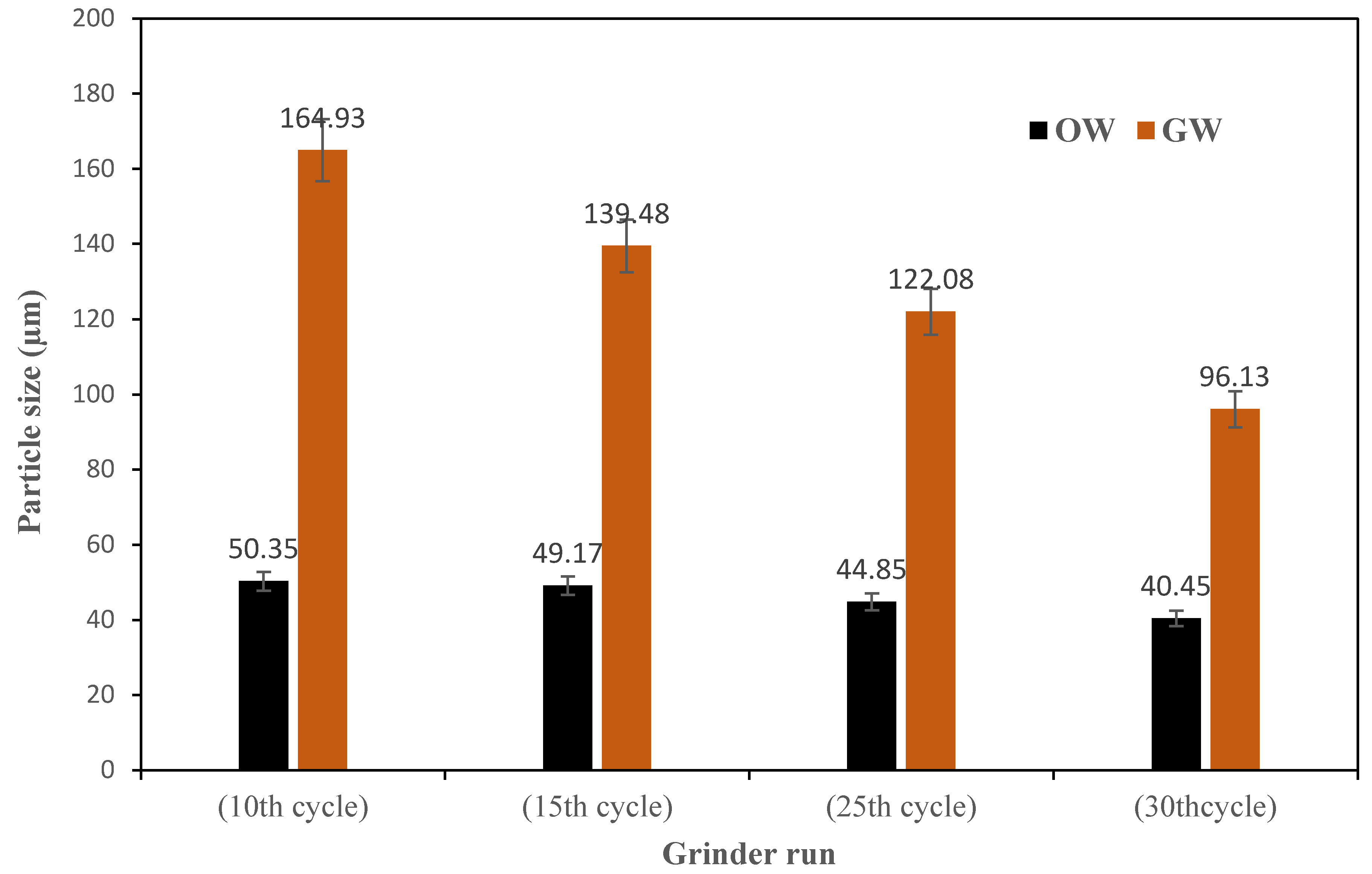1. Introduction
Plastic is a significant material that is commonly used in our daily lives and has a significant impact on our standard of life. Because plastics are mostly generated from fossil fuels today, this has resulted in a serious impact on the environment. However, switching to bio-based plastics from oil-based ones would aid in lowering the amount of greenhouse gas emissions. Finding a way to utilize industrial waste as raw materials for bio-based products would now not only be economical but also environmentally friendly. [
1,
2]
Several research focus on byproducts and waste materials from the food sector, especially fruit and vegetable industries, for bioplastic production [
1,
3,
4,
5,
6]. Orange and ginger have been interesting raw materials for bioplastics production [
1]. According to the European Commission (2020), Europe produced more than 6,550,000 tons orange in the year 2018/2019 and the orange sector remains one of the largest in the world. After pressing orange juice, 50–60% of the original mass remains as peels, pulp, and seeds residue. These orange residues contain valuable polysaccharides, such as pectin, cellulose‚ and hemicellulose as well as di- and monosaccharides in the form of soluble sugars. Pectin has been studied earlier and its gelling ability showed interesting properties in the context of bioplastic production. Pectin was employed as a matrix and cellulosic fibres were used as reinforcement in the development of biocomposite materials [
7]. Orange waste has previously been used as a reinforcement in petrochemical or biometric matrices. In all these cases, the authors reported an increase in the mechanical properties of the products compared to the pure polymer. The increase in mechanical properties is due to the direct effect of cellulose fibers. However, pectin, a major component of orange peel, does not appear to have a significant effect on the above composites [
3,
6,
8]. There are 1% fat, 2% protein, 18% carbs, and 79% water in raw ginger. 1 to 2% volatile oil, 5 to 8% pungent resinous substance, and starch are present in ginger. The cork, which is ginger's outermost covering, is made up of irregular parenchymatous cells and is dark brown in color. The inner cork is made up of radial rows of a few layered, colorless parenchymatous cell. The cortex is made up primarily of thin-walled, spherical cells with an indistinct phellogen, and the intercellular gaps are filled with many starch grains. The starch grains are simple, ovate, or sac shaped. All these characteristics give ginger a fibrous internal structure [
9].
Biofilms and 3D objects are types of biomaterials that are in high demand on the market. Compression molding and solution casting are two of the few straightforward processes used to make such materials. A highly efficient and economical technique for creating thin films in laboratory scale is solution casting, which involves applying biopolymer solutions to a surface and allowing them to dry. Researchers have developed different techniques to cast biofilms. [
1,
4,
11,
12,
13]. Dissolving cellulose or lignin before casting is one way to create thin films from lignocellulosic materials, and a number of solvents have been proposed for this purpose [
10]. We have earlier developed a novel solution casting method to produce pectin-based thin films [
3]. The method involved dissolving pectin and dispersing cellulose fibres in a citric acid solution, which were then dried into thin layers. It is well known that citric acid works as an effective solvent to dissolve pectin and the pectin dissolution facilitate the film formation. The films can be viewed as biocomposite materials made of cellulose fibres that are reinforced into a matrix of pectin and hemicellulose [
14]. The addition of glycerol to the solution could provide a plasticizing effect to the casted films [
15]. In a study, a twin-screw extruder was used to blend pectin, glycerol, starch, and water, and the mixture was extruded as films [
15]. Glycerol plasticization was studied in this work [
15]. Most of the published work is focused on citrus waste, and there are limited publications on ginger waste.
The particle size of the reinforcements plays a crucial role in in the final properties of the biocomposites or reinforced biofilms. Reduction in particle size of the reinforcements would allow them to be an effective reinforcement and provide biofilms good properties. This is because the decreased particle size leads to an increase in the surface area for bonding. Nanoparticle reinforcements offer a large surface area for chemical modifications, and therefore can enhance mechanical and barrier properties that are crucial for packaging applications [
2,
3]. In previous studies, particles from orange peels were reinforced in high density polyethylene (HDPE) and found that the orange particles have positive effect on tensile, bending and hardness properties. The results hint that particles from orange peels waste could be used as a biodegradable eco-friendly reinforcement [
6]. However, the dispersion of nano-sized particles in the matrix could be a challenge as the particles could agglomerate [
16,
17].
In this work, orange (OW) and ginger waste (GW) suspensions were ground using ultrafine grinder to fine particles, before solution casting thin biofilms. The effect of grinding on particle size distribution was studied. Furthermore, the mechanical and thermal characteristics of the biofilms were studied.
2. Materials and Methods
2.1. Materials
OW and GW were provided by Brämhults Juice AB (Borås, Sweden) and they were stored at -20 ℃ until used as shown in
Figure 1. Orange and ginger juice production generates 50-60 % residue of the original mass after juice pressing; residue of peels, pulp, seeds‚ and membrane. Citric acid (anhydrous>99.5%, Sigma-Aldrich) and glycerol (>99%, VWR Chemicals) were used as a solvent for pectin and plasticizer, respectively.
2.2. Preparation of OW and GW Suspensions
The OW and GW were submerged in water overnight in order to be able to extract the soluble sugars (3.2 kg water/kg OW, (3.2 kg water/kg GW). Although GW has a significantly lower sugar content than OW, both materials underwent the same process for better comparison. The insoluble fraction was then recovered using a kitchen sieve. A suspension of washed OW and GW (2 wt%) in water was prepared and subjected to mechanical grinding using a supermasscolloider disc-mill grinder (MKCA6-5J, Masuko Sangyo, Kawaguchi, Japan). The grinder used MKE #46 grinding stones (standard grinding stones for soft materials) which are ultrafine silicon carbide stones for pulverizing and emulsifying fibrous materials. Figures 1(a) and 1(b) show the suspension preparation and ultrafine grinder respectively. The rotation speed of the stones was 1500 rpm and the distance between the stones was gradually adjusted to −70 µm (contact mode) to homogenize the OW and GW suspension. The suspensions of 2% OW and GW in water were run through an ultrafine grinder up to 30 times. This process of the suspension going through the grinder is called fibrillation. Every fifth run, the suspensions' viscosity was measured using a viscometer to determine whether it had been adequately fibrillated.
2.3. Viscosity Measurement
Viscosity measurements were conducted using a Vibro Viscometer SV-10, A&D Company, Ltd, (Japan), at a constant shear rate. The viscosity of a fluid is a measure of its resistance to deformation at a given rate and is defined scientifically as a force multiplied by a time divided by an area. Thus, its SI units are newton-seconds per square meter, or pascal-seconds [
18]. Measurements were taken during the suspensions’ grinding process to assess the rheological behavior of these suspensions during fibrillation. A total of 30 fibrillations were made for both OW and GW suspensions, and samples of 1000 ml were taken after 5, 10, 15, 20, 25 and 30 runs to study the effects of grinding cycles and particle size on the mechanical properties of the biofilms. The measurements were performed at 23°C ±0.5°C. It can be presumed that the cellulose fibers have reached microscopic size (the grinding machine's capacity) when the viscosity stabilizes. This was ascertained using a microscope.
2.4. FluidScopeTM Scanning Technology (oCelloScope)
The automated time lapse microscopy pictures recorded with the FluidScopeTM scanning technology with the oCelloScope (BioSense Solutions ApS, Farum, Denmark) were used to determine the particle size in suspensions after grinding. OW and GW suspensions in water were diluted 500 times before imaging. Images were captured from 3 samples of each suspension, obtained at different grinding cycles and the number of images for each sample was set to 20. EQPC algorithm provided by UniExplorer software was used to illustrate particle size.
2.5. Solution Casting
Biofilms were prepared in accordance with the ASTM-D1708 standard [
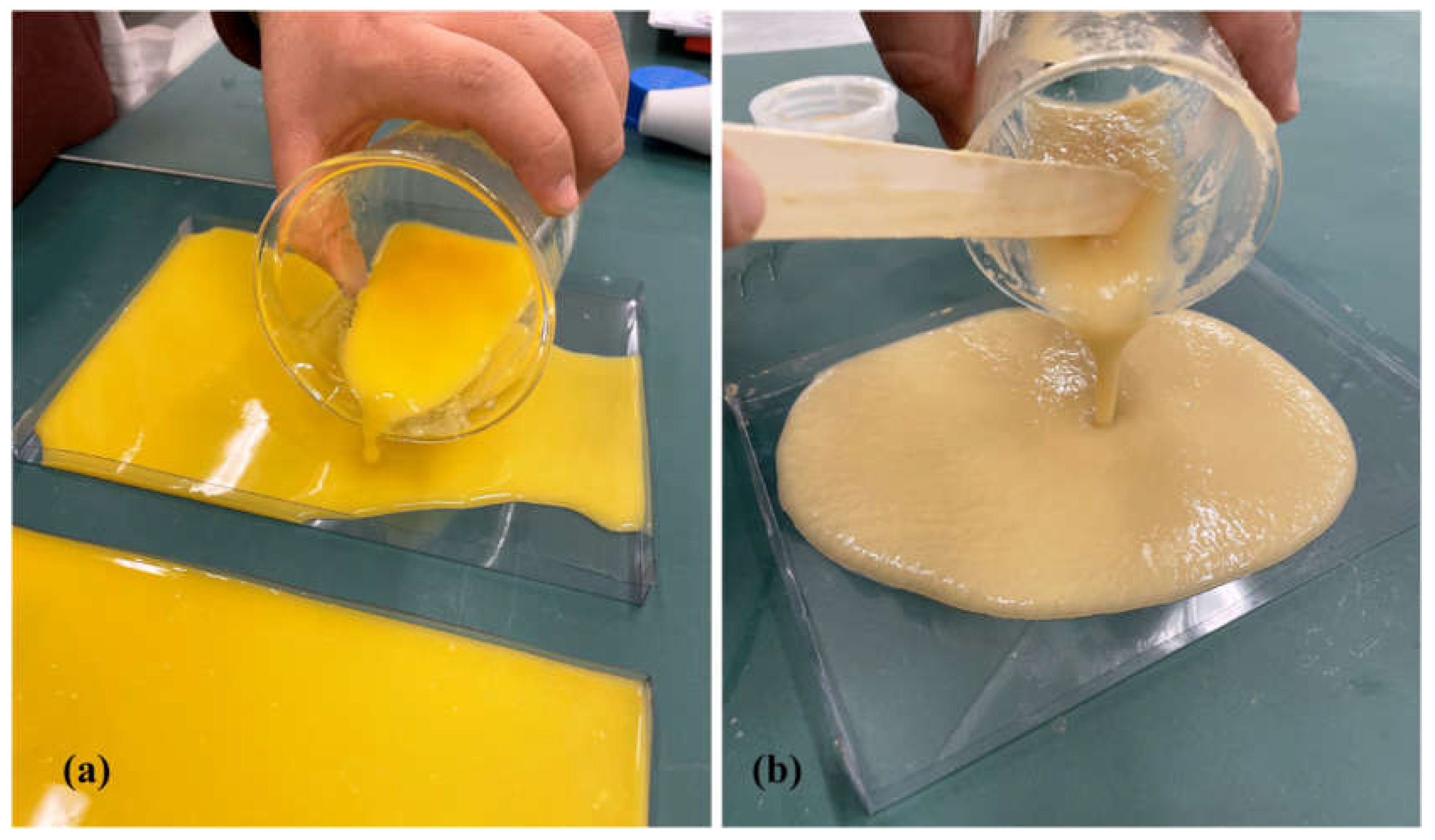
19]. 100 ml of ground OW suspension was mixed with 0.5 g of glycerol, and 0.4 g of citric acid, while 100 ml of ground GW suspension was mixed with 0.5 g of glycerol. The amount of glycerol and citric acid were chosen after preliminary experiments. The mixtures were placed in a shaker at 110 rpm for 10 minutes, before casting in plastic molds (210×148 mm). The suspensions were poured into each mold which were then oven dried at 35°C for 12 hours.
Figure 2 shows the solution casting of OW suspension and the same procedure was followed for casting of GW suspension.
2.6. Tensile Testing
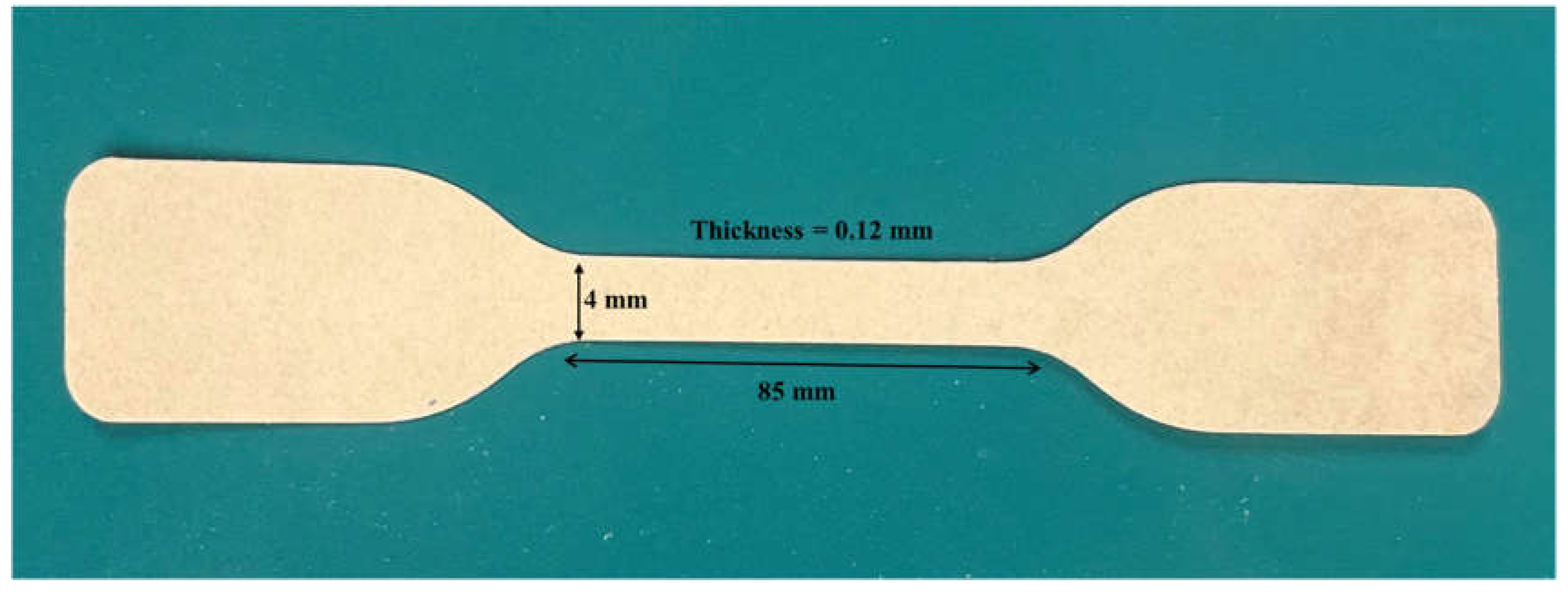
Specimens for tensile test were prepared according to ASTM-D1708. According to the standard, biofilms were cut to dogbone shaped specimens with 85 mm in length, width of 4 mm and 0.12 mm in thickness as shown in
Figure 3. Tests were carried out using universal testing machine (Tinius Olsen H10KT universal, Salfords, UK) equipped with QMAT Professional software. The gauge length and the rate of loading (crosshead speed) were 45 mm and 10 mm/min respectively. A load cell of 100 N was used for the measurement. At least 10 specimens were analyzed for each sample.
2.7. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis of the OW and GW films was carried out using a Q500 thermogravimetric analyzer (TA Instruments, New Castle, DE, USA). All the samples were oven dried and conditioned for 12 hours at 35°C before testing. Approximately 10 mg of each sample placed in platinum pan was heated from room temperature (20±2)°C to 600°C at a heating rate of 10°C/min under nitrogen atmosphere (flow rate of 50 ml/min). Thermal degradation behavior of the biofilms was investigated. The measurements were done at least thrice for each sample.
2.8. Differential Scanning Calorimetry (DSC)
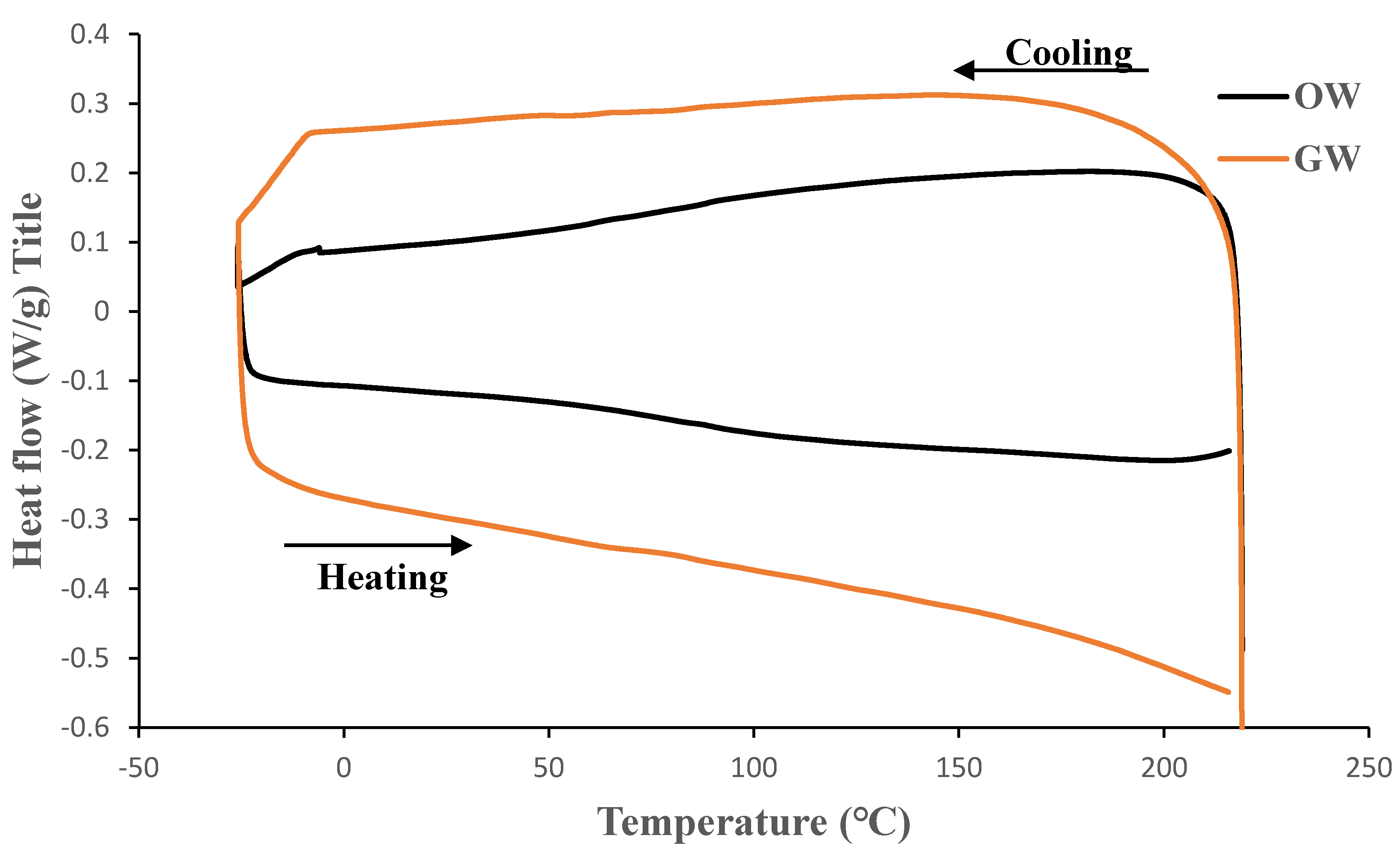
DSC analysis was carried out using a DSC Q2000 supplied by TA Instruments, New Castle, DE, USA. Samples of 7-9 mg were sealed in an aluminum pan and heated from -30°C to 220°C at a rate of 10°C/min. The samples were then cooled to -30°C (cooling cycle) before heating again to 200°C at the same rate of heating (10°C/min). Tests were conducted under nitrogen atmosphere. The first cycle of heating was not used in analysis to eliminate any previous thermal history in the polymer. Glass transition temperature of the biofilms was investigated. The measurements were repeated at least thrice for each sample.
2.9. Dynamic Mechanical Thermal Analysis (DMTA)
A dynamic mechanical thermal analysis (DMTA) (Q800, TA Instruments, Waters LLC, USA) was performed according to standard testing to determine viscoelastic properties of the biofilms [
20]. Tests were conducted using a film tension clamp in a multifrequency strain mode. The specimen dimensions were 10 mm in width, 30 mm in length, and 0.12 mm in thickness. The temperature range was between 30 and 250°C and the heating rate was 5°C/min. Tests were conducted at a strain frequency of 1 Hz, and at amplitude of 5 𝜇m. Modulus (stiffness) and damping (energy dissipation) properties of biofilms were analyzed quantitatively. Experiments were done in triplicate.
3. Results
Several packaging films have been studied extensively. The effect of particle size on the properties of biofilms has, however, rarely been investigated. In this study, orange and ginger waste were subjected to ultrafine grinding at different cycles and biofilms were produced. After 5, 10, 15, 20, 25 and 30 runs, the suspensions' viscosity was examined, along with the effect of particle size on biofilms’ properties.
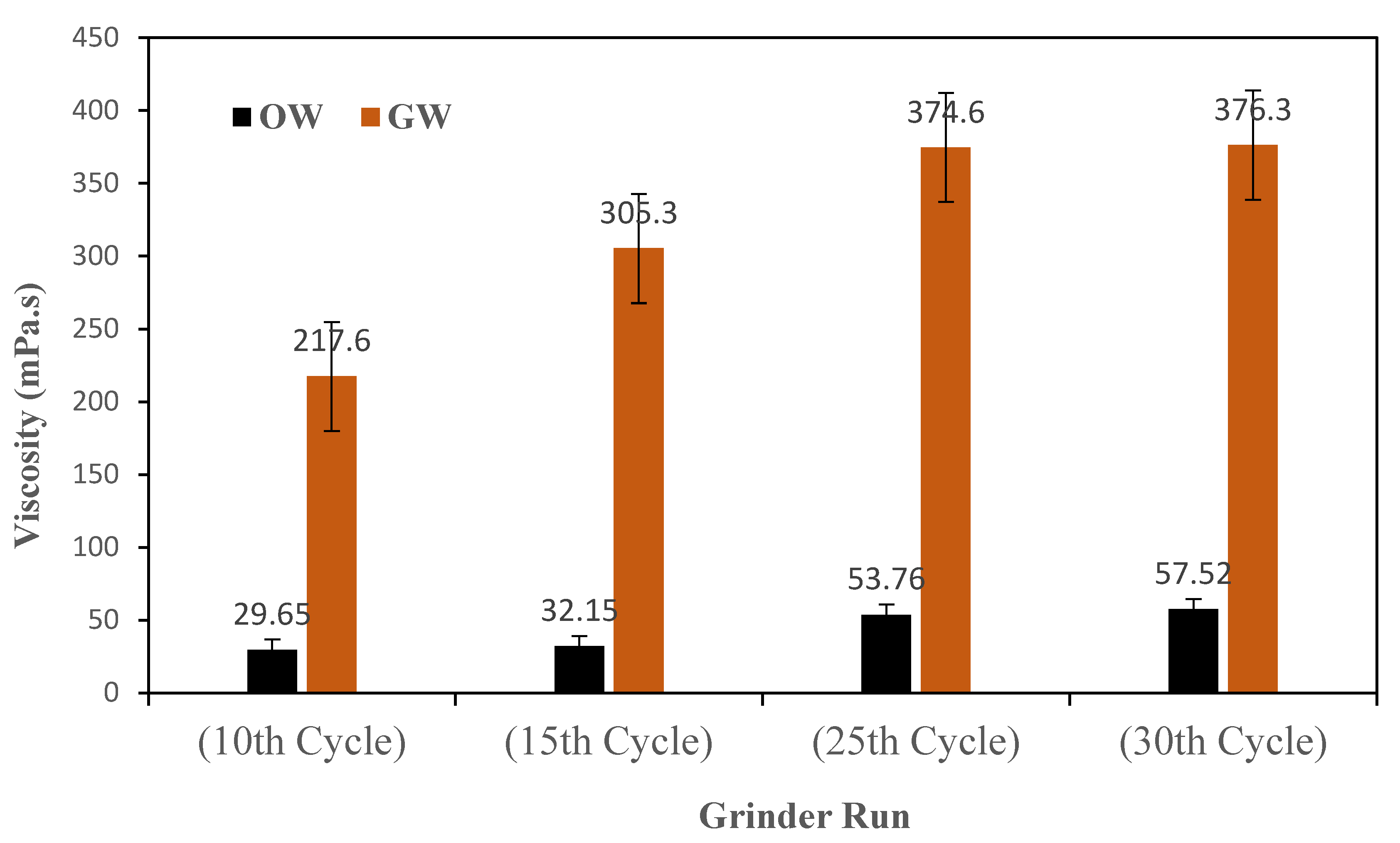
Figure 4 shows the viscosities of OW and GW suspensions after selected number of grinding cycles (10, 15, 25 and 30) to show recognizable differences between viscosities.
The particle size of the solid phase (OW and GW) was decreased while the total mass of the particles in a suspension is maintained; the overall result was an increase in the number of particles in the system.
Figure 4 illustrates how the change in particle size would affect viscosity. As shown in the figure, the viscosities of OW and GW suspensions increased on increasing the grinding cycles (number of runs in ultrafine grinder) due to an increase in number of particles, and therefore an increase in particle-particle interactions.
The viscosity of the OW suspension increased from 29 to 57 mPa.s on increasing the grinding cycle from 10 to 30. From the initial 217 mPa.s on 10 grinding cycles, the viscosity of the GW suspension was increased to 376 mPa.s on 30 grinding cycles. GW suspensions showed much higher viscosity than OW suspensions due to inherent fiber-like structure of GW.
3.1. SubsectionOW and GW Grinding and Determination of Particle Size
After the fibrillation process, the particle size in the suspensions was measured. The decrease in the particle size was evident after each grinding cycle and the trend is shown in
Figure 5. The results align with those of the results from viscosity measurements.
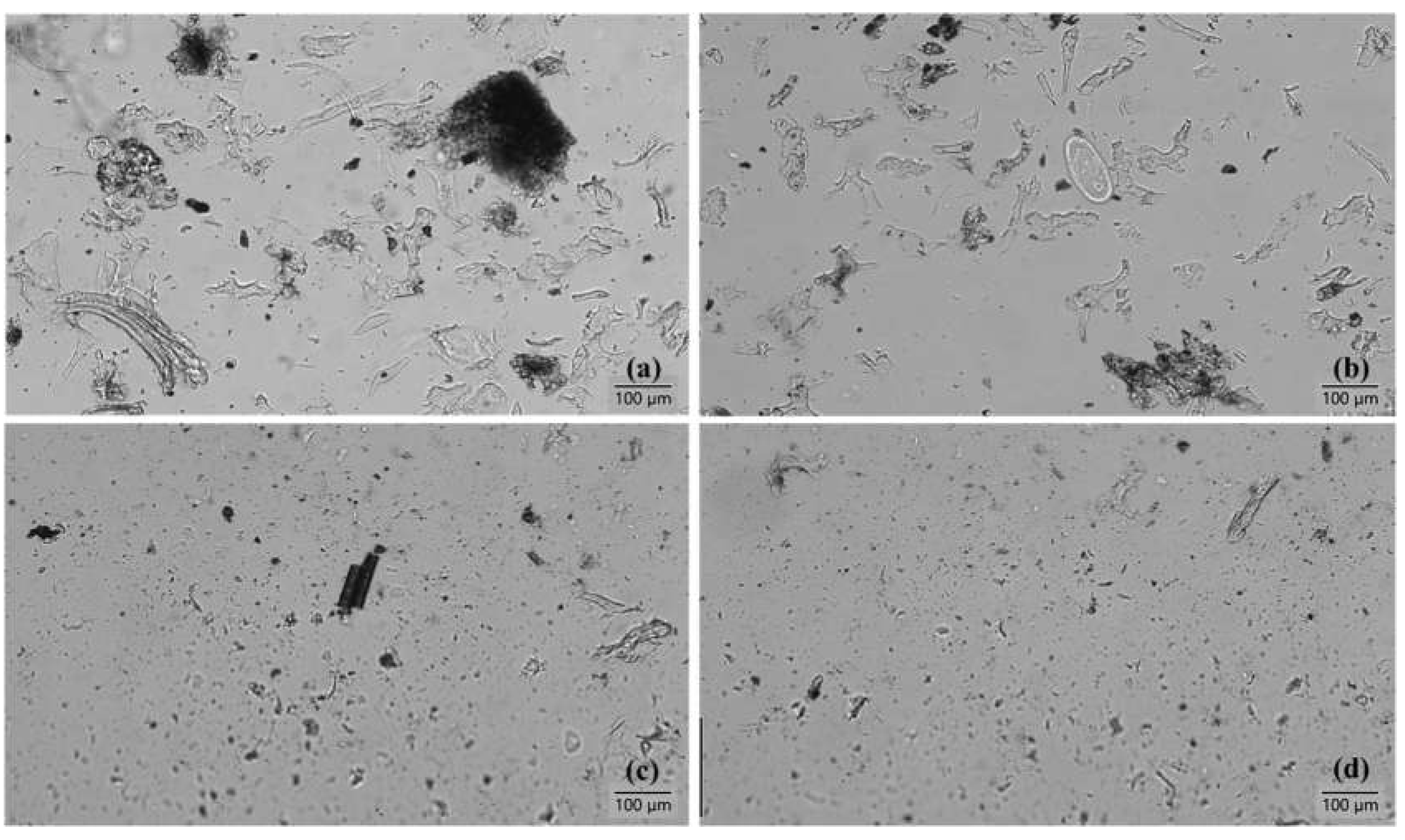
Microscopic images,
Figure 6, shows that the particle size within the suspensions has decreased by each grinding cycle. Comparing the images after 10th and 30th fibrillations show the presence of large fibres and particles after 10 grinding cycles, whereas the particles are smaller and less noticeable after 30 grinding cycles.
3.2. Production of Thin Films from OW and GW by Solution Casting
It is well known that the addition of acid to pectin forms gel due to hydrogen bonding and hydrophobic interactions between pectin chains. The introduction of citric acid to OW suspension helped to bind pectins and form films. The formation of films was be due to gelling of the suspensions and similar effect was noticed by Maitra et.al [
21]. Since GW are not high in pectin content, the addition of citric acid step was ignored while casting GW suspension. The film formation was possible without citric acid addition, and this could be due to high interactions between the particles and high viscosity of GW. Plasticizer (glycerol) was added to both suspensions to make the biofilms flexible, which is necessary for packing applications, and the resulting films were controllable.
After grinding, the orange particles reached microscopic size, resulting in a homogeneous OW suspension. This made it easier to produce biofilms. However, due to the long and thin fiber-like structure of GW, the suspension is not as homogenous after grinding. As a result, the suspension of these particles was not distributed as evenly as OW suspension, which complicated the casting.
Over time the color of the suspension started to change, and the smell of orange and ginger grew stronger indicating the biodegradability. Due to this, the solution casting was performed right after the size reduction by grindings and the molds place in oven dried at 35°C for 12 hours. Due to the complete loss of water inside the structure, the films were completely dried and the decomposition rate was minimalized, but not eliminated. The films started to change its color and started to smell over time indicating the decomposition.
3.3. Mechanical Properties
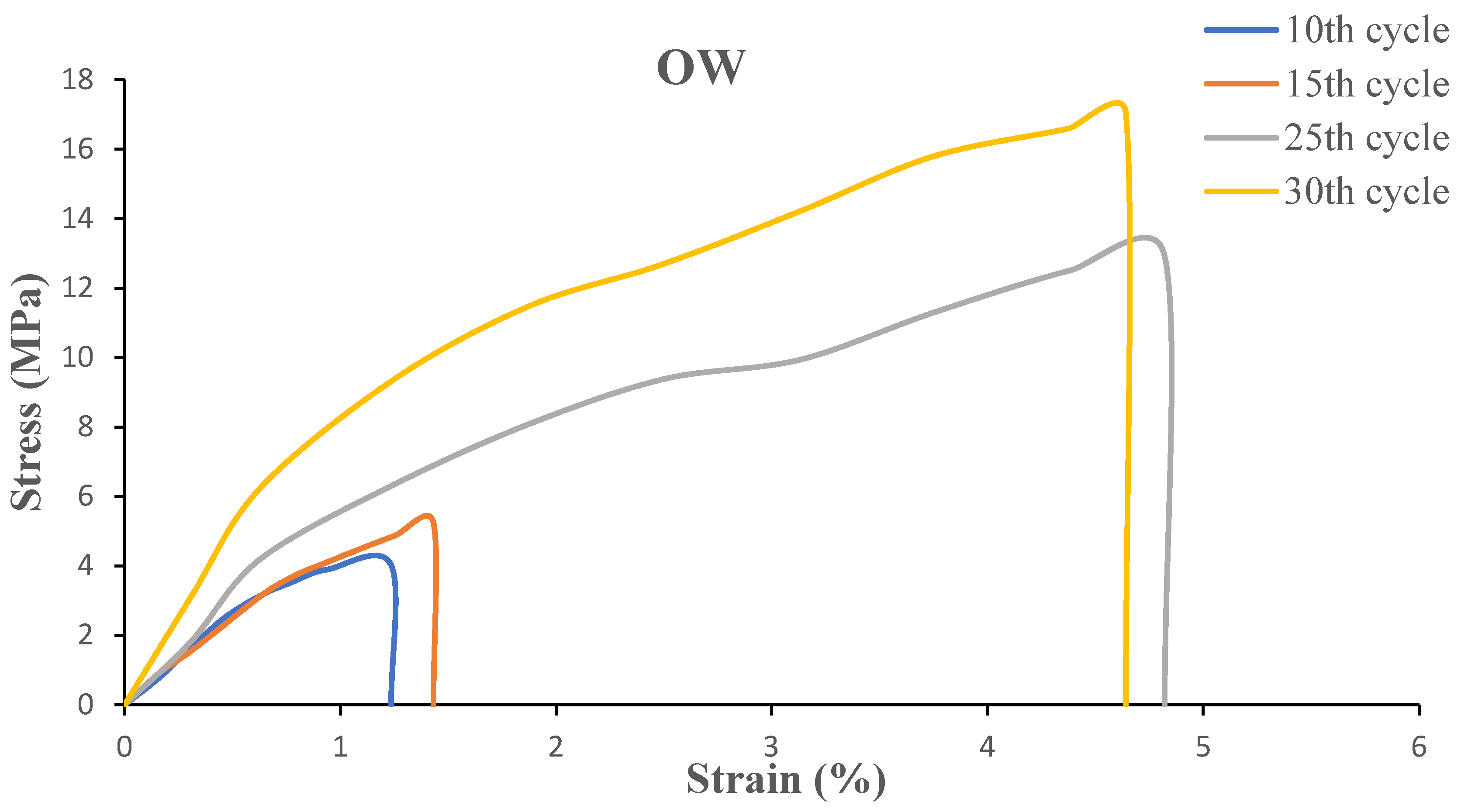
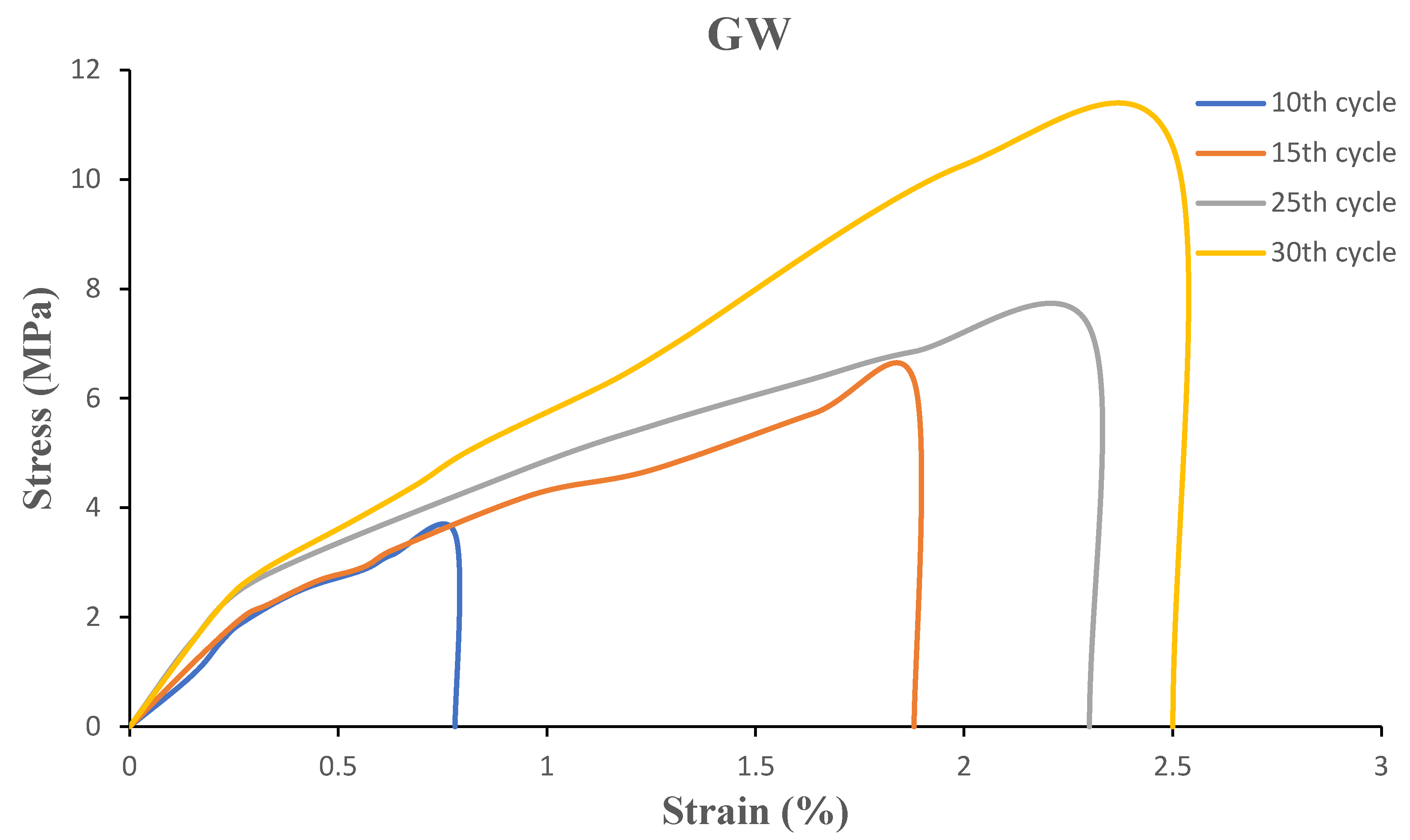
The mechanical performance of biofilms is characterized by tensile strength and elongation at break. To study the effect of grinding cycles, the biofilms were cut into dogbone shape according to the ASTM-D1708 standard, then evaluated by the tensile testing machine. The tensile modulus, tensile strength at break, maximum elongation at break and stress-strain graphs for OW and GW biofilms are shown in
Table 1 and
Figure 7 and
Figure 8.
The strain-stress diagrams show that both biofilm samples exhibit semi-ductile behavior since failure occurs at low strain in both samples, and the addition of glycerol to both suspensions did not increase the strain significantly. However, glycerol facilitated molding and casting as the biofilms could not be removed from the mold for characterization without the addition of the plasticizer.
The tensile strength of the biofilms ranged from 4 to 17 MPa. Both OW and GW based biofilms had a significant increase in tensile strength as a result of the increase in grinding cycles. This is due to strong interactions between the smaller particles caused by an increase in the specific surface area. As a result, the particles were dispersed more evenly throughout the biofilms. Citric acid could have enhanced the interactions between the particles during gelling in the OW-based films. Because GW’s inherent fiber-like structure provides entanglements to withstand force applied to it, one can anticipate a high tensile strength from GW based films. Nevertheless, when the number of grinding cycles increased, the raw materials were reduced to finer particles, producing films with tensile strength comparable to that of OW-based films. As the number of grinding cycles increased, strain also increased. However, the slope in the stress-strain diagram representing the Young’s modulus did not improve on increasing the grinding cycles. Previous studies showed that the biofilms had similar tensile properties [
22].
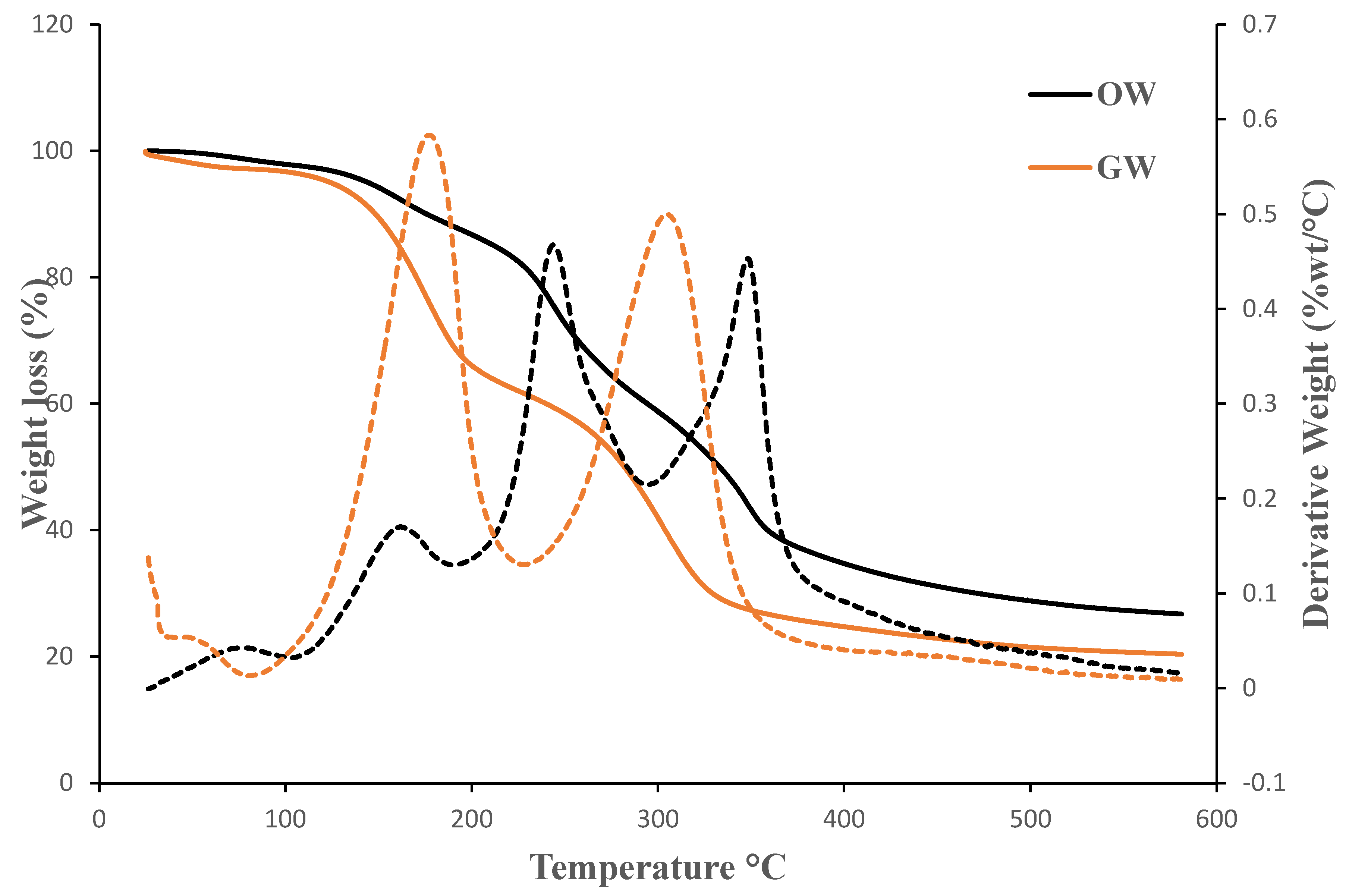
3.4. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis was used to follow the thermal degradation through weight loss of the films over a temperature range. Maximum degradation temperatures of the films were analyzed. The onset temperature, T
10% (the temperature at 10% mass loss), second derivative peak temperature, T
max (maximum decomposition temperature), and the residual mass at 550°C for all the samples reported in
Table 2.
The trend was identical in both cases, and
Figure 9 illustrates the three stages of thermal decomposition. The evaporation of moisture in the samples caused the first thermal event, which was a weight loss of up to 1.79–2.34% at a temperature of 86.38–91.61°C. The second thermal event, which is connected to the degradation of the glycerol and pectin included in the films, took place at a temperature of 175.42–237.66°C, and the weight reduction was 19.75–26.28% [
23]. At a temperature of 303.64–348.31°C, the third thermal event was noticed. The degradation of the films revealed a further 16.83–25.91% by weight, which can be attributed to the breakdown of the cellulose and starch [
24]. The remaining 20.74–27.35 wt% of the biofilms were found as the residual ash at 550°C.
3.5. Differential Scanning Calorimetry (DSC)
DSC was used to determine the glass transition temperature (Tg) of the films. The DSC curves in
Figure 10 demonstrate that the glass transition temperatures fall between 75 and 100°C. The absence of endothermic peaks in the curves suggests that the films have no melting point. Therefore, it is anticipated that these biofilms are mostly amorphous.
3.6. Dynamic Mechanical Thermal Analysis (DMTA)
Because of its inherent sensitivity and clear information on damping, DMTA is a good technique for the determination of the glass transition of polymeric materials. In addition, elastic behavior of the biofilms were analyzed through storage modulus.
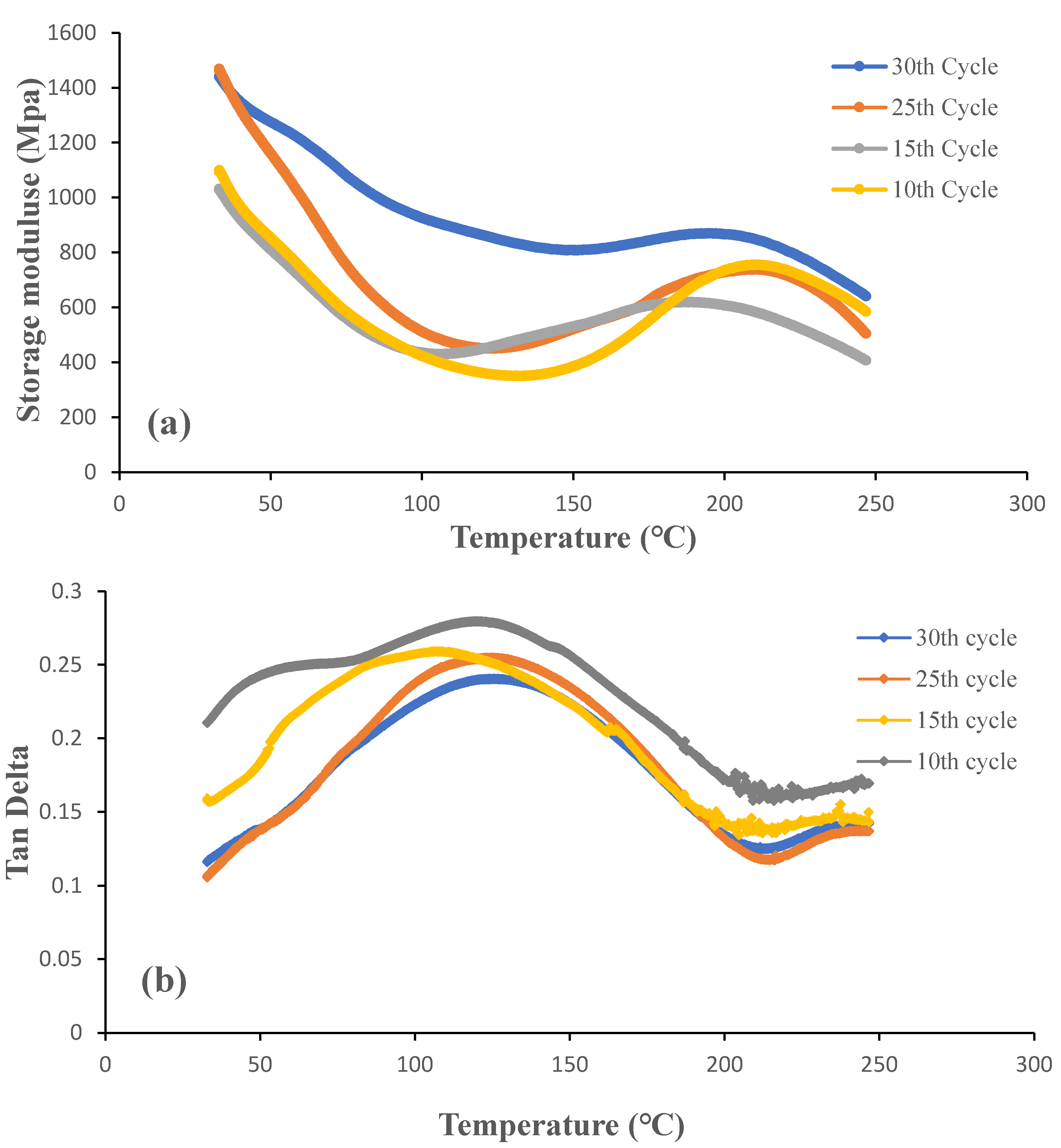
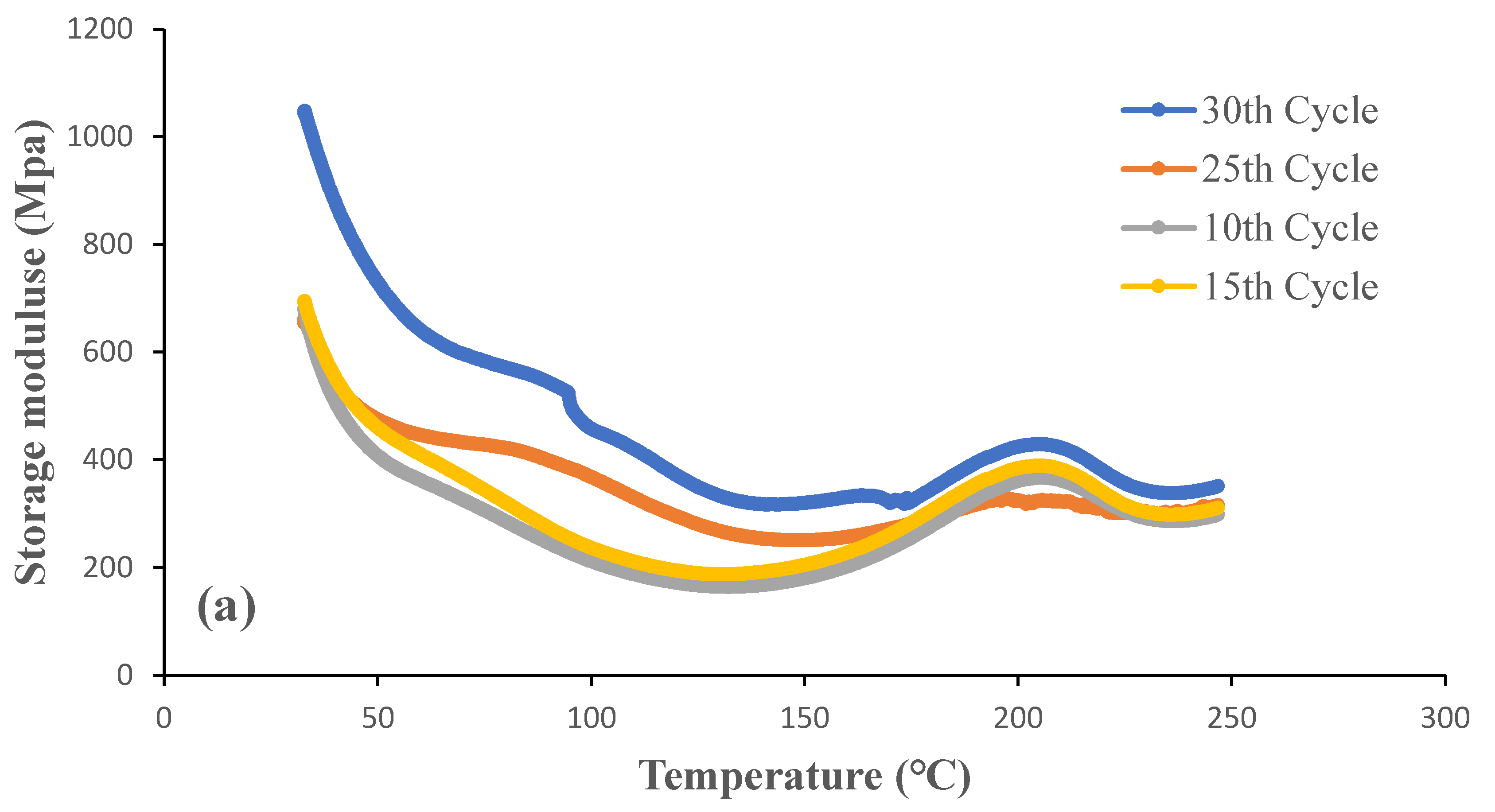
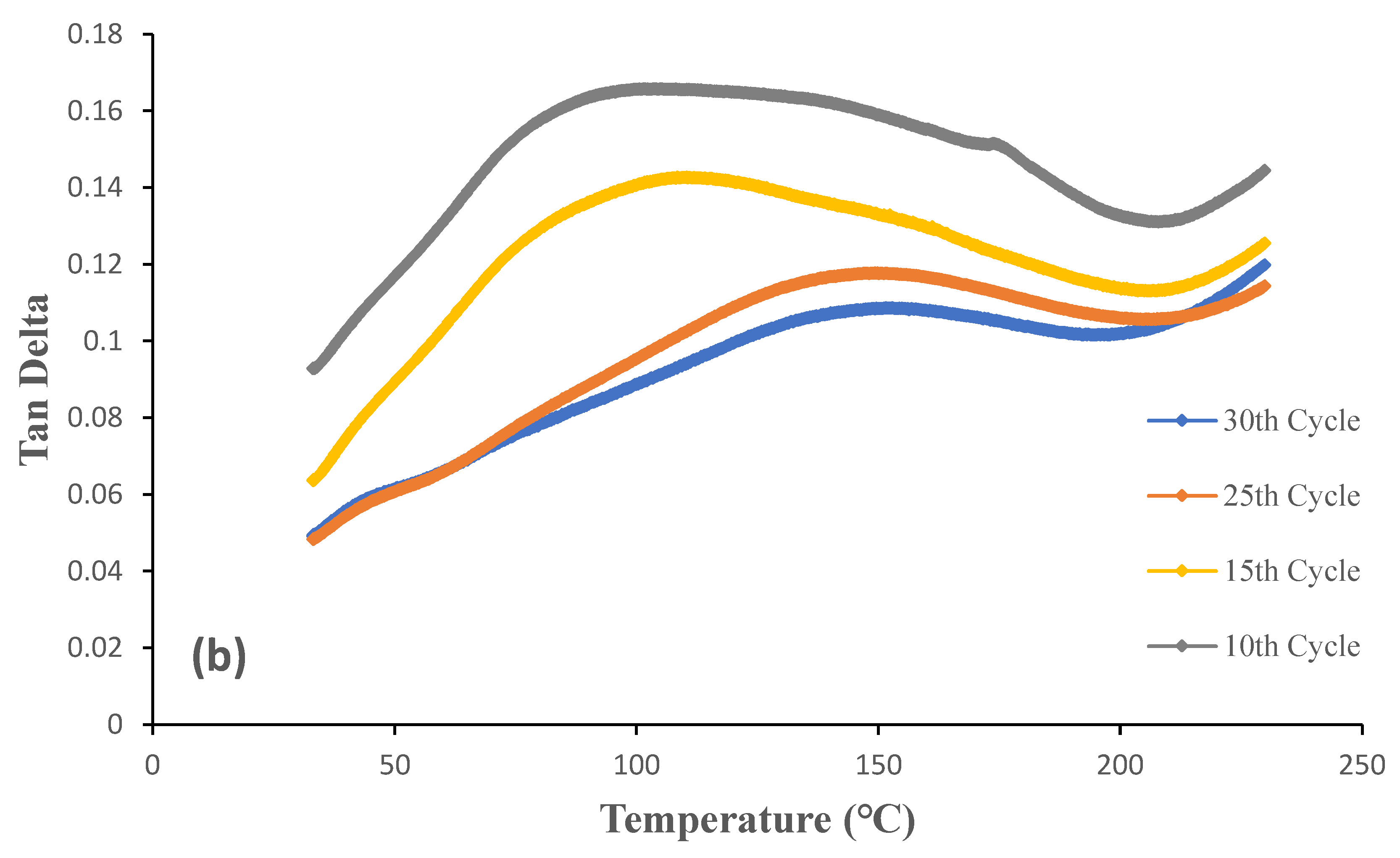
Figure 11 and
Figure 12 show storage modulus and the damping (Tan δ) curves for OW and GW biofilms at different grinding cycles. It was evident that the biofilms produced with 30 grinding cycles had a higher storage modulus than the biofilms produced with less grinding cycles. Both low and high temperatures proved this to be true. The result is consistent with tensile strength, see section 3.3, as expected given that the stiffness of the biofilms increased when the particle size was decreased. Strong interactions between the smaller particles brought on by an increase in the specific surface area are responsible for this. The effect was a more equal distribution of the particles throughout the biofilms. The storage modulus of the OW and GW biofilms at 30°C were 1450 MPa and 1032 MPa, respectively. The outcome is in line with the tensile strength as the OW biofilms showed better properties than GW biofilms, and this could be due to the interactions between the particles during the gelling of the OW-based films on addition of citric acid. The biofilms from our earlier research are similar storage modulus [
2].
The peak in the tan δ curve is taken as the biofilms' glass transition temperature as it has the highest ratio of viscous response compared to elastic response under deformation conditions, indicating the relaxation in the biofilms. According to tan δ curves, the glass transition temperature of the biofilms is around 100°C. High sensitivity of DMA equipment and the sample size used in the equipment are frequently attributed for glass transition temperatures that are higher than those of DSC. The glass transition temperature of the biofilms from our earlier studies is comparable [
2]. It is important to note that the grinding cycles had a minimum or no effect on glass transition temperature.
4. Conclusions
In this study, biofilms were successfully prepared using industrial waste. Orange and ginger residues from juicing industry was used as a primary raw material for film production. Ultrafine grinding was used to produce fine particles from the organic waste, orange and ginger. The grinding process has a significant effect on process and the resulting films. The particle size affected the viscosity and the mechanical properties of the resulting films.
Table 3 compares each experimental data obtained in this work with other biofilms reported in the literature. Results from the tensile test show that 17 MPa tensile strength and about 5% strain can be obtained. Thermal testing and DMTA indicate that these biofilms are stable at room temperature and until about 100°C. The results go hand-in-hand with the literature. However, the properties of these films should be improved to meet the industrial requirements for packaging films.
Over time the color of the films started to change, and the smell of orange or ginger grew stronger indicating the biodegradability of the films. Finally, employing orange and ginger waste for the production of biofilms paves the way for generating eco-conscious materials addressing issues related to plastic pollution and waste disposal simultaneously.
Author Contributions
Conceptualization, A.Z..; methodology, A.Z., S.K.R. and R.M.; validation, R.M., M.G. and S.K.R.; formal analysis, R.M., M.G. and S.K.R.; investigation, R.M., M.G. and S.K.R.; writing—original draft preparation, R.M. and S.K.R..; writing—review and editing, R.M., M.G. and S.K.R.; supervision, S.K.R.; project administration, S.K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct transformation of edible vegetable waste into bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Hall, N. Bioplastics made from coffee and orange. 3D Printing Industry 2016, 13, 10–134. [Google Scholar]
- Bátori, V.; Jabbari, M.; Åkesson, D.; Lennartsson, P.R.; Taherzadeh, M.J.; Zamani, A. Production of pectin-cellulose biofilms: a new approach for citrus waste recycling. International Journal of Polymer Science 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Cecchini, C. Bioplastics made from upcycled food waste. Prospects for their use in the field of design. The Design Journal 2017, 20, S1596–S1610. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Ridout, M.J.; Cross, K.; Brito, E.S.; Silva, L.M.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M. Bionanocomposite films based on polysaccharides from banana peels. International journal of biological macromolecules 2017, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.; Atuanya, C.; Igogori, E.; Ihom, P. Development of high-density polyethylene/orange peels particulate bio-composite. Gazi University Journal of Science 2013, 26, 107–117. [Google Scholar]
- Bátori, V. Fruit wastes to biomaterials: Development of biofilms and 3D objects in a circular economy system. Högskolan i Borås 2018.
- Abass, R. Mechanical behavior of natural material (Orange peel) reinforced polyester composite. International Journal of Engineering Sciences Research Technology 2015, 4, 166–172. [Google Scholar]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochimica Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Xu, A.; Chen, L.; Wang, J. Functionalized imidazalium carboxylates for enhancing practical applicability in cellulose processing. Macromolecules 2018, 51, 4158–4166. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Russo, D.; Penna, I.; Ceseracciu, L.; Palazon, F.; Scarpellini, A.; Cingolani, R.; Bertorelli, R.; Bayer, I.S.; Heredia-Guerrero, J.A. Facile production of seaweed-based biomaterials with antioxidant and anti-inflammatory activities. Algal research 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.; Bayer, I.S.; Athanassiou, A. Bioplastics from vegetable waste via an eco-friendly water-based process. Green Chemistry 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Tran, T.N.; Heredia-Guerrero, J.A.; Mai, B.T.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Bayer, I.S. Bioelastomers based on cocoa shell waste with antioxidant ability. Advanced Sustainable Systems 2017, 1, 1700002. [Google Scholar] [CrossRef]
- Bátori, V.; Lundin, M.; Åkesson, D.; Lennartsson, P.R.; Taherzadeh, M.J.; Zamani, A. The effect of glycerol, sugar, and maleic anhydride on pectin-cellulose thin films prepared from orange waste. Polymers 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.L.; Coffin, D.R.; Onwulata, C.I.; Konstance, R.P. Extrusion of pectin and glycerol with various combinations of orange albedo and starch. Carbohydrate Polymers 2004, 57, 401–413. [Google Scholar] [CrossRef]
- Matteucci, F.; Giannantonio, R.; Calabi, F.; Agostiano, A.; Gigli, G.; Rossi, M. Deployment and exploitation of nanotechnology nanomaterials and nanomedicine. AIP Conference Proceedings 2018, 1, 1–25. [Google Scholar]
- Wang, R.; Rosen, T.; Zhan, C.; Chodankar, S.; Chen, J.; Sharma, P.R.; Sharma, S.K.; Liu, T.; Hsiao, B.S. Morphology and flow behavior of cellulose nanofibers dispersed in glycols. Macromolecules 2019, 52, 5499–5509. [Google Scholar] [CrossRef]
- Urone, P.P., and Hinrichs, R.: ‘12.4 Viscosity and Laminar Flow; Poiseuille’s Law’, College Physics, 2016, 124, 5-68.
- Gooch, J.W. Encyclopedic dictionary of polymers. Springer New York 2011, 1015. [Google Scholar]
- Esmaeili,N,; Bakare, F.O.; Afshar, S.J.; Skrifvars, M.; Åkesson, D. Mechanical properties for bio-based thermoset composites made from a lactic acid, glycerol and viscose fibers. Cellulose 2014, 22, 603-613.
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. American Journal of Polymer Science 2014, 4, 25–31. [Google Scholar]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of bio-based films and 3D objects from apple pomace. Polymers 2019, 11, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Merino D, Bertolacci L, Paul UC, Simonutti R, Athanassiou A. Avocado peels and seeds: processing strategies for the development of highly antioxidant bioplastic films. ACS Applied Materials & Interfaces. 2021 Aug 4;13(32):38688-99. [CrossRef]
- Merino D, Paul UC, Athanassiou A. Bio-based plastic films prepared from potato peels using mild acid hydrolysis followed by plasticization with a polyglycerol. Food Packaging and Shelf Life. 2021 Sep 1;29:100707. [CrossRef]
- Moro TM, Ascheri JL, Ortiz JA, Carvalho CW, Meléndez-Arévalo A. Bioplastics of native starches reinforced with passion fruit peel. Food and Bioprocess Technology. 2017 Oct;10:1798-808. [CrossRef]
- Zubeldía F, Ansorena MR, Marcovich NE. Wheat gluten films obtained by compression molding. Polymer Testing. 2015 May 1;43:68-77. [CrossRef]
- Yaradoddi JS, Banapurmath NR, Ganachari SV, Soudagar ME, Sajjan AM, Kamat S, Mujtaba MA, Shettar AS, Anqi AE, Safaei MR, Elfasakhany A. Bio-based material from fruit waste of orange peel for industrial applications. Journal of Materials Research and Technology. 2022 Mar 1;17:3186-97. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).