Submitted:
11 May 2024
Posted:
13 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CA membranes
2.3. Membrane Structure Characterisation
2.3.1. Porosity and Equilibrium Water Content
2.3.2. Contact Angle
2.3.3. Surface Oxygen Groups
2.3.4. Membrane Performance Characterisation
2.4. Carbonaceous Materials Characerisation
3. Results
3.1. Chracterizastion of the Carbon Materials
3.2. Physicochemical Properties and Adsorption Characteristics of the Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mulungulungu, G.A.; Mao, T.; Han, K. Efficient removal of high-concentration copper ions from wastewater via 2D g-C3N4 photocatalytic membrane filtration. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 623, 126714. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Khoshtinat, F.; Tabatabaie, T.; Ramavandi, B.; Hashemi, S. Phenol removal kinetics from synthetic wastewater by activation of persulfate using a catalyst generated from shipping ports sludge. Chemosphere 2021, 283, 131265. [Google Scholar] [CrossRef]
- Ade, I.A.; Tran, H.N.; Zhang, J-W. ; Wang, Y-C.; Dat, N.D.; Nguyen, D.T.; Chao, H-P. Adsorption characteristics of lead, copper, cadmium, methylene blue, phenol, and toluene in water using composite synthesized from titanium dioxides and carbon spheres through hydrothermal method. Journal of Water Process Engineering 2022, 50, 103221. [Google Scholar] [CrossRef]
- Hamad, H.T. Removal of phenol and inorganic metals from wastewater using activated ceramic. Journal of King Saud University - Engineering Sciences 2021, 33, 221–226. [Google Scholar] [CrossRef]
- Mojiri, A.; Bashir, M.J.K. Wastewater Treatment: Current and Future Techniques. Water 2022, 14, 448. [Google Scholar] [CrossRef]
- Plisko, T.; Karslyan, Y.; Bildyukevich, A. Effect of Polyphenylsulfone and Polysulfone Incompatibility on the Structure and Performance of Blend Membranes for Ultrafiltration. Materials 2021, 14, 5740. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, H.; Liu, Y.; He, D.; Peng, L.E.; Matsuyama, H.; Hu, M.; Li, H. Fabrication of polyethersulfone/sulfonated polysulfone loose nanofiltration membranes for enhanced selectivity of pharmaceuticals and personal care products and minerals. Separation and Purification Technology 2024, 337, 126466. [Google Scholar] [CrossRef]
- Gharbani, P.; Mehrizad, A. Preparation and characterization of graphitic carbon nitrides/polyvinylidene fluoride adsorptive membrane modified with chitosan for Rhodamine B dye removal from water: Adsorption isotherms, kinetics and thermodynamics. Carbohydrate Polymers 2022, 277, 118860. [Google Scholar] [CrossRef]
- Mompó-Curell, R.; Biti, S.; Iborra-Clar, A.; Iborra-Clar, M.I.; Garcia-Castello, E.M.; Fernández-Martín, C. Activated-Carbon-Doped Non-Solvent-Induced Phase-Inversion Membranes: A Comprehensive Study on Synthesis, Characterisation, and Performance Evaluation. Sustainability 2024, 16, 1150. [Google Scholar] [CrossRef]
- Yang, H.; Ye, Q.; Zhou, Y. ; Y. Xiang, Q. Xing, X. Dong, et al., Formation, morphology and control of high-performance biomedical polyurethane porous membranes by water micro-droplet induced phase inversion, Polymer 2004, 55, 5500–5508. [Google Scholar]
- Zaherzadeh, A.; Karimi-Sabet, J.; Mohammad, S.; Mousavian, A.; Ghorbanian, S. Optimization of flat sheet hydrophobic membranes synthesis via supercritical CO2 induced phase inversion for direct contact membrane distillation by using response surface methodology (RSM), The Journal of Supercritical Fluids 2015, 103, 105–114. 103.
- Lazarenko, N.S.; Golovakhin, V.V.; Shestakov, A.A.; Lapekin, N.I.; Bannov, A.G. Recent Advances on Membranes for Water Purification Based on Carbon Nanomaterials. Membranes 2022, 12, 915. [Google Scholar] [CrossRef]
- Ullah, N.; Ali, Z.; Khan, A.S.; Adalat, B.; Nasrullah, A.; Khan, S.B. Preparation and dye adsorption properties of activated carbon/clay/sodium alginate composite hydrogel membranes. RSC Advances 2024, 2), 211–221. [Google Scholar] [CrossRef]
- Raheel, F.; Rafay, A.; Bibi, B.; Ahmad, S.; Ali, Z.; Saleem, M.; Butt, M.S.; Rehman, A.U.; Irfan, M. Synthesis and Characterization of Activated Carbon and Its Application for Wastewater Treatment. Materials Proceedings 2024, 17, 4. [Google Scholar]
- Obayomi, K.S.; Lau, S.Y.; Danquah, M.K.; Zhang, J.; Chiong, T.; Meunier, L.; Rahman, M.M. Selective adsorption of organic dyes from aqueous environment using fermented maize extract-enhanced graphene oxide-durian shell derived activated carbon composite. Chemosphere 2023, 339, 139742. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Machelak, K.; Nosal-Wiercińska, A.; Pietrzak, R. Microwave Heating for Synthesis of Carbonaceous Adsorbents for Removal of Toxic Organic and Inorganic Contaminants. Molecules 2023, 28, 6825. [Google Scholar] [CrossRef]
- Jasiewicz, K; Pietrzak, R. The influence of pore generating agent on the efficiency of copper and iron ions removal from liquid phase by polyethersulfone membranes. Chemical Engineering Journal 2013, 228, 449–454. [Google Scholar] [CrossRef]
- Krason, J.; Pietrzak, R. Removal of Iron and Copper Ions from the Liquid Phase by Modified Polymeric Membranes. Journal of Polymers and the Environment 2018, 26, 3237–3242. [Google Scholar] [CrossRef]
- Hofman, M.; Pietrzak, R. Adsorbents obtained from waste tires for NO2 removal under dry conditions at room temperature. Chemical Engineering Journal 2011, 170, 202–208. [Google Scholar] [CrossRef]
- Di Bella, G.; Trapani, D. A Brief Review on the Resistance-in-Series Model in Membrane Bioreactors (MBRs). Membranes 2019, 9, 24. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors. Preparation, Optimization and Selection. John Wiley & Sons, Ltd, 2011. [Google Scholar]
- Bazan, A.; Nowicki, P.; Pietrzak, R. The influence of activation procedure on the physicochemical and sorption properties of activated carbons prepared from pistachio nutshells for removal of NO2/H2S gases and dyes. Journal of Cleaner Production 2017, 152, 211–222. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
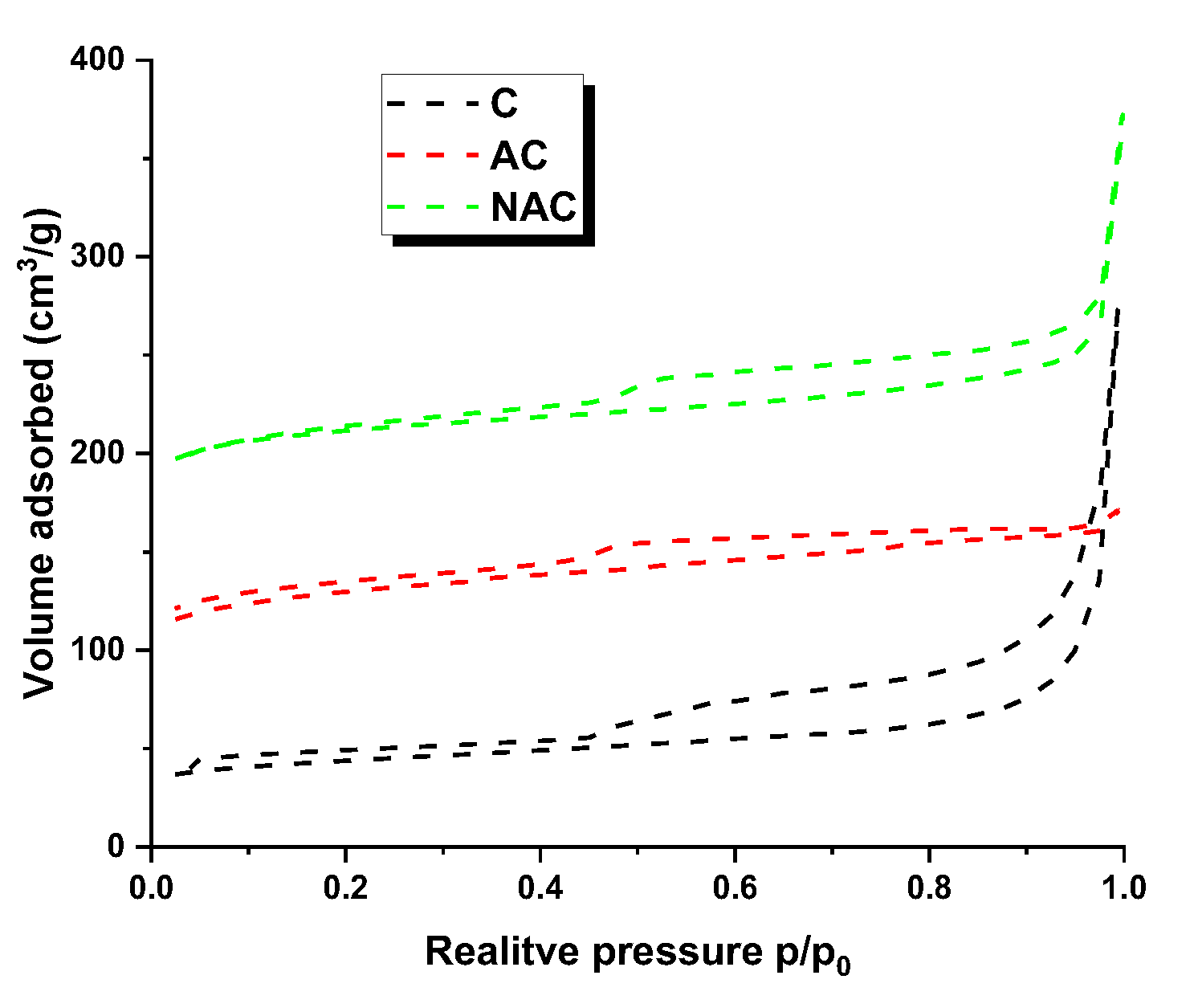

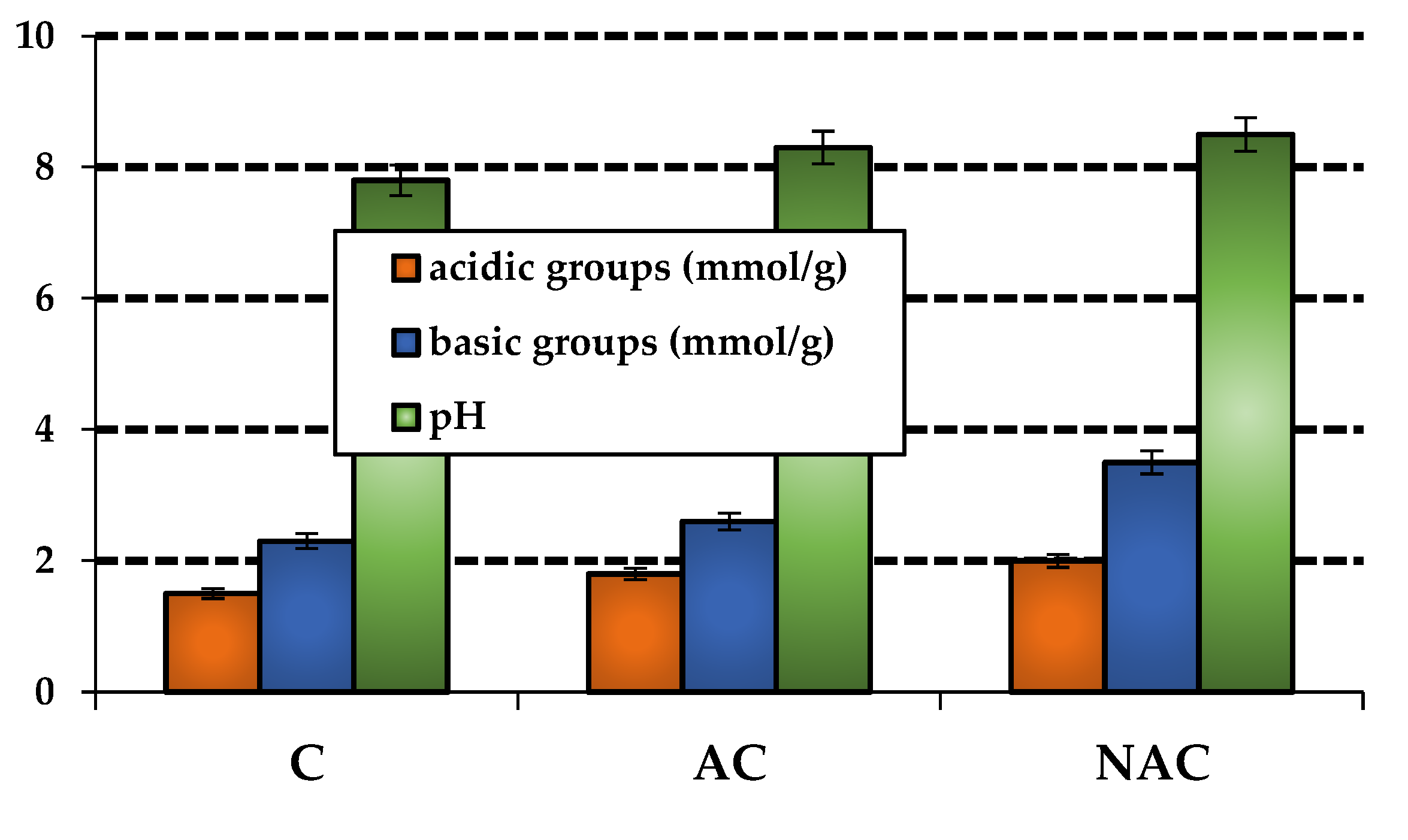
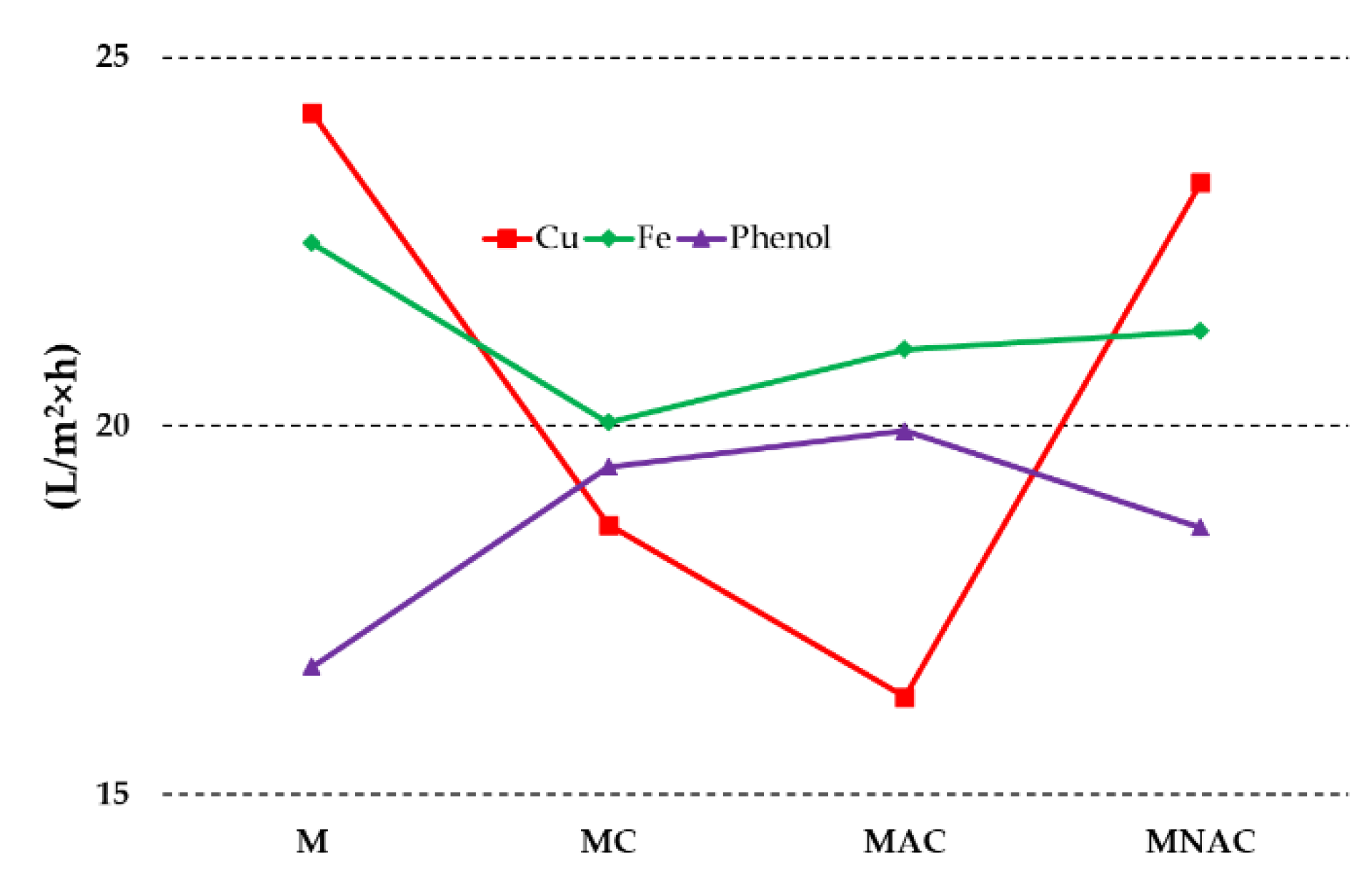
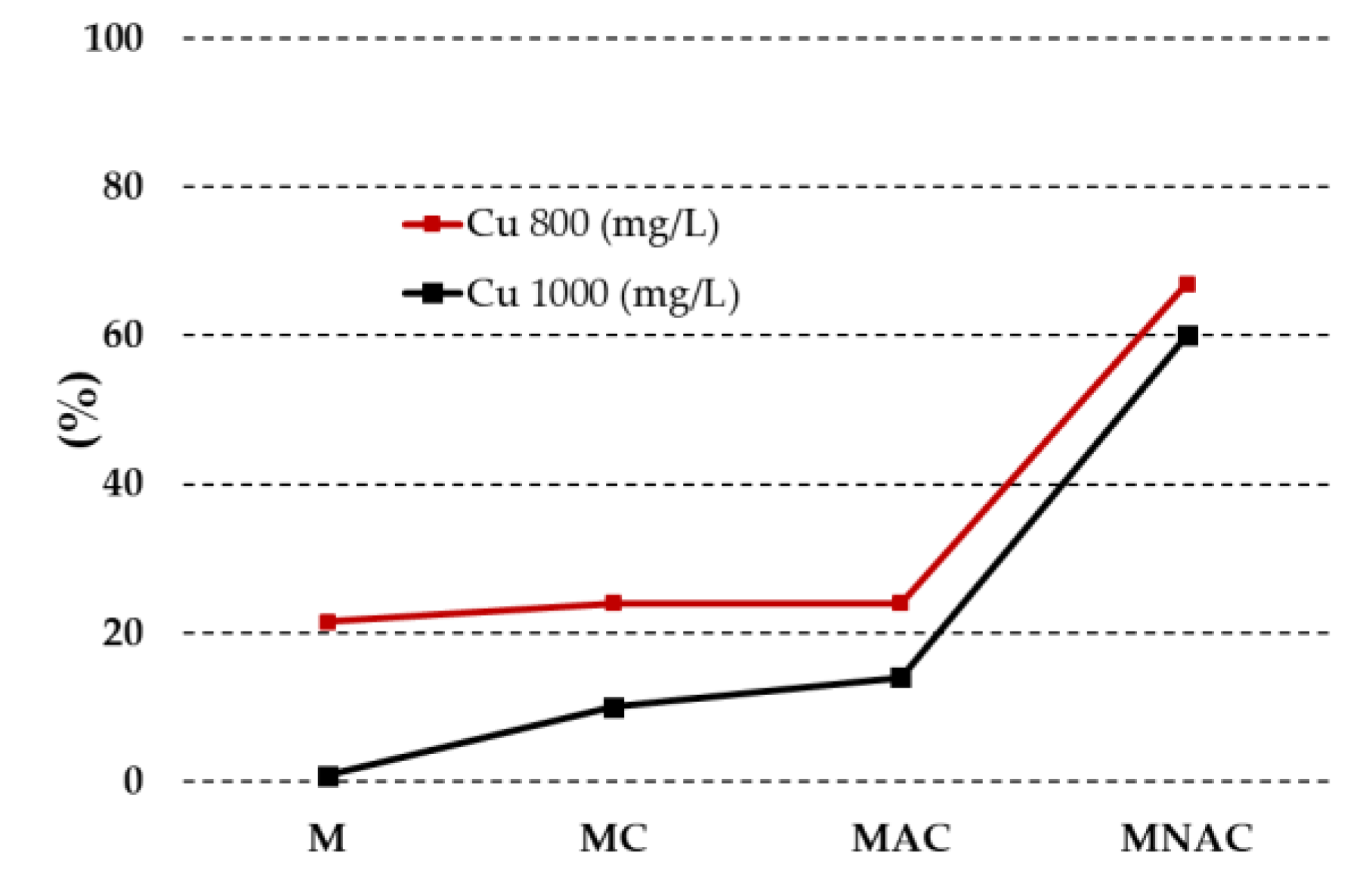
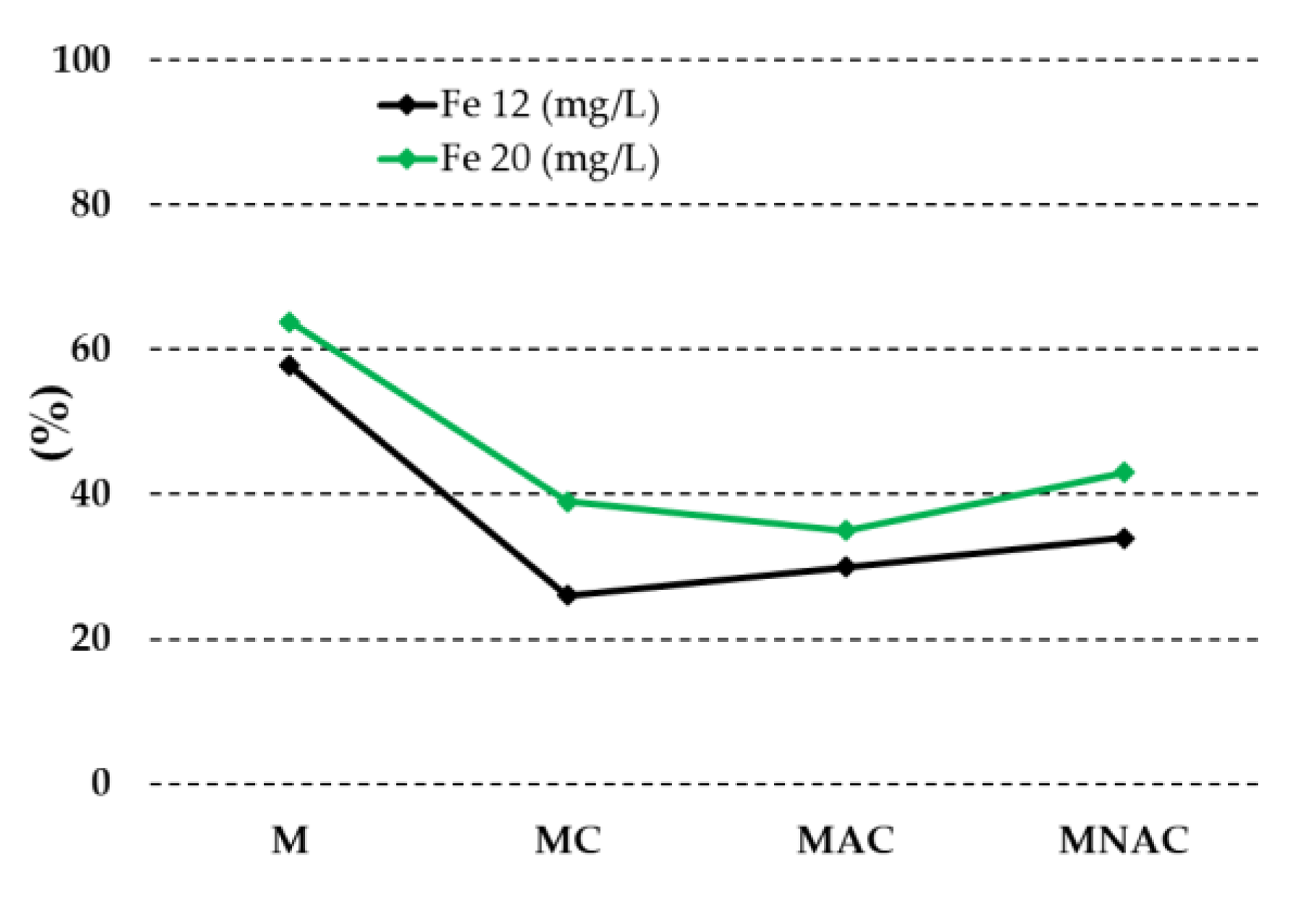
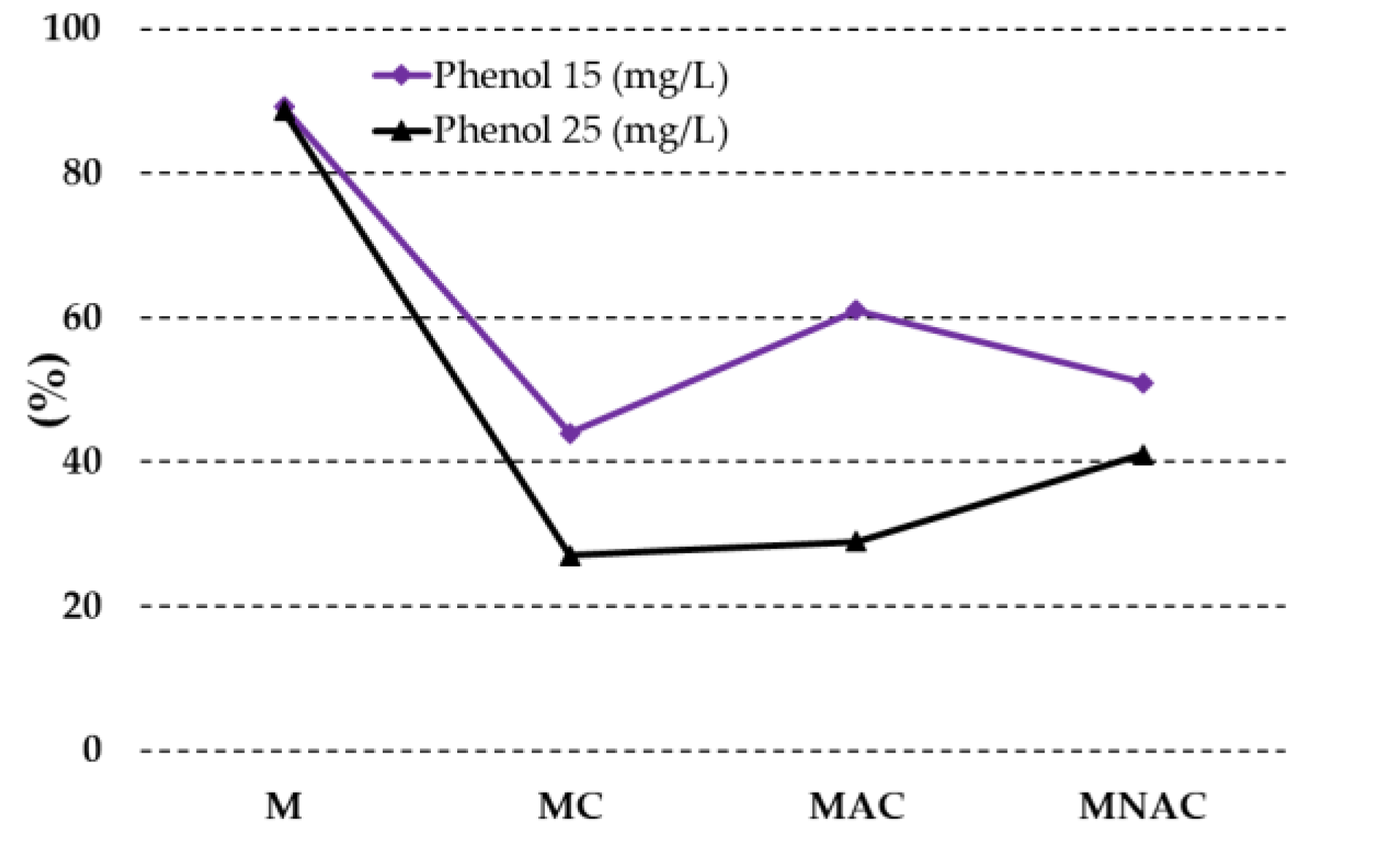
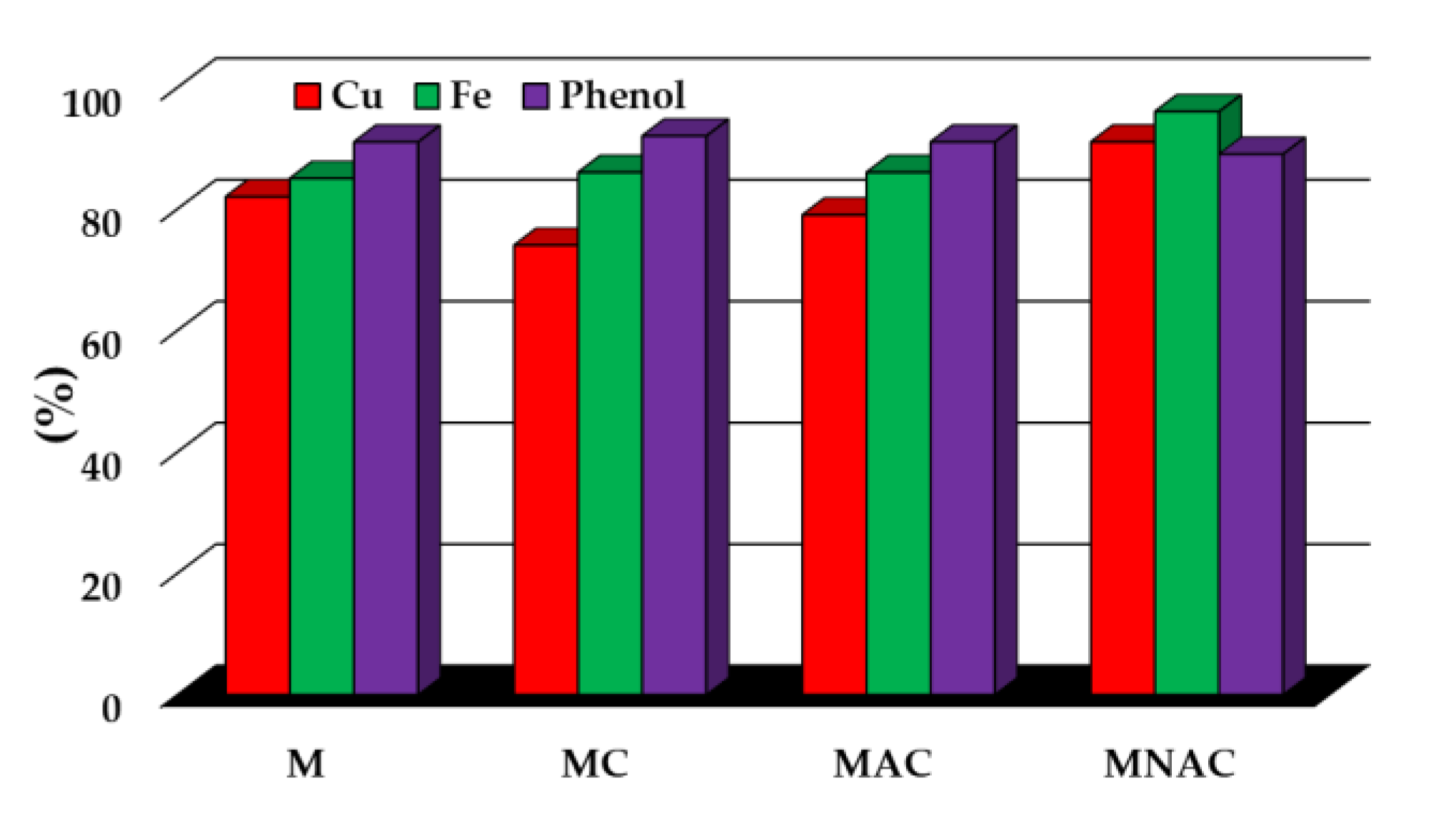
| Carbon materials | Cdaf | Hdaf | Ndaf | Sdaf | Odaf,1 | Ash |
|---|---|---|---|---|---|---|
| C | 69.2 | 2.1 | 2.9 | 0.2 | 25.7 | 12.1 |
| AC | 74.6 | 2.5 | 3.0 | 0.1 | 19.8 | 14.3 |
| NAC | 86.9 | 2.7 | 4.7 | 0.1 | 5.6 | 7.8 |
| Carbon materials | Iodine number (mg/g) | Surface area (m2/g) |
Total pore volume (cm3/g) |
Micropore volume (cm3/g) |
Average pore diameter (nm) |
|---|---|---|---|---|---|
| C | 205 | 125 | 0.38 | 0.04 | 7.32 |
| AC | 356 | 749 | 0.52 | 0.39 | 4.22 |
| NAC | 644 | 888 | 0.60 | 0.44 | 4.15 |
| Membrane | ε (%) | EWC (%) | Contact angle |
|---|---|---|---|
| M | 50.31 | 76.74 | 62.80 ± 1.47 |
| MC | 68.60 | 88.21 | 60.08 ± 4.09 |
| MAC | 38.01 | 80.13 | 57.12 ± 2.97 |
| MNAC | 62.71 | 85.62 | 58.24 ± 3.57 |
| Membrane | Acidic groups | Basic groups | Total content of oxygen groups |
|---|---|---|---|
| M | 3.69 | 1.66 | 5.35 |
| MC | 2.57 | 5.05 | 7.62 |
| MAC | 5.19 | 4.60 | 9.79 |
| MNAC | 2.80 | 5.19 | 7.99 |
| Membrane | Rm (x1013) | Rp (x1013) | Rc (x1013) | Rt (x1013) |
|---|---|---|---|---|
| M | 4.65 | 5.82 | 5.80 | 16.27 |
| MC | 5.79 | 7.80 | 9.07 | 22.66 |
| MAC | 6.64 | 8.42 | 10.20 | 25.26 |
| MNAC | 4.65 | 5.15 | 4.52 | 14.32 |
| Membrane | Rm (x1013) | Rp (x1013) | Rc (x1013) | Rt (x1013) |
|---|---|---|---|---|
| M | 4.87 | 5.71 | 5.25 | 15.83 |
| MC | 5.49 | 6.36 | 6.45 | 18.30 |
| MAC | 5.14 | 5.98 | 6.38 | 17.50 |
| MNAC | 5.09 | 5.34 | 5.08 | 15.51 |
| Membrane | Rm (x1013) | Rp (x1013) | Rc (x1013) | Rt (x1013) |
|---|---|---|---|---|
| M | 6.59 | 7.19 | 6.41 | 17.19 |
| MC | 5.58 | 6.07 | 5.57 | 17.22 |
| MAC | 5.62 | 6.12 | 5.95 | 17.69 |
| MNAC | 5.80 | 6.54 | 6.27 | 18.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





