Introduction
Typhoid fever and malaria are among the most common infections in tropical developing countries such as Ghana, raising major public health problems (Mutua et al., 2015a). Malaria is caused by five different species of protozoan parasites from the genus Plasmodium (Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi) (Talapko et al., 2019). P. falciparum is the leading cause of nearly all malaria-related deaths in humans, with the majority occurring in Sub-Saharan Africa (Batire et al., 2022). The protozoa in the saliva of an infected female Anopheles mosquito gain entry into the circulatory system through a bite and mature and multiply in the liver; this is the most common form of disease transmission (Martens, 2000).
Malaria was regarded as endemic in 91 countries and territories at the beginning of 2016, down from 108 in 2000. Even with these advancements, malaria still has a devastating impact on people worldwide. Globally, there were 212 million cases in 2015, resulting in 429,000 deaths, the majority of which were in children under five in Africa (Shretta et al., 2017). This disease impedes economic growth and the advancement of living standards while causing widespread premature deaths, suffering, and financial hardships for low-income households. The annual economic growth rate has been lowered by 1.3 percent due to malaria infections. The illness primarily affects weak and vulnerable populations, such as newborns and children, and is responsible for about 20% of childhood deaths in Africa (WHO, 2023).
Over a million people die from malaria each year, and that number may reach nearly three million if malaria's contribution to deaths from other illnesses is taken into account. The World Malaria Report, 2011, states that there were approximately 216 million cases of malaria and 655,000 deaths from the disease in 2010 (Birhanie et al., 2014). Most deaths in endemic areas occur in children younger than five. About 25% of all child deaths between the ages of 0 and 4 in areas with stable endemic transmission have been directly linked to malaria (Dasgupta, 2018). Malaria is common in pregnant women and can result in congenital infections, low birth weight, miscarriages, and neonatal and infant mortality. Both acute and chronic malaria infections can impact the immune system and the body's response to immunizations (Sachs & Malaney, 2002). Malaria symptoms include headaches, vomiting, fever, and lethargy. In severe situations, seizures, yellow skin, coma, or even death are possible results (Källander et al., 2004; Sohanang Nodem et al., 2023)
In tropical developing nations, typhoid fever is a severe public health concern, particularly in areas with limited access to clean water and other sanitation measures (Nas et al., 2018). Low- and middle-income nations have a high Salmonella infection rate (>100 per 100,000 infected individuals annually) (Wilairatana et al., 2021). Salmonella Typhi-a Gram-Negative, oxidase-negative bacteria, through contaminated foods and water, causes typhoid fever known as acute systemic infection. Mohammad et al. state that there are approximately 33 million cases of typhoid fever globally, with endemic areas accounting for 216,000 fatalities (Mohammed et al., 2020; Sohanang Nodem et al., 2023). Typhoid fever is caused by Salmonella enterica serotype typhi (S. typhi) and Salmonella enterica serotype paratyphi (S. paratyphi A, S. paratyphi B, and S. paratyphi C), with S. typhi been the most prevalent strain in causing typhoid fever (Crump, 2019). In developing nations, improper handling of human waste, inadequate latrine facilities, inadequate hand-washing practices, and the use of untreated water are the primary factors contributing to the spread of typhoid fever (Bennett et al., 2018). The epidemiological patterns of typhoid and related diseases in third-world countries—including most in Africa, Asia, and Latin America—have changed in recent years (Paul & Bandyopadhyay, 2017). It is estimated that more than 33 million cases and more than 500,000 deaths due to typhoid fever occur each year (Phan et al., 2000). S. typhi and the paratyphoid bacilli thrive in the intestinal tract and cause invasive typhoid diseases, with symptoms ranging from mild to severe. Symptoms become noticeable between six to thirty days after exposure and build up into fever over several days (Ammah et al., 1999).
There have also been reports of transmission by flies, such as Musca domestica. The infection's most noticeable symptom is a fever that progressively increases to a high plateau (Cirillo, 2006). The incidence of typhoid is prevalent in areas with poor sanitation and inadequate water supply, and due to multidrug resistance development and altered presentation patterns in recent times, it is increasingly difficult to diagnose and treat typhoid fever (Chowta & Chowta, 2005). The primary symptoms of the infection are high fever and other unfavorable conditions such as nausea, stomach cramps, and irregular bowel movements (Crump et al., 2015). The diagnosis of typhoid fever is carried out by analyzing blood, stool, or bone marrow samples from infected people, and traditional malaria diagnosis is achieved through microscopy (Cao et al., 2021).
Malaria and Typhoid Co-Infection - Typhomalaria
Typhomalaria was first identified in 1862 among young soldiers afflicted with a feverish illness that appeared to be typhoid (including intestinal lesions discovered at postmortem) but had fever patterns also suggestive of intermittent fever by army physician J J Woodward (1833–1884). He thought it might be a hybrid rather than a novel illness species. However, laboratory testing had disproved this hypothesis by the end of the 19th century, finding that co-infection with both S. typhi and the malaria plasmodium was either rare or nonexistent (Keong & Sulaiman, 2006). Although the two diseases have different causes and transmission modes, intriguing connections between them contribute to public health issues and fall into two categories: False diagnosis and true co-infection (Mutua et al., 2015a). Their mimicking symptomatology frequently results in total misdiagnosis and mistreatment (Ammah et al., 1999). The main challenges in managing malaria and typhoid are true co-infection, false positive results from testing methods, and false diagnoses based on similar signs and symptoms, which result in inappropriate control procedures (Mutua et al., 2015b). Major public health issues arise from the co-occurrence of typhoid fever and malaria, particularly in tropical and subtropical regions where both diseases are co-endemic. Without laboratory confirmation, clinicians frequently treat both infections at the same time. Concurrent treatment, however, has consequences for public health since overuse of antibiotics or antimalarials can result in drug resistance, needless expense, and patient exposure to unneeded side effects (Tanko Rufai et al., 2022).
Both illnesses have been linked to rising rates of poverty, declining levels of sanitation, inadequate public health services, and worsening drug resistance of the two etiological agents. People living in endemic areas can get both infections at the same time. Typhoid fever and malaria share many similar signs and symptoms. As a result, the feverish patient may receive incorrect diagnosis and treatment due to the similar clinical features of the two illnesses (Birhanie et al., 2014; Tanko Rufai et al., 2022). The intertwined challenges of malaria and typhoid fever underscore the importance of accurate diagnosis and appropriate management to mitigate the burden of these diseases in endemic regions. A comprehensive approach involving effective surveillance, improved sanitation, and access to healthcare services is crucial for addressing these public health challenges (Florence et al., 2003).
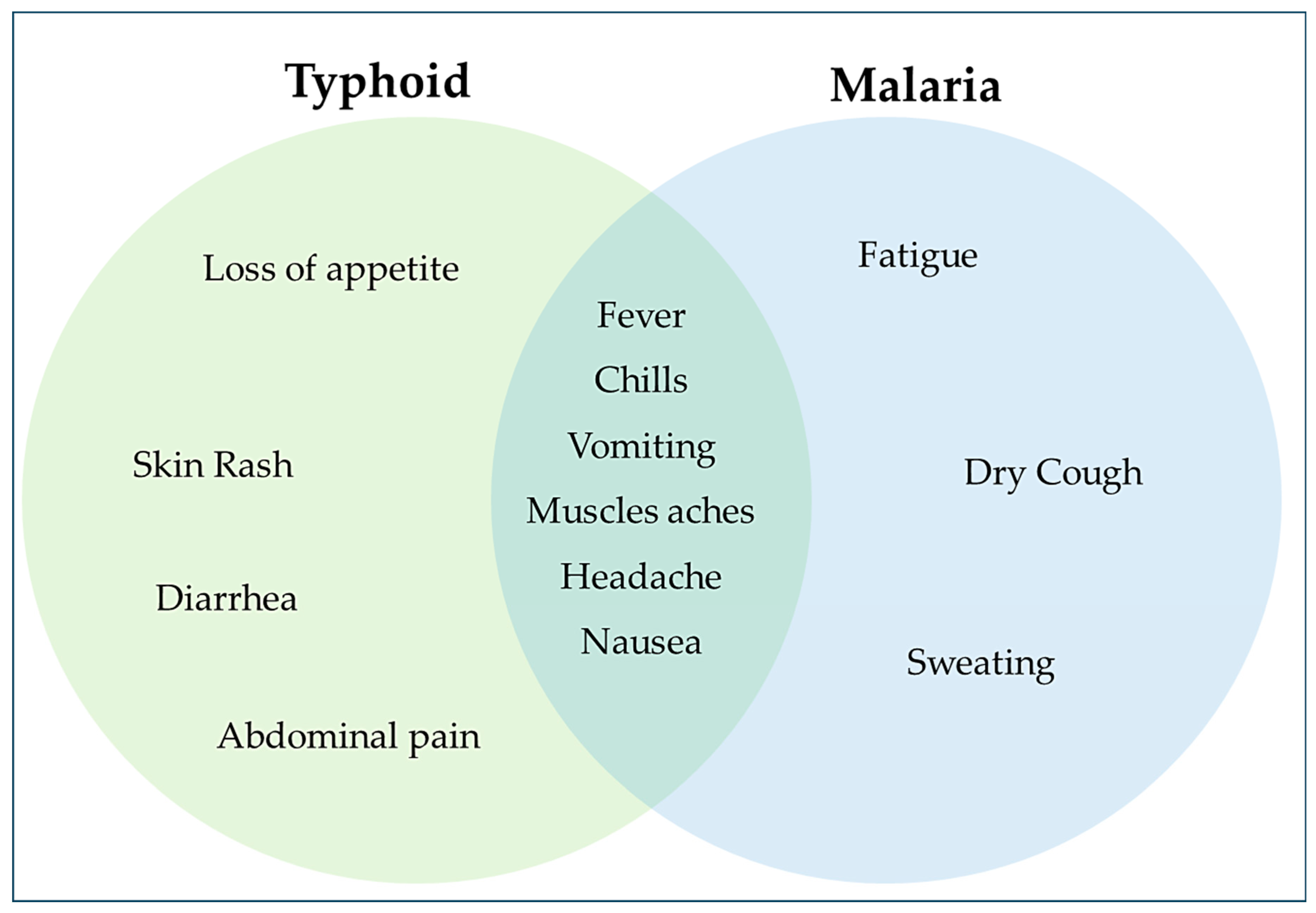
Figure 1.
Venn Diagram showing symptoms of Typhoid, Malaria and those common to them.
Figure 1.
Venn Diagram showing symptoms of Typhoid, Malaria and those common to them.
Both conditions frequently have similar symptoms and share a similar transmission factor. This is why many medical professionals attempt to treat typhoid fever and malaria simultaneously in every suspected Salmonella infection and vice versa (Qureshi et al., 2019). Numerous factors, including similar epidemiological factors like population density and inadequate sanitation practices, predispose to coinfection. Furthermore, the pathophysiology of malaria results in complement factor exhaustion, which puts the patient at risk for Salmonella infection. Additionally, the hemolysis brought on by malaria also causes intracellular iron to deposit inside liver cells, which promotes the growth of intracellular pathogens like Salmonella (Bhawna Sharma et al., 2016). Even though typhoid fever and malaria are frequently found together in tropical regions, the difficulties in diagnosing the condition and its effects on public health have yet to be investigated thoroughly (Adamu et al., 2023).
Pathogenesis and Clinical Presentation of Typhoid Fever and Malaria
Enteric fever, another name for typhoid fever, is a highly deadly illness prevalent in most developing nations, particularly in Africa and some regions of Asia like India (Crump et al., 2004). It has been a significant contributor to human mortality and morbidity for over 200 years since its inception. Salmonella enterica subspecies, gram-negative bacteria, is the primary cause of typhoid fever, an infection that can be contracted through food or water (Sinha et al., 1999). Nonetheless, it has also been noted that S. Paratyphi A can occasionally result in typhoid fever. S. typhi primarily originates from humans, meaning that while the pathogen cannot reproduce outside the human body, it can only slightly proliferate in the environment. S. typhi is primarily spread orally and faecally, with infected food and water serving its main reservoirs (Crump, 2019). In addition to excreta being the primary pathway for S. typhi to leave the body, the bacteria can occasionally be detected in urine (Sears et al., 1924). Due to some overlapping signs and symptoms, it can be challenging to distinguish typhoid from other febrile illnesses, particularly malaria in its early stages. However, the disease's primary symptoms are fever and headache (Nsutebu et al., 2003). Additional signs and symptoms of the illness include meteorism, coated tongue, anorexia, splenomegaly, vomiting, abdominal pain, decreased appetite, splenomegaly, anorexia, and hepatomegaly (Mweu & English, 2008). The bacterium is frequently discovered in contaminated water and food. It passes through the stomach and the distal ileum, entering the gut epithelium. During the invasion process, the bacteria actively adhere to the host cell by interacting with receptors on the host cell via unidentified adhesion molecules on the bacterium. There are 12 fimbrial operons in the chaperone usher assembly class in S. typhi, none of which are specific to the species (Townsend et al., 2001). However, it has a unique combination of fimbrial operons, such as the type IVB pilus operon and the cystic fibrosis transmembrane conductance regulator (Tsui et al., 2003). Using bacterial-mediated endocytosis, which involves cytoskeletal reorganization, disruption of the epithelial cell brush boundary, and the formation of membrane ruffles, Salmonella infects epithelial cells in vitro. Enterica is activated in conditions similar to the human small intestine. This invasive behavior is partly caused by Salmonella pathogenicity island (SPI)-1, which alters the host cell and promotes bacterial uptake (Finlay et al., 1991). Soon after the invasion, Salmonella species come into contact with macrophages in the gut-associated lymphoid tissue. The interaction between Salmonella and macrophages alters the expression of host genes, including pro-inflammatory mediators, adhesion molecules or receptors, and anti-inflammatory mediators. Genes linked to apoptosis or cell death and transcription factors are other genes up regulated when macrophages engulf the bacteria (Arai et al., 1995). It is commonly known that Salmonella needs to be able to live inside macrophages and monocytes (Fields et al., 1986). Without SPI-1 expression, Salmonella-infected macrophages can survive for several hours and serve as a cellular niche that shields the bacteria from the host immune system. As the disease manifests, the percentage of intracellular S. typhi in the human host's bone marrow increases (LaRock et al., 2015).
Malaria is yet another serious global health issue, with the majority of cases occurring in Sub-Saharan Africa and Southeast Asia (Postels & Birbeck, 2013). The Plasmodium parasite-carrying female Anopheles mosquito bite is the only way that malaria, an infectious disease, can spread. Malaria is primarily caused by parasitic plasmodium species, specifically falciparum, vivax, ovale, malariae, and Knowlesi (Nogueira & Lopes, 2011). The two most prevalent species among the five, falciparum and vivax, are responsible for the elevated mortality and morbidity rates associated with malaria. Fever, shivering, and sweating are some initial signs and symptoms of malaria infection (Bria et al., 2021). Malaria shares early symptoms with other feverish illnesses, but it differs from them in that it also presents with additional symptoms like fatigue, headache, diarrhea, limpness, and muscle aches (Barcus et al., 2007). For those who have previously contracted Plasmodium infections, malaria symptoms may be mild; however, malaria can be fatal for newborns, children under five, HIV/AIDS patients, and expectant mothers (WHO, 2023). Plasmodium has two different hosts to complete its life cycle; humans are the vertebrate host in the asexual cycle, known as schizogony. The product of schizogony is called a merozoite. Anopheles, the invertebrate host, performs the sexual cycle, or sporogony, and the end product is called a sporozoite (Sato, 2021). Sporozoites, an infectious form of Plasmodium, pass into human hosts when a female Anopheles mosquito bites them. They enter the hepatocytes through the bloodstream and proliferate there to create merozoites or pre-erythrocytic schizogony (Mota et al., 2001). This fits the description of an incubation phase during which the peripheral blood is sterile, the patient is asymptomatic, and the infection is not contagious. Hepatocyte rupture releases merozoites into the bloodstream, entering red blood cells and starting the erythrocytic schizogony process. A tiny percentage stays latent for years and then causes a malarial relapse, or they re-enter the hepatocytes to perform the exo-erythrocytic schizogony (only in the cases of P. vivax and P. ovale) (Milner, 2018). During erythrocytic schizogony within RBCs, a single merozoite transforms into an early trophozoite (ring form), then a late trophozoite, and finally goes through multiple mitotic divisions to create the schizont. This corresponds to the stage of prodromal symptoms, which involves the release of numerous merozoites, that are transformed from the schizonts, and cause the malarial paroxysm of fever with chills. When the RBC ruptures, this phenomenon occurs and the rupture of red blood cells causes anemia (Menendez et al., 2000). These merozoites then attack other RBCs. Only a tiny portion mature into gametocytes, both male and female. After a female anopheles ingests the blood of an infected human, the plasmodium life cycle is complete. The mosquito consumes the gametocytes and turns them into sporozoites using the sexual cycle (WHO, 2023). The two illnesses have many symptoms in common, even though they are caused by very different pathogens and spread through different routes. This presents a challenge that can result in early diagnostic errors (Ohanu et al., 2003). Therefore, a definitive laboratory-based diagnosis is needed to distinguish between the two disorders and identify coinfections. When a link between typhoid fever and malaria was found in the middle of the 19th century, the United States Army named the illness typho-malarial fever (Uneke, 2008). It is not always true that a high titer antibody against Salmonella means the infection is active. Due to the frequent finding of high antibody titer of these Salmonella serotypes in malaria patients, some people have come to believe that typhoid can develop from malaria or that typhoid and malaria always co-infect all patients. Accordingly, some people treat both typhoid and malaria concurrently if they have a high titer of antibodies to Salmonella serotypes, even in cases where there is insufficient test diagnosis for malaria, and vice versa (Eze et al., 2012). While all 25 subjects had antibody titers against Salmonella serotypes, only 3 (12%) of the malaria patients had isolated Salmonella species from their blood and stool samples, according to the findings of Eze et al. The study found that it is necessary to perform confirmatory tests by isolating Salmonella spp. from stool or blood because the Widal test can identify infections other than Salmonella infections that can increase antibody responses against H and O antigens, which are similar to those of Salmonella serotypes (Eze et al., 2012). When a victim also has malaria and typhoid fever, their health and financial situation worsen. There are negative consequences for the mother, the fetus, and the children from this illness during pregnancy and childhood. These consequences may include fever, anemia in the mother or fetus, abortion, stillbirth, or even the mother's or child's death before or soon after delivery (Mohammed et al., 2020). Additionally, the statistical correlation between continuous fever and chill patterns and malaria-typhoid fever coinfection may aid in the differential diagnosis of malaria-typhoid coinfection (Batire et al., 2022). In a different study, the rates of nausea, vomiting, stomach pain, and diarrhea were significantly higher in subjects who also had coinfection. In addition, compared to the dry seasons, the highest number of malaria and typhoid fever cases occurred during the rainy/wet season (August). This is primarily due to how the parasite Plasmodium and the bacteria Salmonella reproduce and spread (Khan et al., 2009).
Malaria Predisposes Typhoid Fever.
Several theories explain why malaria may increase the risk of contracting Salmonella bacteremia. However, it is still unclear what the precise underlying mechanisms are that explain how typhoid fever infection and malaria are related (Keong & Sulaiman, 2006). One of them is a decreased antibody response to S. typhi during acute malaria episodes. It has been shown that an acute malaria infection reduces the antibody response to the somatic (O) antigen of S. typhi (Greenwood et al., 1972). Moreover, the enzyme heme oxygenase 1, released during malarial hemolysis, demobilizes the granulocytes involved in cellular immunity against Salmonella, raising the risk of bacterial infections (Mooney et al., 2018). Because malaria alters immunological reactivity, it may increase the risk of bacterial super-infection. When malaria reaches severe levels, as when there is hemolysis, impaired leucocyte, and macrophage function due to parasite phagocytosis, accumulation of malaria pigment, and immunosuppression, the invasive bacteria multiply and cause septicemia and bacteremia (Prada et al., 1993). Iron overload in the liver and complement deficiency in malaria predispose a person to typhoid (Sohanang Nodem et al., 2023). It has been shown that anemia due to hemolysis (in the event of Malaria infection) leads to iron deposition in the liver; hence, the extra iron feeds the causative organism that causes typhoid fever (Bashyam, 2007). However, intracellular bacteria such as S. typhi have an obligate requirement for iron to support intracellular growth and survival in their host (Ratledge & Dover, 2000). The complements (C3, C4, and C1q) in our immune system are like superheroes fighting off infections when it comes to coping with both Salmonella and Plasmodium. These complement levels decrease with severe malaria, impacting our immune system. Lack of C1q increases our susceptibility to Salmonella, similar to missing an essential amour piece, according to studies conducted on mice. Children without C4b are more likely to get blood infections, which necessitates the need to look at the relationship between Salmonella and decreased complement levels in malaria (Prasanna Pradhan, 2011).
Diagnostic Challenges and Laboratory Techniques
Due to the difficulty in differentiating coinfection from single infections and the simultaneous presence of multiple pathogens in an individual, coinfection presents significant diagnostic challenges (Bicudo et al., 2020). Their overlapping symptoms and clinical manifestations further compound the burden of infectious diseases on the world's health. When coinfections occur, the clinical presentation becomes complex because the presence of multiple pathogens affects treatment response, changes test results, and masks symptoms. Since many pathogens exhibit similar symptoms, such as fever, exhaustion, and malaise, it is unreliable to differentiate between them only based on clinical presentation (Molaeipoor et al., 2014). For example, bacterial pneumonia and influenza can mimic one another, and HIV and malaria can present with nonspecific complaints that are similar to one another. One infection can mask the signs of another, underestimating the full clinical relevance. HIV infection is a well-known example, as it compromises immunity and can prevent the manifestation of opportunistic infections like tuberculosis (Molaeipoor et al., 2014). Standard diagnostic test accuracy may be affected by coinfection. Cross-reactivity in viral serological testing can produce false-positive results, and the most common pathogen in bacterial cultures can dominate and mask the presence of other pathogens (Bhat et al., 2015). Nonspecific inflammatory markers such as elevated C-reactive protein (CRP) or white blood cell count are frequently triggered by infections. Because of these markers' low discriminatory power, it is difficult to distinguish between coinfections and single infections (Bhat et al., 2015). Pathogens that co-infect can interact, changing the pathogen's virulence and clinical presentation. HIV, for instance, compromises immunity, predisposing one to TB and other opportunistic infections, which makes both conditions manifest more severely (Parker et al., 2020). Advanced diagnostic tools like multiplex PCR may not be available in resource-constrained settings, which makes it more challenging to accurately identify multiple pathogens, especially when there are low bacterial or viral loads (Parker et al., 2020). Identifying the dominant pathogen causing the clinical menace can be difficult, mainly when symptoms are caused by more than one infection. Treatment decisions may result from this inadvertently.
Table 1.
Diagnostic tests for Typhoid fever and Malaria.
Table 1.
Diagnostic tests for Typhoid fever and Malaria.
| Typhoid Fever |
Description |
Pros |
Cons |
References |
| Blood Cultures |
Gold standard for identifying bloodstream infections. |
Conclusive results essential for directing antibiotic treatment. |
Susceptible to contamination, slow turnaround time (24-48 hours), incapacity to identify some cultured organisms. |
Tsalik et al., 2010; Lagier et al., 2015 |
| Typhidot Test |
Serological test detecting IgM and IgG antibodies against S. typhi . |
Sensitive and specific, aids in diagnosing enteric fever. |
Potential for cross-reactivity, especially in cases of previous infections or distinct Salmonella serotypes. |
Khoharo, 2011; Jesudason et al., 2002 |
| Widal Test |
Detects increasing titers of antibodies against S. typhi 's H and O antigens. |
Historical method, discontinued in developed nations due to low sensitivity and specificity. |
Low sensitivity and specificity, dependence on matching samples separated by 10 to 14 days. |
Jason B. Harris & Edward T. Ryan, 2015; Mawazo et al., 2019 |
| TUBEX Test |
Inhibits reaction between patient IgM antibodies and monoclonal antibodies specific to S. typhi.
|
Rapid results, relatively high specificity. |
Limited sensitivity (42%), inability to detect IgG antibodies. |
Khan et al., 2017; Feleszko et al., 2004 |
| Polymerase Chain Reaction (PCR) |
Molecular technique for detecting pathogens from clinical samples. |
Highly sensitive and specific, can detect multiple organisms simultaneously. |
High cost, technical complexity, limited accessibility in resource-limited settings. |
Achonduh-Atijegbe et al., 2016; Fortina et al., 2002 |
| Malaria |
Description |
Pros |
Cons |
References |
| Microscopy Technique |
Examination of blood films stained with Giemsa, Wright's, or Field's stain. |
Recognized laboratory procedure, allows species identification. |
Labor-intensive, requires skilled personnel, low sensitivity in cases of low parasitaemia. |
Warhurst & Williams, 1996; Moody, 2002 |
| Malaria Rapid Diagnostic Tests (RDTs) |
Immunochromatographic tests detecting specific malaria antigens in blood. |
Rapid results (10-15 minutes), suitable for remote locations. |
Limited sensitivity and specificity compared to microscopy, potential for false positives or negatives. |
Obeagu et al., 2018; WHO, 2009 |
| PCR |
Molecular technique offering high sensitivity and species identification capabilities. |
High sensitivity, speed, and species identification, especially useful in cases of low parasitaemia. |
High cost, technical expertise required, longer turnaround time compared to RDTs. |
Hawkes & Kain, 2007; Nandwani et al., 2005 |
Typhoid Fever Treatment Strategies and Antibiotic Resistance
Antimicrobial medication is required for the treatment of enteric fever and associated complications. Penicillins, cephalosporins, aminoglycosides, macrolides, fluoroquinolones, and tetracyclines are examples of common antibiotics (Richard A. Harvey (Ph.D.), 2007). Chloramphenicol, the most effective treatment for typhoid fever, inhibits protein synthesis by attaching to the 50S ribosomal subunit and inhibiting direct bacterial protein synthesis, killing the bacterium. Many fluoroquinolones block DNA gyrase, including Ciprofloxacin, Ofloxacin, Levofloxacin, and Moxifloxacin. Ceftriaxone, cefotaxime, and cefoperazone are examples of third generation cephalosporins that are useful against S. typhi infections resistant to several medications (D. R. Arora & B. Arora, n.d.). These cephalosporins work by inhibiting penicillin-binding proteins, which block the bacterial cell wall and the pathway that leads to the manufacture of peptidoglycan (Hartman et al., 2021; Ramdhani et al., 2021). Nowadays, ofloxacin and ciprofloxacin are the recommended treatments for most instances of typhoid fever. Ofloxacin is an excellent antibiotic for treating typhoidal Salmonella because it has higher bactericidal activity and improved intracellular and plasma penetrations (Veeraraghavan et al., 2018; Wain et al., 2021). Following this, more strains that exhibit plasmid-mediated multidrug resistance (MDR) to ampicillin, cotrimoxazole, and chloramphenicol have been reported. With the emergence of strains with MDR, fluoroquinolone (ciprofloxacin and ofloxacin) has become the preferred medication (Veeraraghavan et al., 2018). Tran et al. conducted a paired, open, randomized study during a multidrug-resistant typhoid outbreak in southern Vietnam. The study compared two short-course ofloxacin regimens for treating uncomplicated typhoid fever: 15 mg/kg daily for three days and 10 mg/kg daily for five days. 228 of the 438 patients who were enrolled—286 of whom were younger than 14—had blood cultures that tested positive for Salmonella species (S. typhi, 207; S. Paratyphi A, 19; and S. Choleraesuis, 2). A patient who experienced one treatment failure took a single ofloxacin dose. Apart from that, both regimens were successful; no carriers were identified, and there was no indication of toxicity, especially in young patients. Simple, multidrug-resistant typhoid fever was successfully treated with a three-day ofloxacin course, which proved safe and highly effective (Tran et al., 1995). Multidrug-resistant typhoid fever (MDRTF) is typhoid fever that is resistant to all three first-line prescribed treatments (chloramphenicol, ampicillin, and cotrimoxazole) (Zaki & Karande, 2011). Pediatricians worldwide prescribed ciprofloxacin at the onset of the illness after it was found less expensive, safe, and effective. However, fluoroquinolones are now the drug of choice for treating MDRTF worldwide. Both industrialized and African countries have reported cases of MDRTF, with most cases involving travelers returning from regions where MDR strains had caused outbreaks or been endemic (Zaki & Karande, 2011). Studies have indicated that drug resistance can develop in clinical isolates of S. typhi obtained before the introduction of antibiotics. Foreign genes acquired through plasmids and chromosomal alterations are the causes of S. typhi resistance (Holt et al., 1994). S. typhi can acquire plasmid-mediated resistance to tetracycline, ampicillin, chloramphenicol, and cotrimoxazole from other food bacteria (Mandal et al., 2012). S. typhi can develop antibiotic resistance through decreased permeability, inactivation, efflux, and target site modification. Drug resistance in S. typhi is primarily caused by genetic changes, such as chromosomal mutations and the acquisition of plasmids or transposons (Denyer S et al., 2004). Plasmid-mediated resistance is often caused by the acquisition of resistance plasmids, leading to drug inactivation, reduced membrane permeability, target site modification, and rapid antibiotic extrusion or efflux (Munita & Arias, 2016). This resistance mechanism is caused by bacteria obstructing medication entrance and preventing antimicrobial drugs from passing across the membrane due to changes in membrane permeability (Zhang & Cheng, 2022). S. typhi and other sulfonamide-resistant bacteria have evolved enzymes with a high affinity for p-aminobenzoic acid (PABA) and a low affinity for sulfonamide in order to survive and flourish in the presence of sulfonamides (Landy et al., 1943). Rapid extrusion, also referred to as efflux, occurs when pathogens pump the antibiotic out of the cell after it has entered. Certain pathogens have plasma membrane translocases, or efflux pumps, responsible for injecting drugs. Resistance to sulfonamides is mediated by a plasmid-encoded transport mechanism that actively exports the drug out of the cell (Andersen et al., 2015). Chromosome resistance presents fewer clinical challenges than plasmid-mediated resistance, brought on by mutations in genes encoding either drug targets or transport systems that control drug uptake (Denyer et al., 2004). Resistance to sulfonamides and fluoroquinolones is primarily caused by mutations in the chromosomal gene encoding for the target enzyme dihydropteroate synthase (Kai et al., 1999).
Table 2.
Malaria treatment strategies and Antibiotic resistance.
Table 2.
Malaria treatment strategies and Antibiotic resistance.
| Treatment Strategy |
Description |
Reference |
| Artemisinin-based Combination Therapies (ACT) |
First-line treatment for acute, uncomplicated malaria. Artemisinin interacts with heme in red blood cells, leading to the formation of free radicals that damage the parasite. |
Meshnick, 2002 |
| Quinine and Clindamycin |
Recommended for pregnant women in the first trimester; injectable artesunate for severe malaria. |
Eastman & Fidock, 2009 |
| Tafenoquine and Primaquine |
Authorized drugs to destroy hypnozoites; primaquine used in clinical settings to prevent P. falciparum transmission and act on stage V gametocytes. |
Markus, 2019 |
| Sulfadoxine-Pyrimethamine (SP) plus Amodiaquine |
Recommended for seasonal malaria in children ages 3 to 59 months. SP-resistant parasites in east and southern Africa pose a threat to intermittent chemoprevention effectiveness. |
van Eijk et al., 2019 |
| Antibiotic Resistance |
Description |
Reference |
| Genetic Modifications |
Primary cause of drug resistance in malaria, leading to inefficiency and emergence of resistance phenotypes. |
Klein, 2013 |
| Poverty and Self-Medication |
Poverty contributes to the inability to eradicate malaria, exposing individuals to counterfeit drugs from neighborhood pharmacies or drug stores. |
Breman et al., 2006; Hyde, 2007 |
| Treatment Noncompliance |
Noncompliance exposes parasites to lower levels of medication, aiding in the selection of resistant parasites. Examples include missing doses or stopping therapy too soon. |
Breman et al., 2006 |
| Asymptomatic Infections |
Asymptomatic individuals carry circulating parasites, encouraging the development of gametocytes with genetic changes that confer drug resistance. |
Sutherland et al., 2002 |
Vaccines as a Public Health Intervention
MALARIA: The World Health Organization (WHO) authorized the RTS, S/AS01 vaccine (MosquirixTM), the first vaccine against malaria, for widespread use in 2021. (El-Moamly & El-Sweify, 2023). According to White et al., RTS, S/AS01, the most promising candidate for a malaria vaccine, partially promotes effectiveness by producing antibodies against the Asn-Ala-Asn-Pro [NANP] core repeat of the circumsporozoite protein. R21 is a novel pre-erythrocytic malaria vaccine candidate. Central repetitions of the CSP and HBsAg attached to the C-terminus are present in both R21 and RTS, S, and they self-assemble into virus-like particles in yeast. R21 lacks the extra HBsAg found in RTS, S. When HBsAg monomers are expressed alone, RTS, S comprises 80% of them, whereas R21 alone has fusion protein moieties. This variation may result in a lower CSP coverage of the surface of the virus-like particle (Regules et al., 2011; Collins et al., 2017; Mehreen S. Datoo et al., 2023). The C-terminal region of the circumsporozoite protein from P falciparum strain NF54 fused to the N-terminus of HBsAg forms the basis of the virus-like particle known as the R21 vaccine. Novavax's Matrix-M (MM) adjuvant produces R21 (Leshem & Wilder-Smith, 2021). Considerable ongoing research and development has benefited malaria vaccination research by advancing the development of circumsporozoite protein-based vaccines like RTS and S; other pre-erythrocytic approaches like whole sporozoite vaccines; and candidates that use different life-cycle stages as their antigenic targets (Chitnis et al., 2020). Creating malaria vaccines has been fraught with difficulties, such as polymorphic antigens, low field-trial efficacy, and currently restricted vaccine supply, which has prevented the vaccine's widespread use. In a licensure trial conducted on African children, RTS, S/AS01, the most effective malaria vaccine candidate, showed 56% efficacy against uncomplicated clinical malaria over 12 months. In a phase IIb trial conducted in Burkina Faso, we discovered that a novel R21 nanoparticle in the saponin adjuvant Matrix demonstrated over 75% efficacy against a similar endpoint with seasonal administration (Datoo et al., 2021). R21 is immunogenic in BALB/c mice at very low doses, as Katharine A. Collins illustrates, and when combined with the adjuvants Abisco-100 and Matrix-M, it produces sterile protection against transgenic sporozoite challenge. Combining R21 with TRAP-based viral vectors also resulted in the simultaneous induction of strong humoral and cellular immune responses, significantly increasing protective efficacy. Furthermore, only a minimal antibody response to the HBsAg carrier was induced, in contrast to RTS, S. These investigations uncover a vaccine component against sporozoites that could enhance the top performing RTS, S malaria vaccine currently in use. Clinical trials conducting Phase 1/2a are currently evaluating R21 (Collins et al., 2017)
TYPHOID: The oral, live attenuated Ty21a vaccine needs to be taken in four doses separated by 48 hours (Booth et al., 2019). If exposure occurs again, this vaccine needs to be administered every two years, and it needs to be repeated every five years. Patients six years and older can receive the vaccination (Qadri et al., 2021). Due to the vaccine's live nature, pregnant or immunocompromised individuals should not receive it (Jackson et al., 2015). In the intestinal tract, the oral, live-attenuated Ty21a vaccine triggers a local immune response (Galen et al., 2021). By causing lipopolysaccharide biosynthesis, the attenuated strain triggers a defensive immune response. The intracellular accumulation of lipopolysaccharide intermediates by the inactivated bacterial cells in the vaccine causes them to lyse prior to virulent infection. Four vaccination doses spaced out over different days are needed for the response (Van Camp & Shorman, 2023). The Typhoid Vi polysaccharide vaccine caused an increase in anti-Vi antibodies following vaccination. During a 20-month follow-up, it was 74% successful in preventing infection in kids between the ages of two and four in Katmandu, Nepal (Theiss-Nyland et al., 2021). The injection of the Vi capsular polysaccharide vaccine is administered intramuscularly; it only needs to be given once, with a booster shot every two years. For use in individuals two years of age and above, a single intramuscular injection of 0.5 mL is administered (Bentsi-Enchill & Pollard, 2018).
Conclusion and Recommendation
Accurate diagnosis of typhoid and malaria is challenging due to their common clinical signs and symptoms. When there is a coinfection, medical practitioners frequently turn to prescribing broad-spectrum antibiotics to treat the illness because appropriate diagnostic tests are not always available. This literature review has determined that new quick and precise diagnostic instruments are needed to identify co-infection between typhoid and malaria. Low sensitivity and specificity were discovered in the RDTs frequently used as point-of-care diagnostic tests. Additionally, healthcare facilities in the endemic countries lacked easy access to molecular techniques with very high sensitivity and specificity, like PCR. As a result, it is advised that if patients exhibit overlapping symptoms and signs, susceptible and specific RDTs should be developed to aid in diagnosing these infections and their coinfections. Effective treatment plans are also required because most antibiotics are susceptible to antimicrobial resistance (AMR), particularly in endemic countries. Effective vaccination programs are necessary to enable people to guard against contracting these illnesses.
References
- Achonduh-Atijegbe, O. A., Mfuh, K. O., Mbange, A. H. E., Chedjou, J. P., Taylor, D. W., Nerurkar, V. R., Mbacham, W. F., & Leke, R. (2016). Prevalence of Malaria, typhoid, toxoplasmosis, and rubella among febrile children in Cameroon. BMC Infectious Diseases, 16(1), 658. [CrossRef]
- Adamu, A. I., Tsakuwa, R. A., Karfi, I. A., Ahmad, J. R., Shehu, M. N., & Muhammad, M. H. (2023). Incidence of malaria and typhoid fever coinfection among patients attending Rimingado Comprehensive Healthcare Centre, Kano State, Nigeria. Dutse Journal of Pure and Applied Sciences, 9(2a), 165–171. [CrossRef]
- Ammah, A., Nkuo-Akenji, T., Ndip, R., & Deas, J. E. (1999). An update on concurrent Malaria and typhoid fever in Cameroon. Transactions of the Royal Society of Tropical Medicine and Hygiene, 93(2), 127–129. [CrossRef]
- Andersen, J., He, G.-X., Kakarla, P., KC, R., Kumar, S., Lakra, W., Mukherjee, M., Ranaweera, I., Shrestha, U., Tran, T., & Varela, M. (2015). Multidrug Efflux Pumps from Enterobacteriaceae, Vibrio cholerae, and Staphylococcus aureus Bacterial Food Pathogens. International Journal of Environmental Research and Public Health, 12(2), 1487–1547. [CrossRef]
- Arai, T., Hiromatsu, K., Nishimura, H., Kimura, Y., Kobayashi, N., Ishida, H., Nimura, Y., & Yoshikai, Y. (1995). Endogenous Interleukin 10 Prevents Apoptosis in Macrophages during Salmonella Infection. Biochemical and Biophysical Research Communications, 213(2), 600–607. [CrossRef]
- Barcus, M. J., Basri, H., Picarima, H., Manyakori, C., Sekartuti, Elyazar, I., Bangs, M. J., Maguire, J. D., & Baird, J. K. (2007). Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. The American Journal of Tropical Medicine and Hygiene, 77(5), 984–991.
- Bashyam, H. (2007). Surviving Malaria, dying of typhoid. The Journal of Experimental Medicine, 204(12), 2774–2774. [CrossRef]
- Batire, S., Yohanes, T., Tadesse, D., Woldemariam, M., Tariku, B., Sanbeto, Z., Dale, D., & Alelign, D. (2022). The magnitude of Malaria-Typhoid Fever Coinfection in Febrile Patients at Arba Minch General Hospital in Southern Ethiopia. Journal of Tropical Medicine, 2022, 1–8. [CrossRef]
- Bennett, S. D., Lowther, S. A., Chingoli, F., Chilima, B., Kabuluzi, S., Ayers, T. L., Warne, T. A., & Mintz, E. (2018). Assessment of water, sanitation, and hygiene interventions in response to an outbreak of typhoid fever in Neno District, Malawi. PLOS ONE, 13(2), e0193348. [CrossRef]
- Bentsi-Enchill, A. D., & Pollard, A. J. (2018). A Turning Point in Typhoid Control. The Journal of Infectious Diseases, 218(suppl_4), S185–S187. [CrossRef]
- Bhat, R., Kodan, P., & Shetty, M. A. (2015). Medley of infections-a diagnostic challenge. Asian Pacific Journal of Tropical Biomedicine, 5(5), 418–420. [CrossRef]
- Bhawna Sharma, Monika Matlani, Rajni Gaind, & Khushbu Pandey. (2016). “Malaria And Typhoid Coinfection In India: A Diagnostic Difficulty’’. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS), 15(9), 2279–0853. [CrossRef]
- Bicudo, N., Bicudo, E., Costa, J. D., Castro, J. A. L. P., & Barra, G. B. (2020). Coinfection of SARS-CoV-2 and dengue virus: a clinical challenge. The Brazilian Journal of Infectious Diseases, 24(5), 452–454. [CrossRef]
- Birhanie, M., Tessema, B., Ferede, G., Endris, M., & Enawgaw, B. (2014). Malaria, Typhoid Fever, and Their Coinfection among Febrile Patients at a Rural Health Center in Northwest Ethiopia: A Cross-Sectional Study. Advances in Medicine, 2014, 1–8. [CrossRef]
- Booth, J. S., Patil, S. A., Goldberg, E., Barnes, R. S., Greenwald, B. D., & Sztein, M. B. (2019). Attenuated Oral Typhoid Vaccine Ty21a Elicits Lamina Propria and Intra-Epithelial Lymphocyte Tissue-Resident Effector Memory CD8 T Responses in the Human Terminal Ileum. Frontiers in Immunology, 10. [CrossRef]
- Breman, J. G., Mills, A., Snow, R. W., Mulligan, J.-A., Lengeler, C., Mendis, K., Sharp, B., Morel, C., Marchesini, P., White, N. J., Steketee, R. W., & Doumbo, O. K. (2006). Conquering Malaria.
- Bria, Y. P., Yeh, C.-H., & Bedingfield, S. (2021). Significant symptoms and nonsymptom-related factors for malaria diagnosis in endemic regions of Indonesia. International Journal of Infectious Diseases, 103, 194–200. [CrossRef]
- Cao, X. E., Kim, J., Mehta, S., & Erickson, D. (2021). Two-Color Duplex Platform for Point-of-Care Differential Detection of Malaria and Typhoid Fever. Analytical Chemistry, 93(36), 12175–12180. [CrossRef]
- Chitnis, C. E., Schellenberg, D., Vekemans, J., Asturias, E. J., Bejon, P., Collins, K. A., Crabb, B. S., Herrera, S., Laufer, M., Rabinovich, N. R., Roestenberg, M., Shirley, A., Tinto, H., Wentworth, M., O’Brien, K., & Alonso, P. (2020). Building momentum for malaria vaccine research and development: key considerations. Malaria Journal, 19(1), 421. [CrossRef]
- Chowta, N., & Chowta, M. (2005). Study of Clinical Profile and Antibiotic Response in Typhoid Fever. Indian Journal of Medical Microbiology, 23(2), 125. [CrossRef]
- Cirillo, V. J. (2006). "Winged Sponges": Houseflies as Carriers of Typhoid Fever in 19th- and Early 20th-Century Military Camps. Perspectives in Biology and Medicine, 49(1), 52–63. [CrossRef]
- Collins, K. A., Snaith, R., Cottingham, M. G., Gilbert, S. C., & Hill, A. V. S. (2017). Enhancing protective immunity to Malaria with a highly immunogenic virus-like particle vaccine. Scientific Reports, 7(1), 46621. [CrossRef]
- Crump, J. A., Luby, S. P., & Mintz, E. D. (2004). The global burden of typhoid fever. Bulletin of the World Health Organization, 82(5), 346–353.
- Crump, J. A., Sjölund-Karlsson, M., Gordon, M. A., & Parry, C. M. (2015). Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clinical Microbiology Reviews, 28(4), 901–937. [CrossRef]
- Crump, J. A. (2019). Progress in Typhoid Fever Epidemiology. Clinical Infectious Diseases, 68(Supplement_1), S4–S9. [CrossRef]
- D. R. Arora, & B. Arora. (n.d.). Textbook of Microbiology: Vol. 771 pages (3rd ed.). CBS Publishers & Distributors, 2008.
- Dasgupta, S. (2018). The burden of climate change on malaria mortality. International Journal of Hygiene and Environmental Health, 221(5), 782–791. [CrossRef]
- Datoo, M. S., Natama, M. H., Somé, A., Traoré, O., Rouamba, T., Bellamy, D., Yameogo, P., Valia, D., Tegneri, M., Ouedraogo, F., Soma, R., Sawadogo, S., Sorgho, F., Derra, K., Rouamba, E., Orindi, B., Ramos Lopez, F., Flaxman, A., Cappuccini, F., … Tinto, H. (2021). Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomized controlled trial. The Lancet, 397(10287), 1809–1818. [CrossRef]
- Denyer S, Hodges N, & Gorman S0. (2004). Hugo and Russell’s Pharmaceutical Microbiology (S. P. Denyer, N. A. Hodges, & S. P. Gorman, Eds.). Wiley. [CrossRef]
- E. Eze, B. Ukwah, P. Okafor, & K. Ugwu. (2012). Prevalence of malaria and typhoid coinfections in the University of Nigeria, Nsukka District of Enugu State, Nigeria. Medicine, Environmental Science.
- Eastman, R. T., & Fidock, D. A. (2009). Artemisinin-based combination therapies: a vital tool in efforts to eliminate Malaria. Nature Reviews Microbiology, 7(12), 864–874. [CrossRef]
- El-Moamly, A. A., & El-Sweify, M. A. (2023). Malaria vaccines: the 60-year journey of hope and final success—lessons learned and prospects. Tropical Medicine and Health, 51(1), 29. [CrossRef]
- Feleszko, W., Maksymiuk, J., Oracz, G., Golicka, D., & Szajewska, H. (2004). The TUBEXTM typhoid test detects current Salmonella infections. Journal of Immunological Methods, 285(1), 137–138. [CrossRef]
- Fields, P. I., Swanson, R. V., Haidaris, C. G., & Heffron, F. (1986). Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proceedings of the National Academy of Sciences, 83(14), 5189–5193. [CrossRef]
- Finlay, B. B., Ruschkowski, S., & Dedhar, S. (1991). Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. Journal of Cell Science, 99(2), 283–296. [CrossRef]
- Florence A. Mbuh, Musa Galadima, & Lucy Ogbadu. (2003). RATE OF COINFECTION WITH MALARIA PARASITES AND SALMONELLA TYPHI IN ZARIA, KADUNA STATE, NIGERIA. Https://Tspace.Library.Utoronto.ca/Handle/1807/3671.
- Fortina, P., Surrey, S., & Kricka, L. J. (2002). Molecular diagnostics: hurdles for clinical implementation. Trends in Molecular Medicine, 8(6), 264–266. [CrossRef]
- Galen, J. E., Wahid, R., & Buskirk, A. D. (2021). Strategies for Enhancement of Live-Attenuated Salmonella-Based Carrier Vaccine Immunogenicity. Vaccines, 9(2), 162. [CrossRef]
- Greenwood, B. M., Palit, A., Bradley-Moore, AliceM., & Bryceson, A. D. M. (1972). IMMUNOSUPPRESSION IN CHILDREN WITH MALARIA. The Lancet, 299(7743), 169–172. [CrossRef]
- Hartman, S. J. F., Upadhyay, P. J., Hagedoorn, N. N., Mathôt, R. A. A., Moll, H. A., van der Flier, M., Schreuder, M. F., Brüggemann, R. J., Knibbe, C. A., & de Wildt, S. N. (2021). Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study. Clinical Pharmacokinetics, 60(10), 1361–1372. [CrossRef]
- Hawkes, M., & Kain, K. C. (2007). Advances in malaria diagnosis. Expert Review of Anti-Infective Therapy, 5(3), 485–495. [CrossRef]
- Holt, Krieg, & Sneath. (1994). Bergey’s Manual of Determinative Bacteriology. Advances in Microbiology, 786–788.
- Hyde, J. E. (2007). Drug-resistant Malaria − an insight. The FEBS Journal, 274(18), 4688–4698. [CrossRef]
- Jackson, B. R., Iqbal, S., Mahon, B., & Centers for Disease Control and Prevention (CDC). (2015). Updated recommendations for the use of typhoid vaccine--Advisory Committee on Immunization Practices, United States, 2015. MMWR. Morbidity and Mortality Weekly Report, 64(11), 305–308.
- Jason B. Harris, & Edward T. Ryan. (2015). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Elsevier. [CrossRef]
- Jesudason, M., Esther, E., & Mathai, E. (2002). Typhidot test to detect IgG & IgM antibodies in typhoid fever. The Indian Journal of Medical Research, 116, 70–72.
- Kai, M., Matsuoka, M., Nakata, N., Maeda, S., Gidoh, M., Maeda, Y., Hashimoto, K., Kobayashi, K., & Kashiwabara, Y. (1999). Diaminodiphenylsulfone resistance of Mycobacterium leprae due to mutations in the dihydropteroate synthase gene. FEMS Microbiology Letters, 177(2), 231–235. [CrossRef]
- Källander, K., Nsungwa-Sabiiti, J., & Peterson, S. (2004). Symptom overlaps for malaria and pneumonia—policy implications for home management strategies. Acta Tropica, 90(2), 211–214. [CrossRef]
- Keong, B. C. M., & Sulaiman, W. (2006). Typhoid and malaria coinfection - an exciting finding in investigating a tropical Fever. The Malaysian Journal of Medical Sciences : MJMS, 13(1), 74–75.
- Khan, F. Y., Lutof, A. K., Yassin, M. A., Khattab, M. A.-, Saleh, M., Rezeq, H. Y., & Almaslamani, M. (2009). Imported Malaria in Qatar: A one-year hospital-based study in 2005. Travel Medicine and Infectious Disease, 7(2), 111–117. [CrossRef]
- Khan, K., Khalid, L., Wahid, K., & Ali, I. (2017). Performance of TUBEX® TF in the diagnosis of enteric fever in private tertiary care Hospital Peshawar, Pakistan. JPMA. The Journal of the Pakistan Medical Association, 67(5), 661–664.
- Khoharo, H. K. (2011). A comparative study of the typhoid (Dot-EIA) and Widal tests in blood culture-positive cases of typhoid fever. Tropical Doctor, 41(3), 136–138. [CrossRef]
- Klein, E. Y. (2013). Antimalarial drug resistance: a review of the biology and strategies to delay emergence and spread. International Journal of Antimicrobial Agents, 41(4), 311–317. [CrossRef]
- Lagier, J.-C., Edouard, S., Pagnier, I., Mediannikov, O., Drancourt, M., & Raoult, D. (2015). Current and Past Strategies for Bacterial Culture in Clinical Microbiology. Clinical Microbiology Reviews, 28(1), 208–236. [CrossRef]
- Landy, M., Larkum, N. W., Oswald, E. J., & Streightoff, F. (1943). Increased Synthesis Of P-Aminobenzoic Acid Associated With The Development Of Sulfonamide Resistance In Staphylococcus Aureus. Science, 97(2516), 265–267. [CrossRef]
- LaRock, D. L., Chaudhary, A., & Miller, S. I. (2015). Salmonellae interactions with host processes. Nature Reviews Microbiology, 13(4), 191–205. [CrossRef]
- Leshem, E., & Wilder-Smith, A. (2021). COVID-19 vaccine impact in Israel and a way out of the pandemic. The Lancet, 397(10287), 1783–1785. [CrossRef]
- Mandal, S., DebMandal, M., & Pal, N. K. (2012). Antibiotic Resistance of Salmonella enterica Serovar Typhi in Kolkata, India, and In Vitro Experiments on Effect of Combined Chemotherapy. The Scientific World Journal, 2012, 1–4. [CrossRef]
- Markus, M. B. (2019). Killing of Plasmodium vivax by Primaquine and Tafenoquine. Trends in Parasitology, 35(11), 857–859. [CrossRef]
- Martens, P. (2000). Malaria on the Move: Human Population Movement and Malaria Transmission. Emerging Infectious Diseases, 6(2), 103–109. [CrossRef]
- Mawazo, A., Bwire, G. M., & Matee, M. I. N. (2019). Performance of Widal test and stool culture in diagnosing typhoid fever among suspected patients in Dar es Salaam, Tanzania. BMC Research Notes, 12(1), 316. [CrossRef]
- Mehreen S. Datoo, Alassane Dicko, Halidou Tinto, Jean-Bosco Ouédraogo, Mainga Hamaluba, Ally Olotu, Emma Beaumont, Fernando Ramos-Lopez, Hamtandi Magloire Natama, Sophie Weston, Mwajuma Chemba, & Yves D. Compaore. (2023). A Phase III Randomised Controlled Trial Evaluating the Malaria Vaccine Candidate R21/Matrix-MTM in African Children. The Lancet, 26 pages.
- Menendez, C., Fleming, A. F., & Alonso, P. L. (2000). Malaria-related Anaemia. Parasitology Today, 16(11), 469–476. [CrossRef]
- Meshnick, S. R. (2002). Artemisinin: mechanisms of action, resistance, and toxicity. International Journal for Parasitology, 32(13), 1655–1660. [CrossRef]
- Milner, D. A. (2018). Malaria Pathogenesis. Cold Spring Harbor Perspectives in Medicine, 8(1), a025569. [CrossRef]
- Mohammed, H. I., Mukhtar, I. M., & Sadiq, H. A. (2020). Malaria and Typhoid Fever: Prevalence, Coinfection and Socio-Demographic Determinants among Pregnant Women Attending Antenatal Care at a Primary Healthcare Facility in Central Nigeria. International Journal of Pathogen Research, 17–24. [CrossRef]
- Molaeipoor, L., Poorolajal, J., Mohraz, M., & Esmailnasab, N. (2014). Predictors of Tuberculosis and Human Immunodeficiency Virus Coinfection: A Case-Control Study. Epidemiology and Health, e2014024. [CrossRef]
- Moody, A. (2002). Rapid Diagnostic Tests for Malaria Parasites. Clinical Microbiology Reviews, 15(1), 66–78. [CrossRef]
- Mooney, J. P., Barry, A., Gonçalves, B. P., Tiono, A. B., Awandu, S. S., Grignard, L., Drakeley, C. J., Bottomley, C., Bousema, T., & Riley, E. M. (2018). Hemolysis and haem oxygenase-1 induction during persistent “asymptomatic” malaria infection in Burkinabé children. Malaria Journal, 17(1), 253. [CrossRef]
- Mota, M. M., Pradel, G., Vanderberg, J. P., Hafalla, J. C. R., Frevert, U., Nussenzweig, R. S., Nussenzweig, V., & Rodrı́guez, A. (2001). Migration of Plasmodium Sporozoites Through Cells Before Infection. Science, 291(5501), 141–144. [CrossRef]
- Munita, J. M., & Arias, C. A. (2016). Mechanisms of Antibiotic Resistance. Microbiology Spectrum, 4(2). [CrossRef]
- Mutua, J. M., Wang, F.-B., & Vaidya, N. K. (2015a). Modeling malaria and typhoid fever coinfection dynamics. Mathematical Biosciences, 264, 128–144. [CrossRef]
- Mutua, J. M., Wang, F.-B., & Vaidya, N. K. (2015b). Modeling malaria and typhoid fever coinfection dynamics. Mathematical Biosciences, 264, 128–144. [CrossRef]
- Mweu, E., & English, M. (2008). Typhoid fever in children in Africa. Tropical Medicine & International Health, 13(4), 532–540. [CrossRef]
- Nandwani, S., Mathur, M., & Rawat, S. (2005). EVALUATION OF THE POLYMERASE CHAIN REACTION ANALYSIS FOR DIAGNOSIS OF FALCIPARUM MALARIA IN DELHI, INDIA. Indian Journal of Medical Microbiology, 23(3), 176–178. [CrossRef]
- Nas, F. S., Ali, M., & Yahaya, A. (2018). Malaria and typhoid fever coinfection among febrile patients in Kumbotso Local Government Area Kano, Nigeria. Bayero Journal of Pure and Applied Sciences, 10(2), 122. [CrossRef]
- Nogueira, C. R., & Lopes, L. M. X. (2011). Antiplasmodial Natural Products. Molecules, 16(3), 2146–2190. [CrossRef]
- Nsutebu, E. F., Martins, P., & Adiogo, D. (2003). Short communication: Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Tropical Medicine & International Health, 8(6), 575–578. [CrossRef]
- Obeagu, E. I., UO, C., & IS, E. (2018). Malaria Rapid Diagnostic Test (RDTs). Annals of Clinical and Laboratory Research, 06(04). [CrossRef]
- Ohanu, M., Mbah, A., Okonkwo, P., & Nwagbo, F. (2004). Interference by Malaria in the diagnosis of typhoid using the Widal test alone. West African Journal of Medicine, 22(3). [CrossRef]
- Parker, A., Shaw, J., Karamchand, S., Lahri, S., Schrueder, N., Chothia, M.-Y., Mowlana, A., Lalla, U., Allwood, B. W., Koegelenberg, C. F. N., & Taljaard, J. J. (2020). HIV and SARS-CoV-2 coinfection: The diagnostic challenges of dual pandemics. South African Medical Journal, 110(6). [CrossRef]
- Paul, U. K., & Bandyopadhyay, A. (2017). Typhoid fever: a review. International Journal of Advances in Medicine, 4(2), 300. [CrossRef]
- Phan, V. B., Nguyen, T. T., Ha, B. K., Dang, D. T., Bryla, D., Robbins, J. B., Lin, F. Y., Vo, A. H., & Tran, C. T. (2000). The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. The American Journal of Tropical Medicine and Hygiene, 62(5), 644–648. [CrossRef]
- Postels, D. G., & Birbeck, G. L. (2013). Cerebral Malaria (pp. 91–102). [CrossRef]
- Prada, J., Alabi, SundayA., Bienzle, U., & Kremsner, PeterG. (1993). Bacterial strains isolated from blood cultures of Nigerian children with cerebral Malaria. The Lancet, 342(8879), 1114. [CrossRef]
- Prasanna Pradhan. (2011). Coinfection of typhoid and Malaria. Journal of Medical Laboratory and Diagnosis, 2(3), 22–26.
- Qadri, F., Khanam, F., Liu, X., Theiss-Nyland, K., Biswas, P. K., Bhuiyan, A. I., Ahmmed, F., Colin-Jones, R., Smith, N., Tonks, S., Voysey, M., Mujadidi, Y. F., Mazur, O., Rajib, N. H., Hossein, M. I., Ahmed, S. U., Khan, A., Rahman, N., Babu, G., … Clemens, J. D. (2021). Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomized trial. The Lancet, 398(10301), 675–684. [CrossRef]
- Qureshi, A. W., Khan, Z.-U., Khan, L., Mansoor, A., & Minhas, R. (2019). Prevalence of Malaria, typhoid and coinfection in District DIR (lower), Pakistan. Biosci. j. (Online), 35(1), 317–325.
- Ramdhani, D., Kusuma, S. A. F., Sediana, D., Bima, A. P. H., & Khumairoh, I. (2021). Comparative study of cefixime and tetracycline as an evaluation policy driven by the antibiotic resistance crisis in Indonesia. Scientific Reports, 11(1), 18461. [CrossRef]
- Ratledge, C., & Dover, L. G. (2000). Iron Metabolism in Pathogenic Bacteria. Annual Review of Microbiology, 54(1), 881–941. [CrossRef]
- Regules, J. A., Cummings, J. F., & Ockenhouse, C. F. (2011). The RTS, S vaccine candidate for Malaria. Expert Review of Vaccines, 10(5), 589–599. [CrossRef]
- Richard A. Harvey (Ph.D.). (2007). Lippincott’s Illustrated Reviews: Microbiology, Second Edition (Vol. 438). https://books.google.com/books/about/Microbiology.html?id=FPd38Gc33gwC.
- Sachs, J., & Malaney, P. (2002). The economic and social burden of Malaria. Nature, 415(6872), 680–685. [CrossRef]
- Sato, S. (2021). Plasmodium—a brief introduction to the parasites causing human Malaria and their basic biology. Journal of Physiological Anthropology, 40(1), 1. [CrossRef]
- Sears, H. J., Garhart, R. W., & Mack, D. W. (1924). A MILK-BORNE EPIDEMIC OF TYPHOID FEVER TRACED TO A URINARY CARRIER. American Journal of Public Health, 14(10), 848–854. [CrossRef]
- Shretta, R., Liu, J., Cotter, C., Cohen, J., Dolenz, C., Makomva, K., Newby, G., Ménard, D., Phillips, A., Tatarsky, A., Gosling, R., & Feachem, R. (2017). Malaria Elimination and Eradication.
- Sinha, A., Sazawal, S., Kumar, R., Sood, S., Reddaiah, V. P., Singh, B., Rao, M., Naficy, A., Clemens, J. D., & Bhan, M. K. (1999). Typhoid fever in children aged less than 5 years. The Lancet, 354(9180), 734–737. [CrossRef]
- Sohanang Nodem, F. S., Ymele, D., Fadimatou, M., & Fodouop, S.-P. C. (2023). Malaria and Typhoid Fever Coinfection among Febrile Patients in Ngaoundéré (Adamawa, Cameroon): A Cross-Sectional Study. Journal of Parasitology Research, 2023, 1–9. [CrossRef]
- Sutherland, C. J., Curtis, J., Alloueche, A., Drakeley, C. J., Ord, R., Greenwood, B. M., Pinder, M., Duraisingh, M., Targett, G. A. T., & Warhurst, D. (2002). Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. The American Journal of Tropical Medicine and Hygiene, 67(6), 578–585. [CrossRef]
- Talapko, Škrlec, Alebić, Jukić, & Včev. (2019). Malaria: The Past and the Present. Microorganisms, 7(6), 179. [CrossRef]
- Tanko Rufai, Enoch Aninagyei, Kwadwo Owusu Akuffo, Christian Teye-Muno Ayin, Priscilla Nortey, Reginald Quansah, Francis Samuel Cudjoe, Ernest Tei-Maya, Isaiah Osei Duah Junior, & Anthony Danso-Appiah. (2022). Malaria and typhoid fever coinfection among patients presenting with febrile illnesses in Ga West Municipality, Ghana. Https://Www.Medrxiv.Org/.
- Theiss-Nyland, K., Shakya, M., Colin-Jones, R., Voysey, M., Smith, N., Karkey, A., Dongol, S., Pant, D., Farooq, Y. G., Mazur, O., Darlow, C., Neuzil, K. M., Shrestha, S., Basnyat, B., & Pollard, A. J. (2021). Corrigendum to: Assessing the Impact of a Vi-polysaccharide Conjugate Vaccine in Preventing Typhoid Infections Among Nepalese Children: A Protocol for a Phase III, Randomized Control Trial. Clinical Infectious Diseases, 73(10), 1950–1950. [CrossRef]
- Townsend, S. M., Kramer, N. E., Edwards, R., Baker, S., Hamlin, N., Simmonds, M., Stevens, K., Maloy, S., Parkhill, J., Dougan, G., & Bäumler, A. J. (2001). Salmonella enterica Serovar Typhi Possesses a Unique Repertoire of Fimbrial Gene Sequences. Infection and Immunity, 69(5), 2894–2901. [CrossRef]
- Tran, T. H., Bethell, D. B., Nguyen, T. T., Wain, J., To, S. D., Le, T. P., Bui, M. C., Nguyen, M. D., Pham, T. T., & Walsh, A. L. (1995). Short course of ofloxacin for treatment of multidrug-resistant typhoid. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 20(4), 917–923.
- Tsalik, E. L., Jones, D., Nicholson, B., Waring, L., Liesenfeld, O., Park, L. P., Glickman, S. W., Caram, L. B., Langley, R. J., van Velkinburgh, J. C., Cairns, C. B., Rivers, E. P., Otero, R. M., Kingsmore, S. F., Lalani, T., Fowler, V. G., & Woods, C. W. (2010). Multiplex PCR To Diagnose Bloodstream Infections in Patients Admitted from the Emergency Department with Sepsis. Journal of Clinical Microbiology, 48(1), 26–33. [CrossRef]
- Tsui, I. S. M., Yip, C. M. C., Hackett, J., & Morris, C. (2003). The Type IVB Pili of Salmonella enterica Serovar Typhi Bind to the Cystic Fibrosis Transmembrane Conductance Regulator. Infection and Immunity, 71(10), 6049–6050. [CrossRef]
- Uneke, C. J. (2008). Concurrent malaria and typhoid fever in the tropics: the diagnostic challenges and public health implications. Journal of Vector Borne Diseases, 45(2), 133–142.
- Van Camp, R. O., & Shorman, M. (2023). Typhoid Vaccine.
- Van Eijk, A. M., Larsen, D. A., Kayentao, K., Koshy, G., Slaughter, D. E. C., Roper, C., Okell, L. C., Desai, M., Gutman, J., Khairallah, C., Rogerson, S. J., Hopkins Sibley, C., Meshnick, S. R., Taylor, S. M., & ter Kuile, F. O. (2019). Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for Malaria in pregnancy in Africa: a systematic review and meta-analysis. The Lancet Infectious Diseases, 19(5), 546–556. [CrossRef]
- Veeraraghavan, B., Pragasam, A. K., Bakthavatchalam, Y. D., & Ralph, R. (2018). Typhoid fever: issues in laboratory detection, treatment options, and management concerns in developing countries. Future Science OA, 4(6), FSO312. [CrossRef]
- Wain, J., Simpson, J. A., Thi Diem Nga, L., Song Diep, T., Thanh Duy, P., Baker, S., Day, N. P. J., White, N. J., & Parry, C. M. (2021). Bactericidal activities and post-antibiotic effects of ofloxacin and ceftriaxone against drug-resistant Salmonella enterica serovar Typhi. Journal of Antimicrobial Chemotherapy, 76(10), 2606–2609. [CrossRef]
- Warhurst, D. C., & Williams, J. E. (1996). ACP Broadsheet no 148. July 1996. Laboratory diagnosis of Malaria. Journal of Clinical Pathology, 49(7), 533–538. [CrossRef]
- WHO. (2009). WHO: Malaria Rapid Diagnostic test performance.
- WHO. (2023). Malaria. World Health Organization: Factsheet on Malaria Https://Www.Who.Int/En/News-Room/Fact-Sheets/Detail/Malaria.
- Wilairatana, P., Mala, W., Klangbud, W. K., Kotepui, K. U., Rattaprasert, P., & Kotepui, M. (2021). Prevalence, probability, and outcomes of typhoidal/non-typhoidal Salmonella and malaria co-infection among febrile patients: a systematic review and meta-analysis. Scientific Reports, 11(1). [CrossRef]
- Zaki, S. A., & Karande, S. (2011). Multidrug-resistant typhoid fever: a review. The Journal of Infection in Developing Countries, 5(05), 324–337. [CrossRef]
- Zhang, F., & Cheng, W. (2022). The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics, 11(9), 1215. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).