Introduction
Intestinal Ischemia is a sudden decline in blood flow through the mesenteric vessels[
1]and based on symptoms it can be stratified into acute, chronic, and acute-on-chronic forms[
2]
Acute Intestinal Ischemia It is a rare abdominal emergency that accounts for 1%-2% of acute gastrointestinal disease [
3,
4,
5], although it carries a high mortality rate due to non-specific presentation and rapid progression of the disease[
1], and it has been reported in up to 90% of cases if the diagnosis is delayed and transmural bowel wall necrosis occurs[
6,
7].Acute Intestinal Ischemia may be caused by arterial or venous occlusion, non-occlusive disease and bowel strangulation[
8].
Clinical presentation and laboratory tests are usually nonspecific [
9]. Patients complain of acute abdominal pain out of proportion to otherwise benign findings on physical examination[
2]. Laboratory parameters, such as l-lactate, leukocytosis, and D-dimer, are often elevated only in advanced disease, suggesting the development of an acute abdominal surgical disease [
10]. New intestinal biomarkers have been proposed such as ischemia modified albumin (IMA), intestinal faty acid binding protein (I-FABP), D lactate i L citrulin although their use in clinical practice is still not diffuse [
10,
11,
12].
Sudden onset of acute abdominal pain and the need for morphine are considered suggestive of AMI, in these patients a prompt multiphasic CT should be performed[
13].
Contrast-enhanced MultiDetector CT Angiography (MDCTA) is the imaging for the diagnosis of intestinal ischemia: MDCTA can detect vascular occlusion of mesenteric arteries or veins and demonstrate downstream bowel wall injury. Moreover, MDCTA allows differential diagnosis between intestinal ischemia subtypes, which is mandatory to guide the correct management [
14]. Therapeutic management requires a multidisciplinary approach of general or abdominal surgeons, vascular surgeons, interventional radiologists, and intensivists, according to the recommendations of WSES (World Society of Emergency Surgery)[
10]. In 2016, the first Intestinal
Stroke Center (Structure d'urgences vasculaires intestinales [SURVI]) was opened in France [
13,
15,
16] with improved survival of patients who underwent multidisciplinary and combined treatment based on the presence of reversible or irreversible lesions. Identification of the subtype of intestinal ischemia is mandatory to achieve the most appropriate management. In intestinal ischemia the bowel involvement is characterized by a three-stage disease progression: at stage I the disease is reversible and pathologically characterized by necrosis, erosion, ulceration, edema, and hemorrhage localized to the mucosa. In stage II, necrosis extends into the submucosal and muscularis propria layers. In stage III, transmural necrosis involves all three layers [
17,
18].
In this scenario, MDCTA findings are fundamental to discriminating reversible ischemia from irreversible transmural necrosis[
19]. While arterial occlusion patterns (atherosclerotic, embolic and superior mesenteric artery dissection) can take advantage of endovascular treatment, venous and non-occlusive ischemia require medical treatment in early phases. Surgery, resections without anastomosis (Damage control surgery), or revascularization, are recommended when transmural necrosis and/or peritonitis occur to remove necrotic bowel loops and to avoid life-threatening systemic complications such as sepsis, disseminated intravascular coagulation, and multi-organ failure [
10,
16,
20].
MDCTA diagnosis requires specific CTA protocol, knowledge of arterial and venous bowel supply and relative ischemic pattern, and confidence with diagnostic pitfalls that may be encountered. Accurate analysis of CT findings supports clinicians, interventional radiologists, and surgeons in staging the disease in reversible and irreversible forms.
MDCTA Technical Consideration
MDCTA is the best imaging modality in the diagnosis of acute mesenteric ischemia with a sensitivity of 94% and a specificity of 95%[
9,
18,
21,
22].CT protocol optimal protocol includes a multiphasic examination: non-enhanced acquisition followed by biphasic contrast-enhanced phases (delay of 30 s for the arterial phase and at 60–70 s for the venous phase). Intravenous administration of nonionic iodinated contrast material at a high flow rate at a rapid injection rate of 4–5 mL/sec, followed by a saline solution flush (1.5 mL/kg ) is administered optimizing scanning times with contrast bolus tracking methods [
18,
23]. The delayed scan phase, at 3 minutes from contrast injection, can be considered in a case-by-base evaluation to detect delayed or decreased enhancement. Multiplanar Reconstruction (MPR) and Maximum Intensity Projection (MIP) images are extremely useful in identifying vessel thrombosis and reduced bowel wall enhancement, the reason why the optimal thickness of image acquisition is 1–2 mm[
24]. Oral contrast is not indicated because it obscures the bowel wall that should be accurately examined to assess hypoperfusion.
Non-enhanced CT images are useful to detect spontaneous bowel wall hyperdensity and/or intraluminal content hyperdensity that are suggestive of bowel ischemia [
25,
26], to assess atherosclerotic calcifications[
27]. Biphasic contrast-enhanced CT allows easy detection of arterial and venous filling defects and bowel wall enhancement. The role of DECT is still controversial[
28,
29,
30] Virtual non-enhanced images can be derived from enhanced scans, although radiologists should be confident to correctly identify hyperdense bowel wall and intraluminal content.
The identification of bowel ischemia can be better identified in the conventional 120 kVp-like images [
28]. Instead, the diagnosis based only on the iodine map can negatively influence the sensitivity [
28]: iodine quantification on an iodine map distribution should be added to the primary evaluation of CT images to increase the diagnostic confidence [
28,
31,
32] because the real role of iodine mapping using a semi-quantitative color scale in the diagnosis of bowel ischemia is still controversial and artifacts such as pseudo hyperenhancement caused by air-filled bowel segments, peristalsis related artifacts, and streak artifacts from metal should be taken into to account[
28,
29,
30].
Intestinal Vascular Supply
Three main branches, originating directly from the abdominal aorta supply the gastrointestinal tract: the celiac trunk, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA).
All of them give a rich net of collateral branches: the pancreaticoduodenal artery between the celiac trunk and SMA, through the common hepatic artery; Riolan arc, and Drummond marginal artery between SMA and IMA. Watershed territories among these three districts are at higher risk of ischemia, particularly in hypoafflux conditions (
Figure 1): the ileocaecal junction, the splenic flexure (Griffith's point), and the rectosigmoid junction (Sudeck’s point)[
33].
SMA is the dominant artery involved in acute intestinal ischemia. SMA is anatomically divided into 3 segments (proximal, middle, and distal, based on the origin of the inferior pancreaticoduodenal artery and ileocolic artery). This classification should be taken into account in CT reports because the site of stenosis/occlusion and corresponding ischemic territories are fundamental in determining therapeutic choices.[
34] .
Intestinal venous return is based on superior and inferior mesenteric veins, the first draining directly in the portal vein, and the second draining in the splenic vein. The mesenteric-portal system presents multiple collateral communications with the systemic venous circulation.
Mesenteric Ischemia CT Pattern
Mesenteric Ischemia CT Pattern can be related to vessel, bowel, and extraintestinal findings.
CT Vessels Findings
There are two main categories of arterial acute intestinal ischemia: occlusive and non-occlusive[
9]. Vascular findings precede bowel wall alterations, the occlusive form includes: embolization (40-50%) or thrombosis (25-30%) of the superior mesenteric artery(AMS) and mesenteric venous thrombosis ( 10-18%)[
10].
Arterial intestinal ischemia is a consequence of the sudden reduction of arterial blood supply that overcomes mesenteric vascularization reserves. It is usually determined by critical stenosis or occlusion of SMA. Ethologically, embolic disease accounts for up to 50% of cases [
2,
35], in the remaining patient’s atherosclerosis SMA dissection and vasculitis.
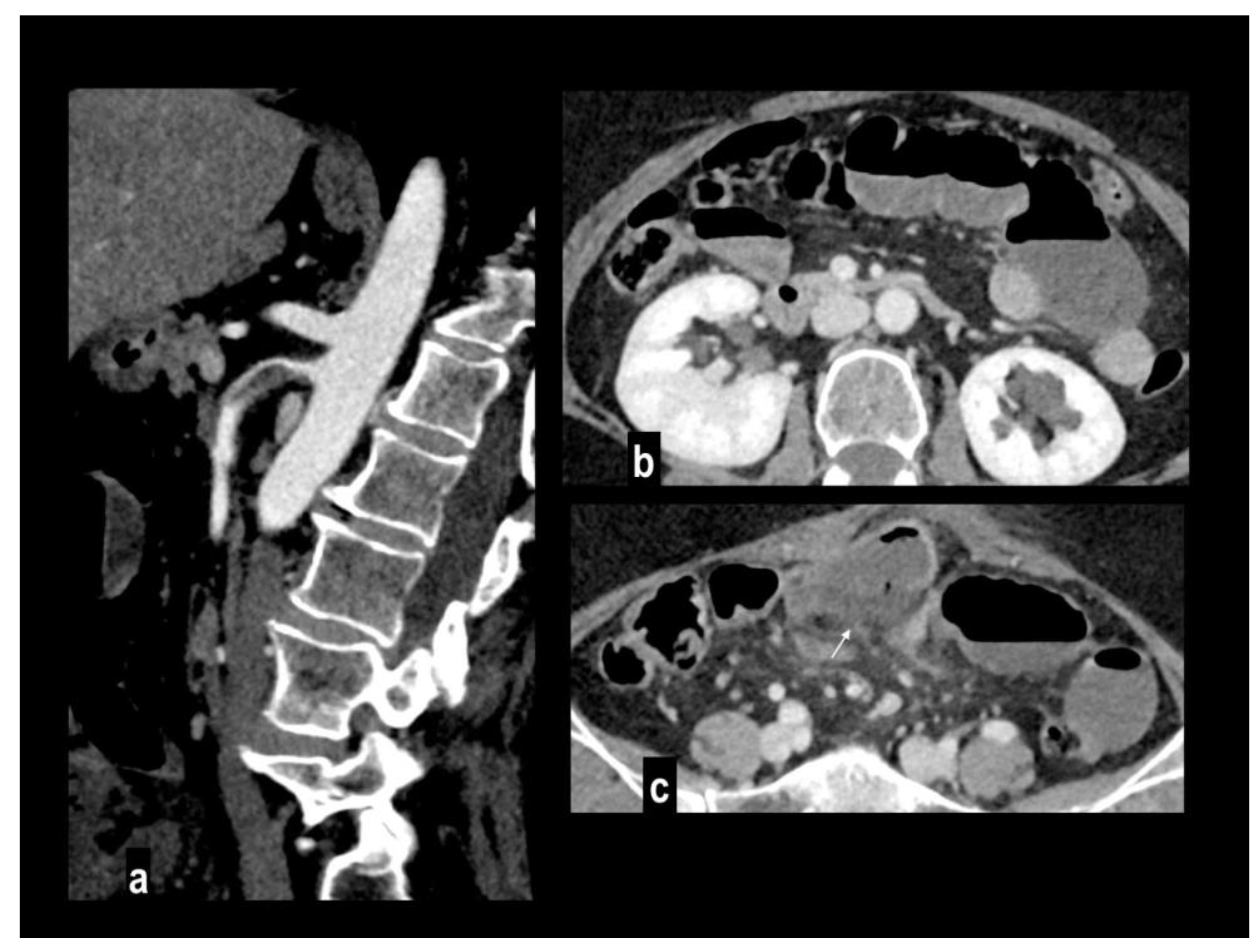
The embolic disease is usually a manifestation of underlying cardiovascular disease [
36] (atrial fibrillation, endocarditis) or, less frequently, from aortic or mesenteric plaques, and it usually involves the high flow SMA due to the narrow take-off angle from the aorta[
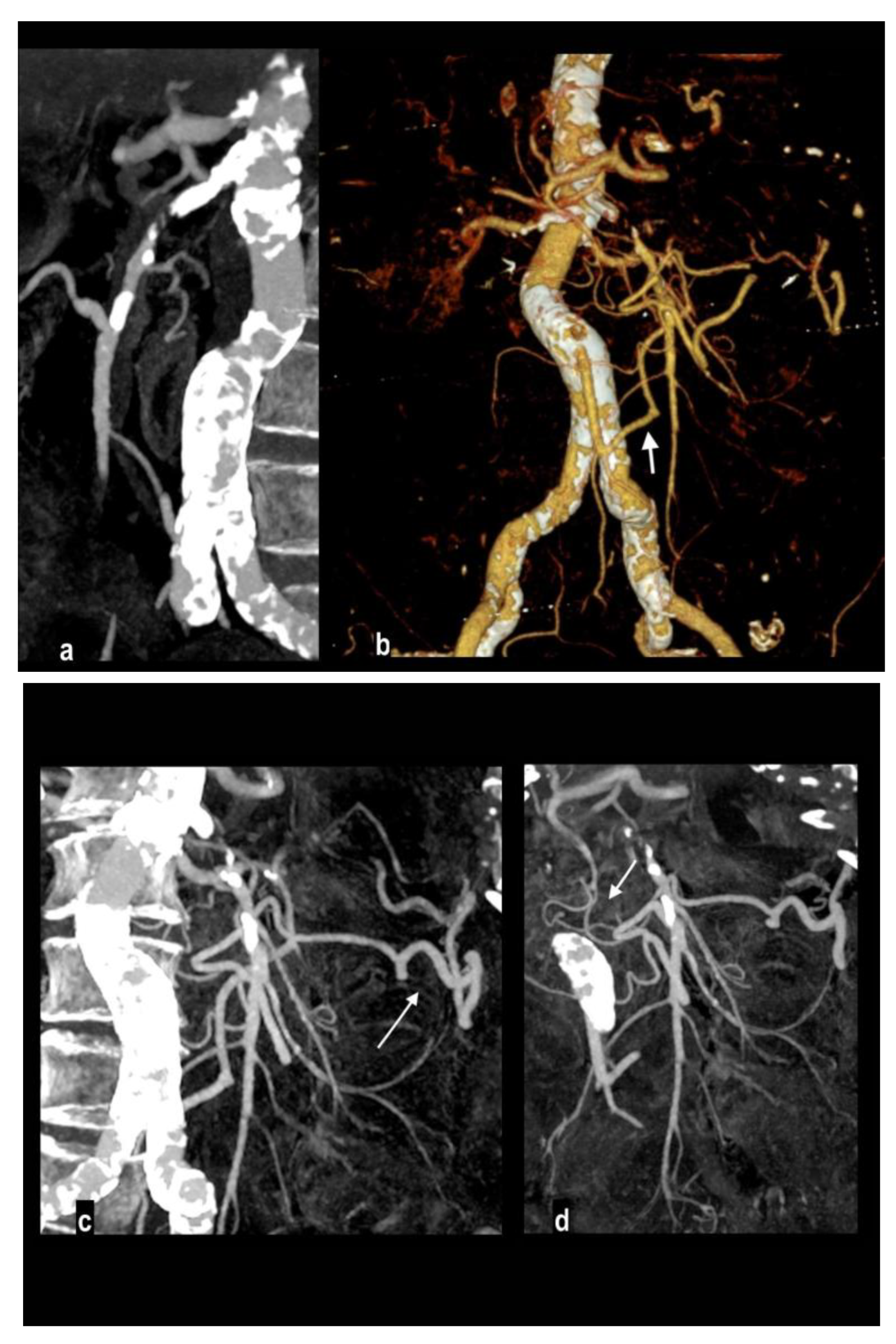
2]. Topography and extension of the involved segment depend on embolus location (
Figure 2): the inflow to the proximal jejunum is preserved if the embolus is located near the takeoff of the middle colic artery with sparing of inferior pancreaticoduodenal branches or almost complete ischemia of the small bowel of the embolus is located close to the SMA orefice[
2,
33,
37,
38].
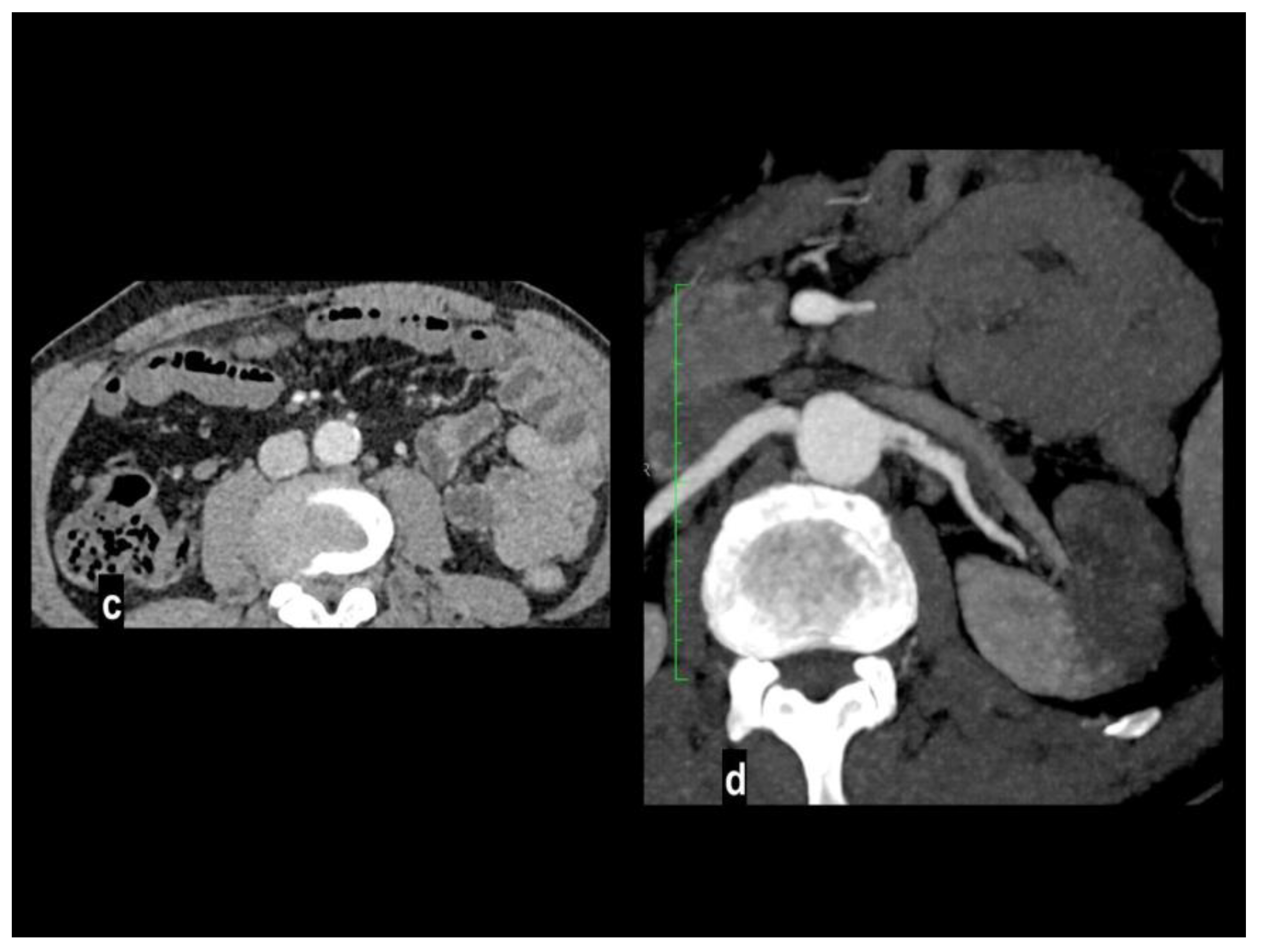
Involved vascular beds are usually healthy and show poor collateralization, so clinical presentation and evolution to transmural necrosis occur earlier, moreover, concurrent emboli can involve other splanchnic arteries, particularly renal and splenic ones, determining parenchymal infarcts (
Figure 3).
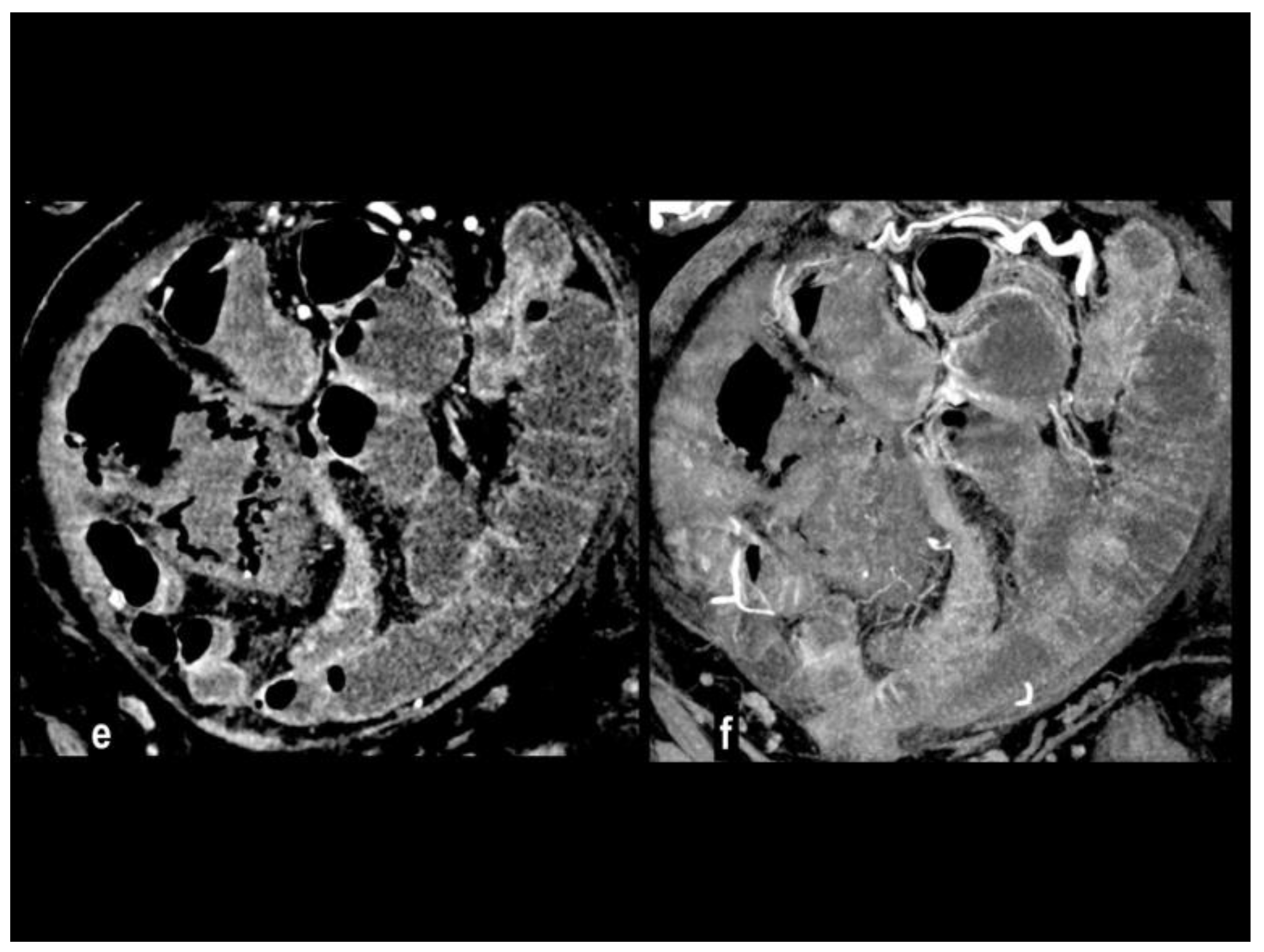
Atherosclerotic steno-occlusion is usually observed in older patients with a history of systemic vasculopathy, and the risk of AMI increases when a critical stenosis involves two of the three major visceral arteries (
Figure 4).
Intestinal ischemia in atherosclerotic disease is frequently associated with a >90% stenosis of the SMA or a >70% stenosis of both celiac artery and SMA [
39]. Because of the long-time progression of atherosclerotic disease, an extensive network of arterial collaterals is recruited. When complete thrombosis occurs it usually involves the ostia determining ischemia of long bowel segment[
38]. Chronic mesenteric ischemia symptoms (postprandial pain, weight loss, etc) can be present in patients’ history before the development of acute ischemia (acute on chronic form). At CT the intra-arterial thromboembolic material can appear hyperdense on unenhanced CT, and high-density
erythrocyte-rich thrombus(>36UH)
have been reported in transmural intestinal necrosis [
40]. After contrast IV administration an abrupt interruption of luminal enhancement is seen in vessel occlusion. The SMV/SMA (SMV superior mesenteric vein) diameter ratio is measured at the level of the upper part of the kidney, a ratio ≥1 is considered normal, indicating the absence of a smaller SMV sign, while a ratio <1 has been reported indicated of acute SMA occlusion[
41,
42]. The sensitivity and specificity of the so-called “smaller SMV sign "have been reported at 70% and 99.2% respectively and it has been assessed in non-contrast images, advocating its utility in case of
unsurpassable contraindications to the use of contrast agents[
41].
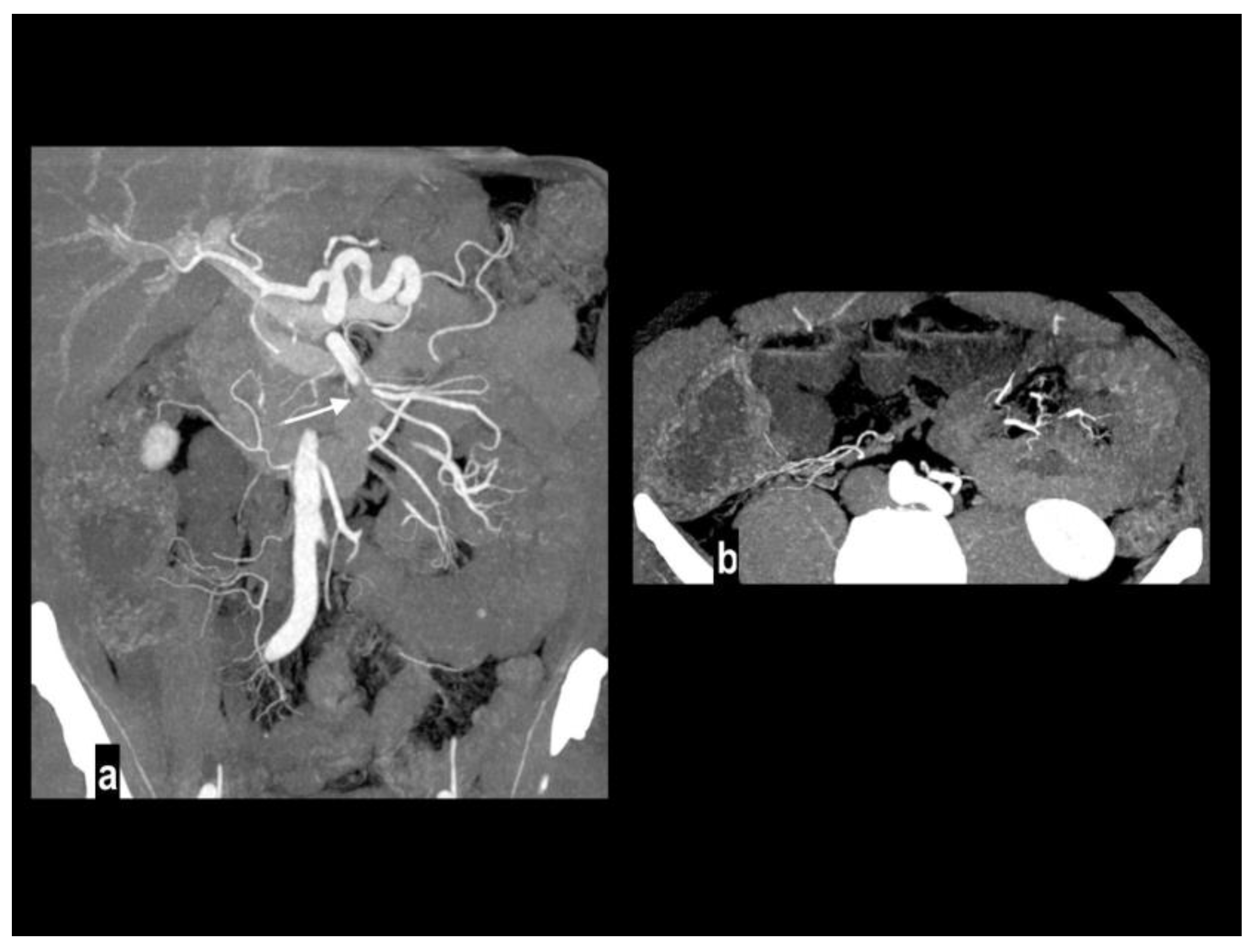
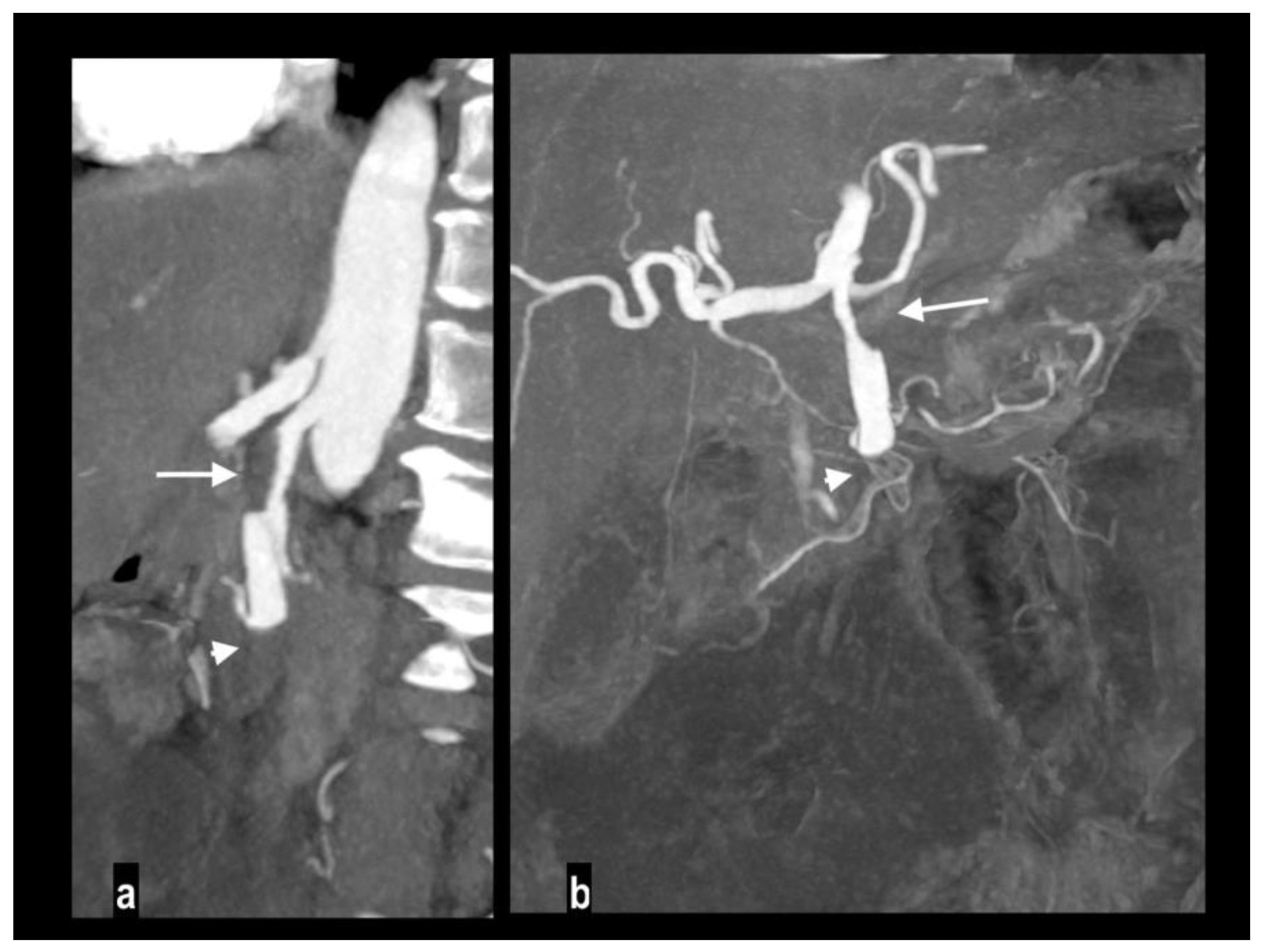
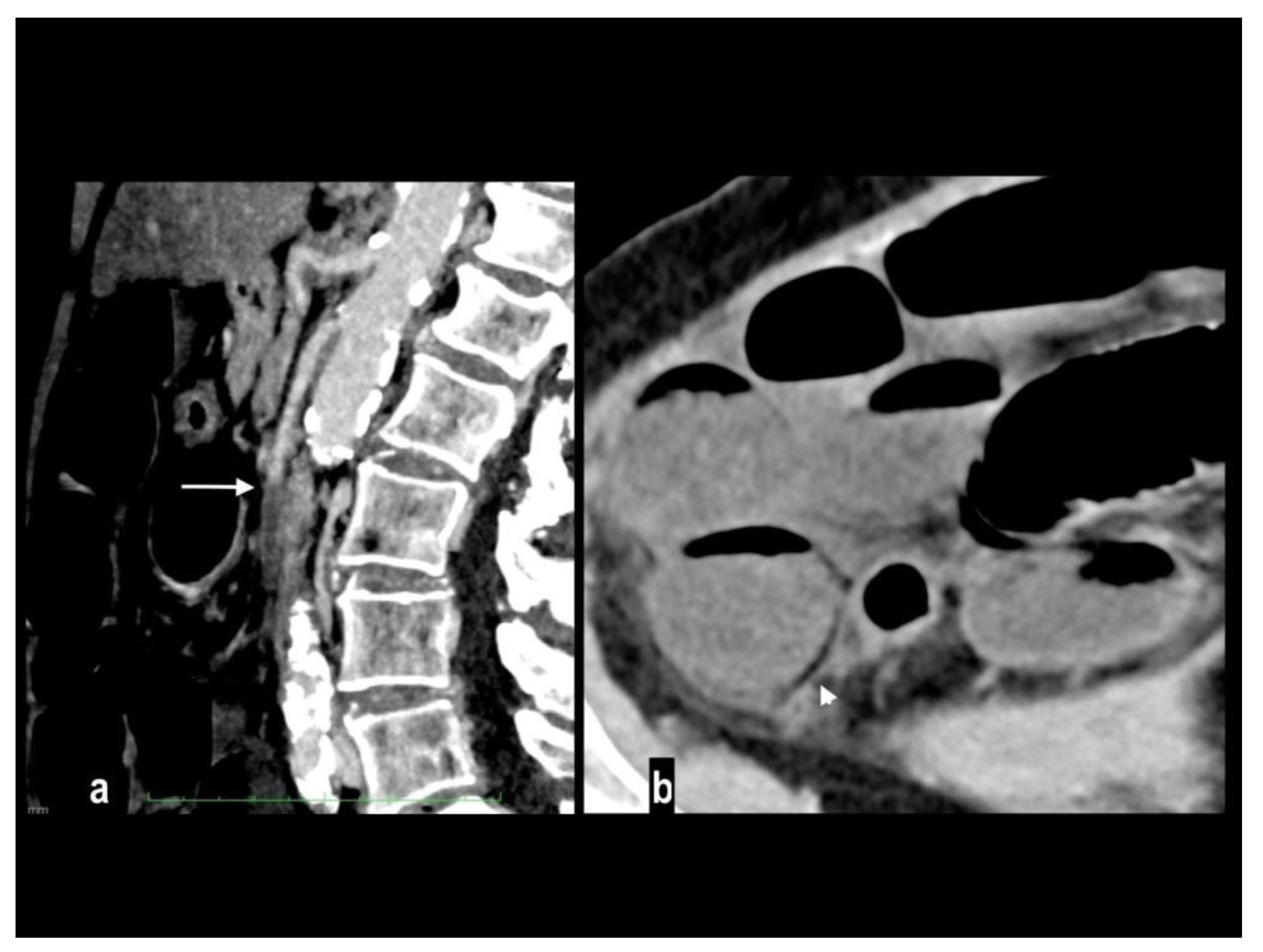
An uncommon cause of arterial thrombosis is SMA dissection, which can occur as a continuation of aortic dissection or in isolation[
43]; spontaneous isolated superior mesenteric artery dissection is defined as superior mesenteric artery (SMA) dissection without the presence of aortic dissection[
44]. SMA spontaneous dissection is a rare cause of mesenteric ischemia (<5%) in males between the fourth and the fifth decade with no particular medical history[
14] and typically affects the convex surface of the SMA trunk, at a distance of 1 to 3 cm away from the root[
45]. Many classifications have been proposed for SMA dissection[
44,
46,
47,
48,
49] to determine the shape, the location, the extent of the false lumen, and whether the false lumen is thrombosed or if the true lumen is stenotic. SMA dissection is responsible for acute pain and may or may not determine intestinal ischemia. Mesenteric ischemia in SMA dissection occurs when critical luminal stenosis or occlusion by false lumen thrombosis determines vessel occlusion and consequent bowel involvement [
50]. CT findings of SMA dissection are focal dilation, intimal flap, intramural hematoma, false lumen thrombosis, increased fat attenuation around the SMA, and mesenteric hematoma (
Figure 5) [
51,
52]. Rarely, large vessels mesenteric arteritis can develop mesenteric ischemia as a devastating complication when parietal inflammation produces critical luminal stenosis or occlusion, presenting acutely and often requiring resection[
53].
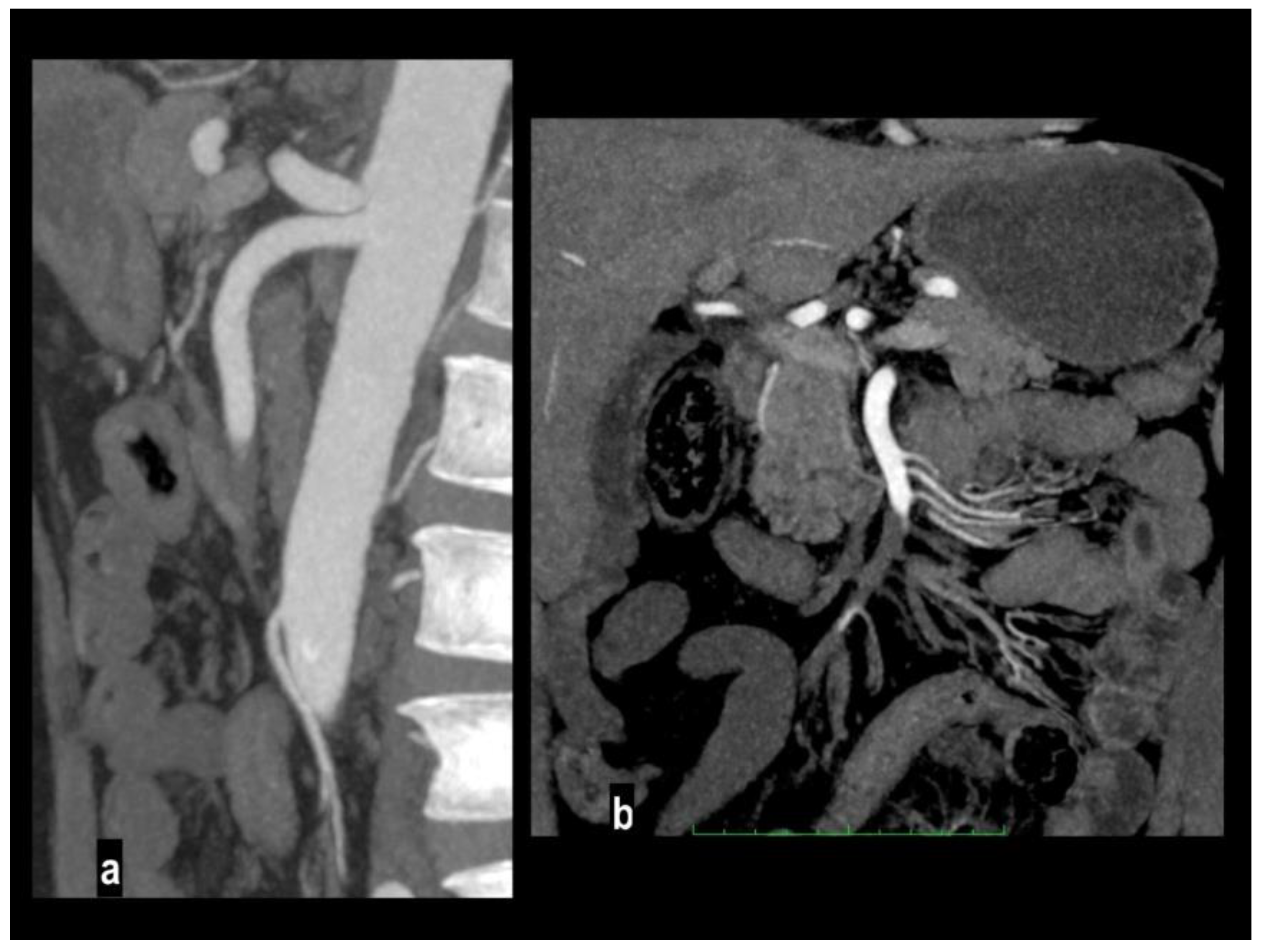
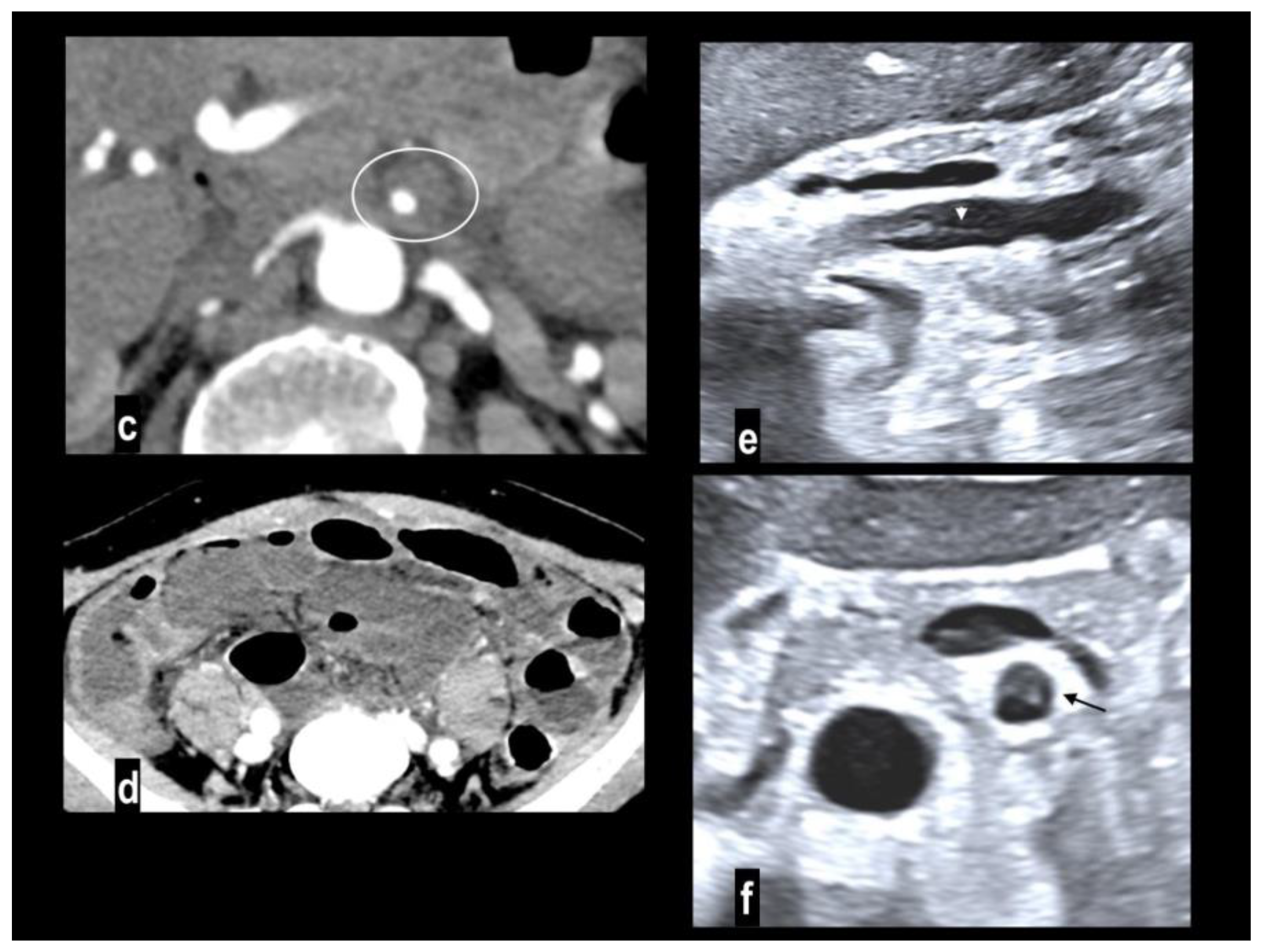
Nonocclusive mesenteric ischemia (NOMI) is an intestinal hypoperfusion in the absence of arterial or venous thromboembolism[
2] accounting for up to 20% of cases of AMI[
9,
54] and atherosclerosis is not considered a risk factor [
55]. The real incidence may be underestimated because this condition occurs in critically ill patients with predisposing conditions such as heart failure, major trauma, the use of vasopressors, and cardiogenic or septic shock (
Figure 6) [
2,
36,
56], NOMI should be considered in the differential diagnosis in these patients because imaging findings may be subtle, and the distribution of affected bowel may be discontinuous or involve multiple vascular territories[
56]. The pathogenetic mechanism is poorly understood it may rely on a protective reflex in which the mesenteric vessels undergo constriction or spasm to preserve blood flow to the cardiac or central nervous systems[
2,
3,
18,
36,
57]. At CT, diagnosis of NOMI can be challenging because vessels are not occluded and may present only subtle luminal narrowing of SMA and its first-order branches. Segmental focal narrowing and dilatation (the so-called "string-of-sausages" sign) can be appreciated along mesenteric vessels. Although large and small bowel can be involved, characteristically the bowel alterations are discontinuous and segmental. Other abdominal signs of the CT hypoperfusion complex can be appreciated such as a small-caliber aorta, a collapsed inferior vena cava, bowel mural hyperenhancement, and hyperenhancement of the kidneys and adrenal glands [
2,
6,
11,
14,
16,
56]. Parenchymal infarcts are often associated with NOMI.
Mesenteric ischemia by venous occlusion is determined by porto-mesenteric vein thrombosis. It is the least common cause of mesenteric ischemia (5-20% of cases) and occurs in younger patients. Hypercoagulability syndromes (antiphospholipid antibody syndrome, protein C and S deficiency, etc ) or states (pregnancy, oral contraceptives); decreased porto-mesenteric blood flow, particularly in chronic liver disease; porto-mesenteric involvement in neoplastic, necrotizing pancreatitis, inflammatory or traumatic concurrent pathologies are the more common risk factors[
58]. Porto-mesenteric thrombosis may occur also for extrinsic compression from tumor encasement[
56]. Primary mesenteric venous thrombosis without an underlying disease has also been described [
59]. The clinical onset of venous thrombosis is sub-acute because ischemia develops gradually and slower than in arterial ischemia. Venous bowel infarct requires extensive involvement of the upstream peripheral arcade of the vasa recta branches. For these reasons, patients complain of subacute long-standing symptoms for about 2-4 weeks and may present nausea and vomiting[
6].
Venous mesenteric thrombi are visualized as filling defects within the venous lumen. The vessel wall can appear as an enhancing rim. The venous outflow is interrupted determining engorgement of mesenteric veins[
23]. Mortality in venoocclusive mesenteric ischemia reaches 44% but is lower than in patients with arterial ischemia. Because intestinal necrosis occurs later due to the underlying pathomechanism, the first therapeutical approach is with anti-coagulation therapy that may improve the clinical picture in 24-48 h. When bowel necrosis occurs, only the damaged bowel loops are resected (
Figure 7) [
9,
54,
60].
Bowel Findings
Immediately after acute arterial obstruction, the bowel shows a spastic reflexus ileus (gasless abdomen in plain X-ray), followed by hypotonia (hypotonic reflexus ileus) and parietal thinning (paper-thin sign)[
38]. At CT, the bowel wall appears thinned (paper thin) with decreased or absent parietal enhancement [
2,
43]. These findings are extremely specific for the diagnosis (up to 97-99%) [
24,
27] but they may be not evident in the early stage of the disease in which accurate analysis of vessels should be carried out (
Figure 8).
The comparison of parietal enhancement between affected and normal bowel in the arterial phase, particularly in segmentary ischemia is extremely useful for the diagnosis [
14].
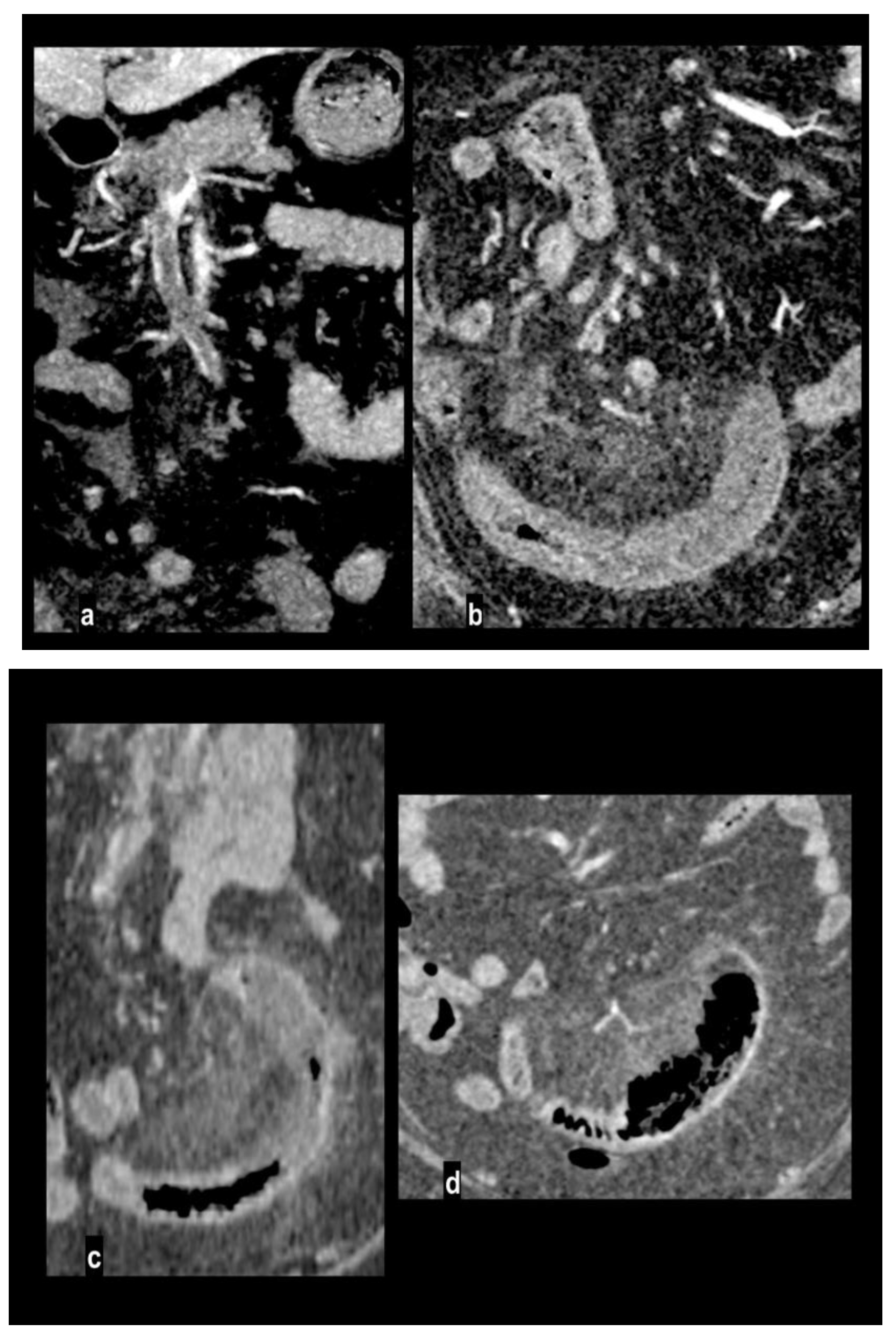
Bowel wall pneumatosis has been reported in only 5% of patients with necrotic bowel at surgery [
61] in advanced mesenteric ischemia, particularly when associated with porto-mesenteric pneumatosis (
Figure 9). Pneumatosis and portomesenteric venous gas are the consequence of transmural necrosis with translocation of luminal gas across the mucosa[
2,
43]. However, bowel pneumatosis is not a specific sign because it has been described also in patients with viable bowel being a generic sign of mucosal integrity loss[
62,
63,
64].
Reperfusion phenomena can be observed peripherally to bowel segments affected by arterial ischemia, thanks to collateralization, or when a partial or complete resolution of arterial obstruction occurs. Reperfusion is characterized by a re-established bowel blood flow after an ischemic injury, with oxidative stress and local inflammation, leading to bowel wall thickening with submucosal edema and mucosal and serosal hyperenhancement. This bull's-eye appearance is similar to venous ischemic bowel wall changes, and similarly, also fat stranding and peritoneal fluid can be observed[
14]. Reperfusion phenomena are not necessarily associated with patients' worsening and can also occur after a successful revascularization[
17,
65].
Oppositely, in venoocclusive ischemia, the bowel walls are thickened with mural stratification due to acute congestion and edema, particularly evident in the submucosal layer[
4,
17]. Mesenteric haziness and stranding and peritoneal fluid are commonly detected due to vascular engorgement; impaired venous outflow with extravasation of fluid into mesentery [
2,
43].
At non-enhanced CT images, spontaneous bowel wall hyperdensity is observed as a consequence of intramural hemorrhagic infarction, while in a late phase decreased enhancement and bowel wall thinning at enhanced phases are indicative of transmural necrosis[
8].
These findings are also observed in NOMI, which is characterized by an association of arterial ischemia and reperfusion phenomena. Because of the segmentary bowel involvement of both small and large bowels, a careful examination should be achieved to identify the segment involved that may correspond to watershed areas between vascular territories[
56].
Conclusions
Acute mesenteric ischemia is a life-threatening condition that requires prompt diagnosis and treatment. MDCTA is mandatory in patients suspected of having mesenteric ischemia because it can confirm the diagnosis and allow a differential diagnosis between different types of intestinal ischemia. MDCTA also depicts several vascular, intestinal, and abdominal signs with prognostic and therapeutic implications. Radiologists should be aware of vessel, bowel, and extraintestinal findings to reach a definitive diagnosis and the knowledge of bowel vascular supply and CT findings is mandatory, especially in the early stage of the disease in which bowel findings could be not evident.
Author Contributions
Conceptualization, F.R., M.D.N, G.B, L.P. G.G, G.P., and Y.Y.; methodology, F.R..; software S.T.; validation, F.R., M.D.N, G.B, L.P. G.G, G.P. Y.Y. and Z.Z.; formal analysis, S.T., P.R, S.M., M.S., F.G.M.; investigation, F.R., M.D.N, G.B, L.P. G.G, G.P..; resources, X.X.; data curation, F.R., T.D.G, ; writing—original draft preparation, F.R, T.D.G, .; writing—review and editing, F.R, T.D.G., ; visualization, S.T., P.R, S.M., M.S., F.G.M. ; supervision, S.T., P.R, S.M., M.S., F.G.M.; project administration, S.T., P.R, S.M., M.S., F.G.M.; funding acquisition,N/A Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
the study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data Available on request.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Monita, M.M.; Gonzalez, L. Acute Mesenteric Ischemia. In: StatPearls. edn. Treasure Island (FL); 2024.
- Olson, M.C.; Bach, C.R.; Wells, M.L.; Andrews, J.C.; Khandelwal, A.; Welle, C.L.; Fidler, J.L. Imaging of Bowel Ischemia: An Update, From the AJR Special Series on Emergency Radiology. AJR Am J Roentgenol 2023, 220, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, W.A.; Lau, L.L.; Rodenberg, T.J.; Edmonds, H.J.; Burger, C.D. Acute mesenteric ischemia: a clinical review. Arch Intern Med 2004, 164, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Cudnik, M.T.; Darbha, S.; Jones, J.; Macedo, J.; Stockton, S.W.; Hiestand, B.C. The Diagnosis of Acute Mesenteric Ischemia: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2013, 20, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, J.F.; Gewertz, B.L. Acute mesenteric ischemia. Surg Clin North Am 1997, 77, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kirkpatrick, I.D.C. An Update on Acute Mesenteric Ischemia. Can. Assoc. Radiol. J. 2022, 74, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Wasnik, A.; Kaza, R.K.; Al-Hawary, M.M.; Liu, P.S.; Platt, J.F. Multidetector CT imaging in mesenteric ischemia—pearls and pitfalls. Emerg. Radiol. 2010, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A.H.; Khameneh, A.G.; Khosravi, B.; Mir, A.; Saffar, H.; Radmard, A.R. Many faces of acute bowel ischemia: overview of radiologic staging. Insights into Imaging 2021, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Catena, F.; Kashuk, J.; De Simone, B.; Gomes, C.A.; Weber, D.; Sartelli, M.; Coccolini, F.; Kluger, Y. ; Abu-Zidan FM et al: Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg 2022, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, Z.; Aleksic, Z. Medical, Surgical and Experimental Approaches to Acute Mesenteric Ischemia and Reperfusion. Mater. Socio Medica 2024, 36, 77–81. [Google Scholar] [CrossRef]
- Treskes, N.; Persoon, A.M.; van Zanten, A.R.H. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern. Emerg. Med. 2017, 12, 821–836. [Google Scholar] [CrossRef]
- Bourcier, S.; Ulmann, G.; Jamme, M.; Savary, G.; Paul, M.; Benghanem, S.; Lavillegrand, J.R.; Schmidt, M.; Luyt, C.E. ; Maury E et al: A multicentric prospective observational study of diagnosis and prognosis features in ICU mesenteric ischemia: the DIAGOMI study. Ann Intensive Care 2022, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Peoc'h, K.; Vaittinada Ayar, P.; Tran-Dinh, A.; Weiss, E.; Panis, Y.; Ronot, M.; Garzelli, L.; Eloy, P. ; Ben Abdallah I et al: Improving clinical suspicion of acute mesenteric ischemia among patients with acute abdomen: a cross-sectional study from an intestinal stroke center. World J Emerg Surg 2023, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Garzelli, L.; Ben Abdallah, I.; Nuzzo, A.; Zappa, M.; Corcos, O.; Dioguardi Burgio, M.; Cazals-Hatem, D.; Rautou, P.E.; Vilgrain, V. ; Calame P et al: Insights into acute mesenteric ischaemia: an up-to-date, evidence-based review from a mesenteric stroke centre unit. Br J Radiol 2023, 96, 20230232. [Google Scholar] [CrossRef]
- Najdawi, M.; Garzelli, L.; Nuzzo, A.; Huguet, A.; Raynaud, L.; Paulatto, L.; Panis, Y.; Ben Abdallah, I.; Castier, Y. ; Sibert A et al: Endovascular revascularization of acute arterial mesenteric ischemia: report of a 3-year experience from an intestinal stroke center unit. Eur Radiol 2022, 32, 5606–5615. [Google Scholar] [CrossRef]
- Nuzzo, A.; Maggiori, L.; Ronot, M.; Becq, A.; Plessier, A.; Gault, N.; Joly, F.; Castier, Y.; Vilgrain, V. ; Paugam C et al: Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol 2017, 112, 597–605. [Google Scholar] [CrossRef]
- Dhatt, H.S.; Behr, S.C.; Miracle, A.; Wang, Z.J.; Yeh, B.M. Radiological Evaluation of Bowel Ischemia. Radiol. Clin. North Am. 2015, 53, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kondo, S.; Narita, K.; Maeda, S.; Fonseca, D.; Honda, Y.; Tani, C.; Fukumoto, W.; Mitani, H. ; Ishibashi M et al: Understanding CT imaging findings based on the underlying pathophysiology in patients with small bowel ischemia. Jpn J Radiol 2023, 41, 353–366. [Google Scholar] [CrossRef]
- Abu-Omar, A.; Murray, N.; Ali, I.T.; Khosa, F.; Barrett, S.; Sheikh, A.; Nicolaou, S.; Tamburrini, S.; Iacobellis, F. ; Sica G et al: Utility of Dual-Energy Computed Tomography in Clinical Conundra. Diagnostics (Basel) 2024, 14(7).
- Tilsed, J.V.; Casamassima, A.; Kurihara, H.; Mariani, D.; Martinez, I.; Pereira, J.; Ponchietti, L.; Shamiyeh, A.; Al-Ayoubi, F. ; Barco LA et al: ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg 2016, 42, 253–270. [Google Scholar] [CrossRef]
- Nakashima, T.; Sagishima, K.; Yamamoto, T. Cervical cord injury complicated by acute mesenteric ischemia. Trauma Case Rep. 2021, 34, 100495. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, M.; Masala, S.; Iacobellis, F.; Tonerini, M.; Sica, G.; Liguori, C.; Saba, L.; Tamburrini, S. Imaging in Non-Traumatic Emergencies. Tomography 2023, 9, 1133–1136. [Google Scholar] [CrossRef]
- Furukawa, A.; Kanasaki, S.; Kono, N.; Wakamiya, M.; Tanaka, T.; Takahashi, M.; Murata, K. CT Diagnosis of Acute Mesenteric Ischemia from Various Causes. Am. J. Roentgenol. 2009, 192, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Yikilmaz, A.; Karahan, O.I.; Senol, S.; Tuna, I.S.; Akyildiz, H.Y. Value of multislice computed tomography in the diagnosis of acute mesenteric ischemia. Eur. J. Radiol. 2011, 80, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Qi, W.J.; Yuan, M.; Wang, H.Y.; Chen, M.; Song, Z.X.; Li, Q.; Li, L.; Jiang, B. ; Ma ZL et al: Increased Attenuation of Intestinal Contents at CT Indicates Bowel Necrosis in Closed-Loop Small Bowel Obstruction. Radiology 2024, 310, e231710. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Qi, W.J.; Yuan, M.; Wang, H.Y.; Chen, M.; Lei, J.A.; Meng, M.; Li, Q.; Li, L. ; Jiang B et al: Prediction of bowel necrosis by reduced bowel wall enhancement in closed-loop small bowel obstruction: Quantitative methods. Eur J Radiol 2024, 173:111363.
- Kirkpatrick, A.W.; McBeth, P.B.; Ball, C.G.; Ejike, J.C.; De Laet, I.E.; Nickerson, D. Mesenteric ischemia, intra-abdominal hypertension, and the abdominal compartment syndrome. Plast Surg (Oakv) 2016, 24, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Ulriksen, P.S.; Jawad, S.; Rohde, Y.Z.; Sejer, M.; Achiam, M.P.; Resch, T.A.; Lonn, L.; Hansen, K.L. Iodine density mapping for the diagnosis of acute bowel ischemia using fast kV-switching dual-energy CT. Abdom Radiol (NY) 2024, 49, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Ulriksen, P.S.; Bjerrum, C.W.; Achiam, M.P.; Resch, T.A.; Lönn, L.; Hansen, K.L. Characterizing incidental mass lesions in abdominal dual-energy CT compared to conventional contrast-enhanced CT. Acta Radiol. 2022, 64, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiographics 2021, 41, E96. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, P.D.M.; Rawski, R.; Mohammed, M.F.; Khosa, F.; Nicolaou, S.; McLaughlin, P. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia. Am. J. Roentgenol. 2018, 211, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Obmann, M.M.; Punjabi, G.; Obmann, V.C.; Boll, D.T.; Heye, T.; Benz, M.R.; Yeh, B.M. Dual-energy CT of acute bowel ischemia. Abdom. Imaging 2021, 47, 1660–1683. [Google Scholar] [CrossRef]
- Navas-Campo, R.; Moreno-Caballero, L.; Ezponda Casajus, A.; Munoz, D.I. Acute mesenteric ischemia: a review of the main imaging techniques and signs. Radiologia (Engl Ed) 2020, 62, 336–348. [Google Scholar] [CrossRef]
- Tual, A.; Garzelli, L.; Nuzzo, A.; Corcos, O.; Castier, Y.; Ben Abdallah, I.; Ronot, M. Strengthening the Description of Superior Mesenteric Artery Occlusions in Acute Mesenteric Ischaemia: Proposition for an Anatomical Classification. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, M.; Koelemay, M.; Acosta, S.; Bastos Goncalves, F.; Kolbel, T.; Kolkman, J.J.; Lees, T.; Lefevre, J.H.; Menyhei, G. ; Oderich G et al: Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017, 53, 460–510. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, S.; Furukawa, A.; Fumoto, K.; Hamanaka, Y.; Ota, S.; Hirose, T.; Inoue, A.; Shirakawa, T.; Nguyen, L.D.H.; Tulyeubai, S. Acute Mesenteric Ischemia: Multidetector CT Findings and Endovascular Management. RadioGraphics 2018, 38, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, J.L. Mesenteric ischemia. Surg Clin North Am 2013, 93, 925–940, ix. [Google Scholar] [CrossRef] [PubMed]
- Garzelli, L.; Nuzzo, A.; Copin, P.; Calame, P.; Corcos, O.; Vilgrain, V.; Ronot, M. Contrast-Enhanced CT for the Diagnosis of Acute Mesenteric Ischemia. Am. J. Roentgenol. 2020, 215, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bordet, M.; Tresson, P.; Huvelle, U.; Long, A.; Passot, G.; Bergoin, C.; Lermusiaux, P.; Millon, A.; Della Schiava, N. Natural History of Asymptomatic Superior Mesenteric Arterial Stenosis Depends on Coeliac and Inferior Mesenteric Artery Status. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, J.; Kuang, L.-Q.; Yi, K.-M.; Li, C.-X.; Wang, Y. Relationship of superior mesenteric artery thrombus density with transmural intestinal necrosis on multidetector computed tomography in acute mesenteric ischemia. Quant. Imaging Med. Surg. 2021, 11, 3120–3132. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ito, T.; Takei, T.; Takemoto, M. Accuracy of the smaller superior mesenteric vein sign for the detection of acute superior mesenteric artery occlusion. Acute Med. Surg. 2018, 5, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S. Superior mesenteric artery thrombosis/embolism. In: A Key to Gastrointestinal Imaging. edn. Edited by Y Y. Tokyo: Gakken Medical; 2015: 162-163.
- Olson, M.C.; Fletcher, J.G.; Nagpal, P.; Froemming, A.T.; Khandelwal, A. Mesenteric ischemia: what the radiologist needs to know. Cardiovasc. Diagn. Ther. 2019, 9, S74–S87. [Google Scholar] [CrossRef]
- Jia, Z.; Tu, J.; Jiang, G. The Classification and Management Strategy of Spontaneous Isolated Superior Mesenteric Artery Dissection. Korean Circ. J. 2017, 47, 425–431. [Google Scholar] [CrossRef]
- Mei, J.; Ding, W.; Yu, H.; Zhao, X.; Xu, H.; Wang, K.; Jia, Z.; Li, B. Different hemodynamic factors cause the occurrence of superior mesenteric atherosclerotic stenosis and superior mesenteric artery dissection. Front. Cardiovasc. Med. 2023, 10. [Google Scholar] [CrossRef]
- Li, D.-L.; He, Y.-Y.; Alkalei, A.M.; Chen, X.-D.; Jin, W.; Li, M.; Zhang, H.-K.; Liang, T.-B. Management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification. J. Vasc. Surg. 2014, 59, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Li, X. Computed Tomography Imaging Features and Classification of Isolated Dissection of the Superior Mesenteric Artery. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, I.; Ogawa, Y.; Sueyoshi, E.; Fukui, K.; Murakami, T.; Uetani, M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur. J. Radiol. 2007, 64, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.-S.; Kim, Y.W.; Park, K.B.; Cho, S.K.; Do, Y.S.; Lee, K.B.; Kim, D.I.; Kim, D.K. Clinical and Angiographic Follow-up of Spontaneous Isolated Superior Mesenteric Artery Dissection. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, I.; Huguet, A.; Nuzzo, A.; Mirault, T.; Roussel, A.; El Batti, S.; Ronot, M.; Castier, Y.; Corcos, O. Acute Isolated Mesenteric Artery Dissection: Four Year Experience From a French Intestinal Stroke Centre. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Eldine, R.N.; Dehaini, H.; Hoballah, J.; Haddad, F. Isolated Superior Mesenteric Artery Dissection: A Novel Etiology and a Review. Ann. Vasc. Dis. 2022, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Furui, S.; Kohtake, H.; Sakamoto, T.; Yamasaki, M.; Furukawa, A.; Murata, K.; Takei, R. Isolated dissection of the superior mesenteric artery: CT findings in six cases. Abdom Imaging 2004, 29, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Krishnan, N.; Gochhait, D.; Emmanuel, D.; Negi, V.S. Takayasu arteritis (TA) first presenting with intestinal ischemia: a case report and review of gastrointestinal tract involvement (ischemic and non-ischemic) associated with TA. Rheumatol. Int. 2016, 37, 169–175. [Google Scholar] [CrossRef]
- Acosta, S. Epidemiology of Mesenteric Vascular Disease: Clinical Implications. Semin. Vasc. Surg. 2010, 23, 4–8. [Google Scholar] [CrossRef]
- Konan, A.; Piton, G.; Ronot, M.; Hassoun, Y.; Winiszewski, H.; Besch, G.; Doussot, A.; Delabrousse, E.; Calame, P. Abdominal atherosclerosis is not a risk factor of nonocclusive mesenteric ischemia among critically ill patients: a propensity matching study. Ann. Intensiv. Care 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A.; Rivers-Bowerman, M.D.; Thipphavong, S.; Clarke, S.E.; Rowe, J.A.; Costa, A.F. Pearls, Pitfalls, and Conditions that Mimic Mesenteric Ischemia at CT. RadioGraphics 2020, 40, 545–561. [Google Scholar] [CrossRef]
- Gnanapandithan, K.; Feuerstadt, P. Review Article: Mesenteric Ischemia. Curr. Gastroenterol. Rep. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Harnik, I.G.; Brandt, L.J. Mesenteric venous thrombosis. Vasc Med 2010, 15, 407–418. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Kim, H.S. Treatment of Acute Superior Mesenteric Vein Thrombosis with Percutaneous Techniques. Am. J. Roentgenol. 2003, 181, 1305–1307. [Google Scholar] [CrossRef]
- Schoots, I.G.; I Koffeman, G.; A Legemate, D.; Levi, M.; van Gulik, T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br. J. Surg. 2003, 91, 17–27. [Google Scholar] [CrossRef]
- Cokkinis, A.J. Observations on the Mesenteric Circulation. . 1930, 64, 200–5. [Google Scholar]
- Milone, M.; Di Minno, M.N.; Musella, M.; Maietta, P.; Iaccarino, V.; Barone, G.; Milone, F. Computed tomography findings of pneumatosis and portomesenteric venous gas in acute bowel ischemia. World J Gastroenterol 2013, 19, 6579–6584. [Google Scholar] [CrossRef]
- Kernagis, L.Y.; Levine, M.S.; Jacobs, J.E. Pneumatosis Intestinalis in Patients with Ischemia: Correlation of CT Findings with Viability of the Bowel. Am. J. Roentgenol. 2003, 180, 733–736. [Google Scholar] [CrossRef]
- Wiesner, W.; Mortele, K.J.; Glickman, J.N.; Ji, H.; Ros, P.R. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol 2001, 177, 1319–1323. [Google Scholar] [CrossRef]
- Garzelli, L.; Nuzzo, A.; Hamon, A.; Ben Abdallah, I.; Gregory, J.; Raynaud, L.; Paulatto, L.; Dioguardi Burgio, M.; Castier, Y. ; Panis Y et al: Reperfusion injury on computed tomography following endovascular revascularization of acute mesenteric ischemia: prevalence, risk factors, and patient outcome. Insights Imaging 2022, 13, 194. [Google Scholar] [CrossRef]
- Sugi, M.D.; Menias, C.O.; Lubner, M.G.; Bhalla, S.; Mellnick, V.M.; Kwon, M.H.; Katz, D.S. CT Findings of Acute Small-Bowel Entities. RadioGraphics 2018, 38, 1352–1369. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).