Submitted:
13 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
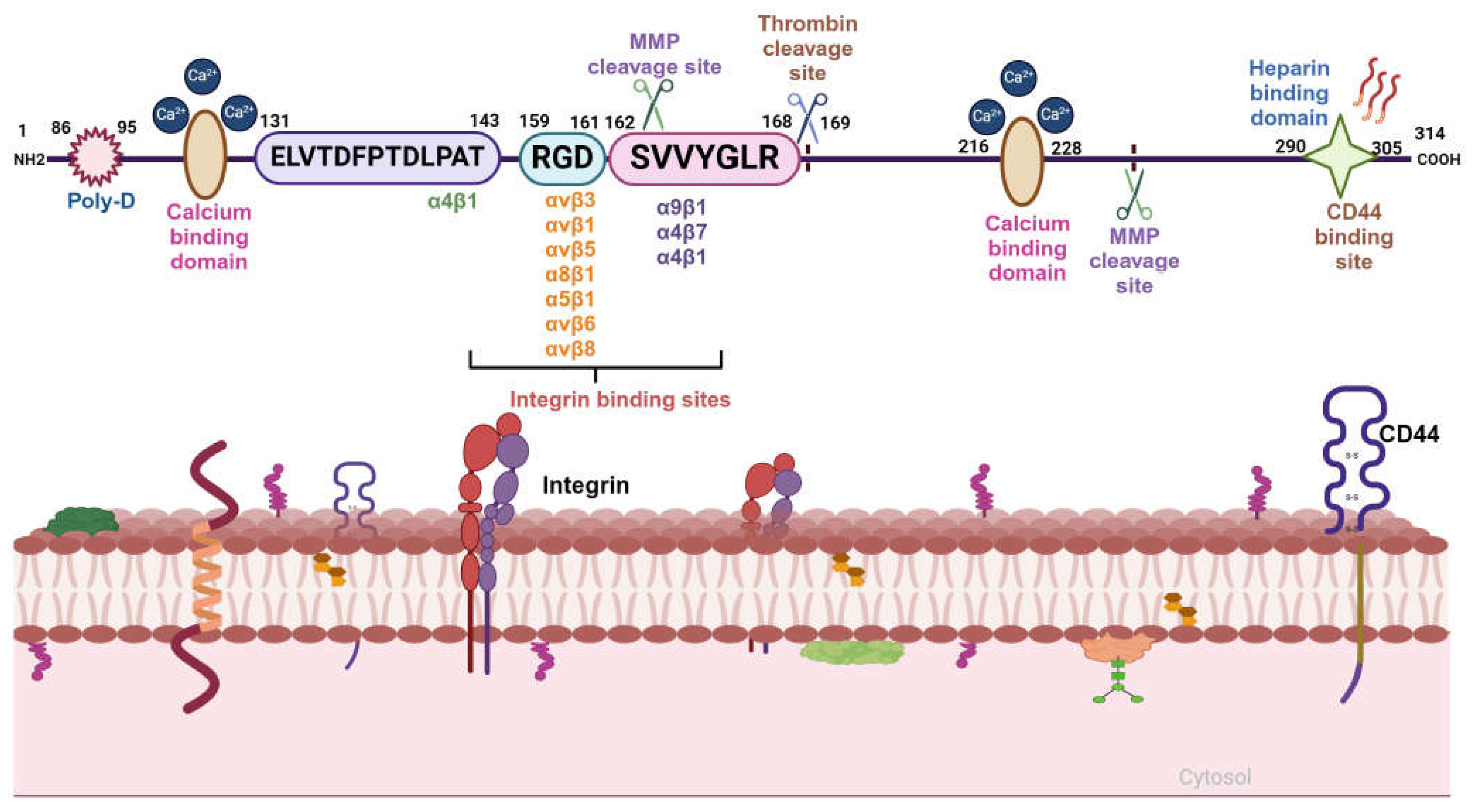
2. OPN Structure and Function
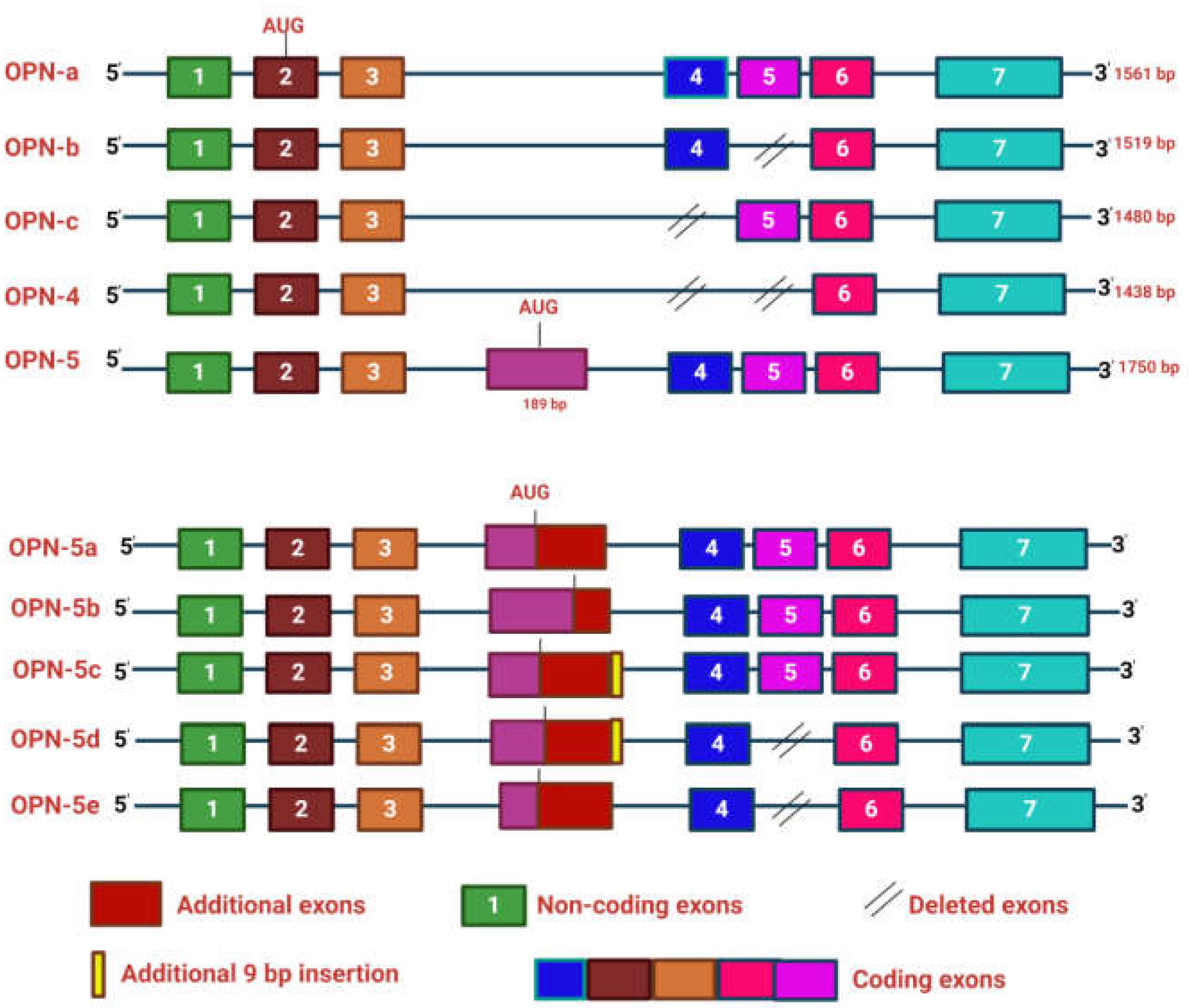
Structural Architecture and Splice Variants of OPN
3. OPN Expression in Various Cancers
4. Role of OPN in Tumor Progression
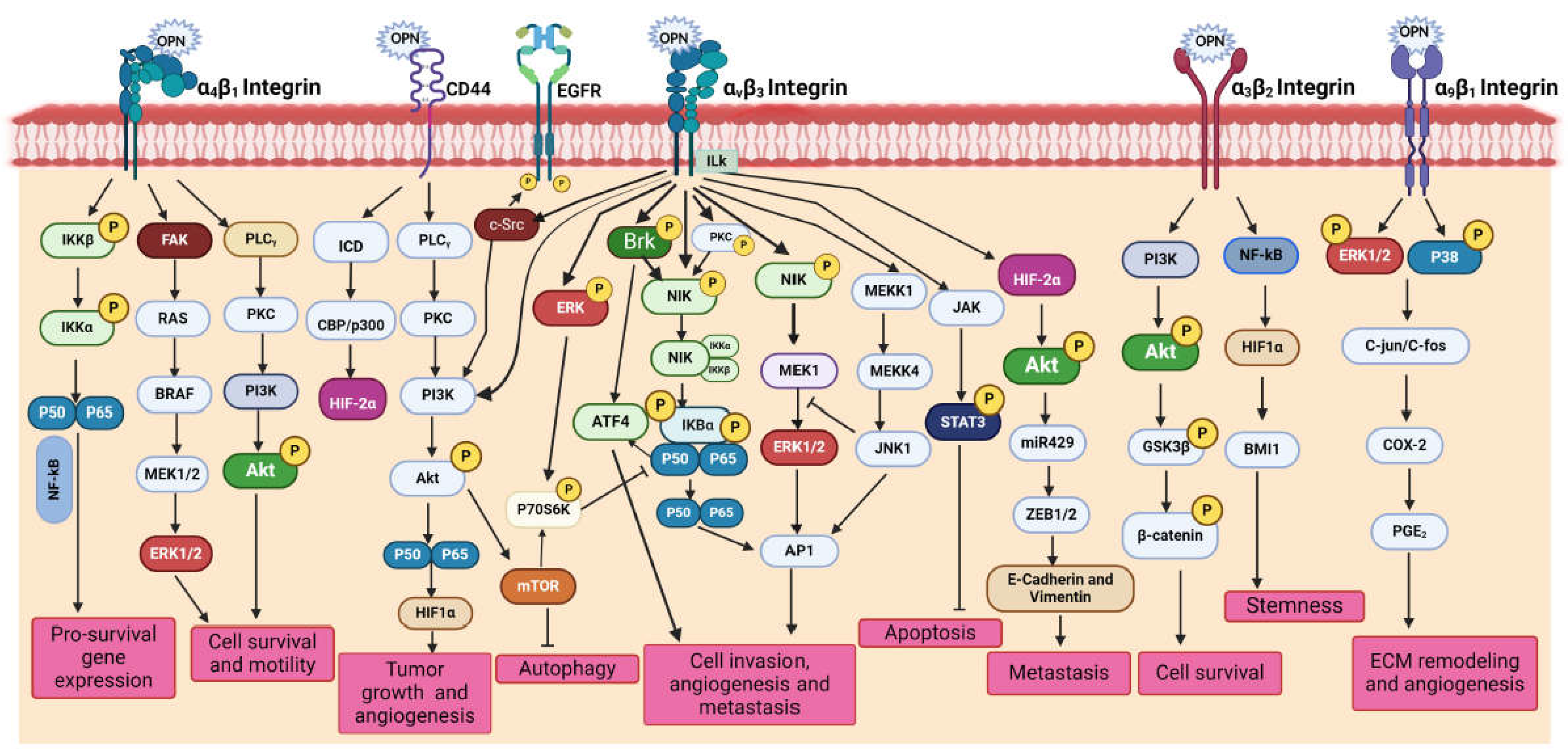
4.1. OPN Receptors
4.1.1. Integrin Receptors
4.1.2. CD44 Receptors
4.1.3. Receptor-Mediated Signalling
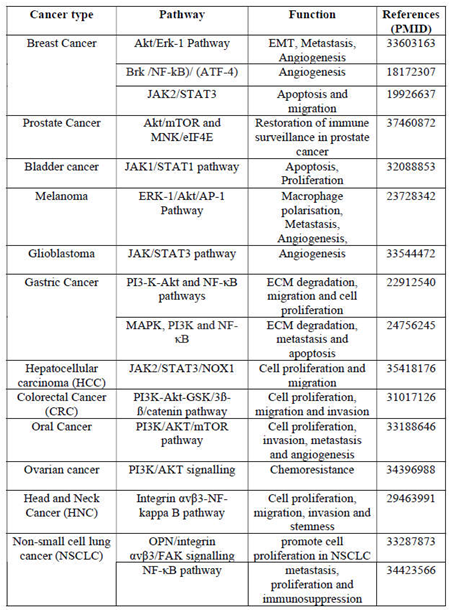
PI3K/Akt Signalling
p38/MAPK Signalling
Other Signalling
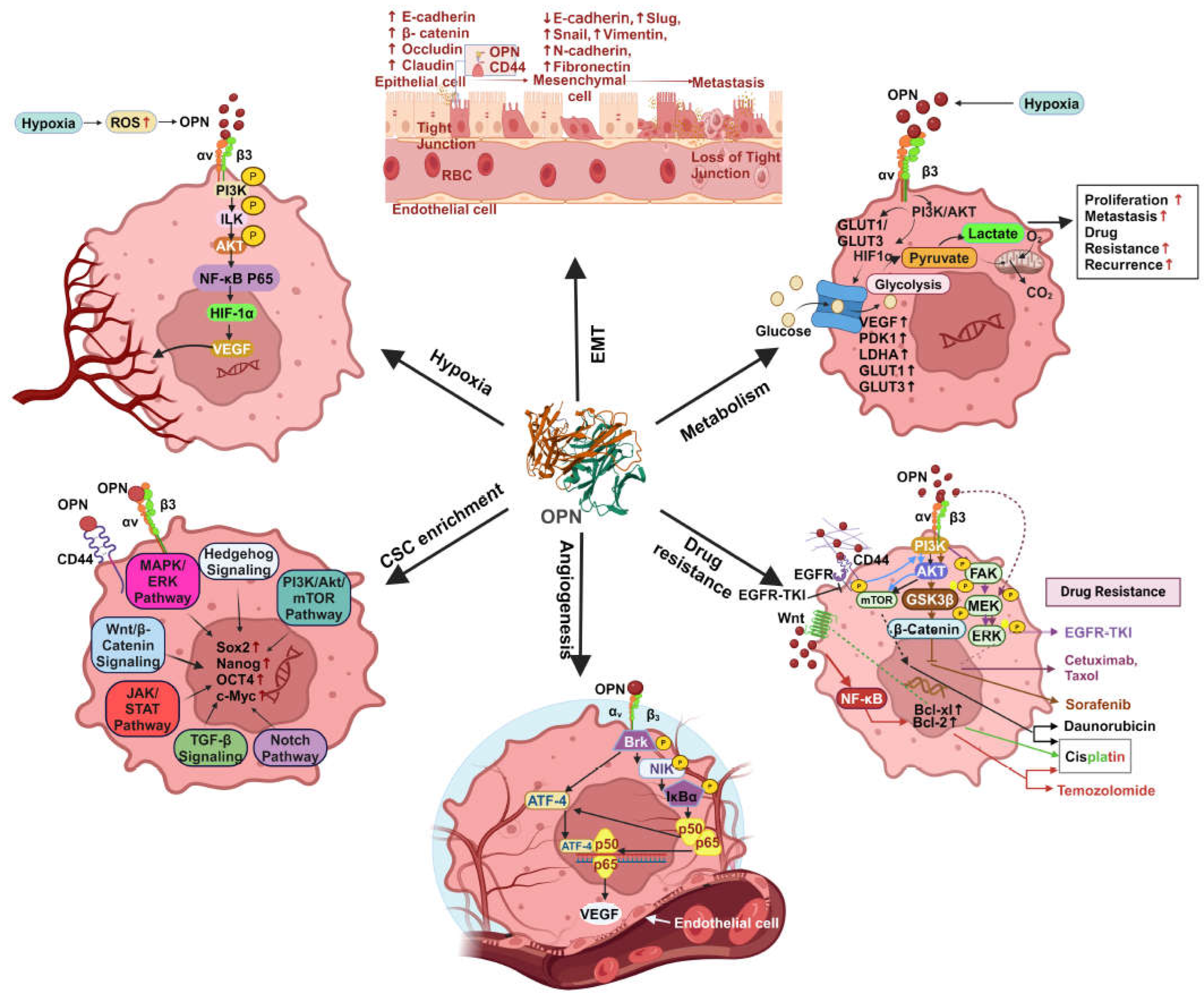
4.2. Multifaceted Functions of OPN in Tumor Progression
4.1.1. EMT
4.1.2. Enrichment of CSC
4.1.3. Chemoresistance
4.1.4. Angiogenesis
4.1.5. Metastasis
4.1.6. Cancer Cell Metabolism
5. OPN-Mediated TME Regulation
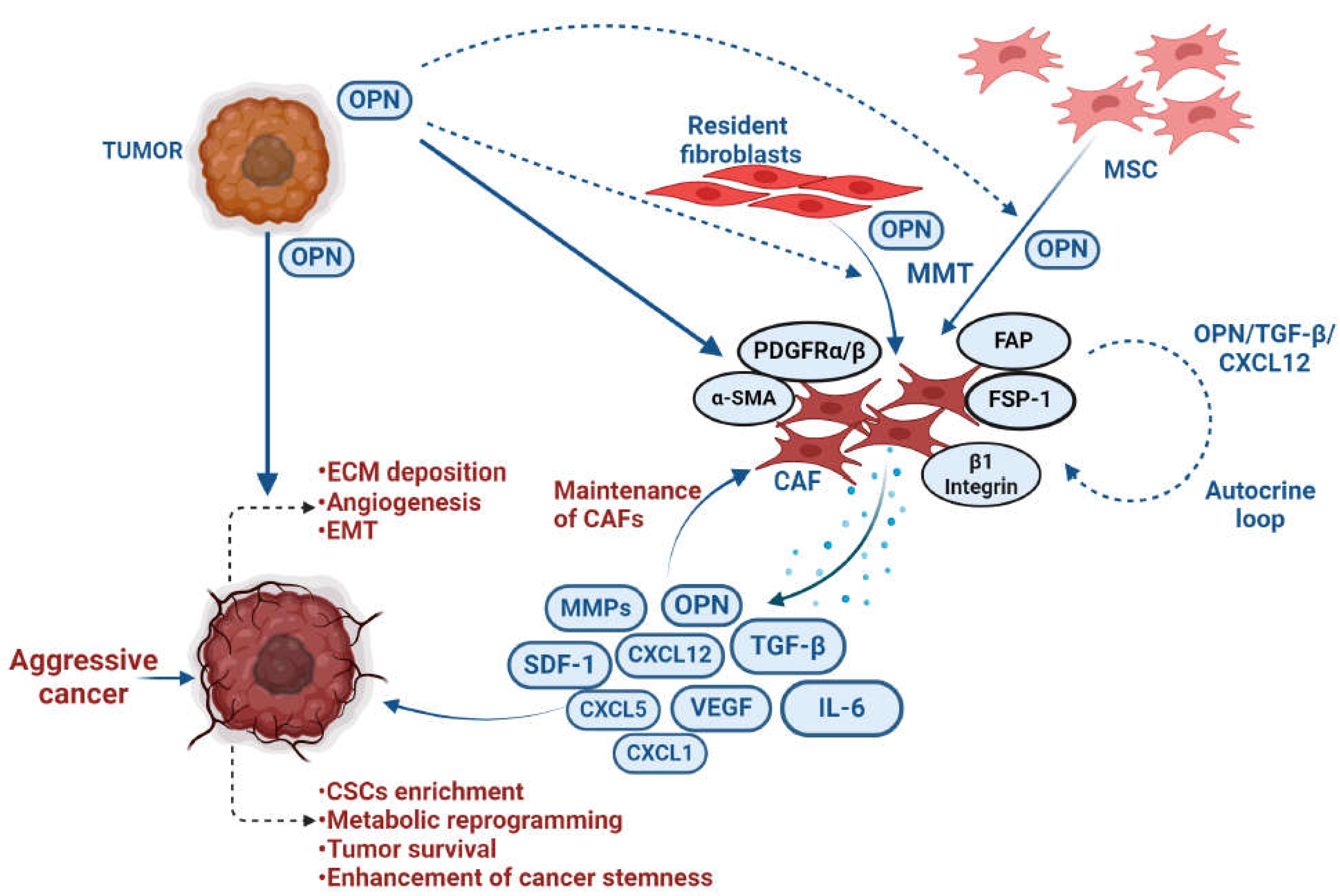
5.1. CAF
5.2. Adipocytes
5.3. Osteoclast
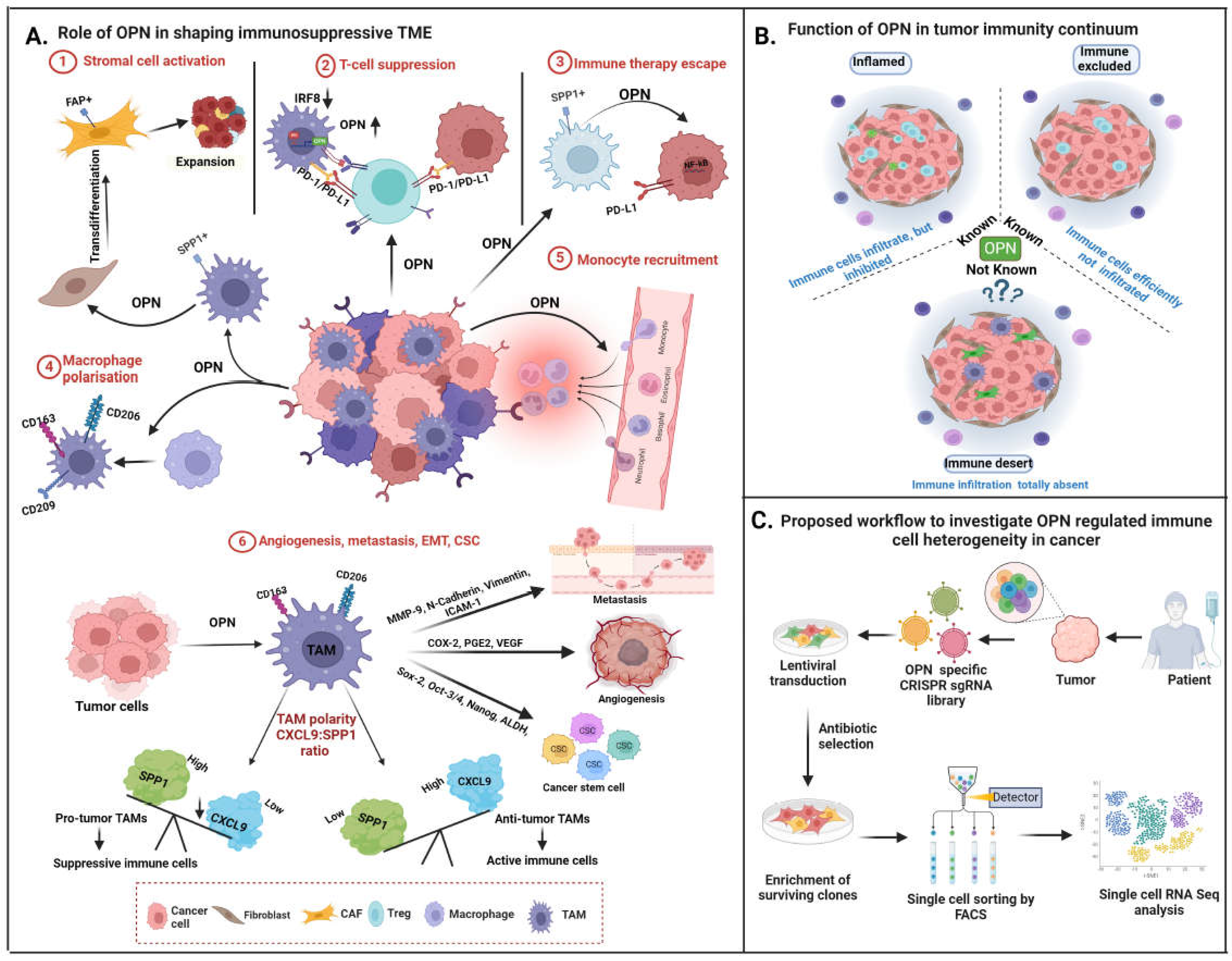
6. OPN in Immunomodulation
6.1. OPN Modulates Macrophages into TAMs
6.2. Role of OPN in TAM-Mediated Tumor Progression
6.3. Role of OPN in Immune Evasion
6.4. OPN Inhibits T Cell Activation
6.5. OPN Regulates Immune Checkpoints
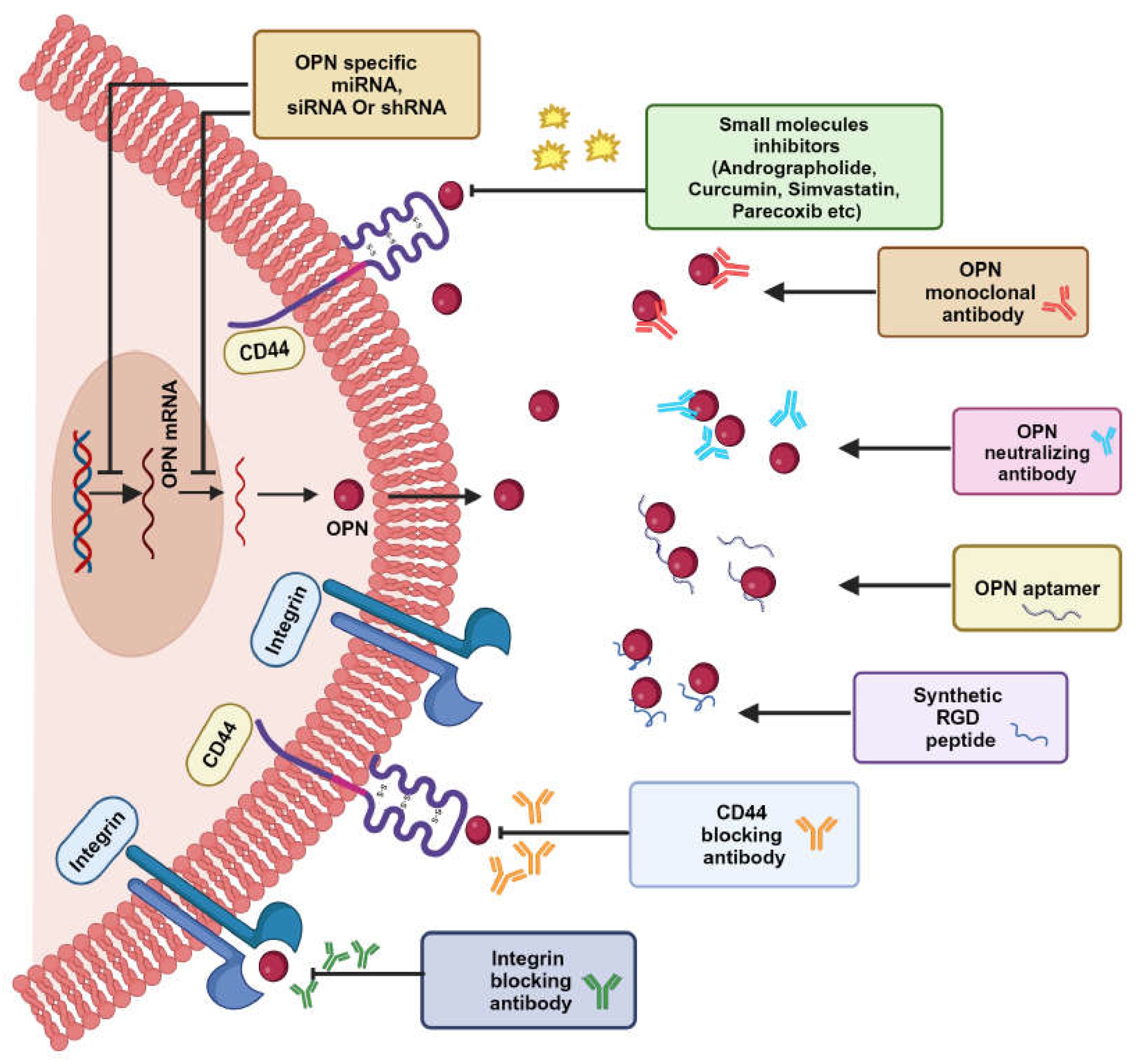
7. Osteopontin as a Therapeutic Target
7.1. OPN Neutralizing Antibody-Mediated Cancer Therapy
7.2. Small Molecule Inhibitors as a Potential Therapeutic Agent
7.3. Epigenetic Approaches
7.4. OPN Aptamer
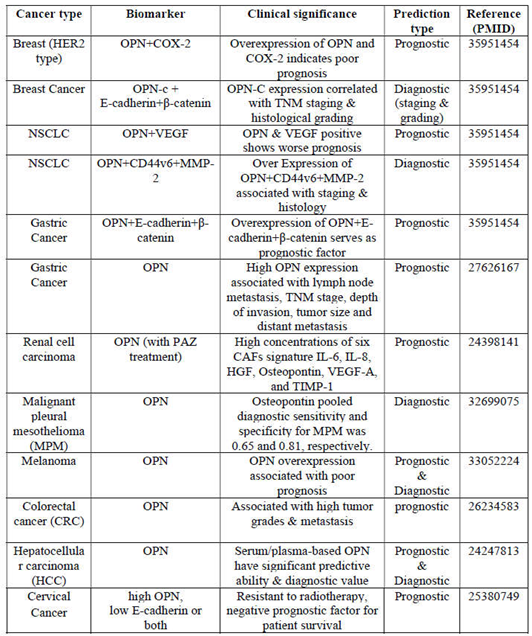
7.5. Biomarker
8. Conclusion and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209-249. [CrossRef]
- Mao X, Xu J, Wang W, Liang C, Hua J, Liu J et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021, 20, 1-30. [CrossRef]
- Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006, 16, 79-87. [CrossRef]
- Cho H-J, Cho H-J, Kim H-S. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009, 11, 206-213. [CrossRef]
- Zhao H, Chen Q, Alam A, Cui J, Suen KC, Soo AP et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 1-15. [CrossRef]
- Moorman HR, Poschel D, Klement JD, Lu C, Redd PS, Liu K. Osteopontin: a key regulator of tumor progression and immunomodulation. Cancers. 2020, 12, 3379. [CrossRef]
- Klement JD, Paschall AV, Redd PS, Ibrahim ML, Lu C, Yang D et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 2018, 128, 5549-5560.
- Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat Commun. 2022, 13, 1742. [CrossRef]
- Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008, 8, 212-226. [CrossRef]
- Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018, 59, 17-24. [CrossRef]
- Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014, 18, 883-895. [CrossRef]
- Lok ZSY, Lyle AN. Osteopontin in vascular disease: Friend or foe? Arterioscler Thromb Vasc Biol. 2019, 39, 613-622.
- Briones-Orta MA, Avendaño-Vázquez SE, Aparicio-Bautista DI, Coombes JD, Weber GF, Syn W-K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim Biophys Acta Rev Cancer. 2017, 1868, 93-108. [CrossRef]
- Shinohara ML, Kim H-J, Kim J-H, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008, 105, 7235-7239. [CrossRef]
- Kariya Y, Kariya Y. Osteopontin in cancer: mechanisms and therapeutic targets. Int J Transl Med. 2022, 2, 419-447.
- Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014, 89, 330-341. [CrossRef]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010, 10, 9-22. [CrossRef]
- Liu Z, Wang F, Chen X. Integrin αvβ3-targeted cancer therapy. Drug Dev Res. 2008, 69, 329-339.
- Ludwig BS, Kessler H, Kossatz S, Reuning U. RGD-binding integrins revisited: how recently discovered functions and novel synthetic ligands (re-) shape an ever-evolving field. Cancers. 2021, 13, 1711. [CrossRef]
- Zargham R, Wamhoff BR, Thibault G. RNA interference targeting α8 integrin attenuates smooth muscle cell growth. FEBS Lett. 2007, 581, 939-943. [CrossRef]
- Baiula M, Spampinato S, Gentilucci L, Tolomelli A. Novel ligands targeting α4β1 integrin: Therapeutic applications and perspectives. Front Chem. 2019, 7, 489. [CrossRef]
- Wei R, Wong JPC, Kwok HF. Osteopontin--a promising biomarker for cancer therapy. J Cancer. 2017, 8, 2173. [CrossRef]
- Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017, 5, 18. [CrossRef]
- Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: A multifunctional mediator of cancer progression. Biomolecules. 2021, 11, 1850. [CrossRef]
- Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K et al. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999, 59, 219-226.
- Fnu G, Agrawal P, Kundu GC, Weber GF. Structural Constraint of Osteopontin Facilitates Efficient Binding to CD44. Biomolecules. 2021, 11, 813. [CrossRef]
- Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014, 14, 357-369. [CrossRef]
- Ahmed M, Sottnik JL, Dancik GM, Sahu D, Hansel DE, Theodorescu D et al. An osteopontin/CD44 axis in RhoGDI2-mediated metastasis suppression. Cancer Cell. 2016, 30, 432- 443. [CrossRef]
- Rao G, Wang H, Li B, Huang L, Xue D, Wang X et al. Reciprocal interactions between tumor- associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res. 2013, 19, 785-797. [CrossRef]
- Cheng Y, Wen G, Sun Y, Shen Y, Zeng Y, Du M et al. Osteopontin promotes colorectal cancer cell invasion and the stem cell-like properties through the PI3K-AKT-GSK/3β-β/catenin pathway. Med Sci Monit. 2019, 25, 3014. [CrossRef]
- Zhang H, Guo M, Chen J-h, Wang Z, Du X-f, Liu P-x et al. Osteopontin knockdown inhibits αv, β3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2014, 33, 991-1002.
- Dos Santos ES, Ramos JC, Roza ALOC, Mariz BALA, Paes Leme AF. The role of osteopontin in oral cancer: a brief review with emphasis on clinical applications. Oral Dis. 2022, 28, 326-335.
- Fan C-S, Chen W-S, Chen L-L, Chen C-C, Hsu Y-T, Chua KV et al. Osteopontin–integrin engagement induces HIF-1α–TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget. 2018, 9, 4998. [CrossRef]
- Zhang G, Zhao Z, Lin X. EGF/PI3K signaling pathway regulates the expression of osteopontin in liver cancer HepG2 cells. Zhonghua Yi Xue Za Zhi. 2003, 83, 1980-1983.
- Yu X, Zheng Y, Zhu X, Gao X, Wang C, Sheng Y et al. Osteopontin promotes hepatocellular carcinoma progression via the PI3K/AKT/Twist signaling pathway. Oncol Lett. 2018, 16, 5299- 5308. [CrossRef]
- Qin Y-C, Yan X, Yuan X-L, Yu W-W, Qu F-J. Osteopontin promotes gastric cancer progression via phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. World J Gastrointest Oncol. 2023, 15, 1544. [CrossRef]
- Chen Y, Wang G, Wang Y, Gao X, Wang K, Li J et al. Capn4 regulates migration and invasion of ovarian carcinoma cells via targeting osteopontin-mediated PI3K/AKT signaling pathway. Oncol Lett. 2019, 17, 564-570. [CrossRef]
- Liu D, Luo M, Hu J, Chen C, Mei H. Osteopontin enhances cisplatin resistance of human A549 lung cancer cells via stimulating the PI3K signaling pathway and upregulating ERCC1 expression. Transl Cancer Res. 2020, 9, 3258. [CrossRef]
- Ding K, Fan L, Chen S, Wang Y, Yu H, Sun Y et al. Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncol Rep. 2015, 34, 3297-3303. [CrossRef]
- Chen J, Shi L, Qian Y, Jin Y, Dong N, Chen C et al. Epithelial-mesenchymal transition is associated with osteopontin-induced EGFR-TKI resistance in EGFR mutant non-small cell lung cancer. J Thorac Dis. 2023, 15, 3359. [CrossRef]
- Kumar D, Haldar S, Gorain M, Kumar S, Mulani FA, Yadav AS et al. Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway. BMC Cancer. 2018, 18, 1-17. [CrossRef]
- Tilli TM, Franco VF, Robbs BK, Wanderley JLM, de Azevedo da Silva FR, de Mello KD et al. Osteopontin-c splicing isoform contributes to ovarian cancer progression. Mol Cancer Res. 2011, 9, 280-293. [CrossRef]
- Shi L, Hou J, Wang L, Fu H, Zhang Y, Song Y et al. Regulatory roles of osteopontin in human lung cancer cell epithelial-to-mesenchymal transitions and responses. Clin Transl Med. 2021, 11, e486. [CrossRef]
- Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL, Zhu XQ et al. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008, 48, 1834-1842. [CrossRef]
- Gupta A, Zhou CQ, Chellaiah MA. Osteopontin and MMP9: associations with VEGF expression/secretion and angiogenesis in PC3 prostate cancer cells. Cancers. 2013, 5, 617-638. [CrossRef]
- Song G, Ouyang G, Mao Y, Ming Y, Bao S, Hu T. Osteopontin promotes gastric cancer metastasis by augmenting cell survival and invasion through Akt-mediated HIF-1α up-regulation and MMP9 activation. J Cell Mol Med. 2009, 13, 1706-1718.
- Pang H, Cai L, Yang Y, Chen X, Sui G, Zhao C. Knockdown of osteopontin chemosensitizes MDA- MB-231 cells to cyclophosphamide by enhancing apoptosis through activating p38 MAPK pathway. Cancer Biother Radiopharm. 2011, 26, 165-173. [CrossRef]
- Kyjacova L, Saup R, Rönsch K, Wallbaum S, Dukowic-Schulze S, Foss A et al. IER2-induced senescence drives melanoma invasion through osteopontin. Oncogene. 2021, 40, 6494-6512. [CrossRef]
- Graessmann M, Berg B, Fuchs B, Klein A, Graessmann A. Chemotherapy resistance of mouse WAP-SVT/t breast cancer cells is mediated by osteopontin, inhibiting apoptosis downstream of caspase-3. Oncogene. 2007, 26, 2840-2850. [CrossRef]
- Belli S, Esposito D, Servetto A, Pesapane A, Formisano L, Bianco R. c-Src and EGFR inhibition in molecular cancer therapy: what else can we improve? Cancers. 2020, 12, 1489. [CrossRef]
- Li NY, Weber CE, Mi Z, Wai PY, Cuevas BD, Kuo PC. Osteopontin up-regulates critical epithelial- mesenchymal transition transcription factors to induce an aggressive breast cancer phenotype. J Am Coll Surg. 2013, 217, 17-26. [CrossRef]
- Butti R, Nimma R, Kundu G, Bulbule A, Kumar TV, Gunasekaran VP et al. Tumor-derived osteopontin drives the resident fibroblast to myofibroblast differentiation through Twist1 to promote breast cancer progression. Oncogene. 2021, 40, 2002-2017. [CrossRef]
- Li NY, Weber CE, Wai PY, Cuevas BD, Zhang J, Kuo PC et al. An MAPK-dependent pathway induces epithelial-mesenchymal transition via Twist activation in human breast cancer cell lines. Surgery. 2013, 154, 404-410. [CrossRef]
- Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem. 2005, 280, 40589-40598. [CrossRef]
- Chua H, Bhat-Nakshatri P, Clare S, Morimiya A, Badve S, Nakshatri H. NF-κB represses E- cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007, 26, 711-724.
- Kale S, Raja R, Thorat D, Soundararajan G, Patil T, Kundu G. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene. 2014, 33, 2295-2306. [CrossRef]
- Takuwa Y, Ikeda H, Okamoto Y, Takuwa N, Yoshioka K. Sphingosine-1-phosphate as a mediator involved in development of fibrotic diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2013, 1831, 185-192. [CrossRef]
- Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun (Lond). 2022, 42, 401-434. [CrossRef]
- Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MA et al. Osteopontin mediates an MZF1– TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015, 34, 4821-4833. [CrossRef]
- Jia R, Liang Y, Chen R, Liu G, Wang H, Tang M et al. Osteopontin facilitates tumor metastasis by regulating epithelial–mesenchymal plasticity. Cell Death Dis. 2016, 7, e2564-e2564. [CrossRef]
- Butti R, Gunasekaran VP, Kumar TV, Banerjee P, Kundu GC. Breast cancer stem cells: Biology and therapeutic implications. Int J Biochem Cell Biol. 2019, 107, 38-52. [CrossRef]
- Butti R, Kumar TV, Nimma R, Banerjee P, Kundu IG, Kundu GC. Osteopontin signaling in shaping tumor microenvironment conducive to malignant progression. Adv Exp Med Biol. 2021, 1329, 419-441.
- Yoshida GJ, Saya H. Molecular pathology underlying the robustness of cancer stem cells. Regen Ther. 2021, 17, 38-50. [CrossRef]
- Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018, 11, 1-23. [CrossRef]
- Pio GM, Xia Y, Piaseczny MM, Chu JE, Allan AL. Soluble bone-derived osteopontin promotes migration and stem-like behavior of breast cancer cells. PLoS One. 2017, 12, e0177640. [CrossRef]
- Sun X, Li K, Hase M, Zha R, Feng Y, Li B-Y et al. Suppression of breast cancer-associated bone loss with osteoblast proteomes via Hsp90ab1/moesin-mediated inhibition of TGFβ/FN1/CD44 signaling. Theranostics. 2022, 12, 929.
- Hu J, Li G, Zhang P, Zhuang X, Hu G. A CD44v+ subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017, 8, e2679-e2679. [CrossRef]
- Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis. 2018, 35, 309-318. [CrossRef]
- Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017, 7, 339. [CrossRef]
- Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019, 114, 108800. [CrossRef]
- Gu M, Zheng X. Osteopontin and vasculogenic mimicry formation are associated with response to neoadjuvant chemotherapy in advanced breast cancer. Onco Targets Ther. 2017, 10, 4121-4127. [CrossRef]
- Kumar S, Sharma P, Kumar D, Chakraborty G, Gorain M, Kundu GC. Functional characterization of stromal osteopontin in melanoma progression and metastasis. PloS One. 2013, 8, e69116. [CrossRef]
- Hsieh I-S, Huang W-H, Liou H-C, Chuang W-J, Yang R-S, Fu W-M. Upregulation of drug transporter expression by osteopontin in prostate cancer cells. Mol Pharmacol. 2013, 83, 968-977. [CrossRef]
- Yi H, Zeng D, Shen Z, Liao J, Wang X, Liu Y et al. Integrin alphavbeta3 enhances β-catenin signaling in acute myeloid leukemia harboring Fms-like tyrosine kinase-3 internal tandem duplication mutations: Implications for microenvironment influence on sorafenib sensitivity. Oncotarget. 2016, 7, 40387.
- Liu G, Fan X, Tang M, Chen R, Wang H, Jia R et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016, 383, 171-182. [CrossRef]
- Sui H, Zhu L, Deng W, Li Q. Epithelial-mesenchymal transition and drug resistance: role, molecular mechanisms, and therapeutic strategies. Oncol Res Treat. 2014, 37, 584-589. [CrossRef]
- Das S, Samant RS, Shevde LA. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to Smoothened-targeting Hedgehog inhibition. J Biol Chem. 2013, 288, 11824-11833. [CrossRef]
- Chakraborty G, Jain S, Behera R, Ahmed M, Sharma P, Kumar V et al. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med. 2006, 6, 819- 830. [CrossRef]
- Krstic M, Hassan HM, Kolendowski B, Hague MN, Anborgh PH, Postenka CO et al. Isoform- specific promotion of breast cancer tumorigenicity by TBX3 involves induction of angiogenesis. Lab Invest. 2020, 100, 400-413. [CrossRef]
- Yang Y-F, Chang Y-C, Jan Y-H, Yang C-J, Huang M-S, Hsiao M. Squalene synthase promotes the invasion of lung cancer cells via the osteopontin/ERK pathway. Oncogenesis. 2020, 9, 78. [CrossRef]
- Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor– dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152-161. [CrossRef]
- Raja R, Kale S, Thorat D, Soundararajan G, Lohite K, Mane A et al. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene. 2014, 33, 2053-2064. [CrossRef]
- Raineri D, Dianzani C, Cappellano G, Maione F, Baldanzi G, Iacobucci I et al. Osteopontin binds ICOSL promoting tumor metastasis. Commun Biol. 2020, 3, 615. [CrossRef]
- Jiang X, Zhang X, Jiang N, Sun Y, Li T, Zhang J et al. The single-cell landscape of cystic echinococcosis in different stages provided insights into endothelial and immune cell heterogeneity. Front Immunol. 2022, 13, 1067338. [CrossRef]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008, 27, 103-118. [CrossRef]
- Rizwan A, Paidi SK, Zheng C, Cheng M, Barman I, Glunde K. Mapping the genetic basis of breast microcalcifications and their role in metastasis. Sci Rep. 2018, 8, 11067. [CrossRef]
- Xu C, Yuan Q, Wang W, Chi C, Zhang Q, Li L et al. Prognostic significance of serum osteopontin levels in small cell lung cancer. BMC Pulm Med. 2020, 20, 1-7. [CrossRef]
- Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014, 14, 342-356. [CrossRef]
- Gu X, Gao X-S, Ma M, Qin S, Qi X, Li X et al. Prognostic significance of osteopontin expression in gastric cancer: a meta-analysis. Oncotarget. 2016, 7, 69666. [CrossRef]
- Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou HJ, Dai C et al. Osteopontin promoter polymorphisms at locus-443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013, 57, 1024-1034. [CrossRef]
- Li Y, Du W, Han J, Ge J. LAMP3 promotes the invasion of osteosarcoma cells via SPP1 signaling. Mol Med Rep. 2017, 16, 5947-5953. [CrossRef]
- Guo J, Tong C-Y, Shi J-G, Li X-J, Chen X-Q. Deletion of osteopontin in non-small cell lung cancer cells affects bone metabolism by regulating miR-34c/Notch1 axis: a clue to bone metastasis. Eur J Histochem. 2023, 67, 3631. [CrossRef]
- Kovacheva M, Zepp M, Schraad M, Berger S, Berger MR. Conditional knockdown of osteopontin inhibits breast cancer skeletal metastasis. Int J Mol Sci. 2019, 20, 4918. [CrossRef]
- Zuo H, Yang D, Wan Y. Fam20C regulates bone resorption and breast cancer bone metastasis through osteopontin and BMP4. Cancer Res. 2021, 81, 5242-5254. [CrossRef]
- Li X-Q, Lu J-T, Tan C-C, Wang Q-S, Feng Y-M. RUNX2 promotes breast cancer bone metastasis by increasing integrin α5-mediated colonization. Cancer Lett. 2016, 380, 78-86. [CrossRef]
- Kuo MC, Kothari AN, Kuo PC, Mi Z. Cancer stemness in bone marrow micrometastases of human breast cancer. Surgery. 2018, 163, 330-335. [CrossRef]
- Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022,34, 355-377. [CrossRef]
- Lei P, Wang W, Sheldon M, Sun Y, Yao F, Ma L. Role of glucose metabolic reprogramming in breast cancer progression and drug resistance. Cancers (Basel). 2023, 15, 3390. [CrossRef]
- Hsieh I-S, Yang R-S, Fu W-M. Osteopontin upregulates the expression of glucose transporters in osteosarcoma cells. PLoS One. 2014, 9, e109550. [CrossRef]
- Bui BP, Nguyen PL, Lee K, Cho J. Hypoxia-inducible factor-1: a novel therapeutic target for the management of cancer, drug resistance, and cancer-related pain. Cancers (Basel). 2022, 14, 6054. [CrossRef]
- Nishikawa M, Inoue A, Ohnishi T, Yano H, Ozaki S, Kanemura Y et al. Hypoxia-induced phenotypic transition from highly invasive to less invasive tumors in glioma stem-like cells: Significance of CD44 and osteopontin as therapeutic targets in glioblastoma. Transl Oncol. 2021, 14, 101137. [CrossRef]
- Zeng K, Ju G, Wang H, Huang J. GLUT1/3/4 as novel biomarkers for the prognosis of human breast cancer. Transl Cancer Res. 2020, 9, 2363.
- Szablewski L. Glucose transporters as markers of diagnosis and prognosis in cancer diseases. Oncol Rev. 2022, 16, 561. [CrossRef]
- Butti R, Kundu GC. The molecular dialogue between the tumor cells and fibroblasts. Oncotarget. 2023, 14, 462. [CrossRef]
- Butti R, Khaladkar A, Bhardwaj P, Prakasam G. Heterotypic signaling of cancer-associated fibroblasts in shaping the cancer cell drug resistance. Cancer Drug Resist. 2023, 6, 182-204. [CrossRef]
- Butti R, Ghosh P, Totakura KV, Venkata RNN, Nimma R, Kundu GC. Role of osteopontin in tumor microenvironment: a new paradigm in cancer therapy. Multi-Targeted Approach to Treatment of Cancer. Springer, 2015, pp 113-125.
- Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, Erez N. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015, 75, 963-973. [CrossRef]
- Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018, 33, 463-479. e410. [CrossRef]
- Muchlińska A, Nagel A, Popęda M, Szade J, Niemira M, Zieliński J et al. Alpha-smooth muscle actin-positive cancer-associated fibroblasts secreting osteopontin promote growth of luminal breast cancer. Cell Mol Biol Lett. 2022, 27, 1-14. [CrossRef]
- Liu J, Xu K, Chase M, Ji Y, Logan JK, Buchsbaum RJ. Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells. J Cell Sci. 2012, 125, 376-386. [CrossRef]
- Mori JO, Elhussin I, Brennen WN, Graham MK, Lotan TL, Yates CC et al. Prognostic and therapeutic potential of senescent stromal fibroblasts in prostate cancer. Nat Rev Urol. 2023, 1-16. [CrossRef]
- Krtolica A, Parrinello S, Lockett S, Desprez P-Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001, 98, 12072-12077. [CrossRef]
- Louault K, Li R-R, DeClerck YA. Cancer-associated fibroblasts: understanding their heterogeneity. Cancers (Basel). 2020, 12, 3108. [CrossRef]
- Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5- mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011, 32, 477-487. [CrossRef]
- Belanger C, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002, 34, 737-745. [CrossRef]
- Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T et al. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008, 149, 1350-1357. [CrossRef]
- Ahmad R, Al-Mass A, Al-Ghawas D, Shareif N, Zghoul N, Melhem M et al. Interaction of osteopontin with IL-18 in obese individuals: implications for insulin resistance. PLoS One. 2013, 8, e63944. [CrossRef]
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16-27. [CrossRef]
- Madel M-B, Ibáñez L, Wakkach A, De Vries TJ, Teti A, Apparailly F, Blin-Wakkach C. Immune function and diversity of osteoclasts in normal and pathological conditions. Front Immunol. 2019, 10, 1408. [CrossRef]
- Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019, 12, 1-16. [CrossRef]
- Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014, 3, 384-393. [CrossRef]
- Lin C-N, Wang C-J, Chao Y-J, Lai M-D, Shan Y-S. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer. 2015, 15, 1-10. [CrossRef]
- Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019, 129, 137-149. [CrossRef]
- Liang K-H, Yeh C-T. OPN sesame. Hepatobiliary Surg Nutr. 2014, 3, 112.
- Komohara Y, Kurotaki D, Tsukamoto H, Miyasato Y, Yano H, Pan C et al. Involvement of protumor macrophages in breast cancer progression and characterization of macrophage phenotypes. Cancer Sci. 2023, 114, 2220. [CrossRef]
- Ramos RN, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Núñez NG et al. Tissue- resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell. 2022, 185, 1189-1207. [CrossRef]
- Wei J, Chen Z, Hu M, He Z, Jiang D, Long J, Du H. Characterizing intercellular communication of pan-cancer reveals SPP1+ tumor-associated macrophage expanded in hypoxia and promoting cancer malignancy through single-cell RNA-seq data. Front Cell Dev Biol. 2021, 9, 749210. [CrossRef]
- Liu Z, Gao Z, Li B, Li J, Ou Y, Yu X et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology. 2022, 11, 2085432. [CrossRef]
- Bill R, Wirapati P, Messemaker M, Roh W, Zitti B, Duval F et al. CXCL9: SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science. 2023, 381, 515-524. [CrossRef]
- Buechler MB, Fu W, Turley SJ. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity. 2021, 54, 903-915. [CrossRef]
- Tokuda K, Morine Y, Miyazaki K, Yamada S, Saito Y, Nishi M et al. The interaction between cancer associated fibroblasts and tumor associated macrophages via the osteopontin pathway in the tumor microenvironment of hepatocellular carcinoma. Oncotarget. 2021, 12, 333. [CrossRef]
- Nakajima T, Uehara T, Iwaya M, Matsuda K, Wada M, Nagaya T et al. Osteopontin expression in the invasive front stroma of colorectal adenocarcinoma is associated with tumor budding and prognosis. Pathol Res Pract. 2022, 240, 154190. [CrossRef]
- Li Y, Guo S, Zhao K, Conrad C, Driescher C, Rothbart V et al. ADAM8 affects glioblastoma progression by regulating osteopontin-mediated angiogenesis. Biol Chem. 2021, 402, 195-206. [CrossRef]
- Radharani N, Yadav AS, Nimma R, Kumar TS, Bulbule A, Chanukuppa V et al. Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway. Cancer Cell Int. 2022, 22, 122. [CrossRef]
- Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021, 21, 298-312. [CrossRef]
- Tan Y, Zhao L, Yang Y-G, Liu W. The role of osteopontin in tumor progression through tumor- associated macrophages. Front Oncol. 2022, 12, 953283. [CrossRef]
- Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013, 123, 4464-4478. [CrossRef]
- Sangaletti S, Tripodo C, Sandri S, Torselli I, Vitali C, Ratti C et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res. 2014, 74, 4706-4719. [CrossRef]
- Allegrezza MJ, Rutkowski MR, Stephen TL, Svoronos N, Perales-Puchalt A, Nguyen JM et al. Trametinib drives T-cell–dependent control of KRAS-mutated tumors by inhibiting pathological myelopoiesis. Cancer Res. 2016, 76, 6253-6265. [CrossRef]
- Klement JD, Poschel DB, Lu C, Merting AD, Yang D, Redd PS, Liu K. Osteopontin blockade immunotherapy increases cytotoxic T lymphocyte lytic activity and suppresses colon tumor progression. Cancers (Basel). 2021, 13, 1006. [CrossRef]
- Zhang Y, Du W, Chen Z, Xiang C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp Cell Res. 2017, 359, 449- 457. [CrossRef]
- Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020, 52, 17-35. [CrossRef]
- Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018, 18, 91-104. [CrossRef]
- Li Y, Liu H, Zhao Y, Yue D, Chen C, Li C et al. Tumor-associated macrophages (TAMs)-derived osteopontin (OPN) upregulates PD-L1 expression and predicts poor prognosis in non-small cell lung cancer (NSCLC). Thorac Cancer. 2021, 12, 2698-2709.
- Zhu Y, Yang J, Xu D, Gao X-M, Zhang Z, Hsu JL et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019, 68, 1653-1666. [CrossRef]
- Yan Z, Hu X, Tang B, Deng F. Role of osteopontin in cancer development and treatment. Heliyon.2023,9, e21055. [CrossRef]
- Dai J, Li B, Shi J, Peng L, Zhang D, Qian W et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010, 59, 355-366. [CrossRef]
- Amilca-Seba K, Tan TZ, Thiery J-P, Louadj L, Thouroude S, Bouygues A et al. Osteopontin (OPN/SPP1), a mediator of tumor progression, is regulated by the mesenchymal transcription factor Slug/SNAI2 in colorectal cancer (CRC). Cells. 2022, 11, 1808. [CrossRef]
- Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012, 31, 1-12. [CrossRef]
- Kumar S, S Patil H, Sharma P, Kumar D, Dasari S, G Puranik V et al. Andrographolide inhibits osteopontin expression and breast tumor growth through down regulation of PI3 kinase/Akt signaling pathway. Curr Mol Med. 2012, 12, 952-966.
- Im E, Yeo C, Lee E-O. Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018, 209, 259-266. [CrossRef]
- Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007, 6, 1-16. [CrossRef]
- Lu C, Liu Z, Klement JD, Yang D, Merting AD, Poschel D et al. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J Immunother Cancer. 2021, 9, e002624. [CrossRef]
- Gao X, Sheng Y, Yang J, Wang C, Zhang R, Zhu Y et al. Osteopontin alters DNA methylation through up-regulating DNMT1 and sensitizes CD133+/CD44+ cancer stem cells to 5 azacytidine in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018, 37, 1-14.
- Deng G, Zeng F, Su J, Zhao S, Hu R, Zhu W et al. BET inhibitor suppresses melanoma progression via the noncanonical NF-κB/SPP1 pathway. Theranostics. 2020, 10, 11428.
- Han B, Huang J, Han Y, Hao J, Wu X, Song H et al. The microRNA miR-181c enhances chemosensitivity and reduces chemoresistance in breast cancer cells via down-regulating osteopontin. Int J Biol Macromol. 2019, 125, 544-556. [CrossRef]
- Ben-David-Naim M, Dagan A, Grad E, Aizik G, Nordling-David MM, Morss Clyne A et al. Targeted siRNA nanoparticles for mammary carcinoma therapy. Cancers (Basel). 2019, 11, 442. [CrossRef]
- Wang S-Y, Chen C-L, Hu Y-C, Chi Y, Huang Y-H, Su C-W et al. High expression of microRNA- 196a is associated with progression of hepatocellular carcinoma in younger patients. Cancers (Basel). 2019, 11, 1549. [CrossRef]
- Lakhin A, Tarantul V, Gening L. Aptamers: problems, solutions and prospects. Acta Naturae. 2013, 5, 34-43.
- Hunter C, Bond J, Kuo PC, Selim MA, Levinson H. The role of osteopontin and osteopontin aptamer (OPN-R3) in fibroblast activity. J Surg Res. 2012, 176, 348-358. [CrossRef]
- Talbot LJ, Mi Z, Bhattacharya SD, Kim V, Guo H, Kuo PC. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells. Surgery. 2011, 150, 224-230. [CrossRef]
- Liu L, Zhang R, Deng J, Dai X, Zhu X, Fu Q et al. Construction of TME and identification of crosstalk between malignant cells and macrophages by SPP1 in hepatocellular carcinoma. Cancer Immunol Immunother. 2022, 71, 121-136. [CrossRef]
- Song H, Lou C, Ma J, Gong Q, Tian Z, You Y et al. Single-cell transcriptome analysis reveals changes of tumor immune microenvironment in oral squamous cell carcinoma after chemotherapy. Front Cell Dev Biol. 2022, 10, 914120. [CrossRef]
- Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020, 181, 442-459. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





