1. Introduction
Autism Spectrum Disorder (ASD) is a chronic, inflammatory, and systemic disease -5that mainly produces gastrointestinal and neurological symptoms [
1]. It is characterized by impairments in language and social interactions, often accompanied by restricted interests and repetitive behaviors [
2]. Like many modern pathological conditions, ASD is an epigenetically initiated disease [
3,
4,
5]. The rate of ASD in the population has been estimated to be almost one in 40, representing a marked increase in occurrence [
6]. Several factors have been proposed to influence the occurrence of ASD in Western societies [
7,
8,
9,
10,
11,
12]. The foremost concern is that the overuse of antibiotics and/or significant dietary shifts have been detrimental to the long-term relationship between homo sapiens and our symbiotic microbiome [
13,
14,
15,
16]. Indeed, the entire human and microbial genetic structures must be considered within an evolutionary framework [
17,
18]. We have evolved as a single unit, the holobiont [
19]. Modern dietary and hygienic practices may have adversely affected this relationship, resulting in the lack of the “Old Friends” and new pathological relationships with bacterial strains previously not part of the evolved holobiont [
20,
21,
22].
In humans, the gut microbiome plays a vital role in many functions, such as modulation of the immune system, production of vitamins and amino acids, detoxifying harmful chemicals, and breaking dietary fiber into short-chain fatty acids (SCFA). Thus, a shift in SCFA production by the gut microbiome may significantly affect the host. Various refined carbohydrate diets may help patients with ASD by reducing the substrates required for SCFA production. Supplementing foods high in complex fibers may exert a therapeutic response in children by preferentially increasing the output of another SCFA, butyrate, over propionic acid [
23]. SCFAs formed by microbial fermentation have an essential effect on colonic health [
24,
25]. MacFabe et al. research associates published several well-designed studies demonstrating a possible link between propionic acid-producing bacteria and the development of autism [
26]. Propionic acid infusions into adult rat cerebral ventricles produce behaviors associated with ASD [
27] and reversible repetitive dystonic behaviors, hyperactivity, turning behavior, retropulsion, caudate spiking, and progressive development of limbic kindled seizures, coupled with neuroinflammatory, metabolic, and epigenetic changes, suggesting that it has central effects [
28,
29]. MacFabe et al. administered propionic acid subcutaneously and then intraperitoneally and reported similar results [
30,
31]. Human lymphoblastoid cell lines exposed to propionic acid elicit atypical immunological responses [
32,
33]. In contrast, propionic acid has positive health effects in adults, including anti-obesity, anti-inflammatory, and cholesterol-lowering effects [
34]. Calcium propionate is used as a food preservative, although its use is decreasing. A large fast-food restaurant chain recently announced the discontinuation of calcium propionate due to concerns about behavioral changes in children consuming calcium propionate-preserved bread [
35].
Xylitol is a naturally occurring nutritional supplement (classified as an alditol, specifically a 5-carbon pentitol) and can directly replace sucrose sugar. The human diet has always included xylitol from fruits, vegetables, and tubers. As much as 15 grams of xylitol is generated daily as a metabolic intermediate linking critically essential pentose shunt (5-carbon sugars) and glucuronic acid (detoxification) pathways [
36]. Because xylitol is isosweet with sucrose but lower in calories, low-glycemic and low-insulinemic, it can displace equivalent amounts of sugar with notable metabolic benefits for diabetic and prediabetic conditions [
37,
38]. As such, xylitol may be considered a dietary supplement.
Xylitol has been extensively researched and demonstrated to be safe and to have notable anti-cariogenic and anti-periodontal disease properties with appropriate use [
39,
40]. Xylitol has been used (for about 50 years) to replace sugar in sweet foods to block tooth enamel demineralization and in the diabetic diet to reduce postprandial blood glucose and insulin excursions [
37]. The benefits of added dietary xylitol go beyond the mere removal of sugar. Emerging evidence demonstrates that xylitol can play several functional roles in actively supporting oral and systemic health maintenance with anti-biofilm, anti-oxidant, anti-inflammatory, and anti-diabetic effects [
36,
37]. Xylitol efficiently stimulates and modulates the immune system. While excess consumption can have a laxative effect, dental amounts of xylitol are well-tolerated, especially after a brief period of adaptation. Xylitol then supports healthy digestion with increased butyrate production. Xylitol helps control blood sugar and obesity, facilitates wound healing, improves skin condition, reduces bone resorption, increases bone strength, and reduces ear and respiratory infections [
41,
42,
43,
44,
45,
46]. Xylitol also inhibits tumor growth in several studied cancer cell lines [
47,
48,
49].
The gastrointestinal microbiome of 30 autistic 5 to 9-year-old children was quantitated by real-time PCR of stool samples, and the gastrointestinal symptoms were assessed [
50]. After the children were supplemented with probiotics, the stool PCR demonstrated increases in Bifidobacteria and Lactobacilli with significant improvements in the severity of autism and gastrointestinal symptoms compared to their baseline evaluation. A review of 16 articles describing gut microbiome interventions in ASD patients (with antibiotics, prebiotics, probiotics, vitamin supplementation, and fecal microbiota transplantation) demonstrated improvements in behavioral and gastrointestinal symptoms [
51]. Another systematic review of 14 articles [
52] concluded that prebiotics and synbiotics were efficacious in behavioral symptoms with ASD. In a rat model of autism, supplementation with
Bifidobacterium longum or fecal matter transplantation restored the calcium propionate acid-induced dysbiosis in a treatment-specific manner [
53]. In another animal model, with germ-free mice, microbiota transplantation from typically developing children did not induce tryptophan and serotonin metabolism changes. However, fecal microbiota from ASD children did, and subsequently, the mice developed ASD-like behavior [
54]. Finally, a double-blind, randomized crossover clinical trial study showed significant improvements after the administration of probiotics in gastrointestinal symptom scale, maladaptive behaviors, communication skills, and perceived parental stress level [
55].
Deep sequencing of the metatranscriptome has provided significantly greater data than was previously possible. Approximately 30,000 strains are included in the database. Metatranscriptomics, also known as RNA-seq or RNA sequencing, can examine the quantity and sequence of RNA in a sample using next-generation sequencing (NGS). RNA-seq analyzes the transcriptome of gene expression patterns encoded within RNA [
56]. The transcriptome is essential for connecting genomic information with functional protein expression. RNA-seq identifies the genes that are turned on in a cell, their level of expression, and at what time they are activated or turned off [
57].
The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a collection of databases dealing with genomes, biological pathways, diseases, drugs, and chemical substances [
58]. KEGG is utilized for bioinformatics research and education, including data analysis in genomics, metagenomics, metabolomics, and other omics studies; modeling and simulation in systems biology; and translational research in drug development. A Review of the KEGG orthology may reveal differences between control subjects from Colombia or the USA and those diagnosed with A.S.D. Additionally, abnormal metabolites, enzymes, or microbiome shifts may be associated with pathological conditions.
This study aimed to determine the difference in the oral transcriptome and mitochondrial status between children from a “blue zone” in Colombia and children from the USA, both healthy and those diagnosed with ASD. The objectives of this study did not include collecting data on cognitive skills, behavioral observations, or gastric symptoms. The long-term intention was to develop a method of objectively diagnosing autism in infancy with oral salivary sampling, allowing for earlier and more effective interventional therapy.
2. Materials and Methods
Institutional Review Board approvals were obtained for both the USA and the Colombia component of the study. Power analysis was required by the IRB before acceptance of the protocol. A biostatistician provided the recommended number of subjects (individuals) to be recruited. However, a possible limitation in the number of ASD and “Blue Zone” recruits was considered in the design of this study. In addition, the prebiotic and probiotic intervention was not approved for the neurotypical groups as that would require treatment without the presence of pathology. However, there were no foreseeable adverse effects from either treatment intervention. The ASD group included 30 children aged 6–21 who were sampled at three different intervals: before the intervention, post-xylitol, and post-probiotic. Complete health and dietary histories were obtained after obtaining guardian consent (assent if possible). Three samples were collected from the ASD group: one before the intervention, 60 days after xylitol, and 60 days after the probiotic. At the appropriate visit, the parents received the intervention materials. For the xylitol intervention, the parents were provided with xylitol toothpaste Spry ®, xylitol suckers/mints, and xylitol mouth rinse (Xlear Inc. American Fork, UT USA). Due to concerns regarding patient cooperation, the parent was advised to use a xylitol product five times a day. The goal was to “strive for five” exposures as recommended by the manufacturer (five grams daily). For the probiotic intervention, the parents were dispensed ProBiora Pro®, a 90-day supply consisting of 2.5 billion CFU of Streptococcus oralis KJ3®, Streptococcus uberis KJ2®, and Streptococcus rattus JH145®, (ProBiora Health®, LLC Tampa, FL USA). The parents daily gave the probiotic tablet to the ASD child, sometimes by masking it in applesauce. Parents already had strategies for dispensing medications to their children and used what had always worked previously. Parents were to report any difficulties in either intervention to the principal investigator. Any parent unable or unwilling to fully participate was excluded from the study per IRB guidelines.
The sampling consisted of buccal swabs for MITOSWAB (Religen Plymouth Meeting, PA, USA) testing using the manufacturer-supplied materials and saliva samples for metatranscriptomics by VIOME using their supplied materials to determine the entire range of microbial species and their biochemical functions. The Colombian component included 30 children, ages 6-16, considered healthy by the study parameters and within usual behavioral standards, except for one who had received systemic antibiotics for an acute skin infection. Buccal swabbing and saliva sampling were performed only once in this group. The 30 children aged 6-16 in the USA healthy group were matched as well as possible for age and gender. Dietary and health information were also obtained for these two “healthy” groups, as were consents and assents per I.R.B. requirements.
Parent interviews and completion of a pediatric diet dairy achieved diet analysis. This was performed for all USA children but not for the Colombian children, who all ate only locally obtained food. The ASD component all reported some dietary intervention, as that is the standard of care for the community. The majority were placed on a gluten-free diet at an early age by parents/guardians, and all were receiving therapy either in school or local facilities. As is typical with Autism, the children were reluctant or resistant to new food introduction. However, the healthy USA child parents reported daily fresh fruit and vegetable servings. Over 75% of those parents reported they avoided ultra-high processed food and prepared meals fresh. However, parental reporting and the completed diet summary are difficult to substantiate. On the other hand, the majority also reported purchasing organic produce or frequenting local farmer’s markets.
The health history was submitted and approved by the IRB. It was a standard form utilized by many pediatric healthcare professionals. The object of the form was to confirm the diagnosis of autism and to confirm the category of “healthy.” For the purpose of this study, the definition of healthy was a child with no chronic disease. Allergies, or history of prescription medications, were an exclusionary condition. Any medical diagnosis of chronic disease and a history of antibiotics was also exclusionary. Other childhood viral infections, such as the common cold, were not exclusionary. In addition, the child could not have a reported BMI (25-29.9), age-adjusted over 85%.
The ASD group was predominantly male, consisting of 28 males and two females, with an average age of 11. It was not possible to exactly match the neurotypical groups with the ASD group, with the USA neurotypical group comprising 18 males and 12 females, with an average age of 10. In comparison, the “Blue Zone” group consisted of 17 males and 13 females, with an average age of 9.
For the metatranscriptomic analysis, saliva was analyzed using a clinically validated Viome Life Sciences CLIA laboratory test, as previously described (
https://www.future-science.com/doi/10.2144/btn-2022-0104). The lab methods are automated and performed on the King Fisher and Hamilton STAR platforms. Samples were lysed using a combination of chemical and mechanical lysis. Total nucleic acids were extracted, and DNA was degraded using the DNase enzyme. Non-informative RNAs (microbial and human ribosomal RNAs and human hemoglobin/myoglobin transcripts) were physically removed using subtractive hybridization. The remaining RNAs were converted to cDNA, labeled with dual unique barcodes, and sequenced on the Illumina NovaSeq 6000 instrument using 2x150 paired-end chemistry. The Taxa were identified using a clinically validated bioinformatic method that uses a catalog of ~50,000 microbial genomes with gene annotations. KEGG Orthology. Reads mapping to the microbial genes were used to quantify KEGG orthologs (KOs). KEGG (Kyoto Encyclopedia of Genes and Genomes) is a database resource for understanding high-level functions and utilities of the biological system, such as the cell, the organism, and the ecosystem, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies. Salivary samples were analyzed at the VIOME Laboratory (Viome Life Sciences, Inc. Los Alamos, NM USA.)
MITOSWAB sampling was done to analyze mitochondrial health. Mitochondria are organelles that produce energy to power cells. They produce ATP, the cell’s energy currency required for all bodily functions. They also contain DNA, which makes them highly dynamic. Otherwise known as the “power plants” of the body, their proper functioning is critical to overall health and longevity. The MITOSWAB is a noninvasive test that quantitatively and qualitatively analyzes the mitochondrial Electron Transport Chain (ETC) to evaluate mitochondrial dysfunction. It reports on the functions of Complexes I and IV, critical components of the mitochondrial ETC. Citrate synthase activity was measured to quantify the number of mitochondria in each sample. Information regarding mitochondrial function is essential and can assist in developing therapeutic strategies for improving mitochondrial health. The samples were analyzed by Religen Labs (USA).
3. Results
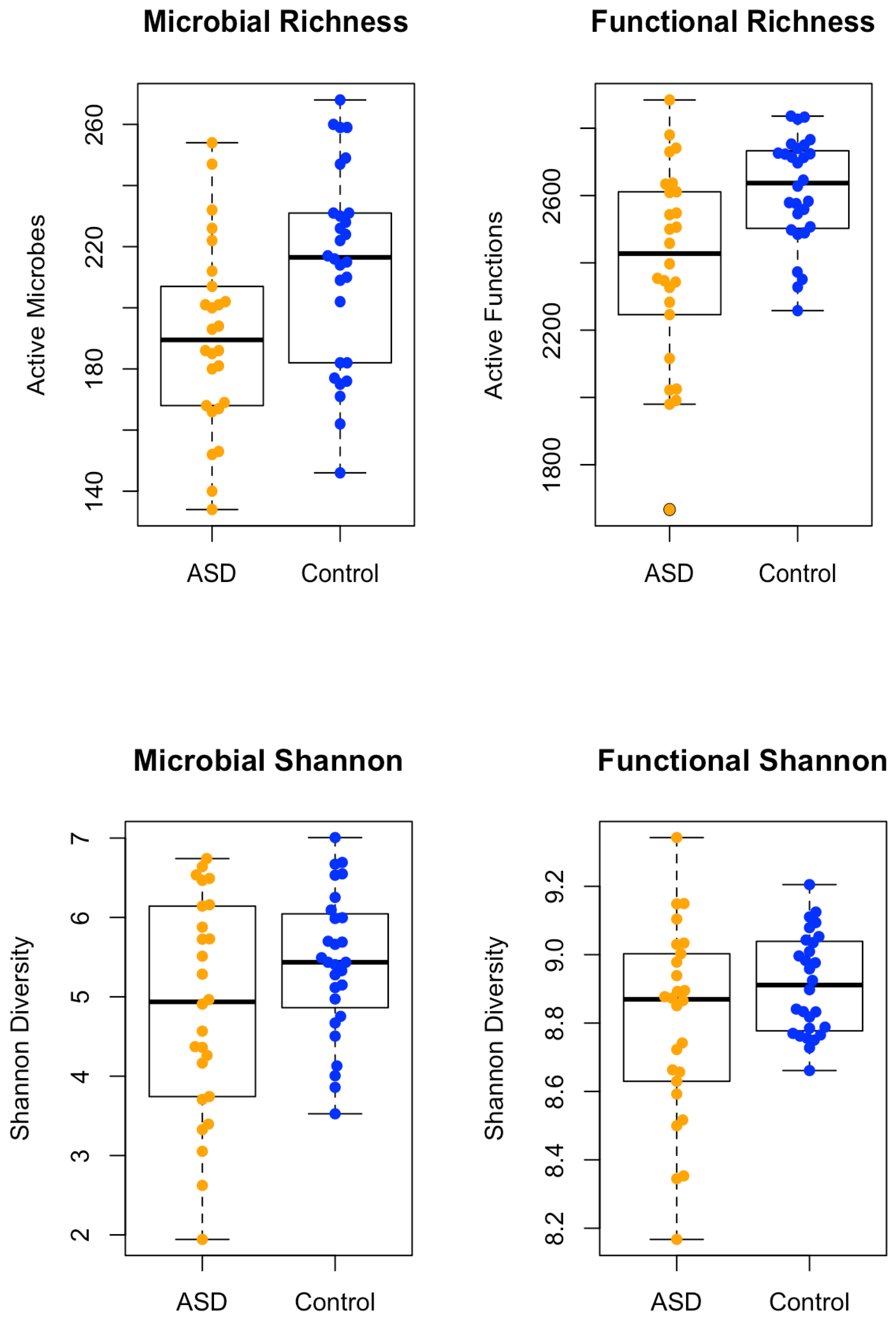
3.1 Spearman correlations demonstrated significant differences between each participant and the intervention group. Neurotypical controls had 645 unique taxa, the ASD pre-treatment group had 609 unique taxa, the ASD post-xylitol group had 629 unique taxa, and the ASD post-probiotics group had 592 unique taxa. A comparison of the Microbial Richness and the Functional Richness of the ASD subjects versus the control children is shown in
Figure 1. A chi-squared test was conducted on taxa or KO expression data. Benjamini-Hochberg correction was applied to the p-value for significance assessment.
The “whole foods children” (healthy children whose parents all reported using organic and fresh foods, no preservatives or fast food if possible) were all reported to be neurotypical and healthy. They presented with greater diversity in richness than the ASD, but the ASD versus Control combined groups were not significantly different in Shannon Diversity of Function.
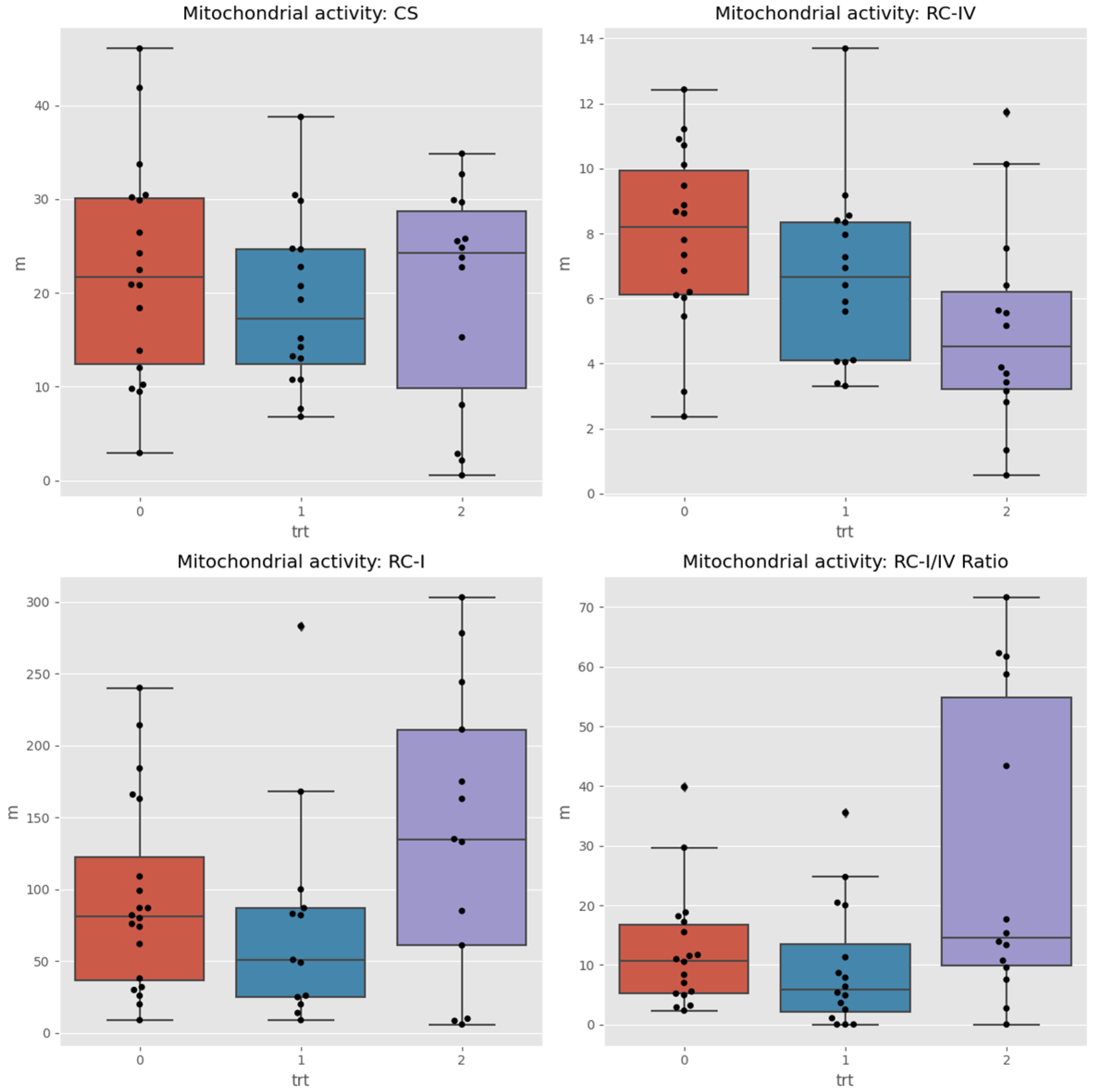
3.2 The MITOSWAB samples from Colombia possibly degraded due to the transit time and climate heat. Although the samples were adequately prepared for shipping and delivered to the local FedEx office, the package was accidentally misplaced by their employee and not dispatched promptly. Because the transportation time was extended, the investigators felt the data obtained may not be accurate. MITOSWAB samples from the neurotypical USA healthy children and the ASD children demonstrated significant differences between individuals and groups in the Respiratory Complex I to IV ratio and citrate synthase levels, as shown in
Figure 2. The Wilcoxon test for changes between times detected significant differences (between paired samples of the same users) in the medians of the RC-IV and RC-I/IV ratios.
3.3 KEGG orthology (KO) analysis demonstrated that, at baseline, there are 37 significantly different taxa proportions in ASD and healthy groups. Only seven of the 30 patients in the ASD group had higher expression levels. For KOs, these numbers were 27 and 1/27, respectively. Of the seven unique taxa, only one enzyme was expressed significantly more than the others. This enzyme was identified as a hydrolase, N-acyl-d-amino-acid deacetylase, produced by a specific bacterial strain typically not found in neurotypical children [
59].
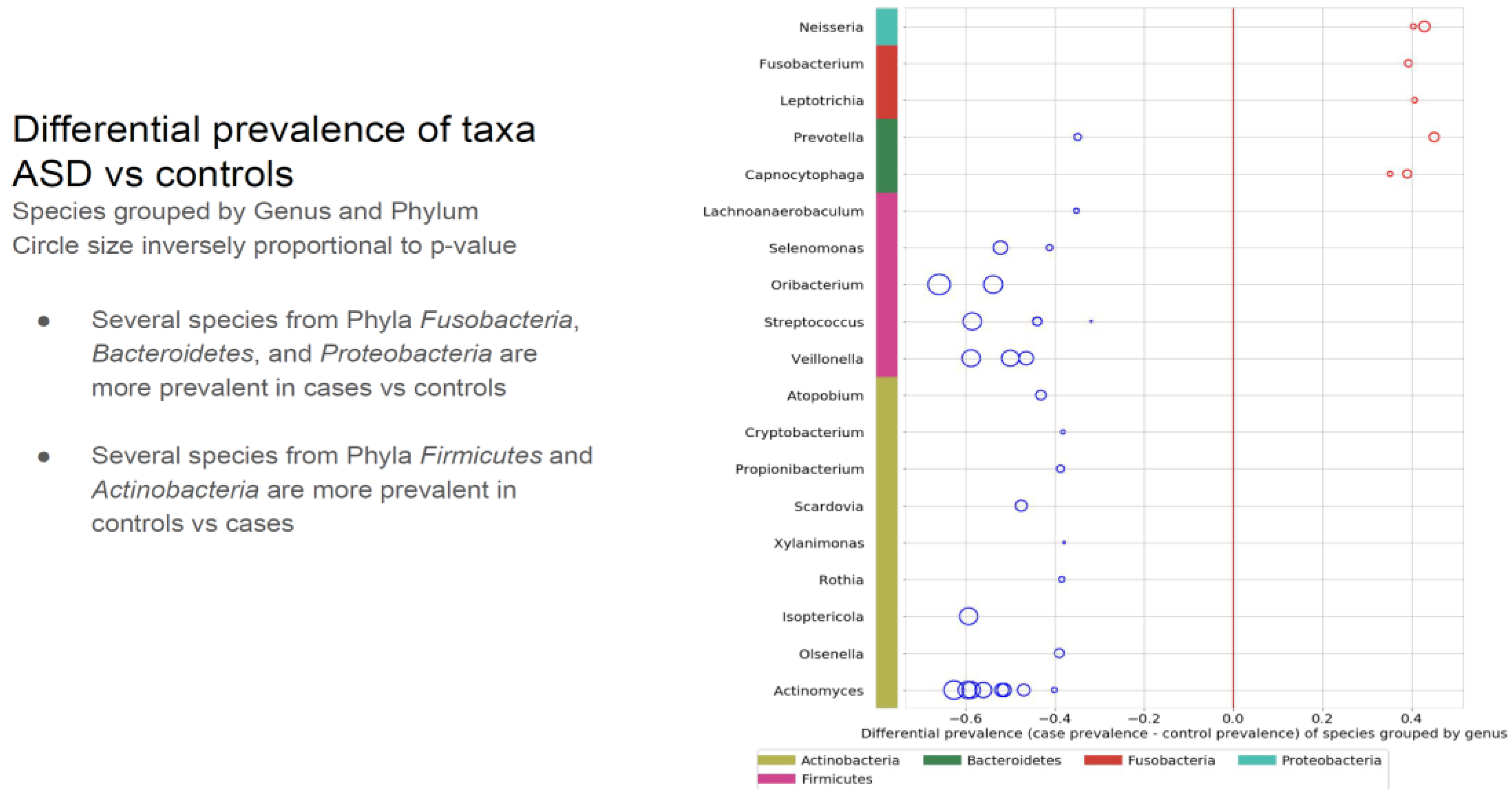
Comparing the ASD subjects to the neurotypical, different species prevalence between the groups was demonstrated in 11 genera. Specific taxa, such as the Prevotella genus, had up-regulated and down-regulated strains in the ASD group. (see
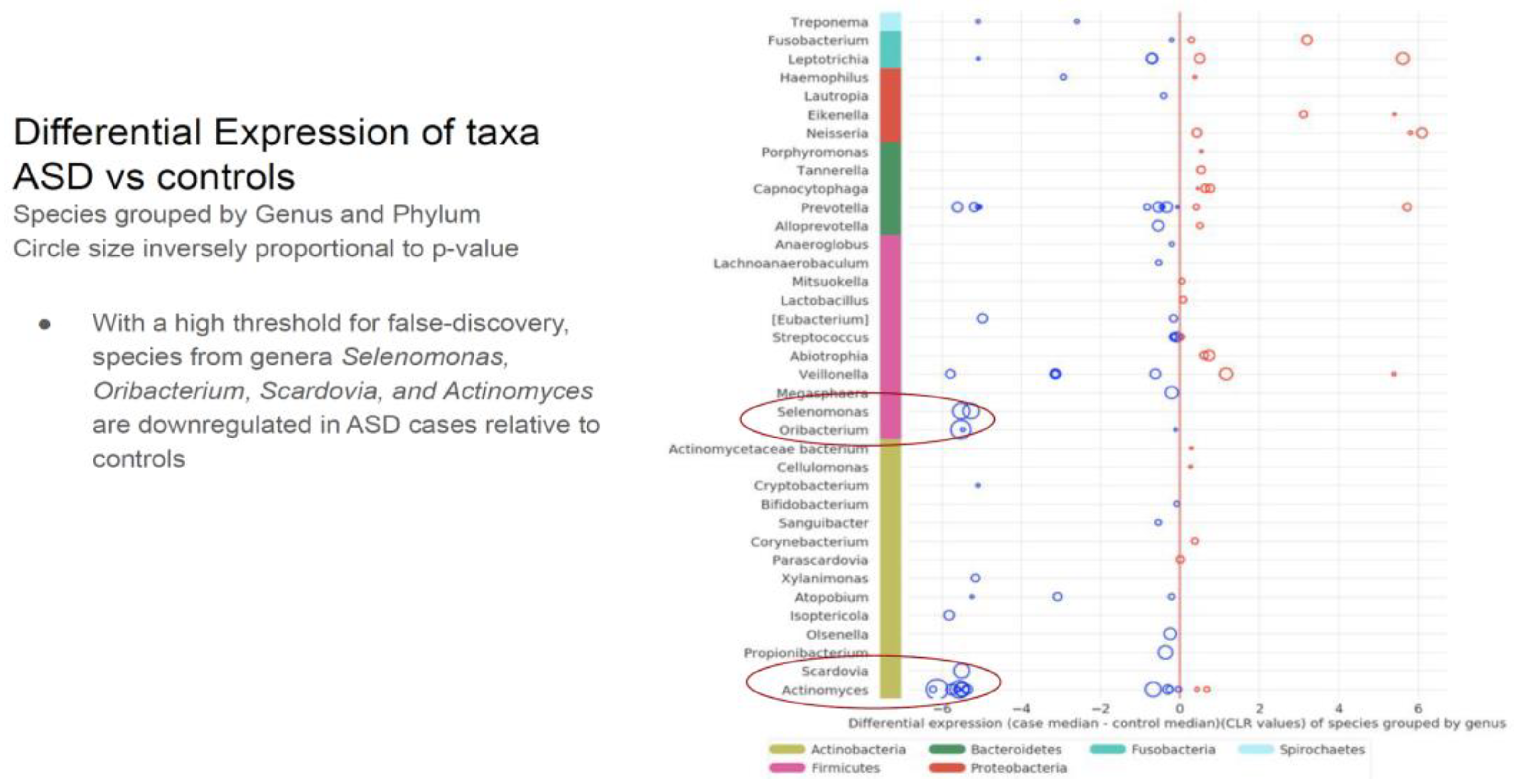
Table 1). Neisseria, Leptotrichia, Prevotella, Capnoctyophaga, and Fusobacterium genera were up-regulated in the ASD group. The oral microbiome of the ASD subjects was significantly different from the neurotypical and “Blue Zone” controls. Species from the genera Treponema, Prevotella, Eubacterium, Selenomonas, Oribacterium, Scardovia, and Actinomyces were increased in the neurotypical (see
Table 2).
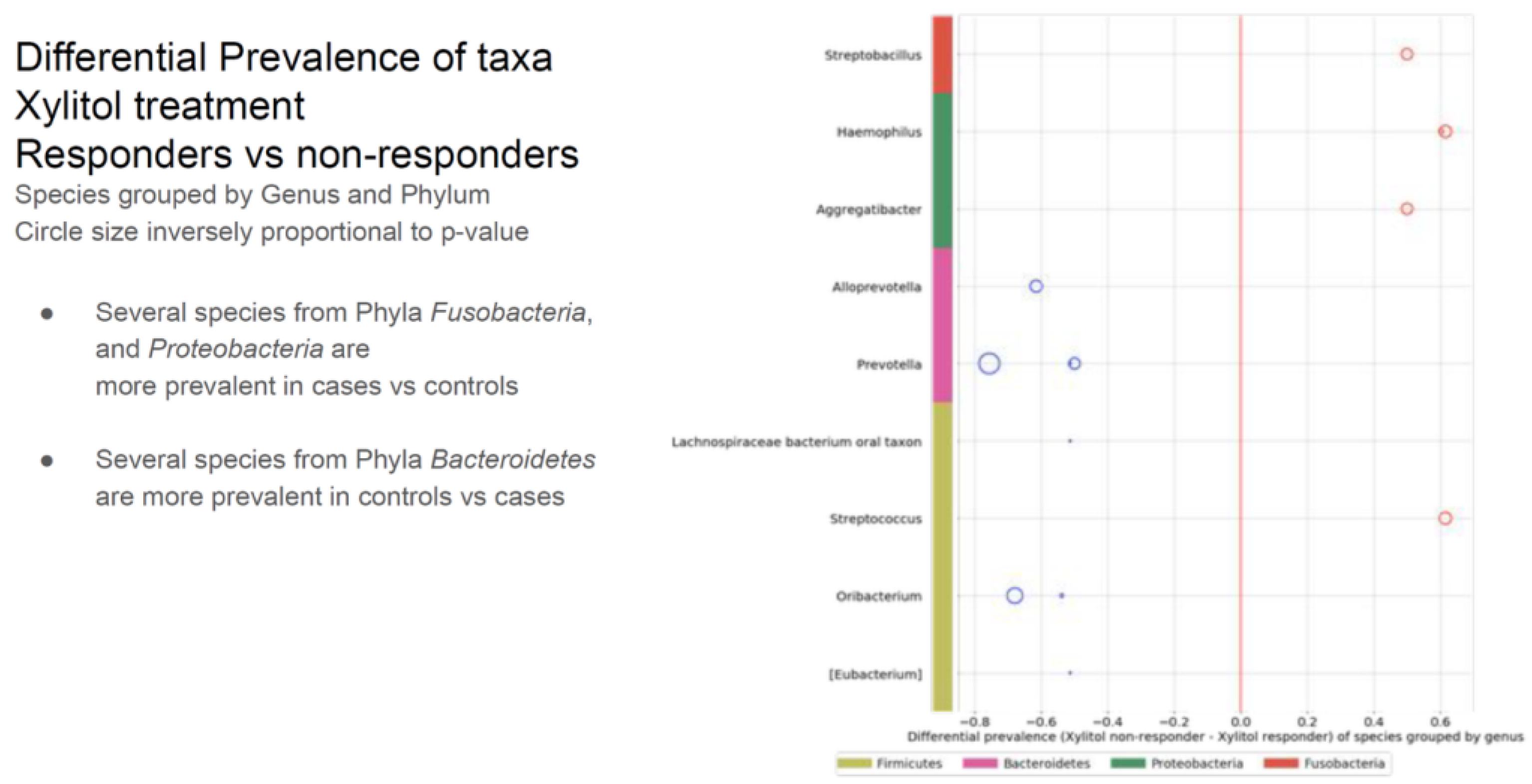
3.4 Within the timeframe of xylitol exposure, six of the 30 ASD participants demonstrated a significant improvement in verbal skills, becoming verbal while previously not being capable of creating sentences. Because this was an incidental finding, verbal data collection was not quantified before or after the xylitol intervention but only as a comment field in the overall health assessment. This was unfortunate because a detailed evaluation by a speech pathologist before and after xylitol intervention would have been invaluable. The improvement in verbal skills was significant for all six participants. An additional three participants showed improved verbal skills after the probiotic challenge. Due to the construction of the study protocol, no objective mechanism for speech evaluation was considered. However, both the parents and healthcare staff reported verbal improvements. In addition, three more became more verbal after the probiotic intervention. Bacterial species from three phyla (four genera) were upregulated in the xylitol responders compared to the non-responders (see
Table 3).
3.5 Figures and Tables
Table 1.
Different prevalence of taxa between the two groups. The larger the circle, the more pronounced the difference. The red circles present the up-regulated in ASD subjects, and the blue circles are up-regulated in the neurotypical children. The Prevotella genus had species and strains that were both up-regulated and down-regulated.
Table 1.
Different prevalence of taxa between the two groups. The larger the circle, the more pronounced the difference. The red circles present the up-regulated in ASD subjects, and the blue circles are up-regulated in the neurotypical children. The Prevotella genus had species and strains that were both up-regulated and down-regulated.
Table 2.
The differential expression of the genus and phylum taxa in the ASD subjects versus the neurotypical “Bule Zone” controls. The oral microbiome of the ASD subjects was significantly different from the neurotypical controls.
Table 2.
The differential expression of the genus and phylum taxa in the ASD subjects versus the neurotypical “Bule Zone” controls. The oral microbiome of the ASD subjects was significantly different from the neurotypical controls.
Table 3.
The verbal xylitol responders were a distinct subset of the ASD group. This may have occurred because of parent-subject compliance, simply due to the prebiotic-specific effects, or even due to the many possible initiating factors of ASD.
Table 3.
The verbal xylitol responders were a distinct subset of the ASD group. This may have occurred because of parent-subject compliance, simply due to the prebiotic-specific effects, or even due to the many possible initiating factors of ASD.
4. Discussion
Deacylase acts on N-acyl conjugates of amino acids and neurotransmitters (NAANs) [
60]. NAANs have recently become prominent because of their potential role in the nervous, vasculature, and immune systems. NAAN are compounds such as glycine, GABA, or dopamine conjugated with long-chain fatty acids [
61]. Interestingly, previous studies have shown that children with autism have decreased GABA concentrations, and many researchers have hypothesized that symptoms of autism are caused by an imbalance between inhibitory and excitatory signals, such as GABA and Glutamate [
62,
63]. It's possible that this enzyme’s effect on NAANs may play a role in the development of some of the symptoms of autism, as many studies have shown that gut bacteria can dramatically alter GABA activity [
64]. Fecal matter transplants have been used to change the gut microbiome in ASD with much-heralded success [
65,
66]. Interestingly, the oral microbiome has been designated a gateway microbiome, influencing the gut microbiome, placental microbiome, and the blood-brain barrier [
67,
68,
69]. A key oral pathogen has been associated with metabolic syndrome, atherosclerosis, and Inflammatory Alzheimer’s [
68,
69,
70,
71,
72]. As used in the study, oral probiotics have documented efficacy against this key oral pathogen,
Porphyromonas gingivalis [
73].
Xylitol acts as a prebiotic, shifting the microbiome by inhibiting pathogenic bacteria and viruses [
74,
75,
76]. Recent research into inhibiting virus pathogens by xylitol has stimulated interest due to the COVID-19 pandemic [
77]. The effect of dietary xylitol on hRSV infection was investigated in a mouse model, and significant results were reported [
78]. The mice received xylitol for 14 days before and three days post-viral exposure. The mice receiving xylitol had significantly reduced viral lung titers than the controls receiving phosphate-buffered saline (PBS). Fewer CD3+ and CD3+CD8+ lymphocytes, whose numbers indicate inflammatory status, were recruited in the mice receiving xylitol. These results demonstrated improved hRSV infection outcomes and reduced inflammation-associated immune responses to hRSV infection with dietary xylitol. The same researchers previously reported positive effects of xylitol on mice with influenza A virus infection (H1N1), as well as a decrease in recruitment of inflammatory lymphocytes. [
79]. The anti-inflammatory and antiviral properties of D-xylose/xylitol in respiratory conditions are the subject of a patent application (number WO1999048361A1) filed in 1998 in the United States [
80]. Subsequently, xylitol is the main active ingredient in nasal spray products, such as Xlear Sinus Care.
The responders were a distinct subset of the ASD group, and several possibilities would explain this. The first possibility is that there are several different autisms, some more environmentally sensitive than others, with subjects responding differently due to background settings. For example, Bis Phenol A's role in autism [
81,
82]. However, the role of the microbiome is well documented, and xylitol did shift the microbiome into the “responder” category. The supplements of prebiotics and probiotics both changed the oral microbiome. Strain shifting occurred within the specific genus Prevotella, with some species/strains more or less prevalent in the ASD subjects than in neurotypicals. This may be evolution-driven, as one critical species or strain is deleted, another takes its’ niche, and the host functions are affected epigenetically by the strain’s metabolites (post probiotics). Bi-directional functions, functioning in two directions, best explain the relationship between the host and the microgenome. The holobiont consists of the host, the host’s genome, the microbiome, and the microgenome, which is the genome of the microbiome. The holobiont has bi-directional influences; all components attempt to regulate each other to maintain the environmentally determined status quo based on the history of successful integration. Unfortunately, many factors, including newer modern-day interventions that were historically not naturally present, may negatively affect the host’s health.
Autism spectrum disorder (ASD) is reported to be associated with dysbiosis in the oral microbiota. Since the oral cavity is the start of the gastrointestinal tract, this supports the theory of a microbial gut-brain axis in ASD and possibly the existence of a microbial oral-brain axis. Oral bacteria may be transported to the brain through pathways, including standard dental procedures. The link between the oral microbiome and neural pathologies has been reported in the literature [
83]. In contrast, a recent publication in Cell presented the hypothesis that ASD influenced the diet, resulting in a significant change in the microbiome [
84]. This article claimed that previous studies were underpowered, too small, and did not adjust for confounders. However, this may be a classic “biofeedback,” whereas dietary preferences may also be driven by the microbiome, such as ASD, influencing dietary choices. An example would be the decrease in the Rothia genus with subjects diagnosed as autistic in the present study, often with symptoms of gluten intolerance [
85,
86,
87]. Their oral microbiome will not degrade gluten, causing discomfort, and they can then preferentially refuse gluten-rich foods, hence being “picky.” The present study included a dietary summary indicating that the healthy USA cohort's rich and diverse diet influenced their overall health. However, prebiotics and probiotic interventions have provided therapeutic benefits without dietary interventions, which would place diet into a more proper perspective [89–91]. A randomized clinical trial recently published reported a significant improvement from baseline in the Vineland-3 Adaptive Behavior Composite score (p = 0.03) during probiotic treatment. The authors reported a trend for increased social/geometric viewing ratio following probiotic treatment compared to placebo. Probiotic-associated directional improvements in adaptive behavior measured by Vineland-3 and social preference measured with eye tracking were noted [92]. Fecal matter transplant treatment for ASD has also been demonstrated to produce significant results in modifying both gut and ASD symptoms, apparently with documented long-term benefits [93]. Microbiome shifts may also have strong epigenetic effects [
54,94]. A recently published study of the gut microbiome using REFS identified a set of bacterial taxa that can be used to predict the ASD status of children in three distinct cohorts. Their results support “that the gut microbiome has a strong association with ASD and should not be disregarded as a potential target for therapeutic interventions “[95].
Previously published studies have demonstrated that the oral microbiome of children with ASD is uniquely different from neurotypical children. A study with RNA extraction and shotgun sequencing of saliva reported that 12 taxa were altered between the developmental groups, and 28 taxa were identified that distinguished ASD patients with and without GI disturbance. In addition, five microbial ratios distinguished ASD from control participants (79.5% accuracy), three distinguished ASD from DD (76.5%), and three distinguished ASD children with/without GI disturbance (85.7%). The Kyoto Encyclopedia of Genes and Genomes microbial database was utilized to assess taxonomic pathways and compared with a one-way analysis of variance [96]. Studies have also been published reporting a gut microbiome shift in other mental disorders, such as anxiety, depression, bipolar, and schizophrenia. In a study published in Molecular Psychiatry, the author’s syntheses identified specific taxa often associated with mental disorders, including lower levels of bacterial genera that produce short-chain fatty acids such as butyrate, higher levels of lactic acid-producing bacteria and higher levels of bacteria associated with glutamate and GABA metabolism {97]. Their results were not dissimilar to those of the current study.
This study determined the differences in the oral transcriptome and mitochondrial status between children from a “blue zone” in Colombia and children from the USA, both healthy and those diagnosed with ASD. Although the objectives of this study did not include collecting data on cognitive skills, behavioral observations, or gastric symptoms, the verbal skills of 6 of the ASD children were reportedly improved with prebiotic and an additional 3 with probiotic intervention. The results should be replicated in more extensive trials to develop a method of objectively diagnosing autism in infancy or young childhood, allowing for earlier and more effective interventional therapy
5. Conclusions
The transcriptomes of children with ASD in the USA are different from those of healthy children in a developing country and neurotypical children in the USA. Intervention with prebiotics (e.g., xylitol) and probiotics may significantly affect the transcriptome.
Author Contributions
Conceptualization, M.C. and G.B.; methodology, G.B., and S. G.; software, G.B. and S. G.; validation, M.V., G.V. and M.C.; formal analysis, G.B.; investigation, M.C. and E.V.; resources, M.C.; data curation, R.T.; writing—original draft preparation, M.C.; writing—review and editing, M.V.; visualization, M.C.; supervision, M.C.; project administration, M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The Swanson Foundation, a Northwestern University private research fund, funded this research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board, Pearl IRB Indianapolis IN, identified as 18-Grov-101 for studies involving humans.
Informed Consent Statement
Informed consent and /or assent was obtained from all subjects involved in the study.
Data Availability Statement
Due to the need to maintain strict privacy of the subjects (children), data was protected, and consent contained specific language assuring complete safeguarding measures.
Acknowledgments
The donors of the Swanson Fund, specifically Drs R William Cornell, Alonzo Jones, and Michael Milligan.
Conflicts of Interest
The authors declare no conflicts of interest. The Swanson Fund had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Pulikkan, J.; Mazumder, A.; & Grace, T. (2019). Role of the Gut Microbiome in Autism Spectrum Disorders. Advances in experimental medicine and biology, 1118, 253–269. [CrossRef]
- Khalil, R.; Tindle, R.; Boraud, T., Moustafa, A. A., & Karim, A. A. (2018). Social decision making in autism: On the impact of mirror neurons, motor control, and imitative behaviors. CNS neuroscience & therapeutics, 24(8), 669–676. [CrossRef]
- Waye, M., & Cheng, H. Y. (2018). Genetics and epigenetics of autism: A Review. Psychiatry and clinical neurosciences, 72(4), 228–244. [CrossRef]
- Siu, M. T., & Weksberg, R. (2017). Epigenetics of Autism Spectrum Disorder. Advances in experimental medicine and biology, 978, 63–90. [CrossRef]
- Duffney, L. J., Valdez, P., Tremblay, M. W., Cao, X., Montgomery, S., McConkie-Rosell, A., & Jiang, Y. H. (2018). Epigenetics and autism spectrum disorder: A report of an autism case with mutation in H1 linker histone HIST1H1E and literature review. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics, 177(4), 426–433. [CrossRef]
- Xu G, Strathearn L, Liu B, et al. Prevalence and Treatment Patterns of Autism Spectrum Disorder in the United States, 2016. JAMA Pediatr. 2019;173(2):153–159. [CrossRef]
- Karimi, P., Kamali, E., Mousavi, S. M., & Karahmadi, M. (2017). Environmental factors influencing the risk of autism. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences, 22, 27. [CrossRef]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:255–74. [PubMed] [Google Scholar]. [CrossRef]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102. [PMC free article] [PubMed] [Google Scholar]. [CrossRef]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–10. [PubMed] [Google Scholar]. [CrossRef]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. [PubMed] [Google Scholar]. [CrossRef]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–73. [PMC free article] [PubMed] [Google Scholar]. [CrossRef]
- Rosenfeld C. S. (2015). Microbiome Disturbances and Autism Spectrum Disorders. Drug metabolism and disposition: the biological fate of chemicals, 43(10), 1557–1571. [CrossRef]
- Desbonnet, L., Clarke, G., Shanahan, F., Dinan, T. G., & Cryan, J. F. (2014). Microbiota is essential for social development in the mouse. Molecular psychiatry, 19(2), 146–148. [CrossRef]
- Golubeva, A. V., Joyce, S. A., Moloney, G., Burokas, A., Sherwin, E., Arboleya, S., Flynn, I., Khochanskiy, D., Moya-Pérez, A., Peterson, V., Rea, K., Murphy, K., Makarova, O., Buravkov, S., Hyland, N. P., Stanton, C., Clarke, G., Gahan, C., Dinan, T. G., & Cryan, J. F. (2017). Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine, 24, 166–178. [CrossRef]
- Sharon, Gil et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell, Volume 177, Issue 6, 1600 - 1618.e17. [CrossRef]
- Lucas P. Henry, Marjolein Bruijning, Simon K.G. Forsberg, Julien F. Ayroles Can the microbiome influence host evolutionary trajectories? bioRxiv 700237. [CrossRef]
- Davenport, E.R., Sanders, J.G., Song, S.J. et al. The human microbiome in evolution. BMC Biol 15, 127 (2017). [CrossRef]
- Zilber-Rosenberg I., Rosenberg E. 2008. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiology Reviews 32: 723–735. [CrossRef]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. [CrossRef]
- Montiel-Castro A.J., Gonzalez-Cervantes R.M., Bravo-Ruiseco G., Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. [CrossRef]
- Arora T., Backhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. 2016;280(4):339–49. [CrossRef]
- McNabney, S. M., Henagan, T. M. (2017). Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients, 9(12), 1348. [CrossRef]
- Cummings J. H., MacFarlane G. T.(1991) The colonic flora, fermentation and large bowel digestive function. in The large intestine: physiology, pathophysiology, and disease. eds Philips S. F., Pemberton J. H., Shorter R. G. (Raven Press, New York, N.Y), pp 51–91.
- Szylit O., Andrieux C. (1993) Physiological and pathophysiological effects of carbohydrate fermentation. World Rev. Nutr. Diet. 74:88–122. [CrossRef]
- Shams, S., Foley K.A., Kavaliers M., MacFabe D.F., Ossenkopp K.P. Systemic treatment with the enteric bacterial metabolic product propionic acid results in reduction of social behavior in juvenile rats: Contribution to a rodent model of autism spectrum disorder. Dev Psychobiol. 2019 Jan 28. [CrossRef]
- MacFabe D. F. , Cain D. P. , Rodriguez-Capote K. et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176(1):149-69. [CrossRef]
- Shultz S. R., MacFabe D. F. , Ossenkopp K. P. et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901-11. [CrossRef]
- Shultz S. R. , Macfabe D. F. , Martin S et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200(1):33-41. [CrossRef]
- MacFabe D. F. , Cain N. E. , Boon F. et al. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47-54. [CrossRef]
- MacFabe D. F. , Cain N. E. , Boon F. et al. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47-54. [CrossRef]
- Frye R. E., Nankova B., Bhattacharyya S., Rose S., Bennuri, S. C., MacFabe D. F. (2017). Modulation of Immunological Pathways in Autistic and Neurotypical Lymphoblastoid Cell Lines by the Enteric Microbiome Metabolite Propionic Acid. Frontiers in immunology, 8, 1670. [CrossRef]
- Rose et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Translational Psychiatry. 2018;8:42. [CrossRef]
- Lin, H. V., Frassetto, A., Kowalik, E. J., Jr, Nawrocki, A. R., Lu, M. M., Kosinski, J. R., Marsh, D. J. (2012). Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PloS one, 7(4), e35240. [CrossRef]
- Dengate S., Ruben A., Controlled trial of cumulative behavioural effects of a common bread preservative. J Paediatr Child Health. 2002 Aug;38(4):373-6. [CrossRef]
- Akram M, Ali Shah SM, Munir N, Daniyal M, Tahir IM, Mahmood Z, Irshad M, Akhlaq M, Sultana S, Zainab R. Hexose mono-phosphate shunt, the role of its metabolites and associated disorders: A review. J Cell Physiol. 2019 Jan 29. Epub ahead of print. [CrossRef] [PubMed]
- Bär A. Sugar alcohols in the diabetic diet. In: Kretchmer N., Hollenbeck C. B., editors. Sugars and Sweeteners. Boca Raton, Fla, USA: CRC Press; 1991. pp. 131–150.
- Meyer-Gerspach AC, Drewe J, Verbeure W, et al. Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study. Nutrients. 2021;13(1):174. Published 2021 Jan 8. [CrossRef]
- Janakiram C., Deepan Kumar C. V., Joseph J., Xylitol in preventing dental caries: A systematic review and meta-analyses. J Nat Sci Biol Med. 2017 Jan-Jun; 8(1): 16–21. [CrossRef]
- Kõljalg S, Smidt I, Chakrabarti A, Bosscher D, Mändar R. Exploration of singular and synergistic effect of xylitol and erythritol on causative agents of dental caries. Sci Rep. 2020 Apr 14;10(1):6297. [CrossRef] [PubMed] [PubMed Central]
- Mäkinen KK (2017) Sugar alcohols and prevention of oral diseases – comments and rectifications. Oral Health Care 2. [CrossRef]
- Salli, K., Lehtinen, M. J., Tiihonen, K., & Ouwehand, A. C. (2019). Xylitol's Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients, 11(8), 1813. [CrossRef]
- Chukwuma CI, Islam MS (2017) Xylitol: one name, numerous benefits. In: Merillon J-M, Ramawat KG (eds) Sweeteners: Pharmacology, Biotechnology, and Applications. Springer International Publishing, Cham, pp 1–27.
- Bettina K. Wölnerhanssen, Anne Christin Meyer-Gerspach, Christoph Beglinger & Md. Shahidul Islam (2020) Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review, Critical Reviews in Food Science and Nutrition, 60:12, 1986-1998. [CrossRef]
- Gasmi Benahmed A, Gasmi A, Arshad M, Shanaida M, Lysiuk R, Peana M, Pshyk-Titko I, Adamiv S, Shanaida Y, Bjørklund G. Health benefits of xylitol. Appl Microbiol Biotechnol. 2020 Sep;104(17):7225-7237. Epub 2020 Jul 7. [CrossRef] [PubMed]
- Cannon ML, Merchant M, Kabat W, Catherine L, White K, Unruh B, Ramones A. In Vitro Studies of Xylitol and Erythritol Inhibition of Streptococcus Mutans and Streptococcus Sobrinus Growth and Biofilm Production. J Clin Pediatr Dent. 2020 Sep 1;44(5):307-314. [CrossRef] [PubMed]
- Park E, Park MH, Na HS, Chung J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol Letters. 2015;37:983-990. [CrossRef]
- Tomonobu N, Komalasari NLGY, Sumardika IW, Jiang F, Chen Y, Yamamoto KI, Sakaguchi M. Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chem Biol Interact. 2020;324:109085. [CrossRef]
- Sahasakul Y, Angkhasirisap W, Lam-Ubol A, Aursalung A, Sano D, Takada K, Trachootham D. Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients. 2022;14(10);2023. [CrossRef]
- Shaaban, S. Y., El Gendy, Y. G., Mehanna, N. S., El-Senousy, W. M., El-Feki, H. S. A., Saad, K., & El-Asheer, O. M. (2018). The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutritional neuroscience, 21(9), 676–681. [CrossRef]
- Yang, J., Fu, X., Liao, X., Li, Y. (2020). Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry Res. 293, 113471. [CrossRef]
- He, X., Liu, W., Tang, F., Chen, X., & Song, G. (2023). Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients, 15(6), 1415. [CrossRef]
- Abujamel, T. S., Al-Otaibi, N. M., Abuaish, S., AlHarbi, R. H., Assas, M. B., Alzahrani, S. A., Alotaibi, S. M., El-Ansary, A., & Aabed, K. (2022). Different Alterations in Gut Microbiota between Bifidobacterium longum and Fecal Microbiota Transplantation Treatments in Propionic Acid Rat Model of Autism. Nutrients, 14(3), 608. [CrossRef]
- Xiao, L., Yan, J., Yang, T., Zhu, J., Li, T., Wei, H. et al. (2021). Fecal microbiome transplantation from children with autism spectrum disorder modulates tryptophan and serotonergic synapse metabolism and induces altered behaviors in Germ-free mice. mSystems. 6, e01343-20. [CrossRef]
- Guidetti, C., Salvini, E., Viri, M., Deidda, F., Amoruso, A., Visciglia, A., Drago, L., Calgaro, M., Vitulo, N., Pane, M., & Caucino, A. C. (2022). Randomized Double-Blind Crossover Study for Evaluating a Probiotic Mixture on Gastrointestinal and Behavioral Symptoms of Autistic Children. Journal of clinical medicine, 11(18), 5263. [CrossRef]
- Malone, J.H., Oliver, B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol 9, 34 (2011). [CrossRef]
- Wang, Z., Gerstein, M., & Snyder, M. (2009). RNA-seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics, 10(1), 57–63. [CrossRef]
- Kanehisa M, Goto S (2000). "KEGG: Kyoto Encyclopedia of Genes and Genomes". Nucleic Acids Res. 28 (1): 27–30. PMC 102409. [CrossRef] [PubMed]
- Wakayama M, Katsuno Y, Hayashi S, Miyamoto Y, Sakai K, Moriguchi M (1995). "Cloning and sequencing of a gene encoding D-aminoacylase from Alcaligenes xylosoxydans subsp. xylosoxydans A-6 and expression of the gene in Escherichia coli". Biosci. Biotechnol. Biochem. 59 (11): 2115–9. [CrossRef] [PubMed]
- Connor, M., Vaughan, C. W., & Vandenberg, R. J. (2010). N-acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets. British journal of pharmacology, 160(8), 1857–1871. [CrossRef]
- Rojas D. C. (2014). The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. Journal of neural transmission (Vienna, Austria : 1996), 121(8), 891–905. [CrossRef]
- Tirouvanziam, R., Obukhanych, T. V., Laval, J., Aronov, P. A., Libove, R., Banerjee, A. G., Parker, K. J., O'Hara, R., Herzenberg, L. A., Herzenberg, L. A., & Hardan, A. Y. (2012). Distinct plasma profile of polar neutral amino acids, leucine, and glutamate in children with Autism Spectrum Disorders. Journal of autism and developmental disorders, 42(5), 827–836. [CrossRef]
- Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., McDonald, D., Dietrich, D., Ramadhar, T. R., Lekbua, A., Mroue, N., Liston, C., Stewart, E. J., Dubin, M. J., Zengler, K., Knight, R., Gilbert, J. A., Clardy, J., & Lewis, K. (2019). GABA-modulating bacteria of the human gut microbiota. Nature Microbiology, 4(3), 396–403. [CrossRef]
- Kang, D., Adams, J.B., Coleman, D. et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep 9, 5821 (2019). [CrossRef]
- Walker H. B., Midvedt T. Microbiota transplant intervention shows promising results in some children with autism spectrum disorder (ASD). Program No. 458.03. 2019 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2019.
- Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. [CrossRef]
- Kobayashi R., Ogawa Y., Hashizume-Takizawa T., Kurita-Ochiai T. Oral bacteria affect the gut microbiome and intestinal immunity, Pathogens and Disease, Volume 78, Issue 3, April 2020, ftaa024. [CrossRef]
- Shoemark, D. K., Allen, S. J. ‘The Microbiome and Disease: Reviewing the Links Between the Oral Microbiome, Aging, and Alzheimer’s Disease’. 1 Jan. 2015 : 725 – 738. [CrossRef]
- Dominy, S. S. et al. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors, Science advances vol. 5,1 eaau3333. 23 Jan. 2019. [CrossRef]
- Kim, H. J., Cha, G. S., Kim, H. J., Kwon, E. Y., Lee, J. Y., Choi, J., & Joo, J. Y. (2018). Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein. Journal of periodontal & implant science, 48(1), 60-68. [CrossRef]
- Kashiwagi, Y., Aburaya, S., Sugiyama, N. et al. Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci Rep 11, 18398 (2021). [CrossRef]
- Hanel, A. N., Herzog, H. M., James, M. G., & Cuadra, G. A. (2020). Effects of Oral Commensal Streptococci on Porphyromonas gingivalis Invasion into Oral Epithelial Cells. Dentistry journal, 8(2), 39. [CrossRef]
- Uebanso, T., Kano, S., Yoshimoto, A., Naito, C., Shimohata, T., Mawatari, K., & Takahashi, A. (2017). Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients, 9(7), 756. [CrossRef]
- Amo K., Arai H., Uebanso T., Fukaya M., Koganei M., Sasaki H., Yamamoto H., Taketani Y., Takeda E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011;49:1–7. [CrossRef]
- Han, S. J., Jeong, S. Y., Nam, Y. J., Yang, K. H., Lim, H. S., & Chung, J. (2005). Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis. Clinical and diagnostic laboratory immunology, 12(11), 1285–1291. [CrossRef]
- Cannon M.L., Westover J., Ferrar G., Bleher R. Anti-viral Potential of nasal spray constituents against SARS-CoV-2. bioRxiv.
- Xu ML, Wi GR, Kim HJ, et al. Ameliorating effect of dietary xylitol on human respiratory syncytial virus (hRSV) infection. Biol Pharm Bull. 2016;39:540-546. [CrossRef]
- Yin SY, Kim HJ, Kim HJ. Protective effect of dietary xylitol on influenza A virus infection. PLoS One. 2014;9:e84633. [CrossRef]
- Jones AH, inventor. Xylitol compositions for treating upper respiratory conditions. World Intellectual Property Organization. International Publication Number: WO 99/48361. September 30, 1999.
- Kaur, K., Chauhan, V., Gu, F., & Chauhan, A. (2014). Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free radical biology & medicine, 76, 25–33. [CrossRef]
- Morimoto, M., Hashimoto, T., Tsuda, Y., Nakatsu, T., Kitaoka, T., & Kyotani, S. (2020). Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PloS one, 15(5), e0233550. [CrossRef]
- Olsen I., Hicks SD. Oral microbiota and autism spectrum disorder (ASD). J Oral Microbiol. 2019;12(1):1702806. Published 2019 Dec 12. [CrossRef]
- Yap, C. X., Henders, A. K., Alvares, G. A., Wood, D. L. A., Krause, L., Tyson, G. W., Restuadi, R., Wallace, L., McLaren, T., Hansell, N. K., Cleary, D., Grove, R., Hafekost, C., Harun, A., Holdsworth, H., Jellett, R., Khan, F., Lawson, L. P., Leslie, J., Frenk, M. L., … Gratten, J. (2021). Autism-related dietary preferences mediate autism-gut microbiome associations. Cell, 184(24), 5916–5931.e17. [CrossRef]
- Feng P, Zhao S, Zhang Y and Li E (2023) A review of probiotics in the treatment of autism spectrum disorders: Perspectives from the gut–brain axis. Front. Microbiol. 14:1123462. [CrossRef]
- Fernandez-Feo, M., Wei, G., Blumenkranz, G., Dewhirst, F. E., Schuppan, D., Oppenheim, F. G., & Helmerhorst, E. J. (2013). The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 19(9), E386–E394. [CrossRef]
- Zamakhchari, M., Wei, G., Dewhirst, F., Lee, J., Schuppan, D., Oppenheim, F. G., & Helmerhorst, E. J. (2011). Identification of Rothia bacteria as gluten-degrading natural colonizers of the upper gastro-intestinal tract. PloS one, 6(9), e24455. [CrossRef]
- Caminero, A., Nistal, E., Herrán, A. R., Pérez-Andrés, J., Ferrero, M. A., Vaquero Ayala, L., Vivas, S., Ruiz de Morales, J. M., Albillos, S. M., & Casqueiro, F. J. (2015). Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. The British journal of nutrition, 114(8), 1157–1167. [CrossRef]
- Yang, J., Fu, X., Liao, X., & Li, Y. (2020). Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry research, 293, 113471. [CrossRef]
- Wang, Y., Li, N., Yang, J. J., Zhao, D. M., Chen, B., Zhang, G. Q., Chen, S., Cao, R. F., Yu, H., Zhao, C. Y., Zhao, L., Ge, Y. S., Liu, Y., Zhang, L. H., Hu, W., Zhang, L., & Gai, Z. T. (2020). Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacological research, 157, 104784. [CrossRef]
- Duque, A. L. R. F., Demarqui, F. M., Santoni, M. M., Zanelli, C. F., Adorno, M. A. T., Milenkovic, D., Mesa, V., & Sivieri, K. (2021). Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food research international (Ottawa, Ont.), 149, 110657. [CrossRef]
- Schmitt, L.M., Smith, E.G., Pedapati, E.V. et al. Results of a phase Ib study of SB-121, an investigational probiotic formulation, a randomized controlled trial in participants with autism spectrum disorder. Sci Rep 13, 5192 (2023). [CrossRef]
- Kang, D. W., Adams, J. B., Vargason, T., Santiago, M., Hahn, J., & Krajmalnik-Brown, R. (2020). Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere, 5(5), e00314-20. [CrossRef]
- Yousefi, B., Kokhaei, P., Mehranfar, F., Bahar, A., Abdolshahi, A., Emadi, A., & Eslami, M. (2022). The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neuroscience and biobehavioral reviews, 132, 998–1009. [CrossRef]
- Peralta-Marzal, L.N., Rojas-Velazquez, D., Rigters, D. et al. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci Rep 14, 814 (2024). [CrossRef]
- Hicks, S. D., Uhlig, R., Afshari, P., Williams, J., Chroneos, M., Tierney-Aves, C., Wagner, K., & Middleton, F. A. (2018). Oral microbiome activity in children with autism spectrum disorder. Autism research : official journal of the International Society for Autism Research, 11(9), 1286–1299. [CrossRef]
- Ling, Z., Cheng, Y., Liu, X., Yan, X., Wu, L., Shao, L., Gao, J., Lei, W., Song, Q., Zhao, L., & Jin, G. (2023). Altered oral microbiota and immune dysfunction in Chinese elderly patients with schizophrenia: a cross-sectional study. Translational psychiatry, 13(1), 383. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).