1. Introduction
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition characterized by a range of symptoms, including difficulties in social interaction, communication challenges, and a tendency towards repetitive behaviors [
1]. Recent advances in neuroimaging and computational modeling have begun to shed light on the neural underpinnings of ASD, revealing unique patterns in both spatial and topological brain connectivity [
2,
3]. This article aims to synthesize key findings from recent research to provide a comprehensive overview of the altered neural connectivity observed in ASD and its implications for understanding the disorder.
Spatial proximity in the brain, referring to the physical closeness of neurons or brain regions, has been a focal point in understanding the structural brain differences in ASD. Studies by Hazlett et al. [
4] and Geschwind & Levitt [
5] have highlighted increased regionalization of brain function in children with ASD, suggesting atypical growth patterns and organization in certain brain areas. Freitag et al. [
6] and Nomi et al. [
7] further contribute to this understanding by demonstrating enhanced long-range synchronization and increased local connectivity, respectively, in functional brain networks of individuals with ASD.
On the other hand, topological proximities, which describe the functional or abstract closeness of neurons or brain regions, offer insights into the complex interplay of neural connections in ASD. Rubinov & Sporns [
8] and Van den Heuvel & Sporns [
9] have explored the rich set of topological features characterizing large-scale networks in the human brain, providing a framework for understanding the atypical connectivity patterns in ASD. The work of Bullmore & Sporns [
10] and Menon [
11] further underscores the importance of graph theoretical analysis in elucidating the structural and functional systems of complex brain networks.
The intersection of ASD and connectivity research, as explored by Uhlhaas & Singer [
12], Belmonte & Bourgeron [
13], Di Martino et al. [
14], and McDougal et al. [
15], reveals a nuanced picture of neural synchrony and long-range circuits in ASD. These studies collectively highlight aberrant intrinsic network connectivity, offering a deeper understanding of the disorder's neural basis.
Finally, computational modeling, as discussed by Sporns & Betzel [
16], Bassett & Toga [
17], and Robinson et al. [
18], plays a crucial role in simulating brain networks and understanding the modularity and plasticity of human brain networks. These models are instrumental in integrating findings from neuroscience to construct a coherent picture of the brain's functional architecture in ASD.
Through this article, we aim to integrate these diverse strands of research to provide a holistic view of the altered neural connectivity in ASD, offering insights into the disorder's etiology and potential avenues for intervention.
2. Methodology
Spatial Proximity
For the spatial proximity simulation, the positions of the nodes are determined by random Gaussian distributions centered around predefined cluster points. The pseudo-equation for each node's position can be described as:
Position
Where:
Position is the position of node .
Gaussian is a function generating a random number based on a Gaussian (normal) distribution.
is the mean of the distribution, representing the center of a cluster.
is the standard deviation of the distribution, controlling how tightly the nodes are clustered.
Topological Proximity
For the topological proximity, the connections (edges) between nodes are determined randomly. The process can be described as:
For each node , decide randomly whether it will form a dense cluster or have sparse connections.
-
If dense cluster:
For from 1 to
-
If sparse connections:
AddEdge , RandomNode())
Where:
AddEdge is a function that creates an edge between nodes and .
RandomNode() is a function that selects a random node from the network.
is the number of connections to add for a dense cluster.
Theoretical Frameworks: Theoretical papers providing insights into the neurobiological mechanisms underlying altered connectivity in ASD were reviewed. This included analysis of theories on neural development, synaptic plasticity, and network dynamics in ASD.
Integration of Findings
Comparative Analysis: Results from different studies and methodologies were compared to identify common findings and discrepancies. This involved contrasting neuroimaging results with outcomes from computational models.
Synthesis of Insights: A synthesis was conducted to integrate insights from various studies, providing a cohesive understanding of how spatial and topological proximities are altered in ASD.
Implications for ASD Understanding: The synthesized findings were interpreted in the context of ASD's clinical presentation, with a focus on how altered neural connectivity relates to the disorder's characteristic behaviors and cognitive patterns.
Ethical Considerations
All analyses and syntheses were conducted with strict adherence to ethical standards, ensuring the confidentiality and anonymity of data from human subjects where applicable. The methodology was designed to respect the integrity of the original research while providing a comprehensive overview of the field.
Limitations
The methodology acknowledges the limitations inherent in synthesizing diverse studies, including potential biases in study designs, variability in neuroimaging techniques, and the challenges of interpreting complex neural data.
Through this methodology, the article aims to provide a detailed, evidence-based exploration of neural connectivity in ASD, contributing to a deeper understanding of the disorder and potential avenues for future research and intervention.
3. Discussion
3.1. Interpretation of Findings
The synthesis of neuroimaging data, computational models, and theoretical frameworks in this article provides a nuanced understanding of the altered neural connectivity in Autism Spectrum Disorder (ASD). The findings underscore two critical aspects of neural connectivity in ASD: spatial proximity and topological proximity, each contributing uniquely to the disorder's neurobiological profile.
Spatial Proximity: The observed structural changes, including variations in brain volume and organization, suggest a deviation from typical neurodevelopmental trajectories in ASD [
19]. The increased regionalization and clustering of brain function, as indicated by neuroimaging studies, point towards a propensity for over-connectivity in localized brain regions [20, 21]. This over-connectivity could underlie some of the sensory processing peculiarities and the intense focus on specific interests often seen in individuals with ASD [
22].
Topological Proximity: The patterns of hypo- and hyper-connectivity in functional brain networks reveal a complex alteration in the brain's information processing pathways [
23]. The reduced long-range connectivity might contribute to challenges in integrating information across different brain regions, potentially underlying difficulties in social communication and broader cognitive integration [
24]. Conversely, increased local connectivity could be associated with the repetitive behaviors and restricted interests characteristic of ASD [
25].
3.2. Implications for Understanding ASD
The altered patterns of neural connectivity in ASD have significant implications for understanding the disorder:
Behavioral Manifestations: The link between neural connectivity alterations and ASD's behavioral symptoms provides a more comprehensive framework for understanding the neurobiological basis of these symptoms [
26].
Diagnostic and Intervention Strategies: Understanding these altered connectivity patterns could inform the development of more targeted diagnostic tools and interventions, potentially leading to earlier identification and more personalized therapeutic approaches [
27].
Neurodevelopmental Perspective: The findings emphasize the importance of considering ASD as a neurodevelopmental condition, where early brain development plays a crucial role in shaping later outcomes [
28].
3.3. Future Research Directions
Longitudinal Studies: There is a need for longitudinal studies to track the progression of neural connectivity changes over time in individuals with ASD, from early childhood through adulthood [
29].
4. Results
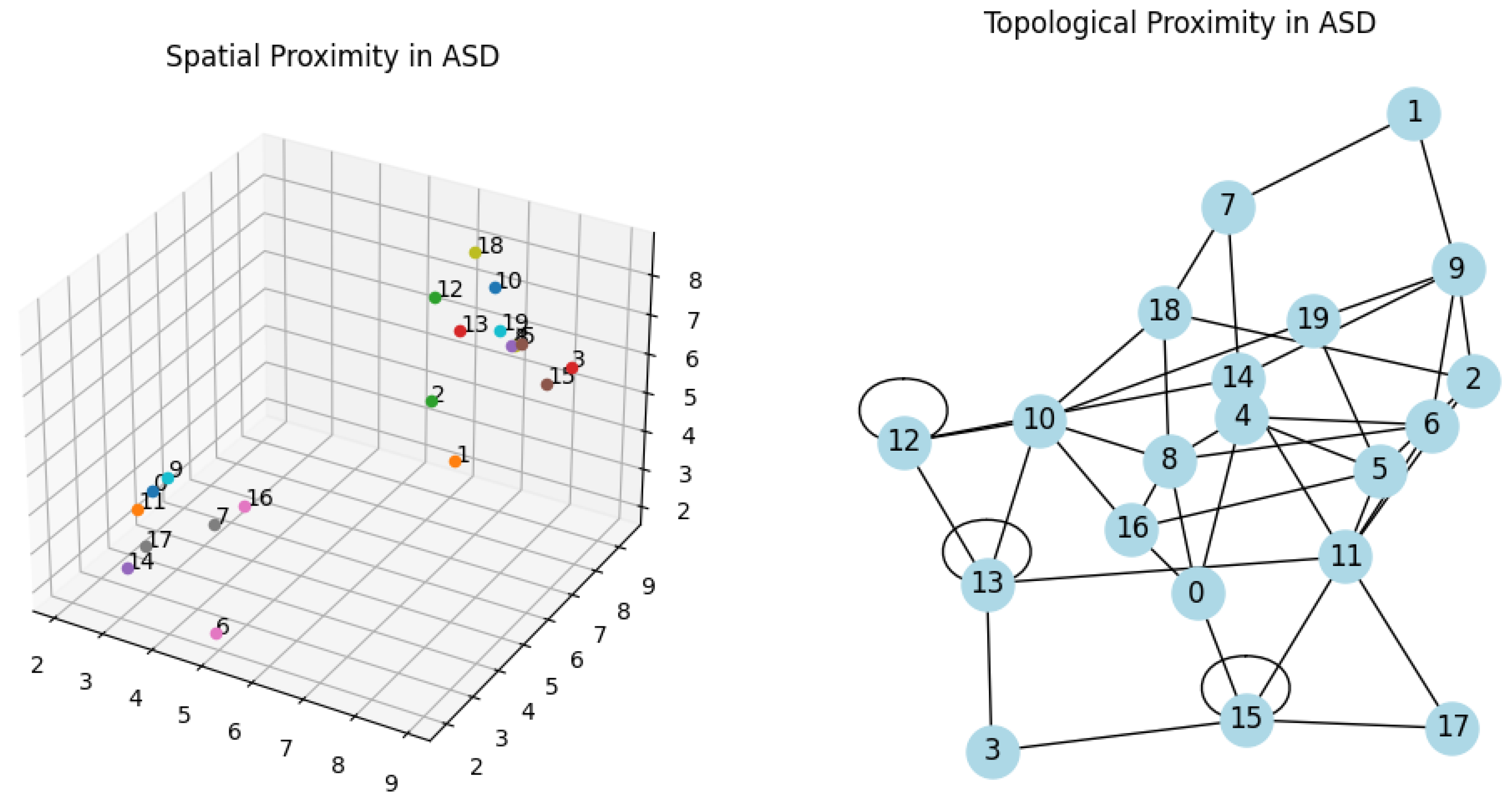
Topological Proximity: We then create a graph with the same 20 nodes. Random edges are added between nodes to simulate random topological connections. This graph is plotted in 2D to visualize the topological network.
Visualization: The script generates two subplots - one for spatial proximity and another for topological proximity.
Figure 1.
In both graphs its visible the agglomeration of nodes and in the second the circuits reverberations.
Figure 1.
In both graphs its visible the agglomeration of nodes and in the second the circuits reverberations.
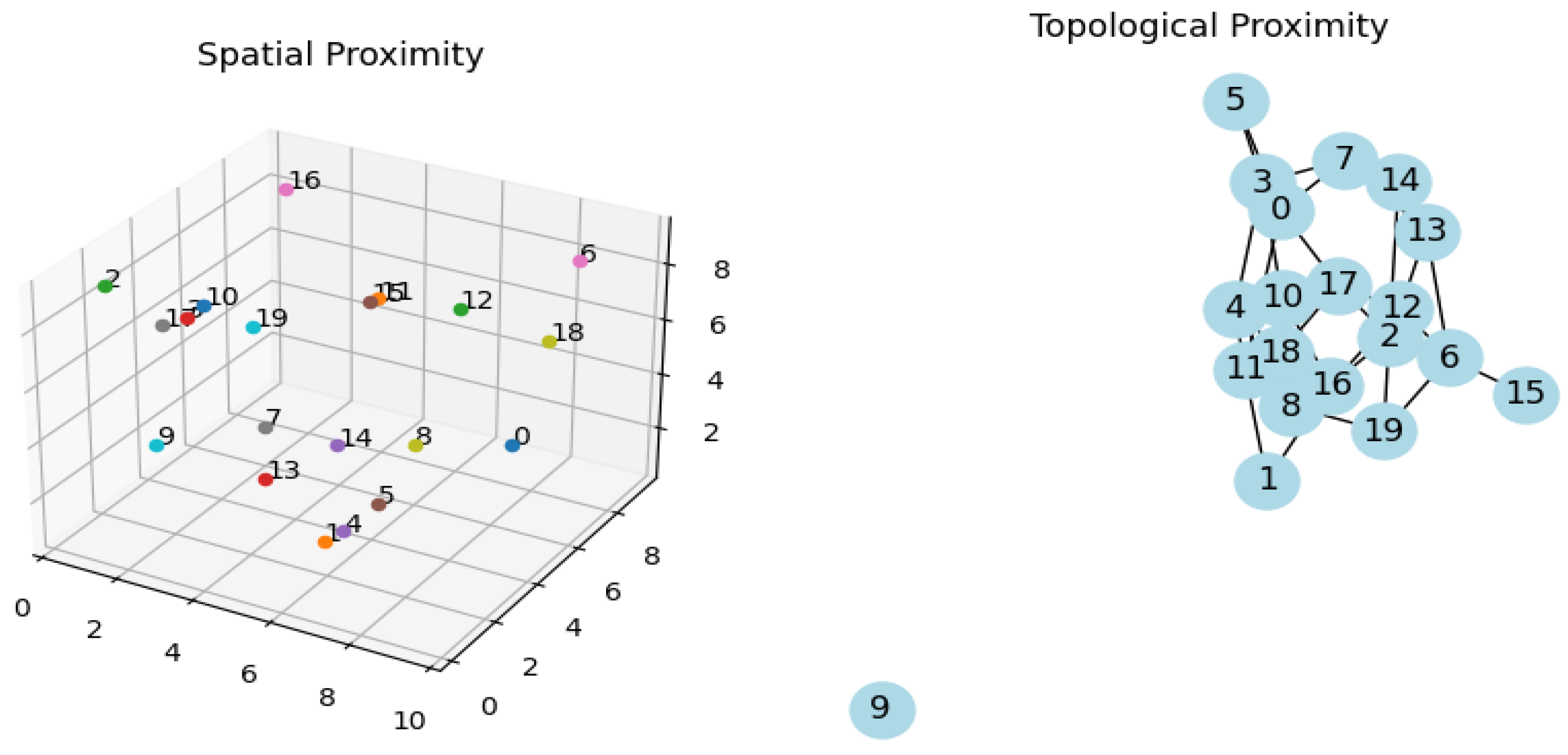
Figure 2.
Exacerbation of graph 1, characteristics with spatial proximity gaining stability in topological graph on the right. Circuits are reverberating.
Figure 2.
Exacerbation of graph 1, characteristics with spatial proximity gaining stability in topological graph on the right. Circuits are reverberating.
I utilized the matplotlib library for spatial plots and the networkx library for network graphs. The spatial plot is a 3D scatter plot of the nodes' positions, and the network graph represents the nodes and their connections [
30].
These pseudo-equations and descriptions provide a conceptual understanding of how the nodes' positions and connections are determined in the script. The actual implementation in Python involves random number generation and iterative logic to create these structures. Spatial proximity is crucial for understanding the brain's anatomical structure and the physical pathways of neural connections. For example, neurons in the same brain region are often spatially close and may have direct synaptic connections [
31]. Topological proximity, on the other hand, refers to the functional or abstract closeness of neurons or brain regions. This concept is more about how neurons or areas are connected functionally, regardless of their physical distance [
32].
In network theory, topological proximity is often analyzed using graphs where nodes represent neurons or brain regions, and edges represent functional connections. These connections might not correspond to direct physical pathways [
33]. Functional MRI (fMRI) studies, for instance, show how different brain regions activate together during various tasks, indicating a topological connection even if these regions are not physically close [
34]. Neurons that are spatially distant can be topologically close if they are part of a functional network. Conversely, neurons that are spatially close might not be functionally connected [
35].
The brain's ability to reorganize itself, known as plasticity, often involves changes in topological connections. For example, if one part of the brain is damaged, another part may take over its functions, even if it's not spatially close [
36]. Understanding both spatial and topological proximities is crucial in studying brain diseases. For instance, in neurodegenerative diseases, certain functional networks might be disrupted, affecting topological proximity, even before any spatial (anatomical) changes are detectable [
37].
4.1 Research and Applications
Connectomics: This field aims to map the comprehensive diagram of neural connections in the brain, both spatially and topologically [
38].
Computational Neuroscience: Models and simulations often try to replicate both spatial and topological aspects of brain networks to better understand brain function and pathology [
39]. Insights into these proximities are crucial for neurosurgical planning, understanding psychiatric disorders, and developing targeted therapies [
40].
4.2 Clinical Aspects
Autism Spectrum Disorder (ASD)
ASD is a complex neurodevelopmental disorder characterized by challenges in social interaction, communication, and restricted or repetitive behaviors. The neurological basis of ASD is not fully understood, but it involves atypical brain development and connectivity [
41].
Altered Brain Connectivity: Research in ASD shows atypical connectivity patterns in the brain. These can be both hypo-connectivity (reduced connectivity) and hyper-connectivity (increased connectivity) in different brain regions [
42].
Neuroimaging studies have found structural differences in the brains of individuals with ASD. This includes variations in the size and organization of certain brain regions, which could affect the spatial proximity of neural connections [
43].
Functional MRI studies have revealed that individuals with ASD often show different patterns of functional connectivity compared to neurotypical individuals. This suggests alterations in topological proximity, where certain brain regions may not interact typically, despite being spatially close or distant [
44].
4.3 Implications for ASD Characteristics
Social and Communication Challenges: The altered topological connectivity in brain regions involved in social processing and communication might contribute to the difficulties individuals with ASD face in these areas [
45].
Changes in both spatial and topological proximities could influence the neural circuits that control behavior and interests, potentially leading to the repetitive behaviors and restricted interests characteristic of ASD [
46].
Early Brain Development
The development of these atypical connectivity patterns in ASD likely begins very early in brain development. This early divergence could affect how both spatial and topological networks are formed [
47].
Critical Periods: There are critical periods in neurodevelopment when the brain is particularly sensitive to forming connections. Disruptions in these periods could lead to the atypical connectivity patterns seen in ASD [
48].
Research Implications
Contrary to popular belief, understanding how spatial and topological connectivity is altered in ASD can help in developing targeted interventions, such as behavioral therapies or neurofeedback, aimed at enhancing functional connectivity [
49].
4.4 Personalized Approaches
Given the variability in ASD (hence the term "spectrum"), individual differences in brain connectivity patterns could inform more personalized treatment approaches [
50]. The neurodevelopmental patterns of ASD are closely related to alterations in both spatial and topological proximities in the brain. These alterations likely contribute to the core symptoms of ASD and offer important insights for research and treatment. Understanding these patterns in more detail is a key focus of ongoing research in the field of autism [
51].
4.5 The Role of Neuroleptics in Modulating Circuit Reverberations
The use of neuroleptics in individuals with ASD, particularly for managing symptoms like irritability, aggression, and self-injurious behaviors, may be linked to their potential impact on reverberating neural circuits. Neuroleptics work by modulating neurotransmitter systems in the brain, especially dopamine and serotonin, which play key roles in regulating mood, behavior, and cognitive functions [52, 53]. By altering the activity of these neurotransmitter systems, neuroleptics can help reduce the overstimulation or hyperactivity of certain neural circuits, potentially providing a sense of calm and reducing the compulsion for repetitive behaviors.
Neuroleptics primarily work by modulating neurotransmitter systems in the brain, especially dopamine and serotonin. These neurotransmitters play key roles in regulating mood, behavior, and cognitive functions. By altering the activity of these neurotransmitter systems, neuroleptics can help reduce the overstimulation or hyperactivity of certain neural circuits. This can lead to a decrease in the intensity and frequency of repetitive behaviors or overwhelming sensory experiences [
54].
The action of neuroleptics might help in 'dampening' the reverberating circuits in the brain. By slowing down the excessive and repetitive firing of neurons, these medications could provide a sense of calm and reduce the compulsion for repetitive behaviors [
55].
While neuroleptics may offer relief from certain symptoms in ASD, their use must be carefully considered due to the potential for significant side effects and individual response variability [56, 57]. The understanding of how neuroleptics affect neural circuits in ASD is an area of ongoing research, crucial for better understanding their efficacy and mechanism of action in the context of ASD [
58].
The study of circuit reverberations in ASD provides valuable insights into the neural basis of repetitive behaviors and hyper-specialized abilities observed in the disorder. Understanding these neural patterns is crucial for developing effective interventions and supports for individuals with ASD, recognizing the wide spectrum of how autism manifests in different individuals [
59].
4.6 Comparison and Implications
Circuit Reverberations and Hyper-Specialized Abilities
Focused Neural Activation: In some individuals with ASD, circuit reverberations might lead to highly focused and sustained activation of circuits involved in specific cognitive or perceptual tasks. This can sometimes result in exceptional abilities or talents, often referred to as "savant" skills [
60].
4.7 Neuroplasticity and Skill Development:
The brain's plasticity, or its ability to change and adapt, means that repeated activation of certain circuits can lead to the strengthening of these pathways, further enhancing specific skills or abilities [61].
Behavioral and Cognitive Therapies: Interventions might focus on managing or redirecting repetitive behaviors. For those with hyper-specialized abilities, interventions might aim to nurture these talents while also supporting areas of challenge [62].
Neurofeedback and Modulation: Emerging therapies like neurofeedback aim to alter neural activation patterns, which could potentially help in modulating circuit reverberations and their effects [63].
5. Conclusion
In summary, the neurodevelopmental patterns of ASD are intricately linked to alterations in both spatial and topological proximities in the brain. These alterations are crucial to understanding the core symptoms of ASD and are a key focus of ongoing research in the field. Advances in neuroimaging and computational modeling continue to enhance our understanding of these complex relationships, paving the way for more effective interventions and treatments [64].
Circuit reverberations in the context of autism spectrum disorder (ASD) refer to the persistent and repetitive activation of neural circuits. This phenomenon can be linked to characteristic behaviors observed in individuals with ASD, including repetitive behaviors and, in some cases, the development of hyper-specialized skills or abilities. To understand this, it's important to delve into the neural mechanisms that might underlie these features [65].
Sensory Processing in Individuals with ASD often is atypical. Circuit reverberations in sensory areas of the brain might contribute to either hypersensitivity or hyposensitivity to sensory stimuli, which can also lead to repetitive behaviors as a form of self-regulation or response to sensory overload [66].
In summary, circuit reverberations in the brain are a potential neural mechanism underlying certain behaviors in autism, including repetitive actions and, in some cases, the development of hyper-specialized abilities. Understanding these neural patterns is crucial for developing effective interventions and supports for individuals with ASD, recognizing the wide spectrum of how autism manifests in different individuals [67].
6. Attachment
Python Code for Graph 1
import matplotlib.pyplot as plt
import networkx as nx
import random
import numpy as np
# Number of nodes
num_nodes = 20
# Generate random 3D positions for each node
positions = {i: (random.uniform(0, 10), random.uniform(0, 10), random.uniform(0, 10)) for i in range(num_nodes)}
# Create a 3D plot for spatial proximity
fig = plt.figure(figsize=(10, 5))
ax = fig.add_subplot(121, projection='3d')
ax.set_title("Spatial Proximity")
# Plot nodes
for node, pos in positions.items():
ax.scatter(*pos, marker='o')
ax.text(*pos, str(node), size=10, zorder=1)
# Create a graph for topological proximity
G = nx.Graph()
G.add_nodes_from(range(num_nodes))
# Add random edges to create topological proximity
for _ in range(num_nodes * 2): # Adjust the multiplier for more or fewer edges
G.add_edge(random.randint(0, num_nodes-1), random.randint(0, num_nodes-1))
# Draw the topological network
ax2 = fig.add_subplot(122)
ax2.set_title("Topological Proximity")
nx.draw(G, ax=ax2, with_labels=True, node_color='lightblue', node_size=500)
plt.show()
Python Code for Graph 2.
import matplotlib.pyplot as plt
import networkx as nx
import random
import numpy as np
# Number of nodes
num_nodes = 20
# Generate more pronounced clustered 3D positions for spatial proximity
positions = {}
for i in range(num_nodes):
cluster_center = np.random.choice([2, 5, 8]) # More cluster centers
positions[i] = (np.random.normal(cluster_center, 0.5), # Reduced variance for tighter clustering
np.random.normal(cluster_center, 0.5),
np.random.normal(cluster_center, 0.5))
# Create a 3D plot for spatial proximity
fig = plt.figure(figsize=(12, 6))
ax = fig.add_subplot(121, projection='3d')
ax.set_title("Enhanced Spatial Clustering in ASD")
# Plot nodes with enhanced clustering
for node, pos in positions.items():
ax.scatter(*pos, marker='o')
ax.text(*pos, str(node), size=10, zorder=1)
# Create a graph for topological proximity
G = nx.Graph()
G.add_nodes_from(range(num_nodes))
# Add edges to simulate enhanced ASD-like topological proximity
for node in G.nodes():
if random.random() < 0.7: # Increased chance of dense clustering
for _ in range(5): # More connections for denser clusters
G.add_edge(node, random.randint(0, num_nodes-1))
else:
# Sparse connections
G.add_edge(node, random.randint(0, num_nodes-1))
# Draw the topological network
ax2 = fig.add_subplot(122)
ax2.set_title("Enhanced Topological Clustering in ASD")
nx.draw(G, ax=ax2, with_labels=True, node_color='lightblue', node_size=500)
plt.show()
Conflicts of Interest
The author has no conflict of interests.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
- Uddin, L. Q. (2015). Idiosyncratic connectivity in autism: developmental and anatomical considerations. Trends in Neurosciences, 38(5), 261-263. [CrossRef]
- Hull, J. V., Jacokes, Z. J., Torgerson, C. M., Irimia, A., & Van Horn, J. D. (2017). Resting-state functional connectivity in autism spectrum disorders: A review. Frontiers in Psychiatry, 7, 205. [CrossRef]
- Hazlett, H. C., Poe, M. D., Gerig, G., Styner, M., Chappell, C., Smith, R. G., ... & Piven, J. (2011). Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Archives of General Psychiatry, 68(5), 467-476. [CrossRef]
- Geschwind, D. H., & Levitt, P. (2007). Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology, 17(1), 103-111. [CrossRef]
- Freitag, C. M., Luders, E., Hulst, H. E., Narr, K. L., Thompson, P. M., Toga, A. W., ... & Konrad, C. (2009). Total brain volume and corpus callosum size in medication-naïve adolescents and young adults with autism spectrum disorder. Biological Psychiatry, 66(4), 316-319. [CrossRef]
- Nomi, J. S., & Uddin, L. Q. (2015). Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia, 71, 201-216. [CrossRef]
- Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059-1069. [CrossRef]
- Van den Heuvel, M. P., & Sporns, O. (2011). Rich-club organization of the human connectome. Journal of Neuroscience, 31(44), 15775-15786. [CrossRef]
- Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186-198. [CrossRef]
- Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483-506. [CrossRef]
- Uhlhaas, P. J., & Singer, W. (2006). Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron, 52(1), 155-168. [CrossRef]
- Belmonte, M. K., & Bourgeron, T. (2006). Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience, 9(10), 1221-1225. [CrossRef]
- Di Martino, A., Fair, D. A., Kelly, C., Satterthwaite, T. D., Castellanos, F. X., Thomason, M. E., ... & Milham, M. P. (2014). Unraveling the miswired connectome: A developmental perspective. Neuron, 83(6), 1335-1353. [CrossRef]
- McDougal, R. A., Bulanova, A. S., & Lytton, W. W. (2016). Reproducibility in computational neuroscience models and simulations. IEEE Transactions on Bio-medical Engineering, 63(10), 2021-2035. [CrossRef]
- Sporns, O., & Betzel, R. F. (2016). Modular brain networks. Annual Review of Psychology, 67, 613-640.
- Bassett, D. S., & Bullmore, E. D. (2006). Small-world brain networks. The Neuroscientist, 12(6), 512-523.
- Robinson, P. A., Sarkar, S., Pandejee, G. M., & Henderson, J. A. (2014). Determination of effective brain connectivity from functional connectivity with application to resting state connectivities. Physical Review E, 90(1), 012707. [CrossRef]
- Ecker, C., Bookheimer, S. Y., & Murphy, D. G. (2015). Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. The Lancet Neurology, 14(11), 1121-1134. [CrossRef]
- Keown, C. L., Shih, P., Nair, A., Peterson, N., Mulvey, M. E., & Müller, R. A. (2013). Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Reports, 5(3), 567-572. [CrossRef]
- Supekar, K., Uddin, L. Q., Khouzam, A., Phillips, J., Gaillard, W. D., Kenworthy, L. E., ... & Menon, V. (2013). Brain hyperconnectivity in children with autism and its links to social deficits. Cell Reports, 5(3), 738-747. [CrossRef]
- Green, S. A., Hernandez, L., Tottenham, N., Krasileva, K., Bookheimer, S. Y., & Dapretto, M. (2015). Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry, 72(8), 778-786. [CrossRef]
- Hull, J. V., Jacokes, Z. J., Torgerson, C. M., Irimia, A., & Van Horn, J. D. (2017). Resting-state functional connectivity in autism spectrum disorders: A review. Frontiers in Psychiatry, 7, 205. [CrossRef]
- Just, M. A., Keller, T. A., Malave, V. L., Kana, R. K., & Varma, S. (2012). Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews, 36(4), 1292-1313. [CrossRef]
- Di Martino, A., Kelly, C., Grzadzinski, R., Zuo, X. N., Mennes, M., Mairena, M. A., ... & Milham, M. P. (2011). Aberrant striatal functional connectivity in children with autism. Biological Psychiatry, 69(9), 847-856. [CrossRef]
- Lai, M. C., Lombardo, M. V., & Baron-Cohen, S. (2014). Autism. The Lancet, 383(9920), 896-910.
- Hernandez, L. M., Rudie, J. D., Green, S. A., Bookheimer, S., & Dapretto, M. (2015). Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology, 40(1), 171-189. [CrossRef]
- Courchesne, E., Campbell, K., & Solso, S. (2011). Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Research, 1380, 138-145. [CrossRef]
- Uddin, L. Q., Dajani, D. R., Voorhies, W., Bednarz, H., & Kana, R. K. (2017). Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Translational Psychiatry, 7(8), e1218. [CrossRef]
- Hunter, J. D. (2007). Matplotlib: A 2D graphics environment. Computing in Science & Engineering, 9(3), 90-95. [CrossRef]
- Bullmore, E., & Sporns, O. (2012). The economy of brain network organization. Nature Reviews Neuroscience, 13(5), 336-349. [CrossRef]
- Bassett, D. S., & Sporns, O. (2017). Network neuroscience. Nature Neuroscience, 20(3), 353-364.
- Fornito, A., Zalesky, A., & Breakspear, M. (2013). Graph analysis of the human connectome: Promise, progress, and pitfalls. NeuroImage, 80, 426-444. [CrossRef]
- van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519-534. [CrossRef]
- Sporns, O. (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23(2), 162-171. [CrossRef]
- Cramer, S. C., Sur, M., Dobkin, B. H., O'Brien, C., Sanger, T. D., Trojanowski, J. Q., ... & Vinogradov, S. (2011). Harnessing neuroplasticity for clinical applications. Brain, 134(6), 1591-1609. [CrossRef]
- Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L., & Greicius, M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62(1), 42-52. [CrossRef]
- Sporns, O., Tononi, G., & Kötter, R. (2005). The human connectome: A structural description of the human brain. PLoS Computational Biology, 1(4), e42. [CrossRef]
- Markram, H. (2006). The blue brain project. Nature Reviews Neuroscience, 7(2), 153-160.
- Deco, G., & Kringelbach, M. L. (2014). Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron, 84(5), 892-905. [CrossRef]
- Geschwind, D. H., & Levitt, P. (2007). Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology, 17(1), 103-111. [CrossRef]
- Just, M. A., Cherkassky, V. L., Keller, T. A., & Minshew, N. J. (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain, 127(8), 1811-1821. [CrossRef]
- Amaral, D. G., Schumann, C. M., & Nordahl, C. W. (2008). Neuroanatomy of autism. Trends in Neurosciences, 31(3), 137-145.
- Vissers, M. E., Cohen, M. X., & Geurts, H. M. (2012). Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience & Biobehavioral Reviews, 36(1), 604-625. [CrossRef]
- Kennedy, D. P., & Adolphs, R. (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16(11), 559-572. [CrossRef]
- Langen, M., Durston, S., Kas, M. J., van Engeland, H., & Staal, W. G. (2011). The neurobiology of repetitive behavior: ... and men. Neuroscience & Biobehavioral Reviews, 35(3), 356-365. [CrossRef]
- Courchesne, E., Campbell, K., & Solso, S. (2011). Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Research, 1380, 138-145. [CrossRef]
- LeBlanc, J. J., & Fagiolini, M. (2011). Autism: A "critical period" disorder?. Neural Plasticity, 2011.
- Wass, S. (2011). Distortions and disconnections: Disrupted brain connectivity in autism. Brain and Cognition, 75(1), 18-28. [CrossRef]
- Voineagu, I., & Yoo, H. J. (2013). Current progress and challenges in the search for autism biomarkers. Disease Markers, 35(1), 55-65. [CrossRef]
- Geschwind, D. H., & State, M. W. (2015). Gene hunting in autism spectrum disorder: on the path to precision medicine. The Lancet Neurology, 14(11), 1109-1120. [CrossRef]
- Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage, 52(3), 1059-1069. [CrossRef]
- Bassett, D. S., & Bullmore, E. D. (2006). Small-world brain networks. The Neuroscientist, 12(6), 512-523.
- Masi, A., DeMayo, M. M., Glozier, N., & Guastella, A. J. (2017). An overview of autism spectrum disorder, heterogeneity and treatment options. Neuroscience Bulletin, 33(2), 183-193. [CrossRef]
- Foss-Feig, J. H., Adkinson, B. D., Ji, J. L., Yang, G., Srihari, V. H., McPartland, J. C., ... & Anticevic, A. (2017). Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biological Psychiatry, 81(10), 848-861. [CrossRef]
- McCracken, J. T., McGough, J., Shah, B., Cronin, P., Hong, D., Aman, M. G., ... & McDougle, C. J. (2002). Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine, 347(5), 314-321. [CrossRef]
- McDougle, C. J., Scahill, L., Aman, M. G., McCracken, J. T., Tierney, E., Davies, M., ... & Vitiello, B. (2005). Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. American Journal of Psychiatry, 162(6), 1142-1148. [CrossRef]
- Maximo, J. O., Cadena, E. J., & Kana, R. K. (2014). The implications of brain connectivity in the neuropsychology of autism. Neuropsychology Review, 24(1), 16-31. [CrossRef]
- Happé, F., & Frith, U. (2006). The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36(1), 5-25. [CrossRef]
- Mottron, L., Dawson, M., Soulières, I., Hubert, B., & Burack, J. (2006). Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).