1. Introduction:
Graphene quantum dots (GQDs) are zero-dimensional graphene-based nanomaterials with unique electronic, optical, and chemical properties[
1,
2]. Their small size (typically less than 10 nm in diameter) endows them with quantum confinement effects, making them highly attractive for a wide range of applications including bioimaging, sensing, energy storage, and catalysis[
3]. The controlled synthesis of GQDs with tunable properties is essential for realizing their full potential in these applications.
In recent years, significant progress has been made in developing various synthesis techniques for GQDs. These techniques can be broadly classified into two categories: top-down and bottom-up approaches[
4]. Top-down approaches involve the exfoliation or fragmentation of larger graphene structures into smaller quantum dots, while bottom-up approaches involve the assembly or growth of GQDs from molecular precursors[
5,
6]. Each approach offers distinct advantages and limitations, and the choice of method depends on the desired properties and intended applications of the GQDs[
7].
Figure 1.
Graphical abstract of various method of synthesis of grapheme quantum dots.
Figure 1.
Graphical abstract of various method of synthesis of grapheme quantum dots.
2. Different Methods of Synthesising of Graphene Quantum Dots:
Graphene quantum dots (GQDs) can be synthesized using various methods, each offering unique advantages and limitations. These methods can be broadly classified into two categories: top-down and bottom-up approaches[
8].
2.1. Top-Down Synthesis Techniques:
Top-down approaches for synthesizing GQDs typically involve the exfoliation or fragmentation of graphene-based materials such as graphene oxide (GO) or graphite into smaller nanoscale structures[
8,
9]. One of the most commonly used methods is the chemical oxidation and subsequent exfoliation of graphite to produce graphene oxide, followed by the reduction of GO to GQDs[
10]. Other top-down techniques include laser ablation, electrochemical exfoliation, and microwave-assisted exfoliation. These methods offer several advantages such as scalability, simplicity, and control over the size and surface chemistry of the resulting GQDs[
5]. However, they often suffer from low quantum yield, limited size control, and the presence of structural defects[
9].
2.1.1. Chemical Oxidation and Exfoliation of Graphene Quantum Dots:
Chemical oxidation and subsequent exfoliation represent one of the most widely employed methods for synthesizing graphene quantum dots (GQDs)[
11]. This technique involves the oxidation of graphite to graphene oxide (GO), followed by the exfoliation of GO sheets into GQDs, which are then reduced to obtain GQDs with desired properties[
11].
2.1.1.1. Chemical Oxidation of Graphite:
The process typically begins with the chemical oxidation of graphite to produce graphene oxide (GO). Hummers' method and modified Hummers' method are two common approaches used for this purpose[
12]. In Hummers' method, a mixture of concentrated sulfuric acid, potassium permanganate, and graphite flakes is stirred at a controlled temperature[
13]. This leads to the oxidation of graphite and the formation of GO, which is characterized by the presence of oxygen-containing functional groups such as hydroxyl, carboxyl, and epoxy groups on its basal plane and edges[
14].
The chemical oxidation method, also known as the oxidation cutting method, is widely employed in the synthesis of graphene quantum dots (GQDs), wherein carbon bonds present in graphene, graphene oxide (GO), or carbon nanotubes are typically broken down using strong oxidants like H
2SO
4, HNO
3, or others[
15].
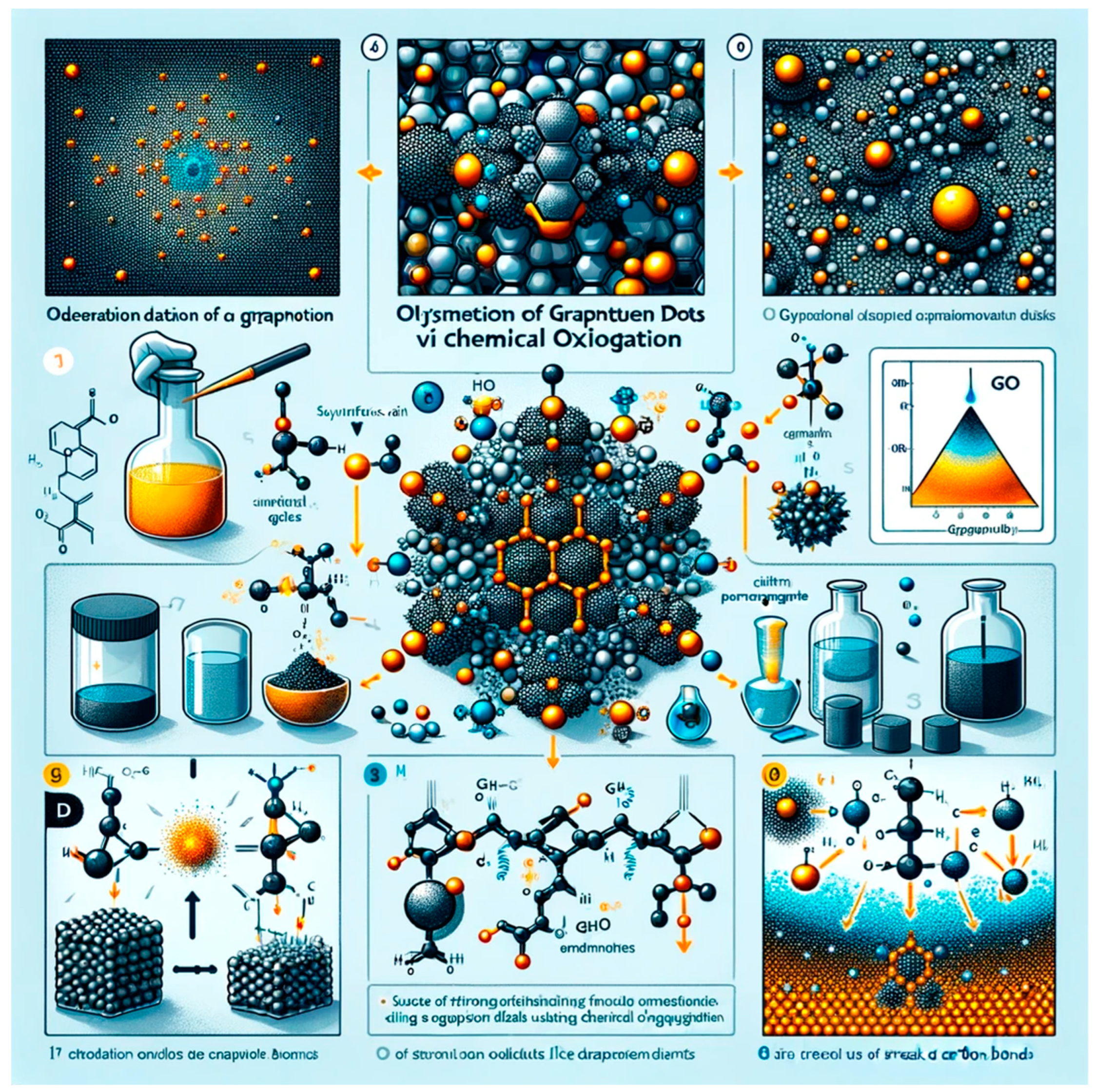
Figure 2.
illustrate the synthesis images of Chemical Oxidation of Graphite.
Figure 2.
illustrate the synthesis images of Chemical Oxidation of Graphite.
2.1.1.2. Exfoliation of Graphene Oxide:
The obtained GO sheets undergo exfoliation into GQDs through various methods, such as ultrasonication of GO suspensions, inducing the exfoliation of GO sheets into smaller fragments due to the cavitation effect, or chemical or thermal reduction of GO, inducing the fragmentation of GO sheets into GQDs, with the reduction process removing oxygen-containing functional groups from GO, leading to the formation of GQDs with sp²-hybridized carbon structures[
16].
Advantages of chemical oxidation and exfoliation methods include scalability, with adaptability for large-scale production of GQDs, control over size and surface chemistry through adjustment of reaction parameters, and versatility allowing application to various carbon precursors[
17].
Limitations include relatively low quantum yields, potential introduction of structural defects and functional groups during oxidation, and environmental and safety concerns due to the use of strong oxidizing agents and harsh reaction conditions[
18].
Applications of GQDs synthesized via chemical oxidation and exfoliation encompass bioimaging, with their biocompatibility, low cytotoxicity, and bright fluorescence, sensing for detection of ions, biomolecules, and environmental pollutants, and photocatalysis for reactions like water splitting, pollutant degradation, and organic synthesis, leveraging their ability to generate reactive oxygen species under light irradiation[
19,
20].
Chemical oxidation and exfoliation represent a versatile and widely used method for synthesizing GQDs with tunable properties, making them valuable materials for various applications in energy, biomedicine, and environmental remediation[
21].
Figure 3.
synthesis method of Exfoliation of Graphene Oxide.
Figure 3.
synthesis method of Exfoliation of Graphene Oxide.
2.1.2. Laser Ablation for Synthesizing Graphene Quantum Dots:
Laser ablation is a powerful technique used for the synthesis of graphene quantum dots (GQDs), offering precise control over the size, structure, and properties of the resulting nanomaterials[
22]. In this method, a high-energy laser beam is focused onto a target material, typically graphite, in a liquid medium, leading to the ablation or fragmentation of the target material and the formation of GQDs[
23].
Laser ablation involves the irradiation of a solid target material, such as graphite, with a high-energy laser beam in a liquid medium, such as water or organic solvents[
24]. The intense laser energy causes the target material to undergo rapid heating and vaporization, leading to the formation of a plasma plume consisting of atoms, ions, and nanoparticles[
25]. As the plasma plume expands and cools, nucleation and growth processes occur, resulting in the formation of GQDs dispersed in the liquid medium[
26].
Laser ablation provides precise control over the size, shape, and structure of synthesized GQDs through parameters such as laser power, pulse duration, and repetition rate, yielding GQDs with high quantum yield and narrow size distribution, minimal defects, and versatility across target materials and liquid media[
27]; however, its complexity, safety concerns, and limited scalability for large-scale production pose challenges, while applications range from optoelectronic devices like LEDs to biomedical imaging and sensing platforms for various analytes[
28].
Figure 4.
synthesis protocol of Laser Ablation for Synthesizing Graphene Quantum Dots.
Figure 4.
synthesis protocol of Laser Ablation for Synthesizing Graphene Quantum Dots.
2.1.3. Electrochemical Exfoliation for Synthesizing Graphene Quantum Dots:
Electrochemical exfoliation is a versatile and scalable technique used for the synthesis of graphene quantum dots (GQDs) from graphite or graphite oxide precursors[
11]. This method offers several advantages, including simplicity, scalability, and control over the size and properties of the resulting GQDs. In this process, graphite-based materials are subjected to an electrochemical reaction in an electrolyte solution, leading to the exfoliation and formation of GQDs[
29].
Electrochemical exfoliation involves the application of an electric potential to a graphite-based electrode immersed in an electrolyte solution[
30]. During the electrochemical reaction, intercalated ions between the graphene layers are removed, leading to the exfoliation of graphite flakes into GQDs[
31]. The exfoliated GQDs are then dispersed in the electrolyte solution, where they can be collected and further processed[
11].
Electrochemical exfoliation offers scalability for large-scale production of GQDs, alongside control over size, morphology, and surface chemistry through parameter adjustments, while being environmentally friendly and cost-effective due to its use of aqueous electrolyte solutions and simple equipment[
32]; however, limitations include variable quality and potential contamination from impurities, as well as electrode degradation from prolonged reactions[
33]. Applications range from energy storage in supercapacitors and batteries to sensing platforms for various analytes and catalysis for chemical reactions like ORR, HER, and photocatalytic water splitting[
33].
Electrochemical exfoliation stands as a promising method for synthesizing graphene quantum dots with tailored properties for a wide range of applications, including energy storage, sensing, and catalysis[
11]. Ongoing research efforts are focused on optimizing this method and exploring new avenues for the application of GQDs in emerging fields[
5].
Figure 5.
Electrochemical Exfoliation for Synthesizing Graphene Quantum Dots synthesis method.
Figure 5.
Electrochemical Exfoliation for Synthesizing Graphene Quantum Dots synthesis method.
2.1.4. Microwave-Assisted Exfoliation for Synthesizing Graphene Quantum Dots:
Microwave-assisted exfoliation is a rapid and efficient technique used for the synthesis of graphene quantum dots (GQDs) from graphite or graphite oxide precursors[
34,
35]. This method harnesses microwave irradiation to induce the exfoliation of graphite-based materials, resulting in the formation of GQDs with controlled properties[
36].
Microwave-assisted exfoliation utilizes microwave irradiation to induce the exfoliation of graphite or graphite oxide precursors in the presence of a suitable solvent[
37]. The microwave energy rapidly heats the precursor material, leading to the expansion and delamination of graphene layers and the formation of GQDs[
38]. The solvent plays a crucial role in facilitating the exfoliation process and stabilizing the resulting GQDs in dispersion[
39].
Microwave-assisted exfoliation facilitates rapid synthesis of GQDs with high yields and reproducibility, alongside control over size, morphology, and properties through adjustable parameters[
40], while employing eco-friendly solvents to minimize environmental impact; however, scalability may be limited due to reactor throughput and precise parameter control requirements, with risks of overheating and dependency on specialized equipment[
41]. Applications span biomedical imaging for fluorescence and bioimaging, sensing platforms for various analytes, and energy conversion/storage in supercapacitors, batteries, and solar cells, leveraging GQDs' optical, electronic, and electrochemical properties[
42].
Microwave-assisted exfoliation represents a promising method for synthesizing graphene quantum dots with tailored properties for various applications in biomedicine, sensing, energy conversion, and beyond[
43]. Further research is needed to optimize this method and explore its full potential in emerging fields[
44].
Figure 6.
Microwave-Assisted Exfoliation for Synthesizing Graphene Quantum Dots.
Figure 6.
Microwave-Assisted Exfoliation for Synthesizing Graphene Quantum Dots.
2.2. Bottom-Up Synthesis Techniques:
Bottom-up approaches involve the synthesis of GQDs from molecular precursors through various chemical or physical processes[
45]. These methods offer precise control over the size, shape, and surface properties of the GQDs, leading to enhanced optical and electronic properties[
46]. Common bottom-up techniques include hydrothermal/solvothermal synthesis, pyrolysis, chemical vapor deposition (CVD), and electrochemical synthesis[
47]. Hydrothermal/solvothermal synthesis, in particular, has emerged as a promising method for producing GQDs with high quantum yield, narrow size distribution, and excellent photoluminescent properties[
5]. However, bottom-up approaches often require complex synthetic routes, expensive precursors, and specialized equipment, limiting their scalability and practicality for large-scale production[
48].
2.2.1. Hydrothermal/Solvothermal Synthesis for Synthesizing Graphene Quantum Dots:
Hydrothermal/solvothermal synthesis is a widely utilized method for the production of graphene quantum dots (GQDs) from carbon precursors under high-temperature and high-pressure conditions in aqueous or organic solvents[
5]. This technique offers precise control over the size, morphology, and surface properties of the resulting GQDs, making it suitable for various applications[
49].
Hydrothermal/solvothermal synthesis involves the reaction of carbon precursors, such as organic molecules or carbon-based materials, in a high-temperature and high-pressure environment, typically in the presence of water (hydrothermal) or organic solvents (solvothermal)[
49]. The carbon precursors undergo nucleation, polymerization, and carbonization processes, leading to the formation of GQDs. The controlled synthesis conditions allow for the precise control over the size, structure, and properties of the synthesized GQDs[
5].
The hydrothermal method offers a straightforward and expeditious approach to fabricate graphene quantum dots (GQDs)[
50,
51]. By employing various macromolecular or small molecular substances as starting materials, GQDs are ultimately obtained through high-temperature and high-pressure conditions[
52]. The fundamental principle involves breaking the bonds between carbon materials to form GQDs via high-temperature processing under elevated pressure[
53].
Hydrothermal/solvothermal synthesis offers a versatile and efficient method for synthesizing graphene quantum dots with tailored properties for various applications in biomedicine, sensing, energy conversion, and beyond[
54]. Further research is needed to optimize this method and explore its full potential in emerging fields.
Figure 7.
Hydrothermal/Solvothermal Synthesis for Synthesizing Graphene Quantum Dots.
Figure 7.
Hydrothermal/Solvothermal Synthesis for Synthesizing Graphene Quantum Dots.
2.2.2. Pyrolysis for Synthesizing Graphene Quantum Dots:
Pyrolysis is a versatile and efficient technique used for the synthesis of graphene quantum dots (GQDs) from carbon-rich precursors through the thermal decomposition and carbonization processes[
55]. This method offers control over the size, structure, and properties of the resulting GQDs, making it suitable for various applications.
Pyrolysis involves the thermal decomposition of carbon-rich precursors, such as small organic molecules, polymers, or carbon nanoparticles, at high temperatures in an inert atmosphere[
43,
56]. During pyrolysis, the carbon precursors undergo decomposition and carbonization processes, leading to the formation of carbonaceous materials, including GQDs[
57]. The controlled synthesis conditions allow for the precise control over the size, morphology, and surface properties of the synthesized GQDs[
43].
The carbonization pyrolysis method, also known as small-molecule carbonization, has emerged as a straightforward approach owing to ongoing research on GQDs[
58,
59]. The fundamental mechanism involves heating the small-molecule precursor carbon source material above its melting point using specialized instruments. Upon reaching a certain temperature, the material undergoes condensation to yield small-molecule graphene quantum dot material[
60].
Figure 8.
Pyrolysis for Synthesizing Graphene Quantum Dots.
Figure 8.
Pyrolysis for Synthesizing Graphene Quantum Dots.
2.2.3. Chemical Vapor Deposition (CVD) for Synthesizing Graphene Quantum Dots:
Chemical Vapor Deposition (CVD) is a versatile and widely employed technique for the synthesis of graphene quantum dots (GQDs) with precise control over their size, structure, and properties[
43,
61]. In this method, carbon-containing precursor gases are thermally decomposed on a catalytic substrate at high temperatures, resulting in the nucleation and growth of GQDs.
CVD involves the deposition of carbon atoms from gas-phase precursor molecules onto a substrate surface under controlled conditions[
62,
63]. In the case of GQD synthesis, carbon-containing precursor gases, such as methane (CH4) or ethylene (C2H4), are introduced into a reaction chamber containing a catalytic substrate, typically a transition metal catalyst like copper (Cu) or nickel (Ni)[
64]. Upon heating to high temperatures (typically above 800°C), the precursor molecules decompose, and carbon atoms reassemble and nucleate on the substrate surface to form GQDs[
65].
Chemical Vapor Deposition (CVD) provides precise control over GQDs' size, morphology, and structure, yielding high crystallinity and scalability for industrial-scale production, while being versatile across substrate materials and catalysts[
66]; however, challenges include equipment complexity, potential catalyst contamination, and substrate compatibility issues, necessitating specialized equipment and optimization for specific applications[
66]. Applications range from optoelectronic devices like LEDs to sensing platforms for analytes detection and catalysis for various chemical reactions, leveraging GQDs' optical, electronic, and catalytic properties[
67].
Chemical Vapor Deposition (CVD) emerges as a powerful method for synthesizing graphene quantum dots with precise control over their properties, enabling various applications in optoelectronics, sensing, catalysis, and beyond[
68]. Further research efforts are focused on optimizing CVD parameters and exploring new applications of GQDs in emerging fields.
Figure 9.
Chemical Vapor Deposition (CVD) for Synthesizing Graphene Quantum Dots.
Figure 9.
Chemical Vapor Deposition (CVD) for Synthesizing Graphene Quantum Dots.
2.2.4. Electrochemical Synthesis of Graphene Quantum Dots:
Electrochemical synthesis is a versatile and efficient method for the production of graphene quantum dots (GQDs), offering precise control over their size, morphology, and properties[
69]. This technique involves the electrochemical reduction of graphite-based precursors in suitable electrolyte solutions, leading to the formation of GQDs.
Electrochemical synthesis of GQDs involves the reduction of graphene oxide (GO) or other graphite-based precursors at the electrode/electrolyte interface under an applied electric potential[
70]. Typically, a conductive substrate, such as indium tin oxide (ITO) or glassy carbon electrode, is employed as the working electrode, while a reference electrode (e.g., Ag/AgCl) and a counter electrode (e.g., platinum) complete the electrochemical cell[
71]. The reduction process results in the formation of GQDs with controlled size and surface chemistry[
72].
The electrochemical oxidation method involves the oxidation and decomposition of carbon-carbon bonds present in graphite, graphene, or carbon nanotubes, leading to the formation of graphene quantum dots (GQDs) at a high redox voltage range of +1.5 to +3 V[
32].
Figure 10.
Electrochemical Synthesis of Graphene Quantum Dots.
Figure 10.
Electrochemical Synthesis of Graphene Quantum Dots.
3. Applications of GQDs:
The unique properties of GQDs make them highly versatile materials with diverse applications[
73]. In electronics, GQDs have been employed in field-effect transistors, light-emitting diodes, and solar cells due to their high carrier mobility, excellent conductivity, and tunable bandgap[
67]. In biomedicine, GQDs have shown great potential as contrast agents for bioimaging, drug delivery vehicles, and theranostic agents for cancer therapy[
74]. Moreover, GQDs have been used in environmental sensing, photocatalysis, and energy storage applications due to their high surface area, chemical stability, and photocatalytic activity[
3].
Figure 11.
various applications of GQD.
Figure 11.
various applications of GQD.
4. Conclusion and Future Perspectives:
In summary, the synthesis of GQDs is a rapidly evolving field with numerous techniques available for producing GQDs with tailored properties. Both top-down and bottom-up approaches offer unique advantages and limitations, and the choice of method depends on the specific requirements of the desired applications. Further research is needed to address the existing challenges in GQD synthesis such as scalability, reproducibility, and cost-effectiveness. Future efforts should focus on developing novel synthesis techniques, optimizing existing methods, and exploring new applications of GQDs in emerging fields such as quantum computing, flexible electronics, and wearable devices.
References
- Kundu, S. and V.K. Pillai, Synthesis and characterization of graphene quantum dots. Physical Sciences Reviews, 2020. 5(4): p. 20190013. [CrossRef]
- Gupta, G.K., et al., In situ fabrication of activated carbon from a bio-waste desmostachya bipinnata for the improved supercapacitor performance. Nanoscale research letters, 2021. 16(1): p. 85. [CrossRef]
- Kumar, Y.R., et al., Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: a review. RSC advances, 2020. 10(40): p. 23861-23898. [CrossRef]
- Biswas, A., et al., Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Advances in colloid and interface science, 2012. 170(1-2): p. 2-27.
- Ghaffarkhah, A., et al., Synthesis, applications, and prospects of graphene quantum dots: a comprehensive review. Small, 2022. 18(2): p. 2102683. [CrossRef]
- Sagar, P., et al., Tagetes erecta as an organic precursor: synthesis of highly fluorescent CQDs for the micromolar tracing of ferric ions in human blood serum. RSC advances, 2021. 11(32): p. 19924-19934. [CrossRef]
- Mansuriya, B.D. and Z. Altintas, Applications of graphene quantum dots in biomedical sensors. Sensors, 2020. 20(4): p. 1072. [CrossRef]
- Bacon, M., S.J. Bradley, and T. Nann, Graphene quantum dots. Particle & Particle Systems Characterization, 2014. 31(4): p. 415-428.
- Chen, Y.-M., et al., Cs4PbBr6/CsPbBr3 perovskite composites with near-unity luminescence quantum yield: large-scale synthesis, luminescence and formation mechanism, and white light-emitting diode application. ACS applied materials & interfaces, 2018. 10(18): p. 15905-15912.
- Gu, S., et al., Optimization of graphene quantum dots by chemical exfoliation from graphite powders and carbon nanotubes. Materials Chemistry and Physics, 2018. 215: p. 104-111. [CrossRef]
- Danial, W.H., et al., A short review on electrochemical exfoliation of graphene and graphene quantum dots. Carbon Letters, 2021. 31: p. 371-388. [CrossRef]
- Lavin-Lopez, M.d.P., et al., Influence of different improved hummers method modifications on the characteristics of graphite oxide in order to make a more easily scalable method. Industrial & Engineering Chemistry Research, 2016. 55(50): p. 12836-12847.
- Esencan Turkaslan, B. and M. Filiz Aydin, Optimizing parameters of graphene derivatives synthesis by modified improved Hummers. Mathematical Methods in the Applied Sciences, 2020. [CrossRef]
- Suhaimin, N.S., et al., The evolution of oxygen-functional groups of graphene oxide as a function of oxidation degree. Materials Chemistry and Physics, 2022. 278: p. 125629. [CrossRef]
- Tajik, S., et al., Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC advances, 2020. 10(26): p. 15406-15429. [CrossRef]
- Chen, W., et al., The Preparation Approaches of Polymer/graphene Nanocomposites and their Application Research Progress as Electrochemical Sensors. Journal of New Materials for Electrochemical Systems, 2017. 20(4).
- Kumar, P., et al., Graphene quantum dots: A contemporary perspective on scope, opportunities, and sustainability. Renewable and Sustainable Energy Reviews, 2022. 157: p. 111993. [CrossRef]
- Dananjaya, V., et al., Synthesis, properties, applications, 3D printing and machine learning of graphene quantum dots in polymer nanocomposites. Progress in Materials Science, 2024: p. 101282. [CrossRef]
- Kansara, V., et al., Graphene quantum dots: Synthesis, optical properties and navigational applications against cancer. Materials Today Communications, 2022. 31: p. 103359. [CrossRef]
- Baruah, S. and J. Dutta, Nanotechnology applications in pollution sensing and degradation in agriculture: a review. Environmental Chemistry Letters, 2009. 7: p. 191-204. [CrossRef]
- Bokare, A., S. Chinnusamy, and F. Erogbogbo, Tio2-graphene quantum dots nanocomposites for photocatalysis in energy and biomedical applications. Catalysts, 2021. 11(3): p. 319. [CrossRef]
- Calabro, R.L., D.-S. Yang, and D.Y. Kim, Liquid-phase laser ablation synthesis of graphene quantum dots from carbon nano-onions: Comparison with chemical oxidation. Journal of colloid and interface science, 2018. 527: p. 132-140. [CrossRef]
- Yogesh, G.K., et al., Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: a review. Applied Physics A, 2021. 127: p. 1-40. [CrossRef]
- Zeng, H., et al., Nanomaterials via laser ablation/irradiation in liquid: a review. Advanced Functional Materials, 2012. 22(7): p. 1333-1353. [CrossRef]
- Voloshko, A. and T.E. Itina, Nanoparticle formation by laser ablation and by spark discharges—properties, mechanisms, and control possibilities. Nanoparticles Technology, 2015: p. 1-12.
- Kim, K. and T. Kim, Nanofabrication by thermal plasma jets: From nanoparticles to low-dimensional nanomaterials. Journal of Applied Physics, 2019. 125(7). [CrossRef]
- Calabro, R.L., Laser Ablation in Liquid for the Controlled Production of Photoluminescent Graphene Quantum Dots and Upconverting Nanoparticles. 2020.
- Bolotsky, A., et al., Two-dimensional materials in biosensing and healthcare: from in vitro diagnostics to optogenetics and beyond. ACS nano, 2019. 13(9): p. 9781-9810. [CrossRef]
- Al Jahdaly, B.A., et al., Outstanding graphene quantum dots from carbon source for biomedical and corrosion inhibition applications: A review. Sustainability, 2021. 13(4): p. 2127. [CrossRef]
- Lei, Y., et al., Electrochemical characterization of graphene-type materials obtained by electrochemical exfoliation of graphite. Journal of Electroanalytical Chemistry, 2021. 887: p. 115084. [CrossRef]
- Low, C., et al., Electrochemical approaches to the production of graphene flakes and their potential applications. Carbon, 2013. 54: p. 1-21. [CrossRef]
- Liu, W., et al., Graphene quantum dots-based advanced electrode materials: design, synthesis and their applications in electrochemical energy storage and electrocatalysis. Advanced Energy Materials, 2020. 10(29): p. 2001275. [CrossRef]
- Radjenovic, J. and D.L. Sedlak, Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental science & technology, 2015. 49(19): p. 11292-11302. [CrossRef]
- Yuan, J.M., et al., graphene oxide quantum dots exfoliated from carbon fibers by microwave irradiation: two photoluminescence centers and self-assembly behavior. Small, 2018. 14(20): p. 1703714. [CrossRef]
- Gupta, G.K., et al., Hydrothermally synthesized nickel ferrite nanoparticles integrated reduced graphene oxide nanosheets as an electrode material for supercapacitors. Journal of Materials Science: Materials in Electronics, 2024. 35(3): p. 255. [CrossRef]
- Zeng, Z., et al., Graphene quantum dots (GQDs) and its derivatives for multifarious photocatalysis and photoelectrocatalysis. Catalysis Today, 2018. 315: p. 171-183. [CrossRef]
- Devi, N., R. Kumar, and R.K. Singh, Microwave-assisted modification of graphene and its derivatives: Synthesis, reduction and exfoliation. Graphene Functionalization Strategies: From Synthesis to Applications, 2019: p. 279-311.
- Schwenke, A.M., S. Hoeppener, and U.S. Schubert, Synthesis and modification of carbon nanomaterials utilizing microwave heating. Advanced Materials, 2015. 27(28): p. 4113-4141. [CrossRef]
- He, P., et al., Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chemistry of Materials, 2015. 27(1): p. 218-226. [CrossRef]
- Li, K., et al., Technical synthesis and biomedical applications of graphene quantum dots. Journal of Materials Chemistry B, 2017. 5(25): p. 4811-4826. [CrossRef]
- Savitskaya, T., et al., Process Technology and Sustainable Development.
- Lightcap, I.V. and P.V. Kamat, Graphitic design: prospects of graphene-based nanocomposites for solar energy conversion, storage, and sensing. Accounts of chemical Research, 2013. 46(10): p. 2235-2243. [CrossRef]
- Kadian, S., S.K. Sethi, and G. Manik, Recent advancements in synthesis and property control of graphene quantum dots for biomedical and optoelectronic applications. Materials Chemistry Frontiers, 2021. 5(2): p. 627-658. [CrossRef]
- Allioui, H. and Y. Mourdi, Exploring the full potentials of IoT for better financial growth and stability: A comprehensive survey. Sensors, 2023. 23(19): p. 8015. [CrossRef]
- Shi, L., B. Wang, and S. Lu, Efficient bottom-up synthesis of graphene quantum dots at an atomically precise level. Matter, 2023. 6(3): p. 728-760. [CrossRef]
- Rani, P., R. Dalal, and S. Srivastava, Effect of surface modification on optical and electronic properties of graphene quantum dots. Applied Surface Science, 2023. 609: p. 155379. [CrossRef]
- de Souza, F. and R.K. Gupta, Bottom-Up Synthesis of 2D Nanomaterials for Energy Applications, in 2D Nanomaterials. 2022, CRC Press. p. 53-70.
- González, C.M.O., et al., Scalable Synthesis of Nanomaterials. Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, 2021: p. 899-921.
- Haque, E., et al., Recent advances in graphene quantum dots: synthesis, properties, and applications. Small Methods, 2018. 2(10): p. 1800050. [CrossRef]
- Tshangana, C.S., et al., The applications of graphene oxide quantum dots in the removal of emerging pollutants in water: An overview. Journal of Water Process Engineering, 2021. 43: p. 102249. [CrossRef]
- Gupta, G.K. and K.K. Shandilya, Hierarchical Ni-Mn Double Layered/Graphene Oxide with Excellent Energy Density for Highly Capacitive Supercapacitors. Mater Sci, 2023. 11: p. 004.
- Iannazzo, D., I. Ziccarelli, and A. Pistone, Graphene quantum dots: multifunctional nanoplatforms for anticancer therapy. Journal of Materials Chemistry B, 2017. 5(32): p. 6471-6489. [CrossRef]
- Shaari, N., S.K. Kamarudin, and R. Bahru, Carbon and graphene quantum dots in fuel cell application: An overview. International Journal of Energy Research, 2021. 45(2): p. 1396-1424. [CrossRef]
- Du, Y. and S. Guo, Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale, 2016. 8(5): p. 2532-2543. [CrossRef]
- Abbas, A., L.T. Mariana, and A.N. Phan, Biomass-waste derived graphene quantum dots and their applications. Carbon, 2018. 140: p. 77-99. [CrossRef]
- Devi, M., S. Rawat, and S. Sharma, A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxford Open Materials Science, 2021. 1(1): p. itab014. [CrossRef]
- Zhang, B., Y. Jiang, and R. Balasubramanian, Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: a critical review. Journal of Materials Chemistry A, 2021. 9(44): p. 24759-24802. [CrossRef]
- Saadiah, M., et al., Functionalization of carbon and graphene quantum dots, in Quantum Dots. 2023, Elsevier. p. 335-381.
- Gupta, G.K., et al., Hierarchical NiMn Double Layered/Graphene with Excellent Energy Density for Highly Capacitive Supercapacitors. 2021.
- Xu, A., et al., Carbon-based quantum dots with solid-state photoluminescent: mechanism, implementation, and application. Small, 2020. 16(48): p. 2004621.
- Gupta, G.K., et al., Excellent supercapacitive performance of graphene quantum dots derived from a bio-waste marigold flower (Tagetes erecta). International Journal of Hydrogen Energy, 2021. 46(77): p. 38416-38424. [CrossRef]
- Creighton, J. and P. Ho, Introduction to chemical vapor deposition (CVD). Chemical vapor deposition, 2001. 2: p. 1-22.
- García-Ruiza, D.L., et al., “Synthesis of carbon nanomaterials by chemical. Handbook of Greener Synthesis of Nanomaterials and Compounds: Volume 1: Fundamental Principles and Methods, 2021. 1: p. 273.
- García-Ruiza, D.L., et al., “Synthesis of carbon nanomaterials by chemical. Handbook of Greener Synthesis of Nanomaterials and Compounds: Volume 1: Fundamental Principles and Methods, 2021. 1: p. 273.
- Tian, P., et al., Graphene quantum dots from chemistry to applications. Materials today chemistry, 2018. 10: p. 221-258. [CrossRef]
- Yan, Y., et al., Recent advances on graphene quantum dots: from chemistry and physics to applications. Advanced materials, 2019. 31(21): p. 1808283. [CrossRef]
- Sheikh Mohd Ghazali, S.A.I., et al., Graphene quantum dots: A comprehensive overview. Open Chemistry, 2023. 21(1): p. 20220285. [CrossRef]
- Lin, L., et al., Bridging the gap between reality and ideal in chemical vapor deposition growth of graphene. Chemical reviews, 2018. 118(18): p. 9281-9343. [CrossRef]
- Nesakumar, N., S. Srinivasan, and S. Alwarappan, Graphene quantum dots: Synthesis, properties, and applications to the development of optical and electrochemical sensors for chemical sensing. Microchimica Acta, 2022. 189(7): p. 258. [CrossRef]
- Shen, T., et al., Quantum dot-carbonaceous nanohybrid composites: preparation and application in electrochemical energy storage. Journal of Materials Chemistry A, 2020. 8(43): p. 22488-22506. [CrossRef]
- Shinwari, M.W., et al., Microfabricated reference electrodes and their biosensing applications. Sensors, 2010. 10(3): p. 1679-1715. [CrossRef]
- Zhu, S., et al., Control the size and surface chemistry of graphene for the rising fluorescent materials. Chemical communications, 2012. 48(38): p. 4527-4539. [CrossRef]
- Wang, Z., H. Zeng, and L. Sun, Graphene quantum dots: versatile photoluminescence for energy, biomedical, and environmental applications. Journal of Materials Chemistry C, 2015. 3(6): p. 1157-1165. [CrossRef]
- Biswas, M.C., et al., Graphene quantum dots (GQDs) for bioimaging and drug delivery applications: a review. ACS Materials Letters, 2021. 3(6): p. 889-911. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).