Submitted:
14 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Postbiotics (MB-2006)
2.2. Cell Line and Cell Culture
2.3. Cell Viability
2.4. Measurement of Cytokines and Chemokines in TNF-α/IFN-γ-induced HaCaT Keratinocytes
2.5. Western-blotting
2.6. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity of MB-2006 and Other Strains
3.2. Inhibition of Cytokines Related to Immune Response by MB-2006 in TNF-α/IFN-γ-induced HaCaT Keratinocytes
 ), 1x108 bacteria/ml (
), 1x108 bacteria/ml ( ) and 1x109 bacteria/ml (
) and 1x109 bacteria/ml ( ). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
 ), 1x108 bacteria/ml (
), 1x108 bacteria/ml ( ) and 1x109 bacteria/ml (
) and 1x109 bacteria/ml ( ). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.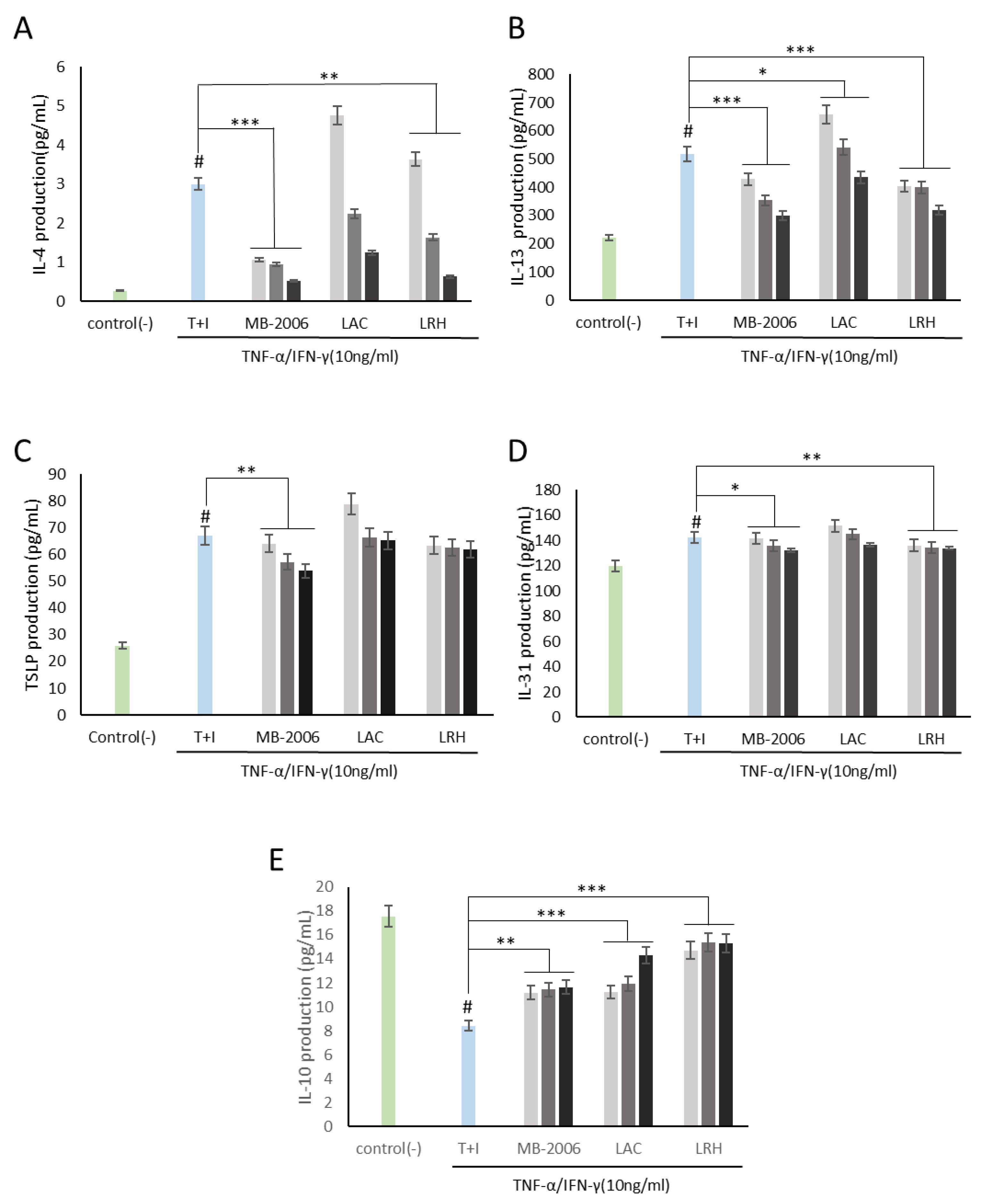
 ), 1x108 bacteria/ml (
), 1x108 bacteria/ml ( ) and 1x109 bacteria/ml (
) and 1x109 bacteria/ml ( ). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
 ), 1x108 bacteria/ml (
), 1x108 bacteria/ml (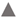 ) and 1x109 bacteria/ml (
) and 1x109 bacteria/ml ( ). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.
). The bars indicate the mean ± SD, and significant differences are shown in comparison to T+I(TNF-α/IFN-γ only treated group). #p<0.001 vs. the negative control; **p<0.01, ***p<0.001 vs. T+I.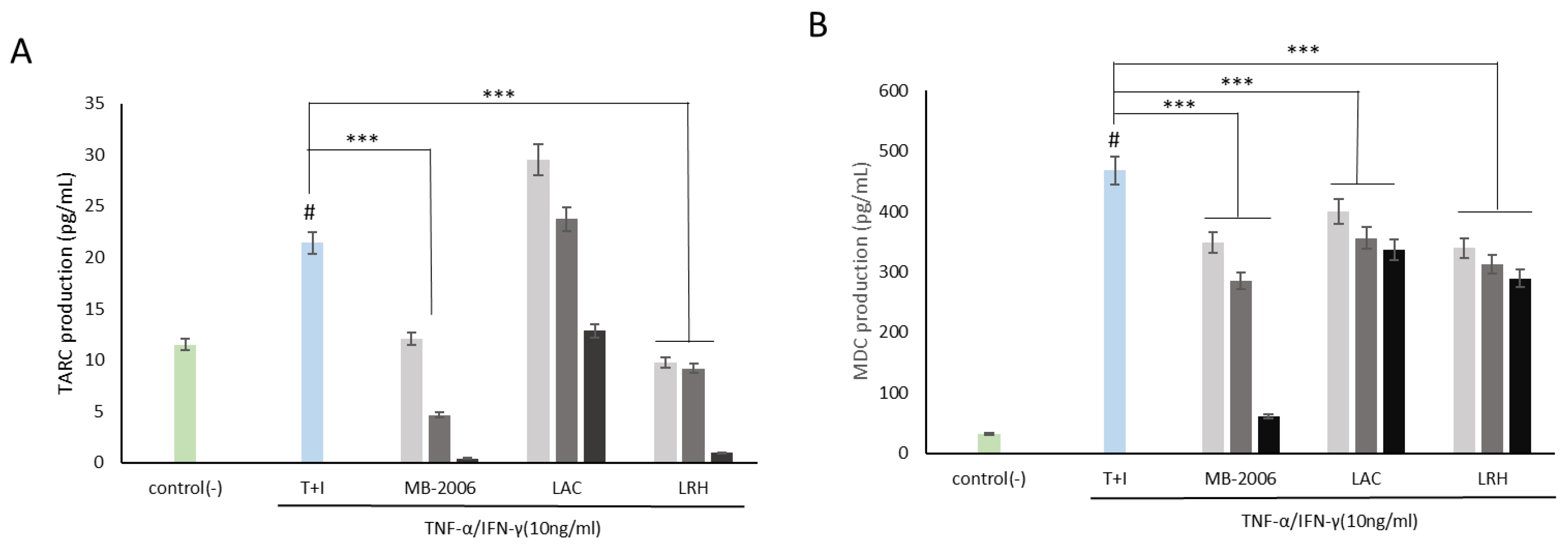
3.3. Inhibition of Chemokines Related to Immune Response by MB-2006 in TNF-α/IFN-γ-induced HaCaT Keratinocytes
3.4. Effect of MB-2006 on NF-κB Signaling Pathway in TNF-α/IFN-γ-induced HaCaT Keratinocytes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Marzio, L.; Centi C Fau - Cinque, B.; Cinque B Fau - Masci, S.; Masci S Fau - Giuliani, M.; Giuliani M Fau - Arcieri, A.; Arcieri A Fau - Zicari, L.; Zicari L Fau - De Simone, C.; De Simone C Fau - Cifone, M.G.; Cifone, M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Experimental Dermatology 2003, 12, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Ichikawa Y Fau - Imokawa, G.; Imokawa, G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. European Journal of Immunology 1998, 28, 769–779. [Google Scholar] [CrossRef]
- Taïeb, A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis 1999, 41, 177–180. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.; Kim, J.-I.; Lee, H.-Y.; Moon, G.-S.; Kang, C.-H. Improvements in Human Keratinocytes and Antimicrobial Effect Mediated by Cell-Free Supernatants Derived from Probiotics. Fermentation 2022, 8. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. In Protocols in In Vitro Hepatocyte Research, Vinken, M., Rogiers, V., Eds.; Springer New York: New York, NY, 2015; pp. 333–348. [Google Scholar]
- Guenounou, M. [Cytokines and allergic response]. Ann Biol Clin (Paris) 1998, 56, 297–304. [Google Scholar] [PubMed]
- Ha, Y.; Lee, W.-H.; Kim, J.K.; Jeon, H.-K.; Lee, J.; Kim, Y.-J. Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Baek, J.; Lee, J.R.; Roh, J.Y.; Jung, Y. Optimization of Cytokine Milieu to Reproduce Atopic Dermatitis-related Gene Expression in HaCaT Keratinocyte Cell Line. Immune Network 2018, 13, e9. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi C Fau - Sebastiani, S.; Sebastiani S Fau - Cavani, A.; Cavani A Fau - Federici, M.; Federici M Fau - Sozzani, S.; Sozzani S Fau - Girolomoni, G.; Girolomoni, G. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. Journal of Leukocyte Biology 2001, 70, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; de Kozak Y Fau - Saoudi, A.; Saoudi A Fau - Goureau, O.; Goureau O Fau - Van der Meide, P.H.; Van der Meide Ph Fau - Druet, P.; Druet P Fau - Bellon, B.; Bellon, B. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. The Journal of Immunology 1996, 1, 2209–2215. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed Pharmacother 2021. [Google Scholar] [CrossRef]
- Man, G.; Hu, L.-Z.; Elias, P.M.; Man, M.-Q. Therapeutic Benefits of Natural Ingredients for Atopic Dermatitis. Chinese journal of integrative medicine 2018, 24, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Challinor, V.L.; Parsons Pg Fau - Chap, S.; Chap S Fau - White, E.F.; White Ef Fau - Blanchfield, J.T.; Blanchfield Jt Fau - Lehmann, R.P.; Lehmann Rp Fau - De Voss, J.J.; De Voss, J.J. Steroidal saponins from the roots of Smilax sp.: structure and bioactivity. Steroids 2012, 77, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Lu, Z.-Q.; Chen, G.-T.; Zhang, J.-Q.; Wang, W.; Yang, M.; Guo, D.-A. Phenylpropanoid-Substituted Catechins and Epicatechins from Smilax china. Helvetica Chimica Acta 2007, 90, 1751–1757. [Google Scholar] [CrossRef]
- Li, Y.; Won, K.J.; Kim, D.Y.; Kim, H.B.; Kang, H.M.; Lee, S.Y.; Lee, H.A.-O. Positive Promoting Effects of Smilax China Flower Absolute on the Wound Healing/Skin Barrier Repair-Related Responses of HaCaT Human Skin Keratinocytes. Chem Biodivers 2021. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: a review. International Journal of Molecular Sciences 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kang, D.-J. Anti-Pollution Activity, Antioxidant and Anti-Inflammatory Effects of Fermented Extract from Smilax china Leaf in Macrophages and Keratinocytes. Cosmetics 2022, 9. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, M.V.P.; Tavaria, F.K. Chapter 19 - Probiotics and skin health. In Probiotics, Brandelli, A., Ed.; Academic Press: 2022; pp. 389-405.
- Lee, J.Y.; Park, J.Y.; Jeong, Y.; Kang, C.H. Anti-Inflammatory Response in TNFα/IFNγ-Induced HaCaT Keratinocytes and Probiotic Properties of Lacticaseibacillus rhamnosus MG4644, Lacticaseibacillus paracasei MG4693, and Lactococcus lactis MG5474. Journal of Microbiology and Biotechnology 2023, 28, 1039–1049. [Google Scholar] [CrossRef]
- Rusu, E.; Enache, G.; Cursaru, R.; Alexescu, A.; Radu, R.; Onila, O.; Cavallioti, T.; Rusu, F.; Posea, M.; Jinga, M.; et al. Prebiotics and probiotics in atopic dermatitis. Experimental and Therapeutic Medicine 2019, 19, 926–931. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: functions and benefits. Journal of Applied Microbiology 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin Infect Dis 2015. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Li N Fau - Wynn, J.L.; Wynn Jl Fau - Neu, J.; Neu, J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev 2009. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.; Graziani C Fau - Guarino, A.; Guarino A Fau - Lamborghini, A.; Lamborghini A Fau - Masi, S.; Masi S Fau - Stanghellini, V.; Stanghellini, V. Gelatin tannate and tyndallized probiotics: a novel approach for treatment of diarrhea. Eur Rev Med Pharmacol Sci 2017. [Google Scholar]
- Vinderola, G.; Sanders, M.E.; Cunningham, M.; Hill, C. Frequently asked questions about the ISAPP postbiotic definition. Front Microbiol 2023, 14, 1324565. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes & Nutrition 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: live and dead cells are biological response modifiers. Nutrition Research Reviews 2010, 23, 37–46. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid Based Complement Alternat Med 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Vasiljevic, T.; Smith, S.C.; Donkor, O.N. Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J Dairy Sci 2014. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz J Fau - Muñoz-Quezada, S.; Muñoz-Quezada S Fau - Gómez-Llorente, C.; Gómez-Llorente C Fau - Gil, A.; Gil, A. Probiotic mechanisms of action. Ann Nutr Metab 2012. [Google Scholar] [CrossRef]
- Ljungh, A.; Wadström, T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 2006, 7(2), 73–89. [Google Scholar] [PubMed]
- Nermes, M.; Salminen S Fau - Isolauri, E.; Isolauri, E. Is there a role for probiotics in the prevention or treatment of food allergy? Curr Allergy Asthma Rep 2013, 13(6), 622–630. [Google Scholar] [CrossRef]
- Gill, P.A.-O.; van Zelm, M.A.-O.; Muir, J.A.-O.; Gibson, P.A.-O. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther 2018, 48(1), 15–34. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol 2021, 29(8), 700–712. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.-O.; Cresci, G.A.-O. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol 2019, 11, 10:277. [Google Scholar]
- Jenab, A.; Roghanian, R.; Emtiazi, G.A.-O. Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review). Drug Des Devel Ther 2020, 18, 14:3787. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed Pharmacother, 1118. [Google Scholar]
- Kim, C.A.-O. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol 2021, 18(5), 1161–1171. [Google Scholar] [CrossRef]
- Blacher, E.A.-O.; Levy, M.; Tatirovsky, E.A.-O.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol.
- Yue, X.; Wen, S.; Long-kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunology 2022, 23, 19. [Google Scholar] [CrossRef]
- Seung-Je, L.; Eun-Gyeong, L.; Ga-Yeon, K.; Mi-Ji, J.; Sang-Yong, K.; Young-Min, K.; Geun, Y. Study of Anti-atopic Dermatitis Effects of Juice of Raphanus sativus var in HaCaT Cell Line. KSBB Journal 2017, 32, 311–318. [Google Scholar] [CrossRef]
- Yan, F.; Li, F.; Liu, J.; Ye, S.; Zhang, Y.; Jia, J.; Li, H.; Chen, D.; Mo, X. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomedicine & Pharmacotherapy 2020, 127, 110142. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Koh, P.O.; Kang, C.; Kim, E. Rosa davurica Pall. improves DNCB-induced atopic dermatitis in mice and regulated TNF-Alpa/IFN-gamma-induced skin inflammatory responses in HaCaT cells. Phytomedicine 2021, 91, 153708. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K.A.-O. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. LID - 10.3390/cells10113000 [doi] LID - 3000. Cells. [CrossRef]
- Datsi, A.A.-O.; Steinhoff, M.A.-O.; Ahmad, F.A.-O.; Alam, M.A.-O.; Buddenkotte, J.A.-O. Interleukin-31: The "itchy" cytokine in inflammation and therapy. European Journal of Allergy and Clinical Immunology 2021, 76, 2982–2997. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol 2016. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 2012. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, S.H.; Kwon, O.K.; Kim, J.H.; Oh, S.R.; Han, S.B.; Park, J.A.-O.; Ahn, K.S. Purpurin suppresses atopic dermatitis via TNF-α/IFN-γ-induced inflammation in HaCaT cells. International Journal of Immunopathology and Pharmacology 2022, 36, 03946320221111135. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hung, Y.-L.; Ko, W.-C.; Tsai, Y.-J.; Chang, J.-F.; Liang, C.-W.; Chang, D.-C.; Hung, C.-F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2017, 2, 17023. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, G.H.; Jin, S.W.; Kim, J.Y.; Hwang, Y.A.-O.; Han, E.A.-O.; Kim, Y.A.-O.; Jeong, H.A.-O. Impressic Acid Ameliorates Atopic Dermatitis-Like Skin Lesions by Inhibiting ERK1/2-Mediated Phosphorylation of NF-κB and STAT1. LID - 10.3390/ijms22052334 [doi] LID - 2334. International Journal of Molecular Sciences 2021, 26, 2334. [Google Scholar] [CrossRef]
- Karin, M.; Hunter, T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Current Biology 1995, 5, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-??B Activity. Annual review of immunology 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



