Preprint
Review
Actional Mechanism of Functional Ingredients in Beer and Barley for Human Health
Altmetrics
Downloads
190
Views
120
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Alerts
Abstract
Nutrition therapy is the best solution to human chronic diseases, especially beer and barley which play an important role in human health and civilization. We demonstrated the actional mechanism of functional ingredients in beer and barley to combat chronic diseases, based on PubMed, google, CNKI, and ISI Web of Science databases from 1997 to 2024. Beer is rich in functional ingredients that is a complex of barley malt and hops; the health effect of beer against 26 chronic diseases is highly similar to that of barley, due to molecular mechanism of polyphenols (phenolic acids, flavonoids), melatonin, minerals, bitter acids, vitamins, and peptides. The ancient German Beer Purity Law provides much scientific basis today, especially indirectly supporting the human one cell disease theory. Low purine beer can be produced by enzymatic and biological degradation and adsorption of purines as well as dandelion addition. Functional beer with low purine and high active ingredients made from Beer Purity and barley malt as well as functional foods addition will be the key and important development direction, such as ginger beer and ginseng beer, especially coix-lily beer at the ancestors ca. 9000 years ago. This review paper not only reveals the actional mechanism of beer overcoming human chronic diseases, but also provides scientific basis for the development of functional beer for the prevention and treatment of human chronic diseases.
Keywords:
Subject: Biology and Life Sciences - Food Science and Technology
1. Introduction
The refined staple foods damage the global economy and threaten human health, such as the global cost of five chronic diseases treatment to reach $47 trillion from 2011 to 2030 [1]. The brewing ingredients of beer drinking in a 9000-year-old in early Holocene southern China may have included red rice, coix seed, acorn, lily bulb, and fungi [2]. Barley plays an important role in health and civilization of human migration from Africa to Asia, later to Eurasia. Barley and hops contain polyphenols for anti- bacterial, anti-inflammatory, anti-oxidative, anti-angiogenic, anti-melanogenic,, anti- cancer and anti-osteoporotic effects, such as kaempferol, quercetin, tyrosol, ferulic acid, xanthohumol, isoxanthohumol, 8-prenylnaringenin, α-bitter acids like humulone and β-bitter acids like lupulone [3]. Yeast transforms polyphenolic compounds in malt wort into healthy hydroxytyrosol and melatonin as well as probiotic drivers [4]. Barley malt and hop become a beverage rich in micronutrients by beer brewing, especially beer polyphenols interact with the intestinal microbiota, such as ferulic acid, yellow phenol, catechins, epicatechin, proanthocyanidin, quercetin, and rutin [5]. German Beer Purity Law from 1516, allows only barley (Hordeum vulgare L.), hops (Humulus lupulus L.), yeasts and water for beer brewing.
We think that this ancient German Beer Purity Law provides much scientific basis today. Beer is not only one of the oldest human inventions for functional foods, but also the most common beverages worldwide, such as 1.89 billion hectoliters in 2022 and USD 721.12 billion. While China built a functional food industry of USD 1.4 billion of ginseng and notoginseng, we forgot that barley malt, one of the raw materials, brewed beer has reached $25 billion. Beer is a future excellent functional food, based on its functional ingredients (barley malt) and traditional Chinese medicine (hops) as well as water, especially polyphenols and dietary fiber with anti-inflammatory and antioxidant effects [6] and medium for live microbe and probiotic delivery [7]. Beer phenolics derives from barley (lignans, and alkylresorcinols) and hops (prenylflavonoids, and stilbenes) and both sides (phenolic acids, flavonoids), such as about 70% of beer polyphenols originate from barley malt, and the remaining 70% from hops, especially 9 compon ents of phenolic acids have been identified in conventional beers, with ferulic acid as the most abundant phenolic acid, followed by caffeic, sinapic, p-coumaric, and vanillic acids [8,9]. Barley is rich in 30 ingredients to combat 28 chronic diseases, due to molecular mechanism of barley grass (GABA, flavonoids, SOD, K-Ca, vitamins, and tryptophan) and grains (β-glucans, polyphenols, arabinoxylan, phytosterols, tocols, and resistant starch) [1]; Purple barley rich in phenols and flavonoids as well as anthocyanins had a good antioxidant impact and α-glucosidase-inhibiting activity [10]. The six natural prenylated flavonoids content (isoxanthohumol, isoxanthohumol-C, 8-prenylnaringenin, 6-prenylnaringenin, xanthohumol, and xanthohumol-C) of beer and hops varied from 0.04 to 3.2 μg/L [11].
Beer is the functional food against chronic diseases that contain lots of functional ingredients, such as phenolic acids, polio phenol, quercetin, kaempferol, tyrosol, flavones bitter acids (humulones and lupulones), vitamin B (VB6, VB12 and folate), 8-prenylnaringenin, xanthohumol, minerals, and complex carbohydrates except ethanol [12,13]. The functional ingredients in beer can mitigate the negative effects of alcohol; Four compounds (total prenylated flavonoids, tyrosol, hydroxytyrosol, and alkylresorcinols) can act synergistically and triggering health effects [14]. Moderate beer consumption of up to 16 g alcohol/day for women and 28 g/day for men associated with decreased incidence of cardiovascular disease and overall mortality, among other metabolic health benefits [12,15], but may vary according to age, sex, genetics and body type, as well as drug or supplement use [16]. There are about 17,000 factories of Craft beer not filtered/not pasteurized in the world, and its innovation concerns aspects, such as ingredients, alcohol content, aging, and packaging, which are beverages rich in health compounds but with a reduced shelf life [17]. Non-alcoholic beer consumption is more effective than conventional beer in preserving the endothelial function and inhibiting thrombogenic activity, but conventional beer with high polyphenols induces greater increases in HDL cholesterol levels [18].
When we solve obesity, diabetes, cardiometabolic and other chronic diseases, we forget that outside our GLP-1 drugs the Breakthrough of the Year, there are lots of functional foods including barley and beer [2]. It is very necessary to clarify the coevolutionary mechanism of disease resistance genes and human functional foods (beer, barley, and rice) as well as GLP-1 receptor agonists [19]. Although beer has contributed greatly to human health and global economic development, it has caused gout to restrict the high-quality development of the industry. To date, there is a lack of systematic reports on human health for active ingredients and the functional mechanism of barley and its brewed beer at home and abroad. This review has important theoretical significance and high practical value for improving the proportion of barley malt in brewage and improving human health.
2. Functional Ingredients of Beer and Its Raw Materials
The quality traits of beer include flavor, texture, foam stability, gushing, peptides and haze formation, especially 7113 proteins (4692 beer proteins, and 3906 foam proteins) including LTP, serpin, hordein, gliadin, and glutenin which may help to evaluate health risks and promote healthy nutrition enrichment [20,21]. The foam-stabilizing proteins in beer from malted barley are Protein Z4 and LTP, which display less foamability but greater foam stability [22]. The foam stability depends on the balance of foaming components (polypeptides, hop bitter acids, metal ions, melanoidins, ethanol, lipids, detergents) [22].
2.1. Contribution of Hops and Barley to Beer Polyphenols
Hops are largely used in traditional medicine with 1000 polyphenolic substances, especially proanthocyanidins (>55%), flavonoid glycosides (>28%) and polyphenols (40–140 mg/g), which became appreciated over the years for the bitterness and aroma they impart to beer [9]. Phenolic antioxidants in beer due to the lemon and hesperidin, however 26 compounds belonging to the different phenolic classes of hydroxybenzoic, hydroxycinnamic and caffeoylquinic acids, flavonoids and prenylflavonoids [23].
In Table 1, the main bioactive phenolic compounds contents from hops and barley found in common beer. Both hops and barley can provide the bioactive phenolic components, such as caffeic, chlorogenic, p-coumaric, ferulic, p-hydroxybenzoic, syringic, gallic, protocatechuic acids for total 8 kinds of phenolic acids; however gentisic acid comes from hops, 2,4-Dihydroxybenzoic and sinapic as well as vanillic acids come from barley [1,8,9,24,25]. Among flavonoids, both hops and barley can provide the bioactive components, such as catechin, kaempferol, naringenin, naringin, quercetin, and rutin; however 10 bioactive components (epigallocatechin, epicatechin, procyanidin B1, procyanidin B2, procyanidin C1, isorhamnetin, isoxanthohumol, 8-prenylnaringenin, xanthohumol,desmethylxanthohumol) come from hops, three bioactive components (myricetin, hesperidin, alkylresorcinols) come from barley [8,9,24,26].
2.2. Contribution of Functional Ingredients of Barley to Beer
Table 2 shows the functional and nutrient compositions of beer and barley grains: Beer water content is up to 88.5% to 97.7%; Barley grains are higher in both protein and 9 mineral elements as well as 4 functional components (polyphenols, flavonoids, FRAP, ABTS) than that of beer, however there are more than 13 nutritional function differences in beer and barley grains. There are the highest anthocyanins (48.07 mg/100 g), total phenols (570.78 mg/100 g) and flavonoids (47.08 mg/100 g) in purple barley [10].
3. Functional Ingredients in Beer
3.1. Functional Ingredients in Common Beer
Beer is not only a popular functional beverage consumed in large amounts all over the world, but also the source of carbohydrates, amino acids, minerals, vitamins, polyphenols (phenolic acids, flavonoids), benzoic and cinnamic-acid derivatives. 26 samples of bottled beer contain significant amounts of biologically active isoflavonoid phytoestrogens, especially four isoflavonoids ranged from 1.26 to 29 nmol/L (isoflavonoid 0.19-14.99 nmol/L, daidzein 0.08-2.5 nmol/L, genistein 0.169-6.74 nmol/L and biochanin A 0.820-4.84 nmol/L) [32].
Many researches focused on the identification of the functional ingredients in conventional beers (see Table 3). Beer contains numerous categories of antioxidants, polyphenols, traces of group B vitamins, minerals (Se, Si, K), soluble fibers, melanoidins, and microorganisms [6] The 13 components of flavonoids and 5 components of prenylflavonoids and 4 components of stilbenes as well as 2 components of phenolic alcohols from hops have been identified in conventional beers [8,9,25]. The 17 components of phenolic acids from barley have been identified in conventional beers, especially most abundant erulic acid and procyanidin B; The 22 components of flavonoids and 12 components of lignan as well as alkylresorcinols from barley have been identified in common beers [1,9,25,26]. In addition, common beer is rich in vitamins and organic acids and its types, including 14 vitamins and 13 organic acids [8,25].
| Potassium D-gluconate [25] |
3.2. Functional Beer
Functional beer is increasingly searching for health and pleasant taste, such as ginger, olive, eggplant, lignans, green beer and so on. The natural ginger beer market is dominated by the United States, supplemented brewing wort with curcumin (25 μg/mL) can increase in the beer's total phenolic and flavonoid content (shagaol, gingerone, zingerone), offering potential antibiofilm and health benefits [39,40]; The ginger beer produced with ginger bug and fermented for 14 days showed better volatile and phenolic compound profiles, physicochemical parameters, microbial diversity, and sensory characteristics [41]. Olive leaves in beer (10 g/L) imparts a sour/astringent taste and herbal aroma, however 5 g/L obtain pleasant sensory profile, it can increase oleuropein and 3-hydroxytyrosol of beers for human health, especially boiling time favored hydrolysis of oleuropein to 3-hydroxytyrosol [42] Beer adding eggplant peel extract (delphinidin-3-rutinoside, delphininidin-3-glucoside and delphinidin-3- rutinoside-5-glucoside) can increase the antioxidant activity , based on its total phenolic content from 0.426 to 0.631 mg /mL and total flavonoid from 0.065 to 0.171 mg/Ml [43]. Lignans are plant phenols for human health, its addition of spruce knot chips or extracts during the wort boiling were produced beer with lignans content ranging from 34 to 174 mg/L [44]. The formulation for green beers can increase the phenolic and antioxidant activity, such as turmeric: black pepper: aroma hops=1.5:1.5:1 or 5:2:3 [45].
Functional beer may be improved through novel brewing approaches, which has become a new field of beer brewing and human health. Recently, characterizations of the functional ingredients and antioxidant properties of beers with added fruit, vegetable, herbs, and natural food; Fruit beers obtained through the addition of 18 fresh fruits during the fermentation process resulted in significant enrichment in phenolic and total flavonoid as well as stilbene molecules compounds in beers, and a similar 20 beers obtained through the addition of vegetables, herbs, and natural foods [9]. In addition to foreign countries to develop functional beer with functional food materials, China also uses 34 functional food materials (Astragalus, barley malt, barbary wolfberry, rugosa rose, rhizoma gastrodiae, polygonatum sibiricum, hawthorn, jujube, lily, fructus mori, yam, papain, lonicerae japonicae, polygonatum odoratum, herba lophatheri, semen cassia, almond, oyster, seabuckthorn, siraitiae fructus, mulberry leaf, chrysanthemum, Perilla frutescens, Puerarialobata, honey, citrus peel, mntha, raspberry, Dendrobium can didum, ginseng, Ganoderma lucidum, cornel, honey,and so on) to develop new functional beer.
3.3. Special Beer
Rice beer is traditionally brewed and consumed by Southeast Asian countries. The metabolite profiles of the rice beer consisted of 18 saccharides, 18 organic acids, 11 sugar alcohols, 8 amino acids, 1 vitamin and nutraceutical compounds thiocoumarine, carotene, oxazolidine-2-one and acetyl tyrosine, however alcoholic content of rice beer varied from 9.41 to 19.33%, phenolic from 2.07 to 5.40 mg gallic acid/ml, DPPH· 1.94-4.14 and ABTS+ 1.69-3.91 mg of Vc /ml, respectively [46]. Non-alcoholic beer before exercise could help maintain electrolyte homeostasis during exercise [47]. In addition, there are some special beers, such as rye, oats, millet, potato, quinoa, tartary buckwheat, jasmine, green tea, coffee, cocoa beans, kelp, pepper, mungbean, Moringa stenopetala and so on.
4. The Health Effects of Raw Materials
4.1. Barley Grains with 28 Health Effects
Barley grains play an important role in health and civilization of human migration from Africa to Asia, later to Eurasia [1], it is the highest comprehensive utilization in grain crop of six in-one with forage feed, wine raw materials, healthy food, functional food, ornamental weaving and Chinese medicinal medicine [48]. To date, 28 human health effects have been reported for barley grains. Except for 22 kinds the chronic diseases of prevention and treatment reported by Zeng et al [1], barley has been found to have some effects in recent years, which has reduced symptoms of COVID-19, treating fatty liver and Alzheimer’s disease, improve glucolipid modulation, cardio-metabolic, and prebiotic formulation.
4.1.1. Barley Grains with 22 health Effects
Zeng et al [1] summarized the health effects of 22 chronic diseases of prevention and treatment, including antidiabetes, antiobesity, anticancer, antioxidants, improve gastrointestinal [49], anti-inflammation, lower blood pressure, hepatoprotection, immunomodulation, alleviates atopic dermatitis, prevent cardiovascular diseases, hyperlipidemia, antiaging, cardioprotection, anti-fatigue, optimize cholesterol, improve bowel health, metabolic syndrome, prevent heart disease, reduce chronic kidney disease [50], accelerate wound healing, decrease stroke and cholelithiasis activities. Barley and adlay are the largest functional food with the use of 110 kinds of homologous substances in China, both barley grasses and grains can prevent and control more than 22 chronic diseases in humans or animals, due to molecular mechanism of barley grass (GABA, flavonoids, SOD, K-Ca, vitamins, and tryptophan) and grains (β-glucans, polyphenols, arabinoxylan, phytosterols, tocols, and resistant starch), especially support that human has only one cell disease theory, which provides a reference for the development of human functional food and animal husbandry production of functional feed [1,51]. The results support highland barley, barley or its malt be selected the 2023 and 2024 Editions of Adult Dietary Guidelines for Hyperlipidemia, Hypertension, Diabetes, Obesity, Chronic Kidney Disease, Hyperuricemia and Gout, as well as Dietary Guidelines for growth retardation and obesity in children and adolescents released by National Health Commission of the People's Republic of China.
4.1.2. Control COVID-19
We report for the first time the role of barley malt in COVID-19 prescribing in eight provinces in China and barley grass powder has a certain effect on COVID-19 treatment [52]; Barley-based remedy could significantly enhance the blood oxygen saturation and reduce fatigue in COVID-19 patients [53].The ricin-based peptide from barley was able to inhibit Mpro in vitro with an IC50 of 0.52 nM, and its low and no cytotoxicity upto 50 µM suggested its therapeutic potential against SARS-CoV-2. Barley and its functional foods to enhance human immunity and control COVID-19 [55], especially the quercetrin in barley should be the best inhibitor for the main protease of SARS-CoV-2 [56]. These new perspectives facilitate the caramel barley malt inclusion of Diagnosis and Treatment Plan for COVID-19 (trial version 10) in 2023 of National Health Commission of the People’s Republic of China [57]. Persian barley water can reduce the length of hospital stay, fever, erythrocyte sedimentation rate, C-reactive protein, and creatinine among hospitalized COVID-19 patients with moderate severity [58,59], such as a treatment protocol approved by Iran's Ministry of Health and Medical Education, consisting of an Iranian regimen, Ficus carica; Vitis vinifera, Safflower, Cicer arietinum, Descurainiasophia seeds, Ziziphus jujuba, chicken soup, barley soup, rose water, saffron, and cinnamon spices, which appears to be effective in the treatment of symptoms as well as inflammatory biomarkers such as C-reactive protein in COVID-19 patients [60].
4.1.3. Treating Fatty Liver
The extract of highland barley Monascus purpureus improves nonalcoholic fatty liver disease through various pathways and targets of body metabolism, and can be used as a functional food for the treatment of liver disease and lipid metabolism disorders [61]. Highland barley β-glucan alleviated western diet-induced NAFLD prevents fat accumulation by increasing energy expenditure, revealing the mechanism associated with changes in hepatic bile acid composition [62].
4.1.4. Treating Alzheimer's Disease
Highland barley contains wide bioactive nutrients, such as carbohydrates, polyphenols, minerals, vitamins, phenolic, flavonoids and β-glucan, which contributes to many health benefits, such as treating Alzheimer’s disease, anti-bacteria, anti-inflammatory, anticancer, antidiabetic, anti-obesity, antifatigue,antiaging; prevent hyperglycaemia, hyperlipidemia, and heart disease as well as cancers [63].
4.1.5. Glucolipid Modulation
A delicious high-energy diet through human brain control of excessive appetite intake leads to obesity, especially highland barley-based functional foods may help manage hyperlipidemia and antiobesity. This work provided a new way of barley functional food and genes to help us overcome obesity [64]. Barley is rich in a variety of functional ingredients with health-promoting effects, especially improve glucolipid modulation mechanisms [65]. Barley 80% reduced blood concentrations of total cholesterol, low-density lipoprotein and triglycerides in fattening pigs, however HDL content increased extremely significantly to 1. 04 mmol / dm3 [66].
4.1.6. Cardio-Metabolic
Bread containing whole-grain red sorghum and barely flours enhanced plasma total polyphenols and antioxidant status, its consumption would modulate biomarkers of cardio-metabolic health [67].
4.1.7. Prebiotic Formulation
Barley by-products are important raw materials for the production of melanoidins. Barley melanoidins prevent lipid peroxidation, oxidative damage of DNA, and induce antioxidant activity, antimicrobial, antihypertensive, antiallergenic, and prebiotic properties [68]. High phenolic compounds and β-glucans and tocols in barley are one of the important substrates of functional foods such as probiotic formulations [69].
4.1.8. Neuroprotection
Barley grains are rich in protein, fiber, minerals, and phenolic compounds, that have potent against neurodegenerative diseases through antioxidant, anti-inflammatory, vasodilatation and restoring neurochemical alterations [70].
4.2. Hops for Human Health
Hops gives the characteristic aroma and bitter taste, based on functional ingredients, such as humulones, lupulones, 8-prenylnaringenin, 6-prenylanaringenin, xanthohumol, 6,8-diprenylnaringenin and 8-geranylnaringenin, its healthy activities including antibiotic, anti-bacterial, anti-molds, anti-virus, anti-fungal, neuropreventive, estrogenic, and antinflamatory properties, especially prevent lots of chronic diseases, such as cancer, arthritis, insulin sensitivity, type II diabetes, metabolic syndrome, and menopause [9,71,72]. The estrogenic effects of hop prenylflavonoids is one of the most potent phytoestrogens identified so far, the yield of 8-prenylnaringenin was 29 mg/100 g of product, which may be alleviated the symptoms of menopausal disorders [73] and over anticancer to estrogenic activity [74]. Xanthohumol in hop possess the synergistic effect against aging, diabetes, inflammation, microbial infection, anti-proliferative, and cancer [75,76].
5. Action of Beer on Human Health
5.1. Healthy Effects of Beer
The 26 health effects of beer are the result of the combined effect of its raw materials brewing into active ingredients (Figure 1). Numerous studies have shown that functional ingredients in beer have many health benefits. Polyphenols have more than 8,000 different types that have been identified so far, which includes phenolic acids, flavonoids, lignans, and stilbenes. Prenylflavonoids from hops can prevent osteoporosis, inhibit cytochrome-P450-mediated activation of procarcinogens, antiproliferative effects on cancer cell lines, and anti-angiogenic activity [9]. The metabolites of hydroxytyrosol and tyrosol exhibits strong anticancer, neuroprotective, cardioprotective, antiatherogenic, antioxidant,and endocrine effects [9]; Lignans from barley have antitumor, antioxidative, antiviral, antibacterial, antifungal, estrogenic, cardioprotective, and cardiovascular activities [9]. The stilbene from hops has antiplatelet, anti-inflammatory, estrogenic, cardioprotective, antitumor, and antiviral properties. The melatonin contained in beer can provide health benefits, due to its neuroprotection, anticancer, antioxidant, anti- inflammatory, and immunomodulatory properties, especially cardiovascular diseases, osteoporosis, control of hypertension and constipation [77,78]; Four phytochemicals from barley (tocopherols, tocotrienols, carotenoids, phytosterols) exhibit strong antioxidant, antiproliferative, and cholesterol-lowering activities, which can prevent some human chronic diseases, such as cancer, cardiovascular disease, diabetes, and obesity [9].
5.1.1. Cardiovascular Disease Prevention
Beer has the protective effects on the cardiovascular and cancer due to alcohol and polyphenols from moderate beer intake [79,80]. Beer phenolics in free and bound forms are mainly originated from barley malt and roasted malts [81]. Beer improves human cardiovascular disease by improving vascular elasticity, flow mediates vascular dilation, and significant increases in and apolipoprotein A1 levels [82]. Higher folic acid in beer improves cardiovascular disease among alcohol consumers [83].
5.1.2. Anticancer
Phenols and melanin in beer have antioxidant properties, which can reduce the content of free radicals in human metabolism, and have obvious effects against cancer or cardiovascular diseases [84]. Moderate intake of beer is attributed to polyphenols, vitamins and fiber; low dose (3 mg / kg) inhibits the formation of lesions, polyps and tumors, while high dose (300 mg / kg) protects against the early cancer [85]. The prenylated flavonoids of beer and hops have anti-cancer and therapeutic metabolic syndrome [11].
5.1.3. Anti-Diabetes
A delicious high-energy diet through human brain control of excessive appetite intake leads to diabetes [86]. Isomaltulose and resistant maltodextrin in alcohol-free beer improve insulin resistance in patients with type 2 diabetes and overweight or obesity [87]. The daily intake of 330 mL alcoholic beer could change the lipid profile and insulin sensitivity of adult men [88]. The beer with low sugar (≤0.75 g/100 ml) and alcohol (<1.2%) contents is a functional food for diabetics [89].
5.1.4. Lipid Deposition Prevention
Human obesity outbreaks owes to the psychological pursuit of taste diverts us from a healthy diet [64]. Moderate intake of beer prevents lipid deposition in the vessel wall, but does not increase body weight in obese healthy individuals [90]. The soluble nitrogen content (74.98%) of barley protein is a dietary secretion stimulating supplement for obesity management [91]. Moderate consumption of beer improves the lipid profile of postmenopausal women, although preventing cardiometabolic effects warrant investigation [92].
5.1.5. Antioxidation
The antioxidants of polyphenols and melanoidins in beer have the effect of protecting against oxidative damage [93]; The majority of beer's antioxidant activity (55-88%) came from ferulic acid (50%) and the five phenolics (syringic acid, catechin, caffeic acid, protocatechuic acid, and epicatechin) [94]. Non-alcoholic beer is more effective in preventing oxidative stress than conventional beer [18]. Moderate intake of beer increases the anti-oxidative properties of HDL and facilitates cholesterol efflux [90].
5.1.6. Anti-Inflammation
Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells [95]. Bitter Iso-α-acids in beer from hops are suppressing both lipid accumulation and brain inflammation [96]. Hop cones are rich in phenolic compounds and have anticancer, antioxidant and anti-inflammatory activities, especially the effects of antiplatelet drugs [97]. The hop metabolite 8-prenylnaringenin stimulates angiogenesis, whereas xanthohumol and isoxanthohumol have anti-angiogenic and anti-inflammatory effects [95].
5.1.7. Immunomodulation
Beer degradation products have anti-inflammatory, anticoagulant, antioxidant, and regulation of glucolipid metabolism, such as anti-cancer, reduced cardiovascular events, and regulatory metabolic syndrome [98]. Beer contains melatonin that is a molecule with antioxidant, oncostatic, immunomodulatory, and cytoprotective properties [78]. The essential oils, bitter acids and flavonoids of hop cones for brewed beer have antioxidant and immunomodulatory as well as neuroprotective effects [99].
5.1.8. Improve Gastrointestinal
Beer polyphenols can reach the large intestine and interact with colonic microbiota [100]. Gastric emptying in full-intensity, low-carbohydrate, and low-alcohol beer determines plasma ethanol response in healthy young, i.e. the most significant negative correlation between plasma ethanol at 15 min and gastric emptying after low-alcohol, AUC 0-180 min for blood glucose was greater for low-alcohol than low-carbohydrate [101]. Moderate beer drinking is conducive to intestinal health due to the higher concentration of butyric acid in beer consumers [102].
5.1.9. Cardioprotection
There may be minor additional benefits associated with drinking beer against coronary disease [103]. The polyphenols and melanoidins in beer have the effect of cardioprotection and anticancer, regulating intestinal microbiota and metabolites, antibacterial, and anti-inflammation [93].
5.1.10. Longevity
The relationship between healthy and longevity of beer drinking was reported in 1884. The main nutrients of beer are polyphenols (30% from hops, 70% from malt), amino acids, carbohydrates, minerals, vitamins and, especially the anti-aging and antioxidant and proliferating effects of brewing components on D-dSCs and Caco-2 cells [104]. The treatment with ethanol and beer before irradiation can enhance longevity in mosquitoes [105]. The iso-α-acids of beer prevents dementia and anti-aging by suppressing neuroinflammation and improving cognitive function [106]. VB2 has anti-oxidant, anti-aging, anti-inflammatory, anti-nociceptive and anti-cancer properties [107].
5.1.11. Improve Skin Health
The health effects of beer substances on skin include the treatment of atopic eczema, contact dermatitis, hyperpigmentation diseases, skin infections, skin aging, skin cancer, and photoprotective measures [4]. The malt and beer by-products (caramalt, aromatic malt, roasted malt, malt sprouts, dark beer spent grain) are antioxidants for whitening cosmetics and so on [108]. Ferulic acid from by-product of beer is an antioxidant with cosmeceutical Nanoferulic with the regeneration of the skin [109].
5.1.12. Prevent Alzheimer’s Disease
The regular beer consumption can prevent Alzheimer and neurodegenerative diseases by reducing the aluminum, and the mineral homeostasis imbalance in the brain [110]. Hop extract had γ-secretase inhibitory activity and significantly reduced Aβ production in cultured cells and Alzheimer’s disease in mouse [111].
5.1.13. Improve Metabolic Syndrome
Polyphenols and bitter acids (iso-α-acids) in hop cones are very effective in treating metabolic syndrome, especially hypoglycemic, antihyperlipidemic, antiobesity, lipid metabolism and glucose tolerance activities [112]; The healthy effects of bitter acids and xanthohumol in beer on chronic diseases have inflammatory and immune diseases, obesity, metabolic disorders, and cancer prevention [113].
5.1.14. Prevent Osteoporosis
Polyphenols of non-alcoholic beer can improve bone health and body water in postmenopausal women [114]. Beer is more effective than spirits in increasing bone mineral density, such as Si in beer, which reveals that components except ethanol may contribute to bone health [115]. The functional components of beer are polyphenols, folic acid and phytoestrogens, which can improve osteoporosis [116].
5.1.15. Improve Cognition
Dementia and cognitive decline are global public health problems. The daily intake of iso-α-acids in beer from hops suppresses inflammations in the hippocampus and improves visual learning and cognitive decline of high fat diet, especially enhances the production of inflammatory cytokines and chemokines as well as improved memory by dopamine release in mice, it could be effective for improvement of working memory in human dementia patients [96]. The supplementation of bitter acids 35 mg/day can stimulate the vagus nerve and enhance cognitive function [117].
5.1.16. Antidepressant
Hop β-bitter acid especially hoperone has potential antidepressant effects in vitro [118]. Iso-α-acids intake of beer prevent brain aging and neurological disorders, which can elucidate the effects of gray matter volume and cognitive function on the Brain Healthcare Quotient [119].
5.1.17. Improve Fatigue or Mood
The non-alcoholic beer in daily life can maintain mood states, including anxiety, depression, fatigue, and vigor, sleep quality and absolute presenteeism [120]. Hop bitter acids in beer might be beneficial for cognition and mood state [117]. Alcoholic beer consumption showed a J-shaped relationship with self-perceived, physical, mental, and social-emotional health, with better values at moderate levels [121].
5.1.18. Blood Pressure Regulation
Beer is major food source of isoxanthohumol for a precursor of 8-prenylnaringenin, the phytoestrogens can reduce perimenopausal symptoms, especially moderate non-alcohol beer consumption improved the lipid profile and decreased blood pressure in postmenopausal women [122]. Five beers led to a significant decrease in systolic blood pressure, heart rate and self-reported feeling of palpitations [123].
5.1.19. Prevent Neurodegenerative Disease
Beer extracts prevent neurodegenerative diseases by regulating adenosine receptor expression and protecting glioma and neuroblastoma cells from oxidation, it is promoting beer consumption on health [124]. Low-moderate 1~2 drink per day of beer reduces the risk of cardiovascular and neurodegenerative disease [125]. Moderate beer consumption was associated with protective cardiovascular function and reduction in the development of neurodegenerative disease [126], and neuroprotective effect by a possible depletion of Aβ aggregation in brain [127].
5.1.20. Promote Sleep
Moderate beer intake can have hepatoprotective effects due to the flavonoids in the hops, and the imperial red ale in eight beers exhibits the highest antioxidant properties and increase enzymatic and non-enzymatic redox status after CCl4 insult [128]. The mechanisms of Xanthohumol with anti-atherosclerotic effect in beer have been proved by decreasing pro-inflammatory factors and improving hepatic lipid metabolism via AMPK activation [129].
5.1.21. Hepatoprotection
Sleep deprivation affects the physiological functions in the human organism. Melatonin is a hormone secreted in the pineal gland with several functions, especially regulation of circadian sleep cycle and the biological processes [77]. The consumption of non-alcoholic beer at dinner time helps to improve the quality of sleep at night [130]. The sleep-promoting effects of α-acids, β-acids, and xanthohumol in beer from hop, especially Simcoe water extract and Saphir ethanol extract modulated GABAergic signaling to improve sleep-related behaviors, including sleep duration [131].
5.1.22. Heart Failure or Stroke Prevention
Oxidative stress of polyphenols plays a central role in cardiovascular disease (myocardial infarction, angina pectoris, cardiac ischemia, heart failure, chronic arrhythmias, and stroke), especially modulating the transcriptomic response of heart oxidative stress by myocardial ischemia in the hypercholesterolemia, however a dose-dependent up-regulation of electron transport chain members and the down-regulation of spliceosome-associated genes linked to beer consumption [132]. Low beer consumption against cardiovascular disease due to its implication on low-grade chronic inflammation [133].
5.1.23. Prevent Gallstone Disease
The alcohol consumption of beer could decrease the risk of gallstone disease, a dose-dependent linear risk reduction and a weakened linear trend between alcohol consumption levels less than and greater than 28 g/day [134]. Regular moderate beer drinking lowers bile concentration and affects cholesterol levels, which lowers the likelihood of gallstone [135].
5.1.24. Reduce Kidney Stones
Kidney stones are a urinary tract disease. Water can maintain blood to produce secretions and to perform metabolic reactions. Barley water is an excellent solution for kidney stones and cysts too, especially children and adult age groups daily till the urine infection subsides [136]. Beer is a diuretic that may aid in the passage of tiny stones that are less than 5 mm in size, however high intakes of Ca, K, and fluids have been shown to be associated with lowered risk of kidney stones, one bottle of beer consumed per day reduce risk by 40% [137,138]. Supplementation of beer with pine shoots can facilitates the dissolution of kidney stones, based on its phenolic acids and flavonoids have antibacterial, expectorant and analgesic properties and is used as an antiseptic for respiratory tract, urinary tract and kidney infections [139].
5.1.25. Wound Healing Acceleration
The dandelion addition improved the beer properties of antioxidant by chlorogenic, caffeic, ferulic, and chicoric acid [140]. Oxidative stress inhibits wound healing, however phytochemicals especially chicoric acid have antioxidant and scavenge reactive oxygen species, thereby promoting wound healing [141].
5.1.26. Prebiotic Action
Lactobacillus fermentum TIU19 isolated from Haria beer can be explored as a potential probiotic with antagonistic activity against MDR uro-pathogenic E. coli and E. faecalis [142]. The polyphenols degradation products from beer have prebiotic action and may combat intestinal dysbiosis [6].
5.2. Beer Hazards to Human Health
5.2.1. Hyperuricemia
Beer has 26 health effects against 26 chronic diseases on the human body (Table 4) is highly similar to that of barley (Figure 1), however the interaction effects between excess adiposity and alcohol use were positively associated with the hyperuricemia [150]. The d-amino acids in beer are the oxidation of Fe2+ to produce hydroxyl radicals, leading to DNA damage and the formation of a large number of purine bases [151]. Uric acid in humans is the end product of purine metabolism due to the loss of hepatic uricase activity during evolution, it associated with oxidative stress and inflammationas well as nephrolithiasis [152]. The purine in beer can be catabolized into uric acid, lead to hyperuricemia and gout [153]. Among the 63 associated foods, beer and liquor with the strongest urate raising effect were associated with a 1.38 μmol/L increase in serum urate per serving per week, equating to a 9.66 μmol/L (0.16 mg/dL) increase per daily serving [154].
5.2.2. Low Purine Beer
The purine content in beer can be reduced by enzymatic and biological degradation or adsorption methods [153]. The purine content in beer after treatment with adenine deaminase and guanine deaminase enzyme was significantly reduced, and two enzymes can work at 5.0-8.0 pH and retain >50% activity at 40°C, which became an effective route of low purine beer production [155]. The dandelion addition improved the beer properties of antioxidant and inhibit uric acid production against xanthine oxidase by chlorogenic, caffeic, ferulic, and chicoric acid [140].
5.2.3. Hyperuricemia and Gout Diet Beer
According to 2024 Editions of Adult Dietary Guidelines for Hyperuricemia and Gout released by National Health Commission of the People's Republic of China, the high frequency of raw materials for hyperuricemia with gout 9 drink prescriptions is in follows: Chicory (7)> orange peel (4) = coix seed (4), four functional foods (Poria cocos, pueraria, Honeysuckle, and lily) were used twice, the 17 functional foods (Amomi Fructus, ginger, chrysanthemum, hawthorn, Chinese yam, jujube, ginseng, Astragalus and so on) were only used once. These materials addition have the potential to develop functional beer for preventing gout and hyperuricemia.
6. Molecular Mechanism of Beer to Human Health
6.1. Polyphenols Mechanism
Polyphenols in beer has 24 health effects such as cardiovascular disease prevention, anticancer, anti-diabetes, lipid deposition prevention, anti-obesity, antioxidation, anti-inflammation, immunomodulation, improve gastrointestinal, cardioprotection, anti-aging, improve skin health, atopic dermatitis alleviation, improve metabolic syndrome, prevent osteoporosis, bone injury recovery, blood pressure regulation, neuroprotection, hepatoprotection, promote sleep, heart failure or stroke prevention, wound healing acceleration, prebiotic action, and so on (Table 4, Figure 2).
Polyphenols can act in the stimulation of β-oxidation and adipocyte differentiation inhibition as well as counteract oxidative stress by modulated physiological and molecular pathways of energy metabolism [156]. Phenolic compounds in beer are important for human health which have antitumor and antioxidant activities [157]. The inhibition of intact human vascular smooth muscle cells for ecto-Alkaline phosphatase activity by polyphenolic compounds and polyphenol-containing beers may contribute to their cardiovascular protective effects [158]. Xanthohumol and 8-prenylnaringenin from beer-derived polyphenols can ameliorate diabetic-associated metabolic disturbances by regulating glucose and lipid pathways, which resulted in skeletal muscle AMPK signaling pathway activation, suppress lipogenesis, prevented body weight gain and improved plasma lipid profile, with significant improvement of insulin resistance and glucose tolerance [159]. The moderate beer has polyphenols and phenolic acids for the anti-inflammatory and antioxidant as well as improve gastrointestinal functions in human health can increase gut microbiota diversity and faecal alkaline phosphatase activity, especially significantly increase the butyric acid and reduce the ammonium content [160,161,162]. The polyphenol intake for moderate beer consumption plays a role in health effects including osteoporosis and cardiovascular risk and the relief of vasomotor symptoms, especially 8-prenylnaringenin, 6-prenylnaringenin, and isoxanthohumol, with intracellular estrogen receptors that leadto the modulation of gene expression, increase in sex hormone plasma concentrations [163]. The 15% colour malt was sweeter, deeper colour, higher bitterness and turbidity, alcohol content (6.2~6.8 %), especially the highest polyphenols (453.8 mg/L) and antioxidant activity (840.1 µmol/L), the lowest foam stability [164].
6.1.1. Phenolic Acids Mechanism
Phenolic acids play a role in anticancer, anti-diabetes, anti-inflammation, antioxidation, improve skin health, Alzheimer’s and Parkinson’s disease, antidepressant, ameliorating ischemia, and reperfusion injury activity, and so on [9] (Table 4, and Figure 2). Phenolic acids lead to the antioxidant and anti-inflammation properties of H-atom through a mechanism of free radical scavenging [165]. Gallic acid has lots of health effects in bacterial or viral infections, cancer, inflammatory, neuropsychological, gastrointestinal, and metabolic disease, it inhibits bacterial growth by altering membrane structure, inhibits cancer cell growth and oncogenes as well as matrix metalloproteinases expression by targeting different signaling pathways in apoptosis, increasing reactive oxygen species production [166]; Protocatechuic acid has anti-allodynic and anti-hyperalgesic effects by KATP channel activation related with A1 receptor stimulation [167]; gentisic acid regulated miR-19b-3p/RAF1 axis to mediate ERK pathway and inhibit the development of rheumatoid arthritis [168]. Chlorogenic acids regulate key targets in the TNF signaling pathway, inhibit the polarization of microglia to the M1 phenotype, and ameliorates neuroinflammation-induced cognitive dysfunction in mice [169]. Vanillic acid has antibacterial and antibiofilm activity against carbapenem-resistant E. hormaechei by ruptured the cell m-embrane integrity, a decrease of intracellular ATP, pH, and membrane potential, inhibited biofilm formation and killed cells within biofilms [170]. Caffeic acid phenylethyl ester have several pharmacological effects such as antibacterial, antitumor, antioxidant and anti-inflammatory activities, however lowering the temperature, adding biosurfactants and sodium deoxycholate and the presence of Cu2+ increased the binding force between caffeic acid phenylethyl ester and Hemoglobin [171]. Ferulic acid has anti-inflammatory, analgesic, anti-radiation, anticancer, and immune-enhancing effects, it can cause mitochondrial apoptosis by the intracellular reactive oxygen, inducing autophagy, a series of cell targets and the regulation of tumor cell signaling pathways [172]. Syringic acid has antioxidant and anti-inflammatory properties that reduced the progression of Parkinson's disease by its neuroprotective, antioxidant and anti-inflammatory effects [173]. 4-Hydroxyphenylacetic acid is the major intestinal metabolite of kaempferol and polymeric proanthocyanidins, its antibacterials can cause cell death through cell membrane damage and decrease the expression of three virulence factors [174]. Sinapic acid is a potent anti-oxidant used for the treatment of cancer, infections, oxidative stress, and inflammation, its mechanism of anticancer due to regulation of multiple proteins (CTNNB1, PRKCA, CASP8, SIRT1) and cytochrome enzymes (CYP1A1, CYP3A4) [175].
6.1.2. Flavonoids Mechanism
The refined staple foods damage the global economy and threaten human health, especially more than half of the global population will be living with overweight or obese by 2035, and over1.3 billion people will have diabetes by 2050 [176]. Flavonoids in beer has 22 health effects, such, as have cardiovascular disease prevention, anticancer, anti-diabetes, lipid deposition prevention, anti-obesity, antioxidation, immunomodul- ation, anti-inflammation, improve gastrointestinal, cardioprotection, anti-aging, improve skin health, improve metabolic syndrome, blood pressure regulation, neuroprotection, hepatoprotection, promote sleep, antibacterial, antiviral, and so on (Table 4 and Figure 2). These healthy roles in beer for human due to reduce free radicals and protect against oxidative stress of hydroxyl group, especially the targeting of multiple genes / pathways including nuclear receptors, the aryl hydrocarbon receptor, kinases, receptor tyrosine kinases and G protein-coupled receptors [177,178]. The prenylated flavonoids in beer from hop such as 8-prenylnaringenin and 6-prenylnaringenin, by spontaneous cyclisation into isoxanthohumol, and subsequently demethylated by gut bacteria, combinations of metabolism by hydroxylation, sulfation, and glucuronidation result in an unknown number of isomers [179]. Xanthohumol in hop treats a variety of cancers, due to the inhibition of cancer cell growth and proliferation by regulating multiple signaling pathways (Akt, AMPK, ERK, IGFBP2, NF-κB, STAT 3) and proteins (Notch1, caspases, MMPs, Bcl-2, cyclin D1, oxidative stress markers, tumor-suppressor proteins, and miRNAs) [75].
Catechins enhance skeletal muscle performance, due to epicatechin gallate with the strongest differentiation, significantly reduced the adhesion force, stiffness, and enhanced C2C12 cell differentiation [180]; The antibacterial activity was hydrogen bonding, hydrophobic and electrostatic interaction between epicatechin gallate and anionic carboxymethyl Poria cocos polysaccharide [181]. Rutin with neuronprotection has an anti-oxidative impact through up-regulating the expression of P-ERK and Nrf2 proteins in the ERK/Nrf2 pathway [182]. Rutin and quercetin with antioxidants can reduce the formation of protein oxidation products and the highest clearance rates for DPPH (62.74 %) and ABTS+ (71.14 %) [183]. The quercetin has anti-inflammatory, anti-oxidative stress, and osteoprotective properties, which reduce 13 indicators (arthritis scores, paw swelling, histopathological scores, interleukin-1β, interleukin-6, interleukin-17, tumor necrosis factor-α, monocyte chemotactic protein-1, C-reactive protein, malondialdehyde, reactive oxygen species, thiobarbituric acid reactive substances, nuclear factor kappB) and increase 6 indicators (interleukin-10, catalase, glutathione peroxidase, SOD, glutathione, and heme oxygenase-1) [184]. Kaempferol alleviating sepsis can reduce inflammatory, ROS production, and cell apoptosis by acting on the HIF-1, NF-κB, and PI3K-Akt signaling pathways [185]. The genistein can induce bone remodeling that involves osteoblasts, osteoclasts, and osteocytes, and different intracellular signaling, through Wnt/β-catenin pathway activation [186]. Luteolin could hinder ovarian cancer cell proliferation and activate the PI3K/AKT pathway to lead to apoptosis [187]. Apigenin has antioxidative, anti-inflammatory and anticancer activities by inhibits GLUT-1 mRNA and protein expression in head and neck cancers [188]. Myricetin inhibited the proliferation and induced the apoptosis of H1975 cells by regulating the expression of MMP 1, MMP 3, MMP 9 and PIK3R1 genes and in various pathways [189]. Nnaringin induced osteoblastic differentiation of BMSCs by activating the BMP2/Runx2/Osterix signaling pathway and promoted the regulation of oestrogen receptor pathway protein expression, a more significant in vivo ectopic osteogenic effect [190].
6.2. Melatonin Mechanism
Melatonin in beer has 14 health effects such as cardiovascular disease prevention, anticancer, antioxidation, anti-inflammation, immunomodulation, atopic dermatitis alleviation, improve gastrointestinal, cardioprotection, prevent osteoporosis, bone injury recovery, blood pressure regulation, neuroprotection, promote sleep, prebiotic action, and so on (Table 4 and Figure 2). Melatonin is a free radical scavenger, enhances antioxidant enzymes, regulates mitochondrial potential, and interferes with pro-inflammatory signaling pathways [191]. The melatonin mechanism is free radical scavenging properties and complex intracellular signaling pathways, limiting the entry of tumor cells into the vascular stream and their distribution in other organs and systems, block the growth of metastases in places far from the original tumor [77,78]. ROS-dependent formation of 2-hydroxymelatonin from melatonin was the major pathway in cancer cells, which could serve as index for the endogenous reactive oxygen level and oxygen-carrying capacity of hemoglobin in human blood [192]. Melatonin with antioxidant and anti-inflammation prosperities alleviated hepatic damage aggravated by PCB126-induced ROS-dependent NETs formation through suppressing excessive ROS production [193]. Melatonin may act indirectly on the immune system through the circadian clock to regulate food allergies [194]. Melatonin protects against ketorolac induced gastric mucosal toxic injuries through molecular mechanism associated with the modulation of Arylakylamine N-Acetyltransferase activity, especially a correlation between depleted gastric melatonin level and ulcer formation unveiled a novel ulcerogenic mechanism [195]. The sleep rhythms of GW117 with antidepressant action may be due to the melatonin system-mediated activation of the Wnt/β-catenin signaling pathway [196].
6.3. Bitter Acids Mechanism
Bitter acids in beer have 15 health effects such as anticancer, anti-diabetes, lipid deposition prevention, anti-obesity, antioxidation, anti-inflammation, allergic rhinitis alleviation, immunomodulation, anti-aging, improve metabolic syndrome, improve cognition, antidepressant, improved fatigue or mood, neuroprotection, promote sleep, and so on (Table 4 and Figure 2). The specific bitter taste receptor activation by matured hop bitter acids drives downstream Ca response and cholecystokinin production in enteroendocrine cells as well as activate the gut-brain axis [197]. The non-alcoholic beer in daily life can maintain mood states by hop bitter acids improved mood and peripheral symptoms as well as enhanced stress resilience-related hippocampal dopaminergic activity in human [120]. Iso-α-acids in beer suppress hippocampal microglial inflammation and improving cognitive decline, however matured hop bitter acids activate the vagus nerve and suppress neuronal damage and depression-like behavior induced by inflammation [198]. The sedation and antidepressant mechanisms of hop bitter acid are the activation of neuron-like Ca2+ channels by lupulones and tricyclolupones represent [118]. Bitter acids of beer enhance memory and cognitive functions by norepinephrine neurotransmission and vagus nerve stimulation; Iso-α-acids enhance hippocampus-dependent memory and prefrontal cortex-associated cognitive function by dopamine neurotransmission activation [117]. The iso-α-acids from hop-derived bitter acids of beer improves spatial and object recognition memory, especially may contribute toward improving obesity-induced cognitive impairments [146]. The hop beta-acids acts like the action mechanism and the spectrum of ionophores and is a useful functional product of ruminants that inhibit rumen bacteria in the classical Gram-positive cell envelope [199].
6.4. Minerals Mechanism
Minerals facilitate various electrical and chemical processes. Minerals in beer have 15 health effects such as cardiovascular disease prevention, anticancer, anti-diabetes, lipid deposition prevention, anti-obesity, antioxidation, longevity, anti-aging, Alzheimer prevention, prevent osteoporosis, bone injury recovery, improve cognition, neuroprotection, hepatoprotection, prevent gallstone, reduce kidney stones, and so on (Table 4 and Figure 2). Se is an indispensible trace element (50-200 μg/day) to human health, more than 40 diseases are highly related to Se deficiency, such as Se supply can maintain glucose and lipid homeostasis in type 2 diabetes patients, its deficiency or excess led to βcell dysfunction [144]; Se deficiency lead to increased vascular endothelial permeability and vascular tissue damage by decreasing SelO expression [200]. Se-rich soy peptides can prevent liver damage caused by heat stress and exercise fatigue by increased glutathione content and glutathioneperoxidase peroxidase activity in rat liver, and protected rat liver by regulating NF-κB/IκB pathway and preventing the release of interleukin-1β, interleukin-6 and tumor necrosis factor α; anticancer SeNPs enhanced the autophagy ability of cancer cells by activating the ROS mediated JNK pathway and inhibiting the PI3K/Akt/mTOR pathway [144]; The fermented beer enriched with organic Se (0.378mg / kg) produce for functional foods [201]. Ca may alter the composition of bile by preventing the reabsorption of secondary bile acids in the colon, thus reducing the deoxycholate and cholesterol content of the bile [149].
Beer contains Si and hop compounds that could play a major role in preventing brain disorders and a potential instrument for protecting against neurodegenerative disease progression [110]. Al (NO3)3 induces metal imbalance, inflammation, and antioxidant damage in mouse brain by significant blocking effects of silicic acid and beer, especially connected the pro-oxidant markers with brain Al content, while brain Zn and Cu levels were closer to antioxidant enzyme expression [202]. Addition 150 g persimmon fruit per 10 L of water could better enrich the nutritional (polyphenol, Mg, K, and Ca), organoleptic, and antioxidant potentials of beer [203]. Mg2+ addition resulted in the pH of the fermenting wort decreasing more quickly, an increase in the level of L-lactic acid and increased concentrations of some volatile compounds, however Zn supplementation resulting in a decrease in the L-lactic acid content and a higher pH in the beer [204]. Light-to-moderate consumption of beer components (calcium ionophore) prevents the coronary endothelial dysfunction against hyperlipemia-induced associated with cardiovascular risk factors by counteracting vascular oxidative damage and preserving the Akt/eNOS pathway [205]. The use of Se-biofortified barley grain as a raw material can produce Se-enriched beer [206].
6.5. Vitamins Mechanism
Vitamins help cellular enzymes to regulate metabolic reactions. Vitamins in beer has 11 health effects such as cardiovascular disease prevention, anticancer, anti-diabetes, antioxidation, anti-inflammation, cardioprotection, longevity, anti-aging, prevent osteoporosis, bone injury recovery, blood pressure regulation, and so on (Table 4 and Figure 2). Beer is rich in vitamins, such as vitamin B family (VB1, VB2, VB3, nicotinamide, VB5, VB6, VB7, VB8, folate, VB12), Vc, fat-soluble vitamin (A, D, E, K) (See Table 4). The ginsenoside Rb1 induced restoration of redox homeostasis was mediated by targeting riboflavin transporters and riboflavin kinase [207]. Nicotinamide may reduce the release of proinflammatory mediators by inhibiting the MAPK and AKT/NF-κB signaling pathways and ultimately alleviate lung injury [208]. Pyridoxal-5-phosphate in cardioprotection and vasorelaxation may be a result of increased expression of KATP channels and H2S production [147]. Myo-inosito may be an insulin sensitizer through insulin-resistant tissues as PCOS-endometrium, SMIT-1, provoking AMPK activation and elevated GLUT-4 levels, thereby increasing glucose uptake by human endometrial cells [145]. Folate plays a protective role against atherosclerosis through regulating DNA methylation, ARID5B expression, and monocyte subsets [209]. High-dose ascorbic acid shows a cytotoxic effect on myelodysplastic syndrome tumor cells, inhibiting cell proliferation, and increasing apoptosis [210]; The protective effect of gallstones for vitamin C modulates the hepatic and biliary pathways of cholesterol homeostasis by promoting the conversion of cholesterol into bile acids through liver 7α-hydroxylation [149].
After one month drank 830 mL of alcoholic beer every day, nine indicators (uric acid, antioxidative capacity, SOD, glutathione reductase, total cholesterol, HDL-cholesterol, Apolipoprotein-AI, LDL-cholesterol and Apolipoprotein B) of 160 male volunteers increased, while vitamin B12 and fibrinogen decreased, especially beer consumption is significantly correlated with uric acid and antioxidative capacity changes [211]. The consumption of industrial beer for preventing cardiovascular disease reduced the serum homocysteine (6.50~7.35 µmol/L) and increased folic acid (3.46~3.94 ng/mL); however craft beer increased gamma-glutamyl transpeptidase (16.6~18.6 U/L) and reduced VB6 (20.9~16.9 ng/mL) [212].
6.6. Active Peptides Mechanism
Beer proteins can be an excellent source of bioactive peptides. Peptides are used as a raw material for human muscles, blood, hormones, and new tissues, it is to exogenous bioactive peptides, such as anticancer, anti-hypertensive, anti-diabetic, anti- inflammatory, immunomodulatory, anti-microbial, neuroprotective and cardio-vascular protective and opioid activities [143]. Peptides in beer has 8 health effects such as anticancer, anti-diabetes, anti-inflammation, allergic rhinitis alleviation, immunomodul- ation, cardioprotection, Blood pressure regulation, neuroprotection, and so on (see Table 4). The inhibitory peptides of the enzyme dipeptidyl-peptidase IV are a pharmacological target in type-2 diabetes therapy since it is involved in the degradation of the insulinotropic incretin hormones [143]. The bioactive peptides have antiinflammation and gut health, due to reduce TNF-α, NF-κB, and TLR4, an improvement in IgA production and in intestinal morphology, with an increase in villi surface area and goblet cell diameter [213]. The beer quality include flavor, texture, foam stability, gushing, and haze formation from beer peptides and 7113 proteins [20]. Over 1900 protein groups in the beer proteome were identified by both approaches of IPG-IEF and LC-MS/MS [214]. Antibodies in PhIP-Seq data with beer peptide serve as excellent records of environmental exposures and immune responses, based on high abundance, relative stability,easy accessibility in peripheral blood [215]. Proteins, peptides, and amino acids were assumed to form disulfide bonds with polyfunctional thiols in malt and hops, especially the release of thiols by the reduction of disulfide-bonded thiols during fermentation [216]. The anxiolytic and antidepressant protein hydrolysates and peptides can exert effects through neurotransmitter systems, neurotrophic functions, neurons, nerves, and the HPA axis mechanisms [217]. Bioactive peptides are short amino acid sequences against a variety of human diseases, such as anti-cancer, anti-hypertensive, anti-diabetic, anti-inflammatory, anti-microbial, immunomodulatory, neuroprotective, and cardio-vascular protective activities [143]. 50 Peptides in Tsingtao draft beer were identified, especially LNFDPNR and LPQQQAQFK peptides could bind angiotensin-converting enzyme and dipeptidyl peptidase IV tightly by hydrogen bonding and hydrophobic interaction as well as their inhibitory activity [218].
In short, barley grains can prevent and control more than 20 chronic diseases in humans or animals, due to molecular mechanism of β-glucans, polyphenols, arabinoxylan, phytosterols, tocols, and resistant starch [1] however barley grass powder can prevent and control more than 20 chronic diseases in humans or animals, due to molecular mechanism of GABA, flavonoids, SOD, K-Ca, vitamins, and tryptophan [219]. Beer contributes to cell protection and health-promoting effects based on lots of functional components against chronic diseases, such as polyphenols (xanthohumol), phenolic acids, melatonin, minerals, bitter acids, kaempferol, quercetin, tyrosol, flavones xanthohumol, 8-prenylnaringenin, the vitamin B complex, citric acid, Vc, silicic acid, etc [78,220]. The prevention and treatment of 26 human chronic diseases by beer is the result of the comprehensive effect of barley grains and its grass as well as hops, due to molecular mechanism of polyphenols (phenolic acids, flavonoids), melatonin, minerals, bitter acids, vitamins, and peptides(Table 4 and Figure 2). Beer styles show wide variation in color, flavor, and clarity, the major flavor compounds are isomerized alpha acids and phenolic compounds [219]. First, the polyphenols mechanism (phenolic acids, flavonoids) of beer in preventing and treating 22 human chronic diseases is the interaction between polyphenols of barley grains and flavonoids of barley malt and hops; Flavonoids especially saponarin and lutonarin in barley grass have similar 11 health effects [221]. Second, the melatonin mechanism of beer in preventing and treating 14 human chronic diseases is the interaction between melatonin and GABA as well as tryptophan of barley malt; In the body, melatonin is synthesized from a tryptophan by leading to competitive O-demethylation and C6-aromatic hydroxylation pathways [222]; Tryptophan in barley grass have similar 4 health effects [219]. Third, 15 health effects of minerals mechanism of beer come from the combined action of barley malt, hops and water; K-Ca mechanism in barley grass have similar 7 health effects [219];11 health effects of vitamins mechanism of beer come from the combined action of barley malt, and hops; Vitamins mechanism in barley grass have similar 7 health effects [219]. Fourth, 15 health effects of bitter acids mechanism of beer from hops; GABA mechanism in barley grass have similar 13 health effects [219].
These results support that human has only one cell disease theory, which provides a reference for the development of human functional food and animal husbandry production of functional feed (1,19,51), nutrition therapy is the best solution to human chronic diseases. High nutrition increases crop yield and leads to human obesity, however adversity increases crop functional composition and human intelligence; the transition from high-yield agriculture and green agriculture to functional agriculture will be a breakthrough cross-domain, and a lot of scientific research needs to be done in the future (176).
7. Conclusion and Future Perspectives
The refined staple foods damage the global economy and threaten human health. Nutrition therapy is the best solution to human chronic diseases, such as beer. Although common beers and special beers are changing rapidly, however functional beer can be limited to four ingredients: barley, hops, water, and yeast. As an excellent functional food in the future, beer is the functional ingredients of barley malt and hops fermented into more absorbable small molecule active ingredients under the action of yeast.
First, beer is rich in active ingredients that is a complex of two functional ingredients of barley malt and hops. Although more than 1,000 polyphenolic substances in hops contribute to the bitterness and aroma of beer, but about 70% of beer polyphenols originate from barley malt, and the remaining 70% from hops. Both hops and barley can provide the 8 phenolic acids and 6 flavonoids, however gentisic acid and 10 flavonoids comes from hops, however 3 flavonoids come from barley malt. Barley grains are higher in both protein and 9 mineral elements as well as 4 functional components than that of beer, however there are more than 13 nutritional function differences in beer and barley grains.
Second, the health effect of beer against 26 chronic diseases is highly similar to that of barley. The healthy effects of beer like barley have 26 health effects, which have cardiovascular disease prevention, anticancer, anti-diabetes, lipid deposition prevention, anti-obesity, antioxidation, anti-inflammation, immunomodulation, improve gastro= intestinal, cardioprotection, longevity, improve skin health, pevent Alzheimer’s disease, improve metabolic syndrome, prevent osteoporosis, improve cognition, antidepressant, improve fatigue or mood, blood pressure regulation, prevent neurodegenerative disease, hepatoprotection, promote sleep, heart failure or stroke prevention, prevent gallstone disease, reduce kidney stones, wound healing acceleration, prebiotic action, and so on.
Third, beer has similar effects and molecular mechanisms to prevent and treat human chronic diseases than that of barley. Barley combats 28 chronic diseases, due to molecular mechanism of barley grass (GABA, flavonoids, SOD, K-Ca, vitamins, and tryptophan) and grains (β-glucans, polyphenols, arabinoxylan, phytosterols, tocols, and resistant starch); beer prevents 26 chronic diseases, due to molecular mechanism of polyphenols (phenolic acids, flavonoids), melatonin, minerals, bitter acids, vitamin, and peptides; The main sources of these functional ingredients are as follows: Four materials for making beer provide mineral elements, three materials expect water provide polyphenols (phenolic acids, flavonoids), vitamin from barley malt and hops, active peptides and melatonin from barley malt and bitter acids from hops. Although barley or its malt be selected the eight Dietary Guidelines released by National Health Commission of the People's Republic of China, but beer was not included in the eight Dietary Guidelines. Low-purine beer and preventive gout beer should become an important development direction of functional beer, such as Chicory beer, orange beer, ginger beer, hawthorn beer, jujube beer, ginseng beer, especially coix-lily beer at the ancestors ca. 9000 years ago.
The complex and variable interactions of such beer and its materials (barley, hops, water, and yeast, or functional foods addition) require a comprehensive discussion to correctly address the management of the human chronic diseases. However, the similarities and differences between the molecular mechanisms of barley and hops as well as beer have broad prospects in future research and development for prevention and treatment of chronic diseases. The beer hurts the human body that excessive drinking beer with high purines can lead to hyperuricemia and even gout, it is the strongest urate raising effect of 63 purine foods. Low purine beer can be produced by enzymatic and biological degradation and adsorption of purines as well as dandelion addition. Therefore, functional beer with low purine and high active ingredient made from Beer Purity and barley malt for functional foods addition will be the key and important development direction to improve the added value and enhance the market competitiveness. In order to reduce the cost, brewers produce beer by used as raw materials of rice and wheat as well as corn with low active ingredients. These beers seriously reduce the health effects of beer, and lower prices and high taxes are recommended.
Author Contributions
Conceptualization, Y.-W.Z. and H.G.M.-D. A; methodology, Y.-W.Z.; validation, Y.-W.Z. and H.G.M.-D. A; formal analysis, X. L.; investigation, Y.-W.Z. and H.G.M.-D. A; resources, X. L. and L.-E. Yang; data curation, Y.-W.Z. and J.-Z. Y.; writing—original draft preparation, Y.-W.Z. and J.-Z. Y.; writing—review and editing, H.G.M.-D. A, and X. L.; visualization, X.-Y. P, and T. Y.; supervision,Y.-W.Z.; project administration, X,-M. Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by China Agriculture Research System (CARS-05-01A-04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ABTS:2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; AMPK: Adenosine 5‘-monophosphate-activated protein kinase; Ca: calcium; DPPH: 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl; ERK: extracellular signal-regulated kinase; FRAP: ferric reducing antioxidant power; Fe: iron; GABA: γ-aminobutyric acid; GLP-1: glucagon-like peptide-1; HDL:high-density lipoprotein; IGFBP2:insulin like growth factor binding protein 2; K: potassium; KATP: adenosine triphosphate-sensitive potassium; LTP: lipid transfer protein; NAFLD: non-alcoholic fatty liver disease; MDR: multi-drug resistance; Mg: magnesium; Mn: manganese; Na: sodium; ORAC:oxygen radical absorbance capacity; P: phosphorous; ROS: reactive oxygen species; Se: selenium; Si: silicon; SOD: superoxide dismutase; TNF: tumor necrosis factor; TEAC: trolox equivalent antioxidant capacity; VB1: thiamin; VB2: riboflavin; VB3: niacin; VB5: pantothenic acid; VB6: pyridoxal; VB7: biotin; VB8: inositol; VB9: folic acid; VB12:vitamin B12; Vc: ascorbic acid; Zn: Zinc.
References
- Zeng, Y.W.; Pu, X.Y.; Du, J.; Yang, X.M.; Li, X.; Mandal, M.S.N.; Yang, T.; Yang, J. Molecular mechanism of functional ingredients in barley to combat human chronic diseases. Oxid. Med. Cell Longev. 2020, 2020, 3836172. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Sun, H.; Chen, X. Serving red rice beer to the ancestors ca. 9000 years ago at Xiaohuangshan early Neolithic site in south China. Holocene 2023, 09596836231169995. [Google Scholar] [CrossRef]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and beer compounds: physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Roldán-López, D.; Muñiz-Calvo, S.; Daroqui, N.; Knez, M.; Guillamón, J.M.; Pérez-Torrado, R. The potential role of yeasts in the mitigation of health issues related to beer consumption. Crit. Rev. Food Sci. Nutr. 2024, 64, 3059–3074. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Molina, M.; Muñoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A new perspective on the health benefits of moderate beer consumption: involvement of the gut microbiota. Metabolites 2019, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Zugravu, C.A.; Medar, C.; Manolescu, L.S.C.; Constantin, C. Beer and microbiota: Pathways for a positive and healthy interaction. Nutrients 2023, 15, 844. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Fox, G.P.; Marco, M.L. Beer for live microbe delivery. J. Funct. Foods 2024, 113, 10598. [Google Scholar] [CrossRef]
- Fang, W. Research progress in beer nutrition and health. Global Alcinfo. 2020, 9–14. [Google Scholar]
- Nardini, M. An overview of bioactive phenolic molecules and antioxidant properties of beer: emerging trends. Molecules 2023, 28, 3221. [Google Scholar] [CrossRef]
- Jin, H.M.; Dang, B.; Zhang, W.G.; Zheng, W.C.; Yang, X.J. Polyphenol and anthocyanin composition and activity of highland barley with different colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef]
- Buckett, L.; Schinko, S.; Urmann, C.; Riepl, H.; Rychlik, M. Stable isotope dilution analysis of the major prenylated flavonoids found in beer, hop tea, and hops. Front. Nutr. 2020, 7, 619921. [Google Scholar] [CrossRef]
- Díaz Prieto, L.E.; Gómez-Martínez, S.; Nova, E.; Marcos, A. Do we know what moderate alcohol consumption is? The particular case of beer. Nutr. Hosp. 2022, 39, 12–16. [Google Scholar] [CrossRef]
- Mikyška, A.; Dušek, M.; Slabý, M. Effects of the mashing process on polyphenols and antiradical activity of beer. Eur, Food Res, Technol, 2023, 249, 71–80. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; de la Torre, R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.; Serra-Majem, L.; Pérez-Jiménez, F.; Pascual, V.; Tinahones, F.J.; Estruch, R. Moderate consumption of beer and its effects on cardiovascular and metabolic health: an updated review of recent scientific evidence. Nutrients 2021, 13, 879. [Google Scholar] [CrossRef]
- Olas, B.; Bryś, M. Beer components and their beneficial effect on the hemostasis and cardiovascular diseases- truth or falsehood. Food Chem. Toxicol. 2020, 146, 111782. [Google Scholar] [CrossRef]
- Baiano, A.; Cr Bamforth, C.W. The physics and chemistry of beer foam: a review. Eur. Food Res. Technol. 2023, 249, 3–11. [Google Scholar]
- Sancén, M.; Léniz, A.; Macarulla, M.T.; González, M.; Milton-Laskibar, I.; Portillo, M.P. Features of non-alcoholic beer on cardiovascular biomarkers. can it be a substitute for conventional beer? Nutrients 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, J.Z.; Yang, X.M.; Li, X.; Pu, X.Y.; Fu, Z.Y.; Yang, L.E. A breakthrough GLP-1Ras and barley control chronic diseases. BMJ 2024, 384, https. [Google Scholar]
- Sun. Z.; Yu. X.; Zhang. Y.; Xu. J.; Li, X. Construction of a comprehensive beer proteome map using sequential filter-aided sample preparation coupled with liquid chromatography tandem mass spectrometry. J. Sep. Sci. 2019, 42, 2835–2841. [Google Scholar] [CrossRef]
- Tachie, C.Y.E.; Onuh, J.O.; Aryee, A.N.A. Nutritional and potential health benefits of fermented food proteins. J. Sci. Food Agric. 2024, 104, 1223–1233. [Google Scholar] [CrossRef]
- Bamforth, C.W. The physics and chemistry of beer foam: a review. Eur. Food Res. Technol. 2023, 249, 3–11. [Google Scholar] [CrossRef]
- Di Matteo, P.; Bortolami, M.; Di Virgilio, L.; Petrucci, R. Targeted phenolic profile of radler beers by HPLC-ESI-MS/MS: the added value of hesperidin to beer antioxidants. J. Food Sci. Technol. 2022, 59, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Liu, C.F.; Li, Q. Study on the effects of beer on human health. Global Alcinfo. 2022, 10–13. [Google Scholar]
- Yang, T.; Zeng, Y.W.; Du, J.; Yang, S.M.; Pu, X.Y. Genetic analysis of four main flavonoids in barley grain. Bangladesh J. Botany 2019, 48, 231–237. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Du, J.; Yang, X.M.; Pu, X.Y.; Wang, L.X.; Yang, J.Z.; Du, L.J.; Yang, T.; Yang, S.M.; Sun, Z.H. Identification of quantitative trait loci for mineral elements in grains and grass powder of barley. Genet. Molecul. Res. 2016, 15, 15049103. [Google Scholar] [CrossRef]
- Thabet, S.G.; Alomari, D.Z.; Brinch-Pedersen, H.; Alqudah, A.M. Genetic analysis toward more nutritious barley grains for a food secure world. Bot. Stud. 2022, 63, 6. [Google Scholar] [CrossRef]
- Jiménez-Pavón, D.; Cervantes-Borunda, M.S.; Díaz, L.E.; Marcos, A.; Castillo, M.J. Effects of a moderate intake of beer on markers of hydration after exercise in the heat: a crossover study. J. Int. Soc. Sports Nutr. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates profile, polyphenols content and antioxidative properties of beer worts produced with different dark malts varieties or roasted barley grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Pietercelie, A.; Allardin, D.; Nedervelde, L.V. Effect of fermentation conditions of brewing yeasts on folate production. Cerevisia 2011, 36, 41–45. [Google Scholar] [CrossRef]
- Lapcík, O.; Hill, M.; Hampl, R.; Wähälä, K.; Adlercreutz, H. Identification of isoflavonoids in beer. Steroids 1998, 63, 14–20. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, J.; Cai, S.; Chen, X.; Quan, X.; Zhang, G. Association mapping for total polyphenol content, total flavonoid content and antioxidant activity in barley. BMC Genomics 2018, 19, 81. [Google Scholar] [CrossRef]
- Liao, Z.; Cai, H.; Xu, Z.; Wang, J.; Qiu, C.; Xie, J.; Huang, W.; Sui, Z. Protective role of antioxidant huskless barley extracts on TNF-α-induced endothelial dysfunction in human vascular endothelial cells. Oxid. Med. Cell Longev. 2018, 2018, 3846029. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Dang, B.; Fan. M.T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Pu, X.Y.; Zhang, J.; Du, J.; Guo, G.G.; Yang, T.; Zhao, C.Y.; Yang, S.M.; Zhao, D.W.; Tang, J.J.; Jia, P. Genetic variation of functional components in grains for barley improved lines from four continents. Agri. Sci. Technol. 2012, 13, 1436–1441. [Google Scholar]
- Li, M.; Du, J.; Zheng, Y. Non-starch polysaccharides in wheat beers and barley malt beers: A comparative study. Foods 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Gribkova, I.N.; Eliseev, M.N.; Lazareva, I.V.; Zakharova, V.A.; Sviridov, D.A.; Egorova, O.S.; Kozlov, V.I. The phenolic compounds' role in beer from various adjuncts. Molecules 2023, 28, 2295. [Google Scholar] [CrossRef]
- Mohammadi, K.; Saris, P.E.J. Antibiofilm effect of curcumin on saccharomyces boulardii during beer fermentation and bottle aging. Biomolecules 2023, 13, 1367. [Google Scholar] [CrossRef]
- Nutakor, C.; Essiedu, J.A.; Adadi, P.; Kanwugu, O.N. Ginger beer: an overview of health benefits and recent developments. Fermentation 2020, 6, 102. [Google Scholar] [CrossRef]
- Iara Gomes de Oliveira, L.; Karoline Almeida da Costa, W.; de Candido de Oliveira, F.; França Bezerril, F.; Priscila Alves Maciel Eireli, L.; Dos Santos Lima, M.; Fontes Noronha, M.; Cabral, L.; Wagner, R.; Colombo Pimentel, T.; et al. Ginger beer derived from back-slopping: volatile compounds, microbial communities on activation and fermentation, metabolites and sensory characteristics. Food Chem. 2024, 435, 137640. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-added lager beer enriched with eggplant (Solanum melongena L.) peel extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef] [PubMed]
- Balík, J.; Híc, P.; Tříska, J.; Vrchotová, N.; Smetana, P.; Smutek, L.; Rohlik, B.A.; Houška, M. Beer and beer-based beverage contain lignans. J. Food Sci. Technol. 2021, 58, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Nunes Filho, R.C.; Galvan, D.; Effting, L.; Terhaag, M.M.; Yamashita, F.; Benassi, M.T.; Spinosa, W.A. Effects of adding spices with antioxidants compounds in red ale style craft beer: a simplex-centroid mixture design approach. Food Chem. 2021, 365, 130478. [Google Scholar] [CrossRef]
- Das, S.; Deb, D.; Adak, A.; Khan, M.R. Exploring the microbiota and metabolites of traditional rice beer varieties of Assam and their functionalities. 3 Biotech. 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sepulveda, M.; Johannsen, N.; Astudillo, S.; Jorquera, C.; Álvarez, C.; Zbinden-Foncea, H.; Ramírez-Campillo, R. Effects of beer, non-alcoholic beer and water consumption before exercise on fluid and electrolyte homeostasis in athletes. Nutrients 2016, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, J.Z.; Pu, X.Y.; Yang, X.M.; Li, X.; Yang, L. E. The role of barley in the nitrogen-hosphorus balance. Science 2022, 75, https. [Google Scholar]
- Matsuoka, T.; Hosomi, K.; Park, J.; Goto, Y.; Nishimura, M.; Maruyama, S.; Murakami, H.; Konishi, K.; Miyachi, M.; Kawashima, H.; et al. Relationships between barley consumption and gut microbiome characteristics in a healthy Japanese population: a cross-sectional study. BMC Nutr. 2022, 8, 23. [Google Scholar] [CrossRef]
- Ishiyama, S.; Kimura, M.; Nakagawa,T. ; Fujimoto, Y.; Uchimura, K.; Kishigami, S.; Mochizuki, K. Development of the dabetic kdney dsease muse mdel clturing ebryos in α-mnimum esential mdium In Vitro, and feeding barley diet attenuated the pathology. Front. Endocrinol. (Lausanne) 2021, 12, 746838. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Fu, Z.Y.; Yang, X.M.; He, X.H.; Yang, L.E.; Li, X.; Pu, X.Y.; Yang, J.Z. Barley-related industry as the cornerstone of health for human and animals. Science 2022, 377, https. [Google Scholar]
- Zeng, Y.W.; Du, J.; Pu, X.Y.; Yang, X.M.; Li,X. ; Yang, J.Z. Remarkable efficacy of COVID-19 treatment by traditional Chinese medicine. Science 2020, 67, https. [Google Scholar]
- Hasheminasab, F.S.; Azimi, M.; Khodadoost, M.; Chouban, B.; Shakeri, N.; Ghasemi, S.; Farokhi, A.; Mokaberinajad, R. Efficacy of the barley-based remedy, a Persian medicine formula, in coronavirus disease 2019 (COVID-19) hospitalized patients: an open-labeled randomized controlled trial. Adv. Integr. Med. 2022, 9, 185–190. [Google Scholar] [CrossRef]
- Kashyap, P.; Bhardwaj, V.K.; Chauhan, M.; Chauhan, V.; Kumar, A.; Purohit, R.; et al. A ricin-based peptide BRIP from Hordeum vulgare inhibits Mpro of SARS-CoV-2. Sci. Rep. 2022, 12, 12802. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, X.M.; Li, X.; Pu, X.Y.; Du, J.; Yang, J.Z. Control COVID-19 by barley-based functional food crops. Science 2020, 367, https. [Google Scholar]
- Zeng, Y.W.; Ahmed, H.G.M.D. ; Zhu,C.F.; Yang, X.M.; Pu, X.Y.; Du, J.; Yang J.Z. Functional foods enhance immunity and COVID-19 resistance. Science, /: https, 1126. [Google Scholar]
- National Health Commission of the People’s Republic of China. Diagnosis and treatment plan for COVID-19 (trial version 10). Chin. J. Clin. Infect. Dis. 2023, 16, 1–9. [Google Scholar]
- Li, Y.; Yang, D.; Gao, X.; Ju, M.; Fang, H.; Yan, Z.; Qu, H.; Zhang, Y.; Xie, L.; Weng, H.; et al. Ginger supplement significantly reduced length of hospital stay in individuals with COVID-19. Nutr. Metab. (Lond) 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Molavi Vardanjani, H.; Namjouyan, F.; Cramer, H.; Pasalar, M. Efficacy of Persian barley water on clinical outcomes of hospitalized moderate-severity COVID-19 patients: a single- blind, add-on therapy, randomized controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1033–1041. [Google Scholar]
- Hajibeygi, R.; Mirghazanfari, S.M.; Pahlavani, N.; Jalil, A.T.; Alshahrani, S.H.; Rizaev, J.A.; Hadi, S.; Hadi, V.; Yekta, N.H.; et al. Effect of a diet based on Iranian traditional medicine on inflammatory markers and clinical outcomes in COVID-19 patients: a double-blind, randomized, controlled trial. Eur. J. Integr. Med. 2022, 55, 102179. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Jiang, W.; Zhu, Y.Y.; Wang, C.Z.; Zhong, W.H.; Wu, G.; Chen, J.; Zhu, M.N.; Wu, Q.L.; Du, X.L.; et al. Highland barley Monascus purpureus Went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J. Ethnopharmacol. 2022, 286, 114922. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Nie, C.; Xie, X.; Yuan, X.; Ma, Q.; Zhang, M.; Chen, Z.; Hu, X.; Li, J. Highland barley β-glucan alleviated western diet-induced non-alcoholic fatty liver disease via increasing energy expenditure and regulating bile acid metabolism in mice. Food Funct. 2022, 13, 11664–11675. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Ahmed, H.G.M.D. Barley functional foods and human genes affect obesity. Science, /: https, 1126. [Google Scholar]
- Zhang, J.; Deng, H.; Bai, J.; Zhou, X.; Zhao, Y.; Zhu, Y.; McClements, D.J.; Xiao, X.; Sun, Q. Health-promoting properties of barley: A review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit. Rev. Food Sci. Nutr. 2022, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Szuba-Trznadel, A.; Hikawczuk, T.; Korzeniowska, M.; Fuchs, B. Effect of different amounts of hybrid barley in diets on the growth performance and selected biochemical parameters of blood serum characterizing health status in fattening pigs. Animals (Basel) 2020, 10, 1987. [Google Scholar] [CrossRef] [PubMed]
- Hajira, B.; Khan, I. Effect of sorghum and barley-containing bread on plasma total polyphenols, antioxidant status and inflammation in healthy subjects. J. Food Sci. Technol. 2022, 59, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Sihmar, M.; Santal, A.R.; Prager, L.; Carbonero, F.; Singh, N.P. Barley melanoidins: key dietary compounds with potential health benefits. Front. Nutr. 2021, 8, 708194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mokhtari, S.; Jafari, S.M.; Sharma, S. Barley-based probiotic food mixture: health effects and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 7961–7975. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Farid, O.A.; Abdelrazek, A.M.; Elwakel, H.; Mohamed, M.M. Hordeum vulgare ethanolic extract mitigates high salt-induced cerebellum damage via attenuation of oxidative stress, neuroinflammation, and neurochemical alterations in hypertensive rats. Metab. Brain. Dis. 2023, 38, 2427–2442. [Google Scholar] [CrossRef] [PubMed]
- Fahle, A.; Bereswill, S.; Heimesaat, M.M. Antibacterial effects of biologically active ingredients in hop provide promising options to fight infections by pathogens including multi-drug resistant bacteria. Eur. J. Microbiol. Immunol. (Bp) 2022, 12, 22–30. [Google Scholar]
- Tronina, T.; Popłoński, J.; Bartmańska,A. Flavonoids as phytoestrogenic components of hops and beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef]
- Karabín, M.; Haimannová, T.; Fialová, K.; Jelínek, L.; Dostálek, P. Preparation of hop estrogen-active material for production of food supplements. Molecules 2021, 26, 6065. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from hop: hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Tyśkiewicz, K.; Miazga-Karska, M.; Dębczak, A.; Rój, E.; Ginalska, G. Bioactive compounds obtained from Polish "Marynka" hop variety using efficient two-step supercritical fluid extraction and comparison of their antibacterial, cytotoxic, and anti-proliferative activities In Vitro. Molecules 2021, 26, 2366. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in wine and beer: beneficial effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Romero-Aibar, J.; Calvo, J. The melatonin contained in beer can provide health benefits, due to its antioxidant, anti-inflammatory and immunomodulatory properties. J. Sci. Food Agr. 2023, 103, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: evidences from human studies. Alcohol 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Guido, L.F. A review on the fate of phenolic compounds during malting and brewing: technological strategies and beer styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef]
- Spaggiari, G.; Cignarelli, A.; Sansone, A.; Baldi, M.; Santi, D. To beer or not to beer: A meta-analysis of the effects of beer consumption on cardiovascular health. PLoS One 2020, 15, e0233619. [Google Scholar] [CrossRef]
- Mayer, O.J.; Simon, J.; Rosolová, H. A population study of the influence of beer consumption on folate and homocysteine concentrations. Eur. J. Clin. Nutr. 2001, 55, 605–609. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Spagnuolo, C.; Bilotto, S.; Izzo, A.A.; Borrelli, F.; Rigano, D.; Russo, M.; Tarricone, F.; Russo, G.L. Antioxidant and chemopreventive effect of Aliophen® formulation based on malts and hops. Antioxidants (Basel) 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Yang, X.M.; Li, X.; Pu, X.Y.; Yang, L. E, Yang JZ. Barley functional foods control human type I diabetes. Science 2021, 373, https://www.science.org/doi/10.1126/science.abg4502.
- Mateo-Gallego, R.; Pérez-Calahorra, S.; Lamiquiz-Moneo, I.; Marco-Benedí, V.; Bea, A.M.; Fumanal, A.J.; Prieto-Martín, A.; Laclaustra, M.; Cenarro, A.; Civeira, F. Effect of an alcohol-free beer enriched with isomaltulose and a resistant dextrin on insulin resistance in diabetic patients with overweight or obesity. Clin. Nutr. 2020, 39, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.C.; do Rio, R.F.; Lollo, P.C.B.; Ferreira, I.M.P.L.V.O. Moderate alcoholic beer consumption: the effects on the lipid profile and insulin sensitivity of adult men. J. Food Sci. 2017, 82, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Franc, A.; Muselík, J.; Vetchý, D. Beer with reduced carbohydrates and alcohol content suitable for diabetics. Ceska Slov. Farm. 2018, 67, 212–215. [Google Scholar]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef]
- Song, H.; Fu, Q.; Huang, K.; Zou, Z.; Chen, L.; Chen, H.; Ge, S.; Wang, J.; Guan, X. Digestion characteristics of quinoa, barley and mungbean proteins and the effects of their simulated gastrointestinal digests on CCK secretion in enteroendocrine STC-1 cells. Food. Funct. 2022, 13, 6233–6243. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Martínez-Carrasco, P.; Tresserra-Rimbau, A.; Moreno, J.J.; Estruch, R.; Lamuela-Raventós, R.M. Effect of moderate beer consumption (with and without ethanol) on cardiovascular health in postmenopausal women. J. Sci. Food Agric. 2023, 103, 7506–7516. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, T.; Wang, Z.; Zhang, X.; Huang, S.; Yu, J. Polyphenols and melanoidins in beer and the antioxidant effect:a review. Food Res. Develop. 2022, 43, 203–209. [Google Scholar]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Negrão, R.; Costa, R.; Duarte, D.; Taveira Gomes, T.; Mendanha, M.; Moura, L.; Vasques, L.; Azevedo, I.; Soares, R. Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells. J. Cell Biochem. 2010, 111, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760. [Google Scholar] [CrossRef] [PubMed]
- Luzak, B.; Kassassir, H.; Rój, E.; Stanczyk, L.; Watala, C.; Golanski, J. Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity. Arch. Physiol. Biochem. 2017, 123, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, S.; Zhang, C.; Hu, S.; Li, H. Beer-gut microbiome alliance: a discussion of beer-mediated immunomodulation via the gut microbiome. Front. Nutr. 2023, 10, 1186927. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Blanco Ayala, T.; Pérez de la Cruz, V.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and anti-inflammatory properties from hop components in beer-related to neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez-Saavedra, M.; Tamargo, A.; Molinero, N.; Relaño de la Guía, E.; Jiménez-Arroyo, C.; Bartolomé, B.; González de Llano, D.; Victoria Moreno-Arribas, M. Simulated gastrointestinal digestion of beer using the simgi® model: investigation of colonic phenolic metabolism and impact on human gut microbiota. Food Res. Int. 2023, 173, 113228. [Google Scholar] [CrossRef]
- Stevens, J.E.; Jalleh, R.J.; Trahair, L.G.; Marathe, C.S.; Horowitz, M.; Jones, K.L. Comparative effects of low-carbohydrate, full-strength and low-alcohol beer on gastric emptying, alcohol absorption, glycaemia and insulinaemia in health. Br. J. Clin. Pharmacol. 2022, 88, 3421–3247. [Google Scholar] [CrossRef] [PubMed]
- González-Zancada, N.; Redondo-Useros, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A.; Nova, E. Association of moderate beer consumption with the gut microbiota and SCFA of healthy adults. Molecules 2020, 25, 4772. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Armstrong, M.A.; Friedman, G.D. Red wine, white wine, liquor, beer, and risk for coronary artery disease hospitalization. Am. J. Cardiol. 1997, 80, 416–420. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant effect of beer polyphenols and their bioavailability in dental-derived stem cells (D-dSCs) and human intestinal epithelial lines (Caco-2) cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.D.; Brar, R.K.; Drake, L.L.; Drumm, H.E.; Price, D.P.; Hammond, J.I.; Urquidi, J.; Hansen, I.A. The effect of the radio-protective agents ethanol, trimethylglycine, and beer on survival of X-ray-sterilized male Aedes aegypti. Parasit Vectors 2013, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, bitter components of beer, prevent inflammation and cognitive decline induced in a mouse model of Alzheimer's disease. J. Biol. Chem. 2017, 29, 3720–3728. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: the health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Almendinger, M.; Rohn, S.; Pleissner, D. Malt and beer-related by-products as potential antioxidant skin-lightening agents for cosmetics. Sustain. Chem. Pharm. 2020, 17. [Google Scholar]
- Bucci, P.L.; Santos, M.V.; Montanari, J.; Zaritzky, N. Nanoferulic: From a by-product of the beer industry toward the regeneration of the skin. J. Cosmet. Dermatol. 2020, 19, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The nutritional components of beer and its relationship with neurodegeneration and Alzheimer's disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Sasaoka, N.; Sakamoto, M.; Kanemori, S.; Kan, M.; Tsukano, C.; Takemoto, Y.; Kakizuka, A. . Long-term oral administration of hop flower extracts mitigates Alzheimer phenotypes in mice. PLoS ONE 2014, 9, e87185. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Iniguez, A.B.; Zhu, M.J. Hop bioactive compounds in prevention of nutrition-related noncommunicable diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 1900–1913. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Vilas-Franquesa, A.; Tresserra-Rimbau, A.; Sasot, G.; Storniolo, C.E.; Estruch, R.; Lamuela-Raventós, R.M. Effects of the non-alcoholic fraction of beer on abdominal fat, osteoporosis, and body hydration in women. Molecules 2020, 25, 3910. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Jugdaohsingh, R.; Powell, J.J.; Qiao, N.; Hannan, M.T.; Sripanyakorn, S.; Cupples, L.A.; Kiel, D.P. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am. J. Clin. Nutr. 2009, 89, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Pérez Medina, T.; de Argila Fernández-Durán, N.; Pereira Sánchez, A.; Serrano González, L. Benefits of moderate beer consumption at different stages of life of women. Nutr. Hosp. 2015, 32, 32–34. [Google Scholar] [PubMed]
- Ayabe, T.; Fukuda, T.; Ano, Y. Improving effects of hop-derived bitter acids in beer on cognitive functions: a new strategy for vagus nerve stimulation. Biomolecules 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Chiheb, C.; Pischetsrieder, M. Quantification of co-, n-, and ad-lupulone in hop-based dietary supplements and phytopharmaceuticals and modulation of their contents by the extraction method. J. Pharm. Biomed. Anal. 2019, 168, 124–132. [Google Scholar] [CrossRef]
- Kita, M.; Yoshida, S.; Kondo, K.; Yamakawa, Y.; Ano, Y. Effects of iso-α-acids, the hop-derived bitter components in beer, on the MRI-based Brain Healthcare Quotient in healthy middle-aged to older adults. Neuropsychopharmacol. Rep. 2019, 39, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Akiyama, S.; Takahashi, K.; Iwadate, Y.; Ano, Y. Effect of non-alcoholic beer containing matured hop bitter acids on mood states in healthy adults: a single-arm pilot study. Nurs. Health Sci. 2022, 24, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Llamas, A.; De la Cruz-Sánchez, E. Moderate beer consumption is associated with good physical and mental health status and increased social support. Nutrients 2023, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Trius-Soler, M.; Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Martínez-Huélamo, M.; Sasot, G.; Storniolo, C.E.; Estruch, R.; Lamuela-Raventós, R.M.; Tresserra-Rimbau, A. Moderate consumption of beer (with and without ethanol) and menopausal symptoms: results from a parallel clinical trial in postmenopausal women. Nutrients 2021, 13, 2278. [Google Scholar] [CrossRef]
- Hartmann, J.P.; Lund, M.T. A randomized, single-blinded study of the effect of intake of repair beer during a hangover. Ugeskr. Laeger. 2017, 179, V69578. [Google Scholar]
- Alonso-Andrés, P.; Martín, M.; Albasanz, J.L. Modulation of adenosine receptors and antioxidative effect of beer extracts in in Vitro Models. Nutrients 2019, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: a consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Ambra, R.; Pastore, G.; Lucchetti, S. The role of bioactive phenolic compounds on the impact of beer on health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.H.; Karppinen, T.T.; Luoto, T.; Alafuzoff, I.; Karhunen, P.J. Beer drinking associates with lower burden of amyloid beta aggregation in the brain: Helsinki sudden death series. Alcohol Clin. Exp. Res. 2016, 40, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Caon, G.; Morrone, M.; Feistauer, L.; Sganzerla, D.; Moreira, J.C.F. Moderate beer consumption promotes silymarin-like redox status without affecting the liver integrity in vivo. Food Biosci. 2021, 43, 10130. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, J.; Wang, B.; Sun, B. Wine, beer and Chinese Baijiu in relation to cardiovascular health: the impact of moderate drinking. Food Sci. Hum. Well. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Franco, L.; Bravo, R.; Galán, C.; Rodríguez, A.B.; Barriga, C.; Cubero, J. Effect of non-alcoholic beer on subjective sleep quality in a university stressed population. Acta Physiol. Hung. 2014, 101, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, Y.; Cho, H.J.; Kwak, W.K.; Jo, K.; Suh, H.J. Chemical compositions and sleep-promoting activities of hop (Humulus lupulus L.) varieties. J. Food Sci. 2023, 88, 2217–2228. [Google Scholar] [CrossRef]
- Gallinat, A.; Vilahur, G.; Padro, T.; Badimon, L. Effects of antioxidants in fermented beverages in tissue transcriptomics: sffect of beer intake on myocardial tissue after oxidative injury. Antioxidants (Basel). 2023, 12, 1096. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Kouli, G.M.; Magriplis, E.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Beer, wine consumption, and 10-year CVD incidence: the ATTICA study. Eur. J. Clin. Nutr. 2019, 73, 1015–1023. [Google Scholar] [CrossRef]
- Cha, B.H.; Jang, M.J.; Lee, S.H. Alcohol consumption can reduce the risk of gallstone disease: a systematic review with a dose-response meta-analysis of case-control and cohort studies. Gut. Liver 2019, 13, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Nehra, M.; Gahlawat, S. K.; Grover, N. Craft Beers: Fortification, Processing, and Production. CRC Press, 2023.
- Pandey, S.; Kunwar, N. Role of barley flour product and its impact on human health. Pharma. Innov. J. 2023, 12, 1500–1502. [Google Scholar]
- Hirvonen, T.; Pietinen, P.; Virtanen, M.; Albanes, D.; Virtamo, J. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am. J. Epidemiol. 1999, 150, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Jie Tang, M. D.; Lynch, M. R. Diet interventions for calcium kidney stone disease. Rhode Island Med. J. 2023, 106, 26–30. [Google Scholar]
- Dziedziński, M.; Stachowiak, B.; Kobus-Cisowska, J.; Kozłowski, R.; Stuper-Szablewska, K.; Szambelan, K.; Górna, B. Supplementation of beer with Pinus sylvestris L. shoots extracts and its effect on fermentation, phenolic content, antioxidant activity and sensory profiles. Electronic J. Biotechnol. 2023, 63, 10–17. [Google Scholar] [CrossRef]
- Yao, J.; Ma, Z.; Wang, Y.; Wang, Y.; Sun, L.; Liu, X. Effects of dandelion addition on antioxidant property, sensory characteristics and inhibitory activity against xanthine oxidase of beer. Curr. Res. Food Sci. 2022, 5, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xu, C.; Gan, J.; Sun, M.; Zhang, X.; Zhao, G.; Lv, C. Chicoric acid inserted in protein Z cavity exhibits higher stability and better wound healing effect under oxidative stress. Int. J. Bio. Macromolecul. 2024, 258, 128823. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vishakha, K.; Banerjee, S.; Bera, T.; Mondal, S.; Ganguli, A. A novel probiotic strain of Lactobacillus fermentum TIU19 isolated from Haria beer showing both in vitro antibacterial and antibiofilm properties upon two multi resistant uro-pathogen strains. Curr. Res. Microb. Sci. 2022, 3, 100150. [Google Scholar] [CrossRef]
- Martini, S.; Tagliazucchi, D. Bioactive peptides in human health and disease. Int. J. Mol. Sci. 2023, 24, 5837. [Google Scholar] [CrossRef]
- Gao, X. Editorial: Selenium and human health. Front. Nutr. 2023, 10, 1269204. [Google Scholar] [CrossRef]
- Cabrera-Cruz, H.; Oróstica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E237–248. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ohya, R.; Ano, Y. Iso-α-acids and matured hop bitter acids in beer improve obesity-induced cognitive impairment. Biosci. Biotechnol. Biochem. 2019, 83, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Strutynskyi, R.B.; Strutynska, N.A.; Piven, O.O.; Mys, L.A.; Goshovska, Y.V.; Fedichkina, R.A.; Okhai, I.Y.; Strutynskyi, V.R.; Dosenko, V.E.; Dobrzyn, P.; et al. Upregulation of ATP-sensitive potassium channels as the potential mechanism of cardioprotection and vasorelaxation under the action of pyridoxal-5-phosphate in old rats. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231213175. [Google Scholar] [CrossRef]
- Ayabe, T.; Ohya, R.; Ano, Y. Hop-derived iso-α-acids in beer improve visual discrimination and reversal learning in mice as assessed by a touch panel operant system. Front. Behav. Neurosci. 2019, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, R. Diet and gallstone. Gallbladder - Anatomy, Pathogenesis, and Treatment, ElGeidie A, editor. IntechOpen Limited, United Kingdom, 2023, 62–150.
- He, H.; Pan, L.; Ren, X.; Wang, D.; Du, J.; Cui, Z.; Zhao, J.; Wang, H.; Wang, X.; Liu, F.; et al. Joint effect of beer,spirits intake, and excess adiposity on hyperuricemia among Chinese male adults: evidence from the China national health survey. Front. Nutr. 2022, 9, 806751. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Zhang, Z.; Yang, K.; Ti, M.; Ke, Y.; Wu, L.; Yang, J.; He, Y. Influence of d-amino acids in beer on formation of uric acid. Food Technol. Biotechnol. 2019, 57, 418–425. [Google Scholar] [CrossRef]
- Anaizi, N. The impact of uric acid on human health: beyond gout and kidney stones. J. Med. Biomed. Sci. 2023, 15, 110–116. [Google Scholar] [CrossRef]
- Almeida, C.; Neves, M.C.; Freire, M.G. Towards the wse of adsorption methods for the removal of purines from beer. Molecules 2021, 26, 6460. [Google Scholar] [CrossRef]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ 2018, 363. [Google Scholar] [CrossRef]
- Mahor, D.; Prasad, G.S. Biochemical characterization of kluyveromyces lactis adenine deaminase and guanine deaminase and their potential application in lowering purine content in beer. Front. Bioeng. Biotechnol. 2018, 6, 180. [Google Scholar] [CrossRef]
- Boccellino, M.; D'Angelo, S. Anti-obesity effects of polyphenol intake: current status and future possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.D.C.; Padilha, F.F. Beer molecules and its sensory and biological properties: a review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [PubMed]
- Negrão, M.R.; Keating, E.; Faria, A.; Azevedo, I.; Martins, M.J. Acute effect of tea, wine, beer, and polyphenols on ecto-alkaline phosphatase activity in human vascular smooth muscle cells. J. Agric. Food Chem. 2006, 54, 4982–4988. [Google Scholar] [CrossRef]
- Costa, R.; Rodrigues, I.; Guardão, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhães, J.; Ferreira-de-Almeida, M.; Negrão, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef]
- Hernández-Quiroz, F.; Nirmalkar, K.; Villalobos-Flores, L.E.; Murugesan, S.; Cruz-Narváez, Y.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Chavez-Carbajal, A.; Pizano-Zárate, M.L.; García-Mena, J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol 2020, 85, 77–94. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.; Barreiros Mota, I.; Morais, J.; Ismael, S.; Pereira-Leal, J.B.; Cardoso, J.; Ribeiro, P.; Beato, H.; Resende, M.; et al. Impact of beer and nonalcoholic beer consumption on the gut microbiota: a randomized, double-blind, controlled trial. J. Agric. Food Chem. 2022, 70, 13062–13070. [Google Scholar] [CrossRef] [PubMed]
- Redondo, N.; Nova, E.; Díaz-Prieto, L.E.; Marcos, A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018, 35, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Lamuela-Raventós, R.M.; Estruch, R.; Sasot, G.; Doménech, M.; Tresserra-Rimbau, A. Beer polyphenols and menopause: effects and mechanisms-a review of current knowledge. Oxid. Med. Cell Longev. 2017, 2017, 4749131. [Google Scholar] [CrossRef]
- Liguori, L.; De Francesco, G.; Orilio, P.; Perretti, G.; Albanese, D. Influence of malt composition on the quality of a top fermented beer. J. Food Sci. Technol. 2021, 58, 2295–303. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotech. Reports 2019, 24, e00370. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An update on the potential mechanism of gallic acid as an antibacterial and anticancer agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Cici, M.O.; Bektas, N. The effect of protocatechuic acid on neuropathic pain and possible mechanism. Indian J. Pharmacol. 2023, 55, 315–321. [Google Scholar] [PubMed]
- Ding, D.; Zhang, Q.; Zeng, F.J.; Cai, M.X.; Gan, Y.; Dong, X.J. Mechanism of gentisic acid on rheumatoid arthritis based on miR-19b-3p/RAF1 axis. Chin. J. Integr. Med. 2023, 29, 508–516. [Google Scholar] [CrossRef]
- Xiong, S.; Su, X.; Kang, Y.; Si, J.; Wang, L.; Li, X.; Ma, K. Effect and mechanism of chlorogenic acid on cognitive dysfunction in mice by lipopolysaccharide-induced neuroinflammation. Front. Immunol. 2023, 14, 1178188. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant enterobacter hormaechei. J.Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Nai, X.; Li, M.; Kong, J.; Chen, Y. ; Liu.; M, Zhang, Q.; Liu, J.; Yan, H. Effects of temperature, metal ions and biosurfactants on interaction mechanism between caffeic acid phenethyl ester and hemoglobin. Molecules, 3440. [Google Scholar]
- Bao, X.; Li, W.; Jia, R.; Meng, D.; Zhang, H.; Xia, L. Molecular mechanism of ferulic acid and its derivatives in tumor progression. Pharmacol. Rep. 2023, 75, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Güzelad, Ö.; Özkan, A.; Parlak, H.; Sinen, O.; Afşar, E.; Öğüt, E.; Yıldırım, F. B.; Bülbül, M.; Ağar, A.; Aslan, M. Protective mechanism of syringic acid in an experimental model of Parkinson's disease. Metab. Brain Dis. 2021, 36, 1003–1014. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, G.; Zhan, H.; Liu, B.; Li, C.; Wang, L.; Wang, H.; Wang, J. Antimicrobial mechanism of 4- hydroxyphenylacetic acid on Listeria monocytogenes membrane and virulence. Biochem. Biophys. Res. Commun. 2021, 572, 145–50. [Google Scholar]
- Dwivedi, P.S.R.; Shastry, C.S. System biology mediated assessment of molecular mechanism for sinapic acid against breast cancer: via network pharmacology and molecular dynamic simulation. Sci. Rep. 2023, 13, 21982. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Fu, Z.Y.; Yang, L.E.; Yang, X.M.; Li, X.; Pu, X.Y.; Yang, J.Z. Functional trust of foods. BMJ 2023, 383, https. [Google Scholar]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Saroha, B.; Kumar, A. Recent advances of flavonoids and their applications. The Flavonoids. Imprint pple Academic Press, USA, 2024, 16. [Google Scholar] [CrossRef]
- Buckett, L.; Schönberger, S.; Spindler, V.; Sus, N.; Schoergenhofer, C.; Frank, J.; Frank, O.; Rychlik, M. Synthesis of human phase I and phase II metabolites of hop (Humulus lupulus) prenylated flavonoids. Metabolites 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Liu, C.; Qu, Z.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Role and mechanism of catechin in skeletal muscle cell differentiation. J. Nutr. Biochem. 2019, 74, 108225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Z.; Wu, W.; He, S.; Xie, B.; Wu, M.; Sun, Z. Molecular mechanism of epicatechin gallate binding with carboxymethyl β-glucan and its effect on antibacterial activity. Carbohydr. Polym. 2022, 298, 120105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Luo, Y.; Liu, S.; Li, S.; Li, L.; Ma, Y.; Liu, J. Protective effect of rutin on spinal motor neuron in rats exposed to acrylamide and the underlying mechanism. Neurotoxicology 2023, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, H.; Chen, J.; Ma, S.; Wang, M.; Zhou, X. The mechanism of in vitro non-enzymatic glycosylation inhibition by Tartary buckwheat's rutin and quercetin. Food Chem. 2023, 406, 134956. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, T.; Yao, H.; Zheng, H.; Wang, F.; Gao, Y. Mechanism of action of quercetin in rheumatoid arthritis models: meta-analysis and systematic review of animal studies. Inflammopharmacology 2023, 31, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Huang, C.; Chen, J.; Zhang, W.; Wang, M.; Ji, X.; Nie, S.; Sun, Z. Exploring the molecular mechanism by which kaempferol attenuates sepsis-related acute respiratory distress syndrome based on network pharmacology and experimental verification. Curr. Comput. Aided. Drug Des. 2024. [CrossRef]
- Mannino, F.; Imbesi, C.; Irrera, N.; Pallio, G.; Squadrito, F.; Bitto, A. Insights into the antiosteoporotic mechanism of the soy-derived isoflavone genistein: modulation of the wnt/beta-catenin signaling. Biofactors 2023. [Google Scholar] [CrossRef]
- Zhu, J.; Han, M.; Yang, Y.; Feng, R.; Hu, Y.; Wang, Y. Exploring the mechanism of brucea javanica against ovarian cancer based on network pharmacology and the influence of luteolin on the PI3K/AKT pathway. Comb. Chem. High Throughput Screen 2024, 27, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, H.; Zhang, X.; Xia, L.; Zhang, J. Research progress on antisepsis effect of apigenin and its mechanism of action. Heliyon 2023, 9, e22290. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhang, C.; Bian, H.; Li, Y. The mechanism of action of myricetin against lung adenocarcinoma based on bioinformatics, in silico and in vitro experiments. N-S Arch. Pharmaco. [CrossRef]
- Li, X.; Zhou, X.; Huang, Z.; Chen, K.; Jiang, X.; Lai, R.; Li, Z. Study on the mechanism of naringin in promoting bone differentiation: In vitro and in vivo study. Heliyon 2024, 10, e24906. [Google Scholar] [CrossRef] [PubMed]
- Alugoju, P. ; Kumaree, K,K.; Prasansuklab, A.; Tencomnao, T. Melatonin as a vital metabolite in medicinal and food plants. Advancement of Melatonin Research in Plants. Roychoudhury A, editor. CRC Press. 2023, 76–92. [CrossRef]
- Tian, X.; Kang, X.; Yan, F.; Feng, L.; Huo, X.; Zhang, H.; Wang, Y.; Lv, X.; Ma, X.; Yuan, J.; et al. ROS-dependent catalytic mechanism of melatonin metabolism and its application in the measurement of reactive oxygen. Front. Chem. 2024, 11, 1229199. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Luo, D.; Xu, A.; Zhao, B.; Lin, H.; Yao, H.; Li, S. Insight into the mechanism of melatonin in attenuating PCB126-induced liver injury: Resistance to ROS-dependent NETs formation to alleviate inflammation and lipid metabolism dysfunction. Ecotoxicol. Environ. Saf, 1159. [Google Scholar]
- Wang, H.; Yan, Y.; Hung, I.; Liu, C.; Yin, J.; Ge, L.; Ren, W. Melatonin in food allergy: Mechanism and potential therapy. J. Pineal. Res. 2023, 75, e12899. [Google Scholar] [CrossRef]
- Majumder, R.; Datta, M.; Banerjee, A.; Bandyopadhyay, D.; Chattopadhyay, A. Melatonin protects against ketorolac induced gastric mucosal toxic injuries through molecular mechanism associated with the modulation of Arylakylamine N-Acetyltransferase (AANAT) activity. Chem. Biol. Interact. 2023, 382, 110611. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Tiliwaerde, M.; Gao, N.N.; Zhang, T.T.; Ji, H.X.; Gu, W.; Jin, Z.L. Mechanism of GW117 antidepressant action: melatonin receptor-mediated regulation of sleep rhythm. Eur. J. Pharmacol. 2024, 964, 176299. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Takahashi, C.; Taniguchi, Y.; Narukawa, M.; Misaka, T.; Ano, Y. Bitter taste receptor activation by hop-derived bitter components induces gastrointestinal hormone production in enteroendocrine cells. Biochem. Biophys. Res. Commun. 2020, 533, 704–709. [Google Scholar] [CrossRef]
- Fukuda, T.; Ohya, R.; Kobayashi, K.; Ano, Y. Matured hop bitter acids in beer improve lipopolysaccharide-induced depression-like behavior. Front. Neurosci. 2019, 13, 41. [Google Scholar]
- Flythe, M.D.; Kagan, I.A.; Wang, Y.; Narvaez, N. Hops (Humulus lupulus L.) bitter acids: modulation of rumen fermentation and potential as an alternative growth promoter. Front. Vet. Sci. 2017, 4, 131. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Liu, T.; Yang, J.; Sun, X.; Gao, X.J. The mechanism of selenium regulating the permeability of vascular endothelial cells through selenoprotein O. Redox Biol. 2024, 70, 103063. [Google Scholar] [CrossRef]
- González-Salitre, L.; Basilio-Cortés, U.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Cardelle-Cobas, A.; González- Olivares, L.G. Physicochemical and microbiological parameters during the manufacturing of a beer-type fermented beverage using selenized Saccharomycesboulardii. Heliyon 2023, 9, e21190. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Garcimartán, A.; Meseguer, I.; Mateos-Vega, C.J.; Orellana, J.M.; Peña-Fernández, A.; Benedí, J.; Sánchez-Muniz, F.J. Silicic acid and beer consumption reverses the metal imbalance and the prooxidant status induced by aluminum nitrate in mouse brain. J. Alzheimers Dis. 2017, 56, 917–927. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.D.; Dhungana, S.K.; Do, H.M.; Shin, D.H. Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 2018, 27, 1067–1073. [Google Scholar] [CrossRef]
- Ciosek, A.; Fulara, K.; Hrabia, O.; Satora, P.; Poreda, A. Chemical composition of sour beer resulting from supplementation the fermentation medium with magnesium and zinc Ions. Biomolecules 2020, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Casani, L.; Mendieta, G.; Lamuela-Raventos, R.M.; Estruch, R.; Badimon, L. Beer elicits vasculoprotective effects through Akt/eNOS activation. Eur. J. Clin. Invest. 2014, 44, 1177–1188. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P.; Poblaciones, M.J. Selenium speciation in malt, wort, and beer made from selenium-biofortified two-rowed barley grain. J. Agric. Food Chem. 2014, 62, 5948–5953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, Y.; Wang, F.; Liu, X.; Wang, J.; Xiao, J.; Zhang, C.; Zhang, Q. A new mechanism for ginsenoside Rb1 to promote glucose uptake, regulating riboflavin metabolism and redox homeostasis. Metabolites 2022, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Zhong, H.; Xu, Y. The mechanism of nicotinamide on reducing acute lung injury by inhibiting MAPK and NF-κB signal pathway. Mol. Med. 2021, 27, 115. [Google Scholar] [CrossRef]
- Xiang, Y.; Liang, B.; Zhang, X.; Qiu, X.; Deng, Q.; Yu, L.; Yu, H.; Lu, Z.; Zheng, F. Atheroprotective mechanism by which folic acid regulates monocyte subsets and function through DNA methylation. Clin. Epigenetics 2022, 14, 32. [Google Scholar] [CrossRef]
- Niu, H.Y.; Shao, Z.H.; Wang, H.Q. Inhibitory effect of ascorbic acid on myelodysplastic syndrome cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1851–1857. [Google Scholar] [PubMed]
- Broz, P.; Rajdl, D.; Racek, J.; Trefil, L.; Stehlík, P. Effect of beer consumption on methylation and redox metabolism. Physiol. Res. 2022, 71, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Spigno, G.; Luzzani, G.; Bozzoni, M.E.; Donadini, G.; Rolla, J.; Bertuzzi, T. Effects of the intake of craft or industrial beer on serum homocysteine. Int. J. Food Sci. Nutr. 2021, 72, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Duarte Villas Mishima, M.; Stampini Duarte Martino, H.; Silva Meneguelli, T.; Tako, E. Effect of food derived bioactive peptides on gut health and inflammatory mediators in vivo: a systematic review. Crit. Rev. Food Sci. Nutr. 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grochalová, M.; Konečná, H.; Stejskal, K.; Potěšil, D.; Fridrichová, D.; Srbová, E.; Ornerová, K.; Zdráhal, Z. Deep coverage of the beer proteome. J. Proteomics 2017, 162, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kammers, K.; Larman, H.B.; Scharpf, R.B.; Ruczinski, I. Detecting and quantifying antibody reactivity in PhIP-Seq data with BEER. Bioinformatics 2022, 38, 4647–4649. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Hisatsune, Y.; Fujita, A.; Yamada, O. Presence of disulfide-bonded thiols in malt and hops as the precursors of thiols in beer. J. Agric. Food Chem. 2022, 70, 13413–13418. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Xi, Y.; Wang, J.R.; Zhang, Y.X.; Li, H.; Liu, X.Q. Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms. Food Sci. Hum. Well. 2024, 13, 1168–1185. [Google Scholar] [CrossRef]
- Tian, W.H.; Hu, S.M.; Zhuang, Y.L.; Sun, L.P.; Yin, H. Identification of in vitro angiotensin-converting enzyme and dipeptidyl peptidase IV inhibitory peptides from draft beer by virtual screening and molecular docking. J. Sci. Food Agric. 2022, 102, 1085–1094. [Google Scholar]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and therapeutic role of functional ingredients of barley grass for chronic diseases in human beings. Oxid. Med. Cell Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 3492–3505. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.E.; Liden, T.; Berger, B.K.; Schug, K.A. Target profiling of beer styles by their iso-α-acid and phenolic content using liquid chromatography-quadrupole time-of-flight-mass spectrometry. J. Sep. Sci. 2021, 44, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Mokkawes, T.; Lim, Z.Q.; de Visser, S.P. Mechanism of melatonin metabolism by CYP1A1: what determines the bifurcation pathways of hydroxylation versus deformylation? J. Phys. Chem. B 2022, 126, 9591–9606. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
The health effect of beer against 26 chronic diseases is highly similar to that of barley:The 26 health effects of beer are the result of the combined effect of barley malt, hops, yeast and water brewing into active ingredients. In particular, the more than 20 health benefits of barley grains and their grass powder coincide with beer. However, the high purine content of beer is easy to cause hyperuricemia and gout. The development of low purine pure barley beer and its functional beer is an important direction of functional foods. These research results support the theory that barley malt, hops and beer brewing as the main nutritional therapy is the best way to treat human chronic diseases and that there is only one cell disease theory for functional foods in humans.
Figure 1.
The health effect of beer against 26 chronic diseases is highly similar to that of barley:The 26 health effects of beer are the result of the combined effect of barley malt, hops, yeast and water brewing into active ingredients. In particular, the more than 20 health benefits of barley grains and their grass powder coincide with beer. However, the high purine content of beer is easy to cause hyperuricemia and gout. The development of low purine pure barley beer and its functional beer is an important direction of functional foods. These research results support the theory that barley malt, hops and beer brewing as the main nutritional therapy is the best way to treat human chronic diseases and that there is only one cell disease theory for functional foods in humans.
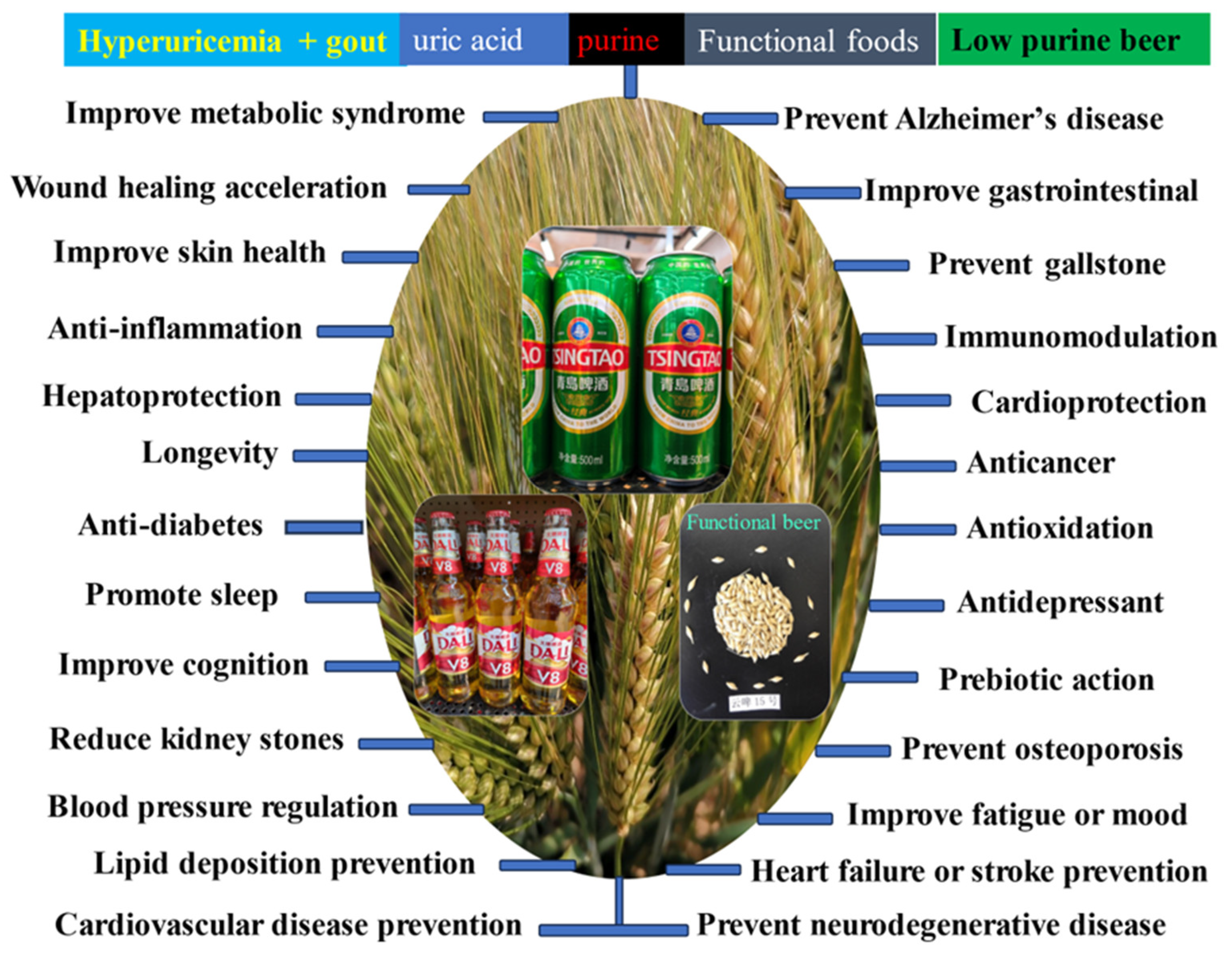
Figure 2.
Actional mechanism of six functional ingredients in beer for human health. There are some differences in the molecular mechanisms of the six functional components of beer on human health, and its health effect is as follows: polyphenols mechanism 22 health effects (phenolic acids 11 effects and flavonoids 22 effects) > bitter acids mechanism 15 health effects ≥ minerals mechanism 15 health effects > melatonin mechanism 14 health effects > vitamins mechanism 11 health effects > active peptides mechanism 8 health effects.
Figure 2.
Actional mechanism of six functional ingredients in beer for human health. There are some differences in the molecular mechanisms of the six functional components of beer on human health, and its health effect is as follows: polyphenols mechanism 22 health effects (phenolic acids 11 effects and flavonoids 22 effects) > bitter acids mechanism 15 health effects ≥ minerals mechanism 15 health effects > melatonin mechanism 14 health effects > vitamins mechanism 11 health effects > active peptides mechanism 8 health effects.
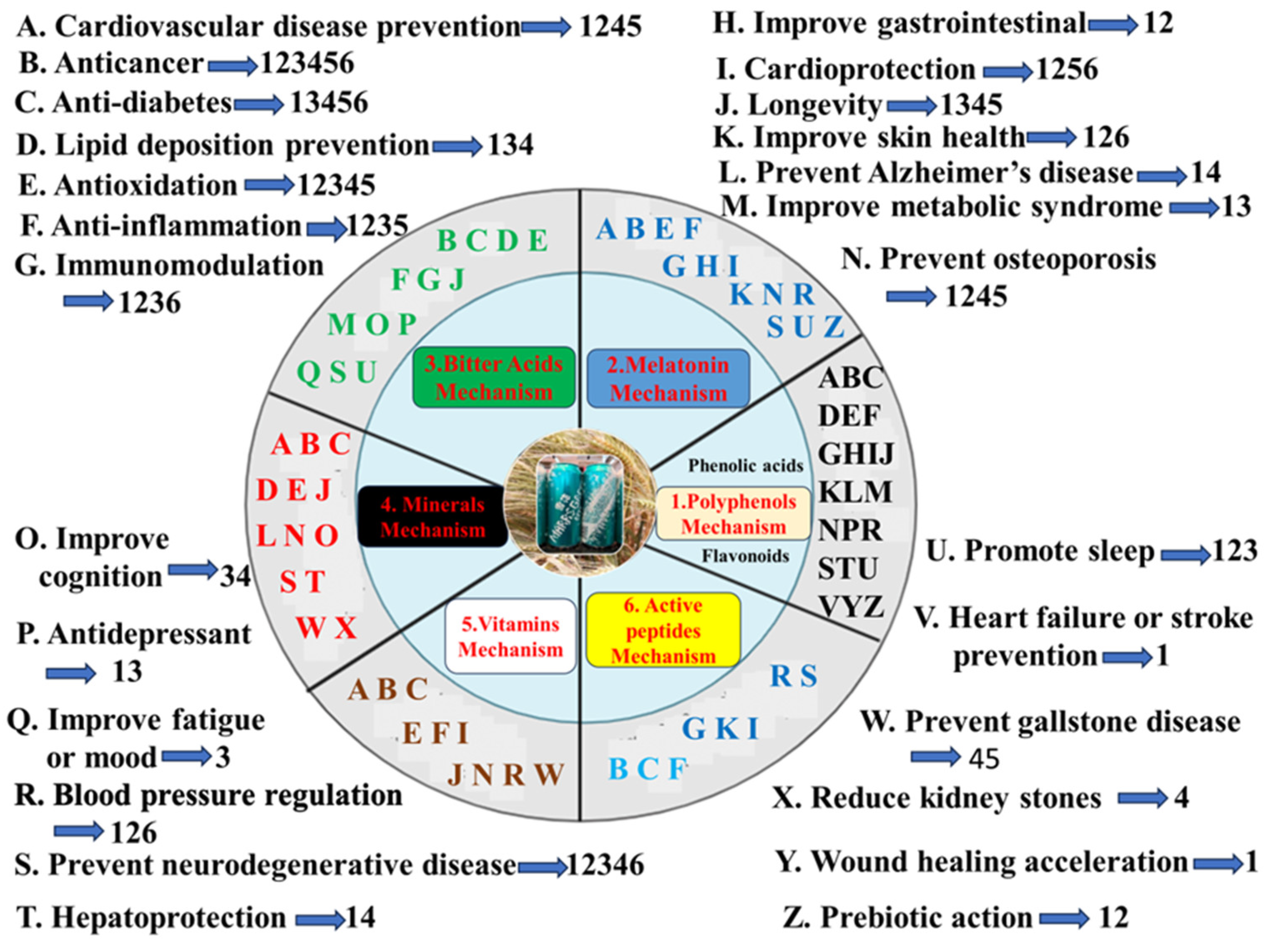
Table 1.
Contents of bioactive phenolic compounds in common beer.
| Hops (mg/100 g) | Barley (mg/100 g) | ||
| Compound [Reference] | Contents | Compound (Reference) | Contents |
| Phenolic acids: | Phenolic acids: | ||
| Caffeic acid [9] | 0.01–15.8 | Caffeic* [1,9] | 0.17 ± 0.01 |
| Chlorogenic acid [9] | 0.47–163.7 | Chlorogenic [9,25] | 0–9.84 |
| p-Coumaric acid [9] | 0.01–28.8 | p-Coumaric [9] | 0.17–58.3 |
| Ferulic acid [9] | 1–10 | Ferulic [9] | 0.59–4.25 |
| p-Hydroxybenzoic acid [9] | 187.0 ±1.0 | p-Hydroxybenzoic [9] | 0.58–2.67 |
| Syringic acid [9] | 3–1290 | Syringic [9,24] | 0.1–91.6 |
| Gallic acid [9] | 8–341 | Gallic [9] | 0.1–136.6 |
| Protocatechuic acid [9] | 42–225 | Protocatechuic* [8,9] | 0.14 ± 0.05 |
| Gentisic acid [9] | 1.5–6.7 | ||
| Flavonoids: | Sinapic acid [9] | 0.14–2.44 | |
| Epigallocatechin [9] | 10.3–28.6 | 2,4-Dihydroxybenzoic [9] | 0.68–6.16 |
| Epicatechin [9] | 0.08–8.4 | Vanillic acid [8,9] | 0.10–3.91 |
| Procyanidin B1 [9] | 1840–5060 | Total [9] | 0.2–67.5 |
| Procyanidin B2 [9] | 840–1460 | Flavonoids: | |
| Procyanidin C1 [9] | 380–1690 | Total [9] | 6.2–30.1 |
| Desmethylxanthohumol [9] | 120.0 | Total * [9] | 16.5–24.1 |
| Catechin [8,9] | 1.2–56.1 | Catechin [9,26] | 0.1–10.5 |
| Kaempferol [9] | 0.44–49.4 | Kaempferol [9,26] | 1.27–19.2 |
| Naringenin [9] | 3.9–11.0 | Naringenin [9] | 4.7–50.2 |
| Naringin [9] | 1.7–3.9 | Naringin [9] | 0.77–6.97 |
| Quercetin [9] | 1.03–111.8 | Quercetin [9,24] | 2.0–8.7 |
| Rutin [9] | 61–88 | Rutin [9] | 1.4–11.8 |
| Isorhamnetin [9] | 0.5–3.3 | Rutin* [9] | 0.07–0.46 |
| Isoxanthohumol [9] | 8.0–35.2 | Quercetin* [9] | 1.5–6.7 |
| 8-Prenylnaringenin [9] | 1.5–23.8 | Myricetin [9,26] | 0–73.3 |
| Xanthohumol [9] | 85.6–480.0 | Myricetin* [9] | 3.1–4.3 |
| Stilbenes*: | Hesperidin [9] | 0.5–24.9 | |
| Total trans-Stilbenes* [9] | 0.05–1.17 | Alkylresorcinols [9] | 3.2–10.3 |
| trans-Resveratrol* [9] | 0.003–0.228 | Alkylresorcinols* [9] | 2.86–3.54 |
| trans-Piceid* [9] | 0.04–1.10 | Total lignans* [9] | 1.25 |
Note: * For fresh weight, the rest is dry weight.
Table 2.
Functional and nutrient compositions of beer and barley grains.
| Beer | Barley grains | ||
| Composition (Reference) | Mean±SD | Composition (Reference) | Mean±SD |
| K mg/L [29] | 543.3±326.5 | K mg/kg [27] | 4802±1839 |
| Na mg/L [8,29] | 66.4±44.79 | Na mg/kg [1,27] | 190.5±104.7 |
| Fe mg/L [8,29] | 0.23±0.19 | Fe mg/kg [1,28] | 43.4±17.6 |
| Mg mg/L [8,29] | 107.0±75.8 | Mg mg/kg [1,27] | 1250± 393 |
| Ca mg/L [29] | 96.67±15.28 | Ca mg/kg [1,27] | 568.3± 235.1 |
| Mn mg/L [8,31] | 0.31±0.41 | Mn mg/kg [1,27] | 29.3±24.8 |
| Zn mg/L [8,29] | 0.17±0.12 | Zn mg/kg [1,28] | 38.1±9.3 |
| P mg/L [29] | 361.7±176.5 | P mg/kg [1,27] | 2593±1046 |
| Se mg/L [29] | 8.33±6.35 | Se mg/kg [28] | 36.03±3.62 |
| Total prenylated flavonoids mg/L [14] | 0.0-9.5 | Flavonoids mg/Kg [24] | 125.1±101.4 |
| Protein % [29] | 0.2-0.6 | Protein % [1] | 14.92±0.13 |
| Polyphenols mg GAE/L [30] | 192.6 ± 8.7 | Polyphenols mg GAE/L [1,33] | 2316±343 |
| FRAP mmol TE/L [30] | 1.23 ± 0.01 | FRAP mmol TE/L [1,34] | 60.36±15.48 |
| ABTS•+mmol TE/L [30] | 1.44 ± 0.09 | ABTS g/L [1,34] | 5.87±0.92 |
| Alcohol % [29] | 3.50-6.15 | β-glucan % [1,35] | 4.61±0.45 |
| Water % [29] | 88.5-97.7 | Resistant starch % [1,36] | 3.63±2.32 |
| Total energy KJ/L [29] | 1410-2780 | Arabinoxylan % [1] | 1.31±0.73 |
| Sugar % [29] | 2.1-5.3 | Phenolic acids mg/100g [35] | 414.70±32.86 |
| Iodide μg/L [29] | 1-8 | Total flavones mg/100g[33,35,36] | 80.64±17.15 |
| Tyrosol mg/L [14] | 0.2-44.4 | Total alkaloid mg/100g [1] | 25.96±1.41 |
| Hydroxytyrosol mg/L [14] | 0.0-0.1 | Total anthocyanin mg/100g [1] | 35.50±23.82 |
| Alkylresorcinols μg/L [14] | 0.02-11.0 | Proanthocyanidin mg/100g [24] | 6.97±3.84 |
| Isoflavonoid nmol/L [32] | 0.19-14.99 | Total tocols mg/100g [1] | 5.85±3.51 |
| Daidzein nmol/L [32] | 0.08-2.5 | Antioxidant activity % [33] | 41.55±7.82 |
| Genistein nmol/L [32] | 0.169-6.74 | GABA mg/100g [36] | 8.00±3.92 |
| Biochanin A nmol/L [32] | 0.820-4.84 | Phytosterols mg/100g [24] | 91.13±21.14 |
Table 3.
Functional ingredients in conventional beers.
| Composition (Reference) | Range | Composition(Reference) | Range | |
| Phenolic acid | mg/L Beer | Flavonoids | mg/L Beer | |
| Gallic acid (free) [8,9] | 0.06–10.4 | Kaempferol [9] | 0.06–16.4 | |
| Protocatechuic acid (free) [9] | 0.02–0.30 | Daidzein [9] | 0.23–0.36 | |
| Protocatechuic acid [8] | 0.80-1.70 | Genistein [9] | 0.06–0.08 | |
| p-Hydroxybenzoic acid (free) [9] | 0.38–9.04 | Formononetin [9] | 0.17–1.30 | |
| Gentisic acid (free) [9] | 0.07–0.30 | Luteolin [9] | 0.10–0.19 | |
| Chlorogenic acid (free) [9] | 0–2.38 | Apigenin [9] | 0.80–0.81 | |
| 2,6-dihydroxybenzoic acid (free) [9] | 2.53 ± 0.11 | Myricetin [9] | 0.15–0.16 | |
| Vanillic (free) ]9] | 0–3.6 | Naringin [9] | 0.70–2.63 | |
| Total vanillic ]9] | 1.17–5.45 | Naringenin[9] | 0.06–2.34 | |
| Homovanillic acid (free) ]9] | 0.41 ± 0.04 | Phenolic alcohols | mg/L beer | |
| Caffeic acid (free) [9] | 0–2.53 | Tyrosol [9,14] | 0.2–44.4 | |
| Total caffeic acid [8,9] | 0.98–6.38 | Hydroxytyrosol [9] | 0.0–0.13 | |
| m-Hydroxybenzoic acid(free)[9] | 0–1.03 | Organic acid | mg/L beer | |
| Syringic acid (free) [9] | 0–1.13 | Lactic acid [8] | 28-700 | |
| Total syringic acid [8,9] | 0–1.23 | Acetic acid [8] | 8-240 | |
| p-Coumaric acid (free) [9] | 0.01–5.58 | Acetylformic acid [8] | 5-330 | |
| Total p-Coumaric acid [8,9] | 0.30–3.10 | Succinate+malic acid [8] | 61-640 | |
| Ferulic acid (free) [8,9] | 0.10–11.03 | Oxalic acid [8) | 2-37 | |
| Total ferulic acid [9] | 9.97–22.60 | Citric acid [8] | 77-590 | |
| 4-hydroxyphenylacetic acid (free) [9] | 0.05–1.47 | Sodium acetate [25] | 0.1171 | |
| Total 4-hydroxyphenylacetic acid [9] | 0.40–1.46 | Sodium 2-oxopropanoate [25] | 0.0494 | |
| Sinapic acid (free) [9] | 0.20–1.39 | Potassium D-gluconate [25] | Potassium D-gluconate [25] | 0.0348 |
| Potassium D-gluconate [25] | ||||
| Total sinapic acid [9] | 2.19–6.16 | DL-Malic acid [25] | 0.1867 | |
| m-Coumaric acid (free) [9] | 0.105±0.006 | Sodium citrate [25] | 0.0595 | |
| Salicylic acid (free) [9] | 0.19–6.66 | Sodium DL-lactate [25] | 0.0348 | |
| o-Coumaric acid (free) [9] | 0.47 ± 0.04 | Prenylflavonoids | mg/L Beer | |
| Flavonoids | mg/L Beer | 8-Prenylnaringenin [9] | 0–0.021 | |
| Catechin [8,9] | 0.03–18.30 | 6-Geranylnaringenin [9] | 0.001–0.074 | |
| Epicatechin [9] | 0.02–11.50 | Isoxanthohumol [9] | 0.04–3.44 | |
| Rutin [9] | 0.06–4.85 | Xanthohumol [9] | 0.002–0.69 | |
| Quercetin [9] | 0.06–2.23 | 6-Prenylnaringenin [9] | 0.011–0.56 | |
| Vitamin | mg/L beer | Alkylresorcinols | mg/L Beer | |
| VB1 [25] | 0.0266 | Total alkylresorcinols [9,14] | 1.01 ± 2.03 | |
| VB2 [8,25] | 0.26-4.03 | Stilbenes | mg/L beer | |
| VB3 [29] | 4.30-8.15 | trans-Resveratrol [9] | 0–0.067 | |
| VB5 [8,25] | 0.033-1.065 | cis-Resveratrol [9] | 0–0.023 | |
| VB7 [8,25] | 0.008-0.022 | cis-Piceid [9] | 0–0.024 | |
| VB6 [8,25,29] | 0.05-0.505 | trans-Piceid [9] | 0–0.009 | |
| VB8 [8,25] | 0-0.048 | Nicotinamide [8,9] | 0.01-0.30 | |
| VB9 [8,25] | 0-0.006 | Amine | mg/L Beer | |
| VB12 [29] | 0.00015-0.00028 | Putrescine [25] | 0.0823 | |
| vitamin(A,D,E,K) [8,25] | 0.398-4.984 | Tyramine [25] | 1.0698 | |
| Dietary fiber | mg/L beer | Histamine [25] | 0.1994 | |
| Non-starchy carbohydrates [6,37] | 1442-2923 | Melanoidins [6] | 0.6-103 | |
| β-glucan [37] | 26-99 | Melanoidins/ malt wort [38] | 125-225 |
Table 4.
Functional ingredients of beers and barley for similar preventive chronic disease.
| Preventive chronic disease | Functional ingredients in grains [grass] [1] | Functional ingredients in beers | Reference in beers |
| Cardiovascular disease prevention | β-glucans, arabinoxylan, lignans, tocols, polyphenols, phytosterols, folate[K,Ca, saponarin,SOD,VB1, vitamins(A,C,E),GABA, tryptophan] | polyphenols, melanin, folate, Mg, prenylated flavonoids, melanoidins | [12,79,80,81,83,84,93] |
| Anticancer | β-glucan,phenolics, arabinoxylan, lignan, phytosterols, resistant starch [Alkaline, flavonoids, chlorophyll, tricin, SOD] | prenylflavonoids,Se,melanin, polyphenols, tyrosol,vitamins, bitter acids,peptides,stilbene, hydroxytyrosol | [9,11,85,113,143,144] |
| Anti-diabetes | β-glucan, phenolic compounds, polysaccharide, phytosterols, tocols, resistant starch [Ca, Saponarin, dietary fibre, AMPK, SOD, polyamines, GABA] | Isomaltulose, phenolic acids, Se, resistant maltodextrin, bitter acids,xanthohumol, VB8, peptides | [9,87,88,112,143,144,145] |
| Lipid deposition prevention oranti-obesity | β-glucan,polysaccharide,resistant starch,polyphenols,dietary fiber,α- tocopherol, tocols, phytosterols [saponarin, 2"-O-glycosyl isovitexin] | soluble nitrogen, Mg, iso-α-acids, xanthohumol, polyphenols | [12,91,146] |
| Antioxidation | polyphenols,GABA,VE,phenolics, anthocyanin,polysaccharide,SOD, tocotrienol [lutonarin,γ-tocopherol, saponarin,orientin, chlorophyll, isoorientin, glutathione, flavonoid] | polyphenols,Se,VB2 ,iso-α-acids ferulic acid, melanoidins, flavonoids | [9,93,94,99,107] |
| Anti-inflammation or allergic rhinitis alleviation | β-glucans, vanillic acid, lignans, arabinoxylan [Chlorophyll, saponarin, SOD, tryptophan, GABA] | iso-α-acids,peptides, VB2, 8-prenylnaringenin, isoxanthohumol,polyphenols, melanoidins ,xanthohumol | [77,78,95,96,97,107,143] |
| Immunomodulation | β-glucans, arabinoxylan [polysaccharide, GABA] | melatonin, bitter acids, flavonoids, bioactive peptides | [78,99,143] |
| Improve gastrointestinal | β-glucans [dietary fiber, Se, GABA] | polyphenols, butyric acid, flavonoids; melanoidins | [77,78,100,102] |
| Cardio-protection | β-d-glucan [K, GABA] | polyphenols,peptides,VB6, melanoidins, flavonoids | [9,93,147] |
| Longevity or Anti-aging | Excavate functional components | polyphenols,minerals,vitamins, Iso-α-acids, flavonoids | [104,106] |
| Improve skin health or atopic dermatitis | GABA, extract P [Metallothioneins, SOD] | flavonoids, by-products, melanoidins | [4,108] |
| Prevent Alzheimer’s disease | flavonoids,minerals,vitamins, phenolic acids in barley grains [63] | Si, hops extract, phenolic acids | [9,110,111] |
| Improve metabolic syndrome | β-glucans in barley grains | polyphenols, bitter acids, xanthohumol | [113] |
| Prevent osteoporosis or bone injury recovery | eExcavate functional components [Ca] | Si, polyphenols, VB9, phytoestrogens, melanoidins | [77,78,114,115,116] |
| Improve cognition | GABA, K, and SOD in barley grass | Iso-α-acids, Se | [96,144,148] |
| Antidepressant | GABA,saponarin, vitamins, and minerals in barley grass | β-bitter acid, iso-α-acids, phenolic acids | [9,118,119] |
Table 4.
Continuation.
| Preventive chronic disease | Functional ingredients in grains [grass] [1] | Functional ingredients in beers | Reference in beers |
| Improve fatigue or mood | Excavate components [Lutonarin, saponarin] | bitter acids | [117,120,121] |
| Blood pressure regulation | β-glucans [saponarin; lutonarin, K,Ca, GABA] | 8-prenylnaringenin, melatonin,peptides, VB6 | [122,143,147] |
| Prevent neurodegenerative disease | protein, fiber, minerals, phenolic compounds in barley grains | flavonoids, minerals, melanoidins, bitter acids, peptides | [70,77,78,119,125,126,127,143] |
| Hepatoprotection | β-glucans,phenolics, pentosan /saponarinm,SOD,GABA[62] | flavonoids, xanthohumol,Se | [128,129,144] |
| Promote sleep | GABA, Ca, K, VC, and tryptophan in barley grass | melatonin, α-acids, β-acids, xanthohumol | [77,130] |
| Heart failure or stroke prevention | β-d-glucan, phenolics, tocols, linoleic acid, extract P, low protein, folate in barley grains | polyphenols | [132,133] |
| Prevent gallstone | Excavate functional components | alcohol, ascorbic acid, Ca | [134,149] |
| Reduce kidney stones | β-glucans in barley grains | Ca, K, Se, fluidslenium | [137,138,144] |
| Wound healing acceleration | β-glucans in barley grains | caffeic acid, ferulic acid, chicoric acid | [140,141] |
| Prebiotic action | melanoidins, tocols, phenolic compounds, β-glucans in barley grains [68,69] | polyphenols, fiber, melanoidins | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








