Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
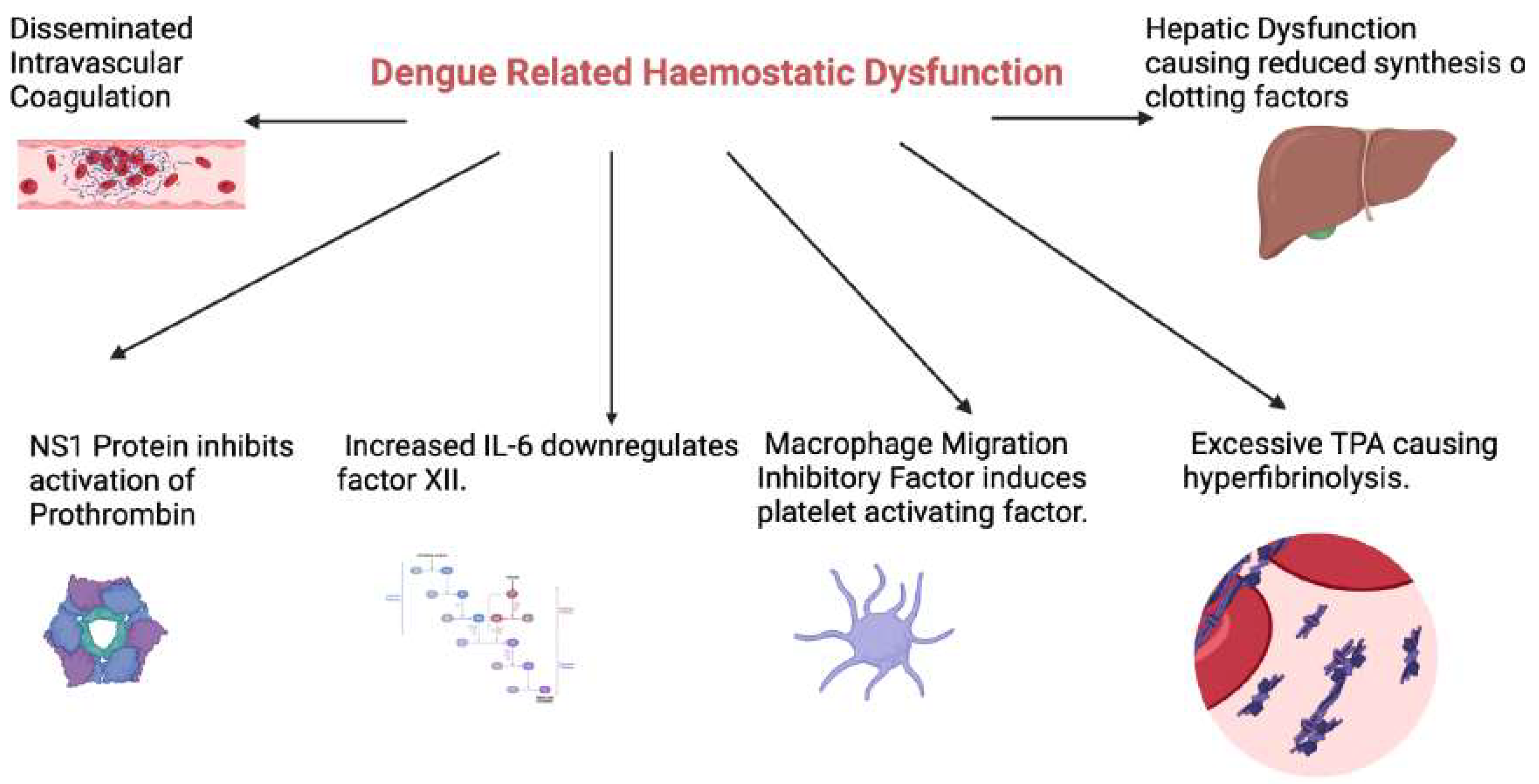
Haemostatic Abnormalities in Dengue Infection
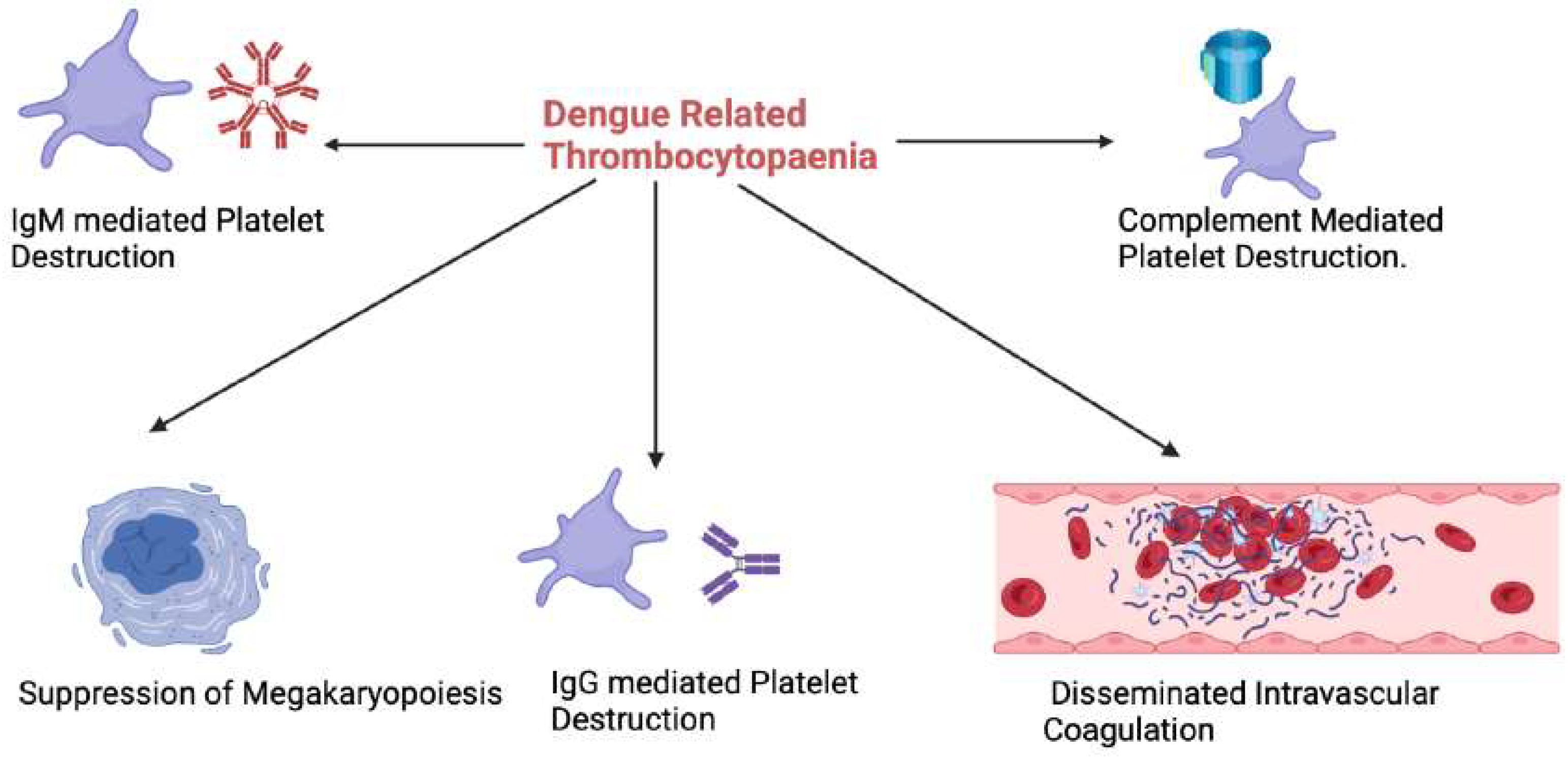
Thrombocytopaenia
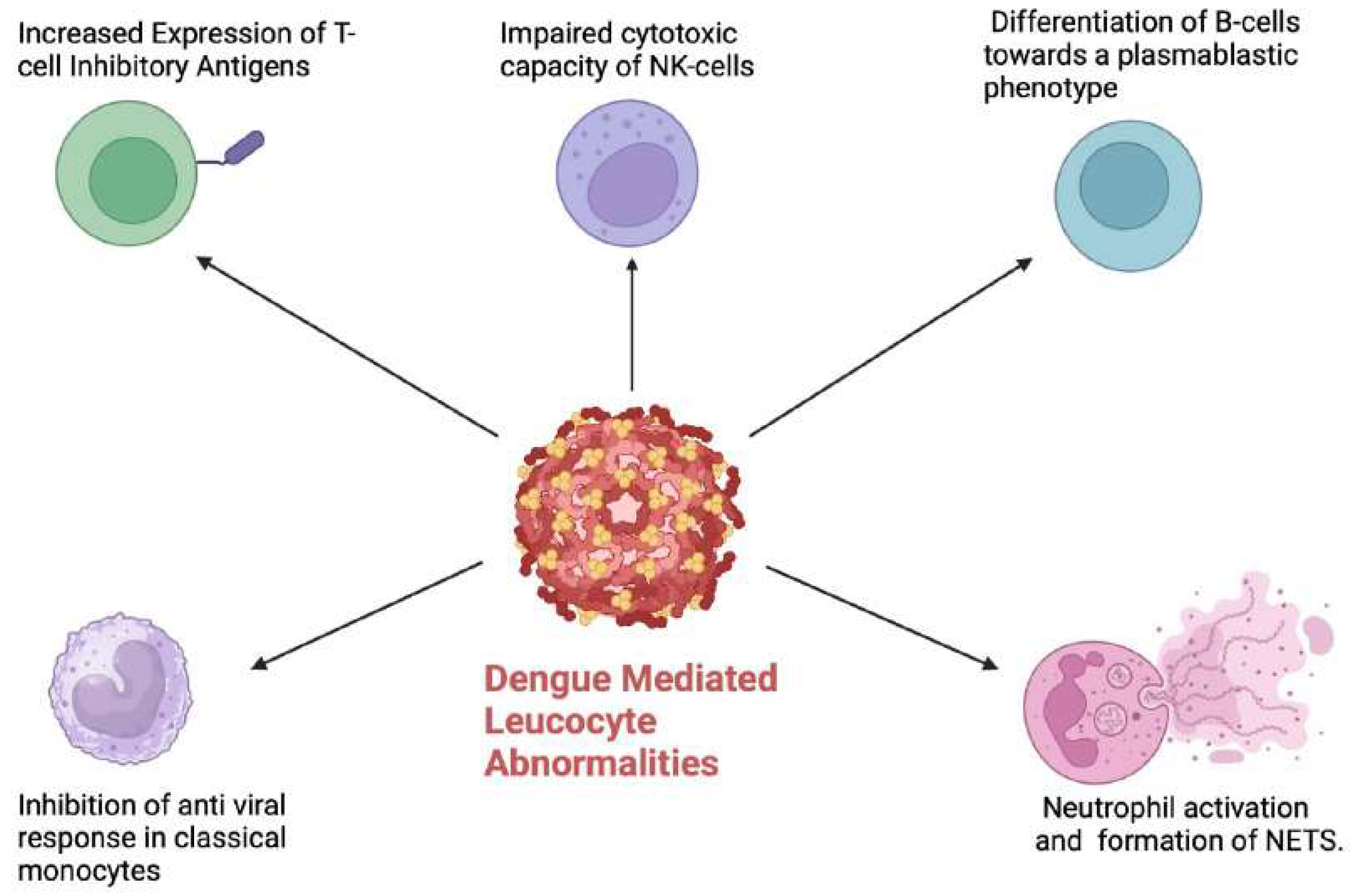
Leucocyte Abnormalities
Monocytes and Macrophages
Lymphocytes
Neutrophils
Mast Cells
Eosinophils and Basophils
Implications for Prognosis and Risk Stratification
Implications for Management
Conclusions and Future Directions
References
- Bhatt, S., Gething, P.W., Brady, O.J., Messina, J.P., Farlow, A.W., Moyes, C.L., Drake, J.M., Brownstein, J.S., Hoen, A.G., Sankoh, O., Myers, M.F., George, D.B., Jaenisch, T., Wint, G.R.W., Simmons, C.P., Scott, T.W., Farrar, J.J. and Hay, S.I. (2013). The global distribution and burden of dengue. Nature, 496(7446), pp.504–507. [CrossRef]
- Murugesan, A. and Manoharan, M. (2020). Dengue Virus. Emerging and Reemerging Viral Pathogens, pp.281–359. [CrossRef]
- Roy, S.K. and Bhattacharjee, S. (2021). Dengue virus: epidemiology, biology, and disease aetiology. Canadian Journal of Microbiology, 67(10), pp.687–702. [CrossRef]
- Shrestha DB, Budhathoki P, Gurung B, Subedi S, Aryal S, Basukala A, Aryal B, Adhikari A, Poudel A, Yadav GK, Khoury M, Rayamajhee B, Shrestha LB. Epidemiology of dengue in SAARC territory: a systematic review and meta-analysis. Parasit Vectors. 2022 Oct 24;15(1):389. [CrossRef] [PubMed] [PubMed Central]
- Kularatne, S.A. and Dalugama, C. (2022). Dengue infection: Global importance, immunopathology, and management. Clinical Medicine, 22(1), pp.9–13. [CrossRef]
- Organization, W.H. (2009). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. [online] Google Books. World Health Organization. Available at:https://books.google.com.sg/books?hl=en&lr=&id=dlc0YSIyGYwC&oi=fnd&pg=PP2&dq=World+Health+Organization+Dengue:+Guidelines+for+Diagnosis [Accessed 30 Dec. 2023].
- Pal, S., Dauner, A.L., Mitra, I., Forshey, B.M., Garcia, P., Morrison, A.C., Halsey, E.S., Kochel, T.J., and Wu, S.-J.L. (2014). Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE, 9(11), p.e113411. [CrossRef]
- Bandyopadhyay, S., Lum, L.C.S. and Kroeger, A. (2006). Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Tropical Medicine and International Health, 11(8), pp.1238–1255. [CrossRef]
- Tayal, A., Kabra, S.K. and Lodha, R. (2022). Management of Dengue: An Updated Review. Indian Journal of Pediatrics, 90(2). [CrossRef]
- Salles T.S., da Encarnação Sá-Guimarães T., de Alvarenga E., Guimarães-Ribeiro V., de Meneses M., de Castro-Salles P.F., et al. History, epidemiology and diagnostics of dengue in the American and Brazilian contexts: a review. Parasit Vectors. 2018;11(1):264. [CrossRef]
- Khanam A, Gutiérrez-Barbosa H, Lyke KE, Chua JV. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses. 2022 Nov 21;14(11):2575. [CrossRef] [PubMed]
- Malavige, G.N., Jeewandara, C. and Ogg, G.S. (2020). Dysfunctional Innate Immune Responses and Severe Dengue. Frontiers in Cellular and Infection Microbiology, [online] 10, p.590004. [CrossRef]
- Adane, T. and Getawa, S. (2021). Coagulation abnormalities in Dengue fever infection: A systematic review and meta-analysis. PLOS Neglected Tropical Diseases, 15(8), p.e0009666. [CrossRef]
- Patel, Govind R.1; Thanvi, Indu2; Nadeem, Mohammad2; Kanwaria, Rahul2. Coagulation abnormalities and their relationship with bleeding manifestations in patients with dengue-A single center observational study. Asian Pacific Journal of Tropical Medicine 16(2):p 65-71, February 2023.
- John, K.J., Gunasekaran, K., Prasad, J.D., Mathew, D., Das, S., Sultan, N., Abraham, A.M. and Iyyadurai, R. (2019). Predictors of Major Bleeding and Mortality in Dengue Infection: A Retrospective Observational Study in a Tertiary Care Centre in South India. Interdisciplinary Perspectives on Infectious Diseases, 2019, pp.1–7. [CrossRef]
- Ruttmann, T. (2006). Coagulation for the clinician. South African Journal of Surgery. Suid-Afrikaanse Tydskrif Vir Chirurgie, [online] 44(1), pp.22, 24–26, 28–30; passim. Available at: https://pubmed.ncbi.nlm.nih.gov/16619987/.
- Lin SW, Chuang YC, Lin YS, Lei HY, Liu HS, Yeh TM. Dengue virus nonstructural protein NS1 binds to prothrombin/thrombin and inhibits prothrombin activation. J Infect. 2012 Mar;64(3):325-34. [CrossRef] [PubMed]
- Isarangkura PB, Pongpanich B, Pintadit P, Phanichyakarn P, Valyasevi A. Hemostatic derangement in dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987 Sep;18(3):331-9. [PubMed]
- Teerasarntipan, T., Chaiteerakij, R., Komolmit, P., Tangkijvanich, P. and Treeprasertsuk, S. (2020). Acute liver failure and death predictors in patients with dengue-induced severe hepatitis. World Journal of Gastroenterology, 26(33), pp.4983–4995. [CrossRef]
- Azeredo, E.L. de, Monteiro, R.Q. and de-Oliveira Pinto, L.M. (2015). Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators of Inflammation, [online] 2015, pp.1–16. [CrossRef]
- Factors contributing to the disturbance of coagulation and fibrinolysis in dengue virus infection. (2013). Journal of the Formosan Medical Association, [online] 112(1), pp.12–17. [CrossRef]
- Azeredo, E.L. de, Monteiro, R.Q. and de-Oliveira Pinto, L.M. (2015). Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators of Inflammation, [online] 2015, pp.1–16. [CrossRef]
- Huang, Y.-H., Lei, H.-Y., Liu, H.-S., Lin, Y.-S., Chen, S.-H., Liu, C.-C. and Yeh, T.-M. (2003). Tissue plasminogen activator induced by dengue virus infection of human endothelial cells. Journal of Medical Virology, 70(4), pp.610–616. [CrossRef]
- Azeredo, E.L. de, Monteiro, R.Q. and de-Oliveira Pinto, L.M. (2015). Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators of Inflammation, [online] 2015, pp.1–16. [CrossRef]
- Sosothikul, D., Seksarn, P., Pongsewalak, S., Thisyakorn, U. and Lusher, J. (2007). Activation of endothelial cells, coagulation, and fibrinolysis in children with Dengue virus infection. Thrombosis and Haemostasis, [online] 97(4), pp.627–634. Available at: https://pubmed.ncbi.nlm.nih.gov/17393026/ [Accessed 29 Dec. 2023].
- Nurnaningsih, null, Sunbanu, S.E., Rusmawatiningtyas, D., Arguni, E., Makrufardi, F. and Kumara, I.F. (2022). Disseminated intravascular coagulation initial score as a predictor of mortality in children with dengue shock syndrome: A retrospective cohort study. Annals of Medicine and Surgery (2012), [online] 79, p.103890. [CrossRef]
- Factors contributing to the disturbance of coagulation and fibrinolysis in dengue virus infection. (2013). Journal of the Formosan Medical Association, [online] 112(1), pp.12–17. [CrossRef]
- Castilho, B.M., Silva, M.T., Freitas, A.R.R., Fulone, I. and Lopes, L.C. (2020). Factors associated with thrombocytopenia in patients with dengue fever: a retrospective cohort study. BMJ Open, [online] 10(9), p.e035120. [CrossRef]
- Ojha, A., Nandi, D., Batra, H., Singhal, R., Annarapu, G.K., Bhattacharyya, S., Seth, T., Dar, L., Medigeshi, G.R., Vrati, S., Vikram, N.K. and Guchhait, P. (2017). Platelet activation determines the severity of thrombocytopenia in dengue infection. Scientific Reports, [online] 7(1). [CrossRef]
- Lam, P.K., Ngoc, T.V., Thu Thuy, T.T., Hong Van, N.T., Nhu Thuy, T.T., Hoai Tam, D.T., Dung, N.M., Hanh Tien, N.T., Thanh Kieu, N.T., Simmons, C., Wills, B. and Wolbers, M. (2017). The value of daily platelet counts for predicting dengue shock syndrome: Results from a prospective observational study of 2301 Vietnamese children with dengue. PLOS Neglected Tropical Diseases, 11(4), p.e0005498. [CrossRef]
- SAITO, M., OISHI, K., INOUE, S., DIMAANO, E.M., ALERA, M.T.P., ROBLES, A.M.P., ESTRELLA, B.D., KUMATORI, A., MOJI, K., ALONZO, M.T., BUERANO, C.C., MATIAS, R.R., MORITA, K., NATIVIDAD, F.F. and NAGATAKE, T. (2004). Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clinical and Experimental Immunology, 138(2), pp.299–303. [CrossRef]
- Bridget A. Wills, Emmanuelle E. Oragui, Alick C. Stephens, Olufunmilayo A. Daramola, Nguyen Minh Dung, Ha Thi Loan, Nguyen Vinh Chau, Mary Chambers, Kasia Stepniewska, Jeremy J. Farrar, Michael Levin, Coagulation Abnormalities in Dengue Hemorrhagic Fever: Serial Investigations in 167 Vietnamese Children with Dengue Shock Syndrome, Clinical Infectious Diseases, Volume 35, Issue 3, 1 August 2002, Pages 277–285. [CrossRef]
- Pan, P., Zhang, Q., Liu, W., Wang, W., Yu, Z., Lao, Z., Zhang, W., Shen, M., Wan, P., Xiao, F., Shereen, M.A., Zhang, W., Tan, Q., Liu, Y., Liu, X., Wu, K., Liu, Y., Li, G. and Wu, J. (2019). Dengue Virus Infection Activates Interleukin-1β to Induce Tissue Injury and Vascular Leakage. Frontiers in Microbiology, [online] 10. [CrossRef]
- Gomes de Azevedo-Quintanilha I, Campos MM, Teixeira Monteiro AP, Dantas do Nascimento A, Calheiros AS, Oliveira DM, Dias SSG, Soares VC, Santos JDC, Tavares I, Lopes Souza TM, Hottz ED, Bozza FA, Bozza PT. Increased platelet activation and platelet-inflammasome engagement during chikungunya infection. Front Immunol. 2022 Sep 15;13:958820. [CrossRef]
- Ojha, A., Nandi, D., Batra, H., Singhal, R., Annarapu, G.K., Bhattacharyya, S., Seth, T., Dar, L., Medigeshi, G.R., Vrati, S., Vikram, N.K. and Guchhait, P. (2017). Platelet activation determines the severity of thrombocytopenia in dengue infection. Scientific Reports, [online] 7(1). [CrossRef]
- La Russa, V.F. and Innis, B.L. (1995). Mechanisms of dengue virus-induced bone marrow suppression. Baillière’s Clinical Haematology, 8(1), pp.249–270. [CrossRef]
- La Russa, V.F. and Innis, B.L. (1995). 11 Mechanisms of dengue virus-induced bone marrow suppression. Baillière’s Clinical Haematology, 8(1), pp.249–270. [CrossRef]
- Abeysuriya V, Seneviratne SL, de Mel P, Clarice CSH, de Mel C, Chandrasena L, Yip C, Yap ES, de Mel S. The immature platelet fraction, a predictive tool for early recovery from dengue-related thrombocytopenia: a prospective study. Trans R Soc Trop Med Hyg. 2022 May 2;116(5):424-432.
- de Mel, S., Thilakawardana, B.U., de Mel, P., Clarice, C.S.H., Shalindi, M., de Mel, C., Chandrasena, L., Yip, C., Yap, E.-S., Seneviratne, S.L. and Abeysuriya, V. (2020). Triple positivity for nonstructural antigen 1, immunoglobulin M and immunoglobulin G is predictive of severe thrombocytopaenia related to dengue infection. Journal of Clinical Virology, 129, p.104509. https://doi.org/10.1016/j.jcv.2020.104509. 1 Rai, Dr.A., Azad, Dr.S., Nautiyal, Dr.S. and Acharya, Dr.S. (2019). Correlation between hematological and serological parameters in dengue patients- an analysis of 2022 cases. Tropical Journal of Pathology and Microbiology, 5(8), pp.547–554. [CrossRef]
- Abeysuriya, V., Choong, C.S.H., Thilakawardana, B.U., de Mel, P., Shalindi, M., de Mel, C., Chandrasena, L., Seneviratne, S.L., Yip, C., Yap, E.-S. and de Mel, S. (2020). The atypical lymphocyte count: a novel predictive factor for severe thrombocytopenia related to dengue. Transactions of The Royal Society of Tropical Medicine and Hygiene, 114(6), pp.424–432. [CrossRef]
- Donaldson CD, De Mel S, Clarice CSH, Thilakawardana BU, De Mel P, Shalindi M, et al. Admission ultrasonography as a predictive tool for thrombocytopenia and disease severity in dengue infection. Trans R Soc Trop Med Hyg. 2021;115: 1396–1402. [PubMed]
- de Mel, S., Thilakawardana, B.U., de Mel, P., Clarice, C.S.H., Shalindi, M., de Mel, C., Chandrasena, L., Yip, C., Yap, E.-S., Seneviratne, S.L. and Abeysuriya, V. (2020). Triple positivity for nonstructural antigen 1, immunoglobulin M and immunoglobulin G is predictive of severe thrombocytopaenia related to dengue infection. Journal of Clinical Virology, 129, p.104509. [CrossRef]
- Sun P, Bauza K, Pal S, Liang Z, Wu SJ, Beckett C, Burgess T, Porter K. Infection and activation of human peripheral blood monocytes by dengue viruses through the mechanism of antibody-dependent enhancement. Virology. 2011 Dec 20;421(2):245-52. https://doi.org/10.1016/j.virol.2011.08.026. Epub 2011 Oct 26. [CrossRef] [PubMed]
- The monocyte-macrophage-mast cell axis in dengue pathogenesis - Journal of Biomedical Science. Wan et al.https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-018-0482-9.
- Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008 Jul 5;376(2):429-35. [CrossRef] [PubMed]
- Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, Pulendran B. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014 Jul 9;16(1):115-27. [CrossRef] [PubMed]
- Singla, M., Kar, M., Sethi, T., Kabra, S.K., Lodha, R., Chandele, A. and Medigeshi, G.R. (2016). Immune Response to Dengue Virus Infection in Pediatric Patients in New Delhi, India—Association of Viremia, Inflammatory Mediators and Monocytes with Disease Severity. PLOS Neglected Tropical Diseases, 10(3), p.e0004497. [CrossRef]
- Tsai, T.-T., Chuang, Y.-J., Lin, Y.-S., Chang, C.-P., Wan, S.-W., Lin, S.-H., Chen, C.-L. and Lin, C.-F. (2014). Antibody-Dependent Enhancement Infection Facilitates Dengue Virus-Regulated Signaling of IL-10 Production in Monocytes. PLoS Neglected Tropical Diseases, 8(11), p.e3320. [CrossRef]
- Malavige, G.N., Jeewandara, C. and Ogg, G.S. (2020). Dysfunctional Innate Immune Responses and Severe Dengue. Frontiers in Cellular and Infection Microbiology, [online] 10, p.590004. [CrossRef]
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004 Apr 15;189(8):1411-8. [CrossRef] [PubMed]
- Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol. 2007 Jun;81(11):5518-26. [CrossRef] [PubMed]
- Wan, SW., Wu-Hsieh, B.A., Lin, YS. et al. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J Biomed Sci 25, 77 (2018). [CrossRef]
- Kan FK, Tan CC, von Bahr Greenwood T, et al. Dengue Infection Complicated by Hemophagocytic Lymphohistiocytosis: Experiences From 180 Patients With Severe Dengue. Clin Infect Dis. 2020;70(11):2247-2255.
- Hasliana Azrah Ab-Rahman, Wong, P.-F., Rahim, H., Juraina Abd-Jamil, Kian Lam Tan, Sulaiman, S., Lum, C.-S., Syarifah-Faridah Syed-Omar and AbuBakar, S. (2015). Dengue death with evidence of hemophagocytic syndrome and dengue virus infection in the bone marrow. SpringerPlus, 4(1). [CrossRef]
- Kan, F.K., Tan, C.C., Von Bahr Greenwood, T., Khalid, K.E., Supramaniam, P., Hed Myrberg, I., Tan, L.H. and Henter, J.-I. (2020). Dengue Infection Complicated by Hemophagocytic Lymphohistiocytosis: Experiences From 180 Patients With Severe Dengue. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, [online] 70(11), pp.2247–2255. [CrossRef]
- Ahmad, R., Suzilah, I., Wan Najdah, W.M.A., Topek, O., Mustafakamal, I. and Lee, H.L. (2018). Factors determining dengue outbreak in Malaysia. PLOS ONE, 13(2), p.e0193326. [CrossRef]
- Kurane, I., Innis, B.L., Nimmannitya, S., Nisalak, A., Meager, A., Janus, J., and Ennis, F.A. (1991). Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. Journal of Clinical Investigation, 88(5), pp.1473–1480. [CrossRef]
- 3 Salvatory Kalabamu, F. and Maliki, S. (2021). Use of Haematological Changes as a Predictor of Dengue Infection among Suspected Cases at Kairuki Hospital in Dar Es Salaam, Tanzania: A Retrospective Cross Sectional Study. East African Health Research Journal, 5(1), pp.91–98. [CrossRef]
- Ananda Rao, A., U, R.R., Gosavi, S. and Menon, S. (2020). Dengue Fever: Prognostic Insights From a Complete Blood Count. Cureus. [CrossRef]
- Salvatory Kalabamu, F. and Maliki, S. (2021). Use of Haematological Changes as a Predictor of Dengue Infection among Suspected Cases at Kairuki Hospital in Dar Es Salaam, Tanzania: A Retrospective Cross Sectional Study. East African Health Research Journal, 5(1), pp.91–98. [CrossRef]
- Tsai, J.-J., Liu, L.-T., Chang, K., Wang, S.-H., Hsiao, H.-M., Clark, K.B. and Perng, G.C. (2011). The importance of hematopoietic progenitor cells in dengue. Therapeutic Advances in Hematology, 3(1), pp.59–71. [CrossRef]
- Thisyakorn, U., Nimmannitya, S., Ningsanond, V. and Soogarun, S. (1984). Atypical lymphocyte in dengue hemorrhagic fever: its value in diagnosis. The Southeast Asian Journal of Tropical Medicine and Public Health, [online] 15(1), pp.32–36. Available at: https://pubmed.ncbi.nlm.nih.gov/6740378/ [Accessed 29 Dec. 2023].
- Clarice, C.S.H., Abeysuriya, V., de Mel, S., Uvindu Thilakawardana, B., de Mel, P., de Mel, C., Chandrasena, L., Seneviratne, S.L., Yip, C., and Yap, E.S. (2019). Atypical lymphocyte count correlates with the severity of dengue infection. PLOS ONE, 14(5), p.e0215061. [CrossRef]
- Chaloemwong, J., Tantiworawit, A., Rattanathammethee, T., Hantrakool, S., Chai-Adisaksopha, C., Rattarittamrong, E. and Norasetthada, L. (2018). Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematology, 18(1). [CrossRef]
- Abeysuriya, V., Choong, C.S.H., Thilakawardana, B.U., de Mel, P., Shalindi, M., de Mel, C., Chandrasena, L., Seneviratne, S.L., Yip, C., Yap, E.-S. and de Mel, S. (2020). The atypical lymphocyte count: a novel predictive factor for severe thrombocytopenia related to dengue. Transactions of The Royal Society of Tropical Medicine and Hygiene, 114(6), pp.424–432. [CrossRef]
- Azeredo, E.L., Zagne, S.M., Alvarenga, A.R., Nogueira, R.M., Kubelka, C.F., and Oliveira-Pinto, L.M. de (2006). Activated peripheral lymphocytes with increased expression of cell adhesion molecules and cytotoxic markers are associated with dengue fever disease. Memórias do Instituto Oswaldo Cruz, 101(4), pp.437–449. [CrossRef]
- Adikari, T.N., Kamaladasa, A., Fernando, R.H., Fernando, S.M., Perera, T.M.K., Gomes, L., Jayaratne, S., Ogg, G.S. and Malavige, G.N. (2014). High CTLA-4 expression in T cells in patients with acute dengue infection. International Journal of Infectious Diseases, 21, p.330. [CrossRef]
- Jayaratne, H.E., Wijeratne, D., Fernando, S., Kamaladasa, A., Gomes, L., Wijewickrama, A., Ogg, G.S. and Malavige, G.N. (2017). Regulatory T-cells in acute dengue viral infection. Immunology, 154(1), pp.89–97. [CrossRef]
- Jantarika Kumar Arora, Anunya Opasawatchai, Tiraput Poonpanichakul, Natnicha Jiravejchakul, Waradon Sungnak, Anavaj Sakuntabhai, Pratap Singhasivanon, Swangjit Suraamornkul, Tawatchai Yingtaweesak, Khajohnpong Manopwisedjaroen, Nada Pitabut, Oranart Matangkasombut, Sarah A. Teichmann, Ponpan Matangkasombut, Varodom Charoensawan, Single-cell temporal analysis of natural dengue infection reveals skin-homing lymphocyte expansion one day before defervescence, iScience,Volume 25, Issue 4,2022,104034,ISSN 2589-0042. [CrossRef]
- Correa, A.R.V., Berbel, A.C.E.R., Papa, M.P., Morais, A.T.S. de, Peçanha, L.M.T. and Arruda, L.B. de (2015). Dengue Virus Directly Stimulates Polyclonal B Cell Activation. PLOS ONE, 10(12), p.e0143391. [CrossRef]
- Zanini, F., Robinson, M.L., Croote, D., Sahoo, M.K., Sanz, A.M., Ortiz-Lasso, E., Albornoz, L.L., Rosso, F., Montoya, J.G., Goo, L., Pinsky, B.A., Quake, S.R. and Einav, S. (2018). Virus-inclusive single-cell RNA sequencing reveals the molecular signature of progression to severe dengue. Proceedings of the National Academy of Sciences, [online] 115(52), pp.E12363–E12369. [CrossRef]
- Carnec, X., Meertens, L., Dejarnac, O., Perera-Lecoin, M., Hafirassou, M.L., Kitaura, J., Ramdasi, R., Schwartz, O. and Amara, A. (2015). The Phosphatidylserine and Phosphatidylethanolamine Receptor CD300a Binds Dengue Virus and Enhances Infection. Journal of Virology, 90(1), pp.92–102. [CrossRef]
- Vinit Upasani, Thi, H., Auerswald, H., Laurent, D., Heng Sothy, Duong, V., Rodenhuis-Zybert, I.A., Philippe Dussart and Tineke Cantaert (2021). Direct Infection of B Cells by Dengue Virus Modulates B Cell Responses in a Cambodian Pediatric Cohort. Frontiers in Immunology, 11. [CrossRef]
- Arora, J.K., Opasawatchai, A., Poonpanichakul, T., Jiravejchakul, N., Sungnak, W., Sakuntabhai, A., Singhasivanon, P., Suraamornkul, S., Yingtaweesak, T., Manopwisedjaroen, K., Pitabut, N., Matangkasombut, O., Teichmann, S.A., Matangkasombut, P. and Charoensawan, V. (2022). Single-cell temporal analysis of natural dengue infection reveals skin-homing lymphocyte expansion one day before defervescence. iScience, [online] 25(4). [CrossRef]
- Upasani, V., Vo, H.T.M., Ung, S., Heng, S., Laurent, D., Choeung, R., Duong, V., Sorn, S., Ly, S., Rodenhuis-Zybert, I.A., Dussart, P. and Cantaert, T. (2019). Impaired Antibody-Independent Immune Response of B Cells in Patients With Acute Dengue Infection. Frontiers in Immunology, [online] 10, p.2500. [CrossRef]
- Vivier, E., Tomasello, E., Baratin, M., Walzer, T. and Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), pp.503–510. [CrossRef]
- Mathew, A. (2018). Defining the role of NK cells during dengue virus infection. Immunology, 154(4), pp.557–562. [CrossRef]
- Costa, V.V., Ye, W., Chen, Q., Teixeira, M.M., Preiser, P., Ooi, E.E. and Chen, J. (2017). Dengue Virus-Infected Dendritic Cells, but Not Monocytes, Activate Natural Killer Cells through a Contact-Dependent Mechanism Involving Adhesion Molecules. mBio, 8(4). [CrossRef]
- Snehal Shabrish, Karnik, N.D., Gupta, V., Priya Bhate and Manisha Madkaikar (2020). Impaired NK cell activation during acute dengue virus infection: A contributing factor to disease severity. Heliyon. [CrossRef]
- McKechnie, J.L., Beltrán, D., Ferreira, A.-M.M., Vergara, R., Saenz, L., Vergara, O., Estripeaut, D., Araúz, A.B., Simpson, L.J., Holmes, S., López-Vergès, S. and Blish, C.A. (2020). Mass Cytometry Analysis of the NK Cell Receptor–Ligand Repertoire Reveals Unique Differences between Dengue-Infected Children and Adults. ImmunoHorizons, 4(10), pp.634–647. [CrossRef]
- Muralidharan, A. and Reid, S.P. (2021). Complex Roles of Neutrophils during Arboviral Infections. Cells, 10(6), p.1324. [CrossRef]
- Thein, T.-L., Wong, J.G.X., Lye, D.C., Hao, Y., Wilder-Smith, A. and Leo, Y.-S. (2014). Severe Neutropenia in Dengue Patients: Prevalence and Significance. The American Journal of Tropical Medicine and Hygiene, [online] 90(6), pp.984–987. [CrossRef]
- Wang SSY, Chng WJ, Liu H, de Mel S. Tumor-Associated Macrophages and Related Myelomonocytic Cells in the Tumor Microenvironment of Multiple Myeloma. Cancers (Basel). 2022 Nov 17;14(22):5654. https://doi.org/10.3390/cancers14225654. [CrossRef] [PubMed]
- Screaton, G., Mongkolsapaya, J., Yacoub, S. and Roberts, C. (2015). New insights into the immunopathology and control of dengue virus infection. Nature Reviews Immunology, [online] 15(12), pp.745–759. [CrossRef]
- Jenne, Craig N., Wong, Connie H.Y., Zemp, Franz J., McDonald, B., Rahman, Masmudur M., Forsyth, Peter A., McFadden, G. and Kubes, P. (2013). Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host & Microbe, 13(2), pp.169–180. [CrossRef]
- Jenne, Craig N., Wong, Connie H.Y., Zemp, Franz J., McDonald, B., Rahman, Masmudur M., Forsyth, Peter A., McFadden, G. and Kubes, P. (2013). Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host & Microbe, 13(2), pp.169–180. [CrossRef]
- Sung, P.-S., Huang, T.-F. and Hsieh, S.-L. (2019). Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nature Communications, [online] 10(1), pp.1–13. [CrossRef]
- Rathore, A.P.S., Mantri, C.K., Aman, S.A.B., Syenina, A., Ooi, J., Jagaraj, C.J., Goh, C.C., Tissera, H., Wilder-Smith, A., Ng, L.G., Gubler, D.J. and St. John, A.L. (2019). Dengue virus–elicited tryptase induces endothelial permeability and shock. Journal of Clinical Investigation, 129(10), pp.4180–4193. [CrossRef]
- Sherif, N.A., Zayan, A.H., Elkady, A.H., Ghozy, S., Ahmed, A.R., Omran, E.S., Taha, E.A., Eldesoky, E.A., Ebied, A., Tieu, T., Maraie, N., Kamel, M.G., Ngo, H.T., Mattar, O.M., Hirayama, K. and Huy, N.T. (2019). Mast cell mediators in relation to dengue severity: A systematic review and meta-analysis. Reviews in Medical Virology, 30(1). [CrossRef]
- Rathore, A.P.S., Mantri, C.K., Aman, S.A.B., Syenina, A., Ooi, J., Jagaraj, C.J., Goh, C.C., Tissera, H., Wilder-Smith, A., Ng, L.G., Gubler, D.J. and St. John, A.L. (2019). Dengue virus–elicited tryptase induces endothelial permeability and shock. Journal of Clinical Investigation, 129(10), pp.4180–4193. [CrossRef]
- Fujimoto, D.E. and Koifman, S. (2014). Clinical and laboratory characteristics of patients with dengue hemorrhagic fever manifestations and their transfusion profile. Revista Brasileira de Hematologia e Hemoterapia, 36(2), pp.115–120. [CrossRef]
- Clarice, C.S.H., Abeysuriya, V., de Mel, S., Uvindu Thilakawardana, B., de Mel, P., de Mel, C., Chandrasena, L., Seneviratne, S.L., Yip, C., and Yap, E.S. (2019). Atypical lymphocyte count correlates with the severity of dengue infection. PLOS ONE, 14(5), p.e0215061. [CrossRef]
- Moallemi S, Lloyd AR, Rodrigo C. Early biomarkers for prediction of severe manifestations of dengue fever: a systematic review and a meta-analysis. Sci Rep. 2023 Oct 14;13(1):17485. [CrossRef] [PubMed]
- Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Hemorrhagic Fever | GHDx. [online] Available at: https://ghdx.healthdata.org/record/comprehensive-guidelines-prevention-and-control-dengue-and-dengue-hemorrhagic-fever [Accessed 29 Dec. 2023].
- Balasubramanian, S., Anandnathan, K., Shivbalan, So., Datta, M. and Amalraj, E. (2004). Cut-off Hematocrit Value for Hemoconcentration in Dengue Hemorrhagic Fever. Journal of Tropical Pediatrics, 50(2), pp.123–124. [CrossRef]
- Balasubramanian, S., Anandnathan, K., Shivbalan, So., Datta, M. and Amalraj, E. (2004). Cut-off Hematocrit Value for Hemoconcentration in Dengue Hemorrhagic Fever. Journal of Tropical Pediatrics, 50(2), pp.123–124. [CrossRef]
- Clarice, C.S.H., Abeysuriya, V., de Mel, S., Uvindu Thilakawardana, B., de Mel, P., de Mel, C., Chandrasena, L., Seneviratne, S.L., Yip, C., and Yap, E.S. (2019). Atypical lymphocyte count correlates with the severity of dengue infection. PLOS ONE, 14(5), p.e0215061. [CrossRef]
- Looi, K.W., Matsui, Y., Kono, M., Samudi, C., Kojima, N., Ong, J.X., Tan, C.A., Ang, C.S., Tan, P.H.Y., Shamnugam, H., Sekaran, S.D., Syed Omar, S.F. and Lum, L.C.S. (2021). Evaluation of immature platelet fraction as a marker of dengue fever progression. International Journal of Infectious Diseases, 110, pp.187–194. [CrossRef]
- Chuansumrit, A., Apiwattanakul, N., Sirachainan, N., Paisooksantivatana, K., Athipongarporn, A., Tangbubpha, N., Kadegasem, P., Tangnararatchakit, K. and Yoksan, S. (2019). The use of immature platelet fraction to predict time to platelet recovery in patients with dengue infection. Paediatrics and International Child Health, 40(2), pp.124–128. [CrossRef]
- Abeysuriya, V., Seneviratne, S.L., de Mel, P., Clarice, C.S.H., de Mel, C., Chandrasena, L., Yip, C., Yap, E.-S. and de Mel, S. (2021). The immature platelet fraction, a predictive tool for early recovery from dengue-related thrombocytopenia: a prospective study. Transactions of The Royal Society of Tropical Medicine and Hygiene, 116(5), pp.424–432. [CrossRef]
- Rai, Dr.A., Azad, Dr.S., Nautiyal, Dr.S. and Acharya, Dr.S. (2019). Correlation between hematological and serological parameters in dengue patients- an analysis of 2022 cases. Tropical Journal of Pathology and Microbiology, 5(8), pp.547–554. [CrossRef]
- de Mel, S., Thilakawardana, B.U., de Mel, P., Clarice, C.S.H., Shalindi, M., de Mel, C., Chandrasena, L., Yip, C., Yap, E.-S., Seneviratne, S.L. and Abeysuriya, V. (2020). Triple positivity for nonstructural antigen 1, immunoglobulin M and immunoglobulin G is predictive of severe thrombocytopaenia related to dengue infection. Journal of Clinical Virology, 129, p.104509. [CrossRef]
- World Health Organisation (2009). Dengue guidelines, for diagnosis, treatment, prevention, and control. [online] www.who.int. Available at: https://www.who.int/publications/i/item/9789241547871.
- Lye, D.C., Archuleta, S., Syed-Omar, S.F., Low, J.G., Oh, H.M., Wei, Y., Fisher, D., Ponnampalavanar, S.S.L., Wijaya, L., Lee, L.K., Ooi, E.-E., Kamarulzaman, A., Lum, L.C., Tambyah, P.A. and Leo, Y.-S. (2017). Prophylactic platelet transfusion plus supportive care versus supportive care alone in adults with dengue and thrombocytopenia: a multicentre, open label, randomised, superiority trial. The Lancet, 389(10079), pp.1611–1618. [CrossRef]
- Assir, M.Z.K., Kamran, U., Ahmad, H.I., Bashir, S., Mansoor, H., Anees, S.B. and Akram, J. (2013). Effectiveness of Platelet Transfusion in Dengue Fever: A Randomized Controlled Trial. Transfusion Medicine and Hemotherapy, 40(5), pp.362–368. [CrossRef]
- Carr, J.M., Kruskall, M.S., Kaye, J.A., and Robinson, S.H. (1986). Efficacy of platelet transfusions in immune thrombocytopenia. The American Journal of Medicine, [online] 80(6), pp.1051–1054. [CrossRef]
- de Mel, S., Thilakawardana, B.U., de Mel, P., de Silva, A.P., de Mel, C., Chandrasena, L., Seneviratne, S.L. and Abeysuriya, V. (2020). The impact of empirical hydrocortisone therapy on clinical outcomes in dengue fever: a retrospective chart review. Transactions of The Royal Society of Tropical Medicine and Hygiene, 114(8), pp.632–634. [CrossRef]
- Tam, D.T.H., Ngoc, T.V., Tien, N.T.H., Kieu, N.T.T., Thuy, T.T.T., Thanh, L.T.C., Tam, C.T., Truong, N.T., Dung, N.T., Qui, P.T., Hien, T.T., Farrar, J.J., Simmons, C.P., Wolbers, M. and Wills, B.A. (2012). Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, [online] 55(9), pp.1216–1224. [CrossRef]
- Kc, S., Ka, M., Gowdappa Hb and A Bhograj (2013). Effect of High Dose of Steroid on Plateletcount in Acute Stage of Dengue Fever with Thrombocytopenia. Journal of Clinical and Diagnostic Research. [CrossRef]
- Kularatne, S. a. M., Walathara, C., Mahindawansa, S.I., Wijesinghe, S., Pathirage, M.M.K., Kumarasiri, P.V.R. and Dissanayake, A.M.S.D.M. (2009). Efficacy of low dose dexamethasone in severe thrombocytopenia caused by dengue fever: a placebo-controlled study. Postgraduate Medical Journal, [online] 85(1008), pp.525–529. [CrossRef]
- Panpanich, R., Sornchai, P. and Kanjanaratanakorn, K. (2006). Corticosteroids for treating dengue shock syndrome. The Cochrane Database of Systematic Reviews, [online] (3), p.CD003488. [CrossRef]
- Low JG, Sung C, Wijaya L, et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis. 2014;14(8):706–15. [CrossRef]
- Palanichamy Kala, M., St. John, A.L. & Rathore, A.P.S. Dengue: Update on Clinically Relevant Therapeutic Strategies and Vaccines. Curr Treat Options Infect Dis 15, 27–52 (2023). [CrossRef]
- Suputtamongkol Y, Avirutnan P, Mairiang D, et al. Ivermectin Accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial. Clin Infect Dis. 2021;72(10):e586–93. [CrossRef]
- Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014;159(4):711–8. [CrossRef]
- Palanichamy Kala, M., St. John, A.L. & Rathore, A.P.S. Dengue: Update on Clinically Relevant Therapeutic Strategies and Vaccines. Curr Treat Options Infect Dis 15, 27–52 (2023). [CrossRef]
- Zou J, Lee LT, Wang QY, et al. Mapping the interactions between the NS4B and NS3 proteins of dengue virus. J Virol. 2015;89(7):3471–83. [CrossRef]
- Kaptein SJF, Goethals O, Kiemel D, Marchand A, Kesteleyn B, Bonfanti JF, Bardiot D, Stoops B, Jonckers THM, Dallmeier K, Geluykens P, Thys K, Crabbe M, Chatel-Chaix L, Münster M, Querat G, Touret F, de Lamballerie X, Raboisson P, Simmen K, Chaltin P, Bartenschlager R, Van Loock M, Neyts J. A pan-serotype dengue virus inhibitor targeting the NS3-NS4B interaction. Nature. 2021 Oct;598(7881):504-509. Epub 2021 Oct 6. Erratum in: Nature. 2021 Nov;599(7883):E2. [CrossRef] [PubMed]
- Wu-Chuang A, Rojas A, Bernal C, Cardozo F, Valenzuela A, Romero C, Mateos-Hernández L, Cabezas-Cruz A. Influence of microbiota-driven natural antibodies on dengue transmission. Front Immunol. 2024 Mar 15;15:1368599. https://doi.org/10.3389/fimmu.2024.1368599. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



