Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fundamentals of Paper Microfluidics
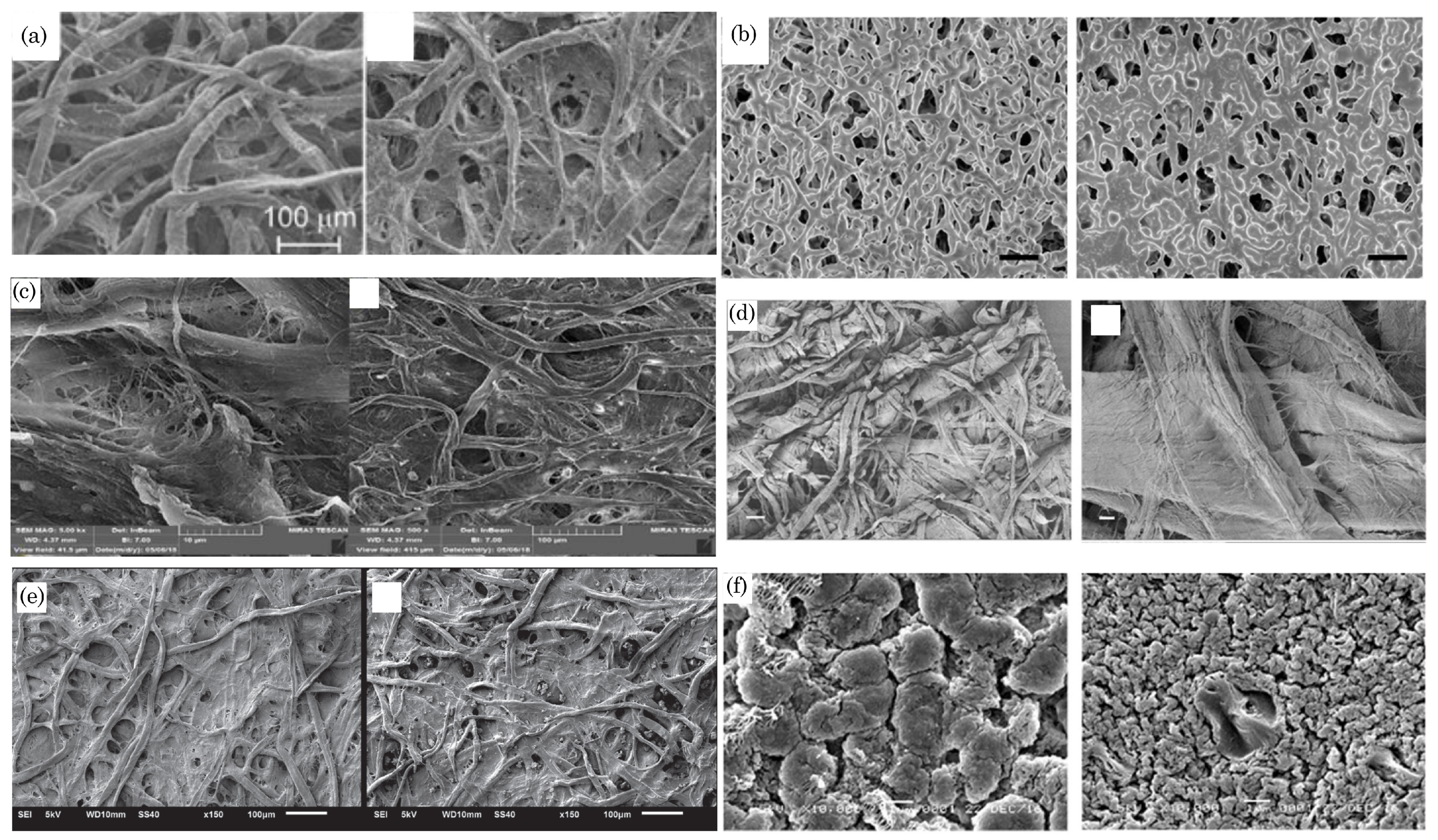
2.1. Paper Types and Their Characteristics
2.2. Paper Selection Factors
2.3. Principles of Fluid Transport in Paper
2.3.1. Classical Lucas-Washburn Equation (Capillary Flow)
2.3.2. Darcy’s Law for Fluid Flow
2.4. Dimensionless Numbers for Fluid Transport
2.4.1. Capillary Number (Ca)
2.4.2. Reynolds Number (Re)
2.4.3. Weber Number (We)
2.4.4. Schmidt Number (Sc)
2.4.5. Number()
3. Classifications of Paper-Based Assays
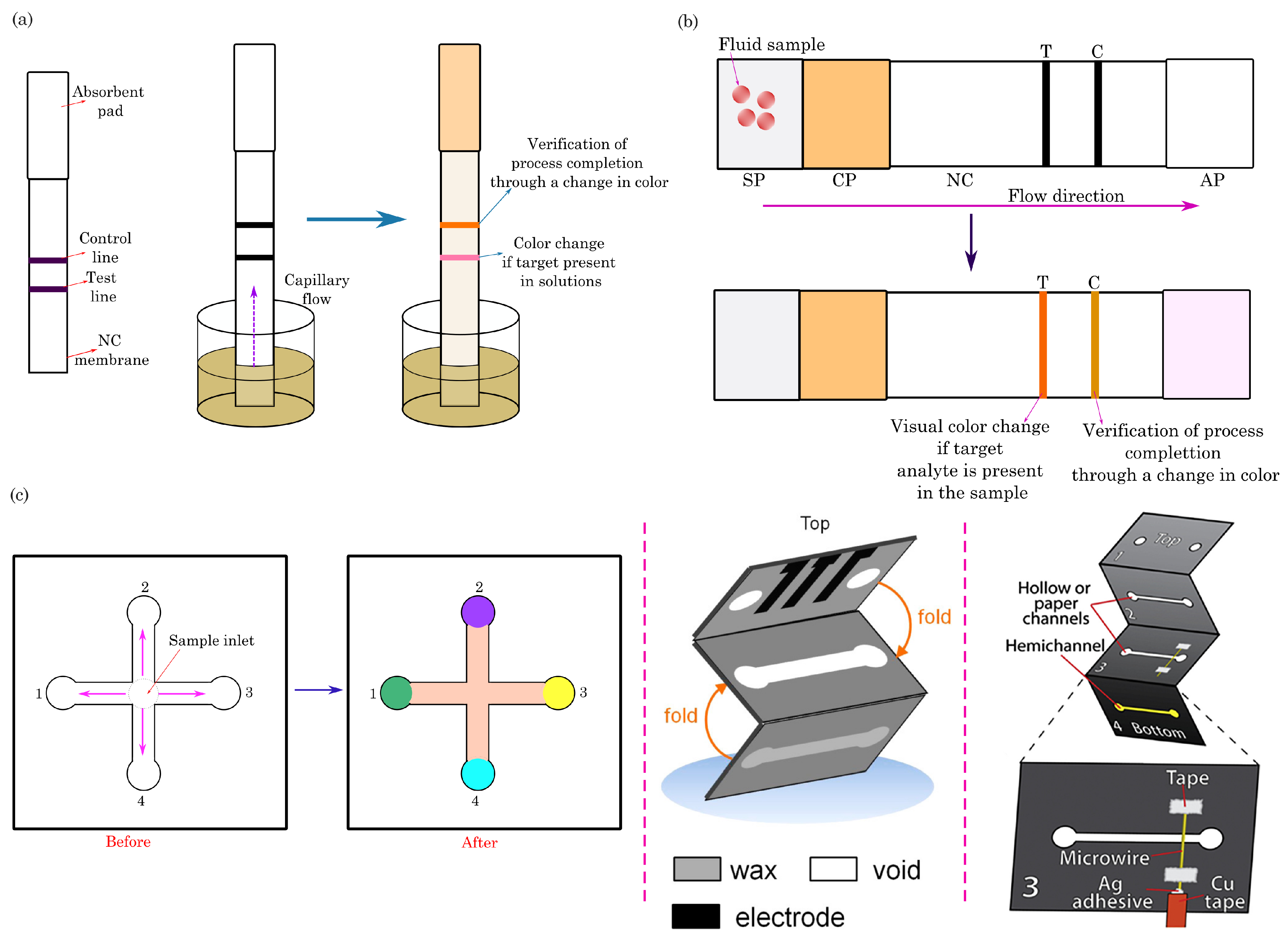
3.1. Dipstick Assays
3.2. Lateral-Flow Assays
3.3. Microfluidic Paper-Based Analytical Devices (PADs)
4. Fabrication Techniques for Paper-Based Devices
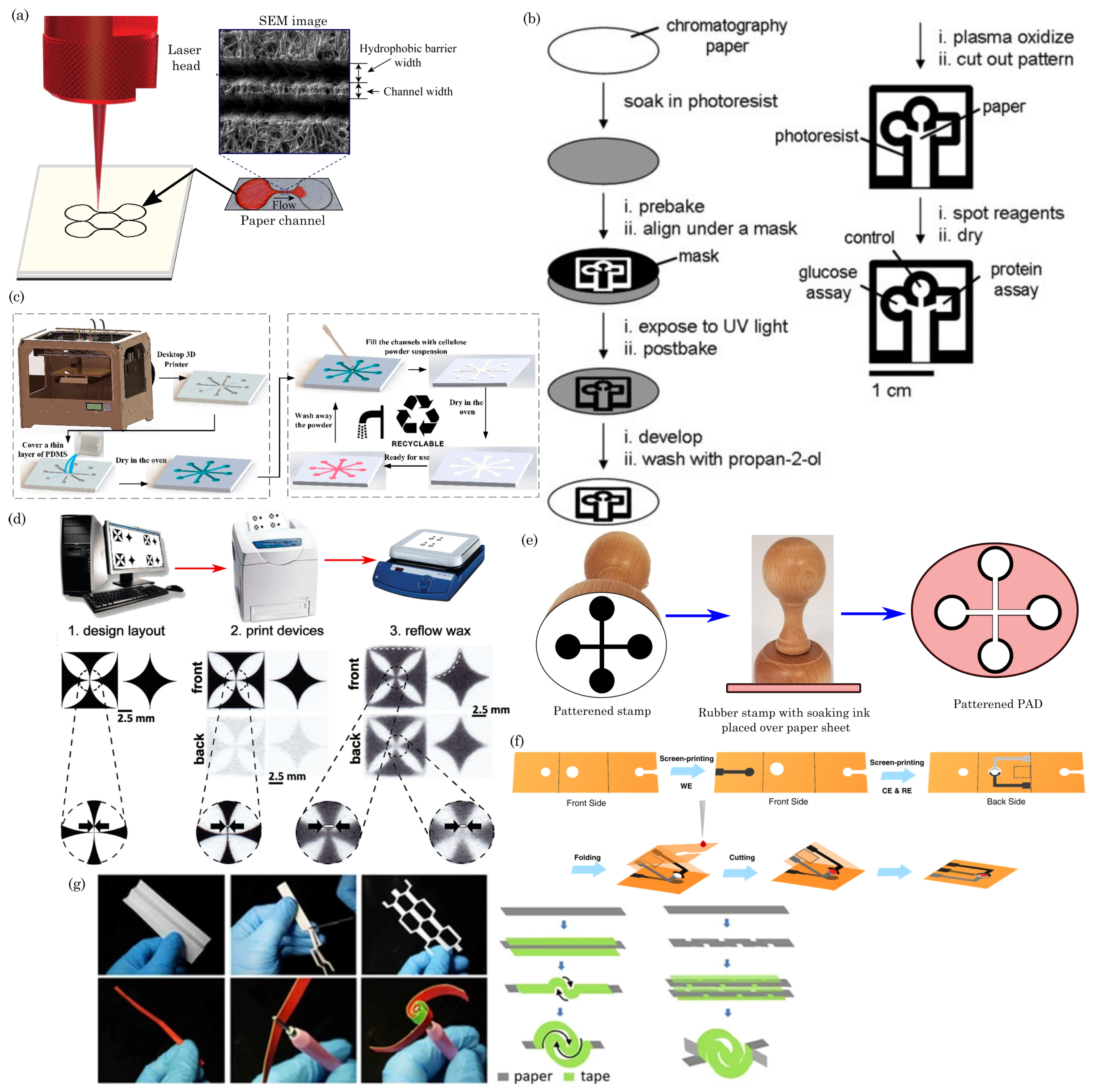
4.1. Blade Cutting/Plotting
4.2. Laser Cutting
4.3. Photolithography
4.4. 3D Printing
4.5. Screen Printing
4.6. Wax Printing
4.7. Inkjet Printing
4.8. Embossing
4.9. Origami, Quilling, and Kirigami
| Fabrication techniques | Equipment and materials requirements | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Blade Cutting/Plotting | X-Y plotter, knife. | Provides sharp features, no chemical required. | Limited to 2D designs | [60,61] |
| Laser cutting | Laser cutter. | Precise, customizable designs, suitable for large-scale production, high resolution (∼60 m) | Requires specialized equipment and polymer films to protect the paper device from damage, may generate debris. | [62,63,64,65,66] |
| Photolithography | UV light, heating plate, photomask, photoresists (positive/negative), mask aligner, chemicals, oxygen plasma. | High resolution (∼200 m), well-established microfabrication technique | Equipment-intensive, may involve multiple complex steps and chances of channel contamination. | [54,67,68,69] |
| 3D printing | 3D printer, inks | Allows for complex, customized designs | Limited resolution compared to traditional microfabrication | [70,71,72,73,74,75,76] |
| Screen printing | Mesh screen, hot plate, transparency film, wax. | Low-cost, scalable for mass production | Resolution may vary, suitable for relatively simple designs, new screens are required for different patterns. | [77,78,79,80,81,82,83] |
| Wax printing | Hot plate, wax printer, solid wax. | Simple, rapid, cost-effective, and suitable for prototyping | Limited resolution (∼550 m), wax spread, limited channel height control, temperature sensitivity | [84,85,86,87,88,89] |
| Inkjet printing | Customized inkjet printer, hydrophobic ink, hot plate, and chemicals. | Non-contact, suitable for rapid prototyping | Resolution may be lower than other techniques, requires multiple steps, and post-printing heating is required for some inks. | [90,91,92,93,94,95,96] |
| Embossing | Embossing tools, adhesives, silane. | Simple, flexible, suitable for rapid prototyping | Limited resolution, may affect paper integrity, susceptible to contamination. | [97,98,99,100] |
| Origami and Kirigami | Paper cutting and folding tools, adhesives. | Foldable Structures, flexible design, enhanced functionality, Scalability. | Precision challenges, design and assembly complexity, limited material compatibility. | [101,102,103,104,105,106,107,108] |
5. Detection Techniques
5.1. Colorimetric Sensing
5.2. Electrochemical Sensing
5.3. Fluorescence
5.4. Chemiluminescence
5.5. Electrochemiluminescence
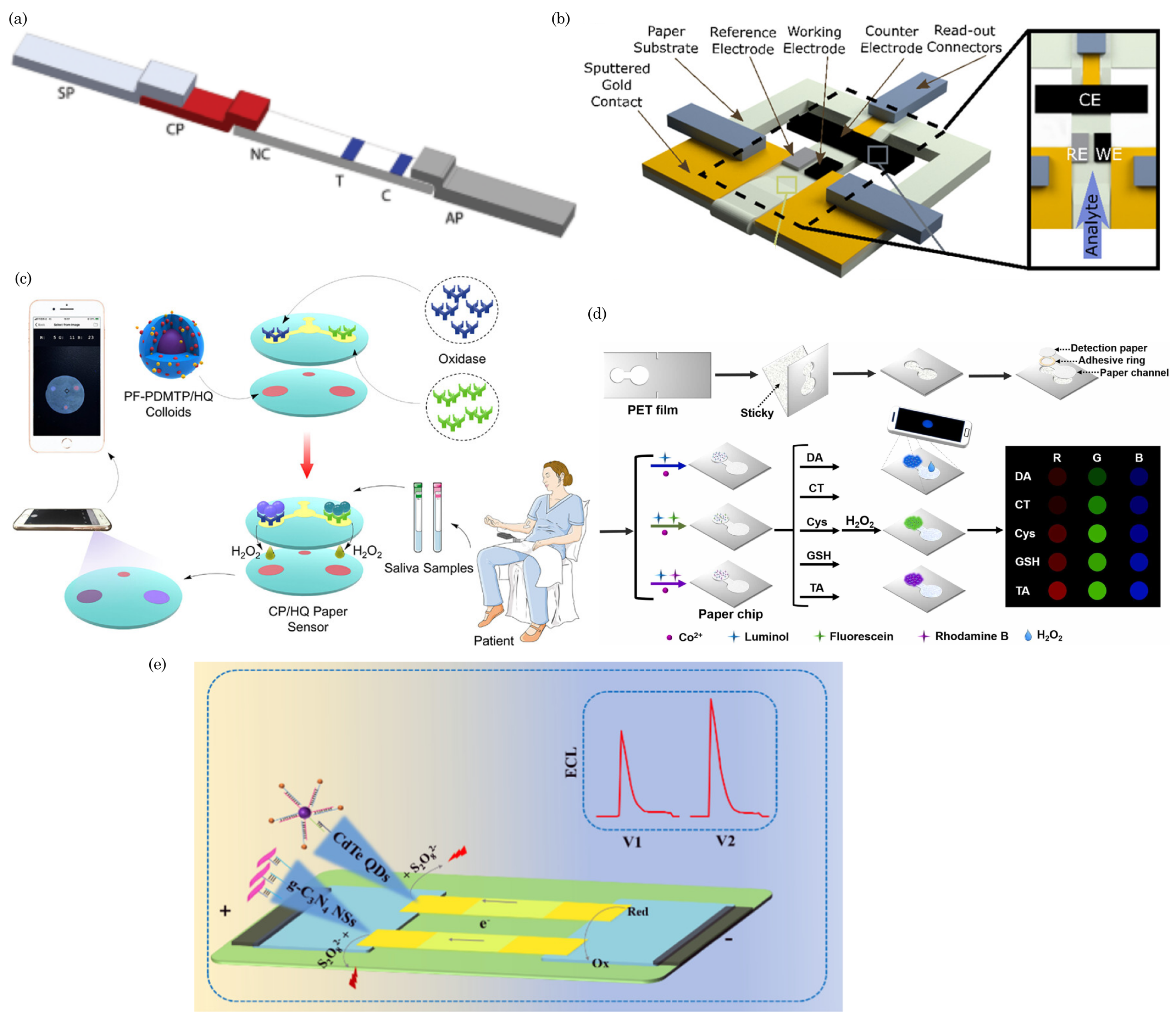
6. Applications in Health Sensing
6.1. Diagnostic Assays for Infectious Diseases and Others Analytes
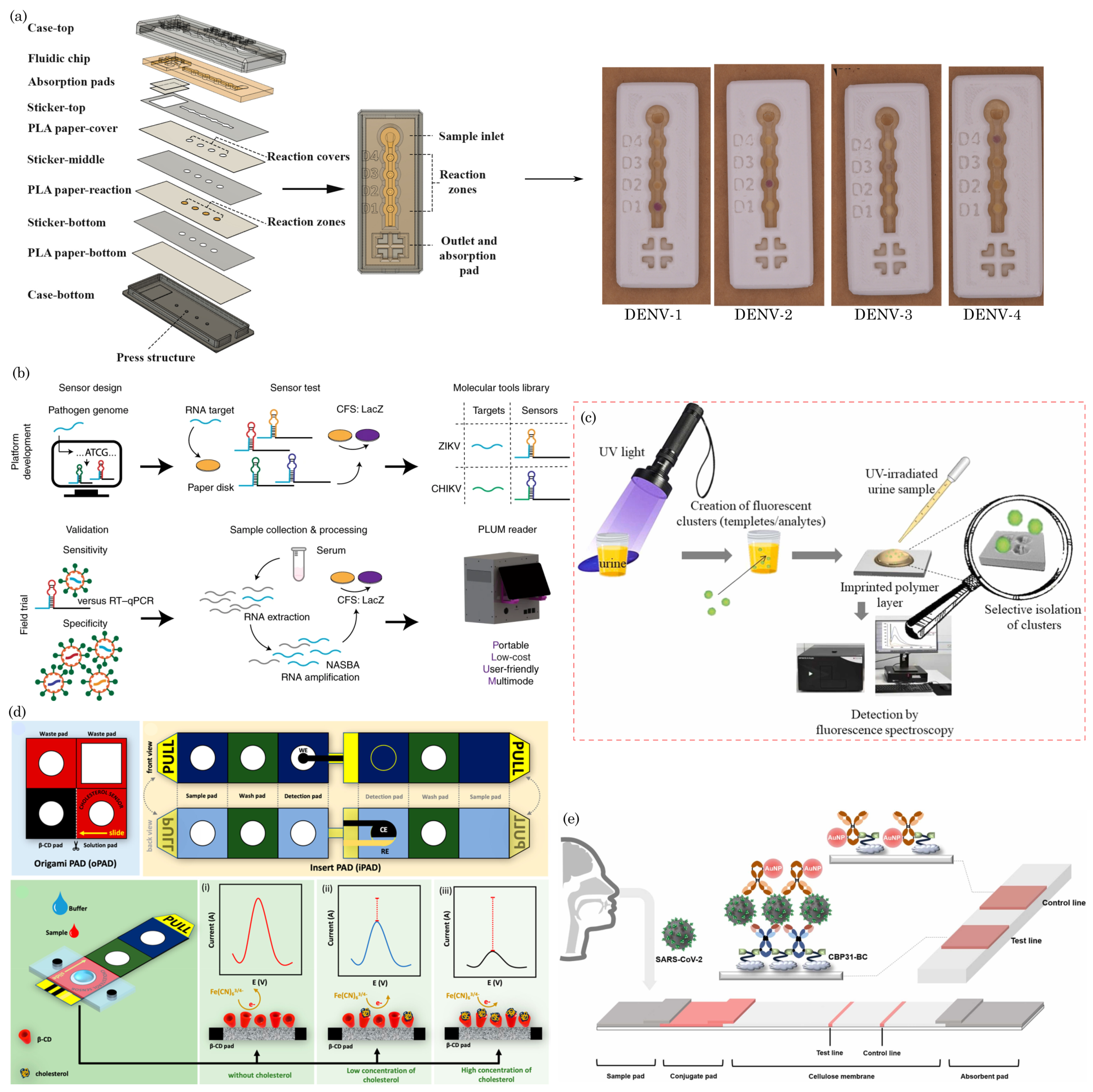
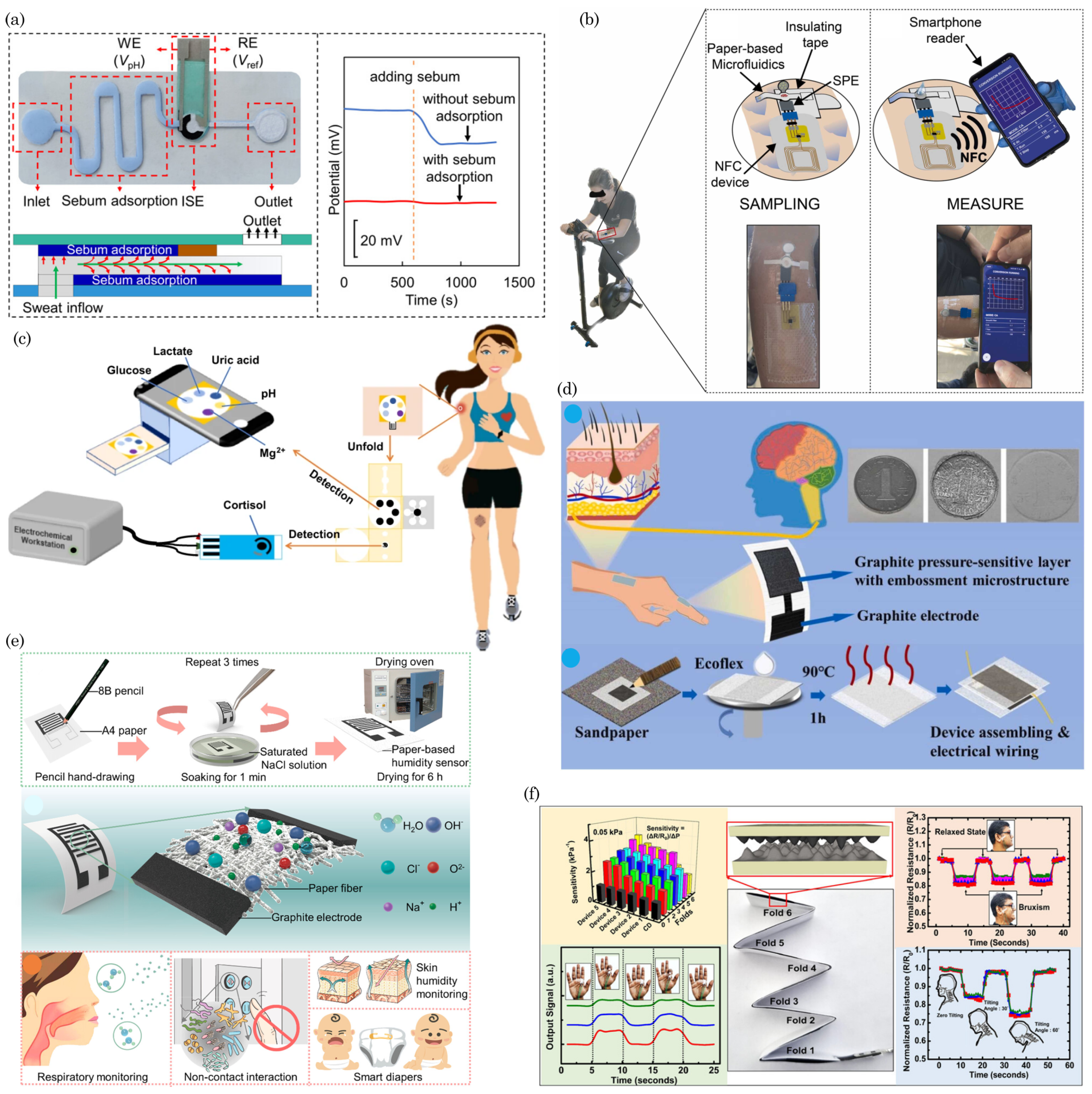
6.2. Wearable and Portable Health Monitoring Devices
7. Environmental Monitoring/Sensing
7.1. Detection of Soil Contaminants
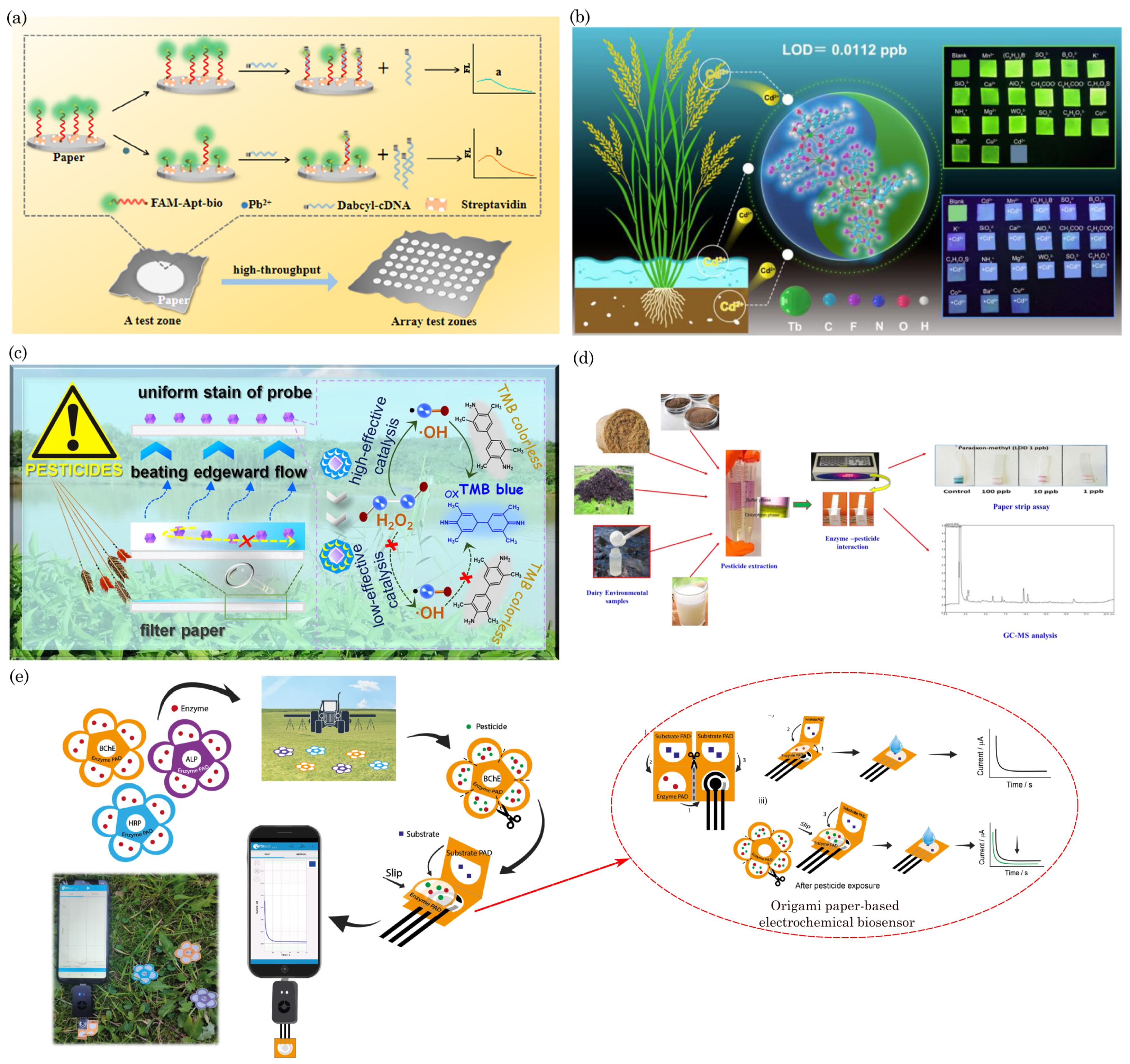
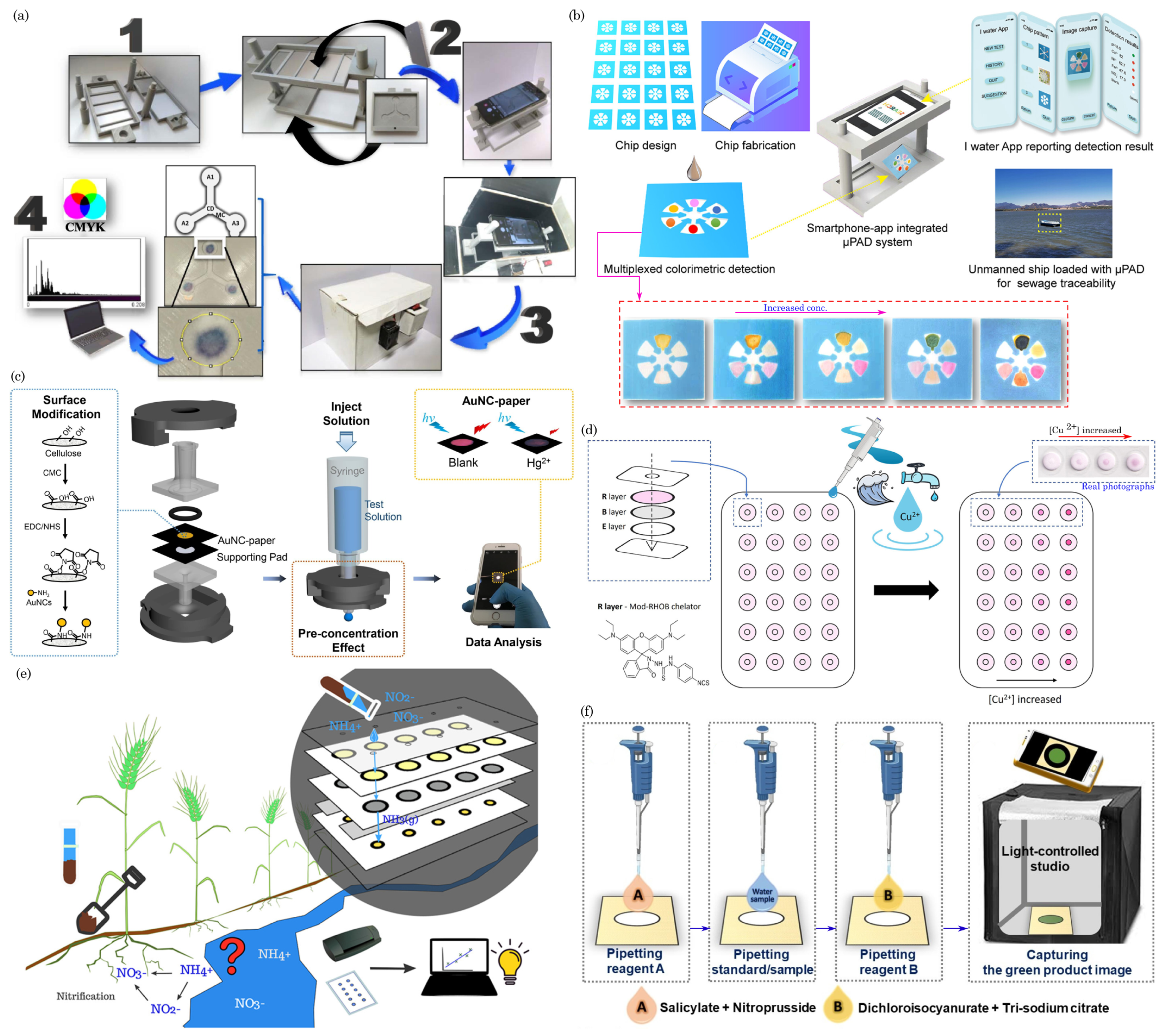
7.2. Water Quality Monitoring
7.3. Air Quality Monitoring/Gas Sensing
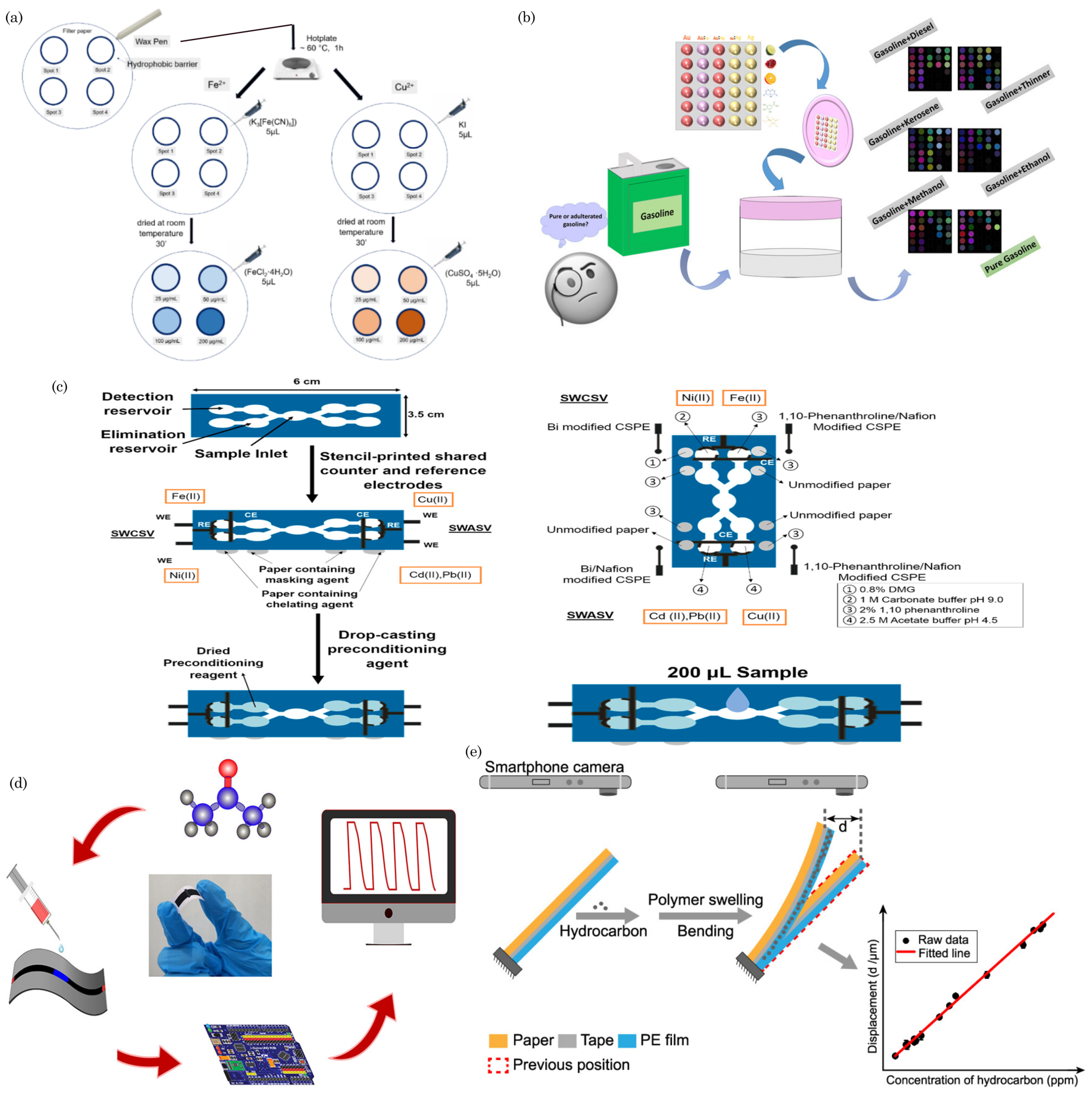
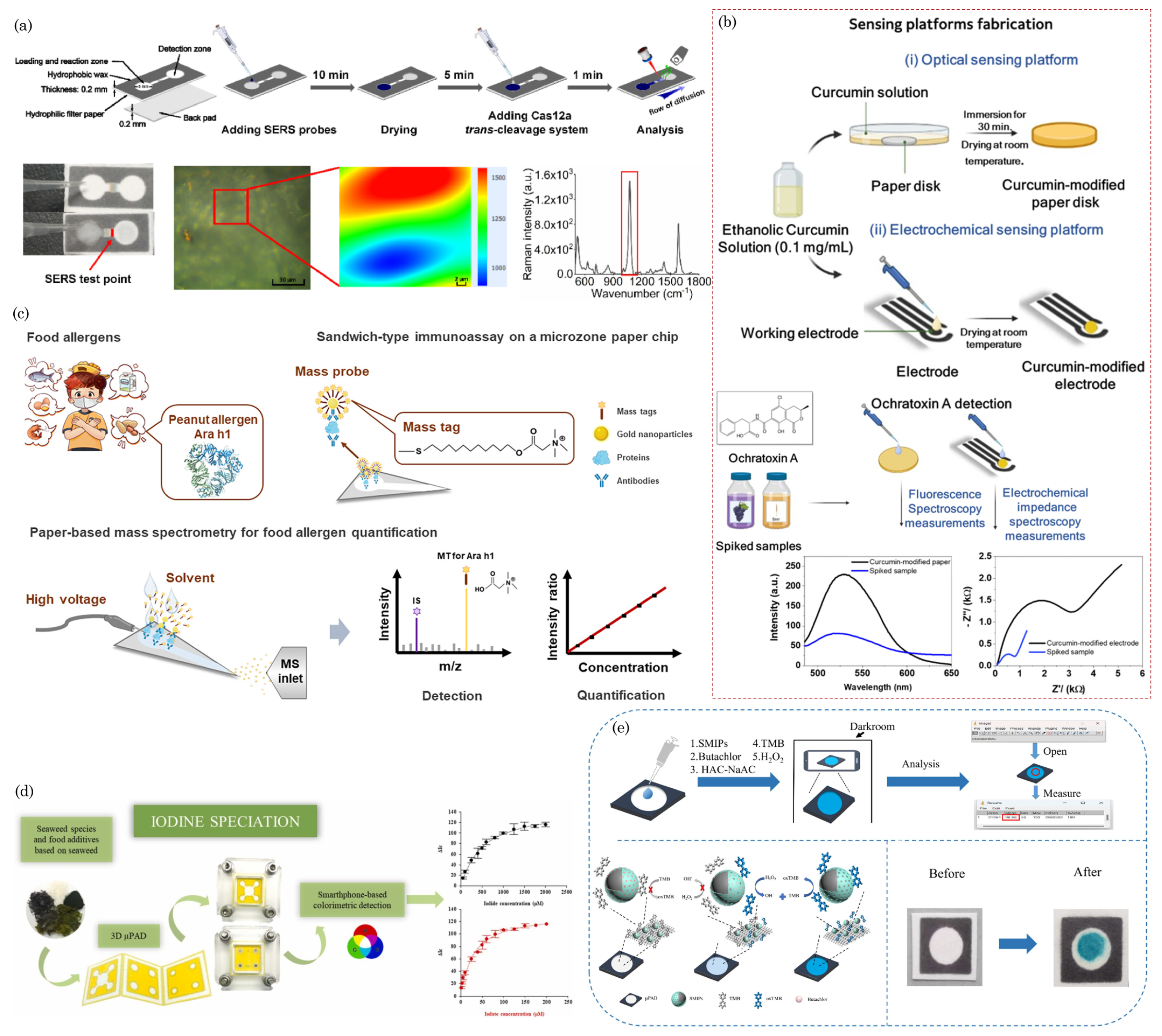
8. Food Safety
| Analytes | paper types | Paper-assay types | sening mechanisms | Ref. |
|---|---|---|---|---|
| Diagnostic assays for infectious diseases | ||||
| Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, | ||
| Wearable and portable health monitoring | ||||
| Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, | ||
| Soil contaminant detections | ||||
| Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, | ||
| Water Quality Monitoring | ||||
| Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, | ||
| Air Pollution Monitoring | ||||
| Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, | ||
| Food safety | ||||
| Milk, | Whatman filter paper Grade1-4, NC membrane | Dipsticks, LFAs, PADs | Colorimetric, enzyme-linked immunosorbent assay (ELISA) | [238] |
9. Biodegradability and Sustainability
9.1. Environmental Impact of Traditional Microfluidic Devices
9.2. Advantages of Biodegradable Paper Microfluidics
10. Challenges and Future Perspectives
10.1. Current Challenges in Paper Microfluidics
10.2. Prospects and Potential Innovations
11. Conclusion
Data Availability Statement
Conflicts of Interest
References
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex. Transm. Dis. 2006, 82, v1–v6. [CrossRef]
- Kosack, C.S.; Page, A.L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 2017, 95, 639–645. [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [CrossRef]
- Vicente, A.T.; Araújo, A.; Gaspar, D.; Santos, L.; Marques, A.C.; Mendes, M.J.; Pereira, L.; Fortunato, E.; Martins, R. Optoelectronics and bio devices on paper powered by solar cells. In Nanostructured solar cells; IntechOpen, 2017. [CrossRef]
- Nontawong, N.; Ngaosri, P.; Chunta, S.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A.; Amatatongchai, M. Smart sensor for assessment of oxidative/nitrative stress biomarkers using a dual-imprinted electrochemical paper-based analytical device. Anal. Chim. Acta 2022, 1191, 339363. [CrossRef]
- Patella, B.; Parisi, A.; Moukri, N.; Gitto, F.; Busacca, A.; Aiello, G.; Russo, M.; O’Riordan, A.; Inguanta, R. Phosphate ions detection by using an electrochemical sensor based on laser-scribed graphene oxide on paper. Electrochim. Acta 2023, 461, 142600. [CrossRef]
- Lu, Y.; Shi, W.; Qin, J.; Lin, B. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal. Chem. 2010, 82, 329–335. [CrossRef]
- Tang, R.H.; Li, M.; Liu, L.N.; Zhang, S.F.; Alam, N.; You, M.; Ni, Y.H.; Li, Z.D. Chitosan-modified nitrocellulose membrane for paper-based point-of-care testing. Cellulose 2020, 27, 3835–3846. [CrossRef]
- Tang, R.; Xie, M.Y.; Li, M.; Cao, L.; Feng, S.; Li, Z.; Xu, F. Nitrocellulose membrane for paper-based biosensor. Appl. Mater. Today 2022, 26, 101305. [CrossRef]
- Tang, R.; Xie, M.; Yan, X.; Qian, L.; Giesy, J.P.; Xie, Y. A nitrocellulose/cotton fiber hybrid composite membrane for paper-based biosensor. Cellulose 2023, 30, 6457–6469. [CrossRef]
- Boonyasit, Y.; Chailapakul, O.; Laiwattanapaisal, W. A multiplexed three-dimensional paper-based electrochemical impedance device for simultaneous label-free affinity sensing of total and glycated haemoglobin: The potential of using a specific single-frequency value for analysis. Anal. Chim. Acta 2016, 936, 1–11. [CrossRef]
- Boonyasit, Y.; Chailapakul, O.; Laiwattanapaisal, W. A folding affinity paper-based electrochemical impedance device for cardiovascular risk assessment. Biosens. Bioelectron. 2019, 130, 389–396. [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [CrossRef]
- Mondal, B.P.; Das, S.; Ranjan, P.; Datta, A. Optimizing Output Performances in Stationery Papers–Based Hybrid Inorganic–Organic Flexible Thermoelectric Generators. physica status solidi (a) 2023, 220, 2300228. [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [CrossRef]
- Smith, S.; Madzivhandila, P.; Ntuli, L.; Bezuidenhout, P.; Zheng, H.; Land, K. Printed paper–based electrochemical sensors for low-cost point-of-need applications. Electrocatalysis 2019, 10, 342–351. [CrossRef]
- Lim, W.Y.; Goh, C.H.; Yap, K.Z.; Ramakrishnan, N. One-step fabrication of paper-based inkjet-printed graphene for breath monitor sensors. Biosensors 2023, 13, 209. [CrossRef]
- Camargo, J.R.; Andreotti, I.A.; Kalinke, C.; Henrique, J.M.; Bonacin, J.A.; Janegitz, B.C. Waterproof paper as a new substrate to construct a disposable sensor for the electrochemical determination of paracetamol and melatonin. Talanta 2020, 208, 120458. [CrossRef]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.Y.; Kameoka, J. A low-cost paper glucose sensor with molecularly imprinted polyaniline electrode. Sensors 2020, 20, 1098. [CrossRef]
- Wu, H.; Chiang, S.W.; Lin, W.; Yang, C.; Li, Z.; Liu, J.; Cui, X.; Kang, F.; Wong, C.P. Towards practical application of paper based printed circuits: capillarity effectively enhances conductivity of the thermoplastic electrically conductive adhesives. Sci. Rep. 2014, 4, 6275. [CrossRef]
- Martins, G.V.; Riveiro, A.; Chiussi, S.; Sales, M. Flexible sensing devices integrating molecularly-imprinted polymers for the detection of 3-nitrotyrosine biomarker. Biosens. Bioelectron. : X 2022, 10, 100107. [CrossRef]
- Qi, J.; Li, B.; Zhou, N.; Wang, X.; Deng, D.; Luo, L.; Chen, L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533. [CrossRef]
- Chen, I.H.; You, M.W.; Tsai, J.H.; Chang, J.H.; Cheng, I.C.; Hsu, C.C.; Luo, S.C.; Chen, C.F.; Chen, J.Z. Feasibility study of dielectric barrier discharge jet-patterned perfluorodecyltrichlorosilane-coated paper for biochemical diagnosis. ECS J. Solid State Sci. Technol. 2021, 10, 037005. [CrossRef]
- Cao, C.X.; Yuan, J.; Cheng, J.P.; Han, B.H. Synthesis of porous polymer/tissue paper hybrid membranes for switchable oil/water separation. Sci. Rep. 2017, 7, 3101. [CrossRef]
- Arena, A.; Donato, N.; Saitta, G.; Bonavita, A.; Rizzo, G.; Neri, G. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens. Actuators B Chem. 2010, 145, 488–494. [CrossRef]
- Jiang, L.; Nelson, G.W.; Kim, H.; Sim, I.; Han, S.O.; Foord, J.S. Cellulose-derived supercapacitors from the carbonisation of filter paper. ChemistryOpen 2015, 4, 586–589. [CrossRef]
- Lin, D.; Li, B.; Fu, L.; Qi, J.; Xia, C.; Zhang, Y.; Chen, J.; Choo, J.; Chen, L. A novel polymer-based nitrocellulose platform for implementing a multiplexed microfluidic paper-based enzyme-linked immunosorbent assay. Microsyst. Nanoeng. 2022, 8, 53. [CrossRef]
- Jabar, A.W.; AL-Bawi, Z.F.; Faris, R.A.; Wahhab, H.K. Plasmonic Nanoparticles Decorated Salty Paper Based on SERS Platform for Diagnostic low-Level Contamination: Lab on Paper. Iraqi J. Laser 2019, 18, 55–61.
- Fernandes, A.R.; Bernardo, R.A.; Carvalho, T.C.d.; Vaz, B.G.; Chaves, A.R. Graphene oxides coated paper as a substrate to paper spray ionization mass spectrometry for creatinine determination in urine samples. J. Braz. Chem. Soc. 2019, 30, 1074–1081. [CrossRef]
- Arahman, N.; Fahrina, A.; Amalia, S.; Sunarya, R.; Mulyati, S. Effect of PVP on the characteristic of modified membranes made from waste PET bottles for humic acid removal. F1000Research 2017, 6, 668. [CrossRef]
- Van der Westhuizen, J.; Du Plessis, J.P. An attempt to quantify fibre bed permeability utilizing the phase average Navier-Stokes equation. Compos. Part A Appl. Sci. Manuf. 1996, 27, 263–269. [CrossRef]
- Nabovati, A.; Llewellin, E.W.; Sousa, A.C. A general model for the permeability of fibrous porous media based on fluid flow simulations using the lattice Boltzmann method. Compos. Part A Appl. Sci. Manuf. 2009, 40, 860–869. [CrossRef]
- Choi, J.R.; Liu, Z.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Yang, H.; Qu, Z.; others. Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing. Anal. Chem. 2016, 88, 6254–6264. [CrossRef]
- Parolo, C.; Medina-Sánchez, M.; De La Escosura-Muñiz, A.; Merkoçi, A. Simple paper architecture modifications lead to enhanced sensitivity in nanoparticle based lateral flow immunoassays. Lab Chip 2013, 13, 386–390. [CrossRef]
- Garnier, G.; Then, W.L. Paper microfluidics: applications and perspectives. Advances in Pulp and Paper Research, Cambridge 2013: Trans. of the 15th Fund. Res. Symp. Cambridge, 2013, 2013, pp. 541–583. [CrossRef]
- Hong, S.; Kim, W. Dynamics of water imbibition through paper channels with wax boundaries. Microfluid. Nanofluid. 2015, 19, 845–853. [CrossRef]
- Conrath, M.; Fries, N.; Zhang, M.; Dreyer, M.E. Radial capillary transport from an infinite reservoir. Transp. Porous Med. 2010, 84, 109–132. [CrossRef]
- Mendez, S.; Fenton, E.M.; Gallegos, G.R.; Petsev, D.N.; Sibbett, S.S.; Stone, H.A.; Zhang, Y.; López, G.P. Imbibition in porous membranes of complex shape: quasi-stationary flow in thin rectangular segments. Langmuir 2010, 26, 1380–1385. [CrossRef]
- Wang, X.; Hagen, J.A.; Papautsky, I. Paper pump for passive and programmable transport. Biomicrofluidics 2013, 7, 014107. [CrossRef]
- Shou, D.; Ye, L.; Fan, J.; Fu, K.; Mei, M.; Wang, H.; Chen, Q. Geometry-induced asymmetric capillary flow. Langmuir 2014, 30, 5448–5454. [CrossRef]
- Elizalde, E.; Urteaga, R.; Berli, C.L. Rational design of capillary-driven flows for paper-based microfluidics. Lab Chip 2015, 15, 2173–2180. [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S., Fluid Transport Mechanisms in Paper-Based Microfluidic Devices. In Paper Microfluidics Theory and Applications; Springer, Singapore, 2019; chapter 2, pp. 7–28.
- Zhong, Z.; Wu, R.; Wang, Z.; Tan, H. An investigation of paper based microfluidic devices for size based separation and extraction applications. J. Chromatogr. B 2015, 1000, 41–48. [CrossRef]
- Garra, R.; Salusti, E. Application of the nonlocal Darcy law to the propagation of nonlinear thermoelastic waves in fluid saturated porous media. Physica D: Nonlinear Phenomena 2013, 250, 52–57.
- Mambatta, A.K.; Jayarajan, J.; Rashme, V.L.; Harini, S.; Menon, S.; Kuppusamy, J. Reliability of dipstick assay in predicting urinary tract infection. J. Family Med. Prim. Care 2015, 4, 265–268. [CrossRef]
- Dadzie, I.; Quansah, E.; Puopelle Dakorah, M.; Abiade, V.; Takyi-Amuah, E.; Adusei, R. The effectiveness of dipstick for the detection of urinary tract infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8642628. [CrossRef]
- Renault, C.; Anderson, M.J.; Crooks, R.M. Electrochemistry in hollow-channel paper analytical devices. J. Am. Chem. Soc. 2014, 136, 4616–4623. [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic paper-based analytical devices: from design to applications. Chem. Rev. 2021, 121, 11835–11885. [CrossRef]
- Peto, T.; Affron, D.; Afrough, B.; Agasu, A.; Ainsworth, M.; Allanson, A.; Allen, K.; Allen, C.; Archer, L.; Ashbridge, N.; others. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine 2021, 36, 100924. [CrossRef]
- Davies, B.; Araghi, M.; Moshe, M.; Gao, H.; Bennet, K.; Jenkins, J.; Atchison, C.; Darzi, A.; Ashby, D.; Riley, S.; others. Acceptability, usability, and performance of lateral flow immunoassay tests for severe acute respiratory syndrome coronavirus 2 antibodies: REACT-2 study of self-testing in nonhealthcare key workers. Open Forum Infect. Dis. Oxford University Press US, 2021, Vol. 8, p. ofab496. [CrossRef]
- Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Han, G.R.; Broto, M.; Estcourt, C.S.; others. Lateral flow test engineering and lessons learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31. [CrossRef]
- Mahmud, M.A.; Blondeel, E.J.; Kaddoura, M.; MacDonald, B.D. Features in microfluidic paper-based devices made by laser cutting: How small can they be? Micromachines 2018, 9, 220. [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angewandte Chemie 2007, 119, 1340–1342. [CrossRef]
- He, Y.; Gao, Q.; Wu, W.B.; Nie, J.; Fu, J.Z. 3D printed paper-based microfluidic analytical devices. Micromachines 2016, 7, 108. [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [CrossRef]
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z.; others. Low sample volume origami-paper-based graphene-modified aptasensors for label-free electrochemical detection of cancer biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [CrossRef]
- Gao, B.; Chi, J.; Liu, H.; Gu, Z. Vertical paper analytical devices fabricated using the principles of quilling and kirigami. Sci. Rep. 2017, 7, 7255. [CrossRef]
- Johnston, M. The Book of Paper Quilling: Techniques & Projects for Paper Filigree; Sterling Publishing Company, Inc., 1995.
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129. [CrossRef]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal. Chem. 2012, 84, 6331–6335. [CrossRef]
- Mahmud, M.A.; Blondeel, E.J.; Kaddoura, M.; MacDonald, B.D. Creating compact and microscale features in paper-based devices by laser cutting. Analyst 2016, 141, 6449–6454. [CrossRef]
- de Araujo, W.R.; Frasson, C.M.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R. Single-step reagentless laser scribing fabrication of electrochemical paper-based analytical devices. Angewandte Chemie 2017, 129, 15309–15313. [CrossRef]
- Theillet, G.; Rubens, A.; Foucault, F.; Dalbon, P.; Rozand, C.; Leparc-Goffart, I.; Bedin, F. Laser-cut paper-based device for the detection of dengue non-structural NS1 protein and specific IgM in human samples. Arch. Virol. 2018, 163, 1757–1767. [CrossRef]
- Zhang, R.; Cai, S.; Wu, Q.; Zhu, Y.; Yin, X.; Xu, Y.; Yang, Y.; Chang, H. Laser cutting assisted fabrication of assembled solid-state supercapacitors based on polypyrrole coated paper. J. Electroanal. Chem. 2022, 919, 116522. [CrossRef]
- Li, X.; Su, D.; Gu, Y.; Zhang, J.; Li, S.; Xiao, Y.; He, J.; Wang, W.; Li, D. Laser fabrication of epidermal paper-based graphene sensors. Appl. Mater. Today 2024, 36, 102051. [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [CrossRef]
- Kakoti, A.; Siddiqui, M.F.; Goswami, P. A low cost design and fabrication method for developing a leak proof paper based microfluidic device with customized test zone. Biomicrofluidics 2015, 9, 026502. [CrossRef]
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [CrossRef]
- Park, C.; Han, Y.D.; Kim, H.V.; Lee, J.; Yoon, H.C.; Park, S. Double-sided 3D printing on paper towards mass production of three-dimensional paper-based microfluidic analytical devices (3D-μPADs). Lab Chip 2018, 18, 1533–1538. [CrossRef]
- Fu, X.; Xia, B.; Ji, B.; Lei, S.; Zhou, Y. Flow controllable three-dimensional paper-based microfluidic analytical devices fabricated by 3D printing technology. Anal. Chim. Acta 2019, 1065, 64–70. [CrossRef]
- He, Y.; Wu, W.b.; Fu, J.z. Rapid fabrication of paper-based microfluidic analytical devices with desktop stereolithography 3D printer. RSC Adv. 2015, 5, 2694–2701. [CrossRef]
- Puneeth, S.; Salve, M.; Akshatha, R.; Goel, S. Realization of microfluidic paper-based analytical devices using a 3-D printer: characterization and optimization. IEEE T. Device Mat. Re. 2019, 19, 529–536. [CrossRef]
- Chiang, C.K.; Kurniawan, A.; Kao, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [CrossRef]
- Silva-Neto, H.A.; Duarte-Junior, G.F.; Rocha, D.S.; Bedioui, F.; Varenne, A.; Coltro, W.K. Recycling 3D Printed Residues for the Development of Disposable Paper-Based Electrochemical Sensors. ACS Appl. Mater. Interfaces 2023, 15, 14111–14121. [CrossRef]
- Zaki, M.F.; Wu, Y.H.; Chen, P.C.; Chen, P.S. Determination of psychoactive substances in one microliter plasma using a novel 3D printing microfluidic paper-based column coupled to liquid chromatography-mass spectrometry. Sens. Actuators B: Chem. 2023, 393, 134243. [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82. [CrossRef]
- Sameenoi, Y.; Nongkai, P.N.; Nouanthavong, S.; Henry, C.S.; Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 2014, 139, 6580–6588. [CrossRef]
- Lamas-Ardisana, P.J.; Casuso, P.; Fernandez-Gauna, I.; Martínez-Paredes, G.; Jubete, E.; Añorga, L.; Cabañero, G.; Grande, H.J. Disposable electrochemical paper-based devices fully fabricated by screen-printing technique. Electrochem. commun. 2017, 75, 25–28. [CrossRef]
- Tasaengtong, B.; Sameenoi, Y. A one-step polymer screen-printing method for fabrication of microfluidic cloth-based analytical devices. Microchem. J. 2020, 158, 105078. [CrossRef]
- Mettakoonpitak, J.; Khongsoun, K.; Wongwan, N.; Kaewbutdee, S.; Siripinyanond, A.; Kuharuk, A.; Henry, C.S. Simple biodegradable plastic screen-printing for microfluidic paper-based analytical devices. Sens. Actuators B: Chem. 2021, 331, 129463. [CrossRef]
- Xiong, C.; Li, M.; Han, Q.; Zhao, W.; Dai, L.; Ni, Y. Screen printing fabricating patterned and customized full paper-based energy storage devices with excellent photothermal, self-healing, high energy density and good electromagnetic shielding performances. J. Mater. Sci. Technol. 2022, 97, 190–200. [CrossRef]
- Oliveira, A.E.; Pereira, A.C.; de Resende, M.A. Fabrication of Low-cost Screen-printed Electrode in Paper Using Conductive Inks of Graphite and Silver/Silver Chloride. Electroanalysis 2023, 35, e202200093. [CrossRef]
- Liu, J.; Kong, X.; Wang, H.; Zhang, Y.; Fan, Y. Roll-to-roll wax transfer for rapid and batch fabrication of paper-based microfluidics. Microfluidics and Nanofluidics 2020, 24, 6. [CrossRef]
- Walia, S.; Bhatnagar, I.; Liu, J.; Mitra, S.K.; Asthana, A. A novel method for fabrication of paper-based microfluidic devices using BSA-ink. Int. J. Biol. Macromol. 2021, 193, 1617–1622. [CrossRef]
- Espinosa, A.; Diaz, J.; Vazquez, E.; Acosta, L.; Santiago, A.; Cunci, L. Fabrication of paper-based microfluidic devices using a 3D printer and a commercially-available wax filament. Talanta open 2022, 6, 100142. [CrossRef]
- Tran, B.T.; Rijiravanich, P.; Puttaraksa, N.; Surareungchai, W. Wax gates in laminated microfluidic paper-based immunosensors. Microchem. J. 2022, 178, 107343. [CrossRef]
- Brito-Pereira, R.; Ribeiro, C.; Costa, P.; Correia, V.; Cardoso, V.F.; Lanceros-Mendez, S. Graphene Based Printable Conductive Wax for Low-Power Thermal Actuation in Microfluidic Paper-Based Analytical Devices. Adv. Mater. Technol. 2023, 8, 2300051. [CrossRef]
- Monju, T.; Hirakawa, M.; Kuboyama, S.; Saiki, R.; Ishida, A. A fabrication technique for paper-based analytical devices via two-sided patterning with thermal-transfer printer and laminator. Sens. Actuators B: Chem. 2023, 375, 132886. [CrossRef]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [CrossRef]
- Paschoalino, W.J.; Kogikoski Jr, S.; Barragan, J.T.; Giarola, J.F.; Cantelli, L.; Rabelo, T.M.; Pessanha, T.M.; Kubota, L.T. Emerging considerations for the future development of electrochemical paper-based analytical devices. ChemElectroChem 2019, 6, 10–30. [CrossRef]
- Ahmad, M.; Costa Angeli, M.A.; Ibba, P.; Vasquez, S.; Shkodra, B.; Lugli, P.; Petti, L. Paper-Based Printed Antenna: Investigation of Process-Induced and Climatic-Induced Performance Variability. Adv. Eng. Mater. 2023, 25, 2201703. [CrossRef]
- Galliani, M.; Ferrari, L.M.; Bouet, G.; Eglin, D.; Ismailova, E. Tailoring inkjet-printed PEDOT: PSS composition toward green, wearable device fabrication. APL Bioeng. 2023, 7, 016101. [CrossRef]
- Le, N.N.; Dinh, D.M.T.; Lam, P.H.; Le, A.V.T.; Le, M.T.; Pham, M.D.; Dang, D.M.T.; Dang, C.M. Fabrication of microfluidic paper-based channels by inkjet printing process for analytical applications. Adv. Nat. Sci: Nanosci. Nanotechnol. 2023, 14, 015015. [CrossRef]
- Ray, A.; Mohan, J.M.; Amreen, K.; Dubey, S.K.; Javed, A.; Ponnalagu, R.; Goel, S. Ink-jet-printed CuO nanoparticle-enhanced miniaturized paper-based electrochemical platform for hypochlorite sensing. Appl. Nanosci. 2023, 13, 1855–1861. [CrossRef]
- Silvestri, A.; Vázquez-Díaz, S.; Misia, G.; Poletti, F.; López-Domene, R.; Pavlov, V.; Zanardi, C.; Cortajarena, A.L.; Prato, M. An Electroactive and Self-Assembling Bio-Ink, based on Protein-Stabilized Nanoclusters and Graphene, for the Manufacture of Fully Inkjet-Printed Paper-Based Analytical Devices. Small 2023, 19, 2300163. [CrossRef]
- de Tarso Garcia, P.; Cardoso, T.M.G.; Garcia, C.D.; Carrilho, E.; Coltro, W.K.T. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. Rsc Adv. 2014, 4, 37637–37644. [CrossRef]
- He, Y.; Wu, Y.; Xiao, X.; Fu, J.; Xue, G. A low-cost and rapid microfluidic paper-based analytical device fabrication method: Flash foam stamp lithography. RSC Adv. 2014, 4, 63860–63865. [CrossRef]
- Guan, Y.; Sun, B. Detection and extraction of heavy metal ions using paper-based analytical devices fabricated via atom stamp printing. Microsyst. Nanoeng. 2020, 6, 14. [CrossRef]
- de Araujo, T.A.; de Moraes, N.C.; Petroni, J.M.; Ferreira, V.S.; Lucca, B.G. Simple, fast, and instrumentless fabrication of paper analytical devices by novel contact stamping method based on acrylic varnish and 3D printing. Microchim. Acta 2021, 188, 437. [CrossRef]
- Kim, M.; Lee, C.; Jeon, K.; Lee, J.Y.; Kim, Y.J.; Lee, J.G.; Kim, H.; Cho, M.; Kim, D.N. Harnessing a paper-folding mechanism for reconfigurable DNA origami. Nature 2023, 619, 78–86. [CrossRef]
- Chen, C.A.; Yeh, W.S.; Tsai, T.T.; Chen, C.F.; others. Three-dimensional origami paper-based device for portable immunoassay applications. Lab Chip 2019, 19, 598–607. [CrossRef]
- Tian, T.; An, Y.; Wu, Y.; Song, Y.; Zhu, Z.; Yang, C. Integrated distance-based origami paper analytical device for one-step visualized analysis. ACS Appl. Mater. Interfaces 2017, 9, 30480–30487. [CrossRef]
- Ding, J.; Li, B.; Chen, L.; Qin, W. A three-dimensional Origami paper-based device for potentiometric biosensing. Angew. Chem. Int. Ed. 2016, 55, 13033–13037. [CrossRef]
- Scida, K.; Li, B.; Ellington, A.D.; Crooks, R.M. DNA detection using origami paper analytical devices. Anal. Chem. 2013, 85, 9713–9720.
- Liu, H.; Xiang, Y.; Lu, Y.; Crooks, R.M. Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angewandte Chemie 2012, 124, 7031–7034. [CrossRef]
- Liu, X.; Sun, J.; Tong, Y.; Zhang, M.; Wang, X.; Guo, S.; Han, X.; Zhao, X.; Tang, Q.; Liu, Y. Calligraphy and Kirigami/Origami-Inspired All-Paper Touch–Temperature Sensor with Stimulus Discriminability. ACS Appl. Mater. Interfaces 2023, 15, 1726–1735. [CrossRef]
- Chen, X.; Li, Y.; Wang, X.; Yu, H. Origami paper-based stretchable humidity sensor for textile-attachable wearable electronics. ACS Appl. Mater. Interfaces 2022, 14, 36227–36237. [CrossRef]
- Kumar, S.; Bhushan, P.; Krishna, V.; Bhattacharya, S. Tapered lateral flow immunoassay based point-of-care diagnostic device for ultrasensitive colorimetric detection of dengue NS1. Biomicrofluidics 2018, 12, 034104. [CrossRef]
- Assi, N.; Rypar, T.; Macka, M.; Adam, V.; Vaculovicova, M. Microfluidic paper-based fluorescence sensor for L-homocysteine using a molecularly imprinted polymer and in situ-formed fluorescent quantum dots. Talanta 2023, 255, 124185. [CrossRef]
- Goncharov, A.; Joung, H.A.; Ghosh, R.; Han, G.R.; Ballard, Z.S.; Maloney, Q.; Bell, A.; Aung, C.T.Z.; Garner, O.B.; Carlo, D.D.; others. Deep Learning-Enabled Multiplexed Point-of-Care Sensor using a Paper-Based Fluorescence Vertical Flow Assay. Small 2023, 19, 2300617. [CrossRef]
- Ren, Y.; Cao, L.; Zhang, X.; Jiao, R.; Ou, D.; Wang, Y.; Zhang, D.; Shen, Y.; Ling, N.; Ye, Y. A novel fluorescence resonance energy transfer (FRET)-based paper sensor with smartphone for quantitative detection of Vibrio parahaemolyticus. Food Control 2023, 145, 109412. [CrossRef]
- Tong, X.; Cai, G.; Xie, L.; Wang, T.; Zhu, Y.; Peng, Y.; Tong, C.; Shi, S.; Guo, Y. Threaded 3D microfluidic paper analytical device-based ratiometric fluorescent sensor for background-free and visual detection of organophosphorus pesticides. Biosens. Bioelectron. 2023, 222, 114981. [CrossRef]
- Yuan, M.; Li, C.; Zheng, Y.; Cao, H.; Ye, T.; Wu, X.; Hao, L.; Yin, F.; Yu, J.; Xu, F. A portable multi-channel fluorescent paper-based microfluidic chip based on smartphone imaging for simultaneous detection of four heavy metals. Talanta 2024, 266, 125112. [CrossRef]
- Liu, W.; Guo, Y.; Li, H.; Zhao, M.; Lai, Z.; Li, B. A paper-based chemiluminescence device for the determination of ofloxacin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 137, 1298–1303. [CrossRef]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 173, 112817. [CrossRef]
- Al Yahyai, I.; Hassanzadeh, J.; Al-Lawati, H.A. A novel and selective multi-emission chemiluminescence system for the quantification of deltamethrin in food samples. Sens. Actuators B: Chem. 2021, 327, 128927. [CrossRef]
- Delaney, J.L.; Hogan, C.F.; Tian, J.; Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011, 83, 1300–1306. [CrossRef]
- Liu, H.; Zhou, X.; Liu, W.; Yang, X.; Xing, D. Paper-based bipolar electrode electrochemiluminescence switch for label-free and sensitive genetic detection of pathogenic bacteria. Anal. Chem. 2016, 88, 10191–10197. [CrossRef]
- Valentine, C.J.; Takagishi, K.; Umezu, S.; Daly, R.; De Volder, M. Paper-based electrochemical sensors using paper as a scaffold to create porous carbon nanotube electrodes. ACS Appl. Mater. Interfaces 2020, 12, 30680–30685. [CrossRef]
- He, Z.; Huang, J.; Shen, W.; Lei, X.; Zhang, Y.; Zhu, L.; Shen, X.; Zhang, D.; Yu, D.; Zhou, M. A Paper-Based Fluorescent Sensor for Rapid Early Screening of Oral Squamous Cell Carcinoma. ACS Appl. Mater. Interfaces 2023, 15, 24913–24922. [CrossRef]
- Li, Z.; Zhu, M.; Li, F.; Li, Z.; Zhao, A.; Haghighatbin, M.A.; Cui, H. Microfluidic paper chip based multicolor chemiluminescence sensing strategy for discrimination of antioxidants. Sens. Actuators B Chem. 2023, 393, 134166. [CrossRef]
- Wang, F.; Liu, Y.; Fu, C.; Li, N.; Du, M.; Zhang, L.; Ge, S.; Yu, J. Paper-based bipolar electrode electrochemiluminescence platform for detection of multiple miRNAs. Anal. Chem. 2020, 93, 1702–1708. [CrossRef]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; others. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [CrossRef]
- Li, J.; Macdonald, J. Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosens. Bioelectron. 2016, 83, 177–192. [CrossRef]
- Arias-Alpízar, K.; Sánchez-Cano, A.; Prat-Trunas, J.; de la Serna Serna, E.; Alonso, O.; Sulleiro, E.; Sánchez-Montalvá, A.; Diéguez, A.; Baldrich, E. Malaria quantitative POC testing using magnetic particles, a paper microfluidic device and a hand-held fluorescence reader. Biosens. Bioelectron. 2022, 215, 114513. [CrossRef]
- Prat-Trunas, J.; Arias-Alpizar, K.; Álvarez-Carulla, A.; Orio-Tejada, J.; Molina, I.; Sánchez-Montalvá, A.; Colomer-Farrarons, J.; Del Campo, F.; Miribel-Català, P.L.; Baldrich, E. Paper-based microfluidic electro-analytical device (PMED) for magneto-assay automation: Towards generic point-of-care diagnostic devices. Biosens. Bioelectron. 2024, 246, 115875. [CrossRef]
- Biswas, P.; Mukunthan Sulochana, G.N.; Banuprasad, T.N.; Goyal, P.; Modak, D.; Ghosh, A.K.; Chakraborty, S. All-Serotype Dengue Virus Detection through Multilayered Origami-Based Paper/Polymer Microfluidics. ACS Sens. 2022, 7, 3720–3729. [CrossRef]
- Le, T.N.; Hsiao, W.W.W.; Cheng, Y.Y.; Lee, C.C.; Huynh, T.T.; Pham, D.M.; Chen, M.; Jen, M.W.; Chang, H.C.; Chiang, W.H. Spin-Enhanced Lateral Flow Immunoassay for High-Sensitivity Detection of Nonstructural Protein NS1 Serotypes of the Dengue Virus. Anal. Chem. 2022, 94, 17819–17826. [CrossRef]
- Trakoolwilaiwan, T.; Takeuchi, Y.; Leung, T.S.; Sebek, M.; Storozhuk, L.; Nguyen, L.; Thanh, N.T.K.; others. Development of a thermochromic lateral flow assay to improve sensitivity for dengue virus serotype 2 NS1 detection. Nanoscale 2023, 15, 12915–12925. [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.Y. Simpler, faster, and sensitive Zika virus assay using smartphone detection of loop-mediated isothermal amplification on paper microfluidic chips. Sci. Rep. 2018, 8, 12438. [CrossRef]
- Seok, Y.; Batule, B.S.; Kim, M.G. Lab-on-paper for all-in-one molecular diagnostics (LAMDA) of zika, dengue, and chikungunya virus from human serum. Biosens. Bioelectron. 2020, 165, 112400. [CrossRef]
- Suvanasuthi, R.; Chimnaronk, S.; Promptmas, C. 3D printed hydrophobic barriers in a paper-based biosensor for point-of-care detection of dengue virus serotypes. Talanta 2022, 237, 122962. [CrossRef]
- Karlikow, M.; da Silva, S.J.R.; Guo, Y.; Cicek, S.; Krokovsky, L.; Homme, P.; Xiong, Y.; Xu, T.; Calderón-Peláez, M.A.; Camacho-Ortega, S.; others. Field validation of the performance of paper-based tests for the detection of the Zika and chikungunya viruses in serum samples. Nat. Biomed. Eng. 2022, 6, 246–256. [CrossRef]
- Gong, H.; Tang, L.; Chen, C.; Chen, F.; Cai, C. Portable paper-based molecularly imprinted sensor for visual real-time detection of influenza virus H5N1. Chem. Eng. J. 2023, 477, 146990. [CrossRef]
- Tavakoli, H.; Hirth, E.; Luo, M.; Timilsina, S.S.; Dou, M.; Dominguez, D.C.; Li, X. A microfluidic fully paper-based analytical device integrated with loop-mediated isothermal amplification and nano-biosensors for rapid, sensitive, and specific quantitative detection of infectious diseases. Lab Chip 2022, 22, 4693–4704. [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B.; others. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 2016, 16, 611–621. [CrossRef]
- Tang, R.; Yang, H.; Gong, Y.; You, M.; Liu, Z.; Choi, J.R.; Wen, T.; Qu, Z.; Mei, Q.; Xu, F. A fully disposable and integrated paper-based device for nucleic acid extraction, amplification and detection. Lab Chip 2017, 17, 1270–1279. [CrossRef]
- Natarajan, S.; Su, F.; Jayaraj, J.; Shah, M.I.I.; Huang, Y. A paper microfluidics-based fluorescent lateral flow immunoassay for point-of-care diagnostics of non-communicable diseases. Analyst 2019, 144, 6291–6303. [CrossRef]
- Shalaby, A.A.; Tsao, C.W.; Ishida, A.; Maeki, M.; Tokeshi, M. Microfluidic paper-based analytical devices for cancer diagnosis. Sens. Actuators B: Chem. 2023, 379, 133243. [CrossRef]
- Sana, T.; Sharma, P.; Khanuja, M.; Narang, J. Detection of Uterine Cancer Biomarker EGFR through an Aptasensor Utilizing a Carbon Electrode Modified with Silver Nanowires. Mater. Chem. Phys. 2024, 319, 129412. [CrossRef]
- Gutiérrez-Capitán, M.; Sanchís, A.; Carvalho, E.O.; Baldi, A.; Vilaplana, L.; Cardoso, V.F.; Calleja, Á.; Wei, M.; de la Rica, R.; Hoyo, J.; others. Engineering a Point-of-Care Paper-Microfluidic Electrochemical Device Applied to the Multiplexed Quantitative Detection of Biomarkers in Sputum. ACS Sens. 2023, 8, 3032–3042. [CrossRef]
- Rohrman, B.A.; Richards-Kortum, R.R. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip 2012, 12, 3082–3088. [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [CrossRef]
- Tsao, Y.T.; Yang, C.Y.; Wen, Y.C.; Chang, T.C.; Matsuura, K.; Chen, Y.; Cheng, C.M. Point-of-care semen analysis of patients with infertility via smartphone and colorimetric paper-based diagnostic device. Bioeng. Transl. Med. 2021, 6, e10176. [CrossRef]
- Sarabi, M.R.; Yigci, D.; Alseed, M.M.; Mathyk, B.A.; Ata, B.; Halicigil, C.; Tasoglu, S. Disposable paper-based microfluidics for fertility testing. Iscience 2022, 25, 104986. [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Development of a paper-based analytical device for colorimetric detection of uric acid using gold nanoparticles–graphene oxide (AuNPs–GO) conjugates. Anal. Methods 2016, 8, 6965–6973. [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Facile synthesis of Au@ Ag–hemin decorated reduced graphene oxide sheets: a novel peroxidase mimetic for ultrasensitive colorimetric detection of hydrogen peroxide and glucose. RSC Adv. 2017, 7, 37568–37577. [CrossRef]
- Pérez-Rodríguez, M.; del Pilar Cañizares-Macías, M. A prototype microfluidic paper-based chromatic device for simultaneous determination of copper (II) and zinc (II) in urine. Talanta Open 2023, 7, 100178. [CrossRef]
- Bezdekova, J.; Plevova, M.; Nejdl, L.; Macka, M.; Masarik, M.; Pacik, D.; Adam, V.; Vaculovicova, M. Prostate cancer diagnosed and staged using UV-irradiated urine samples and a paper-based analytical device. Sens. Actuators B Chem. 2024, 403, 135146. [CrossRef]
- Chaiyo, S.; Kunpatee, K.; Kalcher, K.; Yakoh, A.; Pungjunun, K. 3D Paper-Based Device Integrated with a Battery-Less NFC Potentiostat for Nonenzymatic Detection of Cholesterol. ACS Meas. Sci. Au 2024. [CrossRef]
- Ozer, T.; Henry, C.S. Paper-based analytical devices for virus detection: Recent strategies for current and future pandemics. TrAC, Trends Anal. Chem. 2021, 144, 116424. [CrossRef]
- Adrover-Jaume, C.; Alba-Patino, A.; Clemente, A.; Santopolo, G.; Vaquer, A.; Russell, S.M.; Baron, E.; Del Campo, M.D.M.G.; Ferrer, J.M.; Berman-Riu, M.; others. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B: Chem. 2021, 330, 129333. [CrossRef]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [CrossRef]
- Zhang, T.; Deng, R.; Wang, Y.; Wu, C.; Zhang, K.; Wang, C.; Gong, N.; Ledesma-Amaro, R.; Teng, X.; Yang, C.; others. A paper-based assay for the colorimetric detection of SARS-CoV-2 variants at single-nucleotide resolution. Nat. Biomed. Eng. 2022, 6, 957–967. [CrossRef]
- Fabiani, L.; Mazzaracchio, V.; Moscone, D.; Fillo, S.; De Santis, R.; Monte, A.; Amatore, D.; Lista, F.; Arduini, F. based immunoassay based on 96-well wax-printed paper plate combined with magnetic beads and colorimetric smartphone-assisted measure for reliable detection of SARS-CoV-2 in saliva. Biosens. Bioelectron. 2022, 200, 113909. [CrossRef]
- Wang, Q.; Chen, Z.; Yang, H. Colorimetric detection of SARS-CoV-2 variants with paper-based analytical devices. MedComm–Biomaterials and Applications 2023, 2, e35. [CrossRef]
- Sen, A.; Masetty, M.; Weerakoon, S.; Morris, C.; Yadav, J.S.; Apewokin, S.; Trannguyen, J.; Broom, M.; Priye, A. Paper-based loop-mediated isothermal amplification and CRISPR integrated platform for on-site nucleic acid testing of pathogens. Biosens. Bioelectron. 2024, 257, 116292. [CrossRef]
- Lee, A.S.; Kim, S.M.; Kim, K.R.; Park, C.; Lee, D.G.; Heo, H.R.; Cha, H.J.; Kim, C.S. A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2. Sens. Actuators B: Chem. 2023, 379, 133245. [CrossRef]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 2017, 6, 1700024. [CrossRef]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-based wearable electronics. Iscience 2021, 24, 102736. [CrossRef]
- Deroco, P.B.; Wachholz Junior, D.; Kubota, L.T. Paper-based Wearable Electrochemical Sensors: A New Generation of Analytical Devices. Electroanalysis 2023, 35, e202200177. [CrossRef]
- Bandodkar, A.J.; Hung, V.W.; Jia, W.; Valdés-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. [CrossRef]
- Nassar, J.M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable nonfunctionalized paper-based ultralow-cost wearable health monitoring system. Adv. Mater. Technol. 2017, 2, 1600228. [CrossRef]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [CrossRef]
- Xu, Y.; Zhao, G.; Zhu, L.; Fei, Q.; Zhang, Z.; Chen, Z.; An, F.; Chen, Y.; Ling, Y.; Guo, P.; others. Pencil–paper on-skin electronics. Proc. Natl. Acad. Sci. 2020, 117, 18292–18301. [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [CrossRef]
- Yang, M.; Sun, N.; Lai, X.; Wu, J.; Wu, L.; Zhao, X.; Feng, L. Paper-Based Sandwich-Structured Wearable Sensor with Sebum Filtering for Continuous Detection of Sweat pH. ACS Sens. 2023, 8, 176–186. [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G.; others. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B: Chem. 2023, 379, 133258. [CrossRef]
- Cheng, Y.; Feng, S.; Ning, Q.; Li, T.; Xu, H.; Sun, Q.; Cui, D.; Wang, K. Dual-signal readout paper-based wearable biosensor with a 3D origami structure for multiplexed analyte detection in sweat. Microsyst. Nanoeng. 2023, 9, 36. [CrossRef]
- Lai, Q.T.; Liang, H.Q.; Tang, X.G.; Zhang, D.; Roy, V.A.; Sun, Q.J. Printing Paper-Derived Ultralight and Highly Sensitive E-Skin for Health Monitoring and Information Encryption. J. Alloys Compd. 2024, 976, 173411. [CrossRef]
- Niu, G.; Wang, Z.; Xue, Y.; Yan, J.; Dutta, A.; Chen, X.; Wang, Y.; Liu, C.; Du, S.; Guo, L.; others. Pencil-on-paper humidity sensor treated with NaCl solution for health monitoring and skin characterization. Nano Lett. 2023, 23, 1252–1260. [CrossRef]
- Karmakar, R.S.; Huang, J.F.; Chu, C.P.; Mai, M.H.; Chao, J.I.; Liao, Y.C.; Lu, Y.W. Origami-Inspired Conductive Paper-Based Folded Pressure Sensor with Interconnection Scaling at the Crease for Novel Wearable Applications. ACS Appl. Mater. Interfaces 2024, 16, 4231–4241. [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Analyst 2016, 141, 1874–1887. [CrossRef]
- Colozza, N.; Caratelli, V.; Moscone, D.; Arduini, F. Paper-based devices as new smart analytical tools for sustainable detection of environmental pollutants. Case Stud. Chem. Environ. Eng. 2021, 4, 100167. [CrossRef]
- Alahmad, W.; Cetinkaya, A.; Kaya, S.I.; Varanusupakul, P.; Ozkan, S.A. Electrochemical paper-based analytical devices for environmental analysis: Current trends and perspectives. Trends Environ. Anal. Chem. 2023, p. e00220. [CrossRef]
- Suo, Z.; Liang, R.; Liu, R.; Wei, M.; He, B.; Jiang, L.; Sun, X.; Jin, H. A convenient paper-based fluorescent aptasensor for high-throughput detection of Pb2+ in multiple real samples (water-soil-food). Anal. Chim. Acta 2023, 1239, 340714. [CrossRef]
- Yu, X.; Chang, W.; Zhang, H.; Cai, Z.; Yang, Y.; Zeng, C. Visual and Real-Time Monitoring of Cd2+ in Water, Rice, and Rice Soil with Test Paper Based on [2+ 2] Lanthanide Clusters. Inorg. Chem. 2023, 62, 6387–6396. [CrossRef]
- Wang, S.; Ge, L.; Li, L.; Yan, M.; Ge, S.; Yu, J. Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosens. Bioelectron. 2013, 50, 262–268. [CrossRef]
- Ma, Z.; Li, Y.; Lu, C.; Li, M. On-site Screening Method for Bioavailability Assessment of the Organophosphorus Pesticide, Methyl Parathion, and Its Primary Metabolite in Soils by Paper Strip Biosensor. J. Hazard. Mater. 2023, p. 131725. [CrossRef]
- Cioffi, A.; Mancini, M.; Gioia, V.; Cinti, S. Office paper-based electrochemical strips for organophosphorus pesticide monitoring in agricultural soil. Environ. Sci. Technol. 2021, 55, 8859–8865. [CrossRef]
- Zhang, X.; Wang, Z.; Huang, X.; Huang, Q.; Wen, Y.; Li, B.; Holmes, M.; Shi, J.; Zou, X. Uniform stain pattern of robust MOF-mediated probe for flexible paper-based colorimetric sensing toward environmental pesticide exposure. Chem. Eng. J. 2023, 451, 138928. [CrossRef]
- Ranveer, S.A.; Harshitha, C.; Dasriya, V.; Tehri, N.; Kumar, N.; Raghu, H. Assessment of developed paper strip based sensor with pesticide residues in different dairy environmental samples. Curr. Res. Food Sci. 2023, 6, 100416. [CrossRef]
- Caratelli, V.; Fegatelli, G.; Moscone, D.; Arduini, F. A paper-based electrochemical device for the detection of pesticides in aerosol phase inspired by nature: A flower-like origami biosensor for precision agriculture. Biosens. Bioelectron. 2022, 205, 114119. [CrossRef]
- Taudte, R.V.; Beavis, A.; Wilson-Wilde, L.; Roux, C.; Doble, P.; Blanes, L. A portable explosive detector based on fluorescence quenching of pyrene deposited on coloured wax-printed μPADs. Lab Chip 2013, 13, 4164–4172. [CrossRef]
- Wang, J.; Yang, L.; Liu, B.; Jiang, H.; Liu, R.; Yang, J.; Han, G.; Mei, Q.; Zhang, Z. Inkjet-printed silver nanoparticle paper detects airborne species from crystalline explosives and their ultratrace residues in open environment. Anal. Chem. 2014, 86, 3338–3345. [CrossRef]
- Ueland, M.; Blanes, L.; Taudte, R.V.; Stuart, B.H.; Cole, N.; Willis, P.; Roux, C.; Doble, P. Capillary-driven microfluidic paper-based analytical devices for Lab on a Chip screening of explosive residues in soil. J. Chromatogr. A 2016, 1436, 28–33. [CrossRef]
- de Araujo, W.R.; Cardoso, T.M.; da Rocha, R.G.; Santana, M.H.; Munoz, R.A.; Richter, E.M.; Paixão, T.R.; Coltro, W.K. Portable analytical platforms for forensic chemistry: a review. Anal. Chim. Acta 2018, 1034, 1–21. [CrossRef]
- Raucci, A.; Miglione, A.; Cimmino, W.; Cioffi, A.; Singh, S.; Spinelli, M.; Amoresano, A.; Musile, G.; Cinti, S. Technical Evaluation of a Paper-Based Electrochemical Strip to Measure Nitrite Ions in the Forensic Field. ACS Meas. Sci. Au 2023, 4, 136–143. [CrossRef]
- Hunter, P.R.; Zmirou-Navier, D.; Hartemann, P. Estimating the impact on health of poor reliability of drinking water interventions in developing countries. Science of the total environment 2009, 407, 2621–2624. [CrossRef]
- Adu-Manu, K.S.; Tapparello, C.; Heinzelman, W.; Katsriku, F.A.; Abdulai, J.D. Water quality monitoring using wireless sensor networks: Current trends and future research directions. ACM Trans. Sens. Networks 2017, 13, 1–41. [CrossRef]
- Vikesland, P.J. Nanosensors for water quality monitoring. Nature Nanotec. 2018, 13, 651–660. [CrossRef]
- Liu, F.; Nordin, A.; Li, F.; Voiculescu, I. A lab-on-chip cell-based biosensor for label-free sensing of water toxicants. Lab Chip 2014, 14, 1270–1280. [CrossRef]
- Liu, B.; Lei, Y.; Li, B. A batch-mode cube microbial fuel cell based “shock” biosensor for wastewater quality monitoring. Biosens. Bioelectron. 2014, 62, 308–314. [CrossRef]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [CrossRef]
- İncel, A.; Akın, O.; Çağır, A.; Yıldız, Ü.H.; Demir, M.M. Smart phone assisted detection and quantification of cyanide in drinking water by paper based sensing platform. Sens. Actuators B: Chem. 2017, 252, 886–893. [CrossRef]
- Cho, J.H.; Gao, Y.; Choi, S. A portable, single-use, paper-based microbial fuel cell sensor for rapid, on-site water quality monitoring. Sensors 2019, 19, 5452. [CrossRef]
- Cho, J.H.; Gao, Y.; Ryu, J.; Choi, S. Portable, disposable, paper-based microbial fuel cell sensor utilizing freeze-dried bacteria for in situ water quality monitoring. ACS omega 2020, 5, 13940–13947. [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based microfluidic device for improved detection of nitrate in water. Sensors 2020, 21, 102. [CrossRef]
- Jaballah, M.B.; Karrat, A.; Amine, A.; Dridi, C. Immobilization of diphenylcarbazide on paper-based analytical devices for the pre-concentration and detection of chromium VI in water samples. Talanta 2023, 265, 124889. [CrossRef]
- da Silva, V.A.O.P.; de Freitas, R.C.; de Oliveira, P.R.; Moreira, R.C.; Marcolino-Júnior, L.H.; Bergamini, M.F.; Coltro, W.K.; Janegitz, B.C. Microfluidic paper-based device integrated with smartphone for point-of-use colorimetric monitoring of water quality index. Measurement 2020, 164, 108085. [CrossRef]
- Xiong, X.; Guo, C.; Yan, G.; Han, B.; Wu, Z.; Chen, Y.; Xu, S.; Shao, P.; Song, H.; Xu, X.; others. Simultaneous Cross-type Detection of Water Quality Indexes via a Smartphone-App Integrated Microfluidic Paper-Based Platform. ACS omega 2022, 7, 44338–44345. [CrossRef]
- Lin, J.H.; Chen, S.J.; Lee, J.E.; Chu, W.Y.; Yu, C.J.; Chang, C.C.; Chen, C.F. The detection of Mercury (II) ions using fluorescent gold nanoclusters on a portable paper-based device. Chem. Eng. J. 2022, 430, 133070. [CrossRef]
- Aguiar, J.I.; Ribeiro, S.O.; Leite, A.; Rangel, M.; Rangel, A.O.; Mesquita, R.B. Use of a rhodamine-based chelator in a microfluidic paper-based analytical device for the in-situ copper quantification in natural waters. Talanta 2024, 271, 125683. [CrossRef]
- Uhlikova, N.; Almeida, M.I.G.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the speciation of inorganic nitrogen species. Talanta 2024, 271, 125671. [CrossRef]
- Thangjitsirisin, K.; Seeharaj, P.; Choengchan, N. Superhydrophobic eggshell for fabrication of hydrophobic barrier of paper-based analytical device for colorimetric determination of ammonium ion in water. Microchem. J. 2024, 200, 110464. [CrossRef]
- Sameenoi, Y.; Panymeesamer, P.; Supalakorn, N.; Koehler, K.; Chailapakul, O.; Henry, C.S.; Volckens, J. Microfluidic paper-based analytical device for aerosol oxidative activity. Environ. Sci. Technol. 2013, 47, 932–940. [CrossRef]
- Khachornsakkul, K.; Hung, K.H.; Chang, J.J.; Dungchai, W.; Chen, C.H. A rapid and highly sensitive paper-based colorimetric device for the on-site screening of ammonia gas. Analyst 2021, 146, 2919–2927. [CrossRef]
- Mettakoonpitak, J.; Sawatdichai, N.; Thepnuan, D.; Siripinyanond, A.; Henry, C.S.; Chantara, S. Microfluidic paper-based analytical devices for simultaneous detection of oxidative potential and copper in aerosol samples. Microchim. Acta 2023, 190, 241. [CrossRef]
- De Matteis, V.; Cascione, M.; Fella, G.; Mazzotta, L.; Rinaldi, R. Colorimetric paper-based device for hazardous compounds detection in air and water: A proof of concept. Sensors 2020, 20, 5502. [CrossRef]
- Bordbar, M.M.; Tashkhourian, J.; Hemmateenejad, B. Paper-Based Optical Nose Made with Bimetallic Nanoparticles for Monitoring Ignitable Liquids in Gasoline. ACS Appl. Mater. Interfaces 2022, 14, 8333–8342. [CrossRef]
- Arroyo, P.; Gómez-Suárez, J.; Suárez, J.I.; Lozano, J. Low-Cost Air Quality Measurement System Based on Electrochemical and PM Sensors with Cloud Connection. Sensors 2021, 21, 6228. [CrossRef]
- Li, G.; Yuan, H.; Mou, J.; Dai, E.; Zhang, H.; Li, Z.; Zhao, Y.; Dai, Y.; Zhang, X. Electrochemical detection of nitrate with carbon nanofibers and copper co-modified carbon fiber electrodes. Compos. Commun. 2022, 29, 101043. [CrossRef]
- Mettakoonpitak, J.; Volckens, J.; Henry, C.S. Janus electrochemical paper-based analytical devices for metals detection in aerosol samples. Anal. Chem. 2019, 92, 1439–1446. [CrossRef]
- Davis, D.; Narayanan, S.K.; Ajeev, A.; Nair, J.; Jeeji, J.; Vijayan, A.; Viyyur Kuttyadi, M.; Nelliparambil Sathian, A.; Arulraj, A.K. Flexible Paper-Based Room-Temperature Acetone Sensors with Ultrafast Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 25734–25743. [CrossRef]
- Qin, X.; Wu, T.; Zhu, Y.; Shan, X.; Liu, C.; Tao, N. A paper based milli-cantilever sensor for detecting hydrocarbon gases via smartphone camera. Anal. Chem. 2020, 92, 8480–8486. [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens. Bioelectron. 2022, 207, 114167. [CrossRef]
- Lin, X.; Li, C.; Tong, X.; Duan, N.; Wang, Z.; Wu, S. A portable paper-based aptasensor for simultaneous visual detection of two mycotoxins in corn flour using dual-color upconversion nanoparticles and Cu-TCPP nanosheets. Food Chem. 2023, 404, 134750. [CrossRef]
- He, Y.; Wang, H.; Yu, Z.; Tang, X.; Zhou, M.; Guo, Y.; Xiong, B. A disposable immunosensor array using cellulose paper assembled chemiresistive biosensor for simultaneous monitoring of mycotoxins AFB1 and FB1. Talanta 2024, p. 126145. [CrossRef]
- Xiang, J.; Qi, J.; Hu, D.; Wang, C.; Wang, L.; Wu, Y.; Chen, J.; Zhang, Z.; Wang, X.; Li, B.; others. Molecularly imprinted metal-organic frameworks assisted cloth and paper hybrid microfluidic devices for visual detection of gonyautoxin. J. Hazard. Mater. 2024, 469, 133969. [CrossRef]
- dos Santos, D.M.; Migliorini, F.L.; Coatrini-Soares, A.; Soares, J.C.; Mattoso, L.H.; Oliveira, O.N.; Correa, D.S. Low-cost paper-based sensors modified with curcumin for the detection of ochratoxin a in beverages. Sens. Actuators Rep. 2024, 7, 100184. [CrossRef]
- Hua, M.Z.; Lu, X. Development of a microfluidic paper-based immunoassay for rapid detection of allergic protein in foods. ACS Sens. 2020, 5, 4048–4056. [CrossRef]
- Kunpatee, K.; Panphloi, M.; Charoenkitamorn, K.; Pimpitak, U.; Puthong, S.; Buakeaw, A.; Komolpis, K.; Sain, M.M.; Yakoh, A.; Chaiyo, S. Electrochemical lateral flow immunosensor with enhanced reproducibility for milk allergen detection. Sens. Actuators B Chem. 2024, 401, 135042. [CrossRef]
- Han, X.; Zhang, D.; Xie, M.; Yang, J.; Wang, Y.; Li, H.; Wang, S.; Pan, M. Microfluidic paper-based analysis device applying black phosphorus nanosheets@ MWCNTs-COOH: A portable and efficient strategy for detection of β-Lactoglobulin in dairy products. Food Chem. 2024, 446, 138844. [CrossRef]
- Ortiz-Gómez, I.; Ipatov, A.; Barreiro-Docío, E.; Salinas-Castillo, A.; de Orbe-Payá, I.; Capitán-Vallvey, L.F.; Prado, M. Microfluidic paper-based analytical aptasensor for fluorometric β-lactoglobulin determination. Microchem. J. 2024, 198, 110121. [CrossRef]
- He, Y.; Hua, M.Z.; Feng, S.; Lu, X. Development of a smartphone-integrated microfluidic paper-based optosensing platform coupled with molecular imprinting technique for in-situ determination of histamine in canned tuna. Food Chem. 2024, 451, 139446. [CrossRef]
- Pan, M.; Han, X.; Chen, S.; Yang, J.; Wang, Y.; Li, H.; Wang, S. Paper-based microfluidic device for selective detection of peanut allergen Ara h1 applying black phosphorus-Au nanocomposites for signal amplification. Talanta 2024, 267, 125188. [CrossRef]
- Lu, C.; Xu, S.; Wang, S.; Wang, T.; Wang, W.L.; Yang, C.; Zhang, Y. Facile and Ultrasensitive Food Allergen Quantification Using Microzone Paper-Based Mass Spectrometric Immunoassay. Anal. Chem. 2024, 96, 2387––2395. [CrossRef]
- Martins, T.S.; Machado, S.A.; Oliveira Jr, O.N.; Bott-Neto, J.L. Optimized paper-based electrochemical sensors treated in acidic media to detect carbendazim on the skin of apple and cabbage. Food Chem. 2023, 410, 135429. [CrossRef]
- Zhang, D.; Wang, S.; Yang, F.; Li, Z.; Huang, W. Visual inspection of acidic pH and bisulfite in white wine using a colorimetric and fluorescent probe. Food Chem. 2023, 408, 135200. [CrossRef]
- Placer, L.; Lavilla, I.; Pena-Pereira, F.; Bendicho, C. A 3D microfluidic paper-based analytical device with smartphone-assisted colorimetric detection for iodine speciation in seaweed samples. Sens. Actuators B: Chem. 2023, 377, 133109. [CrossRef]
- Masoomi, S.; Sharifi, H.; Hemmateenejad, B. A paper-based optical tongue for characterization of Iranian honey: identification of geographical/botanical origins and adulteration detection. Food Control 2024, 155, 110052. [CrossRef]
- Selvaraj, S.; Ravi Shankaran, D. Nano Enabled Plasmonic Strips For Colorimetric Detection Of Food Adulterants. ChemistrySelect 2023, 8, e202302027. [CrossRef]
- Andrade, L.M.; Romanholo, P.V.; Ananias, A.C.A.; Venancio, K.P.; Silva-Neto, H.A.; Coltro, W.K.; Sgobbi, L.F. Pocket test for instantaneous quantification of starch adulterant in milk using a counterfeit banknote detection pen. Food Chem. 2023, 405, 134844. [CrossRef]
- Dortez, S.; Crevillen, A.G.; Escarpa, A.; Cinti, S. Electroanalytical paper-based device for reliable detection and quantification of sugars in milk. Sens. Actuators B: Chem. 2024, 398, 134704. [CrossRef]
- Shalileh, F.; Sabahi, H.; Dadmehr, M.; Hosseini, M. Sensing approaches toward detection of urea adulteration in milk. Microchem. J. 2023, 193, 108990. [CrossRef]
- Wu, Y.; Zhang, L.; Zhang, D.; Yu, R. A surface molecularly imprinted microfluidic paper based device with smartphone assisted colorimetric detection for butachlor in mung bean. Food Chem. 2024, 435, 137659. [CrossRef]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [CrossRef]
- Qin, X.; Liu, J.; Zhang, Z.; Li, J.; Yuan, L.; Zhang, Z.; Chen, L. Microfluidic paper-based chips in rapid detection: Current status, challenges, and perspectives. TrAC, Trends Anal. Chem. 2021, 143, 116371. [CrossRef]
- Gautam, N.; Verma, R.; Ram, R.; Singh, J.; Sarkar, A. Development of a biodegradable microfluidic paper-based device for blood-plasma separation integrated with non-enzymatic electrochemical detection of ascorbic acid. Talanta 2024, 266, 125019. [CrossRef]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: state-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





