54.32 (F6C), 53.50 (W8Cα), 53.06 (H12Cα), 52.70 (Q7Cα), 52.61 (K9Cα), 52.30 (K2Cαn), 52.20 (K2Cαm), 51.76 (E1Cα), 51.32 (L14Cα), 50.97 (DOTAcycl.), 50.37 (Sta13Cα), 50.28 (DOTAcycl.), 49.86 (DOTAcycl.), 49.75 (DOTAcycl.), 49.65 (X8Cαn), 48.30 (A9Cα), 47.17 (X8Cαm), 46.88 (K2Cεm), 45.32 (K2Cεn), 42.06 (G11Cα), 40.81 (L14Cβ), 39.87 (Sta13Cγ), 39.62 (Sta13Cδ), 38.64 (Ahx3Cεm), 38.61(Ahx7Cε + Ahx3Cεn), 38.40 (Ahx10Cε), 38.27 (K9Cε), 37.38 (F6(D)Cβ), 37.19 (F6Cβ), 36.83 (F5Cβ), 35.08 (Ahx7Cα), 34.99 (Ahx10Cα), 32.29 (Ahx3Cαn), 31.87 (Ahx3Cαm), 31.83 (K2Cβ + K9Cβ), 31.33 (Q7Cγ), 30.67 (Suc4Cα), 30.55 (Suc4Cβ), 30.50 (V10Cβ), 30.03 (E1Cγ), 29.60 (H12Cβ), 29.05 (Ahx3Cδm), 28.95 (Ahx3Cδn), 28.82 (Ahx7Cδ), 28.68 (Ahx10Cδ), 28.55 (K9Cδ), 27.81 (K2Cδm), 27.66 (E1Cβ), 27.54 (Q7Cβ), 27.18 (W8Cβ), 26.75 (K2Cδn), 26.27 (Ahx3Cγm), 26.21 (Ahx3Cγn), 26.05 (Ahx7Cγ), 25.96 (Ahx10Cγ), 25.01 (Ahx7Cβ), 24.81 (Ahx10Cβ), 24.72 (Ahx3Cβm), 24.58 (AhxCβn), 24.25 (L14Cγ), 24.12 (Sta13Cε), 23.26 (Sta13Cζ(a)), 23.07 (L14Cγ(a)), 22.83 (K9Cγ), 22.50 (K2Cγn), 22.34 (K2Cγm), 22.00 (Sta13Cζ(b)), 21.56 (L14Cγ(b)), 19.23 (V10Cγ(a)), 17.92 (V10Cγ(b)), 17.60 (A9Cβ).
LCMS 88% in positive ion mode, main impurity (compound 26x) - 10% in positive ion mode.
ESI-MS (m/z) C136H19737ClN28O33: calc. for [M + 3H+]3 + : 930.15, found: 930.15.
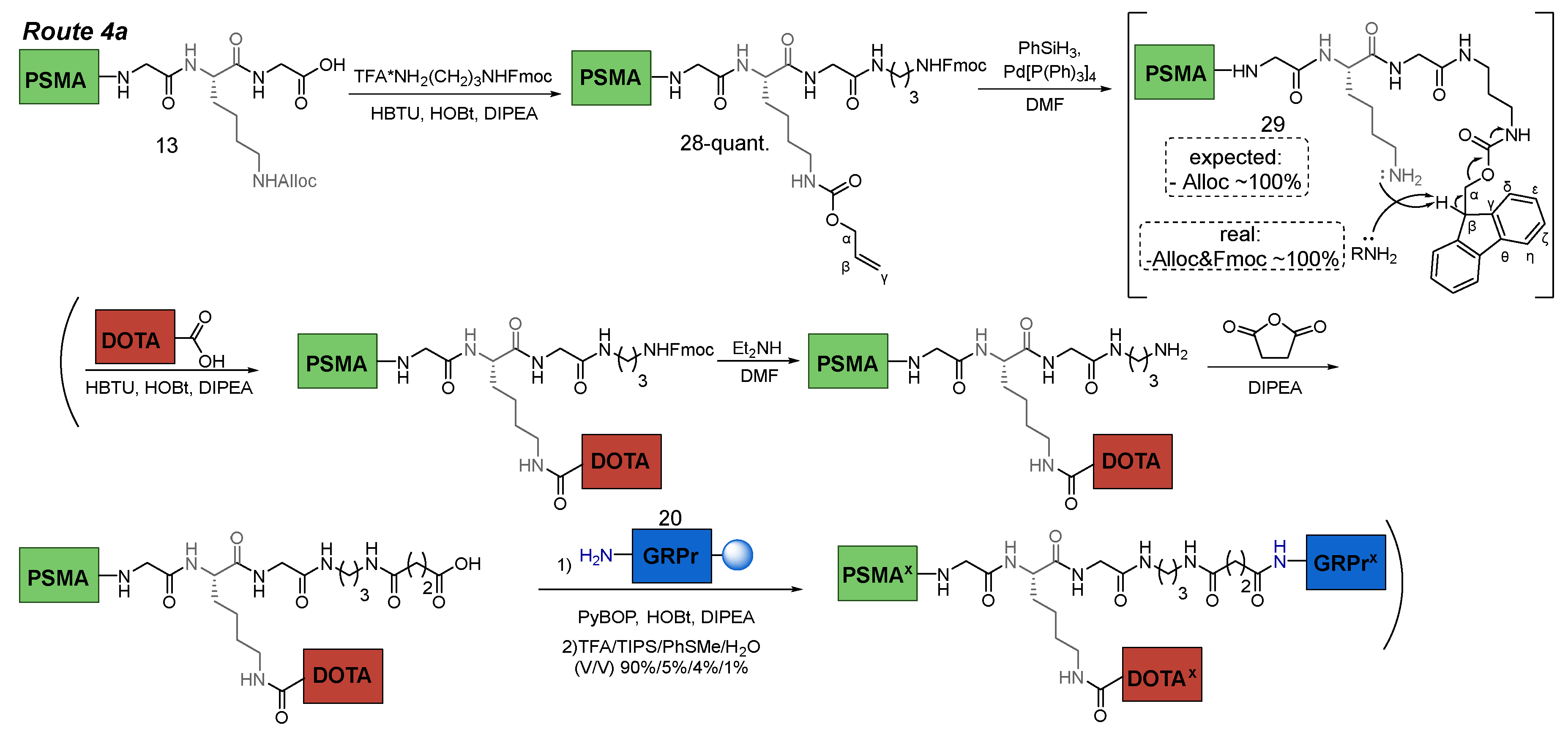
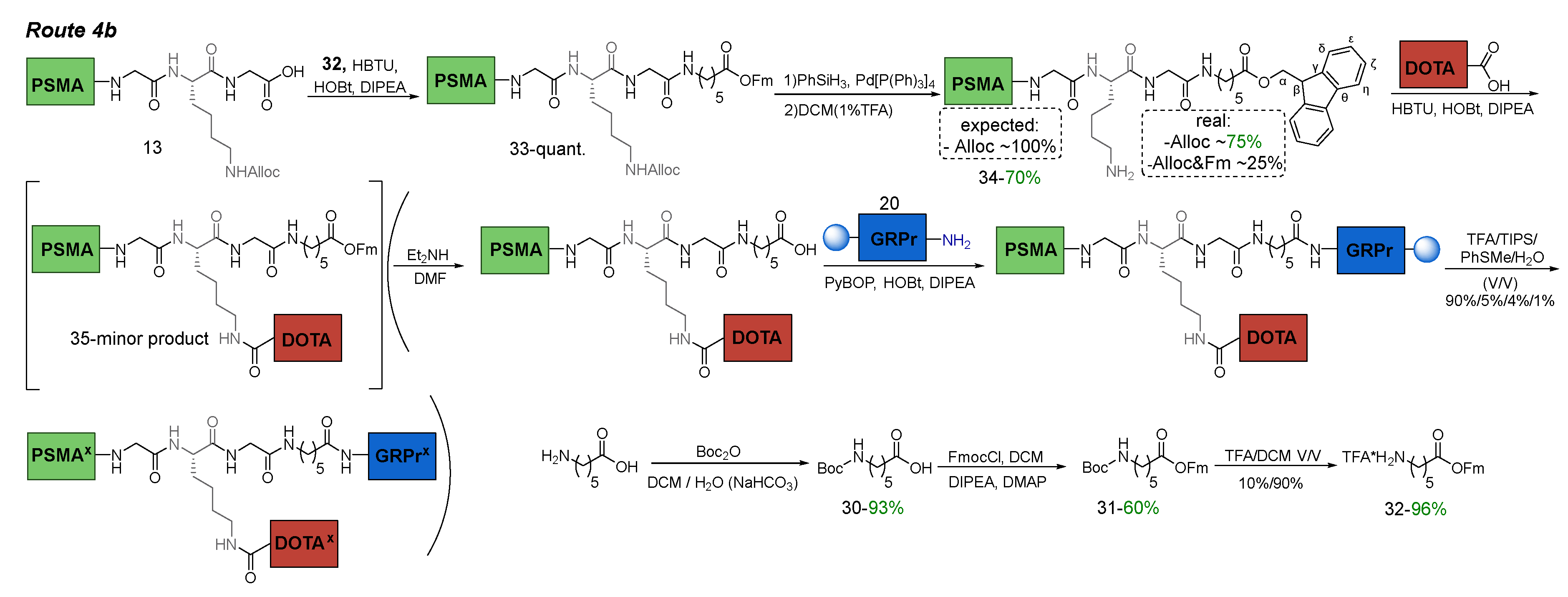
Route 4a
Compound 28
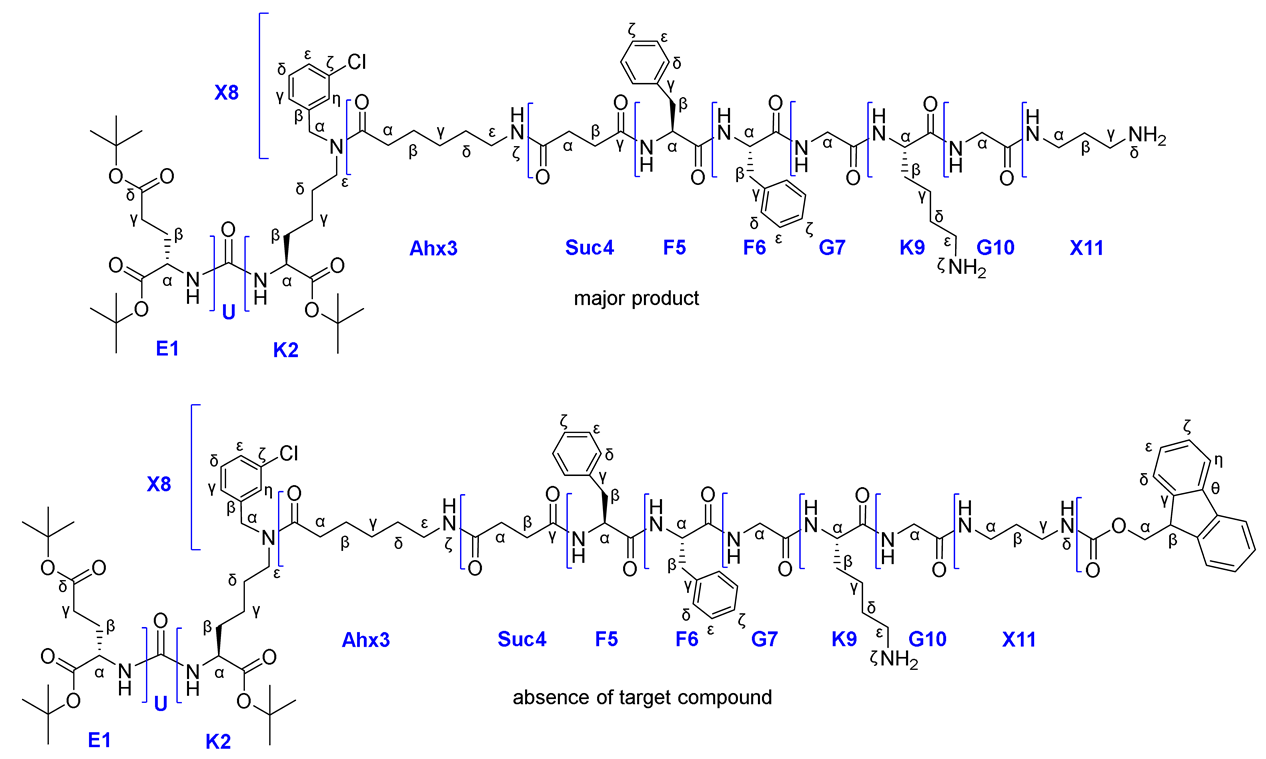
To a solution of compound 13 (1 eq.; 217 mg; 0.15 mmol) in DMF (10 ml) DIPEA (3.5 eq.; 92 μl; 0.525 mmol), HOBt (0.5 eq.; 10 mg; 0.075 mmol) and HBTU (1.1 eq.; 62.6 mg; 0.165 mmol) were added. The mixture was stirred for 10 min under inert atmosphere of Ar. Then a solution of TFA*NH2-(CH2)3-NHFmoc (1.05 eq.; 64.6 mg; 0.158 mmol) in DMF was added and the mixture was stirred for 12 h. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with a mixture of MeOH/H2O (50/50) to completely remove the DMF residue (in some other solvents, the substance forms organogels). The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with H2O (3*3 ml). The resulting precipitate was dried under reduced pressure and washed with P.E. (3*3 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 28 was obtained as a pale yellow powder (258 mg, quant.).
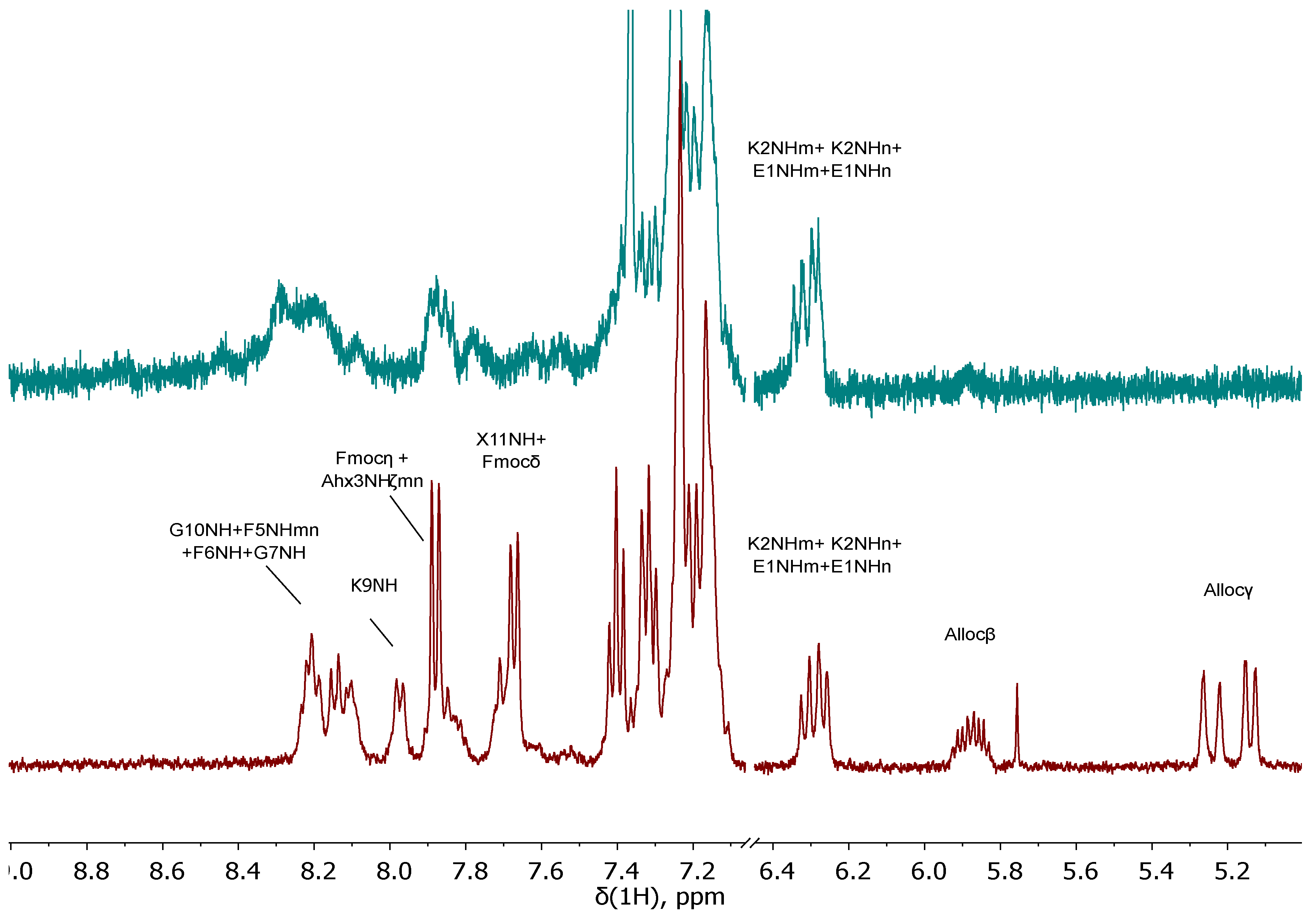
1H NMR (400 MHz, DMSO-d6, δ): 8.26-8.06 (m, 4H, G10NH + F5NHmn + F6NH + G7NH), 7.97 (d, J = 8.0 Hz, 1H, K9NH), 7.92-7.79 (m, 3H, Ahx3NHζmn + Fmocη), 7.74-7.63 (m, 3H, X11NH + Fmocδ), 7.45-7.09 (m, 20H, Fmocζ + X8δn + X8εn + Fmocε + X8δm + X8εm + F6ε + F6δ + X11NHδ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.35 (m, 6H, X8αnm + F6α + Allocα + F5Hα), 4.28 (d, J = 6.9 Hz, 1H, Fmocα), 4.24-4.15 (m, 2H, Fmocβ + K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.60 (m, 4Н, G7α + G9α), 3.21 (t, J = 7.3 Hz, n) & 3.17 (t, J = 7.3 Hz, m) (2H, K2ε, m/n=3/2), 3.12-2.82 (m, 11Н, F6β(a) + X11γ + F6β(b) + X11α + Ahx3ε + K9ε + F5β(a)), 2.71-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + X11β + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.40-1.35 (m, 27H, tBu).
Compound 29
Compound 28 (1 eq.; 120 mg; 69.58 μmol) dissolved in DMF (3 ml), then the system was vacuumate and filled with Ar. Next, PhSiH3 (6 eq.; 52 μl; 418 μmol) was added. After 2 min of stirring Pd[P(Ph)3]4 (0.1 eq.; 8 mg; 7 μmol) in DCM (2 ml) was added using a syringe and the mixture was stirred 90 min under inert atmosphere. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the DMF traces. The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with Et2O (3*2 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. As a result, complete removal of Fmoc protecting group occurred (according to NMR data). Obtained compound 29 was K9NH2ζ&X11NH2δ with two free NH2 group.
Route 4b
Compound 30
Aminohexanoic acid (1 eq.; 2836 mg; 21.6 mmol) was dissolved in a mixture of dioxane/satur. NaHCO3(aq) (37.5/75 ml), then a solution of Boc2O (1.5 eq.; 7500 μl; 32.4 mmol) in dioxane (37.5 ml) was added dropwise. The reaction mixture was stirred overnight. Afterwards, dioxane was removed under reduced pressure, EtOAc (100 ml) was added to the residue and the resulting mixture was acidified to pH = 3 with 0.5 М HCl (dropwise addition). Then the organic layer was separated and aqueous phase was washed with EtOAc (3*100 ml). The combined organic layers were washed with H2O (100 ml) and brine (100 ml). Then the organic layer was dried over Na2SO4. Afterwards, the solvent was removed under reduced pressure. The residue was purified by column chromatography (Puriflash, SIHP-F0120 + SIHP-F0120 (50 μ 116 g + 50 μ 116 g), eluent: DCM(100%)/MeOH(0%) => DCM(90%)/MeOH(10%) for 40 min, after МеOH(100%) for 10 min). Compound 30 was obtained as colorless oil (4630 mg, 93% yield).
1H NMR (400 MHz, DMSO-d6, δ): 11.97 (s, 1H, COOH), 6.76 (t, J = 5.3 Hz, 1H, Ahx11NHζ), 2.88 (td, J = 7.0, 5.3 Hz 2H, Ahx11ε), 2,18 (t, J = 7.4 Hz, 2H, Ahx11α), 1.52-1.41 (m, 2H, Ahx11β), 1.40-1.30 (m, 11H, Ahx11δ + tBu), 1.27-1.18 (m, 2H, Ahx11γ).
Compound 31
To a solution of compound 30 (1 eq., 1876 mg, 8.1 mmol) in DCM (10 ml) DIPEA (1.2 eq., 1690 μl, 9.4 mmol) was added. Then the mixture was cooled to 0 °C and a solution of FmocCl (1.05 eq., 2204 mg, 8.5 mmol) in DCM (25 ml) was added dropwise. The mixture was stirred for 5 min, then DMAP (0.2 eq., 198 mg, 1.6 mmol) was added and the mixture was stirred overnight at room temreture. After completion of the reaction, the mixture was diluted with DCM (65 ml) and washed with: 1) 0.05 М HCl (100 ml); 2) NaHCO3(aq) (1% m/V, 100ml); 3) H2O (100ml); 4) brine (100ml). Then the organic layer was dried over Na2SO4. Afterwards, the solvent was removed under reduced pressure. The residue was purified by column chromatography (Puriflash SI-HP-F0120 (50 μ 116 g), eluent: EtOAc(5%)/P.E(95%) => EtOAc(50%)/P.E(50%) for 40 min, after EtOAc(100%) for 10 min, then МеOH(100%) for 10 min). Compound 31 was obtained as white solid (1994 mg, 60% yield).
1H NMR (400 MHz, DMSO-d6, δ): 7.89 (d, J = 7.4 Hz, 2H, Fmη), 7.65 (d, J = 7.4 Hz, 2H, Fmδ), 7.41 (t, J = 7.4 Hz, 2H, Fmζ), 7.33 (td, J = 7.4, 1.1 Hz, 2H, Fmε), 6.77 (t, J = 5.3 Hz, 1H, Ahx11NHζ), 4.42 (d, J = 6.4 Hz, 2H, Fmα), 4.25 (t, J = 6.4 Hz, 1H, Fmβ), 2.90-2.80 (m, 2H, Ahx11ε), 2.26 (t, J = 7.3 Hz, 2H, Ahx11α), 1.45-1.34 (m, 11H, Ahx11δ + tBu), 1.32-1.25 (m, 2H, Ahx11β), 1.17-1.06 (m, 2H, Ahx11γ).
Compound 32
Compound 31 (1 eq., 1573 mg, 3.8 mmol) was dissolved in system of TFA/DCM (10%/90%, 20 ml) and the mixture was stirred for 2 h. After completion of the reaction, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the TFA excess. The resulting crude product was used without further purification. Compound 32 was obtained as *1TFA form as white solid (1561 mg, 96% yield).
1H NMR (400 MHz, DMSO-d6, δ) for *1TFA form: 7.90 (d, J = 7.4 Hz, 2H, Fmη), 7.71 (br.s., 3H, Ahx11NH3+ζ), 7.65 (d, J = 7.4 Hz, 2H, Fmδ), 7.42 (t, J = 7.4 Hz, 2H, Fmζ), 7.34 (t, J = 7.4, 2H, Fmε), 4.44 (d, J = 6.4 Hz, 2H, Fmα), 4.26 (t, J = 6.4 Hz, 1H, Fmβ), 2.77-2.66 (m, 2H, Ahx11ε), 2.28 (t, J = 7.3 Hz, 2H, Ahx11α), 1.51 - 1.35 (m, 4H, Ahx11β + Ahx11δ), 1.27 - 1.13 (m, 2H, Ahx11γ).
Compound 33
To a solution of compound 13 (1 eq.; 100 mg; 69.15 μmol) in DMF (5 ml) DIPEA (3.5 eq.; 42 μl; 242 μmol), HOBt (0.5 eq.; 4.6 mg; 34.5 μmol) and HBTU (1.1 eq.; 29 mg; 76.07 μmol) were added. The mixture was stirred for 10 min under inert atmosphere of Ar. Then compound 32 (1.05 eq.; 30.7 mg; 72.47 μmol) was added in DMF and the reaction mixture was stirred for 12 h under Ar atmosphere. After completion of the reaction, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the DMF traces. The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with H2O (3*3 ml). The resulting precipitate was dried under reduced pressure and washed with P.E. (3*3 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 33 was obtained as pale-yellow solid (119 mg, quant.).
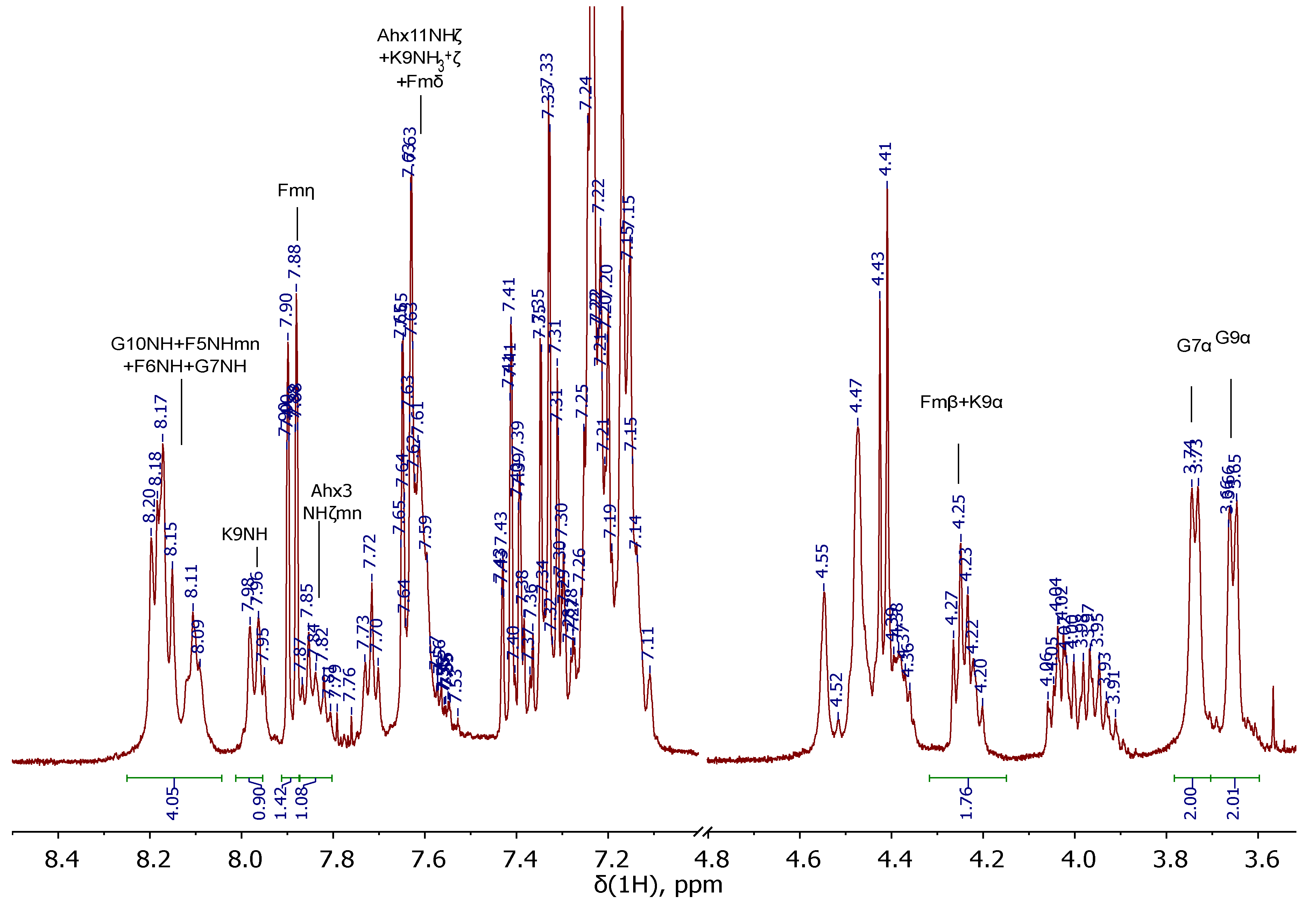
1H NMR (400 MHz, DMSO-d6, δ): 8.23-8.05 (m, 4H, G10NH + F5NHmn + F6NH + G7NH), 7.97 (d, J = 8.0 Hz, 1H, K9NH), 7.89 (d, J = 7.5 Hz, 2H, Fmη), 7.84 (t, J = 5.4 Hz, m) & 7.81 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.70-7.59 (m, 3H, Ahx11NHζ + Fmδ), 7.45-7.09 (m, 19H, Fmζ + X8δn + X8εn + Fmε + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.34-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.34 (m, 8H, X8αnm + F6α + Allocα + Fmα + F5Hα), 4.25 (t, J = 6.5 Hz, 1H, Fmβ), 4.22-4.14 (m, 1H, K9α), 4.08-3.88 (m, 2H, E1α + K2αm + K2αn), 3.80-3.57 (m, 4Н, G7α + G9α), 3.21 (t, J = 7.3 Hz, n) & 3.17 (t, J = 7.3 Hz, m) (2H, K2ε, m/n = 3/2), 3.12-2.82 (m, 11Н, F6β(a) + F6β(b) + Ahx11ε + Ahx3ε + K9ε + F5β(a)), 2.71-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 10H, Ahx3αm + Suc4βmn + Ahx11α + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.05 (m, 25Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx11β + Ahx3δ + K2δ + Ahx11δ + K2γ + K9γ + Ahx3γ + Ahx11γ, m + n), 1.40-1.35 (m, 27H, tBu).
Compound 34
Compound 33 (1 eq.; 119 mg; 69.15 μmol) was dissolved in DMF (3 ml), then the system was vacuumate and filled with Ar. Next PhSiH3 (6 eq.; 51 μl; 415 μmol) was added. After 2 min of stirring Pd[P(Ph)3]4 (0.1 eq.; 8 mg; 7 μmol) in DCM (2 ml) was added using a syringe and the mixture was stirred 90 min under inert atmosphere. Afterwards, 1% TFA solution in DCM (3 ml) was added to the reaction mixture to inactivate free NH2 group. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the DMF traces. The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with Et2O (3*2 ml). Then, residual solvent was removed from the precipitate under reduced pressure. As a result, partial removal of OFm occurred (according to NMR data). The residue was purified by column chromatography (Puriflash SIHP-F0020 (50 μ 20 g), eluent: DCM(100%)/MeOH(0%) => DCM(98%)/MeOH(2%) for 10 min, after DCM(0.1%TFA)(98%)/MeOH(2%) => DCM(0.1%TFA)(90%)/MeOH(10%) for 30 min, then МеOH(100%) for 10 min). Compound 34 was obatined as *1TFA form as pale-yellow amorphous product (85 mg, 70% yield).
1H NMR (400 MHz, DMSO-d6, δ): 8.23-8.05 (m, 4H, G10NH + F5NHmn + F6NH + G7NH), 7.99 (d, J = 8.0 Hz, 1H, K9NH), 7.91-7.81 (m, 3H, Fmη + Ahx3NHζmn), 7.75-7.60 (m, 6H, Ahx11NHζ + K9NH3+ζ + Fmδ), 7.45-7.09 (m, 18H, Fmζ + X8δn + X8εn + Fmε + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + F5δ + X8γmn), 6.34-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.58-4.34 (m, 6H, X8αnm + F6α + Fmα + F5Hα), 4.30-4.17 (m, 2H, Fmβ + K9α), 4.08-3.88 (m, 2H, E1α + K2αm + K2αn), 3.80-3.57 (m, 4Н, G7α + G9α), 3.21 (t, J = 7.3 Hz, n) & 3.17 (t, J = 7.3 Hz, m) (2H, K2ε, m/n = 3/2), 3.12-2.80 (m, 11Н, F6β(a) + F6β(b) + Ahx11ε + Ahx3ε + F5β(a)), 2.80-2.70 (m, 2H, K9ε), 2.71-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 10H, Ahx3αm + Suc4βmn + Ahx11α + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.05 (m, 25Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx11β + Ahx3δ + K2δ + Ahx11δ + K2γ + K9γ + Ahx3γ + Ahx11γ, m + n), 1.40-1.35 (m, 27H, tBu).
Compound 35
 |
To a solution of DOTA(tBu)3-COOH (1.55 eq.; 43 mg; 75.25 μmol) in DMF (5 ml) DIPEA (7 eq.; 59 μl; 338.6 μmol), HOBt (0.75 eq.; 4.9 mg; 36.3 μmol), HBTU (1.75 eq.; 32 mg; 84.7 μmol) were added. After 5 min of stirring compound 34 (1 eq.; 85 mg; 48.4 μmol) was added under inert atmosphere of Ar, and then was stirred for 12 h. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the DMF traces. The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with H2O (3*2 ml). Then, residual solvent was removed from the precipitate under reduced pressure. The residue was purified by column chromatography (Puriflash SIHP-F0020 (50 μ 20 g), eluent: DCM(100%)/MeOH(0%) => DCM(98%)/MeOH(2%) for 10 min, after DCM(0.1%TFA)(98%)/MeOH(2%) => DCM(0.1%TFA)(80%)/MeOH(20%) for 30 min, then МеOH(100%) for 10 min). As a result, the main product of the reaction was the product of the addition of the TFA residue to the amino group K9NH2ζ, the target compound was isolated in trace amounts.
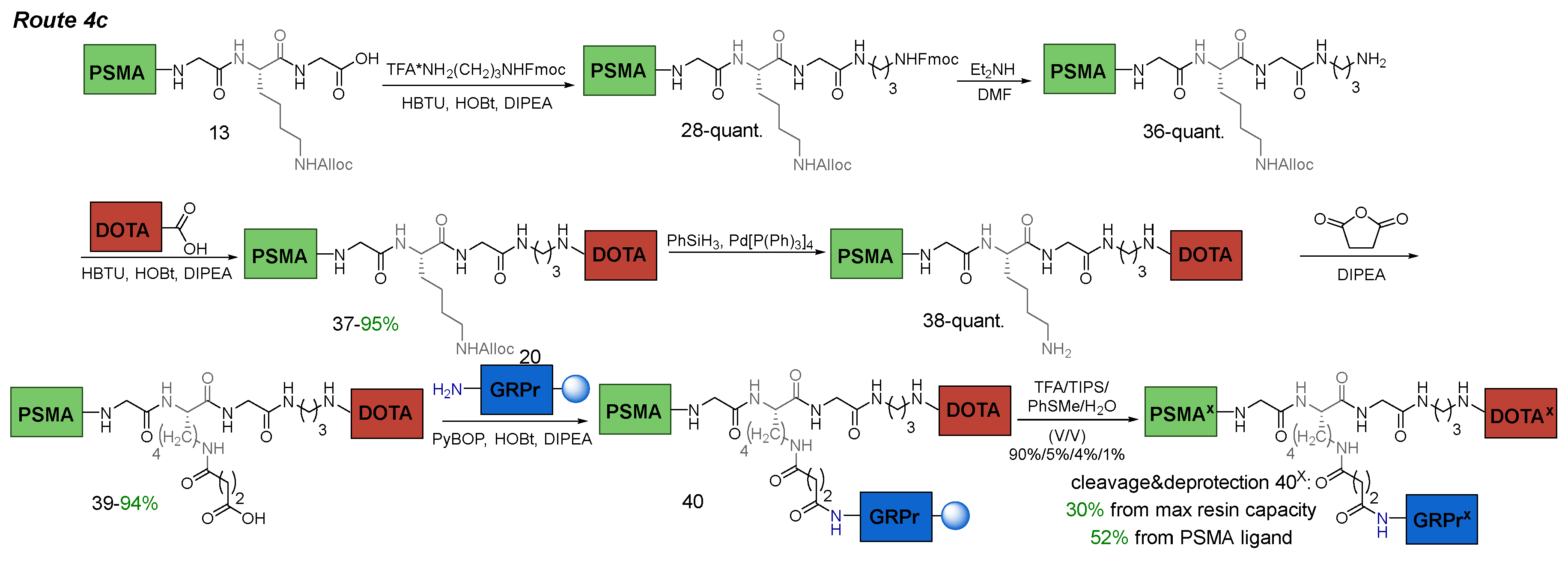
Route 4c
Compound 28
To a solution of compound 13 (1 eq.; 217 mg; 0.15 mmol) in DMF (10 ml) DIPEA (3.5 eq.; 92 μl; 0.525 mmol), HOBt (0.5 eq.; 10 mg; 0.075 mmol) and HBTU (1.1 eq.; 62.6 mg; 0.165 mmol) were added. The mixture was stirred for 10 min under inert atmosphere. Then TFA*NH2-(CH2)3-NHFmoc (1.05 eq.; 64.6 mg; 0.158 mmol) in DMF was added and the mixture was stirred for 12 h. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with a mixture of MeOH/H2O (50/50) to completely remove the DMF residue (in some other solvents the substance forms organogels). The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with H2O (3*3 ml). The resulting precipitate was dried under reduced pressure and washed with P.E. (3*3 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 28 was obtained as pale-yellow solid (258 mg, quant.).
1H NMR (400 MHz, DMSO-d6, δ): 8.26-8.06 (m, 4H, G10NH + F5NHmn + F6NH + G7NH), 7.97 (d, J = 8.0 Hz, 1H, K9NH), 7.92-7.79 (m, 3H, Ahx3NHζmn + Fmocη), 7.74-7.63 (m, 3H, X11NH + Fmocδ), 7.45-7.09 (m, 20H, Fmocζ + X8δn + X8εn + Fmocε + X8δm + X8εm + F6ε + F6δ + X11NHδ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.35 (m, 6H, X8αnm + F6α + Allocα + F5Hα), 4.28 (d, J = 6.9 Hz, 1H, Fmocα), 4.24-4.15 (m, 2H, Fmocβ + K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.60 (m, 4Н, G7α + G9α), 3.21 (t, J = 7.3 Hz, n) & 3.17 (t, J = 7.3 Hz, m) (2H, K2ε, m/n = 3/2), 3.12-2.82 (m, 11Н, F6β(a) + X11γ + F6β(b) + X11α + Ahx3ε + K9ε + F5β(a)), 2.71-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + X11β + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.40-1.35 (m, 27H, tBu).
Compound 36
Compound 28 (1 eq.; 258 mg; 0.15 mmol) was dissolved in a system of Et2NH/DMF (25 eq. Et2NH, 7.5 ml DMF) and the mixture was stirred for 20 min under inert atmosphere of Ar. Afterwards the solvent was removed under reduced pressure and the residue was re-evaporated twice with 0.5% TFA solution in DCM (5 ml) to inactivate free NH2 group. The product was precipitated by Et2O and washed with: 1) Et2O (2*2 ml), 2) P.E. (2*2 ml). Compound 37 was obtained as 1*TFA form as pale-yellow solid (242 mg, quant.).
To obtain free NH2 form the precipitate was dissolved in a mixture of H2O/MeCN (50/50, 8 ml) and charged with a saturated solution of NaHCO3 (2 ml), then the solvent was removed under reduced pressure to obtain a powder, which was grinded using a spatula and washed with H2O (3*3 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 36 was obtained as pale-yellow solid (224 mg, quant.).
1H NMR (400 MHz, DMSO-d6, δ) for *1TFA form: 8.28 (t, J = 6 Hz, 1H, G10NH), 8.23-8.14 (m, 2Н, F5NHmn + F6NH), 8.08 (t, J = 5.9 Hz, 1H, G7NH), 8.01 (d, J = 7.1 Hz, 1H, K9NH), 7.90-7.77 (m, 2H, Ahx3NHζmn + X11NH), 7.63 (br.s., 3H, X11NH3+δ), 7.42-7.09 (m, 15H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.33 (m, 6H, X8αnm + F6α + Allocα + F5Hα), 4.18-4.10 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.58 (m, 4Н, G7α + G9α), 3.25-2.81 (m, 11Н, K2εnm + X11γ + F6β(a) + F6β(b) + Ahx3ε + K9ε + F5β(a)), 2.80-2.72 (m, 2H, X11α), 2.70-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, X11β + E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.40-1.35 (m, 27H, tBu).
1H NMR (400 MHz, DMSO-d6, δ) for free NH2 form: 8.28-8.04 (m, 4H, G10NH + F5NHmn + F6NH + G7NH), 8.04-7.95 (m, 1H, K9NH), 7.88-7.78 (m, 1H, Ahx3NHζmn), 7.72-7.60 (m, 1H, X11NH), 7.42-7.09 (m, 15H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.33 (m, 6H, X8αnm + F6α + Allocα + F5Hα), 4.20-4.10 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.58 (m, 4Н, G7α + G9α), 3.25-2.81 (m, 11Н, K2εnm + F6β(a) + X11α + F6β(b) + Ahx3ε + K9ε + F5β(a)), 2.70-2.60 (m, 1Н, F5β(b)), 2.52-2.45 (m, 2Н, X11γ), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + K9β(b) + X11β + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.40-1.35 (m, 27H, tBu).
Compound 37
To a solution of DOTA(tBu)3-COOH (1 eq.; 86 mg; 150 μmol) in DMF (5 ml) DIPEA (4 eq.; 105 μl; 600 μmol), HOBt (0.5 eq.; 10 mg; 75 μmol), HBTU (1.5 eq.; 85 mg; 225 μmol) were added, and the reaction mixture was stirred for 10 min under inert atmosphere. Then compound 36 (1 eq.; 224 mg; 150 μmol) was added and the mixture was stirred for 12 h. Next, the solvent was removed under reduced pressure, the residue was dissolved in DCM (30 ml) and washed with: 1) H2O (1*30 ml), 2) NaHCO3 (2*30 ml). 2) brine (1*30 ml). Then the organic layer was dried over Na2SO4. Afterwards, the solvent was removed under reduced pressure. The residue was purified by column chromatography (Puriflash (SIHP-F0012 50 μ 20 g); eluent: DCM(100%)/MeOH(0%) => DCM(90%)/MeOH(10%) for 30 min, after МеOH (100%) in 10 min). Compound 37 was obtained as a pale-yellow solid (292 mg, 95% yield).
1H NMR (400 MHz, DMSO-d6, δ): 8.24-8.13 (m, 3Н, F5NHmn + G10NH + F6NH), 8.12-8.02 (m, 2H, G7NH + X11NHδ), 7.95 (d, J = 7.1 Hz, 1H, K9NH), 7.86 (t, J = 5.4 Hz, m) & 7.83 (t, J = 5.4 Hz, n) (1H, Ahx3NHζ, m + n), 7.70 (t, J = 5.5 Hz, X11NH), 7.42-7.08 (m, 15H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + K9NHζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 5.95-5.81 (m, 1Н, Allocβ), 5.30-5.20 (m, 1Н, Allocγ(a)), 5.18-5.10 (m, 1Н, Allocγ(b)), 4.60-4.33 (m, 6H, X8αnm + F6α + Allocα + F5Hα), 4.23-4.13 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.58 (m, 4Н, G7α + G9α), 3.80-1.60 (br.m., 24H, DOTA), 3.25-2.81 (m, 13Н, K2εnm + F6β(a) + X11γ + X11α + F6β(b) + Ahx3ε + K9ε + F5β(a)), 2.70-2.60 (m, 1Н, F5β(b)), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + X11β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.43 (s, 9H, 22), 1.40 (s, 18H, 18+26), 1.40-1.35 (m, 27H, tBu).
13C NMR (101 MHz, DMSO-d6, δ): 172.57 (1), 172.20 (K2C(nm)+16+24), 172.12 (Suc4Cγ(nm) + Ahx3C(nm)), 171.92 (E1C), 171.82 (K9C), 171.47 (Suc4C(mn)), 171.45 (E1Cδ), 171.37 (20+F6C), 171.32 (F5C), 168.93 (G9C), 168.55 (G7C), 157.13 (U), 155. 91 (AllocC), 141.17 (X8Cβ(m)), 140.82 (X8Cβ(n)), 137.99 (F6Cγ), 137.92 (F5Cγ), 133.88 (AlloCβ), 133.42 (X8Cζ(n)), 133.08 (X8Cζ(m)), 130.61 (X8Cδ(n)), 130.25 (X8Cδ(m)), 129.15 (F6Cδ), 129.08 (F5Cδ), 128.12 (F6Cε), 128.03 (F5Cε), 127.20 (X8Cη(m)), 127.15 (X8Cε(n)), 126.86 (X8Cε(m)), 126.30 (X8Cη(n) + F6Cζ), 126.20 (F5Cζ), 126.06 (X8Cγ(m)), 124.97 (X8Cγ(n)), 116.87 (AllocCγ), 81.10 (21), 80.98 (17+25), 80.58 (E1tBu), 80.41 (K2tBu(m)), 80.32 (K2tBu(n)), 79.77 (E1δtBu), 64.14 (AllocCα), 55.84 (2), 55.35 (15+23), 55.24 (19), 54.34 (F5Cα + F6Cα), 52.99 (K2Cα(n)), 52.93 (K9Cα), 52.85 (K2Cα(m)), 52.17 (E1Cα), 49.59 (X8Cα(n)), 47.09 (X8Cα(m)), 46.79 (K2Cε(m)), 45.22 (K2Cε(n)), 43.03 (G7Cα), 42.05 (G9Cα), 40.27 (K9Cε), 39.50 (series of br. peaks, DOTAcyclic), 38.58 (Ahx3Cε(m)), 38.51 (Ahx3Cε(n)), 36.96 (F6Cβ + F5Cβ), 36.38 (X11Cγ + X11Cα), 32.32 (Ahx3Cα(n)), 31.94 (Ahx3Cα(m)), 31.81 (K2Cβ), 31.41 (K9Cβ), 30.90 (E1Cγ), 30.77 (Suc4Cα), 30.64 (Suc4Cβ), 29.19 (Ahx3Cδ(m) + K9Cδ), 29.09 (Ahx3Cδ(n)), 29.04 (X11Cβ), 27.74 (tBuE1), 27.60 (22+18+26+tBuK2 + K2Cδ(m) + tBuE1δ + E1Cβ), 26.73 (K2Cδ(n)), 26.29 (Ahx3Cγ(m)), 26.19 (Ahx3Cγ(n)), 24.75 (Ahx3Cβ(m)), 24.65 (Ahx3Cβ(n)), 22.61 (K9Cγ), 22.40 (K2Cγ(n)), 22.24 (K2Cγ(m)).
HRMS (m/z, ESI): calc. for C104H16335ClN16O24 - [M+2Na+]2+ 1050.5749, found: 1050.5749
Compound 38
Compound 37 (1 eq.; 144 mg; 72 μmol) was dissolved in DMF (3 ml), then the system was vacuumate and filled with Ar. Next PhSiH3 (6 eq.; 52 μl; 420 μmol) was added. After 2 min of stirring Pd[P(Ph)3]4 (0.1 eq.; 8 mg; 7.2 μmol) in DCM (2 ml) was added using a syringe and the mixture was stirred for 90 min under inert atmosphere of Ar. Afterwards, the solvent was removed under reduced pressure and the residue was re-evaporated twice with DCM (3 ml) to completely remove the DMF residue. The resulting amorphous mass was grinded using a spatula to obtain a fine powder, which was washed with Et2O (3*2 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 38 was obtained as pale-yellow solid (138 mg, quant.).
1H NMR (400 MHz, DMSO-d6, δ): 8.31-8.16 (m, 3Н, F5NHmn + G10NH + F6NH), 8.15-8.02 (m, 2H, G7NH + X11NHδ), 8.02-7.92 (m, 1H, K9NH), 7.92-7.81 (m, 1H, Ahx3NHζmn), 7.78-7.65 (m, 1H, X11NH), 7.42-7.08 (m, 14H, X8δn + X8εn + X8δm + X8εm + F6ε + F6δ + X8ηmn + F5ε + F6ζ + F5ζ + F5δ + X8γmn), 6.35-6.21 (m, 2Н, K2NHm + K2NHn + E1NHm + E1NHn), 4.60-4.31 (m, 4H, X8αnm + F6α + Allocα + F5Hα), 4.23-4.13 (m, 1H, K9α), 4.08-3.90 (m, 2H, E1α + K2αm + K2αn), 3.81-3.58 (m, 4Н, G7α + G9α), 3.80-1.60 (br.m., 24H, DOTA), 3.25-2.81 (m, 13Н, K2εnm + F6β(a) + X11γ + X11α + F6β(b) + Ahx3ε + F5β(a)), 2.70-2.60 (m, 1Н, F5β(b)), 2.59-2.51 (m, 2H, K9ε), 2.40-2.10 (m, 8H, Ahx3αm + Suc4βmn + E1γ + Suc4αmn + Ahx3αn), 1.92-1.80 (m, 1Н, E1β(a)), 1.72-1.10 (m, 21Н, E1β(b) + K9β(a) + K2β(a) + Ahx3β + X11β + K9β(b) + K2β(b) + K9δ + Ahx3δ + K2δ + K2γ + K9γ + Ahx3γ, m + n), 1.43 (s, 9H, 22), 1.40 (s, 18H, 18+26), 1.40-1.35 (m, 27H, tBu).
Compound 39
Compound 38 (1 eq.; 138 mg; 70 μmol) was dissolved in DCM (5 ml). Then succinic anhydride (2 eq.; 14 mg; 140 μmol), DIPEA (2.5 eq.; 31 μl; 175 μmol) were added and the reaction mixture was stirred for 6 h under inert atmosphere of Ar. Afterwards, the solvent was removed under reduced pressure. The residue was dissolved in a mixture of H2O/MeCN (50/50, 8 ml), charged with saturated NaHCO3 solution (2 ml) and stirred for 10 min. Next, the solvent was removed under reduced pressure until formation of the powder, which was grinded using a spatula and washed with H2O (3*3 ml). Then, the residual solvent was removed from the precipitate under reduced pressure. Compound 39 was obtaines as Na salt as pale-yellow solid (138 mg, 94% yield) and used without further purification.
ESI-MS C104H16335ClN16O25: m/z calc. for [M+H++Na+]2+: 1047.58, found: 1047.39.
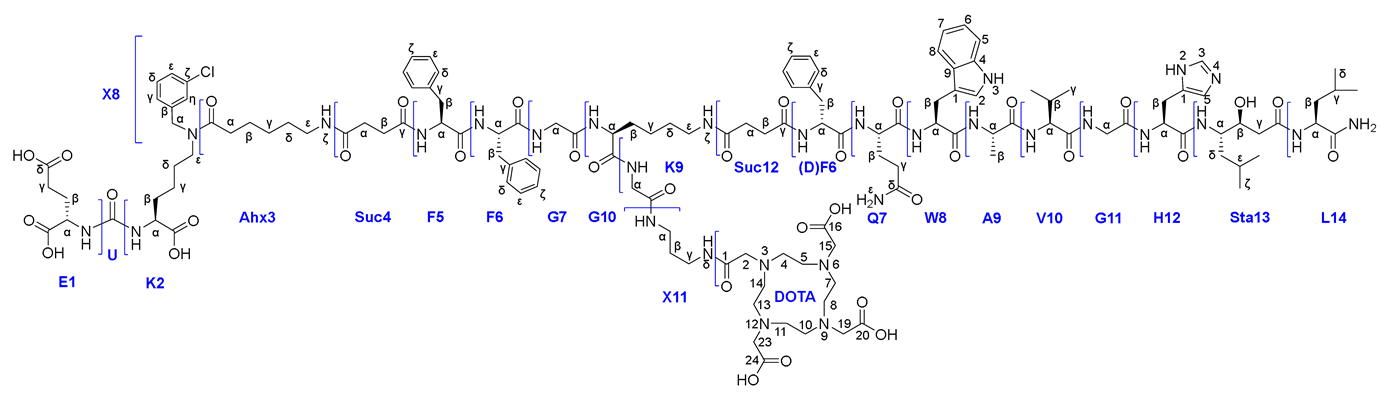
Compound 40x
Compound 39 (1 eq.; 137 mg; 65.4 μmol), HOBt (0.75 eq.; 6.6 mg; 49 μmol), HBTU (3 eq.; 74.4 mg; 196.2 μmol) and DIPEA (6 eq.; 68 μl; 392.4 μmol) were added under Ar atmosphere to a mixture of compound 20 on Rink Amide MBHA resin (1 eq.; 65.4 μmol) in DMF (5 ml) in reactor. The mixture was stirred for 24 h under inert atmosphere of Ar. Then the solvent was removed by filtration on a porous reactor filter and the resin was washed three times with DMF (5 ml), three times with DCM (5 ml), then dried from traces of solvents.
After, the resin was transferred to a 10 ml round-bottomed glass flask, charged with the system of TFA/TIPS/PhSMe/H2O (90%/5%/4%/1%, 7 ml) and stirred for 4 h. Then the resulting solution was separated on a sintered glass filter, washing the residue twice with TFA. The solvent was removed under reduced pressure, the crude product was precipitated by Et2O and purified by column chromatography (Puriflash PF-15C18HP-F0040 (15 μ 60 g), eluent: H2O(95%)/MeCN(5%) => H2O(50%)/MeCN(50%) for 50 min, after MeCN(100%) for 10 min). Compound 40x was obtained as white solid (96.5 mg, 30% yield from max resin capacity, 52% from PSMA fragment).
1H NMR (400 MHz, DMSO-d6, δ): 10.82-10.72 (m, 1H, W8(3)), 8.58-7.89 (m, 13H, F5NHmn + F6NHm + G10NH + Q7NH + G11NH + F6NHn + G7NH + X11NHδ + F6(D)NH + H12NH + W8NH + L14NH + A9NH + Ahx3NHζmn), 7.90-7.48 (m, 4H, V10NH + K9NH + K9NHε + X11NH), 7.62-7.55 (m, 2H, W8(8) + H12(3)), 7.51 (s, 1H, L14NH2(a)), 7.44 (d, J = 8.9 Hz, 1H, Sta13NH), 7.41-7.09 (m, 21H, X8δn + X8εn + W8(5) + X8δm + X8εm + F6ε + F6δ + X8ηmn + F6(D)δ + F6(D)ε + F5ε + F6ζ + F6(D)ζ + F5ζ + W8(2) + X8γm + F5δ + X8γn), 7.09-6.90 (m, 4H, Q7NH2(a) + W8(6) + L14NH2(b) + W8(7)), 6.86 (br.s., 1H, H12(5)), 6.75 (s, 1H, Q7NH2(b)), 6.54-6.22 (m, 2Н, K2NHmn + E1NHmn), 4.60-4.25 (m, 8H, X8αn + W8α + H12α + X8αm + F6(D)α + F6α + A9α + F5αmn), 4.24-3.96 (m, 6H, K9α + V10α + L14α + Q7α + E1α + K2αm + K2αn), 3.90-3.60 (m, 8H, Sta13β + Sta13α + G10α + G11α + G7α), 3.60-2.40 (br.m, 44H, 2+19+15+23+Hcyclic + K2εn + W8β(a) + K2εm + F6β(a) + Hcyclic + X11γ + W8β(b) + Ahx3εm + X11α + Ahx3εn + F6(D)β(a) + K9ε + F6β(b) + H12β(a) + F5β(a) + H12β(b) + F6(D)β(b) + F5β(b)), 2.40-1.95 (m, 15H, Suc4βm + Suc4αm + Ahx3αm + Suc4βn + E1γ + Ahx3αn + Suc4αn + Sta13γ + Suc12α + Suc12β + V10β), 1.94-1.82 (m, 3H, Q7γ + E1β(a)), 1.82-1.69 (m, 6H, Q7β(a) + E1β(b) + L14γ + K2β(a) + Q7β(b) + K9β(a)), 1.57-1.13 (m, 26H, X11β + K9δ + Ahx3βm + K2β(b) + K9β(b) + Ahx3βn + K2δm + L14β + Sta13ε + K2δn + Ahx3δm + Sta13δ(a) + Ahx3δn + Ahx3γmn + K9γ + K2γmn + A9β + Sta13δ(b)), 0.90-0.75 (m, 18H, L14δ(a) + V10γ(a) + L14δ(b) + V10γ(b) + Sta13ζ(a) + Sta13ζ(b)).
LCMS 95% in positive ion mode
ESI-MS C135H19337ClN30O35: m/z calc. for [M+3H+]3+: 944.80, found: 945.05.