Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.2.1. Solution Polycondensation
2.2.2. Small-Scale Melt Polycondensation
2.2.3. Melt Polycondensation
2.2.4. Melt Polycondensation Accompanied with Solid-State Polycondensation
2.3. Comonomer and Polymer Characterization
3. Results and Discussion
3.1. Binary Copolyesters
3.2. Ternary Copolyesters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J.I.; Kang, C.S. Thermotropic main chain polyesters. Progress in Polymer Science 1997, 22, 937–973. [Google Scholar] [CrossRef]
- Jackson, W.J. Liquid Crystal Aromatic Polyesters: Early History and Future Trends. Molecular Crystals and Liquid Crystals Incorporating Nonlinear Optics 1989, 169, 23–49. [Google Scholar] [CrossRef]
- Economy, J. Aromatic Polyesters of p -Hydroxybenzoic Acid. Molecular Crystals and Liquid Crystals Incorporating Nonlinear Optics 1989, 169. [Google Scholar] [CrossRef]
- Deberdeev, T.R.; Akhmetshina, A.I.; Karimova, L.K.; et al. Heat-Resistant Polymer Materials Based on Liquid Crystal Compounds. Polym. Sci. Ser. C 2020, 62, 145–164. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, A.; Shi, D.; et al. Liquid crystalline polymers: Discovery, development, and the future. Polymer 2020, 202, 122740. [Google Scholar] [CrossRef]
- Limeneh, D.Y.; Yilma, K.T. Review on Vectran-Super Fiber from Thermotropic Crystals of Rigid-Rod Polymer. Journal of Engineering 2021, 6646148. [Google Scholar] [CrossRef]
- Roeting, O.; Hinrichsen, G. Blends of thermotropic liquid-crystalline and thermoplastic polymers—a short review. Advances in Polymer Technology 1994, 13, 57–64. [Google Scholar] [CrossRef]
- Han, H.S.; Bhowmik, P.K. Wholly aromatic liquid-crystalline polyesters. Progress in Polymer Science 1997, 22, 1431–1502. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Platé, N.A. Blend composites based on liquid crystal thermoplast. Polymer Science U.S.S.R. 1991, 33, 1–37. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, S.W.; Kim, S.H. Thermotropic liquid crystal polymer reinforced poly(butylene terephthalate) composites to improve heat distortion temperature and mechanical properties. Fibers and Polymers 2006, 7, 358–366. [Google Scholar] [CrossRef]
- Yang, Q.; Hirata, M.; Hsu, Y.-I.; Lu, D.; Kimura, Y. Improved Thermal and Mechanical Properties of Poly(butylene succinate) by Polymer Blending with a Thermotropic Liquid Crystalline Polyester. Journal of Applied Polymer Science 2014, 131, 39952. [Google Scholar] [CrossRef]
- Choi, J.-K.; Lee, B.-W.; Choi, Y.-S.; et al. Reinforcing Properties of Poly(trimethyleneterephthalate) by a Thermotropic Liquid Crystal Polymer. Journal of Applied Polymer Science 2015, 132, 41408. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Pepels, M.P.F.; Spoelstra, A.B.; et al. Improving Stiffness, Strength, and Toughness of Poly(ω-pentadecalactone) Fibers through in Situ Reinforcement with a Vanillic Acid-Based Thermotropic Liquid Crystalline Polyester. Macromolecules 2016, 49, 2228–2237. [Google Scholar] [CrossRef]
- de Kort, G.W.; Bouvrie, L.H.C.; Rastogi, S.; et al. Thermoplastic PLA-LCP Composites: A Route toward Sustainable, Reprocessable, and Recyclable Reinforced Material. ACS Sustainable Chemistry & Engineering 2020, 8, 624–631. [Google Scholar]
- Sirigu, A. Topics in the evolution of liquid crystal polymers. Liquid Crystals 1993, 14, 15–36. [Google Scholar] [CrossRef]
- Economy, J.; Storm, R.S.; Matkovich, V.I.; et al. Synthesis and structure of para-hydroxybenzoic acid polymer. Journal of Polymer Science Part A-Polymer Chemistry 1976, 14, 2207–2224. [Google Scholar] [CrossRef]
- Mikhaylov, P.A.; Zuev, K.V.; Filatova, M.P.; et al. Synthesis and Properties of Thermotropic Copolyesters Based on Poly(ethylene terephthalate) and 4′-Acetoxy-4-biphenyl-carboxylic Acid. Polymers 2021, 13, 1720. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, X.; Liu, J.; et al. Synthesis of a fire-retardant and high Tg biobased polyester from 2,5-furandicarboxylic acid. Polymer Journal 2022, 54, 1–14. [Google Scholar] [CrossRef]
- Bazin, A.; Averous, L.; Pollet, E. Lipase-catalyzed synthesis of furan-based aliphatic-aromatic biobased copolyesters: Impact of the solvent. Eur. Polym. J. 2021, 159, 110717. [Google Scholar] [CrossRef]
- Singh, M.; Takada, K.; Kaneko, T. Biobased liquid crystalline poly(coumarate)s composites and their potential applications. Composites Communications 2020, 22, 100531. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, W.; Zhang, Y. Direct synthesis of potentially biodegradable aromatic-aliphatic thermotropic copolyesters with photocrosslinking properties. Liquid Crystals 2019, 46, 1780–1789. [Google Scholar] [CrossRef]
- Bloom, M.E.; Vicentin, J.; Honeycutt, D.S.; et al. Highly renewable, thermoplastic tetrapolyesters based on hydroquinone, p-hydroxybenzoic acid or its derivatives, phloretic acid, and dodecanedioic acid. Journal of Polymer Science, Part A—Polymer Chemistry 2018, 56, 1498–1507. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Deshmukh, Y.S.; Liu, W. Processing and performance of aromatic-aliphatic thermotropic polyesters based on vanillic acid. Polymer 2015, 60, 198–206. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Verhoeven, J.M.G.A.; Noordover, B.A.J.; et al. Thermotropic Polyesters from 2,5-Furandicarboxylic Acid and Vanillic Acid: Synthesis, Thermal Properties, Melt Behavior, and Mechanical Performance. Macromolecules, 2014, 47, 3306–3316. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Huang, S.; et al. Synthesis and Characterization of Novel Thermotropic Aromatic-Aliphatic Biodegradable Copolyesters Containing D,L-Lactic acid (LA), Poly(butylene terephthalate) (PBT) and Biomesogenic Units. Polymer-Plastics Technology and Engineering 2014, 53, 1697–1705. [Google Scholar] [CrossRef]
- Mialon, L.; Pemba, A.G.; Miller, S.A. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 2010, 12, 1704. [Google Scholar] [CrossRef]
- Imasaka, K.; Nagai, T.; Yoshida, M.; et al. Synthesis and in vitro degradations of low-molecular-weight copolyesters composed of L-lactic acid and aromatic hydroxy acids. Makromol. Chem. 1990, 191, 2077–2082. [Google Scholar] [CrossRef]
- Nicely, V.A.; Dougherty, J.T.; Renfro, L.W. Sequence Distributions and a Phase Diagram for Copolymers Made from Poly(ethy1ene terephthalate) and p-Acetoxybenzoic Acid. Macromolecules 1987, 20, 573–578. [Google Scholar] [CrossRef]
- Jin, X.; Carfagna, C.; Nicolais, L.; et al. Synthesis and characterization of potentially biodegradable thermotropic polyesters based on p-hydroxybenzoic acid and glycolic acid. Journal of Polymer Science Part A: Polymer Chemistry 1994, 32, 3115–3122. [Google Scholar] [CrossRef]
- Jin, X.; Carfagna, C.; Nicolais, L.; et al. Synthesis, Characterization, and in Vitro Degradation of a Novel Thermotropic Ternary Copolyester Based on p-Hydroxybenzoic Acid, Glycolic Acid, and p-Hydroxycinnamic Acid. Macromolecules 1995, 28, 4785–4794. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Schwarz, G. New polymer syntheses: 10. Syntheses of high molecular weight poly(4-hydroxybenzoate)s by bulk condensations of 4-hydroxybenzoic acids. Polymer 1984, 25, 520–528. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Löhden, G. Whisker 11. Poly(ester—amide)s derived from vanillic acid and 4-aminobenzoic acid. Polymer 1995, 36, 1697–1705. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Stukenbrock, T. New polymer syntheses, 92. Biodegradable, thermotropic copolyesters derived from β-(4-hydroxyphenyl)propionic acid. Macromol. Chem. Phys. 1997, 198, 3753–3767. [Google Scholar] [CrossRef]
- Nagata, M. Synthesis, characterization, and hydrolytic degradation of copolyesters of 3-(4-hydroxyphenyl) propionic acid and p-hydroxybenzoic acid, vanilic acid, or syringic acid. Journal of Applied Polymer Science 2000, 78, 2474–2481. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Noordover, B.A.J.; Rastogi, S. Aromatic thermotropic polyesters based on 2,5-furandicarboxylic acid and vanillic acid. Polymer 2014, 55, 2432–2439. [Google Scholar] [CrossRef]
- Mikhaylov, P.A.; Kalita, A.G.; Kulichikhin, V.G. Synthesis of New Thermotropic Fully Aromatic Copolyesters from Hydroxybenzoic and Hydroxybiphenylcarboxylic Acids. Polymer Science, Series B 2022, 64, 393–401. [Google Scholar] [CrossRef]
- Pearl, I.A. Reactions of Vanillin and Its Derived Compounds. IV.1 The Caustic Fusion of Vanillin. J. Am. Chem. Soc. 1946, 68, 2180–2181. [Google Scholar] [CrossRef]
- Schwarz, G.; Kricheldorf, H.R. Whiskers. 12. Whisker-like Crystals of Poly(4′-hydroxybiphenyl-4-carboxylic acid). Macromolecules 1995, 28, 3911–3917. [Google Scholar] [CrossRef]
- Kim, W.N.; Denn, M.M. Properties of blends of a thermotropic liquid crystalline polymer with a flexible polymer (Vectra/PET). Journal of Rheology 1992, 36, 1477–1498. [Google Scholar] [CrossRef]
- Huh, S.M.; Jin, J.I. Synthesis and characterization of wholly aromatic polyesters derived from 6-hydroxy-5-phenyl-2-naphthoic acid or 4′-hydroxy-3′-phenylbiphenyl-4-carboxylic acid and 4-hydroxybenzoic acid. Macromolecules 1997, 30, 3005–3013. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Rastogi, S.; Veld, M.A.J. et al. Liquid crystalline furandicarboxylic acid-based aromatic. polyesters. Patent WO2013/092667, 2013. [Google Scholar]
- Mikhailov, P.A.; Zuev, K.V.; Kulichikhin, V.G. Synthesis and Characterization of Novel Wholly Aromatic Copolyesters Based on 4′-Hydroxybiphenyl-3-Carboxylic and 3-Hydroxybenzoic Acids. Polymers 2023, 15, 2133. [Google Scholar] [CrossRef]
- Fernandez-Blazquez, J.P.; Bello, A.; Perez, E. Observation of Two Glass Transitions in a Thermotropic Liquid-Crystalline Polymer. Macromolecules 2004, 37, 9018–9026. [Google Scholar] [CrossRef]
- Shinn, T.-H.; Lin, C.-C. Co[poly(ethylene terephthalate-p-oxybenzoate)] thermotropic copolyester. II. X-ray diffraction analysis. J. Appl. Polym. Sci. 1993, 47, 1105–1113. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Z.; Zeng, S.; et al. Synthesis and characterization of renewable polyesters based on vanillic acid. J. Appl. Polym. Sci. 2020, 137, 49189. [Google Scholar] [CrossRef]
- Chen, S.-C.; Zhang, X.-M.; Liu, M.; et al. Rheological Characterization and Thermal Stability of Different Intrinsic Viscosity Poly(ethylene terephthalate) in Air and Nitrogen. International Polymer Processing 2016, 31, 292–300. [Google Scholar] [CrossRef]
- Heifferon, K.V.; Spiering, G.A.; Talley, S.J.; et al. Synthesis and Characterization of a Nematic Fully Aromatic Polyester Based on Biphenyl 3,4′-Dicarboxylic Acid. Polymer Chemistry 2019, 10, 4287–4296. [Google Scholar] [CrossRef]
- Park, G.T.; Lee, W.J.; Chang, J.-H.; et al. Dependence of the Physical Properties and Molecular Dynamics of Thermotropic Liquid Crystalline Copolyesters on p-Hydroxybenzoic Acid Content. Polymers 2020, 12, 198. [Google Scholar] [CrossRef]
- de Kort, G.W.; Leone, N.; Stellamanns, E.; et al. Effect of Shear Rate on the Orientation and Relaxation of a Vanillic Acid Based Liquid Crystalline Polymer. Polymers 2018, 10, 935. [Google Scholar] [CrossRef]
- de Kort, G.W.; Saidi, S.; Hermida-Merino, D.; et al. Reactive Processing Route to Thermotropic Polyesters with a Low Processing Temperature and Enhanced Relaxation Time. Macromolecules 2021, 54, 1401–1413. [Google Scholar] [CrossRef]
- Wei, P.; Wang, Y.; Wang, Y.; et al. Synthesis and Properties of Thermotropic Poly(Oxybenzoate-Co-Oxynaphthoate) Copolyester Modified by a Third AB Type Monomer. Journal of Macromolecular Science, Part B 2020, 59, 197–212. [Google Scholar] [CrossRef]
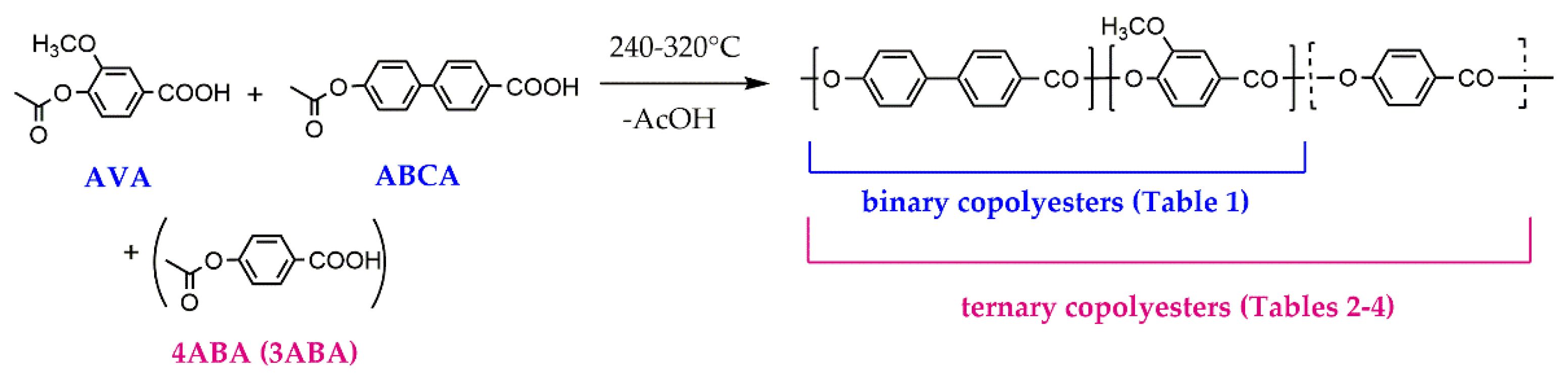
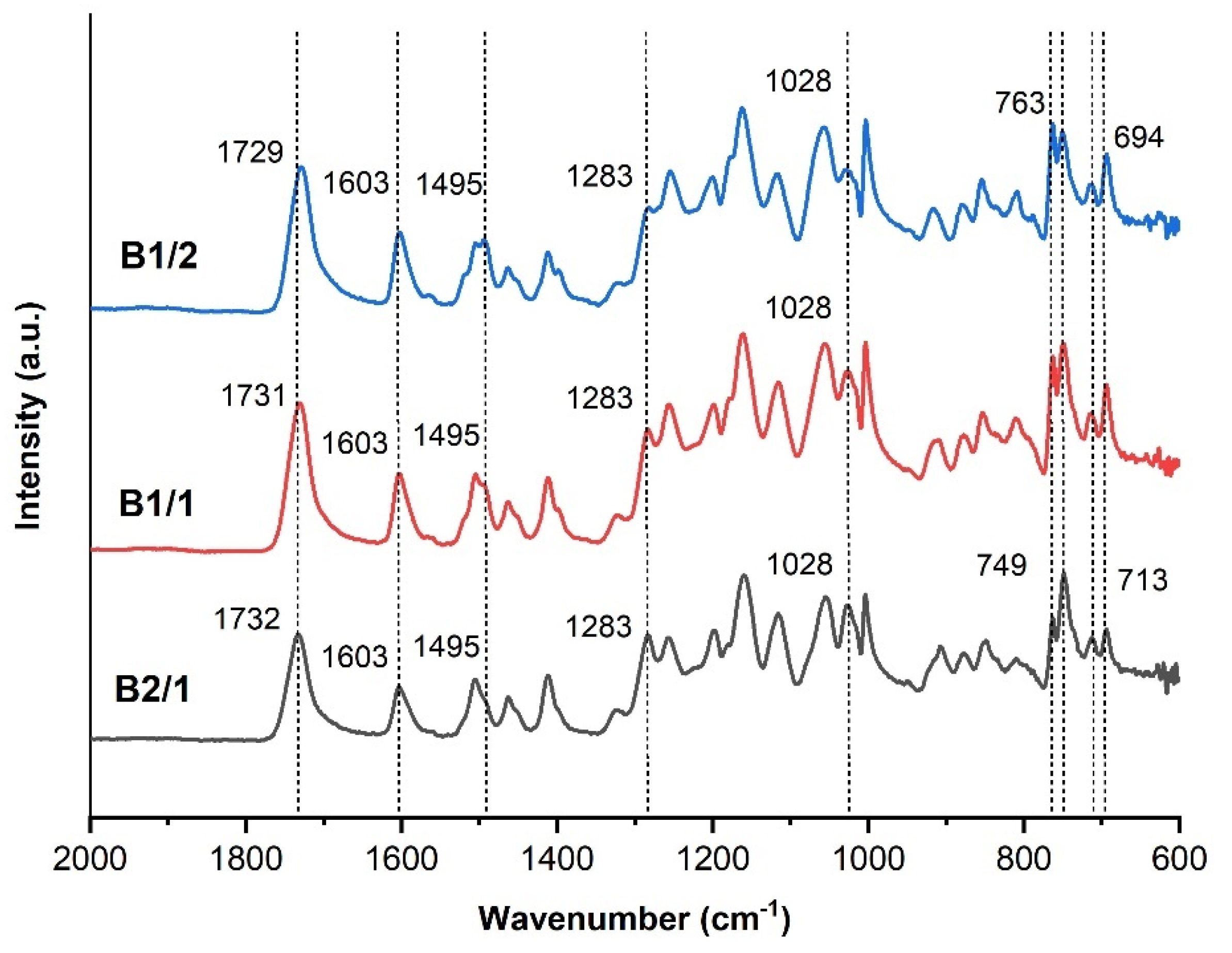
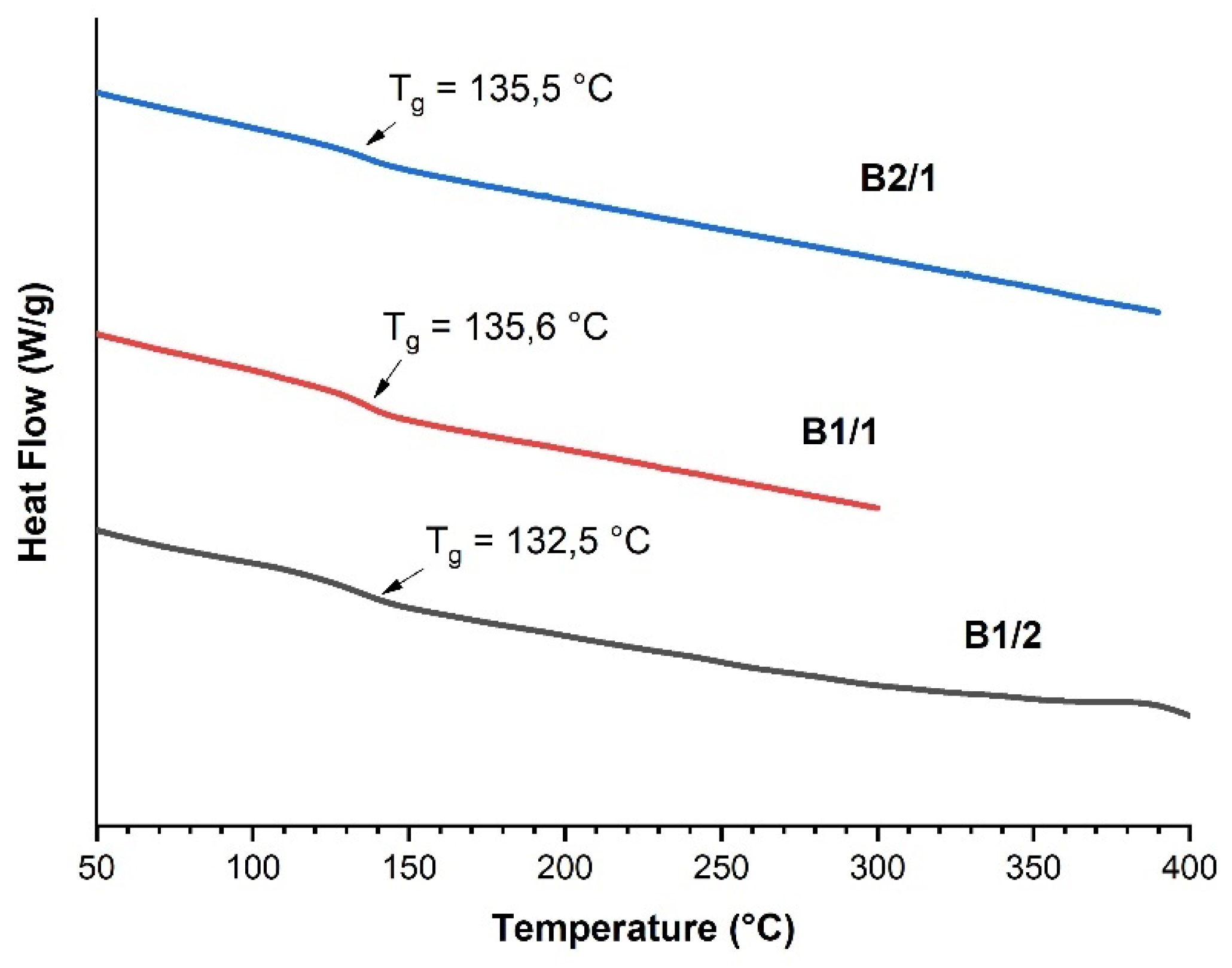
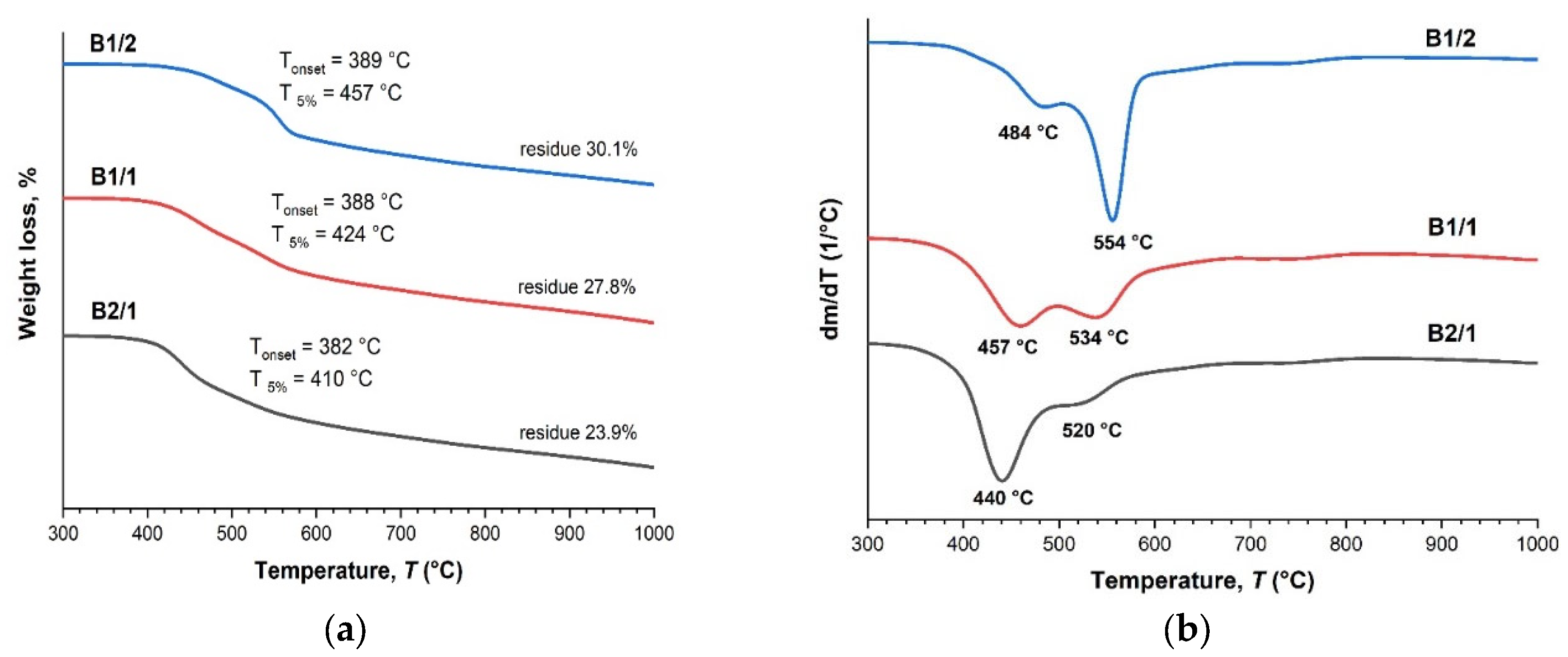
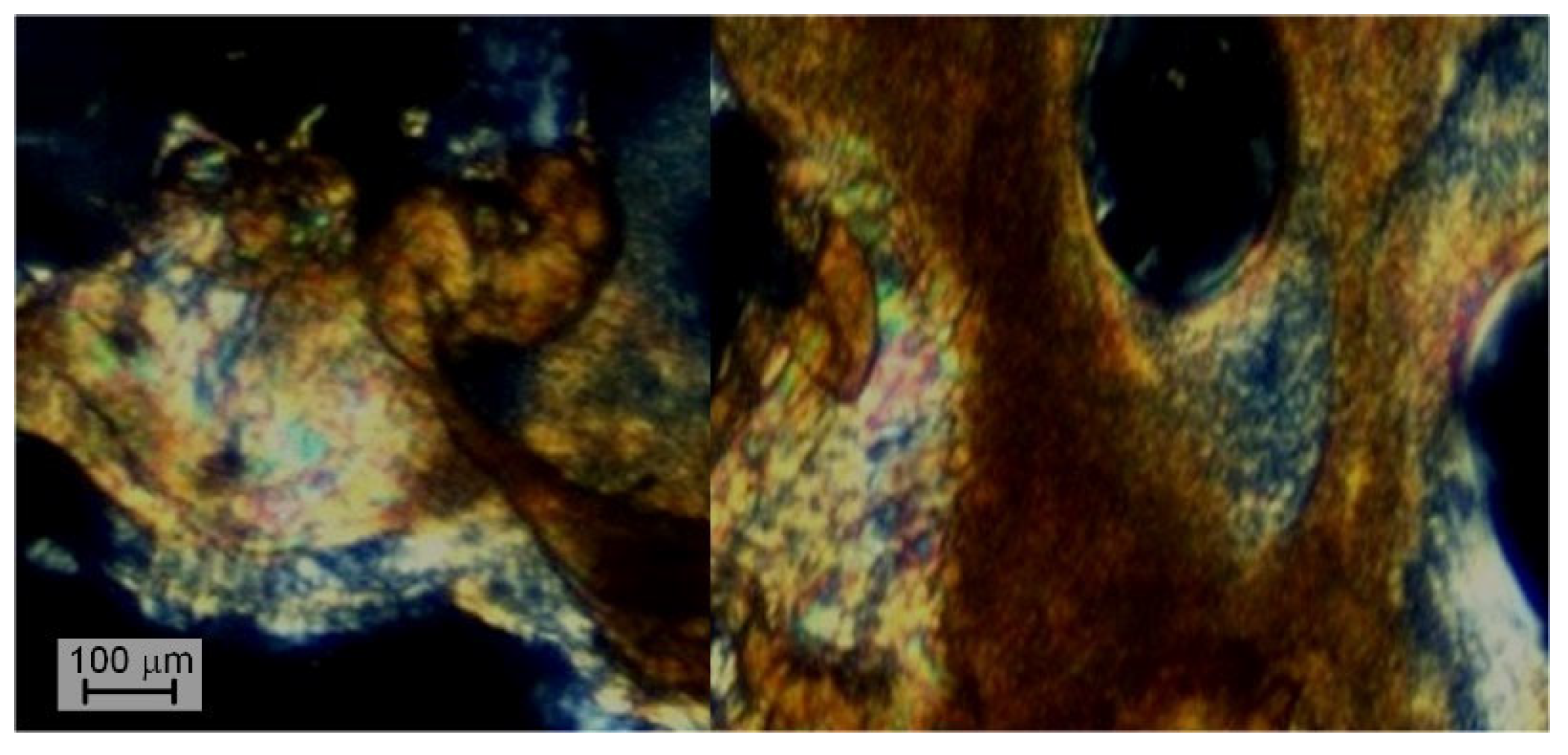
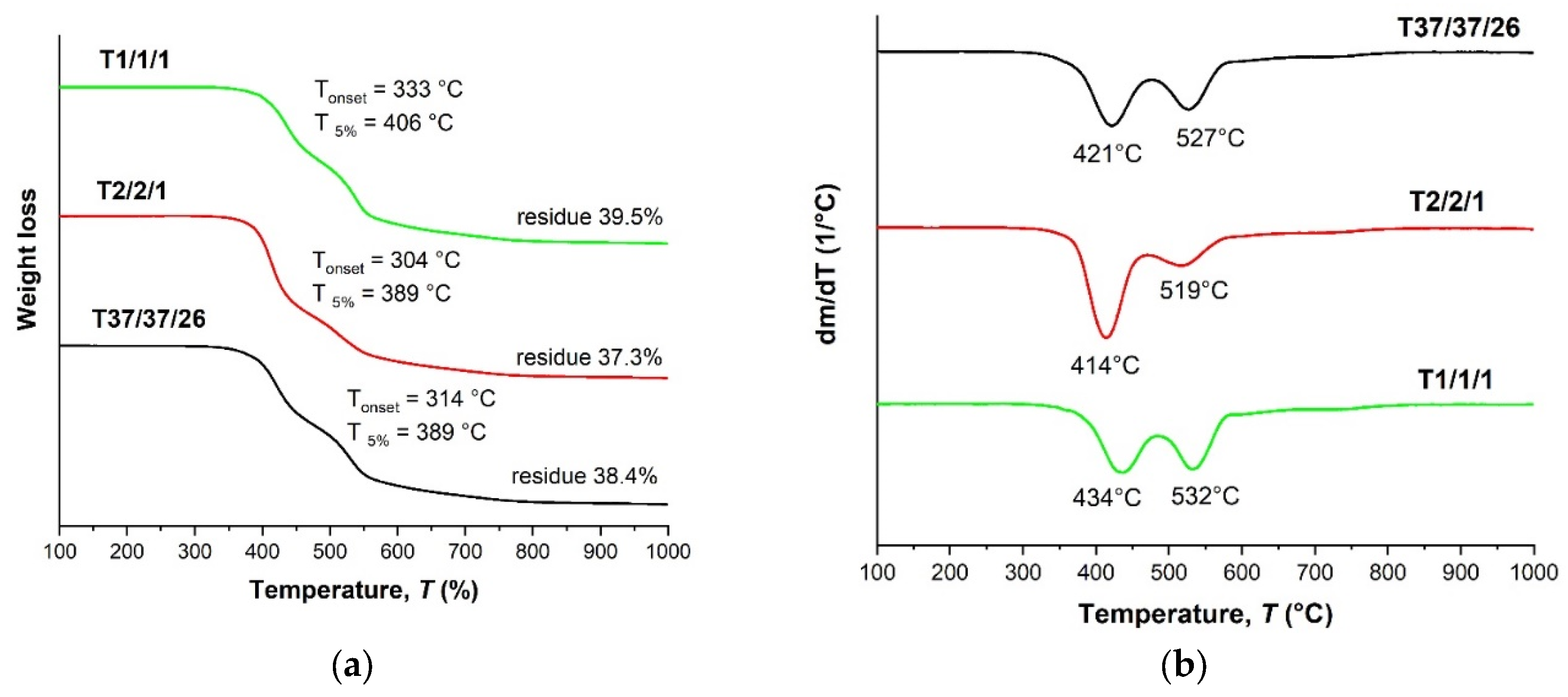
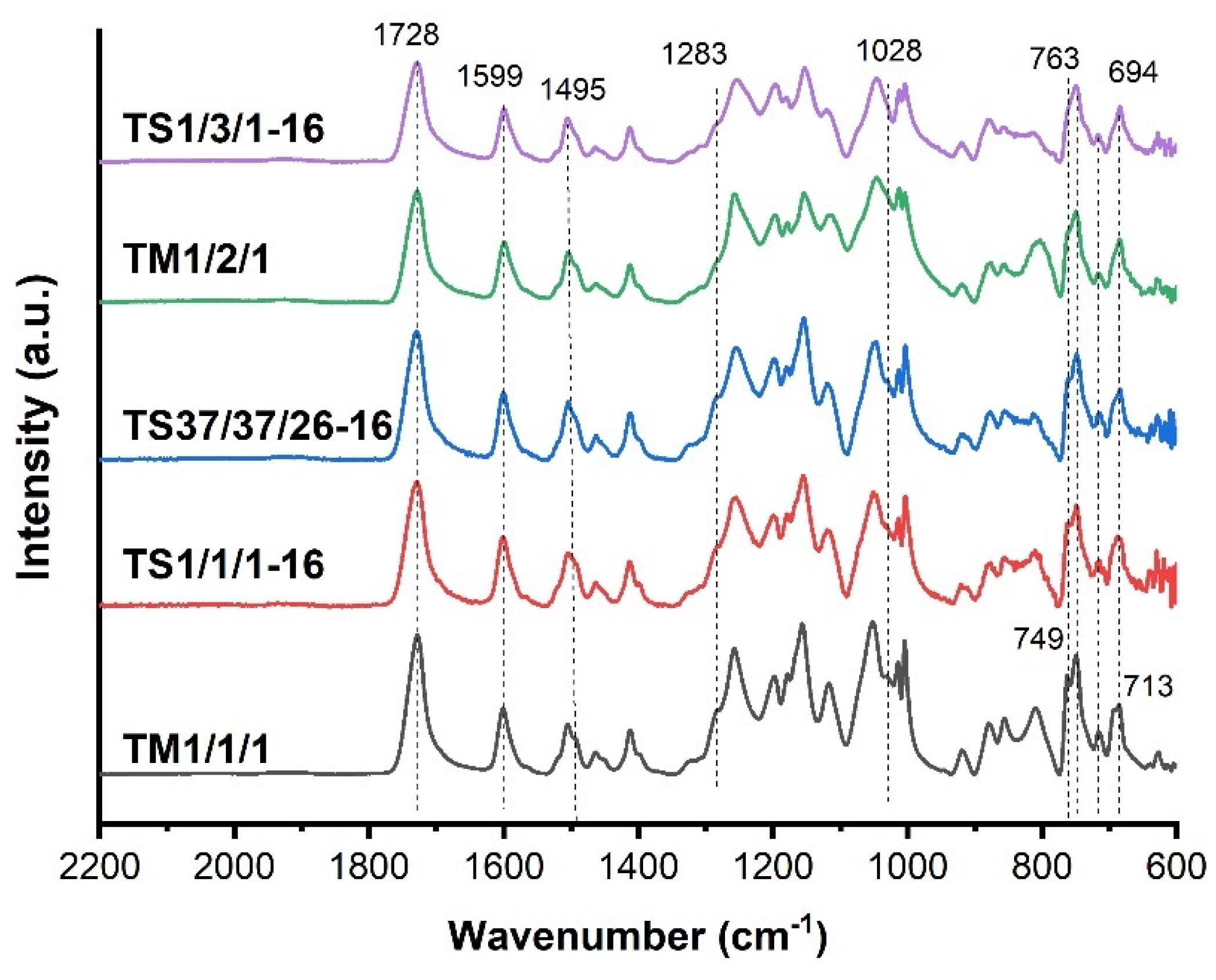
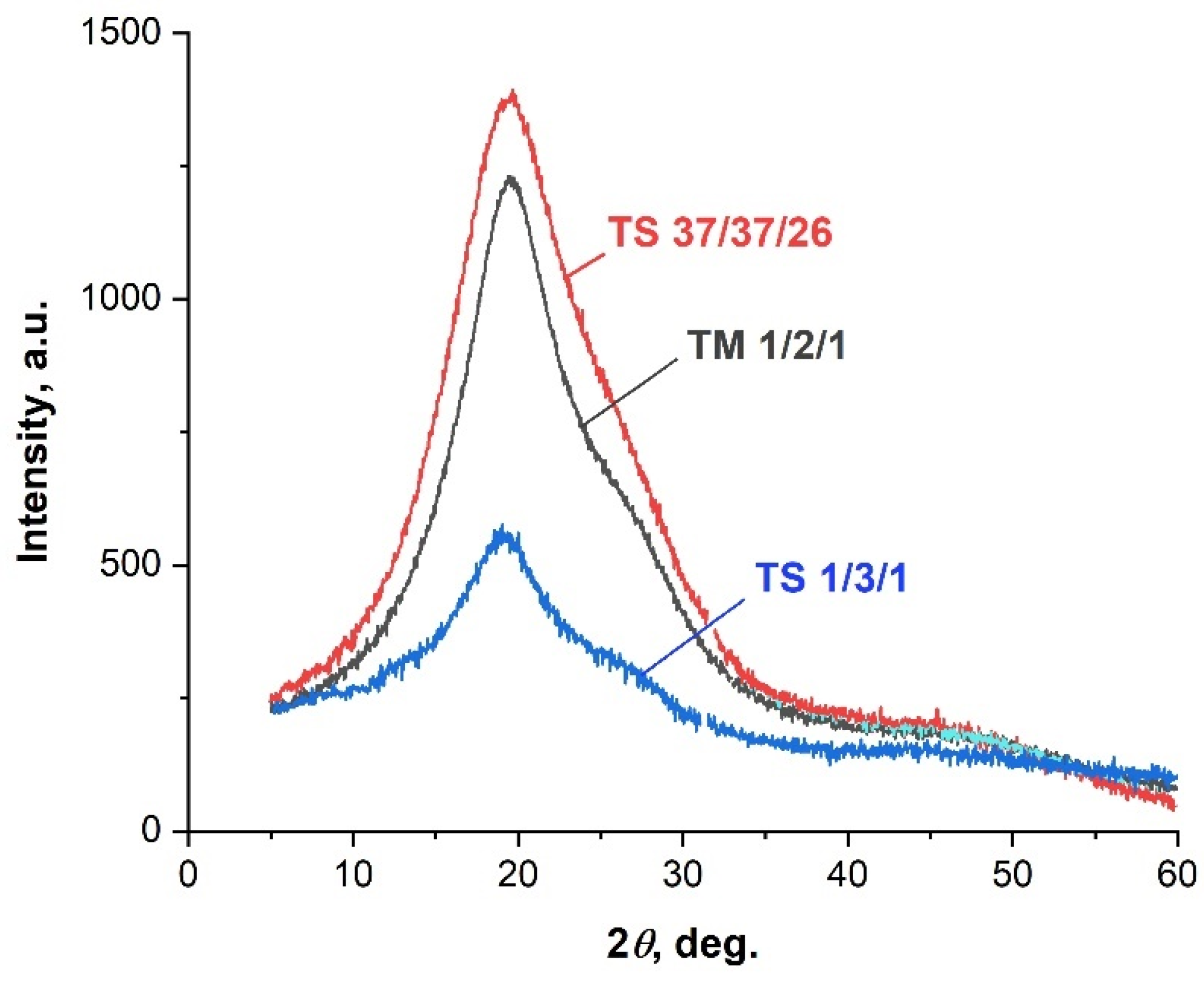
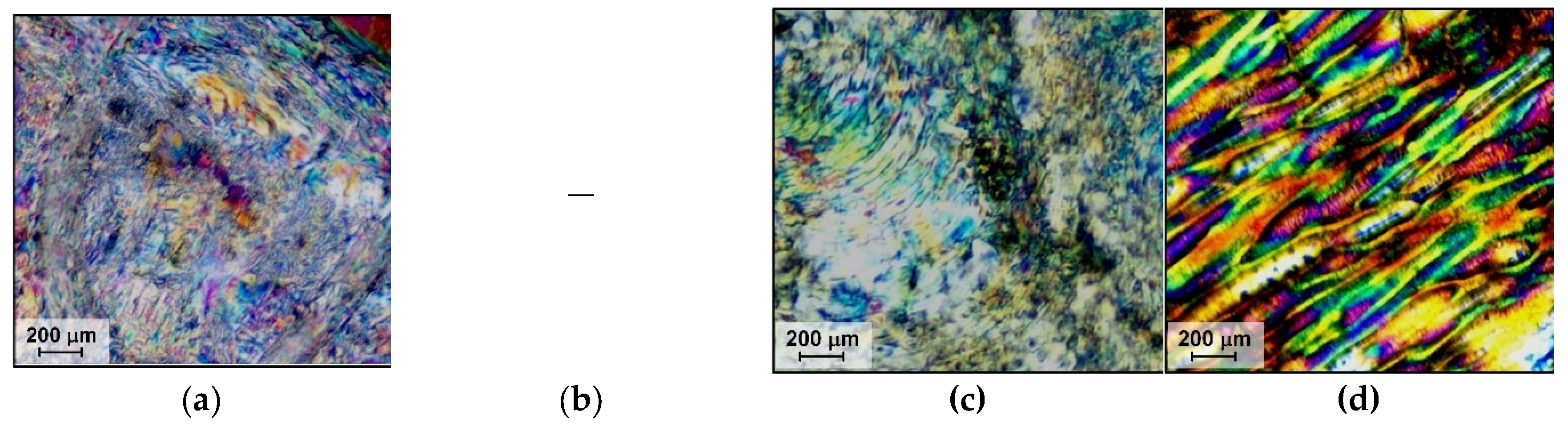
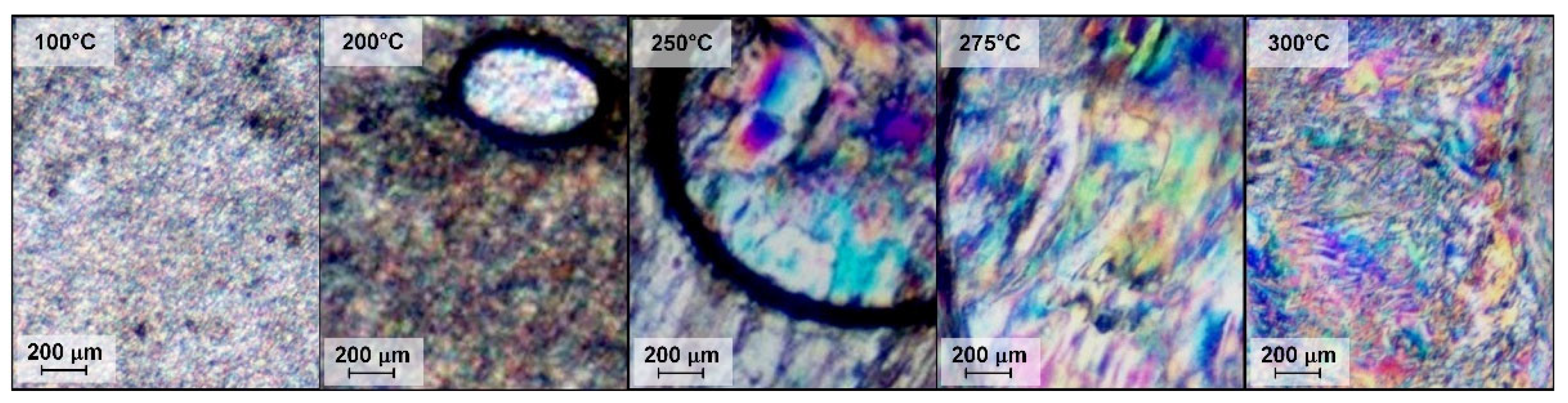
| Copolyester | Molar ratio (AVA / ABCA) |
Tg, °C | Tm, °C | Ton, °C | T5%, °C |
|---|---|---|---|---|---|
| B 1/2 | 1:2 | 135.5 | - | 389 | 457 |
| B 1/1 | 1:1 | 135.6 | - | 388 | 424 |
| B 2/1 | 2:1 | 132.5 | - | 382 | 410 |
| Copolyester | Molar ratio (AVA / ABA / ABCA) |
Tg, °C | Tm, °C | Ton, °C | T5%, °C |
|---|---|---|---|---|---|
| T 1/1/1 | 1:1:1 | 117.5 | - | 332.7 | 405.9 |
| T 2/2/1 | 2:2:1 | 116.4 | - | 304.4 | 389.2 |
| T 37/37/26 | 37:37:26 | 116.8 | - | 313.8 | 388.9 |
| T 68/21/211 | 68:21:11 | - | - | - | - |
| Copolyester | Molar ratio (AVA / ABA / ABCA) |
Tg, °C | Tm, °C | Ton, °C | T5%, °C |
|---|---|---|---|---|---|
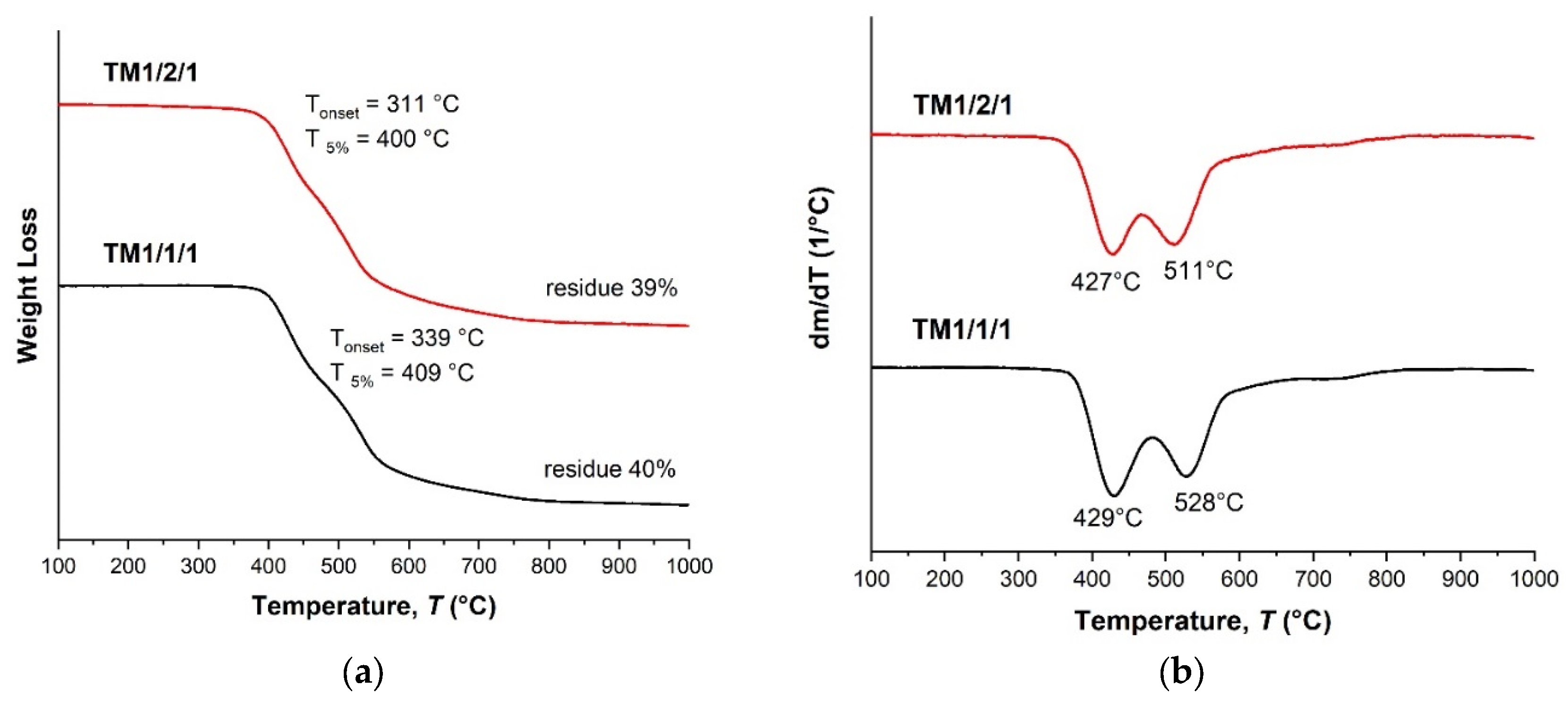
| TM 1/1/1 | 1:1:1 | 120.8 | - | 339.1 | 408.7 |
| TM 1/2/1 | 1:2:1 | 117.0 | - | 310.5 | 399.7 |
| Copolyester | Molar ratio (AVA / ABA / ABCA) |
SSP condition: temperature, °C (time, h) |
[η] | Tg, °C | Tm, °C | Ton, °C | T5%, °C |
|---|---|---|---|---|---|---|---|
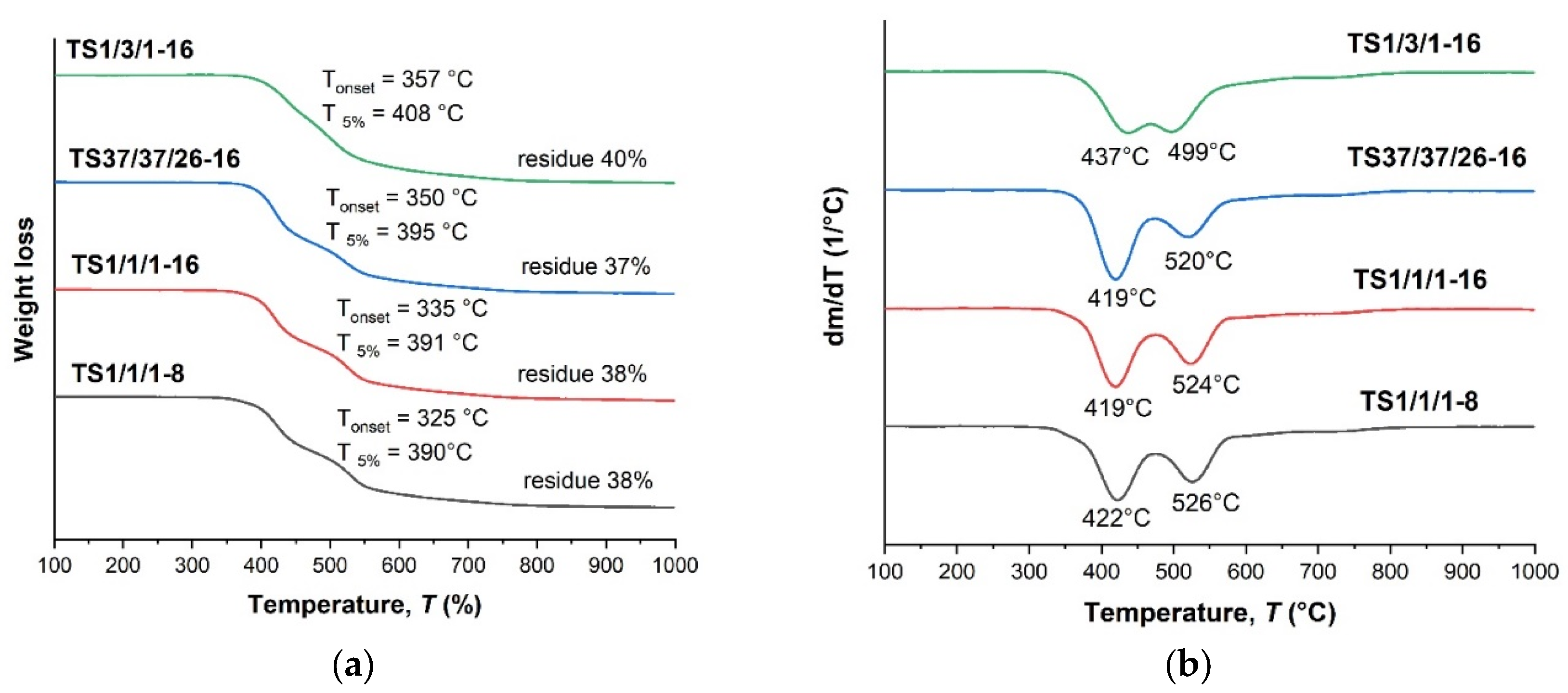
| TS 1/1/1-8 | 1:1:1 | 250 (8) | 6.3 | 114.0 | - | 325.1 | 389.8 |
| TS 1/1/1-16 | 250 (8) + 255 (8) | 8.7 | 116.8 | - | 334.9 | 391.4 | |
| TS 37/37/26-8 | 37:37:26 | 250 (8) | 13.8 | 113.1 | - | n/a | n/a |
| TS 37/37/26-16 | 250 (8) + 255 (8) | insol. | 119.6 | - | 349.7 | 394.9 | |
| TS 1/3/1-8 | 1:3:1 | 250-260 (8) | insol. | 106.7 | - | n/a | n/a |
| TS 1/3/1-16 | 260 (16) | insol. | 108.1 | - | 357.3 | 408.2 |
| Copolyester | Molar ratio (AVA / ABA / ABCA) |
[η] | Tg, °C | Ton, °C | T5%, °C |
residue at 1000°C |
|---|---|---|---|---|---|---|
| T 1/1/1 | 1:1:1 | 1.2 | 118 | 333 | 406 | 40 |
| TM 1/1/1 | insol. | 121 | 339 | 409 | 40 | |
| TS 1/1/1-8 | 6.3 | 114 | 325 | 390 | 38 | |
| TS 1/1/1-16 | 8.7 | 117 | 335 | 391 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





