Submitted:
13 May 2024
Posted:
14 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Bronchoalveolar Lavage and AMs Extraction
2.4. Preparation of CS Extract
2.5. TH-P1 Culture and Cytotoxicity Assay
2.6. AMs Culture and CS Exposure
2.7. Biochemistry Assays and Real Time PCR (RT-PCR)
2.8. In Silico Prediction of hsa-miRs Target Genes
2.9. Statistical Analysis
3. Results
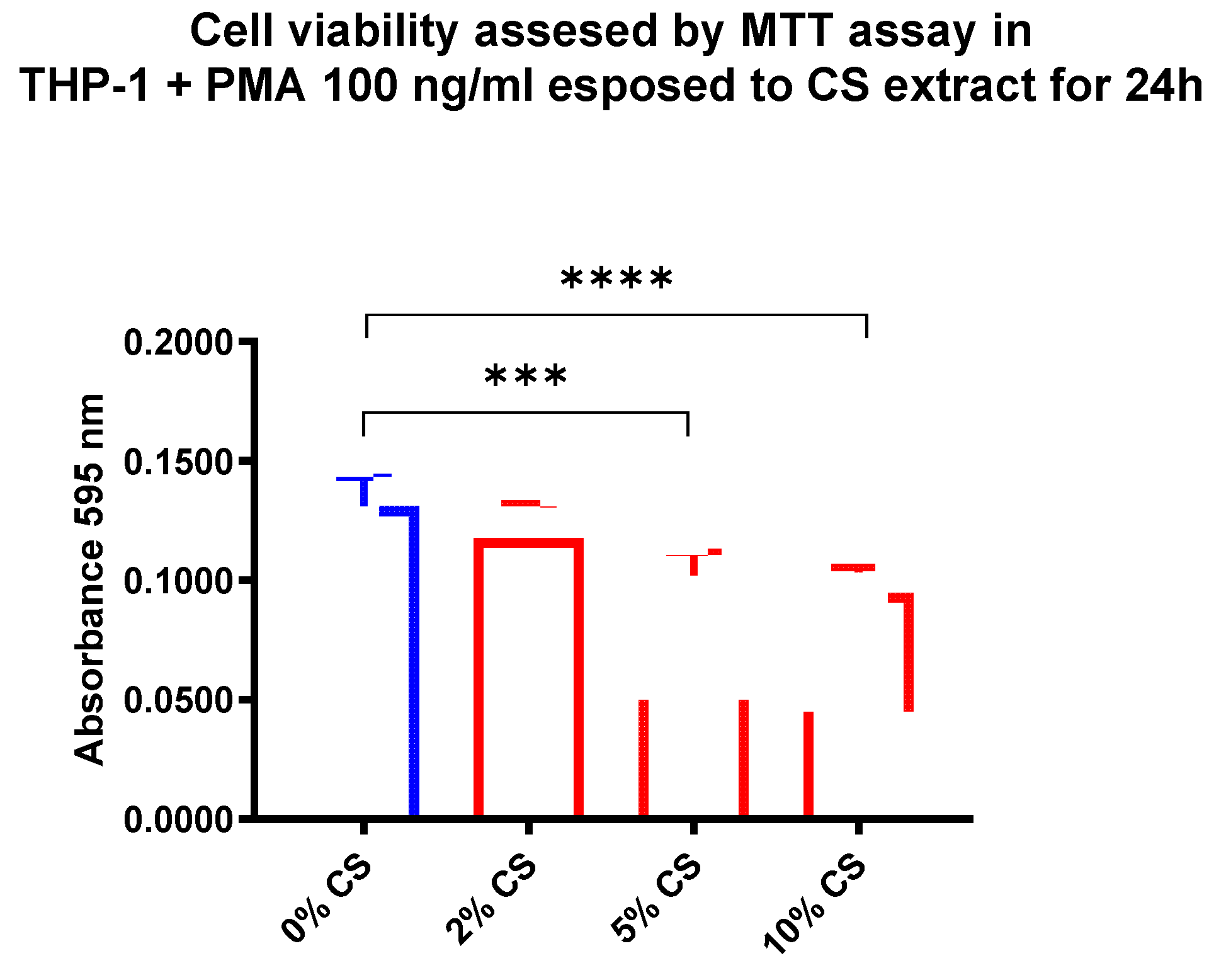
3.1. CS Effect on TH-P1 Viability
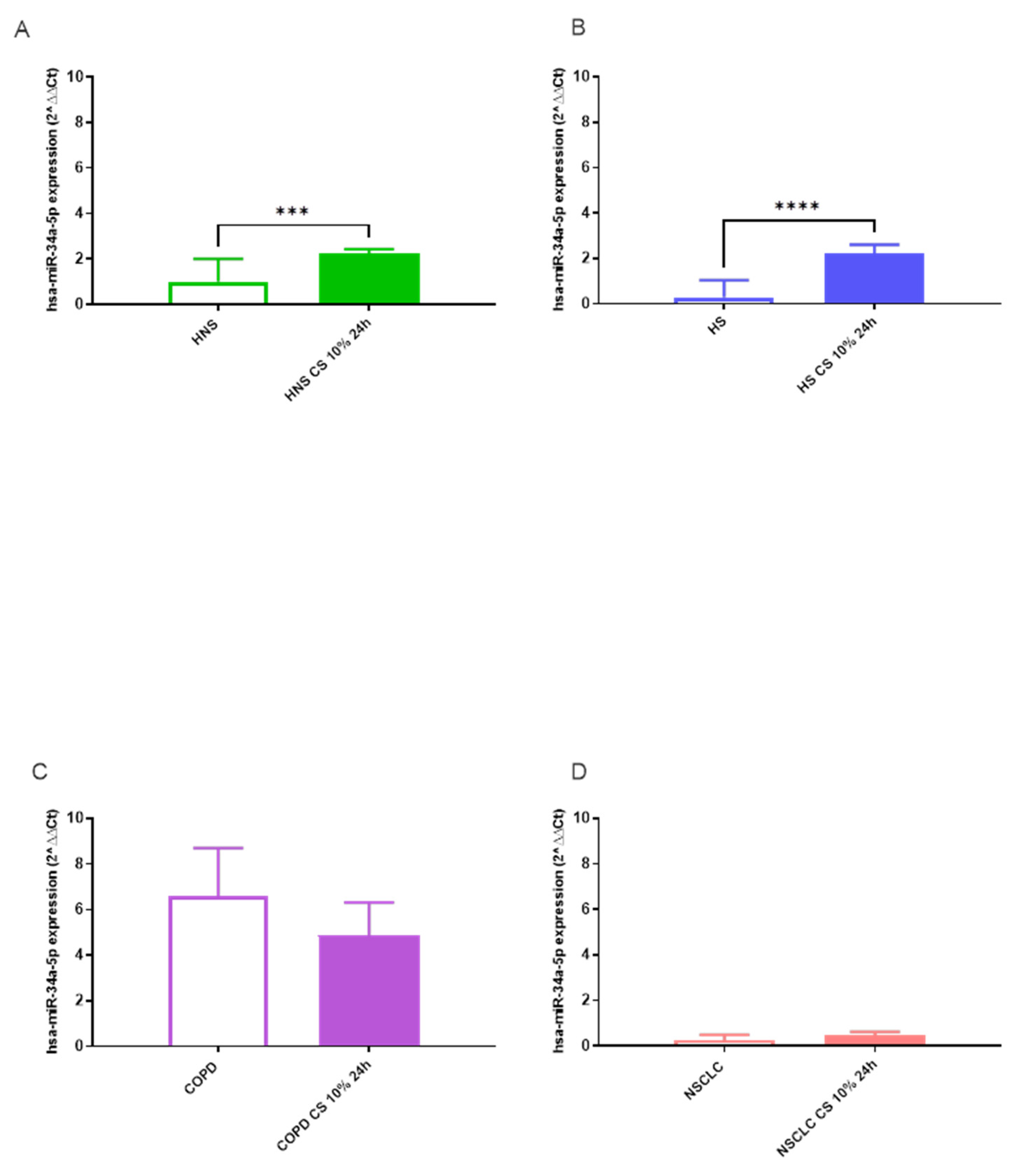
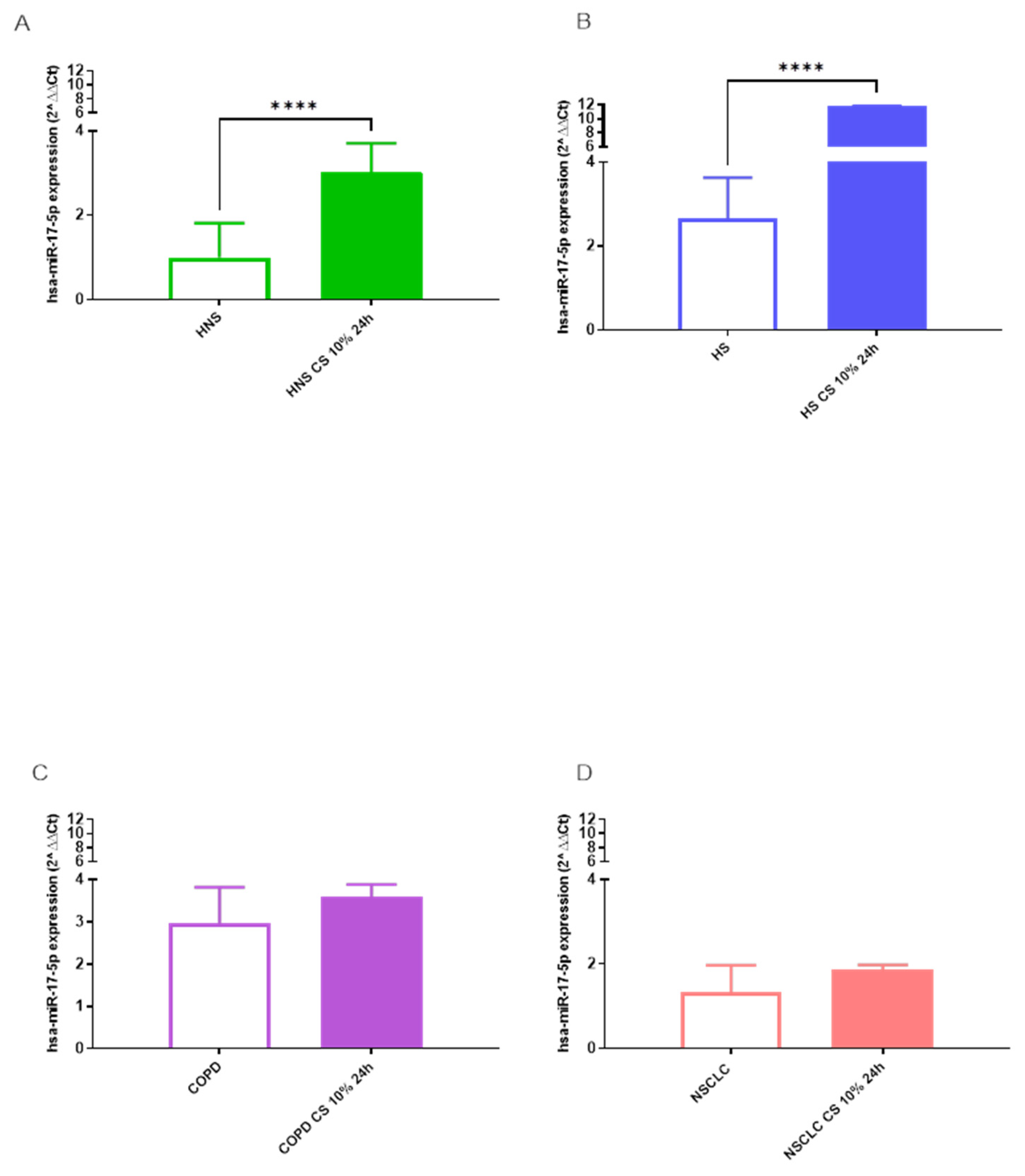
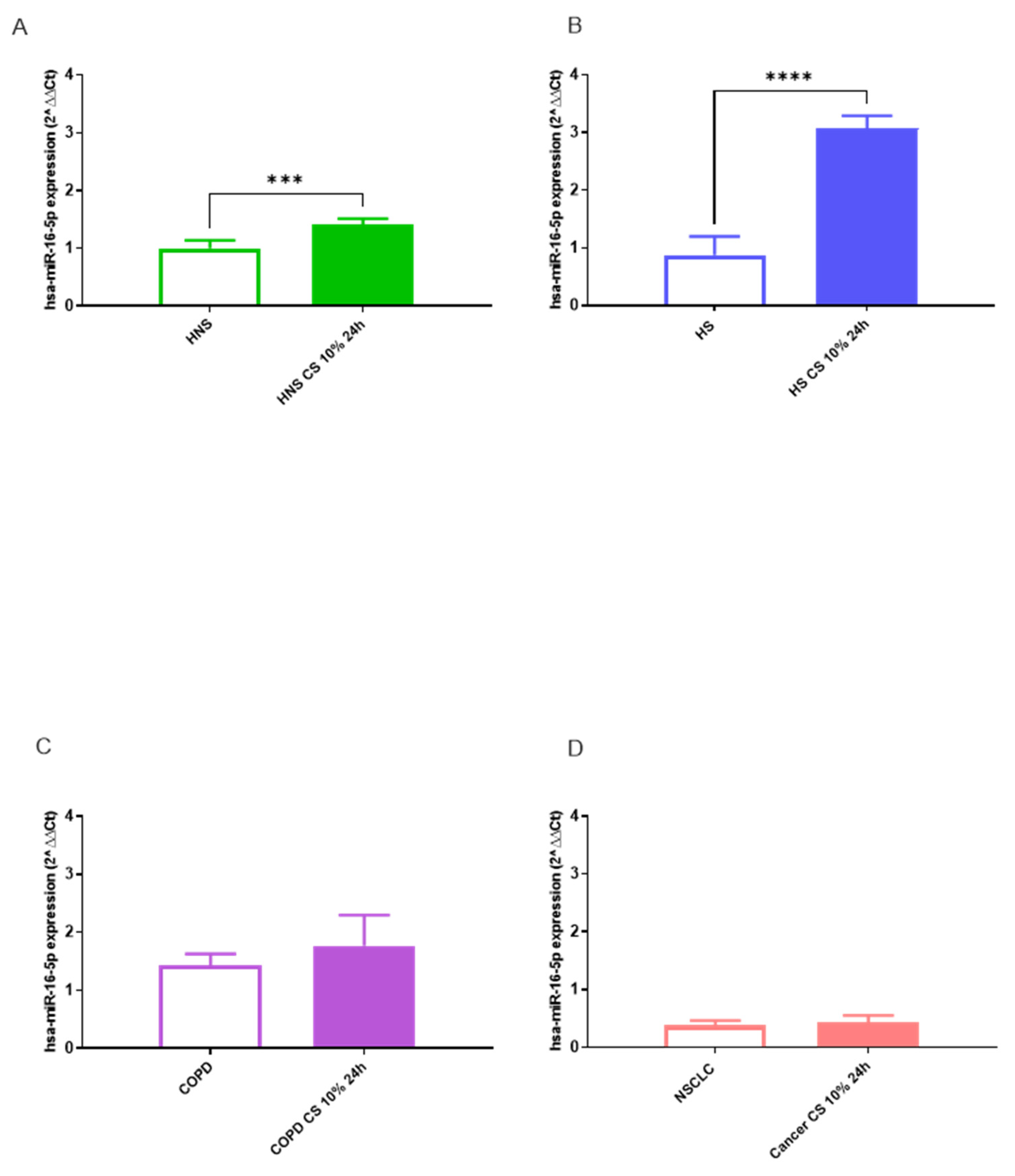
3.2. miRNA Expression Levels
hsa-miR-34a-5p, 17-5p and 16-5p
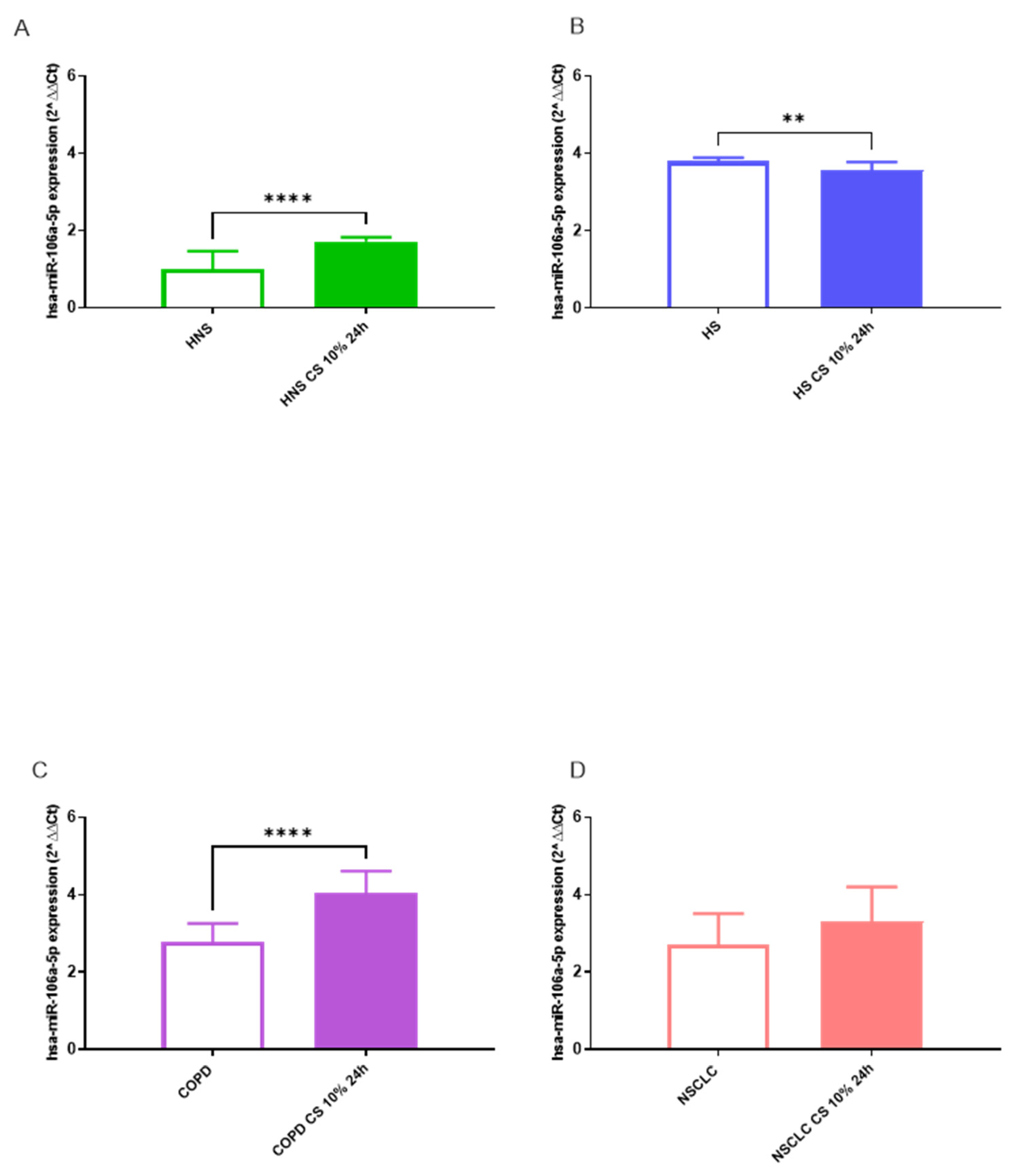
hsa-miR-106a-5p
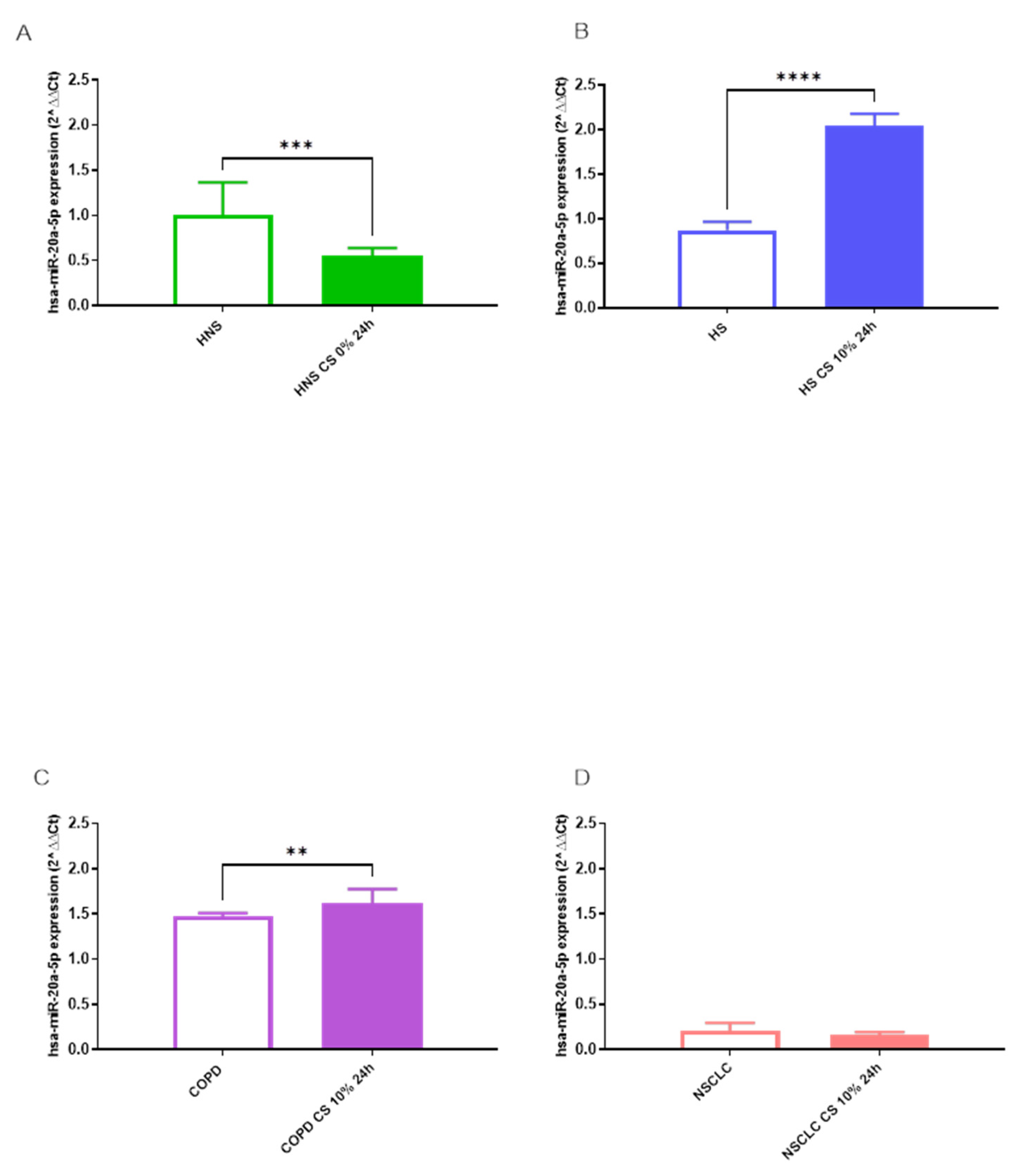
hsa-miR-223-5p and 20a-5p
3.3. In Silico Identification of Target mRNAs
| Number of Target Genes | ||
|---|---|---|
| miRNA | miR Target Link 2.0 | DIANA Tools |
| hsa-miR-223-5p | 551 | 10 |
| hsa-miR-16-5p | 2.279 | 455 |
| hsa-miR-20a-5p | 1.659 | 611 |
| hsa-miR-17-5p | 1.817 | 136 |
| hsa-miR-34a-5p | 968 | 324 |
| hsa-miR-106a-5p | 1.166 | 435 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GBD 2019 Tobacco Collaborators (2021). Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study. Lancet (London, England), 2019, 397, 2337–2360. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.G.; O’Connor, T. P.; Crystal, R. G. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. Journal of immunology 2009, 183, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R. A.; Rabe, K. F. Burden and Clinical Features of Chronic Obstructive Pulmonary Disease (COPD). The Lancet 2004, 364, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. The Role of Smoking in the Mechanisms of Development of Chronic Obstructive Pulmonary Disease and Atherosclerosis. IJMS 2023, 24, 8725. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, K.; De Angelis, A.; Spaziano, G.; Piegari, E.; Matteis, M.; Cappetta, D.; Esposito, G.; Russo, R.; Tartaglione, G.; De Palma, R.; Rossi, F.; D’Agostino, B. Intratracheal Administration of Mesenchymal Stem Cells Modulates Tachykinin System, Suppresses Airway Remodeling and Reduces Airway Hyperresponsiveness in an Animal Model. PLoS ONE 2016, 11, e0158746. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, B.; Advenier, C.; De Palma, R.; Gallelli, L.; Marrocco, G.; Abbate, G. F.; Rossi, F. The Involvement of Sensory Neuropeptides in Airway Hyper-Responsiveness in Rabbits Sensitized and Challenged to Parietaria Judaica: Sensory Neuropeptides in Airway Hyper-Responsiveness. Clinical & Experimental Allergy 2002, 32, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Roviezzo, F.; Sorrentino, R.; Terlizzi, M.; Riemma, M. A.; Iacono, V. M.; Rossi, A.; Spaziano, G.; Pinto, A.; D’Agostino, B.; Cirino, G. Toll-Like Receptor 4 Is Essential for the Expression of Sphingosine-1-Phosphate-Dependent Asthma-Like Disease in Mice. Frontiers in immunology 2017, 8, 1336. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, B.; Marrocco, G.; De Nardo, M.; Calò, G.; Guerrini, R.; Gallelli, L.; Advenier, C.; Rossi, F. Activation of the Nociceptin/Orphanin FQ Receptor Reduces Bronchoconstriction and Microvascular Leakage in a Rabbit Model of Gastroesophageal Reflux: N/OFQ Effects in the Airways in a GER Animal Model. British Journal of Pharmacology 2005, 144, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Cui, Y. Y.; D’Agostino, B.; Faisy, C.; Naline, E.; Bardou, M.; Advenier, C. Nociceptin Inhibits Airway Microvascular Leakage Induced by HCl Intra-Oesophageal Instillation: Nociceptin and Gastro-Oesophageal Reflux. British Journal of Pharmacology 2004, 141, 1077–1083. [Google Scholar] [CrossRef]
- Gallelli, L.; D’Agostino, B.; Marrocco, G.; De Rosa, G.; Filippelli, W.; Rossi, F.; Advenier, C. Role of Tachykinins in the Bronchoconstriction Induced by HCl Intraesophageal Instillation in the Rabbit. Life Sciences 2003, 72, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Young, R. P.; Hopkins, R. J. Link between COPD and Lung Cancer. Respiratory Medicine 2010, 104, 758–759. [Google Scholar] [CrossRef] [PubMed]
- Schetter, A. J.; Heegaard, N. H. H.; Harris, C. C. Inflammation and Cancer: Interweaving MicroRNA, Free Radical, Cytokine and P53 Pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Adcock, I. M.; Casolari, P.; Ito, K.; Jazrawi, E.; Tsaprouni, L.; Villetti, G.; Civelli, M.; Carnini, C.; Chung, K. F.; Barnes, P. J.; Papi, A. Unbalanced Oxidant-Induced DNA Damage and Repair in COPD: A Link towards Lung Cancer. Thorax 2011, 66, 521–527. [Google Scholar] [CrossRef]
- Schaible, A. M.; Filosa, R.; Temml, V.; Krauth, V.; Matteis, M.; Peduto, A.; Bruno, F.; Luderer, S.; Roviezzo, F.; Di Mola, A.; de Rosa, M.; D’Agostino, B.; Weinigel, C.; Barz, D.; Koeberle, A.; Pergola, C.; Schuster, D.; Werz, O. Elucidation of the Molecular Mechanism and the Efficacy in Vivo of a Novel 1,4-Benzoquinone That Inhibits 5-Lipoxygenase. Br J Pharmacol 2014, 171, 2399–2412. [Google Scholar] [CrossRef]
- Schaible, A. M.; Filosa, R.; Krauth, V.; Temml, V.; Pace, S.; Garscha, U.; Liening, S.; Weinigel, C.; Rummler, S.; Schieferdecker, S.; Nett, M.; Peduto, A.; Collarile, S.; Scuotto, M.; Roviezzo, F.; Spaziano, G.; de Rosa, M.; Stuppner, H.; Schuster, D.; D’Agostino, B.; Werz, O. The 5-Lipoxygenase Inhibitor RF-22c Potently Suppresses Leukotriene Biosynthesis in Cellulo and Blocks Bronchoconstriction and Inflammation in Vivo. Biochemical Pharmacology 2016, 112, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic effects of smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef]
- Jensen, E. J.; Pedersen, B.; Frederiksen, R.; Dahl, R. Prospective study on the effect of smoking and nicotine substitution on leucocyte blood counts and relation between blood leucocytes and lung function. Thorax, 1998, 53, 784–789. [CrossRef] [PubMed]
- Kono, Y.; Colley, T.; Masako, To.; Papaioannou, A. I.; Mercado, N.; Baker, J. R.; To, Y.; Abe, S.; Haruki, K.; Ito, K.; Barnes, P. J. Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Scientific reports, 2021, 11, 335. [CrossRef]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. International journal of cancer, 2012, 131, 2724–2732. [CrossRef]
- Mark, N. M.; Kargl, J.; Busch, S. E.; Yang, G. H. Y.; Metz, H. E.; Zhang, H.; Hubbard, J. J.; Pipavath, S. N. J.; Madtes, D. K.; Houghton, A. M. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non–Small Cell Lung Cancer. Am J Respir Crit Care Med 2018, 197, 325–336. [Google Scholar] [CrossRef]
- Punturieri, A.; Szabo, E.; Croxton, T. L.; Shapiro, S. D.; Dubinett, S. M. Lung Cancer and Chronic Obstructive Pulmonary Disease: Needs and Opportunities for Integrated Research. JNCI Journal of the National Cancer Institute 2009, 101, 554–559. [Google Scholar] [CrossRef]
- sbv IMPROVER project team (in alphabetical order), Boue, S.; Fields, B.; Hoeng, J.; Park, J.; Peitsch, M. C.; Schlage, W. K.; Talikka, M.; Challenge Best Performers (in alphabetical order), Binenbaum, I.; Bondarenko, V.; Bulgakov, O. V.; Cherkasova, V.; Diaz-Diaz, N.; Fedorova, L.; Guryanova, S.; Guzova, J.; Igorevna Koroleva, G.; Kozhemyakina, E.; Kumar, R.; Zelikman, M. Enhancement of COPD biological networks using a web-based collaboration interface. F1000Research 2015, 4, 32. [CrossRef]
- Hautamaki, R.D.; Kobayashi, D.K.; Senior, R.M.; Shapiro, S.D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science (New York, N.Y.), 1997, 277, 2002–2004. [CrossRef]
- Shapiro, S.D. COPD unwound. The New England journal of medicine, 2005, 352, 2016–2019. [CrossRef]
- Shapiro, S.D.; Ingenito, E.P. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. American journal of respiratory cell and molecular biology, 2005, 32, 367–372. [CrossRef]
- Hunninghake, G. M.; Cho, M. H.; Tesfaigzi, Y.; Soto-Quiros, M. E.; Avila, L.; Lasky-Su, J.; Stidley, C.; Melén, E.; Söderhäll, C.; Hallberg, J.; Kull, I.; Kere, J.; Svartengren, M.; Pershagen, G.; Wickman, M.; Lange, C.; Demeo, D. L.; Hersh, C. P.; Klanderman, B. J.; Raby, B. A.; Celedón, J. C. MMP12, lung function, and COPD in high-risk populations. The New England journal of medicine, 2009, 361, 2599–2608. [CrossRef]
- Bitterman, P. B.; Rennard, S. I.; Hunninghake, G. W.; Crystal, R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. The Journal of clinical investigation, 1982, 70, 806–822. [CrossRef]
- Chen, H.; Cowan, M. J.; Hasday, J. D.; Vogel, S. N.; Medvedev, A. E. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. Journal of immunology (Baltimore, Md.: 1950), 2007, 179, 6097–6106. [CrossRef]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P. N. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology, 2007, 37, 748–755. [CrossRef]
- Stämpfli, M. R.; Anderson, G. P. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nature reviews. Immunology, 2009, 9, 377–384. [CrossRef]
- Heguy, A.; O’Connor, T. P.; Luettich, K.; Worgall, S.; Cieciuch, A.; Harvey, B. G.; Hackett, N. R.; Crystal, R. G. Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. Journal of molecular medicine (Berlin, Germany), 2006, 84, 318–328. [CrossRef]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B. G.; O’Connor, T. P.; Crystal, R. G. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. Journal of immunology (Baltimore, Md.: 1950), 2009, 183, 2867–2883. [CrossRef]
- Woodruff, P. G.; Koth, L. L.; Yang, Y. H.; Rodriguez, M. W.; Favoreto, S.; Dolganov, G. M.; Paquet, A. C.; Erle, D. J. (2005). A distinctive alveolar macrophage activation state induced by cigarette smoking. American journal of respiratory and critical care medicine, 2005, 172, 1383–1392. [CrossRef]
- Lu, L.-F.; Liston, A. MicroRNA in the Immune System, MicroRNA as an Immune System. Immunology 2009, 127, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Montesanto, A.; Cione, E.; Crocco, P.; Caroleo, M. C.; Dato, S.; Rose, G.; Passarino, G. Expression Patterns of Muscle-Specific MiR-133b and MiR-206 Correlate with Nutritional Status and Sarcopenia. Nutrients 2020, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Caroleo, M. C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and MicroRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Molina-Pinelo, S.; Pastor, M. D.; Suarez, R.; Romero-Romero, B.; Gonzalez De la Pena, M.; Salinas, A.; Garcia-Carbonero, R.; De Miguel, M. J.; Rodriguez-Panadero, F.; Carnero, A.; Paz-Ares, L. MicroRNA Clusters: Dysregulation in Lung Adenocarcinoma and COPD. European Respiratory Journal 2014, 43, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Mirra, D.; Cione, E.; Spaziano, G.; Esposito, R.; Sorgenti, M.; Granato, E.; Cerqua, I.; Muraca, L.; Iovino, P.; Gallelli, L.; D’Agostino, B. Circulating MicroRNAs Expression Profile in Lung Inflammation: A Preliminary Study. JCM 2022, 11, 5446. [Google Scholar] [CrossRef]
- Mirra, D.; Esposito, R.; Spaziano, G.; Sportiello, L.; Panico, F.; Squillante, A.; Falciani, M.; Cerqua, I.; Gallelli, L.; Cione, E.; et al. MicroRNA Monitoring in Human Alveolar Macrophages from Patients with Smoking-Related Lung Diseases: A Preliminary Study. Biomedicines 2024, 12, 1050. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 Family: A Potential Tumor Suppressor and Therapeutic Candidate in Cancer. J Exp Clin Cancer Res 2019, 38, 53. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. MiR-223-5p Suppresses OTX1 to Mediate Malignant Progression of Lung Squamous Cell Carcinoma Cells. Computational and Mathematical Methods in Medicine 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, Y.; Lin, M.; Peng, X.; Liu, M.; Wang, Y.; Li, S.; Yang, D.; Yang, Z. Serum Exosomal miR -16-5p Functions as a Tumor Inhibitor and a New Biomarker for PD-L1 Inhibitor-dependent Immunotherapy in Lung Adenocarcinoma by Regulating PD-L1 Expression. Cancer Medicine 2022, 11, 2627–2643. [Google Scholar] [CrossRef]
- Ye, T.; Changyu, S.; Limeng, Z.; Yuan, P. Clinical Significance of MiRNA - 106a in Non-Small Cell Lung Cancer Patients Who Received Cisplatin Combined with Gemcitabine Chemotherapy. Cancer Biology & Medicine 2018, 15, 157. [Google Scholar] [CrossRef]
- Han, J.; Hu, J.; Sun, F.; Bian, H.; Tang, B.; Fang, X. MicroRNA-20a-5p Suppresses Tumor Angiogenesis of Non-Small Cell Lung Cancer through RRM2-Mediated PI3K/Akt Signaling Pathway. Mol Cell Biochem 2021, 476, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Qi, P.; Ma, Z. Biology of MiR-17-92 Cluster and Its Progress in Lung Cancer. Int. J. Med. Sci. 2018, 15, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Sweat, Y.; Ries, R. J.; Sweat, M.; Su, D.; Shao, F.; Eliason, S.; Amendt, B. A. MiR-17 Acts as a Tumor Suppressor by Negatively Regulating the MiR-17-92 Cluster. Molecular Therapy - Nucleic Acids 2021, 26, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, J.W., Jr.; Burgher, L.W.; Jones, F.L., Jr.; Patterson, J.R.; Selecky, P.A. Guidelines for fiberoptic bronchoscopy in adults. American Thoracic Society guidelines. Medical Section of the American Lung Association. Am. Rev. Respir. Dis. 1987, 136, 1066. [Google Scholar] [CrossRef] [PubMed]
- de Torres, J.P.; Marín, J.M.; Casanova, C.; Cote, C.; Carrizo, S.; Cordoba-Lanus, E.; Baz-Dávila, R.; Zulueta, J.J.; Aguirre-Jaime, A.; Saetta, M.; et al. Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease: Incidence and Predicting Factors. Am. J. Respir. Crit. Care Med. 2011, 184, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kang, D.; Shin, S.H.; Yoo, K.-H.; Rhee, C.K.; Suh, G.Y.; Kim, H.; Shim, Y.M.; Guallar, E.; Cho, J.; et al. Chronic Obstructive Pulmonary Disease and Lung Cancer Incidence in Never Smokers: A Cohort Study. Thorax 2020, 75, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Willinger, C. M.; Rong, J.; Tanriverdi, K.; Courchesne, P. L.; Huan, T.; Wasserman, G. A.; Lin, H.; Dupuis, J.; Joehanes, R.; Jones, M. R.; Chen, G.; Benjamin, E. J.; O’Connor, G. T.; Mizgerd, J. P.; Freedman, J. E.; Larson, M. G.; & Levy, D. MicroRNA Signature of Cigarette Smoking and Evidence for a Putative Causal Role of MicroRNAs in Smoking-Related Inflammation and Target Organ Damage. Circulation. Cardiovascular genetics 2017, 10, e001678. [CrossRef] [PubMed]
- Lofdahl, J. M. Bronchoalveolar Lavage in COPD: Fluid Recovery Correlates with the Degree of Emphysema. European Respiratory Journal 2005, 25, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Aoshiba, K.; Tamaoki, J.; Nagai, A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2001, 281, L1392–401. [Google Scholar] [CrossRef]
- Long, Y. J.; Liu, X. P.; Chen, S. S.; Zong, D. D.; Chen, Y.; Chen, P. miR-34a is involved in CSE-induced apoptosis of human pulmonary microvascular endothelial cells by targeting Notch-1 receptor protein. Respiratory research 2018, 19, 21. [Google Scholar] [CrossRef]
- Zeng, X. L.; Yang, X. N.; Liu, X. J. Resveratrol attenuates cigarette smoke extract induced cellular senescence in human airway epithelial cells by regulating the miR-34a/SIRT1/NF-κB pathway. Medicine 2022, 101, e31944. [Google Scholar] [CrossRef] [PubMed]
- Mirra, D.; Esposito, R.; Spaziano, G.; La Torre, C.; Vocca, C.; Tallarico, M.; Cione, E.; Gallelli, L.; D’Agostino, B. Lung MicroRNAs Expression in Lung Cancer and COPD: A Preliminary Study. Biomedicines 2023, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Danov, O.; Wolff, M.; Bartel, S.; Böhlen, S.; Obernolte, H.; Wronski, S.; Jonigk, D.; Hammer, B.; Kovacevic, D.; Reuter, S.; Krauss-Etschmann, S.; Sewald, K. Cigarette Smoke Affects Dendritic Cell Populations, Epithelial Barrier Function, and the Immune Response to Viral Infection With H1N1. Front. Med. 2020, 7, 571003. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A. N.; Brown, M. H. The SIRP Family of Receptors and Immune Regulation. Nat Rev Immunol 2006, 6, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Pan, C.; Li, L.; Bian, Z.; Lv, Z.; Shi, L.; Zhang, J.; Li, D.; Gu, H.; Zhang, C.-Y.; Liu, Y.; Zen, K. MicroRNA-17/20a/106a Modulate Macrophage Inflammatory Responses through Targeting Signal-Regulatory Protein α. Journal of Allergy and Clinical Immunology 2013, 132, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Lee, C.C.; Avalos, A. M.; Ploegh, H. L. Accessory molecules for Tolllike receptors and their function. Nat. Rev. Immunol.
- Moon, H. G.; Yang, J.; Zheng, Y.; Jin, Y. miR-15a/16 regulates macrophage phagocytosis after bacterial infection. Journal of immunology 2014, (Baltimore, Md.: 1950), 193, 4558–4567. [CrossRef]
- Liu, K.; Hong, D.; Zhang, F.; Li, X.; He, M.; Han, X.; Zhang, G.; Xu, G.; Stonehouse, N. J.; Jiang, Z.; An, W.; Guo, L. MicroRNA-106a Inhibits Autophagy Process and Antimicrobial Responses by Targeting ULK1, ATG7, and ATG16L1 During Mycobacterial Infection. Frontiers in immunology 2021, 11, 610021. [Google Scholar] [CrossRef] [PubMed]
- Vij, N.; Chandramani-Shivalingappa, P.; Van Westphal, C.; Hole, R. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol 2018, 314, C73-C87. [CrossRef]
- Sharma, A.; Kumar, M.; Ahmad, T.; Mabalirajan, U.; Aich, J.; Agrawal, A.; Ghosh, B. Antagonism of Mmu-Mir-106a Attenuates Asthma Features in Allergic Murine Model. Journal of Applied Physiology 2012, 113, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M. P.; Bracke, K. R.; Heijink, I. H.; Maes, T. MiR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef]
- Schembri, F.; Sridhar, S.; Perdomo, C.; Gustafson, A. M.; Zhang, X.; Ergun, A.; Lu, J.; Liu, G.; Zhang, X.; Bowers, J.; Vaziri, C.; Ott, K.; Sensinger, K.; Collins, J. J.; Brody, J. S.; Getts, R.; Lenburg, M. E.; Spira, A. MicroRNAs as Modulators of Smoking-Induced Gene Expression Changes in Human Airway Epithelium. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 2319–2324. [Google Scholar] [CrossRef]
- Lugg, S. T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D. R. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M. P.; Maes, T.; Brandsma, C. A.; van den Berge, M.; Vanaudenaerde, B. M.; Joos, G. F.; Brusselle, G. G.; Heijink, I. H.; Bracke, K. R. MiR-223 is increased in lungs of patients with COPD and modulates cigarette smoke-induced pulmonary inflammation. American journal of physiology. Lung cellular and molecular physiology 2021, 321, L1091–L1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, H.; Guo, Q. MicroRNA-20a promotes inflammation via the nuclear factor-κB signaling pathway in pediatric pneumonia. Molecular medicine reports 2018, 17, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Mirra, D.; Yang, C. X.; Esposito, R.; Spaziano, G.; Rojas-Quintero, J.; Sgambato, M.; Piegari, E.; Cozzolino, A.; Cione, E.; Gallelli, L.; Capuozzo, A.; Santoriello, C.; Berrino, L.; de-Torres, J. P.; Hackett, T. L.; Polverino, M.; D’Agostino, B. Similar Programmed Death Ligand 1 (PD-L1) Expression Profile in Patients with Mild COPD and Lung Cancer. Sci Rep 2022, 12, 22402. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Goswami, S.; Grudo, A.; Song, L.; Bandi, V.; Goodnight-White, S.; Green, L.; Hacken-Bitar, J.; Huh, J.; Bakaeen, F.; Coxson, H. O.; Cogswell, S.; Storness-Bliss, C.; Corry, D. B.; Kheradmand, F. Antielastin Autoimmunity in Tobacco Smoking–Induced Emphysema. Nat Med 2007, 13, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Laucho-Contreras, M.; Rojas Quintero, J.; Divo, M.; Pinto-Plata, V.; Sholl, L.; de-Torres, J. P.; Celli, B. R.; Owen, C. A. Increased Expression of A Proliferation-Inducing Ligand (APRIL) in Lung Leukocytes and Alveolar Epithelial Cells in COPD Patients with Non Small Cell Lung Cancer: A Possible Link between COPD and Lung Cancer? Multidiscip Respir Med 2016, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Chen, G.; Cui, Q. Towards the Understanding of MicroRNA and Environmental Factor Interactions and Their Relationships to Human Diseases. Sci Rep 2012, 2, 318. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S. W.; Choi, A. M. ; Autophagy in the lung. Proceedings of the American Thoracic Society 2010, 7, 13–21. [Google Scholar] [CrossRef]
- Zeng, H.; Li, T.; He, X.; Cai, S.; Luo, H.; Chen, P.; Chen, Y. Oxidative stress mediates the apoptosis and epigenetic modification of the Bcl-2 promoter via DNMT1 in a cigarette smoke-induced emphysema model. Respiratory research 2020, 21, 229. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Gong, J.; Yang, G.; Zhi, S.; Ren, D.; Zhao, H. Cpt1a alleviates cigarette smoke-induced chronic obstructive pulmonary disease. Experimental and therapeutic medicine 2022, 25, 54. [Google Scholar] [CrossRef]
- Xia, S.; Qu, J.; Jia, H.; He, W.; Li, J.; Zhao, L.; Mao, M.; Zhao, Y. Overexpression of Forkhead box C1 attenuates oxidative stress, inflammation and apoptosis in chronic obstructive pulmonary disease. Life sciences 2019, 216, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Dentener, M. A.; Vernooy, J. H.; Hendriks, S.; Wouters, E. F. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax 2005, 60, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hlapčić, I.; Hulina-Tomašković, A.; Grdić Rajković, M.; Popović-Grle, S.; Vukić Dugac, A.; Rumora, L. Association of Plasma Heat Shock Protein 70 with Disease Severity, Smoking and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. Journal of clinical medicine 2020, 9, 3097. [Google Scholar] [CrossRef]
- Hayama, Y.; Kimura, T.; Takeda, Y.; Nada, S.; Koyama, S.; Takamatsu, H.; Kang, S.; Ito, D.; Maeda, Y.; Nishide, M.; Nojima, S.; Sarashina-Kida, H.; Hosokawa, T.; Kinehara, Y.; Kato, Y.; Nakatani, T.; Nakanishi, Y.; Tsuda, T.; Koba, T.; Okada, M.; Kumanogoh, A. Lysosomal Protein Lamtor1 Controls Innate Immune Responses via Nuclear Translocation of Transcription Factor EB. Journal of immunology 2018, (Baltimore, Md.: 1950), 200, 3790–3800. [CrossRef]
- Bewley, M. A.; Preston, J. A.; Mohasin, M.; Marriott, H. M.; Budd, R. C.; Swales, J.; Collini, P.; Greaves, D. R.; Craig, R. W.; Brightling, C. E.; Donnelly, L. E.; Barnes, P. J.; Singh, D.; Shapiro, S. D.; Whyte, M. K. B.; Dockrell, D. H. Impaired Mitochondrial Microbicidal Responses in Chronic Obstructive Pulmonary Disease Macrophages. American journal of respiratory and critical care medicine 2017, 196, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Gregory, A. D.; Pérez Verdaguer, M.; Ware, S. A.; Harvey, H.; DeVallance, E.; Brzoska, T.; Sundd, P.; Zhang, Y.; Sciurba, F. C.; Shapiro, S. D.; Kaufman, B. A. Extracellular Release of Mitochondrial DNA: Triggered by Cigarette Smoke and Detected in COPD. Cells 2022, 11, 369. [Google Scholar] [CrossRef]
- Murray, R. Z.; Stow, J. L. Cytokine Secretion in Macrophages: SNAREs, Rabs, and Membrane Trafficking. Frontiers in immunology, 2014, 5, 538. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, D.; Zhan, W.; He, K.; Yang, H. (2020). Downregulation of SENP1 suppresses LPS-induced macrophage inflammation by elevating Sp3 SUMOylation and disturbing Sp3-NF-κB interaction. American journal of translational research 2020, 12, 7439–7448. [Google Scholar]
- Diao, X.; Zhou, J.; Wang, S.; Ma, X. Upregulation of miR-132 contributes to the pathophysiology of COPD via targeting SOCS5. Experimental and molecular pathology, 2018, 105, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R. K.; Chinnapaiyan, S.; Rasmussen, L.; Raju, S. V.; Unwalla, H. J. (2019). A Neutralizing Aptamer to TGFBR2 and miR-145 Antagonism Rescue Cigarette Smoke- and TGF-β-Mediated CFTR Expression. Molecular therapy: the journal of the American Society of Gene Therapy, 2019 27, 442–455. [CrossRef]
- Michaud, S. E.; Dussault, S.; Groleau, J.; Haddad, P.; Rivard, A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. Journal of molecular and cellular cardiology 2006, 41, 275–284. [Google Scholar] [CrossRef]

| Abbreviation | Gene Name | Methods | Tissues | References (PMID) |
|---|---|---|---|---|
| ATG14 | Autophagy Related 14 | Sequencing, HITS-CLIP |
Embryonic kidney cells, B cells |
20371350 22473208 |
| BCL2 | BCL2 Apoptosis Regulator | Luciferase reporter assay, qRT-PCR, Western blot, Proteomics analysis, Immunohistochemistry, Microarray, Sequencing, HITS-CLIP, Immunoblot, Immunoprecipitaion | Cervix cells, gastric cells, bone cells, marrow cells, spleen, liver, kidney, lymph node, tracheal/bronchial epithelial cells, breast cells, ovary cells, human embryonic kidney cells, B cells, mesothelial cell, glioma cells |
17877811 18449891 18362358 17351108 17707831 20643754 20876285 19269153 16166262 19903841 20371350 23907579 22473208 24148817 25435430 26397135 26722459 |
| CPT1A | Carnitine Palmitoyltransferase 1A | Proteomics HITS-CLIP |
Cervix cells, neuronal cells |
18668040 23313552 |
| FOXC1 | Forkhead Box C1 | PAR-CLIP | Human embryonic kidney cells | 21572407 |
| HAS2 | Hyaluronan Synthase 2 | HITS-CLIP | B-cell | 22927820 |
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | Microarray pSILAC, Proteomics, PAR-CLIP | Leukemic cells, cervix cells, human embryonic stem cells |
18362358 18668040 22012620 |
| LAMTOR1 | Late Endosomal/Lysosomal Adaptor, MAPK And MTOR Activator 1 | Proteomics, PAR-CLIP |
Cervix cells, brain tissue human embryonic kidney cells, B cells |
18668040 24398324 23446348 20371350 |
| MCL1 | MCL1 Apoptosis Regulator, BCL2 Family Member | HITS-CLIP, microarray, Immunohistochemistry, Luciferase reporter assay, qRT-PCR, Western blot, PCR array | Human embryonic kidney cells, leukemic cells, liver | 22473208 18362358 23594563 28097098 |
| MFN2 | Mitofusin 2 | Proteomics, luciferase reporter assay, western blot, CLASH | Breast cells, lungs, human embryonic kidney cells | 18668040 27640178 23622248 |
| SCAMP5 | Secretory Carrier Membrane Protein 5 | HITS-CLIP | B cells | 22473208 22473208 |
| SENP1 | SUMO Specific Peptidase 1 | HITS-CLIP, PAR-CLIP |
Human embryonic kidney cells | 22473208 20371350 21572407 |
| SOCS5 | Suppressor Of Cytokine Signaling 5 | CLASH, PAR-CLIP |
Human embryonic kidney cells, peripheral blood mononuclear cells, macrophages, brain tissue |
23622248 23592263 23446348 |
| TGFBR2 | Transforming Growth Factor Beta Receptor 2 | Immunoblot, Luciferase reporter assay, Microarray, qRT-PCR, Western blot HITS-CLIP PAR-CLIP Immunohistochemistry, In situ hybridization |
Colorectal cancer cells, umbilical cord blood cell, B cells, human embryonic stem cells, human embryonic kidney cells, B cells, epithelial cells of the small and large intestines, esophageal cells |
20940405 19435428 22473208 22012620 21572407 20371350 27080303 27508097 26729221 |
| VEGFA | Vascular Endothelial Growth Factor A | ELISA, Luciferase reporter assay | Kidney cells | 18320040 |
| Biochemical Pathways | miRNA | Validated target genes |
|---|---|---|
| Autophagy- adaptive immune response regulation | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p |
ATG14 |
| Apoptosis- ROS production | hsa-miR-16-5p hsa-miR-17-5p hsa-miR-20a-5p hsa-miR-34a-5p |
BCL2 |
| Apoptosis- inflammatory response regulation | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p hsa-miR-106a-5p |
CPT1A |
| Oxidative stress-inflammation responses- cell apoptosis | hsa-miR-20a-5p hsa-miR-17-5p hsa-miR-223-5p |
FOXC1 |
| Cytokines, chemokines, and matrix metalloproteinase production | hsa-miR-20a-5p hsa-miR-17-5p hsa-miR-106a-5p |
HAS2 |
| Protein folding- prevention of protein aggregation - apoptosis | hsa-miR-16-5p hsa-miR-34a-5p hsa-miR-223-5p |
HSPA1A |
| Macrophages polarization-innate immune response regulation | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p |
LAMTOR1 |
| Apoptosis- bacterial clearance | hsa-miR-16-5p hsa-miR-17-5p hsa-miR-20a-5p hsa-miR-34a-5p |
MCL1 |
| Mitochondrial fusion- mitochondrial membranes regulation | hsa-miR-16-5p hsa-miR-17-5p hsa-miR-34a-5p hsa-miR-106a-5p |
MFN2 |
| TNF secretory pathway | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p |
SCAMP5 |
| Cytokines secretion- NF-κB pathway | hsa-miR-20a-5p hsa-miR-16-5p hsa-miR-34a-5p hsa-miR-223-5p |
SENP1 |
| EGFR signaling pathway | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p |
SOCS5 |
| TGF-β signaling pathway | hsa-miR-20a-5p hsa-miR-17-5p hsa-miR-34a-5p |
TGFBR2 |
| VEGF pathway- ROS generation- Akt/eNOS/NO pathway | hsa-miR-16-5p hsa-miR-20a-5p hsa-miR-17-5p |
VEGFA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





