You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
In Vitro Hemostatic Activity of Novel Fish Gelatin- Alginate Sponge (FGAS) Prototype
Altmetrics
Downloads
97
Views
52
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
A hemostatic sponge prototype was successfully synthesized from fish gelatin as an alternative to mammalian gelatin, which was mixed with alginate in certain combinations and double cross-linking of calcium ions and gamma irradiation at a dose of 20 kGy to improve the characteristics and effectiveness of its function as a local hemostatic agent. There was an improvement in the physicochemical and mechanical properties, porosity index, absorption capacity, biodegradation properties, biocompatibility, and hemocompatibility of the FGAS prototypes compared with the pure fish gelatin sponge. Hemostatic activity tests showed that the means of clotting time, prothrombin time, and activated partial thromboplastin time were shorter in the FGAS prototype than in the negative control, and there was no significant difference compared with the commercial gelatin sponge. The hemostatic mechanism of the FGAS prototype combined a passive mechanism as a concentrator factor and an active mechanism through the release of calcium ions as a coagulation factor in the coagulation cascade process.
Keywords:
Subject: Medicine and Pharmacology - Dentistry and Oral Surgery
1. Introduction
Uncontrolled bleeding is one of the leading causes of death in medical emergencies in civilian and military life. Approximately 80% of deaths in trauma cases in civilian society and 20% in the military are caused by exsanguination. Another cause is complications during surgical procedures, in which uncontrolled bleeding is the most common complication.[1,2] The risk of morbidity and mortality will increase in surgical procedures, especially in cases of uncontrolled bleeding, because it can be life-threatening.[3,4] Despite the proper use of conventional techniques for hemorrhage control to avoid such complications when uncontrolled bleeding occurs, a broad range of hemostatic agents are available as adjunctive measures to enhance hemostasis.[5] To treat excessive bleeding in areas that are difficult to access with conventional methods, a variety of topical hemostatic agents, including gelatin-based hemostatic agents, can be used and are currently available on the market.[6]
One of the most commonly used local hemostatic agents, especially after surgical procedures, is a mechanical hemostatic agent made from gelatin material in the shape of an absorbent sponge.[7] Gelatin is made from the denaturation of collagen-derived proteins through a limited thermos-hydrolysis process and is known as an essential natural biopolymer. It has the property to change shape reversibly between sol and gel.[8,9] The sources of gelatin as raw materials for biomaterials, including hemostatic sponges, are still dominated by mammalian gelatin from pigs and cows. Currently, the sources of gelatin used in the world originate from 46% pork skins, 29.4% cow skins, 23.1% beef bones, and 1.5% other sources such as poultry and fish.[7,9]
Using gelatin from mammals such as pigs and cows is restricted because of religious and infectious disease concerns. Both Muslim and Jewish communities are prohibited from consuming pork-based products, and Hindu communities reject bovine-based products. Both Muslim and Jewish communities are prohibited from consuming pork-based products, and Hindu communities reject bovine-based products. Furthermore, some animal-borne infectious diseases, such as bovine spongiform encephalopathy (BSE) in cows and swine flu in pigs, pose a threat to human health.[10,11] Therefore, another source of gelatin, for example, fish gelatin, has recently been required as an alternative to mammalian gelatin.
Fish gelatin has been widely researched and developed as an alternative source of mammalian (cow and pig) gelatin. However, fish gelatin has shortcomings in terms of physicochemical properties, mechanical strength, and gel stability compared with mammalian gelatin.[12] To improve the weak properties of fish gelatin, the mixing method can be used with other biopolymers, one of which is alginate, to form a gelatin-alginate composite compound. Alginate, which is found in many seaweeds, is also a natural polymer that is widely used in the biomedical field. One of the alginate salts, calcium alginate, is effective as a hemostatic agent. The fish gelatin-alginate composite can be further increased in its physicochemical properties using cross-linking methods, for example, by Ca2+ ionic cross-linking and gamma irradiation.[13,14]
This research aims to synthesize a prototype of a fish gelatin-alginate hemostatic sponge using blending methods, double cross-linking with calcium ions, and gamma irradiation, and then characterize and test the effectiveness of its hemostatic function in the blood coagulation process.
2. Materials and Methods
2.1. Materials
Gelatin (Redman fish gelatin, food grade 200 bloom, and viscosity of 3.45mPa-s) was purchased from Phoon Huat Pte. Ltd. (Singapore). Sodium alginate (analytical grade with a viscosity of 17mPa-s) and Calcium Chloride (CaCl2) were purchased from Sigma-Aldrich Corporation (America), and commercial gelatin hemostatic sponges (Ceraspon) were purchased from PT Swayasa Perkasa (Indonesia). The other reagents used in this study were at least analytically pure.
2.2. Methods
2.2.1. Synthesis of The Fish Gelatin-Alginate Sponge (FGAS) Prototype
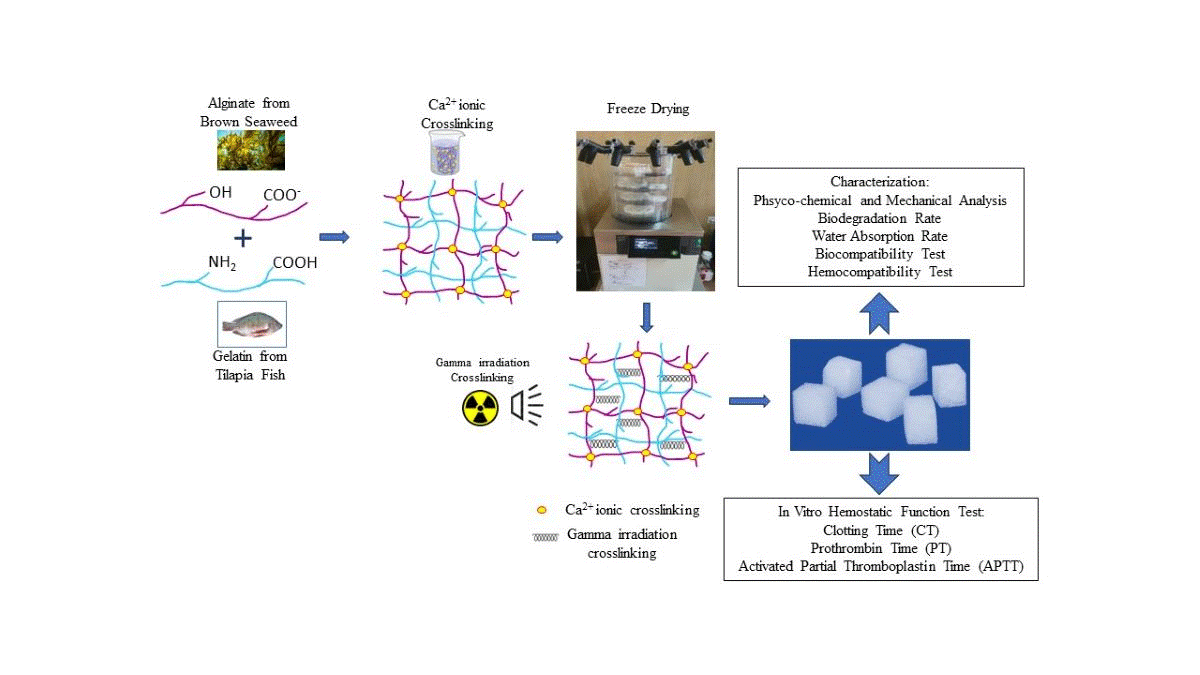
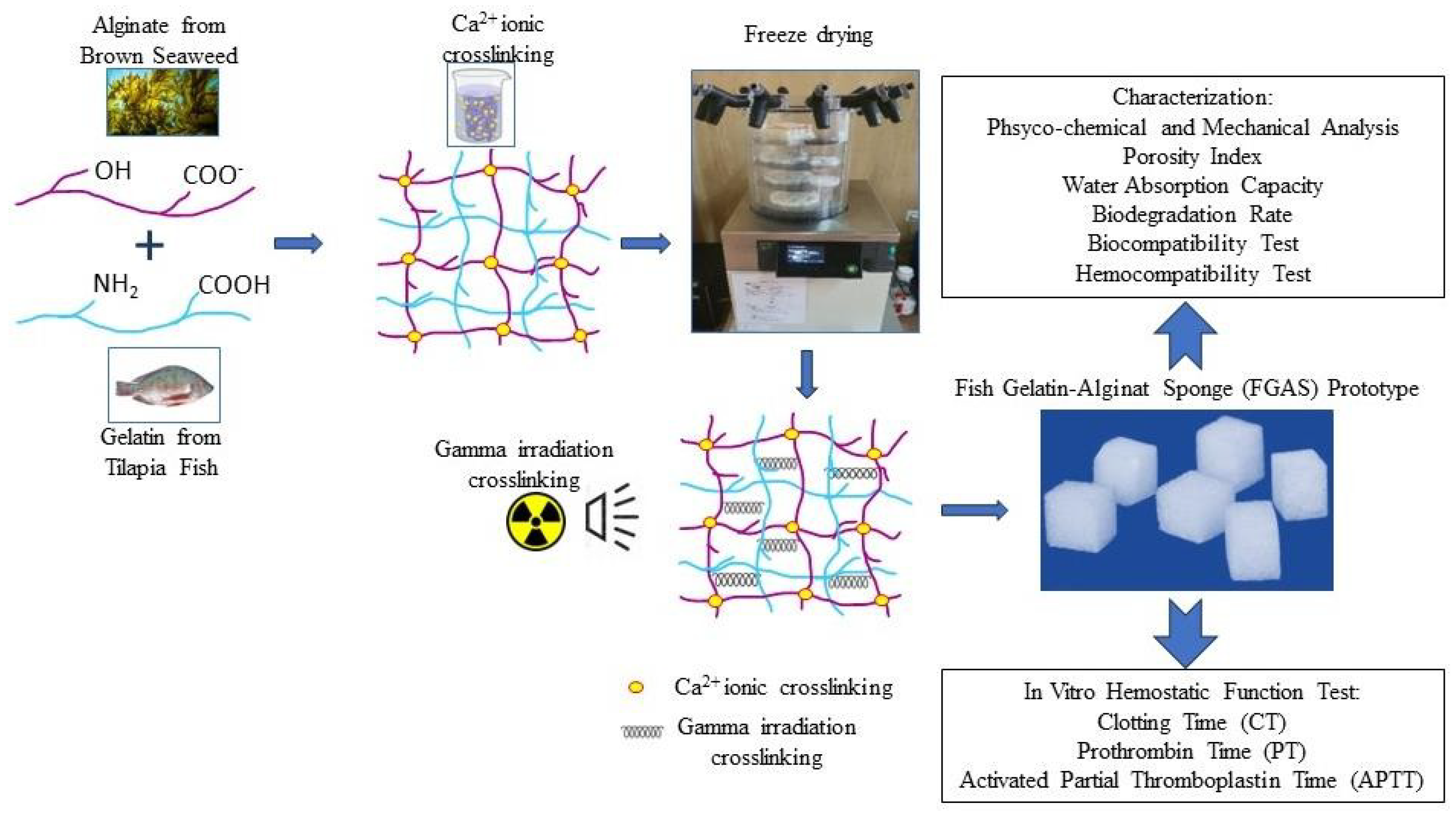
First, fish gelatin and sodium alginate powder were blended in a beaker containing double-distilled water to obtain a 4% (w/v) mixed solution with a certain proportion of fish gelatin (FG) and sodium alginate (SA) of 100/0, 75:25, 50:50, and 25:75. The solutions were then stirred evenly for 2 h at 50oC and then cast in a silicon mold to a size of 1x1x1 cm, and frozen at -20oC. The frozen fish gelatin-alginate composites, except for the composition of 100:0 (pure fish gelatin), were then immersed in 2% CaCl2 to provide ionic crosslinking between alginate and Ca2+ and produce a calcium alginate compound. In the next step, the Ca2+ crosslinked materials were then lyophilized with a freeze dryer at -50 °C for 24 h to obtain the fish gelatin-alginate sponge (FGAS) prototype. The final procedure was gamma irradiation crosslinking of the prototype materials at a 20 kGy dose. The prototype was prepared at the Research Center for Radiation Process Technology, Jakarta. All procedures were performed according to previous studies, with some modifications.[15,16,17,18,19,20,21,22]
Figure 1.
Diagrammatic representation of the research process.
2.2.2. Physicochemical and Mechanical Characterization
A scanning electron microscope (SEM) was used to analyze the surface morphology of the prototype, followed by chemical element analysis using the energy-dispersive X-ray spectroscopy (EDX/EDS) method. The samples were additionally coated with a metal (e.g., gold or platinum) before examination to avoid sample charging and increase contrast when imaging under high vacuum conditions. The analysis of the FGAS prototype functional groups was performed using Fourier transform infrared (FTIR) spectroscopy. All samples were prepared by grinding and mixing with KBr before being analyzed using an FTIR instrument. The mechanical properties were measured using the compressive strength test to determine the elasticity modulus of the FGAS prototype in wet conditions.[23,24,25]
2.2.3. Porosity Index Analysis
Porosity is an essential characteristic of sponge materials because it may directly impact the amount of water or blood they can absorb. There are various methods for measuring the porosity index of materials, including software like OriginPro and ImageJ.[26,27,28] We used OriginPro software to convert the images from SEM to quantitative measurements.
2.2.4. Water Absorption Capacity
The swelling test is a standard procedure for analyzing the absorption capacity of materials. The dry sponge (W0) was weighed and placed in a pH 7.4 PBS solution for 30 min. The sponge gel was then wiped with filter paper to remove the residual solution from the sponge surface and weighed again (W1). The sponge’s water absorption rate was calculated using the following formula:[29]
Water absorption rate (%) = (W1−W0)/W0 x 100
2.2.5. Biodegradation Rate
The dry sponge (W0) was weighed and placed in a pH 7.4 PBS solution at 37 °C for 1, 7, 14, and 30 days. On each day, the sponge was removed to dry, and then the sponge was weighed again (W1). The weight retention (%) was calculated using the following equation:[30]
Weight retention (%) = (W0−W1)/W0 x 100
2.2.6. Biocompatibility (Cytotoxicity Test)
The cytotoxicity test was performed according to the ISO 10993-5 standard. The sponges produced were incubated on BHK-21 fibroblasts for 24, 48, and 72 h to measure cell viability by the MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cell viability was measured by reading the optical density (OD) of the sample, control, and blank with an Elisa reader and converting it to the following formula:[31]
2.2.7. Hemocompatibility (Hemolysis Test)
Hemocompatibility is an essential criterion for any biomaterial that will interact closely with blood. The sponge samples were evaluated by hemolytic tests according to ISO 10993-4. Each of these samples was mixed with 0.4 mL of the incubated blood sample individually. Normal saline and deionized water were used as negative (neg) and positive (pos) controls, respectively. After completing all of the hemolysis assay steps, all samples were centrifuged at 3000 rpm for 5 min. Finally, the absorbance of the supernatants was determined at 545 nm using a UV-Vis spectrophotometer and counted using the following equation:[32]
2.2.8. Clotting Time (CT)
The in vitro clotting time was determined using the previously described method. Each sponge sample was placed in a clean test tube. The tubes were then immersed in a water bath at a constant temperature of 37°C for 1 h. Anticoagulated fresh donor blood (1 mL) was added to each of the test tubes and placed in the water bath. The tubes were tilted at 30° every 30 s until the blood in the tubes stopped flowing.[21] The coagulation times of blood in various tubes were recorded.
2.2.9. Prothrombin Time (PT)
Prothrombin time (PT) is the in vitro clotting time test after placing the PT reagent, which contains thromboplastin (phospholipids with tissue factor), calcium, and citrated plasma.[33] A platelet-poor plasma (PPP) sample was obtained by centrifuging fresh human blood at 3000 g for 10 min. Each sponge sample (1.0 cm3) received 500 µL of PPP and was incubated for 30 min at 37°C. To measure PT, combine 100 µL of PT reagent with 200 µL of PPP in a test tube and incubate at 37°C for 3 min. PT was then measured using an automated blood coagulation analyzer (COAX Bio System, Jerman). To serve as a control group, 500 µL of PPP extracted from fresh human blood was incubated at 37°C for 30 min without any test material.
2.2.10. Activated Partial Thromboplastin Time (APTT)
The APTT test measures the time it takes for plasma to clot in vitro after adding calcium, an activator of the intrinsic pathway, and the APTT reagent, which contains phospholipid, a platelet substitute that lacks tissue factor.[33] A platelet-poor plasma (PPP) sample was obtained by centrifuging fresh human blood at 3000 g for 10 min. Each sponge sample (1.0 cm3) received 500 µL of PPP and was incubated for 30 min at 37°C. To measure APTT, incubate 100 µL of PPP with 100 µL of reagent at 37°C for 3 min. Finally, 100 mL of aqueous calcium chloride (CaCl2) was added to the solution, and the coagulation analyzer was used to determine the APTT value. To serve as a control group, 500 µL of PPP extracted from fresh human blood was incubated at 37°C for 30 min without any test material.
2.3. Statistical Analysis
The experimental results are presented as the mean ± SD. The data was analyzed using the one-way ANOVA method for studies with more than two treatment groups and the independent sample t-test method for studies with only two treatment groups.
3. Results and Discussion
3.1. Synthesis of the Fish Gelatin-Alginate Sponge (FGAS) Prototype
This research produced a fish gelatin-alginate sponge prototype, which was synthesized by mixing and freeze-drying methods from fish gelatin and sodium alginate with Ca2+ ions and gamma irradiation cross-linking, and was proven to have hemostatic characteristics and effectiveness against blood clotting. The FGAS prototype is a white sponge, cube-shaped, and has an interconnected porous structure containing chemical elements and functional groups combined from gelatin and alginate, as well as additional calcium ions.
At a larger gelatin composition (75:25), the sponge prototype material appeared cuboid with flat and straight edges. The addition of alginate compositions (50:50 and 25:75) changed the shape of the sponge prototype to become more rounded at each corner. This is thought to be caused by an increase in the alginate composition, causing more cross-linking between alginate and Ca2+ ions to form an “egg-box model” structure. This structure is formed by an ionic bond between the Ca2+ ions and the carboxyl group on the guluronic block of the alginate polymer chain, thereby forming a stable bond in the alginate gelation process.[34,35] The formation of more egg-box model structures due to increasing alginate concentration can cause shrinkage and compaction of the sponge material, starting at the weakest corner points, so that the sponge containing more alginate becomes rounded at the corners.[36]
3.2. Physicochemical and Mechanical Characterization
The results of the SEM examination in Figure 2 show the surface morphology of the FGAS prototype, consisting of a porous structure with varying porous sizes with an average size between 39.03 – 266.66 µm. These porous sizes qualify as hemostatic sponges, as reported by previous research, which states that a porous size of between 50-100 µm is sufficient to accommodate the concentration of red blood cells and platelets in the initial process of blood clotting.[37] According to the IUPAC (International Union of Pure and Applied Chemistry) criteria, sponge material with a porous size of >50 µm is included in the macroporous category, which is ideal for containing cell concentrations because, in general, the average cell size is <100 µm.[38]
The SEM images can also be used to analyze the chemical element content of the FGAS prototype combined with energy-dispersive X-ray spectroscopy (EDX/EDS) analysis, as shown in Table 1. The main chemical element composition of the FGAS prototype material consists of carbon (C), nitrogen (N), oxygen (O), and calcium (Ca). The element content corresponds to the main elements contained in the raw materials, namely fish gelatin and alginate, which consist of C, N, O, and H. The H element cannot be detected using EDX analysis because it is very light. Calcium (Ca) is an additional element formed by cross-linking sodium alginate with Ca2+ ions in the CaCl2 solution.[39]
The analysis of the FGAS prototype functional groups was carried out using the Fourier transform infrared (FTIR) test to observe the wavelength spectrum in the form of specific peaks that indicate the functional groups possessed by the material, as shown in Figure 3 below.
Figure 3 shows the peaks of the infrared light absorption spectrum for typical functional groups found in gelatin polymers, alginate, and gelatin-alginate composites. Gelatin includes hydroxyl groups (O- H) and amine groups (N-H) at peak 3435.28cm-1 (superimposed) and carbonyl (C=O) at 1653.99cm-1. Alginate includes hydroxyl (O-H) at 3439.14cm-1; carbonyl (C=O) at 1621.20cm-1; carboxyl (C-O) at 1314.51cm-1; guluronate and mannuronate fingerprints at 894.99cm-1 and 819.76cm-1. The FTIR spectrum that appears in the gelatin-alginate composite is a combination of the spectrum of functional groups from gelatin and alginate polymers. The effect of gamma radiation can be seen from the shift in the absorption peaks of functional groups toward larger wavelengths in the group of samples that received gamma irradiation. these findings confirmed the previous studies of Sow et al., Derkach et al., and Hariyanti et al.[18,40,41]
The compressive strength test is a method for determining the mechanical properties of a biomaterial by measuring the elastic modulus, which is an important parameter in analyzing the characteristics of a sponge-shaped biomaterial that will be applied as a local hemostatic agent.[42] Sponge elastic modulus measurement was performed in wet conditions according to the purpose of hemostatic sponge application in bleeding situations.
All sponge samples show greater elasticity modulus mean compared with PFGS and were statistically significant. The FGAS75:25 Nir and FGAS75:25 Ir (157,79±3.99 and 173,86±5.18) have the closest values of elastic modulus to commercial gelatin sponges (133,54±9,79), but there was no significant difference in elastic modulus between irradiated and non-irradiated samples. Fish gelatin has low mechanical strength, especially in a single form, therefore, modifications are needed both in the synthesis method and by adding reinforcement materials to increase it.[43,44]
The increase in the elastic modulus of the FGAS prototype is thought to be due to the addition of alginate, resulting in an increase in density and shrinkage of the polymer chain caused by the formation of a model egg-box structure due to the ionic cross-linking reaction between the guluronate residue of the alginate polymer and the divalent cation Ca2+. As reported by Ma et al., in the preparation of fish gelatin hydrogel mixed with alginate, there was an increase in the elastic modulus.[45]
3.3. Porosity Index Analysis
Porosity is an essential characteristic of sponge materials because it may directly impact the amount of water or blood they can absorb, and these characteristics are strongly reliant on the underlying structure and morphology of sponges.[26,27] We analyzed the porosity index using OriginPro software, and the results are shown in Figure 5. All FGAS samples showed greater porosity index means compared with PFGS and were statistically significant.
The addition of alginate increases the porosity index of the FGAS prototype above the standard value of 60% for all FGAS prototype compositions, both irradiated and non-irradiated, namely between 71.67% and 75.32%.46. The porosity indices of FGAS75:25 Nir and FGAS75:25 Ir have the largest means (75.33%±1.25 and 75.07%±2.15) compared to all samples and have no significant difference compared to CGS. This is thought to be due to the addition of alginate, which is hydrophilic and binds water more strongly than fish gelatin during mixing so that when the lyophilization process (freeze-drying) is performed, it leaves more pores in the FGAS prototype material.[47] Sponge’s highly porous nature makes it easier for them to absorb blood fluid, which in turn minimizes the amount of excess exudate. Similarly, the spongy hemostatic agents and wound dressings rely on the sponge’s absorption properties which depend on the shape and structure of the sponges.[26,27,28] The comparison between irradiated and non-irradiated samples shows that there was no significant difference in the porosity index.
3.4. Water Absorption Capacity
Absorbing fluid or blood is one of the main requirements for materials with high absorption capacity, such as local hemostatic sponges.[29] The results of the water absorption rate of the FGAS prototype are shown in Figure 6, where all FGAS samples show greater water absorption rates than PFGS and are statistically significant.
The addition of alginate can increase the absorption capacity ratio of the FGAS prototype. As seen in Figure 6, the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the largest mean absorption capacity of 3147.45 ±19.87 and 3141.58 ± 34.22, respectively, and a significant difference statistically compared to the mean absorption capacity of PFGS. This is possible because of the addition of alginate, which has greater hydrophilic properties, so it can bind more water molecules. During the lyophilization process, the bound water molecules will be sublimated, leaving many spaces or pores in the material.[48] There was no significant difference in the water absorption capacity between the irradiated and non-irradiated samples.
3.5. Biodegradation Rate
The biodegradation rate of the FGAS prototype can be measured by counting the weight retention after immersing the sponge in a solution for a certain period.[30] The results of the biodegradation rate of the FGAS prototype are shown in Figure 7, where all sponge samples showed slower degradation rates compared with PFGS.
On Day 1 of observation, the FGAS prototype group, both irradiated and non-irradiated, showed a smaller weight loss ratio compared with PFGS, which was completely dissolved (100%) and did not qualify as a hemostatic sponge. The rapid degradation of pure fish gelatin sponge (PFGS) is thought to be due to the amino acid hydroxyproline content in fish gelatin being lower than that of mammalian gelatin, causing a low solubility (melting point) at a temperature of 25 °C–27 °C compared with mammalian gelatin at 32 °C – 35 °C.[49] This was confirmed by Yang et al., who reported that pure gelatin sponges without cross-linking can immediately dissolve when immersed in PBS solution.[50]
The FGAS prototype has a slower biodegradation ratio than PGFS, allegedly due to the addition of alginate and the presence of ionic cross-linking with Ca2+ to form calcium alginate compounds, which are difficult to dissolve in water, plus the combination of hydrogen bonds between gelatin and alginate molecules, which are strengthened by covalent bonds through cross-linking by gamma irradiation.[30,51] On Days 7, 14, and 30, it was shown that FGAS75:25 Nir and FGAS75:25 Ir have similar weight loss rates to CGS. There was also a significant difference in biodegradation rates between irradiated and non-irradiated FGAS samples. This proves that gamma irradiation can strengthen the bonds between peptide molecules in gelatin and between functional groups of gelatin and alginate through covalent bonds.[52,53]
3.6. Biocompatibility (Cytotoxicity Test)
Based on the MTT Assay test results presented in Figure 8, it can be seen that all samples show an average viability of above 70% and no significant differences, which means that all samples meet the biocompatibility requirements according to the ISO 10993-5 standard.[54]
The cytotoxicity test results prove that all FGAS prototypes, both irradiated and non-irradiated have non-cytotoxic properties. Statistical testing using one-way ANOVA revealed no significant differences in the mean cell viability values for all research samples. These results confirmed several previously studies, such as Rezaie et al., who synthesized a highly absorbent sponge from bovine gelatin and sodium alginate by cross-linking using CaCl2. The results of the cytotoxicity test on human fibroblast cells showed that the viability of all test samples was above 80%.[16] Another study reporting the effect of gamma irradiation at a dose of 25 kGy on the cytotoxicity of gelatin-alginate bioadhesive material showed that the average overall fibroblast cell viability value was in the range of 89–100%.[52]
3.7. Hemolysis Test
The hemolysis test results as presented in Figure 9 show that all samples show significant differences in erythrocyte hemolysis rates but are still below 5%, which means that all samples met the hemocompatibility requirements according to the ISO 10993-4 standard.[32]
There was also a significant difference in hemolysis rates between irradiated and nonirradiated FGAS samples. These findings confirmed the results of previous research by Rallapalli et al., who reported the effect of gamma irradiation at a dose of 25 kGy as a sterilization method on bovine pericardium scaffolds on the hemolysis ratio. There was an increase in the average hemolysis ratio from 1.8% before irradiation to 6.32% after irradiation. The increase in the hemolysis ratio is considered due to the irradiation process causing damage to the surface of the biomaterial, which becomes rougher so that it can damage erythrocytes and result in the lysis of hemoglobin.[55]
Hemocompatibility characteristics are an important requirement for assessing interactions between drugs or biomaterials that function or are related to the circulatory system. Hemocompatible drug compositions or materials are capable of interacting with blood components without causing clinically significant adverse reactions such as thrombosis, hemolysis, complement activation, or other adverse side effects.[56]
3.8. Hemostatic Activity Test
At this stage, the hemostatic function effectiveness test was only performed on the best FGAS prototype which resulted from the characterization test in the previous stage, where the FGAS75:25 Nir and FGAS75:25 Ir were found to be the selected prototypes.
3.8.1. Clotting Time (CT)
Based on the results of the blood clotting time test, as shown in Figure 10, the mean clotting time for the FGAS75:25 Nir prototype was 294.33±24.36 seconds and the FGAS75:25 Ir prototype was 297.17±19 seconds, significantly different compared with the negative control (485.00±24.36 seconds), but not significantly different than CGS (272,33±22,47 seconds). There was no significant difference in clotting time between the irradiated and non-irradiated samples.
The FGAS prototype has an effective function as a local hemostatic agent by accelerating the bleeding time due to the porous structure of the hemostatic sponge, which allows it to absorb a lot of blood fluid while concentrating coagulation components, red blood cells, and platelets. Immediately upon contact with blood, the hemostatic sponge causes adhesion and aggregation of platelets, which in turn causes the formation of a platelet plug, thereby preventing blood flow from the wound. It also triggers the release of blood clotting factors involved in the extrinsic and intrinsic coagulation pathways, resulting in formation of a stable blood clot that helps control bleeding from wounds.[37]
Several previous studies have confirmed the use of gelatin and alginate as local hemostatic agents, including research by Dai et al., who reported that the manufacture of gelatin (GA), calcium alginate (CA), and silk fibroin (SF) composite sponges had good effectiveness by speeding up bleeding time.[57] Chen et al. reported that the synthesis of gelatin-alginate hemostatic sponge with the addition of curcumin was effective in accelerating blood clotting time and preventing tumor recurrence.[30]
3.8.2. Prothrombin Time (PT)
Figure 11 shows that the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the shortest mean prothrombin time, 10.9±0.52 and 11.1±0.59 seconds, respectively, and significant difference compared with the negative control (10,9±0,52 seconds) but not significant difference than CGS (11,7±0,48 seconds). There was also no significant difference in prothrombin time between the irradiated and non-irradiated samples.
The effectiveness of the FGAS prototype in accelerating prothrombin time (PT) is considered due to the content of calcium ions bound in alginate. When in contact with blood, the FGAS prototype turns into a hydrogel, and a reaction occurs that encourages the entry of calcium ions into the wound through an ion exchange reaction with sodium ions in the blood. Furthermore, calcium ions trigger the production of coagulation factors VII, IX, and X, as well as platelets, activate the coagulation cascade reaction, and accelerate the hemostasis process.[58]
Previous research has reported the role of calcium alginate in the coagulation process because it contains phytohemagglutinin, which can trigger red blood cell aggregation and change erythrocyte morphology, exposing phosphatidylserine on the surface of erythrocytes and accelerating the conversion of local prothrombin to thrombin.[59] Dai et al. reported the hemostatic effectiveness of a composite sponge made from silk fibroin, gelatin, and calcium alginate by accelerating prothrombin time.[57]
3.8.3. Activated Partial Thromboplastin Time (APTT)
The results of the APTT test, as shown in Figure 12, show that the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the shortest APPT means, namely 32.88 ± 0.78 and 33.18±1.64 seconds, respectively, which is significantly different from the negative control (41,12±0,63), but there is no significant difference with CGS (33,92±0,63). There was also no significant difference in the APTT means between the irradiated and non-irradiated samples.
Similar to the PT test, the APTT test results also suggested that the ability of the FGAS prototype to accelerate APTT was due to the calcium ion content bound in the alginate that was released when the sponge came into contact with the blood and then promoted the cascade coagulation process.[58]
As reported by Che et al. in making a polyelectrolyte multilayer film on a sodium alginate/gelatin sponge, it has the effectiveness of shortening APTT, which is thought to be due to the activity of the calcium ions contained in the sponge.[60] Another study by Kumar et al. stated that the synthesis of calcium alginate- zinc chloride hydrogel is determined by the content of calcium ions, which can trigger clotting factors in the coagulation cascade process.[61]
5. Conclusions
The FGAS prototype has better physicochemical and mechanical characteristics, porosity index, water absorption capacity, biodegradation rate, biocompatibility, and hemocompatibility than PFGS where the FGAS75:25Nir and FGAS75:25Ir are selected as the best prototype composition. It is also proven that the FGAS prototype is effective as a local hemostatic agent, where the hemostatic mechanism is a combination of a passive mechanism as a concentrator factor through superiority in porosity index and absorption capacity, functioning as a matrix to collect blood cells, especially platelets, and coagulation factors, and the active mechanism through the content of calcium ions (Ca2+), which are released in the coagulation cascade process. The gamma irradiation of 20kGy dose only has an effect in the biodegradation and hemolysis rate characteristics of the FGAS prototype.
Author Contributions
Heri Herliana was responsible for conceptualization, formal analysis, and writing. Harmas Yazid was responsible for reviewing and supervising. Avi Laviana was responsible for editing and reviewing. Ganesha Wandawa was responsible for editing and directing. Basril Abbas was responsible for the methodology, resources, and field supervision.
Funding
This study received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Indonesian Marine Hospital (Number: 18/X/2023/RSMC, date of approval: 30th October 2023).
Data Availability
This article includes all data presented or analyzed during the study.
Acknowledgments
We thank the Doctoral Program Faculty of Dentistry, Universitas Padjadjaran, and the National Research and Innovation Agency (NRIA), for their technical support.
Conflicts of Interest
The authors declare that they have no conflicts of interest or personal relationships that could have influenced the work presented in this paper.
References
- Gordy, S.D.; Rhee, P.; A Schreiber, M. Military applications of novel hemostatic devices. Expert Rev. Med Devices 2011, 8, 41–47. [Google Scholar] [CrossRef]
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic strategies for uncontrolled bleeding: A comprehensive update. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef]
- Nagarale, R.; Todkar, M.; Khan, S.; Khan, Y.; Rehan, M.; Rizvi, Q. Assessment of knowledge, importance and management of uncontrolled bleeding in dental surgical procedures among dental professionals. Int. J. Appl. Dent. Sci. 2021, 7, 312–316. [Google Scholar] [CrossRef]
- de Campos, N.; Furlaneto, F.; Buischi, Y.D.P. Bleeding in dental surgery. In Contemporary Applications of Biologic Hemostatic Agents across Surgical Specialties-Volume 2; IntechOpen, 2019. [Google Scholar]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilicò, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 1–20. [Google Scholar] [CrossRef]
- Irfan, N.I.; Zubir, A.Z.M.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef]
- Herliana, H.; Yusuf, H.Y.; Laviana, A.; Wandawa, G.; Cahyanto, A. Characterization and Analysis of Chitosan-Gelatin Composite-Based Biomaterial Effectivity as Local Hemostatic Agent: A Systematic Review. Polymers 2023, 15, 575. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34. [Google Scholar] [CrossRef]
- Alipal, J.; Pu'Ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today: Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Nurilmalaa, M.; Suryamarevita, H.; Hizbullah, H.H.; Jacoeb, A.M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi J. Biol. Sci. 2021, 29, 1100–1110. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Kahtani, H.A.; Jaswir, I.; AbuTarboush, H.; Ismail, E.A. Extraction and characterization of gelatin from camel skin (potential halal gelatin) and production of gelatin nanoparticles. Saudi J. Biol. Sci. 2020, 27, 1596–1601. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Rajeswari, A.; Stobel Christy, E.J.; Pius, A. Biopolymer blends and composites [Internet]. Biopolymers and their Industrial Applications. Elsevier Inc., 2021; pp. 105–147. [Google Scholar] [CrossRef]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Lou, C.W.; Huang, M.S.; Chang, C.Y.; Lu, C.T.; Chen, W.C.; Lin, J.H. Preliminary Study in Cross-Linked Gelatin/Alginate Sponges. Appl. Mech. Mater. 2012, 184–185, 1102–1105. [Google Scholar] [CrossRef]
- Rezaie, A.; Mehdipour, A.; Salmanipour, S.; Alipour, N.; Salehi, R. HIGHLY POROUS ALGINATE/GELATIN SPONGE FOR HEMOSTASIS OF SEVERE FEMORAL BLEEDING IN RATS. Stud. Med Sci. 2023, 33, 839–856. [Google Scholar] [CrossRef]
- Song, Y.; Xu, L.; Deng, L. Radiation cross-linked gelatin/sodium alginate/carboxymethylcellulose sodium hydrogel for the application as debridement glue paste. Polym. Bull. 2021, 79, 725–742. [Google Scholar] [CrossRef]
- Hariyanti, H.; Erizal, E.; Apriyani, R.Z.; Perkasa, D.P.; Lestari, I.; Rahmi, H. Synthesis of Polyvinyl Alcohol (PVA)-Gelatin Hydrogel from White Snapper (Lates calcarifer, Bloch) with Gamma Irradiation and Its Characterizations. At. Indones. 2023, 1, 69–75. [Google Scholar] [CrossRef]
- Lu, H.; Butler, J.A.; Britten, N.S.; Venkatraman, P.D.; Rahatekar, S.S. Natural Antimicrobial Nano Composite Fibres Manufactured from a Combination of Alginate and Oregano Essential Oil. Nanomaterials 2021, 11, 2062. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Hu, Z.; Ouyang, Q.Q.; Cheng, Y.; Hong, P.Z.; Liao, M.N.; Chen, F.J.; Li, S.D. Optimization of preparation process and characterization of carboxymethyl chitosan/sodium alginate hemostatic sponge. IOP Conf. Series: Mater. Sci. Eng. 2017, 213, 012045. [Google Scholar] [CrossRef]
- Kang, H.J.; Jo, C.; Lee, N.Y.; Kwon, J.H.; Byun, M.W. A combination of gamma irradiation and CaCl2 immersion for a pectin-based biodegradable film. Carbohydr. Polym. 2005, 60, 547–551. [Google Scholar] [CrossRef]
- Kuo, Z.-K.; Lai, P.-L.; Toh, E.K.-W.; Weng, C.-H.; Tseng, H.-W.; Chang, P.-Z.; Chen, C.-C.; Cheng, C.-M. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci. Rep. 2016, 6, 32884. [Google Scholar] [CrossRef]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful scanning electron microscopic (SEM) visualization of 3D printed alginate-based scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-Montmorillonite Composite Sponge for Safe and Effective Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef]
- Hojat, N.; Gentile, P.; Ferreira, A.M.; Siller, L. Automatic pore size measurements from scanning electron microscopy images of porous scaffolds. J. Porous Mater. 2023, 30, 93–101. [Google Scholar] [CrossRef]
- Tasya, A.Y.; Kusumawati, D.H. Karakteristik Porositas Wound Dressing Nanofiber PVA-Ekstrak Daun Nangka. J Inov Fis Indonesia [Internet] 2023, 12, 106–112. [Google Scholar]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef]
- Sharifi, S.; Dizaj, S.M.; Ahmadian, E.; Karimpour, A.; Maleki, A.; Memar, M.Y.; Ghavimi, M.A.; Abdolahinia, E.D.; Goh, K.W. A Biodegradable Flexible Micro/Nano-Structured Porous Hemostatic Dental Sponge. Nanomaterials 2022, 12, 3436. [Google Scholar] [CrossRef]
- Hossain, S.; Shaikh, A.A.; Jahan, S.A.; Mahmud, M.; Bin Mobarak, M.; Rahaman, S.; Uddin, N.; Ahmed, S. Exploring the biomedical competency of gamma-radiation aided hydroxyapatite and its composite fabricated with nano-cellulose and chitosan. RSC Adv. 2023, 13, 9654–9664. [Google Scholar] [CrossRef]
- Chee, Y.L. Coagulation. J R Coll Physicians Edinb. 2014, 44, 42–45. [Google Scholar] [CrossRef]
- Pulat, M.; Ozukaya, D. Preparation and Characterization of Na-Alginate Hydrogel Beads. The Eurasia Proceedings of Science Technology Engineering and Mathematics 2019, 6, 32–38. [Google Scholar]
- Kaur, N.; Singh, B.; Sharma, S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharma Innov J [Internet] 2018, 7, 142–148. [Google Scholar]
- Zhang, X.; Wang, K.; Hu, J.; Zhang, Y.; Dai, Y.; Xia, F. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 2020, 8, 25390–25401. [Google Scholar] [CrossRef]
- Nepal, A.; Tran, H.D.N.; Nguyen, N.-T.; Ta, H.T. Advances in haemostatic sponges: Characteristics and the underlying mechanisms for rapid haemostasis. Bioact. Mater. 2023, 27, 231–256. [Google Scholar] [CrossRef]
- Ebrahimi, M. Porosity parameters in biomaterial science: Definition, impact, and challenges in tissue engineering. Front. Mater. Sci. 2021, 15, 352–373. [Google Scholar] [CrossRef]
- Taheraslani, M.; Gardeniers, H. High-Resolution SEM and EDX Characterization of Deposits Formed by CH4+Ar DBD Plasma Processing in a Packed Bed Reactor. Nanomaterials 2019, 9, 589. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron'Ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Yang, G.; Huang, Z.; McCarthy, A.; Huang, Y.; Pan, J.; Chen, S.; Wan, W. Super-Elastic Carbonized Mushroom Aerogel for Management of Uncontrolled Hemorrhage. Adv. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Atma, Y. Synthesis and application of fish gelatin for hydrogels/ composite hydrogels: A review. Biointerface Res Appl Chem. 2022, 12, 3966–3976. [Google Scholar]
- Ma, C.; Choi, J.-B.; Jang, Y.-S.; Kim, S.-Y.; Bae, T.-S.; Kim, Y.-K.; Park, J.-M.; Lee, M.-H. Mammalian and Fish Gelatin Methacryloyl–Alginate Interpenetrating Polymer Network Hydrogels for Tissue Engineering. ACS Omega 2021, 6, 17433–17441. [Google Scholar] [CrossRef] [PubMed]
- Cuicui, D.; Kuan, C.; Yue, W.; Yifan, Y.; Xiaohong, C.; Jingyi, L.; et al. Dual green hemostatic sponges constructed by collagen fibers disintegrated from Halocynthia roretzi by a shortcut method. Mater Today Bio. 2024, 24, 1–12. [Google Scholar]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef] [PubMed]
- Saarai A, Kasparkova V, Sedlacek T, Saha P. A comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. Recent Res Geogr Geol Energy, Environ Biomed - Proc 4th WSEAS Int Conf EMESEG’11, 2nd Int Conf WORLD-GEO’11, 5th Int Conf EDEB’11. 2011;384–9.
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar Drugs 2021, 19. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Junkyu, S. Evaluation of Calcium Alginate Microparticles Prepared Using a Novel Nebulized Aerosol Mediated Interfacial Crosslinking Method. University of Toledo, 2016. [Google Scholar]
- Foox, M.; Ben-Tzur, M.; Koifman, N.; Zilberman, M. Effect of gamma radiation on novel gelatin alginate–based bioadhesives. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 611–618. [Google Scholar] [CrossRef]
- Azmir, M.S.N.A.; Moni, N.; Gobetti, A.; Ramorino, G.; Dey, K. Advances in modulating mechanical properties of gelatin-based hydrogel in tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 1–36. [Google Scholar] [CrossRef]
- Gruber, S.; Nickel, A. Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Front. Med Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Rallapalli, S.; Liman, A.M.; Guhathakurta, S. Hemocompatibility and surface properties of bovine pericardial patches: Effects of gamma sterilization. Curr. Med. Res. Pr. 2016, 6, 224–228. [Google Scholar] [CrossRef]
- Nalezinkova, M. In vitro hemocompatibility testing of medical devices. Thromb. Res. 2020, 195, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, M.; Gong, J.; Meng, L.; Zhang, B.; Zhang, Y.; Yin, Y.; Wang, J. Silk fibroin/gelatin/calcium alginate composite materials: Preparation, pore characteristics, comprehensive hemostasis in vitro. Mater. Des. 2022, 216, 110577. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Qin, S. Three Polymers from the Sea: Unique Structures, Directional Modifications, and Medical Applications. Polymers 2021, 13, 2482. [Google Scholar] [CrossRef] [PubMed]
- Che, C.; Liu, L.; Wang, X.; Zhang, X.; Luan, S.; Yin, J.; Li, X.; Shi, H. Surface-Adaptive and On-Demand Antibacterial Sponge for Synergistic Rapid Hemostasis and Wound Disinfection. ACS Biomater. Sci. Eng. 2020, 6, 1776–1786. [Google Scholar] [CrossRef]
- Kumar, A.; Sah, D.K.; Khanna, K.; Rai, Y.; Yadav, A.K.; Ansari, M.S.; Bhatt, A.N. A calcium and zinc composite alginate hydrogel for pre-hospital hemostasis and wound care. Carbohydr. Polym. 2023, 299, 120186. [Google Scholar] [CrossRef]
Figure 2.
SEM examination of the surface morphology of the FGAS prototype with magnifications of 300, 500, and 700X shows interconnected porous material.
Figure 2.
SEM examination of the surface morphology of the FGAS prototype with magnifications of 300, 500, and 700X shows interconnected porous material.

Figure 3.
FTIR Spectra of Alginate, Gelatin, and The FGAS Prototypes.
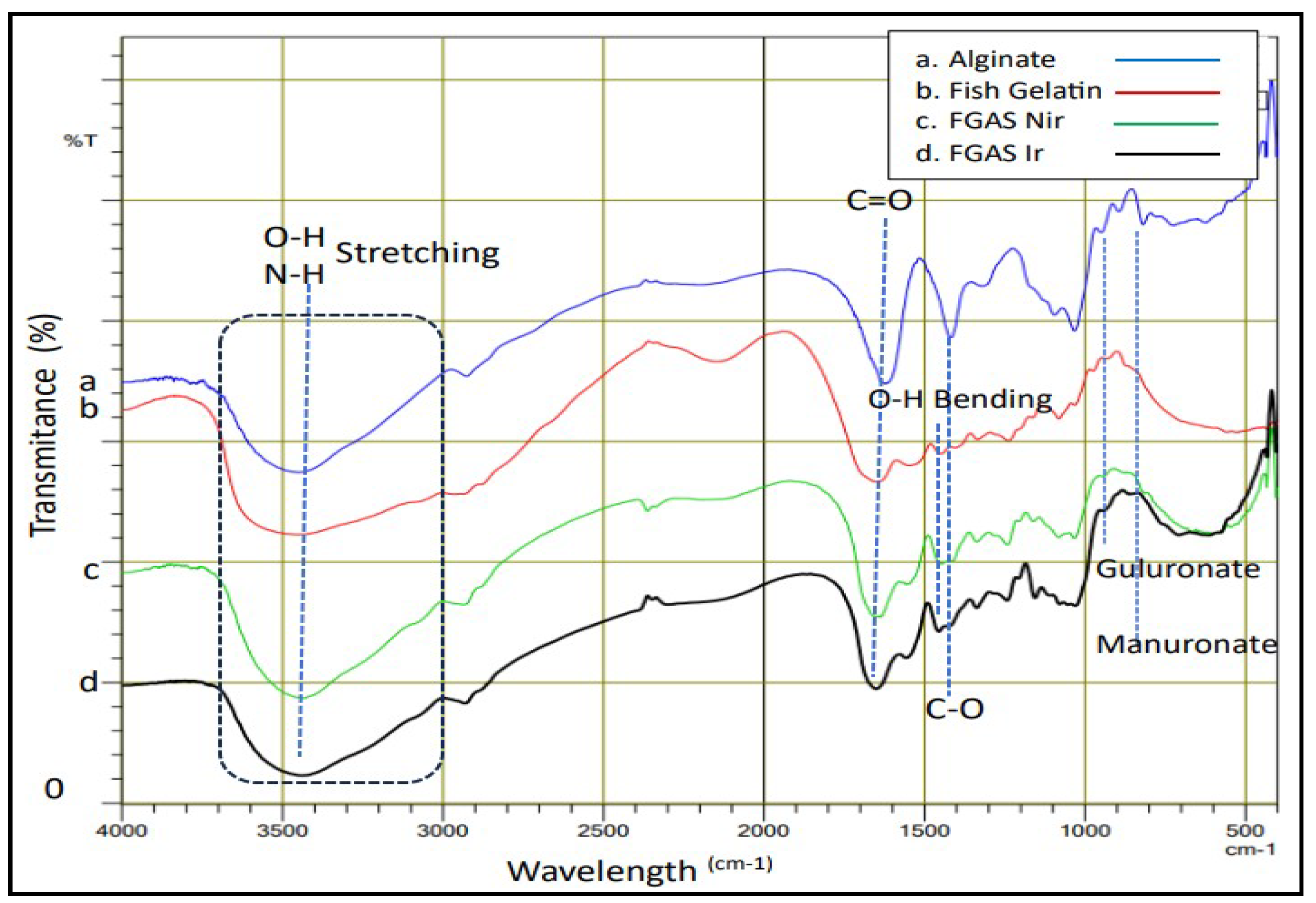
Figure 4.
Modulus Elastic of The FGAS Prototypes in Wet Conditions (**p < 0.01).
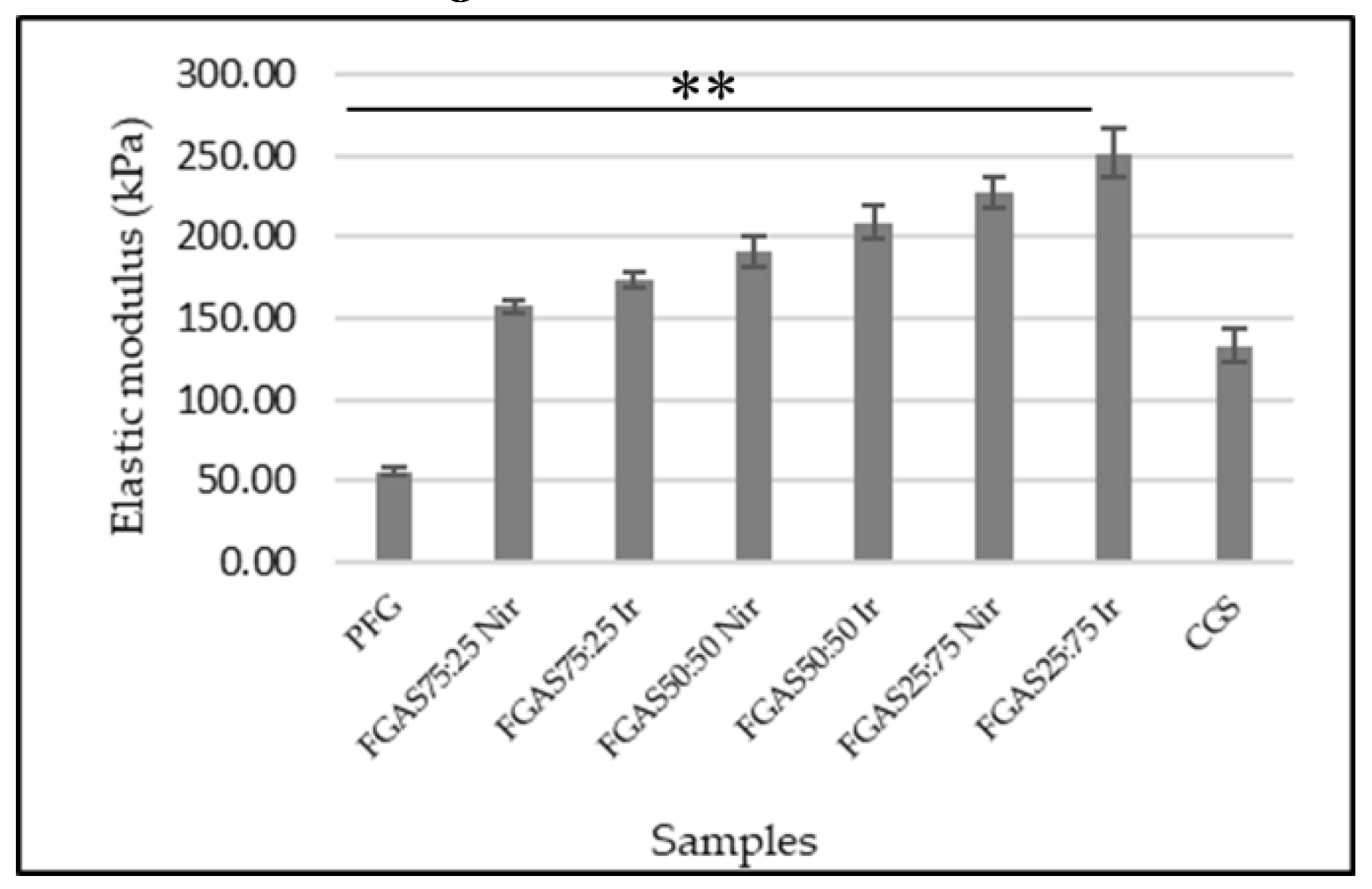
Figure 5.
Porosity Index of FGAS Prototypes (**p < 0.01).
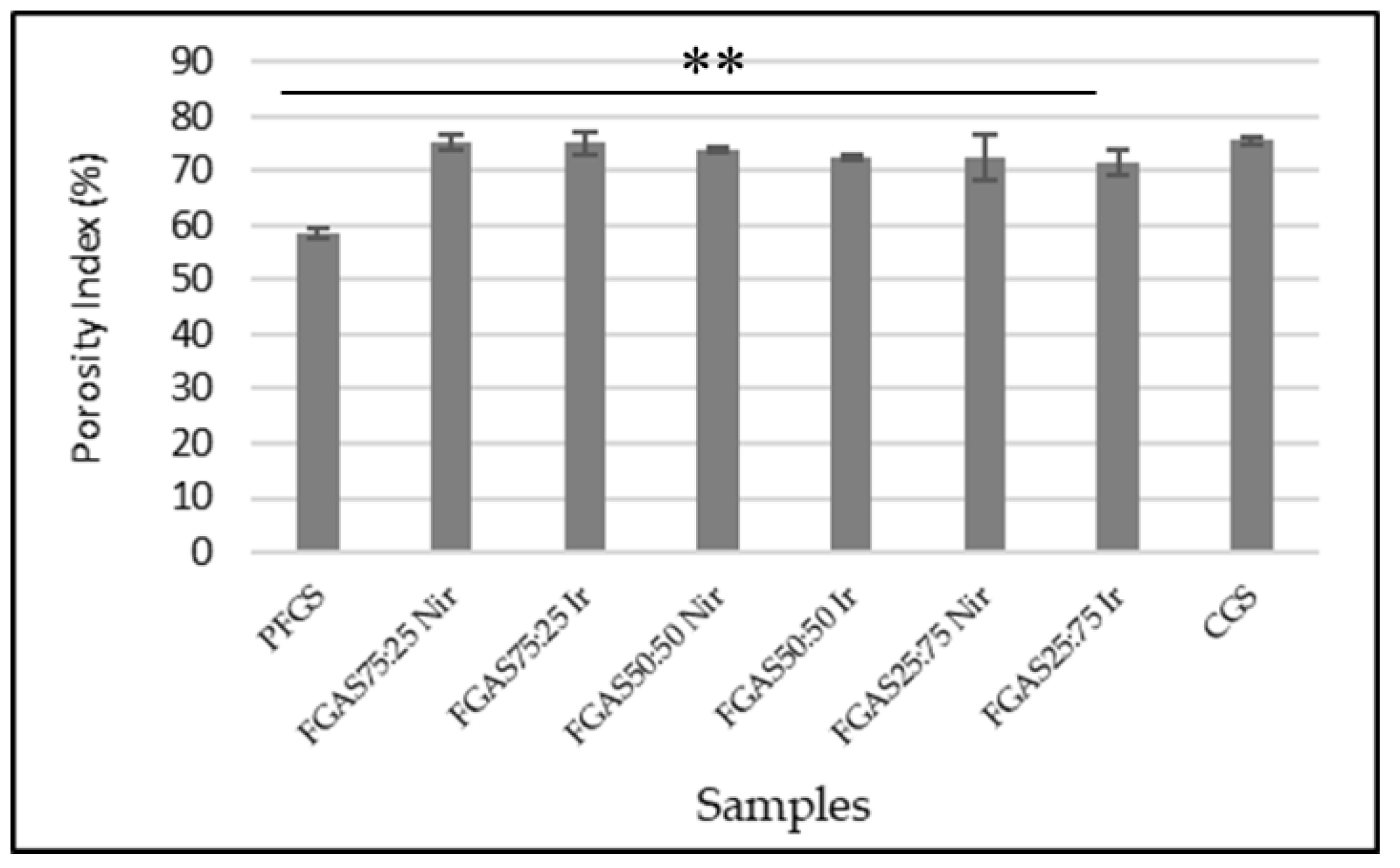
Figure 6.
Water Absorption Capacity of FGAS Prototypes (**p < 0.01).
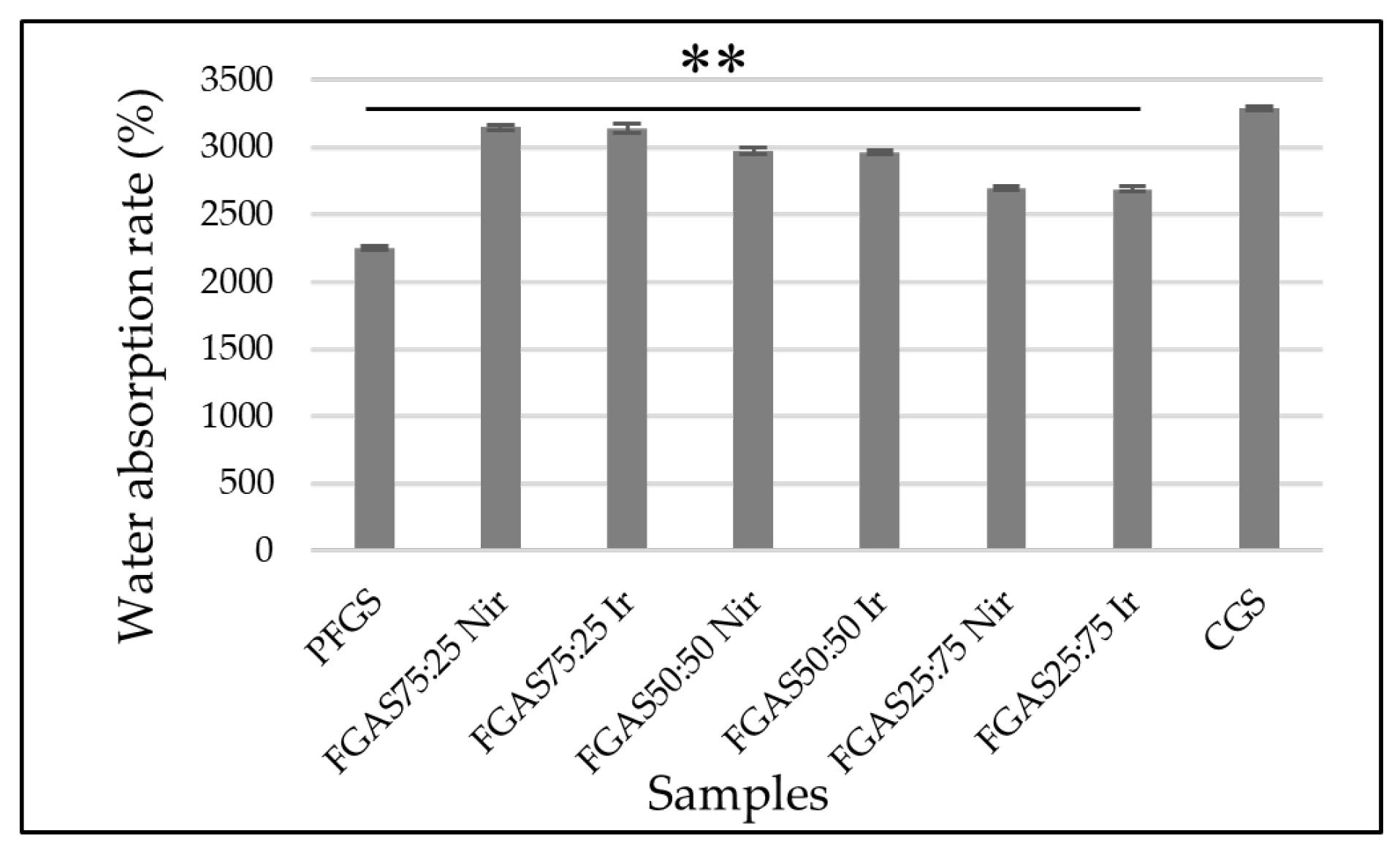
Figure 7.
Biodegradation Rates of FGAS Prototypes.
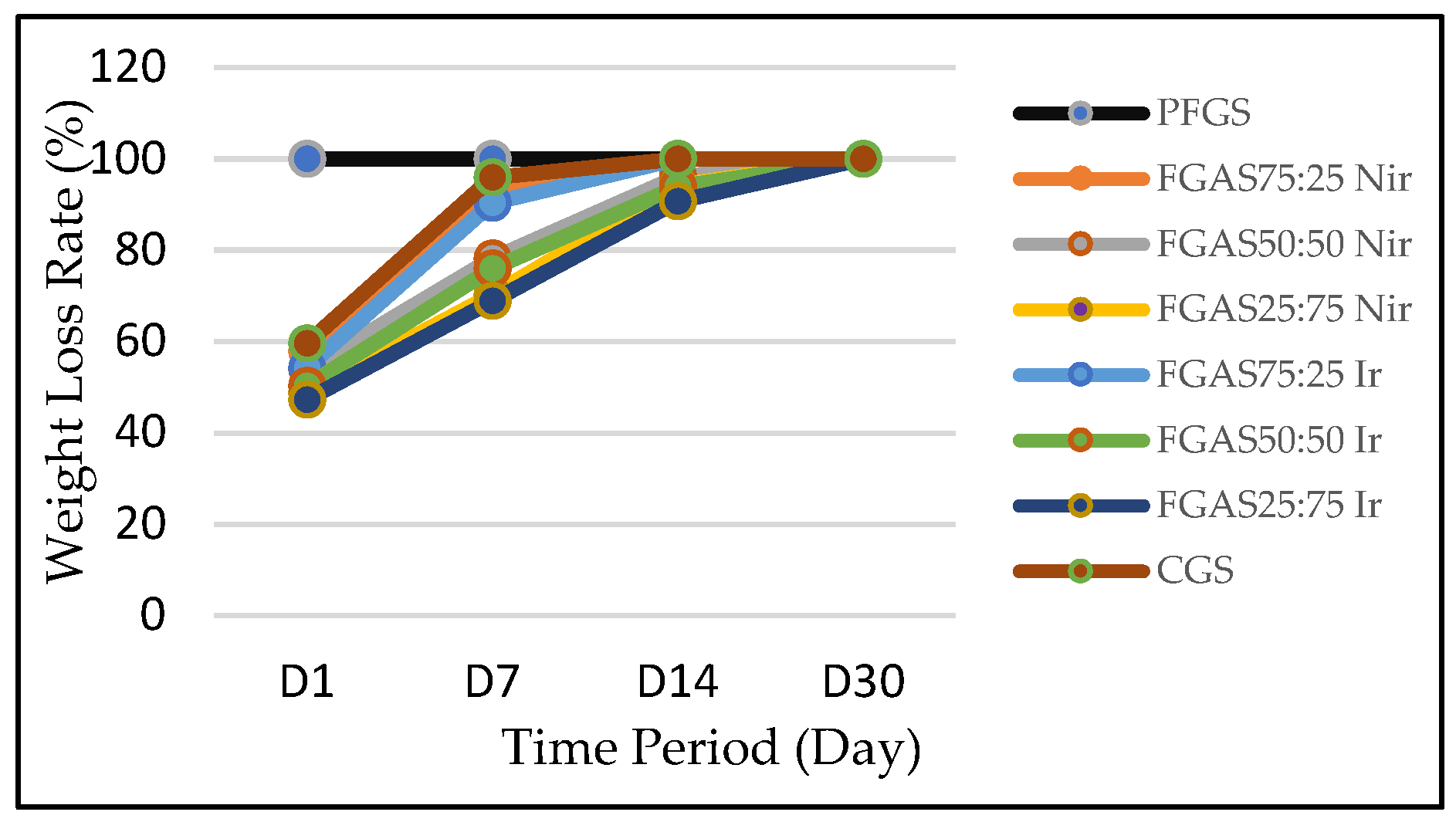
Figure 8.
Cytotoxicity Test of FGAS Prototypes.
Figure 9.
Hemocompatibility Rate of FGAS Prototypes (**p < 0.01).
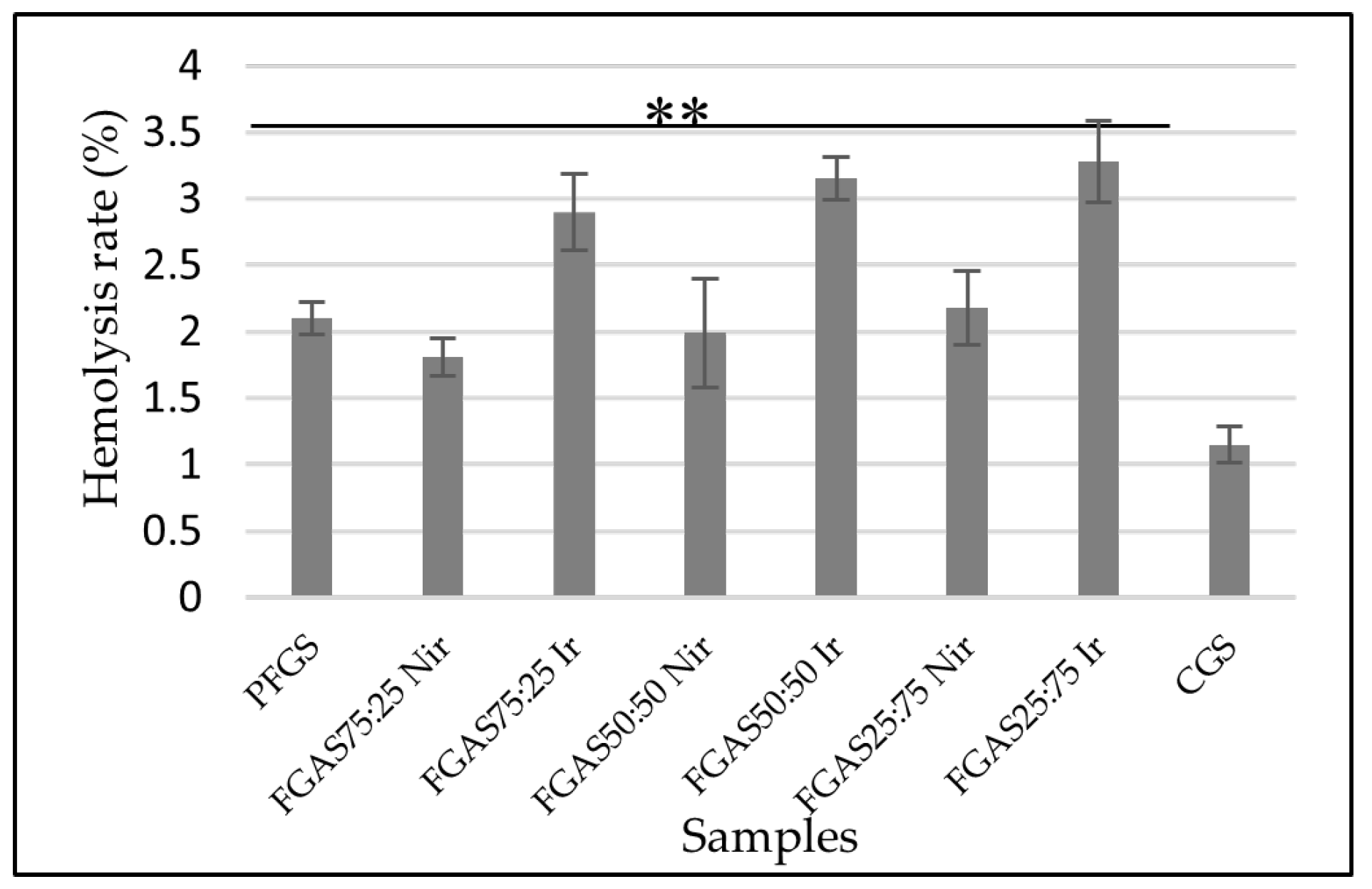
Figure 10.
The Clotting Time of FGAS Prototypes (**p < 0.01).
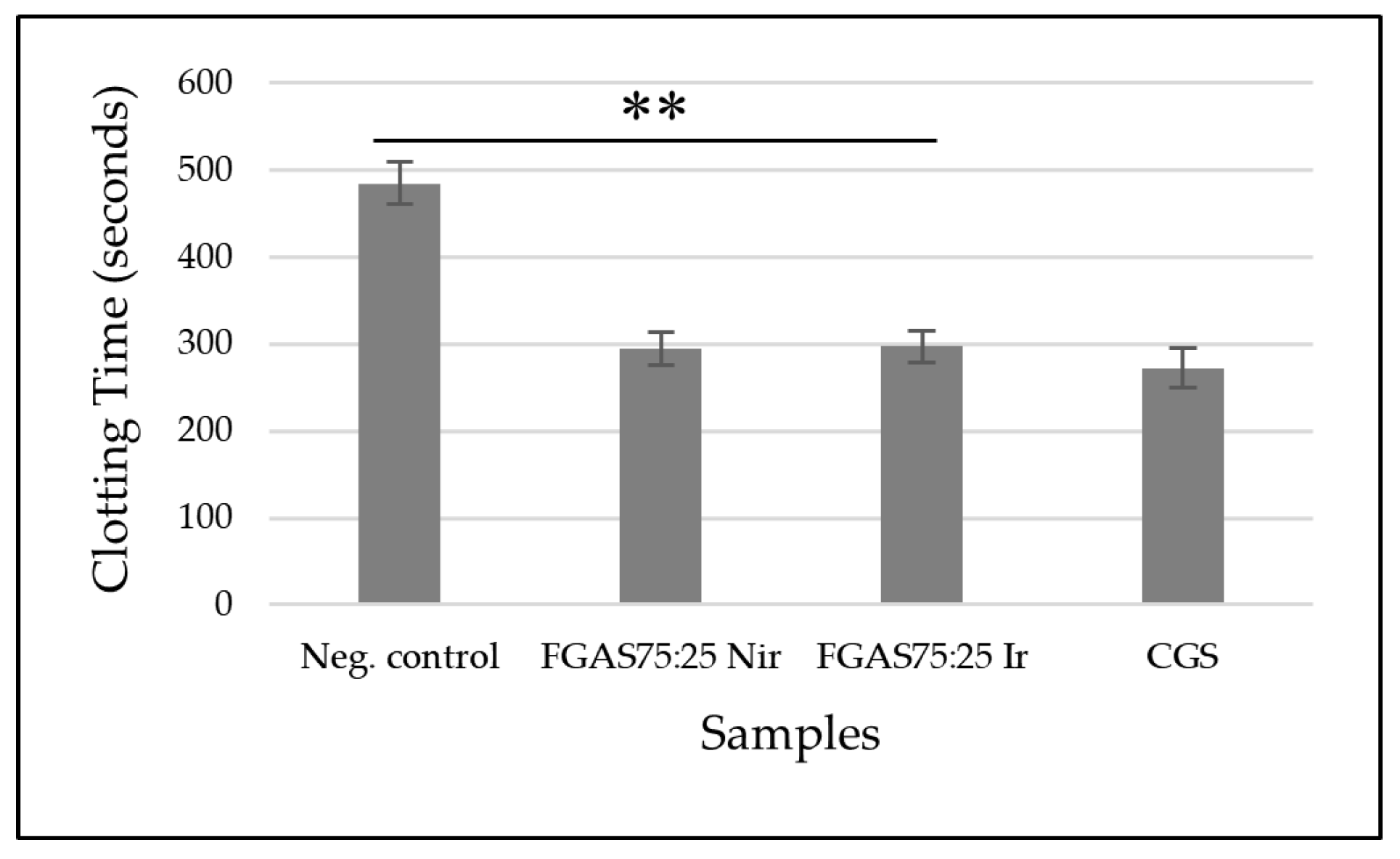
Figure 11.
Prothrombin Time of FGAS Prototypes (**p < 0.01).
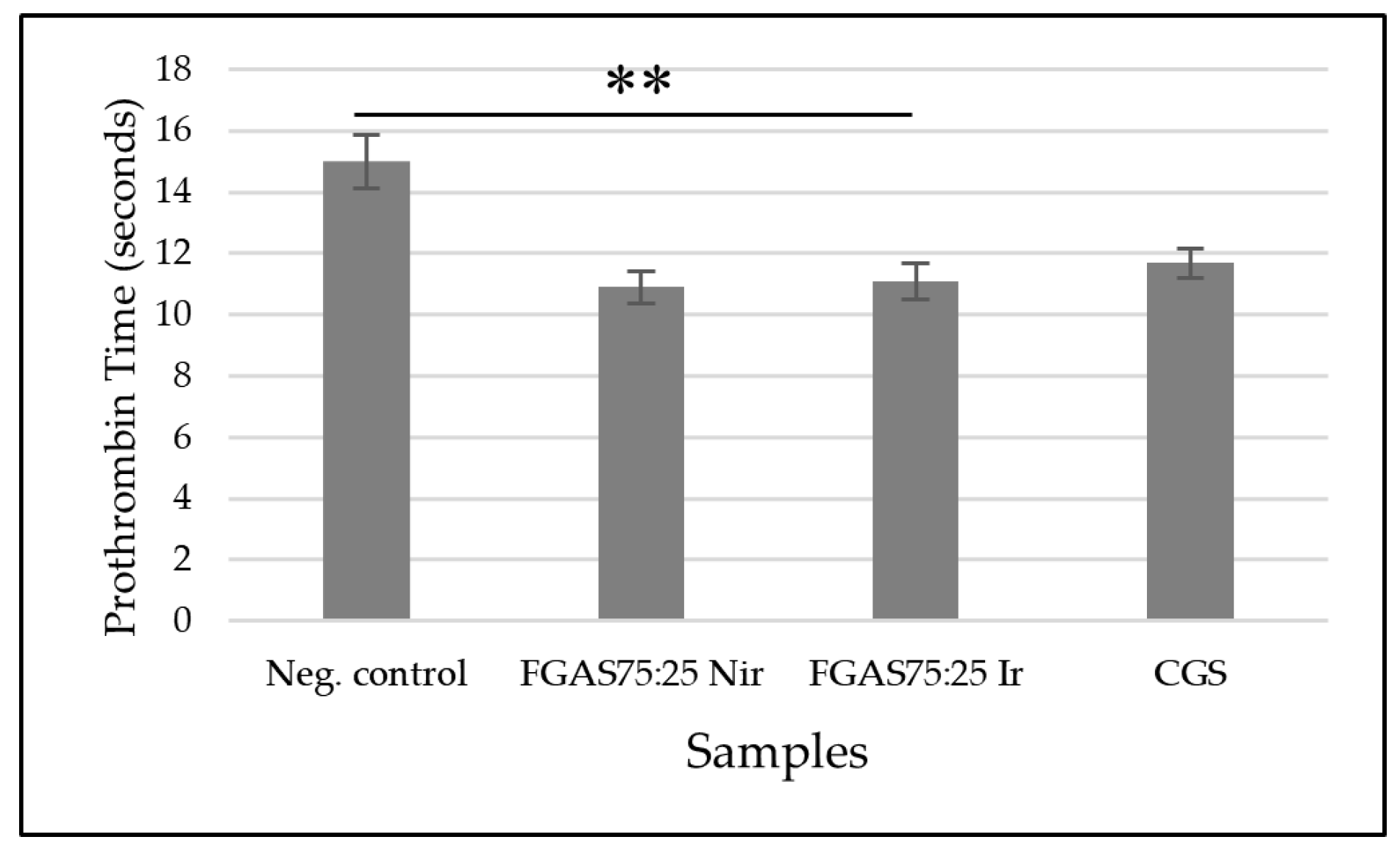
Figure 12.
Activated Partial Thromboplastin Time of FGAS Prototypes (**p < 0.01).
Table 1.
Chemical Elements Composition of The FGAS Prototype.
| Sample | Composition | Chemical Elements (wt%) | |||
| Carbon (C) | Nitrogen (N) | Oxygen (O) | Calcium (Ca) | ||
| 1 | PFGS | 22.478 | 21.578 | 16.583 | - |
| 2 | FGAS75:25 Nir | 5.506 | 12.613 | 10.711 | 30.030 |
| 3 | FGAS50:50 Nir | 6.300 | 4.600 | 9.800 | 25.600 |
| 4 | FGAS25:75 Nir | 1.800 | 1.900 | 5.300 | 18.700 |
| 5 | FGAS75:25 Ir | 20.500 | 27.000 | 8.900 | 22.000 |
| 6 | FGAS50:50 Ir | 18.418 | 15.516 | 14.715 | 19.119 |
| 7 | FGAS25:75 Ir | 17.700 | 6.900 | 16.700 | 17.700 |
| 8 | CGS | 35.200 | 33.900 | 30.900 | - |
PFGS: pure fish gelatin sponge, FGAS: fish gelatin-alginate sponge, Nir: non-irradiated, Ir: Irradiated, CGS: commercial gelatin sponge.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Alerts
Abstract
A hemostatic sponge prototype was successfully synthesized from fish gelatin as an alternative to mammalian gelatin, which was mixed with alginate in certain combinations and double cross-linking of calcium ions and gamma irradiation at a dose of 20 kGy to improve the characteristics and effectiveness of its function as a local hemostatic agent. There was an improvement in the physicochemical and mechanical properties, porosity index, absorption capacity, biodegradation properties, biocompatibility, and hemocompatibility of the FGAS prototypes compared with the pure fish gelatin sponge. Hemostatic activity tests showed that the means of clotting time, prothrombin time, and activated partial thromboplastin time were shorter in the FGAS prototype than in the negative control, and there was no significant difference compared with the commercial gelatin sponge. The hemostatic mechanism of the FGAS prototype combined a passive mechanism as a concentrator factor and an active mechanism through the release of calcium ions as a coagulation factor in the coagulation cascade process.

Keywords:
Subject: Medicine and Pharmacology - Dentistry and Oral Surgery
1. Introduction
Uncontrolled bleeding is one of the leading causes of death in medical emergencies in civilian and military life. Approximately 80% of deaths in trauma cases in civilian society and 20% in the military are caused by exsanguination. Another cause is complications during surgical procedures, in which uncontrolled bleeding is the most common complication.[1,2] The risk of morbidity and mortality will increase in surgical procedures, especially in cases of uncontrolled bleeding, because it can be life-threatening.[3,4] Despite the proper use of conventional techniques for hemorrhage control to avoid such complications when uncontrolled bleeding occurs, a broad range of hemostatic agents are available as adjunctive measures to enhance hemostasis.[5] To treat excessive bleeding in areas that are difficult to access with conventional methods, a variety of topical hemostatic agents, including gelatin-based hemostatic agents, can be used and are currently available on the market.[6]
One of the most commonly used local hemostatic agents, especially after surgical procedures, is a mechanical hemostatic agent made from gelatin material in the shape of an absorbent sponge.[7] Gelatin is made from the denaturation of collagen-derived proteins through a limited thermos-hydrolysis process and is known as an essential natural biopolymer. It has the property to change shape reversibly between sol and gel.[8,9] The sources of gelatin as raw materials for biomaterials, including hemostatic sponges, are still dominated by mammalian gelatin from pigs and cows. Currently, the sources of gelatin used in the world originate from 46% pork skins, 29.4% cow skins, 23.1% beef bones, and 1.5% other sources such as poultry and fish.[7,9]
Using gelatin from mammals such as pigs and cows is restricted because of religious and infectious disease concerns. Both Muslim and Jewish communities are prohibited from consuming pork-based products, and Hindu communities reject bovine-based products. Both Muslim and Jewish communities are prohibited from consuming pork-based products, and Hindu communities reject bovine-based products. Furthermore, some animal-borne infectious diseases, such as bovine spongiform encephalopathy (BSE) in cows and swine flu in pigs, pose a threat to human health.[10,11] Therefore, another source of gelatin, for example, fish gelatin, has recently been required as an alternative to mammalian gelatin.
Fish gelatin has been widely researched and developed as an alternative source of mammalian (cow and pig) gelatin. However, fish gelatin has shortcomings in terms of physicochemical properties, mechanical strength, and gel stability compared with mammalian gelatin.[12] To improve the weak properties of fish gelatin, the mixing method can be used with other biopolymers, one of which is alginate, to form a gelatin-alginate composite compound. Alginate, which is found in many seaweeds, is also a natural polymer that is widely used in the biomedical field. One of the alginate salts, calcium alginate, is effective as a hemostatic agent. The fish gelatin-alginate composite can be further increased in its physicochemical properties using cross-linking methods, for example, by Ca2+ ionic cross-linking and gamma irradiation.[13,14]
This research aims to synthesize a prototype of a fish gelatin-alginate hemostatic sponge using blending methods, double cross-linking with calcium ions, and gamma irradiation, and then characterize and test the effectiveness of its hemostatic function in the blood coagulation process.
2. Materials and Methods
2.1. Materials
Gelatin (Redman fish gelatin, food grade 200 bloom, and viscosity of 3.45mPa-s) was purchased from Phoon Huat Pte. Ltd. (Singapore). Sodium alginate (analytical grade with a viscosity of 17mPa-s) and Calcium Chloride (CaCl2) were purchased from Sigma-Aldrich Corporation (America), and commercial gelatin hemostatic sponges (Ceraspon) were purchased from PT Swayasa Perkasa (Indonesia). The other reagents used in this study were at least analytically pure.
2.2. Methods
2.2.1. Synthesis of The Fish Gelatin-Alginate Sponge (FGAS) Prototype
First, fish gelatin and sodium alginate powder were blended in a beaker containing double-distilled water to obtain a 4% (w/v) mixed solution with a certain proportion of fish gelatin (FG) and sodium alginate (SA) of 100/0, 75:25, 50:50, and 25:75. The solutions were then stirred evenly for 2 h at 50oC and then cast in a silicon mold to a size of 1x1x1 cm, and frozen at -20oC. The frozen fish gelatin-alginate composites, except for the composition of 100:0 (pure fish gelatin), were then immersed in 2% CaCl2 to provide ionic crosslinking between alginate and Ca2+ and produce a calcium alginate compound. In the next step, the Ca2+ crosslinked materials were then lyophilized with a freeze dryer at -50 °C for 24 h to obtain the fish gelatin-alginate sponge (FGAS) prototype. The final procedure was gamma irradiation crosslinking of the prototype materials at a 20 kGy dose. The prototype was prepared at the Research Center for Radiation Process Technology, Jakarta. All procedures were performed according to previous studies, with some modifications.[15,16,17,18,19,20,21,22]
Figure 1.
Diagrammatic representation of the research process.

2.2.2. Physicochemical and Mechanical Characterization
A scanning electron microscope (SEM) was used to analyze the surface morphology of the prototype, followed by chemical element analysis using the energy-dispersive X-ray spectroscopy (EDX/EDS) method. The samples were additionally coated with a metal (e.g., gold or platinum) before examination to avoid sample charging and increase contrast when imaging under high vacuum conditions. The analysis of the FGAS prototype functional groups was performed using Fourier transform infrared (FTIR) spectroscopy. All samples were prepared by grinding and mixing with KBr before being analyzed using an FTIR instrument. The mechanical properties were measured using the compressive strength test to determine the elasticity modulus of the FGAS prototype in wet conditions.[23,24,25]
2.2.3. Porosity Index Analysis
Porosity is an essential characteristic of sponge materials because it may directly impact the amount of water or blood they can absorb. There are various methods for measuring the porosity index of materials, including software like OriginPro and ImageJ.[26,27,28] We used OriginPro software to convert the images from SEM to quantitative measurements.
2.2.4. Water Absorption Capacity
The swelling test is a standard procedure for analyzing the absorption capacity of materials. The dry sponge (W0) was weighed and placed in a pH 7.4 PBS solution for 30 min. The sponge gel was then wiped with filter paper to remove the residual solution from the sponge surface and weighed again (W1). The sponge’s water absorption rate was calculated using the following formula:[29]
Water absorption rate (%) = (W1−W0)/W0 x 100
2.2.5. Biodegradation Rate
The dry sponge (W0) was weighed and placed in a pH 7.4 PBS solution at 37 °C for 1, 7, 14, and 30 days. On each day, the sponge was removed to dry, and then the sponge was weighed again (W1). The weight retention (%) was calculated using the following equation:[30]
Weight retention (%) = (W0−W1)/W0 x 100
2.2.6. Biocompatibility (Cytotoxicity Test)
The cytotoxicity test was performed according to the ISO 10993-5 standard. The sponges produced were incubated on BHK-21 fibroblasts for 24, 48, and 72 h to measure cell viability by the MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cell viability was measured by reading the optical density (OD) of the sample, control, and blank with an Elisa reader and converting it to the following formula:[31]
2.2.7. Hemocompatibility (Hemolysis Test)
Hemocompatibility is an essential criterion for any biomaterial that will interact closely with blood. The sponge samples were evaluated by hemolytic tests according to ISO 10993-4. Each of these samples was mixed with 0.4 mL of the incubated blood sample individually. Normal saline and deionized water were used as negative (neg) and positive (pos) controls, respectively. After completing all of the hemolysis assay steps, all samples were centrifuged at 3000 rpm for 5 min. Finally, the absorbance of the supernatants was determined at 545 nm using a UV-Vis spectrophotometer and counted using the following equation:[32]
2.2.8. Clotting Time (CT)
The in vitro clotting time was determined using the previously described method. Each sponge sample was placed in a clean test tube. The tubes were then immersed in a water bath at a constant temperature of 37°C for 1 h. Anticoagulated fresh donor blood (1 mL) was added to each of the test tubes and placed in the water bath. The tubes were tilted at 30° every 30 s until the blood in the tubes stopped flowing.[21] The coagulation times of blood in various tubes were recorded.
2.2.9. Prothrombin Time (PT)
Prothrombin time (PT) is the in vitro clotting time test after placing the PT reagent, which contains thromboplastin (phospholipids with tissue factor), calcium, and citrated plasma.[33] A platelet-poor plasma (PPP) sample was obtained by centrifuging fresh human blood at 3000 g for 10 min. Each sponge sample (1.0 cm3) received 500 µL of PPP and was incubated for 30 min at 37°C. To measure PT, combine 100 µL of PT reagent with 200 µL of PPP in a test tube and incubate at 37°C for 3 min. PT was then measured using an automated blood coagulation analyzer (COAX Bio System, Jerman). To serve as a control group, 500 µL of PPP extracted from fresh human blood was incubated at 37°C for 30 min without any test material.
2.2.10. Activated Partial Thromboplastin Time (APTT)
The APTT test measures the time it takes for plasma to clot in vitro after adding calcium, an activator of the intrinsic pathway, and the APTT reagent, which contains phospholipid, a platelet substitute that lacks tissue factor.[33] A platelet-poor plasma (PPP) sample was obtained by centrifuging fresh human blood at 3000 g for 10 min. Each sponge sample (1.0 cm3) received 500 µL of PPP and was incubated for 30 min at 37°C. To measure APTT, incubate 100 µL of PPP with 100 µL of reagent at 37°C for 3 min. Finally, 100 mL of aqueous calcium chloride (CaCl2) was added to the solution, and the coagulation analyzer was used to determine the APTT value. To serve as a control group, 500 µL of PPP extracted from fresh human blood was incubated at 37°C for 30 min without any test material.
2.3. Statistical Analysis
The experimental results are presented as the mean ± SD. The data was analyzed using the one-way ANOVA method for studies with more than two treatment groups and the independent sample t-test method for studies with only two treatment groups.
3. Results and Discussion
3.1. Synthesis of the Fish Gelatin-Alginate Sponge (FGAS) Prototype
This research produced a fish gelatin-alginate sponge prototype, which was synthesized by mixing and freeze-drying methods from fish gelatin and sodium alginate with Ca2+ ions and gamma irradiation cross-linking, and was proven to have hemostatic characteristics and effectiveness against blood clotting. The FGAS prototype is a white sponge, cube-shaped, and has an interconnected porous structure containing chemical elements and functional groups combined from gelatin and alginate, as well as additional calcium ions.
At a larger gelatin composition (75:25), the sponge prototype material appeared cuboid with flat and straight edges. The addition of alginate compositions (50:50 and 25:75) changed the shape of the sponge prototype to become more rounded at each corner. This is thought to be caused by an increase in the alginate composition, causing more cross-linking between alginate and Ca2+ ions to form an “egg-box model” structure. This structure is formed by an ionic bond between the Ca2+ ions and the carboxyl group on the guluronic block of the alginate polymer chain, thereby forming a stable bond in the alginate gelation process.[34,35] The formation of more egg-box model structures due to increasing alginate concentration can cause shrinkage and compaction of the sponge material, starting at the weakest corner points, so that the sponge containing more alginate becomes rounded at the corners.[36]
3.2. Physicochemical and Mechanical Characterization
The results of the SEM examination in Figure 2 show the surface morphology of the FGAS prototype, consisting of a porous structure with varying porous sizes with an average size between 39.03 – 266.66 µm. These porous sizes qualify as hemostatic sponges, as reported by previous research, which states that a porous size of between 50-100 µm is sufficient to accommodate the concentration of red blood cells and platelets in the initial process of blood clotting.[37] According to the IUPAC (International Union of Pure and Applied Chemistry) criteria, sponge material with a porous size of >50 µm is included in the macroporous category, which is ideal for containing cell concentrations because, in general, the average cell size is <100 µm.[38]
The SEM images can also be used to analyze the chemical element content of the FGAS prototype combined with energy-dispersive X-ray spectroscopy (EDX/EDS) analysis, as shown in Table 1. The main chemical element composition of the FGAS prototype material consists of carbon (C), nitrogen (N), oxygen (O), and calcium (Ca). The element content corresponds to the main elements contained in the raw materials, namely fish gelatin and alginate, which consist of C, N, O, and H. The H element cannot be detected using EDX analysis because it is very light. Calcium (Ca) is an additional element formed by cross-linking sodium alginate with Ca2+ ions in the CaCl2 solution.[39]
The analysis of the FGAS prototype functional groups was carried out using the Fourier transform infrared (FTIR) test to observe the wavelength spectrum in the form of specific peaks that indicate the functional groups possessed by the material, as shown in Figure 3 below.
Figure 3 shows the peaks of the infrared light absorption spectrum for typical functional groups found in gelatin polymers, alginate, and gelatin-alginate composites. Gelatin includes hydroxyl groups (O- H) and amine groups (N-H) at peak 3435.28cm-1 (superimposed) and carbonyl (C=O) at 1653.99cm-1. Alginate includes hydroxyl (O-H) at 3439.14cm-1; carbonyl (C=O) at 1621.20cm-1; carboxyl (C-O) at 1314.51cm-1; guluronate and mannuronate fingerprints at 894.99cm-1 and 819.76cm-1. The FTIR spectrum that appears in the gelatin-alginate composite is a combination of the spectrum of functional groups from gelatin and alginate polymers. The effect of gamma radiation can be seen from the shift in the absorption peaks of functional groups toward larger wavelengths in the group of samples that received gamma irradiation. these findings confirmed the previous studies of Sow et al., Derkach et al., and Hariyanti et al.[18,40,41]
The compressive strength test is a method for determining the mechanical properties of a biomaterial by measuring the elastic modulus, which is an important parameter in analyzing the characteristics of a sponge-shaped biomaterial that will be applied as a local hemostatic agent.[42] Sponge elastic modulus measurement was performed in wet conditions according to the purpose of hemostatic sponge application in bleeding situations.
All sponge samples show greater elasticity modulus mean compared with PFGS and were statistically significant. The FGAS75:25 Nir and FGAS75:25 Ir (157,79±3.99 and 173,86±5.18) have the closest values of elastic modulus to commercial gelatin sponges (133,54±9,79), but there was no significant difference in elastic modulus between irradiated and non-irradiated samples. Fish gelatin has low mechanical strength, especially in a single form, therefore, modifications are needed both in the synthesis method and by adding reinforcement materials to increase it.[43,44]
The increase in the elastic modulus of the FGAS prototype is thought to be due to the addition of alginate, resulting in an increase in density and shrinkage of the polymer chain caused by the formation of a model egg-box structure due to the ionic cross-linking reaction between the guluronate residue of the alginate polymer and the divalent cation Ca2+. As reported by Ma et al., in the preparation of fish gelatin hydrogel mixed with alginate, there was an increase in the elastic modulus.[45]
3.3. Porosity Index Analysis
Porosity is an essential characteristic of sponge materials because it may directly impact the amount of water or blood they can absorb, and these characteristics are strongly reliant on the underlying structure and morphology of sponges.[26,27] We analyzed the porosity index using OriginPro software, and the results are shown in Figure 5. All FGAS samples showed greater porosity index means compared with PFGS and were statistically significant.
The addition of alginate increases the porosity index of the FGAS prototype above the standard value of 60% for all FGAS prototype compositions, both irradiated and non-irradiated, namely between 71.67% and 75.32%.46. The porosity indices of FGAS75:25 Nir and FGAS75:25 Ir have the largest means (75.33%±1.25 and 75.07%±2.15) compared to all samples and have no significant difference compared to CGS. This is thought to be due to the addition of alginate, which is hydrophilic and binds water more strongly than fish gelatin during mixing so that when the lyophilization process (freeze-drying) is performed, it leaves more pores in the FGAS prototype material.[47] Sponge’s highly porous nature makes it easier for them to absorb blood fluid, which in turn minimizes the amount of excess exudate. Similarly, the spongy hemostatic agents and wound dressings rely on the sponge’s absorption properties which depend on the shape and structure of the sponges.[26,27,28] The comparison between irradiated and non-irradiated samples shows that there was no significant difference in the porosity index.
3.4. Water Absorption Capacity
Absorbing fluid or blood is one of the main requirements for materials with high absorption capacity, such as local hemostatic sponges.[29] The results of the water absorption rate of the FGAS prototype are shown in Figure 6, where all FGAS samples show greater water absorption rates than PFGS and are statistically significant.
The addition of alginate can increase the absorption capacity ratio of the FGAS prototype. As seen in Figure 6, the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the largest mean absorption capacity of 3147.45 ±19.87 and 3141.58 ± 34.22, respectively, and a significant difference statistically compared to the mean absorption capacity of PFGS. This is possible because of the addition of alginate, which has greater hydrophilic properties, so it can bind more water molecules. During the lyophilization process, the bound water molecules will be sublimated, leaving many spaces or pores in the material.[48] There was no significant difference in the water absorption capacity between the irradiated and non-irradiated samples.
3.5. Biodegradation Rate
The biodegradation rate of the FGAS prototype can be measured by counting the weight retention after immersing the sponge in a solution for a certain period.[30] The results of the biodegradation rate of the FGAS prototype are shown in Figure 7, where all sponge samples showed slower degradation rates compared with PFGS.
On Day 1 of observation, the FGAS prototype group, both irradiated and non-irradiated, showed a smaller weight loss ratio compared with PFGS, which was completely dissolved (100%) and did not qualify as a hemostatic sponge. The rapid degradation of pure fish gelatin sponge (PFGS) is thought to be due to the amino acid hydroxyproline content in fish gelatin being lower than that of mammalian gelatin, causing a low solubility (melting point) at a temperature of 25 °C–27 °C compared with mammalian gelatin at 32 °C – 35 °C.[49] This was confirmed by Yang et al., who reported that pure gelatin sponges without cross-linking can immediately dissolve when immersed in PBS solution.[50]
The FGAS prototype has a slower biodegradation ratio than PGFS, allegedly due to the addition of alginate and the presence of ionic cross-linking with Ca2+ to form calcium alginate compounds, which are difficult to dissolve in water, plus the combination of hydrogen bonds between gelatin and alginate molecules, which are strengthened by covalent bonds through cross-linking by gamma irradiation.[30,51] On Days 7, 14, and 30, it was shown that FGAS75:25 Nir and FGAS75:25 Ir have similar weight loss rates to CGS. There was also a significant difference in biodegradation rates between irradiated and non-irradiated FGAS samples. This proves that gamma irradiation can strengthen the bonds between peptide molecules in gelatin and between functional groups of gelatin and alginate through covalent bonds.[52,53]
3.6. Biocompatibility (Cytotoxicity Test)
Based on the MTT Assay test results presented in Figure 8, it can be seen that all samples show an average viability of above 70% and no significant differences, which means that all samples meet the biocompatibility requirements according to the ISO 10993-5 standard.[54]
The cytotoxicity test results prove that all FGAS prototypes, both irradiated and non-irradiated have non-cytotoxic properties. Statistical testing using one-way ANOVA revealed no significant differences in the mean cell viability values for all research samples. These results confirmed several previously studies, such as Rezaie et al., who synthesized a highly absorbent sponge from bovine gelatin and sodium alginate by cross-linking using CaCl2. The results of the cytotoxicity test on human fibroblast cells showed that the viability of all test samples was above 80%.[16] Another study reporting the effect of gamma irradiation at a dose of 25 kGy on the cytotoxicity of gelatin-alginate bioadhesive material showed that the average overall fibroblast cell viability value was in the range of 89–100%.[52]
3.7. Hemolysis Test
The hemolysis test results as presented in Figure 9 show that all samples show significant differences in erythrocyte hemolysis rates but are still below 5%, which means that all samples met the hemocompatibility requirements according to the ISO 10993-4 standard.[32]
There was also a significant difference in hemolysis rates between irradiated and nonirradiated FGAS samples. These findings confirmed the results of previous research by Rallapalli et al., who reported the effect of gamma irradiation at a dose of 25 kGy as a sterilization method on bovine pericardium scaffolds on the hemolysis ratio. There was an increase in the average hemolysis ratio from 1.8% before irradiation to 6.32% after irradiation. The increase in the hemolysis ratio is considered due to the irradiation process causing damage to the surface of the biomaterial, which becomes rougher so that it can damage erythrocytes and result in the lysis of hemoglobin.[55]
Hemocompatibility characteristics are an important requirement for assessing interactions between drugs or biomaterials that function or are related to the circulatory system. Hemocompatible drug compositions or materials are capable of interacting with blood components without causing clinically significant adverse reactions such as thrombosis, hemolysis, complement activation, or other adverse side effects.[56]
3.8. Hemostatic Activity Test
At this stage, the hemostatic function effectiveness test was only performed on the best FGAS prototype which resulted from the characterization test in the previous stage, where the FGAS75:25 Nir and FGAS75:25 Ir were found to be the selected prototypes.
3.8.1. Clotting Time (CT)
Based on the results of the blood clotting time test, as shown in Figure 10, the mean clotting time for the FGAS75:25 Nir prototype was 294.33±24.36 seconds and the FGAS75:25 Ir prototype was 297.17±19 seconds, significantly different compared with the negative control (485.00±24.36 seconds), but not significantly different than CGS (272,33±22,47 seconds). There was no significant difference in clotting time between the irradiated and non-irradiated samples.
The FGAS prototype has an effective function as a local hemostatic agent by accelerating the bleeding time due to the porous structure of the hemostatic sponge, which allows it to absorb a lot of blood fluid while concentrating coagulation components, red blood cells, and platelets. Immediately upon contact with blood, the hemostatic sponge causes adhesion and aggregation of platelets, which in turn causes the formation of a platelet plug, thereby preventing blood flow from the wound. It also triggers the release of blood clotting factors involved in the extrinsic and intrinsic coagulation pathways, resulting in formation of a stable blood clot that helps control bleeding from wounds.[37]
Several previous studies have confirmed the use of gelatin and alginate as local hemostatic agents, including research by Dai et al., who reported that the manufacture of gelatin (GA), calcium alginate (CA), and silk fibroin (SF) composite sponges had good effectiveness by speeding up bleeding time.[57] Chen et al. reported that the synthesis of gelatin-alginate hemostatic sponge with the addition of curcumin was effective in accelerating blood clotting time and preventing tumor recurrence.[30]
3.8.2. Prothrombin Time (PT)
Figure 11 shows that the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the shortest mean prothrombin time, 10.9±0.52 and 11.1±0.59 seconds, respectively, and significant difference compared with the negative control (10,9±0,52 seconds) but not significant difference than CGS (11,7±0,48 seconds). There was also no significant difference in prothrombin time between the irradiated and non-irradiated samples.
The effectiveness of the FGAS prototype in accelerating prothrombin time (PT) is considered due to the content of calcium ions bound in alginate. When in contact with blood, the FGAS prototype turns into a hydrogel, and a reaction occurs that encourages the entry of calcium ions into the wound through an ion exchange reaction with sodium ions in the blood. Furthermore, calcium ions trigger the production of coagulation factors VII, IX, and X, as well as platelets, activate the coagulation cascade reaction, and accelerate the hemostasis process.[58]
Previous research has reported the role of calcium alginate in the coagulation process because it contains phytohemagglutinin, which can trigger red blood cell aggregation and change erythrocyte morphology, exposing phosphatidylserine on the surface of erythrocytes and accelerating the conversion of local prothrombin to thrombin.[59] Dai et al. reported the hemostatic effectiveness of a composite sponge made from silk fibroin, gelatin, and calcium alginate by accelerating prothrombin time.[57]
3.8.3. Activated Partial Thromboplastin Time (APTT)
The results of the APTT test, as shown in Figure 12, show that the FGAS75:25 Nir and FGAS75:25 Ir prototypes have the shortest APPT means, namely 32.88 ± 0.78 and 33.18±1.64 seconds, respectively, which is significantly different from the negative control (41,12±0,63), but there is no significant difference with CGS (33,92±0,63). There was also no significant difference in the APTT means between the irradiated and non-irradiated samples.
Similar to the PT test, the APTT test results also suggested that the ability of the FGAS prototype to accelerate APTT was due to the calcium ion content bound in the alginate that was released when the sponge came into contact with the blood and then promoted the cascade coagulation process.[58]
As reported by Che et al. in making a polyelectrolyte multilayer film on a sodium alginate/gelatin sponge, it has the effectiveness of shortening APTT, which is thought to be due to the activity of the calcium ions contained in the sponge.[60] Another study by Kumar et al. stated that the synthesis of calcium alginate- zinc chloride hydrogel is determined by the content of calcium ions, which can trigger clotting factors in the coagulation cascade process.[61]
5. Conclusions
The FGAS prototype has better physicochemical and mechanical characteristics, porosity index, water absorption capacity, biodegradation rate, biocompatibility, and hemocompatibility than PFGS where the FGAS75:25Nir and FGAS75:25Ir are selected as the best prototype composition. It is also proven that the FGAS prototype is effective as a local hemostatic agent, where the hemostatic mechanism is a combination of a passive mechanism as a concentrator factor through superiority in porosity index and absorption capacity, functioning as a matrix to collect blood cells, especially platelets, and coagulation factors, and the active mechanism through the content of calcium ions (Ca2+), which are released in the coagulation cascade process. The gamma irradiation of 20kGy dose only has an effect in the biodegradation and hemolysis rate characteristics of the FGAS prototype.
Author Contributions
Heri Herliana was responsible for conceptualization, formal analysis, and writing. Harmas Yazid was responsible for reviewing and supervising. Avi Laviana was responsible for editing and reviewing. Ganesha Wandawa was responsible for editing and directing. Basril Abbas was responsible for the methodology, resources, and field supervision.
Funding
This study received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Indonesian Marine Hospital (Number: 18/X/2023/RSMC, date of approval: 30th October 2023).
Data Availability
This article includes all data presented or analyzed during the study.
Acknowledgments
We thank the Doctoral Program Faculty of Dentistry, Universitas Padjadjaran, and the National Research and Innovation Agency (NRIA), for their technical support.
Conflicts of Interest
The authors declare that they have no conflicts of interest or personal relationships that could have influenced the work presented in this paper.
References
- Gordy, S.D.; Rhee, P.; A Schreiber, M. Military applications of novel hemostatic devices. Expert Rev. Med Devices 2011, 8, 41–47. [Google Scholar] [CrossRef]
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic strategies for uncontrolled bleeding: A comprehensive update. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef]
- Nagarale, R.; Todkar, M.; Khan, S.; Khan, Y.; Rehan, M.; Rizvi, Q. Assessment of knowledge, importance and management of uncontrolled bleeding in dental surgical procedures among dental professionals. Int. J. Appl. Dent. Sci. 2021, 7, 312–316. [Google Scholar] [CrossRef]
- de Campos, N.; Furlaneto, F.; Buischi, Y.D.P. Bleeding in dental surgery. In Contemporary Applications of Biologic Hemostatic Agents across Surgical Specialties-Volume 2; IntechOpen, 2019. [Google Scholar]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilicò, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 1–20. [Google Scholar] [CrossRef]
- Irfan, N.I.; Zubir, A.Z.M.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef]
- Herliana, H.; Yusuf, H.Y.; Laviana, A.; Wandawa, G.; Cahyanto, A. Characterization and Analysis of Chitosan-Gelatin Composite-Based Biomaterial Effectivity as Local Hemostatic Agent: A Systematic Review. Polymers 2023, 15, 575. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34. [Google Scholar] [CrossRef]
- Alipal, J.; Pu'Ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today: Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Nurilmalaa, M.; Suryamarevita, H.; Hizbullah, H.H.; Jacoeb, A.M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi J. Biol. Sci. 2021, 29, 1100–1110. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Kahtani, H.A.; Jaswir, I.; AbuTarboush, H.; Ismail, E.A. Extraction and characterization of gelatin from camel skin (potential halal gelatin) and production of gelatin nanoparticles. Saudi J. Biol. Sci. 2020, 27, 1596–1601. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Rajeswari, A.; Stobel Christy, E.J.; Pius, A. Biopolymer blends and composites [Internet]. Biopolymers and their Industrial Applications. Elsevier Inc., 2021; pp. 105–147. [Google Scholar] [CrossRef]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Lou, C.W.; Huang, M.S.; Chang, C.Y.; Lu, C.T.; Chen, W.C.; Lin, J.H. Preliminary Study in Cross-Linked Gelatin/Alginate Sponges. Appl. Mech. Mater. 2012, 184–185, 1102–1105. [Google Scholar] [CrossRef]
- Rezaie, A.; Mehdipour, A.; Salmanipour, S.; Alipour, N.; Salehi, R. HIGHLY POROUS ALGINATE/GELATIN SPONGE FOR HEMOSTASIS OF SEVERE FEMORAL BLEEDING IN RATS. Stud. Med Sci. 2023, 33, 839–856. [Google Scholar] [CrossRef]
- Song, Y.; Xu, L.; Deng, L. Radiation cross-linked gelatin/sodium alginate/carboxymethylcellulose sodium hydrogel for the application as debridement glue paste. Polym. Bull. 2021, 79, 725–742. [Google Scholar] [CrossRef]
- Hariyanti, H.; Erizal, E.; Apriyani, R.Z.; Perkasa, D.P.; Lestari, I.; Rahmi, H. Synthesis of Polyvinyl Alcohol (PVA)-Gelatin Hydrogel from White Snapper (Lates calcarifer, Bloch) with Gamma Irradiation and Its Characterizations. At. Indones. 2023, 1, 69–75. [Google Scholar] [CrossRef]
- Lu, H.; Butler, J.A.; Britten, N.S.; Venkatraman, P.D.; Rahatekar, S.S. Natural Antimicrobial Nano Composite Fibres Manufactured from a Combination of Alginate and Oregano Essential Oil. Nanomaterials 2021, 11, 2062. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Hu, Z.; Ouyang, Q.Q.; Cheng, Y.; Hong, P.Z.; Liao, M.N.; Chen, F.J.; Li, S.D. Optimization of preparation process and characterization of carboxymethyl chitosan/sodium alginate hemostatic sponge. IOP Conf. Series: Mater. Sci. Eng. 2017, 213, 012045. [Google Scholar] [CrossRef]
- Kang, H.J.; Jo, C.; Lee, N.Y.; Kwon, J.H.; Byun, M.W. A combination of gamma irradiation and CaCl2 immersion for a pectin-based biodegradable film. Carbohydr. Polym. 2005, 60, 547–551. [Google Scholar] [CrossRef]
- Kuo, Z.-K.; Lai, P.-L.; Toh, E.K.-W.; Weng, C.-H.; Tseng, H.-W.; Chang, P.-Z.; Chen, C.-C.; Cheng, C.-M. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci. Rep. 2016, 6, 32884. [Google Scholar] [CrossRef]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful scanning electron microscopic (SEM) visualization of 3D printed alginate-based scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-Montmorillonite Composite Sponge for Safe and Effective Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef]
- Hojat, N.; Gentile, P.; Ferreira, A.M.; Siller, L. Automatic pore size measurements from scanning electron microscopy images of porous scaffolds. J. Porous Mater. 2023, 30, 93–101. [Google Scholar] [CrossRef]
- Tasya, A.Y.; Kusumawati, D.H. Karakteristik Porositas Wound Dressing Nanofiber PVA-Ekstrak Daun Nangka. J Inov Fis Indonesia [Internet] 2023, 12, 106–112. [Google Scholar]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef]
- Sharifi, S.; Dizaj, S.M.; Ahmadian, E.; Karimpour, A.; Maleki, A.; Memar, M.Y.; Ghavimi, M.A.; Abdolahinia, E.D.; Goh, K.W. A Biodegradable Flexible Micro/Nano-Structured Porous Hemostatic Dental Sponge. Nanomaterials 2022, 12, 3436. [Google Scholar] [CrossRef]
- Hossain, S.; Shaikh, A.A.; Jahan, S.A.; Mahmud, M.; Bin Mobarak, M.; Rahaman, S.; Uddin, N.; Ahmed, S. Exploring the biomedical competency of gamma-radiation aided hydroxyapatite and its composite fabricated with nano-cellulose and chitosan. RSC Adv. 2023, 13, 9654–9664. [Google Scholar] [CrossRef]
- Chee, Y.L. Coagulation. J R Coll Physicians Edinb. 2014, 44, 42–45. [Google Scholar] [CrossRef]
- Pulat, M.; Ozukaya, D. Preparation and Characterization of Na-Alginate Hydrogel Beads. The Eurasia Proceedings of Science Technology Engineering and Mathematics 2019, 6, 32–38. [Google Scholar]
- Kaur, N.; Singh, B.; Sharma, S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharma Innov J [Internet] 2018, 7, 142–148. [Google Scholar]
- Zhang, X.; Wang, K.; Hu, J.; Zhang, Y.; Dai, Y.; Xia, F. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J. Mater. Chem. A 2020, 8, 25390–25401. [Google Scholar] [CrossRef]
- Nepal, A.; Tran, H.D.N.; Nguyen, N.-T.; Ta, H.T. Advances in haemostatic sponges: Characteristics and the underlying mechanisms for rapid haemostasis. Bioact. Mater. 2023, 27, 231–256. [Google Scholar] [CrossRef]
- Ebrahimi, M. Porosity parameters in biomaterial science: Definition, impact, and challenges in tissue engineering. Front. Mater. Sci. 2021, 15, 352–373. [Google Scholar] [CrossRef]
- Taheraslani, M.; Gardeniers, H. High-Resolution SEM and EDX Characterization of Deposits Formed by CH4+Ar DBD Plasma Processing in a Packed Bed Reactor. Nanomaterials 2019, 9, 589. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron'Ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Yang, G.; Huang, Z.; McCarthy, A.; Huang, Y.; Pan, J.; Chen, S.; Wan, W. Super-Elastic Carbonized Mushroom Aerogel for Management of Uncontrolled Hemorrhage. Adv. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Atma, Y. Synthesis and application of fish gelatin for hydrogels/ composite hydrogels: A review. Biointerface Res Appl Chem. 2022, 12, 3966–3976. [Google Scholar]
- Ma, C.; Choi, J.-B.; Jang, Y.-S.; Kim, S.-Y.; Bae, T.-S.; Kim, Y.-K.; Park, J.-M.; Lee, M.-H. Mammalian and Fish Gelatin Methacryloyl–Alginate Interpenetrating Polymer Network Hydrogels for Tissue Engineering. ACS Omega 2021, 6, 17433–17441. [Google Scholar] [CrossRef] [PubMed]
- Cuicui, D.; Kuan, C.; Yue, W.; Yifan, Y.; Xiaohong, C.; Jingyi, L.; et al. Dual green hemostatic sponges constructed by collagen fibers disintegrated from Halocynthia roretzi by a shortcut method. Mater Today Bio. 2024, 24, 1–12. [Google Scholar]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef] [PubMed]
- Saarai A, Kasparkova V, Sedlacek T, Saha P. A comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. Recent Res Geogr Geol Energy, Environ Biomed - Proc 4th WSEAS Int Conf EMESEG’11, 2nd Int Conf WORLD-GEO’11, 5th Int Conf EDEB’11. 2011;384–9.
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar Drugs 2021, 19. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Junkyu, S. Evaluation of Calcium Alginate Microparticles Prepared Using a Novel Nebulized Aerosol Mediated Interfacial Crosslinking Method. University of Toledo, 2016. [Google Scholar]
- Foox, M.; Ben-Tzur, M.; Koifman, N.; Zilberman, M. Effect of gamma radiation on novel gelatin alginate–based bioadhesives. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 611–618. [Google Scholar] [CrossRef]
- Azmir, M.S.N.A.; Moni, N.; Gobetti, A.; Ramorino, G.; Dey, K. Advances in modulating mechanical properties of gelatin-based hydrogel in tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 1–36. [Google Scholar] [CrossRef]
- Gruber, S.; Nickel, A. Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Front. Med Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Rallapalli, S.; Liman, A.M.; Guhathakurta, S. Hemocompatibility and surface properties of bovine pericardial patches: Effects of gamma sterilization. Curr. Med. Res. Pr. 2016, 6, 224–228. [Google Scholar] [CrossRef]
- Nalezinkova, M. In vitro hemocompatibility testing of medical devices. Thromb. Res. 2020, 195, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, M.; Gong, J.; Meng, L.; Zhang, B.; Zhang, Y.; Yin, Y.; Wang, J. Silk fibroin/gelatin/calcium alginate composite materials: Preparation, pore characteristics, comprehensive hemostasis in vitro. Mater. Des. 2022, 216, 110577. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Qin, S. Three Polymers from the Sea: Unique Structures, Directional Modifications, and Medical Applications. Polymers 2021, 13, 2482. [Google Scholar] [CrossRef] [PubMed]
- Che, C.; Liu, L.; Wang, X.; Zhang, X.; Luan, S.; Yin, J.; Li, X.; Shi, H. Surface-Adaptive and On-Demand Antibacterial Sponge for Synergistic Rapid Hemostasis and Wound Disinfection. ACS Biomater. Sci. Eng. 2020, 6, 1776–1786. [Google Scholar] [CrossRef]
- Kumar, A.; Sah, D.K.; Khanna, K.; Rai, Y.; Yadav, A.K.; Ansari, M.S.; Bhatt, A.N. A calcium and zinc composite alginate hydrogel for pre-hospital hemostasis and wound care. Carbohydr. Polym. 2023, 299, 120186. [Google Scholar] [CrossRef]
Figure 2.
SEM examination of the surface morphology of the FGAS prototype with magnifications of 300, 500, and 700X shows interconnected porous material.
Figure 2.
SEM examination of the surface morphology of the FGAS prototype with magnifications of 300, 500, and 700X shows interconnected porous material.

Figure 3.
FTIR Spectra of Alginate, Gelatin, and The FGAS Prototypes.

Figure 4.
Modulus Elastic of The FGAS Prototypes in Wet Conditions (**p < 0.01).

Figure 5.
Porosity Index of FGAS Prototypes (**p < 0.01).

Figure 6.
Water Absorption Capacity of FGAS Prototypes (**p < 0.01).

Figure 7.
Biodegradation Rates of FGAS Prototypes.

Figure 8.
Cytotoxicity Test of FGAS Prototypes.

Figure 9.
Hemocompatibility Rate of FGAS Prototypes (**p < 0.01).

Figure 10.
The Clotting Time of FGAS Prototypes (**p < 0.01).

Figure 11.
Prothrombin Time of FGAS Prototypes (**p < 0.01).

Figure 12.
Activated Partial Thromboplastin Time of FGAS Prototypes (**p < 0.01).

Table 1.
Chemical Elements Composition of The FGAS Prototype.
| Sample | Composition | Chemical Elements (wt%) | |||
| Carbon (C) | Nitrogen (N) | Oxygen (O) | Calcium (Ca) | ||
| 1 | PFGS | 22.478 | 21.578 | 16.583 | - |
| 2 | FGAS75:25 Nir | 5.506 | 12.613 | 10.711 | 30.030 |
| 3 | FGAS50:50 Nir | 6.300 | 4.600 | 9.800 | 25.600 |
| 4 | FGAS25:75 Nir | 1.800 | 1.900 | 5.300 | 18.700 |
| 5 | FGAS75:25 Ir | 20.500 | 27.000 | 8.900 | 22.000 |
| 6 | FGAS50:50 Ir | 18.418 | 15.516 | 14.715 | 19.119 |
| 7 | FGAS25:75 Ir | 17.700 | 6.900 | 16.700 | 17.700 |
| 8 | CGS | 35.200 | 33.900 | 30.900 | - |
PFGS: pure fish gelatin sponge, FGAS: fish gelatin-alginate sponge, Nir: non-irradiated, Ir: Irradiated, CGS: commercial gelatin sponge.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization
Julia Radwan-Pragłowska
et al.
Molecules,
2019
Preparation of Chitosan-Based Hemostatic Sponges by Supercritical Fluid Technology
Hu-Fan Song
et al.
Materials,
2014
Rapid Hemostatic Biomaterial from a Natural Bath Sponge Skeleton
Qinghua Wang
et al.
Marine Drugs,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






