Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
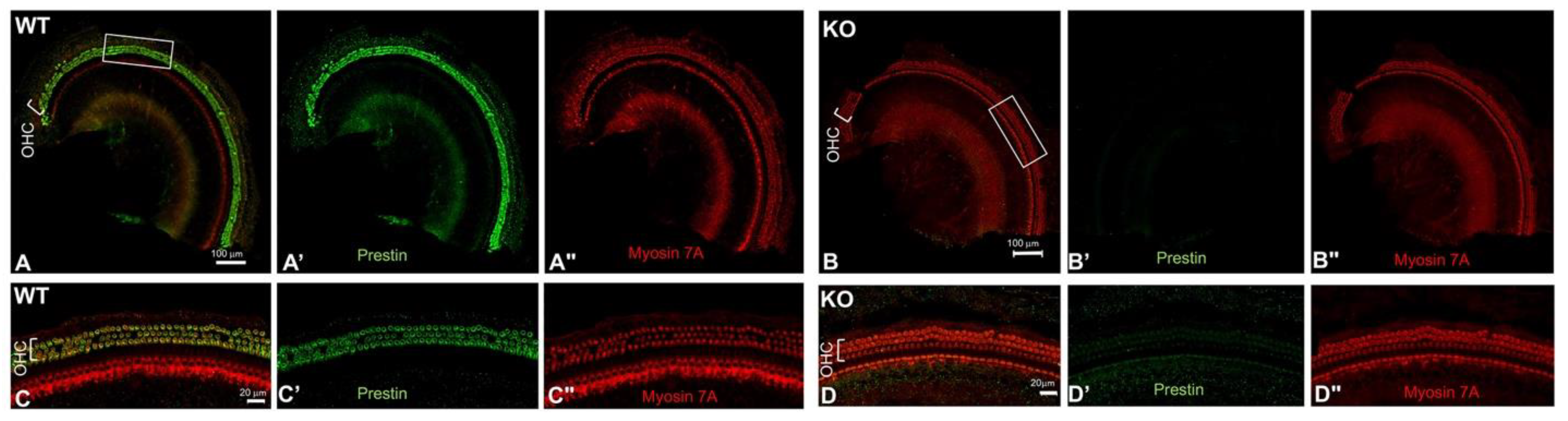
2.1. Confirm the Positive and Negative Control Samples for Prestin-ELISA: Prestin is Expressed in OHCs from WT but not from Prestin-KO Mice
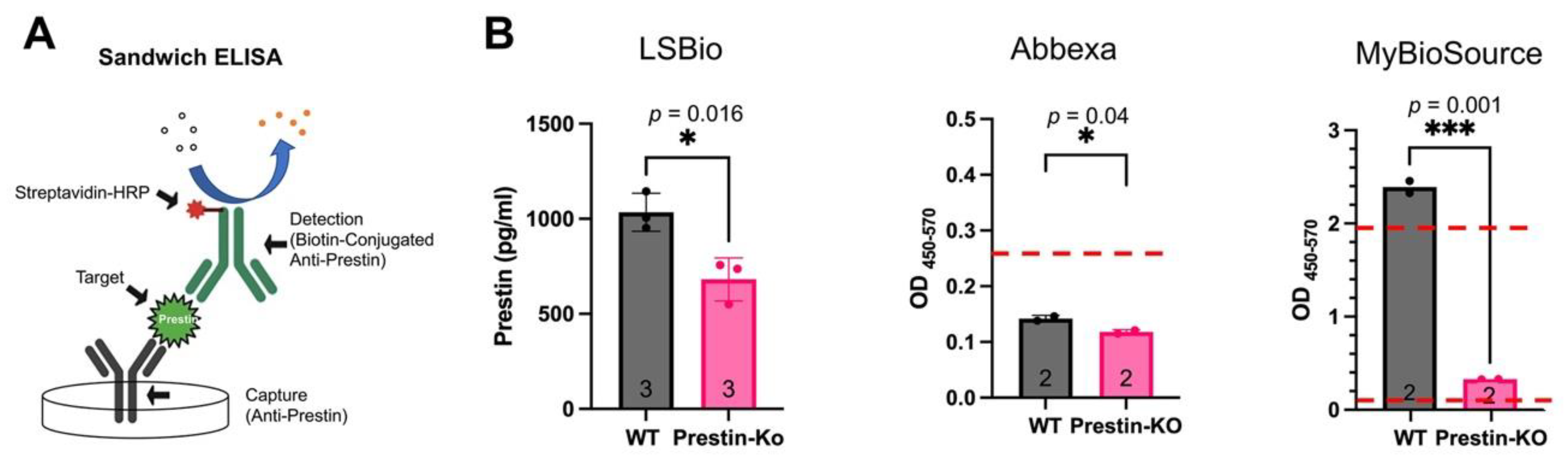
2.2. Test Sensitivities and Specificity of Different Prestin-ELISA Kits
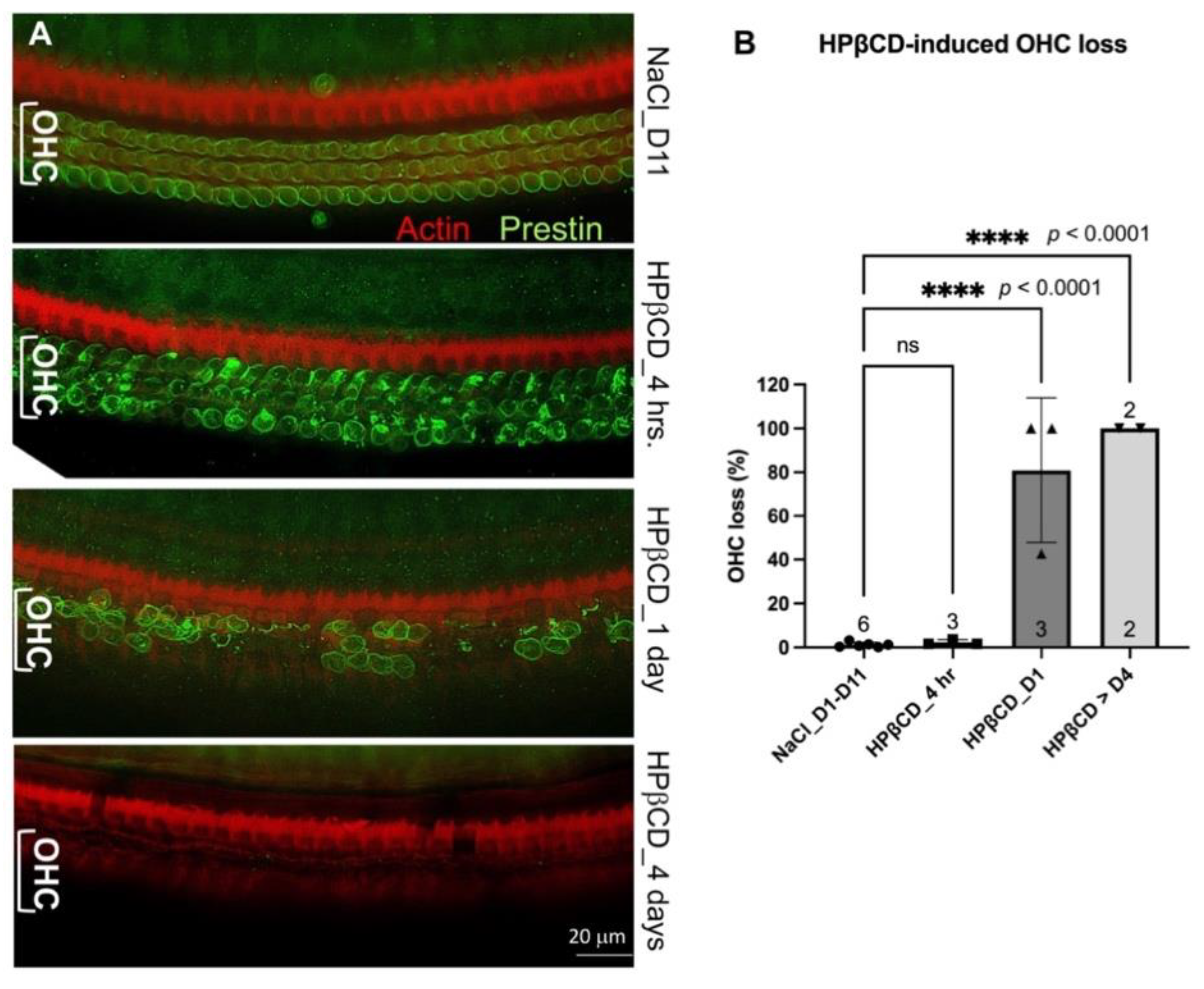
2.3. Establish an OHC Damage Mouse Model to Test whether Prestin from Cochleae can be Detected in the Bloodstream
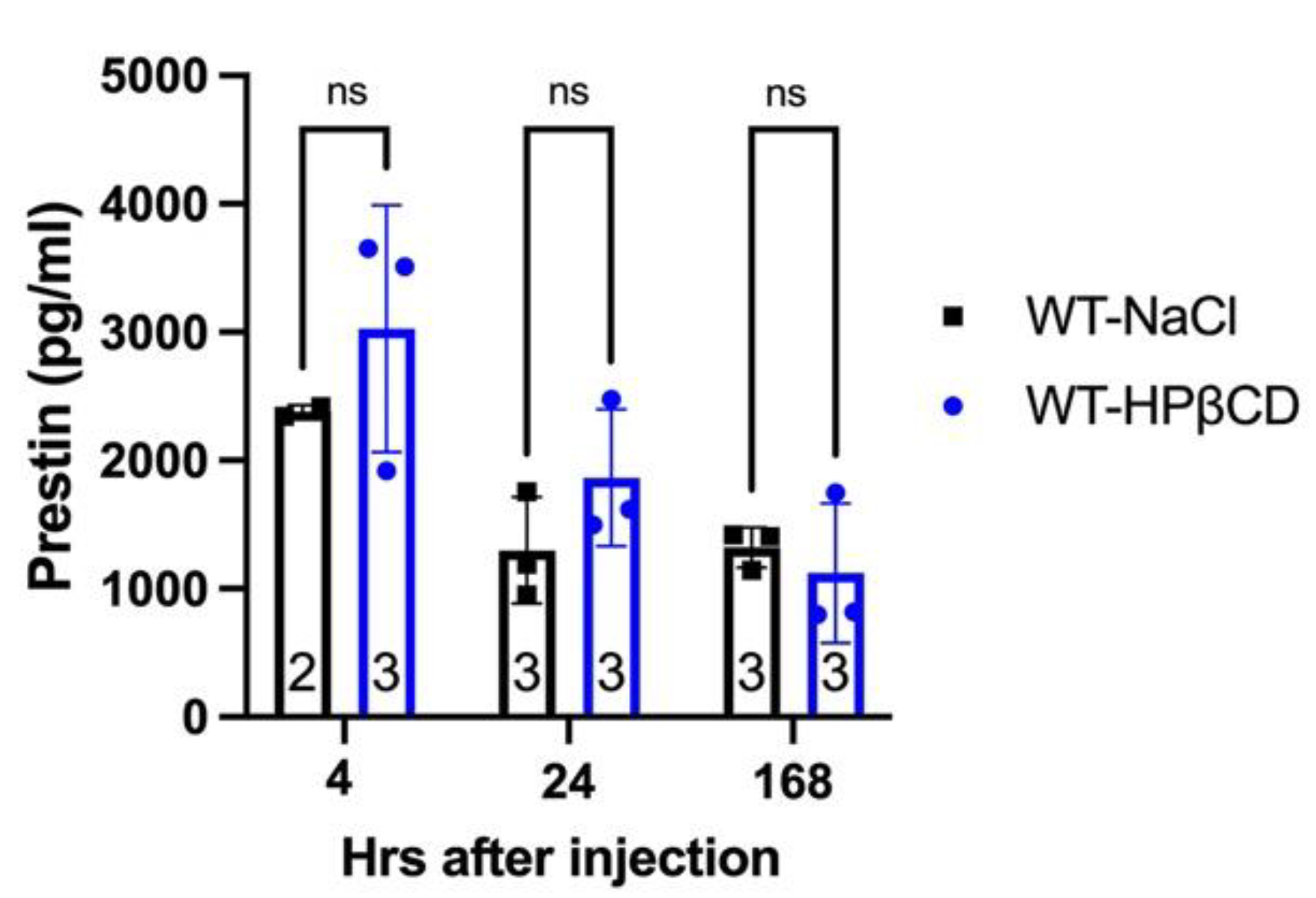
2.4. Prestin Levels Show No Undetectable Difference in the Bloodstream of WT and Prestin-KO Mice Regardless of whether OHCs were Stressed or Damaged
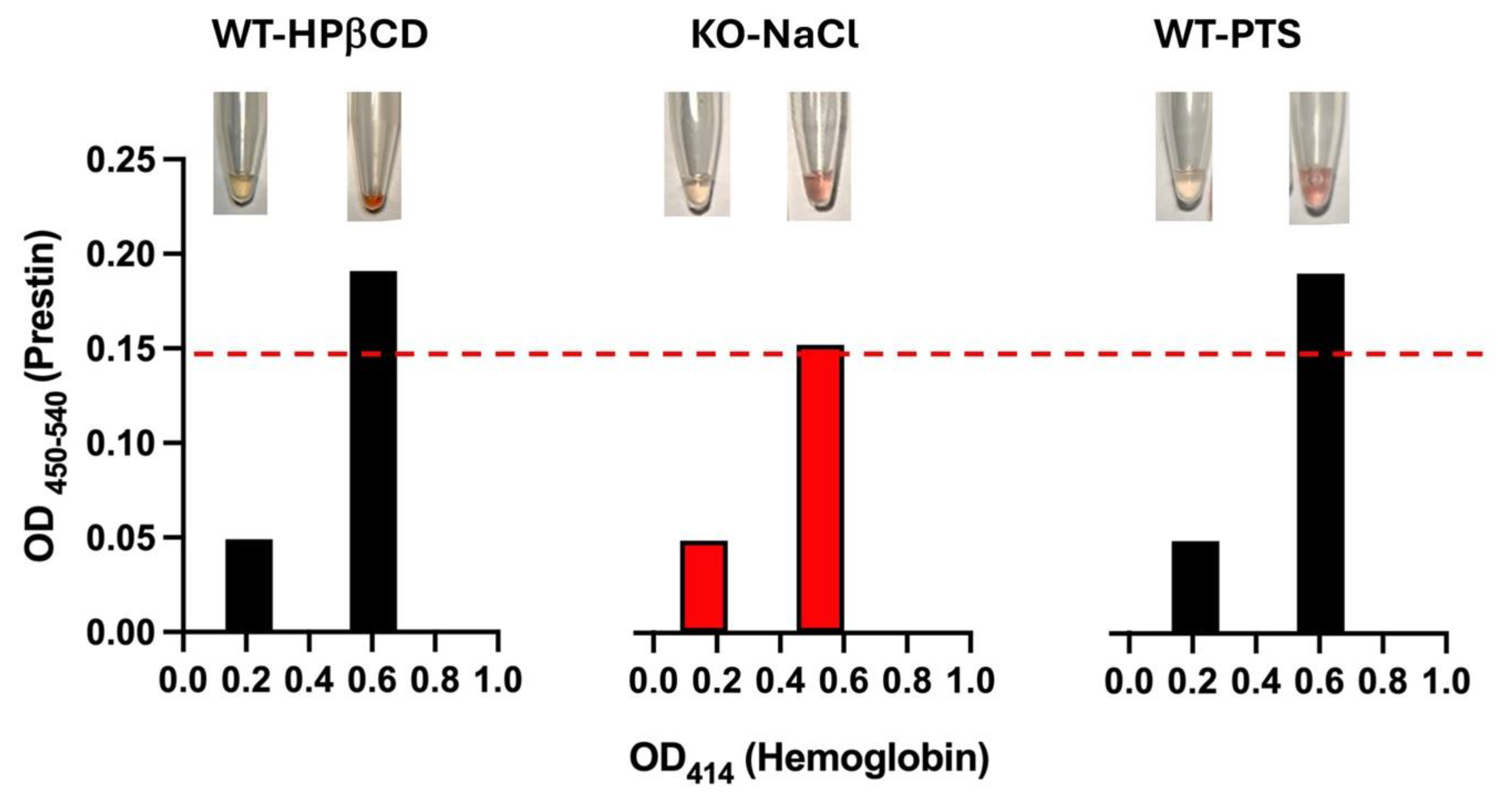
2.5. The Severities of Hemolysis Influence Prestin Quantification Measured by ELISA
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Cochlear Stress Treatment
4.3. Prestin and Hemoglobin Measurement
4.4. Immunofluorescence
4.5. Cochlear Lysate
4.6. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World report on hearing: executive summary; World Health Organization: Geneva, 2021. [Google Scholar]
- Zheng, J.; et al. Prestin is the motor protein of cochlear outer hair cells. Nature 2000, 405, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P.; et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 2008, 58, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; et al. Molecular mechanism of prestin electromotive signal amplification. Cell 2021, 184, 4669–4679.e13. [Google Scholar] [CrossRef] [PubMed]
- Butan, C.; et al. Single particle cryo-EM structure of the outer hair cell motor protein prestin. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bavi, N.; et al. Cryo-EM Structures of Prestin and the Molecular Basis of Outer Hair Cell Electromotility. bioRxiv 2021. [Google Scholar] [CrossRef]
- Futamata, H.; et al. Cryo-EM structures of thermostabilized prestin provide mechanistic insights underlying outer hair cell electromotility. Nature Communications 2022, 13, 6208. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.F.; et al. Elevator-like movements of prestin mediate outer hair cell electromotility. Nature Communications 2023, 14, 7145. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002, 419, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; et al. Cochlear function in Prestin knockout mice. J Physiol 2004, 560 Pt 3, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; et al. Evaluation of an independent prestin mouse model derived from the 129S1 strain. Audiology and Neuro-Otology 2007, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; et al. Prestin-Dependence of Outer Hair Cell Survival and Partial Rescue of Outer Hair Cell Loss in Prestin(V499G/Y501H) Knockin Mice. Plos One 2015, 10. [Google Scholar] [CrossRef]
- Sun, C.; et al. A Preliminary Report on the Investigation of Prestin as a Biomarker for Idiopathic Sudden Sensorineural Hearing Loss. Ear, Nose & Throat Journal 2020, 99, 528–531. [Google Scholar] [CrossRef]
- Tovi, H.; et al. Prestin autoantibodies screening in idiopathic sudden sensorineural hearing loss. Eur Ann Otorhinolaryngol Head Neck Dis 2019, 136, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Saadat, F.; Jalali, M.M.; Akbari, M. Assessment of prestin level changes as an inner-ear biomarker in patients with idiopathic sudden sensorineural hearing loss. J Laryngol Otol 2022, 136, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Parham, K.; Dyhrfjeld-Johnsen, J. Outer Hair Cell Molecular Protein, Prestin, as a Serum Biomarker for Hearing Loss: Proof of Concept. Otol Neurotol 2016, 37, 1217–1222. [Google Scholar] [CrossRef]
- Parham, K.; et al. Noise-induced trauma produces a temporal pattern of change in blood levels of the outer hair cell biomarker prestin. Hear Res 2019, 371, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Naples, J.; et al. Prestin as an Otologic Biomarker of Cisplatin Ototoxicity in a Guinea Pig Model. Otolaryngol Head Neck Surg 2018, 158, 541–546. [Google Scholar] [CrossRef]
- Liba, B.; et al. Changes in Serum Prestin Concentration After Exposure to Cisplatin. Otol Neurotol 2017, 38, e501–e505. [Google Scholar] [CrossRef] [PubMed]
- Generotti, C.; et al. Subclinical diagnosis of cisplatin-induced ototoxicity with biomarkers. Scientific Reports 2022, 12, 18032. [Google Scholar] [CrossRef]
- Yilmazer, A.B.; et al. Evaluation of inner ear damage by mastoid drilling with measurement of serum prestin (SLC26A5) levels. Brazilian Journal of Otorhinolaryngology 2023, 101380. [Google Scholar] [CrossRef]
- Santosa, A.; et al. Potential diagnostic biomarkers for early detection of idiopathic sensorineural hearing loss in type 2 diabetes mellitus patients. Indonesia Journal of Biomedical Science 2022, 16, 37–42. [Google Scholar] [CrossRef]
- Parker, A.; Parham, K.; Skoe, E. Noise exposure levels predict blood levels of the inner ear protein prestin. Sci Rep 2022, 12, 1154. [Google Scholar] [CrossRef]
- Parker, A.; Parham, K.; Skoe, E. Reliability of Serological Prestin Levels in Humans and its Relation to Otoacoustic Emissions, a Functional Measure of Outer Hair Cells. Ear Hear 2021, 42, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, S.; Cazevieille, C. N-Acetylcysteine Treatment Reduces Noise-Induced Hearing Loss in Guinea Pig. Journal of Community and Preventive Medicine 2020, 3, 1. [Google Scholar] [CrossRef]
- Emre, S.; et al. Can prestin level be a biomarker for determining sensorineural hearing loss? Auris Nasus Larynx 2022, 49, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Asli, R.H.; et al. Evaluation of the relationship between prestin serum biomarker and sensorineural hearing loss: a case-control study. Eur Arch Otorhinolaryngol 2023, 280, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Solis-Angeles, S.; et al. Prestin and otolin-1 proteins in the hearing loss of adults chronically exposed to lead. Toxicology and Applied Pharmacology 2021, 426, 115651. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Parham, K.; Skoe, E. Age-related declines to serum prestin levels in humans. Hear Res 2022, 426, 108640. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.S.; et al. Automated Western Blot Analysis of Ototoxin-Induced Prestin Burst in the Blood after Cyclodextrin Exposure. Otology & Neurotology 2023, 44. [Google Scholar] [CrossRef]
- Jalali, M.M.; Saedi, H.S.; Saadat, F. Effect of cisplatin chemotherapy on the inner ear function and serum prestin concentration. European Archives of Oto-Rhino-Laryngology 2022, 279, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Naples, J.G.; et al. Evaluating the Role of Otologic Biomarkers to Differentiate Meniere’s Disease and Vestibular Migraine. Ear and Hearing 2022, 43. [Google Scholar] [CrossRef]
- Turan, M.; et al. Blood prestin levels in COVID-19 patients. Journal of the Chinese Medical Association 2023, 86, 571–576. [Google Scholar] [CrossRef]
- SOLIS-ANGELES, S.; et al. Evaluation of serum prestin as a new potential biomarker for hearing damage due to lead exposure in population from Tlaxcala, Mexico. ISEE Conference Abstracts 2020 2020. [Google Scholar] [CrossRef]
- Zhang, X.D.; et al. Prestin amplifies cardiac motor functions. Cell Rep 2021, 35, 109097. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; et al. Susceptibility of outer hair cells to cholesterol chelator 2-hydroxypropyl-beta-cyclodextrine is prestin-dependent. Scientific Reports 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; et al. The susceptibility of cochlear outer hair cells to cyclodextrin is not related to their electromotile activity. Acta Neuropathologica Communications 2018, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; et al. Analysis of the oligomeric structure of the motor protein prestin. Journal of Biological Chemistry 2006, 281, 19916–19924. [Google Scholar] [CrossRef] [PubMed]
- Crumling, M.A.; et al. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-beta-cyclodextrin. PLoS One 2012, 7, e53280. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Zalewski, C.; Farhat, N.; Keener, L.A.; Hoa, M.; Bianconi, S.; Porter, F.D.; Brewer, C.C. HPβCD Therapy in Humans with NPC1 Disease: Audiological Outcomes. Abstract of 38th Meeting of the Assoc. Res. Otolaryngol. Baltimore, Maryland, 2015.
- Cronin, S.; et al. Hearing Loss and Otopathology Following Systemic and Intracerebroventricular Delivery of 2-Hydroxypropyl-Beta-Cyclodextrin. J Assoc Res Otolaryngol 2015, 16, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; et al. Systemic application of honokiol prevents cisplatin ototoxicity without compromising its antitumor effect. American journal of cancer research 2020, 10, 4416–4434. [Google Scholar]
- Yamashita, T.; et al. Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins. PLoS Genet 2015, 11, e1005500. [Google Scholar] [CrossRef] [PubMed]
- Mahendrasingam, S.; et al. The ultrastructural distribution of prestin in outer hair cells: a post-embedding immunogold investigation of low-frequency and high-frequency regions of the rat cochlea. European Journal of Neuroscience 2010, 31, 1595–1605. [Google Scholar] [CrossRef]
- Ashmore, J. Biophysics of the cochlea - biomechanics and ion channelopathies. Br Med Bull 2002, 63, 59–72. [Google Scholar] [CrossRef]
- Ehret, G.; Frankenreiter, M. Quantitative analysis of cochlear structures in the house mouse in relation to mechanisms of acoustical information processing. Journal of comparative physiology 1977, 122, 65–85. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 1999, 130, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Dépreux, F.; et al. Statins protect mice from high-decibel noise-induced hearing loss. Biomedicine & Pharmacotherapy 2023, 163, 114674. [Google Scholar] [CrossRef]
- Kirschner, M.B.; et al. Haemolysis during Sample Preparation Alters microRNA Content of Plasma. PLOS ONE 2011, 6, e24145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; et al. The C-terminus of prestin influences nonlinear capacitance and plasma membrane targeting. J Cell Sci 2005, 118 Pt 13, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; et al. N-linked glycosylation sites of the motor protein prestin: effects on membrane targeting and electrophysiological function. J Neurochem 2004, 89, 928–938. [Google Scholar] [CrossRef]
- Muller, M.; et al. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 2005, 202, 63–73. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




