Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Host-Based Factors Affecting Pathogen-Intrusion
2.1. Immune Attenuation
2.2. Developmental Stage
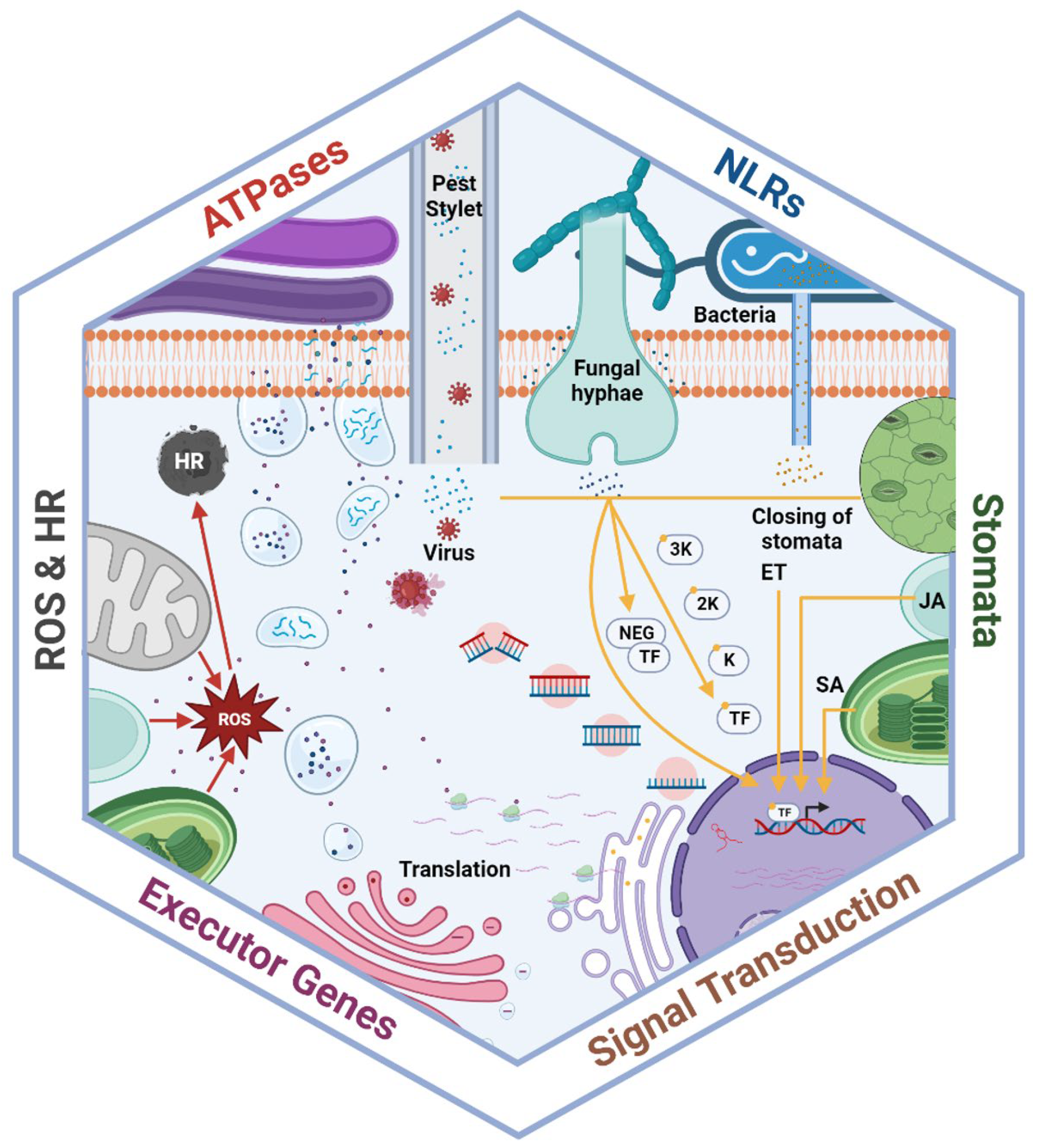
3. Underlying Mechanisms to Counter Pathogen Attack
3.1. Physiological Mechanisms
3.1.1. Stomatal Immunity
3.1.2. Hypersensitive Response, Reactive Oxygen Species Burst, and cell Wall Modifications
3.1.3. Phytohormones
3.2. Molecular Mechanisms Underpinning R Genes
3.2.1. Host Reprogramming by Passive Loss of Susceptibility
3.2.2. Disrupting Interaction with Host Key Targets
3.2.3. NLRs Activation by Direct Intracellular Recognition of Effectors
3.2.4. Active Loss of Susceptibility
3.2.5. TAL Effector-Dependent Expression of Executor Genes
| Crop specie | Gene | Protein type | Disease | Pathogen | Reference |
|---|---|---|---|---|---|
| Barley | Stb6 | Receptor kinase | Septoria tritici blotch | Zymoseptoria tritici | [198] |
| Mla1 | NB-LRR | Powdery mildew | Blumeria graminis | [199] | |
| Mla6 | NB-LRR | Powdery mildew | B. graminis | [200] | |
| Rpg1 | Protein kinase | Stem rust | Puccinia graminis | [201] | |
| Wheat | Pm3 | NB-LRR | Powdery mildew | B. gramini | [202] |
| Lr10 | NB-LRR | Leaf rust | P. triticina | [83] | |
| Lr21 | NB-LRR | Leaf rust | P. triticina | [202] | |
| Maize | ZmTrxh | H-type thioredoxin | Lethal necrosis | Sugarcane mosaic virus | [203] |
| Rp1-D | NB-LRR | Leaf rust | Puccinia sorghi | [204] | |
| Rxo1 | NB-LRR | Bacterial streak | Xanthomonas oryzae | [205] | |
| Hm1 | HC toxin reductase | corn leaf blight | Cochliobolus carbonum | [121] | |
| Hm2 | HC toxin reductase | corn leaf blight | C. carbonum | [206] | |
| Rp3 | NB-LRR | Leaf rust | Puccinia sorghi | [57] | |
| qRfg1 | CCT domain gene | Gibberella stalk rot | Fusariumgraminearum | [208] | |
| qMdr9.02 | Lignin biosynthesis | Multiple | Multiple | [209] | |
| Rice | Piz-t | NB-LRR | Rice blast | Magnaporthe oryzae | [210] |
| Pi-ta | NB-LRR | Rice blast | M. oryzae | [211] | |
| Pi-b | NB-LRR | Rice blast | M. oryzae | [212] | |
| Pi-d2 | B-lectin receptor kinase | Rice blast | M. oryzae | [213] | |
| Pi9 | NB-LRR | Rice blast | M. oryzae | [214] | |
| RGA 5 | NB-LRR | Rice blast | M. oryzae | [215] | |
| Xa1 | NB-LRR | Bacterial blight | X. oryzae | [216] | |
| Xa5 | TFIIA Transcription factor | Bacterial blight | X. oryzae | [217] | |
| Xa7 | Executer R protein | Bacterial blight | X. oryzae | [218] | |
| Xa10 | Executer R protein | Bacterial blight | X. oryzae | [126] | |
| Xa21 | Receptor kinase | Bacterial blight | X. oryzae | [219] | |
| Xa23 | Executer R protein | Bacterial blight | X. oryzae | [127] | |
| Xa26 | Receptor kinase | Bacterial blight | X. oryzae | [220] | |
| Xa27 | No homolog | Bacterial blight | X. oryzae | [128] | |
| Arabidopsis thaliana | FLS2 | NB-LRR | Necrosis | Pseudomonas syringae | [221] |
| RPM1 | NB-LRR | Necrosis | Peronospora parasitica | [222] | |
| RSP2 | NB-LRR | Necrosis | P. syringae | [223] | |
| Tomato | Cf-2 | NB-LRR | Leaf mold | Cladosporium fulvum | [159] |
| Prf | NB-LRR | Necrosis | P. syringae | [157] |
3.3. Metagenomic Dynamics
4. Strategies to Exploit Innate Immune Responses of the Host for Disease Resistance
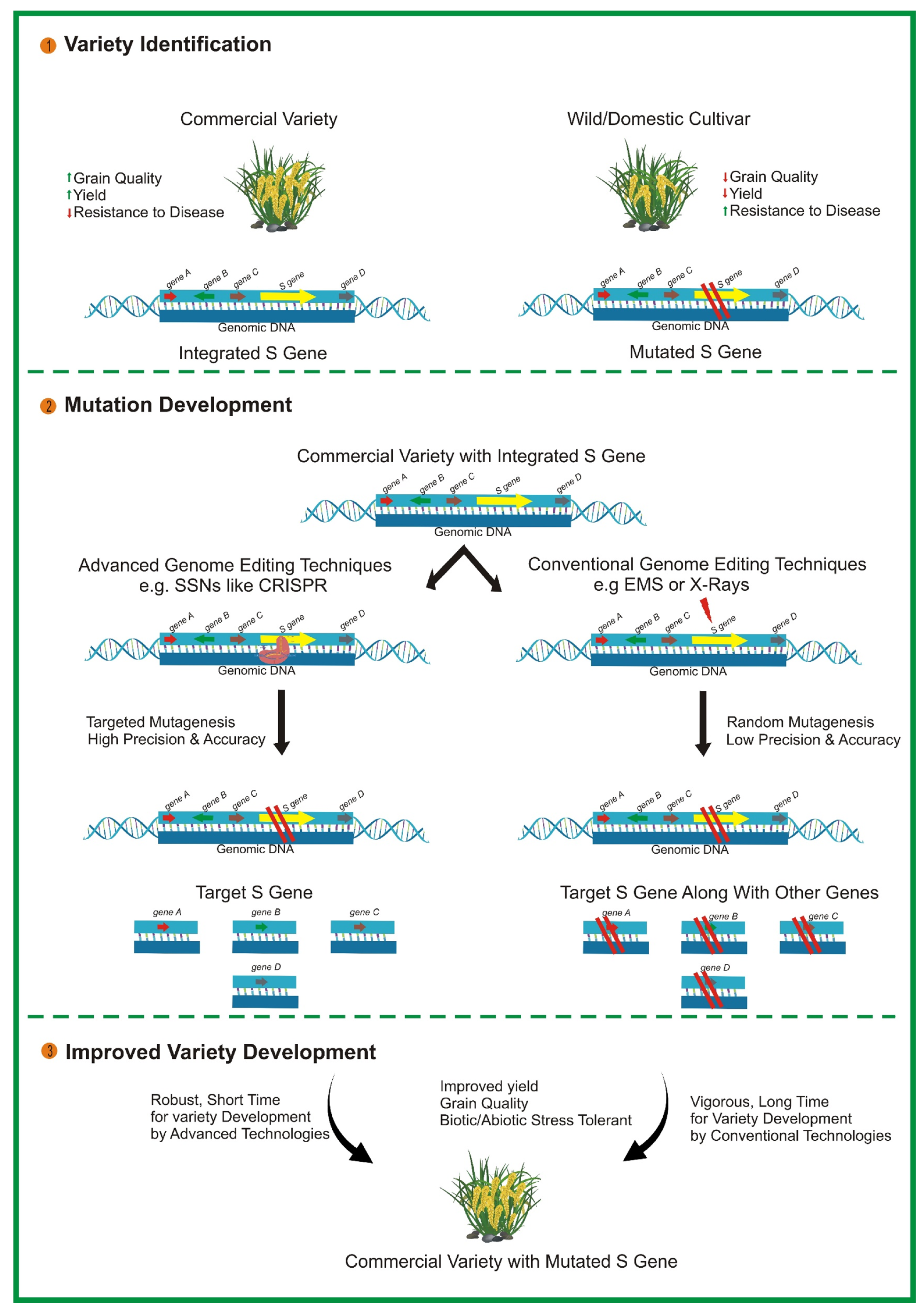
4.1. Introgression of R Genes from Wild Species
4.2. Identification and Acceleration of of R Gene Cloning
4.3. CRISPR-Cas9-Mediated Genome Engineering to Confer Disease Resistance
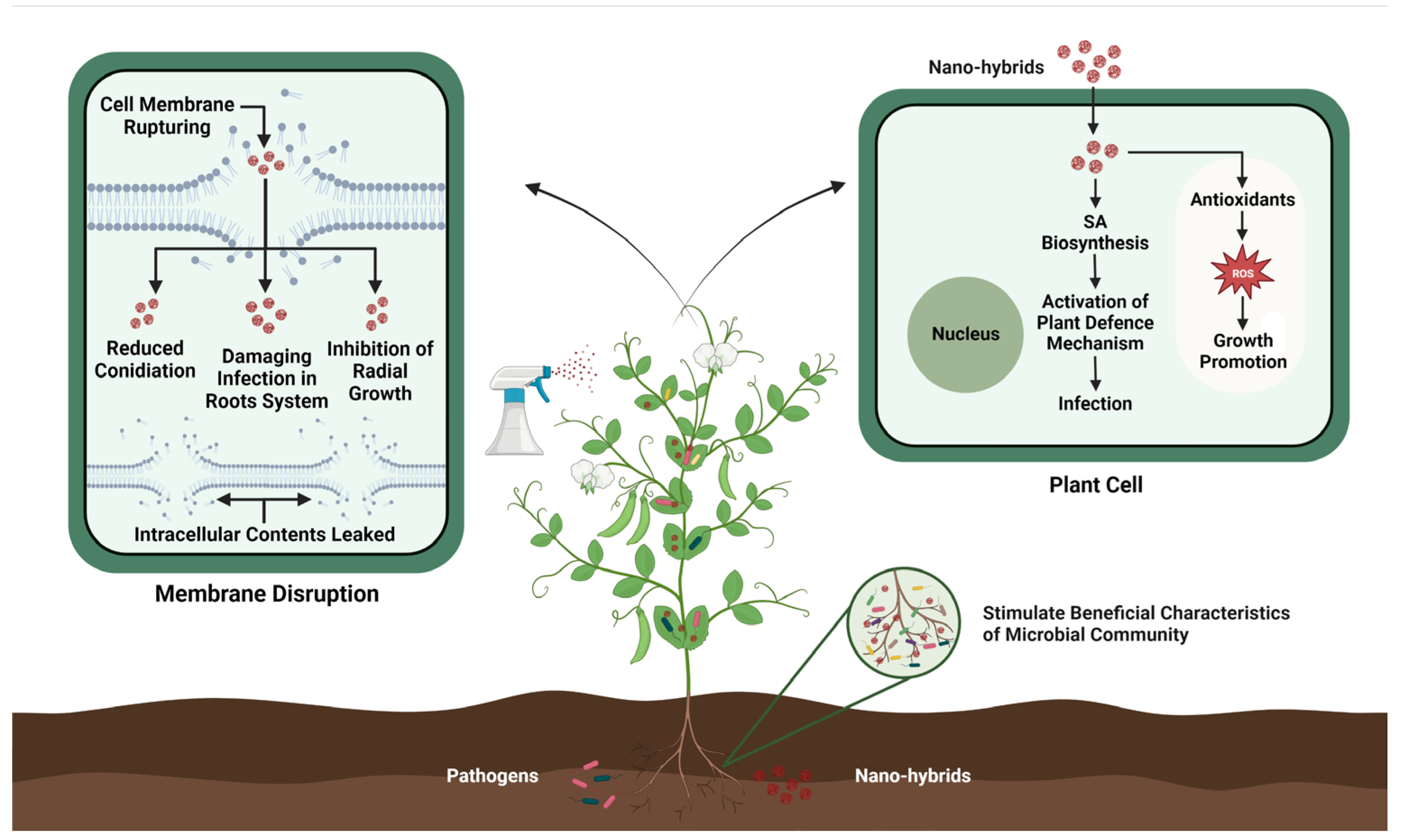
4.4. Nanohyrbid-Induced Plant Disease Resistance
5. Challenges and Future Directions
5.1. Food Safety Issues
5.2. Nano Vehicle-Based Gene Delivery to Enhance Disease Resistance Traits
5.3. Public Acceptance and Awareness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflict of interest
References
- Prospects, U.N. Highlights (ST/ESA/SER. A/423): United Nations, Department of Economic and Social Affairs, Population Division 2019. World Population.
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant. Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bindraban, P.S.; Dimkpa, C.O.; Angle, S.; Rabbinge, R. Unlocking multiple public good services from balanced fertilizers. Food Secur. 2018, 10, 273–285. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Bai, Y.; Wei, Y.; Reiter, R.J.; Shi, H. Phytomelatonin as a central molecule in plant disease resistance. J. Exp. Bot 2022, 73, 5874–5885. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sadat, M.A.; Choi, J. Wheat blast: A new fungal inhabitant to Bangladesh threatening world wheat production. Plant Pathol. J. 2017, 33, 103. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Castroagudin, V.L.; Rodrigues, F.A.; Rios, J.A.; Eduardo Aucique-Pérez, C.; Moreira, S.I.; Maciel, J.L.N. Wheat blast: Past, present, and future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Sawaira, T.; Kareem, A.T.; Hussain, N.; Din, M.I.; Iqbal, H. Occurrence, toxic effects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials—A review. Chemosphere 2022, 293, 133538. [Google Scholar] [CrossRef]
- St. Clair, D.A. Quantitative disease resistance and quantitative resistance loci in breeding. Annu. Rev. Phytopathol. 2010, 48, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Kourelis, J.; Van Der Hoorn, R.A. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell. 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defense responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Niks, R.E.; Qi, X.; Marcel, T.C. Quantitative resistance to biotrophic filamentous plant pathogens: Concepts, misconceptions, and mechanisms. Annu. Rev. Phytopathol. 2015, 53, 445–470. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhou, M.; Yoo, H.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. 2015, 112, 9166–9173. [Google Scholar] [CrossRef]
- David, L.; Harmon, A.C.; Chen, S. Plant immune responses-from guard cells and local responses to systemic defense against bacterial pathogens. Plant Signal. Behav. 2019, 14, e1588667. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays. Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Das, P.; Panda, D.; Xie, K.; Baig, M.J.; Molla, K.A. A detailed landscape of CRISPR-Cas-mediated plant disease and pest management. Plant Sci. 2022, 323, 111376. [Google Scholar] [CrossRef]
- Ganesh Bonagiri, D.D.G.; Kuri, A.; Kumar, V. CRISPR/cas9 gene editing tool for diseases resistant varieties. J. Pharm. Innov. 2022, 11, 2731–2738. [Google Scholar]
- Liu, T.; Ji, J.; Cheng, Y.; Zhang, S.; Wang, Z.; Duan, K.; Wang, Y. CRISPR/Cas9-mediated editing of GmTAP1 confers enhanced resistance to Phytophthora sojae in soybean. J Integr. Plant Biol. 2023, 65, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, S.; Jiang, N.; Zhao, X.; Bai, Z.; Liu, J.; Yang, Y. Engineering of rice varieties with enhanced resistances to both blast and bacterial blight diseases via CRISPR/Cas9. Plant Biotechnol J. 2022, 20, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Macho, A.P.; Han, Z.; Hu, Z.; Zipfel, C.; Chai, J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013, 342, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Xin, X.F. Pattern recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.J.; Chen, H.; Day, B. The lifecycle of the plant immune system. Cri. Rev. plant. Sci. 2020, 39, 72–100. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Song, E.H.; Nguyen, X.C.; Lee, K.; Kim, K.E.; Kim, H.S.; Chung, W.S. Arabidopsis MAP kinase phosphatase 1 is phosphorylated and activated by its substrate AtMPK6. Plant Cell. Rep. 2011, 30, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wan, Y.; Anderson, J.C.; Hou, J.; Islam, S.M.; Cheng, J.; Peck, S.C. Genetic dissection of Arabidopsis MAP kinase phosphatase 1-dependent PAMP-induced transcriptional responses. J. Exp. Bot. 2017, 68, 5207–5220. [Google Scholar] [CrossRef]
- Anderson, J.C.; Bartels, S.; Besteiro, M.A.G.; Shahollari, B.; Ulm, R.; Peck, S.C. Arabidopsis MAP Kinase Phosphatase 1 (AtMKP1) negatively regulates MPK6-mediated PAMP responses and resistance against bacteria. Plant. J. 2011, 67, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Dong, X. Post-translational regulation of plant immunity. Curr. Opin. Plant. Biol. 2017, 38, 124–132. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 410346. [Google Scholar] [CrossRef]
- Guo, D.; Hao, C.; Hou, J.; Zhao, G.; Shan, W.; Guo, H.; Guo, X. The protein phosphatase GhAP2C1 interacts together with GhMPK4 to synergistically regulate the immune response to Fusarium oxysporum in cotton. Int. J. Mol. Sci. 2022, 23, 2014. [Google Scholar] [CrossRef]
- Segonzac, C.; Macho, A.P.; Sanmartín, M.; Ntoukakis, V.; Sánchez-Serrano, J.J.; Zipfel, C. Negative control of BAK 1 by protein phosphatase 2A during plant innate immunity. Plant Biotech. J. 2014, 33, 2069–2079. [Google Scholar] [CrossRef]
- Develey-Rivière, M.P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New. Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, L. Time to fight molecular mechanisms of age-related resistance. Phytopathology 2019, 109, 1500–1508. [Google Scholar] [CrossRef]
- Häffner, E.; Konietzki, S.; Diederichsen, E. Keeping control: The role of senescence and development in plant pathogenesis and defense. Plants 2015, 4, 449–488. [Google Scholar] [CrossRef]
- Glander, S.; He, F.; Schmitz, G.; Witten, A.; Telschow, A.; de Meaux, J. Assortment of flowering time and immunity alleles in natural Arabidopsis thaliana populations suggests immunity and vegetative lifespan strategies coevolve. Gen. Biol. Evol. 2018, 10, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ding, X.; Cai, M.; Zhao, J.; Lin, Y.; Li, X.; Wang, S. The expression pattern of a rice disease resistance gene Xa3/Xa26 is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics 2007, 177, 523–533. [Google Scholar] [CrossRef]
- Paasch, B.C.; Sohrabi, R.; Kremer, J.M.; Nomura, K.; Cheng, Y.T.; Martz, J.; He, S.Y. A critical role of a eubiotic microbiota in gating proper immunocompetence in Arabidopsis. Nat. Plants. 2023, 9, 1468–1480. [Google Scholar] [CrossRef]
- Xu, Y.P.; Lv, L.H.; Xu, Y.J.; Yang, J.; Cao, J.Y.; Cai, X.Z. Leaf stage-associated resistance is correlated with phytohormones in a pathosystem-dependent manner. J. Integr. Plant. Biol. 2018, 60, 703–722. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Rodrigues, O.; Shan, L. Stomata in a state of emergency: H2O2 is the target locked. Trends. Plant Sci. 2022, 27, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Amari, K.; Niehl, A. Nucleic acid-mediated PAMP-triggered immunity in plants. Curr. Opin. Virol. 2020, 42, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Battache, M.; Lebrun, M.H.; Sakai, K.; Soudière, O.; Cambon, F.; Langin, T.; Saintenac, C. Blocked at the stomatal gate, a key step of wheat Stb16q-mediated resistance to Zymoseptoria tritici. Front. Plant Sci. 2022, 13, 921074. [Google Scholar] [CrossRef]
- Lin, P.A.; Chen, Y.; Ponce, G.; Acevedo, F.E.; Lynch, J.P.; Anderson, C.T.; Felton, G.W. Stomata-mediated interactions between plants, herbivores, and the environment. Trends. plant Sci. 2022, 27, 287–300. [Google Scholar] [CrossRef]
- Mott, K.A.; Takemoto, J.Y. Syringomycin, a bacterial phytotoxin, closes stomata. Plant Physiol. 1989, 90, 1435–1439. [Google Scholar] [CrossRef]
- Assmann, S.M.; Jegla, T. Guard cell sensory systems: Recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant. Biol. 2016, 33, 157–167. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Sun, L.R.; Yue, C.M.; Hao, F.S. Update on roles of nitric oxide in regulating stomatal closure. Plant Signal. Behav. 2019, 14, e1649569. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant. Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Takahashi, F.; Hanada, K.; Kondo, T.; Shinozaki, K. Hormone-like peptides and small coding genes in plant stress signaling and development. Cur. Opin. Plant. Biol. 2019, 51, 88–95. [Google Scholar] [CrossRef]
- Olsson, V.; Joos, L.; Zhu, S.; Gevaert, K.; Butenko, M.A.; De Smet, I. Look closely, the beauty may be small: Precursor-derived peptides in plants. Annu. Rev. Plant. Biol. 2019, 70, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, S.; Rodrigues, O.; Wang, P.; Luo, D.; Munemasa, S.; Shan, L. Phytocytokines signaling reopens stomata in plant immunity and water loss. Nature 2022, 605, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, Y.J.; Chen, H.; Day, B. The lifecycle of the plant immune system. Cri. Rev. plant. Sci. 2020, 39, 72–100. [Google Scholar] [CrossRef] [PubMed]
- Survila, M.; Davidsson, P.R.; Pennanen, V.; Kariola, T.; Broberg, M.; Sipari, N.; Palva, E.T. Peroxidase-generated apoplastic ROS impair cuticle integrity and contribute to DAMP-elicited defenses. Front. Plant. Sci. 2016, 7, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Zipfel, C. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell. 2014, 54, 43–55. [Google Scholar] [CrossRef]
- Dat, J.F.; Pellinen, R.; Tom, Beeckman; Van De Cotte, B.; Langebartels, C.; Kangasjärvi, J.; Van Breusegem, F. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 2003, 33, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longe. 2021, 1155, 9912436. [Google Scholar] [CrossRef]
- Lehmann, S.; Serrano, M.; L’Haridon, F.; Tjamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T. Overexpression of barley oxalate oxidase gene induces partial leaf resistance to Sclerotinia sclerotiorum in transgenic oilseed rape. Plant Pathol. 2015, 64, 1407–1416. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, Y.; He, H.; Niu, L.; Guo, D.; Dong, Y. Enhanced resistance to sclerotinia stem rot in transgenic soybean that overexpresses wheat oxalate oxidase. Transgenic Res. 2019, 28, 103–114. [Google Scholar] [CrossRef]
- Verma, R.; Kaur, J. Expression of barley oxalate oxidase confers resistance against Sclerotinia sclerotiorum in transgenic Brassica juncea cv Varuna. Transgenic Res. 2021, 30, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Melida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant. J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Greger, M. A review on Si uptake and transport system. Plants 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Chen, S. Plant silicon-cell wall complexes: Identification, model of covalent bond formation and biofunction. Plant Physiol. Biochem. 2020, 155, 13–19. [Google Scholar] [CrossRef]
- Abdelrhim, A.S.; Mazrou, Y.S.; Nehela, Y.; Atallah, O.O.; El-Ashmony, R.M.; Dawood, M.F. Silicon dioxide nanoparticles induce innate immune responses and activate antioxidant machinery in wheat against Rhizoctonia solani. Plants 2021, 10, 2758. [Google Scholar] [CrossRef]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of phytohormones in plant defense: Signalling and cross talk. Mol. Plant. Pathol. 2018, 3, 159–184. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agricul. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Vos, I.A.; Moritz, L.; Pieterse, C.M.; Van Wees, S.C. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front. Plant. Sci. 2015, 6, 639. [Google Scholar] [CrossRef]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant immunity: The EDS1 regulatory node. Curr. Opin. Plant. Biol. 2005, 8, 383–389. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signaling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Dong, X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; El-Shazly, H.H.; Badr, A. Role of salicylic acid in biotic and abiotic stress tolerance in plants. Plant Phenolics in Sustainable Agriculture 2020, 1, 533–554. [Google Scholar] [CrossRef]
- Hu, C.; Wei, C.; Ma, Q.; Dong, H.; Shi, K.; Zhou, Y.; Yu, J. Ethylene response factors 15 and 16 trigger jasmonate biosynthesis in tomato during herbivore resistance. Plant Physiol. 2021, 185, 1182–1197. [Google Scholar] [CrossRef]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant. 2008, 1, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Tu, Y.; Wang, Y.; Cheng, W.; Yang, Y. Overexpression of an EIN3-binding F-box protein2-like gene caused elongated fruit shape and delayed fruit development and ripening in tomato. Plant Sci. 2018, 272, 131–141. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Kim, J.G.; Stork, W.; Mudgett, M.B. Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host. Micr. 2013, 13, 143–154. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant. Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Madonna, V.; Di Lelio, I.; Esposito, F.; Avitabile, C.; Corrado, G. Plant-to-plant communication triggered by systemin primes anti-herbivore resistance in tomatoes. Sci Rep. 2017, 7, 15522. [Google Scholar] [CrossRef] [PubMed]
- Feuillet, C.; Travella, S.; Stein, N.; Albar, L.; Nublat, A.; Keller, B. Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc. Natl. Acad. Sci. 2003, 100, 15253–15258. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan, E.; Periyannan, S.; Hickey, L.T. Harnessing adult-plant resistance genes to deploy durable disease resistance in crops. Essays. Biochem. 2022, 66, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant. Sci. 2014, 5, 641. [Google Scholar] [CrossRef]
- Piffanelli, P.; Zhou, F.; Casais, C.; Orme, J.; Jarosch, B.; Schaffrath, U.; Schulze-Lefert, P. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plan Physiol. 2002, 129, 1076–1085. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. Mlo-based resistance: A universal “weapon” to defeat powdery mildew disease. Mol. Plant-Micr. Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Yano, M. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef]
- Yang, J.; Fang, Y.; Wu, H.; Zhao, N.; Guo, X.; Mackon, E.; Li, R. Improvement of resistance to rice blast and bacterial leaf streak by CRISPR/Cas9-mediated mutagenesis of Pi21 and OsSULTR3; 6 in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1209384. [Google Scholar] [CrossRef]
- Maqbool, A.; Saitoh, H.; Franceschetti, M.; Stevenson, C.E.M.; Uemura, A.; Kanzaki, H.; Banfield, M.J. Structural basis of pathogen recognition by an integrated HMA domain in a plant NLR immune receptor. Elife 2015, 4, e08709. [Google Scholar] [CrossRef]
- de Guillen, K.; Ortiz-Vallejo, D.; Gracy, J.; Fournier, E.; Kroj, T.; Padilla, A. Structure analysis uncovers a highly diverse but structurally conserved effector family in phytopathogenic fungi. PLoS Pathog. 2015, 11, e1005228. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Foessel, S.A.; Singh, R.P.; Lillemo, M.; Huerta-Espino, J.; Bhavani, S.; Singh, S.; Lagudah, E.S. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 2014, 127, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lagudah, E. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- El-Orabey, W.M.; Hamwieh, A.; Ahmed, S.M. Molecular markers and phenotypic characterization of adult plant resistance genes Lr 34, Lr 46, Lr 67 and Lr 68 and their association with partial resistance to leaf rust in wheat. J. Genet. 2019, 98, 1–12. [Google Scholar] [CrossRef]
- Truniger, V.; Aranda, M.A. Recessive resistance to plant viruses. In Advances in Virus Research: Natural and Engineered Resistance to Plant Viruses, Part I. 2009, 75, 119–231. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, M.; Zhou, Y.A.N.; Li, X.; Xiao, J.; Wang, S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant. Cell. Environ. 2011, 34, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, S.; Zhou, J.; Yang, B. Designer TAL effectors induce disease susceptibility and resistance to Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2013, 6, 781–789. [Google Scholar] [CrossRef]
- Han, J.; Xia, Z.; Liu, P.; Li, C.; Wang, Y.; Guo, L.; Zhai, W. TALEN-based editing of TFIIAy5 changes rice response to Xanthomonas oryzae pv. Oryzae. Sci. Rep. 2020, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ovenden, B.; Milgate, A. Recent insights into barley and Rhynchosporium commune interactions. Mol Plant. Pathol. 2020, 21, 1111–1128. [Google Scholar] [CrossRef]
- Huang, S.; Antony, G.; Li, T.; Liu, B.; Obasa, K.; Yang, B.; White, F.F. The broadly effective recessive resistance gene xa5 of rice is a virulence effector-dependent quantitative trait for bacterial blight. Plant J. 2016, 86, 186–194. [Google Scholar] [CrossRef]
- Hui, S.; Liu, H.; Zhang, M.; Chen, D.; Li, Q.; Tian, J.; Yuan, M. The host basal transcription factor IIA subunits coordinate for facilitating infection of TALEs-carrying bacterial pathogens in rice. Plant Sci. 2019, 284, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lolle, S.; Stevens, D.; Coaker, G. Plant NLR-triggered immunity: From receptor activation to downstream signaling. Cur. Opin. Immun. 2020, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Krasileva, K.V.; Dahlbeck, D.; Staskawicz, B.J. Activation of an Arabidopsis resistance protein is specified by the in-planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell. 2010, 22, 2444–2458. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, A.D.; Goritschnig, S.; Staskawicz, B.J. Recognition and activation domains contribute to allele-specific responses of an Arabidopsis NLR receptor to an oomycete effector protein. PloS Pathog. 2015, 11, e1004665. [Google Scholar] [CrossRef] [PubMed]
- Bernoux, M.; Burdett, H.; Williams, S.J.; Zhang, X.; Chen, C.; Newell, K.; Dodds, P.N. Comparative analysis of the flax immune receptors L6 and L7 suggests an equilibrium-based switch activation model. Plant Cell. 2016, 28, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Rathjen, J.P.; Dodds, P.N. Direct recognition of pathogen effectors by plant NLR immune receptors and downstream signaling. Essays. Biochem. 2022, 66, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.I.A.; Guncˇar, G.; Forwood, J.K.; Teh, T.; Catanzariti, A.M.; Lawrence, G.J.; Kobe, B. Crystal structures of flax rust avirulence proteins AvrL567-A and-D reveal details of the structural basis for flax disease resistance specificity. Plant Cell. 2007, 19, 2898–2912. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Bernoux, M.; Ve, T.; Kobe, B.; Thrall, P.H.; Ellis, J.G.; Dodds, P.N. Intramolecular interaction influences binding of the Flax L5 and L6 resistance proteins to their AvrL567 ligands. PLoS Pathog. 2012, 8, e1003004. [Google Scholar] [CrossRef]
- Adachi, H.; Derevnina, L.; Kamoun, S. NLR singletons, pairs, and networks: Evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr. Opin. Plant. Biol. 2019, 50, 121–131. [Google Scholar] [CrossRef]
- Zhu, M.; Jiang, L.; Bai, B.; Zhao, W.; Chen, X.; Li, J.; Tao, X. The intracellular immune receptor Sw-5b confers broad-spectrum resistance to tospoviruses through recognition of a conserved 21-amino acid viral effector epitope. Plant Cell. 2017, 29, 2214–2232. [Google Scholar] [CrossRef]
- Lu, X.; Kracher, B.; Saur, I.M.; Bauer, S.; Ellwood, S.R.; Wise, R.; Schulze-Lefert, P. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc. Natl. Acad. Sci. 2016, 113, E6486–E6495. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kou, Y.; Bao, J.; Li, Y.; Tang, M.; Zhu, X.; Zhou, B. Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol. 2015, 206, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, A.; Steele, J.F.; Segretin, M.E.; Bozkurt, T.O.; Zhou, J.; Robatzek, S.; Kamoun, S. Tomato I2 immune receptor can be engineered to confer partial resistance to the oomycete Phytophthora infestans in addition to the fungus Fusarium oxysporum. Mol. Plant-Micr. Inter. 2015, 28, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Vossen, J.H.; van Arkel, G.; Bergervoet, M.; Jo, K.R.; Jacobsen, E.; Visser, R.G. The Solanum demissum R8 late blight resistance gene is a Sw-5 homologue that has been deployed worldwide in late blight resistant varieties. Theor. Appl. Genet. 2016, 129, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.; Nishimura, M.T. Structural, functional, and genomic diversity of plant NLR proteins: An evolved resource for rational engineering of plant immunity. Proc. Natl. Acad. Sci. 2018, 109, 20119–20123. [Google Scholar] [CrossRef] [PubMed]
- Anbu, S.; Swart, V.; Van Den Berg, N. Unmasking the invaders: NLR-mal function in plant defense. Front. Plant. Sci. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Marla, S.R.; Chu, K.; Chintamanani, S.; Multani, D.S.; Klempien, A.; DeLeon, A.; Johal, G.S. Adult plant resistance in maize to northern leaf spot is a feature of partial loss-of-function alleles of Hm1. PloS. Pathog. 2018, 14, e1007356. [Google Scholar] [CrossRef] [PubMed]
- Tettey, C.K.; Yan, Z.Y.; ZHAO, M.S.; Chao, G.E.N.G.; TIAN, Y.P.; LI, X.D. Tomato mottle mosaic virus: Characterization, resistance gene effectiveness, and quintuplex RT-PCR detection system. J. Integr. Agricul. 2022, 21, 2641–2651. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, J.; Wu, Z.; VandenLangenberg, K.; Wehner, T.C.; Wen, C.; Weng, Y. STAYGREEN, STAY HEALTHY: A loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum disease resistances for over 50 years of US cucumber production. New Phytol. 2019, 221, 415–430. [Google Scholar] [CrossRef]
- Johal, G.S.; Briggs, S.P. Reductase activity encoded by the HM1 disease resistance gene in maize. Science 1992, 258, 985–987. [Google Scholar] [CrossRef]
- Butterbach, P.; Verlaan, M.G.; Dullemans, A.; Lohuis, D.; Visser, R.G.; Bai, Y.; Kormelink, R. Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. Proc. Natl. Acad. Sci. 2014, 111, 12942–12947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, Z.; White, F. TAL effectors and the executor R genes. Front. Plant Sci. 2015, 6, 641. [Google Scholar] [CrossRef] [PubMed]
- Nowack, M.K.; Holmes, D.R.; Lahaye, T. TALE-induced cell death executors: An origin outside immunity? Trends. Plant. Sci. 2022, 27, 536–548. [Google Scholar] [CrossRef]
- Ji, Z.; Guo, W.; Chen, X.; Wang, C.; Zhao, K. Plant Executor Genes. Int. J. Mol. Sci. 2022, 23, 1524. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wang, J.; Zeng, X.; Gu, K.; Qiu, C.; Yang, X.; Yin, Z. The rice TAL effector–dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell. 2014, 26, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Fan, Y.; Gao, Y.; Zhu, Q.; Zheng, C.; Zhao, K. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol. Plant. 2015, 8, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Yang, B.; Tian, D.; Wu, L.; Wang, D.; Sreekala, C.; Yin, Z. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 2005, 435, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Römer, P.; Hahn, S.; Jordan, T.; Strauss, T.; Bonas, U.; Lahaye, T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 2007, 318, 645–648. [Google Scholar] [CrossRef]
- Strauss, T.; van Poecke, R.M.; Strauß, A.; Römer, P.; Minsavage, G.V.; Singh, S.; Lahaye, T. RNA-seq pinpoints a Xanthomonas TAL-effector activated resistance gene in a large-crop genome. Proc. Natl. Acad. Sci. 2012, 109, 19480–19485. [Google Scholar] [CrossRef]
- Zeng, X.; Tian, D.; Gu, K.; Zhou, Z.; Yang, X.; Luo, Y.; Yin, Z. Genetic engineering of the Xa10 promoter for broad-spectrum and durable resistance to Xanthomonas oryzae pv. oryzae. Plant Biotechnol. J. 2015, 13, 993–1001. [Google Scholar] [CrossRef]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Peng, J. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)—An updated review. Rice 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gallas, N.; Li, X.; von Roepenack-Lahaye, E.; Schandry, N.; Jiang, Y.; Wu, D.; Lahaye, T. An ancient cis-element targeted by Ralstonia solanacearum TALE-like effectors facilitates the development of a promoter trap that could confer broad-spectrum wilt resistance. Plant Biotech. J. 2024, 22, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Vannier, N.; Agler, M.; Hacquard, S. Microbiota-mediated disease resistance in plants. PLoS Pathog. 2019, 15, e1007740. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Ahmed, T.; Ijaz, U.; Shahid, M.; Nazir, M.M.; Azizullah; White, J.C.; Li, D.; Song, F. Bio-functionalized manganese nanoparticles suppress Fusarium wilt in watermelon (Citrullus lanatus L.) by infection disruption, host defense response potentiation, and soil microbial community modulation. Small 2023, 19, 2205687. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, J.; Zhang, H.; Ji, G.; Zeng, L.; Li, Y.; Chen, W. Bacterial blight induced shifts in endophytic microbiome of rice leaves and the enrichment of specific bacterial strains with pathogen antagonism. Front. Plant. Sci. 2020, 11, 963. [Google Scholar] [CrossRef]
- Sahu, K.P.; Kumar, A.; Patel, A.; Kumar, M.; Gopalakrishnan, S.; Prakash, G.; Gogoi, R. Rice blast lesions: An unexplored phyllosphere microhabitat for novel antagonistic bacterial species against Magnaporthe oryzae. Microb. Ecol. 2021, 81, 731–745. [Google Scholar] [CrossRef]
- Tamura, T.; Shinzato, N.; Ito, M.; Ueno, M. Microbial secondary metabolite induction of abnormal appressoria formation mediates control of rice blast disease caused by Magnaporthe oryzae. Phytopathology 2019, 167, 156–162. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods. 2020, 170, 105860. [Google Scholar] [CrossRef]
- Mechan Llontop, M.E.; Sharma, P.; Aguilera Flores, M.; Yang, S.; Pollok, J.; Tian, L.; Vinatzer, B.A. Strain-level identification of bacterial tomato pathogens directly from metagenomic sequences. Phytopathology 2020, 110, 768–779. [Google Scholar] [CrossRef]
- Mendes, L.W.; Raaijmakers, J.M.; De Hollander, M.; Sepo, E.; Gómez Expósito, R.; Chiorato, A.F.; Carrión, V.J. Impact of the fungal pathogen Fusarium oxysporum on the taxonomic and functional diversity of the common bean root microbiome. Environ. Microbiol. 2023, 18, 68. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Kim, J.F. The Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Crews, T.E.; Carton, W.; Olsson, L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Glo. Sustain. 2018, 1, e11. [Google Scholar] [CrossRef]
- Clare, S.J.; Çelik Oğuz, A.; Effertz, K.; Karakaya, A.; Azamparsa, M.R.; Brueggeman, R.S. Wild barley (Hordeum spontaneum) and landraces (Hordeum vulgare) from Turkey contain an abundance of novel Rhynchosporium commune resistance loci. Theor. Appl. Genet. 2023, 136, 15. [Google Scholar] [CrossRef]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Dubcovsky, J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Kong, L. The horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Kawashima, C.G.; Guimarães, G.A.; Nogueira, S.R.; MacLean, D.; Cook, D.R.; Steuernagel, B.; Brommonschenkel, S.H. A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat. Biotechnol. 2016, 34, 661–665. [Google Scholar] [CrossRef]
- Martin, M.J.; Chicaiza, O.; Caffarel, J.C.; Sallam, A.H.; Druka, A.; Waugh, R.; Steffenson, B.J. Development of barley introgression lines carrying the leaf rust resistance genes Rph1 to Rph15. Crop Sci. 2020, 60, 282–302. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; He, Z. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, X.; Shen, Y.; He, Z. Genetic characterization and fine mapping of the blast resistance locus Pigm (t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor. Appl. Genet. 2006, 113, 705–713. [Google Scholar] [CrossRef]
- Jaganathan, D.; Bohra, A.; Thudi, M.; Varshney, R.K. Fine mapping and gene cloning in the post-NGS era: Advances and prospects. Theor. Appl. Genet. 2020, 133, 1791–1810. [Google Scholar] [CrossRef]
- Thoen, M.P.; Davila Olivas, N.H.; Kloth, K.J.; Coolen, S.; Huang, P.P.; Aarts, M.G.; Dicke, M. Genetic architecture of plant stress resistance: Multi-trait genome-wide association mapping. New Phytol. 2017, 213, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wang, Y.; Peng, S.; Zhang, Y.; Xiao, Y.; Wang, D.; Wang, G.L. Dissection of the genetic architecture of rice resistance to the blast fungus Magnaporthe oryzae. Mol Plant. Pathol. 2016, 17, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Kang, H.; Xu, Y.; Peng, Y.; Wang, D.; Gao, L.; Wang, G.L. Genome-wide association study identifies an NLR gene that confers partial resistance to Magnaporthe oryzae in rice. Plant. Biotech. J. 2020, 18, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Genger, R.K.; Nesbitt, K.; Brown, A.H.D.; Abbott, D.C.; Burdon, J.J. A novel barley scald resistance gene: Genetic mapping of the Rrs15 scald resistance gene derived from wild barley, Hordeum vulgare ssp. spontaneum. Plant Breed. 2005, 124, 137–141. [Google Scholar] [CrossRef]
- Hofmann, K.; Silvar, C.; Casas, A.M.; Herz, M.; Büttner, B.; Gracia, M.P.; Schweizer, G. Fine mapping of the Rrs1 resistance locus against scald in two large populations derived from Spanish barley landraces. Theor. Appl. Genet. 2013, 126, 3091–3102. [Google Scholar] [CrossRef]
- Ntoukakis, V.; Saur, I.M.; Conlan, B.; Rathjen, J.P. The changing of the guard: The Pto/Prf receptor complex of tomato and pathogen recognition. Curr. Opin. Plant. Biol. 2014, 20, 69–74. [Google Scholar] [CrossRef]
- Arora, S.; Steuernagel, B.; Gaurav, K.; Chandramohan, S.; Long, Y.; Matny, O.; Wulff, B.B. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat Biotech. 2019, 37, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Steuernagel, B.; Ghosh, S.; Herren, G.; Hurni, S.; Adamski, N.; Wulff, B.B. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell. 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Gao, W.; Long, L.; Tian, X.; Xu, F.; Liu, J.; Singh, P.K.; Song, C. Genome editing in cotton with the CRISPR/Cas9 system. Front. Plant. Sci. 2017, 8, 1364. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Chen, X.; Zhou, J.M. From plant immunity to crop disease resistance. J. Genet. Genom. 2022, 49, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Porteus, M.H.; Carroll, D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005, 23, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kumar, R.; Kumar, V.; Won, S.Y.; Shukla, P. Engineering disease resistant plants through CRISPR-Cas9 technology. GM Crops. Food. 2021, 12, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, M.S.; Yang, Y. Versatile applications of the CRISPR/Cas toolkit in plant pathology and disease management. Phytopathology 2021, 111, 1080–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant. Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Brauer, E.K.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Schernthaner, J.; Subramaniam, R.; Ouellet, T. Genome editing of a deoxynivalenol-induced transcription factor confers resistance to Fusarium graminearum in wheat. Mol. Plant-Micro. Inter. 2020, 33, 553–560. [Google Scholar] [CrossRef]
- Koseoglou, E.; van der Wolf, J.M.; Visser, R.G.; Bai, Y. Susceptibility reversed: Modified plant susceptibility genes for resistance to bacteria. Trends. Plant. Sci. 2022, 27, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Luebbert, C.; Chauhan, R.D.; Vijayaraghavan, A.; Bart, R. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 confers elevated resistance to cassava brown streak disease. BioRxiv 2017, 209874. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Gao, C. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Zeilmaker, T.; Giovannini, O.; Salvagnin, U.; Masuero, D.; Franceschi, P.; Moser, C. Simultaneous editing of two DMR6 genes in grapevine results in reduced susceptibility to downy mildew. Front. Plant. Sci. 2023, 14. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Iqbal, H.M.; Parra-Saldivar, R.; Varjani, S.; Tong, Y.W. Genetic modifications associated with sustainability aspects for sustainable developments. J. Bioeng. 2022, 13, 9509–9521. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, U.; Shafique, A.; Hasnain, N.; Babar, N.I.; Zameer, R.; Azeem, F. Current and future perspectives of genetically modified organisms in North America. GMOs and Political Stance 2023, 151–163. [Google Scholar] [CrossRef]
- Avila-Quezada, G.D.; Golinska, P.; and Rai, M. Engineered nanomaterials in plant diseases: Can we combat phytopathogens? Appl Microb. Biotechol. 2022, 106, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, D.S.; Kumari, R.; Chandra, P. Chemically engineered unzipped multiwalled carbon nanotube and rGO nanohybrid for ultrasensitive picloram detection in rice water and soil samples. Sci. Rep. 2023, 13, 9899. [Google Scholar] [CrossRef]
- Chen, W.X.; Chen, K.F.; Chang, K.L.; Chen, W.H.; Lin, C.H.; Chen, C.H.; and Peng, Y.P. Photoelectrochemical degradation of TMAH by green synthesized silver modified titanate nanotube arrays. J. Environ. Chem. Engin. 2023, 11, 110625. [Google Scholar] [CrossRef]
- Sidhu, A.; Bala, A.; Singh, H.; Ahuja, R.; Kumar, A. Development of MgO-sepoilite nanocomposites against phytopathogenic fungi of rice (Oryzae sativa): A green approach. ACS Omega 2020, 5, 13557–13565. [Google Scholar] [CrossRef] [PubMed]
- Ranpariya, B.; Salunke, G.; Karmakar, S.; Babiya, K.; Sutar, S.; Kadoo, N.; Ghosh, S. Antimicrobial synergy of silver-platinum nanohybrids with antibiotics. Front. Microbiol. 2021, 11, 610968. [Google Scholar] [CrossRef] [PubMed]
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Bucchini, L.; Goldman, L.R. Starlink corn: A risk analysis. Environ. Health. Perspect. 2002, 110, 5–13. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Starlink™ corn regulatory information. Pesticides. 2017, US EPA.
- Fagan, J.; Traavik, T.; Bøhn, T. The Seralini affair: Degeneration of science to re-science? Environ Sci. Eur. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Séralini, G.E.; Clair, E.; Mesnage, R.; Gress, S.; Defarge, N.; Malatesta, M.; de Vendômois, J.S. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ. Sci. Eur. 2014, 26, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Stockhofe, N.; Wal, J.M.; Weesendorp, E.; Lalles, J.P.; van Dijk, J.; Kleter, G. Case studies on genetically modified organisms (GMOs): Potential risk scenarios and associated health indicators. Food. Chem. Toxicol. 2018, 117, 36–65. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, B.; Won, O.J.; Hwang, K.S.; Suh, S.J.; Kim, C.G.; Park, K.W. Gene flows from herbicide resistant genetically modified rice to conventional rice (Oryza sativa L.) cultivars. J. Ecol. Environ. 2015, 38, 397–403. [Google Scholar] [CrossRef]
- Umurzokov, M.; Bo Bo, A.; Ruziev, F.; Jia, W.Q.; Le, T.; Cho, M.K.; Park, K.W. Pollen-mediated exclusive gene flow from transgenic crops. Inter. J. Pest. Manag. 2021, 67, 260–268. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.; Lawton-Rauh, A.; Bagavathiannan, M.V.; Roma-Burgos, N. EPSPS gene amplification primarily confers glyphosate resistance among Arkansas Palmer amaranth (Amaranthus palmeri) populations. Weed Sci. 2018, 66, 293–300. [Google Scholar] [CrossRef]
- Anjanappa, R.B.; Gruissem, W. Current progress and challenges in crop genetic transformation. J. Plant. Physiol. 2021, 261, 153411. [Google Scholar] [CrossRef] [PubMed]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M. Enhancement of tomato resistance to Tuta absoluta using a new efficient mesoporous silica nanoparticle-mediated plant transient gene expression approach. Hortic Sci. 2019, 243, 367–375. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Tuteja, S.K.; Kim, K.H. Nanovehicle for plant modifications towards pest-and disease-resistance traits. Trends. Plant Sci. 2020, 25, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Saintenac, C.; Lee, W.S.; Cambon, F.; Rudd, J.J.; King, R.C.; Marande, W.; Kanyuka, K. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 2018, 50, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Kurth, J.; Wei, F.; Elliott, C.; Valé, G.; Yahiaoui, N.; Keller, B.; Somerville, S.; Wise, R.; Schulze-Lefert, P. Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1 -independent signaling pathway. Plant Cell. 2001, 13, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Halterman, D.; Zhou, F.; Wei, F.; Wise, R.P.; Schulze-Lefert, P. The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J. 2001, 25, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Brueggeman, R.; Druka, A.; Nirmala, J.; Cavileer, T.; Drader, T.; Rostoks, N.; Kleinhofs, A. The stem rust resistance gene Rpg5 encodes a protein with nucleotide-binding-site, leucine-rich, and protein kinase domains. Proc. Natl. Acad. Sci. 2008, 105, 14970–14975. [Google Scholar] [CrossRef]
- Srichumpa, P.; Brunner, S.; Keller, B.; Yahiaoui, N. Allelic series of four powdery mildew resistance genes at the Pm3 locus in hexaploid bread wheat. Plant Physiol. 2005, 139, 885–895. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Gong, Y.; Tao, Y.; Jiang, L.; Zuo, W.; Xu, M. An atypical thioredoxin imparts early resistance to sugarcane mosaic virus in maize. Mol Plant. 2017, 10, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Drake, J.; Ayliffe, M.; Sun, Q.; Ellis, J.; Hulbert, S.; Pryor, T. Molecular characterization of the maize Rp1-D rust resistance haplotype and its mutants. Plant Cell. 1999, 11, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, X.; Poland, J.; Trick, H.; Leach, J.; Hulbert, S. A maize resistance gene functions against bacterial streak disease in rice. Proc. Natl. Acad. Sci. 2005, 102, 15383–15388. [Google Scholar] [CrossRef] [PubMed]
- Chintamanani, S.; Multani, D.S.; Ruess, H.; Johal, G.S. Distinct mechanisms govern the dosage-dependent and developmentally regulated resistance conferred by the maize Hm2 gene. Mol. Plant-Micro. Interact. 2008, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Q.; Wang, W.; Li, Y.; Guo, Y.; Zhang, D.; Xu, M. A transposon-directed epigenetic change in ZmCCT underlies quantitative resistance to Gibberella stalk rot in maize. New Phytol. 2017, 215, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Balint-Kurti, P. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, S.; Liu, G.; Dolan, M.; Sakai, H.; Lu, G.; Wang, G.L. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol. Plant-Microb. Interact. 2006, 19, 1216–1228. [Google Scholar] [CrossRef]
- Bryan, G.T.; Wu, K.S.; Farrall, L.; Jia, Y.; Hershey, H.P.; McAdams, S.A.; Valent, B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell. 2000, 12, 2033–2045. [Google Scholar] [CrossRef]
- Tsunoda, Y.; Jwa, N.S.; Akiyama, K.; Nakamura, S.; Motomura, T.; Kamihara, K.; Kawasaki, S. Cloning of the rice blast resistance gene PI-B. In Advances in rice blast research; Springer: Dordrecht, 2000; pp. 9–16. [Google Scholar] [CrossRef]
- Chen, D.X.; Chen, X.W.; Lei, C.L.; Wang, Y.P.; Li, S.G. Rice blast resistance of transgenic rice plants with Pi-d2 gene. Rice Sci. 2010, 17, 179–184. [Google Scholar] [CrossRef]
- Qu, S.; Liu, G.; Zhou, B.; Bellizzi, M.; Zeng, L.; Dai, L.; Wang, G.L. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site–leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 2006, 172, 1901–1914. [Google Scholar] [CrossRef]
- Cesari, S.; Thilliez, G.; Ribot, C.; Chalvon, V.; Michel, C.; Jauneau, A.; Rivas, S.; Alaux, L.; Kanzaki, H.; Okuyama, Y.; Morel, J.B. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell. 2013, 25, 1463–1481. [Google Scholar] [CrossRef]
- Yoshimura, S.; Yamanouchi, U.; Katayose, Y.; Toki, S.; Wang, Z.X.; Kono, I.; Kurata, N.; Yano, M.; Iwata, N.; Sasaki, T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. 1998, 95, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; McCouch, S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant-Microb. Interact. 2004, 17, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Pan, J.; Gu, Z.; Chen, X.; Jin, Y.; Ma, B. Identification and molecular mapping of the rice bacterial blight resistance gene allelic to Xa7 from an elite restorer line Zhenhui 084. Europ. J. Plant Pathol. 2009, 125, 235–244. [Google Scholar] [CrossRef]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; Fauquet, C. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004, 37, 517–527. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000, 5, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Mackey, D.; Holt III, B.F.; Wiig, A.; Dangl, J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002, 108, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Staskawicz, B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 2003, 112, 369–377. [Google Scholar] [CrossRef]
- Dixon, M.S.; Jones, D.A.; Keddie, J.S.; Thomas, C.M.; Harrison, K.; Jones, J.D. The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell 1996, 84, 451–459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





