You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
UPLC-PDA-ESI-QDA Characterization and Evaluation of the Antioxidant and Anxiolytic Activities of the Ethanolic Extract of Sarcomphalus joazeiro (Mart.) Hauenschild Leaves
Altmetrics
Downloads
98
Views
45
Comments
0
This version is not peer-reviewed
Abstract
Anxiety is a multifactorial pathology associated with oxidative stress and changes in different neurotransmitters in the central nervous system (CNS). Sarcomphalus joazeiro (Rhamnaceae) has a predicted effect on the CNS, being considered an alternative for pre-clinical investigations of anxiolytic drugs. The objective of this study is to evaluate the chemical composition, analyze the antioxidant capacity and the anxiolytic effect of the ethanolic extract of S. joazeiro leaves (EEFSJ) in zebrafish. The chemical profile was analyzed by liquid chromatography coupled to mass spectrometry (UPLC-PDA-ESI-QDA). Antioxidant action by DPPH• elimination and ABTS•⁺ capture assays. The 96 h acute toxicity and open field and light and dark tests, for sedative and anxiolytic evaluation, respectively, were applied in vivo, using zebrafish. It was possible to identify 11 compounds from the flavonoid and saponin class. EEFSJ exhibited a mean inhibitory concentration (IC50) of 185.2 ± 2.2 µg/mL and 74.17 ± 1.5 µg/mL against the DPPH• and ABTS•⁺ radicals, respectively. In vivo, EEFSJ demonstrated reduced locomotor activity and an anxiolytic effect similar to diazepam via GABAergic systems, without presenting toxicity within 96 h. These results suggest the safety of using EEFSJ for the development of clinical trials for the production of new anxiolytic drugs.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Anxiety is considered pathological when it causes psychological and social harm to the individual [1]. In recent years, anxiety has been one of the most prevalent neuropsychiatric disorders in the world [2]. According to the World Health Organization – WHO, around 3.6 % of the world population is affected by the disease, which in Brazil affects approximately 9.3 % of the population, ranking among the countries with the highest rates [3].
The etiology of anxiety is multifactorial and is mainly associated with interactions of genetic, neurobiological, psychological variables and environmental influence [4]. In the central nervous system (CNS), several neurotransmission pathways contribute to the mechanisms that mediate anxiety, especially changes in the serotonergic and GABAergic systems [1]. Furthermore, high levels of polyunsaturated acids and low reserves of antioxidant species in the CNS contribute to making neurons more vulnerable to oxidative stress and triggering neuroinflammation, associated with the development of pathological anxiety [5].
Benzodiazepines (BZDs), γ-aminobutyric acid (GABA) receptor agonists, and serotonin reuptake inhibitors (SSRIs) are commonly used anxiolytic drugs in the treatment of anxiety [3]. However, chronic use of BZD produces tolerance, and abrupt cessation of treatment can lead to a withdrawal syndrome [6]. From another perspective, the chronic use of SSRIs can reduce motor coordination, drowsiness, sedation and, when used for a prolonged period, they can also lead to dependence and withdrawal syndromes, and in high doses they can be fatal [7].
The adverse effects of these medications are the main reason why there is intensified research into the anxiolytic effects of products of natural origin. In general, plants synthesize a diversity of substances derived from their secondary metabolism, which often present satisfactory results in therapy against various psychological disorders [8].
Species from the Rhamnaceae family are commonly used in therapy against neurological diseases [9]. Among these, Sarcomphalus joazeiro (Mart.) Hauenschild, previously classified as Ziziphus joazeiro Mart [10], can be considered an alternative for pre-clinical investigations. Popularly known as “juá”, “joazeiro”, it is native to the Brazilian Northeast and endemic to the caatinga. In traditional medicine, it is used in the therapy of dermatological, respiratory, and digestive problems, in addition, it exhibits antioxidant, anti-inflammatory and antimicrobial properties [11]. Chemical data indicate the presence of flavonoid and saponin derivatives, which have reported and well-established anxiolytic activities [12], [13].
Animal research is being carried out as a model to screen natural products capable of combating anxiety [3]. Zebrafish (Danio rerio) have been a useful model as they possess key neuromediation systems, such as neurotransmitter receptors, transporters, sensitivity to anxiolytics, and behavioral paradigms similar to mammals [14]. Furthermore, they have a signaling system developed with high sensitivity to GABAergic sedatives [15].
Considering the importance of finding new active substances for use as an anxiolytic, this study aimed to identify the chemical components through hyphenated analytical methods and evaluate the antioxidant and anxiolytic capacity of the S. joazeiro leaf extract.
2. Results
2.1. Chemical Characterization
The EEFSJ was analyzed using UPLC-PDA-ESI-QDA in negative ESI mode. The constituents were identified based on their exact masses and comparison with data reported in the literature, allowing the identification of 11 peaks (Table 1). In summary, the peak with tR of 5.63 min refers to a nitrogenous compound, the peaks that ranged from 6.98 to 7.97 min are attributed to phenolic compounds and from 8.30 to 9.88 min to saponins. The structural representations of the identified compounds are illustrated in Figure 1.
The structural representations of the identified compounds are illustrated in Figure 1.
2.2. Antioxidant Activity
The DPPH• radical scavenging assay is routinely applied to evaluate the antiradical properties of different compounds [20]. In this assay, EEFSJ exhibited a mean inhibitory concentration (IC50) of 185.2 ± 28.5 µg/mL and a maximum percentage of DPPH• radical inhibition of 84.13 %, while ascorbic acid exhibited an IC50 of 8.871 ± 0.9480 µg/mL and maximum percentage of 89.75 %. (Figure 2).
In the ABTS•+ radical cation capture assay, EEFSJ presented a maximum percentage of 88.47 % and IC50 of 99.78 ± 25.61 µg/mL. Ascorbic acid presented values greater than 50 % at concentrations greater than 10 µg/mL, as shown in Figure 3.
2.3. 96h Acute Toxicity
Zebrafish is an experimental model that presents genetic and physiological similarity to humans, providing promising results regarding drug safety and anxiolytic evaluation (Caballero e Candiracci, 2018; Kalueff et al., 2014). There were no deaths after oral administration (LD50 ˃ 400 mg/kg), and no apparent anatomical changes in the animals during this period (Table 2) indicating preclinical safety in the use of EEFSJ [21].
2.4. Locomotor Activity (Open Field Test)
The open field test provides simultaneous measurements of zebrafish locomotion, which allow establishing a correlation between behavioral performance and neurobiological mechanisms that mediate behavior [21]. In this study, the locomotion of zebrafish was altered in the treatment with EEFSJ at all doses analyzed, as the animals had reduced locomotor activity when compared to the control group (Figure 4), presenting a statistically significant difference, indicating action on the CNS and sedative effect, similar to DZP (**** p < 0.0001 vs. CONTROL; ### p < 0.001; #### p < 0.0001 vs. DZP).
2.5. Anxiolytic Activity
The light-dark test using the adult zebrafish model is used to screen anxiolytic drugs similar to diazepines [22]. Due to the natural aversion of zebrafish to illuminated environments, this model allows the assessment of the animal’s anxiety level through the frequency of transitions and time spent in the illuminated compartment of the aquarium. (Kalue et al., 2014). Treatment with EEFSJ at the doses tested (40, 200 and 400 mg/kg) increased (* p < 0.05; ** p <0.01; **** p <0.0001 vs CONTROL) the time of permanence of the animals in the light region of the aquarium (Figure 5), demonstrating an anxiolytic effect similar to DZP.
2.6. Involvement of the GABAergic System
Flumazenil (FMZ), a competitive antagonist of the GABAA receptor in the αβγ subunits, antagonizes the sedative effects caused by BZD overdoses and reverses these effects, besides to preventing respiratory depression by blocking GABA receptors [7]. In this study, the lowest dose of EEFSJ (40 mg/kg) caused anxiolytic behavior in the animals and its effect was reversed (#### p <0.0001, vs. FMZ + EEFSJ; Figure 6) by Fmz, indicating that the anxiolytic effect of EEFSJ is related to the GABAergic system.
3. Discussion
In phytochemical screening studies, S. joazeiro extract exhibited classes of secondary metabolites, such as flavonoids, saponins and tannins, which are associated with different biological activities [12]. Previous studies performed UPLC-ESI-QTOF-MS analysis and showed the presence of rutin (2), kaempferol (3), isorhamnetin (4, 5 and 7), quercetin (6) and saponin derivatives (8, 9, 10 and 11), corroborating this study [16], [23].
Compounds 4 and 7 showed the same peak of the deprotonated molecular ion [M−H]− m/z 477 (C29H17O7). Previous research suggests that both are isomers of isorhamnetin-3-O-hexoside, due to the detection of fragment ions m/z 315 and m/z 162 (Matos et al., 2021). From a structural point of view, the flavonoids found (2, 3, 4, 5, 6 and 7) are characterized by the presence of two aromatic rings and a heterocyclic benzopyran ring containing oxygen and hydroxyl substituents [24].
Saponins are widely found in the Rhamanaceae family. In the species S. joazeiro, tetracyclic triterpenes of the dammarane type are frequently reported [25]. Masullo et al., (2019) proved the presence of these saponins through structural elucidation by 2D NMR. From a structural point of view, these derivatives are differentiated by their side chains, which correspond to 16.23:16.30 diepoxidammarane (8, 9, 10 e 11). Previous studies of species from the Rhamnaceae family, including Z. jujuba [19], [26], and Z. mauritiana [27] report the presence of these saponins.
The antioxidant activity of species of the genus Ziziphus reported in the literature agrees with these data, in which Z. cotinifolia exhibited IC50 values of 7.2 ± 1.5 μg/ml and 6.4 ± 0.3 μg/mL, for the DPPH• and ABTS•⁺ method, while Z. jujuba showed IC50 of 47.37 mmol of trolox, for ABTS•⁺. On the other hand, in the DPPH• elimination test, Brito et al., (2015) found higher IC50 values than those found in this study (735,72 μg/mL). In an HPLC/DAD analysis, the authors confirmed a low content of rutin (0.97 %) and quercetin (0.52 %) in the extract.
The high antioxidant action exerted by EEFSJ in this study can be attributed to the higher content of rutin (2) and quercetin (6) in the composition, which may justify the difference in the results found in the studies, considering that these are the main natural flavonoids responsible for enhancing the antioxidant effects of plant extracts. This variation in composition is due to the influence of extractive methods, organic solvents, as well as seasonal factors, such as location, collection period and type of soil [28].
The structures of flavonoids found in the present study allow them to act by combating free radicals through the donation of hydrogen atoms from a hydroxyl group of their aromatic structure, which has the ability to support an unpaired electron through its displacement around the entire molecule electron system [29], [20]. Furthermore, they can also act as scavengers of pro-inflammatory and neurotoxic species in the CNS [9].
The intensity of the anxiolytic effect observed in this study was inversely proportional to the concentration of EEFSJ. Silva et al., (2023) recently reported similar behavior of S. joazeiro extract in the same experimental model of zebrafish. These data propose that EEFSJ when administered in high doses (> 200 mg/kg) may cause tolerance and adverse effects similar to DZP. The administration of high doses of DZP (> 30 mg/kg) is capable of developing tolerance, causing compensatory changes in GABA receptors, which become less responsive, and as a consequence, reduce their inhibitory actions [30]. Furthermore, higher doses of DZP can cause CNS depression, whose additional non-classical BZD binding sites on GABAARs can cause adverse action [15].
EEFSJ also altered the locomotion of zebrafish at all concentrations tested, exhibiting behavior similar to DZP. Recently, studies of Z. cotinifolia (Rhamnaceae) using the same experimental model showed similar results [14]. BZDs produce anxiolytic and sedative effects by binding to high-affinity sites located at the α1/2/3/5+/γ2− interface of synaptic GABAARs to enhance GABA-mediated post-synaptic membrane hyperpolarization [15]. Therefore, the alteration of zebrafish locomotion caused by EEFSJ can be attributed to the high affinity for the GABAA receptor binding sites, which are capable of reducing central activity, causing an anxiolytic and/or sedative action similar to BDZ.
The involvement of GABAergic neurotransmission was evaluated through pre-treatment with flumazenil (Fmz) [7]. EEFSJ demonstrated anxiolytic behavior in animals and its effect was reversed by FMZ (Figure 6), proving that EEFSJ is capable of activating the GABAA receptor in the same region as BZD [31].
Clinical and pre-clinical studies prove that flavonoids and saponins act through selective binding to GABAA receptors, promoting membrane hyperpolarization by allowing the influx of chloride anions, accompanied by inhibition of excitatory transmission, which consequently contribute to the reduction of anxiety disorders [9], [32]. This hypothesis is corroborated by previous studies that show the anxiolytic potential of S. joazeiro, flavonoids and saponins through GABAergic neurotransmission in zebrafish and mice [8], [11], [33].
Quercetin (6) has been widely investigated due to its antagonistic actions on GABAUmρ1R, this effect may be mediated by a redox-independent allosteric mechanism, in addition to affecting the expression of GABAUmα5R, which could be a mechanism to attenuate the severity of crises [34]. In addition, rutin (2) is involved in GABAergic neurotransmission without involvement of BZD receptors [35]. Previous research also shows that rutin (2) can act on different anxiolytic mechanisms, for example, combating oxidative stress in the CNS, modulating the release of the neurotransmitters serotonin, norepinephrine, opioids and activating opioid receptors [8]. Quantitative models prove that the increased affinity of these flavonoids (2, 3, 4, 5, 6, 7) for the BZD receptor binding site is associated with the presence of electronegative substituents at 6 and 3' of the flavone backbone [13].
This study shows the potential of EEFSJ as an antioxidant and anxiolytic agent, making it a promising candidate for therapy against psychological disorders, especially anxiolytics.
4. Materials and Methods
4.1. Collection and Preparation of Extract
The leaves (484 g) were collected in Tabocas, located in the rural area of the Municipality of Exu, Pernambuco, Brazil. The plant material was sanitized, crushed, and subjected to extraction using the cold maceration method for 72 h at room temperature, in hexane to degrease, and subsequently submerged in 99.5 % ethanol to obtain the crude extract. Organic solvents were removed using a rotary evaporator under reduced pressure. The yield obtained from the ethanolic extract of S. joazeiro leaves (EEFSJ) was 4.78 %.
4.2. Chemical Characterization
4.2.1. Extract Preparation
Approximately 4 mg of EEFSJ was dissolved in 1 mL of a 50/50 % methanol/water mixture. The material was filtered through a 0.22 μm PTFE filter, placed in vials and stored at -80 °C for analysis.
4.2.2. Analisys by UPLC-PDA-ESI-QDA
Analysis was performed using an Acquity UPLC system (Waters, USA) coupled to a PDA (210-600 nm) and QDa mass system (Quadrupole, Waters). A Waters Acquity BEH C18 column for separation condition (150 mm × 2,1 mm, 1,7 μm) was set at 40 °C. An injection volume of 5 μL aliquot of EEFSJ was subjected to an exploratory gradient with the mobile phase composed of deionized water (A) and acetonitrile (B), both containing formic acid (0.1 % v/v). The optimized instrumental parameters were as follows for negatives: capillary voltage at 0.8 kV, cone voltage at 15 V, source temperature at 120 °C, desolvation temperature at 350 °C, desolvation gas flow at 500 L/h. The system was controlled using Empower 3 software (Waters Corporation).
4.3. Antioxidant Assays
4.3.1. Determination of Antioxidant Activity by the DPPH● Method
The free radical scavenging activity was determined by the DPPH● (1,1, diphenyl-2-picrylhydrazyl) photocolorimetric method, proposed by Rufino, et al., (2007) with adaptations [36]. EEFSJ concentrations ranged from 10 to 1000 µg/mL. For the test, 20 μL of the sample, 80 μL of 95 % ethanol and 100 μL of the DPPH● radical solution (0.3 mM) were used. After 30 min of incubation at room temperature and protected from light, absorbance measurements were carried out using a UV-visible spectrophotometer at 518 nm. The blank samples and the antioxidant standard (ascorbic acid) were quantified under the same conditions with 20 μL of standards and 180 μL of 95 % ethanol. The results were calculated according to equation 1.
Equation 1
IP % = 100 ‐ {[(Abs Sample – Abs blank) / Abs negative control] x 100}
Where: IP % = Inhibition Percentage; Abs: Absorbances.
4.3.2. ABTS●+ Free Radical Capture
For the ABTS●+ free radical capture assay, the methodology proposed by Rufino, et al., (2006) was used [37]. The ABTS●+ radical was prepared by mixing a 7 mM ABTS diammonium salt solution with a potassium persulfate solution (final concentration of 2.45 mM), both prepared in phosphate buffer saline (pH 7.4). The resulting solution (stock solution) was kept in an amber bottle at room temperature for 16 h to form the free radical ABTS●+. Subsequently, it was diluted with phosphate buffer saline (pH 7.4) until an absorbance value of 0.90-1.0 ± 0.02 was obtained at 734 nm. An aliquot of 2.970 µL of this solution was added with 30 µL of concentrations of 10 to 1.000 µg/mL of the extract, as well as the positive controls of ascorbic acid. The assay was carried out in triplicate and readings were taken 3 min after the start of the reaction in a spectrophotometer adjusted to 734 nm. The results were calculated according to equation 1.
4.4. Toxicity and Anxiolytic Assays
4.4.1. Zebrafish
Zebrafish (Danio rerio) (age 90 to 120 days; 0.4 ± 0.1 g, 3.5 ± 0.5 cm), wild, of both sexes, were purchased commercially in Fortaleza, CE. Animals were maintained in a glass aquarium (30 × 15 × 20 cm) of 10 L (n = 3/L), at a temperature of 25 ± 2°C, in 24-h light-dark cycles with chlorinated water (ProtecPlus®) and air pump with submerged filters, under a temperature of 25 ° C and pH 7.0, Circadian cycle of 10 - 14 h (light/dark). The fish received food (Spirulina®) ad libitum 24 h before the experiments. Before drug applications, the animals were anesthetized in ice-cold water and after the experiments, the animals were sacrificed by immersion in ice-cold water (2 and 4 °C) for 1 min until loss of opercular movements. The work was approved by the Ethics Committee on the Use of Animals of the Universidade Estadual do Ceará (CEUA-UECE; nº 04983945/2021), in accordance with the Ethical Principles of Animal Experimentation.
4.4.2. General Protocol
Zebrafish were randomly selected in the experiments, anesthetized in ice water, and transferred to a damp sponge, treated with 20 µL of EEFSJ (40; 200 and 400 mg/kg) or Diazepam (4 mg/kg), or 3% DMSO (control group – drug diluent) orally (v.o).
4.4.3. Assessment of Locomotor Activity (Open Field Test)
The open field test was performed to evaluate the presence or absence of changes in motor coordination in animals [38], whether due to the anxiolytic effect and/or muscle relaxation. Animals (n = 6/group) were pre-treated (20 µL; p.o.) at the same doses analyzed in the previous section. Diazepam (DZP; 4 mg/kg) and vehicle (DMSO 3 %) were used as positive and negative controls, respectively. After 60 min of treatment, the animals were added individually to glass Petri dishes (10x15 cm; with quadrants at the bottom of the dish), containing the same water as in the aquarium. The number of line crossings was recorded during 0-5 min and the percentage of each group was calculated.
4.4.4. 96. h Acute Toxicity
After the open field test, fish (n = 6 / grupo) treated orally with EEFSJ (40; 200 or 400 mg/kg; 20 µL) or control (vehicle: DMSO 3 %; 20 µL; i.pwere left at rest to analyze the mortality rate for a period of 96 h, recording the number of dead fish in each group every 24 h [39], with the lethal dose capable of killing 50 % of the animals (LD50) determined by the Trimmed Spearman-Karber mathematical method with a 95 % confidence interval.
4.4.5. Anxiolytic Assessment
An animal's anxiety behavior can be observed using the light/dark test. Similar to rodents, zebrafish naturally avoid illuminated areas [40]. The experiment was carried out in a glass aquarium (30 cm x 15 cm x 20 cm) divided into a light area and a dark area. The aquarium was filled to 3 cm with dechlorinated tap water, which simulated a new shallow environment different from the conventional aquarium and capable of inducing anxiety behaviors. In animals (n = 6/group) 20 µL of EEFSJ were administered orally at doses of 40 mg/kg, 200 mg/kg, or 400 mg/kg. The negative and positive control groups consisted of 3 % DMSO and 4 mg/kg Diazepam solution, respectively. After 60 min, the animals were placed individually in the light zone and the anxiolytic effect was measured based on the time spent in the light zone of the aquarium within 5 min of observation [22].
4.4.6. Assessment of GABAergic Neuromodulation
The GABAergic neuromodulation involved in the anxiolytic effect of the extracts was identified through pre-treatment with flumazenil (Fmz) (GABAA antagonist) before the light/dark test [41]. Fish (n = 6/group) were pretreated with Fmz (4 mg/kg; 20 μL; i.p.). After 15 min, the dose with the highest anxiolytic efficacy of the extracts (40 mg/kg; 20 μL; p.o.) found in the previously performed test, was administered. 3 % DMSO (vehicle; 20 μL; i.p.) was used as a negative control. After 60 min of treatments, the animals were subjected to the light/dark test.
4.5. Statistical Analysis
The results were expressed as mean values ± standard error of the mean for each group of 6 animals. After confirming the normality of distribution and homogeneity of the data, the differences between the groups were subjected to analysis of variance - one-way ANOVA, followed by the Tukey’s test. All analysis were performed using GraphPad Prism v software. 8.0.1. The level of statistical significance was set at 5 % (p<0.05).
5. Conclusions
This study showed the presence of flavonoids and saponins in the chemical composition of S. joazeiro, through UPLC-PDA-ESI-QDA. Among the biological assays tested, the extract showed antioxidant potential using the free radical scavenging methods DPPH• and the radical cation capture ABTS•⁺. In in vivo tests with zebrafish, the extract did not show reduced locomotor activity and an anxiolytic effect similar to DZP at the lowest dose tested (40 mg/kg) mediated by the activation of the GABAA receptor, without showing toxicity within 96 h.
These results suggest that S. joazeiro is a promising candidate for the development of specialized CNS-associated clinical trials. Research aimed at evaluating pharmacokinetics/pharmacodynamics and bioavailability, such as the encapsulation of nanoparticles, can help in the controlled delivery of the bioactive compounds present and assist in the progress of research.
Author Contributions
Research, writing, formatting and editing, Natália Kelly Gomes de Carvalho, Johnatan Wellisson da Silva Mendes, Débora Odília Duarte Leite, Mariana Pereira da Silva. Chemical characterization, Kirley Marques Canuto, Paulo Riceli Vasconcelos Ribeiro. Biological assays, Gerson Javier Torres Salazar, Mariana Amanda Maria Barros Alves, Ivana Carneiro Romão, Hélcio Silva dos Santos. Supervision, José Galberto Martins da Costa; the authors have read and agreed to the published version of the manuscript.”
Acknowledgment
This work was carried out at the Laboratório de Pesquisa de Produtos Naturais (LPPN) of the Departamento de Química Biológica at the Universidade Regional do Cariri (URCA), with support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) and Instituto Nacional de Ciência e Tecnologia – Alimentos (INCT-ALIM).
Conflicts of Interest
The authors declare no conflict of interest.
References
- T. I. dos Santos Sampaio et al., “Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio),” J. Ethnopharmacol., vol. 224, no. February, pp. 563–578, 2018. [CrossRef]
- H. P. S. Hozana Patrícia et al., “Anxiolytic-like effect of brominated compounds from the marine sponge Aplysina fulva on adult zebrafish (Danio rerio): Involvement of the GABAergic system,” Neurochem. Int., vol. 146, no. March, 2021. [CrossRef]
- N. C. de Melo et al., “Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) moldenke: A study on zebrafish (Danio rerio),” Pharmaceuticals, vol. 12, no. 3, 2019. [CrossRef]
- E. Palazidou, “The neurobiology of depression,” Br. Med. Bull., vol. 101, no. 1, pp. 127–145, 2012. [CrossRef]
- M. Shafiee et al., “Depression and anxiety symptoms are associated with prooxidant-antioxidant balance: A population-based study,” J. Affect. Disord., vol. 238, pp. 491–498, 2018. [CrossRef]
- G. P. da S. M. Matos, A. F. Dos Santos, D. M. S. Acioli, E. F. Da Silva, and J. I. Guerra Junior, “Benzodiazepínicos: uma revisão de literatura sobre uso indiscriminado, dependência e efeitos colaterais,” Brazilian J. Heal. Rev., vol. 7, no. 2, p. e67735, 2024. [CrossRef]
- J. da C. Xavier et al., “Anxiolytic-like and anticonvulsant effect in adult zebrafish (Danio rerio) through gabaergic system and molecular docking study of chalcone derived from natural products,” Biointerface Res. Appl. Chem., vol. 11, no. 6, pp. 14021–14031, 2021. [CrossRef]
- E. Aguirre-Hernández, M. E. González-Trujano, T. Terrazas, J. H. Santoyo, and P. Guevara-Fefer, “Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation,” Salud Ment., vol. 39, no. 1, pp. 37–46, 2016. [CrossRef]
- M. U. Rehman et al., “Neuroprotective Strategies for Neurological Disorders by Natural Products: An update,” Curr. Neuropharmacol., vol. 17, no. 3, pp. 247–267, 2018. [CrossRef]
- N. J. S. Araújo et al., “Chemical characterization UPLC-ESI-QToF-MSE, antibacterial and antibiofilm potential of Sarcomphalus joazeiro (MART.) Hauenschild,” Food Biosci., vol. 50, no. August, 2022. [CrossRef]
- B. de Souza et al., “Antibacterial activity and anxiolytic-like effect of Ziziphus joazeiro Mart. leaves in adult zebrafish (Danio rerio),” Fish Shellfish Immunol. Reports, vol. 5, no. April, 2023. [CrossRef]
- S. M. O. Brito et al., “Analysis of bioactivities and chemical composition of Ziziphus joazeiro Mart. using HPLC-DAD,” Food Chem., vol. 186, pp. 185–191, 2015. [CrossRef]
- J. R. Hanrahan, M. Chebib, and G. A. R. Johnston, “Flavonoid modulation of GABA A receptors,” Br. J. Pharmacol., vol. 163, no. 2, pp. 234–245, 2011. [CrossRef]
- D. V. de Azevedo et al., “Evaluation of antioxidant, toxicological and anxiolytic-like effect of ethanolic extracts of Ziziphus cotinifolia Reissek in adult zebrafish (Danio rerio),” Phytomedicine Plus, vol. 4, no. 1, 2024. [CrossRef]
- Y. Cao, H. Yan, G. Yu, and R. Su, “Flumazenil-insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immobility in zebrafish larvae,” Life Sci., vol. 239, p. 117033, 2019. [CrossRef] [PubMed]
- J. Cosmo Andrade et al., “Control of bacterial and fungal biofilms by natural products of Ziziphus joazeiro Mart. (Rhamnaceae),” Comp. Immunol. Microbiol. Infect. Dis., vol. 65, no. March, pp. 226–233, 2019. [CrossRef]
- Y. Pu, T. Ding, N. Zhang, P. Jiang, and D. Liu, “Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao,” Int. J. Food Prop., vol. 20, no. 1, pp. S26–S35, 2017. [CrossRef]
- Tools, “Integrated UPLC-HRMS, Chemometric Tools, and Metabolomic Analysis of Forage Palm (,” vol. 32, no. 8, pp. 1617–1627, 2021.
- M. Masullo, A. Cerulli, P. Montoro, C. Pizza, and S. Piacente, “In depth LC-ESIMS n -guided phytochemical analysis of Ziziphus jujuba Mill. leaves,” Phytochemistry, vol. 159, no. October 2018, pp. 148–158, 2019. [CrossRef]
- N. R. Sucupira, A. B. Da Silva, G. Pereira, and J. N. Da Costa, “Métodos Para Determinação da Atividade Antioxidante de Frutos,” UNOPAR Científica Ciências Biológicas e da Saúde, vol. 14, no. 4, pp. 263–269, 2014, [Online]. Available: http://revistas.unopar.br/index.php/biologicas/article/view/442.
- W. M. Arika, C. M. Kibiti, J. M. Njagi, and M. P. Ngugi, “Effects of DCM Leaf Extract of Gnidia glauca (Fresen) on Locomotor Activity, Anxiety, and Exploration-Like Behaviors in High-Fat Diet-Induced Obese Rats,” Behav. Neurol., vol. 2019, 2019. [CrossRef] [PubMed]
- L. Gebauer, N. Pagnussat, Â. L. Piato, I. C. Schaefer, C. D. Bonan, and D. R. Lara, “Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol,” Pharmacol. Biochem. Behav., vol. 99, no. 3, pp. 480–486, 2011. [CrossRef]
- J. C. Andrade et al., “UPLC-MS-ESI-QTOF characterization and evaluation of the antibacterial and modulatory antibiotic activity of Ziziphus joazeiro Mart. aqueous extracts,” South African J. Bot., vol. 123, pp. 105–112, 2019. [CrossRef]
- Z. Liu et al., “Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property,” J. Ethnopharmacol., vol. 267, p. 113630, 2021. [CrossRef] [PubMed]
- S. T. Sakna, Y. R. Maghraby, M. S. Abdelfattah, and M. A. Farag, “Phytochemical diversity and pharmacological effects of triterpenes from genus Ziziphus: a comprehensive review,” Phytochem. Rev., vol. 22, no. 6, pp. 1611–1636, 2023. [CrossRef]
- K. Yoshikawa, N. Shimono, and S. Arihara, “Antisweet substances, jujubasaponins I-III from zizyphus jujuba revised structure of ziziphin,” Tetrahedron Lett., vol. 32, no. 48, pp. 7059–7062, 1991. [CrossRef]
- S. Seri, T. A. Okpekon, P. A. Yao-Kouassi, A. A. Magid, C. Sayagh, and L. Voutquenne-Nazabadioko, “Saponins and flavonoid glycosides from the leaves of Ziziphus mauritiana Lam. native of a forest area of Ivory Coast,” Phytochem. Lett., vol. 37, no. March, pp. 5–9, 2020. [CrossRef]
- J. M. Macedo, L. G. P. J. M. Macedo, L. G. P. Souza, V. del C. Troncozo Valenzuela, A. B. Oliveira, R. Oliveira Castilho, and R. L. Ribeiro Jácome, “Variação sazonal nos teores de flavonoides, taninos e atividade antioxidante de Davilla rugosa poir,” Rev. Ciencias Farm. Basica e Apl., vol. 34, no. 4, pp. 585–590, 2013.
- T. C. de L. E. Silva, “AVALIAÇÃO COMPARATIVA DE CASCAS E FOLHAS DE Ziziphus joazeiro Mart (RHAMNACEAE) EM RELAÇÃO AOS PERFIS FITOQUIMICO E TOXICOLÓGICO E AS ATIVIDADES ANTIOXIDANTE E ANTIMICROBIANA,” p. 73, 2009.
- C. M. F. E Silva, D. I. M. Pereira, and G. V. Mesquita, “Análise do uso indiscriminado de benzodiazepínicos em graduandos de medicina de um centro universitário no Piauí,” Brazilian J. Heal. Rev., vol. 6, no. 6, pp. 28005–28020, 2023. [CrossRef]
- R. B. Lydiard, “The role of GABA in anxiety disorders,” J. Clin. Psychiatry, vol. 64, no. SUPPL. 3, pp. 21–27, 2003.
- S. K. J. Njapdounke et al., “Anxiolytic - Like properties of Hallea ciliata in mice,” African J. Tradit. Complement. Altern. Med., vol. 13, no. 4, pp. 1–7, 2016. [CrossRef]
- N. Bao, J. Ou, W. Shi, N. Li, L. Chen, and J. Sun, “Highly Efficient Synthesis and Structure–Activity Relationships of a Small Library of Substituted 1,4-Naphthoquinones,” European J. Org. Chem., vol. 2018, no. 19, pp. 2254–2258, 2018. [CrossRef]
- C. I. Calero, A. N. B. González, J. Gasulla, S. Alvarez, P. Evelson, and D. J. Calvo, “Quercetin antagonism of GABAAρ1 receptors is prevented by ascorbic acid through a redox-independent mechanism,” Eur. J. Pharmacol., vol. 714, no. 1–3, pp. 274–280, 2013. [CrossRef]
- J. L. Ríos, G. R. Schinella, and I. Moragrega, “Phenolics as GABAA Receptor Ligands: An Updated Review,” Molecules, vol. 27, no. 6, 2022. [CrossRef]
- M. do S. Rufino, R. E. Alves, E. S. De Brito, S. M. De Morais, C. D. G. Sampaio, and F. D. Saura-calixto, “ISSN 1679-6535 Julho, 2007 Fortaleza, CE,” pp. 0–3, 2007.
- M. do S. M. Rufino et al., “Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH.,” Comun. Técnico Online EMBRAPA, vol. 127, pp. 1–4, 2007, [Online]. Available: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/426953/1/Cot127.pdf.
- M. K. A. Ferreira et al., “Chalcones reverse the anxiety and convulsive behavior of adult zebrafish,” Epilepsy Behav, vol. 117, p. 107881, Apr. 2021. [CrossRef]
- Oecd, “Fish, acute toxicity test,” Guidel. Test. Chem., no. July, pp. 1–9, 1992.
- N. G. G. Gonçalves et al., “Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system,” J. Funct. Foods, vol. 66, no. April 2019, p. 103772, 2020. [CrossRef]
- C. K. Benneh, R. P. Biney, P. K. Mante, A. Tandoh, D. W. Adongo, and E. Woode, “Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—Involvement of GABAergic and 5-HT systems,” J. Ethnopharmacol., 2017. [CrossRef]
Figure 1.
Structural representation of the components found in the EEFSJ using the UPLC-PDA-ESI-QDA method.
Figure 1.
Structural representation of the components found in the EEFSJ using the UPLC-PDA-ESI-QDA method.
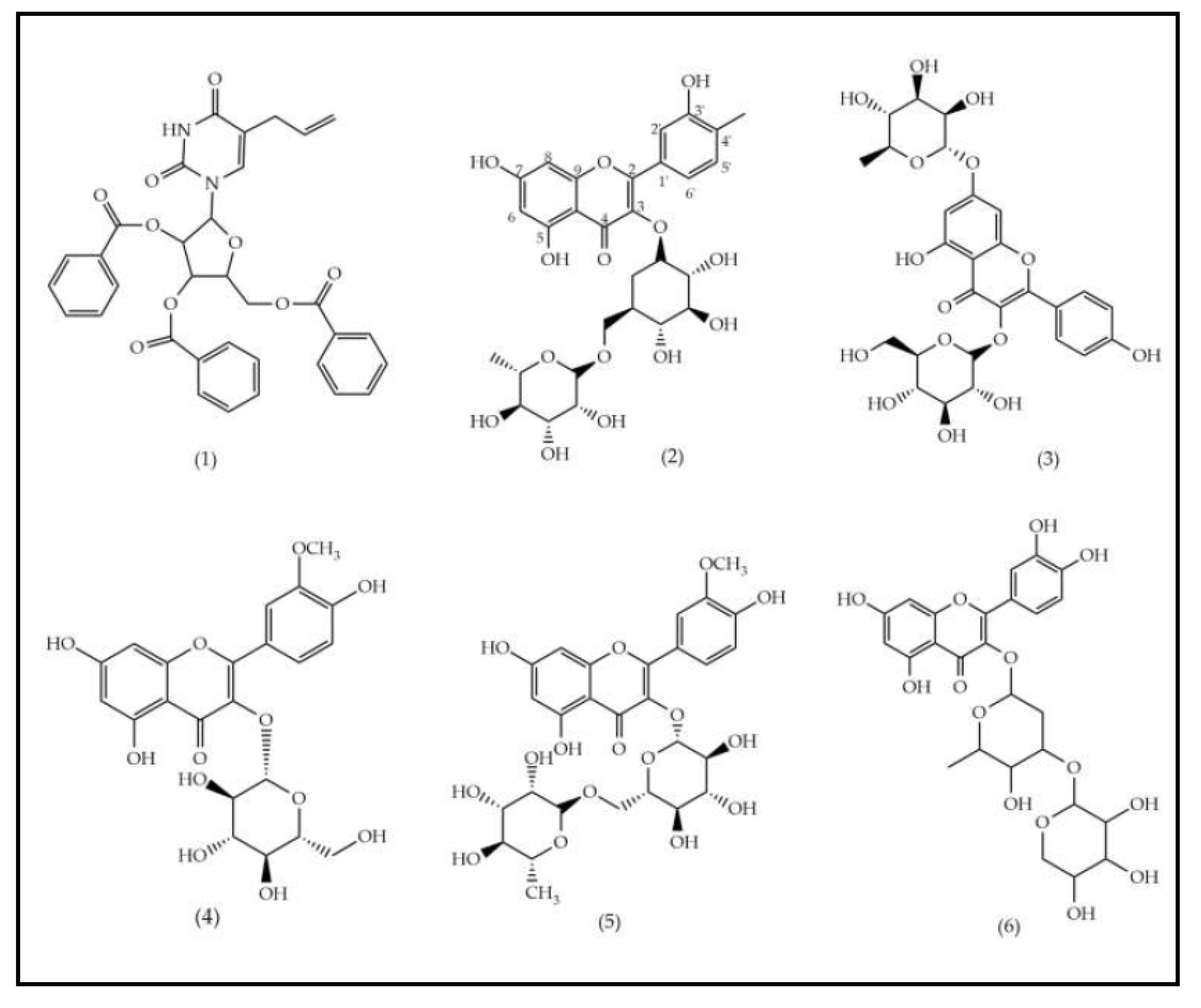
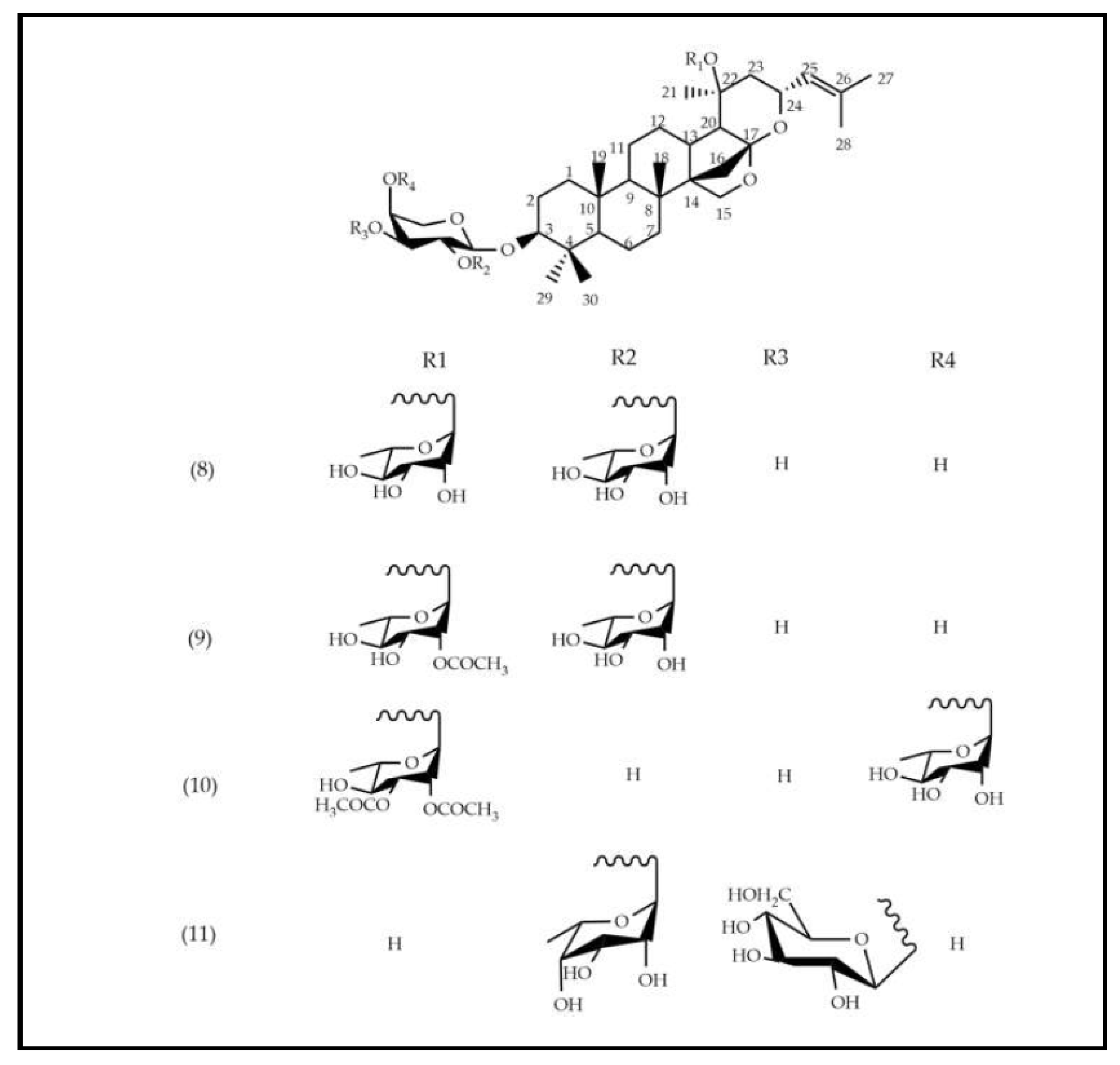
Figure 2.
DPPH• free radical scavenging from EEFSJ and antioxidant control. Souce: Author.
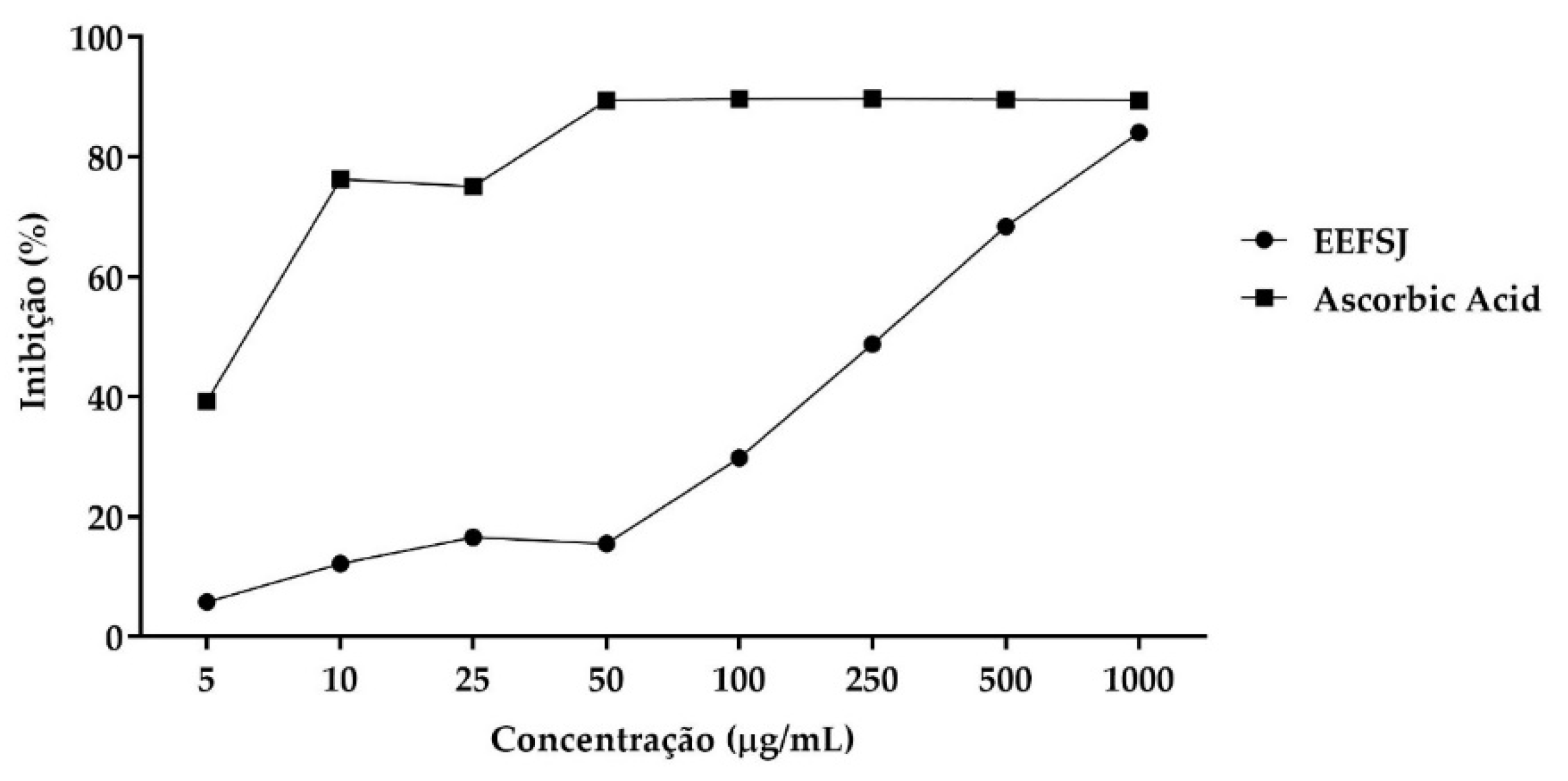
Figure 3.
ABTS•+ radical capture by EEFSJ and antioxidant control. Source: Author.
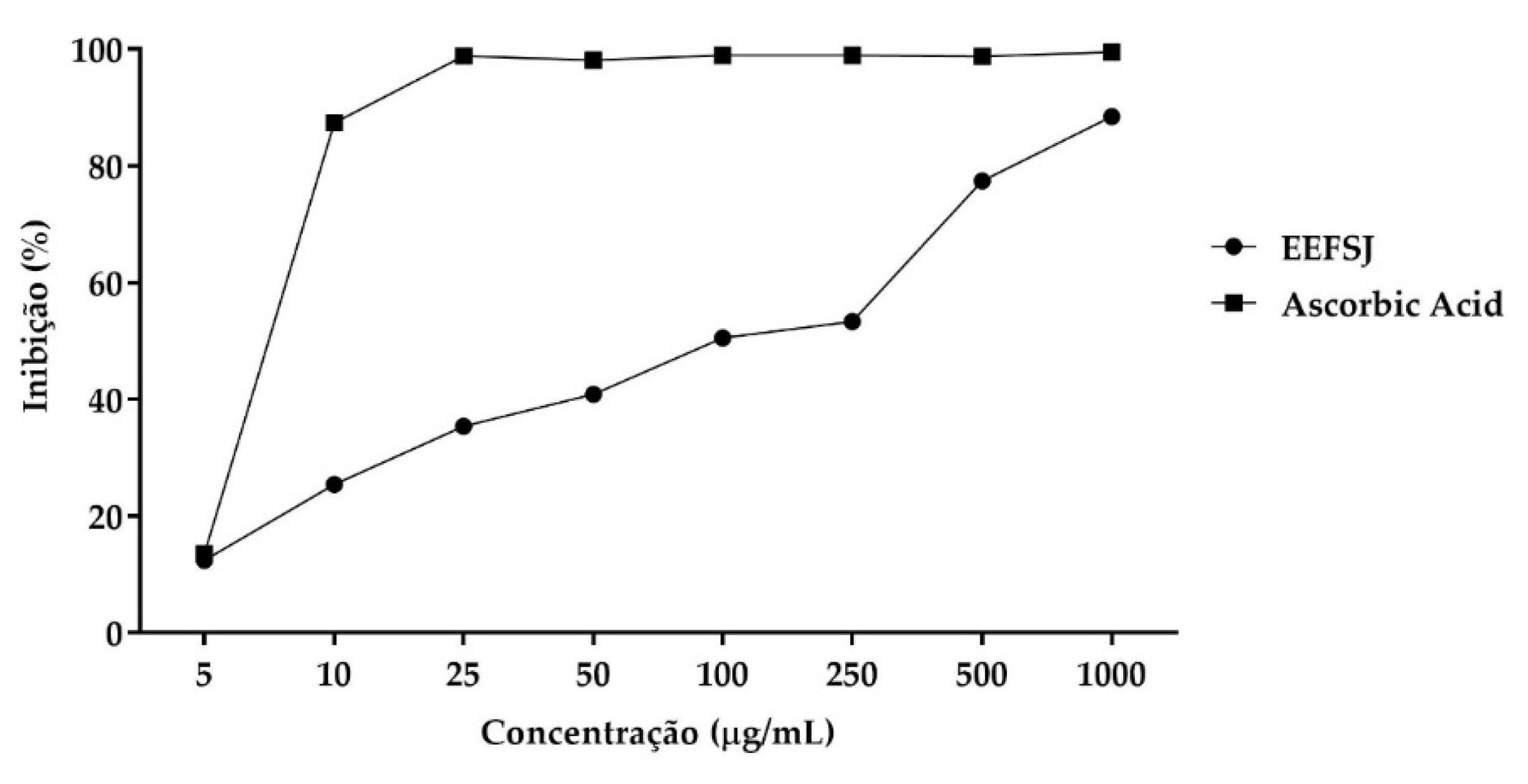
Figure 4.
Effect of EEFSJ on the locomotor behavior of adult zebrafish in the Open Field Test (0–5 min). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (**** p < 0,0001 vs. CONTROL; ### p < 0,001; #### p < 0,0001 vs. DZP).
Figure 4.
Effect of EEFSJ on the locomotor behavior of adult zebrafish in the Open Field Test (0–5 min). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (**** p < 0,0001 vs. CONTROL; ### p < 0,001; #### p < 0,0001 vs. DZP).
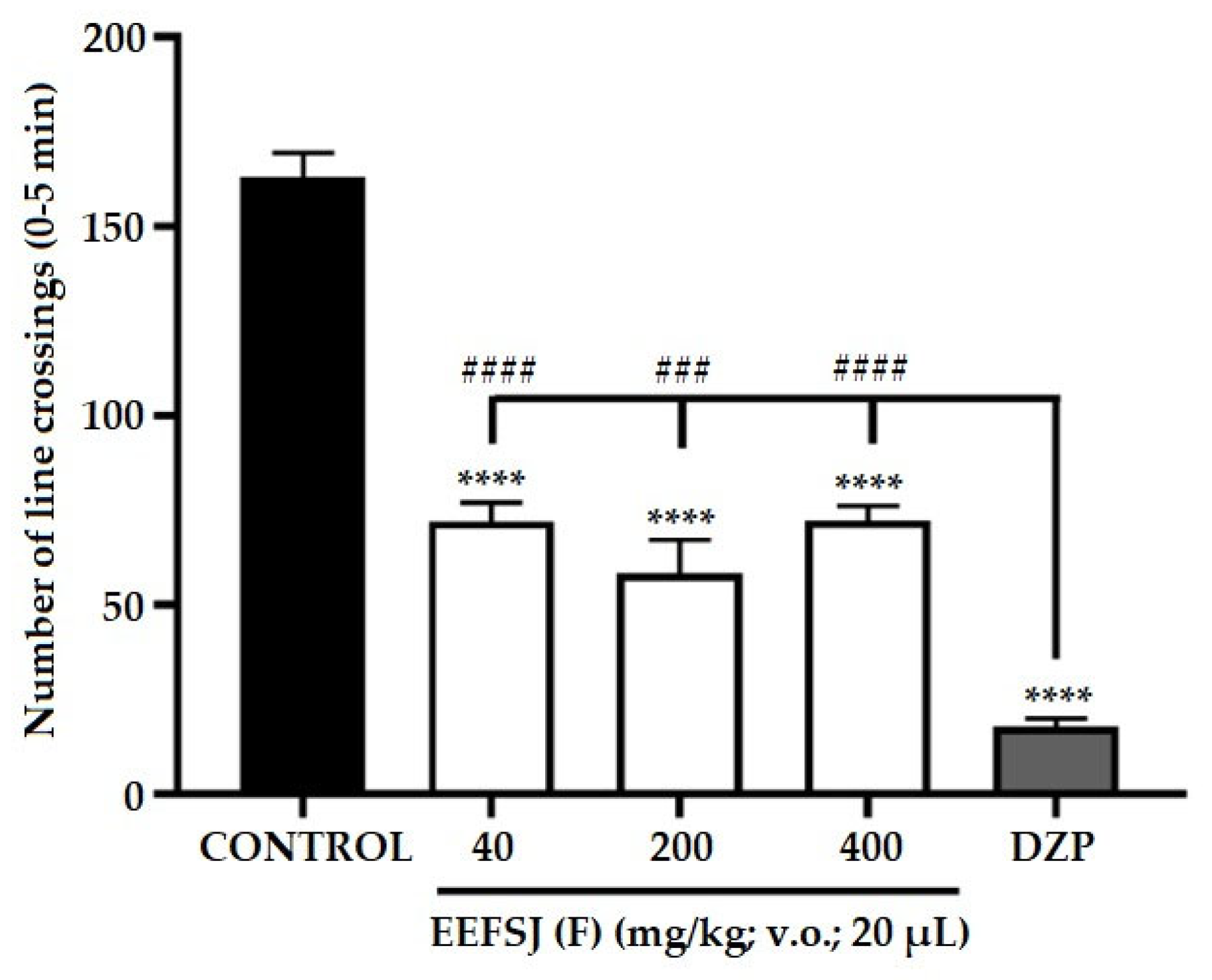
Figure 5.
Effect of EEFSJ on zebrafish anxiety in the light/dark test (0–5 min). CONTROL (DMSO 3%; 20 μl; ip.); DZP (Diazepam 4 mg/kg; ip). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (* p < 0,05; ** p < 0,001; **** p < 0,0001 vs. CONTROL).
Figure 5.
Effect of EEFSJ on zebrafish anxiety in the light/dark test (0–5 min). CONTROL (DMSO 3%; 20 μl; ip.); DZP (Diazepam 4 mg/kg; ip). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (* p < 0,05; ** p < 0,001; **** p < 0,0001 vs. CONTROL).
Figure 6.
Effect of ethanolic extract of S. joazeiro leaves on GABAergic neuromodulation in zebrafish in the light/dark test (0–5 min). Control (DMSO 3 %; 20 μl; ip.); DZP (Diazepam 4 mg/kg; i.p). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey’s Test.
Figure 6.
Effect of ethanolic extract of S. joazeiro leaves on GABAergic neuromodulation in zebrafish in the light/dark test (0–5 min). Control (DMSO 3 %; 20 μl; ip.); DZP (Diazepam 4 mg/kg; i.p). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey’s Test.
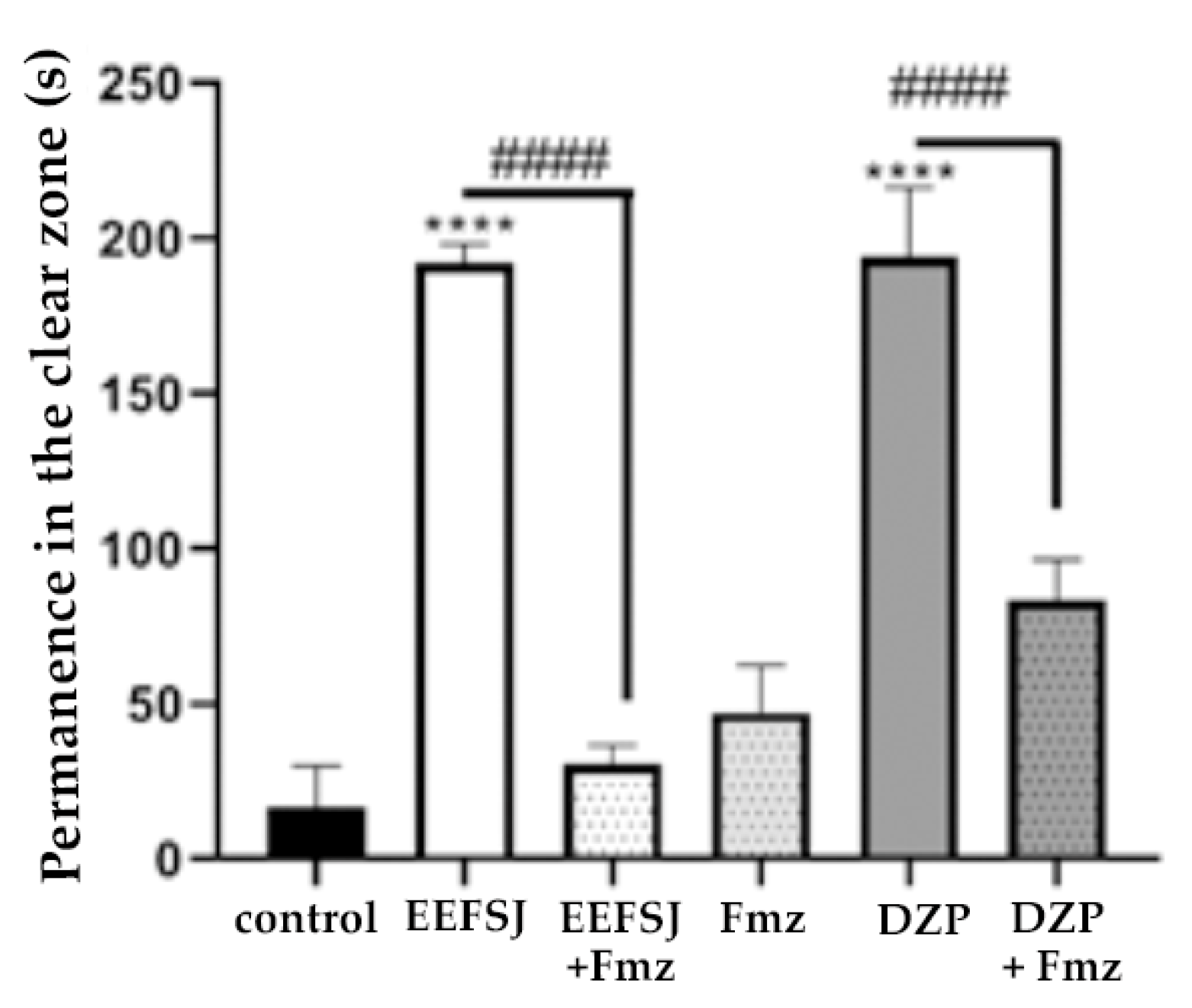
Table 1.
Substances identified in the EEFSJ in negative ESI mode.
| Peak N° | Name | tR(min) | [M-H]-/ [M+HCOOH]- | Empirical formula | Reference |
|---|---|---|---|---|---|
| 1 | 5-allyl-1-(2,3,4,-tris-O-benzoylpentofuranosyl)-2, 4(1H,3H)-pyrimidinedione | 5.63 | 595 | C33H27N2O9 | [16], [17] |
| 2 | Rutin | 6.98 | 609 | C27H29O16 | [17] |
| 3 | Kaempferol O-α-L-rhamnopyranosyl-(1→ 6)-β-D-hexoside | 7.37 | 593 | C27H29O15 | [17] |
| 4 | Isorhamnetin O-hexoside | 7.44 | 477 | C29H17O7 | [16] |
| 5 | Isorhamnetin O-rutinoside | 7.57 | 623 | C28H31O16 | [16] |
| 6 | Quercetin -O-α-L-arabinopyranosyl-(1 → 2)-α-L-rhamnopyranoside | 7.87 | 579 | C26H27O15 | [17] |
| 7 | Isorhamnetin O-hexoside | 7.94 | 477 | C29H17O7 | [18] |
| 8 | Jujubasaponin I | 8.30 | 941 | C48H77O18 | [19] |
| 9 | Jujubasaponin II | 9.28 | 983 | C50H79O19 | [19] |
| 10 | Ziziphin | 9.39 | 979 | C51H79O18 | [19] |
| 11 | Zizyphus saponin I | 9.88 | 911 | C47H75O17 | [19] |
tR: retention time
Table 2.
Results of the acute toxicity test of EEFSJ against adult zebrafish.
| Sample | (mg/kg) | 96hLD50 (mg/kg) | ||
| 40 | 200 | 400 | >400 | |
| EEFSJ | 0 | 0 | 0 | |
LD50: medium lethal dose; Statistical significance (p > 0,05).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Alerts
This version is not peer-reviewed
Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Alerts
Abstract
Anxiety is a multifactorial pathology associated with oxidative stress and changes in different neurotransmitters in the central nervous system (CNS). Sarcomphalus joazeiro (Rhamnaceae) has a predicted effect on the CNS, being considered an alternative for pre-clinical investigations of anxiolytic drugs. The objective of this study is to evaluate the chemical composition, analyze the antioxidant capacity and the anxiolytic effect of the ethanolic extract of S. joazeiro leaves (EEFSJ) in zebrafish. The chemical profile was analyzed by liquid chromatography coupled to mass spectrometry (UPLC-PDA-ESI-QDA). Antioxidant action by DPPH• elimination and ABTS•⁺ capture assays. The 96 h acute toxicity and open field and light and dark tests, for sedative and anxiolytic evaluation, respectively, were applied in vivo, using zebrafish. It was possible to identify 11 compounds from the flavonoid and saponin class. EEFSJ exhibited a mean inhibitory concentration (IC50) of 185.2 ± 2.2 µg/mL and 74.17 ± 1.5 µg/mL against the DPPH• and ABTS•⁺ radicals, respectively. In vivo, EEFSJ demonstrated reduced locomotor activity and an anxiolytic effect similar to diazepam via GABAergic systems, without presenting toxicity within 96 h. These results suggest the safety of using EEFSJ for the development of clinical trials for the production of new anxiolytic drugs.
Keywords:
Subject: Biology and Life Sciences - Biology and Biotechnology
1. Introduction
Anxiety is considered pathological when it causes psychological and social harm to the individual [1]. In recent years, anxiety has been one of the most prevalent neuropsychiatric disorders in the world [2]. According to the World Health Organization – WHO, around 3.6 % of the world population is affected by the disease, which in Brazil affects approximately 9.3 % of the population, ranking among the countries with the highest rates [3].
The etiology of anxiety is multifactorial and is mainly associated with interactions of genetic, neurobiological, psychological variables and environmental influence [4]. In the central nervous system (CNS), several neurotransmission pathways contribute to the mechanisms that mediate anxiety, especially changes in the serotonergic and GABAergic systems [1]. Furthermore, high levels of polyunsaturated acids and low reserves of antioxidant species in the CNS contribute to making neurons more vulnerable to oxidative stress and triggering neuroinflammation, associated with the development of pathological anxiety [5].
Benzodiazepines (BZDs), γ-aminobutyric acid (GABA) receptor agonists, and serotonin reuptake inhibitors (SSRIs) are commonly used anxiolytic drugs in the treatment of anxiety [3]. However, chronic use of BZD produces tolerance, and abrupt cessation of treatment can lead to a withdrawal syndrome [6]. From another perspective, the chronic use of SSRIs can reduce motor coordination, drowsiness, sedation and, when used for a prolonged period, they can also lead to dependence and withdrawal syndromes, and in high doses they can be fatal [7].
The adverse effects of these medications are the main reason why there is intensified research into the anxiolytic effects of products of natural origin. In general, plants synthesize a diversity of substances derived from their secondary metabolism, which often present satisfactory results in therapy against various psychological disorders [8].
Species from the Rhamnaceae family are commonly used in therapy against neurological diseases [9]. Among these, Sarcomphalus joazeiro (Mart.) Hauenschild, previously classified as Ziziphus joazeiro Mart [10], can be considered an alternative for pre-clinical investigations. Popularly known as “juá”, “joazeiro”, it is native to the Brazilian Northeast and endemic to the caatinga. In traditional medicine, it is used in the therapy of dermatological, respiratory, and digestive problems, in addition, it exhibits antioxidant, anti-inflammatory and antimicrobial properties [11]. Chemical data indicate the presence of flavonoid and saponin derivatives, which have reported and well-established anxiolytic activities [12], [13].
Animal research is being carried out as a model to screen natural products capable of combating anxiety [3]. Zebrafish (Danio rerio) have been a useful model as they possess key neuromediation systems, such as neurotransmitter receptors, transporters, sensitivity to anxiolytics, and behavioral paradigms similar to mammals [14]. Furthermore, they have a signaling system developed with high sensitivity to GABAergic sedatives [15].
Considering the importance of finding new active substances for use as an anxiolytic, this study aimed to identify the chemical components through hyphenated analytical methods and evaluate the antioxidant and anxiolytic capacity of the S. joazeiro leaf extract.
2. Results
2.1. Chemical Characterization
The EEFSJ was analyzed using UPLC-PDA-ESI-QDA in negative ESI mode. The constituents were identified based on their exact masses and comparison with data reported in the literature, allowing the identification of 11 peaks (Table 1). In summary, the peak with tR of 5.63 min refers to a nitrogenous compound, the peaks that ranged from 6.98 to 7.97 min are attributed to phenolic compounds and from 8.30 to 9.88 min to saponins. The structural representations of the identified compounds are illustrated in Figure 1.
The structural representations of the identified compounds are illustrated in Figure 1.
2.2. Antioxidant Activity
The DPPH• radical scavenging assay is routinely applied to evaluate the antiradical properties of different compounds [20]. In this assay, EEFSJ exhibited a mean inhibitory concentration (IC50) of 185.2 ± 28.5 µg/mL and a maximum percentage of DPPH• radical inhibition of 84.13 %, while ascorbic acid exhibited an IC50 of 8.871 ± 0.9480 µg/mL and maximum percentage of 89.75 %. (Figure 2).
In the ABTS•+ radical cation capture assay, EEFSJ presented a maximum percentage of 88.47 % and IC50 of 99.78 ± 25.61 µg/mL. Ascorbic acid presented values greater than 50 % at concentrations greater than 10 µg/mL, as shown in Figure 3.
2.3. 96h Acute Toxicity
Zebrafish is an experimental model that presents genetic and physiological similarity to humans, providing promising results regarding drug safety and anxiolytic evaluation (Caballero e Candiracci, 2018; Kalueff et al., 2014). There were no deaths after oral administration (LD50 ˃ 400 mg/kg), and no apparent anatomical changes in the animals during this period (Table 2) indicating preclinical safety in the use of EEFSJ [21].
2.4. Locomotor Activity (Open Field Test)
The open field test provides simultaneous measurements of zebrafish locomotion, which allow establishing a correlation between behavioral performance and neurobiological mechanisms that mediate behavior [21]. In this study, the locomotion of zebrafish was altered in the treatment with EEFSJ at all doses analyzed, as the animals had reduced locomotor activity when compared to the control group (Figure 4), presenting a statistically significant difference, indicating action on the CNS and sedative effect, similar to DZP (**** p < 0.0001 vs. CONTROL; ### p < 0.001; #### p < 0.0001 vs. DZP).
2.5. Anxiolytic Activity
The light-dark test using the adult zebrafish model is used to screen anxiolytic drugs similar to diazepines [22]. Due to the natural aversion of zebrafish to illuminated environments, this model allows the assessment of the animal’s anxiety level through the frequency of transitions and time spent in the illuminated compartment of the aquarium. (Kalue et al., 2014). Treatment with EEFSJ at the doses tested (40, 200 and 400 mg/kg) increased (* p < 0.05; ** p <0.01; **** p <0.0001 vs CONTROL) the time of permanence of the animals in the light region of the aquarium (Figure 5), demonstrating an anxiolytic effect similar to DZP.
2.6. Involvement of the GABAergic System
Flumazenil (FMZ), a competitive antagonist of the GABAA receptor in the αβγ subunits, antagonizes the sedative effects caused by BZD overdoses and reverses these effects, besides to preventing respiratory depression by blocking GABA receptors [7]. In this study, the lowest dose of EEFSJ (40 mg/kg) caused anxiolytic behavior in the animals and its effect was reversed (#### p <0.0001, vs. FMZ + EEFSJ; Figure 6) by Fmz, indicating that the anxiolytic effect of EEFSJ is related to the GABAergic system.
3. Discussion
In phytochemical screening studies, S. joazeiro extract exhibited classes of secondary metabolites, such as flavonoids, saponins and tannins, which are associated with different biological activities [12]. Previous studies performed UPLC-ESI-QTOF-MS analysis and showed the presence of rutin (2), kaempferol (3), isorhamnetin (4, 5 and 7), quercetin (6) and saponin derivatives (8, 9, 10 and 11), corroborating this study [16], [23].
Compounds 4 and 7 showed the same peak of the deprotonated molecular ion [M−H]− m/z 477 (C29H17O7). Previous research suggests that both are isomers of isorhamnetin-3-O-hexoside, due to the detection of fragment ions m/z 315 and m/z 162 (Matos et al., 2021). From a structural point of view, the flavonoids found (2, 3, 4, 5, 6 and 7) are characterized by the presence of two aromatic rings and a heterocyclic benzopyran ring containing oxygen and hydroxyl substituents [24].
Saponins are widely found in the Rhamanaceae family. In the species S. joazeiro, tetracyclic triterpenes of the dammarane type are frequently reported [25]. Masullo et al., (2019) proved the presence of these saponins through structural elucidation by 2D NMR. From a structural point of view, these derivatives are differentiated by their side chains, which correspond to 16.23:16.30 diepoxidammarane (8, 9, 10 e 11). Previous studies of species from the Rhamnaceae family, including Z. jujuba [19], [26], and Z. mauritiana [27] report the presence of these saponins.
The antioxidant activity of species of the genus Ziziphus reported in the literature agrees with these data, in which Z. cotinifolia exhibited IC50 values of 7.2 ± 1.5 μg/ml and 6.4 ± 0.3 μg/mL, for the DPPH• and ABTS•⁺ method, while Z. jujuba showed IC50 of 47.37 mmol of trolox, for ABTS•⁺. On the other hand, in the DPPH• elimination test, Brito et al., (2015) found higher IC50 values than those found in this study (735,72 μg/mL). In an HPLC/DAD analysis, the authors confirmed a low content of rutin (0.97 %) and quercetin (0.52 %) in the extract.
The high antioxidant action exerted by EEFSJ in this study can be attributed to the higher content of rutin (2) and quercetin (6) in the composition, which may justify the difference in the results found in the studies, considering that these are the main natural flavonoids responsible for enhancing the antioxidant effects of plant extracts. This variation in composition is due to the influence of extractive methods, organic solvents, as well as seasonal factors, such as location, collection period and type of soil [28].
The structures of flavonoids found in the present study allow them to act by combating free radicals through the donation of hydrogen atoms from a hydroxyl group of their aromatic structure, which has the ability to support an unpaired electron through its displacement around the entire molecule electron system [29], [20]. Furthermore, they can also act as scavengers of pro-inflammatory and neurotoxic species in the CNS [9].
The intensity of the anxiolytic effect observed in this study was inversely proportional to the concentration of EEFSJ. Silva et al., (2023) recently reported similar behavior of S. joazeiro extract in the same experimental model of zebrafish. These data propose that EEFSJ when administered in high doses (> 200 mg/kg) may cause tolerance and adverse effects similar to DZP. The administration of high doses of DZP (> 30 mg/kg) is capable of developing tolerance, causing compensatory changes in GABA receptors, which become less responsive, and as a consequence, reduce their inhibitory actions [30]. Furthermore, higher doses of DZP can cause CNS depression, whose additional non-classical BZD binding sites on GABAARs can cause adverse action [15].
EEFSJ also altered the locomotion of zebrafish at all concentrations tested, exhibiting behavior similar to DZP. Recently, studies of Z. cotinifolia (Rhamnaceae) using the same experimental model showed similar results [14]. BZDs produce anxiolytic and sedative effects by binding to high-affinity sites located at the α1/2/3/5+/γ2− interface of synaptic GABAARs to enhance GABA-mediated post-synaptic membrane hyperpolarization [15]. Therefore, the alteration of zebrafish locomotion caused by EEFSJ can be attributed to the high affinity for the GABAA receptor binding sites, which are capable of reducing central activity, causing an anxiolytic and/or sedative action similar to BDZ.
The involvement of GABAergic neurotransmission was evaluated through pre-treatment with flumazenil (Fmz) [7]. EEFSJ demonstrated anxiolytic behavior in animals and its effect was reversed by FMZ (Figure 6), proving that EEFSJ is capable of activating the GABAA receptor in the same region as BZD [31].
Clinical and pre-clinical studies prove that flavonoids and saponins act through selective binding to GABAA receptors, promoting membrane hyperpolarization by allowing the influx of chloride anions, accompanied by inhibition of excitatory transmission, which consequently contribute to the reduction of anxiety disorders [9], [32]. This hypothesis is corroborated by previous studies that show the anxiolytic potential of S. joazeiro, flavonoids and saponins through GABAergic neurotransmission in zebrafish and mice [8], [11], [33].
Quercetin (6) has been widely investigated due to its antagonistic actions on GABAUmρ1R, this effect may be mediated by a redox-independent allosteric mechanism, in addition to affecting the expression of GABAUmα5R, which could be a mechanism to attenuate the severity of crises [34]. In addition, rutin (2) is involved in GABAergic neurotransmission without involvement of BZD receptors [35]. Previous research also shows that rutin (2) can act on different anxiolytic mechanisms, for example, combating oxidative stress in the CNS, modulating the release of the neurotransmitters serotonin, norepinephrine, opioids and activating opioid receptors [8]. Quantitative models prove that the increased affinity of these flavonoids (2, 3, 4, 5, 6, 7) for the BZD receptor binding site is associated with the presence of electronegative substituents at 6 and 3' of the flavone backbone [13].
This study shows the potential of EEFSJ as an antioxidant and anxiolytic agent, making it a promising candidate for therapy against psychological disorders, especially anxiolytics.
4. Materials and Methods
4.1. Collection and Preparation of Extract
The leaves (484 g) were collected in Tabocas, located in the rural area of the Municipality of Exu, Pernambuco, Brazil. The plant material was sanitized, crushed, and subjected to extraction using the cold maceration method for 72 h at room temperature, in hexane to degrease, and subsequently submerged in 99.5 % ethanol to obtain the crude extract. Organic solvents were removed using a rotary evaporator under reduced pressure. The yield obtained from the ethanolic extract of S. joazeiro leaves (EEFSJ) was 4.78 %.
4.2. Chemical Characterization
4.2.1. Extract Preparation
Approximately 4 mg of EEFSJ was dissolved in 1 mL of a 50/50 % methanol/water mixture. The material was filtered through a 0.22 μm PTFE filter, placed in vials and stored at -80 °C for analysis.
4.2.2. Analisys by UPLC-PDA-ESI-QDA
Analysis was performed using an Acquity UPLC system (Waters, USA) coupled to a PDA (210-600 nm) and QDa mass system (Quadrupole, Waters). A Waters Acquity BEH C18 column for separation condition (150 mm × 2,1 mm, 1,7 μm) was set at 40 °C. An injection volume of 5 μL aliquot of EEFSJ was subjected to an exploratory gradient with the mobile phase composed of deionized water (A) and acetonitrile (B), both containing formic acid (0.1 % v/v). The optimized instrumental parameters were as follows for negatives: capillary voltage at 0.8 kV, cone voltage at 15 V, source temperature at 120 °C, desolvation temperature at 350 °C, desolvation gas flow at 500 L/h. The system was controlled using Empower 3 software (Waters Corporation).
4.3. Antioxidant Assays
4.3.1. Determination of Antioxidant Activity by the DPPH● Method
The free radical scavenging activity was determined by the DPPH● (1,1, diphenyl-2-picrylhydrazyl) photocolorimetric method, proposed by Rufino, et al., (2007) with adaptations [36]. EEFSJ concentrations ranged from 10 to 1000 µg/mL. For the test, 20 μL of the sample, 80 μL of 95 % ethanol and 100 μL of the DPPH● radical solution (0.3 mM) were used. After 30 min of incubation at room temperature and protected from light, absorbance measurements were carried out using a UV-visible spectrophotometer at 518 nm. The blank samples and the antioxidant standard (ascorbic acid) were quantified under the same conditions with 20 μL of standards and 180 μL of 95 % ethanol. The results were calculated according to equation 1.
Equation 1
IP % = 100 ‐ {[(Abs Sample – Abs blank) / Abs negative control] x 100}
Where: IP % = Inhibition Percentage; Abs: Absorbances.
4.3.2. ABTS●+ Free Radical Capture
For the ABTS●+ free radical capture assay, the methodology proposed by Rufino, et al., (2006) was used [37]. The ABTS●+ radical was prepared by mixing a 7 mM ABTS diammonium salt solution with a potassium persulfate solution (final concentration of 2.45 mM), both prepared in phosphate buffer saline (pH 7.4). The resulting solution (stock solution) was kept in an amber bottle at room temperature for 16 h to form the free radical ABTS●+. Subsequently, it was diluted with phosphate buffer saline (pH 7.4) until an absorbance value of 0.90-1.0 ± 0.02 was obtained at 734 nm. An aliquot of 2.970 µL of this solution was added with 30 µL of concentrations of 10 to 1.000 µg/mL of the extract, as well as the positive controls of ascorbic acid. The assay was carried out in triplicate and readings were taken 3 min after the start of the reaction in a spectrophotometer adjusted to 734 nm. The results were calculated according to equation 1.
4.4. Toxicity and Anxiolytic Assays
4.4.1. Zebrafish
Zebrafish (Danio rerio) (age 90 to 120 days; 0.4 ± 0.1 g, 3.5 ± 0.5 cm), wild, of both sexes, were purchased commercially in Fortaleza, CE. Animals were maintained in a glass aquarium (30 × 15 × 20 cm) of 10 L (n = 3/L), at a temperature of 25 ± 2°C, in 24-h light-dark cycles with chlorinated water (ProtecPlus®) and air pump with submerged filters, under a temperature of 25 ° C and pH 7.0, Circadian cycle of 10 - 14 h (light/dark). The fish received food (Spirulina®) ad libitum 24 h before the experiments. Before drug applications, the animals were anesthetized in ice-cold water and after the experiments, the animals were sacrificed by immersion in ice-cold water (2 and 4 °C) for 1 min until loss of opercular movements. The work was approved by the Ethics Committee on the Use of Animals of the Universidade Estadual do Ceará (CEUA-UECE; nº 04983945/2021), in accordance with the Ethical Principles of Animal Experimentation.
4.4.2. General Protocol
Zebrafish were randomly selected in the experiments, anesthetized in ice water, and transferred to a damp sponge, treated with 20 µL of EEFSJ (40; 200 and 400 mg/kg) or Diazepam (4 mg/kg), or 3% DMSO (control group – drug diluent) orally (v.o).
4.4.3. Assessment of Locomotor Activity (Open Field Test)
The open field test was performed to evaluate the presence or absence of changes in motor coordination in animals [38], whether due to the anxiolytic effect and/or muscle relaxation. Animals (n = 6/group) were pre-treated (20 µL; p.o.) at the same doses analyzed in the previous section. Diazepam (DZP; 4 mg/kg) and vehicle (DMSO 3 %) were used as positive and negative controls, respectively. After 60 min of treatment, the animals were added individually to glass Petri dishes (10x15 cm; with quadrants at the bottom of the dish), containing the same water as in the aquarium. The number of line crossings was recorded during 0-5 min and the percentage of each group was calculated.
4.4.4. 96. h Acute Toxicity
After the open field test, fish (n = 6 / grupo) treated orally with EEFSJ (40; 200 or 400 mg/kg; 20 µL) or control (vehicle: DMSO 3 %; 20 µL; i.pwere left at rest to analyze the mortality rate for a period of 96 h, recording the number of dead fish in each group every 24 h [39], with the lethal dose capable of killing 50 % of the animals (LD50) determined by the Trimmed Spearman-Karber mathematical method with a 95 % confidence interval.
4.4.5. Anxiolytic Assessment
An animal's anxiety behavior can be observed using the light/dark test. Similar to rodents, zebrafish naturally avoid illuminated areas [40]. The experiment was carried out in a glass aquarium (30 cm x 15 cm x 20 cm) divided into a light area and a dark area. The aquarium was filled to 3 cm with dechlorinated tap water, which simulated a new shallow environment different from the conventional aquarium and capable of inducing anxiety behaviors. In animals (n = 6/group) 20 µL of EEFSJ were administered orally at doses of 40 mg/kg, 200 mg/kg, or 400 mg/kg. The negative and positive control groups consisted of 3 % DMSO and 4 mg/kg Diazepam solution, respectively. After 60 min, the animals were placed individually in the light zone and the anxiolytic effect was measured based on the time spent in the light zone of the aquarium within 5 min of observation [22].
4.4.6. Assessment of GABAergic Neuromodulation
The GABAergic neuromodulation involved in the anxiolytic effect of the extracts was identified through pre-treatment with flumazenil (Fmz) (GABAA antagonist) before the light/dark test [41]. Fish (n = 6/group) were pretreated with Fmz (4 mg/kg; 20 μL; i.p.). After 15 min, the dose with the highest anxiolytic efficacy of the extracts (40 mg/kg; 20 μL; p.o.) found in the previously performed test, was administered. 3 % DMSO (vehicle; 20 μL; i.p.) was used as a negative control. After 60 min of treatments, the animals were subjected to the light/dark test.
4.5. Statistical Analysis
The results were expressed as mean values ± standard error of the mean for each group of 6 animals. After confirming the normality of distribution and homogeneity of the data, the differences between the groups were subjected to analysis of variance - one-way ANOVA, followed by the Tukey’s test. All analysis were performed using GraphPad Prism v software. 8.0.1. The level of statistical significance was set at 5 % (p<0.05).
5. Conclusions
This study showed the presence of flavonoids and saponins in the chemical composition of S. joazeiro, through UPLC-PDA-ESI-QDA. Among the biological assays tested, the extract showed antioxidant potential using the free radical scavenging methods DPPH• and the radical cation capture ABTS•⁺. In in vivo tests with zebrafish, the extract did not show reduced locomotor activity and an anxiolytic effect similar to DZP at the lowest dose tested (40 mg/kg) mediated by the activation of the GABAA receptor, without showing toxicity within 96 h.
These results suggest that S. joazeiro is a promising candidate for the development of specialized CNS-associated clinical trials. Research aimed at evaluating pharmacokinetics/pharmacodynamics and bioavailability, such as the encapsulation of nanoparticles, can help in the controlled delivery of the bioactive compounds present and assist in the progress of research.
Author Contributions
Research, writing, formatting and editing, Natália Kelly Gomes de Carvalho, Johnatan Wellisson da Silva Mendes, Débora Odília Duarte Leite, Mariana Pereira da Silva. Chemical characterization, Kirley Marques Canuto, Paulo Riceli Vasconcelos Ribeiro. Biological assays, Gerson Javier Torres Salazar, Mariana Amanda Maria Barros Alves, Ivana Carneiro Romão, Hélcio Silva dos Santos. Supervision, José Galberto Martins da Costa; the authors have read and agreed to the published version of the manuscript.”
Acknowledgment
This work was carried out at the Laboratório de Pesquisa de Produtos Naturais (LPPN) of the Departamento de Química Biológica at the Universidade Regional do Cariri (URCA), with support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) and Instituto Nacional de Ciência e Tecnologia – Alimentos (INCT-ALIM).
Conflicts of Interest
The authors declare no conflict of interest.
References
- T. I. dos Santos Sampaio et al., “Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio),” J. Ethnopharmacol., vol. 224, no. February, pp. 563–578, 2018. [CrossRef]
- H. P. S. Hozana Patrícia et al., “Anxiolytic-like effect of brominated compounds from the marine sponge Aplysina fulva on adult zebrafish (Danio rerio): Involvement of the GABAergic system,” Neurochem. Int., vol. 146, no. March, 2021. [CrossRef]
- N. C. de Melo et al., “Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) moldenke: A study on zebrafish (Danio rerio),” Pharmaceuticals, vol. 12, no. 3, 2019. [CrossRef]
- E. Palazidou, “The neurobiology of depression,” Br. Med. Bull., vol. 101, no. 1, pp. 127–145, 2012. [CrossRef]
- M. Shafiee et al., “Depression and anxiety symptoms are associated with prooxidant-antioxidant balance: A population-based study,” J. Affect. Disord., vol. 238, pp. 491–498, 2018. [CrossRef]
- G. P. da S. M. Matos, A. F. Dos Santos, D. M. S. Acioli, E. F. Da Silva, and J. I. Guerra Junior, “Benzodiazepínicos: uma revisão de literatura sobre uso indiscriminado, dependência e efeitos colaterais,” Brazilian J. Heal. Rev., vol. 7, no. 2, p. e67735, 2024. [CrossRef]
- J. da C. Xavier et al., “Anxiolytic-like and anticonvulsant effect in adult zebrafish (Danio rerio) through gabaergic system and molecular docking study of chalcone derived from natural products,” Biointerface Res. Appl. Chem., vol. 11, no. 6, pp. 14021–14031, 2021. [CrossRef]
- E. Aguirre-Hernández, M. E. González-Trujano, T. Terrazas, J. H. Santoyo, and P. Guevara-Fefer, “Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation,” Salud Ment., vol. 39, no. 1, pp. 37–46, 2016. [CrossRef]
- M. U. Rehman et al., “Neuroprotective Strategies for Neurological Disorders by Natural Products: An update,” Curr. Neuropharmacol., vol. 17, no. 3, pp. 247–267, 2018. [CrossRef]
- N. J. S. Araújo et al., “Chemical characterization UPLC-ESI-QToF-MSE, antibacterial and antibiofilm potential of Sarcomphalus joazeiro (MART.) Hauenschild,” Food Biosci., vol. 50, no. August, 2022. [CrossRef]
- B. de Souza et al., “Antibacterial activity and anxiolytic-like effect of Ziziphus joazeiro Mart. leaves in adult zebrafish (Danio rerio),” Fish Shellfish Immunol. Reports, vol. 5, no. April, 2023. [CrossRef]
- S. M. O. Brito et al., “Analysis of bioactivities and chemical composition of Ziziphus joazeiro Mart. using HPLC-DAD,” Food Chem., vol. 186, pp. 185–191, 2015. [CrossRef]
- J. R. Hanrahan, M. Chebib, and G. A. R. Johnston, “Flavonoid modulation of GABA A receptors,” Br. J. Pharmacol., vol. 163, no. 2, pp. 234–245, 2011. [CrossRef]
- D. V. de Azevedo et al., “Evaluation of antioxidant, toxicological and anxiolytic-like effect of ethanolic extracts of Ziziphus cotinifolia Reissek in adult zebrafish (Danio rerio),” Phytomedicine Plus, vol. 4, no. 1, 2024. [CrossRef]
- Y. Cao, H. Yan, G. Yu, and R. Su, “Flumazenil-insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immobility in zebrafish larvae,” Life Sci., vol. 239, p. 117033, 2019. [CrossRef] [PubMed]
- J. Cosmo Andrade et al., “Control of bacterial and fungal biofilms by natural products of Ziziphus joazeiro Mart. (Rhamnaceae),” Comp. Immunol. Microbiol. Infect. Dis., vol. 65, no. March, pp. 226–233, 2019. [CrossRef]
- Y. Pu, T. Ding, N. Zhang, P. Jiang, and D. Liu, “Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao,” Int. J. Food Prop., vol. 20, no. 1, pp. S26–S35, 2017. [CrossRef]
- Tools, “Integrated UPLC-HRMS, Chemometric Tools, and Metabolomic Analysis of Forage Palm (,” vol. 32, no. 8, pp. 1617–1627, 2021.
- M. Masullo, A. Cerulli, P. Montoro, C. Pizza, and S. Piacente, “In depth LC-ESIMS n -guided phytochemical analysis of Ziziphus jujuba Mill. leaves,” Phytochemistry, vol. 159, no. October 2018, pp. 148–158, 2019. [CrossRef]
- N. R. Sucupira, A. B. Da Silva, G. Pereira, and J. N. Da Costa, “Métodos Para Determinação da Atividade Antioxidante de Frutos,” UNOPAR Científica Ciências Biológicas e da Saúde, vol. 14, no. 4, pp. 263–269, 2014, [Online]. Available: http://revistas.unopar.br/index.php/biologicas/article/view/442.
- W. M. Arika, C. M. Kibiti, J. M. Njagi, and M. P. Ngugi, “Effects of DCM Leaf Extract of Gnidia glauca (Fresen) on Locomotor Activity, Anxiety, and Exploration-Like Behaviors in High-Fat Diet-Induced Obese Rats,” Behav. Neurol., vol. 2019, 2019. [CrossRef] [PubMed]
- L. Gebauer, N. Pagnussat, Â. L. Piato, I. C. Schaefer, C. D. Bonan, and D. R. Lara, “Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol,” Pharmacol. Biochem. Behav., vol. 99, no. 3, pp. 480–486, 2011. [CrossRef]
- J. C. Andrade et al., “UPLC-MS-ESI-QTOF characterization and evaluation of the antibacterial and modulatory antibiotic activity of Ziziphus joazeiro Mart. aqueous extracts,” South African J. Bot., vol. 123, pp. 105–112, 2019. [CrossRef]
- Z. Liu et al., “Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property,” J. Ethnopharmacol., vol. 267, p. 113630, 2021. [CrossRef] [PubMed]
- S. T. Sakna, Y. R. Maghraby, M. S. Abdelfattah, and M. A. Farag, “Phytochemical diversity and pharmacological effects of triterpenes from genus Ziziphus: a comprehensive review,” Phytochem. Rev., vol. 22, no. 6, pp. 1611–1636, 2023. [CrossRef]
- K. Yoshikawa, N. Shimono, and S. Arihara, “Antisweet substances, jujubasaponins I-III from zizyphus jujuba revised structure of ziziphin,” Tetrahedron Lett., vol. 32, no. 48, pp. 7059–7062, 1991. [CrossRef]
- S. Seri, T. A. Okpekon, P. A. Yao-Kouassi, A. A. Magid, C. Sayagh, and L. Voutquenne-Nazabadioko, “Saponins and flavonoid glycosides from the leaves of Ziziphus mauritiana Lam. native of a forest area of Ivory Coast,” Phytochem. Lett., vol. 37, no. March, pp. 5–9, 2020. [CrossRef]
- J. M. Macedo, L. G. P. J. M. Macedo, L. G. P. Souza, V. del C. Troncozo Valenzuela, A. B. Oliveira, R. Oliveira Castilho, and R. L. Ribeiro Jácome, “Variação sazonal nos teores de flavonoides, taninos e atividade antioxidante de Davilla rugosa poir,” Rev. Ciencias Farm. Basica e Apl., vol. 34, no. 4, pp. 585–590, 2013.
- T. C. de L. E. Silva, “AVALIAÇÃO COMPARATIVA DE CASCAS E FOLHAS DE Ziziphus joazeiro Mart (RHAMNACEAE) EM RELAÇÃO AOS PERFIS FITOQUIMICO E TOXICOLÓGICO E AS ATIVIDADES ANTIOXIDANTE E ANTIMICROBIANA,” p. 73, 2009.
- C. M. F. E Silva, D. I. M. Pereira, and G. V. Mesquita, “Análise do uso indiscriminado de benzodiazepínicos em graduandos de medicina de um centro universitário no Piauí,” Brazilian J. Heal. Rev., vol. 6, no. 6, pp. 28005–28020, 2023. [CrossRef]
- R. B. Lydiard, “The role of GABA in anxiety disorders,” J. Clin. Psychiatry, vol. 64, no. SUPPL. 3, pp. 21–27, 2003.
- S. K. J. Njapdounke et al., “Anxiolytic - Like properties of Hallea ciliata in mice,” African J. Tradit. Complement. Altern. Med., vol. 13, no. 4, pp. 1–7, 2016. [CrossRef]
- N. Bao, J. Ou, W. Shi, N. Li, L. Chen, and J. Sun, “Highly Efficient Synthesis and Structure–Activity Relationships of a Small Library of Substituted 1,4-Naphthoquinones,” European J. Org. Chem., vol. 2018, no. 19, pp. 2254–2258, 2018. [CrossRef]
- C. I. Calero, A. N. B. González, J. Gasulla, S. Alvarez, P. Evelson, and D. J. Calvo, “Quercetin antagonism of GABAAρ1 receptors is prevented by ascorbic acid through a redox-independent mechanism,” Eur. J. Pharmacol., vol. 714, no. 1–3, pp. 274–280, 2013. [CrossRef]
- J. L. Ríos, G. R. Schinella, and I. Moragrega, “Phenolics as GABAA Receptor Ligands: An Updated Review,” Molecules, vol. 27, no. 6, 2022. [CrossRef]
- M. do S. Rufino, R. E. Alves, E. S. De Brito, S. M. De Morais, C. D. G. Sampaio, and F. D. Saura-calixto, “ISSN 1679-6535 Julho, 2007 Fortaleza, CE,” pp. 0–3, 2007.
- M. do S. M. Rufino et al., “Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH.,” Comun. Técnico Online EMBRAPA, vol. 127, pp. 1–4, 2007, [Online]. Available: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/426953/1/Cot127.pdf.
- M. K. A. Ferreira et al., “Chalcones reverse the anxiety and convulsive behavior of adult zebrafish,” Epilepsy Behav, vol. 117, p. 107881, Apr. 2021. [CrossRef]
- Oecd, “Fish, acute toxicity test,” Guidel. Test. Chem., no. July, pp. 1–9, 1992.
- N. G. G. Gonçalves et al., “Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system,” J. Funct. Foods, vol. 66, no. April 2019, p. 103772, 2020. [CrossRef]
- C. K. Benneh, R. P. Biney, P. K. Mante, A. Tandoh, D. W. Adongo, and E. Woode, “Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—Involvement of GABAergic and 5-HT systems,” J. Ethnopharmacol., 2017. [CrossRef]
Figure 1.
Structural representation of the components found in the EEFSJ using the UPLC-PDA-ESI-QDA method.
Figure 1.
Structural representation of the components found in the EEFSJ using the UPLC-PDA-ESI-QDA method.


Figure 2.
DPPH• free radical scavenging from EEFSJ and antioxidant control. Souce: Author.

Figure 3.
ABTS•+ radical capture by EEFSJ and antioxidant control. Source: Author.

Figure 4.
Effect of EEFSJ on the locomotor behavior of adult zebrafish in the Open Field Test (0–5 min). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (**** p < 0,0001 vs. CONTROL; ### p < 0,001; #### p < 0,0001 vs. DZP).
Figure 4.
Effect of EEFSJ on the locomotor behavior of adult zebrafish in the Open Field Test (0–5 min). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (**** p < 0,0001 vs. CONTROL; ### p < 0,001; #### p < 0,0001 vs. DZP).

Figure 5.
Effect of EEFSJ on zebrafish anxiety in the light/dark test (0–5 min). CONTROL (DMSO 3%; 20 μl; ip.); DZP (Diazepam 4 mg/kg; ip). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (* p < 0,05; ** p < 0,001; **** p < 0,0001 vs. CONTROL).
Figure 5.
Effect of EEFSJ on zebrafish anxiety in the light/dark test (0–5 min). CONTROL (DMSO 3%; 20 μl; ip.); DZP (Diazepam 4 mg/kg; ip). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey's test (* p < 0,05; ** p < 0,001; **** p < 0,0001 vs. CONTROL).

Figure 6.
Effect of ethanolic extract of S. joazeiro leaves on GABAergic neuromodulation in zebrafish in the light/dark test (0–5 min). Control (DMSO 3 %; 20 μl; ip.); DZP (Diazepam 4 mg/kg; i.p). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey’s Test.
Figure 6.
Effect of ethanolic extract of S. joazeiro leaves on GABAergic neuromodulation in zebrafish in the light/dark test (0–5 min). Control (DMSO 3 %; 20 μl; ip.); DZP (Diazepam 4 mg/kg; i.p). Values represent the mean ± standard error of the mean for 6 animals/group; ANOVA followed by Tukey’s Test.

Table 1.
Substances identified in the EEFSJ in negative ESI mode.
| Peak N° | Name | tR(min) | [M-H]-/ [M+HCOOH]- | Empirical formula | Reference |
|---|---|---|---|---|---|
| 1 | 5-allyl-1-(2,3,4,-tris-O-benzoylpentofuranosyl)-2, 4(1H,3H)-pyrimidinedione | 5.63 | 595 | C33H27N2O9 | [16], [17] |
| 2 | Rutin | 6.98 | 609 | C27H29O16 | [17] |
| 3 | Kaempferol O-α-L-rhamnopyranosyl-(1→ 6)-β-D-hexoside | 7.37 | 593 | C27H29O15 | [17] |
| 4 | Isorhamnetin O-hexoside | 7.44 | 477 | C29H17O7 | [16] |
| 5 | Isorhamnetin O-rutinoside | 7.57 | 623 | C28H31O16 | [16] |
| 6 | Quercetin -O-α-L-arabinopyranosyl-(1 → 2)-α-L-rhamnopyranoside | 7.87 | 579 | C26H27O15 | [17] |
| 7 | Isorhamnetin O-hexoside | 7.94 | 477 | C29H17O7 | [18] |
| 8 | Jujubasaponin I | 8.30 | 941 | C48H77O18 | [19] |
| 9 | Jujubasaponin II | 9.28 | 983 | C50H79O19 | [19] |
| 10 | Ziziphin | 9.39 | 979 | C51H79O18 | [19] |
| 11 | Zizyphus saponin I | 9.88 | 911 | C47H75O17 | [19] |
tR: retention time
Table 2.
Results of the acute toxicity test of EEFSJ against adult zebrafish.
| Sample | (mg/kg) | 96hLD50 (mg/kg) | ||
| 40 | 200 | 400 | >400 | |
| EEFSJ | 0 | 0 | 0 | |
LD50: medium lethal dose; Statistical significance (p > 0,05).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio)
Nayara Costa de Melo
et al.
Pharmaceuticals,
2019
Clitorienolactones and Isoflavonoids of Clitorea ternatea Roots Alleviate Stress-Like Symptoms in a Reserpine-Induced Zebrafish Model
Muhammad Ngadni
et al.
Molecules,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







